Abstract
Introduction
Autoantibody to angiotensin II type 1 receptor (AT1R-Ab) has been recognized as a non-human leukocyte antigen (HLA) antibody relevant in transplantation. Endothelin type A receptor antibody (ETAR-Ab) has been strongly associated with AT1R-Ab, but the data in kidney transplantation are scarce.
Methods
We examined the relationship of ETAR-Ab and AT1R-Ab with clinical outcomes, biopsy findings, inflammatory cytokines, and HLA donor-specific antibody (DSA) in a cohort of pediatric renal transplant recipients. Sixty-five patients were longitudinally monitored for ETAR-Ab, AT1R-Ab, HLA DSA, interleukin (IL)-8, tumor necrosis factor-α, IL-1β, interferon-γ, IL-17, IL-6, renal dysfunction, hypertension, rejection, and allograft loss during the first 2 years post-transplant.
Results
Fifteen patients (23%) had AT1R-Ab alone, 1 (2%) had ETAR-Ab alone, 23 (35%) had both ETAR-Ab and AT1R-Ab, and 26 (40%) were negative for both antibodies at all timepoints. Having both ETAR-Ab and AT1R-Ab was associated with >30% decline in estimated glomerular filtration rate (P = 0.024), arteritis (P = 0.016), and elevated IL-8 levels (P = 0.010), but not rejection, HLA DSA, or allograft loss. Having both antibodies resulted in greater increases in IL-8 compared with AT1R-Ab alone, even when controlled for additional clinical factors, including HLA DSA (P = 0.012).
Conclusion
Our study demonstrates that, in pediatric kidney transplantation, ETAR-Ab is highly associated with AT1R-Ab, but there are a subset of patients with AT1R-Ab alone. Having both antibodies is significantly associated with arteritis, elevated IL-8, and decline in renal function, and our results suggest possible interaction effects. Better understanding of this interaction may be informative in developing protocols for testing, treatment, and prevention of allograft injury.
Keywords: angiotensin II type 1 receptor antibody, cytokine, endothelin type A receptor antibody, human leukocyte antigen donor-specific antibody, pediatric nephrology, transplantation
Graphical abstract
Antibodies against human leukocyte antigens (HLAs) are known to mediate allograft injury, leading to poor clinical outcomes in renal transplantation. Recently, autoantibodies to multiple non-HLA1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 targets are becoming recognized for their role in antibody-mediated renal allograft injury. The activating autoantibody to angiotensin II type 1 receptor (AT1R-Ab)7,8,15, 16, 17, 18, 19, 20 is among the most well described of these non-HLA antibodies in transplantation. Autoantibody to the closely related G protein‒coupled receptor, endothelin type A receptor (ETAR)21,22 has been strongly associated with AT1R-Ab in other disease states, such as systemic sclerosis and preeclampsia.23, 24, 25, 26 The pathophysiology of AT1R-Ab has been linked to increased endothelin-1 and activation of ETAR27,28 and, importantly, clinical disease severity in both systemic sclerosis and preeclampsia has been correlated with the activation of both pathways.25,26 The strong association of AT1R-Ab and ETAR-Ab has also been described in solid-organ transplantation29, 30, 31, 32; however, the data in kidney transplantation19,20 are scarce and limited to adult studies.
Our group33 and others34, 35, 36 recently reported a higher prevalence of AT1R-Ab in pediatric versus adult kidney transplant recipients, highlighting potentially important differences between these 2 populations with respect to the development of non-HLA antibodies. In particular, pediatric kidney transplant patients appear to have a higher rate of de novo AT1R-Ab formation.33 Importantly, AT1R-Ab has been associated with vascular inflammation, decline in estimated glomerular filtration rate (eGFR), and allograft loss33,34 in this vulnerable population. AT1R-Ab has also been associated with elevations in tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin (IL)-8, IL-1β, IL-6, and IL-17 in pediatric kidney transplant recipients,33,37 further supporting an association between vascular inflammation and autoantibody formation.
A better understanding of how autoimmunity to other G protein‒coupled receptors, such as ETAR, may interact with AT1R-Ab, alloimmunity, and inflammatory cytokines is important to understanding the overall impact of these autoantibodies. Moreover, the relationship between non-HLA and HLA DSA is still not well understood. Some studies have supported an interplay between antibodies to G protein‒coupled receptors and HLA DSA in kidney transplantation with the development of non-HLA autoantibodies predisposing recipients to HLA sensitization and together causing worsening allograft dysfunction.8,17,20,34 By contrast, other studies have shown HLA and non-HLA antibodies to have independent effects on renal allografts.18,33 Furthermore, how this interaction may function in the context of a developing immune system has yet to be elucidated. The availability of pharmacologic agents to block activity at these receptors has important therapeutic implications. Therefore, we sought to describe the relationship between ETAR-Ab and AT1R-Ab in pediatric kidney transplant recipients in the first 2 years post-transplant, and the association of these antibodies with clinical outcomes. In addition, we aimed to understand the association of these antibodies with HLA DSA and inflammatory cytokines.
Methods
Patients, Samples, and Study Design
In this retrospective study, 65 pediatric kidney transplant patients were monitored for 2 years posttransplant. From August 2005 to November 2014, 83 patients were enrolled in the UCLA Pediatric Kidney Transplant Immune Monitoring Study, and 18 patients were excluded from analysis secondary to missing more than 1 study sample at the timepoints indicated. This study was approved by the institutional review board of UCLA (#11-002375) and conforms with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards and the Principles of the Declaration of Istanbul. Informed consent and when appropriate patient assent was obtained for all patients. Blood samples were obtained per protocol pretransplant and at 6, 12, and 24 months post-transplant and for indication during episodes of kidney transplant rejection. In longitudinal analyses, blood samples were grouped by periods within 3 months of the timepoint. Demographic and clinical data, including age, race, ethnicity (Hispanic or Latino vs not Hispanic or Latino), etiology of end-stage renal disease, transplant type (deceased/living donor), sensitization history, time on dialysis, delayed graft function (defined as dialysis in the first week post-transplant), immunosuppression regimen, cytomegalovirus, Epstein-Barr virus, and BK virus levels, and blood pressure were collected. Patients were monitored for rejection, allograft loss, and decrease in eGFR as determined by the updated Schwartz equation38 in patients <18 years old at the time of transplant and by the abbreviated Modification of Diet in Renal Disease equation39 in patients ≥18 years of age at the time of transplant. Hypertension was determined by age, sex, and height percentile40 for children <18 years old and based on American Society of Hypertension and the International Society of Hypertension guidelines for patients ≥18 years old.41 Study data were collected and managed using secure REDCap (Research Electronic Data Capture) electronic data capture tools hosted at UCLA.42 Of the 65 patients, 54 had complete 2-year follow-up, 7 patients had graft loss, and 4 patients transferred care to a different institution. No patients died during the study period. A total of 9.1 patient-years of follow-up were analyzed. In addition to analyzing patient-level outcomes, a separate analysis of the 262 blood samples yielded from the 65 patients was undertaken. This sample level analysis allowed for a time-sensitive characterization of the association between AT1R-Ab, ETAR-Ab, and cytokine levels. One patient and 2 blood samples from that patient were positive for ETAR-Ab alone, and were therefore excluded from analyses that compared AT1R-Ab positive only, both AT1R-Ab and ETAR-Ab positive, and antibody-negative patients/samples.
Clinical Protocols and Biopsy Evaluation
UCLA immunosuppressive regimen for pediatric transplant recipients included induction with either antithymocyte globulin for panel-reactive antibodies ≥30%, delayed graft function, or rapid-steroid withdrawal protocol or anti-CD25 monoclonal antibody for those with panel reactive antibodies <30%. Maintenance immunosuppression consisted of steroid-free or steroid-based immunosuppression, a calcineurin inhibitor, and an antimetabolite. Acute and chronic rejection were treated with previously described protocols.43
Patients underwent protocol biopsies at 6, 12, and 24 months post-transplantation or for clinical indication. Biopsies were evaluated based on the 2013 Banff criteria.44 Three patients were not included in the biopsy score data as they had missing values.
Antibody and Cytokine Testing
HLA typing of recipient and donor was performed using molecular methods, as described elsewhere.43 HLA antibodies were detected using a Luminex single-antigen bead assay (Immucor, Stamford, CT) and quantified by mean fluorescence intensity. Antibodies were considered positive when mean fluorescence intensity was ≥1000 for HLA-A, -B, -DR, and -DQ, and ≥2000 for HLA-C and -DP.45 AT1R-Ab and ETAR-Ab were measured by enzyme-linked immunosorbent‒based assay (One Lambda, Canoga Park, CA). Sera were diluted 1:100, tested in duplicate, and AT1R-Ab and ETAR-Ab concentrations were determined by a standard curve. AT1R-Ab IgG >17 U/ml and ETAR-Ab >10 U/ml was considered positive based on other previous studies,30 recommendations from the test manufacturer, and our receiver operating curve analysis. Cytokines were selected based on a literature review of cytokines that have been associated with activation of the AT1R and ETAR.46, 47, 48, 49, 50, 51 A custom magnetic bead kit including IL-8, TNF-α, IL-1β, IFN-γ, IL-17, and IL-6 (EMD Millipore, Darmstadt, Germany) was used per manufacturer's instructions. Fluorescence was quantified using a Luminex 200TM instrument (Thermo Fisher Scientific, Waltham, MA). For the patient-level analysis of cytokines, the median of the post-transplant values of each cytokine across samples was used as representative. Before regression analysis, cytokines were transformed using the log(x + 1) transformation.
Statistical Methods
ETAR-Ab >10 U/ml was considered positive based on other previous studies30 and recommendations from the test manufacturer. We evaluated further by completing a receiver operating curve analysis comparing this cutoff to the optimal threshold as determined by maximizing the Youden index,52 and confirmed that the cutoff of 10 U/ml was within −0.12 on average of the best threshold for all outcomes. AT1R-Ab level >17 U/ml was obtained by a similar process, as described elsewhere.33 Categorical variables were compared between groups using Fisher’s exact test. Continuous variables were compared between groups using either the Kruskal-Wallis test or 1-way analysis of variance, based on the distribution of the data. Correlations between blood sample AT1R-Ab and ETAR-Ab levels and antibody status and cytokine levels were assessed by mixed-effects models, including patient-level random effects to account for repeated measurements. Timing of antibody development was assessed by 1-sample t test. Logistic and linear regression models were used to evaluate the effect of AT1R-Ab and ETAR-Ab positivity on eGFR decline and median patient IL-8 levels, respectively. Odds ratios and regression coefficients are reported along with 95% confidence intervals. Firth’s penalized maximum method was used to stabilize the estimates in the logistic regression model. Analyses were performed using R version 3.6.2 (http://www.r-project.org). P < 0.05 was considered statistically significant and all tests were 2-sided.
Results
Prevalence, Natural History, and Clinical Characteristics
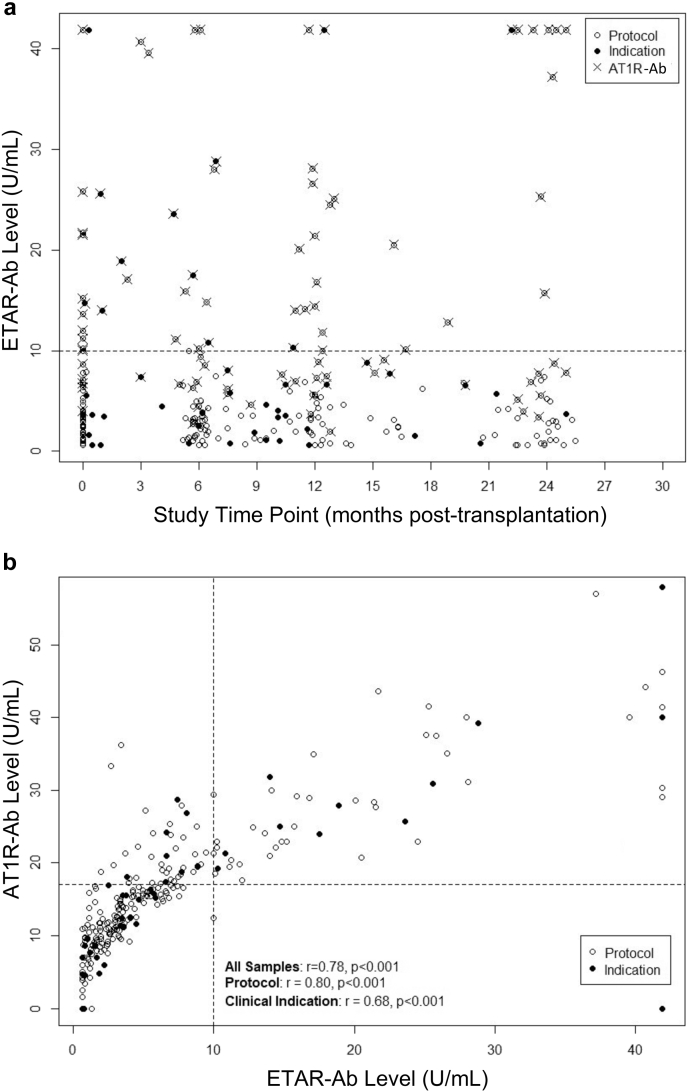
In our population of 65 pediatric renal transplant recipients, 15 (23%) had AT1R-Ab alone, 1 (2%) had ETAR-Ab alone, and 23 (35%) had both ETAR-Ab and AT1R-Ab during at least 1 timepoint during the study period. Twenty-six (40%) patients were negative for both antibodies at all timepoints. All patients (with 1 exception, 96%) positive for ETAR-Ab were also positive for AT1R-Ab. Of the 262 blood samples analyzed from the aforementioned patients, 56 (22%) were positive for ETAR-Ab (Figure 1a). All blood samples (except 2 samples from the same patient) positive for ETAR-Ab were also positive for AT1R-Ab. Notably, 44 blood samples (17%) were positive for AT1R-Ab alone. ETAR-Ab and AT1R-Ab levels were highly correlated (r = 0.78, P < 0.001; Figure 1b). These relationships were observed across timepoints and in both samples associated with biopsies done for clinical indication and protocol samples (Figure 1a and b). Of the 23 patients with both ETAR-Ab and AT1R-Ab, 21 (88%) were positive for ETAR-Ab on a post-transplant sample. Of these 21 patients, 8 (38%) were also positive for ETAR-Ab pretransplant, 9 (43%) were negative pretransplant, and 4 (19%) did not have a pretransplant sample available for analysis. Of the 53 patients with pretransplant samples available, 10 (18.9%) were positive for ETAR-Ab.
Figure 1.
Characteristics of blood samples positive for ETAR-Ab. Two hundred sixty-two blood samples from 65 patients are shown. Sample timing (protocol timepoint vs time of clinically indicated biopsy) is presented. (a) Distribution of ETAR-Ab levels by time post-transplant. The distribution of ETAR-Ab levels over time is shown. The distribution of ETAR-Ab levels in the population remained relatively stable over time. AT1R-Ab positivity (defined as >17 units/ml by ELISA) in individual samples is presented. All (except 2) samples positive for ETAR-Ab, were also AT1R-Ab positive. However, it is of note that there are samples positive for AT1R-Ab but negative for ETAR-Ab. (b) Correlation between blood sample ETAR-Ab and AT1R-Ab levels. ETAR-Ab was highly correlated with AT1R-Ab. This remained true in stratified analyses by protocol versus clinical indication sample. AT1R-Ab, autoantibody to angiotensin II type 1 receptor; ELISA, enzyme-linked immunoassay; ETAR-Ab, endothelin type A receptor antibody.
In all patients with ETAR-Ab and AT1R-Ab, the antibodies appeared simultaneously, or AT1R-Ab developed, on average, 2.8 months earlier (P = 0.003; Table 1). Further analyses were conducted based on the following groups: both ETAR-Ab and AT1R-Ab negative; AT1R-Ab positive only; and both ETAR-Ab and AT1R-Ab positive. One patient with ETAR-Ab alone on 2 blood samples with subsequent blood samples negative for both ETAR-Ab and AT1R-Ab was excluded from further analyses as an outlier. This allowed us to examine risk factors associated with ETAR-Ab while also addressing the potential impact of concurrent AT1R-Ab. We found no demographic or clinical characteristics that were significantly associated with the development of both ETAR-Ab and AT1R-Ab (Table 1). Interestingly, there was a trend toward an association between non-Hispanic ethnicity, antithymocyte globulin induction, and steroid-free immunosuppression at discharge and the development of both ETAR-Ab and AT1R-Ab (Table 1).
Table 1.
Baseline characteristics of population by ETAR-Ab and AT1R-Ab status
| Variable | Negative (n = 26) | AT1R-Ab positive only (n = 15) | ETAR-Ab and AT1R-Ab positive (n = 23) | P value |
|---|---|---|---|---|
| Time (months) from AT1R-Ab to ETAR-Ab, mean (SD) | --- | --- | 2.8 (4.3) | 0.003a |
| Age (years), mean (SD) | 14.7 (4.9) | 16.1 (3.3) | 13.2 (4.2) | 0.12 |
| Sex (% male) | 15 (57.7%) | 6 (40%) | 17 (73.9%) | 0.12 |
| Recipient race, n (% white) | 18 (69.2%) | 8 (53.3%) | 20 (87%) | 0.07 |
| Recipient ethnicity, n (% Hispanic) | 17 (65.4%) | 9 (60%) | 9 (39.1%) | 0.18 |
| Donor type, n (% deceased) | 14 (53.8%) | 13 (86.7%) | 13 (56.5%) | 0.09 |
| Etiology of ESRD, n (%) | 0.59 | |||
| Dysplasia | 4 (15.4%) | 3 (20%) | 2 (8.7%) | |
| FSGS | 2 (7.7%) | 3 (20%) | 3 (13%) | |
| Other glomerulonephritis | 3 (15.3%) | 4 (26.7%) | 1 (4.3%) | |
| Obstructive uropathy | 7 (26.9%) | 2 (13.3%) | 7 (30.4%) | |
| Polycystic kidney disease | 0 (0%) | 1 (6.7%) | 1 (4.3%) | |
| Unknown | 4 (15.4%) | 1 (6.7%) | 5 (21.7%) | |
| Other | 5 (19.2%) | 1 (6.7%) | 4 (17.4%) | |
| Time on dialysis, median (IQR) | 1.9 (1.1‒2.7) | 2 (0.8‒3.2) | 2.5 (1‒2.8) | 0.80 |
| Primary transplant, n (%) | 25 (96.2%) | 13 (86.7%) | 20 (87%) | 0.49 |
| HLA mean mismatch, mean (SD) | 1.2 (0.4) | 1.4 (0.4) | 1.1 (0.7) | 0.46 |
| Baseline PRA class I >20%, n (%) | 1 (3.8%) | 1 (6.7%) | 2 (8.7%) | 0.82 |
| Baseline PRA class II >20%, n (%) | 1 (3.8%) | 4 (26.7%) | 2 (8.7%) | 0.09 |
| EBV serostatus, n (% positive) | 19 (73.1%) | 12 (80%) | 16 (69.6%) | 0.88 |
| CMV serostatus, n (% positive) | 14 (53.8%) | 12 (80%) | 12 (52.2%) | 0.19 |
| Induction ATG (vs. IL-2 inhibitor), n (%) | 0 (0%) | 2 (13.3%) | 4 (17.4%) | 0.07 |
| Steroid-free at hospital discharge, n (%) | 12 (46.2%) | 5 (33.3%) | 16 (69.6%) | 0.07 |
| Delayed graft function, n (%) | 1 (3.8%) | 1 (6.7%) | 2 (8.7%) | 0.82 |
AT1R-Ab, autoantibody to angiotensin II type 1 receptor; ATG, antithymocyte globulin; CMV, cytomegalovirus; EBV, Epstein-Barr virus; ESRD, end-stage renal disease; ETAR-Ab, endothelin type A receptor antibody; FSGS, focal segmental glomerulosclerosis; HLA, human leukocyte antigen; IL, interleukin; IQR, interquartile range; PRA, panel-reactive antibody; SD, standard deviation.
P values represent 3-group comparisons unless otherwise indicated. Fisher’s exact test was used for categorical variables. For continuous variables (age and HLA mismatch), a 1-way analysis of variance was used. One patient with ETAR-Ab alone was excluded from analysis.
P value obtained by 1-sample t test by assessing the probability that mean time from development of AT1R-Ab to ETAR-Ab was >0 month.
Clinical Outcomes
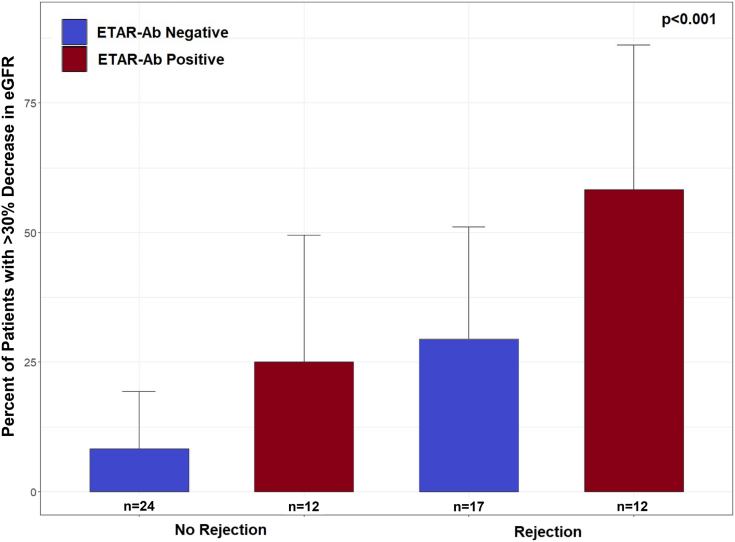
ETAR-Ab and AT1R-Ab were not associated with rejection, proteinuria, hypertension, or allograft loss (Table 2). Notably, we found no association between the development of ETAR-Ab and AT1R-Ab and HLA DSA (Table 2). More severe T-cell‒mediated rejection was more common in the group that was ETAR-Ab and AT1R-Ab positive (Table 2), but this relationship was not statistically significant. Interestingly, ETAR-Ab and AT1R-Ab were associated with a >30% decline in eGFR over the follow-up period (P = 0.024; Table 2). To examine the impact of rejection on this association, we compared patients with ETAR-Ab (all with concurrent AT1R-Ab) versus patients without ETAR-Ab (including both patients with AT1R-Ab alone and negative for both) by rejection status. We found that declines in eGFR were worse for patients with ETAR-Ab both with and without rejection (Figure 2). In an analysis of all 3 antibody groups (ETAR-Ab and AT1R-Ab negative, AT1R-Ab positive only, ETAR-Ab and AT1R-Ab positive) further subdivided by rejection status, a similar trend was observed (P = 0.13, data not shown). To further investigate the relationship between having both ETAR-Ab and AT1R-Ab and decline in eGFR, and to distinguish it from the impact of AT1R-Ab alone, a logistic regression analysis was performed (Table 3). In this analysis, having both ETAR-Ab and AT1R-Ab was significantly associated with a >30% decrease in eGFR. AT1R-Ab alone had borderline significance. These relationships remained true when controlled for age, sex, presence of HLA DSA, rejection, and HLA mismatch (Table 3). Younger age and rejection were also associated with a >30% decline in eGFR in this model (P = 0.021 and P = 0.029, respectively). Interaction effects between HLA DSA and non-HLA antibody status were evaluated and were not significant. Patients with AT1R-Ab alone or both AT1R-Ab and ETAR-Ab also had higher median number of treatment days and more plasmapheresis than patients negative for both antibodies (see Supplementary Table S1).
Table 2.
Clinical outcomes by ETAR-Ab and AT1R-Ab status
| Outcome | Negative (n = 26) | AT1R-Ab positive only (n = 15) | ETAR-Ab and AT1R-Ab positive (n = 23) | P value |
|---|---|---|---|---|
| Any rejection, n (%) | 10 (38.5%) | 7 (46.7%) | 11 (47.8%) | 0.80 |
| Rejection type, n (%) | 0.28 | |||
| Borderline | 6 (60%) | 3 (42.9%) | 3 (27.3%) | |
| TCMR > borderline | 2 (20%) | 2 (28.6%) | 7 (63.6%) | |
| AMR | 1 (10%) | 0 (0%) | 0 (0%) | |
| Mixed (AMR and TCMR) | 1 (10%) | 2 (28.6%) | 1 (9.1%) | |
| Biopsies for cause, median (IQR) | 0 (0‒33.3) | 33.3 (0‒62.5) | 41.6 (0‒66.7) | 0.18 |
| Development of HLA DSA, n (%) | 0.83 | |||
| None | 18 (69.2%) | 11 (73.3%) | 16 (69.6%) | |
| Class I only | 2 (7.7%) | 0 (0%) | 3 (13%) | |
| Class II only | 5 (19.2%) | 3 (20%) | 4 (17.4%) | |
| Class I and II | 1 (3.8%) | 1 (6.7%) | 0 (0%) | |
| EBV, CMV, or BK viremia ever, n (%) | 11 (45.8%) | 9 (69.2%) | 9 (42.9%) | 0.35 |
| Proteinuria, n (%) | 18 (69%) | 11 (73%) | 14 (61%) | 0.70 |
| Hypertension, n (%) | 4 (15.4%) | 3 (20.0%) | 9 (39.1%) | 0.14 |
| >30% decrease in eGFR, n (%) | 2 (7.7%) | 5 (33.3%) | 9 (39.1%) | 0.024 |
| IL-8 level,a median (IQR) | 7.5 (4.5‒18.4) | 12.6 (4.8‒17.8) | 24.4 (9.2‒40.8) | 0.010 |
| Allograft loss, n (%) | 0 | 3 (20.0%) | 4 (17.4%) | 0.07 |
AMR, antibody-mediated rejection; AT1R-Ab, autoantibody to angiotensin II type 1 receptor; CMV, cytomegalovirus; DSA, donor-specific antibody; EBV, Epstein-Barr virus; eGFR, estimated glomerular filtration rate; ETAR-Ab, endothelin type A receptor antibody; HLA, human leukocyte antigen; IL, interleukin; IQR, interquartile range; TCMR, T-cell‒mediated rejection.
P values represent 3-group comparisons. Fisher’s exact test was used for categorical variables. For IL-8 level, a Kruskal-Wallis test was used. One patient with ETAR-Ab alone was excluded from the analysis.
Median IL-8 level for each patient was used as representative.
Figure 2.
Renal function outcome by ETAR-Ab and rejection status. In this analysis, patients with ETAR-Ab and AT1R-Ab are labeled as ETAR-Ab positive and patients with neither antibody or AT1R-Ab alone are labeled ETAR-Ab negative. A higher percentage of patients with ETAR-Ab had a >30% decrease in eGFR over the follow-up period (measured from hospital discharge to last follow-up). This was true in patients both with and without rejection. The 1 ETAR-Ab‒positive, AT1R-Ab‒negative patient excluded in other analyses is included in this analysis (n = 65). AT1R-Ab, autoantibody to angiotensin II type 1 receptor; eGFR, estimated glomerular filtration rate; ETAR-Ab, endothelin type A receptor antibody.
Table 3.
Association of decline in eGFR and IL-8 levels with antibody status
| Outcomes and predictors | OR (95% CI) | P value | Adjusted ORa (95% CI) | P value |
|---|---|---|---|---|
| >30% Decline in eGFR | ||||
| AT1R-Ab alone | 6.00 (0.99‒36.23) | 0.051 | 6.79 (0.96‒48.33) | 0.056 |
| ETAR-Ab and AT1R-Ab | 7.71 (1.46‒40.90) | 0.016 | 6.49 (1.17‒36.00) | 0.032 |
| Age (+1 year) | 0.81 (0.67‒0.97) | 0.021 | ||
| Rejection | 4.73 (1.17‒19.11) | 0.029 | ||
| Median IL-8 levelb | Estimate (95% CI) | Adjusted estimatec (95% CI) | ||
| AT1R-Ab alone | 0.24 (−0.43 to 0.92) | 0.48 | 0.13 (−0.63 to 0.89) | 0.74 |
| ETAR-Ab and AT1R-Ab | 0.98 (0.38‒1.57) | 0.002 | 0.83 (0.21‒1.45) | 0.012 |
AT1R-Ab, autoantibody to angiotensin II type 1 receptor; CI, confidence interval; CMV, cytomegalovirus; ETAR-Ab, endothelin type A receptor antibody; eGFR, estimated glomerular filtration rate; HLA, human leukocyte antigen; IL, interleukin; OR, odds ratio.
The number of patients in each antibody group, the number of events with >30% decline in eGFR, and median IL-8 levels in each antibody group are shown in Table 2. Of note, interaction effects between HLA DSA and non-HLA antibody status were tested with respect to each outcome and were not significant.
Model adjusted for age, sex, presence of HLA DSA, rejection, and HLA mismatch. Variables with significant associations with the outcome are shown.
Median IL-8 level for each patient assessed across all blood samples.
Model adjusted for age, sex, living versus deceased donor, presence of HLA DSA, rejection, HLA mismatch, and viremia (CMV, BK, or EBV). No other variables had significant associations with the outcome.
Vascular Inflammation
Having both ETAR-Ab and AT1R-Ab was associated with arteritis on biopsy (P = 0.016; Supplementary Table S2). There was no association between ETAR-Ab and AT1R-Ab and other acute or chronic vascular or tubulointerstitial findings (Supplementary Table S2). We also analyzed a panel of cytokines in the blood associated with vascular inflammation.46, 47, 48, 49, 50, 51 Differences between the antibody groups evaluated by Kruskal-Wallis test were noted for IL-8 in the patient-level analysis (P = 0.010, n = 64; Table 2 and Supplementary Table S3), but not for TNF-α, IFN-γ, IL-1β, IL-6, and IL-17 (Supplementary Table S3). In the sample-level analysis, consistent with our previous data,37 all blood samples with AT1R-Ab (AT1R-Ab alone and both AT1R-Ab and ETAR-Ab) had higher levels of all cytokines tested (n = 260; Supplementary Table S4). Comparisons between the 3 groups of samples by antibody status were significant for all cytokines except IL-17.
We further examined the effects of ETAR-Ab and AT1R-Ab together and AT1R-Ab alone when compared with having neither antibody on cytokines significant on initial comparison by Kruskal-Wallis test by using linear regression analyses. For the patient-level analysis (n = 64), we found that having both antibodies resulted in greater increases in IL-8 above patients with neither antibody compared with AT1R-Ab alone (P = 0.002; Table 3). This relationship persisted when controlled for age, sex, living versus deceased donor, presence of HLA DSA, rejection, HLA mismatch, and viremia (P = 0.012; Table 3). There was no significant interaction between HLA DSA and non-HLA antibody status on median patient IL-8 level. For the sample-level analysis (n = 260), TNF-α, IFN-γ, IL-1β, IL-6, and IL-8 were significantly elevated in blood samples positive for AT1R-Ab alone and samples positive for both AT1R-Ab and ETAR-Ab when compared with samples having neither antibody. These relationships remained consistent when controlled for age, sex, living versus deceased donor, presence of HLA DSA, rejection, HLA mismatch, and viremia (Supplementary Table S5). Interestingly, the magnitude of the association was higher in the samples positive for both antibodies than in samples with AT1R-Ab alone for all cytokines (Supplementary Table S5). The percent increase in TNF-α, IFN-γ, IL-1β, and IL-6 levels above antibody-negative samples was, on average, 28.2% higher for samples with both antibodies than for samples with AT1R-Ab alone in the adjusted models. This increase in magnitude was larger for IL-8, with an increase of 122.1% (Supplementary Table S5).
Discussion
In this longitudinal cohort of pediatric kidney transplant recipients, we are the first to report the association of ETAR-Ab with decline in renal function and vascular inflammation in the first 2 years post-transplant. Given AT1R-Ab and ETAR-Ab have been highly associated with each other in transplantation20,22,29, 30, 31 and other disease states,23, 24, 25 we also examined the natural history and differing clinical associations of patients with these autoantibodies. We found distinct groups of pediatric kidney transplant patients who developed both ETAR-Ab and AT1R-Ab and AT1R-Ab alone. Importantly, we found that patients with both ETAR-Ab and AT1R-Ab had greater declines in renal function, more arteritis, and higher IL-8 levels. In addition, similar to AT1R-Ab, the development of ETAR-Ab was not associated with HLA DSA. Our findings suggest that testing for ETAR-Ab in addition to AT1R-Ab may be beneficial in clinical decisionmaking and guide potential treatment strategies in pediatric kidney transplant recipients.
Given that ETAR-Ab and AT1R-Ab are highly associated in most, but not all,23 disease states, we measured both antibodies in each sample to describe the timing of antibody development and to assess this correlation. We found that there was a subset of pediatric kidney transplant patients that develop AT1R-Ab without concomitant ETAR-Ab. However, it was uncommon for a patient with ETAR-Ab to not have AT1R-Ab. Banasik et al. found a pretransplant prevalence of ETAR-Ab of 47.4%; however, a cutoff of >2.5 units/ml was used to determine positivity based on their data analysis. This is lower than what has been used in other studies in solid-organ transplantation29, 30, 31, 32 and may overlap with levels in healthy adults.23 In our study, we used a cutoff value of >10 units/ml based on other previous studies,30 recommendations from the test manufacturer, and our receiver operating curve analysis. We and others have described that AT1R-Ab levels are higher in pediatric kidney transplant recipients when compared with adults.33,35 Given AT1R-Ab and ETAR-Ab are highly correlated,23 it is not surprising that we found a higher level to be more informative in our population. In an analysis of adult kidney transplant recipients with similar serial sampling, only 4% of blood samples were positive for ETAR-Ab (vs. 22% in our cohort) and only 56% of these samples were also positive for AT1R-Ab (vs. 96% in our cohort).20 Our data, when compared with adult data, suggest that ETAR-Ab may be more common in pediatric kidney transplant recipients and may have a differing pattern of occurrence with respect to AT1R-Ab. However, the methodologies of measuring ETAR-Ab were different between our study, which utilized enzyme-linked immunoassay, and the adult study that used Luminex, which may potentially affect the comparability.
We also found that patients who developed both AT1R-Ab and ETAR-Ab in the first 2 years posttransplantation tended to do so either simultaneously, or AT1R-Ab preceded ETAR-Ab development. The reason for this pattern remains unclear. It has been suggested that there may be different levels of expression of these receptors during different stages of allograft injury,53 which may help explain the timing of antibody formation, but this hypothesis requires further investigation. Of the patients with ETAR-Ab post-transplant with pretransplant samples available, an approximately equal number of patients had the antibody before transplant, whereas the others developed it presumably de novo. A larger cohort is necessary to further subanalyze potential differences between these populations, and comparable data in solid-organ transplantation are not available. In terms of risk factors for developing both ETAR-Ab and AT1R-Ab, there was an interesting trend toward an association with non-Hispanic ethnicity, steroid-free immunosuppression at hospital discharge, and antithymocyte globulin induction. These relationships require further investigation with larger sample sizes.
Our analysis strategy of comparing patients who were negative for both antibodies versus patients with AT1R-Ab only and both ETAR-Ab and AT1R-Ab was designed to try to distinguish whether these patterns were associated with differing clinical findings. We found that both groups of non‒HLA-antibody-positive patients (AT1R-Ab only and both AT1R-Ab and ETAR-Ab) had more dramatic declines in renal function compared with patients who were antibody negative. The significance in the regression model of having both antibodies above AT1R-Ab alone suggests a potential synergistic interaction. It should be noted, however, that AT1R-Ab alone had borderline significance in these models. It is possible that, with a larger sample size, effects would have been seen in both groups (AT1R-Ab only and both AT1R-Ab and ETAR-Ab) and the power to detect synergistic effects would be enhanced. These results are consistent with an earlier study on ETAR-Ab in adult kidney transplant recipients.21
We have previously shown that AT1R-Ab was associated with glomerulitis or arteritis on kidney biopsy.33 With this study, we are able to further distinguish that all patients with arteritis had both AT1R-Ab and ETAR-Ab. By contrast, glomerulitis occurred in patients with AT1R-Ab alone and with both AT1R-Ab and ETAR-Ab. This suggests the possibility of differing pathophysiologic patterns associated with different antibody combinations, which may be related to receptor expression. Our association of ETAR-Ab with arteritis is consistent with the findings of Banasik et al. in adult kidney transplant recipients.21 Unfortunately, the rarity of these events in our population limited our ability to further analyze this relationship. We did not find any association between having both AT1R-Ab and ETAR-Ab and HLA DSA, rejection, or allograft loss. Although some studies have supported an association between AT1R-Ab and the development of HLA DSA,8,17,20,34 others have not.18,33 The relationship between HLA DSA and the development of ETAR-Ab has not been described previously in kidney transplantation; however, pretransplant ETAR-Ab has been associated with the development of HLA DSA in lung transplantation.30 Elevations in AT1R-Ab and ETAR-Ab have been associated with rejection, microvasculopathy, and allograft loss in heart transplantation,29 and with rejection in lung,30 liver,32 and multivisceral31 transplantation. Interestingly, despite the association with arteritis, ETAR-Ab was not associated with rejection in adult kidney transplant recipients, which is in agreement with our findings.21 It is also notable that more severe forms of T-cell‒mediated rejection (over borderline) were more common in patients with ETAR-Ab and AT1R-Ab in our cohort. Although this relationship did not attain statistical significance, it would be of interest to investigate in a larger cohort. In adult kidney transplantation, allograft loss has been associated with AT1R-Ab and/or ETAR-Ab when assessed cross-sectionally at time of biopsy,54 but not on pretransplant testing.21 Overall, our results are consistent with clinical associations in adult kidney transplant recipients, with the limitations in comparing results using different cutoffs, as noted previously.
In addition, in this study we found that patients with both AT1R-Ab and ETAR-Ab had elevations in median IL-8 levels compared with patients with AT1R-Ab alone or negative for both antibodies (Tables 2 and 3). In the sample-level analysis, blood samples with AT1R-Ab alone and both AT1R-Ab and ETAR-Ab had elevations in TNF-α, IFN-γ, IL-1β, IL-6, and IL-8 (Supplementary Tables S4 and S5). The trend was similar for IL-17 but did not attain statistical significance. We have previously shown AT1R-Ab to be associated with elevations in IL-8, TNF-α, IL-1β, IFN-γ, IL-17, and IL-6 in patient blood samples.33,37 The findings in this analysis are consistent with our previous data, but provide additional insight into the potential role of ETAR-Ab in enhancing these relationships. For all cytokines, the addition of ETAR-Ab increased the magnitude of the association between AT1R-Ab and elevations in each cytokine. Notably, the increase in magnitude with the addition of ETAR-Ab was higher for IL-8 compared with the other cytokines. This likely accounts for why it was only IL-8 that was significant in the patient-level analysis, which had a much smaller sample size (n = 64 vs 260) when controlling for AT1R-Ab alone. It also highlights IL-8 as perhaps the cytokine of most interest in evaluating potential synergistic interactions between AT1R-Ab and ETAR-Ab. Possible synergy between AT1R-Ab and ETAR-Ab has been described in other disease states24,25 in which elevated IL-8 was relevant.46,49,50,55 IL-8 can be secreted by endothelial cells in the context of antibody activation and promotes leukocyte recruitment.56, 57, 58 In kidney transplantation, elevated urinary IL-8 has been associated with early and long-term allograft dysfunction.59 This relationship and its significance in autoantibody pathogenesis in transplantation requires further investigation.
Although we found that ETAR-Ab along with AT1R-Ab may be clinically relevant, there are limitations to our study. Our data support associations between ETAR-Ab, AT1R-Ab, and vascular inflammation and allograft dysfunction; however, additional work is needed to comment on the mechanisms of these relationships. Antibody cross-linking of the 2 receptors24 and enhanced IL-8 production may contribute to the findings we observed. As was the case in our study and in others, not all patients with these antibodies have allograft dysfunction. Studies to assess the role of receptor expression, epitope specificity, antibody kinetics, and antibody subclass in differentiating among phenotypes of transplant patients with ETAR-Ab and AT1R-Ab may be informative. It is noteworthy that our previous work showed elevations in cytokines associated with AT1R-Ab,33,37 and this analysis has added that IL-8, in particular, may increase above the level of AT1R-Ab alone in patients who also develop ETAR-Ab. Based on this and the timing of antibody development, we hypothesize that the development of ETAR-Ab may be a form of progression that occurs in some kidney transplant patients with AT1R-Ab. This intermolecular epitope spreading may be one factor contributing to the varying phenotypes we observed. However, our data still suggest that other factors are likely impacting injury patterns in the context of autoantibody formation post-transplantation. Notably, patients with AT1R-Ab alone in our cohort still had detrimental clinical outcomes. Importantly, our limited sample size did not allow for extensive subanalyses or the inclusion of additional factors in multivariable analyses. Samples were tested retrospectively, and therefore we are unable to meaningfully evaluate the impact of treatment on antibody levels.
Overall, our study suggests that AT1R-Ab and ETAR-Ab may be associated with vascular injury and functional decline in kidney transplantation that may not present with frank rejection or be associated with HLA DSA. There are some reports of success in both preventing and treating AT1R-Ab‒associated rejection with antithymocyte globulin, i.v. Ig, plasmapheresis, and angiotensin receptor blockade.7,60,61 However, the role of ETAR blockade in transplantation has not yet been explored. The successful use of ETAR blockers to treat complications associated with ETAR-Ab has been reported in scleroderma.26,62 Furthermore, the availability of new agents that block both AT1R and ETAR and have been shown to be safe in patients with other forms of chronic kidney disease are of significant interest in this context.63 In our cohort, there were patients with both AT1R-Ab and ETAR-Ab, and others with AT1R-Ab alone. This may have implications for monitoring of AT1R-Ab and ETAR-Ab in addition to HLA DSA to allow for targeted therapeutics, particularly given the availability of multiple angiotensin receptor blockade agents and, more recently, dual AT1R and ETAR blockers. The benefits of this approach require prospective investigation.
In conclusion, our study has demonstrated that, in pediatric kidney transplant patients, ETAR-Ab is highly associated with AT1R-Ab, but there exists a subset of patients with AT1R-Ab alone. Having both antibodies is significantly associated with arteritis, elevated IL-8, and decline in renal function. Our results suggest interaction effects between ETAR-Ab and AT1R-Ab that require further investigation. Better understanding this interaction and the role of these antibodies, may be informative in developing protocols for testing, tailored treatment, and prevention of allograft injury.
Disclosure
MHP reports grants from Veloxis Pharmaceuticals and personal fees from Bristol Myers Squibb outside the submitted work. All the other authors declared no competitng interests.
Acknowledgments
This study was supported by the National Institute of Allergy and Infectious Diseases (K23AI139335 to MHP); Ruth L. Kirschstein National Research Service Award T32 DK104687 UCLA Translational Research Grant in Pediatric Nephrology Program (to MHP); the National Kidney Foundation (to MHP); the American Society of Nephrology (to MHP); the Casey Lee Ball Foundation (to ETC and MHP); the Today’s and Tomorrow’s Children Fund (to ETC); Duke Health Scholars Award (to ETC); the National Institute of Allergy and Infectious Diseases (R01AI135201, NIH PO1 AI120944, 5U19AI128913, and 1U01AI124319 to EFR); and the National Center for Advancing Translational Sciences (UL1TR001881).
Footnotes
Table S1. Immunomodulatory therapies by ETAR-Ab and AT1R-Ab status. Patients with AT1R-Ab alone or both AT1R-Ab and ETAR-Ab had higher median number of treatment days and more plasmapheresis than patients negative for both antibodies.
Table S2. Biopsy characteristics by ETAR-Ab and AT1R-Ab status. Acute and chronic Banff scores are shown. The highest value in each score over the follow-up period was used to represent each patient. All patients with arteritis were positive for both ETAR-Ab and AT1R-Ab. Three patients were excluded because of missing data.
Table S3. Cytokine levels in patients by ETAR-Ab and AT1R-Ab status. ETAR-Ab and AT1R-Ab positivity was associated with elevations in median patient IL-8 level. The median for each patient was taken as represented. Cytokine levels were compared across groups by Kruskal-Wallis test.
Table S4. Cytokine levels in blood samples by ETAR-Ab and AT1R-Ab status (n = 260). When blood samples were compared across 3 groups by antibody status, there were differences noted in all cytokines except IL-17. Cytokines levels were compared by Kruskal-Wallis test. Two samples from 1 patient were removed that had ETAR-Ab alone for this comparison.
Table S5. Cytokine levels in blood samples by ETAR-Ab and AT1R-Ab status (n = 260). AT1R-Ab alone and having both ETAR-Ab and AT1R-Ab positivity were associated with elevations in TNF-α, IFN-γ, IL-1β, IL-6, and IL-8 levels in individual patient blood samples on linear regression analysis. The magnitude of the association appeared higher in samples with both ETAR-Ab and AT1R-Ab. This relationship remained true when adjusted for additional clinical factors. Both models were adjusted for patient-level random effect. Cytokines were log transformed for analysis. The percent increases in each cytokine above having neither antibody on the linear scale with 95% confidence interval are shown. There was a consistent increase in cytokine level in samples with both antibodies above AT1R-Ab alone, the magnitude of which was largest for IL-8.
Supplementary Material
References
- 1.Zhang Q., Reed E.F. The importance of non-HLA antibodies in transplantation. Nat Rev Nephrol. 2016;12:484–495. doi: 10.1038/nrneph.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dragun D., Catar R., Philippe A. Non-HLA antibodies against endothelial targets bridging allo- and autoimmunity. Kidney Int. 2016;90:280–288. doi: 10.1016/j.kint.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Zou Y., Stastny P., Susal C., Dohler B., Opelz G. Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med. 2007;357:1293–1300. doi: 10.1056/NEJMoa067160. [DOI] [PubMed] [Google Scholar]
- 4.Terasaki P.I., Ozawa M., Castro R. Four-year follow-up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am J Transplant. 2007;7:408–415. doi: 10.1111/j.1600-6143.2006.01644.x. [DOI] [PubMed] [Google Scholar]
- 5.Cardinal H., Dieude M., Brassard N. Antiperlecan antibodies are novel accelerators of immune-mediated vascular injury. Am J Transplant. 2013;13:861–874. doi: 10.1111/ajt.12168. [DOI] [PubMed] [Google Scholar]
- 6.Angaswamy N., Klein C., Tiriveedhi V. Immune responses to collagen-IV and fibronectin in renal transplant recipients with transplant glomerulopathy. Am J Transplant. 2014;14:685–693. doi: 10.1111/ajt.12592. [DOI] [PubMed] [Google Scholar]
- 7.Dragun D., Muller D.N., Brasen J.H. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi M., Rebellato L.M., Cai J. Higher risk of kidney graft failure in the presence of anti-angiotensin II type-1 receptor antibodies. Am J Transplant. 2013;13:2577–2589. doi: 10.1111/ajt.12395. [DOI] [PubMed] [Google Scholar]
- 9.Jackson A.M., Sigdel T.K., Delville M. Endothelial cell antibodies associated with novel targets and increased rejection. J Am Soc Nephrol. 2015;26:1161–1171. doi: 10.1681/ASN.2013121277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Divanyan T., Acosta E., Patel D., Constantino D., Lopez-Soler R.I. Anti-vimentin antibodies in transplant and disease. Hum Immunol. 2019;80:602–607. doi: 10.1016/j.humimm.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Rampersad C., Shaw J., Gibson I.W. Early antibody-mediated kidney transplant rejection associated with anti-vimentin antibodies: a case report. Am J Kidney Dis. 2020;75:138–143. doi: 10.1053/j.ajkd.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Kamburova E.G., Gruijters M.L., Kardol-Hoefnagel T. Antibodies against ARHGDIB are associated with long-term kidney graft loss. Am J Transplant. 2019;19:3335–3344. doi: 10.1111/ajt.15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang B., Dieudé M., Hamelin K. Anti-LG3 antibodies aggravate renal ischemia-reperfusion injury and long-term renal allograft dysfunction. Am J Transplant. 2016;16:3416–3429. doi: 10.1111/ajt.13866. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland S.M., Li L., Sigdel T.K. Protein microarrays identify antibodies to protein kinase Czeta that are associated with a greater risk of allograft loss in pediatric renal transplant recipients. Kidney Int. 2009;76:1277–1283. doi: 10.1038/ki.2009.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinsmoen N.L., Lai C.H., Heidecke H. Anti-angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients. Transplantation. 2010;90:1473–1477. doi: 10.1097/TP.0b013e3181fd97f1. [DOI] [PubMed] [Google Scholar]
- 16.Banasik M., Boratynska M., Koscielska-Kasprzak K. The influence of non-HLA antibodies directed against angiotensin II type 1 receptor (AT1R) on early renal transplant outcomes. Transplant Int. 2014;27:1029–1038. doi: 10.1111/tri.12371. [DOI] [PubMed] [Google Scholar]
- 17.Cuevas E., Arreola-Guerra J.M., Hernandez-Mendez E.A. Pretransplant angiotensin II type 1-receptor antibodies are a risk factor for earlier detection of de novo HLA donor-specific antibodies. Nephrol Dial Transplant. 2016;31:1738–1745. doi: 10.1093/ndt/gfw204. [DOI] [PubMed] [Google Scholar]
- 18.Giral M., Foucher Y., Dufay A. Pretransplant sensitization against angiotensin II type 1 receptor is a risk factor for acute rejection and graft loss. Am J Transplant. 2013;13:2567–2576. doi: 10.1111/ajt.12397. [DOI] [PubMed] [Google Scholar]
- 19.Lefaucheur C., Viglietti D., Bouatou Y. Non-HLA agonistic anti-angiotensin II type 1 receptor antibodies induce a distinctive phenotype of antibody-mediated rejection in kidney transplant recipients. Kidney Int. 2019;96:189–201. doi: 10.1016/j.kint.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Gareau A.J., Wiebe C., Pochinco D. Pre-transplant AT1R antibodies correlate with early allograft rejection. Transplant Immunol. 2018;46:29–35. doi: 10.1016/j.trim.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Banasik M., Boratyńska M., Kościelska-Kasprzak K. The impact of non-HLA antibodies directed against endothelin-1 type A receptors (ETAR) on early renal transplant outcomes. Transplant Immunol. 2014;30:24–29. doi: 10.1016/j.trim.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Banasik M., Boratynska M., Koscielska-Kasprzak K. Long-term follow-up of non-HLA and anti-HLA antibodies: incidence and importance in renal transplantation. Transplant Proc. 2013;45:1462–1465. doi: 10.1016/j.transproceed.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Cabral-Marques O., Marques A., Giil L.M. GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis. Nat Commun. 2018;9:5224. doi: 10.1038/s41467-018-07598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabral-Marques O., Riemekasten G. Vascular hypothesis revisited: role of stimulating antibodies against angiotensin and endothelin receptors in the pathogenesis of systemic sclerosis. Autoimmun Rev. 2016;15:690–694. doi: 10.1016/j.autrev.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Velloso E.P., Pimentel R.L., Braga J.F. Identification of a novel agonist-like autoantibody in preeclamptic patients. Am J Hypertens. 2016;29:405–412. doi: 10.1093/ajh/hpv099. [DOI] [PubMed] [Google Scholar]
- 26.Riemekasten G., Philippe A., Nather M. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis. 2011;70:530536. doi: 10.1136/ard.2010.135772. [DOI] [PubMed] [Google Scholar]
- 27.LaMarca B., Parrish M., Ray L.F. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension. 2009;54:905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou C.C., Irani R.A., Dai Y. Autoantibody-mediated IL-6-dependent endothelin-1 elevation underlies pathogenesis in a mouse model of preeclampsia. J Immunol. 2011;186:6024–6034. doi: 10.4049/jimmunol.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiemann N.E., Meyer R., Wellnhofer E. Non-HLA antibodies targeting vascular receptors enhance alloimmune response and microvasculopathy after heart transplantation. Transplantation. 2012;94:919–924. doi: 10.1097/TP.0b013e3182692ad2. [DOI] [PubMed] [Google Scholar]
- 30.Reinsmoen N.L., Mirocha J., Ensor C.R. A 3-center study reveals new insights into the impact of non-HLA antibodies on lung transplantation outcome. Transplantation. 2017;101:1215–1221. doi: 10.1097/TP.0000000000001389. [DOI] [PubMed] [Google Scholar]
- 31.Gerlach U.A., Lachmann N., Ranucci G. Non-HLA antibodies may accelerate immune responses after intestinal and multivisceral transplantation. Transplantation. 2017;101:141–149. doi: 10.1097/TP.0000000000001439. [DOI] [PubMed] [Google Scholar]
- 32.OʼLeary J.G., Demetris A.J., Philippe A. Non-HLA antibodies impact on C4d staining, stellate cell activation and fibrosis in liver allografts. Transplantation. 2017;101:2399–2409. doi: 10.1097/TP.0000000000001853. [DOI] [PubMed] [Google Scholar]
- 33.Pearl M.H., Zhang Q., Palma Diaz M.F. Angiotensin II Type 1 receptor antibodies are associated with inflammatory cytokines and poor clinical outcomes in pediatric kidney transplantation. Kidney Int. 2018;93:260–269. doi: 10.1016/j.kint.2017.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fichtner A., Süsal C., Schröder C. Association of angiotensin II type 1 receptor antibodies with graft histology, function and survival in paediatric renal transplant recipients. Nephrol Dial Transplant. 2018;33:1065–1072. doi: 10.1093/ndt/gfy008. [DOI] [PubMed] [Google Scholar]
- 35.Bjerre A., Tangeraas T., Heidecke H., Dragun D., Dechend R., Staff A.C. Angiotensin II type 1 receptor antibodies in childhood kidney transplantation. Pediatr Transplant. 2016 doi: 10.1111/petr.12728. [DOI] [PubMed] [Google Scholar]
- 36.Hesemann L.E., Subramanian V., Mohanakumar T., Dharnidharka V.R. De novo development of antibodies to kidney-associated self-antigens angiotensin II receptor type I, collagen IV, and fibronectin occurs at early time points after kidney transplantation in children. Pediatr Transplant. 2015;19:499–503. doi: 10.1111/petr.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearl M.H., Grotts J., Rossetti M. Cytokine profiles associated with angiotensin II type 1 receptor antibodies. Kidney Int Rep. 2019;4:541–550. doi: 10.1016/j.ekir.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz G.J., Munoz A., Schneider M.F. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levey A.S., Coresh J., Greene T. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 40.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(Suppl. 4th Report):555–576. [PubMed] [Google Scholar]
- 41.Weber M.A., Schiffrin E.L., White W.B. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014;32:3–15. doi: 10.1097/HJH.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 42.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)---a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearl M.H., Nayak A.B., Ettenger R.B. Bortezomib may stabilize pediatric renal transplant recipients with antibody-mediated rejection. Pediatr Nephrol. 2016;31:1341–1348. doi: 10.1007/s00467-016-3319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haas M., Sis B., Racusen L.C. Banff 2013 Meeting report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272‒283. doi: 10.1111/ajt.12590. [DOI] [PubMed] [Google Scholar]
- 45.Blumberg J.M., Gritsch H.A., Reed E.F. Kidney paired donation in the presence of donor-specific antibodies. Kidney Int. 2013;84:1009–1016. doi: 10.1038/ki.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunther J., Kill A., Becker M.O. Angiotensin receptor type 1 and endothelin receptor type A on immune cells mediate migration and the expression of IL-8 and CCL18 when stimulated by autoantibodies from systemic sclerosis patients. Arthritis Res Ther. 2014;16:R65. doi: 10.1186/ar4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamat N.V., Thabet S.R., Xiao L. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-γ-/- and interleukin-17A-/- mice. Hypertension. 2015;65:569–576. doi: 10.1161/HYPERTENSIONAHA.114.04975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie P., Joladarashi D., Dudeja P., Sun L., Kanwar Y.S. Modulation of angiotensin II-induced inflammatory cytokines by the Epac1-Rap1A-NHE3 pathway: implications in renal tubular pathobiology. Am J Physiol Renal Physiol. 2014;306:F1260–F1274. doi: 10.1152/ajprenal.00069.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kauma S., Takacs P., Scordalakes C., Walsh S., Green K., Peng T. Increased endothelial monocyte chemoattractant protein-1 and interleukin-8 in preeclampsia. Obstet Gynecol. 2002;100:706–714. doi: 10.1016/s0029-7844(02)02169-5. [DOI] [PubMed] [Google Scholar]
- 50.Jonsson Y., Ruber M., Matthiesen L. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006;70:83–91. doi: 10.1016/j.jri.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Sprague A.H., Khalil R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 53.Philogene M.C., Johnson T., Vaught A.J., Zakaria S., Fedarko N. Antibodies against angiotensin II type 1 and endothelin A receptors: relevance and pathogenicity. Hum Immunol. 2019;80:561–567. doi: 10.1016/j.humimm.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banasik M., Boratynska M., Koscielska-Kasprzak K. Non-HLA antibodies: angiotensin II type 1 receptor (anti-AT1R) and endothelin-1 type A receptor (anti-ETAR) are associated with renal allograft injury and graft loss. Transplant Proc. 2014;46:2618–2621. doi: 10.1016/j.transproceed.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 55.Hasegawa M., Asano Y., Endo H. Serum chemokine levels as prognostic markers in patients with early systemic sclerosis: a multicenter, prospective, observational study. Mod Rheumatol. 2013;23:1076–1084. doi: 10.1007/s10165-012-0795-6. [DOI] [PubMed] [Google Scholar]
- 56.Russo R.C., Garcia C.C., Teixeira M.M., Amaral F.A. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol. 2014;10:593–619. doi: 10.1586/1744666X.2014.894886. [DOI] [PubMed] [Google Scholar]
- 57.Utgaard J.O., Jahnsen F.L., Bakka A., Brandtzaeg P., Haraldsen G. Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells. J Exp Med. 1998;188:1751–1756. doi: 10.1084/jem.188.9.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Naemi F.M., Carter V., Kirby J.A., Ali S. Anti-donor HLA class I antibodies: pathways to endothelial cell activation and cell-mediated allograft rejection. Transplantation. 2013;96:258–266. doi: 10.1097/TP.0b013e3182985504. [DOI] [PubMed] [Google Scholar]
- 59.Kwiatkowska E., Domanski L., Bober J. Urinary IL-8 is a marker of early and long-term graft function after renal transplantation. Ren Fail. 2017;39:484–490. doi: 10.1080/0886022X.2017.1323644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carroll R.P., Riceman M., Hope C.M. Angiotensin II type-1 receptor antibody (AT1Rab) associated humoral rejection and the effect of peri operative plasma exchange and candesartan. Hum Immunol. 2016;77:1154–1158. doi: 10.1016/j.humimm.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 61.Carroll R.P., Deayton S., Emery T. Proactive treatment of angiotensin receptor antibodies in kidney transplantation with plasma exchange and/or candesartan is safe and associated with excellent graft survival at 4 years: a single centre Australian experience. Hum Immunol. 2019;80:573–578. doi: 10.1016/j.humimm.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Denton C.P., Pope J.E., Peter H.H. Long-term effects of bosentan on quality of life, survival, safety and tolerability in pulmonary arterial hypertension related to connective tissue diseases. Ann Rheum Dis. 2008;67:1222–1228. doi: 10.1136/ard.2007.079921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trachtman H., Nelson P., Adler S. DUET: A phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. J Am Soc Nephrol. 2018;29:2745–2754. doi: 10.1681/ASN.2018010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.