Introduction
Immune checkpoint inhibitors (ICPIs) are monoclonal antibodies that trigger increased antitumor immunity by blocking the intrinsic downregulators of immunity, such as programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T-lymphocyte antigen 4. The broad clinical activity has transformed the lives of many cancer patients, leading to significant, durable responses, especially in advanced melanoma, but it comes at the cost of a unique spectrum of side effects termed immune-related adverse events (irAEs). Immune checkpoint inhibitor–associated acute kidney injury (AKI) is well-described irAEs in which acute tubulointerstitial nephritis (ATIN) is the most common histopathological finding.1 The incidence of ICPI-AKI is estimated to be 0.5–6%.1 Median time from ICPI initiation to AKI onset is estimated to be 14 weeks (Figure 1).1, 2, 3, 4, 5, 6 We report a case of unusually rapid-onset oliguric AKI within 3 days of receiving the first dose of ipilimumab (cytotoxic T-lymphocyte antigen 4 inhibitor) and nivolumab (PD-1 inhibitor) in a patient with metastatic melanoma. Urine-microscopy findings of a white blood cell (WBC) cast prompted kidney biopsy on presentation. The biopsy confirmed ATIN and led to the successful management of ICPI-AKI. Immunoperoxidase staining demonstrated PD-L1 expression in the tubular epithelial cell (TEC) membrane and peritubular capillary, which might suggest some mechanistic role for this atypical presentation. Therefore, knowledge of the heterogeneous presentation of ICPI-AKI, diagnostic tests, and underlying pathogenesis is required for early recognition and management to minimize the development of chronic kidney disease and risk for rare fatal events.
Figure 1.
Median time to onset of immune checkpoint inhibitor (ICPI)–acute kidney injury (AKI) after initiation of ICPI. Circles represent medians and bars indicate ranges.
Case Presentation
A 67-year-old man was admitted to the hospital with severe weakness and diarrhea. Nephrology was consulted for doubling of serum creatinine within 3 days of the patient receiving the first dose of ICPI. His medical history included a diagnosis of type 2 diabetes mellitus, hypertension, coronary artery disease, and metastatic melanoma. Melanoma in situ was diagnosed during the evaluation of a skin lesion on the back in July 2017. It was treated with local excision with negative margins. In February 2020, imaging studies revealed multiple metastatic lesions in the brain, right lung, gastric body, and upper pole of the left kidney during the evaluation of right-hand clumsiness and dizziness. Multiple biopsies confirmed the diagnosis of melanoma in the lung and stomach. Once completing stereotactic radiosurgery to the brain lesions and a course of steroids for brain edema in March 2020, he received the first dose of nivolumab (1 mg/kg) and ipilimumab (3 mg/kg). Three days later, he presented to the clinic with worsening fatigue, nausea, and reduced oral intake, for which a comprehensive metabolic panel was obtained that revealed a serum creatinine of 2.30 mg/dl (estimated glomerular filtration rate of 28 ml/min). Baseline creatinine was 1.17–1.39 mg/dl over the past 6 months. He was hospitalized and started on fluid resuscitation because of relative hypotension. Angiotensin-converting enzyme inhibitor, hydrochlorothiazide, and proton-pump inhibitor (PPI) were discontinued. The patient had received a stable dose of each of these medications for at least the past 6 years. There were no recent changes in the dose of PPI. Nephrology was consulted on the third day of hospitalization to address worsening renal function. Upon examination, body temperature was 36.4 °C, heart rate was 65 beats/min, respiratory rate was 18 breaths/min, and blood pressure was 113/62 mm Hg. Physical examination revealed dry mucus membranes, regular work of breathing, clear lung fields on auscultation, regular heart rate and rhythm, soft and nontender abdomen, normal neurologic examination, and no skin rash or facial or pedal edema. The infectious workup was negative. Urinalysis demonstrated trace protein, red blood cell count of 4–10/high power field, and WBC count of 6–10/high power field. Urine sediments revealed granular and WBC casts (Figure 2). There was no history of exposure to new medications or radiocontrast study. The patient did not report an allergy to medications. Renal ultrasound showed normal kidney size; a small lesion in the upper pole of the left kidney demonstrated on previous images, and there was no hydronephrosis. After multidisciplinary ICPI toxicity team discussion regarding the progressive deterioration of renal function despite aggressive intravenous fluid replacement and suspicion for ATIN, a decision was made to perform a kidney biopsy and hold off on the empiric use of steroids. On the fifth day of hospitalization, serum creatinine was 7.23 mg/dl (estimated glomerular filtration rate of 8 ml/min), and the patient underwent an ultrasound-guided kidney biopsy. Hemodialysis had to be initiated on the same day because of hyperkalemia and anuria. The kidney biopsy contained 23 glomeruli, 3 of which were globally sclerosed (Figure 3). The remaining glomeruli appeared normal with no significant inflammatory cell infiltration. There was a patchy to diffuse interstitial lymphocytic cellular infiltrate with a focal increase in eosinophils, associated with mild interstitial edema. Also, a few multinucleated giant cells were present. However, no well-defined granulomas were seen. Special stains for acid-fast bacilli and fungal organisms were negative. Tubules displayed moderate degenerative changes, including attenuation of brush borders, tubulitis, and some cytoplasmic vacuolization and sloughing of tubular epithelial cells. There was no significant interstitial fibrosis or tubular atrophy (5–10% in aggregate). Immunofluorescence staining revealed no significant immune deposits except tubular basement membranes, demonstrating a focal deposition of C3 (+). Electron microscopy examination revealed no electron-dense deposits.
Figure 2.
Urine microscopy demonstrating white blood cell cast (arrow).
Figure 3.
Kidney biopsy. Photomicrographs depicting morphologic features of the renal biopsy. (a) Intense cellular infiltrates composed of lymphocytes, a few monocytes, and eosinophils. Focal areas of tubulitis (white arrowhead) and a few multinucleated giant cells (asterisk) are present (hematoxylin-eosin stain; bar = 150 μm). (b) Intense anti-CD3 immunoreactivity with interstitial cellular infiltrates (immunoperoxidase stain; bar = 150 μm). (c) Focal immunoreactivity of anti-PD-L1 with cellular infiltrates (arrows) and tubular epithelial (arrowheads) and few of the peritubular capillaries (immunoperoxidase stain; bar = 150 μm). (d) No significant immunoreactivity of anti-CD20 with the interstitial cellular infiltrates (immunoperoxidase stain; bar = 150 μm).
The patient was treated with pulsed steroids, 500 mg methylprednisolone intravenously for 3 days, followed by 60 mg oral prednisone daily. He was maintained on hemodialysis 3 times per week and urine output started to improve. However, he had severe hyperglycemia requiring a high dose of insulin. We performed further immunohistochemical testing on kidney biopsy tissue (Figure 3). The cellular infiltrates had intense diffuse anti-CD3 reactivity. No significant immunoreactivity was observed with anti-CD19 or -20. Intense but focal immunoreactivity with anti-PD-L1 antibody was observed within the cellular infiltrates, some of the tubular epithelia, and a few peritubular capillaries. We decided to continue with 60 mg prednisone for 4 weeks and then taper slowly over the next 1–3 months. Fortunately, within 2 weeks of steroid treatment, urine output started to improve. Hemodialysis was safely discontinued after a total of 3 weeks. After 6 weeks of initial presentation, creatinine was down to 2.2 mg/dl. Mycophenolate mofetil was added to allow for the rapid taper of steroids.
Discussion
Immune checkpoint inhibitors “remove the brakes” on the immune system, leading to loss of tolerance and allowing T cells to become activated against tumor and nontumor cells. The heightened inflammatory response to ICPI results in favorable tumor destruction and unfortunate irAEs. The toxic effects most frequently involve skin, gastrointestinal tract, and endocrine system. Immune-related adverse events usually occur within the first few weeks to months (medians of 1–6 months) after treatment, but they can occur anytime. Most often occur after several infusions.5,7 Dermatitis and colitis are usually the first to appear within 4–6 weeks.5
Compared with monotherapy, combination immunotherapy has more severe or fatal toxic effects (24% and 54%, respectively) and tends to occur early in treatment (median of 40 vs. 14.5 days, respectively).8,9 In pharmacovigilance, time to onset (time from the start of drug administration to the onset of reaction) is one of the most fundamental criteria when assessing the likelihood of a causal relationship between a suspected adverse drug reaction.S1 It has been suggested that time to onset from individual case reports can be used to detect safety signals.S1
In the largest multicenter study of ICPI-AKI, Cortazar et al.1 identified the concomitant use of PPIs, combination treatment with anti–cytotoxic T-lymphocyte antigen 4 and anti–PD-1/PD-L1 agents, and lower baseline estimated glomerular filtration rate as independent risk factors for ICPI-AKI. The presence of concomitant or prior extrarenal irAEs was also an important clinical clue that should heighten suspicion for ICPI-AKI.1 The patient in the current report also presented with acute gastroenteritis as a probable feature of extrarenal irAE, was receiving a PPI, received a combination of ICPIs, and had a baseline GFR of 55 ml/min. Hence, he was at high risk for ICPI-AKI. However, based on current literature, ICPI-AKI is assumed to occur at a median of 14 weeks (Figure 1).1, 2, 3, 4, 5, 6 Atypical time to onset of AKI in this patient on the third day after receiving the first dose of ipilimumab and nivolumab might mislead many clinicians and nephrologists to consider a more traditional diagnosis of prerenal ischemia or acute tubular necrosis. Abnormal urine dipstick analysis and urine microscopy findings of leukocyturia and WBC cast, respectively, helped us to consider ATIN in the differential diagnosis despite the atypical timing of presentation of ICPI-AKI (Table 1). Prompt kidney biopsy led to the diagnosis of ATIN in this patient. Hence, ICPI-AKI should be suspected as a cause of AKI even within 3 days or at any time after initiation of immune-checkpoint inhibitor therapy. Unfortunately, in our case, no clinical features except the administration combination immunotherapy versus monotherapy with anti-PD1/PD-L1 reliably help distinguish ICPI-AKI from alternative etiologies of AKI. Urinalysis and urine microscopy with sediment examination are vital for evaluating patients with AKI, particularly while receiving ICPI, although they are insufficiently sensitive or specific to confirm or rule out ICPI-AKI. Rapid identification of irAEs and prompt management can optimize outcomes, as described in this patient.
Table 1.
Teaching points
| ICPI-AKI should be suspected as a cause of AKI at any time after initiation of ICPI therapy. |
| Urine dipstick analysis and urine microscopy should be performed in every case of AKI while patients are receiving ICPI. |
| Patients receiving proton-pump inhibitors or nonsteroidal antiinflammatory drugs, those with underlying chronic kidney disease, those who have or had concomitant and immune-related adverse events, and those receiving dual-checkpoint blockade are at high risk for ICPI-AKI. |
AKI, acute kidney injury; ICPI, immune checkpoint inhibitor.
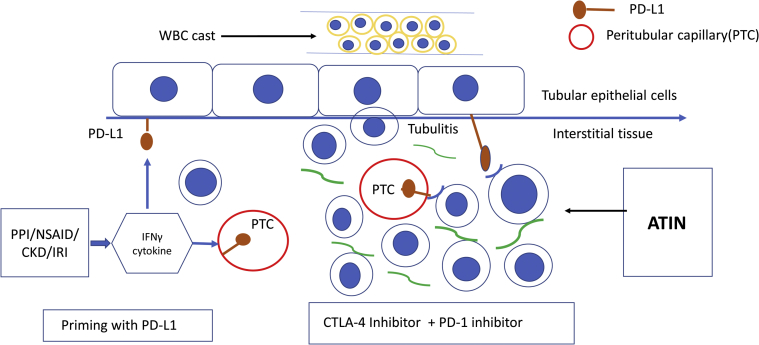
The renal biopsy confirmed the diagnosis of ATIN. The immunohistochemistry staining in kidney biopsy demonstrated intense diffuse anti-CD3 reactivity within the interstitial cellular infiltrates. Focal intense tubular epithelium and peritubular capillary immunoreactivity with anti-PD-L1 antibody were observed. Cassol et al.S2 noted immunohistochemistry staining of PD-L1 on TEC only from renal biopsy specimens from patients with anti–PD-1 therapy associated ATIN, and not in patients with anti–PD-1 therapy associated acute tubular necrosis, and not in those with AIN resulting from other medications, or patients with lupus nephritis. PD-L1 expression has shown to be induced in tumor cells and TEC after interferon-gamma–elicited T-cell responses.S3–S6 PD-L1 in TEC thus helps to downregulate T-cell responses and protect epithelium from potential immune-mediated tubulointerstitial injury.S5,S6 We speculate that established risk factors for PPI and underlying chronic kidney disease in our case could be the inflammatory stimulus for priming of TEC and peritubular capillary with PD-L1 expression as a defense mechanism. After the introduction of dual ICPI, unchecked T-cell activation ensued, leading to hyperacute ATIN (Figure 4). We are limited by a lack of information on clonality and antigen specificity of tissue-infiltrating cells to make a definitive conclusion
Figure 4.
Immune checkpoint inhibitor–related acute tubulointerstitial nephritis (ATIN) mechanism. Risk factors proton-pump inhibitor (PPI), nonsteroidal antiinflammatory drugs (NSAID0, and chronic kidney disease (CKD) potentially upregulate tubulointerstitial cytokines leading to tubular epithelial cell and peritubular capillary expression of PD-L1. Upon exposure to dual ICPI, unchecked T-cell activation ensues, triggering ATIN. CTLA-4, cytotoxic T-lymphocyte antigen 4; IFNγ, interferon-gamma; IRI, ischemia-reperfusion injury; WBC, white blood cell.
This case experience will add to current knowledge on the kinetics of appearance, the clinical presentation, and underlying mechanisms of ICPI-AKI. Patients at high risk for ICPI-AKI should be under close surveillance for AKI as early as within the first week of ICPI, and the development of AKI should prompt evaluation with urine microscopy and renal biopsy.
Disclosure
JAS is on the advisory board for Array, BMS, Nektar, and Genentech. All other authors declared no competing interests.
Footnotes
Supplementary References.
Supplementary Material
References
- 1.Cortazar F.B., Kibbelaar Z.A., Lezerman I.G. Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: a multicenter study. J Am Soc Nephrol. 2020;31:435–446. doi: 10.1681/ASN.2019070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mamlouk O., Selamet U., Machado S. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: a single-center experience. J Immunother Cancer. 2019;7:2. doi: 10.1186/s40425-018-0478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manohar S., Ghamrawi R., Chengappa M. Acute interstitial nephritis and checkpoint inhibitor therapy: single-center experience of management and drug rechallenge. Kidney360. 2020;1:16–24. doi: 10.34067/KID.0000152019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Izzedine H., Mathian A., Champiat S. Renal toxicities associated with pembrolizumab. Clin Kidney J. 2019;12:81–88. doi: 10.1093/ckj/sfy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber J.S., Hodi F.S., Wolchok J.D. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 6.Shirali A.C., Perazella M.A., Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis. 2016;68:287–291. doi: 10.1053/j.ajkd.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 7.Johnson D.B., Chandra S., Sosman J.A. Immune checkpoint inhibitor toxicity in 2018. JAMA. 2018;320:1702–1703. doi: 10.1001/jama.2018.13995. [DOI] [PubMed] [Google Scholar]
- 8.Postow M.A., Chesney J., Pavlick A.C. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Zhou S., Yang F. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5:1008–1019. doi: 10.1001/jamaoncol.2019.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.