Abstract
To date, studies of patients with islet transplantation addressing intermittently scanned continuous glucose monitoring profile and the flexibility of the graft islet function under different doses of insulin administration, both of which reflect the real daily life of patients, are quite limited. Here, we report a case of a 46‐year‐old woman who received islet transplantation after kidney transplantation. The patient was followed up over a period of 2 years after initial islet transplantation. Our results show that intermittently scanned continuous glucose monitoring can be useful for monitoring the reduction of glycemic variability, and suggest the appropriate regulation of insulin secretion from graft islets during mixed‐meal test by using different doses of exogenous insulin administration. Additionally, during the 2‐year observational period, glucagon elevation was detected only at hypoglycemia, whereas the level was within the normal range at normoglycemia or hyperglycemia.
Keywords: Islet transplantation, Intermittently scanned continuous glucose monitoring, Mixed‐meal test
Our study shows that intermittently scanned continuous glucose monitoring can be useful for monitoring the reduction of glycemic variability after islet transplantation, and suggests the appropriate regulation of insulin secretion from graft islets during mixed‐meal test by using different doses of exogenous insulin administration. During the 2‐year observation period, glucagon elevation was detected only at hypoglycemia, whereas the level was within the normal range at normoglycemia or hyperglycemia.

Introduction
There have been several reports regarding the measurement of glycemic variability with a subcutaneous glucose monitoring system after islet transplantation (ITx) 1 , 2 , 3 , although the monitoring period of these reports was limited to no longer than 1 week using continuous glucose monitoring (CGM). Recently, intermittently scanned continuous glucose monitoring (isCGM), also known as flash glucose monitoring (FGM), has enabled monitoring of glycemic variability up to 3 months, which can better reflect the daily life of a patient.
We present the case of a 46‐year‐old woman who received islet transplantation after kidney transplantation, and was followed with CGM for 2 years after the first ITx and 28‐day isCGM at 2 years. Mixed‐meal test, which is known to be useful for the assessment of β‐cell function in type 1 diabetes 4 and ITx patient 5 , was also carried out using different doses of exogenous insulin to ascertain the flexibility of the graft insulin secretion.
Case Report
The patient was diagnosed with type 1 diabetes at the age of 12 years. She had been treated with multiple daily injections and was treated with continuous subcutaneous insulin infusion from the age of 29 years. Although her glycated hemoglobin (HbA1c) level was approximately 7–9%, she suffered from severe hypoglycemia. After gradual deterioration of renal function, she started peritoneal dialysis at the age of 36 years, and then received a living‐donor kidney transplantation from her mother at the age of 37 years, followed by a maintenance regimen of cyclosporin, mycophenolate mofetil and methylprednisolone. The patient became steroid‐free 3 years later. Her serum creatinine level was ameliorated, and reached a normal level after the kidney transplantation.
The first ITx was carried out at the age of 43 years with 356,200 islet equivalents (IEQ; 6,772 IEQ/kg), and the second ITx with 548,992 IEQ (10,807 IEQ/kg) was carried out 13 months after the first ITx. Donor islets of both ITx were from donation after brain death. The induction regimen was anti‐thymocyte globulin and etanercept for the first ITx, and basiliximab and etanercept for the second ITx. The maintenance regimen was cyclosporin with a trough level of 150–200 ng/mL and 500–1,500 mg/day mycophenolate mofetil. Fluconazole and acyclovir were used for 3 months, and trimethoprim‐sulfamethoxazole was used for 6 months as prophylactic antibiotics.
As to the graft function, the Secretory Units of Islets in Transplantation index, which is an index to assess β‐cell function calculated by (fasting serum C‐peptide [ng/mL] × 1,500) / (fasting plasma glucose [mg/dL] − 61.7) 6 , was maintained for 2 years after the first transplantation (Figure S1). At the time that the glucagon level was measured during the observational period, glucagon elevation was captured only at hypoglycemia. In contrast, it was not seen at normoglycemia or hyperglycemia (Figure 1). Improvement of glycemic control (Figure 2) and reduction of the insulin dose (Figure S2) after ITx were observed concomitantly.
Figure 1.
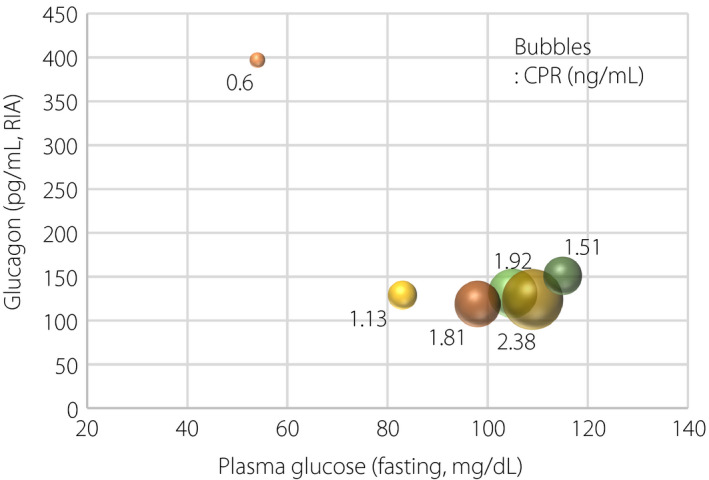
Relationships between fasting plasma glucose, glucagon and C‐peptide reactivity (CPR). Size of bubbles reflects CPR level as noted beside each bubble. RIA, radioimmunoactivity.
Figure 2.
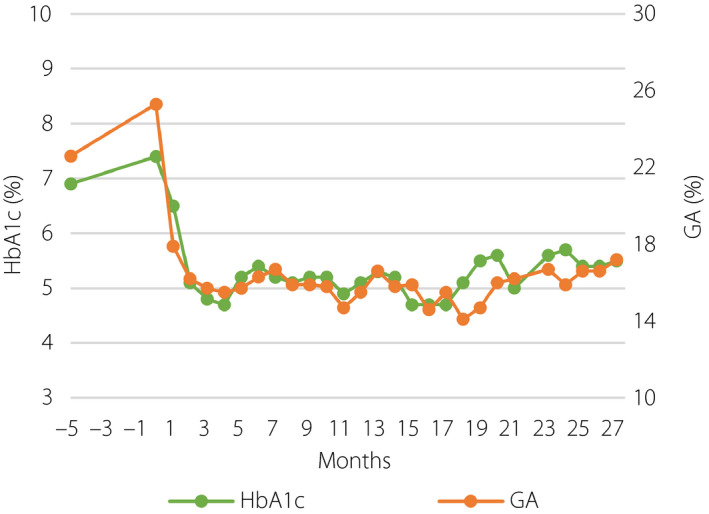
Glycemic control before and after islet transplantation. GA, glycoalbumin; HbA1c, glycated hemoglobin.
We followed glucose variability before and after ITx. We used iPro2™ (Medtronic, Northridge, CA, USA) for CGM, and Freestyle Libre™ (Abbott Diabetes Care, Alameda, CA, USA) for isCGM. CGM data were collected for 5–7 days in hospital, and isCGM data were collected for 28 days of daily life. CGM data showed an improvement of glycemic control and glycemic fluctuation: 180 ± 43 mg/dL at 6 months before the first ITx, 105 ± 22 mg/dL at 1 week after the first ITx, 98 ± 18 mg/dL at 6 months, 101 ± 17 mg/dL at 14 months (2 weeks after the second ITx), 121 ± 19 mg/dL without insulin at 17 months (4 months after the second ITx) and 132 ± 25 mg/dL at 25 months (12 months after the second ITx; Figure S3). At 2 years after the first ITx, we evaluated glucose variability with isCGM, which showed an almost flat glucose pattern that maintained an average glucose level of 114 mg/dL with only a few hypoglycemic events. Estimated HbA1c was 5.6%. Under the target glucose range of 100–140 mg/dL, time above range was 16%, time in range was 51% and time below range was 33% (Figure 3).
Figure 3.
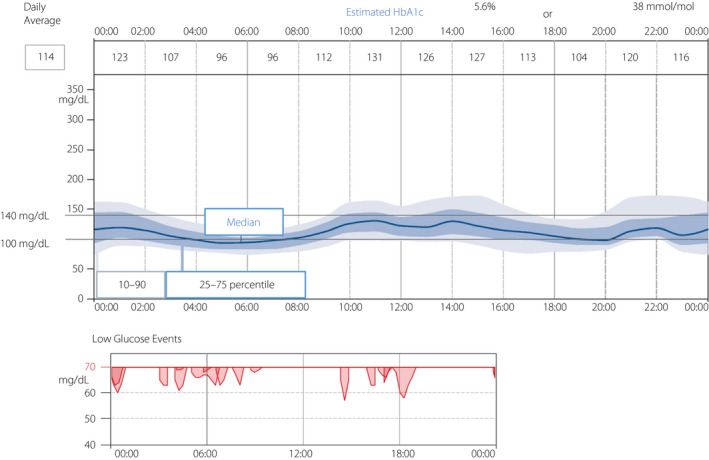
Intermittently scanned continuous glucose monitoring (isCGM) profile including low glucose events at 2 years after the first islet transplantation. HbA1c, glycated hemoglobin.
To ascertain that the adequate insulin secretion from the graft improved glycemic variability, we carried out the mixed‐meal tolerance test twice using different doses of insulin on different days 14 months after the first ITx. The test followed CGM carried out several days before the test in the hospital. The average glucose level was 101 ± 17 mg/dL, and estimated HbA1c was 5.1%. The test meal has 575 kcal: 52.0% carbohydrate, 32.2% fat and 15.8% protein. A low dose (carbohydrate‐to‐insulin ratio 30) and high dose (carbohydrate‐to‐insulin ratio 15) of insulin lispro were injected subcutaneously just before the mixed‐meal test. Interestingly, the former showed that insulin secretion from graft islets appeared to be accelerated in response to the glucose elevation after taking the test meal, and thus avoiding further glucose elevation. On the contrary, the latter showed that insulin secretion of the graft islets appeared appropriately suppressed (Figure 4).
Figure 4.
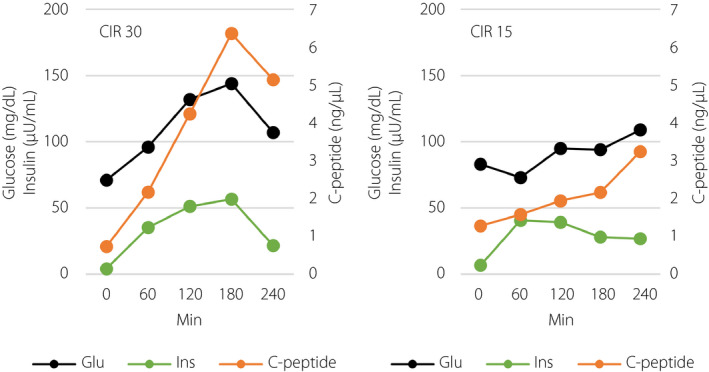
Glucose, insulin and C‐peptide level in the mixed‐meal test with different doses of insulin. CIR, carbohydrate‐to‐insulin ratio; Glu, glucose; Ins, insulin.
Islet transplantations in this study were performed following the clinical trial “Islet transplantation using brain‐dead donors and donors after cardiac death for patients with insulin‐dependent diabetes mellitus suffering from complicating hypoglycemia unawareness” (UMIN000003977). The protocol for this research project was approved by the institutional review boards, the ethics committee of Kyoto University Graduate School and Faculty of Medicine, and Kyoto University Specially Certified Committee for Regenerative Medicine. CGM/isCGM, mixed‐meal test and glucagon measurement are not included in the protocol of the clinical trial. Written informed consent for this publication was obtained from the participant of this case report independently of that for the clinical trial.
Discussion
The present case suggests that the appropriate “flexibility” of insulin secretion from graft islets can contribute to the reduction of glycemic variability, which was shown in both CGM and isCGM profiles, thereby accomplishing better HbA1c level with less hypoglycemia even with exogenous insulin administration. It indicates that isCGM is thought to be applicable to the evaluation of glycemic variability in the real daily life of ITx patients. Reactive elevation of glucagon to hypoglycemia might also have contributed to the reduction of glycemic variety. We also find it invaluable to capture the suppression of endogenous insulin secretion when enough exogenous insulin is administered during a mixed‐meal test. As for the long‐term outcome, glycemic control and endogenous insulin secretion of this case were well maintained at least during the 2‐year observation period compared with results of ITx patients in our previous cohort study 7 . Improvement of the immunosuppressive regimen using anti‐thymocyte globulin might have lead to the successful outcome of the present case because the immunosuppressive regimen used in the previous cohort study was based on the modified Edmonton Protocol 8 not using anti‐thymocyte globulin. Further cases are required to consolidate the conclusion regarding long‐term outcome.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 | Secretory Units of Islets in Transplantation (SUIT) index after islet transplantation.
Figure S2 | Insulin doses before and after islet transplantation. GA, glycoalbumin; TDD, total daily dose of insulin.
Figure S3 | Average glucose level and standard deviation of continuous glucose monitoring (CGM) profile before and after islet transplantation. Av, average glucose; SD, standard deviation.
Acknowledgments
The islet transplantation was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP16lk0201042 as a project for the promotion of clinical research “pancreatic islet transplantation for severe type 1 diabetic patients both from deceased (donation after brain death and DCD) donors.” The authors thank all clinical staff who participated in the treatment of the patient.
J Diabetes Investig. 2020
References
- 1. Bertuzzi F, De Carlis L, Marazzi M, et al Long‐term effect of islet transplantation on glycemic variability. Cell Transplant 2018; 27: 840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holmes‐Walker DJ, Gunton JE, Hawthorne W, et al Islet transplantation provides superior glycemic control with less hypoglycemia compared with continuous subcutaneous insulin infusion or multiple daily insulin injections. Transplantation 2017; 101: 1268–1275. [DOI] [PubMed] [Google Scholar]
- 3. Kessler L, Passemard R, Oberholzer J, et al Reduction of blood glucose variability in pancreatic islet transplantation type 1 diabetic patients treated by pancreatic islet transplantation. Diabetes Care 2002; 25: 2256–2262. [DOI] [PubMed] [Google Scholar]
- 4. Greenbaum CJ, Mandrup‐Poulsen T, McGee PF, et al Mixed‐meal tolerance test versus glucagon stimulation test for the assessment of β‐cell function in therapeutic trials in type 1 diabetes. Diabetes Care 2008; 31: 1966–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tekin Z, Garfinkel MR, Chon WJ, et al Outcomes of pancreatic islet allotransplantation using the edmonton protocol at the University of Chicago. Transplant Direct 2016; 2: e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamada Y, Fukuda K, Fujimoto S, et al SUIT, secretory units of islets in transplantation: an index for therapeutic management of islet transplanted patients and its application to type 2 diabetes. Diabetes Res Clin Pract 2006; 74: 222–226. [DOI] [PubMed] [Google Scholar]
- 7. Nakamura T, Fujikura J, Anazawa T, et al Long‐term outcome of islet transplantation on insulin‐dependent diabetes mellitus: an observational cohort study. J Diabetes Investig 2020; 11: 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saito T, Gotoh M, Satomi S, et al Islet transplantation using donors after cardiac death: report of the Japan Islet Transplantation Registry. Transplantation 2010; 90: 740–747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Secretory Units of Islets in Transplantation (SUIT) index after islet transplantation.
Figure S2 | Insulin doses before and after islet transplantation. GA, glycoalbumin; TDD, total daily dose of insulin.
Figure S3 | Average glucose level and standard deviation of continuous glucose monitoring (CGM) profile before and after islet transplantation. Av, average glucose; SD, standard deviation.


