Abstract
Introduction
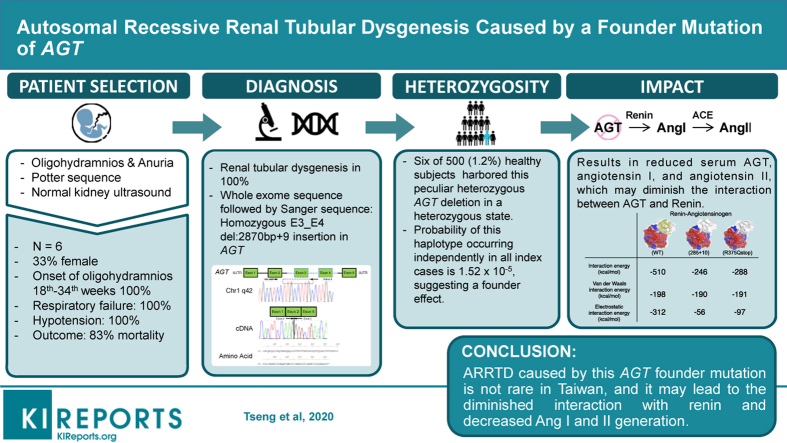
Autosomal recessive renal tubular dysgenesis (ARRTD) caused by inactivation mutations in AGT, REN, ACE, and AGTR is a very rare but fatal disorder with an unknown prevalence.
Methods
We report 6 Taiwanese individuals with ARRTD from 6 unrelated families diagnosed by renal histology. Clinical features, outcome, and prevalence of carrier heterozygosity were examined.
Results
All patients exhibited antenatal oligohydramnios, postnatal anuria, pulmonary hypoplasia, and profound hypotension refractory to interventions. Angiotensinogen (AGT) protein levels were diminished in the liver, along with reduced serum AGT, angiotensin I (Ang I) and angiotensin II (Ang II) levels. Neonatal demise occurred in all but 1 case. All individuals carried the same homozygous E3_E4 del:2870bp deletion+9bp insertion in AGT, which led to a truncated protein (1-292 amino acid). The allelic frequency of this heterozygous AGT mutation was approximately 1.2% (6/500), suggesting that ARRTD may not be exceedingly rare in Taiwan. This mutation results in skipping of exons encoding the serpin domain of AGT, which is important for renin interaction and the generation of truncated protein. In silico modeling revealed a diminished interaction between mutant AGT and renin. One patient survived after responding to high-dose hydrocortisone therapy, with resolution of profound hypotension, accompanied by an increase in serum AGT, Ang I, and Ang II levels.
Conclusion
This AGT mutation may lead to the diminished interaction with renin and decreased Ang I and Ang II generation. Hydrocortisone may potentially rescue cases of ARRTD caused by this truncated AGT.
Keywords: angiotensinogen, founder effect, hydrocortisone, hypotension, renal tubular dysgenesis, renin
Graphical abstract
Autosomal recessive renal tubular dysgenesis (ARRTD) is an inherited disorder characterized by the absence or poor differentiation of proximal convoluted tubules, profound arterial hypotension, pulmonary hypoplasia, and anuria, with a very high mortality.1, 2, 3, 4, 5, 6, 7, 8, 9 Nearly all affected individuals die either in utero or within the first few postnatal days, secondary to refractory hypotension and/or pulmonary hypoplasia. Because of its extreme rarity, its exact prevalence is unknown. In 2005, Gribouval et al described mutations in 4 different genes encoding proteins of the renin–angiotensin system (RAS) resulting in ARRTD.10 These mutations were identified as AGT-encoding angiotensinogen, REN-encoding renin, ACE-encoding angiotensin-converting enzyme (ACE), or AGTR-encoding angiotensin II (Ang II) receptor. The RAS is critical in maintenance of blood pressure and blood perfusion of vital organs including the kidneys during fetal life and is also a key regulator of kidney development.11, 12, 13 A defect in RAS has been proposed to be responsible for the development of renal tubular dysgenesis and ARRTD via compromised renal perfusion14,15 and defective metanephritic kidney development. Without a clear understanding of how mutations affecting the RAS can contribute to the pathogenesis of this disease, specific therapeutic strategies cannot be developed.
Recently, we identified 6 patients with ARRTD from 6 unrelated Taiwanese families. In this study, we examined their clinical features and outcomes, identified the mutations and calculated the estimated prevalence, and proposed a potential therapy.
Materials and Methods
Patients
This study was approved by the ethics committee on human studies at Chang Gung Memorial Hospital in Taiwan (IRB105-6067C). Neonates with ARRTD caused by large deletion of AGT, their parents, and 500 healthy adult subjects (>18 years of age) were enrolled in this study. Written informed consent was obtained from all participants.
Diagnosis of ARRTD
Neonates with maternal oligohydramnios, normal fetal kidneys, and postnatal refractory hypotension underwent renal biopsy for histological evidence of renal tubular dysgenesis, and molecular sequencing was conducted for the diagnosis of ARRTD.
Whole Exome Sequencing and Direct Sanger Sequencing
Genomic DNA was isolated from peripheral venous blood sample. We performed exome capture using the Agilent SureSelect v6 and massively parallel sequencing using the HiSeq 4000 platform (Illumina, San Diego, CA) as previously reported.16,17 ANNOVAR was used to catalog the detected variations.18 Then, we filtered variations that had a homo-polymer length >6 (and synonymous substitutions) or that were common (>2%) in dbSNP150 (http://www.ncbi.nlm.nih.gov/projects/SNP/), HapMap, the 1000 Genomes Project (http://www.1000 genomes.org), Exome Aggregation Consortium (ExAC) database and the Genome Aggregation Database (gnomAD, https://gnomad.broadinstitute.org). Direct Sanger sequencing was performed for all patients and their parents to verify the genetic variants detected by whole exome sequencing (WES).
Haplotype Analysis for Founder Effect
Haplotype analysis using extragenic microsatellite markers (D1S2847, D1S2631, D1S2805, D1S3462, and D1S1656), intragenic single nucleotide polymorphism markers (D1S103), and coding single nucleotide polymorphisms from WES was performed in patients and their parents. Haplotype blocks were called using Haploview version v4.1.
Immunohistochemistry and Immunofluorescence Staining of Liver and Kidney Tissues
The kidney specimens obtained from patients with ARRTD, normal controls (those with traumatic kidney injury), and disease controls (those with minimal change disease and acquired ARRTD) were fixed by paraffin-embedded tissue sections with paraformaldehyde. Deparaffinization and antigen unmasking were performed using Trilogy (cell marque, MilliporeSigma). A monoclonal anti−CD 10, anti-EMA, anti-renin, and anti-AGT antibodies (target epitope: amino acids 2−135) were used to recognize the proximal tubular cells, distal tubules/collecting ducts, renin expression, and AGT expression, respectively. The biopsied liver specimens were fixed and prepared as paraffin-embedded tissue sections, and immunofluorescence staining for the expression of AGT obtained from patients with, normal controls (traumatic liver injury), and disease controls (hepatocellular carcinoma and ARRTD caused by ACE mutation) was performed.
Analysis of Prevalence of AGT Heterozygosis Analysis
Reverse transcription−polymerase chain reaction analysis using exon 2- to 5-specific primers (forward, TTCCATGGAGCTTTGAATCCA; reverse, AGCACTTTCGTTTGCACAGT) was performed to detect the heterozygosity of 500 healthy subjects.
Determination of Levels of AGT, Ang I and II, and Renin
Serum levels of AGT, renin, Ang I, ACE, and Ang II from patients, their parents, and healthy subjects were determined by enzyme-linked immunosorbent assay (ELISA; Cusabio Corporation).
Simulation of the Complex Models
The models of renin in complex with wild-type or mutant AGT were built using the Homology Modeling protocol (Biovia Discovery Studio 2017). The resolved structures of human AGT in complex with renin, human, mouse, and rat AGT (PDB code: 2X0B, 2WXW, 2WXY, and 2WXZ) were used as templates.19 The model with the best probability density function energy and discrete optimized protein energy score was chosen for energy minimization using the CHARM (Chemistry at Harvard Macromolecular Mechanics) as the applied force field.
Statistical Analysis
The resulting mean data were compared with a Student 2-tailed t test. The coefficient of variation or the percent relative SD was calculated as 100 multiplied by SD divided by the mean.
Results
Clinical Characteristics and Outcomes
Four neonates and 2 fetuses (4 male and 2 female) with ARRTD were studied. As shown in Table 1, patients were born between 22 and 35 weeks of gestation with a birth bodyweight ranging from 700 to 2205 g. Pregnancy was terminated in 2 cases because of severe oligohydramnios. All patients shared the same clinical features: oligohydramnios, pulmonary hypoplasia, joint contractures, skull ossification defects, wide fontanelles, and normal kidney size with increased echogenicity. In addition to profound hypotension refractory to fluid resuscitation, inotropic agents, and physiologic dosing of hydrocortisone, all neonates presented with respiratory failure and anuria after birth. Of note, infusion of fresh frozen plasma transiently improved the blood pressure to normotensive range (defined as mean blood pressure greater than that for gestational age). All but 1 neonate died at a postnatal age of 2 to 8 days because of severe pulmonary hypoplasia and profound hypotension.
Table 1.
Clinical characteristics and outcomes of patients with autosomal recessive renal tubular dysgenesis caused by AGT deletion
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|---|---|---|---|---|---|
| Parental consanguinity | No | No | No | No | No | No |
| Sex | Male | Female | Male | Female | Male | Male |
| Gestational age (wk) | 33 | 33 | 35 | 26 | 35 | 22 |
| Birth weight (g) | 1615 | 2094 | 2205 | 804 | 2670 | 700 |
| Onset of oligohydramnio (wk) | 30 | 23 | 24 | 22 | 34 | 18 |
| Respiratory failure | Yes | Yes | Yes | - | Yes | - |
| Hypotension (BP, mm Hg) | Yes (29/13) | Yes (28//12) | Yes (34/23) | Not available | Yes (40/22) | Not available |
| Anuria at birth | Yes | Yes | Yes | Yes | Yes | Yes |
| Joint contractures | Yes | Yes | Yes | No | No | Yes |
| Wide fontanel | Yes | Yes | Yes | Yes | Yes | Yes |
| Renal ultrasound | ||||||
| Size | Normal | Normal | Normal | Normal | Normal | Normal |
| Echogenicity | Increased | Increased | Increased | Increased | Increased | Increased |
| Pulmonary hypoplasia | Yes | Yes | Yes | Not available | Yes | Not available |
| Respond to fluid challenge | No | No | No | Not perform | No | Not perform |
| Respond to inotropic agents | No | No | No | Not perform | No | Not perform |
| Respond to plasma infusion | Not perform | Yes | Yes | Not perform | Yes | Not perform |
| Outcome (age at death) | Death (8 days) | Death (3 days) | Death (2 days) | Death/ termination of pregnancy | Survival | Death/ termination of pregnancy |
Renal Histological Characteristics
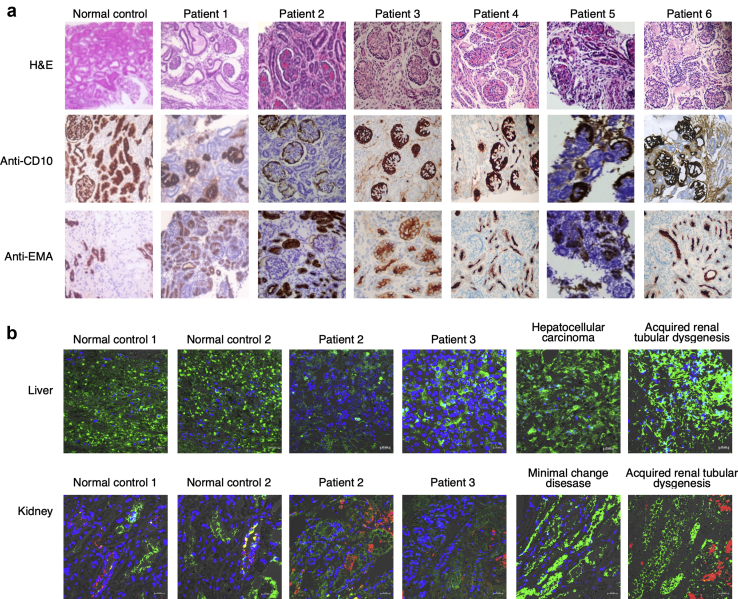
Renal tissues were obtained by percutaneous fine needle biopsy in all cases. Immunolabeling with anti-CD10 and anti-EMA was used to identify proximal tubules and distal tubules, respectively. There was strong staining of proximal and distal tubules in disease controls with minimal change disease. Although there was intense staining of podocytes and diffuse tubular staining with anti-EMA of renal tissues, no discernible tubular labeling with anti-CD 10 antibody was observed. This is indicative of the absence of proximal tubular development (Figure 1a).
Figure 1.
Differentiation of proximal convoluted tubules and expression of angiotensinogen (AGT) in kidney and liver in patients with autosomal recessive renal tubular dysgenesis and normal control. (a) Light microscopy shows lack of tubular labeling with anti-CD10 antibody in contrast to positive staining of podocytes and demonstration of anti-epithelial membrane antigen stains of distal tubules and collecting ducts. (b) Immunofluorescence of AGT and renin on liver and kidney. There is faint expression of AGT protein (green) detected in proximal tubules of kidney and hepatocytes in liver. High renin expressions (red) were noticed in patients 2 and 3 (autosomal recessive renal tubular dysgenesis) and also acquired renal tubular dysgenesis. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). H&E, hematoxylin and eosin.
Molecular Analysis by Whole Exome Sequencing
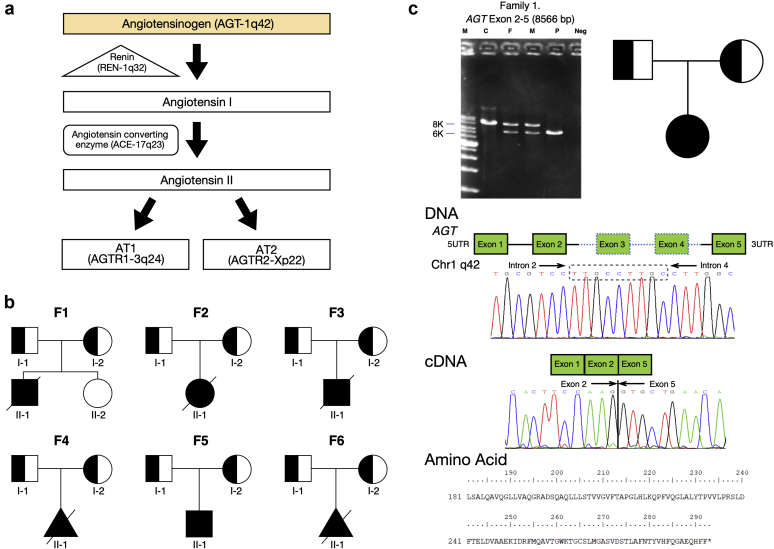
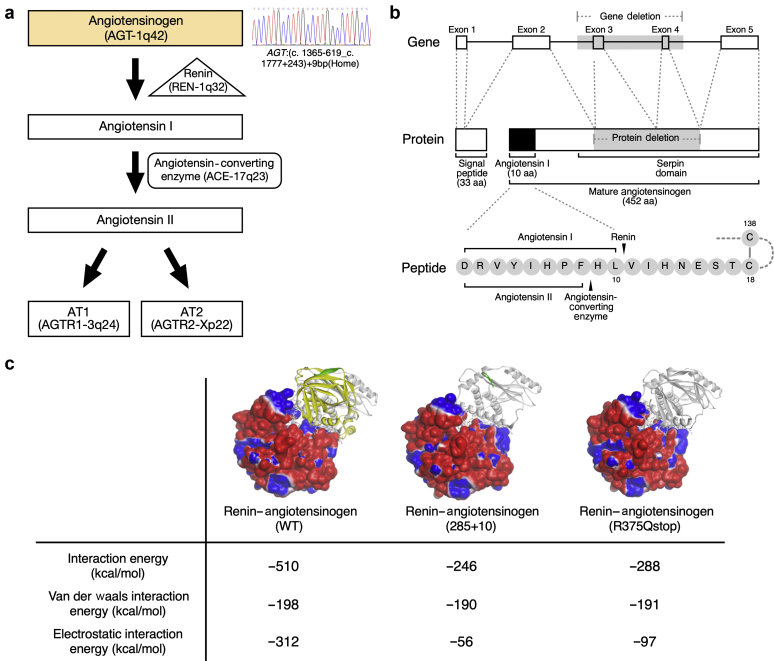
Whole exome sequencing followed by validation with Sanger sequencing was performed in all 6 patients and their parents (Figure 2a). The WES did not reveal pathogenic mutations in the coding regions of REN, ACE, and AGTR1, but did identify a large homozygous deletion of exons 3 and 4 of AGT in all 6 patients that cosegregated with the disease. Further analysis using PCR primers flanking the possible deletion breakpoints revealed a 2800-bp deletion shared by all 6 patients and their parents (Figure 2b and c). Direct sequencing of the PCR product uncovered a novel deletion E3_E4 del:2870bp deletion (NM_000029.3:c.857-619_C1269+243del) +9bp insertion in AGT gene. This deletion included parts of intron 2 (619 bp), exon 3 (268 bp), intron 3 (1595 bp), exon 4 (145 bp), and intron 4 (243 bp). This mutation causes a frame shift and a subsequent premature stop codon in exon 5 (NP_000020.1:p.Gly286Valfs∗6), leading to a product shorter by 292 amino acids, containing intact N-terminal 285 amino acids of AGT (Figure 2c). This AGT deletion has not been reported previously and is absent from the gnomad and other databases. Using software (AnnotSV), prediction for this AGT mutation was severely pathogenic. We performed analysis of linkage disequilibrium and organization in haplotypes of single nucleotide polymorphisms adjacent to the AGT gene in all of the patients and their parents (Supplementary Figure S1). The probability that this haplotype occurred independently in all index cases was of 1.52 × 10−5, suggesting a founder effect.
Figure 2.
Genetic characteristics, pedigree, Sanger sequence, and cDNA of patients with autosomal recessive renal tubular dysgenesis caused by AGT deletion. (a) Illustration of genes responsible for proteins involving the renin−angiotensin system. (b) Pedigree showing individuals from 6 unrelated families with homozygous AGT deletion with filled black symbol with a short lower arrow. (c) Illustration of large deletion of AGT by electrophoresis. E3_E4 del:2870bp deletion with 9-bp insertion (dashed box) detected by Sanger sequencing leads to a truncated protein (292 amino acids).
Prevalence of Heterozygous AGT Deletion
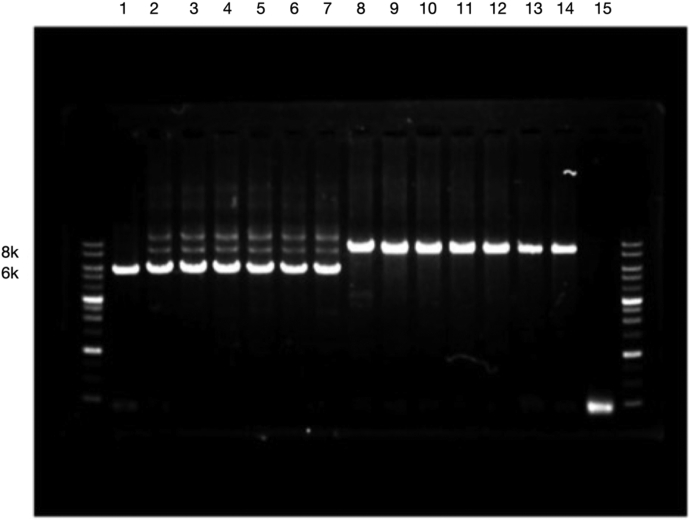
Because of the nature of rare disease and identical deletion breakpoint of the AGT gene in patients and their parents, we assessed the frequency of the peculiar AGT deletion in 500 healthy and unrelated Taiwanese individuals. Six of 500 healthy subjects harbored this peculiar heterozygous AGT deletion in a heterozygous state, as detected by pulse field gel electrophoresis and confirmed by direct Sanger sequencing (Figure 3). Thus, in Taiwan, the heterozygous carrier rate was approximately 1.2% (6/500).
Figure 3.
Prevalence of angiotensinogen (AGT) heterozygosity. Reverse transcription−polymerase chain reaction analysis using exon 2- to 5-specific primers detected comparatively small bands (6K) from 6 carriers of heterozygosity of AGT deletion (lanes 2−7) in contrast to relatively large bands (8K, lanes 8−14) in normal controls. Lanes 1 and 15 are positive and negative controls, respectively.
Tissue AGT Expression
We examined AGT expression in liver and kidney tissues obtained from autopsy of deceased patients 2 and 3, disease controls, and normal controls. As shown in Figure 1b, AGT expression in liver and kidney, as compared to control, was very faint in patients 2 and 3, indicating that the deletion of AGT diminished the expression of AGT both in liver and in the kidney.
Serum Levels of AGT, Renin, ACE, and Ang I and II
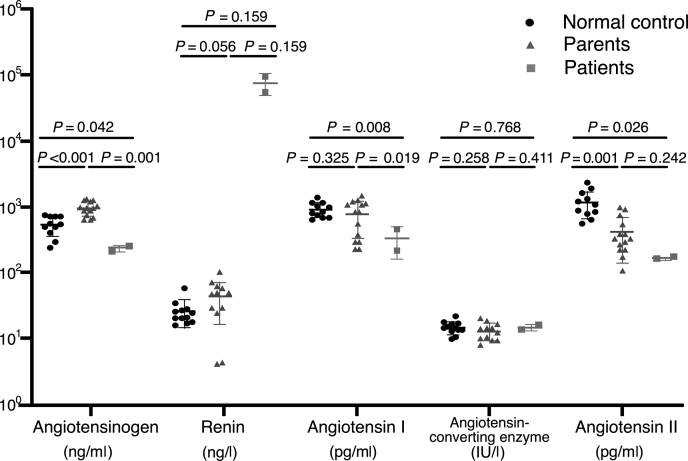
As the genetic defect of AGT resulted in decreased tissue expression of AGT, serum AGT, renin, Ang I, ACE, and Ang II levels were measured in affected individuals, their parents, and healthy subjects of similar age. Compared to healthy neonates, serum AGT, Ang I, and Ang II concentrations were markedly reduced in ARRTD patients (P = 0.042, P = 0.008, and P = 0.026, respectively) (Figure 4). In contrast, the serum renin levels of patients were much higher than those of healthy subjects and their parents. Relative increase in serum AGT and higher renin levels in their parents were observed. These findings indicated that the homozygous AGT deletion resulted in the reduction of serum AGT and its downstream end-product, serum Ang II.
Figure 4.
Serum angiotensinogen (AGT), renin, angiotensin I (Ang I), angiotensin-converting enzyme (ACE), and angiotensin II (Ang II) levels in patients, their parents, and healthy neonates.
Simulation and Measurement of Renin−AGT Interaction
As shown in Figure 5a, truncated AGT (AA 1−295) lacks the serpin domain of the AGT protein, which is critical for the interaction with renin for cleavage.20 The simulation models were first examined to evaluate whether this AGT deletion would abolish the interaction between AGT and renin. As shown in Figure 5b, the resolved structures of human AGT in complex with renin, human, mouse, and rat AGT (PDB code: 2X0B, 2WXW, 2WXY, and 2WXZ) were used as a template. A salt bridge (R:LYS286:HZ3 − A:GLU338:OE2) of wild-type AGT (amino acids 1−485) protein was not found in the 2 truncated AGT (AA 1−295 and AA 1−375/R375Q) proteins. Alternative hydrogen bonds were formed at the interface of the truncated AGT proteins and renin as compared to wild-type AGT. The calculated interaction energy at the interface of the truncated AGT (AA 1295 and AA R375Q) and renin was reduced by about 2-fold compared to that of the wild type.
Figure 5.
Simulation models of renin in complex with wild-type and truncated angiotensinogen (AGT) proteins. (a) Illustration of proteins involving the renin−angiotensin system. (b) Deleted region of AGT and its encoded proteins involving serpin domain. (c) Renin is present as a molecular surface model colored by the electrostatic potential. Wild-type and truncated AGT are shown as a ribbon model in white. Residues involved in interactions at the interface are shown as a stick model. Missing segments in the truncated AGT proteins (amino acids 1−292 and amino acids 1−375/R375Q) in the wild-type AGT are highlighted in yellow. Green color in the wild type represents the replaced sequence (amino acids 286−295) in truncated AGT.
Hydrocortisone May Exert Therapeutic Effects in ARRTD
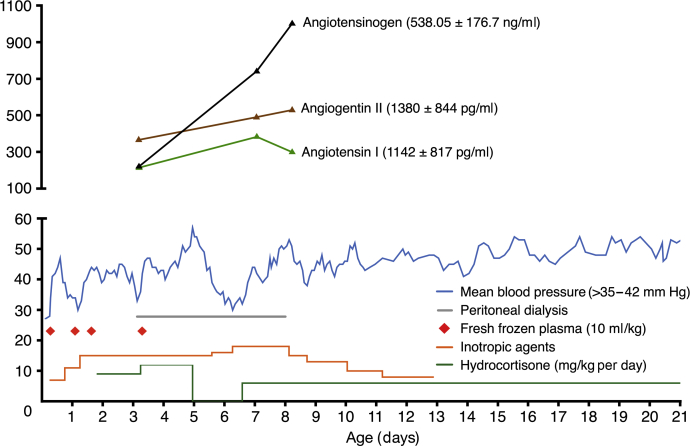
Patient 5 was born after a pregnancy complicated by oligohydramnios at 34 weeks of gestational age. He presented with profound hypotension and primary anuria. Hypotension was refractory to i.v. saline challenges and vasopressors but transiently responsive to fresh frozen plasma. Peritoneal dialysis was initiated for his persistent anuria. Because of his refractory hypotension and in light of glucocorticoids as the regulators of hepatic AGT generation due to its stimulation of AGT mRNA transcription,21,22 we administered i.v. hydrocortisone (2 mg/kg every 6 hours) 40 hours after birth and continued hydrocortisone but not mineralocorticoid thereafter. His blood pressure, as shown in Figure 6, increased significantly after hydrocortisone treatment and the fresh frozen plasma infusion was discontinued at the age of 3 days. He remained normotensive until hydrocortisone was discontinued on day 5 of postnatal life. This was followed by hypotension, which was rescued again only by restarting hydrocortisone. Furthermore, we observed a much improved urinary output allowing cessation of renal replacement therapy on day 8 of postnatal life. Further analysis demonstrated that serum Ang II level was significantly increased in parallel with serum AGT levels after administration of hydrocortisone (Figure 6). At the age of 28 days, parenteral hydrocortisone was switched to oral hydrocortisone. At last observation at the age of 12 months, he had chronic kidney disease, stage III, but still remained normotensive on oral hydrocortisone (1.5 mg/kg per day) treatment.
Figure 6.
Rescue autosomal recessive renal tubular dysgenesis neonate with hydrocortisone infusion. Hypotension was refractory to an inotropic agent but responded transiently to plasma infusion. Blood pressure stabilized with the use of high-dose hydrocortisone without the need for plasma infusion, then declined and returned to normal after withdraw and re-administration of hydrocortisone.
Discussion
In this study, we identified a novel founder deletion mutation of AGT in 6 patients from 6 unrelated Taiwanese families. This homozygous deletion of AGT led to truncated protein (292 amino acids), less detectable AGT protein in liver and kidneys, and diminished serum AGT, Ang I, and Ang II levels. The heterozygous carrier rate in Taiwan was approximately 1.2% (6/500). A simulation model demonstrated the attenuated interaction of this truncated protein with renin.
This novel AGT mutation is most likely pathogenic, based on aberrant protein expression (Figure 1), and also the absence of other genetic defects (ACE, REN, and AGTR) on WES that could cause ARRTD. The identification of this identical AGT mutation in our patients and parents urged us to assess the prevalence of this peculiar mutation in healthy individuals in Taiwan. Surprisingly, we found that the allelic frequency of this AGT mutations in a heterozygous state was 1.2%, and the expected incidence of patients with ARRTD would be approximately 3.6 per 100,000 pregnancies (1.2/100 × 1.2/100 × 1/4) is higher than expected. This indicates that the risk for ARRTD is higher in the Taiwanese population than in other parts of the world.8 Because heterozygous carriers of the AGT mutation are asymptomatic, molecular analysis of parents with offspring complicated by oligohydramnios and profound hypotension may help to uncover undiagnosed cases, enhance genetic counseling for heterozygous carriers, and provide earlier prenatal diagnosis of ARRTD for subsequent pregnancies.
In this study, ARRTD patients with truncation of AGT had low amounts of AGT protein in the liver and kidneys, resulting in decreased serum AGT and its downstream products of serum Ang I and Ang II levels. Lower expression of truncated AGT has been shown in an in vitro study.19 These findings resembled those in AGT null and hypomorph mice,23,24 and were consistent with a previous report in two patients with homozygous missense mutation (R375Q) in AGT.25 The source of AGT in the kidney is both liver and kidney derived,26,27 and a marked reduction in AGT expression in the liver and kidney indicates that the low hepatic expression of AGT might at least play an important role. The remarkably attenuated expression of truncated AGT protein in the liver caused by this AGT mutation may be related to either reduced hepatic production or enhanced hepatic degradation of truncated AGT.
Higher serum renin activity and enhanced tissue renin expression uniformly found in patients with ARRTD caused by mutations in RAS other than REN were thought to be secondary to the loss of negative Ang II−mediated regulation of renin synthesis.10,14,25 Of interest, our ARRTD patients with AGT truncation exhibited extraordinarily high serum renin levels (300-fold higher than normal control). Prior studies have demonstrated that the functional conserved sequences in the core serpin domain of AGT are necessary for AGT−renin interaction.28,29 The large deletion per se on AGT excluding this serpin domain of AGT may reduce renin cleavage and thus contribute to this markedly higher serum renin concentration. The results of a simulation model showing the diminishment of interaction between renin and truncated AGT may support this notion.
In parallel to an elevated serum AGT, the increased serum Ang II with simultaneous correction of hypotension was notably found in patient 5 after persistent and higher-dose hydrocortisone treatment. The spontaneous recovery of hypotension and renal hypoperfusion in 2 cases of ARRTD caused by a nonhomozygous truncated AGT mutation have been reported previously.19,30 The presence of at least 1 nontruncating variant may allow survival.8 The rapidly fatal course in all other similar patients and the recurrence and resumption of profound hypotension after discontinuation and re-delivery of hydrocortisone in patient 5 was intriguing. Indeed, no ARRTD patients with homozygous truncating variants have survived to date.8 These findings argue against the possibility of spontaneous recovery in our surviving patient. Glucocorticoid has been shown to be the regulator of generation of AGT in liver by increasing the abundance mRNA of AGT in in vivo and in vitro studies.21,22 The mechanisms underlying the enhancement of serum AGT and Ang II concentrations by supraphysiological dosing of hydrocortisone require further elucidation.
Because glucocorticoid receptor is abundant in the proximal convoluted tubule during development,11,30 antenatal hydrocortisone may exert an effect on the expression of glucocorticoid receptor. The synthetic human Ang II might also be used for reversing the profound hypotension and might act as a growth factor during the development of proximal tubular cells.31, 32, 33, 34, 35 The creation of ARRTD-causing knock-in mice may help to clarify the effects of glucocorticoid and synthetic Ang II in patients with ARRTD.
In conclusion, we have identified a novel founder mutation of AGT in 6 ARRTD patients from 6 unrelated families and have discovered a higher frequency of this heterozygous mutation, suggesting that ARRTD may be underdiagnosed in Taiwan. This AGT E3_E4 del:2870bp deletion+9bp insertion mutation leads to the production of truncated AGT and a decreased detectable AGT protein in the liver with diminished serum AGT, Ang I, and Ang II levels. Prolonged and high-dose hydrocortisone may have a role in maintaining hemodynamic stability in ARRTD patients without severe pulmonary hypoplasia.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by research fund of Chang Gung Memorial Hospital (CMRPG3H0361, CMRPG3H0911, CMRPG3I0021), Tri-Service General Hospital (TSGH-C108-132), and the Ministry of Science and Technology (MOST 106-2314-B-182A-123-MY3, MOST 109-2314-B-182A-134-MY3). We are grateful to the participating patients and their families. We would like to thank to Yu-Ching Chou for statistical advice, Hsiao-Tzu Yang and Feng-Chun Hung for help with ascertainment of the family, and Che-Chung Huang for technical support in sample preparation.
Footnotes
Figure S1. Haplotype analysis of AGT deletion. The analysis of linkage disequilibrium and organization in haplotypes of single nucleotide polymorphisms adjacent to the AGT gene in all of the patients and their parents. The probability that this haplotype occurs independently in all index cases was of 1.52 x 10-5, suggesting a founder effect.
STREGA Checklist.
Supplementary Material
References
- 1.Allanson J.E., Pantzar J.T., Macleod P.M. Possible new autosomal recessive syndrome with unusual renal histopathological changes. Am J Med Genet. 1983;16:57–60. doi: 10.1002/ajmg.1320160110. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz B.R., Lage J.M., Pober B.R., Driscoll S.G. Isolated congenital renal tubular immaturity in siblings. Hum Pathol. 1986;17:1259–1263. doi: 10.1016/s0046-8177(86)80570-6. [DOI] [PubMed] [Google Scholar]
- 3.Swinford A.E., Bernstein J., Toriello H.V., Higgins J.V. Renal tubular dysgenesis: delayed onset of oligohydramnios. Am J Med Genet. 1989;32:127–132. doi: 10.1002/ajmg.1320320127. [DOI] [PubMed] [Google Scholar]
- 4.Allanson J.E., Hunter A.G.W., Mettler G.S., Jimenez C. Renal tubular dysgenesis: a not uncommon autosomal recessive syndrome: a review. Am J Med Genet. 1992;43:811–814. doi: 10.1002/ajmg.1320430512. [DOI] [PubMed] [Google Scholar]
- 5.Ariel I., Wells T.R., Landing B.H. Familial renal tubular dysgenesis: a disorder not isolated to proximal convoluted tubules. Fetal Pediatr Pathol. 1995;15:915–922. doi: 10.3109/15513819509027027. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein J., Barajas L. Renal tubular dysgenesis: evidence of abnormality in the renin-angiotensin system. J Am Soc Nephrol. 1994;5:224–227. doi: 10.1681/ASN.V52224. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber R., Gubler M.C., Gribouval O. Inherited renal tubular dysgenesis may not be universally fatal. Pediatr Nephrol. 2010;25:2531–2534. doi: 10.1007/s00467-010-1584-0. [DOI] [PubMed] [Google Scholar]
- 8.Gribouval O., Morinière V., Pawtowski A. Spectrum of mutations in the renin-angiotensin system genes in autosomal recessive renal tubular dysgenesis. Hum Mutat. 2012;33:316–326. doi: 10.1002/humu.21661. [DOI] [PubMed] [Google Scholar]
- 9.Bacchetta J., Dijoud F., Bouvier R. Dysgénésie tubulaire proximale et mutation du gène de la rénine. Arch Pediatr. 2007;14:1084–1087. doi: 10.1016/j.arcped.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Gribouval O., Gonzales M., Neuhaus T. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet. 2005;37:964–968. doi: 10.1038/ng1623. [DOI] [PubMed] [Google Scholar]
- 11.Yosypiv I.V. Renin-angiotensin system in ureteric bud branching morphogenesis: implications for kidney disease. Pediatr Nephrol. 2014;29:609–620. doi: 10.1007/s00467-013-2616-3. [DOI] [PubMed] [Google Scholar]
- 12.Niimura F., Labosky P.A., Kakuchi J. Gene targeting in mice reveals a requirement for angiotensin in the development and maintenance of kidney morphology and growth factor regulation. J Clin Invest. 1995;96:2947–2954. doi: 10.1172/JCI118366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliverio M.I., Kim H.S., Ito M. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc Natl Acad Sci U S A. 1998;95:15496–15501. doi: 10.1073/pnas.95.26.15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gubler M.C., Antignac C. Renin-angiotensin system in kidney development: renal tubular dysgenesis. Kidney Int. 2010;77:400–406. doi: 10.1038/ki.2009.423. [DOI] [PubMed] [Google Scholar]
- 15.Pierre C., Annie M., Olivier G. Can we live without a functional renin-angiotensin system? Clin Exp Pharmacol Physiol. 2008;35:431–433. doi: 10.1111/j.1440-1681.2008.04891.x. [DOI] [PubMed] [Google Scholar]
- 16.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DePristo M.A., Banks E., Poplin R. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–501. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang K., Li M., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:1–7. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uematsu M., Sakamoto O., Nishio T. A case surviving for over a year of renal tubular dysgenesis with compound heterozygous angiotensinogen gene mutations. Am J Med Genet Part A. 2006;140A:2355–2360. doi: 10.1002/ajmg.a.31448. [DOI] [PubMed] [Google Scholar]
- 20.Streatfeild-James R.M.A., Williamson D., Pike R.N. Angiotensinogen cleavage by renin: importance of a structurally constrained N-terminus. FEBS Lett. 1998;436:267–270. doi: 10.1016/s0014-5793(98)01145-4. [DOI] [PubMed] [Google Scholar]
- 21.Deschepper C.F., Dallman M.F. The stimulation of liver angiotensinogen by glucocorticoids depends on the type of steroid and its mode of administration. Endocrinology. 1992;131:2371–2377. doi: 10.1210/endo.131.5.1425436. [DOI] [PubMed] [Google Scholar]
- 22.Deschepper C.F., Hong-Brown L.Q. Hormonal regulation of the angiotensinogen gene in liver and other tissues. In: Raizada M.K., Phillips M.I., Sumners C., editors. Cellular and Molecular Biology of the Renin-Angiotensinogen System. CRC Press; Boca Raton, FL: 1993. pp. 149–166. [Google Scholar]
- 23.Ishida J., Sugiyama F., Tanimoto K. Rescue of angiotensinogen-knockout mice. Biochem Biophys Res Commun. 1998;252:610–616. doi: 10.1006/bbrc.1998.9707. [DOI] [PubMed] [Google Scholar]
- 24.Tanimoto K., Sugiyama F., Goto Y. Angiotensinogen-deficient mice with hypotension. J Biol Chem. 1994;269:31334–31337. [PubMed] [Google Scholar]
- 25.Lacoste M., Cai Y., Guicharnaud L. Renal tubular dysgenesis, a not uncommon autosomal recessive disorder leading to oligohydramnios: role of the renin-angiotensin system. J Am Soc Nephrol. 2006;17:2253–2263. doi: 10.1681/ASN.2005121303. [DOI] [PubMed] [Google Scholar]
- 26.Matsusaka T., Niimura F., Shimizu A. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23:1181–1189. doi: 10.1681/ASN.2011121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingelfinger J.R., Zuo W.M., Fon E.A. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C., Lu H., Cassis L.A., Daugherty A. Molecular and pathophysiological features of angiotensinogen: a mini review. N Am J Med Sci (Boston) 2011;4:183–190. doi: 10.7156/v4i4p183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu H., Cassis L.A., Vander Kooi C.W., Daugherty A. Structure and functions of angiotensinogen. Hypertens Res. 2016;39:492–500. doi: 10.1038/hr.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zingg-Schenk A., Bacchetta J., Corvol P. Inherited renal tubular dysgenesis: the first patients surviving the neonatal period. Eur J Pediatr. 2008;167:311–316. doi: 10.1007/s00431-007-0492-1. [DOI] [PubMed] [Google Scholar]
- 31.Khalique S.C., Ferguson N. Angiotensin II (Giapreza): a distinct mechanism for the treatment of vasodilatory shock. Cardiol Rev. 2019;27:167–169. doi: 10.1097/CRD.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 32.Johal J.S., Thorp J.W., Oyer C.E. Neonatal hemochromatosis, renal tubular dysgenesis, and hypocalvaria in a neonate. Pediatr Dev Pathol. 1998;1:433–437. doi: 10.1007/s100249900059. [DOI] [PubMed] [Google Scholar]
- 33.Schütz S., Le Moullec J.M., Corvol P., Gasc J.M. Early expression of all the components of the renin-angiotensin-system in human development. Am J Pathol. 1996;149:2067–2079. [PMC free article] [PubMed] [Google Scholar]
- 34.Tufro-McReddie A., Johns D.W., Geary K.M. Angiotensin II type 1 receptor: role in renal growth and gene expression during normal development. Am J Physiol. 1994;266:F911–F918. doi: 10.1152/ajprenal.1994.266.6.F911. [DOI] [PubMed] [Google Scholar]
- 35.Zhang S.-L., Moini B., Ingelfinger J.R. Angiotensin II increases Pax-2 expression in fetal kidney cells via the AT2 receptor. J Am Soc Nephrol. 2004;15:1452–1465. doi: 10.1097/01.asn.0000130567.76794.58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.