Abstract
Introduction
The term “acute tubular injury” (ATI) represents histopathologic renal tubular injury and often manifests clinically as acute kidney injury (AKI). Studies systematically summarizing the clinical presentation and histological changes in human ATI are limited.
Methods
We used a comprehensive search strategy to search human studies of ATI from 1936 to July 2019. We extracted study characteristics, clinical characteristics, and histologic descriptions of ATI by bright field, immunofluorescence, electron microscopy, and immunohistochemistry. We compared ATI histology as a function of tissue procurement type, timing, and etiologies.
Results
We included 292 studies comprising a total of 1987 patients. The majority of studies (222 of 292, 76%) were single-center case reports. The mean age of included patients was 47 years. In native kidney biopsy cases, baseline, peak, and latest creatinine were 1.3 mg/dl, 7.19 mg/dl, and 1.85 mg/dl respectively, and biopsy was performed mostly after peak creatinine (86.7%, 391 of 451). We identified 16 histologic descriptions of tubular injury, including tubular cell sloughing (115 of 292, 39.4%), tubular epithelial flattening/simplification (110 of 292, 37.7%), tubular dilatation (109 of 292, 37.3%), and tubular cell necrosis (93 of 292, 31.8%). There was no difference in tubular injury histology among different tissue procurement types (native kidney biopsy, transplant kidney biopsy, and autopsy), among different etiologies, or between different tissue procurement timing (before or after creatinine peaks in native kidneys). Electron microscopy and immunohistochemistry were used in a minority of studies.
Conclusion
ATI manifests with diverse histologic changes. Efforts to establish protocols to harmonize biopsy practices, to handle kidney biopsy for tissue interrogation, and to report results across clinical practice are needed to improve our understanding of this complex disease.
Keywords: acute kidney injury, acute tubular injury, acute tubular necrosis, histology, pathology
Graphical abstract
Acute kidney injury (AKI) is common in hospitalized patients and is associated with significantly higher morbidity and mortality.1 It represents the acute decline in kidney function, often measured by serum creatinine, with or without structural changes in the kidney. The morphologic and structural changes in the kidney vary widely, depending on the nature and mechanism of injury.
The histopathologic correlate of AKI can often be captured in the term “acute tubular injury” (ATI), which reflects intrinsic kidney damage as a result of either ischemic or toxic insult affecting the functional and morphologic integrity of renal tubules.2 Its manifestation as AKI includes rapid decline in glomerular filtration rate and frequently with oliguria. The prevalence of AKI attributable to ATI and the clinical presentation of ATI is difficult to evaluate because of the lack of large human tissue cohort studies correlating tubular injury patterns with clinical AKI.3 However, it is clear that either AKI or ATI itself carries a high risk of mortality and morbidity, especially in the aging patient population.4 Acute tubular injury is well recognized both in clinical and mechanistic studies as an important driver of chronic kidney disease (CKD) and progression to end-stage kidney disease (ESKD).5,6 Unfortunately, promising therapies for ATI based on animal models have failed to translate into success in human trials.7, 8, 9, 10 In addition, most preclinical ATI studies use animal models in which the pathogenesis and histopathology may be distinct from human ATI.11,12 Therefore, it is essential to establish a large collection of human ATI biopsy samples to systematically establish and then link pathophysiology with potential therapeutic options in ATI.
For many decades, ATI was synonymous with acute tubular necrosis (ATN). However, frank tubular epithelial necrosis is only 1 histologic pattern observed in clinical ATI and may reflect particular etiologies and/or severity of injury. The diverse pathologic changes are often dynamic and may differ by the timing of kidney tissue procurement and etiology.3,13 However, there are limited studies systematically profiling and relating the histologic changes in human ATI with each other and with the etiology. In this report, we systematically reviewed studies of human ATI with the goal of summarizing the clinical presentation and the histologic description of ATI, and we explored the histologic diversity as a function of timing of kidney tissue procurement during the clinical course and clinical etiologies.
Methods
Data Source
We performed a comprehensive systematic PubMed search of articles published through July 2019. The earliest study was published in 1936. The search strategy included articles indexed under both the “kidney tubular necrosis, acute” (Medical Subject Headings), “acute tubular injury”, “ATI”, “acute tubular necrosis”, or “ATN” under title/abstract and “kidney biopsy”, “renal biopsy”, “pathology”, “histopathology”, “histology” , or “morphology” under title/abstract. A group of high-performance AKI search filters that includes >100 terms used in combination, including “acute kidney injury,” “tubular necrosis,” “azotemia,” and “ischemic injury,” were developed to identify articles to AKI in PubMed, Ovid Medline, and Embase.14 We applied the sensitive filter for PubMed, which has a sensitivity of 96.1% and specificity of 95%, to the above relevant terms to increase search sensitivity. Studies with unavailable abstracts or full abstracts were then searched with the assistance of librarians from the Icahn School of Medicine at Mount Sinai and Johns Hopkins University School of Medicine. We limited our search to studies published in English. A full list of study reports is available in Supplementary Material.S1–S292
Study Selection
Studies reporting histology of ATI were included. We excluded studies that did not include human subjects, did not have ATI on the pathology report, did not report a histologic description of tubular injury, or reported imaging tools without a pathology report, as well as review articles. We did not find any donor kidney biopsy studies reporting histologic description of tubular injury. Two of these 3 authors (YW, CY, SPM) reviewed the abstracts and full texts for all studies, and disagreement was resolved upon discussion.
Data Extraction
We extracted publication year, study type (case report, case series, or cohort study), single- versus multi-center study, country of first author, clinical information, and histology description. Clinical information included subjects’ age, sex, ethnicity, comorbidities, baseline kidney function, peak creatinine, latest creatinine, suspected etiologies, descriptive kidney outcome, timing of biopsy (before vs. after creatinine peak, after kidney transplantation), and type of tissue procurement (native kidney biopsy, transplant kidney biopsy, and autopsy). Histology descriptions of tubular injury included descriptions under light microscopy, immunofluorescence, electron microscopy, and immunohistochemistry. We extracted all morphologic descriptions of tubular injury reported, and provided the definition of these descriptions based on prior studies, review, and books (Supplementary Table S1).15, 16, 17, 18, 19, 20 Examples of these histologic descriptions can be found in included studies.16,21, 22, 23, 24, 25, 26, 27
Statistical Analysis
We used descriptive statistics such as means and proportions. Because of the large number of missing data points from underreporting, we reported both the percentage of each clinical feature and the overall availability of data across included studies and by tissue procurement types. We reported timing of kidney biopsy, biochemical characteristics, and clinical outcome (last serum creatinine or descriptive outcome if last serum creatinine was not reported) in native kidney biopsy cases. We reported the percentage of each histologic description based on the number of studies rather than patients, as it was not feasible to extract discrete histologic features at the patient level in the majority of case series and large cohorts. We compared the histologic descriptions among different types of tissue procurement (native kidney biopsy, transplant kidney biopsy, and autopsy) and among different etiologies using 1-way analysis of variance. We then compared the histologic descriptions based on timing of biopsy (before or after serum creatinine peaks) in native kidney biopsy cases using the χ2 test. We performed all analyses using Stata Version 14.2 (StataCorp, College Station, TX). We considered P values <0.05 as statistically significant.
Results
Our PubMed search identified 1823 articles, of which 1070 were excluded and 753 were selected for full text screen (Figure 1). Reasons for exclusion included nonhuman studies (n = 534), no ATI on biopsy (n = 198), review articles (n = 142), imaging reports without pathology findings (n = 68), and non−English language (n = 128). Following a full text screening, an additional 461 studies were excluded because they did not describe the histologic patterns of ATI. For the purposes of this systematic review, 292 articles were used for further analysis (Supplementary References S1−S292).
Figure 1.
Study flowchart. ATI, acute tubular injury.
The majority of these articles consisted of case reports (222, 76%), followed by case series (66, 22.6%) and cohort studies (4, 1.4%) cohorts. In total, these reports and studies reflected 1987 patients with a combined 2022 kidney biopsies and autopsies. Patient characteristics and the availability of data overall and in native kidney biopsy, transplant kidney biopsy, and autopsy studies are shown in Table 1. The mean age of the patients was 47 years, and 40.8% were female. Of the patients, 60.9% were white and 28% were black. In all, 38.2% patients had hypertension and 20.8% were diabetic. A total of 71.7% (1450 of 2022) were native kidney biopsies, 17.4% (351 of 2022) of cases were biopsies performed after kidney transplantation, and 10.9% (221 of 2022) were autopsies. Among native kidney biopsy cases, ATI was often associated with severe AKI (mean peak creatinine of 7.19 mg/dl), and incomplete recovery with a mean creatinine increase to 1.85 mg from 1.3 mg/dl at baseline. In postdischarge follow-up, descriptive kidney outcomes were reported in 109 patients. A total of 44 patients were reported to have normal kidney function, 11 patients developed CKD, 40 patients developed ESKD, 1 patient subsequently received a kidney transplant, and 13 patients died. Among native kidney biopsy cases for which the timing of kidney biopsy is available (451 of 1450, 31.1%), 13.3% (60 of 451) were biopsies performed before creatinine peaked, and 86.7% (391 of 451) were biopsies performed after creatinine peaked. A summary of the biochemical characteristics, timing of biopsy, descriptive clinical outcome. and data availability in all native kidney biopsy studies are shown in Table 2.
Table 1.
Patient characteristics and data availability in included studies
| Variable | Characteristics |
|||
|---|---|---|---|---|
| Overall N = 2022 |
Native kidney biopsy n = 1450 | Transplant kidney biopsy n = 351 | Autopsy n = 221 | |
| Age, yr, mean (data available, N) | 47 (1389) | 45.5 (961) | 51.4 (328) | 41 (87) |
| Female, % (data available, n/N) | 40.8 (561/1376) | 39.9 (383/961) | 41.7 (137/328) | 47 (41/87) |
| Ethnicity, % (data available, n/N) | ||||
| White | 60.9 (198/325) | 60.5 (181/299) | 0 | 70.8 (17/24) |
| Black | 28 (91/325) | 28.1 (84/299) | 50 (1/2) | 25 (6/24) |
| Hispanic | 2.5 (8/325) | 2.3 (7/299) | 50(1/2) | 0 |
| Asian | 5.2 (17/325) | 5.4 (16/299) | 0 | 4.2 (1/24) |
| Other | 3.4 (11/325) | 3.7 (11/299) | 0 | 0 |
| Hypertension, % (data available, n/N) | 38.2 (221/578) | 40 (208/516) | 50 (3/6) | 17.9 (10/56) |
| Diabetes mellitus, % (data available, n/N) | 20.8 (110/530) | 22.2 (104/468) | 16.7 (1/6) | 8.9 (5/56) |
Table 2.
Timing of kidney biopsy, biochemical characteristics, descriptive clinical outcomes, and data availability in native kidney biopsy studies
| Variable | Data available, % (n/N) | |
|---|---|---|
| Timing/type of kidney tissue procurement, % (n/N) | 31.1 (451/1450) | |
| Before serum creatinine peaked | 13.3% (60/451) | |
| After serum creatinine peaked | 86.7% (391/451) | |
| Baseline serum creatinine, mg/dl | 1.3 | 28.1 (407/1450) |
| Peak serum creatinine, mg/dl | 7.19 | 26.7 (387/1450) |
| Last serum creatinine, mg/dl | 1.85 | 25.7 (372/1450) |
| Descriptive clinical outcome, N | 7.5 (109/1450) | |
| Normal kidney function | 44 | |
| Chronic kidney disease | 11 | |
| End-stage renal disease | 40 | |
| Kidney transplant | 1 | |
| Death | 13 |
The various etiologies of ATI were described in 95.2% of studies (278 of 292). These included medication (n = 78), infection (n = 33), hypoperfusion (n = 4), hyperbilirubinemia (n = 12), rejection in transplanted kidneys (n = 5), toxins (n = 47), hemolysis (n = 11), rhabdomyolysis (n = 8), glomerular disease (n = 19), oxalate nephropathy (n = 10), a combination of more than 1 etiology (n = 6), and other etiologies (n = 39). Six studies included series of patients with different etiologies.
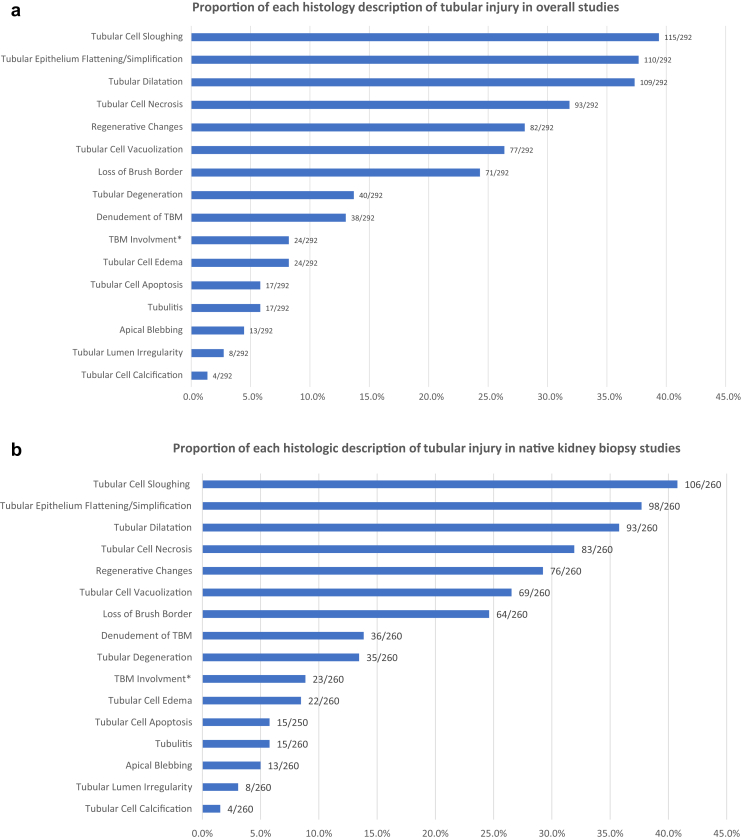
All studies reported histologic findings; however, only 34.2% of studies (100 of 292) reported a tubular injury pattern, whereas the rest did not report which proportion of tubules was involved. Proximal tubular injury was reported in 92% of studies (92 of 100), and involvement of the loop of Henle and distal tubules/collecting duct were reported in 7% (7 of 100) and 33% of studies (33 of 100), respectively. The extent of tubular injury was mostly semiquantitative but was reported in only 10.3% of studies (30 of 292). Figure 2a summarizes the results of tubular injury descriptions found throughout the included studies. The most common histologic descriptors of tubular injury included tubular cell sloughing (115 of 291, 39.4%), tubular epithelial flattening/simplification (110 of 292, 37.7%), tubular dilatation (109 of 292, 37.3%), tubular cell necrosis (93 of 292, 31.8%), regenerative changes (82 of 292, 28.1%), and tubular cell vacuolization (77 of 292, 26.4%). Other descriptors included loss of brush border, tubular degeneration, tubular cell apical blebbing, tubular basement membrane denudation, tubular basement membrane involvement (rupture, thickening, or thinning), tubular cell edema, tubular cell calcification, tubular cell apoptosis, tubulitis, and tubular lumen irregularity. Granular casts and epithelial casts were reported in 5.5% (16 of 292) and 7.2% of studies (21 of 292). A separate analysis of histologic descriptions of tubular injury in native kidney biopsy studies showed similar results (Figure 2b).
Figure 2.
(a) Reporting of histologic descriptors of tubular injury in overall studies. (b) Reporting of histologic descriptors of tubular injury in native kidney biopsy studies. ∗TBM involvement includes tubular basement membrane rupture, duplication, thickening, or thinning.
There was no difference in histologic descriptions of ATI among tissue procurement types or among different etiologies (Supplementary Tables S2 and S3). Tubular lumen irregularity was more commonly reported in native kidney biopsies performed before creatinine peaked, but this was likely clinically nonsignificant, given the low incidence of this description (Supplementary Table S4).
None of the studies reported positive findings on immunofluorescence in the tubular cells or interstitium. A subset of studies (123 of 292; 42.1%) reported ultrastructural results by electron microscopy. Apart from histologic changes consistent with findings from light microscopy, additional ultrastructural changes noted within tubular cells were also reported. These included changes in tubular cytoplasm, nucleus, and various organelles such as endoplasmic reticulum, endosome, lysosome, autophagosome, and mitochondria (Supplementary Table S5). Of the studies, 53 of 292 (18.2%) performed immunohistochemistry (IHC) or staining for other receptors. Among these studies, 14 studies used IHC to identify lymphocytes and macrophages, 6 studies stained for myoglobin, 4 studies stained for the proliferation marker Ki-67, 3 studies stained for kidney injury molecule−1 (KIM-1), and 2 studies stained for apoptosis (i.e., cleaved caspase-3).
Discussion
In this systematic review, we identified 292 human studies with reports of histologic manifestations of ATI in 1987 participants. Most studies were single-center studies and case reports. Clinical information such as demographic characteristics, comorbidities, baseline kidney function, and clinical outcomes were not universally reported. Overall, native kidney biopsies were performed in middle-aged men with mild baseline kidney impairment, presenting with severe AKI and with poor clinical outcomes. Native kidney biopsies were often performed after serum creatinine peaked in the course of AKI. On histological reporting, proximal tubules were commonly involved with 16 different histologic descriptions, such as tubular cell sloughing, tubular epithelium flattening/simplification, tubular dilatation, tubular cell necrosis, regenerative and tubular cell vacuolization. Among these qualitative studies, there was no difference in tubular injury histology among native kidney biopsies, transplant kidney biopsies, and autopsies, based on timing of biopsy or among different etiologies. Electron microscopy and immunohistochemistry were used in a minority of studies.
The need for better understanding of ATI clinically and histologically in aging patients with more advanced CKD is unmet. Contemporary AKI is much more common in older patients and occurs in 25% of patients older than 75 years in the intensive care setting.4,5 However, even in this relative younger population in the present study, ATI caused severe AKI in most cases, often with incomplete recovery. This is consistent with previous studies of long-term outcomes of AKI including significant risk for CKD, ESKD, and mortality.28 However, notably absent in the literature are biopsy findings in patients with mild AKI, which is 3- to 4-fold more common than severe AKI in clinical practice.29
Histologically, ATI most commonly involves proximal tubular injury, which is consistent with morphologies in animal models of toxic and septic injury.30 The primary location of injury in states of hypoperfusion is still a matter of debate. Early human studies demonstrated a relatively greater extent of injury to the distal nephrons, such as the medullary thick ascending limbs (mTAL) and medullary collecting ducts, than to the proximal tubules.31,32 However, a warm ischemia reperfusion model demonstrated primarily proximal (S3 segment) tubular injury, whereas cold ischemia models commonly resulted in mTAL injury.13,30,33 Although our study demonstrated almost universal involvement of proximal tubules, the analysis could be limited by sampling bias, limited appreciation on routine histologic stains of the various tubular segments, and limited morphologic changes that are appreciated by anatomic pathologists in the affected segments.
Tubular injury is a dynamic disease process. Tubular cells may involve a series of histological changes and transitions from an injury phase to a maintenance phase and finally to repair/recovery or chronic injury.34 Brush border loss, tubular simplification, flattening, and resultant dilatation and irregularity of tubular lumen represent a series of perhaps sequential events during this process of tubular injury.16 However, this chain of events is poorly established in the clinical literature. Notably, we have demonstrated that there were no significant histologic differences between biopsies performed before versus after serum creatinine peaked, further reinforcing the notion that serum creatinine is an insensitive marker for the extent of tubular injury.35 The increasingly recognized roles of urinary biomarkers in AKI will hopefully facilitate early detection of tubular injury and enhance the understanding of the disease process and its correlation with histological changes.35
Numerous medications, including anticoagulants, antibiotics, and immune check point inhibitors, have been found to be associated with ATI with different proposed mechanisms.36, 37, 38 Our knowledge of certain tubular diseases, such as light chain tubulopathy, bilirubin cast nephropathy, and phosphate nephropathy, have also been emerging.39, 40, 41 Although tubular injury can be caused by hypoperfusion or toxicities from medications, crystal, or pigment casts, they can also occur in primary glomerular diseases and acute interstitial nephritis and can even be part of antibody-mediated rejection in transplant kidneys. The mechanisms of ATI associated with these diseases were diverse, likely involving ischemic injury from hemodynamic compromise in nephrotic syndrome, and tubular toxicity from proteinuria or hemoglobin cast, or immune-mediated tubular injury.42, 43, 44 However, our study failed to demonstrate any significant difference in tubular cell injury between various etiologies, possibly due to lack of power in certain disease groups. Patients with ATI secondary to well-established etiologies such as hypoperfusion rarely undergo a kidney biopsy in clinical practice, which may result in selection bias. These clinical cases are also unlikely to be published, leading to publication bias. The majority of included studies were case reports and single-center case series, which are inherently subject to low reproducibility, accuracy, and interobserver variability.45, 46, 47 The lack of high-quality human ATI studies therefore highlights the importance of establishing multicenter studies with uniform sampling methods and standardized reporting system to make meaningful subgroups of ATI based on pathophysiology and disease severity.
In our assessment, a small proportion of studies applied immunohistochemistry stains. These additional stains were used to differentiate inflammatory cell infiltration or to facilitate the understanding of the pathogenesis. Several tissue markers, such as KIM-1, neutrophil gelatinase− associated lipocalin (NGAL), hemoxygenase-1, and osteopontin, have been shown to correlate with stage of ATI, to differentiate etiologies, to identify pathogenic pathways, and to provide prognoses.48, 49, 50, 51, 52, 53, 54, 55 Because little is known about the expression of tissue markers in human ATI and their correlation with histological changes, the utility of these markers requires further investigation.54, 55, 56, 57, 58, 59 Similarly, only less than half of the included studies performed electron microscopy. Ischemic and toxic ATI may have different patterns of injury, with ischemic ATI marked by autophagy pathway with extensive autophagosomes, and toxic ATI marked by extensive necrosis, dilation of endoplasmic reticulum, and mitochondria swelling with inclusions.60, 61, 62 Incorporating electron microscopy in a standardized protocol for diagnosing and studying human ATI may improve the global understanding of ATI pathophysiology, link histology to etiologies, and advance precision medicine in kidney diseases.
The underreporting of demographic information, clinical presentations, and patterns of tubular injury in a number of included studies may have contributed to potential selection bias and sampling biases. In addition, as different histologic changes may describe the same disease process, there may have been redundancy in the nomenclature. This highlights the importance of standardized case descriptions and pathology reports in both clinical practice and research, where harmonization of the ontologies for histologic description of ATI would be most useful. Standardized descriptions of ATI histology to include severity and phase of damage have not been established to correlate disease severity clinically and to provide prognosis.16 A recent study of pathology reports on glomerulonephritis proposed a standardized classification to facilitate diagnosis, to make treatment decisions, and to evaluate prognosis.63 The introduction of the Banff classification has also benefited clinical decision making in the transplantation setting.64 Tubular cell simplification, sloughing, and regeneration in native kidney biopsies were shown to correlate well with clinical AKI. There are also scoring systems developed to evaluate transplant ATI and its prognostic value.65,66 However, these scoring systems have not been widely assessed in large cohorts of native kidney biopsies, and the prognostic value of each histologic description remains unclear. Standardizing pathology reporting may help to objectively quantify the extent and severity of tubular injury, to predict renal recovery, to stimulate epidemiologic and mechanistic research, and eventually to shed light on therapeutic options for ATI.
This current systematic review is the first study to systematically summarize the histologic manifestation of human ATI. We used a comprehensive search strategy with a sensitive filter for AKI to maximize our search.14 With the assistance of librarians in the Icahn School of Medicine at Mount Sinai and Johns Hopkins University School of Medicine, we were able to include studies as early as 1936. However, we do acknowledge several important limitations. The current review focused only on human ATI studies, and animal studies were excluded. This may underestimate the variety of histological changes in tubular injury. As mentioned above, this analysis was also subject to publication bias, as patients with mild AKI or AKI from well-established etiologies are less likely to undergo kidney biopsy. There could also be selection bias and sampling bias reflected by the amount of missing information in clinical characteristics and injury pattern. However, the results of our study highlighted the importance of standardized case presentation and pathology report. With the efforts of large collaborative, multi-center studies such as the Kidney Precision Medicine Project within which biopsies are being performed in participants with ATI, our knowledge of this common disease will hopefully improve in this decade.67
In conclusion, ATI biopsies were performed in relatively young patients with underlying mild CKD. Injury is located primarily in the proximal tubules, with many histological manifestations. However, there is a significant underreporting of clinical details and histology descriptions. We urge establishing protocols to harmonize biopsy practices, handling of kidney biopsy samples for tissue interrogation, and reporting of results across clinical centers. This will facilitate knowledge generation and the development of meaningful subgroups to link histological changes on biopsy to clinical etiologies so as to guide prognosis and practice.
Disclosure
CRP is a member of the advisory board of RenalytixAI and owns equity in the same. He also serves as a DSMB member for Genfita. All the other authors declared no competing interests.
Acknowledgments
CRP was supported by the National Institutes of Health grants R01DK-93770 and R01HL-085757. He is also participating in the NIDDK sponsored Kidney Precision Medicine Project UH3DK114866. We appreciate the assistance from the librarians in the Icahn School of Medicine at Mount Sinai and Johns Hopkins University School of Medicine in obtaining study report with unavailable abstract or full text.
Author Contributions
YW, CY, and CRP participated in the study design. YW, CY, and SPM participated in abstract and full text review. YW carried out statistical analysis. YW, CY, SPM, AZR, and CRP drafted and revised the paper, and all authors approved the final version.
Footnotes
Table S1. Histologic descriptions of tubular injury and definition based on prior studies.
Table S2. Tubular injury histologic descriptors in ATI in native kidney biopsy, transplant kidney biopsy, and autopsy studies.
Table S3. Histologic description of tubular injury in ATI based on categories of etiologies.
Table S4. Histologic description of tubular injury in ATI before and after creatinine peaked in native kidney biopsies.
Table S5A. Histologic descriptions of tubular cell ultrastructure reported in studies of ATI.
Table S5B. Histologic descriptions of tubular cell ultrastructure seen both under electron microscopy and brightfield.
Supplementary References.
Supplementary Material
References
- 1.Xue J.L., Daniels F., Star R.A. Incidence and mortality of acute renal failure in medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 2.Ricci Z., Cruz D.N., Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nat Rev Nephrol. 2011;7:201. doi: 10.1038/nrneph.2011.14. [DOI] [PubMed] [Google Scholar]
- 3.Moeckel G.W. Pathologic perspectives on acute tubular injury assessment in the kidney biopsy. Semin Nephrol. 2018;38:21–30. doi: 10.1016/j.semnephrol.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Joannidis M., Metnitz B., Bauer P. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35:1692–1702. doi: 10.1007/s00134-009-1530-4. [DOI] [PubMed] [Google Scholar]
- 5.Hsu C., Chertow G.M., McCulloch C.E. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4:891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuk A., Bonventre J.V. Acute kidney injury. Annu Rev Med. 2016;67:293–307. doi: 10.1146/annurev-med-050214-013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharp C.N., Siskind L.J. Developing better mouse models to study cisplatin-induced kidney injury. Am J Physiol Renal Physiol. 2017;313:F835–F841. doi: 10.1152/ajprenal.00285.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allgren R.L., Marbury T.C., Rahman S.N. Anaritide in acute tubular necrosis. N Engl J Med. 1997;336:828–834. doi: 10.1056/NEJM199703203361203. [DOI] [PubMed] [Google Scholar]
- 9.Pickkers P., Mehta R.L., Murray P.T. Effect of human recombinant alkaline phosphatase on 7-day creatinine clearance in patients with sepsis-associated acute kidney injury: a randomized clinical trial. JAMA. 2018;320:1998–2009. doi: 10.1001/jama.2018.14283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumlin J.A., Finkel K.W., Murray P.T. Fenoldopam mesylate in early acute tubular necrosis: a randomized, double-blind, placebo-controlled clinical trial. Am J Kidney Dis. 2005;46:26–34. doi: 10.1053/j.ajkd.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Heyman S.N., Lieberthal W., Rogiers P., Bonventre J.V. Animal models of acute tubular necrosis. Curr Opin Crit Care. 2002;8 doi: 10.1097/00075198-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Rosen S., Samuel N.H. Difficulties in understanding human acute tubular necrosis: limited data and flawed animal models. Kidney Int. 2001;60:1220–1224. doi: 10.1046/j.1523-1755.2001.00930.x. [DOI] [PubMed] [Google Scholar]
- 13.Heyman S.N., Rosenberger C., Rosen S. Experimental ischemia–reperfusion: biases and myths—the proximal vs. distal hypoxic tubular injury debate revisited. Kidney Int. 2010;77:9–16. doi: 10.1038/ki.2009.347. [DOI] [PubMed] [Google Scholar]
- 14.Hildebrand A.M., Iansavichus A.V., Haynes R.B. High-performance information search filters for acute kidney injury content in PubMed, Ovid Medline and Embase. Nephrol Dial Transplant. 2014;29:823–832. doi: 10.1093/ndt/gft531. [DOI] [PubMed] [Google Scholar]
- 15.Toback F.G. Regeneration after acute tubular necrosis. Kidney Int. 1992;41:226–246. doi: 10.1038/ki.1992.32. [DOI] [PubMed] [Google Scholar]
- 16.Kudose S., Hoshi M., Jain S., Gaut J.P. Renal histopathologic findings associated with severity of clinical acute kidney injury. Am J Surg Pathol. 2018;42:625–635. doi: 10.1097/PAS.0000000000001028. [DOI] [PubMed] [Google Scholar]
- 17.Frazier K.S., Seely J.C., Hard G.C. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol Pathol. 2012;40(4 suppl):14S–86S. doi: 10.1177/0192623312438736. [DOI] [PubMed] [Google Scholar]
- 18.Jennette J.C., Heptinstall R.H., editors. Heptinstall’s pathology of the kidney. 2007. http://www.r2library.com/marc_frame.aspx?ResourceID=584 Available at: Accessed October 14, 2020. [Google Scholar]
- 19.Roufosse C.A., Becker J., Clahsen van Groningen M. A 2018 reference guide to the Banff Classification of Renal Allograft Pathology. Transplantation. 2018;102:1795–1814. doi: 10.1097/TP.0000000000002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brito P.L., Fioretto P., Drummond K. Proximal tubular basement membrane width in insulin-dependent diabetes mellitus. Kidney Int. 1998;53:754–761. doi: 10.1046/j.1523-1755.1998.00809.x. [DOI] [PubMed] [Google Scholar]
- 21.Pleros C., Stamataki E., Papadaki A. Dapagliflozin as a cause of acute tubular necrosis with heavy consequences: a case report. CEN Case Reports. 2018;7:17–20. doi: 10.1007/s13730-017-0283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markowitz G.S., Fine P.L., Stack J.I. Toxic acute tubular necrosis following treatment with zoledronate (Zometa) Kidney Int. 2003;64:281–289. doi: 10.1046/j.1523-1755.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- 23.Minkara A.A., Lin A.Y., Vitale M.G., Roye D.P., Jr. Acute kidney injury secondary to cell saver in posterior spinal fusion. Spine Deform. 2017;5:430–434. doi: 10.1016/j.jspd.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Lee J.-J., Chen H.-C. Flavonoid-induced acute nephropathy by Cupressus funebris Endl (mourning cypress) Am J Kidney Dis. 2006;48:e81–e85. doi: 10.1053/j.ajkd.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Markowitz G.S., Nasr S.H., Klein P. Renal failure due to acute nephrocalcinosis following oral sodium phosphate bowel cleansing. Hum Pathol. 2004;35:675–684. doi: 10.1016/j.humpath.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Robert R., Frasca D., Souweine B. Histologically proven acute tubular necrosis in a series of 27 ICU patients. J Crit Care. 2018;48:130–134. doi: 10.1016/j.jcrc.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 27.George N., Basu G., Mohapatra A. Adefovir nephrotoxicity in a renal allograft recipient. Indian J Nephrol. 2015;25:180–183. doi: 10.4103/0971-4065.144423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coca S.G., Yusuf B., Shlipak M.G. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansour S.G., Zhang W.R., Moledina D.G. The association of angiogenesis markers with acute kidney injury and mortality after cardiac surgery. Am J Kidney Dis. 2019;74:36–46. doi: 10.1053/j.ajkd.2019.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desanti De Oliveira B., Xu K., Shen T.H. Molecular nephrology: types of acute tubular injury. Nat Rev Nephrol. 2019;15:599–612. doi: 10.1038/s41581-019-0184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solez K.I.M., Morel-Maroger L., Sraer J.-D. The morphology of “acute tubular necrosis” in man: analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine (Baltimore) 1979;58 [PubMed] [Google Scholar]
- 32.Olsen T.S., Hansen H.E. Ultrastructure of medullary tubules in ischemic acute tubular necrosis and acute interstitial nephritis in man. APMIS. 1990;98:1139–1148. doi: 10.1111/j.1699-0463.1990.tb05046.x. [DOI] [PubMed] [Google Scholar]
- 33.Lieberthal W., Nigam S.K. Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am J Physiol Physiol. 1998;275:F623–F632. doi: 10.1152/ajprenal.1998.275.5.F623. [DOI] [PubMed] [Google Scholar]
- 34.Sharfuddin A.A., Molitoris B.A. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7:189. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 35.Moledina D.G., Hall I.E., Thiessen-Philbrook H. Performance of serum creatinine and kidney injury biomarkers for diagnosing histologic acute tubular injury. Am J Kidney Dis. 2017;70:807–816. doi: 10.1053/j.ajkd.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan M., Ware K., Qamri Z. Warfarin-related nephropathy is the tip of the iceberg: direct thrombin inhibitor dabigatran induces glomerular hemorrhage with acute kidney injury in rats. Nephrol Dial Transplant. 2014;29:2228–2234. doi: 10.1093/ndt/gft380. [DOI] [PubMed] [Google Scholar]
- 37.Luque Y., Louis K., Jouanneau C. Vancomycin-associated cast nephropathy. J Am Soc Nephrol. 2017;28:1723–1728. doi: 10.1681/ASN.2016080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izzedine H., Mathian A., Champiat S. Renal toxicities associated with pembrolizumab. Clin Kidney J. 2019;12:81–88. doi: 10.1093/ckj/sfy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stokes M.B., Valeri A.M., Herlitz L. Light chain proximal tubulopathy: clinical and pathologic characteristics in the modern treatment era. J Am Soc Nephrol. 2016;27:1555–1565. doi: 10.1681/ASN.2015020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Slambrouck C.M., Salem F., Meehan S.M., Chang A. Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney Int. 2013;84:192–197. doi: 10.1038/ki.2013.78. [DOI] [PubMed] [Google Scholar]
- 41.Markowitz G.S., Stokes M.B., Radhakrishnan J., D’Agati V.D. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389–3396. doi: 10.1681/ASN.2005050496. [DOI] [PubMed] [Google Scholar]
- 42.Kim S., Chang W. Atypical triad of IgA nephropathy: reversible acute kidney injury, gross hematuria, and severe bilateral flank pain. CEN Case Rep. 2014;3:145–147. doi: 10.1007/s13730-013-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jennette J.C., Falk R.J. Adult minimal change glomerulopathy with acute renal failure. Am J kidney Dis. 1990;16:432–437. doi: 10.1016/s0272-6386(12)80055-2. [DOI] [PubMed] [Google Scholar]
- 44.Haas M., Loupy A., Lefaucheur C. The Banff 2017 Kidney Meeting report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18:293–307. doi: 10.1111/ajt.14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elmore J.G., Barnhill R.L., Elder D.E. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ. 2017;357:j2813. doi: 10.1136/bmj.j2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peck M., Moffat D., Latham B., Badrick T. Review of diagnostic error in anatomical pathology and the role and value of second opinions in error prevention. J Clin Pathol. 2018;71:995. doi: 10.1136/jclinpath-2018-205226. [DOI] [PubMed] [Google Scholar]
- 47.Gru A.A., Kim J., Pulitzer M. The use of central pathology review with digital slide scanning in advanced-stage mycosis fungoides and Sézary syndrome: a multi-institutional and international pathology study. Am J Surg Pathol. 2018;42:726–734. doi: 10.1097/PAS.0000000000001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Supavekin S., Zhang W., Kucherlapati R. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63:1714–1724. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 49.Yuen P.S.T., Jo S.-K., Holly M.K. Ischemic and nephrotoxic acute renal failure are distinguished by their broad transcriptomic responses. Physiol Genomics. 2006;25:375–386. doi: 10.1152/physiolgenomics.00223.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshida T., Kurella M., Beato F. Monitoring changes in gene expression in renal ischemia-reperfusion in the rat. Kidney Int. 2002;61:1646–1654. doi: 10.1046/j.1523-1755.2002.00341.x. [DOI] [PubMed] [Google Scholar]
- 51.Kashiwagi E., Tonomura Y., Kondo C. Involvement of neutrophil gelatinase-associated lipocalin and osteopontin in renal tubular regeneration and interstitial fibrosis after cisplatin-induced renal failure. Exp Toxicol Pathol. 2014;66:301–311. doi: 10.1016/j.etp.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 52.Jiang Y., Jiang T., Ouyang J. Cell atavistic transition: paired Box 2 re-expression occurs in mature tubular epithelial cells during acute kidney injury and is regulated by angiotensin II. PLoS One. 2014;9:e93563. doi: 10.1371/journal.pone.0093563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu-Dubois Y.-C., Baugey E., Peltier J. Epithelial phenotypic changes are associated with a tubular active fibrogenic process in human renal grafts. Hum Pathol. 2013;44:1251–1261. doi: 10.1016/j.humpath.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 54.Wichapoon B., Punsawad C., Viriyavejakul P. Expression of cleaved caspase-3 in renal tubular cells in Plasmodium falciparum malaria patients. Nephrology. 2017;22:79–84. doi: 10.1111/nep.12715. [DOI] [PubMed] [Google Scholar]
- 55.Honarpisheh M., Desai J., Marschner J.A. Regulated necrosis-related molecule mRNA expression in humans and mice and in murine acute tissue injury and systemic autoimmunity leading to progressive organ damage, and progressive fibrosis. Biosci Rep. 2016;36 doi: 10.1042/BSR20160336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nadasdy T., Laszik Z., Blick K.E. Human acute tubular necrosis: a lectin and immunohistochemical study. Hum Pathol. 1995;26:230–239. doi: 10.1016/0046-8177(95)90042-x. [DOI] [PubMed] [Google Scholar]
- 57.Parasuraman R., Zhang P.L., Samarapungavan D. Primary nonfunction of renal allograft secondary to acute oxalate nephropathy. Case Rep Transplant. 2011;2011:876906. doi: 10.1155/2011/876906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cez A., Brocheriou I., Lescure F.-X. Decreased expression of megalin and cubilin and altered mitochondrial activity in tenofovir nephrotoxicity. Hum Pathol. 2018;73:89–101. doi: 10.1016/j.humpath.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 59.García-Camín RM. Molecular mediators of favism-induced acute kidney injury. Clin Nephrol. 81:203–209. [DOI] [PubMed]
- 60.Ishmael J., Pratt I., Lock E.A. Necrosis of the pars recta (S3 segment) of the rat kidney produced by hexachloro 1:3 butadiene. J Pathol. 1982;138:99–113. doi: 10.1002/path.1711380202. [DOI] [PubMed] [Google Scholar]
- 61.Isaka Y., Suzuki C., Abe T. Bcl-2 protects tubular epithelial cells from ischemia/reperfusion injury by dual mechanisms. Transplant Proc. 2009;41:52–54. doi: 10.1016/j.transproceed.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 62.Wakabayashi T. Light and electron microscopical changes seen in acute tubular necrosis in renal allografts. Acta Pathol Jpn. 1988;38:435–444. doi: 10.1111/j.1440-1827.1988.tb02317.x. [DOI] [PubMed] [Google Scholar]
- 63.Sethi S., Fervenza F.C. Standardized classification and reporting of glomerulonephritis. Nephrol Dial Transplant. 2018;34:193–199. doi: 10.1093/ndt/gfy220. [DOI] [PubMed] [Google Scholar]
- 64.Solez K i m, Axelsen R.A., Benediktsson H. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int. 1993;44:411–422. doi: 10.1038/ki.1993.259. [DOI] [PubMed] [Google Scholar]
- 65.Oppong Y.D., Farber J.L., Chervoneva I., Martinez Cantarin M.P. Correlation of acute tubular injury in reperfusion biopsy with renal transplant outcomes. Clin Transplant. 2016;30:836–844. doi: 10.1111/ctr.12757. [DOI] [PubMed] [Google Scholar]
- 66.Pieters T.T., Falke L.L., Nguyen T.Q. Histological characteristics of acute tubular injury during delayed graft function predict renal function after renal transplantation. Physiol Rep. 2019;7 doi: 10.14814/phy2.14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.National Institute of Diabetes and Digestive and Kidney Diseases Kidney Precision Medicine Project. 2018. https://www.niddk.nih.gov/research-funding/research-programs/kidneyprecision-medicine-project-kpmp Available at: Accessed October 14, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.