Abstract
Numerous recent studies have sought to determine the developmental trajectories of motor-related oscillatory responses from youth to adulthood. However, most of this work has relied on simple movements, and rarely have these studies linked developmental neural changes with maturational improvements in motor performance. In this study, we recorded magnetoencephalography during a complex finger-tapping task in a large sample of 107 healthy youth aged 9–15 years old. The relationships between region-specific neural activity, age, and performance metrics were examined using structural equation modeling. We found strong developmental effects on behavior and beta oscillatory activity during movement planning, as well as associations between planning-related beta activity and activity within the same region during the movement execution period. However, when all factors were tested, we found that only right parietal cortex beta dynamics mediated the relationship between age and performance on the task. These data suggest that strong, sustained beta activity within the right parietal cortex enhances motor performance, and that these sustained oscillations develop through childhood into early adolescence. In sum, these are the first data to link developmental trajectories in beta oscillatory dynamics with distinct motor performance metrics and implicate the right parietal cortex as a crucial hub in movement execution.
Keywords: beta, magnetoencephalography, motor control, movement, neural dynamics
Introduction
The planning and execution of movements are known to be served by a specific pattern of oscillatory activity throughout the cortical motor network, particularly in the beta (~15–30 Hz) band. Specifically, there is a significant beta event-related desynchronization (ERD) that occurs from about 1 s prior to movement onset and persists until about 0.5 s after movement, termed the peri-movement beta ERD (Pfurtscheller and Lopes da Silva 1999; Kaiser et al. 2003; Doyle et al. 2005; Cheyne et al. 2006; Jurkiewicz et al. 2006; Gaetz et al. 2010; Tzagarakis et al. 2010; Wilson et al. 2010, 2011, 2014; Grent-'t-Jong et al. 2014; Heinrichs-Graham et al. 2014, 2016, 2018b; Heinrichs-Graham and Wilson 2015, 2016). The peri-movement beta ERD has been widely studied and is associated with movement planning and execution processes (Kaiser et al. 2001, 2003; Doyle et al. 2005; Gross et al. 2005; Jensen et al. 2005; Pogosyan et al. 2009; Engel and Fries 2010; Tzagarakis et al. 2010; Park et al. 2013; Grent-'t-Jong et al. 2014; Kurz et al. 2014; Heinrichs-Graham and Wilson 2015, 2016; Heinrichs-Graham et al. 2016). For example, the peri-movement beta ERD is known to be modulated by the certainty of the pending movement (Kaiser et al. 2003; Doyle et al. 2005; Tzagarakis et al. 2010; Heinrichs-Graham et al. 2016), the similarity in potential movement options (Praamstra et al. 2009; Grent-'t-Jong et al. 2014), the complexity of the movement to be performed (Heinrichs-Graham and Wilson 2015), and other high-order movement planning-related factors. Interestingly, beta ERD response amplitude does not seem to be impacted by parameters of the movement itself (e.g., force or speed of the muscles employed (Fry et al. 2016)), suggesting that the response is much more sensitive to the goal of the movement than actual motor output. Of note, other significant and robust motor responses include a postmovement beta rebound (PMBR), which occurs approximately 0.8 s after movement termination and lasts for at least 1.0 s. The PMBR response has been less widely studied but is generally thought to reflect sensory feedback to the motor cortices and/or active inhibition following movement (Pfurtscheller et al. 1996; Cassim et al. 2001; Alegre et al. 2008; Reyns et al. 2008; Pfurtscheller and Solis-Escalante 2009; Fry et al. 2016; Tan et al. 2016; Heinrichs-Graham et al. 2017). There is also a strong movement-related gamma synchronization (MRGS) that occurs from approximately 50 ms before to 100 ms after movement onset (Cheyne et al. 2008; Muthukumaraswamy 2010; Wilson et al. 2010, 2011; Gaetz et al. 2011; Hall et al. 2011; Shaw et al. 2015; Heinrichs-Graham et al. 2018a; Trevarrow et al. 2019; Spooner et al. 2020).
A limited number of studies have sought to determine how these motor-related oscillations change as a function of age. A classic paper by Wilson and colleagues used magnetoencephalography (MEG) and a simple finger tapping task and found that beta ERD, PMBR, and MRGS amplitude all linearly increased as a function of age (Wilson et al. 2010), though most of these results were localized to secondary motor cortices (e.g., supplementary motor area, cerebellum). A later study using a similar movement paradigm showed that the peri-movement beta ERD and PMBR were significantly stronger in the primary motor cortices of older children (11–13 years) and adults relative to younger children (4–6 years), with the youngest children showing no significant PMBR response during a simple finger tapping task (Gaetz et al. 2010). Such a developmental increase in beta ERD amplitude was similarly found in a recent MEG investigation of knee movements (Kurz et al. 2016). Finally, another recent developmental study characterized the beta ERD, PMBR, and gamma ERS amplitude changes in the primary motor cortices in a large sample of children and adolescents and found a significant relationship between age and the PMBR and gamma ERS, but no such relationship with the beta ERD (Trevarrow et al. 2019). Of note, there are also substantial changes in beta activity during healthy aging, such that both baseline beta levels and peri-movement beta ERD power have been shown to significantly and dramatically increase with age (Rossiter et al. 2014; Heinrichs-Graham and Wilson 2016; Heinrichs-Graham et al. 2018a).
While these studies have provided major insight on the age-related trajectories of motor oscillations, they have also largely relied on simple movements (e.g., single finger taps), which has limited their ability to link developmental oscillatory changes to behavioral performance. A benefit of more complex movement paradigms is that they provide multiple behavioral output metrics, which can be critical for understanding responses like the beta ERD, as it has been implicated in both motor planning and execution. While a small number of studies have attempted to dissociate these concepts in the context of movement inhibition (Maimon and Assad 2006; Liebrand et al. 2017; Kaiser and Schutz-Bosbach 2019), there has only been one study to date that investigated how cortical beta ERD dynamics change from movement planning to execution during complex movements (Heinrichs-Graham and Wilson 2015). In that study, three numbers were presented on the screen, each corresponding to a finger on the hand, and healthy adults were instructed to tap the sequence in order. While there were no neural differences during the planning period, there were significant beta oscillatory and connectivity differences in parietal and frontal regions during complex movement execution (Heinrichs-Graham and Wilson 2015). This suggests that secondary motor regions, such as the parietal cortices, may be especially pertinent to the execution of complex movements in the healthy adult motor system.
The current study sought to determine the developmental trajectory of the motor-related oscillatory dynamics serving complex movement processing in a large cohort of youth. We hypothesized that there would be robust effects of age on motor performance and that these differences would be reflected in altered beta dynamics during motor planning and execution. Further, we hypothesized that the most robust developmental changes would occur in secondary motor regions such as the parietal cortices.
Methods
Subject Selection
A total of 107 healthy youth (55 females, mean age: 11.74 years, range 9–15 years, 8 left-handed) were recruited from the local community. Exclusionary criteria, based on parent/guardian report, included any diagnosed neurological or psychiatric disorder, other illness affecting CNS function (e.g., HIV infection), presence of a learning disability, history of head trauma, current substance abuse, and the presence of any irremovable type of ferromagnetic material. After complete description of the study was given to participants, written informed consent was obtained from the parent/guardian, and informed assent was obtained from the participant, following the guidelines of the University of Nebraska Medical Center’s Institutional Review Board, which approved the study protocol.
Experimental Paradigm and Stimuli
During MEG recording, participants were seated in a nonmagnetic chair within a magnetically shielded room. Each participant rested their right hand on a custom five-finger button pad while fixating on a crosshair presented centrally. This response pad was connected directly to the MEG system, and each button sent a TTL pulse to the acquisition computer in real-time. Behavioral responses were thus temporally synced with the MEG data, which allowed accuracy, reaction times, and movement durations (in ms) to be computed offline. After an initial baseline period of 3.75 s, a series of three numbers, each corresponding to a finger on the right hand, were presented simultaneously in black for 0.5 s. After 0.5 s, the numbers changed color, signaling the participant to complete the motor sequence by pressing the corresponding buttons as quickly and accurately as possible. The participant was given 2.25 s to complete the motor plan and return to rest. Figure 1 depicts the slides constituting one trial. A total of 160 trials were completed, making overall MEG recording time about 16 minutes for the task.
Figure 1 .
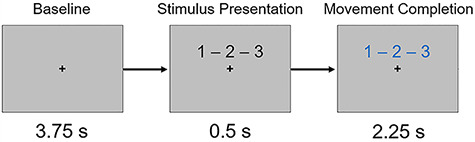
Task paradigm. Following a baseline period, three numbers corresponding to fingers on the right hand were presented. After 0.5 s, the numbers changed color, which prompted the participant to tap the sequence in order as quickly and accurately as possible.
MEG Data Acquisition & Coregistration with Structural MRI
All recordings were conducted in a one-layer magnetically shielded room with active shielding engaged. Neuromagnetic responses were sampled continuously at 1 kHz with an acquisition bandwidth of 0.1–330 Hz using an MEG system with 306 magnetic sensors (Elekta, Helsinki, Finland). MEG data from each subject were individually corrected for head motion (MaxFilter v2.2; Elekta) and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu et al. 2005; Taulu and Simola 2006). For motion correction, the position of the head throughout the recording was aligned to the individual’s head position at the start of the MEG recording. Additionally, a high-resolution structural T1-weighted MRI was collected from each participant using a 3 T Siemens Skyra scanner equipped with a 32-channel head coil (TR: 24.0 ms; TE: 1.94 ms; field of view: 256 mm; slice thickness: 1 mm with no gap; in-plane resolution: 1.0 × 1.0 mm). The structural volumes were aligned parallel to the anterior and posterior commissures and transformed into standardized Talairach space. Each participant’s MEG data were coregistered with their MRI data using BESA MRI (Version 2.0). After source imaging (beamformer), each subject’s functional images were also transformed into standardized space using the transform that was previously applied to the structural MRI volume, and spatially resampled.
MEG Time-Frequency Transformation and Statistics
Cardiac and ocular artifacts were removed from the data using signal-space projection, which was accounted for during source reconstruction (Uusitalo and Ilmoniemi 1997). The continuous magnetic time series was divided into epochs of 6.4 s duration, with 0.0 s defined as movement onset (i.e., first button press) and the baseline defined as the −2.25 to −1.75 s time window (i.e., before movement onset; Fig. 1). Only correct trials were used for analysis. Epochs containing artifacts were rejected based on a fixed threshold method, supplemented with visual inspection. There were an average of 119.71 (SD: 10.83) trials per person included after artifact rejection.
Artifact-free epochs were then transformed into the time-frequency domain using complex demodulation (resolution: 1.0 Hz, 50 ms; Papp and Ktonas 1977; Kovach and Gander 2016), whereby the single-trial data was subject to a discrete Fourier decomposition to determine the signal power in each 1 Hz frequency bin in overlapping 50 ms time windows for the length of the epoch. The resulting spectral power estimations per sensor were averaged over trials to generate time-frequency plots of mean spectral density. These sensor-level data were normalized per frequency bin using the mean power during the −2.25 to −1.75 s baseline period. The specific time-frequency windows used for imaging were determined by statistical analysis of the sensor-level spectrograms across the entire array of gradiometers. Each data point in the spectrogram was initially evaluated using a mass univariate approach based on the general linear model. To reduce the risk of false positive results while maintaining reasonable sensitivity, a two-stage procedure was followed to control for Type 1 error. In the first stage, paired-samples t-tests against baseline were conducted on each data point and the output spectrogram of t-values was thresholded at P < 0.05 to define time-frequency bins containing potentially significant oscillatory deviations across all participants. In stage two, time-frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also below the (P < 0.05) threshold, and a cluster value was derived by summing all of the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster-values, and the significance level of the observed clusters (from stage one) were tested directly using this distribution (Ernst 2004; Maris and Oostenveld 2007). For each comparison, 10 000 permutations were computed to build a distribution of cluster values. Based on these analyses, time-frequency windows that corresponded to events of a priori interest (i.e., the peri-movement beta ERD) and contained a significant oscillatory event across all participants were subjected to the beamforming analysis. Further details of our analysis pipeline are available (Wiesman and Wilson 2020).
MEG Imaging & Statistics
Cortical networks were imaged through an extension of the linearly constrained minimum variance vector beamformer (Gross et al. 2001; Hillebrand et al. 2005) using the Brain Electrical Source Analysis (BESA 7.0) software. Following convention, we computed noise-normalized source power per voxel in each participant using active (i.e., task) and passive (i.e., baseline) periods of equal duration and bandwidth. These images are typically referred to as pseudo-t maps, with units (pseudo-t) that reflect noise-normalized power differences (i.e., active vs. passive) per voxel. These maps were computed for the selected time-frequency bands over the entire brain volume per participant at 4.0 × 4.0 × 4.0 mm resolution. As stated above, the maps were then transformed into standardized Talairach space using the transform previously applied to the structural MRI volume and spatially resampled. These images were then averaged across participants, and source power from peak voxels was identified and extracted per location, time bin (i.e., planning vs. execution), and participant. These data were used for all statistical modeling.
Our primary hypotheses were that beta oscillations during the planning and execution periods would predict task performance and that these dynamics would follow distinct developmental trends. This overall conceptual model is shown in Figure 2. Specifically, we tested a multiple mediation model by which beta oscillatory activity during motor planning predicted reaction time and beta oscillatory activity during motor execution. All variables during planning and execution then predicted movement duration (i.e., time to completion). Age served as a control variable on all measures. All analyses were conducted with and without full information maximum likelihood estimation for missing data. Conclusions were the same, so we report results using missing data estimation for increased statistical power. Statistical significance was determined using the false-discovery rate (FDR) to correct for multiple comparisons. All statistical modeling was performed with MPlus (v.8.1).
Figure 2 .
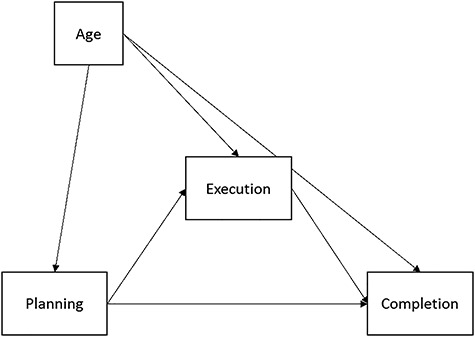
The conceptual mediation model tested. Execution variables (oscillatory activity and reaction time) were tested as potential mediators of the effects of neural oscillatory planning mechanisms on behavioral performance (i.e., successful movement completion). We also probed potential developmental effects on the neural and behavioral measures of interest via chronological age.
Results
Behavioral Results
Eleven participants were excluded from all analyses: seven were excluded for behavioral performance (i.e., < 70% accuracy on the task), two participants for technical difficulties during MEG acquisition, and two participants for excessive noise in their MEG data. The remaining 96 participants performed generally well, with an average accuracy of 92.12% (SD: 5.68%). Average reaction time (i.e., first button press) was 786.31 ms (SD: 242.54 ms), and average movement duration (i.e., how long it took to complete the tapping sequence) was 963.77 ms (SD: 220.76). To initially probe for developmental behavioral effects, we computed correlations between age (calculated to the hundredth of a year) and performance measures. These revealed significant correlations between age and accuracy, r(96) = 0.326, P = 0.001, reaction time, r(96) = −0.466, P < 0.001, and movement duration, r(96) = −0.396, P < 0.001, suggesting that with older age, participants were more accurate, responded faster, and had shorter movement durations (Fig. 3). We synced our MEG data analyses with movement onset in each trial and focused all analyses on the time windows preceding and during the early stages of movement execution (i.e., before any participant completed the movement sequence).
Figure 3 .
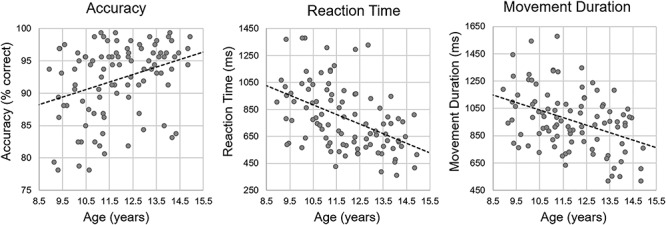
Behavioral results. Age (in years) is shown on the x axis, while accuracy (% correct) is shown on the left, reaction time (in ms) is shown in the middle, and movement duration (in ms) is shown on the right. There were significant correlations between age and all behavioral metrics (all Ps < 0.001).
Identification of the Task-Induced Cortical Motor Network
Sensor-level time–frequency spectrograms were statistically examined to derive the time-frequency bins to be imaged. Significant peri-movement beta ERD responses were found across a large number of gradiometers near the sensorimotor cortex in the 17–23 Hz range, and this response extended from about 0.5 s before movement onset until about 0.75 s after average movement offset (P < 0.0001, corrected). Of note, there was also a significant alpha ERD and a small, yet significant PMBR response found in the sensor-level data (both Ps < 0.0001, corrected), but these responses were outside the scope of the study and thus were not imaged. Sensor-level activity is shown in Figure 4A.
Figure 4 .
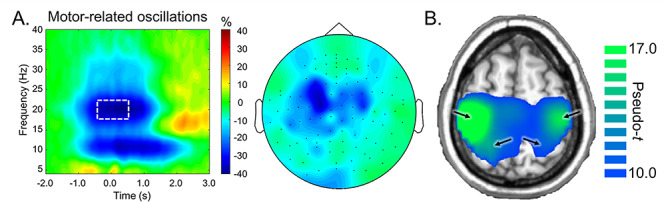
(A) Left: Grand-averaged time-frequency spectrogram from a sensor near the primary motor cortex. Time is denoted on the x-axis (0.0 s = movement onset), while frequency (in Hz) is shown on the y-axis. The white box denotes the time-frequency window that was imaged using beamforming. The color bar shows change in power (% change from baseline). Right: Sensor-level distribution of 17–23 Hz activity from −0.4 to 0.4 s (0.0 s = movement onset) across the entire array of gradiometers. (B) Grand-averaged beamformer image of beta ERD activity (17–23 Hz, −0.4 to 0.4 s). Color bar represents source power (pseudo-t). Arrows denote neural peaks.
In order to distinguish differences in beta activity related to movement planning versus execution, the significant beta ERD response period was divided into two temporally distinct 17–23 Hz windows (i.e., motor planning: −0.4 to 0.0 s; motor execution: 0.0 to 0.4 s; 0.0 s defined as movement onset), and these windows were independently imaged in each participant using a baseline period of −2.15 to −1.75 s. We then averaged the resulting images in each participant and then across participants and determined the peak motor-related responses induced by the task. This investigation yielded four distinct peaks, including the left precentral gyrus (coordinates: −38, −28, 49), right precentral gyrus (coordinates: 38, −28, 49), left superior parietal cortex (coordinates: −18, −44, 65), and right superior parietal cortex (coordinates: 18, −44, 65; Fig. 4B). Peak voxel values (pseudo-t) were then extracted from each brain region per time bin (i.e., planning and execution). Of note, we also averaged the planning and execution windows individually, and determined that the source peak locations were identical across the two time windows.
Statistical Modeling of Developmental Effects on Motor Network Dynamics
As described above, we tested a multiple mediation model by which beta oscillatory activity during the planning period (−0.4 to 0.0 s, 0.0 s = movement onset) predicted reaction time, as well as beta oscillatory activity during execution (0.0–0.4 s). All variables during both planning and execution then predicted movement duration (i.e., time to complete the movement). Age served as a control variable on all measures. The full model that was tested is shown in Figure 5. Briefly, planning variables included beta ERD power (pseudo-t) during the planning period (−0.4–0.0 s) in the left and right precentral gyri, as well as the left and right parietal cortices. Execution variables included reaction time (in ms) and beta ERD power (pseudo-t) during the execution period (0.0–0.4 s) in the same four brain regions. Movement duration (in ms) served as the completion variable.
Figure 5 .
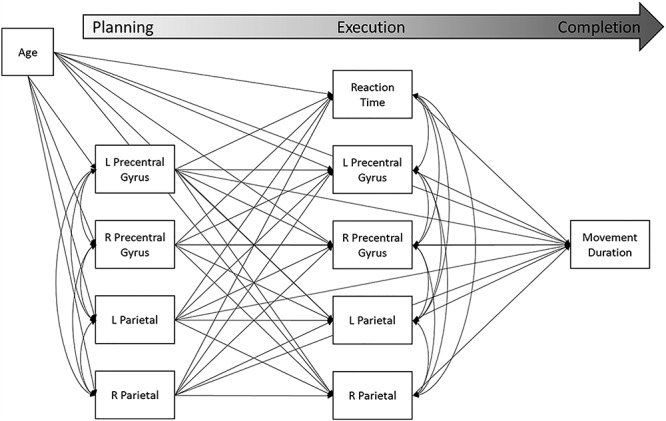
Full mediation model tested. Planning variables included source activity (pseudo-t) during the planning period (−0.4 to 0.0 s, 0.0 s = movement onset) for the left and right precentral gyri and parietal cortices. Planning variables predicted execution variables, which included source activity (pseudo-t) during the execution period (0.0 to 0.4 s) for the bilateral precentral and parietal cortices, as well as reaction time (in ms). Planning and execution variables predicted movement duration (in ms). Age (in years) acted as a control variable.
Results of the final model are displayed in Figure 6. For simplicity and visibility, only significant and trending associations are reported in the figure. A full table of parameter estimates from both significant and nonsignificant effects can be found in Supplemental Table S1. In total, the model accounted for a significant proportion of the variance in movement duration (R2 = 0.42, P < 0.001). Beta ERD activity was significantly correlated across the four neural regions during the planning phase, with correlations ranging from 0.39 to 0.74. There were fewer significant correlations within this motor circuit during the execution phase, though the effects that did hold were moderate-to-strong (range: 0.42–0.64). Beta ERD activity in each brain region during the planning period strongly and significantly predicted activity during execution within the same region, such that greater beta ERD during planning predicted greater subsequent beta ERD during execution (β’s = 0.78–0.93, Ps < 0.001). As shown, there were additional significant network-level associations. Briefly, greater planning-related beta activity within the left precentral gyrus predicted greater execution-related activity in the right precentral gyrus (β = 0.17, P = 0.007), as well as trended toward predicting greater execution-related activity within the left parietal cortex (β = 0.11, P = 0.054). Left parietal planning-related activity similarly predicted execution-related beta ERD activity within the left precentral gyrus (β = 0.12, P = 0.026), though this effect did not survive after FDR correction. There was a trend toward planning-related activity within the right parietal cortex negatively predicting left precentral gyrus activity during execution (β = −0.084, P = 0.075).
Figure 6 .
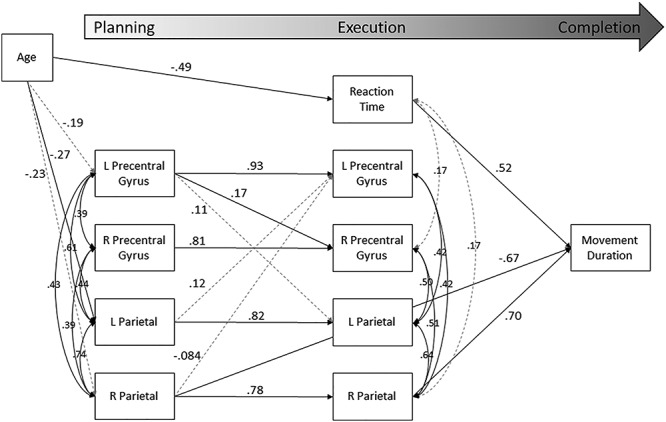
Results of the full mediation model. Statistically significant estimates (P < 0.05) are denoted with a solid black line, while those that were approaching significance (P < 0.10) are shown by dashed gray lines. Nonsignificant estimates are not shown. All listed parameters are standardized coefficients.
Considering task behavior, we did not detect any statistically significant predictions from planning-related beta oscillatory activity to reaction time. However, both planning- and execution-related beta activity within the right parietal cortex significantly predicted movement duration. Interestingly, these predictions followed opposite patterns. Stronger planning-related beta ERD in the right parietal cortex was associated with longer overall movement duration (β = −0.67, P = 0.009). Conversely, a greater execution-related beta ERD predicted shorter movement durations (β = 0.70, P = 0.014). The only other metric that significantly predicted movement duration was reaction time; individuals with faster reaction times tended to have shorter overall movement durations (β = 0.52, P < 0.001).
We detected several statistically significant developmental effects within the model. Age was associated with planning-related beta activity in bilateral parietal areas, indicating that older participants exhibited stronger beta ERD activity compared with younger participants (βleft = −0.26, P = 0.015; βright = −0.23, P = 0.040), though the latter did not survive following FDR correction. There was a similar trend with respect to planning-related beta activity in the left precentral gyrus (β = −0.20, P = 0.062). In addition, age was significantly associated with reaction time, indicating that older participants initiated the movement more quickly than their younger peers (β = −0.50, P < 0.001).
Of note, we also tested a simplified model in which all nonsignificant parameters were omitted from the model estimation. Although our conclusions held in the simplified model, fit statistics suggested that removing nonsignificant parameters markedly harmed overall model fit (ΔAIC = 346.26; ΔBIC = 287.28). Thus, the complete model (Figs 5 and 6, Supplemental Table S1) provided a superior structure for explaining variance in motor planning and execution in these data.
Mediating Effects
We examined all potential mediating effects of age, planning-related neural activity, reaction time, and execution-related neural activity on movement duration. Statistical significance of indirect effects was determined using bias-corrected bootstrapped confidence intervals (Efron and Tibshirani 1986; Fritz and MacKinnon, 2007) around all indirect effects using 1000 bootstrapped samples. Note that because of the use of confidence intervals to determine significance, exact p-values are not available for indirect effects. In total, we were able to detect four statistically significant indirect effects at the P < 0.05 level. These significant mediating effects are illustrated in Figure 7.
Figure 7 .
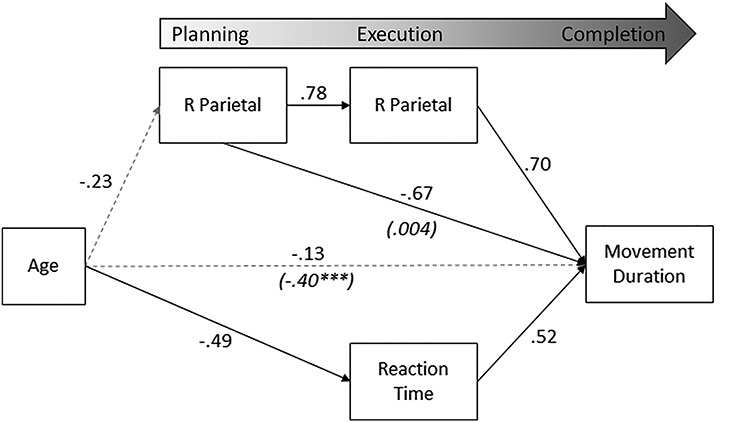
Statistically significant mediating effects. Significant estimates (P < 0.05) are denoted with a solid black line. Note that the total effect of age on movement duration was significant (P < 0.001; shown in parentheses), but this relationship was fully mediated (dashed gray line denoting nonsignificance after taking into account mediating factors). All listed parameters are standardized coefficients.
There was a significant indirect effect of right parietal planning-related activity on movement duration via right parietal execution-related activity (β = 0.54, P < 0.05). Greater beta ERD within the right parietal cortex during planning predicted subsequent greater execution-related beta ERD in the right parietal cortex, which ultimately predicted shorter overall movement duration. The direct effect of right parietal planning-related activity was statistically significant (β = −0.67, P = 0.033), with greater planning-related ERD predicting longer movement duration. The model suggests only a partial mediation via execution-related parietal activity.
The total effect of age on movement duration was also statistically significant (β = −0.40, P < 0.001). However, the direct effect was not significant in the final model (β = −0.14, P = 0.18), suggesting that the relationship was fully mediated. In fact, there were three statistically significant mediating paths. First, there was an overall negative indirect effect of age on movement duration via reaction time (β = −0.25, P < 0.01); older participants were faster to initiate a response, and faster initial responses predicted shorter movement durations. Second, planning-related beta oscillatory activity in the right parietal cortex mediated the effect of age on movement duration (β = 0.16, P < 0.01). Older participants exhibited stronger planning-related beta ERD, which then predicted longer movement durations. Finally, we detected a significant multiple mediation via the path through planning-related right parietal activity to execution-related right parietal activity (β = −0.13, P < 0.01). Older participants had greater planning-related beta ERD in the right parietal cortex, which predicted greater execution-related beta ERD, and shorter subsequent movement durations.
Discussion
In this study, we investigated how the relationship between age and motor performance was mediated by neural dynamics in a large developmental sample of youth aged 9–15 years old. Specifically, we sought to identify (a) how the neural dynamics of the motor system change as a function of age, (b) how these dynamics are altered between motor planning and execution, and (c) which brain areas predict motor performance metrics (i.e., reaction time and movement duration) that change as a function of age. As expected, the degree of beta ERD activity within a given region during the planning period predicted beta ERD activity during execution. Additionally, activity in each region was associated with activity in other regions during the same time period (though less so during execution). Surprisingly, we found no significant relationships between beta oscillatory activity and reaction time, though execution-related parietal and precentral activity showed positive trends. In addition, a unique and interesting relationship between age, right parietal dynamics, and movement duration emerged. We found that the relationship between age and movement duration was fully mediated by beta oscillatory dynamics in right parietal cortex, although planning and execution-related activity showed distinct and opposite effects on movement duration. Below we discuss the implications of these results for our understanding of development in the human motor system.
Within the entire model, we found a number of interesting developmental relationships. Age significantly predicted beta ERD power during the planning period in the parietal cortices but not the primary motor cortices (though the left precentral gyrus did show a trend toward significance). The lack of significant age-related effects on activity in the primary motor cortex is not entirely surprising. For example, Wilson and colleagues found a trending relationship between beta ERD power and age in the right but not left primary motor cortex during right finger movements (Wilson et al. 2010). Gaetz and colleagues showed a significant linear trend in left precentral beta ERD power in 4–6 year-old children, but not 11–13 year-olds (Gaetz et al. 2010). Trevarrow et al. who used an overlapping study population (though an entirely different task), found no relationship between left precentral beta ERD activity and age (Trevarrow et al. 2019). The variability of these findings, with a tendency toward nonsignificance, is likely due to the fact that the primary motor cortices mature relatively early. In fact, some structural MRI research demonstrates maturation of the precentral gyri by 9 years of age (Gogtay et al. 2004; Shaw et al. 2008), but that other regions of the motor network, including the parietal cortices, continue to develop into adolescence. Nonetheless, planning-related activity in the left precentral gyrus not only predicted execution-related activity in the left precentral gyrus, but was also the only region to affect execution-related activity in other regions; namely in the right precentral gyrus, with trending effects on the left parietal cortex. This suggests a downstream effect whereby the left precentral gyrus guides activity throughout the motor network. It was also particularly interesting that there were no unique developmental effects on execution-related beta activity, above and beyond those found in the planning period. Much of this is likely due to the strong relationship between planning and execution-related activity; in other words, there was not much variance left over to be taken into account. It should also be noted that in this particular study sample, other common motor-related regions (e.g., the premotor cortices, supplementary motor area, etc.) were not significantly active. It is possible that these regions are not “tuned” to such tasks at this age, or that this sequencing task does not require these regions to be involved.
Perhaps most importantly, we found that right parietal cortex dynamics uniquely mediated the relationship between age and motor performance. Interestingly, our mediation analyses showed that the developmental changes in planning and execution-related activity had opposite effects on movement duration. Age was positively associated with planning-related beta oscillations, which negatively predicted movement duration, such that greater activity was associated with longer movements. However, there was also a double mediation effect by which age positively predicted planning-related activity, which positively predicted execution-related beta activity, and this pattern positively predicted movement duration; in other words, stronger beta responses were associated with shorter movement duration. While these effects may seem initially counterintuitive, we posit that they emphasize the importance of strong, sustained beta oscillatory activity in the right parietal cortex during complex movement processing. Basically, in line with previous research in adults (Heinrichs-Graham and Wilson 2015), we find that execution-related beta oscillations in the right parietal cortex are crucial for proper complex movement completion. If there is strong planning-related beta activity in the right parietal cortex, but it is not sustained into movement execution, it is detrimental to motor performance. However, if robust activity during the planning period is sustained or increases during execution in this region (i.e., does not decrease), this pattern predicts better motor performance. The regional specificity of these findings align with other studies that have shown the importance of the parietal cortices in motor planning and execution, especially in the context of sequence-related movement and visuomotor transformations (Harrington et al. 2000; Thoenissen et al. 2002; Cui and Andersen 2011; Convento et al. 2014; Battaglia-Mayer et al. 2015; Yokoi and Diedrichsen 2019), and solidify the right parietal cortex as an important motor-network hub. Of note, none of the neural metrics in our model predicted reaction time; however, reaction time mediated the relationship between age and movement duration, such that as youth get older, both reaction time and movement duration become faster. Future studies should attempt to clarify which neural regions best predict reaction time differences throughout development, as this information could be utilized for simpler motor tasks such as single movement protocols.
In sum, we modeled the relationship between age, planning and execution-related neural dynamics within the motor network and task performance in a large developmental cohort of healthy youth. We found that the relationship between age and motor performance was uniquely mediated by neural dynamics within the right parietal cortex. Importantly, our data suggest that stronger beta oscillatory activity in the right parietal cortex during movement execution, especially if preceded by an increase in planning-related activity, is crucial to successful motor performance, and these dynamics are developing throughout childhood and adolescence. Future studies should extend this investigation into late adolescence and adulthood to identify when these developmental changes peak. Additionally, future work could utilize neuromodulatory techniques to probe whether execution-related parietal activity can be optimized to maximize motor performance. Finally, given the data-driven approach employed here, other studies should attempt to reproduce these findings in a larger sample and/or age range. Future studies should also determine the impact of other oscillatory responses (e.g., alpha ERD, PMBR, and gamma ERS), as well as the interaction between these neural responses, on complex movement metrics.
Supplementary Material
Acknowledgements
The authors thank the participants for volunteering. Any inquiries can be sent to the corresponding author at heinrichs.graham@gmail.com or to: Elizabeth Heinrichs-Graham, Cognitive Neuroscience of Development and Aging (CoNDA) Center, University of Nebraska Medical Center, 985505 Nebraska Medical Center, Omaha, NE 68198-5505.
Funding
This work was supported by the National Science Foundation (#1539067 to Y-PW, JMS, VDC, and TWW), the National Institutes of Health (U54-GM115458 to EHG, R01-MH121101 to TWW, R01-MH116782 to TWW, and P20-GM130447 to TWW and EHG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Alegre M, Alvarez-Gerriko I, Valencia M, Iriarte J, Artieda J. 2008. Oscillatory changes related to the forced termination of a movement. Clin Neurophysiol. 119(2):290–300. [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Ferrari-Toniolo S, Visco-Comandini F. 2015. Timing and communication of parietal cortex for visuomotor control. Curr Opin Neurobiol. 33:103–109. [DOI] [PubMed] [Google Scholar]
- Cassim F, Monaca C, Szurhaj W, Bourriez JL, Defebvre L, Derambure P, Guieu JD. 2001. Does post-movement beta synchronization reflect an idling motor cortex? Neuroreport. 12(17):3859–3863. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Bakhtazad L, Gaetz W. 2006. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach. Hum Brain Mapp. 27(3):213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D, Bells S, Ferrari P, Gaetz W, Bostan AC. 2008. Self-paced movements induce high-frequency gamma oscillations in primary motor cortex. Neuroimage. 42(1):332–342. [DOI] [PubMed] [Google Scholar]
- Convento S, Bolognini N, Fusaro M, Lollo F, Vallar G. 2014. Neuromodulation of parietal and motor activity affects motor planning and execution. Cortex. 57:51–59. [DOI] [PubMed] [Google Scholar]
- Cui H, Andersen RA. 2011. Different representations of potential and selected motor plans by distinct parietal areas. J Neurosci. 31(49):18130–18136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LM, Yarrow K, Brown P. 2005. Lateralization of event-related beta desynchronization in the EEG during pre-cued reaction time tasks. Clin Neurophysiol. 116(8):1879–1888. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. 1986. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat. Sci. 1:54–75. [Google Scholar]
- Engel AK, Fries P. 2010. Beta-band oscillations--signalling the status quo? Curr Opin Neurobiol. 20(2):156–165. [DOI] [PubMed] [Google Scholar]
- Ernst MD. 2004. Permutation methods: a basis for exact inference. Stat Sci. 19:676–685. [Google Scholar]
- Fritz MS, MacKinnon DP. 2007. Required sample size to detect the mediated effect. Psychol Sci. 18:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry A, Mullinger KJ, O'Neill GC, Barratt EL, Morris PG, Bauer M, Folland JP, Brookes MJ. 2016. Modulation of post-movement beta rebound by contraction force and rate of force development. Hum Brain Mapp. 37(7):2493–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Edgar JC, Wang DJ, Roberts TP. 2011. Relating MEG measured motor cortical oscillations to resting gamma-aminobutyric acid (GABA) concentration. Neuroimage. 55(2):616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Macdonald M, Cheyne D, Snead OC. 2010. Neuromagnetic imaging of movement-related cortical oscillations in children and adults: age predicts post-movement beta rebound. Neuroimage. 51(2):792–807. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, et al. 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 101(21):8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grent-'t-Jong T, Oostenveld R, Jensen O, Medendorp WP, Praamstra P. 2014. Competitive interactions in sensorimotor cortex: oscillations express separation between alternative movement targets. J Neurophysiol. 112(2):224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. 2001. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci U S A. 98(2):694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Pollok B, Dirks M, Timmermann L, Butz M, Schnitzler A. 2005. Task-dependent oscillations during unimanual and bimanual movements in the human primary motor cortex and SMA studied with magnetoencephalography. Neuroimage. 26(1):91–98. [DOI] [PubMed] [Google Scholar]
- Hall SD, Stanford IM, Yamawaki N, McAllister CJ, Ronnqvist KC, Woodhall GL, Furlong PL. 2011. The role of GABAergic modulation in motor function related neuronal network activity. Neuroimage. 56(3):1506–1510. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Rao SM, Haaland KY, Bobholz JA, Mayer AR, Binderx JR, Cox RW. 2000. Specialized neural systems underlying representations of sequential movements. J Cogn Neurosci. 12(1):56–77. [DOI] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Arpin DJ, Wilson TW. 2016. Cue-related temporal factors modulate movement-related Beta oscillatory activity in the human motor circuit. J Cogn Neurosci. 28(7):1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Hoburg JM, Wilson TW. 2018a. The peak frequency of motor-related gamma oscillations is modulated by response competition. Neuroimage. 165:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Kurz MJ, Gehringer JL, Wilson TW. 2017. The functional role of post-movement beta oscillations in movement termination. Brain Struct Funct. 222(7):3075–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, McDermott TJ, Mills MS, Wiesman AI, Wang YP, Stephen JM, Calhoun VD, Wilson TW. 2018b. The lifespan trajectory of neural oscillatory activity in the motor system. Dev Cogn Neurosci. 30:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW. 2015. Coding complexity in the human motor circuit. Hum Brain Mapp. 36(12):5155–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW. 2016. Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. Neuroimage. 134:514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW, Santamaria PM, Heithoff SK, Torres-Russotto D, Hutter-Saunders JA, Estes KA, Meza JL, Mosley RL, Gendelman HE. 2014. Neuromagnetic evidence of abnormal movement-related beta desynchronization in Parkinson's disease. Cereb Cortex. 24(10):2669–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR. 2005. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp. 25(2):199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Goel P, Kopell N, Pohja M, Hari R, Ermentrout B. 2005. On the human sensorimotor-cortex beta rhythm: sources and modeling. Neuroimage. 26(2):347–355. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D. 2006. Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. Neuroimage. 32(3):1281–1289. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Birbaumer N, Lutzenberger W. 2001. Event-related beta desynchronization indicates timing of response selection in a delayed-response paradigm in humans. Neurosci Lett. 312(3):149–152. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Schutz-Bosbach S. 2019. Proactive control without midfrontal control signals? The role of midfrontal oscillations in preparatory conflict adjustments. Biol Psychol. 148:107747. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Ulrich R, Lutzenberger W. 2003. Dynamics of sensorimotor cortex activation to spatial sounds precueing ipsi- versus contralateral manual responses. Brain Res Cogn Brain Res. 17(3):573–583. [DOI] [PubMed] [Google Scholar]
- Kovach CK, Gander PE. 2016. The demodulated band transform. J Neurosci Methods. 261:135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Becker KM, Heinrichs-Graham E, Wilson TW. 2014. Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Dev Med Child Neurol. 56(11):1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Proskovec AL, Gehringer JE, Becker KM, Arpin DJ, Heinrichs-Graham E, Wilson TW. 2016. Developmental trajectory of Beta cortical oscillatory activity during a knee motor task. Brain Topogr. 29(6):824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebrand M, Pein I, Tzvi E, Kramer UM. 2017. Temporal dynamics of proactive and reactive motor inhibition. Front Hum Neurosci. 11:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimon G, Assad JA. 2006. A cognitive signal for the proactive timing of action in macaque LIP. Nat Neurosci. 9(7):948–955. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. 2007. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 164(1):177–190. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD. 2010. Functional properties of human primary motor cortex gamma oscillations. J Neurophysiol. 104(5):2873–2885. [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P. 1977. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum. 13:135–145. [PubMed] [Google Scholar]
- Park H, Kim JS, Chung CK. 2013. Differential beta-band event-related desynchronization during categorical action sequence planning. PLoS One. 8(3):e59544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. 1999. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 110(11):1842–1857. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Solis-Escalante T. 2009. Could the beta rebound in the EEG be suitable to realize a brain switch? Clin Neurophysiol. 120(1):24–29. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A Jr, Neuper C. 1996. Post-movement beta synchronization. A correlate of an idling motor area? Electroencephalogr Clin Neurophysiol. 98(4):281–293. [DOI] [PubMed] [Google Scholar]
- Pogosyan A, Gaynor LD, Eusebio A, Brown P. 2009. Boosting cortical activity at Beta-band frequencies slows movement in humans. Curr Biol. 19(19):1637–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praamstra P, Kourtis D, Nazarpour K. 2009. Simultaneous preparation of multiple potential movements: opposing effects of spatial proximity mediated by premotor and parietal cortex. J Neurophysiol. 102(4):2084–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyns N, Houdayer E, Bourriez JL, Blond S, Derambure P. 2008. Post-movement beta synchronization in subjects presenting with sensory deafferentation. Clin Neurophysiol. 119(6):1335–1345. [DOI] [PubMed] [Google Scholar]
- Rossiter HE, Davis EM, Clark EV, Boudrias MH, Ward NS. 2014. Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. Neuroimage. 91:360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AD, Saxena N, L EJ, Hall JE, Singh KD, Muthukumaraswamy SD. 2015. Ketamine amplifies induced gamma frequency oscillations in the human cerebral cortex. Eur Neuropsychopharmacol. 25(8):1136–1146. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, et al. 2008. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 28(14):3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, Prosksovec AL, Heinrichs-Graham E, Wilson TW. 2020. Prefrontal theta modulates sensorimotor gamma networks during the reorienting of attention. Hum Brain Mapp. 41(2):520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Wade C, Brown P. 2016. Post-movement Beta activity in sensorimotor cortex indexes confidence in the estimations from internal models. J Neurosci. 36(5):1516–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Simola J. 2006. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol. 51(7):1759–1768. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M. 2005. Applications of the signal space separation method (SSS). IEEE Trans Signal Process. 53(9):3359–3372. [Google Scholar]
- Thoenissen D, Zilles K, Toni I. 2002. Differential involvement of parietal and precentral regions in movement preparation and motor intention. J Neurosci. 22(20):9024–9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevarrow MP, Kurz MJ, McDermott TJ, Wiesman AI, Mills MS, Wang YP, Calhoun VD, Stephen JM, Wilson TW. 2019. The developmental trajectory of sensorimotor cortical oscillations. Neuroimage. 184:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G. 2010. Beta-band activity during motor planning reflects response uncertainty. J Neurosci. 30(34):11270–11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ. 1997. Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput. 35(2):135–140. [DOI] [PubMed] [Google Scholar]
- Wiesman AI, Wilson TW. 2020. Attention modulates the gating of primary somatosensory oscillations. Neuroimage. 211:116610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Becker KM. 2014. Circadian modulation of motor-related beta oscillatory responses. Neuroimage. 102(2):531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Reite ML, Teale PD, Rojas DC. 2010. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn. 73(2):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Teale PD, Reite ML, Rojas DC. 2011. Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Dev Neuropsychol. 36(5):596–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi A, Diedrichsen J. 2019. Neural Organization of Hierarchical Motor Sequence Representations in the human neocortex. Neuron. 103(6):1178–1190 e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


