Abstract
This review focuses on recent clinical and translational discoveries in severe and uncontrolled asthma that now enable phenotyping and personalized therapies in these patients. Although asthma is common in both children and adults and typically responds to standard therapies, a subset of individuals with asthma experience severe and/or persistent symptoms despite appropriate therapies. Airflow obstruction leading to frequent symptoms requiring higher levels of controller therapy is the cardinal feature of severe asthma, but the underlying molecular mechanisms, or endotypes, are diverse and variable between individuals. Two major risk factors that contribute to severe asthma are genetics and environmental exposures that modulate immune responses, and although these often interact in complex manners that are not fully understood, certain endotypes converge in severe asthma. A number of studies have evaluated various features of patients with severe asthma and classified patients into phenotypes with clinical relevance. This phenotyping is now incorporated into clinical practice and can be used to guide advanced biological therapies that target specific molecules and inflammatory pathways that contribute to asthma pathogenesis.
Key Words: asthma, endotype, phenotype, severe asthma
Abbreviations: GWAS, genome-wide association study; ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; NSAID, nonsteroidal antiinflammatory drug; SARP, Severe Asthma Research Program
Asthma is the most common chronic respiratory illness, and 8.2% of the US population has asthma.1 Although recurrent wheezing and airflow obstruction are hallmarks of asthma, other features associated with asthma are variable, including age of onset, comorbidities, laboratory abnormalities, and reversibility of airflow obstruction. These observations indicate that asthma is a heterogenous disease, which is underscored by differences in the exacerbation rate, response to therapy, and remission rate. The heterogeneity of asthma extends beyond clinical phenotypes, and over the last two decades, translational research has established a number of genetic, immunologic, and environmental factors that contribute to asthma risk and asthma pathogenesis. The identification of specific immune pathways related to certain clinical phenotypes has enabled targeted therapy for a subset of patients with asthma and continues to be an area of active research.
The clinical course for individuals diagnosed with asthma is highly variable. Remission is common, and rates of remission are higher in children than adults.2, 3, 4 The persistence of asthma symptoms has been associated with a number of relevant clinical features, including recent symptoms, a reduced FEV1, and female sex.5 In 1999, the European Respiratory Society6 defined therapy-resistant asthma as persistent symptoms or evidence of obstruction despite 6 months of appropriate guideline-based asthma management. This definition has been updated in a joint statement by the European Respiratory Society and American Thoracic Society7 and expanded to include severe asthma, which is defined as asthma requiring high-dose inhaled corticosteroids (ICSs) plus a second controller medication. Persistent and severe asthma frequently occur concurrently with a prevalence of 5% to 10% of all patients with asthma and consume a large proportion of overall asthma resources.8
This review focuses on recent clinical and translational advances related to persistent and severe asthma, with a focus on emerging classification of patients by phenotypes and endotypes and the new therapies that have been developed based on these insights.
Assessment of Asthma and Related Conditions
Accurate Asthma Diagnosis
Symptoms of intermittent dyspnea, wheezing, and cough are classically associated with asthma, but these are nonspecific and presentation with atypical asthma symptoms such as cough is common. Therefore, asthma should be considered whether classical symptoms are present or not, and evaluation for other nonasthma diagnoses is warranted, even when patients present with classical symptoms. Although the diagnosis of asthma is based on clinical findings, objective measurements, such as reversible airflow obstruction, can be used to support a diagnosis. Guidelines for the diagnosis of asthma have been published by several organizations for both children and adults.9,10 These guidelines emphasize the need for a careful history, including identifying common triggers, work exposures, personal history of wheezing, symptoms with exercise, and family history of asthma symptoms. Evaluation in patients > 5 years of age should include spirometry with evaluation for a bronchodilator response and in patients without airflow obstruction consideration of bronchoprovocation testing. Further testing may include measurement of exhaled fractional excretion of nitric oxide, CBC count with differential to evaluate for eosinophilia, serum IgE levels, and allergy testing. In cases of persistent or severe asthma, all of these tests and imaging (chest radiograph and/or chest CT scan) and alpha-1 antitrypsin level and phenotype are indicated. Figure 1 provides a step-by-step approach to evaluation, diagnostic testing, and interventions when severe asthma is considered.
Figure 1.
Overview of evaluation of patients with severe asthma. Flowchart of clinical evaluation (left boxes) with indicated diagnostic testing (right boxes). Tests in italics may be necessary depending on the clinical context and other diagnostic testing results. ANCA = antineutrophil cytoplasmic antibodies; ICS = inhaled corticosteroid.
Alternate diagnoses need to be considered, particularly in circumstances of persistent or severe asthma. Airflow obstruction in the upper airway may be suggested by auscultation and distinguished from asthma with spirometry and otolaryngologic evaluation. However, COPD, bronchiectasis, bronchiolitis obliterans, sarcoidosis, and lower airway obstruction because of masses or foreign objects can mimic spirometry patterns seen in asthma. Dyspnea, particularly when other asthma symptoms are minimal, warrants a broad evaluation; cardiovascular disease, obesity, and anemia are common conditions that can cause persistent dyspnea. Additionally, interstitial lung disease should be considered, particularly in settings of hypoxia. Many years ago, it was recognized that chronic cough in the absence of wheezing or dyspnea can be caused by asthma, but a primary complaint of cough may also be caused by acute infection, chronic infection, acid reflux, aspiration, and interstitial lung disease.11 Medication usage should be carefully reviewed because some asthma symptoms can be induced by over-the-counter and prescription medications. Although these considerations apply generally to both children and adults, particular considerations in children include cystic fibrosis, immunodeficiency, and foreign body aspiration.11 Bronchoscopy with BAL, endobronchial biopsies, transbronchial biopsies, and/or endobronchial ultrasound-guided biopsies of lymph nodes should also be considered in cases of severe asthma to both exclude alternative diagnoses and confirm an accurate diagnosis of asthma.
Comorbid Diseases
Comorbid diseases are common in patients with asthma, and can substantially worsen asthma symptoms. Allergic rhinitis is present in over one-half of children with asthma, and is present at an increased frequency in both adults and children with asthma compared with those without asthma, reaching as high as 82% of nonsmoker adults with severe asthma in one cohort.12,13 Allergic sensitization also frequently co-occurs with asthma, particularly in children. Obesity has an inverse relationship with lung residual volume which can exacerbate dyspnea symptoms in individuals with asthma. However, inflammatory states in the lung and of lung immune cells and adipose tissue are also altered in subjects who are obese, including increased levels of the proinflammatory molecules IL-6, leptin, and tumor necrosis factor-alpha.14, 15, 16 The presence of OSA should also be considered in patients with asthma. Gastroesophageal reflux disease is also a common condition and contributes to worsened symptoms in both adults and children with asthma. In adults who have smoked, asthma and COPD can coexist and has been described as asthma-COPD overlap syndrome.17
Asthma-Associated Diseases
In addition to the comorbid diseases previously described, several diseases contribute to asthma symptoms, but are asthma-associated, rarely occurring in the absence of asthma. Allergic bronchopulmonary aspergillosis occurs in 1% to 2% of patients with asthma, but in patients with persistent asthma, significantly higher rates have been observed, including as high as 38.6% in patients with acute severe asthma exacerbations.18,19 This condition is associated with elevated IgE levels and aspergillus-specific antibodies. Clinically, patients frequently have mucoid impaction and may expectorate airway casts, whereas chest radiographs can reveal transient or migratory opacities. Aspirin sensitivity and chronic rhinosinusitis with nasal polyposis in the setting of asthma has been described as nonsteroidal antiinflammatory drug (NSAID)-exacerbated respiratory disease and classically results in bronchospasm within a few hours of ingestion of aspirin or NSAIDs. Eosinophilic granulomatosis with polyangiitis is a vasculitis, but is almost always preceded by many years of asthma symptoms that do not respond well to ICSs.20 Treatment of eosinophilic granulomatosis with polyangiitis often requires systemic corticosteroids with or without additional immunosuppression. Patients with these conditions will often require additional nonasthma medications, or in the case of NSAID-exacerbated respiratory disease (also known as aspirin-exacerbated respiratory disease), desensitization with high-dose daily aspirin, before their asthma symptoms improve.21 Exercise-induced bronchoconstriction may also contribute to severe and persistent asthma symptoms, and leukotriene receptor antagonists have been shown to improve symptoms in a large portion of patients with exercise-induced bronchoconstriction.22
Accurate diagnosis of asthma and identification of comorbid diseases is crucial for patients who do not demonstrate a rapid response to standard asthma therapies. All patients, but in particular those with refractory or severe symptoms, should have any comorbid conditions treated when possible. In patients where these measures fail, we recommend that physicians refer patients to an asthma specialist with experience in persistent and refractory asthma management.
Asthma Classifications
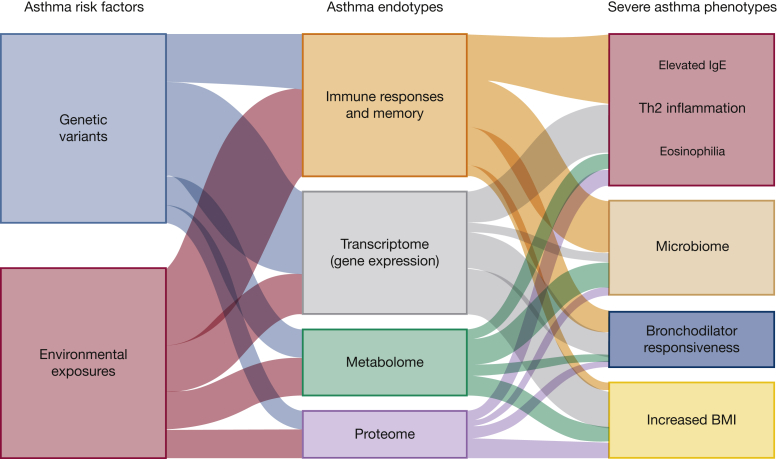
A number of asthma classifications have been described based on the onset of disease, phenotypes, and molecular features. Many of these have overlapping features, and persistent and severe asthma transgress all of these classifications. Here, we highlight aspects within these classifications that are associated with persistent and severe asthma. Figure 2 illustrates the interplay between risk factors, endotypes, and phenotypes.
Figure 2.
Interplay between risk factors, endotypes, and phenotypes in severe asthma. Both genetics and environment contribute to asthma risk and interact in complex ways to influence asthma endotypes or biological processes. Size of the lines indicates the relative proportion to severe asthma phenotype.
Asthma Phenotypes
Recognizing that asthma is a heterogenous disease, asthma phenotypes have been proposed as a way of distinguishing groups of patients with asthma. Phenotypes are features shared by some but not all patients with asthma and can incorporate clinical, physiological, laboratory, and/or molecular data. One advantage of classifying patients into specific asthma phenotypes is that it can provide personalized care.
Childhood-onset and adult-onset asthma differ regarding sex ratios, exacerbation triggers, comorbidities and severity, and genetics, suggesting that these may have nonoverlapping features.23, 24, 25 In childhood-onset asthma, older age of diagnosis (> 15 years of age), sensitization as measured by elevated serum IgE levels, low lung function, and increased airway hyperresponsiveness have been associated with more severe or persistent asthma.26,27 Overall, adult-onset asthma is more severe, has a lower remission rate, and is not as frequently associated with allergy when compared with childhood-onset asthma.28,29 Recently, a study was conducted comparing the genetic architecture of childhood-onset and adult-onset asthma using genome-wide association studies (GWASs) and found that the genetic risk for adult-onset asthma is largely a subset of the genetic risk for childhood-onset asthma but with overall smaller effect sizes. The 17q12-23 locus that has had the strongest association with asthma in many prior GWASs was highly significant in the childhood-onset GWAS but not the adult-onset GWAS. In contrast, the HLA region on chromosome 6 was strongly associated with both childhood-onset and adult-onset asthma.25 In another study, differences in gene expression in several tissues have suggested that severe asthma in children and severe asthma in adults differ regarding gene expression and inflammatory pathways.30 In combination, these studies suggest that genetic risk is larger in childhood-onset asthma, and that adult-onset asthma is influenced to a greater extent by environmental exposures with immune mechanisms contributing to both.
The Severe Asthma Research Program (SARP) has identified five phenotypic clusters of severe asthma, including three clusters in adults and two clusters in children. These clusters are distinguished by the age of onset, allergen sensitization, lung function, medications, health-care utilization, and comorbidities.31,32 Unsupervised clustering using machine learning has been applied to the SARP cohort to identify additional variables that define severe asthma clusters and those who are likely to have responses to corticosteroid treatment.33,34 Within the SARP cohort, patients with severe asthma and frequent exacerbations have been reported to have higher blood eosinophil count, higher BMI, and bronchodilator responsiveness.35 Although SARP phenotypes have been important in identifying features that distinguish different clusters of patients with asthma, the phenotypes cannot be easily incorporated into clinical decision-making. Using a smaller cohort of patients, a different group has reported that frequent exacerbators were more likely to have higher fractional excretion of nitric oxide and a history of smoking.36 A number of other studies have also evaluated different phenotypes in severe asthma, including Unbiased biomarkers in prediction of respiratory disease outcomes (U-BIOPRED) and the Severe and Uncontrolled Asthma Registry from Italy, and have contributed to our understanding of asthma phenotypes and endotypes.
Ongoing clinical trials have been designed to target specific phenotypes or compare responses between patients grouped into different phenotypes.
Asthma Endotypes
Although phenotypes focus on observed or measured features, endotypes subset individuals based on distinct functional or pathobiologic mechanisms. This frequently involves multiomic characterization, and asthma endotypes have been described according to genomic, transcriptomic, epigenomic, proteomic, and/or metabolomic profiles. Additionally, immune profiling enables phenotypes to be linked with endotypes.
Th2 inflammation has been linked to a subset of patients with severe asthma and has primarily focused on IgE levels and blood eosinophil count. One retrospective analysis of patients with asthma, comparing high vs low airway reversibility with bronchodilators suggested that low airway reversibility has higher biomarkers of Th2 immune responses and better disease control.37 Response to mepolizumab, an anti-IL-5 antibody, has also been used to phenotype patients retrospectively, and the predictors of response included blood eosinophil count, airway reversibility, and BMI.38 Limited studies have expanded beyond evaluating Th2 inflammation in severe asthma. One suggested that high soluble ST2, the IL-33 receptor, in patients with stable asthma predicted a severe asthma exacerbation within 3 months.39 Collectively, supervised and unsupervised phenotyping of persistent and severe asthma suggests that patients have higher BMI and higher markers of Th2 inflammation. Not surprisingly, persistent and severe asthma phenotypes have more frequent exacerbations and higher corticosteroid use.
Over 60 genetic loci have been associated with asthma.25 Some of these have been linked to severe asthma. GWASs in children and adults have identified five loci associated with severe exacerbations, and implicated several genes involved in immune responses, including IL33, IL1RL1, and CDHR3.40,41 A GWAS reported 24 loci that were associated with moderate-to-severe asthma, and further analysis suggested that one of the risk loci was associated with increased mucin production.42 A number of studies have investigated gene expression in persistent and severe asthma. These studies have been conducted in various cell types, including airway epithelial cells, whole blood, sputum, and BAL, and it is important to recognize that gene expression is tissue-specific.30,43, 44, 45, 46, 47 Moreover, some studies have compared gene expression response differences after specific treatments. Not surprisingly, several gene expression signatures have been identified, and the signatures implicate that diverse mechanisms contribute to persistent and severe asthma. Many of these mechanisms imply altered immune responses. Although sputum proteomics has been an emerging area of research in asthma, only a few studies have investigated proteomics in severe asthma. In the largest proteomic study of patients with severe asthma, sputum supernatants were compared between patients with severe asthma who smoked and those who were ex-smokers or never smokers, and distinct proteomic patterns were observed between all groups. Smokers in this study had increased levels of colony-stimulating factor 2 protein in their sputum, and ex-smokers had increased levels of CXCL8, neutrophil elastase, and azurocidin 1.48 Metabolomic differences between persistent and severe asthma have been reported in both children and adults, but the significance of these findings is unclear because no single pathway was identified, and there was a high coincidence of corticosteroid use that confounds the results.49, 50, 51 In addition, the airway microbiome in patients with severe asthma has been associated with several clinical phenotypes, including increased Pseudomonadaceae and Enterobacteriaceae in the sputum of patients with severe asthma and corticosteroid responsiveness with Actinobacteria in bronchial brushings.52, 53, 54 Collectively, endotyping in severe asthma has highlighted the prominent role of several immune mechanisms. Further integration of the various observations from different cell types is likely to clarify severe asthma pathobiology.
Current Strategies for Management of Persistent and Severe Asthma
The Expert Panel Report 3 guidelines9 categorize persistent asthma as mild, moderate, or severe based on symptom frequency and spirometry and can be used to direct escalation of therapy. Importantly, these guidelines recommend frequent reevaluation, and when patients meet the European Respiratory Society and American Thoracic Society criteria6,7 for persistent asthma, they have been continued on appropriate therapy, including high-dose ICSs and a long-acting beta-agonist (LABA), for at least 6 months. Often leukotriene receptor antagonists are added if symptoms persist despite high-dose ICS and LABA therapy; however, the benefit of this therapy in severe asthma is unclear. The Global Initiative for Asthma55 was updated in early 2019 and provides a detailed framework for the evaluation and management of asthma.
Two studies evaluated the impact of increasing the ICS dose in children or adults to prevent exacerbations, but most subjects in these studies did not appear to meet the criteria for persistent asthma. In children 5 to 11 years of age with mild-to-moderate persistent asthma on low-dose ICSs, quintupling the dose of ICSs did not change the rate of severe asthma exacerbations compared with control subjects assigned to usual controller medications and a self-management plan.56 In a separate study of adults who had an exacerbation within the previous 12 months, quadrupling the dose of ICSs resulted in a modest reduction of severe exacerbations after 1 year (45% for the intervention group vs 52% for the control group).57 These studies suggest that in children and most adults, increasing ICS dosing does not reduce exacerbation rate. However, in a small subset of adult with asthma, aggressive increases in ICS dosing may reduce exacerbation rate as demonstrated by the modest reduction in severe exacerbations.
Oral corticosteroids were first reported to be of benefit in treating severe asthma exacerbations in 1956, and they continue to be the mainstay of pharmacotherapy for persistent and severe asthma treatment.58 Corticosteroids elicit potent antiinflammatory responses from a number of different cell types that can dramatically alter asthma symptoms. Although most patients with severe asthma respond to systemic corticosteroid therapy, albeit sometimes only at high doses, short-term and long-term adverse effects of corticosteroid therapy often complicate their chronic use in severe asthma.
The identification of altered immune responses in subsets of patients with asthma has led to phenotyping and now the regular use of targeted immunomodulatory agents. Severe and persistent asthma with concurrent allergen sensitization and an elevated IgE level warrants consideration of omalizumab therapy (Table 1 summarizes features of omalizumab and other severe asthma biologic therapies, including mechanisms, patient considerations, routes of administration, dosing frequencies, and contraindications). Omalizumab is an anti-IgE antibody administered subcutaneously that has been approved for the treatment of moderate-to-severe persistent asthma in both children ≥ 6 years of age and adults who have also demonstrated allergic sensitization. It is dosed based on serum IgE levels and weight and is administered every 2 to 4 weeks. Omalizumab has not been shown to be effective in acute asthma exacerbations and does not have a role in the management of acute bronchospasm or status asthmaticus; however, one case report has described clinical improvement and reduced serum IgE level for a patient on salvage therapy for several days after presenting with status asthmaticus.59 In the first two randomized controlled trials of omalizumab, adults with moderate-to-severe asthma on ICSs and SABA (short-acting beta agonist; not on LABA) had fewer exacerbations per patient from > 5% to < 2.3% on stable ICS dosing during a 16-week period of treatment. Omalizumab also reduced the number of exacerbations in patients reducing ICS dosing because of improved control of asthma symptoms during a 12-week period of treatment.60,61 A third study in adults that did not exclude patients on LABA did not show a difference in the percent of patients with one or more exacerbations.62 In adults with severe asthma on high-dose ICSs and LABA, the incidence of exacerbations was reduced by 25% in patients receiving omalizumab compared with placebo; however, the rates of exacerbations per subject during the 12-month study were relatively low at 0.66 and 0.88, respectively.63 In a study of a limited number of children with severe asthma (n = 34), treatment with omalizumab was associated with improved symptoms, improved FEV1, and a reduction in oral corticosteroid dose.64 A number of other clinical trials in both children and adults have shown omalizumab to be beneficial in severe asthma and mild-to-persistent asthma. A retrospective analysis of data from two clinical trials in adults with moderate-to-severe asthma suggests that patients with higher blood eosinophil counts have a higher percent reduction in asthma exacerbations65; however, a recent study evaluating the course of asthma symptoms and biomarkers in adults while on omalizumab treatment did not find any association between exacerbation rates and elevated fractional excretion of nitric oxide of ≥ 25 parts per billion or eosinophil count of ≥ 300 cells/μL.66 Therefore, although children and adults with severe asthma and elevated IgE levels appear to benefit from omalizumab treatment, further subphenotyping of patients to predict response is not currently warranted.
Table 1.
Immunomodulatory Biologic Agents Approved for Use in Asthma
| Pathway | IgE | IL-4 and IL-13 | IL-5 | ||
|---|---|---|---|---|---|
| Mechanism | Blocks IgE-mediated immune stimulation | Binds to IL-4R alpha subunit and blocks IL-4 and IL-13 cytokine-induced inflammatory responses | Block IL-5 binding to the receptor and reduces survival of eosinophils | ||
| Medication | Omalizumab | Dupilumab | Mepolizumab | Benralizumab | Reslizumab |
| Target | Anti-IgE monoclonal antibody | Anti-IL-4R alpha monoclonal antibody | Anti-IL-5 monoclonal antibody | Anti-IL-5 alpha monoclonal antibody | Anti-IL-5 receptor monoclonal antibody |
| Considerations | Elevated IgE | Atopic dermatitis and/or eosinophilia | Eosinophilia | Eosinophilia | Eosinophilia |
| Indications | Add-on therapy for patients ≥ 6 y old with moderate-to-severe persistent asthma inadequately controlled on ICS and a total serum IgE level between 30 and 700 units/mL and a positive allergen test | Moderate to severe asthma in patients ≥ 12 y old; oral corticosteroid-dependent asthma or asthma with severe atopic dermatitis or chronic rhinosinusitis with nasal polyps | Severe asthma in patients ≥ 12 y old with eosinophilia | Severe asthma in patients ≥ 12 y old with eosinophilia | Severe asthma in patients ≥ 18 y old with eosinophilia |
| Dosing route | Subcutaneous | Subcutaneous | Subcutaneous | Subcutaneous | IV |
| Dosing interval | Every 2-4 wk depending on pretreatment serum IgE level | Every 2 wk | Every 4 wk | Every 4 wk for the first three doses, then once every 4 or 8 wk | Every 4 wk |
| Outcomes observed in clinical trials | Reduced exacerbations by approximately 25%-50% in subjects with an FEV1 between 40% and 80% predicted | Reduced exacerbations by approximately 50% in patients with severe asthma compared with placebo and improvement in FEV1 Among patients on oral glucocorticoids, 70% had a reduction in the dose, compared with 42% in placebo |
Fewer exacerbations compared with placebo and reduced corticosteroid dose in patients requiring maintenance corticosteroids | Reduced exacerbation rate in moderate or severe asthma. In patients with eosinophil counts ≥ 300 cells/μL, rate ratio of < 0.55 for both dosing regimens and improved prebronchodilator FEV1. Reduced glucocorticoid use with an odds of reduction of 4.09 compared with placebo |
Decreased asthma exacerbations by as much as 59%. Improvement in lung function. Improvement in asthma symptoms and asthma-related quality of life |
| Common (> 3%) or severe side effects | Headache (6%-12%) Arthralgias (3%-8%) Anaphylaxis (0.3%) – black box warning Serum sickness-like reaction Cardiovascular events, including transient ischemic attack and ischemic stroke Eosinophilic granulomatosis and polyangiitis |
Injection site reaction (10%-18%) Oral herpes simplex infection (4%) Antibody response with neutralizing activity (2%-4%) Conjunctivitis (10%) Eosinophilic granulomatosis with polyangiitis and eosinophilic pneumonia Hypersensitivity reactions |
Headache (19%) Injection site reaction (8%-15%) |
Antibody response with neutralizing activity (12%) Headache (8%) Pharyngitis (5%) |
Antibody to medication (5%) Transient increased creatine phosphokinase (20%) Oropharyngeal pain (3%) Increased malignancies observed at 6 mo (diverse types) Anaphylaxis (0.3%) – black box warning |
Serious side effects are in bold font.
Dupilumab is another monoclonal antibody that has been developed and was recently approved for treatment of moderate-to-severe asthma. Similar to omalizumab, dupilumab targets a molecule in the Th2 pathway, but dupilumab targets the IL-4 receptor alpha molecule and blocks both IL-4 and IL-13 signaling. In a study of subjects with glucocorticoid-dependent asthma, dupilumab resulted in a decrease in the corticosteroid dose of 70% compared with a decrease of 42% in the placebo group. Treated subjects had a 59% lower rate of severe asthma exacerbation compared with the placebo group.67 Dupilumab was also evaluated in subjects with moderate-to-severe uncontrolled asthma at two different doses. Both doses (200 and 300 mg every 2 weeks) reduced the rate of asthma exacerbations by nearly 50% compared with placebo groups, and the largest reduction in exacerbations was seen in those with elevated eosinophil counts (≥ 300 cells/μL) with an OR of 0.34 compared with placebo.68
A subset of patients with asthma have increased blood and/or sputum eosinophil counts and this has been identified as a risk factor for asthma exacerbations.69,70 IL-5 is a cytokine that potently enhances eosinophil viability, and mepolizumab was the first anti-IL-5 therapy to be approved for severe asthma. A clinical trial of mepolizumab in subjects with asthma with a history of two or more exacerbations in the previous year while on high-dose ICSs plus an additional inhaler demonstrated a reduction in the number of exacerbations requiring corticosteroid therapy and/or ED visits compared with a placebo group. In a separate clinical trial of mepolizumab in subjects who required oral corticosteroids at the time of enrollment, daily corticosteroid dose was decreased compared with placebo.
Two additional therapies, reslizumab and benralizumab, block IL-5 activity and are approved for use in severe asthma. Reslizumab has been shown to reduce the exacerbation rate and increase the time to exacerbation when compared with control subjects.71 Another trial of reslizumab included subjects with blood eosinophil counts of ≥ 400 cells/μL and demonstrated an increase in prebronchodilator FEV1 at 16 weeks compared with baseline FEV1 values, and the magnitude of effect was dose-dependent.72 Benralizumab has also shown to be of benefit in subsets of patients with severe asthma. In one clinical trial of subjects with two exacerbations while on high-dose ICSs and LABA, subjects were assigned to either 4- or 8-week dosing intervals and stratified based on blood eosinophil counts. Patients with blood eosinophil counts ≥ 300 cells/μL had fewer exacerbations than the placebo group (also with elevated eosinophil counts) in both the 4- and 8-week dosing groups and had an improvement in prebronchodilator FEV1, whereas patients with blood eosinophil counts < 300 cells/μL did not have a difference in exacerbation frequency for either dosing interval compared with the placebo-treated group.73 Concurrently, a separate clinical trial evaluating benralizumab in a similar study design with enrollment criteria targeting patients with more severe asthma also demonstrated a decrease in annual exacerbations and prebronchodilator FEV1 in patients on ICSs and LABA with blood eosinophil counts ≥ 300 cells/μL with either 4- or 8-week dosing intervals.74 This study also observed a decreased rate of exacerbations in subjects with blood eosinophil counts < 300 cells/μL as well, but there was no difference in prebronchodilator FEV1 in this group compared with the placebo group. These clinical trials indicate that patients with severe asthma and elevated blood eosinophil counts are most likely to benefit from the addition of anti-IL-5 therapies, and those without elevated blood eosinophil counts may also have fewer exacerbations with this medication.
Azithromycin, a macrolide antibiotic that inhibits bacterial protein synthesis and also has antiinflammatory mechanisms of action, has been studied in clinical trials of asthma. In the largest randomized trial, addition of 500 mg three times per week of azithromycin to ICSs and long-acting bronchodilators reduced asthma exacerbations with an incident rate ratio of 0.59.75 Asthma-related quality of life was also improved in this study. Subgroup analysis suggested that subjects with eosinophilia responded better than subjects without eosinophilia. However, this result is overshadowed by other smaller studies and meta-analyses that have failed to demonstrate the efficacy of azithromycin in severe or poorly controlled asthma. Because of these conflicting results, azithromycin has not been added to guidelines for asthma treatment. Several ongoing studies of azithromycin are focusing on how it alters the microbiome and whether these are clinically significant.
Limiting the effects of smooth muscle contraction has been targeted by bronchial thermoplasty, which delivers radiofrequency energy to the bronchial walls. Interestingly, endobronchial biopsies taken after bronchial thermoplasty have shown a reduction in the number of nerve fibers in airway smooth muscle and epithelium. Bronchial thermoplasty was approved by the Food and Drug Administration nearly a decade ago and clinical trials indicate that it may have a role in management of adults with severe persistent asthma.
Potential Future Targets
Therapies that target other inflammatory molecules are currently being evaluated for potential use in asthma. IL-13 is a chemokine that elicits cell-specific responses in a number of cell types that are relevant in asthma, including airway smooth muscle contraction, class switching to IgE in B cells, and eosinophil trafficking. However, randomized controlled trials of two monoclonal antibodies that block IL-13 have demonstrated only modest or no benefit in severe and persistent asthma. Blocking thymic stromal lymphopoietin, a cytokine that initiates allergic inflammation and is secreted by epithelial cells, has been evaluated in limited clinical trials and is undergoing further consideration in clinical trials of severe asthma (tezepelumab76, 77, 78). IL-2 is a potent autocrine chemokine that promotes the proliferation of antigen-specific T cells, and blocking IL-2 is being investigated in asthma. The cytokine IL-33 activates ILC2 cells and promotes Th2 responses, and biological agents that inhibit IL-33 activity are being studied (REGN350079 and etokimab80). Other targets being investigated in early clinical trials include the transcription factor GATA3 (SB01081), prostaglandin D2 receptor (fevipiprant/QAW039A82, 83, 84), tyrosine kinases (imatinib85), and endothelin-A receptor (sitaxenten86). Although there is strong evidence that all of these pathways can contribute to severe asthma, targeted therapies are limited by the underlying phenotypic and endotypic variation between patients. Therefore, although some targeted therapies may be of limited utility in the general asthma population, they may be of substantial benefit to a subset of patients with asthma.
Conclusions
As physicians have recognized for many years, severe and persistent asthma arises from heterogenous conditions that result in shared symptoms. More recently, a number of studies have reinforced the heterogeneity at the level of asthma phenotypes and asthma endotypes. These findings and in particular the delineation of distinct immune phenotypes have led to the development and testing of new classes of asthma treatments that target specific immune pathways. Emerging therapies now enable physicians to provide personalized medicine to patients with severe asthma.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This work was funded by the National Institues of Health [grant NHLBI T32 HL007605].
Supplementary Data
References
- 1.Centers for Disease Control and Prevention Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001--2009. MMWR Morb Mortal Wkly Rep. 2011;60(17):547–552. [PubMed] [Google Scholar]
- 2.Holm M., Omenaas E., Gislason T. Remission of asthma: a prospective longitudinal study from northern Europe (RHINE study) Eur Respir J. 2007;30(1):62–65. doi: 10.1183/09031936.00121705. [DOI] [PubMed] [Google Scholar]
- 3.Ronmark E., Jonsson E., Lundback B. Remission of asthma in the middle aged and elderly: report from the Obstructive Lung Disease in Northern Sweden study. Thorax. 1999;54(7):611–613. doi: 10.1136/thx.54.7.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vonk J.M., Postma D.S., Boezen H.M. Childhood factors associated with asthma remission after 30 year follow up. Thorax. 2004;59(11):925–929. doi: 10.1136/thx.2003.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tai A., Tran H., Roberts M. Outcomes of childhood asthma to the age of 50 years. J Allergy Clin Immunol. 2014;133(6):1572–1578.e1573. doi: 10.1016/j.jaci.2013.12.1033. [DOI] [PubMed] [Google Scholar]
- 6.Chung K.F., Godard P., Adelroth E. Difficult/therapy-resistant asthma: the need for an integrated approach to define clinical phenotypes, evaluate risk factors, understand pathophysiology and find novel therapies. ERS Task Force on Difficult/Therapy-Resistant Asthma. European Respiratory Society. Eur Respir J. 1999;13(5):1198–1208. doi: 10.1034/j.1399-3003.1999.13e43.x. [DOI] [PubMed] [Google Scholar]
- 7.Chung K.F., Wenzel S.E., Brozek J.L. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 8.Hekking P.P., Wener R.R., Amelink M., Zwinderman A.H., Bouvy M.L., Bel E.H. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi: 10.1016/j.jaci.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 9.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 10.Lougheed M.D., Lemiere C., Ducharme F.M. Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults. Can Respir J. 2012;19(2):127–164. doi: 10.1155/2012/635624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corrao W.M., Braman S.S., Irwin R.S. Chronic cough as the sole presenting manifestation of bronchial asthma. N Engl J Med. 1979;300(12):633–637. doi: 10.1056/NEJM197903223001201. [DOI] [PubMed] [Google Scholar]
- 12.Hamouda S., Karila C., Connault T., Scheinmann P., de Blic J. Allergic rhinitis in children with asthma: a questionnaire-based study. Clin Exp Allergy. 2008;38(5):761–766. doi: 10.1111/j.1365-2222.2008.02953.x. [DOI] [PubMed] [Google Scholar]
- 13.Wilson S.J., Ward J.A., Sousa A.R. Severe asthma exists despite suppressed tissue inflammation: findings of the U-BIOPRED study. Eur Respir J. 2016;48(5):1307–1319. doi: 10.1183/13993003.01129-2016. [DOI] [PubMed] [Google Scholar]
- 14.Leiria L.O., Martins M.A., Saad M.J. Obesity and asthma: beyond T(H)2 inflammation. Metabolism. 2015;64(2):172–181. doi: 10.1016/j.metabol.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Shore S.A. Obesity, airway hyperresponsiveness, and inflammation. J Appl Physiol (1985) 2010;108(3):735–743. doi: 10.1152/japplphysiol.00749.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters M.C., McGrath K.W., Hawkins G.A. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4(7):574–584. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postma D.S., Rabe K.F. The asthma-COPD overlap syndrome. N Engl J Med. 2015;373(13):1241–1249. doi: 10.1056/NEJMra1411863. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal R. Allergic bronchopulmonary aspergillosis. Chest. 2009;135(3):805–826. doi: 10.1378/chest.08-2586. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal R., Nath A., Aggarwal A.N., Gupta D., Chakrabarti A. Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with acute severe asthma in a respiratory intensive care unit in North India. Mycoses. 2010;53(2):138–143. doi: 10.1111/j.1439-0507.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- 20.Cottin V., Bel E., Bottero P. Revisiting the systemic vasculitis in eosinophilic granulomatosis with polyangiitis (Churg-Strauss): a study of 157 patients by the Groupe d'Etudes et de Recherche sur les Maladies Orphelines Pulmonaires and the European Respiratory Society Taskforce on eosinophilic granulomatosis with polyangiitis (Churg-Strauss) Autoimmun Rev. 2017;16(1):1–9. doi: 10.1016/j.autrev.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Fahrenholz J.M. Natural history and clinical features of aspirin-exacerbated respiratory disease. Clin Rev Allergy Immunol. 2003;24(2):113–124. doi: 10.1385/CRIAI:24:2:113. [DOI] [PubMed] [Google Scholar]
- 22.Edelman J.M., Turpin J.A., Bronsky E.A. Oral montelukast compared with inhaled salmeterol to prevent exercise-induced bronchoconstriction. A randomized, double-blind trial. Exercise Study Group. Ann Intern Med. 2000;132(2):97–104. doi: 10.7326/0003-4819-132-2-200001180-00002. [DOI] [PubMed] [Google Scholar]
- 23.Bush A., Menzies-Gow A. Phenotypic differences between pediatric and adult asthma. Proc Am Thorac Soc. 2009;6(8):712–719. doi: 10.1513/pats.200906-046DP. [DOI] [PubMed] [Google Scholar]
- 24.Busse W., Banks-Schlegel S.P., Larsen G.L. Childhood- versus adult-onset asthma. Am J Respir Crit Care Med. 1995;151(5):1635–1639. doi: 10.1164/ajrccm.151.5.7735626. [DOI] [PubMed] [Google Scholar]
- 25.Pividori M., Schoettler N., Nicolae D.L., Ober C., Im H.K. Shared and distinct genetic risk factors for childhood-onset and adult-onset asthma: genome-wide and transcriptome-wide studies. Lancet Respir Med. 2019;7(6):509–522. doi: 10.1016/S2213-2600(19)30055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzpatrick A.M., Teague W.G. Severe asthma in children: insights from the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. Pediatr Allergy Immunol Pulmonol. 2010;23(2):131–138. doi: 10.1089/ped.2010.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dodge R., Martinez F.D., Cline M.G., Lebowitz M.D., Burrows B. Early childhood respiratory symptoms and the subsequent diagnosis of asthma. J Allergy Clin Immunol. 1996;98(1):48–54. doi: 10.1016/s0091-6749(96)70225-7. [DOI] [PubMed] [Google Scholar]
- 28.De Marco R., Locatelli F., Cerveri I. Incidence and remission of asthma: a retrospective study on the natural history of asthma in Italy. J Allergy Clin Immunol. 2002;110(2):228–235. doi: 10.1067/mai.2002.125600. [DOI] [PubMed] [Google Scholar]
- 29.Shaaban R., Zureik M., Soussan D. Rhinitis and onset of asthma: a longitudinal population-based study. Lancet. 2008;372(9643):1049–1057. doi: 10.1016/S0140-6736(08)61446-4. [DOI] [PubMed] [Google Scholar]
- 30.Hekking P.P., Loza M.J., Pavlidis S. Pathway discovery using transcriptomic profiles in adult-onset severe asthma. J Allergy Clin Immunol. 2018;141(4):1280–1290. doi: 10.1016/j.jaci.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 31.Fitzpatrick A.M., Teague W.G., Meyers D.A. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011;127(2):382–389. doi: 10.1016/j.jaci.2010.11.015. e381-e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore W.C., Meyers D.A., Wenzel S.E. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu W., Bleecker E., Moore W. Unsupervised phenotyping of Severe Asthma Research Program participants using expanded lung data. J Allergy Clin Immunol. 2014;133(5):1280–1288. doi: 10.1016/j.jaci.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu W., Bang S., Bleecker E.R. Multiview cluster analysis identifies variable corticosteroid response phenotypes in severe asthma. Am J Respir Crit Care Med. 2019;199(11):1358–1367. doi: 10.1164/rccm.201808-1543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denlinger L.C., Phillips B.R., Ramratnam S. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195(3):302–313. doi: 10.1164/rccm.201602-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kupczyk M., ten Brinke A., Sterk P.J. Frequent exacerbators--a distinct phenotype of severe asthma. Clin Exp Allergy. 2014;44(2):212–221. doi: 10.1111/cea.12179. [DOI] [PubMed] [Google Scholar]
- 37.Busse W.W., Holgate S.T., Wenzel S.W. Biomarker profiles in asthma with high vs low airway reversibility and poor disease control. Chest. 2015;148(6):1489–1496. doi: 10.1378/chest.14-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortega H., Li H., Suruki R., Albers F., Gordon D., Yancey S. Cluster analysis and characterization of response to mepolizumab. A step closer to personalized medicine for patients with severe asthma. Ann Am Thorac Soc. 2014;11(7):1011–1017. doi: 10.1513/AnnalsATS.201312-454OC. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe M., Nakamoto K., Inui T. Serum sST2 levels predict severe exacerbation of asthma. Respir Res. 2018;19(1):169. doi: 10.1186/s12931-018-0872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonnelykke K., Sleiman P., Nielsen K. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014;46(1):51–55. doi: 10.1038/ng.2830. [DOI] [PubMed] [Google Scholar]
- 41.Wan Y.I., Shrine N.R., Soler Artigas M. Genome-wide association study to identify genetic determinants of severe asthma. Thorax. 2012;67(9):762–768. doi: 10.1136/thoraxjnl-2011-201262. [DOI] [PubMed] [Google Scholar]
- 42.Shrine N., Portelli M.A., John C. Moderate-to-severe asthma in individuals of European ancestry: a genome-wide association study. Lancet Respir Med. 2019;7(1):20–34. doi: 10.1016/S2213-2600(18)30389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bigler J., Boedigheimer M., Schofield J.P.R. A severe asthma disease signature from gene expression profiling of peripheral blood from U-BIOPRED cohorts. Am J Respir Crit Care Med. 2017;195(10):1311–1320. doi: 10.1164/rccm.201604-0866OC. [DOI] [PubMed] [Google Scholar]
- 44.Modena B.D., Bleecker E.R., Busse W.W. Gene expression correlated with severe asthma characteristics reveals heterogeneous mechanisms of severe disease. Am J Respir Crit Care Med. 2017;195(11):1449–1463. doi: 10.1164/rccm.201607-1407OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singhania A., Rupani H., Jayasekera N. Altered epithelial gene expression in peripheral airways of severe asthma. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0168680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hekking P.P., Loza M.J., Pavlidis S. Transcriptomic gene signatures associated with persistent airflow limitation in patients with severe asthma. Eur Respir J. 2017;50(3) doi: 10.1183/13993003.02298-2016. [DOI] [PubMed] [Google Scholar]
- 47.Rossios C., Pavlidis S., Hoda U. Sputum transcriptomics reveal upregulation of IL-1 receptor family members in patients with severe asthma. J Allergy Clin Immunol. 2018;141(2):560–570. doi: 10.1016/j.jaci.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi K., Pavlidis S., Ng Kee Kwong F. Sputum proteomics and airway cell transcripts of current and ex-smokers with severe asthma in U-BIOPRED: an exploratory analysis. Eur Respir J. 2018;51(5) doi: 10.1183/13993003.02173-2017. [DOI] [PubMed] [Google Scholar]
- 49.Fitzpatrick A.M., Park Y., Brown L.A., Jones D.P. Children with severe asthma have unique oxidative stress-associated metabolomic profiles. J Allergy Clin Immunol. 2014;133(1):258–261. doi: 10.1016/j.jaci.2013.10.012. e251-e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park Y.H., Fitzpatrick A.M., Medriano C.A., Jones D.P. High-resolution metabolomics to identify urine biomarkers in corticosteroid-resistant asthmatic children. J Allergy Clin Immunol. 2017;139(5):1518–1524.e1514. doi: 10.1016/j.jaci.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 51.Reinke S.N., Gallart-Ayala H., Gomez C. Metabolomics analysis identifies different metabotypes of asthma severity. Eur Respir J. 2017;49(3) doi: 10.1183/13993003.01740-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang Y.J., Nariya S., Harris J.M. The airway microbiome in patients with severe asthma: associations with disease features and severity. J Allergy Clin Immunol. 2015;136(4):874–884. doi: 10.1016/j.jaci.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li N., Qiu R., Yang Z. Sputum microbiota in severe asthma patients: relationship to eosinophilic inflammation. Respir Med. 2017;131:192–198. doi: 10.1016/j.rmed.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 54.Millares L., Bermudo G., Perez-Brocal V. The respiratory microbiome in bronchial mucosa and secretions from severe IgE-mediated asthma patients. BMC Microbiol. 2017;17(1):20. doi: 10.1186/s12866-017-0933-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Global Initiative for Asthma . 2019. Global Strategy for Asthma Management and Prevention. www.ginasthma.org. Accessed November 13, 2019. [Google Scholar]
- 56.Jackson D.J., Bacharier L.B., Mauger D.T. Quintupling inhaled glucocorticoids to prevent childhood asthma exacerbations. N Engl J Med. 2018;378(10):891–901. doi: 10.1056/NEJMoa1710988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McKeever T., Mortimer K., Wilson A. Quadrupling inhaled glucocorticoid dose to abort asthma exacerbations. N Engl J Med. 2018;378(10):902–910. doi: 10.1056/NEJMoa1714257. [DOI] [PubMed] [Google Scholar]
- 58.CONTROLLED trial of effects of cortisone acetate in status asthmaticus; report to the Medical Research Council by the subcommittee on clinical trials in asthma. Lancet. 1956;271(6947):803–806. [PubMed] [Google Scholar]
- 59.Milger K., Schroeder I., Behr J., Meis T., Wulffen W.V., Kneidinger N. Omalizumab rescue therapy for refractory status asthmaticus. Ann Intern Med. 2019;170(2):351–352. doi: 10.7326/L18-0359. [DOI] [PubMed] [Google Scholar]
- 60.Busse W., Corren J., Lanier B.Q. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 61.Soler M., Matz J., Townley R. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18(2):254–261. doi: 10.1183/09031936.01.00092101. [DOI] [PubMed] [Google Scholar]
- 62.Holgate S.T., Chuchalin A.G., Hebert J. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy. 2004;34(4):632–638. doi: 10.1111/j.1365-2222.2004.1916.x. [DOI] [PubMed] [Google Scholar]
- 63.Hanania N.A., Alpan O., Hamilos D.L. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154(9):573–582. doi: 10.7326/0003-4819-154-9-201105030-00002. [DOI] [PubMed] [Google Scholar]
- 64.Brodlie M., McKean M.C., Moss S., Spencer D.A. The oral corticosteroid-sparing effect of omalizumab in children with severe asthma. Arch Dis Child. 2012;97(7):604–609. doi: 10.1136/archdischild-2011-301570. [DOI] [PubMed] [Google Scholar]
- 65.Casale T.B., Chipps B.E., Rosen K. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy. 2018;73(2):490–497. doi: 10.1111/all.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casale T.B., Luskin A.T., Busse W. Omalizumab effectiveness by biomarker status in patients with asthma: evidence from PROSPERO, a prospective real-world study. J Allergy Clin Immunol Pract. 2019;7(1):156–164.e151. doi: 10.1016/j.jaip.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 67.Rabe K.F., Nair P., Brusselle G. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475–2485. doi: 10.1056/NEJMoa1804093. [DOI] [PubMed] [Google Scholar]
- 68.Castro M., Corren J., Pavord I.D. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi: 10.1056/NEJMoa1804092. [DOI] [PubMed] [Google Scholar]
- 69.Malinovschi A., Fonseca J.A., Jacinto T., Alving K., Janson C. Exhaled nitric oxide levels and blood eosinophil counts independently associate with wheeze and asthma events in National Health and Nutrition Examination Survey subjects. J Allergy Clin Immunol. 2013;132(4):821–827. doi: 10.1016/j.jaci.2013.06.007. e821-e825. [DOI] [PubMed] [Google Scholar]
- 70.Zeiger R.S., Schatz M., Li Q. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J Allergy Clin Immunol Pract. 2014;2(6):741–750. doi: 10.1016/j.jaip.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 71.Castro M., Zangrilli J., Wechsler M.E. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–366. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 72.Bjermer L., Lemiere C., Maspero J., Weiss S., Zangrilli J., Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150(4):789–798. doi: 10.1016/j.chest.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 73.Bleecker E.R., FitzGerald J.M., Chanez P. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 74.FitzGerald J.M., Bleecker E.R., Nair P. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 75.Gibson P.G., Yang I.A., Upham J.W. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10095):659–668. doi: 10.1016/S0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]
- 76.National Institutes of Health. Long-term safety of tezepelumab in Japanese subjects with inadequately controlled severe asthma (NOZOMI). NCT04048343. ClinicalTrials.gov. Bethesda, MD; National Institutes of Health; 2019. https://clinicaltrials.gov/ct2/show/NCT04048343. Updated November 7, 2019.
- 77.National Institutes of Health. Study to evaluate tezepelumab in adults and adolescents with severe uncontrolled asthma (NAVIGATOR). NCT03347279. ClinicalTrials.gov. Bethesda, MD; National Institutes of Health; 2017. https://clinicaltrials.gov/ct2/show/NCT03347279. Updated October 24, 2019.
- 78.National Institutes of Health. Study to evaluate tezepelumab in adults with severe uncontrolled asthma (DIRECTION). NCT03927157. ClinicalTrials.gov. Bethesda, MD; National Institutes of Health; 2019. https://clinicaltrials.gov/ct2/show/NCT03927157. Updated October 25, 2019.
- 79.National Institutes of Health. Study of REGN3500 and dupilumab in patients with asthma. NCT03112577. ClinicalTrials.gov. Bethesda, MD; National Institutes of Health; 2017. https://clinicaltrials.gov/ct2/show/NCT03112577. Updated July 19, 2019.
- 80.National Institutes of Health. A study investigating the efficacy, safety and PK profile of ANB020 administered to adult subjects with moderate-to-severe AD (ATLAS). NCT03533751. ClinicalTrials.gov. Bethesda, MD; National Institutes of Health; 2018. https://clinicaltrials.gov/ct2/show/NCT03533751. Updated May 20, 2019.
- 81.National Institutes of Health. Safety, efficacy, PK, and PD characteristics of orally inhaled SB010 in male patients with mild asthma. NCT01743768. ClinicalTrials.gov. Bethesda, MD; National Institutes of Health; 2012. https://clinicaltrials.gov/ct2/show/NCT01743768. Updated May 20, 2015.
- 82.National Institutes of Health. Systemic corticosteroids avoidance study in severe asthma patients. NCT03629249. ClinicalTrials.gov. Bethesda, MD; National Institutes of Health; 2018. https://clinicaltrials.gov/ct2/show/NCT03629249. Updated June 6, 2019.
- 83.National Institutes of Health. Study of efficacy and safety of QAW039 in patients with severe asthma inadequately controlled with standard of care asthma treatment. NCT02563067. ClinicalTrials.gov. Bethesda, MD; National Institutes of Health; 2015. https://clinicaltrials.gov/ct2/show/NCT02563067. Updated August 28, 2019.
- 84.National Institutes of Health. Study of efficacy and safety of QAW039 in patients with severe asthma inadequately controlled with standard of care asthma treatment. NCT02555683. ClinicalTrials.gov. Bethesda, MD; National Institutes of Health; 2015. https://clinicaltrials.gov/ct2/show/NCT02555683. Updated December 16, 2019.
- 85.National Institutes of Health. Effects of cKit inhibition by imatinib in patients with severe refractory asthma. NCT01097694. ClinicalTrials.gov. Bethesda, MD; National Institutes of Health; 2017. https://clinicaltrials.gov/ct2/show/NCT01097694. Updated May 19, 2017.
- 86.National Institutes of Health. Study on the effects of sitaxsentan on airway remodeling in patients with severe asthma. NCT01050491. ClinicalTrials.gov. Bethesda, MD; National Institutes of Health; 2010. https://clinicaltrials.gov/ct2/show/NCT01050491. Updated May 10, 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.