Abstract
Good syndrome (GS) is a primary immunodeficiency (PID) that presents in middle aged to older adults with features of thymoma, hypogammaglobulinemia, CD4 T lymphopenia, inverted CD4/CD8+ ratio, and impaired T-cell mitogen proliferative responses. We present a patient, a 62-year-old female, who first presented with disease manifestation of acute hepatitis from hepatitis B virus (HBV) reactivation, which was subsequently complicated by recurrent hospitalizations for recurrent pneumonia and concomitant Helicobacter pylori and cytomegalovirus enteritis. She was later found to have thymoma and hypogammaglobulinemia and was diagnosed with GS. Although the well-known importance of T cell is in directing B-cell responses in the immunopathology of thymoma, low levels of natural killer and CD4+ γδ T cells may also be the cause of both low immune surveillance of tumor development and weak clearance of viral infection. Hence, the temporal sequence of opportunistic infections following HBV reactivation and thymoma discovery may reflect a loss of immune surveillance as the first manifestation of PID.
Keywords: Good syndrome, Thymoma and hypogammaglobulinemia, HBV reactivation, Acute hepatitis
INTRODUCTION
Since the first description of adult-onset hypogammaglobulinemia with the presence of thymoma in 1954 by Dr Robert Good [1], there have been numerous descriptions of Good syndrome (GS) but no formal diagnostic criteria. To date, knowledge of the pathogenesis of GS remains incomplete. Aside from hypogammaglobulinemia, there have been other immunological impairments described: low or absent B cells, variable defects in cell-mediated immunity with a CD4 T lymphopenia, inverted CD4/CD8+ T-cell ratio and reduced T-cell mitogen proliferative responses [1]. Physicians must be aware that GS is a combined immunodeficiency with both T- and B-cell defects and that pooled immunoglobulin (Ig) replacement therapy may not sufficiently prevent all infective complications. We describe a unique case of GS with an atypical presentation, the evaluation, and complex subsequent disease course.
CASE HISTORY
In July 2018, a 62-year-old Chinese woman retired seamstress presented to the general medicine department of an acute hospital. She complained of acute epigastric pain, nausea, and jaundice for 1 week. Her past medical history was significant for hepatitis B virus (HBV) carriage, chronic rhinitis, previous tonsillectomy, right hemithyroidectomy for benign goitre, and uterine fibroids status post hysterectomy. She had no family history of primary immunodeficiency (PID) or systemic autoimmunity and was uncertain about her vaccination status. She was the eldest of 8 siblings born to nonconsanguineous parents, she was married and nulliparous.
On admission, the clinical findings were those of a petite 39-kg female who was jaundiced; there was no hepatomegaly, no stigmata of chronic liver disease were noted. She was not in acute hepatic failure. She did not have oral thrush, nor signs of onychomycosis. Examination of the cardiorespiratory systems were unremarkable. Laboratory evaluation revealed severe transaminitis of aspartate aminotransferase 480 U/L (10–30 U/L), alanine transaminase 632 U/L (10–36 U/L), alkaline phosphatase 148 U/L (22–104 U/L), gamma-glutamyl transferase 177 U/L (7–32 U/L). HBV viral load was elevated at 101,000,000 IU/mL (8.00 Log IU/mL); with positive serologic markers of hepatitis B surface antigen, hepatitis B core antibody, and hepatitis B envelope antigen. Serology for human immunodeficiency virus (HIV) and hepatitis C virus infection were negative. An ultrasound of the abdomen showed concomitant acute acalculous cholecystitis without evidence of liver cirrhosis. Other significant negatives in the evaluation for hepatitis included negative antinuclear antibody; anti-M2; anti-smooth muscle; and anti-LKM. IgG was low at <3.00 g/L (7.00–16.00) and caerulopasmin levels were normal 22.6 mg/dL (15.0–45.0). In view of persistent dyspepsia, an oesophago-duodenoscope (OGD) was performed. This revealed oesophageal candidiasis without gastritis. Retrospective history taking was negative for previous blood transfusions or history of HBV infection in her mother, spouse or siblings. She was treated with a course of antibiotics for cholecystitis, started on entecavir for acute hepatitis B flare and oral nystatin. She made good improvement and was discharged. She was later put on long-term tenofovir for antiviral prophylaxis.
In early August 2018, the patient was readmitted for a 3-week history of cough with white sputum production and diarrhoea of 1 day. A chest radiograph (CXR) revealed a left lower zone consolidation with small pleural effusion and bronchiectatic changes over the right suprahilar region (Fig. 1). She was treated for community-acquired pneumonia with a course of clarithromycin and coamoxiclav. Antibiotic therapy was escalated empirically to meropenem when she continued to be febrile despite initial treatment. Blood and sputum as well as stool microbiology were unyielding. She showed response to empiric antimicrobial therapy and was discharged.
Fig. 1. Chest radiograph findings in August 2018 (left) showed left lower zone consolidation with small pleural effusion and bronchiectatic changes over the right suprahilar region. In September 2018 (right), there was persistence of air space changes in the left lower zone and cystic bronchiectatic changes in the right hilar and suprahilar region.
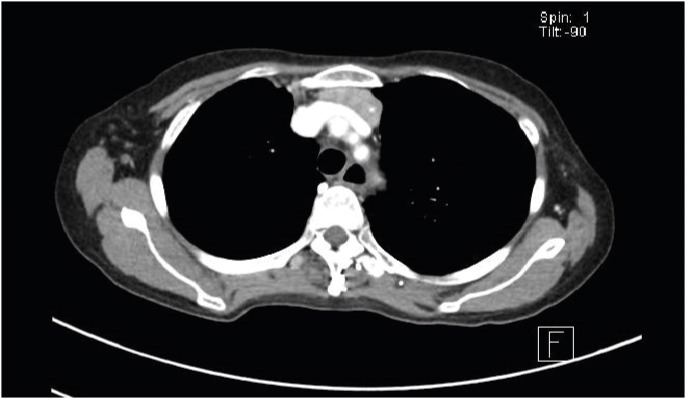
Two weeks later in the same month, the patient was readmitted for persistent cough and recurrence of diarrhoea, she also complained of dyspepsia unresolved since July 2018. A repeat CXR showed persistence of air space changes in the left lower zone and cystic bronchiectatic changes in the right hilar and supra-hilar region (Fig. 1). A high resolution computed tomographic (CT) scan of the thorax revealed bronchiectasis in the right upper lobe with associated volume loss and left lower lobe consolidation (Fig. 2). There was an incidental finding of a retrosternal mass noted in relation to the left lobe of the thyroid gland which was further evaluated with a contrast-enhanced CT scan of the thorax (Fig. 3). The patient had 2 days of piperacillin-tazobactam then 5 days of levofloxacin to complete a 1-week course of antibiotics. Persistent dyspepsia was presumed to be due to failure of nystatin and the patient was started on a 2-week course of oral fluconazole. Repeat stool Clostridioides difficile toxin was negative and further evaluation was not pursued. The cause of diarrhoea was attributed to prolonged antibiotic use. Further imaging on neck computed tomography (CT) scan (Fig. 4) showed that the retrosternal mass appeared to be in continuation with the lower pole of the left thyroid. Ultrasound-guided fine needle aspiration cytology was attempted but unsuccessful due to poor yield. The patient was clinically and biochemically euthyroid.
Fig. 2. High resolution computed tomographic scan findings of cystic and tubular bronchiectatic changes in right upper lobe with associated volume loss with patchy areas of consolidation seen in left lower lobe. There are also scattered areas of centrilobular nodules seen scattered in the right lung and left lower lobe with associated bronchial dilatation and bronchial wall thickening.
Fig. 3. Contrast-enhanced computed tomographic scan of the thorax showing a well-defined lobulated heterogeneously enhancing soft tissue mass noted in relation to the lower pole of left thyroid gland extending inferiorly into the superior mediastinum in the retrosternal region with foci of calcification within.
Fig. 4. Computed tomographic scan of the neck image describing a retrosternal mass that appeared to be in continuation with the lower pole of the left thyroid.
In October 2018, she was admitted once again for dyspepsia and recurrence of diarrhoea. The clinical assessment did not suggest sepsis or dysentery, stool studies were negative for bacterial or a parasitic aetiology. The patient was given omeprazole and symptomatic therapy.
One month later in November 2018, the patient once again presented to the Emergency Department with acute vomiting and exacerbation of chronic diarrhoea, she now reported inability to tolerate oral feeds. This was her 5th hospitalization of the year, and by now her body weight had decreased to 34.4 kg. This time, the patient underwent both OGD and colonoscopic evaluation. There was evidence of candidiasis in the upper oesophagus, erosive pan-gastritis, and punctate erythema in the cecum, the rest of the colon and rectum were unremarkable (Fig. 5). The rapid urease test was positive for Helicobacter pylori infection, viral cytopathic change, and positive cytomegalovirus (CMV) immunostains were seen in the gastric and caecal mucosa on histologic evaluation. Whilst there was evidence of chronic inflammation in the gastric biopsy, no evidence of colitis or granulomatous inflammation were detected on histology from the ileum, caecum, and random colonic biopsy samples. An ophthalmic screen for CMV retinitis was negative. The patient was initiated on eradication therapy for H. pylori infection with a course of triple therapy: amoxicillin, clarithromycin and omeprazole, oral fluconazole for oesophageal candidiasis and valganciclovir for CMV enteritis.
Fig. 5. Images of oesophago-duodenoscope and colonoscopy respectively showing evidence of candidiasis in the upper oesophagus, erosive pan-gastritis. Besides punctate erythema in the cecum, the rest of the colon and rectum were unremarkable.
Further history was sought and this revealed past pulmonary tuberculosis in the patient's 20s, for which she had completed treatment. She also had an episode of zoster although she was unable to recall an earlier episode of varicella infection. She had recurrent lip ulcers suggestive of herpes liabialis. There was no past history of chronic rhinosinusitis, or recurrent pneumonia prior to 2018. A diagnosis of GS was now suspected based on cumulative evidence of gastrointestinal candidiasis (1), CMV enteritis in the absence of HIV infection recurrent pneumonia with bronchiectasis (2), retrosternal mass with suspicions of a thymoma (3), and hypogammaglobulinemia (4).
IMMUNOLOGICAL EVALUATION
At onset of disease suspicion, repeat Ig levels were sought, this revealed pan-hypogammaglobinaemia with IgG < 3.0 g/L (normal range, 0.7–4.0 g/L), IgA < 0.5 g/L (7.0–16.0 g/L), and IgM < 0.25 g/L (0.4–2.3 g/L) (Table 1). There was no significant proteinuria (24-hour urinary protein 0.15 g/day), or signs of liver dysfunction. T lymphocyte subset evaluation via flow cytometry showed marked reduction in CD19 lymphocytes (absolute count of <5 cells/μL; normal range, 65–620 cells/μL) with normal levels of CD3, CD4, CD8 lymphocytes and natural killer (NK) cells. Vaccine stimulatory study of B-cell function with 23 polyvalent pneumococcal vaccines and tetanus toxoid was subsequently performed. Antibody levels were assessed at baseline and 4-week postvaccination. For pneumococcus, all but 2 of 23 serotypes had antibody levels below reference range prestimulation and all 23 serotypes had antibody levels below reference range post vaccination. Antibody levels to tetanus toxoid were undetectable both pre- and postvaccination. Isohaemagglutinins (anti-A, anti-B) were low at titres of 1:4 (normal range, ≥1:8). Lymphocyte proliferation assay showed mildly depressed lymphoproliferative response to phytohaemagglutinin. The overall evaluation was consistent with a combined immunodeficiency with a predominance of reduced B-cell numbers and defective B-cell response to vaccine stimulation.
Table 1. Summary of immunological workup comprising first set of immunoglobulins levels, T lymphocyte subset evaluation, lymphocyte proliferation assay, vaccine stimulation studies to pneumococcus, tetanus, and isohaemagglutinins.
| Variable | Results (unit) | Reference range | |
|---|---|---|---|
| Immunoglobulins levels | |||
| IgG (g/L) | <3.0 | 0.7–4.0 | |
| IgA (g/L) | <0.5 | 7.0–16.0 | |
| IgM (g/L) | <0.25 | 0.4–2.3 | |
| Tlymphocyte subset evaluation | |||
| CD3 abs (cells/μL) | 1,994 | 600–2,500 | |
| CD4 abs (cells/μL) | 877 | 280–1,430 | |
| CD8 abs (cells/μL) | 1,010 | 165–1,045 | |
| CD19 abs (cells/μL) | <5↓ | 65–620 | |
| NK cells abs (cells/μL) | 438 | 90–960 | |
| CD4/8 ratio | 0.87 | 0.50–2.50 | |
| Lymphocytes abs (cells/μL) | 2,441 | 1,000–3,500 | |
| Total WBC (cells/μL) | 7,900 | 4,000–10,000 | |
| Lymphocyte proliferation assay | |||
| Phytohaemagglutinin (μg/mL) | 0.5: 41,726 cpm | Control: 79,235 cpm | |
| 0.1: 12,425 cpm | Control: 43,503 cpm | ||
| Concanavalin A | 7.5: 103,482 cpm | Control: 75,338 cpm | |
| Vaccine stimulation studies to S. pneumoniae IgG Ab at baseline* | |||
| Serotype 1 (μg/mL) | <0.4 | ≥2.3 | |
| Serotype 2 (μg/mL) | <0.4 | ≥1.0 | |
| Serotype 3 (μg/mL) | <0.4 | ≥1.8 | |
| Serotype 4 (μg/mL) | <0.4 | ≥0.6 | |
| Serotype 5 (μg/mL) | <0.4 | ≥10.7 | |
| Serotype 8 (μg/mL) | <0.4 | ≥2.9 | |
| Serotype 9N (μg/mL) | <0.4 | ≥9.2 | |
| Serotype 12F (μg/mL) | <0.4 | ≥0.6 | |
| Serotype 14 (μg/mL) | <0.4 | ≥7.0 | |
| Serotype 17F (μg/mL) | <0.4 | ≥7.8 | |
| Serotype 19F (μg/mL) | <0.4 | ≥15.0 | |
| Serotype 20 (μg/mL) | <0.4 | ≥1.3 | |
| Serotype 22F (μg/mL) | 0.6 | ≥7.2 | |
| Serotype 23F (μg/mL) | 0.6 | ≥8.0 | |
| Serotype 6B (μg/mL) | <0.4 | ≥4.7 | |
| Serotype 10A (μg/mL) | <0.4 | ≥2.9 | |
| Serotype 11A (μg/mL) | <0.4 | ≥2.4 | |
| Serotype 7F (μg/mL) | <0.4 | ≥3.2 | |
| Serotype 15B (μg/mL) | <0.4 | ≥3.3 | |
| Serotype 18C (μg/mL) | <0.4 | ≥3.3 | |
| Serotype 19A (μg/mL) | <0.4 | ≥17.1 | |
| Serotype 9V (μg/mL) | <0.4 | ≥2.6 | |
| Serotype 33F (μg/mL) | <0.4 | ≥1.7 | |
| Vaccine stimulation studies to tetanus toxoid IgG Ab† | |||
| Tetanus toxoid IgG Ab | 0.00 IU/mL | ≥0.01 | |
| Isohaemagglutinin levels (blood group: O+) | |||
| Anti-A isoantibody | 1:4 | ≥1:8 | |
| Anti-B isoantibody | 1:4 | ≥1:8 | |
NK, natural killer; WBC, white blood cell; Ab, antibody.
*Repeat values post vaccination 1 month later remained the same except that of serotype 23F which showed a modest increase to 0.8 μg/mL. †Repeat values post vaccination 1 month later remained below detection limit.
DEFINITVE MANAGEMENT
In late November 2018, she underwent surgical and excision of the anterior mediastinal tumor. Intraoperatively, the remnant left thyroid and anterior mediastinal tumor were resected, there was no evidence of local tumor invasion. The tumor on histological analysis was revealed to be type A thymoma with focally present transcapsular invasion and tumor extension into mediastinal fat (Masaoka stage IIA), remnant thyroid on histology was consistent with a multinodular goitre. The patient was started on lifelong thyroxine replacement. In January 2019, she was commenced on intravenous pooled immunoglobulin (IVIg) infusion, replacement doses of 0.4-g/kg body weight were administered once monthly. Additionally, the patient underwent a course of radiation therapy from February to March 2019.
CLINICAL PROGRESSION
From April to May 2019, she suffered complications of infective exacerbation of bronchiectasis, oral thrush, and oesophageal stricture as a result of CMV oesophagitis. She had a further 5-kg weight loss as a result of dysphagia and poor oral intake. Aspiration was suspected as the trigger of chest infection despite appropriate IVIg therapy. CMV infection was treated with IV ganciclovir and the patient underwent a series of endoscopic oesophageal dilatation. From June 2019 till date, the patient has not developed any further episodes of infection, she now tolerates a soft diet and her body weight has stabilised at 30 kg (body mass index, 13.9 kg/m2). She continues with tenofovir for HBV prophylaxis and IVIG replacement therapy achieving a trough IgG level of 5.53 to 6.23 g/L.
DISCUSSION
Definition
Although GS is classified as a distinct entity by the expert committee of the World Health Organisation/International Union of Immunological Societies on PID [2], no formal diagnostic criteria have been proposed to date. GS is a PID that presents in the 5th–6th decade of life [1,3,4,5] with features of thymoma and hypogammaglobulinemia [6]. The focus on low immunoglobulins can be misleading, for aside from defects in humoral immunity, cell-mediated immunity can also be afflicted, with reports of CD4 T lymphopenia; inverted CD4/CD8+ T-cell ratio; and reduced T-cell mitogen proliferative responses [1,7]. GS seems to be better described as a broader classification of thymoma associated with immunodeficiency, as described in the European Society for Immunodeficiencies (ESID) registry [6]. The ESID registry also defines clinical criteria of GS as the presence of thymoma associated with reduced serum IgG (<2 standard deviation below the mean reference for age) [6].
Pathogenesis
The association of thymoma and hypogammaglobulinemia leading to infective complications is not well understood. Initially thought to be secondary to hypogammaglobulinemia, thymic pathology was later thought to have consequences on B-cell activation through loss of self-tolerance [8]. The thymus has been known to play a critical role in T-cell education, influencing the balance of immune reactivity essential to host-defense and self-tolerance [9]. Thymic pathology thus explains the observation of coincident autoimmunity and immunodeficiency, reflecting T cells that are both overreactive to self and underresponsive to pathogens [9]. In GS, low levels of NK, and CD4+ γδ T cells may be the cause of both low immune surveillance of tumor development and weak clearance of viral infection [10]. The importance of T cell directing B-cell responses is also apparent in the immunopathology of thymoma, leading to hypogammaglobulinemia as a cardinal manifestation of GS [8,11].
However, in a study by Przemyslaw and colleagues, 2 observations suggest that thymic pathology is a consequence of central tolerance defects and marrow autoimmune processes rather than the etiology [10,11]. One, that follow-up of a patient postsurgical excision of a thymoma resulted in neither IgG nor B/NK cell count increase but induces permanent decrease of all subpopulation of T cells during 4-year observation period. Further, significantly lower IgG level, percentage of peripheral blood B lymphocytes, CD4/CD8 ratio, and expression of CD28 in thymectomized patients have also been described [12]. Secondly, that B cells in patients with severe form of GS were defective and failed to produce normal amount of Ig even when the influence of T cells was inhibited by immunosuppressive therapy (for example, cyclosporine) [10]. These suggest that both T- and B-cell defects are not a result of thymic pathology but are a consequence of abnormal differentiation of lymphoid precursor cell subset in the bone marrow [8].
In recent years, there has also been growing interest in the genetic polymoprhism of cell signaling pathways linked to GS. One such mechanism highlighted is that of weak interaction between CXCL12 (formerly called pre-B-cell growth-stimulating factor), and its ligand CXCR4 on B-cell precursors resulting in low B-cell expression and maturation, and possibly NK cells differentiation arrest [10]. Another pathway to mention is that of the B-cell activating factor (BAFF): BAFF receptor signaling [13,14]. It has been demonstrated in mice that high levels of BAFF can support B-cell survival [9,13] and increase numbers of mature B cells but this also leads to progressive autoimmune disease similar to SLE [15]. A case report by Lougaris et al. [16] describes the first patient affected with GS to carry 2 missense mutations in BAFF-R, P21R/H159Y, associated with low BAFF-R expression and low B-cell counts.
Indeed, delineating well-defined clinical and immune parameters with a better understanding of the genetic basis of immune mechanisms will aid in further differentiation of GS from other PIDs and allow better prognostication of disease. One such observation thus far has been the lower expression of NK cells in GS and the association with a poorer prognosis than X-linked agammaglobinaemia (XLA) and common variable immunodeficiency (CVID) [10,11].
Clinical presentation
GS is distinctive in that onset of PID occurs in the 5th–6th decade of life [3,4,5]. This is in stark contrast to severe combined immunodeficiency (SCID) and XLA [6] which present in the 1st decade of life and CVID which presents from 20–40 years of age [5,17]. Unlike most other PIDs which present at a young age or in childhood, our patient did not have a clinical profile suspicious for PID before age 62 years old.
GS renders patients susceptible to a spectrum of characteristic bacterial, viral, fungal, and opportunistic infections [18] reflecting dysfunction in humoral and cellular arms of immune defense [19]. GS patients are commonly afflicted with severe infections by encapsulated bacterial such as Streptococcus pneumoniae and Haemophilus influenzae [3,19,20], causing recurrent and severe ear, sinus, and pulmonary infections [1,3,4,20]. This has also been observed in other disorders with predominantly antibody dysfunction [6]. In contrast, conditions with defective cell-mediated immunity such as SCID [20] give rise to opportunistic viral and fungal infections. A similar observation has also been described in thymoma patients [7,21,22,23]. Of the viral pathogens that have been reported in GS patients, CMV appears to be the most common pathogen [1,3,24], others include varicella zoster virus, herpes simplex virus, HHV8, human papilloma virus, Echovirus 22, and JC virus [1,7]. Kelleher and Misbah [3] described CMV colitis and retinitis and mucocutaneous Candida infection as prominent features of GS. Manifestations of CMV disease reported are retinitis, pneumonitis, encephalitis, colitis, enteritis, adrenalitis, and gastritis [1]. In terms of fungal infections, common pathogens are those of Candida, pneumocystis jiroveci, and Aspergillus [1,11].
In a systemic review of GS, Kelesidis and Yang [1] also described the temporal relationship of thymoma diagnosis with onset of hypogammaglobulinemia or infection. In 42.4% of patients, diagnosis of thymoma precedes hypogammaglobulinemia or infections, whilst 20% were diagnosed subsequently, and in 37.9% diagnosis was made simultaneously. In the same systemic review, spindle cell thymomas made up half of all thymomas compared to other histologic types.
Autoimmune manifestations commonly described in association with thymoma include pure red cell aplasia (PRCA) (34.8%), myasthenia gravis (15.7%), and oral lichen planus (12.4%) [1,7]. One-third of patients had PRCA in a study by Malphettes et al. [5] Other manifestations also described are Sjogren's syndrome and systemic lupus erythematous [4,5].
The patient had an atypical presentation with HBV reactivation [25] as the initial event leading to eventual PID diagnosis. She had a serious infection (acute liver failure) with a common pathogen (HBV) preceding a chain of recurrent fungal and opportunistic infections (CMV, candidiasis). The patient did not have other risk factors for HBV reactivation such as the presence of cirrhosis or ongoing steroid and other immunosuppressive therapy. Although speculative, the temporal sequence of opportunistic infections following HBV reactivation and thymoma discovery may reflect a loss of immune surveillance as the first manifestation of PID [10]. A novel feature in our patient was the concomitant H. pylori infection in addition to CMV gastritis. Lower serum bactericidal activity to H. pylori have been observed in primary antibody deficiency disorders like XLA and CVID, leading some to postulate that hypogammaglobulinemic states, causes immune dysregulation and predisposes to chronic infections like H. pylori. However, to our best knowledge, H. pylori is not commonly reported as an associated infection in GS patients [26].
Principles of management
The management of GS is challenging, as it requires urgent surgical management in the setting of a malignant thymoma, with the need for appropriate and often prolonged antimicrobial therapy in a chronically ill patient. In the long term, there is a need for pooled Ig replacement and in select cases, antimicrobial prophylaxis to address defects in T-cell immunity.
A thymectomy is often required and its completeness is the most important indicator of long-term prognosis [27,28]. Surgical removal or debulking can prevent locally invasive growth and metastasis with advanced staged tumors requiring radiotherapy and combination chemotherapy [1]. However, it is also worth noting that surgical removal of the thymoma does not reverse immunological abnormalities [11,29,30].
Ig replacement has been shown to improve infection control, reduce hospitalization, and decrease use of antibiotics [1,11]. Kelesidis and Yang [1] found that approximately 38% of patients with GS had reduced incidence of infections after treatment with pooled gamma globulin also known as IVIg. The likelihood of IVIg replacement is inversely related to the baseline IgG levels (particularly with levels below 4 g/L) [31]. However, IVIg therapy has been associated with risks of anaphylaxis, acute renal failure, thromboembolism, and exposure to blood borne pathogens [32]. In a review article, Jolles proposed 3 key factors in determining the need for IVIg. These include impairment of vaccine responses (1), degree of infection burden (2), and risk of potential end organ damage (3) [31].
There are no fixed guidelines on the dosing regime of IVIg but most consensus guidelines recommend starting in doses between 400 and 600 mg/kg every 3–4 weeks intravenously (or the equivalent given in divided doses once or twice a week subcutaneously) to achieve trough IgG serum levels around 6–8 g/L [27]. Registry data from the European PID [33] also demonstrated increased episodes of serious infections and prolonged hospitalization in PID patients with median IgG levels of <4 g/L. Ultimately, IVIg therapy should be titrated based on effective reduction in frequency of infections requiring antibiotics and hospitalization [28]. In Singapore, long-term IVIg can be costly and patients often require assistance with public funding.
Serious infections despite adequate IVIg replacement, the extent of end organ damage from recurrent infection, are potential indications for antimicrobial prophylaxis in GS [34]. There is wide variation in prescribing practice for the use of prophylactic antibiotics across PIDs [34]. One observed trend has been the use of macrolide antibiotic as prophylaxis in the management of bronchiectasis in cystic fibrosis patients. It was found that long-term macrolide use resulted in reduced mortality, improved lung function and fewer exacerbations in randomized trials studying erythromycin, clarithromycin, and azithromycin [34]. In chronic HBV carriage, antiviral prophylaxis is recommended for patients undergoing immunosuppressive therapy in the form of cytotoxic chemotherapy, antitumor necrosis factor therapy, antirejection therapy for solid organ transplants, anti-CD20 therapy, or glucocorticoids ≥20-mg day for a minimum of 4 weeks [25,35]. However, there is no guidance as to the duration of anti-HBV prophylaxis in the patient with PID. In our patient who has preserved liver function without cirrhosis, lifelong antiviral prophylaxis is a strong consideration.
In summary, we describe an atypical presentation of GS, with first disease manifestation of acute hepatitis from HBV reactivation, complicated by thymoma, recurrent pneumonia, concomitant H. pylori, and CMV enteritis. The diagnosis of GS requires a high index of suspicion, with profound hypogammaglobinemia being an important clue. Further, the temporal sequence of opportunistic infections following HBV reactivation and thymoma discovery may reflect a loss of immune surveillance as the first manifestation of PID. The management of GS is multidisciplinary in nature and there is a need for lifelong IVIg replacement.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Tan Teck Choon.
- Data curation: Tan Teck Choon.
- Formal analysis: Tan Teck Choon, Lai Yi Wye.
- Methodology: Tan Teck Choon, Lai Yi Wye.
- Project administration: Lai Yi Wye.
- Visualization: Lai Yi Wye.
- Writing - original draft: Lai Yi Wye.
- Writing - review & editing: Tan Teck Choon.
References
- 1.Kelesidis T, Yang O. Good's syndrome remains a mystery after 55 years: a systematic review of the scientific evidence. Clin Immunol. 2010;135:347–363. doi: 10.1016/j.clim.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Picard C, Bobby Gaspar H, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Crow YJ, Cunningham-Rundles C, Etzioni A, Franco JL, Holland SM, Klein C, Morio T, Ochs HD, Oksenhendler E, Puck J, Tang MLK, Tangye SG, Torgerson TR, Sullivan KE. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J Clin Immunol. 2018;38:96–128. doi: 10.1007/s10875-017-0464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelleher P, Misbah SA. What is Good's syndrome? Immunological abnormalities in patients with thymoma. J Clin Pathol. 2003;56:12–16. doi: 10.1136/jcp.56.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaman M, Huissoon A, Buckland M, Patel S, Alachkar H, Edgar JD, Thomas M, Arumugakani G, Baxendale H, Burns S, Williams AP, Jolles S, Herriot R, Sargur RB, Arkwright PD. Clinical and laboratory features of seventy-eight UK patients with Good's syndrome (thymoma and hypogammaglobulinaemia) Clin Exp Immunol. 2019;195:132–138. doi: 10.1111/cei.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malphettes M, Gérard L, Galicier L, Boutboul D, Asli B, Szalat R, Perlat A, Masseau A, Schleinitz N, Le Guenno G, Viallard JF, Bonnotte B, Thiercelin-Legrand MF, Sanhes L, Borie R, Georgin-Lavialle S, Fieschi C, Oksenhendler E DEFicit Immunitaire de l'adulte Study Group. Good syndrome: an adult-onset immunodeficiency remarkable for its high incidence of invasive infections and autoimmune complications. Clin Infect Dis. 2015;61:e13–9. doi: 10.1093/cid/civ269. [DOI] [PubMed] [Google Scholar]
- 6.Tarr PE, Sneller MC, Mechanic LJ, Economides A, Eger CM, Strober W, Cunningham-Rundles C, Lucey DR. Infections in patients with immunodeficiency with thymoma (Good syndrome). Report of 5 cases and review of the literature. Medicine (Baltimore) 2001;80:123–133. doi: 10.1097/00005792-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Dong JP, Gao W, Teng GG, Tian Y, Wang HH. Characteristics of Good's syndrome in China. Chin Med J (Engl) 2017;130:1604–1609. doi: 10.4103/0366-6999.208234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shelly S, Agmon-Levin N, Altman A, Shoenfeld Y. Thymoma and autoimmunity. Cell Mol Immunol. 2011;8:199–202. doi: 10.1038/cmi.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez B, Browne SK. Good syndrome, bad problem. Front Oncol. 2014;4:307. doi: 10.3389/fonc.2014.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zdziarski P, Dworacki G, Korzeniowska-Kowal A, Ziemnicka K. Role of chemokine signalling in the pathogenesis of Good's syndrome-case reports, clinical characterization from single-centre perspective. Immunome Res. 2016;12:10000119 [Google Scholar]
- 11.Joven MH, Palalay MP, Sonido CY. Case report and literature review on Good's syndrome, a form of acquired immunodeficiency associated with thymomas. Hawaii J Med Public Health. 2013;72:56–62. [PMC free article] [PubMed] [Google Scholar]
- 12.Krawczyk P, Adamczyk-Korbel M, Kieszko R, Korobowicz E, Milanowski J. Immunological system status and the appearance of respiratory system disturbances in thymectomized patients. Arch Immunol Ther Exp (Warsz) 2007;55:49–56. doi: 10.1007/s00005-007-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pieper K, Grimbacher B, Eibel H. B-cell biology and development. J Allergy Clin Immunol. 2013;131:959–971. doi: 10.1016/j.jaci.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 14.Khan WN. B cell receptor and BAFF receptor signaling regulation of B cell homeostasis. J Immunol. 2009;183:3561–3567. doi: 10.4049/jimmunol.0800933. [DOI] [PubMed] [Google Scholar]
- 15.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lougaris V, Vitali M, Baronio M, Tampella G, Plebani A. BAFF-R mutations in Good's syndrome. Clin Immunol. 2014;153:91–93. doi: 10.1016/j.clim.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Tam JS, Routes JM. Common variable immunodeficiency. Am J Rhinol Allergy. 2013;27:260–265. doi: 10.2500/ajra.2013.27.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jansen A, van Deuren M, Miller J, Litzman J, de Gracia J, Sáenz-Cuesta M, Szaflarska A, Martelius T, Takiguchi Y, Patel S, Misbah S, Simon A Good syndrome Study Group. Prognosis of Good syndrome: mortality and morbidity of thymoma associated immunodeficiency in perspective. Clin Immunol. 2016;171:12–17. doi: 10.1016/j.clim.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 19.Madkaikar M, Mishra A, Ghosh K. Diagnostic approach to primary immunodeficiency disorders. Indian Pediatr. 2013;50:579–586. doi: 10.1007/s13312-013-0171-4. [DOI] [PubMed] [Google Scholar]
- 20.Reust CE. Evaluation of primary immunodeficiency disease in children. Am Fam Physician. 2013;87:773–778. [PubMed] [Google Scholar]
- 21.Kirkpatrick CH. Mucocutaneous candidiasis and thymoma. Clin Microbiol Newsl. 1980;2:5. [Google Scholar]
- 22.Moysset I, Lloreta J, Miguel A, Vadell C, Ribalta T, Estrach T, Serrano S. Thymoma associated with CD4+ lymphopenia, cytomegalovirus infection, and Kaposi's sarcoma. Hum Pathol. 1997;28:1211–1213. doi: 10.1016/s0046-8177(97)90261-6. [DOI] [PubMed] [Google Scholar]
- 23.Sicherer SH, Cabana MD, Perlman EJ, Lederman HM, Matsakis RR, Winkelstein JA. Thymoma and cellular immune deficiency in an adolescent. Pediatr Allergy Immunol. 1998;9:49–52. doi: 10.1111/j.1399-3038.1998.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 24.Sun X, Shi J, Wang M, Xu K, Xiao Y. Good's syndrome patients hospitalized for infections. A single-center retrospective study. Medicine. 2015;94:e2090. doi: 10.1097/MD.0000000000002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pol S. Management of HBV in immunocompromised patients. Liver Int. 2013;33(Suppl 1):182–187. doi: 10.1111/liv.12055. [DOI] [PubMed] [Google Scholar]
- 26.Desar IM, van Deuren M, Sprong T, Jansen JB, Namavar F, Vandenbroucke-Grauls CM, van der Meer JW. Serum bactericidal activity against Helicobacter pylori in patients with hypogammaglobulinaemia. Clin Exp Immunol. 2009;156:434–439. doi: 10.1111/j.1365-2249.2009.03909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson SB, Eng TY, Giaccone G, Thomas CR., Jr Thymoma: update for the new millennium. Oncologist. 2001;6:239–246. doi: 10.1634/theoncologist.6-3-239. [DOI] [PubMed] [Google Scholar]
- 28.Cooper JD. Current therapy for thymoma. Chest. 1993;103(4 Suppl):334S–336S. doi: 10.1378/chest.103.4_supplement.334s. [DOI] [PubMed] [Google Scholar]
- 29.Souadjian JV, Enriquez P, Silverstein MN, Pépin JM. The spectrum of diseases associated with thymoma. Coincidence or syndrome? Arch Intern Med. 1974;134:374–379. [PubMed] [Google Scholar]
- 30.Qu J, Lü X, Gao Q, Zhang Y. Good Syndrome, a rare cause of refractory chronic diarrhea and recurrent pneumonia in a Chinese patient after thymectomy. Clin Vaccine Immunol. 2013;20:1097–1098. doi: 10.1128/CVI.00141-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirk BW, Freedman SO. Hypogammaglobulinemia, thymoma and ulcerative colitis. Can Med Assoc J. 1967;96:1272–1277. [PMC free article] [PubMed] [Google Scholar]
- 32.Katz U, Achiron A, Sherer Y, Shoenfeld Y. Safety of intravenous immunoglobulin (IVIG) therapy. Autoimmun Rev. 2007;6:257–259. doi: 10.1016/j.autrev.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Gathmann B, Mahlaoui N, Gérard L, Oksenhendler E, Warnatz K, Schulze I, Kindle G, Kuijpers TW, van Beem RT, Guzman D, Workman S, Soler-Palacín P, De Gracia J, Witte T, Schmidt RE, Litzman J, Hlavackova E, Thon V, Borte M, Borte S, Kumararatne D, Feighery C, Longhurst H, Helbert M, Szaflarska A, Sediva A, Belohradsky BH, Jones A, Baumann U, Meyts I, Kutukculer N, Wågström P, Galal NM, Roesler J, Farmaki E, Zinovieva N, Ciznar P, Papadopoulou-Alataki E, Bienemann K, Velbri S, Panahloo Z, Grimbacher B CEREDIH; Dutch WID; European Society for Immunodeficiencies Registry Working Party. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;134:116–126. doi: 10.1016/j.jaci.2013.12.1077. [DOI] [PubMed] [Google Scholar]
- 34.Kuruvilla M, de la Morena MT. Antibiotic prophylaxis in primary immune deficiency disorders. J Allergy Clin Immunol Pract. 2013;1:573–582. doi: 10.1016/j.jaip.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Di Bisceglie AM, Lok AS, Martin P, Terrault N, Perrillo RP, Hoofnagle JH. Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: just the tip of the iceberg? Hepatology. 2015;61:703–711. doi: 10.1002/hep.27609. [DOI] [PMC free article] [PubMed] [Google Scholar]