See Clinical Research on Page 1914
In the field of kidney transplantation, baseline biopsies of the donated kidney, including biopsies obtained before perfusion (0 hours; T0) and at 1 hour after perfusion, and a combination of both these biopsies, is used to confirm the quality of the donated organ. The information obtained from these baseline biopsies is necessary to determine if subsequent histopathologic changes in the allograft after transplantation have originated de novo, or were already present in the donated kidney.
IgA nephropathy (IgAN) is one of the most commonly diagnosed forms of glomerulonephritis worldwide. It is characterized by predominant mesangial IgA and C3 deposition related to mesangial proliferative changes. Latent mesangial IgA deposition can be observed in donated kidneys, including kidneys from living donors with no signs of abnormal urinalysis or kidney dysfunction.1, 2, 3, 4, 5, 6 Latent mesangial IgA deposition, determined as mesangial IgA deposition detected by immunofluorescence microscopy and paramesangial dense deposits detected by electron microscopy,1 is distinguished from IgAN by the absence of proteinuria or hematuria.7,8 Asymptomatic latent IgA deposition has been reported in 13%–29% of kidneys donated by Asian living donors1,3, 4, 5, 6; however, the precise prevalence in an ethnically diverse US population is unknown.
In this light, Gaber and colleagues recently analyzed the prevalence and prognosis of IgA deposition in donated kidneys in a large, retrospective, single-center cohort in the United States in this issue of Kidney International Reports.9 Although half of the cases in the cohort did not undergo T0 biopsy and mesangial immune deposits were not confirmed by electron microscopy, notably 13.2% of living donor kidneys and 24.5% of cadaver donor kidneys that underwent T0 biopsy had IgA deposition. The generally high prevalence of IgA deposition in living-related donor kidneys is probably associated with intrafamilial accumulation, and the lower prevalence of IgA deposition among living donors in the report by Gaber et al. may have been due to differences in the proportions of living-related and living-unrelated donors between Asian countries and the United States. In contrast, the prevalence of IgA deposition generally tends to be lower among cadaveric donations and autopsy specimens, reflecting the general population.S1 However, the prevalence of IgA deposition in Gaber and colleagues’ report was higher among cadaver donor kidneys. Although we cannot exclude the possibility of selection bias among donors who underwent T0 biopsy, these results suggest that the prevalence of asymptomatic IgA deposition in the general US population is higher than expected.
An important strength of the Gaber et al. study is that it reports the prevalence of IgA deposition according to ethnicity: the prevalence was low among blacks (9.7%) but higher among Asians (25.0%), Hispanics (25.9%), and non-Hispanic whites (18.2%). Thus, even in the ethnically diverse United States, the prevalence of IgA deposition was relatively constant. Similar to previous reports,1,3, 4, 5 Gaber et al. failed to identify any predictive factors for IgA deposition before donation. The prevalence of IgA deposition in this report thus needs to be interpreted with caution, because of potential selection bias, and the results may not reflect the actual general population.
What then is the clinical significance of latent IgA deposition in donated kidneys? A short-term analysis showed that latent IgA deposition did not affect the prognosis of the recipients in terms of allograft survival rate, allograft function, and urinalysis, irrespective of the presence of mesangial expansion.1,5 However, Gaber et al. found that IgA deposition in donated kidneys was associated with an increased frequency of acute rejection during the first 6 months posttransplantation and a lower estimated glomerular filtration rate at discharge; however, IgA deposition in donated kidneys had no significant impact on 5-year graft or patient survival. Only 1 previous report identified latent IgA deposition in the donated kidney as a major risk factor for the recurrence of IgAN after kidney transplantation,4 whereas another report showed no association between IgA deposition and recurrent IgAN.1 Unfortunately, the association between IgA deposition in donated kidneys and recurrent IgAN in the allograft was not determined in Gaber and colleagues’ study.
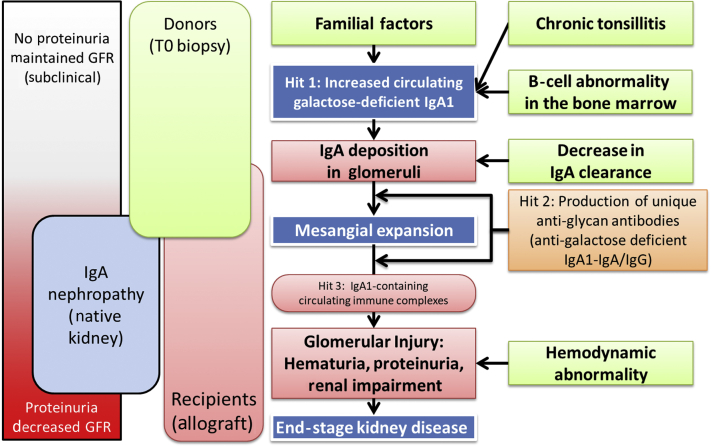
It is necessary to ensure that IgA deposition in donor kidneys will not result in abnormal urinalysis and reduced remnant kidney function after donation; however, the outcomes for living donors were not well studied in the report by Gaber et al. A previous study showed that IgA deposition did not affect remnant kidney function or urinalysis after donation, irrespective of mesangial expansion,1 implying that not all cases of asymptomatic IgA deposition progress to symptomatic IgAN.2 The pathophysiology of IgAN has recently been elucidated in native kidneys,7,S2,S3 and translation of these concepts to kidney allografts may substantially improve our understanding of the mechanisms involved in the pathogenesis of IgAN (Figure 1). The onset of IgAN needs additional stimulation by environmental factors, such as tonsillitis.S2 A previous report of familial IgA nephropathy suggested that high serum concentrations of galactose-deficient IgA1 alone were not sufficient to induce renal injury and abnormal urinalysis.S3 Another study showed that serum IgA O-glycosylation patterns were similar in donors with and without IgA deposition, but different from those in recipients with IgAN.6 Progression from latent IgA deposition to IgAN thus requires the additional appearance of IgG antibodies specific for galactose-deficient IgA1.S4 These reports suggest that IgA deposition in the remnant kidney in living donors will not progress to IgAN in the absence of additional stimuli. However, long-term follow-up of living donors is desirable to determine the safety of donations from donors with IgA depositions.
Figure 1.
Pathophysiology of IgA deposition and IgA nephropathy after kidney transplantation. Gd-IgA1, galactose-deficient IgA1; GFR, glomerular filtration rate.
Regarding the subclass of IgA, all cases with latent IgA deposition in the donated kidney showed IgA1-predominant mesangial deposition on immunofluorescence microscopy.S5 Furthermore, co-deposition of C3 and IgG accompanied by IgA deposition is considered to be an indicator of progression from IgA deposition to IgAN.8 In the report by Gaber et al., only 9.1% of donated kidneys with IgA deposition had co-deposition of IgG. In contrast, another recent study of symptomatic IgAN in the native kidney detected IgG antibodies against galactose-deficient IgA1 in samples using confocal immunofluorescence microscopy, even in kidneys negative for IgG by routine immunofluorescence.S6 Further investigation of the prevalence of IgG antibodies specific for galactose-deficient IgA1 in donated kidneys with IgA deposition is therefore needed.
Latent IgA deposition from donated kidneys was reported to disappear in 70% of allografts by 1 year after transplantation, irrespective of mesangial expansion.1 Gaber et al. also showed that IgA deposition disappeared in 76% (68 of 90) of patients within 1 year after kidney transplantation: 91% of biopsies with 1+ IgA staining became negative at follow-up, compared with 63% of biopsies with 2+ IgA staining and 40% of biopsies with 3+ IgA staining. The precise mechanisms by which IgA deposits disappear from mesangial cells have not been established, and the effects of immunosuppressant dosages and regimens on the rate of disappearance have not been reported. A previous report from Japan showed an association between the disappearance of IgA deposition and sufficient allograft function.S7 Human kidneys are thought to have a self-purifying ability, allowing them to remove IgA deposits from the glomeruli and excrete them via the lymphatic vessels; alternatively, IgA deposits may be digested by serine proteases in mesangial cells.S8 Furthermore, reduced kidney function is thought to lead to the deterioration of its lymphatic vessels.S9 I therefore hypothesized that reduced allograft function would be associated with reduced clearance of IgA deposits from the mesangial domain. In addition to the recipient’s immunologic status, this reduced ability to clear IgA deposits from the mesangial domain would affect the disappearance of IgA deposition.
In conclusion, Gaber and colleagues clearly showed that IgA deposition in donated kidneys is not limited to Asians and Hispanics but is similarly prevalent among non-Hispanic whites in an ethnically diverse population. Combined with the results of previous reports, IgA deposition from the donated kidney appears to have little effect on the prognoses of kidney donors and recipients. However, the long-term prognosis of donors has not been reported, and future large-scale analyses are required.
Disclosure
The author declared no competing interests.
Acknowledgments
This study was supported by JSPS KAKENHI Grant Numbers JP18K09195. I thank Susan Furness, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Footnotes
Supplementary References.
Supplementary Material
References
- 1.Sofue T., Inui M., Hara T. Latent IgA deposition from donor kidneys does not affect transplant prognosis, irrespective of mesangial expansion. Clin Transplant. 2013;27:14–21. doi: 10.1111/ctr.12158. [DOI] [PubMed] [Google Scholar]
- 2.Hara S., Ichimaru N., Kyo M. Latent mesangial immunoglobulin A deposition in long-term functioning kidney does not correlate with disease progression and may exhibit fluctuating patterns. Transplant Proc. 2014;46:124–129. doi: 10.1016/j.transproceed.2013.07.072. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki K., Honda K., Tanabe K. Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int. 2003;63:2286–2294. doi: 10.1046/j.1523-1755.63.6s.2.x. [DOI] [PubMed] [Google Scholar]
- 4.Moriyama T., Nitta K., Suzuki K. Latent IgA deposition from donor kidney is the major risk factor for recurrent IgA nephropathy in renal transplantation. Clin Transplant. 2005;19(suppl 14):41–48. doi: 10.1111/j.1399-0012.2005.00403.x. [DOI] [PubMed] [Google Scholar]
- 5.Ji S., Liu M., Chen J. The fate of glomerular mesangial IgA deposition in the donated kidney after allograft transplantation. Clin Transplant. 2004;18:536–540. doi: 10.1111/j.1399-0012.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- 6.Nakazawa S., Imamura R., Kawamura M. Difference in IgA1 O-glycosylation between IgA deposition donors and IgA nephropathy recipients. Biochem Biophys Res Commun. 2019;508:1106–1112. doi: 10.1016/j.bbrc.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Silva F.G., Chander P., Pirani C.L. Disappearance of glomerular mesangial IgA deposits after renal allograft transplantation. Transplantation. 1982;33:241–246. [PubMed] [Google Scholar]
- 8.Sofue T., Suzuki H., Ueda N. Post-transplant immunoglobulin A deposition and nephropathy in allografts. Nephrology. 2018;23(suppl 2):4–9. doi: 10.1111/nep.13281. [DOI] [PubMed] [Google Scholar]
- 9.Gaber L., Khan F.N., Graviss E.A. Prevalence, characteristics, and outcomes of incidental IgA glomerular deposits in donor kidneys. Kidney Int Rep. 2020;5:1914–1924. doi: 10.1016/j.ekir.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.