Abstract
Biomarkers, especially biochemical markers, are important in osteoarthritis (OA) research, clinical trials, and drug development and have potential for more extensive use in therapeutic monitoring. However, they have not yet had any significant impact on disease diagnosis and follow-up in a clinical context. Nevertheless, the development of immunoassays for the detection and measurement of biochemical markers in OA research and therapy is an active area of research and development. The evaluation of biochemical markers representing low-grade inflammation or extracellular matrix turnover may permit OA prognosis and expedite the development of personalized treatment tailored to fit particular disease severities. However, currently detection methods have failed to overcome specific hurdles such as low biochemical marker concentrations, patient-specific variation, and limited utility of single biochemical markers for definitive characterization of disease status. These challenges require new and innovative approaches for development of detection and quantification systems that incorporate clinically relevant biochemical marker panels. Emerging platforms and technologies that are already on the way to implementation in routine diagnostics and monitoring of other diseases could potentially serve as good technological and strategic examples for better assessment of OA. State-of-the-art technologies such as advanced multiplex assays, enhanced immunoassays, and biosensors ensure simultaneous screening of a range of biochemical marker targets, the expansion of detection limits, low costs, and rapid analysis. This paper explores the implementation of such technologies in OA research and therapy. Application of novel immunoassay-based technologies may shed light on poorly understood mechanisms in disease pathogenesis and lead to the development of clinically relevant biochemical marker panels. More sensitive and specific biochemical marker immunodetection will complement imaging biomarkers and ensure evidence-based comparisons of intervention efficacy. We discuss the challenges hindering the development, testing, and implementation of new OA biochemical marker assays utilizing emerging multiplexing technologies and biosensors.
Keywords: osteoarthritis (OA), biochemical marker, multiplexing technologies, biosensors, nanotechnology, immunodetection, magnetic resonance imaging (MRI)
Introduction
Osteoarthritis (OA) is the most common form of joint disease and a major cause of pain and chronic disability in older individuals (1). Although OA is primarily associated with aging in the human population (2), there are other key contributing risk factors, including obesity, gender, a history of joint trauma or repetitive use, genetics, heritable metabolic disorders, muscle weakness, underlying anatomical and orthopedic disorders (i.e., congenital hip dislocation), previous joint infection, crystal deposition (i.e., gout), previous rheumatoid arthritis (RA), and various disorders of bone turnover and blood clotting (3).
Biomarkers have been defined in the literature with considerable overlap (4). In general, biomarkers are any quantifiable measurement that can be objectively assessed as an indicator of a biological process, including anatomic, physiologic, biochemical, or molecular parameters. These markers can be associated not only with the presence and severity of specific diseases but also the effects of medical treatments and interventions. The use of laboratory-based biochemical markers in clinical settings is relatively new, and the best strategies to this application are still being developed at medical research level and as well at technological level (i.e., development of reliable detection methods).
Currently, there are no reliable, quantifiable, and easily measured biochemical markers capable of providing an earlier diagnosis of OA, inform on the prognosis of OA disease, and monitor responses to emerging therapeutic modalities (5). The evaluation of structural changes in articular damage via imaging biomarkers [as determined by radiograph or magnetic resonance imaging (MRI)] is the most frequently used in clinical trials to evaluate subject eligibility, and/or efficacy of intervention, supporting decision making in OA drug development by ascertaining treatment effects on joint structure (6). The European League Against Rheumatism (EULAR) has formulated a set of guidelines for imaging applications for the clinical management of OA in peripheral joints (7). However, radiography seems inadequate in the case of OA, as the utility for objective clinical picture evaluation is limited, and in most of the cases, there is no correlation between radiographical and clinical features, especially during early OA stages. This is an example of how radiographic imaging has hampered OA research endeavors (8, 9). MRI thanks to its high sensitivity in showing all involved joint tissues that are clinically relevant at a much earlier disease stage is gaining greater recognition. The importance of MRI in OA diagnosis and prognosis is increasingly emphasized (10–12). However, early detection of structural changes in the joint by MRI does not necessarily serve as an indicator of the existence of clinically defined OA, especially in the absence of symptoms. The potential outcome domains to assess for early OA include patient reported outcomes, features of clinical examination, objective measures of physical function and pain, levels of physical activity, imaging, and biochemical markers in body fluids.
In addition to imaging biomarkers, biochemical markers of joint tissue turnover have the capacity to reflect disease-relevant biological activity and provide useful diagnostic and therapeutic information, enabling a more rational and personalized approach to healthcare management (13). Expert consensus groups have proposed a generally accepted classification of OA biochemical markers according to the disease pathogenesis (14, 15). They include markers of cartilage, bone, and synovial metabolism, which comprise several collagenous proteins, their epitopes and cleavage peptides, various enzymes and non-collagenous proteins, as well as markers of low-grade inflammation: cytokines, chemokines, lipid mediators, and other biochemicals (15). The ratio between the synthesis and breakdown of both articular cartilage and bone can provide an insight into the underlying pathological processes involved in OA, albeit this process may be detected only in late stages of disease pathogenesis. The importance of inflammatory response in OA has also been increasingly highlighted during the past decade, shifting the focus of research investigations on the correlation between biochemical markers of “low-grade inflammation” and disease progression, especially in the context of emerging inflammatory endotypes and phenotypes of OA (16).
The complex evaluation of biochemical markers of extracellular matrix (ECM) turnover, low-grade inflammation, and other biological processes may lead to more specific evaluation of the catabolic and inflammatory aspects of OA. However, most of the studies carried out with biochemical markers to date have focused on late stages of the disease in humans or animal models. Studies in early stages of OA are rare due to the lack of biochemical markers that permit detection of early OA stages.
Early OA refers to the earliest disease stage characterized by emerging clinical symptoms. Early OA does not have a mutually agreed-upon definition, but a group of specialists have proposed a draft classification based on the existence of patient-reported symptoms, clinical examination findings, and minimal to no radiographic signs (17). The definition of early OA is still evolving, and there are ongoing efforts to move toward a consensus definition. Although there is no consensus on an internationally accepted classification, it is generally agreed that identification of the early stages would enable the development of new therapeutics, allowing more targeted and efficacious clinical intervention. However, this requires establishing a clear link between molecular changes in the preradiographic stages, before the onset of clinical and radiographic manifestation of OA (18). Biomarkers indicative of early disease processes and the criteria by which OA is stratified into “early clinical” and “early OA” stages require validation by extensive longitudinal studies that take into account different risk factors, patient subgroups, and types of affected joints (18).
At the present time, none of the currently available biochemical markers are sufficiently capable of discriminating OA diagnosis or prognosis in individuals or provide a consistent outcome measure in OA clinical trials (19). The need for stratification of distinct OA subtypes (the so-called clinical phenotypes) has been recently highlighted (20). This relates to the need for gaining a better understanding of the pathogenesis of OA, the identification of molecular endotypes, and the prospect of developing personalized treatments. Hence, stratifying OA biochemical markers for the detection of molecular endotypes and the enhanced definition of the clinical phenotypes will inevitably expedite the development of personalized OA treatment approaches, as proposed recently (11, 21, 22).
The presence of inflammation has been recognized as a leading component of different OA subtypes (23). Contrast-enhanced MRI of pre-radiographic OA joints revealed that presence of moderate synovitis is very common in knees with clinical OA and is also commonly found in joints without exhibiting concomitant joint effusion (24). Systemic inflammatory mediators, adipokines, released by the adipose tissue, are also involved in the pathogenesis of OA (25), which suggests the convergence, overlap, and interaction of these phenotypes, especially in older individuals with multiple comorbidities.
The inability to assess changes in the joint in the early stages of OA is a major obstacle to making further progress in OA diagnosis and prognosis. Moreover, this stagnancy in biochemical marker development is hampering drug development and thwarting efforts to identify the disease early and mitigate its huge socioeconomic impacts. Therefore, there is an acute need to establish biochemical marker panels or methods for efficient detection to facilitate earlier diagnosis of OA, inform OA prognosis, and monitor therapeutic efficacy of the disease (16, 26). The development of biochemical marker immunoassays has been an ongoing area of activity in OA research and clinical development, but it is still far from being incorporated into a unified framework for disease characterization.
The present article reviews the relevant literature and outlines the main challenges faced by the scientific, medical, and engineering communities in the establishment of the relationship between biochemical markers and OA, as well as the development and design of emerging immunodetection methods such as multiplexing technologies, biosensors, and nanotechnology platforms.
Biochemical Markers for OA Diagnosis and Prognosis
The currently accepted classification criteria for OA biochemical markers developed by the OA Biomarkers Network has assigned biomarkers into five categories, including burden of disease, investigative, prognostic, efficacy of intervention, diagnostic, and safety (BIPEDs) (27, 28). This categorization provides a framework to describe the potential application of biochemical markers as tools for the early identification of the disease, differentiation of patients based on the extent of disease severity, pre-symptomatic identification of individuals with the disease, or a clinical endpoint used to determine the efficacy of treatment. Biochemical marker detection in serum or urine samples is the least invasive procedure, therefore standing in the first line of clinical interest, while the synovial fluid (SF) biochemical markers are expected to be more reflecting local processes in the joint but have gained more traction in recent years with the increased developmental focus on intra-articular therapies. Although a series of different biochemical markers were assessed for their potential utility, only minimal success was achieved in their clinical validation, highlighting the need for new biochemical markers, representative of joint tissue damage or even linked to particular joints (22, 29). Nevertheless, the major challenge for OA drug development remains the lack of biochemical markers indicating the efficacy of treatment and OA progression (30).
The establishment of normal range intervals of well-phenotyped age-matched controls for 18 separate biochemical markers that were used by the Foundation for the National Institutes of Health/Osteoarthritis Research Society International (FNIH/OARSI) consortium project was an important step in the development of biochemical marker for the diagnosis of OA (31). However, even though some of these biochemical markers showed different distributions between OA subjects and non-OA controls, the overlap of concentration values remains a problem for diagnostic and prognostic applications, as well as the differences between reference intervals based on race, gender, and age, which might necessitate further research into factors that might affect biochemical marker concentrations (31). The variables in baseline levels between individuals pose a significant challenge for biochemical marker development, especially since OA pathogenesis does not embody a singular etiological trajectory, as previously indicated (i.e., inflammatory, bone, metabolic, or age).
Biochemical Markers of Cartilage Turnover in OA
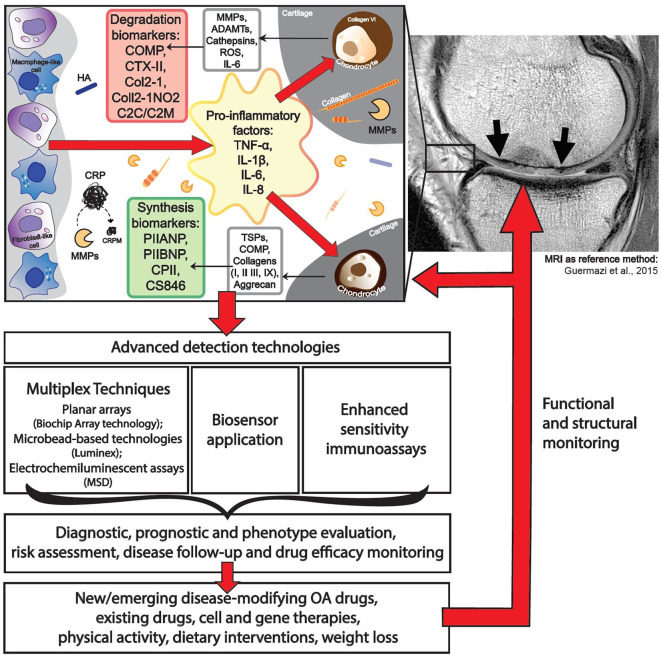
Most OA biochemical markers are dedicated to characterizing cartilage turnover. The most commonly investigated biochemical markers includes the following: ECM degradation—CTX-II, Coll2-1, C2C, C2M, Coll2-1NO2, cartilage oligomeric matrix protein (COMP), aggrecan epitopes (ARGS, TEGE, FFGV), fibulin-3 epitopes (Fib3-1, Fib3-2, Fib3-3), etc.; ECM synthesis—PIIANP, PIIBNP, CPII, CS846, and many others (32) (Figure 1). Some of those cartilage metabolism biochemical markers have gained recognition in the field. For instance, urinary CTX-II (C-telopeptide fragments of type II collagen) is one of the better-known OA biochemical markers that has achieved a superior predictive profile when compared to others (33). Both urine and SF CTX-II were found to be associated with radiographic severity (34), while urine CTX-II was associated with pain in patients with early OA (35).
Figure 1.
Application of advanced technologies for immunodetection of osteoarthritis biomarkers.
Another potential biochemical marker widely reported in the OA literature is COMP. Serum COMP is a biochemical marker that has been used in numerous studies because of its ability to distinguish between healthy subjects and OA patients. Furthermore, in the case of COMP, possible prognostic capabilities have been observed (36). Although some studies reported conflicting results, a meta-analysis showed that these biochemical markers (i.e., COMP and CTX-II) could be effective for OA diagnosis and prognosis of progression and differentiation between healthy groups and individuals affected with OA (37). Variation in COMP levels seems more pronounced in SF samples, as compared to serum levels (36). In addition, levels of COMP in SF showed a strong correlation with OA severity, while only a weak inverse correlation to serum COMP levels was found, reinforcing that SF samples are strongly reflective of COMP as an OA biochemical marker (36). Levels of COMP in SF, specifically intact forms of the molecule, were found to be higher in OA patients than in those suffering from other articular disorders, like RA, reactive arthritis, or acute trauma (38). As such, intact COMP appears to better associate with slow progressing and chronic joint pathogenesis, rather than acute proteolytic processes that are associated with severe inflammation in other arthritides, which cause COMP degradation and cleavage. This also highlights the fact that neo-epitopes from the same protein could be generated in diverse manners and thus serve as indicators of various pathological mechanisms related to distinct diseases or disease subtypes (39). Such phenomena should be considered when designing OA analysis platforms, as appropriate antibody selection (with respect to targeted regions) could potentially be linked to better pathogenetic profile validation.
Biochemical Markers of Low-Grade Inflammation in OA
The most recent definition of OA describes it as disease characterized by low-grade inflammation, distinguishing it from other inflammatory joint diseases. Identification of biochemical markers of low-grade inflammation may identify subtypes of OA, thus contributing to the choice of treatment strategies, particularly with non-steroidal anti-inflammatory drugs, which currently remains among the primary options from the scarce choice of clinically relevant therapies for OA. Notable inflammation-related biochemical markers of OA include CRP, tumor necrosis factor-α (TNF-α), IL-6, and IL-1β, recurring in multiple study reports and systematic reviews (15, 22, 29). The concentrations of these biochemical markers are low, especially compared with other inflammatory diseases of the joint, with TNF-α, IL-6, and IL-1β levels detected at picogram-scale quantities (40), making it challenging to detect their fluctuations in the circulation. For example, CRP levels are consistently elevated in OA patients, with modest variations in its levels among various studies (41). CRP levels were shown to differ between OA patients and non-OA controls and correlated with symptoms of OA, such as pain and function loss; however, these were not found to reflect radiographic findings such as joint space narrowing and KL scores (41). Measurement of hsCRP is a routine laboratory test and the gold standard for evaluation of inflammation (15), making it a relatively translational and accessible transition to OA diagnosis. It is important to note that more attention should be given to known confounding factors of CRP such as body mass index (BMI), race, and gender when interpreting data (42–44). Distinct from full-length CRP are fragments derived from CRP, such as CRPM, which are generated by endopeptidases, such as matrix metalloproteinases (MMPs), which are activated during OA. Indeed, CRPM has been evaluated in RA (45), axial spondyloarthritis (46), and OA (47–49) as a marker of inflammation. The Rotterdam cohort study revealed for the first time that CRPM predicts the risk of OA progression independently of the established biochemical markers uCTX-II and COMP (50).
Elevated levels of IL-1β, IL-5, IL-6, IL-10, IL-13, and TNF-α have been observed in the plasma of patients with knee OA, as compared to SF samples showing unchanged levels (27). Authors of the study attribute either the fluctuation in cytokines in the circulation to the systemic nature of OA or the enhanced permeability of the synovial membrane (40). Similarly, serum TNF-α concentrations were shown to be predictive for radiographic knee OA progression (41) and together with IL-6, were indicative of cartilage loss and joint space narrowing (51). On the other hand, increased SF IL-6, IL-8, and TNF-α levels were associated with pain during movement, while only TNF-α correlated with pain at rest (34).
The selection of inflammatory biochemical markers for OA assessment remains challenging and inconsistent and depends on the purpose, hypothesis, and concept of each study. As there is no consensus on the parameters for the evaluation of low-grade inflammatory activity in OA, a combination of inflammatory and cartilage turnover biochemical markers, in addition to imaging biomarkers, may be the best choice to characterize OA (see section Choice of the Reference Method for Biochemical Marker Detection).
Emerging Exploratory and Investigative Biochemical Markers of OA: From Mice to Large Animal Models of OA
As previously stated, there is a lack of novel biochemical markers to predict OA clinical status. Moreover, fewer biochemical marker candidates enable clinical decision making about the efficacy of various therapies. The current situation requires pairing between investigative knowledge regarding the disease pathogenesis and the utility of this knowledge to develop novel biochemical markers and therapies.
Searching the current literature for the following phrases including “investigative biomarkers” “osteoarthritis,” and “mice” showed 48 results. Among some of the reports, higher serum levels of FGF21 were displayed in an ACL model of mice, which appeared to be markedly higher in adipsin-deficient mice (52). Another interesting therapeutic target is transglutaminase (TG2), which was shown in various preclinical models to mediate chondrocyte hypertrophy and interleukin-1-induced calcification (53–56). As such, TG2 protein levels in synovial fluid protein have been reported to correlate with the histological grade of OA (57). As the activity of TG2 may be altered in non-OA-related synovitis, better characterization of its levels and bioavailability are needed to assess its potential utility as an OA biochemical marker for human subjects (58). MMP3, an enzyme that degrades proteoglycans and collagens, was associated with early structural OA changes in STR/ort mice (59), which are consistent with human studies showing that its levels correlate with joint width narrowing (60). Another catabolic matrix modifier, cathepsin B, was shown to display increased activity in synovial fluid in various studies (61, 62) and could be paired with imaging of the joint using activity-based probes. Mechanistically, cathepsin B is linked with Sirt1 cleavage and inactivation (63), as well as collagen degradation in cartilage ECM (64). Recently, fragments of Sirt1 were shown to be predictive of chondrosenescence and OA severity using an enzyme-linked immunosorbent assay (ELISA)-based method (65), linking the loss of SIRT1 activity in OA cartilage to its emergence in serum. Lipid profiles in plasma are also reported as biochemical markers of pain and cartilage destruction using the DMM mice models (66). In particular, Pousinis and colleagues, reported six lipid species that increased during posttraumatic OA, identified as cholesterol esters-CE(18:2), CE(20:4), CE(22:6), phosphocholine-PC(18:0/18:2), PC(38:7), and sphingomyelins-SM(d34:1) (66). Phospholipid species were also shown to be increased in human OA plasma (67, 68), indicating that these lipid species may undergo aberrant biosynthesis during OA pathogenesis. The study of lipid mediators as potential marker of OA is an important and promising area of research that highlights the potential for using lipidomics in future studies. However, at the present time, there are no standardized low-cost platforms and easy-to-perform assays for identifying a wide-range of lipid biomarkers.
The use of mouse models for biomarker screening is often advantageous over large animals due to its capacity to undergo skeletal maturation or develop OA more rapidly than larger animals. Moreover, the abundance of genetic mouse models for the validation of pathways and biomarkers related to OA pathophysiology make them good cost-efficient models for initial screening. Indeed, there are many novel findings related to biomarker discovery in mouse models as compared to those available in large animals. Conversely, the use of large animals is justified because they are translational models due to their physiological and anatomical biomechanical similarity to load-bearing human joints and is documented mostly in dogs, sheep, and horses, which exhibit naturally occurring OA (69). When assessing dogs undergoing surgical induction of OA, a significant decrease in TIMP-2 in SF and serum is reported, while MMP-2 was elevated in SF during the progression of OA (70). Coll2-1 and Coll2-1 NO2 were correlated with OA changes in the canine ACLT model (71), consistent with the decrease in these serum fragments after HA intra-articular administration to OA patients in a separate study (72).
Assessment of spatial changes in serum proinflammatory cytokines and ECM-related biomarkers was reported for a 4-year training period of posttraumatic OA racehorses. While COMP and CTX-II showed an early increase in serum already at year 2 of training, IL-1β, TNF-α, and IL-6 were mostly elevated at the fourth year of training (73). These data are in line with the approximate 4-fold increase reported for lame training horses vs. non-lame training horses in a separate study using ELISA for the detection of COMP neoepitope (74). NMR analysis of synovial fluid from sheep subjected to anterior cruciate ligament (ACL) reconstruction injury vs sham, revealed significant changes in the following metabolites: isobutyrate, glucose, hydroxyproline, asparagine, serine, and uridine (75). Another study examining the synovial fluid of sheep with OA of the temporomandibular joint detected elevated levels of the active MMP2 in pathogenic SF samples (76). Overall, large animals screened for OA biomarkers exhibit increased levels of matrix-degrading enzymes and ECM fragments (MMP2, COMP, and CTXII), associated with early stages of the disease, while cytokines emerge in the circulation at latter stages, which resembles some of the findings reported for human cohorts (77). In summary, changes in biomarkers are more readily apparent in the synovial fluid of large animals compared to that in serum, as is the case in humans.
Altogether, these biochemical markers, and possibly more, discovered primarily in animal models could contribute to our understanding of OA pathogenesis, become potent drug targets, and serve as potential biochemical markers of OA. The capacity to use genetic mice models as affirmation of a biochemical marker is particularly an important approach for validating candidate investigative and exploratory biochemical markers to associate them with OA and its accompanied joint tissue damage.
Conventional Immunoassays and Emerging Multiplexing Immunodetection-Based Technologies
Disease prediction by biochemical marker analysis is one of the most promising research areas nowadays. By far, the most widely applied method for biochemical marker quantification is ELISA, which are frequently used to quantify antibodies, peptides, proteins, and hormones in the plasma, serum, urea, or supernatants. ELISA is a highly sensitive, specific, and accurate method. However, ELISAs can only measure a single molecule of interest at a time, which is a major challenge for simultaneous quantification of multiple antigens in huge cohorts of patient samples, in terms of both workload and sample amount. The detection of a single biochemical marker in the serum, intracranial fluid, synovial fluid, urine, or other bodily fluids is not a reliable prognostic or diagnostic indicator in the vast majority of diseases. Single analyte assays are widely applied in protein measurements, while running at least a couple of experiments in parallel increases the time consumption and risk for an error to occur, and a larger volume of analytical samples are needed (78, 79).
Analysis of multiple biochemical marker molecules in every patient could potentially shed better light on disease characteristics and status. However, the measurement of multiple parameters in a single precious sample from a patient in a single run has challenged the scientific and medical community and led to the development and design of assays utilizing multiplexing technologies. Multiplex immunoassays have gained traction in recent years and have already been successfully used in diagnostic tests and cohort screening. Although the majority of developed platforms is still under investigation in the preclinical phase, these techniques can lead to a strong technological revolution in the future as reliable and cost-efficient systems for diagnosis, disease prediction, and monitoring. Two basic assay formats have been developed to facilitate simultaneous detection of multiple analytes: planar arrays (i.e., biochip array technology) and bead-based arrays (e.g., Luminex Technology), and the applications of these assays has been extensively reviewed (78, 80).
The basic principles of biochip planar arrays based on ligand-binding assays were first described in the 1980s (81). Since then, the technology has advanced and attracted the attention from biochemical marker research to clinical diagnoses and prognoses. In antibody microarray systems, large amounts of different antibodies are printed on the planar microarray surface where multiplexed affinity reagents are used to detect and quantify proteins in complex with biological samples (82). In addition to the advantage of detecting multiple biochemical markers, antibody microarrays assays are high throughput and highly sensitive by using small sample volumes and delivering fast results (<24 h from sample preparation to data analysis). Despite the remaining challenges associated with multiplex immunoassay platforms, such as cross-reactivity to off-target biochemical markers and their clinical applicability, there are several systems commercially available (83). For instance, the company MesoScale Discovery® (MSD) offers a multiplex immunoassay platform built upon planar array technology utilizing electrochemiluminescence detection (83, 84). The platform is made in a 96-well format with integrated electrodes to deliver an electric impulse to each well and specific antibodies spotted at the bottom. The detection reagent contains electrochemiluminescent labels that bind to the detection antibody and are only activated by an electric charge, eliminating any background interference by non-specific label detection. This kind of detection system was validated and the sensitivity compared favorably to well-validated single-plex ELISAs (84).
Among planar-array-based technologies, bead-based or cytometric bead array (CBA) platform has also been developed by combining ELISA-based technology with flow cytometry. Beads of different sizes or colors are used for those multiplexed immunoassays (85). Initially, the assay was developed for conventional flow cytometers, but the design of a CBA analyzer by Luminex Company (Luminex Corporation, Austin, TX, USA) enabled streamlining of the workflow and data analysis, making it more accessible. Luminex technology has been adopted by many leading bioscience companies, and various biochemical marker panels have been developed. The Luminex xMAP (multianalyte profiling) technology is a bead-based flow cytometric platform for multiplex analysis. It uses magnetic or polystyrene particles, incorporating two fluorophores in 100 different ratios, giving the possibility to detect 100 analytes (86). The technology offers a greater reproducibility, as compared to the planar arrays, and sensitivity comparable to that of ELISA (87, 88).
The development of a multiplex assay requires overcoming many difficulties, including insufficient detection limits and standardization of the biochemical marker panels for diagnosis and treatment monitoring (89). At the research level, non-traditional methods in clinical settings have been considered as novel strategies to enable high sensitivity and simultaneous detection of a multitude of biochemical markers. Proximity ligation assay (PLA) and, more recently, the proximity extension assay (PEA) have been reported as a sensitive and selective immunoassay method for protein quantification using a pair of DNA oligonucleotides linked to antibodies against the target molecule (90). A multiplexed platform containing 96-plex PEA-based immunoassay was developed to achieve simultaneous measurement of 92 biochemical markers related to cancer, and its performance was evaluated in comparison with benchmark bead-based immunoassays (91, 92). Very recently, a digital PLA (dPLA) has been proposed to simultaneously detect Gram-negative and Gram-positive bacterial DNA as well as the inflammatory biochemical markers IL-6 and TNF-α from patient samples (93). A major advantage of this innovative platform is the use of a digital amplification method, which enables the quantification of very small changes in concentration of the biochemical markers (i.e., subfemtomolar resolution for protein targets). As an outcome, those analyses showed that temporal changes in several biochemical markers, rather than the absolute concentrations, are reliable predictors of patient outcomes.
Surface-enhanced Raman scattering (SERS) has also been considered as a potential detection method of multiple biochemical makers. SERS combines nanostructures made of noble metals (e.g., silver and gold) with Raman spectroscopy, providing a dramatic increase in the characteristic molecular fingerprint offered by Raman spectrum (94). Particular attention has been given to SERS immunoassays, which ensure high sensitivity by SERS detection and high specificity from the antigen–antibody binding. Furthermore, the combination of nanotags and characteristic spectrum of the target molecule makes SERS detection a very attractive strategy to achieve multiplexing as recently demonstrated (94, 95). Photon-upconversion nanoparticles have been used to develop a microtiter plate immunoassay capable of detecting PSA at 1.2 pg/ml, which is 10 times more sensitive than commercial ELISA and covers a dynamic range of three orders of magnitude (96). Such technology has the potential to develop into a new generation of digital immunoassays.
A multiplexed protein detection technology resembling an immunodetection method was developed by SomaLogic Company. The SOMAScan assay utilizes nucleic acid ligands with protein-like side chains—aptamers (SOMAmers) (97). The principle resembles antibody–antigen interaction detection, with affinities comparable and often superior to those of antibodies. SOMAmers have distinct advantages for such applications, including selection conditions not tied to in vivo immunization, thermal and chemical stability, smaller size, ease of manufacturing, reliable supply, and full control of lot-to-lot variability (98).
Development of the multiplexed detection assays should inevitably focus on the selection of the detection system/technology and reagents that are the key factors in obtaining sensitivity and specificity. For example, four high-sensitivity cytokine multiplex assays on a Luminex or electrochemiluminescence (MSD) platform were evaluated for their ability to detect circulating concentrations of 13 cytokines as well as for laboratory and lot variability (99). The study showed that no single multiplex panel detected all cytokines, and there were highly significant differences between laboratories and/or lots with all kits. The detection of single IL-6 cytokine was assessed by means of four different immunoassays/platforms (100). IL-6 was measurable in all plasma samples by MSD, while 35, 1, and 4.3% of samples were out of range when measured by Luminex assay, Ultrasensitive Luminex assay (Invitrogen), and High-Sensitivity ELISA (R&D), respectively. Again, it emphasizes the importance of the reagents used for the detection, not only the detection platform.
Application of Multiplexing Technologies in Diagnostics of Various Diseases
This section describes the lessons from research experiences and challenges observed in various diseases applying multiplexing and diverse innovative technologies for biochemical marker identification and analysis. One of the main challenges is the choice of the most relevant biochemical markers for the prediction and monitoring of a disease. For instance, automated and multiplex biochemical marker assay has been developed to reliably distinguish between RA patients and healthy individuals (101). This study included serum samples from 120 patients. The multiplexed assay was considered to be a relevant and specific method to diagnose RA by using a biochemical marker panel with three biochemical markers yielding a sensitivity of 84.2% and a specificity of 93.8% and using four biochemical markers a sensitivity of 59.2% and a specificity of 96.3%. In another study, quantification of 12 biochemical markers was performed, utilizing a multiplexed sandwich immunoassay in three panels for RA diagnosis (102). This study has demonstrated that the 12 individually selected biochemical markers exhibit a high level of precision with minimal cross-reactivity and interference by substances commonly seen in RA patients. Interestingly, among these two multiplex panels, only two biochemical markers, IL-6 and TNF-α, were included in both studies, while analysis of other biochemical markers generated inconsistent results. Despite that, both studies conclude that their methods provide highly reproducible results, are effective, and can even stratify RA patients into clinically relevant subtypes (102, 103).
Additional analytical capacity has been introduced to the RA biochemical marker field when the recently developed multibiomarker disease activity (MBDA) test was validated in a clinical study on RA patients. The MBDA scores (from 1 to 100) were capable of monitoring changes in disease activity over time and effectively discriminating clinical responders from non-responders in diverse RA cohorts (104). This scoring system is most commonly used to assess the response of biological therapy in RA, however with varying success and utility for the process of tapering and the ceasing of disease-modifying anti-rheumatic drugs (DMARDs). In the AMPLE study of the biological agents Abatacept and Adalimumab, the MBDA score did not reflect clinical disease activity (105), while in the post hoc analysis of three cohort studies on Rituximab, the same score was confirmed to represent the clinical response to treatment in RA patients (106). Currently, a prospective, a randomized study of the Vectra DA MBDA blood test is under investigation for Food and Drug Administration (FDA) authorization (107) in diverse RA cohorts.
Disease phenotyping in multiple sclerosis (MS) served as another example of biochemical marker selection. Cases of different subtypes of MS were compared by simultaneous analysis of serum IL-1β, IL-6, IL-8, and TNF-α levels via comparison of two commercially available multiplex platforms (i.e., Luminex-xMAP and Meso Scale Discovery) (108). Although the presence of these biochemical markers was detected in all the subtypes of MS, the levels varied. The significant increase in IL-6 and IL-8 in all the MS subtypes was determined, but a significant increase in TNF-α was observed only in one of the subtypes, as compared to the controls. In addition to the biochemical markers that can be used to diagnose MS, this parameter could be included into the biochemical marker panel for specification of the MS subtype.
Protein array chip immunotechnology has been applied in osteoporosis diagnosis as an alternative method for single biomarker concentration evaluations (109). Individual biomarker assays in osteoporosis, similarly to other multimodal diseases, fail to describe such complex diseases. Single biomarker concentration measurement is currently used to evaluate the progression of osteoporosis (OP) as well as the measurement of bone mineral density (BMD) by dual-energy X-ray absorptiometry (110). The same Immunological Multiparameter Chip Technology (IMPACT) platform that has been applied in RA diagnosis (101) is used to simultaneously measure OP biomarkers CTX-I, procollagen type I N-terminal propeptide (PINP), osteocalcin, and intact parathyroid hormone (PTH). The choice to measure these specific biomarkers was made due to their high sensitivity and suitability to evaluate bone resorption and formation changes. Although the results demonstrated similar analytical performance characteristics to single biomarker assays with an increased sensitivity, the necessity for larger numbers of OP patients as well as inclusion of more biomarkers associated with bone metabolism change indicators in the panel is further needed (109, 111).
Biochemical marker analysis is also becoming a crucial step in cancer diagnostic and predictive/prognostic characterization of the disease stages. The 96-plex PEA immunoassay has been developed and shown to be both sensitive and specific, as well as more scalable, in comparison to traditional immunoassays (91). Such PEA immunoassay has been applied for multiplex analysis of patients with colorectal cancer, which determined the significant correlation of the expression of CEA, IL-8, and prolactin with specific colorectal cancer stage (92). The identical set of 1 μl of plasma samples from patients with colorectal cancer or unaffected controls was run for both assays. Similar quantitative expression patterns were determined for 13 plasma antigens common to both platforms, while the potential efficacy of proximity extension assay was endorsed, as it only demonstrated that the expression of CEA, IL-8, and prolactin are significantly correlated with colorectal cancer stage. Later, another PEA platform for an expanded panel of 275 biochemical markers has been developed and produced a 12 biochemical marker signature algorithm that was comparable to a clinically approved blood-based screening test (112).
Antibody array systems have also been applied in cancer biomarker screening, where simultaneous detection of multiple breast cancer and ovarian cancer biochemical markers, relevant to clinical diagnosis was achieved (113, 114).
It is evident that biochemical marker research in cancer is highly advanced compared to other disease areas, and these studies may serve as good examples of simplified ways for sensitive and specific detection of different cancer types, for instance breast, colorectal, etc. Such state-of-the art technologies as multiplexing a combination of biochemical markers and implementation of biosensors save time and resources for the prediction of treatment response. Other kits mostly cover single-antigen protocols that have been implemented in most clinical laboratories. Cancer biochemical markers profile a panel of different cancer subtypes, where a single biochemical marker might indicate a particular subtype of a disease. However, only 15–20% of patients develop a response to biochemical markers of different cancer subtypes (115). Therefore, larger numbers of biochemical markers can be included, more sensitive and specific diagnostic tool can be developed, and there will be a higher likelihood of positive responses for at least one of the biochemical markers (116). Taken together, the application of multiplex biochemical marker technologies in other disease areas may offer insights that could be implemented as a framework for clinically important OA biochemical marker combination research.
Multiplexing Advances in OA Research
Due to the capability of measuring up to 100 analytes in one relatively small sample, many different companies have utilized the Luminex xMAP technology platform and created different panels of multiplex assays. Although only a few studies applied this or other multiplex technologies in screening samples from OA patients so far, they generated important data on disease pathogenesis and progression. These data are summarized in Table 1.
Table 1.
Multiplex assays-based studies in OA.
| Assay | Platform | Samples tested | Detected valuable markers in OA (correlations, associations etc.) | References |
|---|---|---|---|---|
| Milliplex MAP human cytokine/chemokine panel (42 analytes) | Luminex | Serum and synovial fluid, hip and knee | IL-6, MDC and IP-10 correlated with hip OA. IL-6, MDC, and IP10 were associated with pain in the hip cohort. MCP-1 and MIP-1β were highly expressed in the knee OA | (117) |
| LINCOplex™ Immunoassay (21 analytes) | Luminex | Knee synovial fluid | MCP-1, MIP-1, IL-2, IL-5 elevated in advanced OA (ICRS scale) | (118) |
| Pro-human cytokine multiplex assay (33 analytes) | Luminex | Knee synovial fluid | IL-10, IL-12, IL-13, SCGF-β, VEGF correlated with knee pain and function. IL-6, IL-8, IFN-γ, SCGF-β, VEGF, CXCL1 were associated with OA severity (KL scoring) | (119) |
| Human Luminex Screening Assay (10 analytes) | Luminex | Knee synovial protein extracts | VEGF was decreased in symptomatic OA vs. asymptomatic OA patients' samples. MMP-1 protein increased in OA vs. postmortem controls | (30) |
| Myriad Human InflammationMAP® 1.0 multiplex immunoassay (47 analytes) | Luminex | Synovial fluid | VEGF, MMP-3, TIMP-1, sICAM-1, sVCAM-1, MCP-1 related to synovial inflammation in OA, radiographic and symptom severity | (47) |
| BioLegend LEGENDplex human adipokine flow cytometry-based ELISA (13 analytes) | Flow cytometer | Knee synovium cells (24 h cultures in vitro/supernatants) | IL-6 expression was highest in mesenchymal cells vs. hemopoietic. One of the patient-specific inflammatory clusters identified had high tissue and mesenchymal cell IL-6 and IL-8 release | (120) |
| SPRi multiplex assay (9 analytes) | IBIS MX96 | Serum and synovial fluid | Early OA markers—IL-1β, IL-6, TNF-α, IFN-γ, IL-10, CCL2, IL-8, IL-4, and C3F high sensitivity (low pg/ml) detection system. Undergoing validation in patient cohort | (121) |
| Microfluidic FMGC (2 analytes) | Microfluidics | Serum and urine | Simultaneous detection of sCTX-II and uCTX-II. 24-fold and 3.5-fold shorter completion time than the ELISA for urinary and serum CTX-II | (122) |
IL-1β, 2, 4, 5, 6, 8, 10, 12, 13, interleukin 1β, 2, 4, 5, 6, 8, 10, 12, 13; MDC, macrophage-derived chemokine; IP-10, interferon gamma-induced protein 10; MCP-1, monocyte chemoattractant protein-1; MIP-1, 1β, macrophage inflammatory protein 1, 1β; ICRS, International Cartilage Repair Society; SCGF-β, stem cell growth factor β; TIMP-1, metallopeptidase inhibitor 1; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1; VEGF, vascular endothelial growth factor; IFN-γ, interferon gamma; CXCL1, C–X–C motif chemokine ligand 1; KL score, Kellgren and Lawrence score; MMP-1, 3, matrix metallopeptidase 1, 3; TNF-α, tumor necrosis factor-α; CCL2, C–C motif chemokine ligand 2; C3F, complement C3 peptide fragment; FMGC, microfluidic fluoro-microbeads guiding chip; sCTX-II and uCTX-II, serum/urinary C-telopeptide fragments of type II collagen.
A cytokine/chemokine panel was measured in serum from patients with hip and knee OA and compared with that in healthy controls using Luminex platform (117). Endothelial growth factor (EGF), FGF2, MCP-3, MIP-1α, and IL-8 were differentially expressed between hip and knee OA cohorts. In the knee OA samples, EGF was undetectable while MCP-1 and MIP-1β were highly expressed compared with that in hip OA and control samples, suggesting specific differences that may be related to differential disease processes within a given joint. Thus, different inflammatory biochemical marker combinations may represent OA lesions of different joints. These data support findings from an earlier pilot study in human knee synovial fluid (118), showing that among 21 cytokines screened, elevated MCP-1 and MIP-1 in SF were also increased in subjects with advanced arthritis, based on the International Cartilage Repair Society (ICRS) criteria.
Recently, five of the biochemical markers examined in synovial fluid, using cytokine multiplex assay (Luminex), significantly correlated with both knee pain and function (119) (Table 1). Furthermore, significant associations between OA radiographic severity (KL scoring) and some molecules in the synovial fluid were observed. Another 10-plex Luminex assay was applied not on SF but on synovial protein extracts of OA patients undergoing knee replacement surgery (30). Noteworthy, among the proteins analyzed, vascular endothelial growth factor (VEGF) was decreased in the synovium of symptomatic compared with asymptomatic OA samples, which is in contrast to the results of previously mentioned study on synovial fluid (117). Additionally, MMP-1 protein expression was increased in OA compared to postmortem synovial tissue controls.
The importance of evaluating SF biochemical markers as indicators of a symptomatic inflammatory OA endotype has been highlighted in a recent study on 25 patients with radiographic knee OA (47). Levels of 47 different cytokines, chemokines, and growth factors related to inflammation were screened using multiplex immunoassay (Luminex technology). A subset of six SF biochemical markers (Table 1) was associated with synovial inflammation, as well as radiographic and clinical severity, in OA. These six OA-related SF biochemical markers were specifically linked to indicators of activated macrophages and neutrophils. These results attest to an inflammatory OA endotype that may serve as the basis for therapeutic targeting of a subset of individuals at high risk for knee OA progression.
A first detailed comparison between Luminex and MSD multiplex platforms for the analysis of real clinical SF samples from end-stage knee OA was performed on inflammatory cytokines IL-1β, TNF-α, IL-6, and IL-8 (123). Both systems were capable of detecting the selected cytokines, while the MSD platform had a significantly lower limit of detection (LOD) for all four analytes. The authors concluded that MSD platform was better able to detect and quantify low-level analytes (IL-1β and TNF-α) in OA SF samples compared to Luminex, but due to the differences in the antibody pairs and their affinities, such comparisons of technologies are not very conclusive. Noteworthy, the cytokine measurements in OA samples were at best semi-quantitative and depended on the applied platform, assay, and its manufacturer, thus making the comparisons between the technologies complicated.
Flow cytometry and multiplex flow cytometry-based ELISA were employed for the analysis of cell composition and soluble protein production in synovium collected from OA patients undergoing knee replacement surgery (120). Here, IL-6 expression was highest in mesenchymal cells, although in a handful of patients, hematopoietic immune cell (mainly macrophage) expression was more dominant. Using a novel approach, patient-specific inflammatory clusters were identified: they broadly separated into T cell/lymphocyte (1) and myeloid (2 and 3) clusters, with cluster 3, in particular, associated with high tissue and mesenchymal cell IL-6 and IL-8 release. There are preliminary suggestions that these clusters reflect different patient phenotypes, with cluster 2 trending with female sex and cluster 3 with a history of prior joint surgery (arthroscopy/arthroplasty). It remains to be determined if these clusters can be better defined and how they are related to disease progression and clinical phenotypes.
A highly sensitive multiplex assay based on surface plasmon resonance imaging (SPRi) was first developed for the analysis of four cytokines in synovial fluid (IL-1β, IL-6, IFN-α, and TNF-α) (124). Later, by adding several early OA biochemical markers, including complement C3 peptide fragment (C3F), the assay was applied to detect early OA (121). Technologically, specific capture antibodies were spotted on a gold sensor and loaded into the SPRi machine (IBIS MX96). A sample with biochemical markers reached the sensor through a flow cell, and the interactions with the antibodies were measured in real time. The signal was enhanced by adding biotinylated detection antibodies, followed by neutravidin and biotinylated gold nanoparticles, resulting in a signal improvement of over 200 times and an increased sensitivity of more than 10,000 times. The assay is currently undergoing validation in a small patient cohort.
There is a growing evidence supporting the importance of biochemical markers reflecting metabolic changes in cartilage and bone during OA. The panel of metabolic products of cartilage and bone ECM molecules, representing the processes of breakdown (catabolism) or synthesis (anabolism), has been extensively reviewed (28, 32, 33). The prognostic value of the peptides arising from molecular breakdown or synthesis of cartilage ECM is still under investigation. An attempt to multiplex such markers via sandwich and competition immunoassays has been made (122). The new strategy aimed to simultaneously detect the C-telopeptide fragments of type II collagen (CTX-II), which has heterogeneous epitope structure in serum (sCTX-II; homodimers) and urine (uCTX-II; monomers or variant monomers). For the detection of both serum and urinary CTX-II peptides, a microfluidic fluoro-microbeads guiding chip (FMGC) was developed. It has one inlet for sample insertion and four separate chambers, two of which are dedicated for the sandwich-based detection, while the other two were for the competitive immunoassay. The proposed FMGC-based multiple sensing system accurately detected CTX-II, and the results obtained using this assay correlated well with those obtained using commercial ELISA kits. A combination of inflammatory cytokines/chemokines/MMPs together with cartilage/bone synthesis/degradation markers in a single multiplex assay would provide a powerful tool for the classification of OA subtypes and individualized OA treatment strategies.
Taken together, although several novel multiplexing technologies have been tested for biochemical markers associated with OA, the number of such studies remain very limited. The ability to measure the inflammatory cytokines in a multiplexed manner is not yet translated to the combined multiplex assays for cartilage/bone metabolic markers and not well-controlled with a sensitive reference method as MRI. These data could lead to understanding the role and importance of inflammation in the processes of cartilage breakdown and regeneration and specify the need for intervention. Moreover, most of the multiplexed OA biochemical marker studies to date were performed on SF samples, while only few studies involved the analyses of serum or urine, which would arguably better serve the major clinical need for OA monitoring.
Nanotechnology Strategies to Enhance Selectivity and Sensitivity of Biochemical Marker Detection
The major drawback, particularly related to serum or urinary biochemical markers, is that some of them are found at lower than picomolar concentrations, which are too low to be detected by conventional methods such as ELISA. Therefore, the analytical techniques, offering high degrees of sensitivity and specificity, such as those employing nanomaterials, proximity ligation, or digital platforms (e.g., digital ELISA), might appear useful for the analysis of ultralow concentrations of biochemical markers in a clinical setting (125). The recent advances in material science, nanotechnology, and bioconjugation techniques have enabled the application of a large diversity of nanomaterials to enhance the sensitivity of advanced immunoassays (126). Only a few studies have been reported about enhanced immunoassays specifically designed for OA biochemical markers (Table 2). For instance, a quantitative lateral-flow immunoassay technique with antibodies conjugated to gold nanoparticles was used for the detection of COMP in the synovial fluid. The proposed method showed similar results to corresponding ELISA, with an average difference of <7% without the need of expensive equipment or complex procedures (127). However, most of the reported studies have investigated the use of advanced nanomaterial-enhanced immunoassays toward inflammatory biochemical markers.
Table 2.
Enhanced immunoassays for detection of biochemical markers relevant to OA.
| Method | Samples tested | Biomarker | Advantages | Characteristics | References |
|---|---|---|---|---|---|
| Quantitative lateral flow immunoassay using antibody-conjugated gold nanoparticles | OA patient SF | COMP | Cost effectiveness | Dynamic detection range: 0.6–20 μg/mL | (127) |
| Quantum dot-linked immunosorbent assay (with immobilized orientation-directed half-part antibodies) | Antigen solution | IL-6 | High sensitivity | Lower LOD: 50 pg/Ml | (128) |
| SENSIA | Pooled human serum | 15-Plex (including IL-1β, IL-2, IL-4, IL-6, IL-10, MMP-9, TNF-α) | Cost effectiveness | IL-1β, IL-2, IL-6, and 2 other markers were in good agreement with FLISA (>0.9R2) | (129) |
| Surface-enhanced Raman scattering based immunoassay | Healthy volunteer blood samples | IL-6, IL-8, and IL-18 | High sensitivity | LOD: IL-6, 3.8 pg/ml; IL-8, 7.5 pg/ml; and IL-18, 5.2 pg/ml | (130) |
| Electrochemiluminescence-based system | Serum samples | CRP | High sensitivity, good selectivity, good reproducibility, and low cost | Range: 0.05–6.25 ng | (131) |
| Microfluidic immunoassay with streptavidin–biotin–peroxidase nanocomplex | Unspecified patient serum samples | IL-6 (multiplexed with procalcitonin) | High sensitivity | Detection range, 5–1,280 pg/ml; LOD, 1.0 pg/ml | (132) |
| Combined electrochemiluminescent and electrochemical immunoassay | Serum samples | IL-6 | Broad dynamic range, high sensitivity, and selectivity | Detection range, 10 ag/ml−90 ng/ml | (133) |
COMP, cartilage oligomeric matrix protein; IL-1β, 2, 4, 6, 8, 10, 18, interleukin 1β, 2, 4, 6, 8, 10, 18; LOD, limit of detection; SENSIA, silver-enhanced sandwich immunoassay; MMP-9, matrix metalloproteinase 9; TNF-α, tumor necrosis factor-α; FLISA, fluorescence-linked immunosorbent assay; CRP, C-reactive protein.
Markers of inflammatory activity have been at the forefront of detection limit improvement, as the concentrations of immune analytes are notoriously low, particularly in the serum or urine (134). For instance, an ELISA-like method based on the nanometer-sized fluorescent semiconductor particles called quantum dots has detected concentrations of IL-6 as low as 50 pg/ml (128). Application of silver-enhanced sandwich immunoassay (SENSIA) showed comparable results to the fluorophore-linked immunosorbent assay for the detection of IL-6, IL-2, and IL-1β in serum samples while being more cost effective (129). IL-6 and IL-8 showed improved detection limits (2.3 and 6.5 pg/ml, respectively) with sensitive surface-enhanced Raman scattering-based immunoassays in comparison to ELISA counterparts (130).
Electrochemiluminescence-based systems have also been applied for the analysis of inflammatory markers. Application of such label-free electrochemiluminescent immunosensor that utilizes the poor conductivity of CRP molecules bound to antibodies has enabled its detection at the limit of 0.011 ng/ml (131). An immunoassay based on mesocrystal nanoarchitectures combined with electrochemiluminescent and electrochemical detection has been presented to quantify IL-6 (133). The results showed high sensitivity by achieving a broad linear dynamic range of 10 ag/ml−90 ng/ml.
A quantitative microfluidic immunoassay combined with the streptavidin–biotin–peroxidase (SA-B-HRP) nanocomplex-signal amplification system (MIS) has also been presented to detect IL-6 simultaneously to a second inflammatory biochemical markers (i.e., procalcitonin). In this case, the linear range for IL-6 detections was 5–1,280 pg/ml, and the limit of detection was 1.0 pg/mL, which was significantly improved compared with microfluidic immunoassays without amplification systems. Despite these promising outcomes toward inflammatory biochemical markers, to our knowledge, the enhanced-detection immunoassays mentioned above have not yet been applied toward OA inflammatory response.
Novel Strategies to Enable Diagnostic and Prognostic Monitoring Via Biosensors
Biochemical markers have the potential to be used as indicators of changes in the course of the disease, which might signal the need for additional imaging tests or changes in the course of OA, which could require treatment option re-evaluation (11). However, as a monitoring tool, they will only be considered if there are methods available to easily detect the subtle changes in concentration values while maintaining consistency in data collection. A potential solution to this is the application of biosensors, which are analytical devices that analyze biological responses and convert them into measurable physicochemical signals, typically exhibiting high specificity and reusability (135). Biosensors are an expanding field toward rapid, easy, and reliable detection of relevant biochemical marker that have been implemented in the diagnosis of various diseases and have also started to be considered as potential tools for OA research and clinical application (Table 3).
Table 3.
Biosensors for detection of biochemical markers relevant to OA.
| Method | Samples tested | Biochemical marker | Advantages | Characteristics | References |
|---|---|---|---|---|---|
| Quartz crystal microbalance biosensor | Urine of OA patients and healthy controls* | COMP | Reaction time advantage, high sensitivity | Detection range: 1–200 ng/ml | (136) |
| Nanoparticle amplified SPRi aptasensor | Human serum | CRP | High sensitivity | LOD: 5 fg/ml | (137) |
| Quartz crystal microbalance biosensor | MMP-1 controls | MMP-1 | Reaction time advantage | Detection range: 2–2,000 nM | (138) |
| Fiber optic-particle plasmon resonance biosensor integrated with microfluidic chip | OA patient SF* | MMP-3 | Cost-effectiveness, portability, high sensitivity | – | (139) |
| Fiber-optic particle plasmon resonance biosensor | OA patient SF* | TNF-α and MMP-3 | Reaction time advantage, simple usage, high sensitivity, high selectivity | LOD: TNF-α, 8.2 pg/ml; MMP-3, 8.2 pg/ml | (140) |
| Fluoromicrobeads guiding chip-based system | Human SF and serum* | COMP | Reaction time advantage | Detection range: 4 and 128 ng/ml | (141) |
| Fluoromicrobeads guiding chip-based system | Human urine-based controls and artificial serum | uCTX-II and sCTX-II | Simultaneous detection, reaction time advantage | Linear detection range: sCTX-II, 0.1–2.0 ng/mL; uCTX-II, 200–2,800 ng/mmol | (122) |
| Ultraviolet–visible spectroscopy | uCTX-II controls | CTX-II (multiplexed with glucose) | Cost effectiveness and simple manufacturing | Detection range: 1.3–10 ng/ml | (142) |
| Ambient light-based optical biosensor | uCTX-II epitope controls | uCTX-II | Cost effectiveness, simple usage | LOD: 0.2 ng/ml | (143) |
Analyzed in OA patient samples.
MMP-1, 3, matrix metallopeptidase 1, 3; TNF-α, tumor necrosis factor-α; sCTX-II and uCTX-II, serum/urinary C-telopeptide fragments of type II collagen; LOD, limit of detection; SENSIA, silver-enhanced sandwich immunoassay; COMP, cartilage oligomeric matrix protein; CRP, C-reactive protein.
COMP has been measured in both OA patient's synovial fluid and serum with an immunosensing device using FMGC technology that has a reaction time advantage over conventional ELISA (141). As it shows good correlation to results obtained by conventional ELISA (coefficient of variation was only within 7%), it could potentially be used in clinical settings, more so if the technology advances to a multiplex format. FMGC-based system has also been used for uCTX-II and sCTX-II quantification, which not only analyzed both factors simultaneously but was also faster than the conventional ELISA by 25- and 3.5-fold, respectively (122). Both systems exhibited high sensitivity and very similar LODs to their respective ELISAs.
Several optics-based biosensing platforms have also been investigated. Low-cost optical biosensing platforms, based on ultraviolet–visible spectroscopy, have been developed for uCTX-II using just common office supplies for data reading (142). As the detection range falls into the clinically relevant intervals, this method of analysis could be introduced into clinical immunoanalysis. The same biochemical marker, uCTX-II, was analyzed using a smartphone-embedded illumination biosensor that had a high accuracy under various lighting conditions (143). Due to its low-cost fabrication requirements and satisfactory detection capabilities in both indoor lighting and in sunlight, it has the potential of being used as a point-of-care diagnostic tool. Real-time multiplexed analysis of MMP-3 and TNF-α was achieved using a single fiber-optic particle plasmon resonance biosensor, which could be used for monitoring both inflammatory and cartilage breakdown activity simultaneously (140). The same type of biosensor has been used for the detection of inflammatory biochemical marker IL-1β with results comparable to a corresponding ELISA method (limit of detection, 21 pg/ml), which, together with the relatively low cost, shorter analysis time, and small sample requirements, seems very attractive for the use in a clinical setting (144).
Other potential candidates for innovative biosensing approaches are technologies that use the piezoelectric effect and transform mechanical stress into quantifiable electrical current. For instance, a quartz crystal microbalance (QCM)-based biosensor for COMP was developed and, compared to ELISA data, showed high accuracy in a shorter analysis time frame (136). Similar biosensor has been demonstrated to detect MMP-1 levels at concentrations between 2 and 2,000 nM in <10 min with a lower detection limit of 2 nM (138). While the clinical relevance of COMP was demonstrated due to its association to the OARSI grades of OA progression, MMP-related biochemical markers might be useful in determining the activity of the processes of cartilage degradation. QCM-based strategies are user friendly and quicker than ELISA, making it a suitable application as a homecare device, comparable to those used for blood glucose monitoring.
Seeking to overcome the problems faced by conventional ELISA method, such as high cost and long process duration, electrochemical-impedance-based immunoassays have been applied to determine levels of the bone-related degradation biochemical marker CTX-I (145).
Advanced biosensors technology has also exploited nanotechnology to enable high sensitivity detection of biochemical markers, potentially leading to applications in clinical settings. Nanoparticle-enhanced plasmonic biosensor have been demonstrated to detect inflammatory marker CRP in only 2 h at concentrations four orders of magnitude lower than the clinically relevant concentrations (146). This ultrasensitive biosensor is fabricated using scalable and low-cost manufacturing, providing a powerful platform for multiplexed biochemical marker detection in several settings. Nanoplasmonic biosensor microarrays have been demonstrated for parallel multiplex immunoassays of six cytokines (i.e., IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ) in a complex serum matrix on a single device chip (147). The device was fabricated using easy-to-implement, one-step microfluidic patterning and antibody conjugation of gold nanorods. The proposed biosensor showed the capability to measure cytokine at concentrations down to 5–20 pg/ml from a 1-μl serum sample within 40 min. Electrochemical immunobased biosensors in combination with carbon nanomaterials have also been reported to detect inflammatory biochemical markers (148, 149). Simultaneous detection of IL-1β and TNF-α using human serum and saliva was achieved using dual-screen printed electrodes modified with carbon nanotubes (148). The proposed biosensor showed improved analytical performance with respect to previous approaches and ELISA methods by achieving limits of detection at 0.38 pg/ml (IL-1β) and 0.85 pg/ml (TNF-α), within 2 h and 30 min and significantly less reagents consumptions.
Diverse cost-effective biosensors for OA that minimize the duration of analysis or in other ways outperforming conventional methods are under development, with a subset of them proposed as clinically suitable biochemical marker detection tools or even potential point-of-care monitoring devices. Although there are multiple technological approaches that are currently under investigation to determine the applicability of various OA biochemical markers, studies that sensitively and consistently follow the changes in levels of OA biochemical markers throughout the course of the disease and elucidate diurnal variation, responses to physical activity, anti-inflammatory medication, or nutrition are still a lacking. For instance, despite that large diversity of biosensors have been developed to detect the inflammatory biochemical marker CRP as reviewed in Vashist et al. (150), CRP biosensors were not tested on OA patient serum samples so far. CRP biosensors created have already been designed reaching detection levels in the zeptomolar concentrations (137). Of note, it is likely that monomeric and multimeric forms of CRP may possess different catabolic and inflammatory profiles. Therefore, new assays need to be developed to distinguish between monomeric and multimeric forms of CRP and other proteins. The application of biosensors, ideally for several multiplexed biochemical markers, might lead to new insights into the role of inflammation in the pathological processes of cartilage turnover in OA.
Choice of the Reference Method for Biochemical Marker Detection
One of the major drawbacks in biochemical marker development for OA is a lack of relevant reference methods to validate their efficacy. Most studies so far have employed radiographic assessment of KL grade for scoring of OA stages, which is neither sensitive, specific, nor easily reproducible in longitudinal clinical trial (10). The lack of a sensitive reference biochemical marker (either imaging or biochemical marker) have likely led to difficulties in proving the utility of biochemical markers in OA. The close correlation between “wet” (biochemical analyte, genomic, etc.) and “dry” (radiography, MRI, or clinical evaluation finding, etc.) as a reference method in each case is of major importance for the successful implementation of biochemical markers (Figure 1). The prospective CHECK study investigates five clusters of biochemical markers, related to specific pathogenic processes: “bone,” “inflammation,” “synovium,” “adipokines,” and “cartilage synthesis,” which will be validated via early radiographic KL scoring of OA status as a reference method (151). Duration of this study is planned for 10 years, and it will finish in 2022.
MRI technology is increasingly implemented as a reference method for evaluation of OA status (152). Several studies involving the analysis of multiple biochemical markers in serum and synovial fluid of knee OA patients (22) use MRI as a reference method for knee damage evaluation. The relation of biochemical serum markers to MRI data in studies performed in 2018 and 2019 are summarized in Table 4.
Table 4.
Correlation between biochemical marker levels and MRI data.
| Study design, number of patients | MRI scoring system | Detection method/biochemical marker panel | Biochemical markers association with MRI scoring data | References |
|---|---|---|---|---|
| Case–control (n = 600) Follow-up points: baseline, 12 and 24 months |
MOAKS: Hoffa synovitis and effusion synovitis | ELISA: HA, MMP-3, Coll2–1NO2 | HA and MMP-3 were modestly associated with effusion-synovitis at baseline | (153) |
| Cross-sectional (n = 89) | WORMS | ELISA: COMP, MMP-3, Coll2-1, Turbidimetric analysis: CRP | COMP correlated positively with WORMS and MMP-3. WORMS scoring data are not provided | (154) |
| Case control (n = 141) Follow-up: 2 years |
WORMS | ELISA: HA, MMP-3, COMP, Coll2-NO2, uCTX-II, PIIANP, CTXI, CS846, C2C, CPII, NTXI/uNTXI, C12C/uC12C | MRI data associated between HA, COMP, and MMP-3 biochemical markers of OA. The biochemical cartilage ECM (Coll2-NO2) degeneration reflects MRI T2 measures | (155) |
| Cross-sectional (n = 160) Follow-up: 2 years |
WORMS | ELISA: IL-8, COMP, CTXI, NTXI, PIIINP, MMP-3, MMP-10, MMP-13 | The positive association was between IL-8 and infrapatellar fat pad signal intensity | (156) |
| Cross-sectional (n = 141) Follow-up: 2 years |
WORMS | ELISA: 100A8/A9, MMP-3, MMP-10, MMP-13 | The levels of alarmins 100A8/A9 had positive associations with MRI score for total and local cartilage defects (lateral femoral, lateral tibial, and medial femoral sites) | (157) |
| Osteoarthritis Initiative Progression subcohort (n = 583) Follow-up: 2 years |
MRI quantitative cartilage volume measurement | ELISA: adiponectin LUMINEX: adipsin chemerin, leptin, visfatin, IL-8, MCP-1, CRP |
The ratio of adipsin/MCP-1 was associated with the MRI knee structural changes, and CRP/MCP-1 with symptoms in obese OA subjects | (158) |
| Cross-sectional (n = 16) Follow-up: 5 years |
MRI semiautomatic segmentation method | ELISA: COMP, C1,2C, CS846 | Long-term mechanical stimuli increase the cartilage degradation markers as C1,2C and CS846. Those biochemical markers correlate with cartilage damage (MRI) | (159) |
| Multicenter, double-blind, phase III clinical trial (n = 163) Follow-up: 1 year |
WORMS | ELISA: CTX-I, COMP, PIIANP, MMP-3, C1M, C3M, C2M, CS846, CTX-II, uCTX-II/creatinine ratio | Clinical study of cell and gene therapy: no significant differences in MRI between the groups of treatment vs. placebo | (160) |
| Randomized, double-blinded, sham-controlled trial (n = 55) Follow-up: 3 months |
MOAKS: Hoffa synovitis and effusion synovitis | ESR ELISA: hsCRP |
Low-dose radiation therapy does not induce significant effects on inflammatory signs assessed by MRI, ultrasound and serum inflammatory markers | (161) |
s100A8/A9, myeloid related protein 8/14, calprotectin; ESR, erythrocyte sedimentation rate; COMP, cartilage oligomeric matrix protein; Coll2-1, type II collagen degradation biomarker 1; Coll2–1NO2, nitrated epitope of the α-helical region of type II collagen; C12C/uC12C, collagen type I and II cleavage product and its urine form; C2C, type II collagen degradation biomarker, generated from C-terminus fragment; C1M, C2M, C3M, matrix metalloproteinase (MMP) mediated type I, II, III collagen degradation biomarker; CPII, C-propeptide of type II procollagen; CRP, C-reactive protein; CTX-I, serum C-terminal telopeptide of type I collagen; CS846, chondroitin sulfate synthesis marker; sCTX-II and uCTX-II, serum/urinary C-telopeptide fragments of type II collagen; HA, hyaluronic acid; IL-8, interleukin 8; MMP-3, 10, 13, matrix metalloproteinase 3, 10, 13; MCP-1, monocyte chemoattractant protein-1; MOAKS, MRI OA Knee Score; NTXI/uNTXI, crosslinked N-telopeptide of type I collagen and its urine form; PIIANP, serum N-propeptide of collagen IIA; PIIINP, N-terminal procollagen III propeptide; WORMS, whole organ magnetic resonance imaging score.
The listed studies revealed associations of different biochemical markers with MRI scoring of synovial inflammation and cartilage degradation. It is important to note that these MRI techniques can detect early bone marrow lesions that may be associated with the onset of cartilage degradation and correlate with inflammation in joint tissue (162). As these data are ambiguous and associated with various aspects of synovial inflammation and/or cartilage damages, no perfect scoring system exists to date. In terms of complexity, heterogeneity, and size of knee OA-related data, it is considered a “big data” issue; therefore, machine learning and application of computer algorithms has attracted significant interest for the evaluation of biochemical and imaging markers (163). A recent publication by Emery et al. described outcome measures for early OA that could be useful in clinical practice and/or the research setting (12). A consensus-based OA phenotype framework was created with intent to facilitate research on OA phenotypes and increase combined efforts to attain effective OA phenotype classification, by providing a number of coherent definitions and statements and a set of reporting recommendations that were supported by a panel of experts in OA research field (164). Many other research groups are currently working with regulatory agencies across the world seeking to clinically qualify confirmed new biochemical markers and imaging biomarkers (165, 166).
Development of Reference Methods
Looking into the future of radiological reference method development, more attention will be focused on morphological MRI, observing “premorphological” biochemical compositional changes of articular tissues (12, 18). Compositional MRI techniques evaluate cartilage composition [glycosaminoglycans (GAGs), proteoglycans, or collagens] and hydration. Compositional MRI of cartilage matrix changes can be performed using advanced MRI techniques such as delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), T1 rho, and T2 mapping (152). The chemical exchange saturation transfer of GAG (GagCEST) can detect cartilage endogenous GAG content without the need of intravenous contrast injections or special hardware (167). Negatively charged GAGs attract sodium cations and their distribution in sodium MRI correlates with GAG content in cartilage (152). It is consequently important to harness morphological MRI, compositional changes of the cartilage (GAGs, proteoglycans, or collagens), and hydration from images and to establish a correlation between them and quantitative biochemical markers.
In differential diagnosis for OA, it is important to evaluate the intensity of inflammatory component expression, which usually is not as high as in other inflammatory arthritides. The hybrid imaging techniques as the positron emission tomography (PET) method with 18F-fluorodeoxyglucose (FDG) uptake reflected the inflammatory activity, associated with elevated inflammatory cytokine levels, suggesting that FDG-PET may be effective for quantification of the inflammatory activity in different rheumatic diseases (168, 169). The inflammation may contribute to the increased FDG uptake in OA, reflecting the rate of disease progression and inflammatory phenotype. Single-photon emission computed tomography combined with the high-resolution computed tomography (SPECT/CT) technique can visualize folate receptor positive cells, representing activated macrophages and neutrophils, which is associated with the inflammation in the joints of OA patients (47).
These new technological approaches are may be systematically applied for the identification and characterization of OA phenotypes and, together with the relevant biochemical and imaging markers, might contribute to a better understanding of the role of low-grade inflammation in the development of OA, as well as facilitate the identification of the need for anti-inflammatory medication (11). The local inflammatory activity associated with elevated inflammatory cytokine levels (169) and folate receptor positive cells, representing activated macrophages and neutrophils, can already be visualized (47). The rapid progress of such sophisticated imaging methods should lead to the precision in imaging biomarker choice and implementation.
Conclusions
In this article, we have provided an overview of several technologies, platforms, and strategies that facilitate and improve biochemical marker detection. Application of such novel techniques in imaging and biochemical marker identification may lead to better definition of OA phenotypes and categories and add complementary value to radiologically validated outcome measures in clinical practice and/or the evidence-based comparisons into the effectiveness of therapeutic interventions.
OA biochemical marker immunoassays are potentially viable tools for OA evaluation, monitoring, and drug development. However, specific problems, such as low biochemical marker concentrations, patient-specific variation, limited utility of single biochemical markers to get definitive characterization of OA status, and application of relevant reference methods, require innovative approaches to produce clinically relevant biochemical marker biosensors. Such technologies are already on the way to establishment in routine diagnostics and monitoring of other diseases and could potentially serve as good technological platforms for early OA characterization. Inflammatory markers are monitored in many inflammatory diseases, and novel technologies like biosensors dedicated to reducing costs or time of their detection may also be successfully implemented in OA. Multiplexing inflammatory biochemical markers in combination with biochemical markers of cartilage matrix turnover may shed light on poorly understood mechanisms involved in OA pathogenesis and lead to a better understanding of the role of low-grade inflammation in OA pathogenesis and better classify different clinical phenotypes and molecular endotypes of OA, leading to personalized therapeutic approaches for OA.
Author Contributions
EBe: conceptualization and review methodology. EBa, GK, PB, IU, and UK: acquisition of resources. EBe, EBa, GK, PB, IU, and UK: writing—original draft. EBe, EBa, GK, PB, IU, UK, MD-G, and AM: writing—revision. EBe, EBa, GK, CT, HH, GL, AG, MD-G, and AM: review and further editing. EBe and AM: supervision. AM: final submission and funding acquisition. All authors contributed to the article and approved the submitted version.
Conflict of Interest
AG is a shareholder of BICL, LLC and consultant to Pfizer, Galapagos, TissueGene, MerckSerono, AstraZeneca, and Roche. AM has consulted for Abbvie, AlphaSights, Artialis SA, Flexion Therapeutics, Galapagos, Guidepoint Global, IAG, Kolon TissueGene, Pacira Biosciences Inc., Pfizer Consumer Healthcare (PCH), Servier, and Science Branding Communications and received research funding from the European Commission (FP7, IMI, Marie Skłodowska-Curie, ES Strukturines Paramos), Versus Arthritis (Arthritis Research UK), Pfizer Inc., Kolon TissueGene, and Merck KGaA. CT is an employee of Nordic Bioscience. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank our collaborators and members of our research teams for useful discussions about the emerging applications of biochemical marker platform and nanotechnologies in OA research and therapy.
Glossary
Glossary
- ACLT
anterior cruciate ligament transection
- ARGS, TEGE, FFGV
Aggrecan epitopes
- BIPEDs
burden of disease, investigative, prognostic, efficacy of intervention, diagnostic and safety
- C12C/uC12C
collagen type I and II cleavage product and its urine form
- C1M, C2M, C3M
matrix metalloproteinase (MMP) mediated types I, II, III collagen degradation biomarker
- C2C
type II collagen degradation biomarker, generated from C-terminus fragment
- C3F
complement C3 peptide fragment
- CA153, CA125
cancer antigen 153, 125
- CBA
cytometric bead array
- CCL2
C–C motif chemokine ligand 2
- CEA
carcinoembryonic antigen
- CHECK
Cohort Hip and Cohort Knee
- Coll2-1
type II collagen degradation biomarker 1
- Coll2-NO2
nitrated form of type II collagen degradation
- COMP
cartilage oligomeric matrix protein
- CPII
C-propeptide of type II procollagen
- CRP
C-reactive protein
- CRPM
neo-epitope of MMP-1 and MMP-8 mediated degradation of C-reactive protein
- CS846
chondroitin sulfate epitope 846
- CXCL1
C–X–C motif chemokine ligand 1
- dGEMRIC
delayed gadolinium-enhanced MRI of cartilage
- ECM
extracellular matrix
- EGF
endothelial growth factor
- ELISA
enzyme-linked immunosorbent assay
- ESR
erythrocyte sedimentation rate
- EULAR
European League Against Rheumatism
- FDA
Food and Drug Administration
- FDG
18F-fluorodeoxyglucose
- FGF2
fibroblast growth factor 2
- Fib3-1,2,3
fibulin-3 epitopes 1,2,3
- FLISA
fluorescence-linked immunosorbent assay
- FMGC
fluoro-microbeads guiding chip
- FNIH
Foundation for the National Institutes of Health
- GagCEST
chemical exchange saturation transfer of GAG
- GAGs
glycosaminoglycans
- HA
hyaluronic acid
- hsCRP
high-sensitivity CRP
- ICRS
International Cartilage Repair Society
- IFN-γ
interferon gamma
- IL-1β
2, 4, 5, 6, 8, 10, 12, 13, interleukin-1β, 2, 4, 5, 6, 8, 10, 12, 13
- IP-10
interferon gamma-induced protein-10
- KL
Kellgren and Lawrence
- LOD
limit of detection
- MAP
multianalyte profiling
- MCP-1,3
monocyte chemoattractant protein-1,3
- MBDA
multibiomarker disease activity
- MDC
macrophage-derived chemokine
- MIP-1
1α, 1β, macrophage inflammatory protein-1, 1α, 1β
- MMPs
matrix metalloproteinases
- MOAKS
MRI OA Knee Score
- MRI
magnetic resonance imaging
- MS
multiple sclerosis
- MSD
Meso Scale Discovery
- MSP
methylation-specific PCR
- NTXI/uNTXI
crosslinked N-telopeptide of type I collagen and its urine form
- OA
osteoarthritis
- OARSI
Osteoarthritis Research Society International
- OPG
osteoprotegerin
- PDGFR
platelet-derived growth factor receptors
- PET
positron emission tomography
- PIIANP
serum N-propeptide of collagen IIA
- PIIBNP
serum N-propeptide of collagen IIB
- RA
rheumatoid arthritis
- SCGF-β
stem cell growth factor-β
- sCTX-II and uCTX-II
serum/urinary C-telopeptide fragments of type II collagen
- SENSIA
silver-enhanced sandwich immunoassay
- SERS
surface enhanced Raman scattering
- SF
synovial fluid
- sICAM-1
soluble intercellular adhesion molecule-1
- SIRT1
deacetylase sirtuin-1
- SPECT/CT
single-photon emission computed tomography combined with high-resolution computed tomography
- SPRi
surface plasmon resonance imaging
- sVCAM-1
soluble vascular cell adhesion molecule-1
- TIMP-1
metallopeptidase inhibitor-1
- TNF-α
tumor necrosis factor-α;VEGF, vascular endothelial growth factor
- WORMS
whole-organ magnetic resonance imaging score.
Footnotes
Funding. We wish to acknowledge financial support from the European Structural and Social Funds through the Research Council of Lithuania (Lietuvos Mokslo Taryba) according to the Programme Attracting Foreign Researchers for Research Implementation, Grant No. 01.2.2-LMT-K-718-02-0022. The research underpinning some of the work reviewed has received funding from a number of sources including The European Commission Framework 7 program (EU FP7; HEALTH.2012.2.4.5-2, project number 305815; Novel Diagnostics and Biomarkers for Early Identification of Chronic Inflammatory Joint Diseases); the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115770, resources of which are composed of financial contribution from the European Union's Seventh Framework program (FP7/2007-2013), EFPIA companies' in-kind contribution and the H2020 projects RESTORE (project number 814558; topic NMBP-22-2018 RIA), and MIRACLE (project number 780598; topic ICT-30-2017). Details of the D-BOARD FP7 Consortium are available at: http://www.d-board.eu. Details of the H2020-RESTORE Consortium are available at restoreproject.eu. Details on the H2020-MIRACLE Consortium are available at miracleproject.eu. AM was the coordinator of the D-BOARD Consortium funded by European Commission Framework 7 program (EU FP7; HEALTH.2012.2.4.5–2, project number 305815, Novel Diagnostics and Biomarkers for Early Identification of Chronic Inflammatory Joint Diseases). AM is a member of the Applied Public-Private Research enabling OsteoArthritis Clinical Headway (APPROACH) consortium, a 5-year project funded by the European Commission's Innovative Medicines Initiative (IMI). Details of the APPROACH IMI Consortium are available at: https://www.approachproject.eu. AM has also received financial support from the European Commission through a Marie Skłodowska-Curie Intra-European Fellowship for career development awarded to Dr. Csaba Matta (project number: 625746; acronym: CHONDRION; FP7-PEOPLE-2013-IEF). MD-G was supported by Rosetrees Trust (PGS19-2/10029) and the Israeli Science foundation (370/17).
References
- 1.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. (2013) 21:1145–53. 10.1016/j.joca.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shane Anderson A, Loeser RF. Why is osteoarthritis an age-related disease? Best Pract Res Clin Rheumatol. (2010) 24:15–26. 10.1016/j.berh.2009.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mobasheri A. Osteoarthritis year 2012 in review: biomarkers. Osteoarthritis Cartilage. (2012) 20:1451–64. 10.1016/j.joca.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 4.Biomarkers Definitions Working G . Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. (2001) 69:89–95. 10.1067/mcp.2001.113989 [DOI] [PubMed] [Google Scholar]
- 5.Mobasheri A. Biomarkers of osteoarthritis: translating information from in vitro culture systems to human patients. BMC Genomics. (2014) 15:O16 10.1186/1471-2164-15-S2-O16 [DOI] [Google Scholar]
- 6.Eckstein F, Guermazi A, Gold G, Duryea J, Hellio Le Graverand MP, Wirth W, et al. Imaging of cartilage and bone: promises and pitfalls in clinical trials of osteoarthritis. Osteoarthritis Cartilage. (2014) 22:1516–32. 10.1016/j.joca.2014.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakellariou G, Conaghan PG, Zhang W, Bijlsma JWJ, Boyesen P, D'Agostino MA, et al. EULAR recommendations for the use of imaging in the clinical management of peripheral joint osteoarthritis. Ann Rheum Dis. (2017) 76:1484–94. 10.1136/annrheumdis-2016-210815 [DOI] [PubMed] [Google Scholar]
- 8.Roemer FW, Kwoh CK, Hayashi D, Felson DT, Guermazi A. The role of radiography and MRI for eligibility assessment in DMOAD trials of knee OA. Nat Rev Rheumatol. (2018) 14:372–80. 10.1038/s41584-018-0010-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guermazi A, Roemer FW, Burstein D, Hayashi D. Why radiography should no longer be considered a surrogate outcome measure for longitudinal assessment of cartilage in knee osteoarthritis. Arthritis Res Ther. (2011) 13:247 10.1186/ar3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guermazi A. Osteoarthritis: you can rely on radiography when managing OA, but not too much! Nat Rev Rheumatol. (2017) 13:394–6. 10.1038/nrrheum.2017.77 [DOI] [PubMed] [Google Scholar]
- 11.Van Spil WE, Kubassova O, Boesen M, Bay-Jensen AC, Mobasheri A. Osteoarthritis phenotypes and novel therapeutic targets. Biochem Pharmacol. (2019) 165:41–8. 10.1016/j.bcp.2019.02.037 [DOI] [PubMed] [Google Scholar]
- 12.Emery CA, Whittaker JL, Mahmoudian A, Lohmander LS, Roos EM, Bennell KL, et al. Establishing outcome measures in early knee osteoarthritis. Nat Rev Rheumatol. (2019) 15:438–48. 10.1038/s41584-019-0237-3 [DOI] [PubMed] [Google Scholar]
- 13.Bay-Jensen AC, Sondergaard BC, Christiansen C, Karsdal MA, Madsen SH, Qvist P. Biochemical markers of joint tissue turnover. Assay Drug Dev Technol. (2010) 8:118–24. 10.1089/adt.2009.0199 [DOI] [PubMed] [Google Scholar]
- 14.Lotz M, Martel-Pelletier J, Christiansen C, Brandi ML, Bruyere O, Chapurlat R, et al. Republished: value of biomarkers in osteoarthritis: current status and perspectives. Postgrad Med J. (2014) 90:171–8. 10.1136/postgradmedj-2013-203726rep [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen LT, Sharma AR, Chakraborty C, Saibaba B, Ahn ME, Lee SS. Review of prospects of biological fluid biomarkers in osteoarthritis. Int J Mol Sci. (2017) 18:601. 10.3390/ijms18030601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mobasheri A, Saarakkala S, Finnila M, Karsdal MA, Bay-Jensen AC, van Spil WE. Recent advances in understanding the phenotypes of osteoarthritis. F1000Res. (2019) 8:F1000. 10.12688/f1000research.20575.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luyten FP, Bierma-Zeinstra S, Dell'Accio F, Kraus VB, Nakata K, Sekiya I, et al. Toward classification criteria for early osteoarthritis of the knee. Semin Arthritis Rheum. (2018) 47:457–63. 10.1016/j.semarthrit.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 18.Kraus VB. Preclinical and Early Osteoarthritis. Rheumatology. 7 ed Philadelphia, PA: Elsevier; (2018). p. 1576–81. [Google Scholar]
- 19.van Spil WE, DeGroot J, Lems WF, Oostveen JC, Lafeber FP. Serum and urinary biochemical markers for knee and hip-osteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthritis Cartilage. (2010) 18:605–12. 10.1016/j.joca.2010.01.012 [DOI] [PubMed] [Google Scholar]
- 20.Mobasheri A, van Spil WE, Budd E, Uzieliene I, Bernotiene E, Bay-Jensen AC, et al. Molecular taxonomy of osteoarthritis for patient stratification, disease management and drug development: biochemical markers associated with emerging clinical phenotypes and molecular endotypes. Curr Opin Rheumatol. (2019) 31:80–9. 10.1097/BOR.0000000000000567 [DOI] [PubMed] [Google Scholar]
- 21.Bay-Jensen AC, Thudium CS, Mobasheri A. Development and use of biochemical markers in osteoarthritis: current update. Curr Opin Rheumatol. (2018) 30:121–8. 10.1097/BOR.0000000000000467 [DOI] [PubMed] [Google Scholar]
- 22.van Spil WE, Szilagyi IA. Osteoarthritis year in review 2019: biomarkers (biochemical markers). Osteoarthritis Cartilage. (2020) 28:296–315. 10.1016/j.joca.2019.11.007 [DOI] [PubMed] [Google Scholar]
- 23.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. (2013) 21:16–21. 10.1016/j.joca.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 24.Roemer FW, Kassim Javaid M, Guermazi A, Thomas M, Kiran A, Keen R, et al. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthritis Cartilage. (2010) 18:1269–74. 10.1016/j.joca.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 25.Berenbaum F, Eymard F, Houard X. Osteoarthritis, inflammation and obesity. Curr Opin Rheumatol. (2013) 25:114–8. 10.1097/BOR.0b013e32835a9414 [DOI] [PubMed] [Google Scholar]
- 26.Mobasheri A, Bay-Jensen AC, van Spil WE, Larkin J, Levesque MC. Osteoarthritis year in review 2016: biomarkers (biochemical markers). Osteoarthritis Cartilage. (2017) 25:199–208. 10.1016/j.joca.2016.12.016 [DOI] [PubMed] [Google Scholar]
- 27.Bauer DC, Hunter DJ, Abramson SB, Attur M, Corr M, Felson D, et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. (2006) 14:723–7. 10.1016/j.joca.2006.04.001 [DOI] [PubMed] [Google Scholar]
- 28.Kraus VB, Burnett B, Coindreau J, Cottrell S, Eyre D, Gendreau M, et al. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthritis Cartilage. (2011) 19:515–42. 10.1016/j.joca.2010.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saberi Hosnijeh F, Bierma-Zeinstra SM, Bay-Jensen AC. Osteoarthritis year in review 2018: biomarkers (biochemical markers). Osteoarthritis Cartilage. (2019) 27:412–23. 10.1016/j.joca.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 30.Wyatt LA, Nwosu LN, Wilson D, Hill R, Spendlove I, Bennett AJ, et al. Molecular expression patterns in the synovium and their association with advanced symptomatic knee osteoarthritis. Osteoarthritis Cartilage. (2019) 27:667–75. 10.1016/j.joca.2018.12.012 [DOI] [PubMed] [Google Scholar]
- 31.Kraus VB, Hargrove DE, Hunter DJ, Renner JB, Jordan JM. Establishment of reference intervals for osteoarthritis-related soluble biomarkers: the FNIH/OARSI OA biomarkers consortium. Ann Rheum Dis. (2017) 76:179–85. 10.1136/annrheumdis-2016-209253 [DOI] [PubMed] [Google Scholar]
- 32.Henrotin Y, Sanchez C, Bay-Jensen AC, Mobasheri A. Osteoarthritis biomarkers derived from cartilage extracellular matrix: current status and future perspectives. Ann Phys Rehabil Med. (2016) 59:145–8. 10.1016/j.rehab.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 33.Kraus VB, Collins JE, Hargrove D, Losina E, Nevitt M, Katz JN, et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA biomarkers consortium. Ann Rheum Dis. (2017) 76:186–95. 10.1136/annrheumdis-2016-209252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung YY, Huebner JL, Haaland B, Wong SBS, Kraus VB. Synovial fluid pro-inflammatory profile differs according to the characteristics of knee pain. Osteoarthritis Cartilage. (2017) 25:1420–7. 10.1016/j.joca.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 35.Ishijima M, Watari T, Naito K, Kaneko H, Futami I, Yoshimura-Ishida K, et al. Relationships between biomarkers of cartilage, bone, synovial metabolism and knee pain provide insights into the origins of pain in early knee osteoarthritis. Arthritis Res Ther. (2011) 13:R22. 10.1186/ar3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arellano RD, Aguilar LS, Arguello R, Hernadez F, Gonzalez FF, Moran J. Cartilage oligomeric matrix protein levels in synovial fluid in patients with primary knee osteoarthritis and healthy controls: a preliminary comparative analysis with serum cartilage oligomeric matrix protein. Arch Rheumatol. (2017) 32:189–96. 10.5606/ArchRheumatol.2017.6220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hao HQ, Zhang JF, He QQ, Wang Z. Cartilage oligomeric matrix protein, C-terminal cross-linking telopeptide of type II collagen, and matrix metalloproteinase-3 as biomarkers for knee and hip osteoarthritis (OA) diagnosis: a systematic review and meta-analysis. Osteoarthritis Cartilage. (2019) 27:726–36. 10.1016/j.joca.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 38.Lorenzo P, Aspberg A, Saxne T, Onnerfjord P. Quantification of cartilage oligomeric matrix protein (COMP) and a COMP neoepitope in synovial fluid of patients with different joint disorders by novel automated assays. Osteoarthritis Cartilage. (2017) 25:1436–42. 10.1016/j.joca.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 39.Bay-Jensen AC, Engstroem A, Sharma N, Karsdal MA. Blood and urinary collagen markers in osteoarthritis: markers of tissue turnover and disease activity. Expert Rev Mol Diagn. (2020) 20:57–68. 10.1080/14737159.2020.1704257 [DOI] [PubMed] [Google Scholar]
- 40.Mabey T, Honsawek S, Tanavalee A, Yuktanandana P, Wilairatana V, Poovorawan Y. Plasma and synovial fluid inflammatory cytokine profiles in primary knee osteoarthritis. Biomarkers. (2016) 21:639–44. 10.3109/1354750X.2016.1171907 [DOI] [PubMed] [Google Scholar]
- 41.Bastick AN, Belo JN, Runhaar J, Bierma-Zeinstra SM. What are the prognostic factors for radiographic progression of knee osteoarthritis? A meta-analysis. Clin Orthop Relat Res. (2015) 473:2969–89. 10.1007/s11999-015-4349-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kraus VB, Jordan JM. Serum c-reactive protein (CRP), target for therapy or trouble? Biomark Insights. (2007) 1:77–80. 10.1177/117727190600100020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dietrich M, Jialal I. The effect of weight loss on a stable biomarker of inflammation, C-reactive protein. Nutr Rev. (2005) 63:22–8. 10.1111/j.1753-4887.2005.tb00107.x [DOI] [PubMed] [Google Scholar]
- 44.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. (2005) 46:464–9. 10.1016/j.jacc.2005.04.051 [DOI] [PubMed] [Google Scholar]
- 45.Bay-Jensen AC, Byrjalsen I, Siebuhr AS, Christiansen C, Platt A, Karsdal MA. Serological biomarkers of joint tissue turnover predict tocilizumab response at baseline. J Clin Rheumatol. (2014) 20:332–5. 10.1097/RHU.0000000000000150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siebuhr AS, Husakova M, Forejtova S, Zegzulkova K, Tomcik M, Urbanova M, et al. Metabolites of C-reactive protein and vimentin are associated with disease activity of axial spondyloarthritis. Clin Exp Rheumatol. (2019) 37:358–66. Available online at: https://www.clinexprheumatol.org/ [PubMed] [Google Scholar]
- 47.Haraden CA, Huebner JL, Hsueh MF, Li YJ, Kraus VB. Synovial fluid biomarkers associated with osteoarthritis severity reflect macrophage and neutrophil related inflammation. Arthritis Res Ther. (2019) 21:146. 10.1186/s13075-019-1923-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loeser RF, Beavers DP, Bay-Jensen AC, Karsdal MA, Nicklas BJ, Guermazi A, et al. Effects of dietary weight loss with and without exercise on interstitial matrix turnover and tissue inflammation biomarkers in adults with knee osteoarthritis: the intensive diet and exercise for Arthritis trial (IDEA). Osteoarthritis Cartilage. (2017) 25:1822–8. 10.1016/j.joca.2017.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siebuhr AS, Petersen KK, Arendt-Nielsen L, Egsgaard LL, Eskehave T, Christiansen C, et al. Identification and characterisation of osteoarthritis patients with inflammation derived tissue turnover. Osteoarthritis Cartilage. (2014) 22:44–50. 10.1016/j.joca.2013.10.020 [DOI] [PubMed] [Google Scholar]
- 50.Saberi Hosnijeh F, Siebuhr AS, Uitterlinden AG, Oei EH, Hofman A, Karsdal MA, et al. Association between biomarkers of tissue inflammation and progression of osteoarthritis: evidence from the Rotterdam study cohort. Arthritis Res Ther. (2016) 18:81. 10.1186/s13075-016-0976-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, et al. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. (2010) 18:1441–7. 10.1016/j.joca.2010.08.016 [DOI] [PubMed] [Google Scholar]
- 52.Pare F, Tardif G, Fahmi H, Ouhaddi Y, Pelletier JP, Martel-Pelletier J. In vivo protective effect of adipsin-deficiency on spontaneous knee osteoarthritis in aging mice. Aging. (2020) 12:2880–96. 10.18632/aging.102784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aeschlimann D, Mosher D, Paulsson M. Tissue transglutaminase and factor XIII in cartilage and bone remodeling. Semin Thromb Hemost. (1996) 22:437–43. 10.1055/s-2007-999043 [DOI] [PubMed] [Google Scholar]
- 54.Aeschlimann D, Thomazy V. Protein crosslinking in assembly and remodelling of extracellular matrices: the role of transglutaminases. Connect Tissue Res. (2000) 41:1–27. 10.3109/03008200009005638 [DOI] [PubMed] [Google Scholar]
- 55.Nurminskaya M, Magee C, Faverman L, Linsenmayer TF. Chondrocyte-derived transglutaminase promotes maturation of preosteoblasts in periosteal bone. Dev Biol. (2003) 263:139–52. 10.1016/S0012-1606(03)00445-7 [DOI] [PubMed] [Google Scholar]
- 56.Johnson KA, Terkeltaub RA. External GTP-bound transglutaminase 2 is a molecular switch for chondrocyte hypertrophic differentiation and calcification. J Biol Chem. (2005) 280:15004–12. 10.1074/jbc.M500962200 [DOI] [PubMed] [Google Scholar]
- 57.Johnson K, Svensson CI, Etten DV, Ghosh SS, Murphy AN, Powell HC, et al. Mediation of spontaneous knee osteoarthritis by progressive chondrocyte ATP depletion in Hartley guinea pigs. Arthritis Rheum. (2004) 50:1216–25. 10.1002/art.20149 [DOI] [PubMed] [Google Scholar]
- 58.Tarantino U, Ferlosio A, Arcuri G, Spagnoli LG, Orlandi A. Transglutaminase 2 as a biomarker of osteoarthritis: an update. Amino Acids. (2013) 44:199–207. 10.1007/s00726-011-1181-y [DOI] [PubMed] [Google Scholar]
- 59.Braza-Boils A, Ferrandiz ML, Terencio MC, Alcaraz MJ. Analysis of early biochemical markers and regulation by tin protoporphyrin IX in a model of spontaneous osteoarthritis. Exp Gerontol. (2012) 47:406–9. 10.1016/j.exger.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 60.Lohmander LS, Brandt KD, Mazzuca SA, Katz BP, Larsson S, Struglics A, et al. Use of the plasma stromelysin (matrix metalloproteinase 3) concentration to predict joint space narrowing in knee osteoarthritis. Arthritis Rheum. (2005) 52:3160–7. 10.1002/art.21345 [DOI] [PubMed] [Google Scholar]
- 61.Lai WF, Chang CH, Tang Y, Bronson R, Tung CH. Early diagnosis of osteoarthritis using cathepsin B sensitive near-infrared fluorescent probes. Osteoarthritis Cartilage. (2004) 12:239–44. 10.1016/j.joca.2003.11.005 [DOI] [PubMed] [Google Scholar]
- 62.Ben-Aderet L, Merquiol E, Fahham D, Kumar A, Reich E, Ben-Nun Y, et al. Detecting cathepsin activity in human osteoarthritis via activity-based probes. Arthritis Res Ther. (2015) 17:69. 10.1186/s13075-015-0586-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dvir-Ginzberg M, Gagarina V, Lee EJ, Booth R, Gabay O, Hall DJ. Tumor necrosis factor alpha-mediated cleavage and inactivation of SirT1 in human osteoarthritic chondrocytes. Arthritis Rheum. (2011) 63:2363–73. 10.1002/art.30279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dvir-Ginzberg M, Reich E. Chopping off the chondrocyte proteome. Biomarkers. (2015) 20:526–32. 10.3109/1354750X.2014.955884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Batshon G, Elayyan J, Qiq O, Reich E, Ben-Aderet L, Kandel L, et al. Serum NT/CT SIRT1 ratio reflects early osteoarthritis and chondrosenescence. Ann Rheum Dis. (2020) 79:1370–80. 10.1136/annrheumdis-2020-217072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pousinis P, Gowler PRW, Burston JJ, Ortori CA, Chapman V, Barrett DA. Lipidomic identification of plasma lipids associated with pain behaviour and pathology in a mouse model of osteoarthritis. Metabolomics. (2020) 16:32. 10.1007/s11306-020-01652-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sluzalska KD, Liebisch G, Lochnit G, Ishaque B, Hackstein H, Schmitz G, et al. Interleukin-1beta affects the phospholipid biosynthesis of fibroblast-like synoviocytes from human osteoarthritic knee joints. Osteoarthritis Cartilage. (2017) 25:1890–9. 10.1016/j.joca.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 68.Kosinska MK, Liebisch G, Lochnit G, Wilhelm J, Klein H, Kaesser U, et al. Sphingolipids in human synovial fluid–a lipidomic study. PLoS ONE. (2014) 9:e91769. 10.1371/journal.pone.0091769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuyinu EL, Narayanan G, Nair LS, Laurencin CT. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res. (2016) 11:19. 10.1186/s13018-016-0346-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alam MR, Ji JR, Kim MS, Kim NS. Biomarkers for identifying the early phases of osteoarthritis secondary to medial patellar luxation in dogs. J Vet Sci. (2011) 12:273–80. 10.4142/jvs.2011.12.3.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henrotin Y, Martel-Pelletier J, Msika P, Guillou GB, Deberg M. Usefulness of specific OA biomarkers, Col12-1 and Col12-1NO(2), in the anterior cruciate ligament OA canine model. Osteoarthr Cartilage. (2012) 20:787–90. 10.1016/j.joca.2012.03.016 [DOI] [PubMed] [Google Scholar]
- 72.Henrotin Y, Chevalier X, Deberg M, Balblanc JC, Richette P, Mulleman D, et al. Early decrease of serum biomarkers of type II collagen degradation (Coll2-1) and joint inflammation (Coll2-1 NO2) by hyaluronic acid intra-articular injections in patients with knee osteoarthritis: A research study part of the Biovisco study. J Orthop Res. (2013) 31:901–7. 10.1002/jor.22297 [DOI] [PubMed] [Google Scholar]
- 73.Bertuglia A, Pagliara E, Grego E, Ricci A, Brkljaca-Bottegaro N. Pro-inflammatory cytokines and structural biomarkers are effective to categorize osteoarthritis phenotype and progression in standard bred racehorses over five years of racing career. BMC Vet Res. (2016) 12:246. 10.1186/s12917-016-0873-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ekman S, Lindahl A, Ruetschi U, Jansson A, Bjorkman K, Abrahamsson-Aurell K, et al. Effect of circadian rhythm, age, training and acute lameness on serum concentrations of cartilage oligomeric matrix protein (COMP) neo-epitope in horses. Equine Vet J. (2019) 51:674–80. 10.1111/evj.13082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mickiewicz B, Heard BJ, Chau JK, Chung M, Hart DA, Shrive NG, et al. Metabolic profiling of synovial fluid in a unilateral ovine model of anterior cruciate ligament reconstruction of the knee suggests biomarkers for early osteoarthritis. J Orthop Res. (2015) 33:71–7. 10.1002/jor.22743 [DOI] [PubMed] [Google Scholar]
- 76.Miyamoto K, Ishimaru JI, Kurita K, Goss AN. Synovial matrix metalloproteinase-2 in different stages of sheep temporomandibular joint osteoarthrosis. J Oral Maxil Surg. (2002) 60:66–72. 10.1053/joms.2002.29077 [DOI] [PubMed] [Google Scholar]
- 77.Watt FE. Osteoarthritis biomarkers: year in review. Osteoarthr Cartilage. (2018) 26:312–8. 10.1016/j.joca.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 78.Ellington AA, Kullo IJ, Bailey KR, Klee GG. Antibody-based protein multiplex platforms: technical and operational challenges. Clin Chem. (2010) 56:186–93. 10.1373/clinchem.2009.127514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lea P, Keystone E, Mudumba S, Kahama A, Ding SF, Hansen J, et al. Advantages of multiplex proteomics in clinical immunology: the case of rheumatoid arthritis: novel IgXPLEX: planar microarray diagnosis. Clin Rev Allergy Immunol. (2011) 41:20–35. 10.1007/s12016-009-8189-z [DOI] [PubMed] [Google Scholar]
- 80.Adamcova M, Simko F. Multiplex biomarker approach to cardiovascular diseases. Acta Pharmacol Sin. (2018) 39:1068–72. 10.1038/aps.2018.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ekins RP. Ligand assays: from electrophoresis to miniaturized microarrays. Clin Chem. (1998) 44:2015–30. 10.1093/clinchem/44.9.2015 [DOI] [PubMed] [Google Scholar]
- 82.Chen Z, Dodig-Crnkovic T, Schwenk JM, Tao SC. Current applications of antibody microarrays. Clin Proteomics. (2018) 15:7. 10.1186/s12014-018-9184-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang T, O'Malley TT, Kanmert D, Jerecic J, Zieske LR, Zetterberg H, et al. A highly sensitive novel immunoassay specifically detects low levels of soluble Abeta oligomers in human cerebrospinal fluid. Alzheimers Res Ther. (2015) 7:14. 10.1186/s13195-015-0100-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bastarache JA, Koyama T, Wickersham NE, Ware LB. Validation of a multiplex electrochemiluminescent immunoassay platform in human and mouse samples. J Immunol Methods. (2014) 408:13–23. 10.1016/j.jim.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, et al. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol. (2004) 110:252–66. 10.1016/j.clim.2003.11.017 [DOI] [PubMed] [Google Scholar]
- 86.Vostry M. Multiplex immunoassays: chips and beads. EJIFCC. (2010) 20:162–5. Available online at: https://europepmc.org/ [PMC free article] [PubMed] [Google Scholar]
- 87.dupont NC, Wang K, Wadhwa PD, Culhane JF, Nelson EL. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol. (2005) 66:175–91. 10.1016/j.jri.2005.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baker HN, Murphy R, Lopez E, Garcia C. Conversion of a capture ELISA to a Luminex xMAP assay using a multiplex antibody screening method. J Vis Exp. (2012) 6:4084. 10.3791/4084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pincus T, Castrejon I, Yazici Y, Gibson KA, Bergman MJ, Block JA. Osteoarthritis is as severe as rheumatoid arthritis: evidence over 40 years according to the same measure in each disease. Clin Exp Rheumatol. (2019) 37:7–17. Available online at: https://www.clinexprheumatol.org/ [PubMed] [Google Scholar]
- 90.Lundberg M, Eriksson A, Tran B, Assarsson E, Fredriksson S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. (2011) 39:e102. 10.1093/nar/gkr424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Assarsson E, Lundberg M, Holmquist G, Bjorkesten J, Thorsen SB, Ekman D, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE. (2014) 9:e95192. 10.1371/journal.pone.0095192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mahboob S, Ahn SB, Cheruku HR, Cantor D, Rennel E, Fredriksson S, et al. A novel multiplexed immunoassay identifies CEA, IL-8 and prolactin as prospective markers for Dukes' stages A-D colorectal cancers. Clin Proteomics. (2015) 12:10. 10.1186/s12014-015-9081-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abasiyanik MF, Wolfe K, Van Phan H, Lin J, Laxman B, White SR, et al. Ultrasensitive digital quantification of cytokines and bacteria predicts septic shock outcomes. Nat Commun. (2020) 11:2607. 10.1038/s41467-020-16124-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smolsky J, Kaur S, Hayashi C, Batra SK, Krasnoslobodtsev AV. Surface-enhanced raman scattering-based immunoassay technologies for detection of disease biomarkers. Biosensors. (2017) 7:7. 10.3390/bios7010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gellner M, Kompe K, Schlucker S. Multiplexing with SERS labels using mixed SAMs of Raman reporter molecules. Anal Bioanal Chem. (2009) 394:1839–44. 10.1007/s00216-009-2868-8 [DOI] [PubMed] [Google Scholar]
- 96.Farka Z, Mickert MJ, Hlavacek A, Skladal P, Gorris HH. Single molecule upconversion-linked immunosorbent assay with extended dynamic range for the sensitive detection of diagnostic biomarkers. Anal Chem. (2017) 89:11825–30. 10.1021/acs.analchem.7b03542 [DOI] [PubMed] [Google Scholar]
- 97.Candia J, Cheung F, Kotliarov Y, Fantoni G, Sellers B, Griesman T, et al. Assessment of variability in the SOMAscan assay. Sci Rep. (2017) 7:14248. 10.1038/s41598-017-14755-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rohloff JC, Gelinas AD, Jarvis TC, Ochsner UA, Schneider DJ, Gold L, et al. Nucleic acid ligands with protein-like side chains: modified aptamers and their use as diagnostic and therapeutic agents. Mol Ther Nucleic Acids. (2014) 3:e201. 10.1038/mtna.2014.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Breen EC, Reynolds SM, Cox C, Jacobson LP, Magpantay L, Mulder CB, et al. Multisite comparison of high-sensitivity multiplex cytokine assays. Clin Vaccine Immunol. (2011) 18:1229–42. 10.1128/CVI.05032-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thompson DK, Huffman KM, Kraus WE, Kraus VB. Critical appraisal of four IL-6 immunoassays. PLoS ONE. (2012) 7:e30659. 10.1371/journal.pone.0030659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chandra PE, Sokolove J, Hipp BG, Lindstrom TM, Elder JT, Reveille JD, et al. Novel multiplex technology for diagnostic characterization of rheumatoid arthritis. Arthritis Res Ther. (2011) 13:R102. 10.1186/ar3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eastman PS, Manning WC, Qureshi F, Haney D, Cavet G, Alexander C, et al. Characterization of a multiplex, 12-biomarker test for rheumatoid arthritis. J Pharm Biomed Anal. (2012) 70:415–24. 10.1016/j.jpba.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 103.Fernandez-Puente P, Calamia V, Gonzalez-Rodriguez L, Lourido L, Camacho-Encina M, Oreiro N, et al. Multiplexed mass spectrometry monitoring of biomarker candidates for osteoarthritis. J Proteomics. (2017) 152:216–25. 10.1016/j.jprot.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 104.Rech J, Hueber AJ, Finzel S, Englbrecht M, Haschka J, Manger B, et al. Prediction of disease relapses by multibiomarker disease activity and autoantibody status in patients with rheumatoid arthritis on tapering DMARD treatment. Ann Rheum Dis. (2016) 75:1637–44. 10.1136/annrheumdis-2015-207900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fleischmann R, Connolly SE, Maldonado MA, Schiff M. Brief report: estimating disease activity using multi-biomarker disease activity scores in rheumatoid arthritis patients treated with abatacept or adalimumab. Arthritis Rheumatol. (2016) 68:2083–9. 10.1002/art.39714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roodenrijs NMT, de Hair MJH, Wheater G, Elshahaly M, Tekstra J, Teng YKO, et al. The multi-biomarker disease activity score tracks response to rituximab treatment in rheumatoid arthritis patients: a post hoc analysis of three cohort studies. Arthritis Res Ther. (2018) 20:256. 10.1186/s13075-018-1750-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Impact of Guided Care With the Vectra DA Multi-biomarker Disease Activity (MBDA) Blood Test. National Library of Medicine (US) (2019). Available online at: https://ClinicalTrials.gov/show/NCT03810144 (accessed October 22, 2019).
- 108.Malekzadeh A, Twaalfhoven H, Wijnstok NJ, Killestein J, Blankenstein MA, Teunissen CE. Comparison of multiplex platforms for cytokine assessments and their potential use for biomarker profiling in multiple sclerosis. Cytokine. (2017) 91:145–52. 10.1016/j.cyto.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 109.Claudon A, Vergnaud P, Valverde C, Mayr A, Klause U, Garnero P. New automated multiplex assay for bone turnover markers in osteoporosis. Clin Chem. (2008) 54:1554–63. 10.1373/clinchem.2008.105866 [DOI] [PubMed] [Google Scholar]
- 110.Greenblatt MB, Tsai JN, Wein MN. Biochemical markers of bone turnover. In: Leder BZ, Wein MN. editors. Osteoporosis. Contemporary Endocrinology. Cham: Springer International Publishing; (2020) p. 169–84. [Google Scholar]
- 111.Wild N, Karl J, Risse B. From research to clinical application multi-parameter testing: marker panels for the early detection of complex diseases. J Med Biochem. (2009) 28:279–84. 10.2478/v10011-009-0024-9 [DOI] [Google Scholar]
- 112.Bhardwaj M, Weigl K, Tikk K, Benner A, Schrotz-King P, Brenner H. Multiplex screening of 275 plasma protein biomarkers to identify a signature for early detection of colorectal cancer. Mol Oncol. (2020) 14:8–21. 10.1002/1878-0261.12591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zheng Z, Wu L, Li L, Zong S, Wang Z, Cui Y. Simultaneous and highly sensitive detection of multiple breast cancer biomarkers in real samples using a SERS microfluidic chip. Talanta. (2018) 188:507–15. 10.1016/j.talanta.2018.06.013 [DOI] [PubMed] [Google Scholar]
- 114.Jiang W, Huang R, Duan C, Fu L, Xi Y, Yang Y, et al. Identification of five serum protein markers for detection of ovarian cancer by antibody arrays. PLoS ONE. (2013) 8:e76795. 10.1371/journal.pone.0076795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Anderson KS, LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J Proteome Res. (2005) 4:1123–33. 10.1021/pr0500814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wallstrom G, Anderson KS, LaBaer J. Biomarker discovery for heterogeneous diseases. Cancer Epidemiol Biomarkers Prev. (2013) 22:747–55. 10.1158/1055-9965.EPI-12-1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ren G, Lutz I, Railton P, Wiley JP, McAllister J, Powell J, et al. Serum and synovial fluid cytokine profiling in hip osteoarthritis: distinct from knee osteoarthritis and correlated with pain. BMC Musculoskelet Disord. (2018) 19:39. 10.1186/s12891-018-1955-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vangsness CT, Jr., Burke WS, Narvy SJ, MacPhee RD, Fedenko AN. Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis–a pilot study. Bull NYU Hosp Jt Dis. (2011) 69:122–7. [PubMed] [Google Scholar]
- 119.Nees TA, Rosshirt N, Zhang JA, Reiner T, Sorbi R, Tripel E, et al. Synovial cytokines significantly correlate with osteoarthritis-related knee pain and disability: inflammatory mediators of potential clinical relevance. J Clin Med. (2019) 8:1343. 10.3390/jcm8091343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Labinsky H, Panipinto PM, Ly KA, Khuat DK, Madarampalli B, Mahajan V, et al. Multiparameter analysis identifies heterogeneity in knee osteoarthritis synovial responses. Arthritis Rheumatol. (2020) 72:598–608. 10.1002/art.41161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hendriks J, Sharif M, Saris DB, Karperien M. A highly sensitive multiplex biomarker assay for the early diagnosis of osteoarthritis. Osteoarthr Cartilage. (2019) 27:S108 10.1016/j.joca.2019.02.161 [DOI] [Google Scholar]
- 122.Park YM, Kim SJ, Lee KJ, Yang SS, Min BH, Yoon HC. Detection of CTX-II in serum and urine to diagnose osteoarthritis by using a fluoro-microbeads guiding chip. Biosens Bioelectron. (2015) 67:192–9. 10.1016/j.bios.2014.08.016 [DOI] [PubMed] [Google Scholar]
- 123.Jayadev C, Snelling S, Price A, Hulley P. Multiplex analysis of osteoarthritic synovial fluid: a comparison of luminex & mesoscale discovery. Osteoarthr Cartilage. (2013) 21:S73 10.1016/j.joca.2013.02.159 [DOI] [Google Scholar]
- 124.Hendriks J, Stojanovic I, Saris DB, Karperien M. A new highly sensitive method for multiplex measurements of biomarkers in synovial fluid aspirates. Osteoarthr Cartilage. (2017) 25:S99 10.1016/j.joca.2017.02.159 [DOI] [Google Scholar]
- 125.Yang X, Tang Y, Alt RR, Xie X, Li F. Emerging techniques for ultrasensitive protein analysis. Analyst. (2016) 141:3473–81. 10.1039/C6AN00059B [DOI] [PubMed] [Google Scholar]
- 126.Hu W, Li CM. Nanomaterial-based advanced immunoassays. Wiley Interdiscip Rev Nanomed Nanobiotechnol. (2011) 3:119–33. 10.1002/wnan.124 [DOI] [PubMed] [Google Scholar]
- 127.Hong SY, Park YM, Jang YH, Min BH, Yoon HC. Quantitative lateral-flow immunoassay for the assessment of the cartilage oligomeric matrix protein as a marker of osteoarthritis. Biochip J. (2012) 6:213–20. 10.1007/s13206-012-6303-4 [DOI] [Google Scholar]
- 128.Suzuki M, Udaka H, Fukuda T. Quantum dot-linked immunosorbent assay (QLISA) using orientation-directed antibodies. J Pharm Biomed Anal. (2017) 143:110–5. 10.1016/j.jpba.2017.05.014 [DOI] [PubMed] [Google Scholar]
- 129.Zhou G, Bergeron S, Juncker D. High-performance low-cost antibody microarrays using enzyme-mediated silver amplification. J Proteome Res. (2015) 14:1872–9. 10.1021/pr501259e [DOI] [PubMed] [Google Scholar]
- 130.Kaminska A, Winkler K, Kowalska A, Witkowska E, Szymborski T, Janeczek A, et al. SERS-based immunoassay in a microfluidic system for the multiplexed recognition of interleukins from blood plasma: towards picogram detection. Sci Rep. (2017) 7:10656. 10.1038/s41598-017-11152-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rong Z, Chen F, Jilin Y, Yifeng T. A C-reactive protein immunosensor based on platinum nanowire / titania nanotube composite sensitized electrochemiluminescence. Talanta. (2019) 205:120135. 10.1016/j.talanta.2019.120135 [DOI] [PubMed] [Google Scholar]
- 132.Wu J, Chen Y, Yang M, Wang Y, Zhang C, Yang M, et al. Streptavidin-biotin-peroxidase nanocomplex-amplified microfluidics immunoassays for simultaneous detection of inflammatory biomarkers. Anal Chim Acta. (2017) 982:138–47. 10.1016/j.aca.2017.05.031 [DOI] [PubMed] [Google Scholar]
- 133.Liu N, Yi H, Lin Y, Zheng H, Zheng X, Lin D, et al. Combined electrochemiluminescent and electrochemical immunoassay for interleukin 6 based on the use of TiO2 mesocrystal nanoarchitectures. Mikrochim Acta. (2018) 185:277. 10.1007/s00604-018-2802-x [DOI] [PubMed] [Google Scholar]
- 134.Corrie SR, Plebanski M. The emerging role of nanomaterials in immunological sensing - a brief review. Mol Immunol. (2018) 98:28–35. 10.1016/j.molimm.2017.12.017 [DOI] [PubMed] [Google Scholar]
- 135.Mehrotra P. Biosensors and their applications - a review. J Oral Biol Craniofac Res. (2016) 6:153–9. 10.1016/j.jobcr.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang SH, Shen CY, Weng TC, Lin PH, Yang JJ, Chen IF, et al. Detection of cartilage oligomeric matrix protein using a quartz crystal microbalance. Sensors. (2010) 10:11633–43. 10.3390/s101211633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vance SA, Sandros MG. Zeptomole detection of C-reactive protein in serum by a nanoparticle amplified surface plasmon resonance imaging aptasensor. Sci Rep. (2014) 4:5129. 10.1038/srep05129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ahmad N, Colak B, Zhang DW, Gibbs MJ, Watkinson M, Becer CR, et al. Peptide cross-linked poly (ethylene glycol) hydrogel films as biosensor coatings for the detection of collagenase. Sensors. (2019) 19:1677. 10.3390/s19071677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hsu WT, Hsieh WH, Cheng SF, Jen CP, Wu CC, Li CH, et al. Integration of fiber optic-particle plasmon resonance biosensor with microfluidic chip. Anal Chim Acta. (2011) 697:75–82. 10.1016/j.aca.2011.04.023 [DOI] [PubMed] [Google Scholar]
- 140.Huang YC, Chiang CY, Li CH, Chang TC, Chiang CS, Chau LK, et al. Quantification of tumor necrosis factor-alpha and matrix metalloproteinases-3 in synovial fluid by a fiber-optic particle plasmon resonance sensor. Analyst. (2013) 138:4599–606. 10.1039/c3an00276d [DOI] [PubMed] [Google Scholar]
- 141.Song SY, Han YD, Hong SY, Kim K, Yang SS, Min BH, et al. Chip-based cartilage oligomeric matrix protein detection in serum and synovial fluid for osteoarthritis diagnosis. Anal Biochem. (2012) 420:139–46. 10.1016/j.ab.2011.09.012 [DOI] [PubMed] [Google Scholar]
- 142.Duk Han Y, Jin Chun H, Yoon HC. The transformation of common office supplies into a low-cost optical biosensing platform. Biosens Bioelectron. (2014) 59:259–68. 10.1016/j.bios.2014.03.037 [DOI] [PubMed] [Google Scholar]
- 143.Park YM, Han YD, Chun HJ, Yoon HC. Ambient light-based optical biosensing platform with smartphone-embedded illumination sensor. Biosens Bioelectron. (2017) 93:205–11. 10.1016/j.bios.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 144.Chiang CY, Hsieh ML, Huang KW, Chau LK, Chang CM, Lyu SR. Fiber-optic particle plasmon resonance sensor for detection of interleukin-1beta in synovial fluids. Biosens Bioelectron. (2010) 26:1036–42. 10.1016/j.bios.2010.08.047 [DOI] [PubMed] [Google Scholar]
- 145.Yun YH, Bhattacharya A, Watts NB, Schulz MJ. A label-free electronic biosensor for detection of bone turnover markers. Sensors. (2009) 9:7957–69. 10.3390/s91007957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Belushkin A, Yesilkoy F, Altug H. Nanoparticle-enhanced plasmonic biosensor for digital biomarker detection in a microarray. ACS Nano. (2018) 12:4453–61. 10.1021/acsnano.8b00519 [DOI] [PubMed] [Google Scholar]
- 147.Chen P, Chung MT, McHugh W, Nidetz R, Li Y, Fu J, et al. Multiplex serum cytokine immunoassay using nanoplasmonic biosensor microarrays. ACS Nano. (2015) 9:4173–81. 10.1021/acsnano.5b00396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sanchez-Tirado E, Salvo C, Gonzalez-Cortes A, Yanez-Sedeno P, Langa F, Pingarron JM. Electrochemical immunosensor for simultaneous determination of interleukin-1 beta and tumor necrosis factor alpha in serum and saliva using dual screen printed electrodes modified with functionalized double-walled carbon nanotubes. Anal Chim Acta. (2017) 959:66–73. 10.1016/j.aca.2016.12.034 [DOI] [PubMed] [Google Scholar]
- 149.Yuan G, Yu C, Xia C, Gao L, Xu W, Li W, et al. A simultaneous electrochemical multianalyte immunoassay of high sensitivity C-reactive protein and soluble CD40 ligand based on reduced graphene oxide-tetraethylene pentamine that directly adsorb metal ions as labels. Biosens Bioelectron. (2015) 72:237–46. 10.1016/j.bios.2015.04.088 [DOI] [PubMed] [Google Scholar]
- 150.Vashist SK, Venkatesh AG, Marion Schneider E, Beaudoin C, Luppa PB, Luong JH. Bioanalytical advances in assays for C-reactive protein. Biotechnol Adv. (2016) 34:272–90. 10.1016/j.biotechadv.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 151.van Spil WE, Jansen NW, Bijlsma JW, Reijman M, DeGroot J, Welsing PM, et al. Clusters within a wide spectrum of biochemical markers for osteoarthritis: data from check, a large cohort of individuals with very early symptomatic osteoarthritis. Osteoarthritis Cartilage. (2012) 20:745–54. 10.1016/j.joca.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 152.Guermazi A, Roemer FW, Alizai H, Winalski CS, Welsch G, Brittberg M, et al. State of the art: MR imaging after knee cartilage repair surgery. Radiology. (2015) 277:23–43. 10.1148/radiol.2015141146 [DOI] [PubMed] [Google Scholar]
- 153.Deveza LA, Kraus VB, Collins JE, Guermazi A, Roemer FW, Nevitt MC, et al. Is synovitis detected on non-contrast-enhanced magnetic resonance imaging associated with serum biomarkers and clinical signs of effusion? Data from the osteoarthritis initiative. Scand J Rheumatol. (2018) 47:235–42. 10.1080/03009742.2017.1340511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Georgiev T, Ivanova M, Kopchev A, Velikova T, Miloshov A, Kurteva E, et al. Cartilage oligomeric protein, matrix metalloproteinase-3, and Coll2-1 as serum biomarkers in knee osteoarthritis: a cross-sectional study. Rheumatol Int. (2018) 38:821–30. 10.1007/s00296-017-3887-y [DOI] [PubMed] [Google Scholar]
- 155.Guimaraes JB, Nevitt MC, McCulloch CE, Schwaiger BJ, Gersing AS, Facchetti L, et al. Association of weight change with progression of meniscal intrasubstance degeneration over 48 months: Data from the Osteoarthritis Initiative. Eur Radiol. (2018) 28:953–62. 10.1007/s00330-017-5054-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ruan G, Xu J, Wang K, Zheng S, Wu J, Bian F, et al. Associations between serum IL-8 and knee symptoms, joint structures, and cartilage or bone biomarkers in patients with knee osteoarthritis. Clin Rheumatol. (2019) 38:3609–17. 10.1007/s10067-019-04718-8 [DOI] [PubMed] [Google Scholar]
- 157.Ruan G, Xu J, Wang K, Zheng S, Wu J, Ren J, et al. Associations between serum S100A8/S100A9 and knee symptoms, joint structures and cartilage enzymes in patients with knee osteoarthritis. Osteoarthritis Cartilage. (2019) 27:99–105. 10.1016/j.joca.2018.08.020 [DOI] [PubMed] [Google Scholar]
- 158.Martel-Pelletier J, Tardif G, Rousseau Trepanier J, Abram F, Dorais M, Raynauld JP, et al. The ratio adipsin/MCP-1 is strongly associated with structural changes and CRP/MCP-1 with symptoms in obese knee osteoarthritis subjects: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. (2019) 27:1163–73. 10.1016/j.joca.2019.04.016 [DOI] [PubMed] [Google Scholar]
- 159.Chu CR, Sheth S, Erhart-Hledik JC, Do B, Titchenal MR, Andriacchi TP. Mechanically stimulated biomarkers signal cartilage changes over 5 years consistent with disease progression in medial knee osteoarthritis patients. J Orthop Res. (2018) 36:891–7. 10.1002/jor.23720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim MK, Ha CW, In Y, Cho SD, Choi ES, Ha JK, et al. A multicenter, double-blind, phase III clinical trial to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients. Hum Gene Ther Clin Dev. (2018) 29:48–59. 10.1089/humc.2017.249 [DOI] [PubMed] [Google Scholar]
- 161.Mahler EAM, Minten MJ, Leseman-Hoogenboom MM, Poortmans PMP, Leer JWH, Boks SS, et al. Effectiveness of low-dose radiation therapy on symptoms in patients with knee osteoarthritis: a randomised, double-blinded, sham-controlled trial. Ann Rheum Dis. (2019) 78:83–90. 10.1136/annrheumdis-2018-214104 [DOI] [PubMed] [Google Scholar]
- 162.Alliston T, Hernandez CJ, Findlay DM, Felson DT, Kennedy OD. Bone marrow lesions in osteoarthritis: what lies beneath. J Orthop Res. (2018) 36:1818–25. 10.1002/jor.23844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kokkotis C, Moustakidis S, Papageorgiou E, Giakas G, Tsaopoulos DE. Machine learning in knee osteoarthritis: a review. Osteoarthritis Cartilage Open. (2020) 2020:100069 10.1016/j.ocarto.2020.100069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van Spil WE, Bierma-Zeinstra SMA, Deveza LA, Arden NK, Bay-Jensen AC, Kraus VB, et al. A consensus-based framework for conducting and reporting osteoarthritis phenotype research. Arthritis Res Ther. (2020) 22:54. 10.1186/s13075-020-2143-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hunter DJ, Eckstein F, Kraus VB, Losina E, Sandell L, Guermazi A. Imaging biomarker validation and qualification report: sixth OARSI workshop on imaging in osteoarthritis combined with third OA biomarkers workshop. Osteoarthritis Cartilage. (2013) 21:939–42. 10.1016/j.joca.2013.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hunter DJ, Nevitt M, Losina E, Kraus V. Biomarkers for osteoarthritis: current position and steps towards further validation. Best Pract Res Clin Rheumatol. (2014) 28:61–71. 10.1016/j.berh.2014.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Krishnamoorthy G, Nanga RPR, Bagga P, Hariharan H, Reddy R. High quality three-dimensional gagCEST imaging of in vivo human knee cartilage at 7 Tesla. Magn Reson Med. (2017) 77:1866–73. 10.1002/mrm.26265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hayashi D, Roemer FW, Guermazi A. Imaging of osteoarthritis by conventional radiography, MR imaging, pet-computed tomography, and PET-MR Imaging. PET Clin. (2019) 14:17–29. 10.1016/j.cpet.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 169.Kubota K, Yamashita H, Mimori A. Clinical Value of FDG-PET/CT for the evaluation of rheumatic diseases: rheumatoid arthritis, polymyalgia rheumatica, and relapsing polychondritis. Semin Nucl Med. (2017) 47:408–24. 10.1053/j.semnuclmed.2017.02.005 [DOI] [PubMed] [Google Scholar]