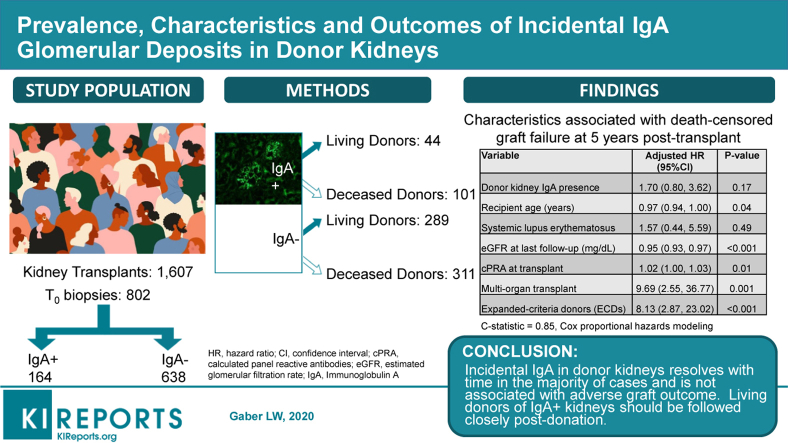
Abstract
Introduction
Incidental IgA deposits in donor kidneys have unknown sequelae and may predate clinical kidney disease if primed by adverse immunologic or hemodynamic stimuli or may remain dormant.
Methods
The presence of incidental IgA in post-implantation (T0) biopsies from living (LDK) and deceased donor (DDK) kidneys, and its relationship to post-transplant patient and graft outcomes was investigated in an ethnically diverse US population at a large transplant center.
Results
Mesangial IgA was present in 20.4% of 802 T0 biopsies; 13.2% and 24.5% of LDK and DDK, respectively. Donors with incidental IgA deposits were more likely to have hypertension and be of Hispanic or Asian origin. Intensity of IgA staining was 1+ (57.3%), 2+ (26.8%), or 3+ (15.8%) of the T0 IgA+ biopsies. Mesangial pathology correlated with higher-intensity IgA staining with less clearance on follow-up (53.8%) versus 79.2% without mesangial pathology. IgA cleared in 91%, 63%, and 40% of follow-up biopsies with 1+, 2+, and 3+ IgA staining, respectively. Early post-transplant rejection and rejection-related graft loss occurred more frequently in IgA+ kidney recipients; however, 5-year kidney function and graft survival were comparable to kidneys without IgA.
Conclusion
This first and largest report of incidental IgA in T0 biopsies of LDK and DDK in a US ethnically diverse population demonstrated no adverse association between the presence of IgA in donor kidneys and graft or patient survival. Whether IgA in donor kidneys represents latent IgA nephropathy (IgAN) is uncertain; nevertheless, living donors who demonstrate IgA on T0 biopsy deserve careful follow-up.
Keywords: biopsy, glomerulonephritis, IgA, kidney transplantation, living donor, pathology
Graphical abstract
See Commentary on Page 1853
IgA as an incidental finding on immunofluorescence or immunohistochemistry in biopsies performed immediately following reperfusion (T0) of transplanted kidneys has been reported.1, 2, 3 Most reports of incidental IgA from T0 biopsies originate from China and Japan,4,5 with single reports from Belgium3 and Chile6 but none from the United States. The larger studies came from Japan and China, populations known to have a higher incidence of IgAN.7 Although 1 study reported an increased incidence of acute rejection in recipients of kidneys with incidental IgA deposits,4 studies providing outcome information report resolution of the deposits and graft survival comparable to grafts without IgA.4,5
IgAN, a leading cause of glomerulonephritis worldwide, has complex epidemiology with variable clinical and histological presentations.8 A higher prevalence of IgAN has been reported in Asia compared with North America, whereas the lowest prevalence is identified in Africa.9 Genetic susceptibility to IgAN may explain the heterogeneity of the disease between populations of different ancestries. Genome-wide association studies conducted in Han Chinese and European populations uncovered several susceptibility loci for IgAN and provided evidence that linked various IgAN susceptibility alleles to altered mucosal immunity, innate immune responses, and inflammation.10,11 Reported frequencies of IgAN are also influenced by socioeconomic and health policies in different regions of the world. Similar to other glomerulonephritides, IgAN is either a primary kidney disease, often thought of as an autoimmune disease, or it is secondary to other systemic disorders, most notably IgAN secondary to liver diseases.12,13 Incidental IgA without clinical evidence of kidney disease may represent a latent or third form of IgAN. Incidental IgA deposition has also been reported in autopsies of individuals without known kidney disease. The significance of incidental IgA deposition in kidneys is unknown. It is also unknown whether these deposits predate the development of clinical kidney disease if primed by adverse immunologic or hemodynamic stimuli or, alternatively, if the IgA deposits remain dormant without adverse consequences. The current study aimed to determine the frequency, demographics, and histopathologic characteristics of donor kidneys with incidental IgA deposits and to evaluate the clinical course of the transplanted kidneys in an ethnically diverse US population.
Methods
Population
All kidney transplants at the J.C. Walter Jr. Transplant Center, Houston Methodist Hospital, Houston, Texas, USA (United Network for Organ Sharing region 4) between 2009 and 2016 with a T0 transplantation biopsy were examined for IgA mesangial deposits and for histopathologic characteristics according to the Banff recommendations. Adequacy of time-zero (T0) biopsy required a satisfactory sample for comprehensive evaluation by light microscopy and immunofluorescence techniques.
Donor Evaluation
Clinical and laboratory evaluation of living and deceased donors was carried out according to generally accepted protocols. Demographic and clinical characteristics of the donors and recipients were collected from clinical records and the Centers for Medicare and Medicaid Services form 2728.14 Living donors were evaluated for the presence of proteinuria and microscopic hematuria on more than one occasion before kidney donation. Microalbuminuria was defined as the urine microalbumin-to-creatinine ratio >30 mg/g and microscopic hematuria was defined as the presence of 2 or more red blood cells per high-power field on urinalysis testing. Follow-up data of the studied patients included estimated glomerular filtration rate (calculated by the Chronic Kidney Disease Epidemiology Collaboration equation15) at discharge from hospital; serum creatinine at 6 months, and 1 year; histological follow-up of subsequent biopsies undertaken for indication, and clinical follow-up of patient survival and death-censored graft survival at 1 and 5 years post transplantation. Institutional Review Board approval was received from the Houston Methodist Research Institute Institutional Review Board under protocol Pro00000587, approval number IRB0507-0053.
Kidney Transplant Pathology
Wedge biopsies averaging 1 cm3 were obtained immediately after reperfusion of the donated kidney. The freshly biopsied sample was divided into 2 portions: one placed in formalin for light microscopy and electron microscopy processing, and a second portion placed in “Zeus solution” for direct immunofluorescence testing (DIF). The kidney samples were processed and evaluated according to standard histology procedures.16 Serial sections of the formalin-fixed paraffin-embedded portion were stained with hematoxylin and eosin, periodic acid–Schiff, and trichrome. The following features were evaluated by light microscopy: number of glomeruli, number of globally sclerotic glomeruli, number of glomeruli with focal segmental glomerulosclerosis, mesangial sclerosis, mesangial hypercellularity, endocapillary hypercellularity, chronic tubulo-interstitial fibrosis (% total biopsy surface, and interstitial fibrosis-tubular atrophy Banff grade), hyaline arteriolar sclerosis, and arterial sclerosis. Pathology findings related to terminal events such as acute tubular injury, myoglobin casts, and glomerular thrombosis were reported. DIF testing for immunoglobins A, G, and M (IgA, IgG, IgM), complement components 3, 4, 1q (C3, C4, C1q), kappa light chain, lambda light chain, and complement component 4d (C4d) was performed on snap-frozen sections according to standard techniques.17 Positive fluorescence was graded on a scale of 0 to 3 by a single expert transplant pathologist (LWG) and the distribution of the staining (mesangial and capillary wall) was documented. Subsequent for indication, kidney allograft biopsies were evaluated for IgA presence and staining intensity. The fate of the IgA deposits in the posttransplant biopsies was documented for up to 7 years post-transplant.
Statistical Analysis
Baseline data were reported as medians and interquartile ranges for continuous variables, and as frequencies and proportions for categorical variables. Differences in baseline data across groups were compared using the χ2 or Fisher's exact tests for categorical variables and Wilcoxon rank-sum test for continuous variables, as appropriate. Patient survival was estimated using Kaplan-Meier statistics. Cox proportional-hazard modeling was used to determine the contribution of potential prognostic variables to recipient outcomes. Variables selected for the multivariable Cox proportional-hazard models were based on clinical importance and cross-validation.18,19 The performance of the predictive models was determined by calculating Harrell’s C-statistic. The best final model was selected based on the smallest Bayesian information criterion and largest C-statistic. All analyses were performed on Stata v16.1 (StataCorp LP, College Station, TX). A P value < 0.05 was considered statistically significant.
Results
Study Population
From 2009 to 2016, a total of 1607 patients received kidney allografts from 1430 donors at the transplant center (Table 1; Supplementary Figure S1). Of these, 805 recipients were excluded from the analysis: no T0 biopsy (n = 726), inadequate or unsatisfactory T0 biopsies (n = 76), and cases with only trace glomerular IgA deposits (n = 3). Thus, 802 kidneys having T0 biopsies, derived from 745 donors, were included in the analysis and divided into 2 groups: IgA-positive (IgA+, n = 164) and IgA-negative (IgA−, n = 638) according to the presence or absence of IgA glomerular deposits (intensity ≥1) by DIF on snap-frozen tissue. The studied biopsied kidney cohort included 41.5% (333/802) living donor kidneys (LDKs) and 58.5% (469/802) deceased donor kidneys (DDKs); the latter derived from 412 deceased donors. DDKs were classified as follows: standard-criteria donors or expanded-criteria donors (ECDs) and classified further as kidneys donated after circulatory death or after brain death.20 The clinical and immunologic profiles of the recipients and donors are reported according to whether or not a T0 biopsy was available (Table 1). Kidney transplant recipients who had a T0 biopsy were more likely to have had native kidney failure secondary to hypertension and a body mass index ≥30 kg/m2. The difference in age between the groups with and without a T0 biopsy was statistically, but not clinically, significant (50 vs. 49 years, respectively). Biopsies were less likely to be performed in the setting of multiorgan transplants and kidney retransplants. Biopsies were more likely to be performed in donors with older age, female gender, higher body mass index, history of hypertension, and serum creatinine ≥132.6 μmol/l (1.5 mg/dl). ECD and donated after circulatory death kidneys and LDKs were significantly more likely to have T0 biopsies than standard-criteria donors kidneys (Table 1).
Table 1.
Demographics and clinical characteristics of kidney donors and transplant recipients having T0 biopsy and included in the analysis versus recipients who did not have baseline biopsy and excluded from the analysis (2009–2016)
| Donor characteristics |
Total |
No T0 biopsy |
T0 biopsy |
P value |
|---|---|---|---|---|
| n | 1430 | 713 | 717 | |
| Donor age, y, median (IQR) | 37.5 (25.0, 49.0) | 33.0 (21.0, 46.0) | 41.0 (30.0, 50.0) | <0.001 |
| Donor male gender, n (%) | 719 (50.3) | 363 (50.9) | 356 (49.7) | 0.63 |
| Donor ethnicity, n (%) | 0.66 | |||
| White | 771 (53.9) | 377 (52.9) | 394 (55.0) | |
| Black | 192 (13.4) | 104 (14.6) | 88 (12.3) | |
| Hispanic/Latino | 399 (27.9) | 201 (28.2) | 198 (27.6) | |
| Asian | 50 (3.5) | 22 (3.1) | 28 (3.9) | |
| Other | 18 (1.3) | 9 (1.3) | 9 (1.3) | |
| Donor BMI, kg/m2, median (IQR) | 25.8 (22.5, 29.3) | 25.1 (22.0, 28.3) | 26.6 (23.4, 30.3) | <0.001 |
| Donor hypertension, n (%) | 178 (12.5) | 71 (10.0) | 107 (15.0) | 0.004 |
| Donor diabetes, n (%) | 53 (6.3) | 25 (5.5) | 28 (7.3) | 0.28 |
| Donor terminal serum creatinine ≥132.6 μmol/l (1.5 mg/dl), n (%) | 104 (12.4) | 45 (9.8) | 59 (15.4) | 0.02 |
| Donor type, n (%) | <0.001 | |||
| Living donor | 589 (41.2) | 256 (35.9) | 333 (46.4) | |
| Deceased donor | 841 (58.8) | 457 (64.1) | 384 (53.6) | |
| Deceased donor, ECD vs. SCD, n/N (%) | 0.056 | |||
| SCD | 782/841 (93.0) | 432/457 (94.5) | 350/384 (91.1) | |
| ECD | 59/841 (7.0) | 25/457 (5.5) | 34/384 (8.9) | |
| Deceased donor, DCD vs. DBD, n/N (%) | 0.003 | |||
| DBD | 746/841 (88.7) | 419/457 (91.7) | 327/384 (85.2) | |
| DCD | 95/841 (11.3) | 38/457 (8.3) | 57/384 (14.8) | |
| Cold ischemic time, hours, median (IQR) | 10.8 (1.0, 19.6) | 11.1 (1.2, 19.0) | 9.7 (1.0, 20.1) | 0.03 |
| Recipient characteristics |
Total |
No T0 Biopsy |
T0 Biopsy |
P value |
|---|---|---|---|---|
| n | 1607 | 805 | 802 | |
| Age, years, median (IQR) | 50.0 (39.0, 60.0) | 49.0 (39.0, 59.0) | 50.0 (40.0, 60.0) | 0.03 |
| Male gender, n (%) | 958 (59.6) | 470 (58.4) | 488 (60.8) | 0.31 |
| Ethnicity, n (%) | 0.51 | |||
| White | 721 (44.9) | 373 (46.3) | 348 (43.4) | |
| Black | 404 (25.1) | 201 (25.0) | 203 (25.3) | |
| Hispanic/Latino | 366 (22.8) | 175 (21.7) | 191 (23.8) | |
| Asian | 109 (6.8) | 51 (6.3) | 58 (7.2) | |
| Other | 7 (0.4) | 5 (0.6) | 2 (0.2) | |
| BMI, kg/m2, median (IQR) | 26.8 (23.4, 30.6) | 26.4 (22.7, 30.1) | 27.3 (23.9, 31.0) | 0.003 |
| Cause of end-stage kidney disease, n (%) | ||||
| Hypertensive nephrosclerosis | 384 (24.5) | 172 (22.3) | 228 (28.8) | 0.003 |
| Diabetes | 584 (36.4) | 342 (42.6) | 242 (30.2) | <0.001 |
| IgA nephropathy | 61 (3.9) | 29 (3.8) | 32 (4.0) | 0.77 |
| Multiorgan transplant, n (%) | 98 (6.1) | 71 (8.8) | 27 (3.4) | <0.001 |
| Kidney retransplant, n (%) | 143 (8.9) | 83 (10.4) | 60 (7.5) | 0.04 |
| End cPRA %, median (IQR) | 3.0 (0.0, 57.0) | 2.0 (0.0, 52.5) | 3.0 (0.0, 62.0) | 0.1 |
| eGFR at discharge, ml/min per 1.73 m2, median (IQR) | 50.8 (28.2, 73.6) | 54.3 (31.4, 77.7) | 48.3 (25.3, 71.1) | <0.001 |
| eGFR at last follow-up, ml/min per 1.73 m2, median (IQR) | 60.5 (44.4, 77.9) | 61.4 (45.7, 82.0) | 59.9 (43.6, 75.2) | 0.02 |
| Recipient and donor | ||||
| HLA mismatch level ≥5, n (%) | 744 (46.4) | 393 (48.9) | 351 (43.9) | 0.04 |
BMI, body mass index; DBD, donor after brain death; DCD, donor after cardiac death; eGFR, estimated glomerular filtration rate; HLA, human leukocyte antigen; IQR, interquartile range; SCD, standard-criteria donor.
Note: 1607 kidneys were donated from 1430 donors. There is a difference in the number of deceased donors in the T0 biopsy group between Table 1 (n = 384) and Table 2 (n = 412) with 28 donors not included in the analysis in Table 1 as they were shared donors with recipients in the nonbiopsy group. Difference between groups compared by Pearson's χ2 or Fisher's exact tests for categorical variables or Wilcoxon rank-sum test for continuous variables, as appropriate.
The number of participants with missing variables for each category is provided in Supplementary Table S1.
ECDs (expanded-criteria donors) refer to older kidney donors (>60 years) or donors who are aged 50 to 59 years and have 2 of the following 3 features: hypertension, terminal serum creatinine >1.5 mg/dl, or death from cerebrovascular accident.13
Donor Demographics and Clinical Characteristics of the T0 Biopsy Cohort
The 802 kidney transplants having T0 biopsies were from 745 donors of the following ethnicity: 54.6% non-Hispanic White, 27.9% Hispanic, 12.5% non-Hispanic Black, and 3.8% Asian (Table 2). IgA mesangial deposits ≥1 were detected by standard DIF in 20.5% (164/802) of T0 biopsies or in 19.5% (145/745) of the donors. The prevalence of IgA positivity by donor ethnicity was as follows: 25.9% (54/208) Hispanic, 25.0% (7/28) Asian, 18.2% (74/407) non-Hispanic White, and 9.7% (9/93) Black (P = 0.01). The prevalence of IgA in T0 biopsies by donor type (DDK vs LDK) was 24.5% (101/412) vs 13.2% (44/333), respectively (P < 0.001). The prevalence of IgA positivity was not different in kidneys from standard-criteria donors versus ECD or from donated after circulatory death versus donated after brain death. Hypertension was significantly more prevalent in donors of kidneys with IgA deposits (P = 0.04). A longer cold-ischemia time occurred in IgA+ versus IgA− groups: median 15.8 hours (interquartile range 1.1, 22.9) versus 9.0 hours (interquartile range 1.0, 20.9) respectively; P = 0.01, Table 2).
Table 2.
Demographic and clinical characteristics of recipients and their kidney donors according to the presence of IgA on biopsy at implantation
| Donor characteristics |
All donors |
Donor IgA (−) |
Donor IgA (+) |
P value |
|---|---|---|---|---|
| n | 745 | 600 | 145 | |
| Donor age, years, median (IQR) | 41.0 (30.0, 50.0) | 41.0 (30.0, 50.0) | 43.0 (27.0, 50.0) | 0.96 |
| Donor male gender, n (%) | 373 (50.1) | 289 (48.2) | 84 (57.9) | 0.04 |
| Donor ethnicity, n (%) | 0.01 | |||
| White | 407 (54.6) | 333 (55.5) | 74 (51.0) | |
| Black | 93 (12.5) | 84 (14.0) | 9 (6.2) | |
| Hispanic/Latino | 208 (27.9) | 154 (25.7) | 54 (37.2) | |
| Asian | 28 (3.8) | 21 (3.5) | 7 (4.8) | |
| Other | 9 (1.2) | 8 (1.3) | 1 (0.7) | |
| Donor BMI, kg/m2, median (IQR) | 26.5 (23.3, 30.2) | 26.6 (23.2, 30.4) | 26.1 (23.6, 29.8) | 0.64 |
| Donor hypertension, n (%) | 114 (15.3) | 84 (14.0) | 30 (21.0) | 0.04 |
| Donor diabetes, n (%) | 28 (6.8) | 17 (5.5) | 11 (11.0) | 0.06 |
| Donor terminal serum creatinine, mg/dl, median (IQR) | 0.9 (0.7, 1.3) | 0.9 (0.7, 1.3) | 0.9 (0.7, 1.3) | 0.90 |
| Donor type, n (%) | <0.001 | |||
| Living donor | 333 (44.7) | 289 (48.2) | 44 (30.3) | |
| Deceased donor | 412 (55.3) | 311 (51.8) | 101 (69.7) | |
| Donor cause of death, n (%) | 0.89 | |||
| Anoxia | 93 (24.1) | 73 (24.6) | 20 (22.5) | |
| CNS tumor | 4 (1.0) | 3 (1.0) | 1 (1.1) | |
| Cerebrovascular/stroke | 126 (32.6) | 94 (31.6) | 32 (36.0) | |
| Head trauma | 163 (42.2) | 127 (42.8) | 36 (40.4) | |
| Deceased donor, ECD vs. SCD, n/N (%) | 0.08 | |||
| SCD | 373/412 (90.5) | 286/311 (92.0) | 87/101 (86.1) | |
| ECD | 39/412 (9.5) | 25/311 (8.0) | 14/101 (13.9) | |
| Deceased donor, DCD vs. DBD, n/N (%) | 0.16 | |||
| DBD | 352/412 (85.4) | 270/311 (86.8) | 82/101 (81.2) | |
| DCD | 60/412 (14.6) | 41/311 (13.2) | 19/101 (18.8) | |
| Cold ischemic time (h), median (IQR) | 11.0 (1.0, 21.0) | 9.0 (1.0, 20.9) | 15.8 (1.1, 22.9) | 0.01 |
| Recipient characteristics |
All recipients |
Donor kidney IgA (−) |
Donor kidney IgA (+) |
P value |
|---|---|---|---|---|
| n | 802 | 638 | 164 | |
| Age (y), median (IQR) | 50.0 (40.0, 60.0) | 50.0 (40.0, 60.0) | 51.0 (39.0, 61.0) | 0.89 |
| Male gender, n (%) | 486 (60.6) | 389 (61.0) | 97 (59.1) | 0.67 |
| Ethnicity, n (%) | 0.60 | |||
| White | 348 (43.4) | 274 (42.9) | 74 (45.1) | |
| Black | 203 (25.3) | 167 (26.2) | 36 (22.0) | |
| Hispanic/Latino | 191 (23.8) | 147 (23.0) | 44 (26.8) | |
| Asian | 58 (7.2) | 48 (7.5) | 10 (6.1) | |
| Other | 2 (0.2) | 2 (0.3) | 0 (0.0) | |
| BMI, kg/m2, median (IQR) | 27.3 (23.9, 31.0) | 27.4 (24.0, 31.0) | 26.4 (23.4, 31.0) | 0.31 |
| Cause of end-stage kidney disease, n (%) | ||||
| Hypertensive nephrosclerosis | 213 (26.9) | 166 (26.4) | 47 (28.8) | 0.53 |
| Diabetes | 242 (30.2) | 192 (30.1) | 50 (30.5) | 0.92 |
| Polycystic kidneys | 84 (10.6) | 70 (11.1) | 14 (8.6) | 0.35 |
| Retransplant/graft failure | 57 (7.2) | 46 (7.3) | 11 (6.7) | 0.80 |
| Focal glomerular sclerosis | 35 (4.4) | 29 (4.6) | 6 (3.7) | 0.61 |
| IgA nephropathy | 32 (4.0) | 24 (3.8) | 8 (4.9) | 0.53 |
| Systemic lupus erythematosus | 31 (3.9) | 25 (4.0) | 6 (3.7) | 0.86 |
| Malignant hypertension | 15 (1.9) | 9 (1.4) | 6 (3.7) | 0.06 |
| Chronic glomerulonephritis unspecified | 13 (1.6) | 12 (1.9) | 1 (0.6) | 0.25 |
| Calcineurin inhibitor nephrotoxicity | 13 (1.6) | 11 (1.7) | 2 (1.2) | 0.64 |
| Membranous glomerulonephritis | 8 (1.0) | 3 (0.5) | 5 (3.1) | 0.003 |
| End cPRA (%), median (IQR) | 3.0 (0.0, 62.0) | 4.0 (0.0, 62.0) | 3.0 (0.0, 65.0) | 0.98 |
| Creatinine at transplant, μmol/l, median (IQR) mg/dl, median (IQR) | 645 (469,884) 7.3 (5.3,10.0) |
637 (469,875) 7.2 (5.3,9.9) |
663 (469,919) 7.5 (5.3,10.4) |
0.36 |
| Creatinine at discharge, μmol/l, median (IQR) mg/dl, median (IQR) | 141 (97,230) 1.6 (1.1, 2.6) |
133 (97,212) 1.5 (1.1, 2.4) |
159 (97,309) 1.8 (1.1, 3.5) |
0.02 |
| eGFR at discharge, ml/min per 1.73 m2, median (IQR) | 48.3 (25.3, 70.9) | 50.0 (28.1, 71.1) | 42.7 (17.3, 67.5) | 0.01 |
| eGFR at last follow-up, ml/min per 1.73 m2, median (IQR) | 59.9 (43.6, 74.8) | 59.9 (44.2, 74.0) | 59.0 (41.6, 76.8) | 0.79 |
| CMV+, n (%) | 451 (97.0) | 337 (97.1) | 114 (96.6) | 0.78 |
| Multiorgan transplant, n (%) | 0.80 | |||
| Kidney alone | 775 (96.6) | 616 (96.6) | 159 (97.0) | |
| Kidney-Pancreas | 27 (3.4) | 22 (3.4) | 5 (3.0) | |
| Kidney retransplant (n = 60) | 60 (7.5) | 48 (7.5) | 12 (7.3) | 0.93 |
| Recipient and donor | ||||
| HLA mismatch level ≥5, n (%) | 351 (43.9) | 263 (41.4) | 88 (53.7) | 0.01 |
| A locus mismatch level | 0.62 | |||
| 0 | 127 (15.9) | 103 (16.2) | 24 (14.6) | |
| 1 | 348 (43.5) | 280 (44.0) | 68 (41.5) | |
| 2 | 325 (40.6) | 253 (39.8) | 72 (43.9) | |
| B locus mismatch level | 0.15 | |||
| 0 | 102 (12.8) | 82 (12.9) | 20 (12.2) | |
| 1 | 262 (32.8) | 218 (34.3) | 44 (26.8) | |
| 2 | 436 (54.5) | 336 (52.8) | 100 (61.0) | |
| DR locus mismatch level | 0.13 | |||
| 0 | 133 (16.6) | 107 (16.8) | 26 (15.9) | |
| 1 | 340 (42.5) | 280 (44.0) | 60 (36.6) | |
| 2 | 327 (40.9) | 249 (39.2) | 78 (47.6) | |
| DR gender mismatch, n (%) | 412 (51.4) | 335 (52.5) | 77 (47.0) | 0.20 |
BMI, body mass index; CMV, cytomegalovirus; cPRA, calculated panel reactive antibodies; IQR, interquartile range; eGFR, estimated glomerular filtration rate; HLA, human leukocyte antigens.
Note: 802 kidneys were donated by 745 donors. Difference between groups compared by Pearson's χ2 or Fisher's exact tests for categorical variables or Wilcoxon rank-sum test for continuous variables, as appropriate.
Urinary Findings in Living Donors
Findings on urinalysis and microalbumin assay in living donors with IgA+ T0 biopsies (n = 44) were as follows: microalbuminuria was unmeasurable or normal in 43 of the 44; 1 donor had no microalbuminuria testing, but proteinuria was negative by dipstick. Microscopic hematuria, on 2 or more urinalyses, was noted in only 2 men and 5 women.
Immunohistological Features of IgA+ T0 Biopsies
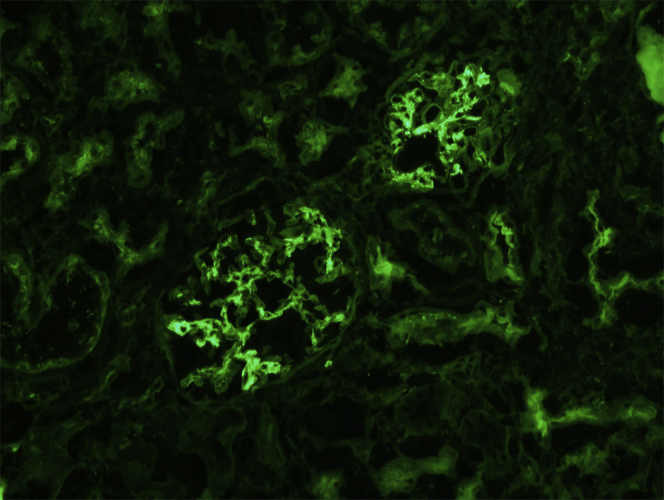
IgA staining intensity was 1+ in 57.3% and 2+ and 3+ in 26.8% and 15.8% of biopsies, respectively (Figure 1). IgM in the mesangium, the most common Ig found, accompanied IgA in 90% of the IgA+ biopsies and was present in 42.9% of IgA− T0 biopsies. In contrast, IgG was present in only 9% of IgA+ biopsies and 0.6% of IgA− biopsies. C3 deposits in the mesangium were detected in 81% of IgA+ biopsies (Table 3). Light microscopy was normal in most biopsies. Vascular pathology (arterial and arteriolar sclerosis) was present in 28% of the IgA+ biopsies and in only 20.7% of the IgA− biopsies, P = 0.04 (Table 3). The rare finding of vascular thrombosis was only observed in the IgA− donor kidneys and 10 of 18 such kidneys had undergone extracorporeal perfusion. Mesangial hypercellularity (7.9% vs. 0.9%) and mesangial sclerosis (11.0% vs. 4.7%) were more prevalent in IgA+ versus IgA− biopsies (P < 0.001 and P = 0.003, respectively). Mesangial pathology (defined as mesangial hypercellularity, sclerosis, or both) correlated with the intensity of IgA staining in the T0 biopsy, with 7.5% of the IgA 1+, 17.7% of the IgA 2+, and 23% of the IgA 3+ demonstrating mesangial pathology. None of the biopsies displayed endocapillary proliferation or crescents. Focal segmental glomerulosclerosis was a significant but infrequent finding in IgA+ biopsies (3%) and IgA− biopsies (0.09%, P = 0.04). Interstitial fibrosis was not significantly different between the 2 groups (Table 3). Except for the isolated presence of segmental glomerulosclerosis in 5 cases with IgA+T0 biopsies (Oxford classification S1) none of the other histopathologic findings met minimum criteria for grading by the Oxford MEST-C criteria.21
Figure 1.
Direct immunofluorescence testing. Immediately post-transplantation, T0 biopsy showing IgA-positive granular deposits in mesangial areas (intensity, 3+; scale, 0–3). Original magnification ×20. The donor was a 39-year-old white man, extended criteria donor who had no hypertension or diabetes but experienced acute kidney injury with increased serum creatinine elevation to 282.9 μmol/l (3.2 mg/dl). Terminal serum creatinine at time of kidney procurement improved to 150.3 μmol/l (1.7 mg/dl). The recipient experienced slow recovery of graft function due to confounding acute tubular necrosis and myoglobin casts. Serum creatinine of the recipient 1-year post-transplantation is 88.3 μmol/l (1.0 mg/dl). T0, time-zero biopsy, performed intraoperatively, post-revascularization of the transplanted kidney.
Table 3.
Biopsy findings of living and deceased kidney donors at the time of implantation
| Biopsy parameter |
Total kidneys |
Donor kidney IgA (−) |
Donor kidney IgA (+) |
P value |
|---|---|---|---|---|
| n | 802 | 638 | 164 | |
| Number of glomeruli in the biopsy, median (IQR) | 20.0 (15.0, 35.0) | 20.0 (15.0, 35.0) | 20.0 (15.0, 34.0) | 0.49 |
| Number of glomeruli with global sclerosis, median (IQR) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.33 |
| Glomeruli with focal segmental sclerosis, n (%) | 11 (1.4) | 6 (0.9) | 5 (3.1) | 0.04 |
| Mesangial cell proliferation, n (%) | 19 (2.4) | 6 (0.9) | 13 (7.9) | <0.001 |
| Mesangial sclerosis, n (%) | 48 (6.0) | 30 (4.7) | 18 (11.0) | 0.003 |
| Interstitial fibrosis, n (%) | 234 (29.3) | 185 (29.1) | 49 (30.1) | 0.81 |
| Vascular lesions (arterial and arteriolar sclerosis), n (%) | 178 (22.2) | 132 (20.7) | 46 (28.0) | 0.04 |
| Ischemia reperfusion injury, n (%) | 94 (11.7) | 79 (12.4) | 15 (9.1) | 0.25 |
| Acute tubular necrosis, n (%) | 65 (8.1) | 62 (9.7) | 3 (1.8) | <0.001 |
| Thrombosis, n (%) | 18 (2.2) | 18 (2.8) | 0 (0.0) | 0.03 |
| Diabetic renal disease, n (%) | 5 (0.6) | 5 (0.8) | 0 (0.0) | 0.59 |
| Immune complex-mediated glomerulonephritis (ICGN) | 4 (0.5) | 3 (0.5) | 1 (0.6) | 0.82 |
| Immunofluorescence testing – IgG | 19 (2.4) | 4 (0.6) | 15 (9.1) | <0.001 |
| Immunofluorescence testing – IgM | 422 (52.6) | 274 (42.9) | 148 (90.2) | <0.001 |
| Immunofluorescence testing – Complement C3 | 316 (39.4) | 183 (28.7) | 133 (81.1) | <0.001 |
| Immunofluorescence testing – Complement C1qa | 20 (87.0) | 8 (72.7) | 12 (100.0) | 0.09 |
BMI, body mass index; cPRA, calculated panel reactive antibodies; eGFR, estimated glomerular filtration rate; HLA, human leukocyte antigens; IQR, interquartile range;
Note: Difference between groups compared by Pearson's χ2 or Fisher's exact tests for categorical variables or Wilcoxon rank-sum test for continuous variables, as appropriate.
Immunofluorescence testing for C1q was performed on 23 samples (12 IgA+ and 11 IgA−); 3 of the IgA− biopsies were negative for C1q.
Recipient Demographic and Clinical Characteristics
Recipients of IgA+ and IgA− kidneys were similar in age, gender, ethnic distribution, and underlying cause of kidney failure (Table 2). IgAN was the cause of kidney failure in 32 patients, of whom 25% (8/32) received IgA+ kidneys. The proportion of recipients with ≥5 human leukocyte antigen (HLA) mismatches was significantly higher in recipients of IgA+ kidneys (P < 0.01).
Recipient Clinical and Histological Outcomes
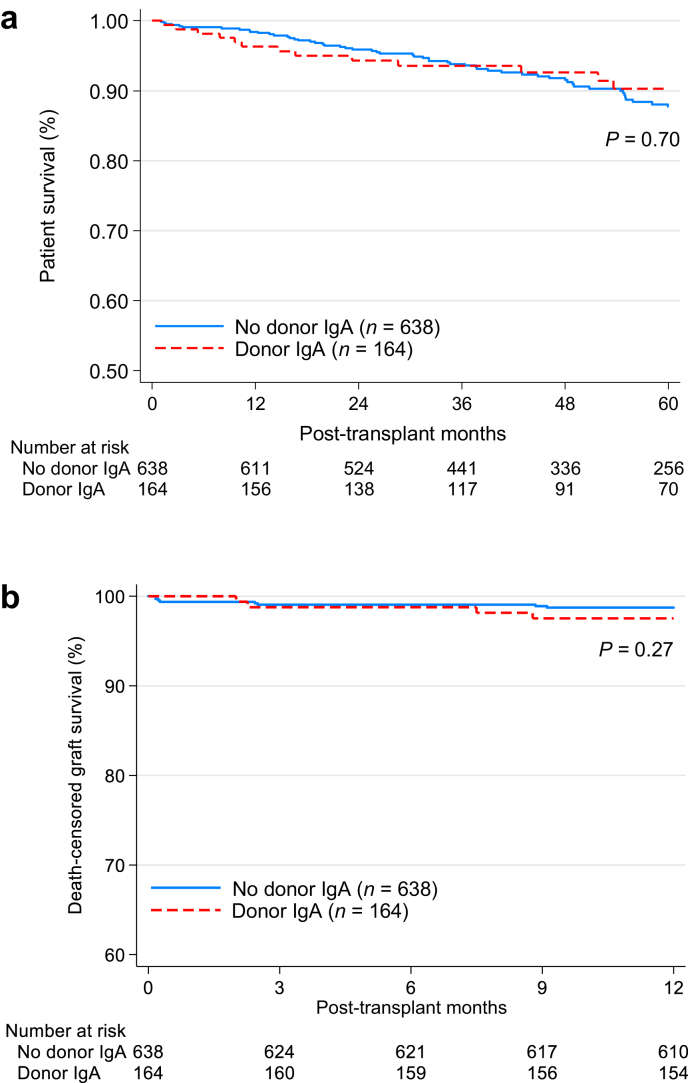
The median follow-up time for all kidney recipients who underwent a T0 biopsy was 4 years. There was no significant difference in patient survival between recipients of IgA+ and IgA− kidneys (Figure 2a). Kidney function, assessed as estimated glomerular filtration rate, at discharge was significantly lower in the IgA+ group; however, the median serum creatinine concentration at 6 months and 1-year post transplantation was not significantly different (106.1 μmol/l [1.2 mg/dl] vs. 114.9 μmol/l [1.3mg/dl], for IgA+ vs IgA−, respectively). Fifteen recipients (9.1%) of IgA+ kidneys versus 5% (n = 32) recipients of IgA− kidneys were treated for acute rejection in the first 6 months posttransplantation (P = 0.04). The estimated incidence of 5-year death-censored graft failure was similar for both groups, 9.4% and 5.6% for IgA+ and IgA− grafts, respectively, P = 0.15 (Figure 2b). Rejection (hyperacute, acute, or chronic) was the listed cause of graft failure in 52.9% (9/17) of the IgA+ recipients and in 48.6% (17/35) of the IgA− recipients experiencing graft loss not due to death (P = 0.77).
Figure 2.
Kaplan-Meier survival curves of kidney transplants with or without IgA deposition present on T0 biopsy at the time of transplantation. (a) Five-year patient survival; (b) 5-year death-censored graft survival.
Multivariable Cox regression analyses showed no difference in the risk of 5-year death-censored graft failure by IgA status. Younger age, lower estimated glomerular filtration rate at last follow-up, higher calculated panel reactive antibodies at transplant, multiorgan transplantation, and ECD kidneys were associated with a higher risk of 5-year death-censored graft failure (Table 4).
Table 4.
Characteristics associated with death-censored graft failure at 5 years post-transplant, Cox proportional hazards modeling
| Variable | Adjusted HR |
P value |
|---|---|---|
| (95% CI) | ||
| Donor kidney IgA presence | 1.70 (0.80–3.62) | 0.17 |
| Recipient age (y) | 0.97 (0.94–1.00) | 0.04 |
| Systemic lupus erythematosus | 1.57 (0.44–5.59) | 0.49 |
| eGFR at last follow-up (mg/dl) | 0.95 (0.93–0.97) | <0.001 |
| cPRA at transplant | 1.02 (1.00–1.03) | 0.01 |
| Multiorgan transplant | 9.69 (2.55–36.77) | 0.001 |
| Expanded-criteria donors | 8.13 (2.87–23.02) | <0.001 |
CI, confidence interval; cPRA, calculated panel reactive antibodies; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
C-statistic = 0.85.
Post-transplant Biopsies
Of 164 IgA+ T0 biopsies, 90 kidney transplant recipients had 1 or more post-transplant biopsies with evaluable DIF. Of those, 13 displayed mesangial pathology on the T0 biopsy and were analyzed for clearance of IgA separately from the remaining 77. Clearance of IgA was judged to have occurred on the first biopsy showing negative DIF, which persisted in any subsequent biopsies. Persistence of IgA was defined as positive IgA staining on the last available biopsy. IgA clearance was determined at 3 time frames: 0 to 30 days, >30 to 180, and >180 days post-transplant. In biopsies in which mesangial pathology was absent from the T0 biopsy, 81.8% (9/11) cleared within 30 days, 73% (19/26) cleared within >30to 180 days, and 82.5% (33/40) cleared at >180 days. Thus 79.2% (61/77) of previously IgA+ recipients without mesangial pathology had cleared IgA over the course of follow-up. Of the 13 patients with mesangial pathology on the T0 biopsy, only 2 of 5 cleared IgA within 30 days, none of 3 cleared at >30 to 180 days; the remaining 5 cleared IgA after >180 days. Thus, 54% (7/13) of IgA+ recipients with mesangial pathology on the T0 biopsy cleared the IgA on follow-up (P < 0.001, with and without mesangial pathology).
Similarly, follow-up biopsies were evaluated for clearance of donor-derived IgA according to the intensity of the IgA staining on T0 biopsy. Whereas 91% of T0 biopsies showing 1+ IgA staining cleared on follow-up biopsy, only 63% and 40% of T0 biopsies staining 2+ and 3+ for IgA, respectively, cleared on follow-up biopsy (P < 0.001).
Discussion
This is the first and largest report of the clinical and immunohistologic features of transplanted donor kidneys with incidental mesangial IgA deposits in an ethnically diverse US population.22 Although both recipients and donors were ethnically diverse, differences in ethnic proportions between the 2 groups reflected differences in the prevalence of kidney disease, and socioeconomic and cultural differences. Previously published studies reported single-center experiences in more homogeneous populations.
The present study identified an overall prevalence of incidental IgA deposits in one-fifth of donor kidneys, as revealed by examination of T0 biopsies using snap-frozen tissue. The frequency of incidental IgA mesangial deposits in this cohort is higher than anticipated and falls within the range reported from regions with a known high incidence of clinical IgAN. In a series of predominantly live donor kidney transplants from Japan, incidental IgA (from paraffin-embedded tissue) was reported in 16.1% (82/510) of T0 biopsies with no difference between LDK and DDK.23 In another study from Japan,5 an incidence of 29.4% (20/68) in LDKs was reported. A study of only DDKs with T0 biopsies from China4 identified incidental IgA deposits in 24% (83/342) of T0 biopsies. Studies from other regions in the world have reported a lower frequency for incidental IgA. T0 biopsies from 70 LDKs reported from Chile identified incidental IgA deposits in 12.9% of the biopsies.6 In a study from Belgium,3 incidental moderate or severe (2+ to 3+) mesangial IgA, considered indicative of IgAN, was reported in 9% (10/114) of T0 biopsies in DDKs. This latter observation is consistent with the findings in the present cohort wherein 10.9% (51/469) of DDK donors exhibited 2+ to 3+ IgA staining on T0 biopsies. Surprisingly, postmortem studies on victims of suicide and trauma from Finland24 and Singapore25 reveal inordinately low prevalence of IgA deposits (6.9% and 4.0%, respectively), possibly due to deterioration of kidney tissue or technique.
It would be informative to examine whether the ethnic prevalence of IgA deposits in kidney donors in the present cohort reflects the prevalence of IgAN in the US population. The findings of the present study confirmed an ethnicity preponderance pattern similar to that reported for IgAN in the US population. The prevalence of IgA deposition in this cohort was greatest in Hispanic and Asian donors: 25.9% and 25.0%, respectively. Whereas non-Hispanic White individuals comprised most of our donor population, both biopsied and unbiopsied (53.9%), this group had a lower incidence of IgA deposits (18.2%). A review of registries26 and reports of case series from US centers9,26, 27, 28, 29 indicates that IgAN is the third most common form of glomerulonephritis (19.7%)28 and occurs with greatest frequency in Hispanic, Asian, and American Indian individuals, with lower frequencies in non-Hispanic White and non-Hispanic Black individuals. The ethnic distribution of kidney donors with IgA+ kidneys in the present cohort is comparable to the ethnic distribution of IgAN in the US population.
In addition to ethnicity, deceased donor status, history of donor hypertension, and a lower estimated glomerular filtration rate at discharge were significantly associated with incidental IgA deposits.
Morphologic alterations in the present study were far less than described in previous reports of biopsied donor kidneys. In those with IgA deposits in the present study, the most common lesion seen was arteriolar sclerosis (28%) of a mild grade with 12.8% demonstrating mesangial pathology, which is commonly present in IgAN.30 Mesangial lesions were noted in more than 50% of biopsies reported from Japanese donors, and their incidence of vascular pathology was 45%.5 Finding some degree of glomerular and vascular pathology in kidneys with incidental IgA deposits invites a hypothetical concern that IgA deposits in these instances represent latent IgAN. This would be of particular concern in the case of live donors. However, the presence of moderate- or severe-IgA deposition (2+ to 3+), considered a marker of true IgAN and correlated with the frequency of mesangial pathology in this cohort, was present in <9% of all T0 biopsies. Although, when viewed by confocal microscopy, IgG accompanies galactose-deficient IgA1,31 IgG detected by routine immunofluorescence has been reported in only 20% of IgAN.32 However, in the present series, only 9.1% of all IgA+ biopsies had detectable IgG. Finally, none of the live donors of IgA+ kidneys had abnormal microalbumin excretion and only 7 of the 44 had microhematuria. Thus, if IgA deposition represents latent IgAN, it must involve only a minority of those donors with IgA in their T0 biopsies. It may have been useful to test for abnormal IgA in the T0 biopsies using the new monoclonal antibody against galactose-deficient IgA1. However, 2 recent groups of investigators have found the test does not discriminate IgAN from other secondary causes of IgA deposition in the kidney.33,34 Alternatively, these deposits may be insignificant, resulting from compromised mesangial functions for clearing and degrading the IgA molecules in the donated kidneys. This possibility is supported by the greater prevalence of incidental IgA in biopsies from DDK than from LDK, in ECD versus standard-criteria donors, and in donated after circulatory death vs donated after brain death. “Secondary IgAN” has been documented in patients with chronic liver disease, gastrointestinal malabsorption and inflammatory diseases, infectious and inflammatory disorders, and neoplasms.35 By inference, pathogenic mechanisms involved in secondary IgAN might account for incidental IgA mesangial deposits. The recently proposed paradigm for IgAN immunopathogenesis recognizes a “multi-hits” hypothesis, whereby injury is initiated by the production of galactose-deficient IgA1 and the formation of autoantibodies to this aberrant IgA1 in genetically predisposed individuals.36 Localization of the IgA-containing immune complexes in the glomerular mesangium occurs either by recruitment of the deposits from the circulation or in situ binding of trapped aberrant IgA1 to its autoantibody. IgA immune complexes possessing nephritogenic properties activate complement-dependent immune pathways, induce oxidative stress, and promote local inflammatory responses that ultimately lead to mesangial activation and injury.37,38 Complex interactions among genetics, immunobiology, and environmental factors influence the progression through these mechanistic steps and presumably account for the varying phenotypic expression of IgAN, ranging from asymptomatic hematuria to acute or chronic progressive glomerulonephritis.39 By inference, aborting or arresting any of these steps could lead to latent IgAN.
The collective experience with clinical and histologic outcomes of kidneys with incidental IgA deposits after placement in a new host supports resolution of the immune deposits within the first year after transplantation and without adverse impact on long-term graft survival.5 Complications in the immediate posttransplant period, including edema, hypertension, hematuria, and delayed graft function, were reported in one study.4 The same study reported a 30% incidence of acute rejection in recipients of IgA+ kidneys without compromising long-term graft survival. Similarly, in the present cohort a higher incidence of early acute rejection that responded to therapy, and was not associated with graft loss, was seen in the IgA+ versus IgA− kidney recipients (9.1 vs 5.0%, respectively). Potential causes for the increased incidence of early rejection in the IgA+ group included longer ischemia time and greater frequency of high-grade (≥5) HLA mismatch for the IgA+ group. The present study supports a benign outcome of donor kidneys with incidental IgA deposits that was similar to kidneys without such deposits, within the duration of follow-up. However, future studies will need to determine whether a different outcome is encountered after a longer follow-up. In the present cohort, resolution of IgA deposits was observed in 77.6% of the biopsied grafts, although clearance appeared to be less frequent and to occur later in kidneys with mesangial pathology and with higher-intensity (2+ and 3+) IgA staining. Conflicting data exist regarding the impact of transplanting kidneys with IgA deposits into patients with IgAN as the underlying cause of their kidney failure. In the present study cohort, only a small number of patients with underlying IgAN (n = 8) received IgA+ kidneys, thus precluding meaningful analysis.
In conclusion, the present study identified for the first time a high frequency of incidental IgA deposits in a US cohort of transplanted kidneys donated by both deceased and living donors, albeit the incidence was less in the latter. The frequency of IgA deposits in different ethnic groups reflected the frequency of IgAN reported in those groups in the United States. The presence of these deposits did not compromise graft outcome for the duration of follow-up for this study. However, it is disturbing that, despite rigorous pretransplant evaluation, 13% of the LDKs had incidental IgA deposits, possibly representing clinically silent IgAN. Whereas the relatively infrequent finding of IgG in the T0 biopsy is reassuring, the high frequency of concurrent IgM mesangial deposits, a finding that has been associated with worse outcomes in IgAN, is disturbing.40 Although the significance of these findings and their underlying mechanisms are unclear, their presence in living donors necessitates long-term close follow-up of blood pressure and kidney function in these individuals.
Disclosure
All the authors declared no competing interests.
Footnotes
Figure S1. Disposition of participants for the study of kidney transplant recipients with or without T0 biopsy and with or without IgA deposits on T0 biopsy.
Table S1. Number of participants with missing data for each variable of interest in the included patients.
STROBE Statement.
Supplementary Material
References
- 1.Gaber L.W., Moore L.W., Alloway R.R. Glomerulosclerosis as a determinant of posttransplant function of older donor renal allografts. Transplantation. 1995;60:334–339. doi: 10.1097/00007890-199508270-00006. [DOI] [PubMed] [Google Scholar]
- 2.Mirza M.K., Kim L., Kadambi P.V. Membranous nephropathy transplanted in the donor kidney: observations of resolving glomerulopathy in serial allograft biopsies. Nephrol Dial Transplant. 2014;29:2343–2347. doi: 10.1093/ndt/gfu333. [DOI] [PubMed] [Google Scholar]
- 3.Cosyns J.P., Malaise J., Hanique G. Lesions in donor kidneys: nature, incidence, and influence on graft function. Transpl Int. 1998;11:22–27. doi: 10.1007/s001470050097. [DOI] [PubMed] [Google Scholar]
- 4.Ji S., Liu M., Chen J. The fate of glomerular mesangial IgA deposition in the donated kidney after allograft transplantation. Clin Transplant. 2004;18:536–540. doi: 10.1111/j.1399-0012.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- 5.Sofue T., Inui M., Hara T. Latent IgA deposition from donor kidneys does not affect transplant prognosis, irrespective of mesangial expansion. Clin Transplant. 2013;27(Suppl 26):14–21. doi: 10.1111/ctr.12158. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg H.G., Martínez P.S., Vaccarezza A.S. Morphological findings in 70 kidneys of living donors for renal transplant. Pathol Res Pract. 1990;186:619–624. doi: 10.1016/S0344-0338(11)80225-6. [DOI] [PubMed] [Google Scholar]
- 7.Schena F.P., Nistor I. Epidemiology of IgA nephropathy: a global perspective. Semin Nephrol. 2018;38:435–442. doi: 10.1016/j.semnephrol.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 8.O'Shaughnessy M.M., Hogan S.L., Thompson B.D. Glomerular disease frequencies by race, sex and region:results from the International Kidney Biopsy Survey. Nephrol Dial Transplant. 2018;33:661–669. doi: 10.1093/ndt/gfx189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Shaughnessy M.M., Hogan S.L., Poulton C.J. Temporal and demographic trends in glomerular disease epidemiology in the southeastern United States, 1986–2015. Clin J Am Soc Nephrol. 2017;12:614–623. doi: 10.2215/CJN.10871016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiryluk K., Li Y., Sanna-Cherchi S. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M., Foo J.N., Wang J.Q. Identification of new susceptibility loci for IgA nephropathy in Han Chinese. Nat Commun. 2015;6:7270. doi: 10.1038/ncomms8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saha M.K., Julian B.A., Novak J. Secondary IgA nephropathy. Kidney Int. 2018;94:674–681. doi: 10.1016/j.kint.2018.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suganuma T. [Glomerular IgA deposits in an autopsy study] Nihon Jinzo Gakkai Shi. 1994;36:813–822. [PubMed] [Google Scholar]
- 14.Lentine K.L., Kasiske B.L., Levey A.S. KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Transplantation. 2017;101:S1–S109. doi: 10.1097/TP.0000000000001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker P.D., Cavallo T., Bonsib S.M. Practice guidelines for the renal biopsy. Mod Pathol. 2004;17:1555–1563. doi: 10.1038/modpathol.3800239. [DOI] [PubMed] [Google Scholar]
- 17.Coppo R., Troyanov S., Camilla R. The Oxford IgA nephropathy clinicopathological classification is valid for children as well as adults. Kidney Int. 2010;77:921–927. doi: 10.1038/ki.2010.43. [DOI] [PubMed] [Google Scholar]
- 18.Hastie T., Tibshirani R., Wainwright M. CRC Press; Boca Raton, FL: 2015. Statistical Learning With Sparsity: The Lasso and Generalizations. [Google Scholar]
- 19.Lasso Reference Manual. Stata Press; College Station, TX: 2019. [Google Scholar]
- 20.Metzger R.A., Delmonico F.L., Feng S. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3(Suppl 4):114–125. doi: 10.1034/j.1600-6143.3.s4.11.x. [DOI] [PubMed] [Google Scholar]
- 21.Trimarchi H., Barratt J., Cattran D.C. Oxford classification of IgA nephropathy. 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91:1014–1021. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 22.US Census Bureau Texas Census. 2010. https://www.census.gov/content/census/en/data/tables/time-series/demo/popest/2010s-counties-detail.html Available at:
- 23.Suzuki K., Honda K., Tanabe K. Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int. 2003;63:2286–2294. doi: 10.1046/j.1523-1755.63.6s.2.x. [DOI] [PubMed] [Google Scholar]
- 24.Varis J., Rantala I., Pasternack A. Immunoglobulin and complement deposition in glomeruli of 756 subjects who had committed suicide or met with a violent death. J Clin Pathol. 1993;46:607–610. doi: 10.1136/jcp.46.7.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinniah R. Occurrence of mesangial IgA and IgM deposits in a control necropsy population. J Clin Pathol. 1983;36:276–279. doi: 10.1136/jcp.36.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Shaughnessy M.M., Montez-Rath M.E., Lafayette R.A. Differences in initial treatment modality for end-stage renal disease among glomerulonephritis subtypes in the USA. Nephrol Dial Transplant. 2016;31:290–298. doi: 10.1093/ndt/gfv386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sim J.J., Batech M., Hever A. Distribution of biopsy-proven presumed primary glomerulonephropathies in. 2000–2011 among a racially and ethnically diverse US population. Am J Kidney Dis. 2016;68:533–544. doi: 10.1053/j.ajkd.2016.03.416. [DOI] [PubMed] [Google Scholar]
- 28.Murugapandian S., Mansour I., Hudeeb M. Epidemiology of glomerular disease in southern Arizona:review of 10-year renal biopsy data. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair R., Walker P.D. Is IgA nephropathy the commonest primary glomerulopathy among young adults in the USA? Kidney Int. 2006;69:1455–1458. doi: 10.1038/sj.ki.5000292. [DOI] [PubMed] [Google Scholar]
- 30.Roberts I.S., Cook H.T., Troyanov S. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 31.Rizk D.V., Saha M.K., Hall S. Glomerular immunodeposits of patients with IgA nephropathy are enriched for IgG autoantibodies specific for galactose-deficient IgA1. J Am Soc Nephrol. 2019;30:2017–2026. doi: 10.1681/ASN.2018111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellur S.S., Troyanov S., Cook H.T. Immunostaining findings in IgA nephropathy:correlation with histology and clinical outcome in the Oxford classification patient cohort. Nephrol Dial Transplant. 2011;26:2533–2536. doi: 10.1093/ndt/gfq812. [DOI] [PubMed] [Google Scholar]
- 33.Wang M., Lv J., Zhang X. Secondary IgA nephropathy shares the same immune features with primary IgA nephropathy. Kidney Int Rep. 2020;5:165–172. doi: 10.1016/j.ekir.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassol CA, Bott C, Nadasdy GM, et al. Immunostaining for galactose-deficient immunoglobulin A is not specific for primary immunoglobulin A nephropathy [e-pub ahead of print]. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfz152. Accessed October 22, 2020. [DOI] [PubMed]
- 35.Waldherr R., Rambausek M., Duncker W.D. Frequency of mesangial IgA deposits in a non-selected autopsy series. Nephrol Dial Transplant. 1989;4:943–946. doi: 10.1093/ndt/4.11.943. [DOI] [PubMed] [Google Scholar]
- 36.Lai K.N., Tang S.C., Schena F.P. IgA nephropathy. Nat Rev Dis Primers. 2016;2:16001. doi: 10.1038/nrdp.2016.1. [DOI] [PubMed] [Google Scholar]
- 37.Wu M.Y., Chen C.S., Yiang G.T. The emerging role of pathogenesis of IgA nephropathy. J Clin Med. 2018;7:225. doi: 10.3390/jcm7080225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wyatt R.J., Julian B.A. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 39.Feehally J., Barratt J. The genetics of IgA nephropathy:an overview from western countries. Kidney Dis (Basel) 2015;1:33–41. doi: 10.1159/000381738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heybeli C., Oktan M.A., Yıldız S. Clinical significance of mesangial IgM deposition in patients with IgA nephropathy. Clin Exp Nephrol. 2019;23:371–379. doi: 10.1007/s10157-018-1651-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.