Abstract
Introduction
Narsoplimab is a human monoclonal antibody against mannan-associated lectin-binding serine protease−2 (MASP-2). Now in a phase 3 study, narsoplimab was evaluated in a staged phase 2 study assessing safety and effectiveness in high-risk patients with IgA nephropathy (IgAN).
Methods
Substudy 1 was a single-arm open-label study of 12 weekly infusions and tapered corticosteroids, with 6 weeks of follow-up. In substudy 2, patients were randomized 1:1 to receive a course of treatment consisting of once-weekly narsoplimab or vehicle infusions for 12 weeks. After 6 weeks of follow-up, both substudy 2 groups could continue in an open-label extension, receiving 1 or more narsoplimab courses at the investigator’s discretion.
Results
The most commonly reported adverse events (AEs) included headache, upper respiratory infection, and fatigue. Most AEs were mild or moderate and transient. No treatment-related serious AEs were reported. All 4 patients who were enrolled in substudy 1 had reductions in 24-hour urine protein excretion (UPE) at week 18, ranging from 54% to 95% compared with baseline. In substudy 2, the vehicle and narsoplimab groups had similar proteinuria reductions at week 18. Eight patients (3 vehicle, 5 narsoplimab) continued in the dosing extension; all received narsoplimab. Median reduction in 24-hour UPE in these 8 patients was 61.4% at 31 to 54 weeks postbaseline. Estimated glomerular filtration rates (eGFR) remained stable in both substudies.
Conclusion
This interim analysis suggests that narsoplimab treatment is safe, is well tolerated, and may result in clinically meaningful reductions in proteinuria and stability of eGFR in high-risk patients with advanced IgAN.
Keywords: complement system, IgA nephropathy, lectin pathway, mannan-associated lectin-binding serine protease-2, MASP-2, narsoplimab
Graphical abstract
IgA nephropathy (IgAN) is the most common primary glomerular disease, with a global incidence of at least 2.5 per 100,000 per year.1 The clinical course of IgAN is heterogenous, but up to 40% of IgAN patients develop end-stage kidney disease (ESKD) within 20 years of diagnosis.2 There are no therapies approved specifically for the treatment of IgAN. Given the conflicting results of many studies of this disease, treatment is customized to individual patients depending on clinical presentation. Currently, management of patients includes treatment with renin−angiotensin system blockade to lower blood pressure and to decrease proteinuria. Corticosteroids and other immunosuppressive agents are variably used when significant proteinuria persists despite renin−angiotensin system blockade, with inconsistent results and significant toxicity.
To improve IgAN outcomes, targeted interventions are necessary. Mounting pathologic, biochemical, experimental, and genetic findings support a pivotal role of complement activation in disease onset and progression of IgAN. Activation of the alternative and lectin pathways have been implicated in IgAN.3 Immunohistochemical findings of C3, properdin, C4d, mannan-binding lectin (MBL), and C5b-9 deposits in the mesangium of IgAN biopsy samples, coupled with the general absence of C1q, strongly indicates activation of alternative and lectin pathways rather than the classical pathway.1,4,5 Deposition of MBL and C4—both lectin pathway−associated proteins—in the absence of C1q have been repeatedly reported in biopsies from patients with IgAN and seem to correlate with worse outcomes.6, 7, 8, 9 These findings further suggest that activation of the lectin pathway contributes to disease progression of IgAN. Mannan-binding lectin-associated serine proteinase 2 (MASP-2), an effector enzyme, is essential for activation of the lectin pathway of complement and is therefore a potential drug target. Narsoplimab is a fully human monoclonal antibody designed to treat diseases mediated by the lectin pathway of complement through inhibition of MASP-2. This is the first study to report the targeting of the lectin pathway of complement through MASP-2 inhibition as a novel approach for the treatment of IgAN.
The objectives of this phase 2 study were to assess the safety and effectiveness of narsoplimab in patients with IgAN. The study includes 2 substudies, and an outline of each substudy is provided in Figure 1. This report presents results from both substudies of patients based on a planned interim analysis conducted in December 2018.
Figure 1.
Overall study designs for (a) substudy 1 and (b) substudy 2. UPE, urine protein excretion.
Materials and Methods
The results of a Phase 2, multicenter clinical trial (NCT02682407, clinicaltrials.gov) of narsoplimab (Figure 1) in patients with IgAN consisting of 2 substudies are reported. The substudies were conducted at 9 centers in the United States. The protocol was approved by the institutional review boards of the participating institutions, conducted in accordance with the Declaration of Helsinki and national guidelines, and all patients provided written informed consent. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE v4.03), as follows: Grade 1 Mild: asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated. Grade 2 Moderate: minimal, local or noninvasive intervention indicated; limiting age-appropriate instrumental activities of daily living. Grade 3: Severe or medically significant but not immediately life threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care activities of daily living. Grade 4 Life-threatening consequences; urgent intervention indicated. Grade 5 Death related to AE.10
The key inclusion criteria for both substudies included: age ≥18 years at screening visit 1; kidney biopsy−confirmed IgAN diagnosis; 24-hour urine protein excretion (UPE) >1 g/d; estimated glomerular filtration rate (eGFR) (Modification of Diet in Renal Disease [MDRD]) >30 ml/min per 1.73 m2; treatment with physician-directed, stable, optimized angiotensin-converting enzyme inhibitors and/or angiotensin II receptor blockers; and systolic blood pressure of <150 mm Hg and a diastolic blood pressure of <90 mm Hg at rest.
Intervention
Narsoplimab is a fully human antibody. In functional assays, narsoplimab inhibits the human lectin pathway with nanomolar (nM) potency but has no effect on the classical or alternative complement pathways. In substudy 1, narsoplimab was dosed at 4 mg/kg, and in substudy 2 it was dosed at a 370-mg fixed-dose. Both doses were administered as an i.v. infusion over 30 minutes.
Trial Design
Substudy 1
Substudy 1 enrolled patients with corticosteroid-dependent IgAN. All patients were receiving a corticosteroid dose of >10 mg/d of prednisone or equivalent dose for at least 12 weeks prior to study initiation. During the initial 4 weeks of narsoplimab treatment, patients were maintained on their prestudy dose of corticosteroids. Investigators were instructed to maintain patients’ dose of corticosteroids during the first 4 weeks of narsoplimab treatment unless an increase in corticosteroid dose was medically indicated. At the end of the initial 4 weeks of narsoplimab treatment, patients underwent corticosteroid taper for the next 4 weeks of narsoplimab treatment. The target was to taper to ≤6 mg prednisone (or equivalent dose) daily. The tapering schedule was at the discretion of the investigator. The taper was discontinued if patients had deterioration of renal function as determined by the investigator.
All patients received narsoplimab at 4 mg/kg i.v. once weekly for 12 weeks. Patients were required to be on physician-directed, stable, optimized treatment with angiotensin-converting enzyme inhibitors and/or angiotensin II receptor blockers and to have a systolic blood pressure of <150 mm Hg and a diastolic blood pressure of <90 mm Hg at rest. No patients in substudy 1 were administered vaccinations or prophylactic antibiotics during the study. An additional inclusion criterion for substudy 1 was treatment with >10 mg of prednisone or equivalent dose for at least 12 weeks prior to screening visit 1.
Substudy 2
Substudy 2 enrolled patients with IgAN who were not receiving corticosteroids. Patients in substudy 2 received either narsoplimab 370 mg i.v. or 5% dextrose in water (D5W) vehicle in a randomized double-blind design. As in substudy 1, patients were required have a systolic blood pressure of <150 mmHg and a diastolic blood pressure of <90 mm Hg at rest, controlled with angiotensin-converting enzyme inhibitors and/or angiotensin II receptor blockers. Regardless of treatment assignment, patients in substudy 2 were eligible to enter an open-label dosing extension after completion of the initial 12 weeks of dosing plus a 6-week follow-up period. Patients in the dosing extension were eligible for narsoplimab treatment if their 24-hour UPE was >1 g/d or ≥50% of baseline. No patients in substudy 2 were administered vaccinations or prophylactic antibiotics during the study.
Randomization was achieved using a trial supply management tool (RTSM) (Medidata Solutions, Inc, New York, NY) which generated a schedule with a block size of 4 and was stratified by region. When a patient was ready for treatment the RTSM system automatically randomized the patient to 1 of the 2 treatment groups while minimizing selection and accidental bias. Treatment assignments were blinded to all trial personnel, with the following exceptions: study site pharmacist; an independent site monitor who tracked study drug accountability; an Omeros Corporation chemistry, manufacturing, and controls (CMC) representative who managed drug supply; and an Omeros Corporation clinical data manager overseeing the implementation of the randomization module.
Outcome Measures
The primary objective of this study was to describe the safety and tolerability of narsoplimab in patients with IgAN as assessed by adverse events (AEs), vital signs, clinical laboratory tests, and electrocardiograms. Other objectives of this study were to assess the effect of narsoplimab on proteinuria measured by 24-hour UPE (reported in this interim report), urine albumin/creatinine ratio, and urine protein/creatinine ratio. Serum creatinine and eGFR calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation using serum creatinine was also assessed. Efficacy results and other measures not presented in this interim report are intended to be published after the study is complete.
In substudy 1, patients had their initial 24-hour UPE measured at screening visit 2 followed by 12 weeks of treatment with narsoplimab, which included 4 weeks on a maintained steroid dose, 4 weeks of steroid taper, and 4 weeks on the tapered steroid dose. At the final study visit (∼6 weeks after the final dose of narsoplimab), 24-hour UPE was assessed to evaluate the effect of narsoplimab treatment on proteinuria.
In substudy 2, patients were randomized 1:1 to receive either blinded narsoplimab 370 mg or vehicle (5% dextrose in water) for 12 weeks (once-weekly administrations) followed by an additional 6 weeks of observation and then an open-label extension period for potential dosing up to 2 years. As in substudy 1, 24-hour UPE was assessed ∼6 weeks after the final dose of narsoplimab. After week 18, patients were eligible to roll over into the dosing extension with open-label narsoplimab regardless of initial treatment group. Patients were eligible for additional 12-week cycles of narsoplimab treatment if they failed to achieve a 24-hour UPE <50% of baseline or if their 24-hour UPE was >1 g/24 h. Interim data analyses were planned for substudy 2 after all patients had completed the assessment through week 18. All data analyses were prespecified as descriptive only and were not powered for statistical comparisons.
Results
The clinical trial includes separate, sequentially initiated substudies that differ by design, treatment duration, and/or concurrent use of corticosteroids. This article presents data from 1 substudy and the planned interim analysis of a second substudy. The first substudy enrolled 4 patients with biopsy-proven IgAN who were treated with oral prednisolone (30−80 mg daily) at the time of enrollment, whereas the second substudy enrolled 12 patients with biopsy-confirmed IgAN who were not receiving corticosteroids for at least the 3 months prior to enrollment.
Substudy 1: Open-Label Narsoplimab With Corticosteroid Tapering
Study Population
Substudy 1 included 4 patients with biopsy-proven IgAN who were treated with a single course of once-weekly infusions of narsoplimab for 12 weeks plus daily corticosteroids (Figure 1a). Patients entered the study on stable regimens of corticosteroids, which were tapered according to a pre-determined protocol over the study period. Patients were followed for up to 6 weeks after the last narsoplimab dose, and 24-hour UPE was assessed by Week 18. The baseline characteristics of the 4 patients in this substudy are presented in Table 1. Median baseline 24-hour UPE was 4.2 g/d, and eGFR was 43 ml/min per 1.7 3m2. The median time from IgAN diagnosis to study entry was 1.3 years.
Table 1.
Baseline characteristics of patients enrolled in substudy 1
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | All patients median (N = 4) |
|---|---|---|---|---|---|
| Age, yr | 35 | 32 | 55 | 45 | 40 |
| Gender | M | F | F | F | – |
| Race | W | A | W | W | – |
| Time since IgAN diagnosis, yr | 8 | 0.4 | 0.4 | 2.2 | 1.3 |
| Proteinuria, 24-h UPE, g/d | 3.9 | 2.4 | 4.9 | 4.6 | 4.2 |
| eGFR (MDRD), ml/min per 1.73 m2 | 47.1 | 35.1 | 44.5 | 44.8 | 44.6 |
| Systolic blood pressure, mm Hg | 126 | 106 | 116 | 162 | 121 |
| Diastolic blood pressure, mm Hg | 88 | 60 | 72 | 84 | 78 |
| Corticosteroida use, mg/d | 60 | 30 | 60 | 30 | 45 |
A, Asian; eGFR, estimated glomerular filtration rate; F, female; M, male; MDRD, Modification of Diet in Renal Disease; UPE, urine protein excretion; W, white.
Prednisone.
Mean daily corticosteroid (prednisone) dose decreased from 45 mg at baseline to 5 mg by week 18. Three of 4 patients discontinued steroids by the end of the study, and the remaining patient discontinued steroid use during subsequent follow-up.
Substudy 1: Safety
Consistent with the Phase 1 data in healthy volunteers (Omeros Corporation, data on file), narsoplimab was well tolerated. No grade 3 or higher AEs were observed. The reported AEs were mild or moderate (grade 1 or 2) in severity and reversible (Table 2). No drug-related AEs were reported. No meningococcal or other bacterial infections were reported.
Table 2.
Treatment-emergent adverse events that occurred at least twice in the total safety population of substudy 1
| Adverse events (preferred term) | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Fatigue | ✓ | ✓ | – | ✓ |
| Upper respiratory tract infection | – | – | ✓ | ✓ |
| Headache | – | ✓ | – | ✓ |
| Alopecia | – | ✓ | ✓ | – |
Both systolic and diastolic blood pressure values either remained similar or improved at post-treatment follow-up compared with baseline (Table 3).
Table 3.
Blood pressure at post-treatment follow-up, substudy 1
| Systolic and diastolic blood pressure | Patient 1 | Patient 2 | Patient 3 | Patient 4 | All patients median (N = 4) |
|---|---|---|---|---|---|
| Systolic blood pressure, mm Hg at follow-up | 106 | 100 | 118 | 120 | 112 |
| Diastolic blood pressure, mm Hg at follow-up | 70 | 70 | 70 | 80 | 70 |
Proteinuria and Kidney Function
In substudy 1, 24-hour UPE was substantially reduced in all 4 patients, with an overall median reduction of 72% at the last follow-up visit relative to baseline (Figure 2). The mean change from baseline in 24-hour UPE was −2.87 ± 1.08 g. The eGFR levels remained stable in all 4 patients (Figure 3). At week 18, the median eGFR was 40 ml/min per 1.73 m2 compared with 43 ml/min per 1.73 m2 at baseline.
Figure 2.
Twenty-four-hour urine protein excretion (UPE) at baseline and last follow-up visit in substudy 1. The percent reductions in 24-hour UPE were 54% for patient 1, 81% for patient 2, 63% for patient 3, and 95% for patient 4.
Figure 3.
Estimated glomerular filtration rate (eGFR) over time for individual patients in substudy 1 from screening through week 18. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Substudy 2: Randomized, Vehicle-Controlled, Followed by Open-Label Therapy With Narsoplimab
Study Population
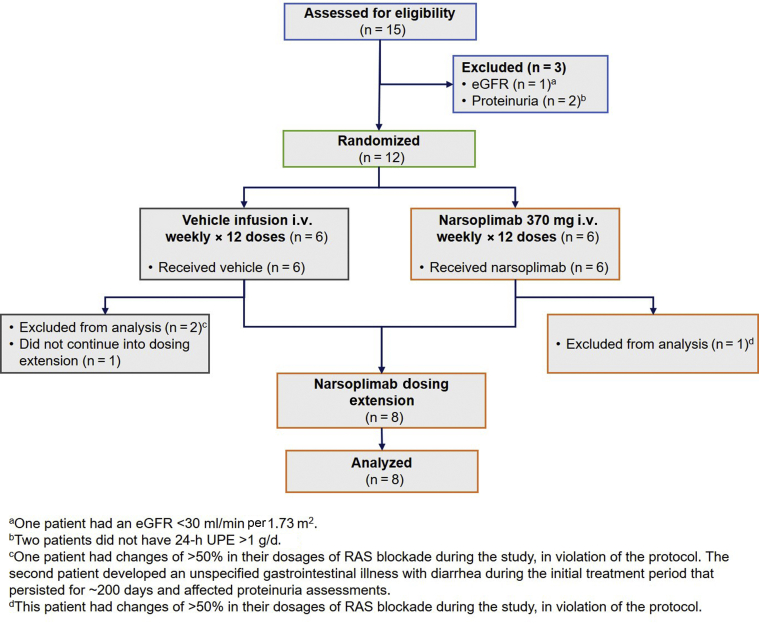
The design of substudy 2 is provided in Figure 1b. A total of 12 patients with biopsy-proven IgAN who were not on corticosteroids were randomized 1:1 to either a course of once-weekly narsoplimab infusions or once-weekly vehicle (5% dextrose in water) infusions for 12 weeks. Follow-up evaluations were performed by week 18. After week 18, patients could enter an open-label dosing extension and receive narsoplimab. Patients in the dosing extension who had 24-hour UPE >1 g/d or 24-hour UPE ≥50% of baseline anytime between weeks 18 and 91 during the dosing extension could receive a first course of narsoplimab (if the patient had previously been in the vehicle group) and/or 1 or more additional courses of narsoplimab treatment (each course constituting once-weekly infusions for 12 weeks) at the investigator’s discretion. The flow of patients through the treatment protocol and analysis is reported in the Consolidated Standards of Reporting Trials (CONSORT) diagram in Figure 4.
Figure 4.
Consolidated Standards of Reporting Trials (CONSORT) diagram for substudy 2. eGFR, estimated glomerular filtration rate; RAS, renin−angiotensin system; UPE, urine protein excretion.
The characteristics of the patients enrolled in substudy 2 are provided in Table 4. Patients in this substudy generally had long-standing IgAN, with a median time from diagnosis to study entry of 9.3 years. The duration of time since kidney biopsy-proven diagnosis was not balanced between the treatment groups of substudy 2; disease duration was a median of 6.9 years in those initially treated with vehicle versus a median of 17 years in the group receiving narsoplimab. The median age of patients was 33 years in vehicle group and 43.5 years in the narsoplimab group. Median baseline 24-hour UPE was 4.0 g in vehicle group and 2.4 g in the narsoplimab group.
Table 4.
Baseline characteristics of patients enrolled in substudy 2
| Characteristic | Vehicle (n = 6) | Narsoplimab (n = 6) | All patients (N = 12) |
|---|---|---|---|
| Median age (range), yr | 33 (29–50) | 43.5 (24–60) | 36 (24–60) |
| Gender, male/female | 4/2 | 5/1 | 9/3 |
| Race, Asian/white | 1/5 | 0/1 | 1/11 |
| Median time (range) since IgAN diagnosis, yr | 6.9 (1.4–18.5) | 17 (1.5–27.5) | 9.3 (1.4–27.5) |
| Median (range) 24-h UPE, g/d | 4 (1.5–11.9) | 2.4 (1.3–5.7) | 3.0 (1.3–11.9) |
| Median (range) eGFR, ml/min per 1.73 m2 | 44 (25.4–63.5) | 35.6 (30.6–76.5) | 37.6 (25.4–76.5) |
| Median systolic blood pressure (range), mm Hg | 126 (101–147) | 135 (126–148) | 129 (101–148) |
| Median diastolic blood pressure (range), mm Hg | 78.5 (62–104) | 84 (76–89) | 83 (62–104) |
eGFR, estimated glomerular filtration rate; IgAN, IgA nephropathy. UPE, UPE, urine protein excretion.
Safety
Narsoplimab was safe and well tolerated in substudy 2, with no drug-related AEs reported (Table 5). Three patients in substudy 2 had a total of 5 grade 3 AEs, all of which were reversible: 1 patient randomized to vehicle experienced 2 grade 3 AEs (urinary tract infection and acute kidney injury); the second patient randomized to narsoplimab had a known psychiatric illness and experienced an acute psychotic event that required hospitalization; and the third patient randomized to vehicle and continuing with narsoplimab in the dosing extension was diagnosed with hyperkalemia at the beginning of narsoplimab treatment. No meningococcal or other bacterial infections were reported. At both the follow-up after completion of the randomized treatment period and at the last available follow-up during the extension phase, systolic and diastolic blood pressure values were similar compared with baseline (Table 6).
Table 5.
Treatment-emergent adverse events that occurred at least twice in the total safety population of substudy 2
| Adverse events (preferred term) | Vehicle (n = 6) | Narsoplimab (n = 6) | All (N = 12) |
|---|---|---|---|
| Fatigue | 2 (33.3) | 1 (16.7) | 3 (25.0) |
| Upper respiratory tract infection | 1 (16.7) | 1 (16.7) | 2 (16.7) |
| Metabolic acidosis | 2 (33.3) | 1 (16.7) | 3 (25) |
| Hyperkalemia | 1 (16.7) | 1 (16.7) | 2 (16.7) |
| Gout | 1 (16.7) | 1 (16.7) | 2 (16.7) |
| Diarrhea | 1 (16.7) | 1 (16.7) | 2 (16.7) |
| Hematochezia | 1 (16.7) | 1 (16.7) | 2 (16.7) |
| Ligament sprain | 1 (16.7) | 1 (16.7) | 2 (16.7) |
Data are n (%).
Table 6.
Blood pressure at week 18 and week 31 to 54 follow-up visits, substudy 2
| Systolic and diastolic blood pressure | Vehicle (n = 6) | Narsoplimab (n = 6) | All (N = 12) |
|---|---|---|---|
| Week 18 follow-up | |||
| Median systolic blood pressure (range), mm Hg | 130 (120–169) | 132 (120–159) | 130 (120–169) |
| Median diastolic blood pressure (range), mm Hg | 80 (72–116) | 79 (70–100) | 80 (70–116) |
| Week 31–54 follow-up | |||
| Median systolic blood pressure (range), mm Hg | 133.5 (116–154) | 129 (114–164) | 130.5 (114–164) |
| Median diastolic blood pressure (range), mm Hg | 79.5 (56–109) | 86.5 (66–95) | 81.5 (56–109) |
Proteinuria and Kidney Function
Nine patients were evaluable in substudy 2. Three patients (patients 8, 9, and 14) were excluded from the 24-UPE efficacy evaluation in substudy 2 due to factors that made meaningful interpretation of 24-hour UPE changes difficult and/or for protocol violations that confound data interpretation: 2 patients (1 randomized to vehicle, 1 randomized to narsoplimab) had changes of >50% in their dosages of renin−angiotensin system blockade during the study, in violation of the protocol. The patient who was randomized to vehicle also developed metabolic acidosis and hyperkalemia, accompanied by a reduction in eGFR. The third excluded patient, randomized to vehicle, developed an unspecified gastrointestinal illness with diarrhea during the initial treatment period that persisted for ∼200 days and affected proteinuria assessments.
For the 9 evaluable patients in substudy 2 who completed the initial 12-week course of treatment per protocol, median reductions in 24-hour UPE by week 18 were similar between the vehicle and narsoplimab groups. The median reductions in proteinuria were 18.0% and 18.4% for vehicle- and narsoplimab-treated patients, respectively.
Eight of the 9 evaluable patients continued in the narsoplimab dosing extension: 3 patients who initially received vehicle and 5 who initially received narsoplimab. For these 8 patients, there was an overall median reduction in proteinuria of 61.4% (range, 7.3 to −77.3) compared to baseline as assessed at 31 to 54 weeks postbaseline (Figure 5). In 2 patients, proteinuria decreased from levels >5 g/d to ∼2 g/d. At the time of the interim analysis, all 8 patients in the dosing extension completed at least 1 course of narsoplimab, with 5 patients receiving 1 course, 2 patients receiving 2 courses, and 1 patient receiving 3 courses.
Figure 5.
Twenty-four-hour urine protein excretion (UPE) at baseline through last available follow-up visit for evaluable patients in substudy 2. Dashed lines represent patients who were initially randomized to vehicle; solid lines represent patients who were initially randomized to narsoplimab. By week 18 of follow-up, the median reduction in proteinuria was 18% and 18.4% for the vehicle- and narsoplimab-treated patients, respectively. All patients entering the dosing extension period beyond week 18 received narsoplimab. The median reduction in proteinuria was 61.4% at weeks 31 to 54 for patients in the dosing extension.
The eGFR levels remained stable in 7 of the 8 evaluable patients in substudy 2 (Figure 6). One patient (patient 6) who was initially randomized to vehicle and then received 1 course of narsoplimab in the dosing extension had a decline in eGFR from approximately 32 to 15 ml/min to 1.73 m2 at last observation.
Figure 6.
Estimated glomerular filtration rate (eGFR) over time for individual evaluable patients in substudy 2 from screening through last measurement during the ongoing dosing extension. Dashed lines represent patients who were initially randomized to vehicle; solid lines represent patients who were initially randomized to narsoplimab.
Discussion
The primary objective of this study was to evaluate the safety and tolerability of narsoplimab in patients with biopsy-confirmed IgAN. Narsoplimab was well tolerated, and no drug-related serious AEs were reported. Most AEs were mild to moderate and transient. C5-targeted agents are associated with an increased risk of infection due to encapsulated bacteria, particularly Neisseria species11; however, in this study with a MASP-2 inhibitor, no meningococcal or other bacterial infections occurred.
Although this study was not primarily designed to assess the efficacy of narsoplimab, the final proteinuria data from substudy 1 and longer-term follow-up proteinuria data from substudy 2 suggest a substantial and clinically meaningful therapeutic effect in patients with IgAN. In substudy 1, all 4 patients achieved significant reductions in 24-hour UPE and were able to taper completely off their corticosteroid therapy, either during or shortly after completion of treatment with narsoplimab. A modest reduction in proteinuria from baseline was seen after the initial 12 weeks of treatment in each of the vehicle and narsoplimab groups in the substudy 2 patients. However, the findings of the open-label dosing extension of substudy 2 provided important indications of a meaningful clinical effect. Narsoplimab treatment was associated with a substantial (61.4%) reduction of 24-hour UPE among the 8 patients who were in the dosing extension. Given the fact that most patients enrolled in this pilot study had longstanding disease, high levels of proteinuria, and low eGFR values, the findings suggest that blocking MASP-2 may halt disease progression and preserve otherwise declining renal function even in patients with longstanding and advanced IgAN.
The study has important limitations. The sample size was small in both substudies, with unbalanced demographics between groups in substudy 2, and the duration of the vehicle-controlled period of the study was relatively short to account for confounders. Variation in the time to response with proteinuria as a marker of treatment effect has been observed with other proteinuria treatment modalities in other glomerular diseases.12 It is well recognized that IgAN is a heterogenous disease and thus the response to any treatment might easily be confounded if treatment groups are not balanced for factors known to influence disease progression.13 In particular, the difference in disease duration may account for the variable and more extended time observed in substudy 2 for the treatment effect to become detectable in terms of proteinuria reduction, in contrast to substudy 1. Proteinuria in IgAN may result not only from active inflammatory lesions but also from sclerotic glomerular lesions with hyperfiltration and tubular damage.14 Although biopsy-confirmed IgAN was an eligibility criterion for enrollment into the study, the pathological diagnosis of IgAN was available only from the medical history of patients and ranged from 1.3 to 27 years prior to enrollment in this study. No histology materials were collected for standardized or central reading or were accessible for further evaluation. However, progressive glomerulosclerosis and tubular atrophy/interstitial fibrosis had likely developed with extended disease duration, potentially rendering patients less responsive to treatment. Furthermore, it is unrealistic to expect normalization of proteinuria in patients with long-standing disease in whom fibrotic kidney changes have already occurred. The markedly longer median disease duration (17 vs. 7 years) and the higher median age in patients initially randomized to narsoplimab compared to those randomized to vehicle in substudy 2 likely confounded the first early assessment at week 18, whereas protein reduction became obvious with time and repeated treatment during the open-label extension. The observed changes in proteinuria from baseline in the extended period are unlikely to be explained by spontaneous remission in this high-risk group with advanced disease. In addition, although steroids could potentially be implicated in substudy 1, patients were not responding to steroid treatment prior to narsoplimab treatment, and steroid dosing was rapidly tapered; steroids were not allowed in substudy 2. The results clearly highlight the need for stratification of risk factors for disease progression, as they likely also determine the potential rapidity to responsiveness to therapeutic intervention. Such an approach, however, requires a larger population than that in the current study.
The demonstrated safety and tolerability of narsoplimab in clinical studies so far are important features of a potential therapy for IgAN, as patients may require repeated dosing to achieve meaningful and sustained responses. Unlike inhibition of other complement enzymes (e.g., C5, C3), inhibition of MASP-2 does not interfere with the antibody-dependent classical complement activation pathway, an important component of the acquired immune response to infection. Furthermore, abnormal MASP-2 function has not been associated with increased infections or autoimmune disorders.15, 16, 17, 18
IgA nephropathy is characterized by a highly variable clinical course with progression to end-stage kidney disease in approximately 20% to 40% of patients over 10 years.2 Several studies have demonstrated increased urinary protein as the strongest predictor of an unfavorable outcome.19, 20, 21, 22 The Kidney Health Initiative reported an analysis based on epidemiologic data that indicates a strong and consistent relationship between the level and duration of proteinuria and loss of kidney function. The authors concluded that these data support the use of proteinuria reduction as a reasonably likely surrogate endpoint for a treatment effect on progression to ESKD in patients with IgAN.21 Indeed, proteinuria itself may contribute to progressive renal damage through tubulointerstitial inflammation, fibrosis, and tubular cell apoptosis,23,24 and overall mortality.25
In conclusion, the interim safety, tolerability, and efficacy data from this ongoing phase 2 study support further clinical development of narsoplimab as a potentially first-in-class intervention to blunt a critical pathological disease pathway in patients with IgAN for whom no specific therapy currently exists.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was funded by Omeros Corporation (Seattle, WA). Louis DeTulleo, Marither Chuidian, Steve Whitaker, and Gregory Demopulos contributed to manuscript revisions.
Author Contributions
RAL and JB wrote the first draft of the manuscript. BHR, HNR, JF and JAT provided critical review, feedback, and revisions to the manuscript. All authors reviewed and approved of the final manuscript.
Footnotes
CONSORT Checklist.
Supplementary Material
References
- 1.Wyatt R.J., Julian B.A. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 2.Berthoux F.C., Mohey H., Afiani A. Natural history of primary IgA nephropathy. Semin Nephrol. 2008;28:4–9. doi: 10.1016/j.semnephrol.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Rizk D.V., Maillard N., Julian B.A. The Emerging Role of Complement Proteins as a Target for Therapy of IgA Nephropathy. Front Immunol. 2019;10:504. doi: 10.3389/fimmu.2019.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Floege J., Daha M.R. IgA nephropathy: new insights into the role of complement. Kidney Int. 2018;94:16–18. doi: 10.1016/j.kint.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Maillard N., Wyatt R.J., Julian B.A. Current understanding of the role of complement in IgA Nephropathy. J Am Soc Nephrol. 2015;26:1503–1512. doi: 10.1681/ASN.2014101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra P. C4d in native glomerular diseases. Am J Nephrol. 2019;49:81–92. doi: 10.1159/000496059. [DOI] [PubMed] [Google Scholar]
- 7.Endo M., Ohi H., Ohsawa I. Glomerular deposition of mannose-binding lectin (MBL) indicates a novel mechanism of complement activation in IgA nephropathy. Nephrol Dial Transplant. 1998;13:1984–1990. doi: 10.1093/ndt/13.8.1984. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda M., Shikata K., Wada J. Deposition of mannan binding protein and mannan binding protein-mediated complement activation in the glomeruli of patients with IgA nephropathy. Nephron. 1998;80:408–413. doi: 10.1159/000045212. [DOI] [PubMed] [Google Scholar]
- 9.Roos A., Rastaldi M.P., Calvaresi N. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724–1734. doi: 10.1681/ASN.2005090923. [DOI] [PubMed] [Google Scholar]
- 10.US Department of Health and Human Services: National Institutes of Health NCI . US Department of Health and Human Services, National Institutes of Health; Bethesda, MD: 2009. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. [Google Scholar]
- 11.Winthrop K.L., Mariette X., Silva J.T. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors) Clin Microbiol Infect. 2018;24(Suppl 2):S21–S40. doi: 10.1016/j.cmi.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Dahan K., Debiec H., Plaisier E. Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. J Am Soc Nephrol. 2017;28:348–358. doi: 10.1681/ASN.2016040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiryluk K., Novak J. The genetics and immunobiology of IgA nephropathy. J Clin Invest. 2014;124:2325–2332. doi: 10.1172/JCI74475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coppo R. Towards a personalized treatment for IgA nephropathy considering pathology and pathogenesis. Nephrol Dial Transplant. 2019;34:1832–1838. doi: 10.1093/ndt/gfy338. [DOI] [PubMed] [Google Scholar]
- 15.Ali Y.M., Lynch N.J., Haleem K.S. The lectin pathway of complement activation is a critical component of the innate immune response to pneumococcal infection. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dommett R.M., Klein N., Turner M.W. Mannose-binding lectin in innate immunity: past, present and future. Tissue Antigens. 2006;68:193–209. doi: 10.1111/j.1399-0039.2006.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Laorden M.I., Sole-Violan J., Rodriguez de Castro F. Mannose-binding lectin and mannose-binding lectin-associated serine protease 2 in susceptibility, severity, and outcome of pneumonia in adults. J Allergy Clin Immunol. 2008;122:368–374. doi: 10.1016/j.jaci.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 18.Mayilyan K.R. Complement genetics, deficiencies, and disease associations. Protein Cell. 2012;3:487–496. doi: 10.1007/s13238-012-2924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Descamps-Latscha B., Witko-Sarsat V., Nguyen-Khoa T. Early prediction of IgA nephropathy progression: proteinuria and AOPP are strong prognostic markers. Kidney Int. 2004;66:1606–1612. doi: 10.1111/j.1523-1755.2004.00926.x. [DOI] [PubMed] [Google Scholar]
- 20.Inker L.A., Mondal H., Greene T. Early change in urine protein as a surrogate end point in studies of IgA nephropathy: an individual-patient meta-analysis. Am J Kidney Dis. 2016;68:392–401. doi: 10.1053/j.ajkd.2016.02.042. [DOI] [PubMed] [Google Scholar]
- 21.Thompson A., Carroll K., L A.I. Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin J Am Soc Nephrol. 2019;14:469–481. doi: 10.2215/CJN.08600718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trimarchi H., Barratt J., Cattran D.C. Oxford classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91:1014–1021. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Eddy A.A. Proteinuria and interstitial injury. Nephrol Dial Transplant. 2004;19:277–281. doi: 10.1093/ndt/gfg533. [DOI] [PubMed] [Google Scholar]
- 24.Schnaper H.W. The tubulointerstitial pathophysiology of progressive kidney disease. Adv Chronic Kidney Dis. 2017;24:107–116. doi: 10.1053/j.ackd.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anyanwagu U., Donnelly R., Idris I. Albuminuria regression and all-cause mortality among insulin-treated patients with type 2 diabetes: analysis of a large UK primary care cohort. Am J Nephrol. 2019;49:146–155. doi: 10.1159/000496276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.