Abstract
Introduction
Alterations in oxalate homeostasis are associated with kidney stone disease and progression of chronic kidney disease (CKD). However, accurate measurement of plasma oxalate (POx) concentrations in large patient cohorts is challenging as prompt acidification of samples has been deemed necessary. In the present study, we investigated the effects of variations in sample handling on POx results and examined an alternative strategy to the established preanalytical procedures.
Methods
The effect of storage time at room temperature (RT) and maintenance of samples at −80°C was tested. POx was measured in 1826 patients enrolled in the German Chronic Kidney Disease (GCKD) study, an ongoing multicenter, prospective, observational cohort study.
Results
We demonstrate that POx concentrations increased rapidly when samples were maintained at RT. This was most relevant for POx <10 μM, as concentrations more than doubled within a few hours. Immediate freezing on dry ice and storage at −80°C provided stable results and allowed postponement of acidification for >1 year. In the patients of the lowest estimated glomerular filtration rate (eGFR) quartile, median POx was 2.7 μM (interquartile range [IQR] <2.0–4.2) with a median eGFR of 25.1 ml/min per 1.73 m2 (IQR 20.3–28.1).
Conclusion
We conclude that immediate freezing and maintenance of plasma samples at -80°C facilitates the sample collection process and allows accurate POx assessment in large cohorts. The present study may serve as a reference for sample handling to assess POx in clinical trials and to determine its role in CKD progression.
Keywords: chronic kidney disease, clinical trials, POx concentration, POx measurement, preanalytical conditions
Graphical abstract
During the past decade, there has been growing evidence suggesting a role for oxalate in systemic inflammation,1,2 renal allograft failure,3 and cardiovascular complications.4, 5, 6 Moreover, a recent study demonstrated an association between urinary oxalate excretion and progression of CKD.7 In advanced stages of CKD, urinary oxalate declines and POx concentrations start to rise.8, 9, 10 Whereas in healthy adults and children POx concentrations have been reported to be in the range of 1–5 μM,11, 12, 13 an increase of POx is generally described in CKD.9,14,15 However, a systematic assessment of POx as a function of eGFR is lacking. Therefore, evaluation of POx concentrations in a large representative cohort of patients with CKD is needed.
It is widely accepted that measurement of POx concentration is challenging due to the conversion of ascorbate to oxalate. Ex vivo in blood samples during processing, only a small percentage of oxalate is presumed to be generated from sources other than ascorbate.16 As ascorbate converts nonenzymatically to oxalate at pH >4, it is recommended that samples are immediately cooled and acidified to lower pH to halt this biochemical process.12,16,17 Samples are also deproteinized to prevent production of denatured proteins, which otherwise might lead to turbidity of the samples and a distortion of the photometric measurement.17
When blood samples are obtained for POx measurements in larger patient cohorts as part of prospective clinical trials, variations in the sample collection process might occur. Specifically, adherence to complex handling instructions, including acidification of samples with high-molarity HCl is difficult in outpatient settings.17 Therefore, the present study aimed to examine the effect of preanalytical conditions on POx results and investigate whether immediate freezing of samples may be sufficient to facilitate the sample collection process. Based on our findings, we developed a new standardized procedure of sample handling and processing that allowed us to perform systematic measurement of POx concentrations in 1826 patients with CKD enrolled in the GCKD study.18
Methods
Study Populations
For determining the effect of variations in preanalytical conditions on POx and serum oxalate (SOx) results, blood samples were obtained from 40 healthy volunteers and patients with CKD stages 1 to 5D at Charité, Berlin, Germany. Thus, eGFR levels from <15 to >120 ml/min per 1.73 m2 and associated oxalate concentrations were evaluated in our experiments. All participants provided written informed consent. Study protocols were approved by the local authorities (local Ethics Committee of Charité, Berlin Study No. EA2/242/17 and EA2/176/19).
For systematic measurement of POx concentrations in patients with CKD, we measured POx in 1826 patients from the GCKD study. The GCKD study is a multicenter, prospective, observational cohort study to identify risk factors and markers for CKD progression and to improve the understanding of the underlying pathophysiology. A detailed description of the GCKD study design has been published previously.18 The GCKD study protocol was approved by the leading ethical review committee of the medical faculty of the University of Erlangen-Nürnberg and all local institutional review boards of the regional centers. All participants provided written informed consent. All data were pseudonymized. All clinical experimentation for the current study occurred in adherence to the Declaration of Helsinki of 1975, as revised in 2013. Of the 1826 patients included in our analysis, there was no patient who had an established diagnosis of primary hyperoxaluria.
Sample Collection and Handling
Blood samples were collected via a venous blood draw or, in dialysis patients, from the arterial line into Vacuette EDTA plasma tubes or serum tubes (Greiner Bio-One GmbH, Kremsmünster, Austria), immediately put on wet ice, and centrifuged in a precooled Centrifuge 5810R (Eppendorf, Wesseling-Berzdorf, Germany) at 2000g for 10 minutes at 4 °C. Subsequently, 400 μl of the separated supernatant plasma/serum is deproteinized by filtration through Vivaspin 500 30,000 MWCO PES filter units (Sartorius, Goettingen, Germany) and acidified with 16 μl 1N hydrochloric acid. The eluate and remaining plasma/serum were aliquoted and stored at −80°C within 2 hours.
The separated supernatant was frozen on dry ice and shipped to our laboratory where samples were stored at −80°C. Samples were commonly sent in groups of 5. Within a maximum of 2 weeks after the initial blood draw, the plasma was deproteinized, acidified, and measured. For assessment of the variability of oxalate results, preanalytical conditions were adjusted as follows: (i) storage of plasma and serum at RT for 6 to 8 hours, (ii) storage of plasma on dry ice for 6 hours, and (iii) storage of plasma and eluate at −80 °C for 16.5 to 21.0 months before deproteinization and acidification.
Measurement of POx and SOx Concentrations
For measurement of POx/SOx, frozen acidified plasma/serum eluates were slowly thawed on ice. Subsequently, 6 μl of 5 NaNO2 solution was added per 100 μl plasma/serum. For measurement of oxalate, an enzymatic method with oxalate oxidase has been conducted based on a publication of Ladwig et al.17 using a commercial assay from Trinity Biotech (Bray, Co., Wicklow, Ireland). The protocol has been adapted by Litholink Corp. (Chicago, IL). The samples were measured in a 1:2 dilution in 96-well plates in an Ultrospec 1000 Spectrophotometer (Pharmacia Biotech Inc., Piscataway, NJ) at 590 nm wavelength (no background wavelength). A 0.5-mM oxalate standard (Trinity Biotech) was used to obtain a new standard curve for every set of measurements and an independent 10 μM and 50 μM sodium oxalate solution was used as a quality control standard. Each sample was measured twice (in 2 wells), and the mean value was calculated from both results. Usage of a 96-well plate for measurement of oxalate oxidase activity led to lower values for intra- and interassay variability than previously described.19 The mean coefficient of variation (CV) was 2% for within-run variation and 4% (high oxalate concentrations ∼50 μM), respectively, 8% (low oxalate concentrations ∼10 μM) for between-run variation. Our assay was validated by cross-checking results with those obtained via ion chromatography. The lower limit of detection was determined at 2 μM and validated by the accuracy of both ion chromatography and the oxalate oxidase assay.19 All measurements less than 2 μM were set to 1.9 μM.
Statistical Analysis
For analyzing the effect of preanalytical conditions on POx and SOx results, statistical analyses were conducted using IBM SPSS Statistics, Version 25 (IBM Corp, Armonk, NY). Values were compared using paired t-test. Mean CV and mean ± SD were used to compare degrees of variations between different series of POx and SOx results. All statistical tests were 2-sided and a P value less than 0.05 was considered statistically significant.
Data Transformation and Statistical Analysis in the GCKD Cohort
Due to skewed nature of POx values, we divided all patients of the GCKD cohort into 4 groups based on their POx concentrations as follows: group 1 (reference): <2.0 μM; group 2: 2.0 to 3.0 μM; group 3: > 3.0 to 4.2 μM; and group 4: >4.2 μM. For each POx concentration, corresponding eGFR was estimated using the CKD-Epidemiology Collaboration formula as follows:
where Scr is serum creatinine (mg/dl), κ is 0.7 for women and 0.9 for men, α is −0.329 for women and −0.411 for men, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1.
Clinical data are presented as median (IQR) for continuous variables and as frequency and percentage for categorical variables. Due to skewed nature of POx values, we divided all patients of the GCKD cohort into 4 groups based on their POx concentrations as follows: group 1 (reference): <2.0 μM; group 2: 2.0 – 3.0 μM; group 3: >3.0 – 4.2 μM; and group 4: >4.2 μM. We compared the baseline characteristics of the cohort stratified by POx concentrations using the Kruskal-Wallis nonparametric, Pearson χ2, and Fisher exact tests, as appropriate. All statistical tests were 2-sided and a P value less than 0.05 was considered statistically significant. SAS statistical software version 9.4 (Statistical Analyses System Inc, Cary, NC) was used for analyses.
Results
POx and SOx Concentrations Rapidly Rise When Samples Are Maintained at RT
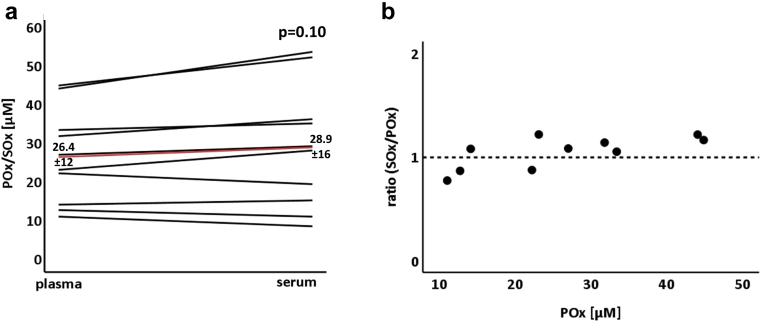
In a first series of experiments, we compared oxalate concentrations in plasma and serum. Following the blood draw, samples were both immediately processed and measured. As shown in Figure 1, oxalate concentrations tended to be slightly higher in serum as compared with plasma, but this trend did not reach significance.
Figure 1.
Measurement of oxalate concentrations in plasma versus serum. Oxalate concentrations were measured in plasma (POx) and serum (SOx). For each patient, an EDTA tube and a serum tube were drawn at the same time, immediately put on ice and processed. (a) SOx concentrations tend to be slightly higher than POx concentrations (n = 10, P = 0.10, mean coefficient of variation 0.10). The red line indicates the mean values of all POx/SOx concentrations. (b) SOx/POx ratio depending on the POx concentration.
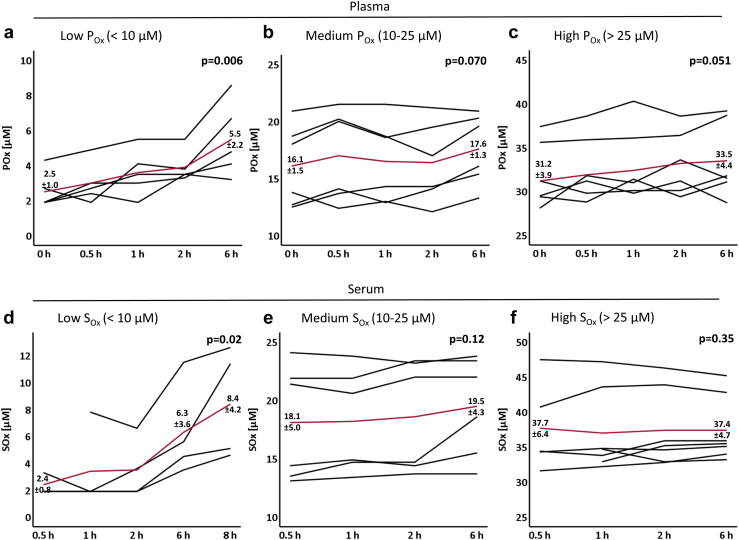
We next examined the effect of storage at RT on POx and SOx concentrations. As shown in Figure 2a–c, POx concentrations increased rapidly when samples were maintained at RT. This increase was most relevant for POx concentrations <10 μM, as the mean POx more than doubled from 2.5 ± 1.0 μM to 5.5 ± 2.2 μM within 6 hours. For POx concentrations >10 μM, POx concentrations increased yet the effect was smaller in relation to total oxalate concentration.
Figure 2.
Plasma (POx) and serum (SOx) oxalate concentrations following storage at room temperature (RT). Oxalate concentrations were measured in plasma (a–c) and serum (d–f) samples with a concentration range of POx/SOx < 10 μM (a+d), POx/SOx 10–25 μM (b+e), and POx/SOx >25 μM (c+f). For each patient, 4 to 5 EDTA or serum tubes were drawn. Each tube was processed at a different timepoint following storage at RT as indicated. Each black line represents POx/SOx results of one patient. The red line indicates the mean values of all POx/SOx concentrations. (a–c) The P value compares POx concentrations at 0 h versus 6 h (a: n = 5, P = 0.006; b: n = 6, P = 0.07, mean coefficient of variation [CV] 0.08; c: n = 6, P = 0.051, mean CV 0.04). (d–f) The P value compares SOx concentrations at 0 h versus 6 h (d: n = 3, P = 0.02; e: n = 6, P = 0.12, mean CV 0.06; f: n = 6, P = 0.35, mean CV 0.03).
As demonstrated in Figure 2d–f, the effect of storage at RT on SOx concentration was similar as observed for plasma. For SOx <10 μM, mean SOx concentration increased continuously from 2.4 ± 0.8 μM to 6.3 ± 3.6 μM after 6 hours, and up to 8.4 ± 4.2 μM after 8 hours. For SOx concentrations >10 μM, the increase in relation to total SOx concentration was again less pronounced (Figure 2e and f).
POx Concentrations Are Stable Following Freezing on Dry Ice and Storage at −80 °C
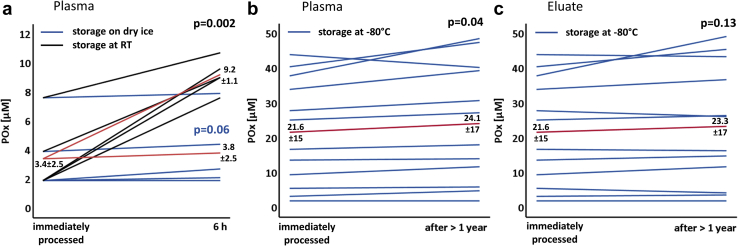
In a next series of experiments, we tested if storage on dry ice can prevent the increase of POx concentrations. POx concentrations were immediately measured following a blood draw. In addition, aliquots of unprocessed plasma were stored on dry ice and at RT. As shown in Figure 3a, POx concentrations remained stable over 6 hours when maintained on dry ice with the mean POx being almost unchanged after 6 hours (3.4 vs. 3.8 μM). In contrast, POx rapidly increased in the samples stored at RT. To further investigate stability of oxalate in blood samples, we examined POx concentrations after a minimum of 1 year of storage at −80°C of plasma or the eluate remaining after immediate protein separation and acidification. As illustrated in Figure 3b, POx concentrations obtained from the stored plasma samples remained relatively stable as compared with immediate measurement with Δ mean POx being 2.5 μM for all samples and only 1 μM for the susceptible POx range <10 μM. Accordingly, the mean CV was 0.10, which is around interassay variability. When measurement was repeated in the acidified and deproteinized eluate that had been stored at −80 °C for >1 year, the least increase of POx concentration could be detected (Figure 3c). Hence, both plasma and eluate can be safely stored at −80 °C for >1 year before acidification and deproteinization and/or final measurement. Based on these findings, we suggest a simplification of the standard preanalytical protocol for POx measurement, as schematically summarized in Figure 4.
Figure 3.
Plasma oxalate (POx) measurement following freezing on dry ice and storage at −80 °C. (a) POx concentrations were measured immediately after blood draw. Aliquots of unprocessed plasma were stored on dry ice and at RT. After 6 hours, POx concentrations were measured and compared with POx concentrations obtained at 0 h. The blue lines indicate POx concentrations after storage on dry ice (n = 5, P = 0.06), the black lines indicate POx results after storage at RT (n = 5, P = 0.002). The red lines indicate the mean values of POx concentrations maintained on dry ice (lower line) versus at room temperature (RT) (upper line). (b,c) POx concentrations were measured immediately after blood draw and following storage at −80 °C and compared via P value. Each blue line represents POx results of 1 patient after storage at −80 °C. The red line indicates the mean value of all POx concentrations. (b) Unprocessed plasma was compared (n = 12, P = 0.04, mean coefficient of variation [CV] 0.10) with (c) eluate obtained following protein separation and acidification (n = 12, P = 0.13, mean CV 0.08).
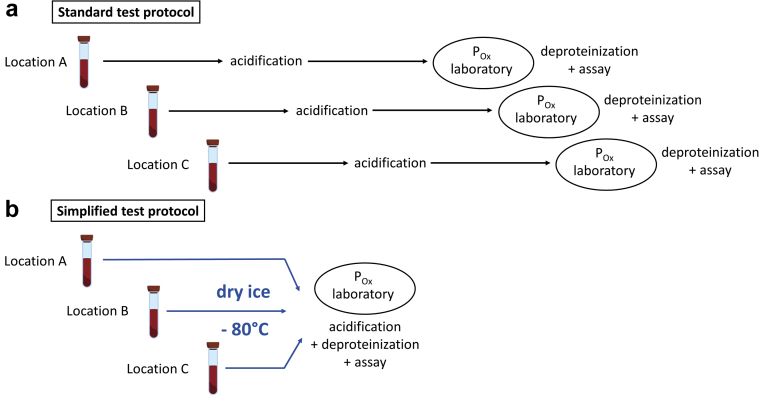
Figure 4.
Schematic of standard and simplified test protocol for measurement of plasma oxalate (POx) concentration. The preanalytical steps for processing of blood samples to determine POx concentration are shown. (a) According to the standard test protocol, blood samples need to be centrifuged and the supernatant acidified within a total of 2 hours. In contrast, we demonstrate that (b) the test protocol can be simplified by placement of plasma on dry ice followed by storage at −80 °C. This prevents addition of high-molarity HCl in outpatient settings, and allows collection of large amounts of samples for efficient processing with low intra-and interassay variability.
Measurement of POx Concentrations in the GCKD Cohort
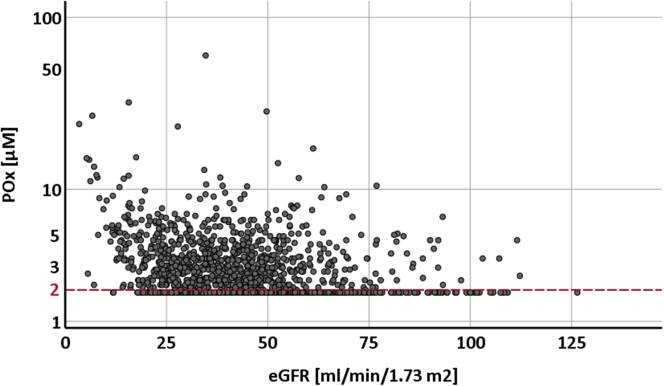
Following our observations regarding sample handling, we next applied our method of rapid sample freezing to systematically examine POx concentrations in 1826 patients of the GCKD cohort: after centrifugation, the supernatant plasma was immediately put on dry ice, strictly maintained at −80°C, and shipped to our laboratory for further acidification/deproteinization and measurement. Table 1 presents the baseline characteristics of these patients stratified by POx. Mean (SD) eGFR was 44.0 (17.9) ml/min per 1.73 m2 at the time the blood was drawn for POx measurement. As expected, POx correlated inversely with eGFR (median in group 1: 50.3, IQR 40.7–60.9 vs. median in group 4: 40.6, IQR 32.1–52.8, P < 0.0001). As illustrated in Figure 5, POx concentrations less than 2.0 μM were observed in more than 50% of the whole GCKD cohort and in more than 30% of patients with an eGFR below 30 ml/min per 1.73 m2. When comparing POx concentrations in patients with lowest versus highest eGFR, median POx was 2.7 μM (IQR <2–4.2) in the lowest eGFR quartile with a median eGFR of 25.1 ml/min per 1.73 m2 (IQR 20.3–28.1) versus median POx being <2 μM (IQR <2.0–2.8) in the highest eGFR quartile with a median eGFR of 60.6 ml/min per 1.73 m2 (IQR 55.7–71.4).
Table 1.
POx concentrations and baseline characteristics in 1826 patients with CKD stages 1–5 (GCKD study cohort)a
| Clinical characteristics | Oxalate <2.0 | Oxalate 2.0 to 3.0 | Oxalate >3.0 to 4.2 | Oxalate >4.2 | P value |
|---|---|---|---|---|---|
| Total | 953 | 378 | 261 | 234 | |
| Creatinine mg/dl | 1.4 (1.1–1.6) | 1.4 (1.2–1.7) | 1.5 (1.3–1.8) | 1.6 (1.3–2.0) | <0.0001 |
| eGFR, median (IQR) | 50.3 (40.7–60.9) | 47.2 (37.2–58.2) | 44.4 (36.6 –54.4) | 40.6 (32.1– 52.8) | <0.0001 |
| Age, median (IQR) | 63 (53–69) | 63.5 (54–70) | 63 (55–69) | 62 (51–69) | 0.8 |
| Gender | 0.6 | ||||
| Male | 565 (59.3) | 219 (57.9) | 152 (58.2) | 148 (63.3) | |
| Female | 388 (40.7) | 159 (42.1) | 109 (41.8) | 86 (36.8) | |
| Albumin to creatinine ratio in mg albumin (urine)/g creatinine (urine) | 30.4 (8.0–249.9) | 43.5 (9.0–222.7) | 55.1 (9.8–333.7) | 101.2 (14.7–611.2) | 0.0001 |
| Systolic BP, median (IQR) | 137 (125–151) | 138 (127–154) | 139 (127–152) | 136 (126–152) | 0.4 |
| Diastolic BP, median (IQR) | 79 (71–86) | 79 (72–87) | 78 (70–87) | 79 (72–87) | 0.4 |
| BMI, median (IQR) | 29.0 (26.1–33.2) | 28.5 (25.6–32.7) | 28.2 (24.8–31.5) | 28.6 (25.4–33.0) | 0.2 |
| Comorbidities | |||||
| Diabetes | 326 (34.2) | 110 (29.1) | 85 (32.6) | 77 (32.9) | 0.4 |
| Hypertension | 907 (95.2) | 364 (96.3) | 254 (97.3) | 223 (95.3) | 0.4 |
| CHD | 186 (19.5) | 63 (16.7) | 53 (20.3) | 32 (13.7) | 0.1 |
| Diabetic nephropathy | 245 (25.7) | 97 (25.7) | 67 (25.7) | 56 (23.9) | 1 |
| Vascular nephropathy | 412 (43.2) | 165 (43.7) | 121 (46.4) | 89 (38.0) | 0.3 |
| Systemic diseases | 92 (9.7) | 44 (11.6) | 33 (12.6) | 33 (14.1) | 0.2 |
| Primary glomerular nephropathy | 204 (21.4) | 81 (21.4) | 62 (23.8) | 63 (26.9) | 0.3 |
| Interstitial nephropathy | 91 (9.6) | 29 (7.7) | 18 (6.9) | 21 (9.0) | 0.5 |
| Acute kidney failure | 42 (4.4) | 17 (4.5) | 12 (4.6) | 14 (6.0) | 0.8 |
| Nephrectomy (single kidney) | 69 (7.2) | 21 (5.6) | 19 (7.3) | 8 (3.4) | 0.1 |
| Hereditary diseases | 29 (3.0) | 17 (4.5) | 11 (4.2) | 20 (8.6) | 0.003 |
| Post renal diseases | 84 (8.8) | 30 (7.9) | 23 (8.8) | 16 (6.8) | 0.8 |
| Other diseases | 39 (4.1) | 31 (8.2) | 18 (6.9) | 11 (4.7) | 0.02 |
| Unkown diseases | 78 (8.2) | 28 (7.4) | 9 (3.5) | 11 (4.7) | 0.03 |
| Medications | |||||
| ACE-Inhibitors or ARB | 705 (74.0) | 280 (74.1) | 186 (71.3) | 171 (73.1) | 0.8 |
| Antihypertensives | 886 (93.0) | 355 (93.9) | 246 (94.3) | 220 (94.0) | 0.8 |
| Diuretics | 535 (56.1) | 210 (55.6) | 144 (55.2) | 147 (62.8) | 0.2 |
| Beta blockers | 493 (51.7) | 204 (54.0) | 143 (54.8) | 117 (50.0) | 0.6 |
| HbA1c, mmol/mol | 41.7 (38.5–47.8) | 42.2 (38.5–46.9) | 41.7 (38.3–48.0) | 40.7 (37.9–47.8) | 0.3 |
| Uric acid, mg/dl | 7.0 (5.8–8.2) | 6.8 (5.7–8.0) | 7.0 (6.1–8.2) | 7.0 (6.1–8.4) | 0.7 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; GCKD, German Chronic Kidney Disease; POx, plasma oxalate.
Continuous variables are presented as median [interquartile range (IQR)] and categorical variables as frequency and percentage. P values <0.05 were considered statistically significant.
Figure 5.
Plasma oxalate (POx) concentrations and corresponding estimated glomerular filtration rate (eGFR) levels in 1826 patients with chronic kidney disease (CKD) (German Chronic Kidney Disease [GCKD] study population). POx concentrations were measured in 1826 patients of the GCKD study and plotted against the corresponding calculated eGFR. Values below the validated lower limit of detection of 2 μM (red dashed line) were set to 1.9 μM. The y-axis is log-scaled.
Discussion
In the present study, we demonstrated that prolonged storage at RT caused most relevant changes for POx concentrations <10 μM. Therefore, assessing accurate POx concentrations is most challenging in study cohorts with expectedly low POx concentrations (<10 μM) as in patients with CKD. Previous reports concluded that prompt acidification is mandatory to prevent spontaneous generation of oxalate.16 However, acidification requiring 12 mol/l HCl17 is not feasible in outpatient settings. Our results suggest that immediate freezing on dry ice and storage at −80°C likely halts nonenzymatic conversion from ascorbate to oxalate, allows postponement of acidification to a more suitable timepoint, and thus enables valid and highly efficient assessment of POx concentrations. We could further demonstrate that both plasma and serum can be used for assessment of oxalate concentrations, depending on study material available.
To date, only a few studies have investigated POx in the general CKD population,14, 15, 16 which may be due to the complex preanalytical sample preparation. Based on our findings, we established a simplified standardized procedure for sample handling and applied it to POx measurement in 1826 patients with common forms of CKD. Performing the oxalate assay in 96-well plates allowed efficient measurement of a large number of samples with low intra-and interassay variability (see the Methods section). The accuracy of our oxalate oxidase assay was cross-checked by comparing our test results with concentrations measured via ion chromatography; 2 μM was determined as the lower limit of detection of both assays.19 We could demonstrate that in patients with a mean eGFR of 44.0 ml/min per 1.73 m2, more than half had a POx below 2 μM. This is in accordance with previous studies describing POx values of 1 to 3 μM in healthy adults11,12,17 and an increase of POx when eGFR falls below 20 to 30 ml/min per 1.73 m2.14,15 However, previous studies examining POx in CKD comprised only small cohorts (<80 patients) with the mean GFR being at least 14 ml/min per 1.73 m2 higher than in our study.14,15 By assessing POx in a large representative cohort (>1800 participants), we noted POx concentrations below 2 μM were not limited to CKD stages 1 to 3, but could also be found in a notable proportion of patients with an eGFR <30 ml/min per 1.73 m2. In contrast to a recent study,15 we could further demonstrate that POx concentrations >5 μM were not limited to eGFR levels < 30 ml/min per 1.73 m2, but also could be detected in some individuals with an eGFR of approximately 50 ml/min per 1.73 m2. Such variations in POx results within a heterogeneous CKD cohort may have several reasons. We measured POx only once and did not perform repeated measurements. Intraindividual fluctuations in POx concentrations could therefore not be taken into account. Moreover, POx concentration is not only determined by elimination via the kidney, but also by the rate of production resulting from dietary intake, relative gastrointestinal absorption, and endogenous production of oxalate. However, we did not examine urinary oxalate excretion or dietary contribution in our analysis. Additional underlying conditions that might lead to increased oxalate absorption or increased oxalate generation have not been assessed. Genetic testing for primary hyperoxaluria in patients with elevated POx concentrations in relation to well-preserved eGFR has not been performed to date. Therefore, further studies are needed to identify the various determinants of POx concentration in CKD.
In conclusion, our study demonstrated that immediate freezing on dry ice and maintenance at −80°C provide stable POx results. By establishing a simple and feasible protocol for rapid standardized sample processing, we were able to perform the first systematic survey of POx concentrations in a large, general CKD cohort. The present study may serve as a reference for sample handling to assess POx in clinical trials and to determine its role in CKD progression.
Disclosure
SGC has received personal fees from Boehringer-Ingelheim, Bayer, Takeda, CHF Solutions, and Relypsa, and personal fees and other from RenalytixAI, outside the submitted work. K-UE has received personal fees from Akebia, Bayer, Fresenius, and Vifor, and grant support from Amgen, Genzyme, and Shire, outside the submitted work. FK has received personal fees from Allena, Oxthera, Fresenius, and Sanofi, outside the submitted work. All the other authors declared no competing interests.
Acknowledgments
We thank John R. Asplin for his help in establishing the POx assay. We are grateful to A. Schäfer, M. Arend, and S. Rößler for their technical assistance in measuring the samples. We are very grateful for the willingness and time of all study participants of the GCKD study. We also thank the large number of nephrologists for their support of the GCKD study (list of nephrologists currently collaborating with the GCKD study is available at http://www.gckd.org).
Current GCKD investigators and collaborators are as follows: University of Erlangen-Nürnberg: Kai-Uwe Eckardt, Heike Meiselbach, Markus P. Schneider, Mario Schiffer, Thomas Dienemann, Hans-Ulrich Prokosch, Barbara Bärthlein, Andreas Beck, Detlef Kraska, André Reis, Arif B. Ekici, Susanne Avendaño, Dinah Becker-Grosspitsch, Ulrike Alberth-Schmidt, Birgit Hausknecht, Anke Weigel; University of Freiburg: Gerd Walz, Anna Köttgen, Ulla T. Schultheiß, Fruzsina Kotsis, Simone Meder, Erna Mitsch, Ursula Reinhard; RWTH Aachen University: Jürgen Floege, Georg Schlieper, Turgay Saritas; Charité, University Medicine Berlin: Elke Schaeffner, Seema Baid-Agrawal, Kerstin Theisen; Hannover Medical School: Hermann Haller, Jan Menne; University of Heidelberg: Martin Zeier, Claudia Sommerer, Rebecca Woitke; University of Jena: Gunter Wolf, Martin Busch, Rainer Paul; Ludwig-Maximilians University of München: Thomas Sitter; University of Würzburg: Christoph Wanner, Vera Krane, Antje Börner-Klein, Britta Bauer; Medical University of Innsbruck, Division of Genetic Epidemiology: Florian Kronenberg, Julia Raschenberger, Barbara Kollerits, Lukas Forer, Sebastian Schönherr, Hansi Weissensteiner; University of Regensburg, Institute of Functional Genomics: Peter Oefner, Wolfram Gronwald, Helena Zacharias; and Department of Medical Biometry, Informatics and Epidemiology (IMBIE), University Hospital of Bonn: Matthias Schmid, Jennifer Nadal.
The GCKD study is funded by grants from the German Ministry of Education and Research (www.gesundheitsforschung-bmbf.de; grant numbers 01ER 0804, 01ER 0818, 01ER 0819, 01ER 0820, 01ER 0821, and 01ER 0122), the KfH Foundation for Preventive Medicine (http://www.kfhstiftung-praeventivmedizin.de/), and corporate sponsors. It is conducted under the auspices of the German Society of Nephrology (http://www.dgfn.eu). In addition, this study was supported by grants to FK and KUE by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Projektnummer 394046635 – SFB 1365, Sachbeihilfe KN 1148/4–1, the Oxalosis and Hyperoxaluria Foundation, a thematic network grant of the Deutscher Akademischer Austauschdienst (TRENAL) and Dicerna Pharmaceuticals, Cambridge, Massachusetts, USA. PSA was supported by National Institutes of Health grant DK33793. The funders had no role in study design, collection, analysis, and interpretation of data, writing the report, and the decision to submit the report for publication.
Author Contributions
FK, PSA, AP, and K-UE researched the idea and designed the study. AP, MW, and MR acquired the data. AP, KC, SGC, FK, and MW analyzed and interpreted the data. AP, KC, and SGC analyzed the statistics. FK, PSA, and K-UE supervised and mentored. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
References
- 1.Knauf F., Asplin J.R., Granja I. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int. 2013;84:895–901. doi: 10.1038/ki.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X., Bhutani G., Vaughan L.E. Urinary monocyte chemoattractant protein 1 associated with calcium oxalate crystallization in patients with primary hyperoxaluria. BMC Nephrol. 2020;21:133. doi: 10.1186/s12882-020-01783-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palsson R., Chandraker A.K., Curhan G.C. The association of calcium oxalate deposition in kidney allografts with graft and patient survival. Nephrol Dial Transplant. 2020;35:888–894. doi: 10.1093/ndt/gfy271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kusumi K., Barr-Beare E., Saxena V. Renal calcium oxalate deposits induce a pro-atherosclerotic and pro-osteoporotic response in mice. J Cell Biochem. 2017;118:2744–2751. doi: 10.1002/jcb.25924. [DOI] [PubMed] [Google Scholar]
- 5.Saito T., Ikeda M., Asai K. Crystalline cardiomyopathy due to secondary oxalosis after short-bowel syndrome and end-stage renal failure. Clin Res Cardiol. 2016;105:714–716. doi: 10.1007/s00392-016-0981-1. [DOI] [PubMed] [Google Scholar]
- 6.Gulhan B., Turkmen K., Aydin M. The relationship between serum oxalic acid, central hemodynamic parameters and colonization by Oxalobacter formigenes in hemodialysis patients. Cardiorenal Med. 2015;5:164–174. doi: 10.1159/000381219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waikar S.S., Srivastava A., Palsson R. Association of urinary oxalate excretion with the risk of chronic kidney disease progression. JAMA Intern Med. 2019;179:542–551. doi: 10.1001/jamainternmed.2018.7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elgstoen K.B., Johnsen L.F., Woldseth B. Plasma oxalate following kidney transplantation in patients without primary hyperoxaluria. Nephrol Dial Transplant. 2010;25:2341–2345. doi: 10.1093/ndt/gfq065. [DOI] [PubMed] [Google Scholar]
- 9.Chen S.M., Chen T.W., Lee Y.H. Renal excretion of oxalate in patients with chronic renal failure or nephrolithiasis. J Formos Med Assoc. 1990;89:651–656. [PubMed] [Google Scholar]
- 10.Worcester E.M., Nakagawa Y., Bushinsky D.A. Evidence that serum calcium oxalate supersaturation is a consequence of oxalate retention in patients with chronic renal failure. J Clin Invest. 1986;77:1888–1896. doi: 10.1172/JCI112516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fry I.D., Starkey B.J. The determination of oxalate in urine and plasma by high performance liquid chromatography. Ann Clin Biochem. 1991;28:581–587. doi: 10.1177/000456329102800607. [DOI] [PubMed] [Google Scholar]
- 12.Wilson D.M., Liedtke R.R. Modified enzyme-based colorimetric assay of urinary and plasma oxalate with improved sensitivity and no ascorbate interference:reference values and sample handling procedures. Clin Chem. 1991;37:1229–1235. [PubMed] [Google Scholar]
- 13.Porowski T., Zoch-Zwierz W., Konstantynowicz J. Reference values of plasma oxalate in children and adolescents. Pediatr Nephrol. 2008;23:1787–1794. doi: 10.1007/s00467-008-0889-8. [DOI] [PubMed] [Google Scholar]
- 14.Perinpam M., Enders F.T., Mara K.C. Plasma oxalate in relation to eGFR in patients with primary hyperoxaluria, enteric hyperoxaluria and urinary stone disease. Clin Biochem. 2017;50:1014–1019. doi: 10.1016/j.clinbiochem.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selistre L.D.S., Cochat P., Rech D.L. Association between glomerular filtration rate (measured by high-performance liquid chromatography with iohexol) and plasma oxalate. J Bras Nefrol. 2018;40:73–76. doi: 10.1590/1678-4685-JBN-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasidas G.P., Rose G.A. Measurement of plasma oxalate in healthy subjects and in patients with chronic renal failure using immobilised oxalate oxidase. Clin Chim Acta. 1986;154:49–58. doi: 10.1016/0009-8981(86)90087-2. [DOI] [PubMed] [Google Scholar]
- 17.Ladwig P.M., Liedtke R.R., Larson T.S. Sensitive spectrophotometric assay for plasma oxalate. Clin Chem. 2005;51:2377–2380. doi: 10.1373/clinchem.2005.054353. [DOI] [PubMed] [Google Scholar]
- 18.Eckardt K.U., Barthlein B., Baid-Agrawal S. The German Chronic Kidney Disease (GCKD) study: design and methods. Nephrol Dial Transplant. 2012;27:1454–1460. doi: 10.1093/ndt/gfr456. [DOI] [PubMed] [Google Scholar]
- 19.Ermer T., Kopp C., Asplin J.R. Impact of regular or extended hemodialysis and hemodialfiltration on plasma oxalate concentrations in patients with end-stage renal disease. Kidney Int Rep. 2017;2:1050–1058. doi: 10.1016/j.ekir.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]