Abstract
Administration of an sodium–glucose cotransporter 2 inhibitor to individuals with insulin resistance reduces the level of glucose disposal necessary to maintain glycemia as a result of the induced increase in urinary glucose excretion. The amount of insulin required for a certain level of glucose disposal, on which the evaluation of insulin resistance is usually based, might thus decrease even without amelioration of insulin resistance.
Both genetic and environmental factors contribute to the development of insulin resistance. Whereas variations in the function of multiple genes determine predisposition to insulin resistance in the general population, rare cases of severe insulin resistance are triggered by single‐gene defects. Type A insulin resistance syndrome, caused by abnormalities of the insulin receptor gene (INSR), is the most common form of such genetic insulin resistance. The extent of insulin resistance in this syndrome is dependent on the type of INSR mutation 1 . Donohue and Rabson–Mendenhall syndromes, which are usually triggered by abnormalities in both alleles of INSR, show more severe phenotypes compared with type A insulin resistance syndrome, and often result in death in infancy or childhood 1 .
Several years ago, mutations in the gene encoding a regulatory subunit of phosphatidylinositol 3‐kinase (PIK3R1), a key molecule in insulin signaling, were found to give rise to severe insulin resistance in white people 2 . Such cases were recently also identified in individuals of East Asian ethnicity 1 , 3 . A nationwide survey in Japan found 23 and five patients with genetic insulin resistance associated with mutations in INSR and PIK3R1, respectively 1 , suggesting that PIK3R1 mutations are relatively common among individuals with such insulin resistance. Mutations in PIK3R1 were first identified in individuals with SHORT syndrome 2 , which was originally defined by characteristic body features including Short stature, Hyperextensivity/Hernia, Ocular depression, Rieger anomaly and Teething delay. However, individuals with PIK3R1 mutations do not always manifest all of these features 1 .
Cases of severe genetic insulin resistance are often difficult to treat. In addition to a combination of oral drugs and large doses of insulin 1 , 4 , recombinant human insulin‐like growth factor‐1is often administered – in particular, to patients with Donohue or Rabson–Mendenhall syndromes 1 . Given that sodium–glucose cotransporter 2 (SGLT2) inhibitors lower glycemia through a mechanism independent of insulin action, these drugs are a potentially promising option for the treatment of patients with severe genetic insulin resistance. Recent reports have indeed shown the efficacy of SGLT2 inhibitors for the treatment of individuals with insulin resistance triggered by mutations in INSR or PIK3R1 3 , 5 . Lipodystrophy, characterized by the severe loss or complete absence of adipose tissue, is also accompanied by severe insulin resistance. Whereas leptin supplementation is a standard treatment for this condition, a thiazolidinedione also appears to exert beneficial effects in some cases 6 . Successful treatment with SGLT2 inhibitor was also reported for a patient with genetic lipodystrophy who was not able to tolerate leptin supplementation as a result of pain at the injection site 7 . Although their long‐term safety remains to be evaluated, SGLT2 inhibitors appear to be a useful option for diabetes with severe insulin resistance.
In contrast to that triggered by profound genetic defects, insulin resistance in type 2 diabetes patients is more amenable to amelioration by lifestyle modification or medication. SGLT2 inhibitors efficiently lower blood glucose levels in type 2 diabetes patients with insulin resistance. However, do these drugs ameliorate insulin resistance in such individuals?
It is not a simple task to evaluate insulin resistance during treatment with SGLT2 inhibitors. Several investigators have examined the effect of these drugs on insulin sensitivity in individuals with type 2 diabetes with the use of hyperinsulinemic‐euglycemic clamp analysis, the gold standard for assessment of insulin sensitivity or resistance. Merovci et al. 8 and Latva‐Rasku et al. 9 carried out such analysis before and 2 or 8 weeks, respectively, after the onset of treatment with dapagliflozin. The former investigators found a significant improvement in insulin‐induced whole‐body glucose disposal and glucose uptake in peripheral organs, whereas the latter did not. The reason for this apparent discrepancy is unknown, but it might be due to differences in the duration of treatment, the protocol of the clamp or the characteristics of the patients.
Several studies have shown that readily available indexes of insulin resistance, such as homeostasis model assessment of insulin resistance (HOMA‐IR) and the Matsuda Index, were improved after treatment of type 2 diabetes patients with SGLT2 inhibitors 10 , 11 , 12 . However, these indexes were developed on the basis of the notion that circulating levels of insulin and glucose are linked through a negative feedback relationships with a relatively fixed constant. Given that SGLT2 inhibitors induce the excretion of a large amount of glucose in urine, such a constant is likely altered by these drugs. It has therefore been unclear whether these indexes appropriately reflect insulin resistance in individuals treated with SGLT2 inhibitors.
A recent study found that HOMA‐IR and a clamp‐derived insulin sensitivity index showed a linear correlation in individuals with type 2 diabetes, even during treatment with SGLT2 inhibitor 13 , indicating that HOMA‐IR reflects insulin sensitivity, even in the presence of such drugs. However, the value of HOMA‐IR corresponding to a certain level of the clamp‐derived insulin sensitivity index was smaller in patients receiving the SGLT2 inhibitor than in control patients treated without this class of drugs 13 . This finding is reasonable, given that less insulin is required during treatment with SGLT2 inhibitor not only because of a potential improvement in insulin sensitivity, but because of a reduction in the demand for glucose disposal as a result of increased urinary glucose excretion (Figure 1). Care should thus be taken not to overestimate the effect of SGLT2 inhibitors on insulin resistance on the basis of HOMA‐IR determination.
Figure 1.
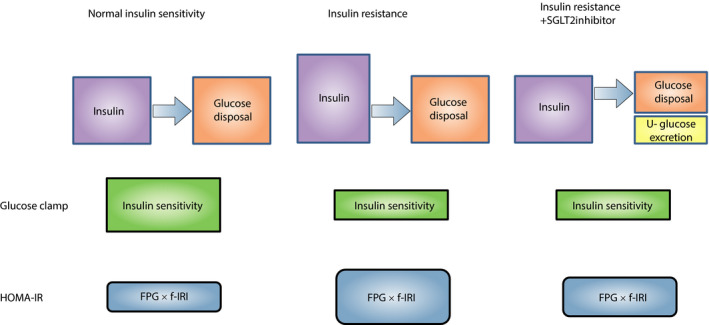
Relationship between circulating insulin levels and body glucose disposal in the absence or presence of sodium–glucose cotransporter 2 (SGLT2) inhibitor. Individuals with insulin resistance require larger amounts of insulin to maintain a certain level of glucose disposal. Administration of SGLT2 inhibitor to such individuals reduces the level of glucose disposal necessary to maintain glycemia as a result of the induced increase in urinary glucose excretion. The amount of insulin required for a certain level of glucose disposal, on which the evaluation of insulin resistance is usually based, might thus decrease, even without amelioration of insulin resistance. f‐IRI, fasting immunoreactive insulin; FPG, fasting plasma glucose; HOMA‐IR, homeostasis model assessment–insulin resistance; U‐glucose, urinary glucose.
Treatment with SGLT2 inhibitors results in a loss of body and fat mass 8 , 9 , 10 , 11 , 12 , 14 , 15 , as well as in amelioration of fatty liver 7 , 9 , all of which potentially contribute to an improvement in insulin resistance. Treatment with SGLT2 inhibitor has been shown to reduce serum leptin levels, even after standardization by body mass 14 . In obese individuals, circulating leptin levels are increased as a result of impairment of leptin action (recognized as leptin resistance), which is thought to be related to the development of insulin resistance. The decrease in serum leptin levels observed in response to SGLT2 inhibitor treatment was accompanied by an increase in appetite 14 , suggesting that the decrease in the circulating leptin concentration was not attributable to amelioration of leptin resistance, rather the decrease results in a reduction in anorexigenic signaling in the hypothalamus, which is well known to be stimulated by leptin, and a consequent increase in appetite. However, a change in appetite is not always observed during treatment with SGLT2 inhibitors 15 .
SGLT2 inhibitors exert multiple beneficial effects in the treatment of diabetes, and such effects are likely mediated by humoral and metabolic changes induced secondarily to inhibition of SGLT2. Further study is required to fully understand these effects of this new class of drugs, as well as to obtain a solid basis for their appropriate use.
Disclosure
WO has received endowment, research funding or honoraria from Astellas Pharma, Abbott Japan, Mitsubishi Tanabe Pharma Corporation, Nippon Boehringer Ingelheim Co., Novartis Pharma, MSD, Sanofi K.K., Sumitomo Dainippon Pharma Co., Takeda Pharmaceutical Co., Eli Lilly Japan, Novo Nordisk Pharma Ltd., Ono Pharmaceutical Co., Daiichi Sankyo Co., Kyorin Pharmaceutical Co., AstraZeneca and Boehringer Ingelheim Pharm GmbH & Co. KG. YH declares no conflict of interest.
Acknowledgment
The authors thank Kazuhiko Sakaguchi for helpful discussion.
J Diabetes Investig. 2020
References
- 1. Takeuchi T, Ishigaki Y, Hirota Y, et al Clinical characteristics of insulin resistance syndromes: a nationwide survey in Japan. J Diabetes Investig 2020; 11: 603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avila M, Dyment DA, Sagen JV, et al Clinical reappraisal of SHORT syndrome with PIK3R1 mutations: toward recommendation for molecular testing and management. Clin Genet 2016; 89: 501–506. [DOI] [PubMed] [Google Scholar]
- 3. Hamaguchi T, Hirota Y, Takeuchi T, et al Treatment of a case of severe insulin resistance as a result of a PIK3R1 mutation with a sodium‐glucose cotransporter 2 inhibitor. J Diabetes Investig 2018; 9: 1224–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang Z, Liu J, Ng K, et al Glimepiride treatment in a patient with type A insulin resistance syndrome due to a novel heterozygous missense mutation in the insulin receptor gene. J Diabetes Investig 2018; 9: 1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nagashima S, Wakabayashi T, Saito N, et al Long‐term efficacy of the sodium‐glucose cotransporter 2 inhibitor, ipragliflozin, in a case of type A insulin resistance syndrome. J Diabetes Investig 2020; 11: 1363–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baba Y, Kaneko H, Takemoto M, et al Case of lipoatrophic diabetes induced by juvenile dermatomyositis. J Diabetes Investig 2018; 9: 632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kawana Y, Imai J, Sawada S, et al Sodium‐glucose cotransporter 2 inhibitor improves complications of lipodystrophy: a case report. Ann Intern Med 2017; 166: 450–451. [DOI] [PubMed] [Google Scholar]
- 8. Merovci A, Solis‐Herrera C, Daniele G, et al Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014; 124: 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Latva‐Rasku A, Honka MJ, Kullberg J, et al The SGLT2 inhibitor dapagliflozin reduces liver fat but does not affect tissue insulin sensitivity: a randomized, double‐blind, placebo‐controlled study with 8‐week treatment in type 2 diabetes patients. Diabetes Care 2019; 42: 931–937. [DOI] [PubMed] [Google Scholar]
- 10. Hattori S. Empagliflozin decreases remnant‐like particle cholesterol in type 2 diabetes patients with insulin resistance. J Diabetes Investig 2018; 9: 870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Osonoi T, Nakamoto S, Saito M, et al Efficacy of ipragliflozin as monotherapy or as add‐on therapy with other oral antidiabetic medications for treating type 2 diabetes in Japanese patients with inadequate glycemic control: a subgroup analysis based on patient characteristics. J Diabetes Investig 2018; 9: 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seino Y, Yabe D, Sasaki T, et al Sodium‐glucose cotransporter‐2 inhibitor luseogliflozin added to glucagon‐like peptide 1 receptor agonist liraglutide improves glycemic control with bodyweight and fat mass reductions in Japanese patients with type 2 diabetes: a 52‐week, open‐label, single‐arm study. J Diabetes Investig 2018; 9: 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. So A, Sakaguchi K, Okada Y, et al Relation between HOMA‐IR and insulin sensitivity index determined by hyperinsulinemic‐euglycemic clamp analysis during treatment with a sodium‐glucose cotransporter 2 inhibitor. Endocr J 2020; 67: 501–507. [DOI] [PubMed] [Google Scholar]
- 14. Miura H, Sakaguchi K, Okada Y, et al Effects of ipragliflozin on glycemic control, appetite and its related hormones: a prospective, multicenter, open‐label study (SOAR‐KOBE Study). J Diabetes Investig 2019; 10: 1254–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Inoue H, Morino K, Ugi S, et al Ipragliflozin, a sodium‐glucose cotransporter 2 inhibitor, reduces bodyweight and fat mass, but not muscle mass, in Japanese type 2 diabetes patients treated with insulin: a randomized clinical trial. J Diabetes Investig 2019; 10: 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]


