Abstract
Background:
Statins, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, are common lipid-lowering agents and may reduce the risk of several cancer types including pancreatic cancer. However, the association between statin use and pancreatic cancer risk has not been fully evaluated in prospective studies.
Methods:
We studied the association between statin use and incident pancreatic cancer in 113,059 participants from the prospective Nurses’ Health Study and Health Professionals Follow-up Study. Statin use was self-reported via study questionnaires and updated biennially. Hazard ratios (HRs) and 95% confidence intervals (CIs) for incidence of pancreatic cancer were estimated using multivariable Cox proportional hazards models with adjustment for potential confounders.
Results:
In total, 583 participants developed incident pancreatic cancer during 1.4 million person-years of follow-up. No difference was identified in pancreatic cancer risk for regular versus non-regular statin users (multivariable-adjusted HR, 0.98; 95% CI, 0.82-1.16). There was no significant heterogeneity in the association of statin use with pancreatic cancer risk between the cohorts. Similarly, longer duration of regular statin use was not associated with decreased risk of pancreatic cancer (Ptrend = 0.67). The results remained similar when we examined statin use status at baseline or accounting for 4-year latency period. We observed no statistically significant effect modification for the association of statin use with pancreatic cancer risk by body mass index, smoking status, or diabetes mellitus status (all Pinteraction > 0.21).
Conclusions:
Regular statin use was not associated with pancreatic cancer risk in two large prospective cohort studies in the U.S..
Keywords: Chemoprevention, Cohort studies, Hydroxymethylglutaryl-CoA reductase inhibitors, Pancreatic neoplasms, Risk factors
Introduction
Pancreatic cancer is a top cause of cancer-related mortality worldwide and the third leading cause in the United States (U.S.) [1]. Given that pancreatic ductal adenocarcinoma is often diagnosed at advanced stages leading to high mortality with an overall 5-year survival rate of < 10% [1], identification of chemopreventive agents is of considerable importance to reduce mortality associated with this malignancy.
Statins, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, are commonly used for their lipid-lowering properties, and the prevention and treatment of cardiovascular disease. This class of medication inhibits cholesterol synthesis by blocking the mevalonate pathway [2]. Evidence supports that statins may also carry anti-neoplastic properties through alterations in inflammation, angiogenesis, and cellular apoptosis and proliferation [3-7]. However, studies have yielded conflicting results regarding the effect of statin use on pancreatic cancer risk [8-12]. Prospective studies have not evaluated the association of statin use with pancreatic cancer risk using a large number of cases with long duration of follow-up. Given the inconclusive findings from prior studies and limited preventive strategies for pancreatic cancer, an important need remains for prospective studies investigating statin use as a potential preventative approach for pancreatic cancer. In the current study, we prospectively examine the association of statin use and risk of pancreatic adenocarcinoma in two large U.S. cohorts.
Patients and methods
Study population
We studied participants from two prospective U.S. cohorts, the Nurses’ Health Study (NHS, 121,700 women aged 30-55 years with study enrollment in 1976) and the Health Professionals Follow-up Study (HPFS, 51,529 men aged 40-75 years with study enrollment in 1986) [13-16]. Information on demographics, medical and social history, and health outcomes such as cancer diagnoses was collected via biennial questionnaires. Participants were followed until death from any cause or the end of follow-up (NHS: May 31, 2014; HPFS: January 31, 2014), whichever occurred first. Follow-up questionnaires were completed with follow-up rates exceeding 90% [17].
This study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants, and the studies were approved by the institutional review board at Harvard T.H. Chan School of Public Health and the Human Research Committee at Brigham and Women’s Hospital (Boston, MA, USA).
Ascertainment of pancreatic cancer cases
We identified patients diagnosed with pancreatic adenocarcinoma by study physicians’ review of medical records, death certificates, and/or cancer registry data. Physicians blinded to exposure status confirmed the diagnosis of pancreatic cancer by review of medical records, death certificates, or cancer registry data. Deaths were ascertained based on information from next of kin, U.S. postal service, and/or the National Death Index; this method has been shown to capture >98% of deaths [18]. We excluded participants with history of cancer (exception of non-melanoma skin cancer) prior to 2000 as the baseline of this study and those without available data on statin use. Date of pancreatic cancer diagnosis and stage at diagnosis were determined through physician review of medical records. Cancer stage was classified as: localized (amenable to surgical resection); locally advanced (unresectable due to extrapancreatic extension but no d6istant metastases); metastatic; or unknown.
Ascertainment of statin use
Information on statin use was first assessed by the 2000 questionnaire and updated biennially. In 2000, participants were asked if they regularly took statins (yes/no) and their duration of statin use (0-2, 3-5, or ≥ 6 years in NHS; and 1-2, 3-5, 6-9, or ≥ 10 years in HPFS). Current statin use was updated by self-report in each follow-up questionnaire. Using the response from the 2000 questionnaire in combination with the subsequent response to current use on the 2002 questionnaire onward, duration of statin use was calculated. Beginning in 2004, participants were additionally asked to report type of statin used including atorvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin. Further details of statin use ascertainment within these cohorts have been previously described [19, 20].
Statistical analysis
We examined the association between statin use (regular vs. non-regular use) and risk of pancreatic cancer. We used Cox proportional hazards regression models to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for incidence of pancreatic cancer by statin use. In the current analyses, follow-up time was defined as the period from the participant’s first report of statin use status to diagnosis of pancreatic adenocarcinoma, death from any cause, or the end of follow-up, whichever occurred first. Statin use was analyzed as a simple update taken from the most recent questionnaire cycle before each follow-up interval. We also evaluated statin use at the time of the baseline questionnaire in 2000, as well as with a latency exposure analysis with a 4-year lag period between statin exposure and cancer diagnosis. This latency analysis was performed to account for a potential lag time between normal pancreatic parenchyma and development of cancer [21]. The multivariable Cox regression models were adjusted for age, sex (cohort), race/ethnicity, smoking status, history of diabetes mellitus (DM) [22], body mass index (BMI), physical activity, alcohol intake, and multivitamin use. Statin use and covariates were treated as time-dependent to account for changes over time, if applicable. We evaluated between-cohort heterogeneity in the association of statin use with pancreatic cancer risk using the Cochran’s Q statistic [23]. In a meta-analysis, we computed a pooled HR for incidence of pancreatic cancer using the DerSimonian and Laird random-effects model [24]. We assessed statistical interaction by entering main effect terms and the cross-product of statin use and a stratification variable into the model and evaluating likelihood ratio tests. In all analyses, two-sided P values < 0.05 were considered statistically significant, and SAS statistical software (version 9.4, SAS Institute, Cary, NC, USA) was used.
Results
Among 113,059 study participants in NHS and HPFS, 583 developed incident pancreatic adenocarcinoma (391 women and 192 men) during ~1.4 million person-years of follow-up. Table 1 shows age-standardized baseline characteristics of participants. Regular statin users were more likely to have history of diabetes than non-regular users.
Table 1.
Age-standardized baseline characteristics of participants by statin use in NHS and HPFS (2000)
| Characteristica | NHS |
HPFS |
||
|---|---|---|---|---|
| Statin use |
Statin use |
|||
| Non-regular use |
Regular use |
Non-regular use |
Regular use |
|
| No. of participants | 55,694 | 15,401 | 22,259 | 6,681 |
| Age, years | 65.8 (7.1) | 68.0 (6.7) | 65.7 (9.0) | 67.1 (8.4) |
| Race | ||||
| White | 97.4% | 97.4% | 91.8% | 90.8% |
| Black | 1.5% | 1.5% | 0.6% | 0.6% |
| Other | 1.1% | 1.1% | 2.8% | 3.6% |
| Unknown | 0.0% | 0.0% | 4.8% | 5.0% |
| Body mass index, kg/m2 | 25.5 (4.6) | 26.7 (4.6) | 25.7 (3.4) | 26.2 (3.2) |
| Smoking status | ||||
| Current smoker | 9.5% | 9.4% | 4.7% | 3.8% |
| Pack-years in smokers | 24.2 (21.4) | 26.0 (21.4) | 23.7 (18.9) | 24.9 (18.5) |
| History of diabetes | 7.1% | 15.8% | 6.4% | 12.2% |
| Physical activity, MET-hours/week | 17.8 (16.9) | 15.8 (14.8) | 34.1 (28.7) | 30.5 (24.6) |
| Alcohol intake, g/day | 5.8 (8.6) | 5.0 (8.2) | 10.8 (13.2) | 10.9 (12.6) |
| Regular multivitamin use | 67.4% | 67.5% | 65.0% | 66.9% |
All variables other than age were standardized to age distribution of the study population. Mean (standard deviation) was presented for continuous variables.
HPFS, Health Professionals Follow-up Study; MET, metabolic equivalent of task; NHS, Nurses’ Health Study.
Regular statin use was not associated with pancreatic cancer risk in the combined population (multivariable-adjusted HR 0.98; 95% confidence interval [CI] 0.82-1.16; Table 2). There was no significant evidence of between-cohort heterogeneity in the association of statin use with pancreatic cancer risk (Pheterogeneity = 0.34; Fig. 1). Among 424 pancreatic cancer cases with available data on cancer stage at diagnosis, the distribution of cancer stage did not differ significantly by statin use (Table 3). When we examined types of statins, we observed no decreased risk of pancreatic cancer among regular users of a specific type of statin compared with non-regular users of any statins, but statistical power was limited in this analysis (Table S1). In addition, no association was found between duration of regular statin use and risk of pancreatic cancer (Ptrend = 0.65; Table 4). We similarly observed no significant association between statin use and pancreatic cancer risk assessing statin exposure at baseline or latency analysis with 4-year lag period between statin exposure and cancer diagnosis (Table 5). We observed no statistically significant effect modification for the association of statin use with pancreatic cancer risk by BMI, smoking status, or DM status (Pinteraction > 0.21, Table 6).
Table 2.
Risk of incident pancreatic cancer by statin use
| NHS |
HPFS |
Combined |
||||
|---|---|---|---|---|---|---|
| Statin use |
Statin use |
Statin use |
||||
| Non-regular use |
Regular use |
Non-regular use |
Regular use |
Non-regular use |
Regular use |
|
| No. of cases | 248 | 143 | 107 | 85 | 355 | 228 |
| Person-years | 634,974 | 344,203 | 239,714 | 150,774 | 874,688 | 494,978 |
| Age-adjusted HR (95% CI) | 1 (referent) | 1.02 (0.82-1.25) | 1 (referent) | 1.18 (0.88-1.58) | 1 (referent) | 1.07 (0.90-1.27) |
| Multivariable HR (95% CI)a | 1 (referent) | 0.92 (0.74-1.14) | 1 (referent) | 1.10 (0.81-1.47) | 1 (referent) | 0.98 (0.82-1.16) |
| Multivariable HR (95% CI)b | 1 (referent) | 0.92 (0.74-1.14) | 1 (referent) | 1.10 (0.82-1.49) | 1 (referent) | 0.98 (0.82-1.16) |
The Cox proportional hazards regression models were adjusted for age (continuous), sex (cohort), calendar year of questionnaire cycle (continuous), smoking in pack-years (never, 0.1-4.9, 5-19.9, 20-39.9, or ≥ 40), and history of diabetes (yes/no).
Further adjusted for race/ethnicity (white, black, other, or unknown), body mass index (< 25, 25-29.9, 30-34.9, or ≥ 35 kg/m2), physical activity (MET-hours per week in quintiles), alcohol intake (0, 0.1-4.9, 5-14.9, 15-29.9, or ≥ 30 g/day), and regular multivitamin use (yes/no).
CI, confidence interval; HPFS, Health Professionals Follow-up Study; HR, hazard ratio; MET, metabolic equivalent of task; NHS, Nurses’ Health Study.
Fig. 1.
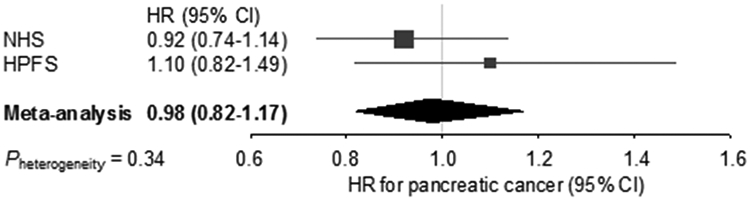
Forest plot and meta-analysis of HRs for pancreatic cancer, comparing regular statin users with non-regular users in the NHS and HPFS cohorts. Squares and horizontal lines indicate cohort-specific multivariable-adjusted HRs and 95% CIs, respectively. Area of the square reflects cohort-specific weight (inverse of the variance). Diamond indicates pooled multivariable-adjusted HR (center) and 95% CI (width). HRs were adjusted for age (continuous), sex (cohort), race/ethnicity (white, black, other, or unknown), calendar year of questionnaire cycle (continuous), smoking in pack-years (never, 0.1-4.9, 5-19.9, 20-39.9, or ≥ 40), history of diabetes (yes/no), body mass index (< 25, 25-29.9, 30-34.9, or ≥ 35 kg/m2), physical activity (MET-hours per week in quintiles), alcohol intake (0, 0.1-4.9, 5-14.9, 15-29.9, or ≥ 30 g/day), and regular multivitamin use (yes/no).
CI, confidence interval; HPFS, Health Professionals Follow-up Study; HR, hazard ratio; MET, metabolic equivalent of task; NHS, Nurses’ Health Study.
Table 3.
Distribution of cancer stage among pancreatic cancer cases by statin use in the combined population.
| Cancer stage | Statin use |
Pa | |
|---|---|---|---|
| Non-regular use (n = 257) |
Regular use (n = 167) |
||
| Localized | 48 (18.7%) | 40 (24.0%) | 0.37 |
| Locally advanced | 24 (9.3%) | 17 (10.2%) | |
| Metastatic | 185 (72.0%) | 110 (65.9%) | |
P value was calculated using the chi-square test.
Table 4.
Risk of incident pancreatic cancer by duration of regular statin use in the combined population
| Duration of regular statin use (years) |
Ptrendc | ||||
|---|---|---|---|---|---|
| Non-regular use |
> 0-2 | 3-5 | ≥ 6 | ||
| No. of cases | 355 | 73 | 46 | 108 | |
| Person-years | 874,688 | 153,181 | 104,602 | 234,328 | |
| Age-adjusted HR (95% CI) | 1 (referent) | 1.14 (0.89-1.47) | 1.01 (0.74-1.37) | 1.05 (0.84-1.31) | 0.66 |
| Multivariable HR (95% CI)a | 1 (referent) | 1.04 (0.81-1.35) | 0.93 (0.68-1.27) | 0.96 (0.77-1.20) | 0.65 |
| Multivariable HR (95% CI)b | 1 (referent) | 1.04 (0.81-1.35) | 0.93 (0.68-1.27) | 0.96 (0.77-1.20) | 0.65 |
The Cox proportional hazards regression models were adjusted for age (continuous), sex (cohort), calendar year of questionnaire cycle (continuous), smoking in pack-years (never, 0.1-4.9, 5-19.9, 20-39.9, or ≥ 40), and history of diabetes (yes/no).
Further adjusted for race/ethnicity (white, black, other, or unknown), body mass index (< 25, 25-29.9, 30-34.9, or ≥ 35 kg/m2), physical activity (MET-hours per week in quintiles), alcohol intake (0, 0.1-4.9, 5-14.9, 15-29.9, or ≥ 30 g/day), and regular multivitamin use (yes/no).
Ptrend was calculated using a linear tend test and ordinal categories of duration of regular statin use.
CI, confidence interval; HR, hazard ratio; MET, metabolic equivalent of task.
Table 5.
Risk of incident pancreatic cancer by statin use at baseline and with 4-year lag time in the combined population
| Baseline exposure |
Latency exposurec |
|||
|---|---|---|---|---|
| Non-regular use |
Regular use |
Non-regular use |
Regular use |
|
| No. of cases | 400 | 142 | 244 | 133 |
| Person-years | 984,338 | 272,522 | 901,328 | 337,073 |
| Age-adjusted HR (95% CI) | 1 (referent) | 1.14 (0.94-1.38) | 1 (referent) | 1.12 (0.91-1.40) |
| Multivariable HR (95% CI)a | 1 (referent) | 1.05 (0.86-1.28) | 1 (referent) | 1.02 (0.82-1.27) |
| Multivariable HR (95% CI)b | 1 (referent) | 1.06 (0.87-1.29) | 1 (referent) | 1.02 (0.82-1.27) |
The Cox proportional hazards regression models were adjusted for age (continuous), sex (cohort), calendar year of questionnaire cycle (continuous), smoking in pack-years (never, 0.1-4.9, 5-19.9, 20-39.9, or ≥ 40), and history of diabetes (yes/no).
Further adjusted for race/ethnicity (white, black, other, or unknown), body mass index (< 25, 25-29.9, 30-34.9, or ≥ 35 kg/m2), physical activity (MET-hours per week in quintiles), alcohol intake (0, 0.1-4.9, 5-14.9, 15-29.9, or ≥ 30 g/day), and multivitamin use (non-regular use or regular use).
Accounted for 4-year lag period between statin exposure and pancreatic cancer diagnosis.
CI, confidence interval; HR, hazard ratio; MET, metabolic equivalent of task.
Table 6.
Risk of incident pancreatic cancer by statin use in the combined population, stratified by covariates
| Statin use |
|||
|---|---|---|---|
| Non-regular use | Regular use | Pinteractionb | |
| Body mass index < 25 kg/m2 | 0.72 | ||
| No. of cases | 171 | 88 | |
| Person-years | 447,460 | 202,690 | |
| Multivariable HR (95% CI)a | 1 (referent) | 1.02 (0.78-1.32) | |
| Body mass index ≥ 25 kg/m2 | |||
| No. of cases | 183 | 140 | |
| Person-years | 426,081 | 291,637 | |
| Multivariable HR (95% CI)a | 1 (referent) | 0.95 (0.76-1.20) | |
| Never smoker | 0.22 | ||
| No. of cases | 157 | 82 | |
| Person-years | 413,774 | 215,544 | |
| Multivariable HR (95% CI)a | 1 (referent) | 0.87 (0.66-1.14) | |
| Ever smoker | |||
| No. of cases | 194 | 144 | |
| Person-years | 450,900 | 273,141 | |
| Multivariable HR (95% CI)a | 1 (referent) | 1.07 (0.86-1.34) | |
| No diabetes | 0.99 | ||
| No. of cases | 296 | 155 | |
| Person-years | 802,584 | 400,191 | |
| Multivariable HR (95% CI)a | 1 (referent) | 0.98 (0.80-1.19) | |
| Diabetes | |||
| No. of cases | 59 | 73 | |
| Person-years | 71,750 | 94,575 | |
| Multivariable HR (95% CI)a | 1 (referent) | 0.98 (0.69-1.39) | |
The Cox proportional hazards regression models were adjusted for the following covariates except for the stratification variable: age (continuous), sex (cohort), race/ethnicity (white, black, other, or unknown), calendar year of questionnaire cycle (continuous), smoking in pack-years (never, 0.1-4.9, 5-19.9, 20-39.9, or ≥ 40), history of diabetes (yes/no), body mass index (< 25, 25-29.9, 30-34.9, or ≥ 35 kg/m2), physical activity (MET-hours per week in quintiles), alcohol intake (0, 0.1-4.9, 5-14.9, 15-29.9, or ≥ 30 g/day), and regular multivitamin use (yes/no).
Pinteraction was calculated by entering main effect terms and the cross-product of statin use and a stratification variable into the model and evaluating likelihood ratio tests.
CI, confidence interval; HR, hazard ratio; MET, metabolic equivalent of task.
Discussion
To test the hypothesis that regular statin use might be associated with lower risk of pancreatic cancer, we conducted this prospective study based on two large U.S. cohorts. No significant association between regular statin use and risk of pancreatic cancer was identified, including in analyses with different timing between statin exposure and cancer risk. The results remained largely unchanged after adjustment for multiple potential confounding covariates in regression models and after consideration of the duration of statin use or types of statins. Our findings suggest that statins, at least when administered at usual clinical doses, are not associated with future risk of pancreatic cancer.
Statins inhibit the conversion of HMG-CoA to mevalonate and decrease levels of intermediate isoprenoids in the mevalonate pathway, which modulate post-translational regulation of proteins involved in cell proliferation and cell cycle progression [2, 7]. Consequently, statins potentially suppress downstream proteins including RAS and the Rho family of proteins, which are strongly implicated in carcinogenesis [3, 4]. In mouse models of pancreatic cancer, simvastatin therapy has been shown to suppress development of pancreatic intraepithelial neoplasia and invasive carcinoma [25]. Mouse studies also suggest that atorvastatin can suppress the activation of the KRAS gene [26], which is a major driver oncogene in pancreatic adenocarcinoma [27], and block downstream RAF-MAPK and PI3K-AKT-MTOR signaling pathways which are involved in cell growth [28, 29]. In addition, the immunomodulatory effects of statins may act as an alternative mechanism for cancer prevention [5, 30].
Despite the plausible biological mechanisms by which statins may suppress the development and progression of cancer cells, our large prospective population-based study does not support that real-world statin use is associated with the risk of developing pancreatic cancer. While prior studies have suggested a decreased incidence of pancreatic cancer with statin use [10, 11], these findings may be limited by retrospective case-control study designs with potential exposure misclassification, selected populations (male smokers [10] or veterans [11]), and referral patterns. A meta-analysis of 16 cohort studies comprised of 1,692,863 participants and 7,807 pancreatic cancer cases failed to show an association between statin use and pancreatic cancer risk (pooled relative risk, 0.89; 95% CI, 0.74-1.07) [12]. This meta-analysis may be limited by the small number of pancreatic cancer cases in prospective studies (n < 10 in randomized controlled trials and n = 168 in a prospective cohort study [31]), relatively short follow-up duration, and heterogeneous study designs. Therefore, there is a great need for prospective studies including a sufficient number of pancreatic cancer cases on the topic. In a large prospective study of postmenopausal U.S. women, statin use was not associated with lower risk of pancreatic cancer (HR, 0.92; 95% CI, 0.57-1.48) [32], which was consistent with the current findings.
Our study has several limitations, including limited statistical power in analyses of specific statin types and no data on the statin dosage ingested by participants. Experimental and clinical studies suggest that anti-tumor profiles may not be the same across the different statin compounds (e.g., hydrophilic or lipophilic) and at different medication doses [33-37]. Therefore, a prospective study including more detailed information on type and dose of statins used would be of interest. The majority of our study population was white, and additional studies in a more ethnically diverse cohort are warranted. Our study also has several notable strengths, including well-defined longitudinal cohorts, prospective study design, and large sample size with over 500 pancreatic cancer cases. Data on statins and covariates were prospectively collected and biennially updated, which allowed us to investigate the long-term effects of statin use without substantial recall bias and to rigorously adjust for potential confounders.
In summary, statin use was not associated with incidence of pancreatic cancer in two large prospective cohort studies in the U.S.. Additional studies designed to identify prediagnosis exposures that impact pancreatic cancer development should be considered.
Supplementary Material
Acknowledgements
The Nurses’ Health Study is supported by U.S. National Institutes of Health (NIH) grants: UM1 CA186107, P01 CA87969, and R01 CA49449. The Health Professionals Follow-up Study is supported by NIH UM1 CA167552. This work was additionally supported by NIH R01 CA205406 and the Broman Fund for Pancreatic Cancer Research to K.N.; by the Robert T. and Judith B. Hale Fund for Pancreatic Cancer, Perry S. Levy Fund for Gastrointestinal Cancer Research, Pappas Family Research Fund for Pancreatic Cancer, NIH R01 CA124908, and NIH P50 CA127003 to C.S.F.; by NIH R35 CA197735 to S.O.; and by Hale Center for Pancreatic Cancer Research, NIH/National Cancer Institute (NCI) U01 CA210171, Department of Defense CA130288, Lustgarten Foundation, Pancreatic Cancer Action Network, Noble Effort Fund, Peter R. Leavitt Family Fund, Wexler Family Fund, and Promises for Purple to B.M.W.. T.H. was supported by a fellowship grant from the Mitsukoshi Health and Welfare Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of the data.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Gazzerro P, Proto MC, Gangemi G, et al. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol Rev. 2012;64:102–46. [DOI] [PubMed] [Google Scholar]
- 3.Demierre MF, Higgins PD, Gruber SB, et al. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–42. [DOI] [PubMed] [Google Scholar]
- 4.Gronich N, Rennert G. Beyond aspirin-cancer prevention with statins, metformin and bisphosphonates. Nat Rev Clin Oncol. 2013;10:625–42. [DOI] [PubMed] [Google Scholar]
- 5.Pisanti S, Picardi P, Ciaglia E, et al. Novel prospects of statins as therapeutic agents in cancer. Pharmacol Res. 2014;88:84–98. [DOI] [PubMed] [Google Scholar]
- 6.Matusewicz L, Meissner J, Toporkiewicz M, et al. The effect of statins on cancer cells--review. Tumour Biol. 2015;36:4889–904. [DOI] [PubMed] [Google Scholar]
- 7.Mullen PJ, Yu R, Longo J, et al. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer. 2016;16:718–31. [DOI] [PubMed] [Google Scholar]
- 8.Miller MS, Allen P, Brentnall TA, et al. Pancreatic Cancer Chemoprevention Translational Workshop: Meeting Report. Pancreas. 2016;45:1080–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen MJ, Tsan YT, Liou JM, et al. Statins and the risk of pancreatic cancer in Type 2 diabetic patients--A population-based cohort study. Int J Cancer. 2016;138:594–603. [DOI] [PubMed] [Google Scholar]
- 10.Carey FJ, Little MW, Pugh TF, et al. The differential effects of statins on the risk of developing pancreatic cancer: a case-control study in two centres in the United Kingdom. Dig Dis Sci. 2013;58:3308–12. [DOI] [PubMed] [Google Scholar]
- 11.Khurana V, Sheth A, Caldito G, et al. Statins reduce the risk of pancreatic cancer in humans: a case-control study of half a million veterans. Pancreas. 2007;34:260–5. [DOI] [PubMed] [Google Scholar]
- 12.Cui X, Xie Y, Chen M, et al. Statin use and risk of pancreatic cancer: a meta-analysis. Cancer Causes Control. 2012;23:1099–111. [DOI] [PubMed] [Google Scholar]
- 13.Birmann BM, Barnard ME, Bertrand KA, et al. Nurses' Health Study Contributions on the Epidemiology of Less Common Cancers: Endometrial, Ovarian, Pancreatic, and Hematologic. Am J Public Health. 2016;106:1608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovannucci E, Ascherio A, Rimm EB, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–34. [DOI] [PubMed] [Google Scholar]
- 15.Bao Y, Nimptsch K, Wolpin BM, et al. Dietary insulin load, dietary insulin index, and risk of pancreatic cancer. Am J Clin Nutr. 2011;94:862–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bao Y, Ng K, Wolpin BM, et al. Predicted vitamin D status and pancreatic cancer risk in two prospective cohort studies. Br J Cancer. 2010;102:1422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Y, Nishihara R, Wu K, et al. Population-wide Impact of Long-term Use of Aspirin and the Risk for Cancer. JAMA Oncol. 2016;2:762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–9. [DOI] [PubMed] [Google Scholar]
- 19.Lee JE, Baba Y, Ng K, et al. Statin use and colorectal cancer risk according to molecular subtypes in two large prospective cohort studies. Cancer Prev Res (Phila). 2011;4:1808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai CJ, Leitzmann MF, Willett WC, et al. Statin use and the risk of cholecystectomy in women. Gastroenterology. 2009;136:1593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Wu F, Saito E, et al. Association between type 2 diabetes and risk of cancer mortality: a pooled analysis of over 771,000 individuals in the Asia Cohort Consortium. Diabetologia. 2017;60:1022–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 25.Fendrich V, Sparn M, Lauth M, et al. Simvastatin delay progression of pancreatic intraepithelial neoplasia and cancer formation in a genetically engineered mouse model of pancreatic cancer. Pancreatology. 2013;13:502–7. [DOI] [PubMed] [Google Scholar]
- 26.Liao J, Chung YT, Yang AL, et al. Atorvastatin inhibits pancreatic carcinogenesis and increases survival in LSL-KrasG12D-LSL-Trp53R172H-Pdx1-Cre mice. Mol Carcinog. 2013;52:739–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammed A, Qian L, Janakiram NB, et al. Atorvastatin delays progression of pancreatic lesions to carcinoma by regulating PI3/AKT signaling in p48Cre/+ LSL-KrasG12D/+ mice. Int J Cancer. 2012;131:1951–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corcos L, Le Jossic-Corcos C. Statins: perspectives in cancer therapeutics. Dig Liver Dis. 2013;45:795–802. [DOI] [PubMed] [Google Scholar]
- 30.Thurnher M, Nussbaumer O, Gruenbacher G. Novel aspects of mevalonate pathway inhibitors as antitumor agents. Clin Cancer Res. 2012;18:3524–31. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs EJ, Newton CC, Thun MJ, et al. Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res. 2011;71:1763–71. [DOI] [PubMed] [Google Scholar]
- 32.Simon MS, Desai P, Wallace R, et al. Prospective analysis of association between statins and pancreatic cancer risk in the Women's Health Initiative. Cancer Causes Control. 2016;27:415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeon CY, Pandol SJ, Wu B, et al. The association of statin use after cancer diagnosis with survival in pancreatic cancer patients: a SEER-medicare analysis. PLoS One. 2015;10:e0121783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu BU, Chang J, Jeon CY, et al. Impact of statin use on survival in patients undergoing resection for early-stage pancreatic cancer. Am J Gastroenterol. 2015;110:1233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gbelcova H, Lenicek M, Zelenka J, et al. Differences in antitumor effects of various statins on human pancreatic cancer. Int J Cancer. 2008;122:1214–21. [DOI] [PubMed] [Google Scholar]
- 36.Huang BZ, Chang JI, Li E, et al. Influence of Statins and Cholesterol on Mortality Among Patients With Pancreatic Cancer. J Natl Cancer Inst. 2017;109:djw275. [DOI] [PubMed] [Google Scholar]
- 37.Lee HS, Lee SH, Lee HJ, et al. Statin Use and Its Impact on Survival in Pancreatic Cancer Patients. Medicine (Baltimore). 2016;95:e3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


