Abstract
Background
The most important infectious trigger of asthma is the virus and patients with immunoglobulin deficiencies are prone to recurrent respiratory infections.
Objective
We investigated the relationship between immunoglobulin G subclass and recurrent respiratory symptom exacerbation and explored possible therapeutic effects of intravenous immunoglobulin administration.
Methods
Twenty-eight infants less than 24 months old with 2 or more recurrent wheezing episodes (infantile wheezer group) and 29 asthmatic children aged 24 months to 15 years (bronchial asthma [B-asthma] group) visited our hospital from October 2010 to January 2018. Serum immunoglobulin G, A, M, E, G1, G2, G3, and G4 were measured in each group and compared. In both groups, serum immunoglobulin and symptoms were compared before and after intravenous immunoglobulin administration.
Results
The 2 study groups exhibited several statistically significant differences when comparing respiratory virus infection rate (p < 0.001), coinfection rate (p < 0.0001), most commonly found viral infection (human bocavirus vs. human rhinovirus), and immunoglobulin A (p < 0.001), E (p = 0.008), G2 (p < 0.001), and G4 (p = 0.011) levels. In the infantile wheezer group, there was an inverse correlation between immunoglobulin G4 levels and wheezing numbers (R = -0.5538, P = 0.0022). Both groups showed significant changes in immunoglobulin levels and respiratory symptom exacerbations (recurrent wheezing, shortness of breath, chest tightness, cough, and fever) over 1 year after intravenous immunoglobulin administration.
Conclusion
There was an association between recurrent wheezing and specific immunoglobulin G deficiencies. We suggest that intravenous immunoglobulin therapy significantly elevates specific immunoglobulin G levels though it lasts only for short term and might be associated with decreased respiratory symptoms. Therefore, low IgG4 levels among infants with recurrent wheezing may be indicative for intravenous immunoglobulin therapy.
Keywords: Child, Immunodeficiency, Intravenous immunoglobulins, Intrinsic asthma, Respiratory tract infections, Viruses
INTRODUCTION
Asthma can be classified into extrinsic asthma and intrinsic asthma, depending on the cause. Extrinsic asthma is an immunoglobulin E (IgE)-mediated allergic asthma that is symptomatic when exposed to the causative antigen and is positive in skin tests or bronchial challenge tests for allergens. Intrinsic asthma is clinically evident in patients with asthma who are unresponsive to skin prick tests and have not been shown to be (IgE) mediated [1]. These patients are susceptible to viral respiratory infections, exercise, anxiety, and changes in climate and humidity, which trigger or aggravate their asthma. Intrinsic asthma is often induced in young children under 2 years old.
The most significant contributory factor to bronchial asthma in children is the respiratory infection [2], and a deficiency of immunoglobulin G (IgG) subclass may contribute to recurrent respiratory infections [3]. However, the IgG subclass differs greatly in normal values according to age and individual immunophysiology. In particular, since IgG2 and IgG4 increase rapidly after infancy to levels found in adults, the relationship between IgG subclass deficiency and bronchial asthma exacerbation is highly dependent on age [4].
The most important infectious trigger of asthma is the virus, with respiratory syncytial virus (RSV) and parainfluenza virus (PIV) in younger children and influenza virus in older children [5]. Studies have shown that chronic bronchial infections occur frequently in young immunodeficient children and that these bronchial infections may contribute substantially to asthma development [6]. It is known that one or more blood immunoglobulins are associated with severe chronic asthma, including one or more IgG subclass deficiencies in children with asthma and nonallergic chronic respiratory symptoms [7]. Intravenous immunoglobulin (IVIG) contains all subclasses of IgG and has been used as a supplementary therapy in primary and secondary immunodeficiency diseases, as well as in many autoimmune diseases in clinical trials [8,9]. Most adverse events (AEs) associated with IVIG are mild and transient. Late-occurring AEs are rare [10].
In this study, we investigated the relationship between IgG subclass and recurrent wheezing and/or asthma exacerbation by comparing levels of immunoglobulins G, A, M, E, and IgG subclasses in 2 groups: infantile wheezers and older children with bronchial asthma. In addition, IVIG was administered to patients with recurrent asthma exacerbation due to respiratory infections as a supplementary therapy and their IgG subclasses were also compared.
MATERIALS AND METHODS
The subjects were 28 infants less than 24 months who had 2 or more recurrent wheezing episodes (infantile wheezer group) and 29 asthmatic children aged 24 months to 15 years (bronchial asthma [B-asthma] group) who visited the Asthma and Allergy Center at Inje University Sanggye Paik Hospital, Seoul, South Korea from October 2010 to January 2018. The inclusion criteria for the infantile wheezer group are infants under 24 months of age who had wheezing more than 2 times regardless of fever, and the inclusion criteria for the B-asthma group included symptomatic or asymptomatic asthma. Exclusion criteria included congenital hypogammaglobulinemia, active malignancy or hematologic disease, protein-losing enteropathy, nephrotic syndrome, or treatment with systemic corticosteroids > 20 mg/day of methylprednisolone or equivalent doses.
Asthma diagnoses were established on a history of respiratory symptoms, such as wheezing, shortness of breath, chest tightness, and coughing associated with variable expiratory airflow limitation before hospitalization, according to the Global Initiative for Asthma [11]. Allergic rhinitis was defined as a disease of the nasal lining presenting with symptoms of nasal itch, sneezing, runny and/or blocked nose with no cold. A child was considered to have had allergic rhinitis in the first year of life if their mother reported at least one episode without a cold [12,13]. Atopic dermatitis was diagnosed by clinical examination mainly based on morphological features and distribution of lesions. Number of wheezing episodes from birth was determined by recording the doctor's auscultation findings when visiting the clinic and the number of times parents observed an episode.
A multiplex reverse transcription-polymerase chain reaction (RT-PCR) test (The Anyplex II RV16 Detection kit, Seegen Inc., Seoul, Korea) of nasopharyngeal aspirates was conducted for 15 items, as follows: influenza virus A and B (IFA, IFB), RSV A and B (RSV A, RSV B), PIV 1-4 (PIV 1, PIV 2, PIV 3, PIV 4), human coronavirus 229E and OC43 (hCV-229E, hCV-OC43), human rhinovirus (hRV), human enterovirus (hEV), adenovirus (AdV), human bocavirus (hBoV), and human metapneumovirus (hMPV).
Serum IgG, IgA, IgM, IgE, IgG1, IgG2, IgG3, and IgG4 were measured in each group and compared. In addition, the correlation between the number of wheezing episodes from birth and serum Ig levels were analyzed and compared among the groups.
Serum total IgE levels were measured by IgE fluorometric enzyme immunoassay (Stratus, Boxter Diagnostic Inc., Farmers Branch, TX, USA), and, for the measurement of IgG, IgA, IgM, and IgG subclass, the human IgG subclasses monoclonal radial immunodiffusion kit from the Green Cross Clinical Research Institute (Yongin, Korea) was used. Results were compared by finding the mean and standard deviation of each group, while statistical analyses were done using the Mann-Whitney U test and χ2 calculations (2-way contingency table tests) by SPSS ver. 10.0 (SPSS Inc., Chicago, IL, USA). A p value of <0.05 was considered statistically significant.
For subjects in which informed consent was given (nonrandomized), IVIG was administered (a total of 2 infusions at 1 g/kg per day) (Green Cross Corp., Yongin, Korea).
This study was approved by the Institutional Review Board of Inje University (#INJE201707011-UE002).
RESULTS
Subject characteristics
Subject characteristics are shown in Table 1. The infantile wheezer group had a mean age of 1.6 years, while the B-asthma group was 6.5 years old (p < 0.01). Regarding concomitant allergic diseases, the B-asthma group had a higher incidence of rhinitis (p < 0.001). In the PCR test for respiratory virus, the positive rate was 65.8% in the infantile wheezer group and 37.9% in the B-asthma group (p < 0.001). In the coinfection rate of respiratory virus, the figure was 66.7% in the younger infantile wheezer group and 16.7% in the B-asthma group (p < 0.0001). There was no gender ratio difference between the 2 groups, and there was no difference in the prevalence of other allergic diseases such as atopic dermatitis and food allergy.
Table 1. Subject characteristics.
| Characteristic | Infantile wheezer (n = 28) | B-asthma (n = 29) | p value | |
|---|---|---|---|---|
| Age (yr) | 1.6 (1–2) | 6.5 (3–15) | < 0.01 | |
| Sex, male:female | 18:10 | 14:15 | NS | |
| Additional history | ||||
| Food allergy | 2 (7.1) | 2 (6.9) | NS | |
| Atopic dermatitis | 2 (7.1) | 3 (10.3) | NS | |
| Allergic rhinitis | 1 (3.5) | 12 (41.4) | < 0.001 | |
| Virus-PCR* | ||||
| Positive | 18 (65.8) | 12 (37.9) | < 0.001 | |
| Coinfection | 12/18 (66.7) | 2/12 (16.7) | < 0.0001 | |
Values are presented as median (range) or number (%).
PCR, polymerase chain reaction; NS, not significant.
*Virus-PCR positive at presentation.
Virus profiles
Table 2 shows virus profiles of the 2 groups derived from the RT-PCR. A multiplex RT-PCR test of nasopharyngeal aspirates was conducted for 15 items, as follows: IFA, IFB, RSV A, RSV B, PIV 1, PIV 2, PIV 3, PIV 4, hCV-229E, hCV-OC43, hRV, hEV, AdV, hBoV, and hMPV. Total viral infection rate was higher in infantile wheezing group than B-asthma group (64.2% vs. 41.3%, p < 0.001).
Table 2. Virus profiles of infantile wheezer and B-asthma groups.
| Virus | Infantile wheezer (n = 18/28) | B-asthma (n = 12/29) | p value |
|---|---|---|---|
| hCV | 2 (5.4) | 1 (6.3) | NS |
| hRV | 5 (13.5) | 7 (43.8) | < 0.0001 |
| RSV | 6 (16.2) | 2 (12.5) | NS |
| hBoV | 10 (27.0) | 1 (6.3) | < 0.001 |
| hEV | 5 (13.5) | 1 (6.3) | NS |
| PIV | 4 (10.8) | 1 (6.3) | NS |
| AdV | 4 (10.8) | 2 (12.5) | NS |
| hMPV | 1 (2.7) | 1 (6.3) | NS |
Nasopharyngeal aspirates from all patients were obtained within 48 hours of admission for multiplex RT-PCR assay to detect the following 15 common respiratory viruses: influenza virus A and B (IFA, IFB), respiratory syncytial virus A and B (RSV A, RSV B), parainfluenza virus 1-4 (PIV 1, PIV 2, PIV 3, PIV 4), human coronavirus 229E and OC43 (hCV-229E, hCV-OC43), human rhinovirus (hRV), human enterovirus (hEV), adenovirus (AdV), human bocavirus (hBoV), and human metapneumovirus (hMPV).
NS, not significant.
χ2 calculations: 2-way contingency table tests.
In the infantile wheezer group, hBoV was the most common virus, and the infection rate was significantly higher (27.0% vs. 6.3%, p < 0.001) when compared with the B-asthma group, followed by RSV, HRV, and hEV. However, hRV was the most common infection in the B-asthma group and the infection rate was significantly higher than that of the infantile wheezer group (43.8% vs 13.5%, p < 0.0001). There was no significant difference in the infection rates of hCV, RSV, hEV, PIV, AdV, or hMPV between the 2 groups.
Immunoglobulin levels
The basic immunoglobulin and IgG subclass values of the infantile wheezer and B-asthma groups are shown in Table 3. IgA level in the infantile wheezer group was 59.0 ± 30.6 mg/mL, which was significantly different from the 118.5 ± 30.6 mg/mL found in the B-asthma group (p < 0.001). The infantile wheezer group had an IgE level of 113.6 ± 233.1 IU/L, which was significantly lower (p = 0.008) than that of the B-asthma group (180.0 ±2 74.4 IU/L). Comparing IgG2 levels, the results were 167.6 ± 1391 mg/mL and 329.4 ± 273.9 mg/mL, respectively (p < 0.001). In the comparison of serum IgG4 concentration, the result of infantile wheezer group was 17.4 ± 16.7, which was significantly lower than 29.1 ± 20.2 of the B-asthma (p = 0.011). There was no significant difference in total IgG, IgM, IgG1, or IgG3 between the 2 groups.
Table 3. Baseline serum Ig and IgG subclass levels in infantile wheezer and B-asthma groups.
| Ig and IgG subclass | Infantile wheezer (n = 28) | B-asthma (n = 29) | p value |
|---|---|---|---|
| IgG (mg/mL) | 822.0 ± 305.2 | 997.2 ± 495.3 | NS |
| IgA (mg/mL) | 59.0 ± 30.6 | 118.5 ± 30.6 | < 0.001 |
| IgM (mg/mL) | 112.0 ± 44.3 | 109.6 ± 32.6 | NS |
| IgE (IU/L) | 113.6 ± 233.1 | 180.0 ± 274.4 | 0.008 |
| IgG1 (mg/mL) | 577.1 ± 278.0 | 761.6 ± 421.3 | NS |
| IgG2 (mg/mL) | 167.6 ± 139.1 | 329.4 ± 273.9 | < 0.001 |
| IgG3 (mg/mL) | 39.6 ± 18.6 | 45.2 ± 35.3 | NS |
| IgG4 (mg/mL) | 17.4 ± 16.7 | 29.1 ± 20.2 | 0.011 |
Values are presented as mean ± standard deviation.
NS, not significant.
Respiratory symptoms, immunoglobulin levels, and immunoglobulin therapy
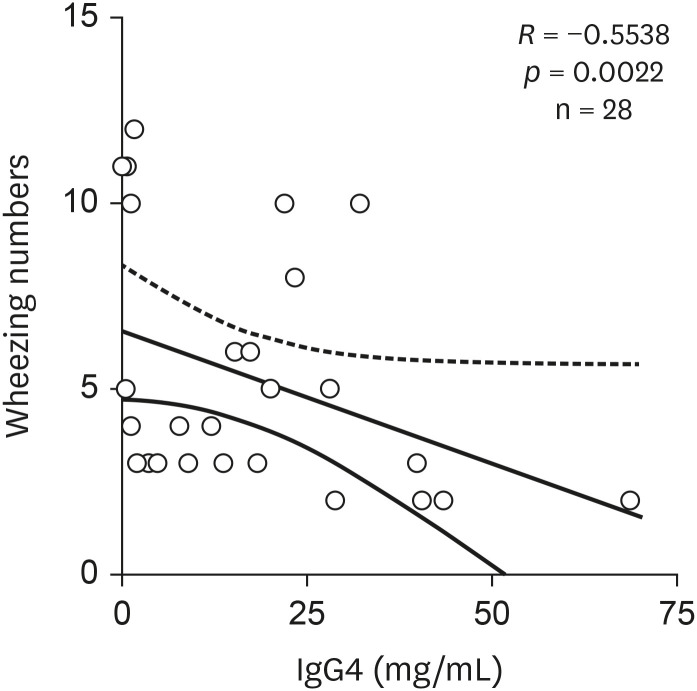
The association between wheezing number and immunoglobulin and IgG subclass levels showed a significant inverse correlation in IgG4, indicating a tendency of increasing wheezing number with lower serum IgG4 levels in infantile wheezer group (Fig. 1) (R = -0.5538, p = 0.0022).
Fig. 1. Correlation between serum IgG4 and wheezing numbers in infantile wheezer group.
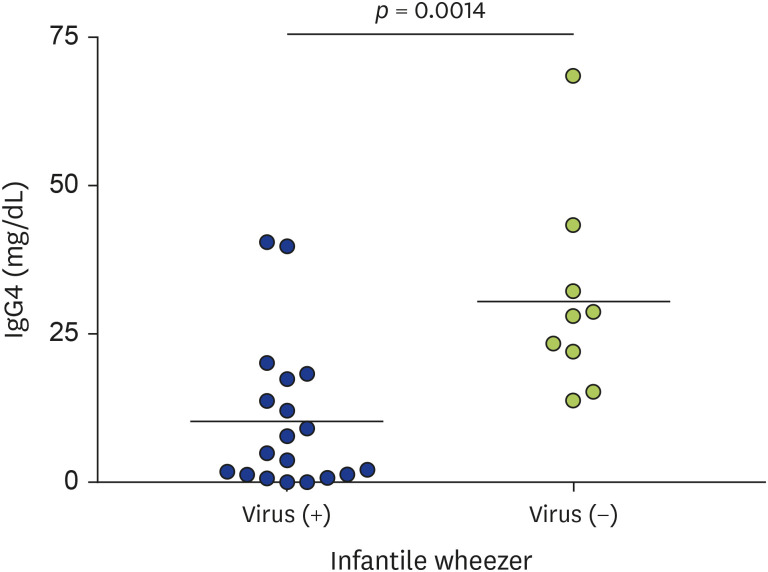
Within the infantile wheezer group, serum IgG4 levels were compared between children positive for respiratory virus infection and children negative for viral infection (mean ± standard deviation: 10.27±12.44 mg/mL vs. 30.62 ± 16.82 mg/mL, respectively; p = 0.0014) (Fig. 2). In addition, there were no differences in IgG4 levels when comparing virus positive and virus negative subgroups within the B-asthma group.
Fig. 2. comparison of serum IgG4 levels between virus positive and virus negative subjects in infantile wheezer group.
IVIG administration was conducted on 18 patients in the infantile wheezer group, and the respective differences in Ig and IgG subclass levels are shown in Table 4. Ig and IgG subclass tests were conducted within 1 week after IVIG administration. In the infantile wheezer group, total IgG was 759.5 ± 171.2 mg/mL and 2588.6 ± 457.2 mg/mL, respectively before and after administration of IVIG, indicating a significant increase (p < 0.0001). Serum IgG1 showed a significant increase after IVIG administration (from 558.4 ± 136.7 to 1,873.9 ± 359 mg/mL, p < 0.0001). Serum IgG2 also showed a significant increase after administration (from 164.5 ± 101.4 to 862.7 ± 245.7 mg/mL, p < 0.0001). Serum IgG3 (from 43.6 ± 18.9 to 97.4 ± 23.2 mg/mL, p < 0.0001) and IgG4 (from 19.0 ± 14.1 to 29.2 ± 13.5 mg/mL, p = 0.025) also showed significant increases after administration. There were no significant changes in the concentrations of IgA, IgM, and IgE after the IVIG administration.
Table 4. Serum Ig and IgG subclass levels in infantile wheezer and B-asthma groups before and 1 week after IVIG administration.
| Ig and IgG subclass | Infantile wheezer (n = 19) | B-asthma (n = 13) | ||||
|---|---|---|---|---|---|---|
| Before IVIG | 1 Week after IVIG | p value | Before IVIG | 1 Week after IVIG | p value | |
| IgG (mg/mL) | 759.5 ± 171.2 | 2,588.6 ± 457.2 | < 0.0001 | 998.8 ± 613.2 | 2,239.2 ± 982.3 | < 0.0001 |
| IgA (mg/mL) | 61.2 ± 27.4 | 56.7 ± 25.1 | NS | 101.2 ± 44.8 | 98.4 ± 41.4 | NS |
| IgM (mg/mL) | 110.3 ± 44.4 | 107.0 ± 33.3 | NS | 118.9 ± 35.2 | 131.5 ± 43.8 | NS |
| IgE (IU/L)† | 45.6 ± 79.4 | 70.6 ± 191.3 | NS | 198.6 ± 340.5 | 196.7 ± 335.7 | NS |
| IgG1 (mg/mL) | 558.4 ± 136.7 | 1,873.9 ± 359 | < 0.0001 | 721.2 ± 479.2 | 1,564.1 ± 701.3 | < 0.001 |
| IgG2 (mg/mL) | 164.5 ± 101.4 | 862.7 ± 245.7 | < 0.0001 | 271.5 ± 223.8 | 768.2 ± 333.9 | < 0.001 |
| IgG3 (mg/mL) | 43.6 ± 18.9 | 97.4 ± 23.2 | < 0.0001 | 35.2 ± 17.5 | 68.5 ± 26.7 | < 0.01 |
| IgG4 (mg/mL) | 19.0 ± 14.1 | 29.2 ± 13.5 | 0.025 | 26.7 ± 20.5 | 33.8 ± 19.1 | NS |
Values are presented as mean ± standard deviation.
IVIG, intravenous immunoglobulin; NS, not significant.
†Median before 25.0 after 20.7.
In the B-asthma group, IVIG administration was performed on 13 patients, and the corresponding changes in Ig and IgG subclass levels are shown in Table 4. Ig and IgG subclass tests were conducted within 1 week after IVIG administration. Total IgG was 998.8 ± 613.2 mg/mL and 22,392 ± 982.3 mg/mL before and after IVIG administration, respectively, indicating a significant increase (p < 0.0001). Serum IgG1 showed a significant difference before and after IVIG administration (from 721.2 ± 479.2 to 1,564.1 ± 701.3 mg/mL, p < 0.001). Serum IgG2 (from 271.5 ± 223.8 to 768.2 ± 333.9 mg/mL, p < 0.001) and IgG3 (from 35.2 ± 17.5 to 68.5 ± 26.7 mg/mL, p < 0.01) also showed significant increases after administration. However, serum IgG4 did not show any significant difference before and after administration (from 26.7 ± 20.5 to 33.8 ± 19.1 mg/mL, p = not significant). There was no significant difference in the IgA, IgM, and IgE concentrations before and after the IVIG administration.
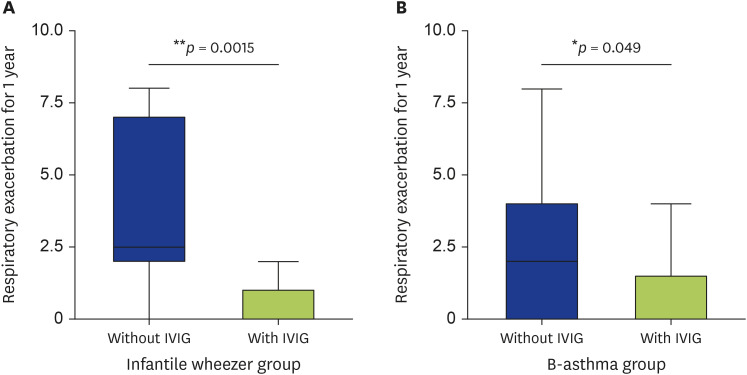
After IVIG infusion, respiratory symptom exacerbations were recorded in a diary and collected prospectively. In the infantile wheezer group, the clinical difference in respiratory symptoms (recurrent wheezing, shortness of breath, chest tightness, cough, and fever) for 1 year between the group receiving IVIG administration and the group without administration is shown in Fig. 3A. The IVIG-administered group (n = 19) had 0.5 ± 0.7 respiratory symptom exacerbations in 1 year, which was significantly lower than the 3.9 ± 3.0 respiratory symptom exacerbations in the group without IVIG administration (n = 8, p = 0.0015).
Fig. 3. Respiratory exacerbations in 1 year with and without intravenous immunoglobulin (IVIG): infantile wheezer group (A) and B-asthma group (B).
In the B-asthma group, the clinical difference in respiratory symptom exacerbation for 1 year between the IVIG-administered group and the group without administration is shown in Fig. 3B. The IVIG-administered group (n = 11) had 0.7 ± 1.3 respiratory symptom exacerbations in 1 year, which was significantly lower than the 2.5 ± 2.6 respiratory symptom exacerbations in the group without IVIG administration (n = 17, p = 0.049).
It was found that the clinical effect of IVIG administration was greater in the infantile wheezer group than in the B-asthma group. It should be noted that the infantile wheezer group is significantly younger than the B-asthma group (p < 0.01).
DISCUSSION
In this study, we observed a significant inverse correlation between wheezing episodes and IgG4 concentration in the infantile wheezer group, who are young children (younger than 24 months old). It was also observed that within the infantile wheezer group, those who were positive for respiratory viral infection had significantly lower IgG4 levels than those who were negative for viral infection. Within 1 week after IVIG administration, there were increases in total IgG and IgG subclasses, including IgG4. These results suggest that viral infection due to low IgG4 (blocking antibody) concentration is a potential reason for wheezing in young children. Extrapolating from this result, delayed maturation of the immune system in children younger than 2 years could lead to virus-induced recurrent wheezing.
IgG2 and IgG4 concentrations reach adult levels later in life unlike IgG1 and IgG3; however, isolated IgG4 deficiency is difficult to confirm [14]. Heiner et al. [15] found that IgG4 deficiency is associated with important clinical disease, and that most IgG4 deficiency cases (69%) are associated with other Ig deficiencies. Moss et al. [16] also reported an association of isolated IgG4 deficiency with respiratory infections. IgG4-committed B cells are abundant in mucous membranes, and IgG4 is distributed around the mucous secretion rather than in serum, which indicates the biologic role of IgG4 in secretory immunity [17,18].
Reports on IgG subclass deficiency began in the late 1960s and these deficiencies are related to immunodeficiency such as ataxia telangiectasia and common variable hypogammaglobulinemia. After these initial reports, numerous cases of IgG subclass deficiency with normal serum total IgG concentration have been described. However, even among normal subjects, there have been selective deficiency of IgG1 and IgG2, as well as reports of selective deficiency of IgG3 among healthy families. In 1981, Beck and Heiner [19] reported 4 cases of recurrent respiratory infections with isolated IgG4 deficiency, 3 of which were shown to have IgG2 deficiency. Smith et al. [20] reported IgG2 and IgG4 deficiency in the majority of the 37 children with asthma and chronic respiratory infections. However, IgG subclass concentrations can differ greatly among individuals and by age. It should be noted that the immunodiffusion method used in the past is unable to measure IgG4 levels in 20%–30% of the entire population [21].
According to our previous study [22], IgM concentrations in wheezing children and normal control group at the age of 24 months or younger were respectively 150.0 ± 4.1 mg/dL and 97.9 ± 4.6 mg/dL, which shows a significant increase in the wheezing children. This result was in line with the results of Lin et al. [23] and Berger et al. [24]. While there was no investigation as to the reason for these increases, it was suggested that the increases may have been due to repeated viral infections. However, in our current study, no difference could be found in IgM level, according to age or disease. Therefore, the contribution of IgM to the wheezing is unclear. It should be noted, however, that in our 1995 study [18] the geometric means of IgG4 concentration in wheezing children and normal control group of the age 24 months or younger were 0.039 ± 0.067 g/L and 0.128 ± 0.110 g/L, respectively, indicating a significant decrease in the wheezing children (p < 0.05). In wheezing children at the age of 24 months or older, the figure was 0.206 ± 0.181 g/L, which is within the normal range for the age. This is an example of the relationship between repeated infection and wheezing in children 24 months or younger. On the other hand, IgE concentrations in the wheezing children and normal control group of 24 months old or younger were respectively 20.9 ± 5.3 IU and 26.9 ± 4.7 IU, indicating no statistically significant difference between the 2 groups. However, wheezing children of 24 months old or older showed an increased value of 368.6 ± 55.1 IU, which was above the normal range, indicating that the IgE-mediated response is becoming a major mechanism. In our current study, IgE concentration was higher in the older B-asthma group with a lower viral infection rate than in the younger age group, indicating that, rather than lower IgG4 levels, higher IgE levels may contribute to asthma exacerbation.
IVIG prevents bacterial infections, reduces the number of infections, and shortens the duration of treatment in patients with X-linked agammaglobulinemia or common variable immunodeficiency [25]. So far, however, except for severe bacterial infections in IgG subclass deficiency, specific antibody deficiency, and transient hypogammaglobulinemia in infants, the therapeutic mechanisms are unclear, which makes it difficult to choose treatment. Ten percent liquid IVIG, which has recently been developed, is known to possess antibodies against respiratory viruses, a major cause of infection [26]. Although IVIGs have proven to be effective and tolerable, various side effects have been reported. Minor side effects include flushing, headache, discomfort, fever, chills, and fatigue, but some rare side effects, including kidney damage, thrombosis, arrhythmia, aseptic meningitis, and anaphylactic reactions, especially in IgA-deficient patients are severe [10]. If viral infection due to IgG subclass deficiency is a major cause of recurrent wheezing in young children, we suggest a selective use of IVIG in severe wheezing diseases that cannot be treated with other medications [27,28].
In this study, there was a significant inverse correlation between wheezing number and IgG4 concentration in the infantile wheezer group, which is comprised of children 24 months old or younger. The lower the serum IgG4 level, the more wheezing number increases. In addition, in the infantile wheezer group, those positive for respiratory viral infection showed a significantly lower IgG4 level than those confirmed negative. Within 1 week after administration of IVIG, total IgG and IgG subclasses (including IgG4) increased. There were fewer respiratory symptoms during the 1 year in the group administered IVIG than in the group without administration, and the difference was more prominent in the younger infantile wheezer group. It should be noted that the increase in IgG levels post IVIG are most likely temporary. Although we did not measure these levels over the long term, a recent study found the half-life of total IgG was 36.1 days (range, 18.5–65.9 days), with similar values reported for IgG1, IgG2, and IgG4 (median, 30.7–38.0 days), and a lower value for IgG3 (26.7 days). For IgG3 and IgG4, there was a greater degree of variability in half-life among patients than for IgG1 and IgG2 [29].
The above results show that viral infection due to low IgG4 (blocking antibody) may be associated with infantile wheezing, and delayed maturation of the immune system at the age of 2 years old or younger may contribute to wheezing later in life. Therefore, low IgG4 levels among infants with recurrent wheezing may be indicative for intravenous immunoglobulin therapy.
ACKNOWLEDGEMENTS
This study was supported by GC Pharma and the authors wish to thank Inje University for the funding provided by a 2018 Inje University Industry-Academia Collaboration Foundation grant.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Chang-Keun Kim, Jin-Sung Park, Hanna Kim.
- Data curation: Jin-Sung Park, Shou-Yu Chu, EunMi Kwon.
- Formal analysis: Zak Callaway, EunMi Kwon.
- Funding acquisition: Chang-Keun Kim.
- Methodology: Chang-Keun Kim, Jin-Sung Park.
- Project administration: EunMi Kwon.
- Visualization: EunMi Kwon.
- Writing - original draft: Chang-Keun Kim, Hanna Kim, Zak Callaway.
- Writing - review & editing: Chang-Keun Kim, Hanna Kim, Zak Callaway.
References
- 1.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 2.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JH, Park S, Hwang YI, Jang SH, Jung KS, Sim YS, Kim CH, Kim C, Kim DG. Immunoglobulin g subclass deficiencies in adult patients with chronic airway diseases. J Korean Med Sci. 2016;31:1560–1565. doi: 10.3346/jkms.2016.31.10.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z, Deng C, Li P, Wang J, Ma L, Li Y, Xu Y. A reference interval for serum IgG subclasses in Chinese children. PLoS One. 2018;13:e0192923. doi: 10.1371/journal.pone.0192923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim CK, Callaway Z, Gern JE. Viral infections and associated factors that promote acute exacerbations of asthma. Allergy Asthma Immunol Res. 2018;10:12–17. doi: 10.4168/aair.2018.10.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Touw CM, van de Ven AA, de Jong PA, Terheggen-Lagro S, Beek E, Sanders EA, van Montfrans JM. Detection of pulmonary complications in common variable immunodeficiency. Pediatr Allergy Immunol. 2010;21:793–805. doi: 10.1111/j.1399-3038.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 7.Whelan MA, Hwan WH, Beausoleil J, Hauck WW, McGeady SJ. Infants presenting with recurrent infections and low immunoglobulins: characteristics and analysis of normalization. J Clin Immunol. 2006;26:7–11. doi: 10.1007/s10875-006-8144-1. [DOI] [PubMed] [Google Scholar]
- 8.Abdou NI, Greenwell CA, Mehta R, Narra M, Hester JD, Halsey JF. Efficacy of intravenous gammaglobulin for immunoglobulin G subclass and/or antibody deficiency in adults. Int Arch Allergy Immunol. 2009;149:267–274. doi: 10.1159/000199723. [DOI] [PubMed] [Google Scholar]
- 9.Abrahamian F, Agrawal S, Gupta S. Immunological and clinical profile of adult patients with selective immunoglobulin subclass deficiency: response to intravenous immunoglobulin therapy. Clin Exp Immunol. 2010;159:344–350. doi: 10.1111/j.1365-2249.2009.04062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orbach H, Katz U, Sherer Y, Shoenfeld Y. Intravenous immunoglobulin: adverse effects and safe administration. Clin Rev Allergy Immunol. 2005;29:173–184. doi: 10.1385/CRIAI:29:3:173. [DOI] [PubMed] [Google Scholar]
- 11.Global Initiative for Asthma (GINA) 2020 GINA Report, Global Strategy for Asthma Management and Prevention [Internet] Fontana (WI): GINA; 2020. [cited 2020 Jul 30]. Available from: www.ginasthma.org. [Google Scholar]
- 12.Keil T, Bockelbrink A, Reich A, Hoffmann U, Kamin W, Forster J, Schuster A, Willich SN, Wahn U, Lau S. The natural history of allergic rhinitis in childhood. Pediatr Allergy Immunol. 2010;21:962–969. doi: 10.1111/j.1399-3038.2010.01046.x. [DOI] [PubMed] [Google Scholar]
- 13.Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, Lang DM, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph CC, Schuller D, Spector SL, Tilles SA. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122:S1–84. doi: 10.1016/j.jaci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Heiner DC. IgG4 immunodeficiency. N Engl Reg Allergy Proc. 1988;9:43–50. doi: 10.2500/108854188778984509. [DOI] [PubMed] [Google Scholar]
- 15.Heiner DC, Lee SI, Short JA. IgG4 subclass deficiency syndromes. Monogr Allergy. 1986;20:149–156. [PubMed] [Google Scholar]
- 16.Moss RB, Carmack MA, Esrig S. Deficiency of IgG4 in children: association of isolated IgG4 deficiency with recurrent respiratory tract infection. J Pediatr. 1992;120:16–21. doi: 10.1016/s0022-3476(05)80590-6. [DOI] [PubMed] [Google Scholar]
- 17.Merrill WW, Naegel GP, Olchowski JJ, Reynolds HY. Immunoglobulin G subclass proteins in serum and lavage fluid of normal subjects. Quantitation and comparison with immunoglobulins A and E. Am Rev Respir Dis. 1985;131:584–587. doi: 10.1164/arrd.1985.131.4.584. [DOI] [PubMed] [Google Scholar]
- 18.Keller MA, Gendreau-Reid L, Heiner DC, Rodriguez A, Short JA. IgG4 in human colostrum and human milk: continued local production or selective transport from serum. Acta Paediatr Scand. 1988;77:24–29. doi: 10.1111/j.1651-2227.1988.tb10592.x. [DOI] [PubMed] [Google Scholar]
- 19.Beck CS, Heiner DC. Selective immunoglobulin G4 deficiency and recurrent infections of the respiratory tract. Am Rev Respir Dis. 1981;124:94–96. doi: 10.1164/arrd.1981.124.1.94. [DOI] [PubMed] [Google Scholar]
- 20.Smith TF, Morris EC, Bain RP. IgG subclasses in nonallergic children with chronic chest symptoms. J Pediatr. 1984;105:896–900. doi: 10.1016/s0022-3476(84)80073-6. [DOI] [PubMed] [Google Scholar]
- 21.Jefferis R, Kumararatne DS. Selective IgG subclass deficiency: quantification and clinical relevance. Clin Exp Immunol. 1990;81:357–367. doi: 10.1111/j.1365-2249.1990.tb05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MY, Kim CK, Hahm YB, Chung CY. Serum IgG subclasses in children with recurrent wheezy bronchitis. Pediatr Allergy Respir Dis. 1995;5:42–48. Korea. [Google Scholar]
- 23.Lin MS, Rabin BS, LaNeve R, Fireman P. Hyper-M-immunoglobulinemia in children with bronchial asthma. J Pediatr. 1977;91:222–227. doi: 10.1016/s0022-3476(77)80816-0. [DOI] [PubMed] [Google Scholar]
- 24.Berger W, Pollock J, Kiechel F. Immunoglobulin levels in children with chronic severe asthma. Ann Allergy. 1978;41:67–74. [PubMed] [Google Scholar]
- 25.Albin S, Cunningham-Rundles C. An update on the use of immunoglobulin for the treatment of immunodeficiency disorders. Immunotherapy. 2014;6:1113–1126. doi: 10.2217/imt.14.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wasserman RL, Lumry W, Harris J, 3rd, Levy R, Stein M, Forbes L. Efficacy, safety, and pharmacokinetics of a new 10% liquid intravenous immunoglobulin containing high titer neutralizing antibody to RSV and other respiratory viruses in subjects with Primary Immunodeficiency Disease. J Clin Immunol. 2016;36:590–599. doi: 10.1007/s10875-016-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spahn JD, Leung DY, Chan MT, Szefler SJ, Gelfand EW. Mechanisms of glucocorticoid reduction in asthmatic subjects treated with intravenous immunoglobulin. J Allergy Clin Immunol. 1999;103:421–426. doi: 10.1016/s0091-6749(99)70466-5. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Ye YM, Ban GY, Shin YS, Lee HY, Nam YH, Lee SK, Cho YS, Jang SH, Jung KS, Park HS. Effects of immunoglobulin replacement on asthma exacerbation in adult asthmatics with IgG subclass deficiency. Allergy Asthma Immunol Res. 2017;9:526–533. doi: 10.4168/aair.2017.9.6.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melamed IR, Borte M, Trawnicek L, Kobayashi AL, Kobayashi RH, Knutsen A, Gupta S, Smits W, Pituch-Noworolska A, Strach M, Pulka G, Ochs HD, Moy JN. Pharmacokinetics of a novel human intravenous immunoglobulin 10% in patients with primary immunodeficiency diseases: analysis of a phase III, multicenter, prospective, open-label study. Eur J Pharm Sci. 2018;118:80–86. doi: 10.1016/j.ejps.2018.03.007. [DOI] [PubMed] [Google Scholar]