Abstract
Hemodialysis has saved many lives, albeit with significant residual mortality. Although poor outcomes may reflect advanced age and comorbid conditions, hemodialysis per se may harm patients, contributing to morbidity and perhaps mortality. Systemic circulatory “stress” resulting from hemodialysis treatment schedule may act as a disease modifier, resulting in a multiorgan injury superimposed on preexistent comorbidities. New functional intradialytic imaging (i.e., echocardiography, cardiac magnetic resonance imaging [MRI]) and kinetic of specific cardiac biomarkers (i.e., Troponin I) have clearly documented this additional source of end-organ damage. In this context, several factors resulting from patient-hemodialysis interaction and/or patient management have been identified. Intradialytic hypovolemia, hypotensive episodes, hypoxemia, solutes, and electrolyte fluxes as well as cardiac arrhythmias are among the contributing factors to systemic circulatory stress that are induced by hemodialysis. Additionally, these factors contribute to patients’ symptom burden, impair cognitive function, and finally have a negative impact on patients’ perception and quality of life. In this review, we summarize the adverse systemic effects of current intermittent hemodialysis therapy, their pathophysiologic consequences, review the evidence for interventions that are cardioprotective, and explore new approaches that may further reduce the systemic burden of hemodialysis. These include improved biocompatible materials, smart dialysis machines that automatically may control the fluxes of solutes and electrolytes, volume and hemodynamic control, health trackers, and potentially disruptive technologies facilitating a more personalized medicine approach.
Keywords: hidden risks of dialysis, intradialytic morbidity, circulatory stress systemic stress, personalized medicine
The development of hemodialysis was a medical breakthrough that has since saved many lives. Technological progress in the design and manufacture of equipment over the past 70 years has resulted in dialysis becoming the most prevalent form of renal replacement therapy, currently sustaining life in almost 3 million patients with chronic kidney disease stage 5 worldwide.1,2 However, dialysis is imperfect and patients who are dependent on it face a number of challenges such as poor quality of life, high disease burden, and increased mortality rates.3, 4, 5, 6, 7, 8, 9 In addition, the high treatment costs of dialysis confer a significant health economic burden,10, 11, 12 making dialysis unaffordable to a proportion of patients in need.13
A growing body of evidence indicates that the intermittent nature of traditional thrice-weekly hemodialysis with intensive 4-hour treatments may be harmful to patients by contributing to systemic circulatory stress and intradialytic ischemic end-organ damage. This, in turn, leads to poor quality of life and augments the chronic health risks associated with uremia.14, 15, 16
In recent years, research has corroborated the need to deliver efficient and adequately dosed dialysis therapy on a regular basis to avoid adverse patient outcomes.17 Recently, studies using new imaging technologies and cardiac biomarkers have documented the systemic circulatory stress induced by hemodialysis; they have also shown how dialysis-based interventions can reduce this risk.8,18,19 However, in this context, recent reports have not focused on the role of dialysis therapy as a potential disease modifier in an integrated way.20, 21, 22, 23
The objective of our in-depth review is, first, to summarize the undesirable systemic effects of intermittent hemodialysis therapy and, second, to discuss approaches to develop more-cardioprotective and better-tolerated hemodialysis.
Undesirable Effects of Intermittent Hemodialysis
The “unphysiological” nature of intermittent hemodialysis has long been recognized as a leading cause of dialysis intolerance.24 This phenomenon was worsened by operational changes that resulted in shorter-duration dialysis treatments.25 Intermittent hemodialysis (e.g., a standard thrice-weekly 4-hour schedule) generates cyclical fluctuations in volume status and blood pressure, osmotic shifts, and swings in solutes and electrolyte levels. For example, cyclical volemic changes (hypervolemia alternating with hypovolemia) that result in chronic cardiac loading and acute unloading are responsible for repetitive myocardial stretching and shortening that lead to release of inflammatory mediators, a recognized promoter of cardiac fibrosis.26,27 These treatment-induced disturbances are in stark contrast to the highly regulated stability of the internal milieu in healthy subjects.22,28,29 Furthermore, the limited efficiency of intermittent hemodialysis only partially restores the internal milieu composition and does not achieve efficient removal of medium- and high-molecular-weight uremic compounds, giving rise to interest in convective therapies and higher-cutoff dialyzer membranes.28 This incomplete removal of uremic toxins by intermittent hemodialysis, as summarized by the “residual syndrome,” is another potential contributor to patient morbidity and mortality.30,31
Despite substantial progress, the intermittency of treatment and regular exposure to extracorporeal circuit still elicits bioincompatibility reactions that results in the periodic activation of serum proteins (e.g., clotting cascades, complement activation, surface contact, and kallikrein-kinin system)32, 33, 34, 35, 36, 37, 38 and blood cells (e.g., platelets, leukocytes, and monocytes). The induction of proinflammatory mediators39,40 is further amplified by microbial-derived products from the dialysate.41, 42, 43, 44 Activation of monocytes and macrophages in turn triggers the release of various proinflammatory cytokines (such as interleukin-1, interleukin-6, and tumor necrosis factor-α).45,46 Furthermore, acute-phase inflammatory reactions are amplified by oxidative stress in a self-amplifying loop.45 Close interplay between biological reactions has been the rationale for improving the hemocompatibility of the extracorporeal circuit (e.g., synthetic polymer membrane, plasticizer, bioengineering design) and the use of ultrapure dialysis fluid in most countries.47,48 These improvements aim to reduce low-grade inflammation in maintenance hemodialysis patients and prevent its effects on the cardiovascular system.20,46,49
Dialysis-Induced Systemic Circulatory Stress and Multiorgan Damage: Understanding the Determinants of a Complex Interplay
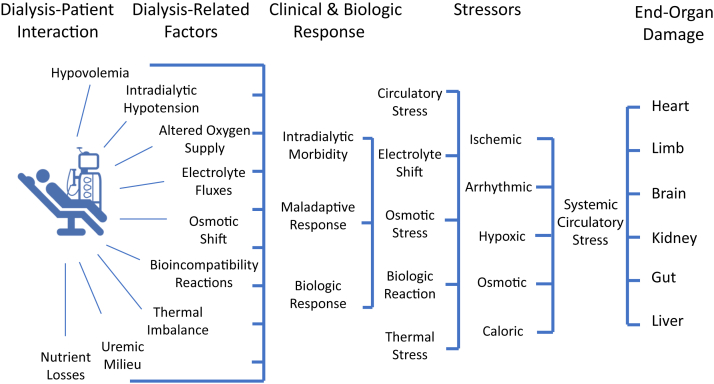
Intradialytic hypovolemia, hypotension, and hypoxia may result in a systemic multifactorial circulatory “stress” that acts as a disease modifier resulting in a multiorgan injury superimposed on preexistent comorbidities as graphically summarized in Figure 1.
Figure 1.
Dialysis-induced systemic stress (DISS) acting as disease modifier and resulting in a multiorgan injury superimposed on preexistent comorbidities and affecting outcomes.
Cardiac and Circulatory Stress
Innovative functional imaging techniques (e.g., echocardiography, cardiac MRI) and sensitive cardiac biomarkers (e.g., troponin I) have revealed that cardiac stress is exacerbated by the dialysis procedure. Adverse cardiac impact starts very early into a hemodialysis session and augments over the course of the treatment.8,50, 51, 52 Several factors contribute to cardiac stress, including treatment time, ultrafiltration volume and rate, and electrolyte flux. Although cardiac stunning, a temporary reduction in myocardial perfusion and contractility, can occur in the absence of ultrafiltration,53 it is recognized that rapid fluid removal by ultrafiltration—expressed in volume per time scaled to body weight—is a key factor.54,55 Briefly, ultrafiltration tends to reduce the circulating blood volume. This decline in blood volume is in part compensated by blood redistribution from venous capacitance vessels (e.g., by means of the De Jager-Krogh phenomenon)56 and vascular refilling with fluid from the interstitial space.57,58 Vascular refilling is driven by several factors, such as a rise in plasma protein concentration and oncotic pressure. Nevertheless, the vascular refill rate during most dialysis sessions does not fully compensate for the ultrafiltration rate, resulting in a decline of the effective blood volume. Furthermore, detailed intradialytic imaging studies have shown that cardiac stunning is directly related to the ultrafiltration rate and occurs even in the absence of coronary artery disease.17,55,59 In the face of ongoing ultrafiltration, arterial blood pressure and tissue perfusion are maintained physiologically by an increase in vascular tone, mainly in alpha-adrenoceptor territories, and venous return.60,61 However, vasoconstriction may be inhibited by a dialysis-induced rise in core temperature.62 Importantly, dialysis has negative effects not only on the perfusion of the heart but also on other organs, such as kidney, gut, liver, and brain, as shown by various imaging techniques of positron emission tomography, computed tomography, and MRI.63, 64, 65 Apart from direct organ damage by tissue ischemia, hepato-splanchnic circulatory stress may also induce bacterial translocation, endotoxin release, and the increased release of gut-derived uremic toxins such as indoxyl sulphate into the systemic circulation.66, 67, 68, 69 The importance of these observations is shown by studies reporting an association between mortality and ultrafiltration rate/volume and change in blood pressure and end-organ ischemic injury.8
A recently introduced general measure of dialysis-related circulatory stress, which is easy to assess in hemodialysis patients with a central venous catheter as vascular access in place, is central venous oxygen saturation (Scvo2). Scvo2 is physiologically linked to upper body blood flow,70 and a decline in Scvo2 is associated with higher mortality in dialysis patients.71 Similar to the findings of organ-specific imaging techniques, the decline in Scvo2 during dialysis was found to be related to ultrafiltration volume.72,73
Patient Factors
The systemic response to hemodialysis and fluid removal is even more complex because it involves others factors such as thermal balance reflecting the dialysate–patient temperature gradient, electrolyte fluxes that depend on dialysate–patient gradients, and also the individual patient’s baseline cardiac and hemodynamic reserve as well as the neurohumoral stress response.74, 75, 76 Furthermore, this response may be modulated by additional factors (e.g., age, gender, comorbidity, and medication) that may explain individual or temporal variations in hemodynamic adaptation.77,78 Whatever the reason, the hemodynamic stress of dialysis must be considered as a potent disease modifier in an already highly vulnerable population.79 This presents an important therapeutic dilemma, as insufficient fluid removal contributes to chronic fluid overload, which is an important risk factor for mortality in dialysis patients.80,81
Hypoxemia
In addition to the circulatory stress that reduces tissue perfusion,82, 83, 84 hypoxemia can be observed in hemodialysis patients. This phenomenon has been known since the early days of hemodialysis but only recently has hypoxemia has been associated with increased mortality. Hypoxemia is particularly marked in the first 30-60 minutes after dialysis initiation, suggestive of impaired pulmonary gas exchange85,86 and/or reduced respiratory drive. The pathogenesis of intradialytic hypoxemia is likely multifactorial, but leukocyte trapping in the lung due to bioincompatibility has been identified as a factor in the early days of dialysis. With the advent of bicarbonate buffered dialysis, a reduced ventilatory drive due to a rapid increase in plasma bicarbonate is likely to play a more prominent role.87 Prolonged intradialytic hypoxemia is likely to aggravate end-organ damage by reducing oxygen delivery to tissues and organs. However, it is difficult to differentiate the effects of intra-dialytic hypoxemia from those of preexisting pulmonary pathologies, sleep apnea, and fluid overload.84,85
Solute Fluxes
Solute fluxes and biologic fluctuations reflect the amplitude of body composition changes that patients face with each hemodialysis treatment. These fluctuations may interact with and aggravate circulatory stress consequences.22,88, 89, 90 Solute flux is related to dialysate-to-blood solute concentration gradients.88 According to the type of exchanges and gradient direction, solutes may be classified as following either negative or positive gradients, whereby the gradient is conventionally defined as dialysate minus serum solute concentration. Uremic retention solutes are removed along negative gradients, whereas selected electrolytes (e.g., bicarbonate, calcium, magnesium) or glucose may diffuse in the opposite direction, from the dialysate into the blood. Although a detailed description of intradialytic biochemical changes is beyond the scope of this review, we wish to highlight the fact that patients are challenged by large osmotic fluctuations due to shifts of urea, electrolyte, and water and acid-base changes; at the same time, patients are losing amino acids and other salutary compounds.91, 92, 93, 94 Clinical manifestations of these shifts range from none, through minor (fatigue, headache) to severe symptoms (impaired cognition, arrhythmias) including the dialysis disequilibrium syndrome.95,96
Cardiac Arrhythmias
Cardiac arrhythmias (CAs) and sudden cardiac death are leading causes of mortality in hemodialysis patients, accounting for a quarter of deaths in prevalent dialysis patients (US Renal Data System).7 It has been recognized for many years that dialysis patients are at an increased risk of CA and sudden cardiac death. However, the underlying mechanisms are not completely understood.97,98 It is also important to note that in contrast to the general population, bradycardia leading to asystole is more frequent in hemodialysis patients.99 Electrolyte abnormalities and cardiac structural changes likely contribute this. Dialysis patients can present with so-called uremic cardiomyopathy, an ill-defined entity characterized by various degrees of left ventricular hypertrophy and dilation, systolic and diastolic dysfunction, and histopathologically, fibrosis and capillary rarefaction.100 Whereas structural cardiac changes may predispose to arrhythmias, both interdialytic events and dialysis per se appear to be risk factors for arrhythmias. In addition to the well-documented effects of potassium concentration, calcium levels may also affect cardiac rhythm in dialysis patients.101 The prevalence of arrhythmias in intermittent hemodialysis patients is likely to be underestimated when based on symptomatic episodes.102 As documented by implantable loop recorders, the prevalence of significant asymptomatic arrhythmias is substantially higher than expected. In a recent prospective study of 66 hemodialysis patients of whom 94% completed the study,99 a total of 1,678 CA events were recorded in 44 patients. The majority were bradycardias (87%; N = 1,461). Fourteen episodes of asystole and only 1 episode of sustained ventricular tachycardia were documented. Atrial fibrillation, though not defined as a clinically significant CA, was detected in 41% of patients. The timing of arrhythmias relative to dialysis treatment schedule is also of interest. With thrice-weekly dialysis, the CA rate was highest during the first dialysis session of the week and increased during the last 12 hours of each interdialytic interval, particularly the long interval. The incidence of atrial fibrillation was highest during hemodialysis. Warm dialysate ≥37°C (hyperthermic dialysis), low dialysate calcium (<1.25 mmol/l), low dialysate potassium (<2 mmol/l), and sodium modeling were associated with a higher CA rate.99,103 Implications of sodium modeling are not discussed but is likely reflecting a confounder by indication rather an a pathogenic factor.
Cognitive Function
Cognitive function is frequently impaired in hemodialysis patients,104,105 and this may be directly linked to the treatment itself. Recent studies using neuropsychological tests and sophisticated tools (transcranial doppler ultrasonography, cerebral MRI) have identified a link between the deleterious effects of hemodialysis on cognitive function and changes in cerebral arterial flow velocity.106,107 In a prospective observational cohort study of 97 adults receiving hemodialysis, the degree of decline in flow velocity in the middle cerebral arteries correlated with the decline in cognitive function.107 Further, transient cognitive impairment has a negative impact on the patients’ experience, but also on their health literacy and ability to process medical information, possibly affecting their ability to adhere to their treatment regimen. Moreover, repetitive hemodialysis-induced brain insults may result in ischemic brain and white matter damage (leukoaraiosis) as shown in a prospective cerebral MRI study.108,109
Quality of Life
Alongside this, dialysis-related morbidity (intra- and interdialytic) aggravates the symptom burden and has a negative impact on patients’ perception of their quality of life.15,79,86,110,111 This can be expressed by symptoms scored according to type, frequency, and intensity, or more specifically by integrative scaling systems such as patient-reported outcome measures or patient-reported experience measures.112, 113, 114 Among the most frequently reported symptoms are hypotensive episodes, cramps, headache, fatigue, pruritus, and sleep disorders.115 Patient-reported outcome measures, patient-reported experience measures, and various domains of health-related quality of life are significantly lower in patients treated by conventional hemodialysis and are improved by daily or longer treatment schedules, for example, nocturnal dialysis.116, 117, 118 Furthermore, dialysis symptom burden is associated with poor outcomes. Indeed, these clinical performance indicators are now recommended as part of the assessment of dialysis adequacy.112,119, 120, 121, 122
Summary
In summary, hemodialysis, although live-saving, also exposes the patient to hemodynamic and ventilatory stress as well as osmotic and electrolyte shifts. Moreover, blood contact with the extracorporeal circuit may induce proinflammatory cytokines and complement activation. Multifactorial, repetitive hemodialysis-induced stress globally reduces tissue perfusion and oxygenation, all of which may have deleterious long-term consequences on the function of vital organs such as the heart, brain, liver, and kidneys. Some of these effects also result in chronic low-grade inflammation, and this may further contribute to poor outcomes.8,18,64,106,108 In addition, the combined effects of cardiocirculatory stress, hypovolemia, and electrolyte changes may create proarrhythmogenic conditions.99,123, 124, 125 Cardiac changes as a result of myocardial stunning, cardiac remodeling in response to cyclical pressure and volume overload, fibrotic scarring, and loss of contractile function with aberrant electrical conductivity are all pathways that conceivably increase the sudden cardiac death risk.59,98,99,126, 127, 128 These findings have similarities with the extreme physiologic demands that are experienced by healthy subjects under adverse environmental conditions, with the difference that they must be endured repeatedly by a vulnerable patient population over months and years.129
Therefore, in order to prevent dialysis-induced organ damage, it is imperative to challenge traditional hemodialysis treatment paradigms.
Developing More-Cardioprotective and Better-Tolerated Hemodialysis
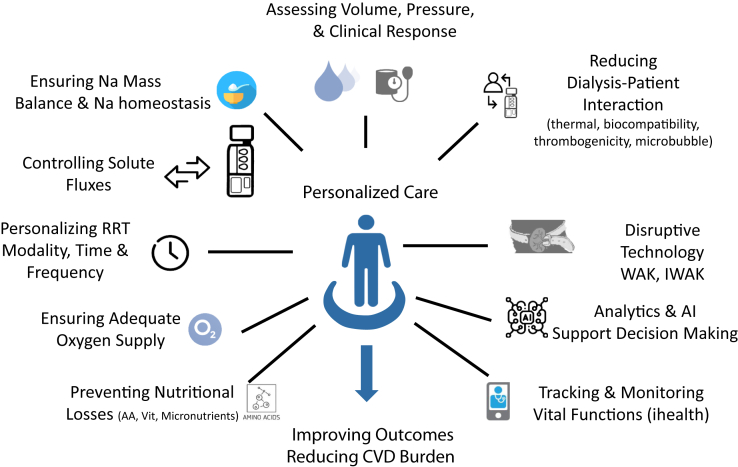
Reducing and preventing dialysis-induced hemodynamic stress is crucial in the quest for cardio- and multiorgan-protective therapy and improving patient experience. We will briefly address these issues and explore various approaches. These include improved biocompatible materials, smart dialysis machines that automatically control the fluxes of solutes and electrolytes, volume and hemodynamic control, health trackers, and potentially disruptive technologies that are envisioned to lower or prevent dialysis-associated injury graphically summarized in Figure 2.
Figure 2.
Various actions envisaged to personalize patient care and reduce dialysis-induced systemic stress (DISS). AA, amino acids; AI, artificial intelligence; CVD, cardiovascular disease; IWAK, implantable and wearable artificial kidney; RRT, renal replacement therapy; Vit, vitamins; WAK, wearable artificial kidney.
Extracorporeal Circuit
Reducing the effects of blood-extracorporeal circuit interaction is of tremendous importance for improving hemoincompatibility reactions while minimizing thrombosis risk and preventing microinflammation. Although major progress has already been made with less reactive biomaterials including synthetic polymer hemodialyzers, less bioreactive tubing material, improved circuitry geometry, and ultrapure dialysis fluid, further advances are possible. Several areas are currently being explored. For example, minimization of the blood-air interface in the extracorporeal circuit is possible by combining a very short circuit with a blood cassette and avoidance of the venous bubble trap.130,131 Incorporation of antithrombotic agents within the core polymer bulk or on the surface of dialyzer fibers and blood lines is also being explored,132,133 as is the use of citric-acidified bicarbonate dialysate as an adjunct to antithrombotic-treated polymer.134, 135, 136 Finally, methods are being developed to prevent or reduce microbubble formation within the extracorporeal circuit.137, 138, 139 By combining these different approaches, the bioreactivity of extracorporeal circuit can be minimized and its contribution to inflammation and/or end-organ damage may be prevented.
Solute Flux
Another important contributor to systemic stress is the magnitude of solute fluxes.140, 141, 142, 143 Reducing instantaneous solute fluxes while keeping total solute mass transfer equivalent to attain dialysis adequacy goals is an important target to improve dialysis tolerance and to reduce patient burden. One approach is to reduce blood flow and increase treatment time and/or frequency (e.g., nocturnal dialysis, alternate day, or daily dialysis) in order to compensate for the reduction of instantaneous solute fluxes. Although a standard clinical practice in Japan, it is generally not well accepted by patients in Western countries.144
Longer and/or more frequent dialysis schedules are generally better tolerated and result in less circulatory stress compared to short dialysis schedules.145,146 This reflects reduced solute gradients and fluxes, including electrolytes and ultrafiltration rate.54 Importantly, fluid removal and solute and electrolytes fluxes are potentially modifiable factors of the dialysis prescription.
Certain dialysate electrolyte levels, in particular low dialysate potassium, alone or in combination with low dialysate calcium and magnesium, are associated with a higher incidence of atrial fibrillation.99 This finding corroborates several retrospective studies indicating that hemodialysis against low potassium dialysate (i.e., ≤2 mmol/l) might increase the risk of sudden cardiac death, mainly in patients without pre-dialysis hyperkalemia.123 The prescription of low dialysate potassium (<2 mmol/l) should therefore be avoided from a safety standpoint. Aside from electrolytes fluxes, cardiac medication (beta-blockers)147,148 clearance induced by high-efficiency renal replacement therapy modalities may further aggravate arrhythmia risk and require dose adaptation.149 Interestingly, the incidence of clinically significant arrhythmias was associated with increased risk of hospitalization with cardiovascular disease. A clinically significant arrhythmia during the past 14 days was associated with 8.8-fold risk of cardiovascular hospitalization, though no sudden cardiac death occurred during the 6 months’ follow-up.99
Intradialytic Hemodynamic Stability
From a mechanistic view, preservation of hemodynamic status in response to fluid removal during dialysis requires a synergistic increase of cardiac output (stroke volume, ejection fraction, contractility, heart rate), total peripheral vascular resistance, and venous tone.150 As discussed in a previous paragraph, the hemodynamic response to fluid removal in dialysis is complex and involves a combination of the patient characteristics, dialysis conditions, and the neurohumoral stress response.151 Considering the complexity of the hemodynamic response to ultrafiltration, an intervention focusing only on 1 specific component may fall short in most patients. This has been shown in a randomized interventional study that has focused exclusively on a single component (intravascular volume control guided by relative blood volume changes), the results of which have been largely disappointing.152 An alternative approach consisting of measuring the absolute blood volume based on an online dilution method,153,154 and then ensuring automated feedback control of the ultrafiltration rate to prevent critical reductions in circulating blood volume,154 may hold the prospect of reducing intradialytic morbid events.155 Sensors measuring relative blood volume during dialysis sessions may also be useful tools to detect critical changes in intravascular volume status, estimate remaining fluid in the interstitium (e.g., rebound after stopping ultrafiltration) or quantify vascular refilling rate capacity,156 but they are not considered as the ultimate tool to manage patient hemodynamics.157 Alternatively, routine monitoring of Scvo2 could indicate critical changes in organ perfusion before they result in clinical symptoms, but Scvo2 can only be measured in patients with a central venous catheter as vascular access, a patient population at a particularly high risk of premature death. For patients with arteriovenous vascular access, noninvasive methods such as near-infrared spectroscopy could be of relevance to estimate tissue oxygenation.158
Closed-loop feedback control of ultrafiltration rate based on changes of absolute or relative blood volume has resulted in improved hemodynamic stability and reduced incidence of hypotension in some,155,159 but not all,157,160 studies. However, no studies have explored the effect of ultrafiltration feedback control on patient outcomes.155,161,162 In addition, these “intelligent” systems should be used with caution, and some researchers advocate cross-checking with other technologies (e.g., bioimpedance) to prevent exposing the patient to chronic fluid overload.163 Other studies have suggested that sodium and ultrafiltration profiling may partly preserve intradialytic hemodynamic status, but sodium profiling may aggravate intradialytic salt loading, resulting in thirst and high interdialytic weight gain with chronic fluid overload.142
Intradialytic Thermal Balance / Temperature
As shown in several studies and confirmed by a meta-analysis, adjusting dialysis thermal balance is a simple strategy to improve hemodynamic tolerance with a low risk of patient discomfort.164 The main objective is to deliver iso- or hypothermic dialysis to prevent the patient from warming up during a dialysis session, because a rise in core temperature may induce an undesired hemodynamic response, such as vasodilation, tachycardia or a fall in ejection fraction.103 Hypothermic dialysis can be easily achieved by setting dialysate temperature 0.5° to 1°C below the patient’s core temperature. Isothermic dialysis requires the use of a blood temperature monitor device embedded on the dialysis machine that can control precisely the patient’s thermal balance by adjusting the dialysate temperature in response to the blood temperature in the extracorporeal circuit.165 Both hypothermic and isothermic dialysis reduce hypotension rates.166
A Personalized Medicine Approach
Recently, a noninvasive whole body bioimpedance cardiography (BIC) device has been tested in hemodialysis patients to measure the intradialytic hemodynamic response to fluid removal including cardiac output, total peripheral vascular resistance, and cardiac power index. Initial results have been promising and suggest that dialysis patients might be divided into different categories based on their predominant hemodynamic response to fluid removal (e.g., low or high cardiac output, low or high total peripheral vascular resistance or normal hemodynamics).150,167 This approach has the potential to help physicians define individualized actions based on the patient’s hemodynamic category, although interventional studies now needed to define its clinical value.167 The same applies to other methods aiming to categorize individual hemodynamic characteristics. These studies need to focus on accuracy and reproducibility of measurements and explore the impact of interventions on patient outcomes.76
Although several tools are currently available to facilitate hemodynamic management, none have been shown individually to improve patient outcomes. One may speculate that combining tools supported by advanced analytics and artificial intelligence will have the potential to address this challenge more effectively in the future.168 Of note, preliminary trials provide some early encouragement.169 Such tools could also be combined with approaches to reduce instantaneous solute fluxes while keeping total solute mass transfer and dialysis adequacy equivalent in an attempt to reduce intradialytic morbidity.140, 141, 142, 143
It is clear from past experience that a one-size-fits-all approach to reducing the physiological stresses of dialysis does not work. It is important to keep this in mind when novel developments in dialysis treatment are considered. In other words, dialysis modality, dialysis dose and efficiency, treatment time, frequency,117,170 and electrolyte prescription should be tailored to the patient’s specific clinical profile.171,172 Furthermore, treatment prescription should be adapted over time in response to the patient’s metabolic changes, treatment tolerance, and symptoms.173 The approach to prescribing hemodialysis should return to basics; it should not be the patient who must adapt (or not) to a fixed treatment but instead, the treatment should be tailored for the patient. Several studies have established the predictive value of stratifying patients according to their risk of adverse short- or medium-term outcomes.174,175 As an example of how better understanding of a patient’s risks could help to define more appropriate and individualized therapy, scoring systems could be tested and implemented as triggers to alter specific treatment features (e.g., incremental dialysis, nocturnal dialysis, and adapted dialysis schedule) in order to reduce early mortality of chronic kidney disease stage 5 patients transitioning to dialysis.
Automated and self-adapting systems embedded in smart dialysis machines, governed by adaptive algorithms coupled to feedback control loops, may offer interesting innovative solutions. One example of such an innovative technology is a sodium control module that has been validated recently in clinical trials.176, 177, 178 Based on continuous dialysis inlet and outlet conductivity measurements, an algorithm controls plasma sodium concentration (e.g., tonicity) changes and allows a precise prescription of dialytic sodium mass transfer. These monitoring and controlling actions on sodium mass transfer and tonicity rely on a fully automated function that follow targeted prescription settings. Outcome studies are warranted to explore benefits to patients and the device’s clinical value.179 Other technical feedback control loops are available to reduce cardiocirculatory stress in dialysis patients (e.g., thermocontrolled or volume-controlled dialysis), but clinical studies are warranted to better understand technical solutions and their combinations with respect to cardiac outcomes. The development of noninvasive methods for continuous intradialytic blood pressure monitoring will enhance early detection of intradialytic hypotension and may even facilitate prediction.180
The use of advanced analytics and remote biosensor devices to support medical decision making may offer innovative steps regarding prescription and monitoring of personalized hemodialysis therapy. Personalized medicine based on mathematical models and artificial intelligence tools (neuronal networks, machine learning) combined with remote wearable biosensors may support physicians’ decision making for individual patients regarding the selection of appropriate treatment modalities and technical options such as control of ultrafiltration rate and dialysate sodium and electrolytic concentrations.168,169 Furthermore, continuous 24/7 monitoring of vital parameters via wearable sensors (e.g., heart rate, blood pressure, oxygen saturation) in the most fragile patients may facilitate an early detection or even prediction of serious intra- and/or interdialytic morbid events. As suggested by recent reports, new remote sensing technology, so-called ihealth tracker connected devices, offers novel and convenient tools for monitoring in a fully automated, ambulatory and unobtrusive way in high-risk dialysis patients through the entire nychthemeral cycle.181
New disruptive artificial kidney devices such as wearable and implantable artificial kidneys (IAK) are further downstream in the pipeline.182,183 Wearable artificial kidneys take advantage of sorbent technology to regenerate dialysate and use microtechnology to miniaturize hardware and incorporate all components in a wearable belt device.184, 185, 186 Wearable artificial kidneys have undergone small trials in humans.187,188 Implantable artificial kidney is a biohybrid combining artificial filters and living cells to mimic native kidney functions. Implantable artificial kidney devices are currently in preclinical testing.183 In theory, these portable devices offer several advantages (e.g., logistical, infrastructure, cost, self-care treatment), including continuous ambulatory treatment, but additional clinical studies are needed to obtain regulatory approval and cost-effectiveness before widespread clinical use can be considered.182
Conclusions and Perspectives on the Future of Renal Replacement Therapy
The paradigm of personalized or precision medicine is highly relevant to renal replacement therapy. It is the basis for designing more-effective, better-tolerated, and more-acceptable hemodialysis treatments.23
To that end, the planning of dialysis therapy should focus on optimizing cardio- and multiorgan protection by fine-tuning sodium, volume, and blood pressure management as well as through customizing electrolytic prescription. In addition, there are opportunities for improved monitoring of vital functions, including arrhythmias and oxygen saturation, with remote connected sensor devices.
Finally, to optimize care of hemodialysis patients, personalized monitoring that incorporates the interdialytic period utilizing ihealth trackers may allow event prediction and timely intervention.189
Progress in achieving the goals discussed in the article will help to develop hemodialysis from a vital but poorly tolerated therapy to one that is personalized and aims to minimize organ damage, reduce adverse clinical outcomes, in particular cardiovascular mortality and morbidity as well as optimize health-related quality of life and patient-reported outcomes. In addition, the use of embarked monitoring tools, such as a sodium balancing module, will support caregivers and dietitians to guide more precisely patient’s diet.
Disclosure
BC, AM, PKop, AC, and PKot are consultants and/or employees of FMC. All the other authors declared no competing interests.
References
- 1.Liyanage T., Ninomiya T., Jha V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 2.Thomas B., Wulf S., Bikbov B. Maintenance dialysis throughout the world in years 1990 and 2010. J Am Soc Nephrol. 2015;26:2621–2633. doi: 10.1681/ASN.2014101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson B.M., Akizawa T., Jager K.J. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet. 2016;388:294–306. doi: 10.1016/S0140-6736(16)30448-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopes A.A., Bragg-Gresham J.L., Satayathum S. Health-related quality of life and associated outcomes among hemodialysis patients of different ethnicities in the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2003;41:605–615. doi: 10.1053/ajkd.2003.50122. [DOI] [PubMed] [Google Scholar]
- 5.Couillerot-Peyrondet A.L., Sambuc C., Sainsaulieu Y. A comprehensive approach to assess the costs of renal replacement therapy for end-stage renal disease in France: the importance of age, diabetes status, and clinical events. Eur J Health Econ. 2017;18:459–469. doi: 10.1007/s10198-016-0801-6. [DOI] [PubMed] [Google Scholar]
- 6.Rayner H.C., Pisoni R.L., Bommer J. Mortality and hospitalization in haemodialysis patients in five European countries: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2004;19:108–120. doi: 10.1093/ndt/gfg483. [DOI] [PubMed] [Google Scholar]
- 7.USRDS. Mortality Chapter 5. USRDS Annual Data Report 2018;2:411-426.
- 8.McIntyre C.W. Recurrent circulatory stress: the dark side of dialysis. Semin Dial. 2010;23:449–451. doi: 10.1111/j.1525-139X.2010.00782.x. [DOI] [PubMed] [Google Scholar]
- 9.McIntyre C., Crowley L. Dying to feel better: the central role of dialysis-induced tissue hypoxia. Clin J Am Soc Nephrol. 2016;11:549–551. doi: 10.2215/CJN.01380216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eriksson J.K., Neovius M., Jacobson S.H. Healthcare costs in chronic kidney disease and renal replacement therapy: a population-based cohort study in Sweden. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanholder R., Davenport A., Hannedouche T. Reimbursement of dialysis: a comparison of seven countries. J Am Soc Nephrol. 2012;23:1291–1298. doi: 10.1681/ASN.2011111094. [DOI] [PubMed] [Google Scholar]
- 12.Vanholder R., Annemans L., Brown E. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol. 2017;13:393–409. doi: 10.1038/nrneph.2017.63. [DOI] [PubMed] [Google Scholar]
- 13.ISN Global Kidney Health Atlas. ISN. https://www2.theisn.org/GKHA Available at: Accessed August 17, 2020.
- 14.Shoji T., Tsubakihara Y., Fujii M., Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66:1212–1220. doi: 10.1111/j.1523-1755.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- 15.Flythe J.E., Xue H., Lynch K.E. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol. 2015;26:724–734. doi: 10.1681/ASN.2014020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assimon M.M., Wang L., Flythe J.E. Cumulative exposure to frequent intradialytic hypotension associates with new-onset dementia among elderly hemodialysis patients. Kidney Int Rep. 2019;4:603–606. doi: 10.1016/j.ekir.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maduell F., Ramos R., Varas J. Hemodialysis patients receiving a greater Kt dose than recommended have reduced mortality and hospitalization risk. Kidney Int. 2016;90:1332–1341. doi: 10.1016/j.kint.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 18.McIntyre C.W., Burton J.O., Selby N.M. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3:19–26. doi: 10.2215/CJN.03170707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eldehni M.T., Odudu A., McIntyre C.W. Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol. 2015;26:957–965. doi: 10.1681/ASN.2013101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freemont A.J. The pathology of dialysis. Semin Dial. 2002;15:227–231. doi: 10.1046/j.1525-139x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 21.Cambi V., Arisi L., Bignardi L. Preliminary results obtained with short dialysis schedules. Ateneo Parmense Acta Biomed. 1975;46:349–358. [PubMed] [Google Scholar]
- 22.Kjellstrand C.M., Evans R.L., Petersen R.J. The “unphysiology” of dialysis: a major cause of dialysis side effects? Kidney Int Suppl. 1975;2:30–34. [PubMed] [Google Scholar]
- 23.Canaud B., Collins A., Maddux F. The renal replacement therapy landscape in 2030: reducing global cardiovascular burden in dialysis patients. Nephrol Dial Transplant. 2020;35:ii51–ii57. doi: 10.1093/ndt/gfaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kjellstrand C.M., Evans R.L., Petersen R.J. The ‘‘unphysiology’’ of dialysis: a major cause of dialysis side effects? Hemodial Int. 2004;8:24–29. doi: 10.1111/j.1492-7535.2004.00083.x. [DOI] [PubMed] [Google Scholar]
- 25.Cambi V., Savazzi G., Arisi L. Short dialysis schedules (SDS)—finally ready to become routine? Proc Eur Dial Transplant Assoc. 1975;11:112–120. [PubMed] [Google Scholar]
- 26.Chirakarnjanakorn S., Navaneethan S.D., Francis G.S., Tang W.H. Cardiovascular impact in patients undergoing maintenance hemodialysis: clinical management considerations. Int J Cardiol. 2017;232:12–23. doi: 10.1016/j.ijcard.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herum K.M., Choppe J., Kumar A. Mechanical regulation of cardiac fibroblast profibrotic phenotypes. Mol Biol Cell. 2017;28 doi: 10.1091/mbc.E17-01-0014. 1871-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ledebo I. Does convective dialysis therapy applied daily approach renal blood purification? Kidney Int Suppl. 2001;78:S286–S291. doi: 10.1046/j.1523-1755.2001.59780286.x. [DOI] [PubMed] [Google Scholar]
- 29.Modell H., Cliff W., Michael J. A physiologist's view of homeostasis. Adv Physiol Educ. 2015;39:259–266. doi: 10.1152/advan.00107.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Depner T.A. Uremic toxicity: urea and beyond. Semin Dial. 2001;14:246–251. doi: 10.1046/j.1525-139x.2001.00072.x. [DOI] [PubMed] [Google Scholar]
- 31.Meyer T.W., Hostetter T.H. Approaches to uremia. J Am Soc Nephrol. 2014;25:2151–2158. doi: 10.1681/ASN.2013121264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hörl W.H., Jochum M., Heidland A., Fritz H. Release of granulocyte proteinases during hemodialysis. Am J Nephrol. 1983;3:213–217. doi: 10.1159/000166713. [DOI] [PubMed] [Google Scholar]
- 33.Muñoz de Bustillo E., Alvarez Chiva V. Leukocyte—endothelial cell interactions in haemodialysis-induced neutropenia. Nephrol Dial Transplant. 1996;11:572–574. doi: 10.1093/oxfordjournals.ndt.a027343. [DOI] [PubMed] [Google Scholar]
- 34.Windus D.W., Atkinson R., Santoro S. The effects of hemodialysis on platelet activation with new and reprocessed regenerated cellulose dialyzers. Am J Kidney Dis. 1996;27:387–393. doi: 10.1016/s0272-6386(96)90362-5. [DOI] [PubMed] [Google Scholar]
- 35.Schoorl M. Activation of platelets and coagulation during haemodialysis. Ned Tijdschr Klin Chem Labgeneesk. 2016;41:17–27. [Google Scholar]
- 36.Coppo R., Amore A., Cirina P. Bradykinin and nitric oxide generation by dialysis membranes can be blunted by alkaline rinsing solutions. Kidney Int. 2000;58:881–888. doi: 10.1046/j.1523-1755.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- 37.Krishnan A., Vogler E.A., Sullenger B.A., Becker R.C. The effect of surface contact activation and temperature on plasma coagulation with an RNA aptamer directed against factor IXa. J Thromb Thrombolysis. 2013;35:48–56. doi: 10.1007/s11239-012-0778-7. [DOI] [PubMed] [Google Scholar]
- 38.Marney A.M., Ma J., Luther J.M. Endogenous bradykinin contributes to increased plasminogen activator inhibitor 1 antigen following hemodialysis. J Am Soc Nephrol. 2009;20:2246–2252. doi: 10.1681/ASN.2009050505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber M., Steinle H., Golombek S. Blood-contacting biomaterials: in vitro evaluation of the hemocompatibility. Front Bioeng Biotechnol. 2018;6:99. doi: 10.3389/fbioe.2018.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doyle A.J., Hunt B.J. Current understanding of how extracorporeal membrane oxygenators activate haemostasis and other blood components. Front Med. 2018;5:352. doi: 10.3389/fmed.2018.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaefer R.M., Heidland A., Hörl W.H. Effect of dialyzer geometry on granulocyte and complement activation. Am J Nephrol. 1987;7:121–126. doi: 10.1159/000167446. [DOI] [PubMed] [Google Scholar]
- 42.Taylor J.E., McLaren M., Mactier R.A. Effect of dialyzer geometry during hemodialysis with cuprophane membranes. Kidney Int. 1992;42:442–447. doi: 10.1038/ki.1992.307. [DOI] [PubMed] [Google Scholar]
- 43.Cheung A.K. Biocompatibility of hemodialysis membranes. J Am Soc Nephrol. 1990;1:150–161. doi: 10.1681/ASN.V12150. [DOI] [PubMed] [Google Scholar]
- 44.Schindler R., Beck W., Deppisch R. Short bacterial DNA fragments: detection in dialysate and induction of cytokines. J Am Soc Nephrol. 2004;15:3207–3214. doi: 10.1097/01.ASN.0000145049.94888.26. [DOI] [PubMed] [Google Scholar]
- 45.Morena M., Delbosc S., Dupuy A.M. Overproduction of reactive oxygen species in end-stage renal disease patients: a potential component of hemodialysis-associated inflammation. Hemodial Int. 2005;9:37–46. doi: 10.1111/j.1492-7535.2005.01116.x. [DOI] [PubMed] [Google Scholar]
- 46.Cobo G., Lindholm B., Stenvinkel P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transplant. 2018;33:iii35–iii40. doi: 10.1093/ndt/gfy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koga Y., Fujieda H., Meguro H. Biocompatibility of polysulfone hemodialysis membranes and its mechanisms: involvement of fibrinogen and its integrin receptors in activation of platelets and neutrophils. Artif Organs. 2018;42:E246–E258. doi: 10.1111/aor.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohlová M., Amorim C.G., Araújo A. The biocompatibility and bioactivity of hemodialysis membranes: their impact in end-stage renal disease. J Artif Organs. 2019;22:14–28. doi: 10.1007/s10047-018-1059-9. [DOI] [PubMed] [Google Scholar]
- 49.Eswari J.S., Naik S. A critical analysis on various technologies and functionalized materials for manufacturing dialysis membranes. Mater Sci Energy Tech. 2020;3:116–126. [Google Scholar]
- 50.McIntyre C.W. Effects of hemodialysis on cardiac function. Kidney Int. 2009;76:371–375. doi: 10.1038/ki.2009.207. [DOI] [PubMed] [Google Scholar]
- 51.Assa S., Hummel Y.M., Voors A.A. Hemodialysis-induced regional left ventricular systolic dysfunction and inflammation: a cross-sectional study. Am J Kidney Dis. 2014;64:265–273. doi: 10.1053/j.ajkd.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Buchanan C., Mohammed A., Cox E. Intradialytic cardiac magnetic resonance imaging to assess cardiovascular responses in a short-term trial of hemodiafiltration and hemodialysis. J Am Soc Nephrol. 2017;28:1269–1277. doi: 10.1681/ASN.2016060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Assa S., Kuipers J., Ettema E. Effect of isolated ultrafiltration and isovolemic dialysis on myocardial perfusion and left ventricular function assessed with (13)N-NH(3) positron emission tomography and echocardiography. Am J Physiol Renal Physiol. 2018;314:F445–F452. doi: 10.1152/ajprenal.00368.2017. [DOI] [PubMed] [Google Scholar]
- 54.Flythe J.E., Brunelli S.M. The risks of high ultrafiltration rate in chronic hemodialysis: implications for patient care. Semin Dial. 2011;24:259–265. doi: 10.1111/j.1525-139X.2011.00854.x. [DOI] [PubMed] [Google Scholar]
- 55.Flythe J.E., Assimon M.M., Wang L. Ultrafiltration rate scaling in hemodialysis patients. Semin Dial. 2017;30:282–283. doi: 10.1111/sdi.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daugirdas J.T. Intradialytic hypotension and splanchnic shifting: integrating an overlooked mechanism with the detection of ischemia-related signals during hemodialysis. Semin Dial. 2019;32:243–247. doi: 10.1111/sdi.12781. [DOI] [PubMed] [Google Scholar]
- 57.de los Reyes V.A., Fuertinger D.H., Kappel F. A physiologically based model of vascular refilling during ultrafiltration in hemodialysis. J Theor Biol. 2016;390:146–155. doi: 10.1016/j.jtbi.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 58.Schneditz D., Roob J., Oswald M. Nature and rate of vascular refilling during hemodialysis and ultrafiltration. Kidney Int. 1992;42:1425–1433. doi: 10.1038/ki.1992.437. [DOI] [PubMed] [Google Scholar]
- 59.Burton J.O., Jefferies H.J., Selby N.M., McIntyre C.W. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levin N.W., de Abreu M., Borges L.E. Hemodynamic response to fluid removal during hemodialysis: categorization of causes of intradialytic hypotension. Nephrol Dial Transplant. 2018;33:1643–1649. doi: 10.1093/ndt/gfy048. [DOI] [PubMed] [Google Scholar]
- 61.McGuire S., Horton E.J., Renshaw D. Hemodynamic instability during dialysis: the potential role of intradialytic exercise. Biomed Res Int. 2018;2018:8276912. doi: 10.1155/2018/8276912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Sande F.M., Gladziwa U., Kooman J.P. Energy transfer is the single most important factor for the difference in vascular response between isolated ultrafiltration and hemodialysis. J Am Soc Nephrol. 2000;11:1512–1517. doi: 10.1681/ASN.V1181512. [DOI] [PubMed] [Google Scholar]
- 63.Marants R., Qirjazi E., Grant C.J. Renal perfusion during hemodialysis: intradialytic blood flow decline and effects of dialysate cooling. J Am Soc Nephrol. 2019;30:1086–1095. doi: 10.1681/ASN.2018121194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grant C.J., Huang S.S., McIntyre C.W. Hepato-splanchnic circulatory stress: an important effect of hemodialysis. Semin Dial. 2019;32:237–242. doi: 10.1111/sdi.12782. [DOI] [PubMed] [Google Scholar]
- 65.Polinder-Bos H.A., García D.V., Kuipers J. Hemodialysis induces an acute decline in cerebral blood flow in elderly patients. J Am Soc Nephrol. 2018;29:1317–1325. doi: 10.1681/ASN.2017101088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McIntyre C.W., Harrison L.E., Eldehni M.T. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:133–141. doi: 10.2215/CJN.04610510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szeto C.C., McIntyre C.W., Li P.K. Circulating bacterial fragments as cardiovascular risk factors in CKD. J Am Soc Nephrol. 2018;29:1601–1608. doi: 10.1681/ASN.2018010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anders H.J., Andersen K., Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013;83:1010–1016. doi: 10.1038/ki.2012.440. [DOI] [PubMed] [Google Scholar]
- 69.Chen Y.Y., Chen D.Q., Chen L. Microbiome-metabolome reveals the contribution of gut-kidney axis on kidney disease. J Transl Med. 2019;17:5. doi: 10.1186/s12967-018-1756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosales L.M., Zhang H., Mateo M. Tracking arteriovenous fistula maturation: a novel approach. Blood Purif. 2019;47:240–245. doi: 10.1159/000494742. [DOI] [PubMed] [Google Scholar]
- 71.Chan L., Zhang H., Meyring-Wosten A. Intradialytic central venous oxygen saturation is associated with clinical outcomes in hemodialysis patients. Sci Rep. 2017;7:8581. doi: 10.1038/s41598-017-09233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang H., Chan L., Meyring-Wosten A. Association between intradialytic central venous oxygen saturation and ultrafiltration volume in chronic hemodialysis patients. Nephrol Dial Transplant. 2018;33:1636–1642. doi: 10.1093/ndt/gfx271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harrison L.E., Selby N.M., McIntyre C.W. Central venous oxygen saturation: a potential new marker for circulatory stress in haemodialysis patients? Nephron Clin Pract. 2014;128:57–60. doi: 10.1159/000362557. [DOI] [PubMed] [Google Scholar]
- 74.Baldamus C.A., Ernst W., Frei U., Koch K.M. Sympathetic and hemodynamic response to volume removal during different forms of renal replacement therapy. Nephron. 1982;31:324–332. doi: 10.1159/000182675. [DOI] [PubMed] [Google Scholar]
- 75.Baldamus C.A., Ernst W., Kachel H.G. Hemodynamics in hemofiltration. Contrib Nephrol. 1982;32:56–60. doi: 10.1159/000406905. [DOI] [PubMed] [Google Scholar]
- 76.Kolb J., Kitzler T.M., Tauber T. Proto-dialytic cardiac function relates to intra-dialytic morbid events. Nephrol Dial Transplant. 2011;26:1645–1651. doi: 10.1093/ndt/gfq599. [DOI] [PubMed] [Google Scholar]
- 77.Jefferies H.J., Virk B., Schiller B. Frequent hemodialysis schedules are associated with reduced levels of dialysis-induced cardiac injury (myocardial stunning) Clin J Am Soc Nephrol. 2011;6:1326–1332. doi: 10.2215/CJN.05200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Canaud B., Chazot C., Koomans J., Collins A. Fluid and hemodynamic management in hemodialysis patients: challenges and opportunities. J Bras Nefrol. 2019;41:550–559. doi: 10.1590/2175-8239-JBN-2019-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chou J.A., Kalantar-Zadeh K., Mathew A.T. A brief review of intradialytic hypotension with a focus on survival. Semin Dial. 2017;30:473–480. doi: 10.1111/sdi.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dekker M.J., Marcelli D., Canaud B.J. Impact of fluid status and inflammation and their interaction on survival: a study in an international hemodialysis patient cohort. Kidney Int. 2017;91:1214–1223. doi: 10.1016/j.kint.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 81.Flythe J.E., Assimon M.M., Overman R.A. Target weight achievement and ultrafiltration rate thresholds: potential patient implications. BMC Nephrol. 2017;18:185. doi: 10.1186/s12882-017-0595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Romaldini H., Rodriguez-Roisin R., Lopez F.A. The mechanisms of arterial hypoxemia during hemodialysis. Am Rev Respir Dis. 1984;129:780–784. doi: 10.1164/arrd.1984.129.5.780. [DOI] [PubMed] [Google Scholar]
- 83.Cardoso M., Vinay P., Vinet B. Hypoxemia during hemodialysis: a critical review of the facts. Am J Kidney Dis. 1988;11:281–297. doi: 10.1016/s0272-6386(88)80133-1. [DOI] [PubMed] [Google Scholar]
- 84.Campos I., Chan L., Zhang H. Intradialytic hypoxemia in chronic hemodialysis patients. Blood Purif. 2016;41:177–187. doi: 10.1159/000441271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meyring-Wosten A., Zhang H., Ye X. Intradialytic hypoxemia and clinical outcomes in patients on hemodialysis. Clin J Am Soc Nephrol. 2016;11:616–625. doi: 10.2215/CJN.08510815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chou J.A., Streja E., Nguyen D.V. Intradialytic hypotension, blood pressure changes and mortality risk in incident hemodialysis patients. Nephrol Dial Transplant. 2018;33:149–159. doi: 10.1093/ndt/gfx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Broe M.E., De Backer W.A. Pathophysiology of hemodialysis-associated hypoxemia. Adv Nephrol Necker Hosp. 1989;18:297–315. [PubMed] [Google Scholar]
- 88.Lopot F., Válek A. Mathematical concept of dialysis unphysiology. Home Hemodial Int (1997) 1998;2:18–21. doi: 10.1111/hdi.1998.2.1.18. [DOI] [PubMed] [Google Scholar]
- 89.Lopot F., Nejedlý B., Sulková S. Physiology in daily hemodialysis in terms of the time average concentration/time average deviation concept. Hemodial Int. 2004;8:39–44. doi: 10.1111/j.1492-7535.2004.00073.x. [DOI] [PubMed] [Google Scholar]
- 90.Saha M., Allon M. Diagnosis, treatment, and prevention of hemodialysis emergencies. Clin J Am Soc Nephrol. 2017;12:357–369. doi: 10.2215/CJN.05260516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burmeister J.E., Scapini A., da Rosa Miltersteiner D. Glucose-added dialysis fluid prevents asymptomatic hypoglycaemia in regular haemodialysis. Nephrol Dial Transplant. 2007;22:1184–1189. doi: 10.1093/ndt/gfl710. [DOI] [PubMed] [Google Scholar]
- 92.Abe M., Kalantar-Zadeh K. Haemodialysis-induced hypoglycaemia and glycaemic disarrays. Nat Rev Nephrol. 2015;11:302–313. doi: 10.1038/nrneph.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chazot C., Shahmir E., Matias B. Dialytic nutrition: provision of amino acids in dialysate during hemodialysis. Kidney Int. 1997;52:1663–1670. doi: 10.1038/ki.1997.500. [DOI] [PubMed] [Google Scholar]
- 94.Raimann J.G., Kruse A., Thijssen S. Metabolic effects of dialyzate glucose in chronic hemodialysis: results from a prospective, randomized crossover trial. Nephrol Dial Transplant. 2012;27:1559–1568. doi: 10.1093/ndt/gfr520. [DOI] [PubMed] [Google Scholar]
- 95.Sahani M.M., Daoud T.M., Sam R. Dialysis disequilibrium syndrome revisited. Hemodial Int. 2001;5:92–96. doi: 10.1111/hdi.2001.5.1.92. [DOI] [PubMed] [Google Scholar]
- 96.Zepeda-Orozco D., Quigley R. Dialysis disequilibrium syndrome. Pediatr Nephrol. 2012;27:2205–2211. doi: 10.1007/s00467-012-2199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Samanta R., Chan C., Chauhan V.S. Arrhythmias and sudden cardiac death in end stage renal disease: epidemiology, risk factors, and management. Can J Cardiol. 2019;35:1228–1240. doi: 10.1016/j.cjca.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 98.Kalra P.A., Green D., Poulikakos D. Arrhythmia in hemodialysis patients and its relation to sudden death. Kidney Int. 2018;93:781–783. doi: 10.1016/j.kint.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 99.Charytan D.M., Foley R., McCullough P.A. Arrhythmia and sudden death in hemodialysis patients: protocol and baseline characteristics of the monitoring in dialysis study. Clin J Am Soc Nephrol. 2016;11:721–734. doi: 10.2215/CJN.09350915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amann K., Ritz E. Cardiac structure and function in renal disease. Curr Opin Nephrol Hypertens. 1996;5:102–106. doi: 10.1097/00041552-199601000-00017. [DOI] [PubMed] [Google Scholar]
- 101.Loewe A., Lutz Y., Nairn D. Hypocalcemia-induced slowing of human sinus node pacemaking. Biophys J. 2019;117:2244–2254. doi: 10.1016/j.bpj.2019.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.El Hage N., Jaar B.G., Cheng A. Frequency of arrhythmia symptoms and acceptability of implantable cardiac monitors in hemodialysis patients. BMC Nephrol. 2017;18:309. doi: 10.1186/s12882-017-0740-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schneditz D. Temperature and thermal balance in hemodialysis. Semin Dial. 2001;14:357–364. doi: 10.1046/j.1525-139x.2001.00088.x. [DOI] [PubMed] [Google Scholar]
- 104.Drew D.A., Weiner D.E., Tighiouart H. Cognitive decline and its risk factors in prevalent hemodialysis patients. Am J Kidney Dis. 2017;69:780–787. doi: 10.1053/j.ajkd.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Drew D.A., Tighiouart H., Duncan S. Blood pressure and cognitive decline in prevalent hemodialysis patients. Am J Nephrol. 2019;49:460–469. doi: 10.1159/000500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McIntyre C.W., Goldsmith D.J. Ischemic brain injury in hemodialysis patients: which is more dangerous, hypertension or intradialytic hypotension? Kidney Int. 2015;87:1109–1115. doi: 10.1038/ki.2015.62. [DOI] [PubMed] [Google Scholar]
- 107.Findlay M.D., Dawson J., Dickie D.A. Investigating the relationship between cerebral blood flow and cognitive function in hemodialysis patients. J Am Soc Nephrol. 2019;30:147–158. doi: 10.1681/ASN.2018050462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Odudu A., Eldehni M.T., McCann G.P., McIntyre C.W. Randomized controlled trial of individualized dialysate cooling for cardiac protection in hemodialysis patients. Clin J Am Soc Nephrol. 2015;10:1408–1417. doi: 10.2215/CJN.00200115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jhamb M., Weisbord S.D., Steel J.L., Unruh M. Fatigue in patients receiving maintenance dialysis: a review of definitions, measures, and contributing factors. Am J Kidney Dis. 2008;52:353–365. doi: 10.1053/j.ajkd.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Johnson S., Crane P.B., Neil J., Christiano C. Coping with intradialytic events and stress associated with hemodialysis. Nephrol Nurs J. 2019;46:13–21. [PubMed] [Google Scholar]
- 111.Kuipers J., Oosterhuis J.K., Paans W. Association between quality of life and various aspects of intradialytic hypotension including patient-reported intradialytic symptom score. BMC Nephrol. 2019;20:164. doi: 10.1186/s12882-019-1366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van der Willik E.M., Meuleman Y., Prantl K. Patient-reported outcome measures: selection of a valid questionnaire for routine symptom assessment in patients with advanced chronic kidney disease—a four-phase mixed methods study. BMC Nephrol. 2019;20:344. doi: 10.1186/s12882-019-1521-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Loon I.N., Bots M.L., Boereboom F.T.J. Quality of life as indicator of poor outcome in hemodialysis: relation with mortality in different age groups. BMC Nephrol. 2017;18:217. doi: 10.1186/s12882-017-0621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nair D., Finkelstein F.O. Toward developing a patient-reported outcome measure for fatigue in hemodialysis. Am J Kidney Dis. 2019;74:151–154. doi: 10.1053/j.ajkd.2019.03.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Flythe J.E., Hilliard T., Castillo G. Symptom prioritization among adults receiving in-center hemodialysis: a mixed methods study. Clin J Am Soc Nephrol. 2018;13:735–745. doi: 10.2215/CJN.10850917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Finkelstein F.O., Finkelstein S.H. Time to rethink our approach to patient-reported outcome measures for ESRD. Clin J Am Soc Nephrol. 2017;12:1885–1888. doi: 10.2215/CJN.04850517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Finkelstein F.O., Schiller B., Daoui R. At-home short daily hemodialysis improves the long-term health-related quality of life. Kidney Int. 2012;82:561–569. doi: 10.1038/ki.2012.168. [DOI] [PubMed] [Google Scholar]
- 118.Kliger A.S., Finkelstein F.O. Can we improve the quality of life for dialysis patients? Am J Kidney Dis. 2009;54:993–995. doi: 10.1053/j.ajkd.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 119.Jaar B.G., Chang A., Plantinga L. Can we improve quality of life of patients on dialysis? Clin J Am Soc Nephrol. 2013;8:1–4. doi: 10.2215/CJN.11861112. [DOI] [PubMed] [Google Scholar]
- 120.Jaber B.L., Lee Y., Collins A.J. Effect of daily hemodialysis on depressive symptoms and postdialysis recovery time: interim report from the FREEDOM (Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements) Study. Am J Kidney Dis. 2010;56:531–539. doi: 10.1053/j.ajkd.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 121.Mapes D.L., Bragg-Gresham J.L., Bommer J. Health-related quality of life in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44:54–60. doi: 10.1053/j.ajkd.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 122.Mapes D.L., Lopes A.A., Satayathum S. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2003;64:339–349. doi: 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 123.Karaboyas A., Zee J., Brunelli S.M. Dialysate potassium, serum potassium, mortality, and arrhythmia events in hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2017;69:266–277. doi: 10.1053/j.ajkd.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pun P.H., Middleton J.P. Dialysate potassium, dialysate magnesium, and hemodialysis risk. J Am Soc Nephrol. 2017;28:3441–3451. doi: 10.1681/ASN.2017060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rhee C.M., Chou J.A., Kalantar-Zadeh K. Dialysis prescription and sudden death. Semin Nephrol. 2018;38:570–581. doi: 10.1016/j.semnephrol.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gorcsan J., 3rd, Haugaa K.H. Ventricular arrhythmias and reduced echocardiographic inferior wall strain: is regional function an important risk marker? Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.005900. [DOI] [PubMed] [Google Scholar]
- 127.Nguyen M.N., Kiriazis H., Gao X.M., Du X.J. Cardiac fibrosis and arrhythmogenesis. Compr Physiol. 2017;7:1009–1049. doi: 10.1002/cphy.c160046. [DOI] [PubMed] [Google Scholar]
- 128.Nguyen T.P., Qu Z., Weiss J.N. Cardiac fibrosis and arrhythmogenesis: the road to repair is paved with perils. J Mol Cell Cardiol. 2014;70:83–91. doi: 10.1016/j.yjmcc.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kooman J.P., Katzarski K., van der Sande F.M. Hemodialysis: a model for extreme physiology in a vulnerable patient population. Semin Dial. 2018;31:500–506. doi: 10.1111/sdi.12704. [DOI] [PubMed] [Google Scholar]
- 130.Schleser A., Fleck N., Tsobanelis T. The impact of disposables towards more eco-friendly and less costly haemodialysis. Nephrol Dial Transplant. 2016;31:i494. [Google Scholar]
- 131.Carr B.D., Johnson T.J., Gomez-Rexrode A. Inflammatory effects of blood-air interface in a porcine cardiopulmonary bypass model. ASAIO J. 2020;66:72–78. doi: 10.1097/MAT.0000000000000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Amiji M., Park K. Surface modification of polymeric biomaterials with poly(ethylene oxide), albumin, and heparin for reduced thrombogenicity. J Biomater Sci Polym Ed. 1993;4:217–234. doi: 10.1163/156856293x00537. [DOI] [PubMed] [Google Scholar]
- 133.Rudolph A., Teske M., Illner S. Surface modification of biodegradable polymers towards better biocompatibility and lower thrombogenicity. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gabutti L., Lucchini B., Marone C. Citrate- vs. acetate-based dialysate in bicarbonate haemodialysis: consequences on haemodynamics, coagulation, acid-base status, and electrolytes. BMC Nephrol. 2009;10:7. doi: 10.1186/1471-2369-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jung S.W., Kim D.R., Cho K.S. Effects of dialysate acidification with citrate versus acetate on cell damage, uremic toxin levels, and inflammation in patients receiving maintenance hemodialysis. Am J Kidney Dis. 2019;73:432–434. doi: 10.1053/j.ajkd.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 136.Kossmann R.J., Gonzales A., Callan R., Ahmad S. Increased efficiency of hemodialysis with citrate dialysate: a prospective controlled study. Clin J Am Soc Nephrol. 2009;4:1459–1464. doi: 10.2215/CJN.02590409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Barak M., Nakhoul F., Katz Y. Pathophysiology and clinical implications of microbubbles during hemodialysis. Semin Dial. 2008;21:232–238. doi: 10.1111/j.1525-139X.2008.00424.x. [DOI] [PubMed] [Google Scholar]
- 138.Stegmayr B., Brännström T., Forsberg U. Microbubbles of air may occur in the organs of hemodialysis patients. ASAIO J. 2012;58:177–179. doi: 10.1097/MAT.0b013e318245d0dd. [DOI] [PubMed] [Google Scholar]
- 139.Wagner S., Rode C., Wojke R., Canaud B. Observation of microbubbles during standard dialysis treatments. Clin Kidney J. 2015;8:400–404. doi: 10.1093/ckj/sfv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brunelli S.M., Spiegel D.M., Du Mond C. Serum-to-dialysate potassium gradient and its association with short-term outcomes in hemodialysis patients. Nephrol Dial Transplant. 2018;33:1207–1214. doi: 10.1093/ndt/gfx241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hecking M., Karaboyas A., Saran R. Dialysate sodium concentration and the association with interdialytic weight gain, hospitalization, and mortality. Clin J Am Soc Nephrol. 2012;7:92–100. doi: 10.2215/CJN.05440611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Trinh E., Weber C. The dialysis sodium gradient: a modifiable risk factor for fluid overload. Nephron Extra. 2017;7:10–17. doi: 10.1159/000453674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Basile C., Rossi L., Lomonte C. Dialysate bicarbonate concentration: too much of a good thing? Semin Dial. 2018;31:576–582. doi: 10.1111/sdi.12716. [DOI] [PubMed] [Google Scholar]
- 144.Nitta K., Masakane I., Hanafusa N. Annual dialysis data report 2017, JSDT Renal Data Registry. Renal Replace Ther. 2019;5 [Google Scholar]
- 145.Tentori F., Zhang J., Li Y. Longer dialysis session length is associated with better intermediate outcomes and survival among patients on in-center three times per week hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2012;27:4180–4188. doi: 10.1093/ndt/gfs021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fotheringham J., Sajjad A., Stel V.S. The association between longer haemodialysis treatment times and hospitalization and mortality after the two-day break in individuals receiving three times a week haemodialysis. Nephrol Dial Transplant. 2019;34:1577–1584. doi: 10.1093/ndt/gfz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lin Y.H., Lin C., Ho Y.H. Heart rhythm complexity impairment in patients undergoing peritoneal dialysis. Sci Rep. 2016;6:28202. doi: 10.1038/srep28202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tieu A., Velenosi T.J., Kucey A.S. β-Blocker dialyzability in maintenance hemodialysis patients: a randomized clinical trial. Clin J Am Soc Nephrol. 2018;13:604–611. doi: 10.2215/CJN.07470717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Roberts P.R., Green D. Arrhythmias in chronic kidney disease. Heart. 2011;97:766–773. doi: 10.1136/hrt.2010.208587. [DOI] [PubMed] [Google Scholar]
- 150.Doenyas-Barak K., de Abreu M., Borges L.E. Non-invasive hemodynamic profiling of patients undergoing hemodialysis—a multicenter observational cohort study. BMC Nephrol. 2019;20:347. doi: 10.1186/s12882-019-1542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Daugirdas J.T. Eliminating the need for routine monthly postdialysis serum urea nitrogen measurement: a method for monitoring Kt/V and normalized protein catabolic rate using conductivity determined dialyzer clearance. Semin Dial. 2018;31:633–636. doi: 10.1111/sdi.12750. [DOI] [PubMed] [Google Scholar]
- 152.Reddan D.N., Szczech L.A., Hasselblad V. Intradialytic blood volume monitoring in ambulatory hemodialysis patients: a randomized trial. J Am Soc Nephrol. 2005;16:2162–2169. doi: 10.1681/ASN.2004121053. [DOI] [PubMed] [Google Scholar]
- 153.Kron J., Schneditz D., Leimbach T. A simple and feasible method to determine absolute blood volume in hemodialysis patients in clinical practice. Blood Purif. 2014;38:180–187. doi: 10.1159/000368157. [DOI] [PubMed] [Google Scholar]
- 154.Kron S., Schneditz D., Leimbach T. Determination of the critical absolute blood volume for intradialytic morbid events. Hemodial Int. 2016;20:321–326. doi: 10.1111/hdi.12375. [DOI] [PubMed] [Google Scholar]
- 155.Kron S., Schneditz D., Leimbach T., Kron J. Feedback control of absolute blood volume: a new technical approach in hemodialysis. Hemodial Int. 2020;24:344–350. doi: 10.1111/hdi.12826. [DOI] [PubMed] [Google Scholar]
- 156.Sinha A.D., Light R.P., Agarwal R. Relative plasma volume monitoring during hemodialysis AIDS the assessment of dry weight. Hypertension. 2010;55:305–311. doi: 10.1161/HYPERTENSIONAHA.109.143974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Leung K.C.W., Quinn R.R., Ravani P. Randomized crossover trial of blood volume monitoring-guided ultrafiltration biofeedback to reduce intradialytic hypotensive episodes with hemodialysis. Clin J Am Soc Nephrol. 2017;12 doi: 10.2215/CJN.01030117. 1831-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Polinder-Bos H.A., Elting J.W.J., Aries M.J. Changes in cerebral oxygenation and cerebral blood flow during hemodialysis—a simultaneous near-infrared spectroscopy and positron emission tomography study. J Cereb Blood Flow Metab. 2020;40:328–340. doi: 10.1177/0271678X18818652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gabrielli D., Krystal B., Katzarski K. Improved intradialytic stability during haemodialysis with blood volume-controlled ultrafiltration. J Nephrol. 2009;22:232–240. [PubMed] [Google Scholar]
- 160.Hecking M., Schneditz D. Feedback control in hemodialysis-much ado about nothing? Clin J Am Soc Nephrol. 2017;12:1730–1732. doi: 10.2215/CJN.09770917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Santoro A., Mancini E., Paolini F. Blood volume regulation during hemodialysis. Am J Kidney Dis. 1998;32:739–748. doi: 10.1016/s0272-6386(98)70128-3. [DOI] [PubMed] [Google Scholar]
- 162.Beaubien-Souligny W., Denault A., Robillard P., Desjardins G. The role of point-of-care ultrasound monitoring in cardiac surgical patients with acute kidney injury. J Cardiothorac Vasc Anesth. 2019;33:2781–2796. doi: 10.1053/j.jvca.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 163.Song J.H., Park G.H., Lee S.Y. Effect of sodium balance and the combination of ultrafiltration profile during sodium profiling hemodialysis on the maintenance of the quality of dialysis and sodium and fluid balances. J Am Soc Nephrol. 2005;16:237–246. doi: 10.1681/ASN.2004070581. [DOI] [PubMed] [Google Scholar]
- 164.Selby N.M., Burton J.O., Chesterton L.J., McIntyre C.W. Dialysis-induced regional left ventricular dysfunction is ameliorated by cooling the dialysate. Clin J Am Soc Nephrol. 2006;1:1216–1225. doi: 10.2215/CJN.02010606. [DOI] [PubMed] [Google Scholar]
- 165.Maggiore Q., Pizzarelli F., Santoro A. The effects of control of thermal balance on vascular stability in hemodialysis patients: results of the European randomized clinical trial. Am J Kidney Dis. 2002;40:280–290. doi: 10.1053/ajkd.2002.34506. [DOI] [PubMed] [Google Scholar]
- 166.Selby N.M., McIntyre C.W. A systematic review of the clinical effects of reducing dialysate fluid temperature. Nephrol Dial Transplant. 2006;21:1883–1898. doi: 10.1093/ndt/gfl126. [DOI] [PubMed] [Google Scholar]
- 167.Feng Y., Zou Y., Zheng Y. The value of non-invasive measurement of cardiac output and total peripheral resistance to categorize significant changes of intradialytic blood pressure: a prospective study. BMC Nephrol. 2018;19:310. doi: 10.1186/s12882-018-1087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Barbieri C., Cattinelli I., Neri L. Development of an artificial intelligence model to guide the management of blood pressure, fluid volume, and dialysis dose in end-stage kidney disease patients: proof of concept and first clinical assessment. Kidney Dis (Basel) 2019;5:28–33. doi: 10.1159/000493479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bucalo M.L., Barbieri C., Roca S. The anaemia control model: does it help nephrologists in therapeutic decision-making in the management of anaemia? Nefrologia. 2018;38:491–502. doi: 10.1016/j.nefro.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 170.Kjellstrand C.M., Buoncristiani U., Ting G. Short daily haemodialysis: survival in 415 patients treated for 1006 patient-years. Nephrol Dial Transplant. 2008;23:3283–3289. doi: 10.1093/ndt/gfn210. [DOI] [PubMed] [Google Scholar]
- 171.Peters S.A., Bots M.L., Canaud B. Haemodiafiltration and mortality in end-stage kidney disease patients: a pooled individual participant data analysis from four randomized controlled trials. Nephrol Dial Transplant. 2016;31:978–984. doi: 10.1093/ndt/gfv349. [DOI] [PubMed] [Google Scholar]
- 172.Davenport A., Peters S.A., Bots M.L. Higher convection volume exchange with online hemodiafiltration is associated with survival advantage for dialysis patients: the effect of adjustment for body size. Kidney Int. 2016;89:193–199. doi: 10.1038/ki.2015.264. [DOI] [PubMed] [Google Scholar]
- 173.Barth C., Boer W., Garzoni D. Characteristics of hypotension-prone haemodialysis patients: is there a critical relative blood volume? Nephrol Dial Transplant. 2003;18:1353–1360. doi: 10.1093/ndt/gfg171. [DOI] [PubMed] [Google Scholar]
- 174.Floege J., Gillespie I.A., Kronenberg F. Development and validation of a predictive mortality risk score from a European hemodialysis cohort. Kidney Int. 2015;87:996–1008. doi: 10.1038/ki.2014.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Couchoud C.G., Beuscart J.B., Aldigier J.C. Development of a risk stratification algorithm to improve patient-centered care and decision making for incident elderly patients with end-stage renal disease. Kidney Int. 2015;88:1178–1186. doi: 10.1038/ki.2015.245. [DOI] [PubMed] [Google Scholar]
- 176.Kuhlmann U., Maierhofer A., Canaud B. Zero diffusive sodium balance in hemodialysis provided by an algorithm-based electrolyte balancing controller: a proof of principle clinical study. Artif Organs. 2019;43:150–158. doi: 10.1111/aor.13328. [DOI] [PubMed] [Google Scholar]
- 177.Sagova M., Wojke R., Maierhofer A. Automated individualization of dialysate sodium concentration reduces intradialytic plasma sodium changes in hemodialysis. Artif Organs. 2019;43:1002–1013. doi: 10.1111/aor.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ponce P., Pinto B., Wojke R. Evaluation of intradialytic sodium shifts during sodium controlled hemodialysis. Int J Artif Organs. 2020;43:620–624. doi: 10.1177/0391398820903055. [DOI] [PubMed] [Google Scholar]
- 179.Canaud B., Kooman J., Selby N.M. Sodium and water handling during hemodialysis: new pathophysiologic insights and management approaches for improving outcomes in end-stage kidney disease. Kidney Int. 2019;95:296–309. doi: 10.1016/j.kint.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 180.Stewart J, Stewart P, Walker T, et al. A feasibility study of non-invasive continuous estimation of brachial pressure derived from arterial and venous lines during dialysis. IEEE J Trans Eng Health Med, in press. [DOI] [PMC free article] [PubMed]
- 181.Dias D., Paulo Silva Cunha J. Wearable health devices—vital sign monitoring, systems and technologies. Sensors (Basel) 2018;18:2414. doi: 10.3390/s18082414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hojs N., Fissell W.H., Roy S. Ambulatory hemodialysis-technology landscape and potential for patient-centered treatment. Clin J Am Soc Nephrol. 2020;15:152–159. doi: 10.2215/CJN.01970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Salani M., Roy S., Fissell W.H. Innovations in wearable and implantable artificial kidneys. Am J Kidney Dis. 2018;72:745–751. doi: 10.1053/j.ajkd.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 184.Gura V., Davenport A., Beizai M. Beta2-microglobulin and phosphate clearances using a wearable artificial kidney: a pilot study. Am J Kidney Dis. 2009;54:104–111. doi: 10.1053/j.ajkd.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 185.Gura V., Macy A.S., Beizai M. Technical breakthroughs in the wearable artificial kidney (WAK) Clin J Am Soc Nephrol. 2009;4:1441–1448. doi: 10.2215/CJN.02790409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gura V., Ronco C., Davenport A. The wearable artificial kidney, why and how: from holy grail to reality. Semin Dial. 2009;22:13–17. doi: 10.1111/j.1525-139X.2008.00507.x. [DOI] [PubMed] [Google Scholar]
- 187.Davenport A., Gura V., Ronco C. A wearable haemodialysis device for patients with end-stage renal failure: a pilot study. Lancet. 2007;370:2005–2010. doi: 10.1016/S0140-6736(07)61864-9. [DOI] [PubMed] [Google Scholar]
- 188.Gura V., Rivara M.B., Bieber S. A wearable artificial kidney for patients with end-stage renal disease. JCI Insight. 2016;1 doi: 10.1172/jci.insight.86397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shafi T., Guallar E. Mapping progress in reducing cardiovascular risk with kidney disease: sudden cardiac death. Clin J Am Soc Nephrol. 2018;13:1429–1431. doi: 10.2215/CJN.02760218. [DOI] [PMC free article] [PubMed] [Google Scholar]