Abstract
Impaired awareness of hypoglycemia (IAH) is a reduction in the ability to recognize low blood glucose levels that would otherwise prompt an appropriate corrective therapy. Identified in approximately 25% of patients with type 1 diabetes, IAH has complex pathophysiology, and might lead to serious and potentially lethal consequences in patients with diabetes, particularly in those with more advanced disease and comorbidities. Continuous glucose monitoring systems can provide real‐time glucose information and generate timely alerts on rapidly falling or low blood glucose levels. Given their improvements in accuracy, affordability and integration with insulin pump technology, continuous glucose monitoring systems are emerging as critical tools to help prevent serious hypoglycemia and mitigate its consequences in patients with diabetes. This review discusses the current knowledge on IAH and effective diagnostic methods, the relationship between hypoglycemia and cardiovascular autonomic neuropathy, a practical approach to evaluating cardiovascular autonomic neuropathy for clinicians, and recent evidence from clinical trials assessing the effects of the use of CGM technologies in patients with type 1 diabetes with IAH.
Keywords: Cardiovascular autonomic neuropathy, Impaired awareness of hypoglycemia, Type 1 diabetes
Hypoglycemia can induce hypoglycemia unawareness, which puts patients at higher risk for developing severe hypoglycemia. Hypoglycemia and its relationship with cardiovascular autonomic neuropathy are complex and remain to be further elucidated. Continuous glucose monitoring systems (CGMs) can help reduce hypoglycemia for those with hypoglycemia unawareness.
Introduction
For almost 100 years, insulin has been the fundamental therapy for type 1 diabetes 1 . By suppressing ketogenesis, insulin mitigates the risk for the development of diabetic ketoacidosis, a life‐threatening acute complication of diabetes. The Diabetes Control and Complications Trial 2 and Epidemiology of Diabetes Interventions and Complications study 3 further established the use of intensive insulin therapy to prevent or delay the development of chronic microvascular and macrovascular complications. Based on recent updates, the impacts of this relatively short‐term glucose control appear to confer durable metabolic benefits for at least 30 years 4 , 5 , 6 , 7 , 8 . However, intensive insulin therapy comes at a price. Intensive insulin treatment almost invariably increases the incidence of severe hypoglycemia 9 , 10 , which is associated with altered mental state, seizures, cardiac arrhythmias and even death 11 , 12 , 13 , 14 .
Hypoglycemia has traditionally been defined by blood glucose levels of <70 mg/dL (recently termed level 1 hypoglycemia 15 , 16 ), as these levels trigger the normal physiology of counterregulatory responses to hypoglycemia 17 . Recent revisions of hypoglycemia definitions also include glucose levels <54 mg/dL (i.e., level 2 hypoglycemia 16 ) for its associations with major comorbidities, such as increased mortality, cognitive dysfunction and the development of impaired awareness of hypoglycemia (IAH) 18 , a condition in which patients have diminished or lost ability to perceive the onset of hypoglycemia 19 . The Diabetes Control and Complications Trial study defined severe hypoglycemia as hypoglycemic episodes requiring assistance of another person for recovery 9 . This definition was subsequently adopted as the universal definition of severe (or level 3) hypoglycemia 11 , 15 , 16 .
Iatrogenic hypoglycemia is not restricted to type 1 diabetes patients. Both sulfonylurea use and insulin therapy in patients with type 2 diabetes result in increased risks for hypoglycemia 20 , 21 . Interestingly, there has been intensive debate as to whether severe hypoglycemic events in type 2 diabetes patients is merely a marker of, or indeed causal of, the well‐documented increased risk of cardiovascular events and mortality after hypoglycemia 22 , 23 , 24 , 25 .
Continuous glucose monitoring systems (CGMs, or real‐time CGMs) are devices that measure subcutaneous interstitial glucose to estimate blood glucose levels, and report the glucose levels and trends to patients in real time 26 . CGMs can also generate audible or vibrating alarms for low/high glucose levels, based on the settings customized by patients or healthcare providers, to alert the patients to hypo/hyperglycemic events. Based on their capability to (i) improve hemoglobin A1C (HbA1c) and average glucose levels; (ii) reduce the risk for serious hypoglycemic complications 27 , 28 , 29 ; and (iii) reduce the burden of repetitive fingerstick glucose monitoring 30 , CGMs are now considered the standard of care for type 1 diabetes patients 31 , 32 , 33 . CGM use has also been further established with improvements in accuracy 34 , feasibility for patients of various ages 35 , 36 and diabetes duration 37 , and the standardization of metrics for quantifying hypoglycemia 18 , 38 . The interest and availability of CGMs that are integrated to sensor‐augmented insulin pumps is also rapidly expanding 39 . For patients with type 2 diabetes, data showing the beneficial roles of CGM technology for glucose control 40 , weight control and lifestyle adherence 41 are also emerging.
The current review gives a brief overview of the current knowledge of the IAH, and its assessment methods, the relationships between hypoglycemia and cardiovascular autonomic neuropathy (CAN), a practical approach on CAN evaluations in clinical care, and the recent clinical trial evidence on the role of CGMs use in the IAH population.
Impaired Awareness of Hypoglycemia as a Barrier for Glucose Control
Patients with IAH develop unrecognized hypoglycemic events and thereby can often miss the opportunity to treat their hypoglycemia in a timely manner 19 . Commonly co‐existing with IAH is the attenuation or loss of sympathoadrenal mechanisms, which limits the endogenous glucoregulatory recovery from hypoglycemia (specifically, catecholaminergic stimulation of hepatic glucose output and restraint of muscle glucose uptake) 42 . Thus, for people with type 1 diabetes, who have already lost the ability to decrease endogenous insulin secretion and increase glucagon production as counterregulatory mechanisms, IAH and impaired adrenomedullary responses result in a further significant loss of defense mechanisms to avoid severe hypoglycemia (Figure 1) 19 . Indeed, IAH is associated with an approximately sixfold increased risk of developing severe hypoglycemia 43 , 44 . Clinically, because of the risk of developing dangerously low glucose levels, patients and healthcare providers alike are often reluctant to practise/advocate tight glucose control to achieve proposed glycemic targets 45 .
Figure 1.
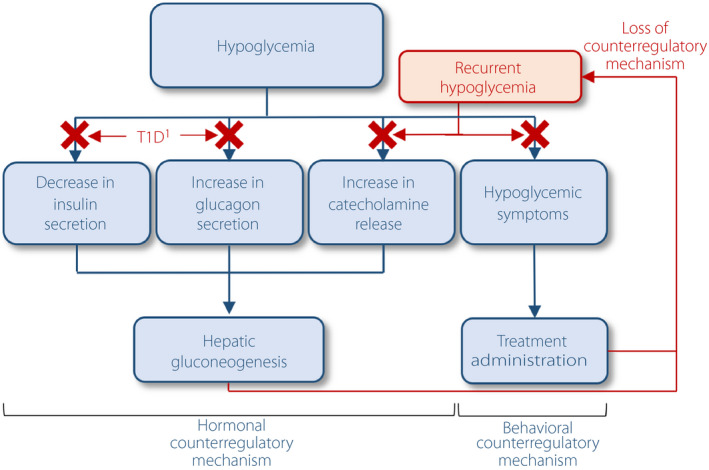
Hypoglycemia counterregulatory mechanisms and the impacts of type 1 diabetes (T1D) and recurrent hypoglycemia on these mechanisms. 1Or advanced type 2 diabetes.
Approximately 25–40% of type 1 diabetes patients were found to have IAH, with a stable prevalence over the past two decades 43 , 44 , 46 , 47 . This value is most certainly an underestimation, as even patients who report having intact hypoglycemia awareness are indeed unaware of CGM‐confirmed hypoglycemia 48 . In the type 2 diabetes population, the IAH prevalence ranges from approximately 6 to 17% in those using insulin injection programs, and the IAH status is associated with 9–17‐fold increased risk for severe hypoglycemia 49 , 50 , 51 .
A major cause of IAH and impaired adrenomedullary responses to hypoglycemia is recurrent episodes of hypoglycemia, which (as part of a vicious cycle) perpetuate these conditions 52 , 53 , 54 . There is also evidence that IAH can be induced by sleep 55 , 56 , psychological stress 57 and alcohol 58 , yet there are still controversies as to whether exercise 59 , 60 and beta‐adrenergic blockers 61 , 62 have detrimental or beneficial effects on hypoglycemia awareness status.
The mechanisms for the development of IAH remain to be elucidated 63 . Earlier studies evaluated the relationships between this condition and adrenal medulla destruction 64 , cortisol (as a systemic mediator) 65 or CAN 66 . Some studies focused on the glucose sensing in the brain and how it is altered with antecedent hypoglycemia. Consistent with this central nervous system‐impaired glucose sensing, recent studies have implicated the use of alternative fuels (e.g., lactate 67 or monocarboxylic acids 68 ) and changes in the neurotransmitter signaling in the brain (e.g., GABAergic 69 , glutaminergic and opioidergic 70 signaling) as likely causes for IAH and the impaired sympathoadrenal response to hypoglycemia.
As these impaired responses are purported to be caused by recurrent antecedent hypoglycemia, it is logical that a reduction in the incidence of hypoglycemia would be expected to improve hypoglycemia awareness and adrenomedullary responses. In support of this notion, studies have shown that strict hypoglycemia avoidance with rigorous monitoring and behavioral modifications can help improve hypoglycemia awareness in as little as 2 weeks 71 , 72 , 73 , 74 . Additionally, blood glucose awareness training 75 , education to optimize insulin dosing 76 and hypoglycemia avoidance motivational programs 77 have also been shown to improve hypoglycemia awareness.
Hypoglycemia and Cardiovascular Autonomic Neuropathy
Diabetic CAN, defined as the impairment of autonomic control of the cardiovascular system in the setting of diabetes after exclusion of other causes 78 , is a major diabetic comorbidity that has been associated with a significant increase in mortality in both patients with type 1 diabetes 79 , 80 , 81 and type 2 diabetes 82 , 83 , 84 . Despite the association between CAN and increased mortality, currently there is no effective therapy to prevent or reverse this condition beyond glycemic control 6 , 85 , 86 and symptomatic management 87 . The role of autonomic dysfunction as a risk factor for IAH had been studied quite extensively. Particularly as a hallmark of IAH is the loss of sympathetic symptoms (e.g., palpitation, tremor and anxiety) and the epinephrine responses to hypoglycemia, it was postulated that autonomic dysfunction including CAN might directly contribute to the development of IAH 88 . However, more recent evidence showed that in some patients IAH can be induced by a single episode of hypoglycemia 53 . This suggests that although autonomic dysfunction and CAN might further impact IAH risk and consequences 89 , 90 , it is unlikely to be the main mechanism involving its development 66 , 91 , 92 . Furthermore, it appears that self‐reported IAH does not predict CAN 93 . Yet, the associations between hypoglycemia and CAN in particular are quite complex, and remain to be further elucidated. There is ample evidence that CAN is independently associated with hypoglycemia in patients with diabetes 25 , 94 , 95 . Several studies have also shown that hypoglycemia can promote reductions in heart rate variability and the baroreflex sensitivity in both patients with diabetes 96 , 97 and healthy controls 98 that might last for many hours after euglycemia is restored 97 . In addition, our group has reported that increased glucose variability, particularly with a predominance of hypoglycemic stress measures, was associated with blunting in measures of heart rate variability in type 1 diabetes patients 94 . These data lend support to a potential role of hypoglycemia in the development of CAN and the loss of the protective cardiovagal mechanisms, which might directly impact cardiac electrical activities and thus eventually increase the risk of cardiac arrhythmias in these patients 94 , 97 , 99 , 100 , 101 . Experimental evidence reported that hypoglycemia might lead to peripheral nerve axonal degeneration, possibly through alterations in the glucose uptake, depletion of energy substrates and changes in Schwann cell metabolism affecting particularly the large myelinated fibers 102 , 103 , although the exact mechanisms and whether these hypoglycemia‐associated changes are functional 104 , 105 , reversible 106 or permanent are still unclear 107 , 108 . An additional example of the complex interactions between hypoglycemia, CAN and neuropathy is treatment‐induced neuropathy. Treatment‐induced neuropathy is a condition described in patients who have experienced a rapid decline in blood glucose levels after the use of insulin, oral hypoglycemic medications, or even diet only to control hyperglycemia, and often manifests as a painful sensory and autonomic neuropathy, often with a dramatic onset and course 109 , 110 .
Assessment of Impaired Awareness of Hypoglycemia and Impaired Adrenomedullary Responses to Hypoglycemia
The hyperinsulinemic hypoglycemic clamp technique is the gold standard of assessing hypoglycemia awareness and hormonal responses to hypoglycemia 17 , 111 . This validated tool assesses the hypoglycemia awareness status by collecting hypoglycemic symptoms during the clamp procedure at specified intervals to determine at what level of glucose hypoglycemic symptoms are experienced 112 , 113 . Information is captured on several domains that include: difficulty thinking/confused, warm, shaky/tremulous, nausea, tired/drowsy, hungry, weak, sweaty, headache, heart‐pounding, difficulty speaking, nervous/anxious, dizzy, faint, tingling and blurred vision 112 . In general, it is accepted that individuals who do not develop significant hypoglycemic symptoms around glucose levels of 50–54 mg/dL are considered to have IAH 114 . Additional methods include the assessment of epinephrine levels and other counterregulatory hormones (norepinephrine, glucagon, cortisol, growth hormone, pancreatic polypeptide) during the various stages of hypoglycemia 17 . Techniques in measuring the endogenous glucose production for the assessment of hepatic glucose output can also be incorporated into hypoglycemic clamps 115 . Both single‐step 116 (from baseline to one single hypoglycemia glucose level target) or step‐wise 117 (from baseline to sequentially lower hypoglycemic level targets) clamps are commonly used. Some studies also carry out additional hyperinsulinemic‐euglycemic clamps 117 , in randomized orders with the hypoglycemic clamps, to blind the participants, so that the participants’ hypoglycemic symptoms and hormonal measures would not be confounded by the knowledge of an anticipated hypoglycemic event or insulin administration. Although the hypoglycemic clamp is a well‐established method to objectively measure the status of counterregulatory mechanisms, the pitfalls of clamp studies are the invasiveness, expense and the significant time commitment from the patients, and thus these studies are often restricted to a small patient cohort. The interlaboratory variabilities in epinephrine assays also prohibit the comparison among studies (Table 1) 118 .
Table 1.
Current measures for assessing hypoglycemia awareness
| Measurements | Advantages | Disadvantages | |
|---|---|---|---|
| Outpatient |
Questionnaires: |
|
|
| Inpatient | Edinburgh Hypoglycemia Scores 112 determined during the hyperinsulinemic hypoglycemic clamp. |
|
|
In the outpatient setting, methods to assess hypoglycemia awareness based on questionnaires (i.e., “self‐reported hypoglycemia awareness”) have also been developed and widely utilized, particularly for studies requiring larger sample sizes. The Gold questionnaire 43 contains a single question (besides two questionnaire‐validation questions) asking individuals to report their experience in detecting hypoglycemic events with scores ranging from 1 (always aware) to 7 (never aware) on a Likert‐type scale. In contrast, the Clarke questionnaire 44 is comprised of eight questions evaluating participants’ prior hypoglycemia experiences, such as the history of severe hypoglycemia developments and the glucose levels at which patients start to detect hypoglycemic symptoms, and generates a score (0–7) based on the responses. Scores ≥4 are indicative of IAH, and ≤2 indicates normal awareness for both the Gold and Clark questionnaires. The Pedersen‐Bjergaard questionnaire 46 asks individuals to report whether they recognize symptoms during hypoglycemic events and, based on the answer, the hypoglycemia awareness status is categorized as “normal,” “impaired awareness,” “unawareness” and :undetermined.” All of these questionnaires have been previously validated based on their associations with severe hypoglycemia. The Clarke questionnaire has also been validated with hypoglycemic clamps 114 . HypoA‐Q 119 is a 33‐item questionnaire assessing hypoglycemia awareness when awake/sleep, and the hypoglycemia frequency, severity and impacts on patients. This questionnaire was validated with strong correlations with the Gold and Clarke questionnaires, together with weak correlations with diabetes‐related distress and HbA1c. Other than wide usability with their non‐invasiveness and no/minimal cost, self‐reported hypoglycemia awareness assessments might also benefit from the direct reporting of patients’ experiences in real life 120 , rather than in highly controlled inpatients settings of hypoglycemic clamps. In contrast, the subjectivity of the experience (e.g., possibly influenced more by the recent events) or lack of a controlled environment might generate biases for the awareness reporting.
Diagnosis of Diabetic Cardiovascular Autonomic Neuropathy in Clinical Care
The American Diabetes Association recommends that screening for CAN should be carried out for patients with evidence of other chronic complications, such as nephropathy, peripheral neuropathy, retinopathy and cardiovascular disease, as well as for patients with IAH 121 , with high glucose variability, before insulin dose adjustments and/or perioperatively 79 . The symptoms of CAN are less prevalent in contemporary cohorts of patients with diabetes, and most patients with CAN are completely asymptomatic 101 , 121 . Weakness, lightheadedness, palpitations, syncope with standing or exercise intolerance are usually associated with advanced CAN 6 , 85 , 122 .
Clinical signs, such as resting tachycardia (>100 b.p.m.) and orthostatic hypotension (a fall in systolic or diastolic blood pressure by >20 mmHg or >10 mmHg, respectively, on standing without an appropriate increase in heart rate) are both easy to document in an office 78 , 123 , but in general present in later stages of CAN 121 , 124 . A decrease in heart rate variability is the earliest sign of CAN 78 , 125 , 126 , and can be assessed in an office by obtaining an electrocardiogram during 1–2 min of deep breathing and calculating indices of heart rate variability 127 , 128 . However, given that both the symptoms and signs described are non‐specific, a careful differential diagnosis is required to exclude other common medical causes (e.g., hyperthyroidism, anemia, dehydration, adrenal insufficiency, arrhythmic disorders), prescription medications effects (e.g., antihypertensive agents, antimuscarinic agents, diuretics), over‐the‐counter supplements and recreational agents 121 .
The cardiovascular reflex tests that assess changes in heart rate and blood pressure in response to several simple physiological maneuvers, such as deep breathing, standing or Valsalva, remain the gold standard diagnostic for autonomic testing in both clinical care and research settings, although these are more expensive and add burden for both clinicians and patients 121 .
Clinical Trials Testing the Use of Continuous Glucose Monitoring Systems in Type 1 Diabetes Patients with Impaired Awareness of Hypoglycemia
Early CGM clinical trials primarily focused on the CGMs’ impact on glucose control, hypoglycemia reduction and quality of life 129 . Additional questions were raised regarding the potential benefits of the CGM technology in improving the hypoglycemia awareness and epinephrine responses in patients with IAH. Below we summarize some of the most relevant trials that have addressed these questions.
In 2011, Ly et al. 130 carried out a small group randomized clinical trial study to evaluate whether the use of CGMs versus self‐monitoring of blood glucose (SMBG) might improve epinephrine responses during hypoglycemic clamps in adolescents with type 1 diabetes and IAH (Table 2). The target glucose levels were 108–180 mg/dL in both groups, and the CGM group had the hypoglycemia alarm thresholds set at 108 mg/dL. Although after 4 weeks the CGM group had greater epinephrine responses during the hypoglycemic clamps (Table 3), suggesting a potential benefit of CGMs in improving hypoglycemia awareness, these findings were limited by the small sample size and to a group with relatively short diabetes duration.
Table 2.
Clinical trials evaluating continuous glucose monitoring use in type 1 diabetes patients with impaired awareness of hypoglycemia
| Authors (year) | Main objective | Trial design and targeted population | Primary outcome(s) | Baseline population characteristics | CGM models (active usage time) |
|---|---|---|---|---|---|
| Ly et al. (2011) 130 | Assess if the use of CGMs with preset hypo alarms (at glucose 108 mg/dL) improves counterregulatory response to hypoglycemia. |
Randomized, controlled. Two arms (CGM vs SMBG). Duration: 4 weeks. Adolescents (aged 12–18 years) with IAH defined per modified Clarke (n = 11). |
Epinephrine response to hypoglycemia measured during hypoglycemia clamp study. |
CGM n = 6; SMBG n = 5 Female: Not reported Age:
DoD:
HbA1c:
MDI: Not reported |
Medtronic Minimed paradigm real‐time system (not reported) |
| Little et al. (HypoCOMPaSS; 2014) 131 ; Leelarathna et al. (HypoCOMPaSS clamp sub‐cohort study; 2013) 132 | Determine if rigorous hypoglycemia prevention improves hypoglycemia awareness and prevents SH development in patients with IAH, independent of insulin delivery and glucose monitoring modalities. |
Randomized, controlled. 2 × 2 factorial (CGM vs SMBG, CSII vs MDI). Duration: 24 weeks. Patients with IAH defined per Gold. (n = 96) |
Difference in hypoglycemia awareness (assessed with Gold) between the CGM and SMBG groups, and between the MDI and CSII groups. Clamp subcohort study: the glucose concentration at which participants felt hypoglycemic during progressive hypoglycemia. |
83 patients completed study; CGM n = 42 and SMBG n = 41 Female: 64% Age: 48.6 ± 12.2 years DoD: 28.9 ± 12.3 years HbA1c: 8.2 ± 1.2% MDI: 97% Clamp Subcohort n = 18 (CGM n = 11, SMBG n = 7) Female: 66.7% Age: 50 ± 9 years DoD: 35 ± 10 years HbA1c: 8.1 ± 1% MDI: 50% |
Medtronic (median 57%) |
| van Beers et al. (IN CONTROL; 2016) 133 | Assess whether CGM use improves glycemia control and prevents severe hypoglycemia in patients with IAH. |
Randomized, cross‐over. Two arms (CGM vs SMBG). Duration: 16‐week intervention with 12‐week washout. Patients with IAH defined per Gold, either on CSII or MDI. (n = 52) |
Mean difference in the percentages of time in normoglycemia. |
CGM n = 26, SMBG n = 26 Female: 46% Age: 48.6 ± 11.6 years DoD: 30.5 ± 40.8 years HbA1c: 7.5 ± 0.8% MDI: 56% |
Medtronic Enlite glucose sensor (median 89.4; IQR 80.8–95.5); |
| Rickels et al. (2018) 134 | Assess if hypoglycemia avoidance with CGMs improves glucose counterregulation in patients with long‐standing diabetes and IAH. |
Single arm (CGM). Duration: 18 months. Patients with IAH defined per Clarke and other criteria † . (n = 11) |
Difference in the endogenous glucose production response during stepped‐hypoglycemic and euglycemic clamps. |
Female: 55% Age: 44 ± 4 years DoD: 31 ± 4 years HbA1c: 7.2 ± 0.2% MDI: 27% |
Dexcom seven plus/G4 or Medtronic Sof‐Sensor (n = 7/4) (median 100%) |
| Heinemann et al. (HypoDE; 2018) 135 | Ascertain whether the incidence and severity of hypoglycemia can be reduced through CGM use in patients on MDI and with high risk for developing SH. |
Randomized, controlled. Two arms (CGM vs SMBG). Duration: 22‐week intervention and 4‐week follow up. Patients on MDI with SH within the last year or IAH defined per Clarke. (n = 149) |
The mean difference in the number of hypoglycemic events (defined as CGM glucose ≤54 mg/dL for ≥20 min) between baseline and the follow up phase. |
141 patients in final analysis; CGM n = 75, SMBG n = 66 Female:
Age:
DoD:
HbA1c:
MDI: 100% |
Dexcom G5 (mean 90.7%) |
| Reddy et al. (I‐HART; 2018) 141 | Assess the impacts of CGMs and FGMs on hypoglycemia reduction in patients on MDI with high risk for developing SH. |
Randomized. Two arms (CGM vs FGM). Duration: 8 weeks. Patients on MDI with SH within the last year or IAH defined per Gold (n = 40) |
The median difference between the change of time in hypoglycemia (<59 mg/dL) from baseline to end‐point. |
CGM n = 20, SMBG n = 20 Female: 40% Age: 49.5 years (37.5–63.5) DoD: 30.0 years (21.0–36.5) HbA1c: 7.3% (6.5–7.8) MDI: Not reported ‡ |
Dexcom G5 (not reported) |
Data presented in mean ± standard deviation or median (interquartile range [IQR]).
AUC, area under the curve; CSII, continuous subcutaneous insulin infusion; DoD, duration of diabetes; HbA1c, hemoglobin A1C; IAH, impaired awareness of hypoglycemia; SH, severe hypoglycemia; SMBG, self‐monitoring of blood glucose; T1D, type 1 diabetes.
Severely problematic hypoglycemia (hypoglycemia [hypo] score ≥1,047), marked glycemic lability (glycemic lability index ≥433 mmol/L2/h/week or a composite of HYPO score ≥423 and glycemic liability index ≥329 mmol/L2/h/week, and either at least one episode of severe hypoglycemia in the past 12 months or the presence of >5% of time spent at <60 mg/dL by 72‐h blinded continuous glucose monitoring (CGM).
The study aimed to assess the CGM effects on multiple daily injection (MDI)‐using population; actual percentage not reported.
Table 3.
Reported time in hypoglycemia, hypoglycemia awareness and autonomic response outcomes in clinical trials evaluating continuous glucose monitoring use in type 1 diabetes patients with impaired awareness of hypoglycemia
| Author | Time in hypoglycemia at study end † (%) | Hypoglycemia awareness outcomes | Endogenous Glucoregulatory Response Outcomes |
|---|---|---|---|
| Ly et al. (2011) 130 | NA | NA |
Changes in epinephrine levels during hypoglycemic clamps compared with euglycemic clamps (%) Baseline:
Study end (4 weeks):
Changes in epinephrine levels during hypoglycemic clamps at baseline vs study end:
|
| Little et al. (HypoCOMPaSS; 2014) 131 ; Leelarathna, et al. (HypoCOMPaSS clamp subcohort study; 2013) 132 |
Glucose <72 mg/dL
Glucose ≤54 mg/dL
Clamp Study Subcohort – AUC of the % of time spent with glucose <54 mg/dL (mean ± standard error):
|
Gold scores
Clarke scores
HypoA‐Q scores
No differences in hypoglycemia awareness scores between the CGM vs SMBG and CSII vs MDI models. Clamp Study Subcohort Plasma glucose level of first felt hypoglycemia
Symptom score AUC Baseline: 500 (364–685) Study end: 650 (365–1,285) (P = 0.02) No differences in the above measures between CGM vs SMBG and CSII vs MDI models. |
Clamp Study Subcohort –AUC of incremental metanephrine levels
Glucose thresholds for metanephrine response
No differences in the above measures between the CGM vs SMBG and CSII vs MDI models. |
| van Beers et al. (IN CONTROL; 2016) 133 |
Glucose ≤70 mg/dL
|
Gold scores
Change in Gold scores from baseline
Clarke scores
Change in Clarke scores from baseline
|
NA |
| Rickels et al. (2018) 134 |
Glucose <60 mg/dL
|
Clark scores
Clamp Study Autonomic symptoms during hypoglycemic vs euglycemic clamps:
No statistical significance when comparing the symptom scores at 6 and 18 months to baseline. |
Epinephrine levels during hypoglycemia
Norepinephrine levels during hypoglycemia
Endogenous glucose production (compared to baseline): ‡
|
| Heinemann et al. (HypoDE; 2018) 135 |
Glucose ≤70 mg/dL
Glucose ≤54 mg/dL
|
Clark scores Baseline
Follow up
Adjusted between‐group differences: P = 0.7662 |
NA |
| Reddy et al. (I‐HART; 2018) 141 |
Glucose <70 mg/dL
Glucose <50 mg/dL
|
Gold scores Baseline:
Study end (8 weeks):
Median change from baseline:
Differences in median changes from baseline to study end: P = 0.23 |
NA |
Data presented in mean ± standard deviation or median (interquartile range) or mean/median [95% confidence interval], unless noted otherwise.
AUC, area under the curve; CSII, continuous subcutaneous insulin infusion; FGM, flash glucose monitoring; HypoCOMPaSS, comparison of optimised MDI versus pumps with or without sensors in severe hypoglycaemia; HypoDE, hypoglycemia in Deutschland; IAH, impaired awareness of hypoglycemia; I‐HART, impact on hypoglycaemia awareness of real time CGM and intermittent continuous glucose data; IN CONTROL, effects of RT‐CGM on glycemia and QoL in patients with T1DM and IHA; MDI, multiple daily injections; NA, not available; NS, not significant; T1D, type 1 diabetes.
Variable definitions for hypoglycemia were used. These trials were performed prior to the current continuous glucose monitoring (CGM)/hypoglycemia guidelines. For self‐monitoring of blood glucose level (SMBG) groups or run‐in phase, time in hypoglycemia were assessed with blinded CGMs.
Primary outcomes of the trials.
Subsequently, the comparison of optimised MDI versus pumps with or without sensors in severe hypoglycaemia group 131 carried out a 2 × 2 factorial (SMBG vs CGM; multiple daily injections, MDI vs continuous subcutaneous insulin infusion) randomized trial to assess whether hypoglycemia avoidance with intensive education could improve hypoglycemia awareness regardless of the glucose monitoring and insulin delivery models. At the study end, the incidence of hypoglycemia was reduced in all study arms, and the degree of hypoglycemia awareness improvements was similar between the CGM and SMBG groups, including the hypoglycemia symptoms scores during the hypoglycemic clamps in a subcohort study 132 . However, the low CGM use time (<50%) in approximately 40% of the participants could have significantly confounded the results.
The effects of RT‐CGM on glycemia and QoL in patients with T1DM and IHA study group 133 evaluated glucose control (CGM vs SMBG) in IAH patients with a cross‐over trial. The CGM phase was related to 15% more time‐in‐range (72–180 mg/dL), and 41% and 55% reduction of the time in hypoglycemia and the number of patients who developed severe hypoglycemia, respectively. The Gold scores at the end of the CGM phase were lower, and tended to be lower compared with the end of the SMBG phase and to the baseline, respectively. Similar findings, however, were not observed in the Clarke scores. Although the cross‐over design allows more “individualized” comparisons to evaluate CGMs’ impact, it was unclear if a 16‐week CGM intervention was long enough to significantly improve self‐reported hypoglycemia awareness, and whether the 12‐week washout period could sufficiently “reset” the hypoglycemia awareness to the baseline.
In 2018, Rickels et al. 134 carried out a small cohort, 18‐month pre‐post trial evaluating the changes in the endogenous glucose production and epinephrine responses with CGM interventions. In this IAH population with severely problematic hypoglycemia, the incidence of severe hypoglycemia decreased nearly 60% during the intervention. The hypoglycemic clamps also showed a doubled endogenous glucose production at 18 months, with no statistically significant improvements in epinephrine responses. Improvements in autonomic symptom scores and self‐reported hypoglycemia awareness were also observed.
The HypoDE (or "Hypoglycemia in Deutschland") study 135 is the largest randomized trial (CGM vs SMBG) to date testing CGMs’ effects in patients with IAH or severe hypoglycemia history. The CGM group showed 72% fewer hypoglycemic episodes with glucose ≤54 mg/dL, along with 64% fewer severe hypoglycemic episodes. The entire cohort also had a 40% improvement in hypoglycemia awareness scores, although no difference was found between the CGM and SMBG groups.
Flash glucose monitoring systems (e.g., FreeStyle LibreTM), like CGMs, can provide glucose levels and trends, but without the feature of automated low/high glucose alarms 136 . Flash glucose monitoring systems have been documented to reduce the time in hypoglycemia 137 and severe hypoglycemia 138 for type 1 diabetes patients, and reduce hypoglycemia 139 and improve HbA1c 140 in the type 2 diabetes population. Reddy et al. compared the efficacy of CGMs versus Flash glucose monitoring systems in reducing hypoglycemia in type 1 diabetes patients with IAH or severe hypoglycemia history 141 . The CGM group showed greater hypoglycemia reduction, particularly at night, attributed to the low glucose alarm systems. However, the improvements in hypoglycemia awareness in these two groups were statistically indistinguishable. Potential confounders include flash glucose monitoring systems’ lower glucose accuracy in the low glucose range 136 , 142 , 143 that might have falsely reported more hypoglycemia.
Although CGMs have clearly shown the benefit of hypoglycemia reduction without compromising the overall glycemic control, the extent to which CGMs can help improve hypoglycemia awareness and epinephrine responses remains unclear. Although meticulous avoidance of hypoglycemia has been shown to improve hypoglycemia awareness within 2–16 weeks 71 , 72 , 73 , 74 , none of the aforementioned studies showed an absolute avoidance of hypoglycemia, which could explain this finding. Recent observational data 144 , 145 , 146 show that IAH is still common and problematic in type 1 diabetes patients, despite CGM use, and thus IAH might unfortunately remain an important clinical obstacle in diabetes management for CGM users.
To definitively determine whether CGMs/diabetes technologies could improve hypoglycemia awareness, more optimal trial design that eliminates confounders and provides sufficient intervention duration is important 131 . This includes matching individuals for age, duration of diabetes, HbA1c, hypoglycemia awareness scores and hypoglycemia cognition 145 to reduce some effects from the individual variabilities. It would also be of interest whether a treat‐to‐target approach (e.g., time in hypoglycemia targets of <4% 147 or even <1% 148 ), with techniques such as more rigorous strategies to engage patients to CGMs 149 or CGM alarm setting adjustments 150 , 151 , could improve hypoglycemia awareness or epinephrine responses to hypoglycemia.
Conclusion
CGM is an effective tool to help reduce hypoglycemia and severe hypoglycemic episodes in type 1 diabetes patients, including those with IAH. Whether CGMs could help improve hypoglycemia awareness, and how CAN and IAH are interrelated, remain to be determined or further elucidated.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
SJF is supported by the NIH R01DK118082. RPB is supported by NIH 1R01DK107956‐01 and U01DK119083, and the JDRF Center of Excellence at the University of Michigan.
J Diabetes Investig. 2020
References
- 1. Katsarou A, Gudbjörnsdottir S, Rawshani A, et al Type 1 diabetes mellitus. Nat Rev Dis Primers 2017; 3: 17016. [DOI] [PubMed] [Google Scholar]
- 2. Nathan DM, Genuth S, Lachin J et al The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 3. Nathan DM, Cleary PA, Backlund JY, et al Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014; 37: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30‐year follow‐up. Diabetes Care 2016; 39: 686–693.26861924 [Google Scholar]
- 6. Martin CL, Albers JW, Pop‐Busui R. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014; 37: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Boer IH. Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014; 37: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aiello LP. Diabetic retinopathy and other ocular findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014; 37: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The Diabetes Control and Complications Trial Research Group . Hypoglycemia in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes 1997; 46: 271–286. [PubMed] [Google Scholar]
- 10. Gubitosi‐Klug RA, Braffett BH, White NH, et al Risk of severe hypoglycemia in type 1 diabetes over 30 years of follow‐up in the DCCT/EDIC Study. Diabetes Care 2017; 40: 1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seaquist ER, Anderson J, Childs B, et al Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36: 1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amiel SA, Aschner P, Childs B. Minimizing hypoglycemia in diabetes. Diabetes Care 2015; 38: 1583–1591. [DOI] [PubMed] [Google Scholar]
- 13. McCoy RG, Van Houten HK, Ziegenfuss JY, et al Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012; 35: 1897–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lung TW, Petrie D, Herman WH, et al Severe hypoglycemia and mortality after cardiovascular events for type 1 diabetic patients in Sweden. Diabetes Care 2014; 37: 2974–2981. [DOI] [PubMed] [Google Scholar]
- 15. Agiostratidou G, Anhalt H, Ball D, et al Standardizing Clinically Meaningful Outcome Measures Beyond HbA1c for Type 1 Diabetes: A Consensus Report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017; 40: 1622–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. 6. Glycemic Targets. Standards of medical care in diabetes—2020. Diabetes Care 2020; 43(Supplement 1): S66–S76. [DOI] [PubMed] [Google Scholar]
- 17. Mitrakou A, Ryan C, Veneman T, et al Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol 1991; 260(1 Pt 1): E67–E74. [DOI] [PubMed] [Google Scholar]
- 18. International Hypoglycaemia Study Group . Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint Position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017; 40: 155–157. [DOI] [PubMed] [Google Scholar]
- 19. Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes 2008; 57: 3169–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zammitt NN, Frier BM. Hypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care 2005; 28: 2948–2961. [DOI] [PubMed] [Google Scholar]
- 21. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 22. Bonds DE, Miller ME, Bergenstal RM, et al The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010; 340: b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee AK, Warren B, Lee CJ, et al The association of severe hypoglycemia with incident cardiovascular events and mortality in adults with type 2 diabetes. Diabetes Care 2018; 41: 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zinman B, Marso SP, Christiansen E, et al Hypoglycemia, cardiovascular outcomes, and death: the LEADER experience. Diabetes Care 2018; 41: 1783–1971. [DOI] [PubMed] [Google Scholar]
- 25. Davis SN, Duckworth W, Emanuele N, et al Effects of severe hypoglycemia on cardiovascular outcomes and death in the veterans affairs diabetes trial. Diabetes Care 2019; 42: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodbard D. Continuous glucose monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol Ther 2017; 19(S3): S25–s37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tamborlane WV, Beck RW, Bode BW, et al Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008; 359: 1464–1476. [DOI] [PubMed] [Google Scholar]
- 28. Beck RW, Riddlesworth T, Ruedy K, et al Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 2017; 317: 371–378. [DOI] [PubMed] [Google Scholar]
- 29. Lind M, Polonsky W, Hirsch IB, et al Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: The GOLD randomized clinical trial. JAMA 2017; 317: 379–387. [DOI] [PubMed] [Google Scholar]
- 30. Aleppo G, Ruedy KJ, Riddlesworth TD, et al REPLACE‐BG: A randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well‐controlled type 1 diabetes. Diabetes Care 2017; 40: 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peters AL, Ahmann AJ, Battelino T, et al Diabetes technology—continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2016; 101: 3922–3937. [DOI] [PubMed] [Google Scholar]
- 32. Fonseca VA, Grunberger G, Anhalt H, et al Continuous glucose monitoring: a consensus conference of the american association of clinical endocrinologists and american college of endocrinology. Endocr Pract 2016; 22: 1008–1021. [DOI] [PubMed] [Google Scholar]
- 33. 7. Diabetes technology: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020; 43(Supplement 1): S77–S88. [DOI] [PubMed] [Google Scholar]
- 34. Reiterer F, Polterauer P, Schoemaker M, et al Significance and reliability of MARD for the accuracy of CGM systems. J Diabetes Sci Technol 2017; 11: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lal RA, Maahs DM. Clinical use of continuous glucose monitoring in pediatrics. Diabetes Technol Ther 2017; 19(S2): S37–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Volcansek S, Lunder M, Janez A. Acceptability of continuous glucose monitoring in elderly diabetes patients using multiple daily insulin injections. Diabetes Technol Ther 2019; 21: 566–574. [DOI] [PubMed] [Google Scholar]
- 37. Prahalad P, Addala A, Scheinker D, et al CGM initiation soon after type 1 diabetes diagnosis results in sustained CGM Use And Wear Time. Diabetes Care 2020; 43: e3–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Danne T, Nimri R, Battelino T, et al International consensus on use of continuous glucose monitoring. Diabetes Care 2017; 40: 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kravarusic J, Aleppo G. Diabetes technology use in adults with type 1 and type 2 diabetes. Endocrinol Metab Clin North Am 2020; 49: 37–55. [DOI] [PubMed] [Google Scholar]
- 40. Beck RW, Riddlesworth TD, Ruedy K, et al Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med 2017; 167: 365–374. [DOI] [PubMed] [Google Scholar]
- 41. Taylor PJ, Thompson CH, Brinkworth GD. Effectiveness and acceptability of continuous glucose monitoring for type 2 diabetes management: a narrative review. J Diabetes Investig 2018; 9: 713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cryer PE. Mechanisms of hypoglycemia‐associated autonomic failure in diabetes. N Engl J Med 2013; 369: 362–372. [DOI] [PubMed] [Google Scholar]
- 43. Gold AE, Macleod KM, Frier BM. Frequency of severe hypoglycemia in patients with type i diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994; 17: 697–703. [DOI] [PubMed] [Google Scholar]
- 44. Clarke WL, Cox DJ, Gonder‐Frederick LA, et al Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care 1995; 18: 517–522. [DOI] [PubMed] [Google Scholar]
- 45. Smith CB, Choudhary P, Pernet A, et al Hypoglycemia unawareness is associated with reduced adherence to therapeutic decisions in patients with type 1 diabetes. Evidence from a clinical audit. Diabetes Care 2009; 32: 1196–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pedersen‐Bjergaard U, Pramming S, Thorsteinsson B. Recall of severe hypoglycaemia and self‐estimated state of awareness in type 1 diabetes. Diabetes Metab Res Rev 2003; 19: 232–240. [DOI] [PubMed] [Google Scholar]
- 47. Geddes J, Schopman JE, Zammitt NN, et al Prevalence of impaired awareness of hypoglycaemia in adults with type 1 diabetes. Diabet Med 2008; 25: 501–504. [DOI] [PubMed] [Google Scholar]
- 48. Kubiak T, Hermanns N, Schreckling HJ, et al Assessment of hypoglycaemia awareness using continuous glucose monitoring. Diabet Med 2004; 21: 487–490. [DOI] [PubMed] [Google Scholar]
- 49. Henderson JN, Allen KV, Deary IJ, et al Hypoglycaemia in insulin‐treated Type 2 diabetes: frequency, symptoms and impaired awareness. Diabet Med 2003; 20: 1016–1021. [DOI] [PubMed] [Google Scholar]
- 50. Schopman JE, Geddes J, Frier BM. Prevalence of impaired awareness of hypoglycaemia and frequency of hypoglycaemia in insulin‐treated type 2 diabetes. Diabetes Res Clin Pract 2010; 87: 64–68. [DOI] [PubMed] [Google Scholar]
- 51. Alkhatatbeh MJ, Abdalqader NA, Alqudah MAY. Impaired awareness of hypoglycaemia in insulin‐treated type 2 diabetes mellitus. Curr Diabetes Rev 2019; 15: 407–413. [DOI] [PubMed] [Google Scholar]
- 52. Heller SR, Cryer PE. Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after 1 episode of hypoglycemia in nondiabetic humans. Diabetes 1991; 40: 223–226. [DOI] [PubMed] [Google Scholar]
- 53. Davis SN, Mann S, Galassetti P, et al Effects of differing durations of antecedent hypoglycemia on counterregulatory responses to subsequent hypoglycemia in normal humans. Diabetes 2000; 49: 1897–1903. [DOI] [PubMed] [Google Scholar]
- 54. Davis SN, Shavers C, Mosqueda‐Garcia R, et al Effects of differing antecedent hypoglycemia on subsequent counterregulation in normal humans. Diabetes 1997; 46: 1328–1335. [DOI] [PubMed] [Google Scholar]
- 55. Jones TW, Porter P, Sherwin RS, et al Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med 1998; 338: 1657–1662. [DOI] [PubMed] [Google Scholar]
- 56. Banarer S, Cryer PE. Sleep‐related hypoglycemia‐associated autonomic failure in type 1 diabetes: reduced awakening from sleep during hypoglycemia. Diabetes 2003; 52: 1195–1203. [DOI] [PubMed] [Google Scholar]
- 57. Pohl J, Frenzel G, Kerner W, et al Acute stress modulates symptom awareness and hormonal counterregulation during insulin‐induced hypoglycemia in healthy individuals. Int J Behav Med 1998; 5: 89–105. [DOI] [PubMed] [Google Scholar]
- 58. Kerr D, Macdonald IA, Heller SR, et al Alcohol causes hypoglycaemic unawareness in healthy volunteers and patients with type 1 (insulin‐dependent) diabetes. Diabetologia 1990; 33: 216–221. [DOI] [PubMed] [Google Scholar]
- 59. Galassetti P, Mann S, Tate D, et al Effects of antecedent prolonged exercise on subsequent counterregulatory responses to hypoglycemia. Am J Physiol Endocrinol Metab 2001; 280: E908–E9017. [DOI] [PubMed] [Google Scholar]
- 60. Potashner D, Brown RE, Li A, et al Paradoxical rise in hypoglycemia symptoms with development of hyperglycemia during high‐intensity interval training in type 1 diabetes. Diabetes Care 2019; 42: 2011–2014. [DOI] [PubMed] [Google Scholar]
- 61. Ramanathan R, Cryer PE. Adrenergic mediation of hypoglycemia‐associated autonomic failure. Diabetes 2011; 60: 602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Farhat R, Su G, Sejling AS, et al Carvedilol prevents counterregulatory failure and impaired hypoglycaemia awareness in non‐diabetic recurrently hypoglycaemic rats. Diabetologia 2019; 62: 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cryer PE. Mechanisms of sympathoadrenal failure and hypoglycemia in diabetes. J Clin Invest 2006; 116: 1470–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cryer PE Hypoglycemia. Pathophysiology, Diagnosis and Treatment. New York: Oxford University Press, 1997. [Google Scholar]
- 65. Goldberg PA, Weiss R, McCrimmon RJ, et al Antecedent hypercortisolemia is not primarily responsible for generating hypoglycemia‐associated autonomic failure. Diabetes 2006; 55: 1121–1126. [DOI] [PubMed] [Google Scholar]
- 66. Dagogo‐Jack SE, Craft S, Cryer PE. Hypoglycemia‐associated autonomic failure in insulin‐dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest 1993; 91: 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chan O, Sherwin R. Influence of VMH fuel sensing on hypoglycemic responses. Trends Endocrinol Metab 2013; 24: 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mason GF, Petersen KF, Lebon V, et al Increased brain monocarboxylic acid transport and utilization in type 1 diabetes. Diabetes 2006; 55: 929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hedrington MS, Tate DB, Younk LM, et al Effects of antecedent GABA A receptor activation on counterregulatory responses to exercise in healthy man. Diabetes 2015; 64: 3253–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vele S, Milman S, Shamoon H, et al Opioid receptor blockade improves hypoglycemia‐associated autonomic failure in type 1 diabetes mellitus. J Clin Endocrinol Metab 2011; 96: 3424–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cranston I, Lomas J, Maran A, et al Restoration of hypoglycaemia awareness in patients with long‐duration insulin‐dependent diabetes. Lancet 1994; 344: 283–287. [DOI] [PubMed] [Google Scholar]
- 72. Fanelli C, Pampanelli S, Epifano L, et al Long‐term recovery from unawareness, deficient counterregulation and lack of cognitive dysfunction during hypoglycaemia, following institution of rational, intensive insulin therapy in IDDM. Diabetologia 1994; 37: 1265–1276. [DOI] [PubMed] [Google Scholar]
- 73. Dagogo‐Jack S, Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes 1994; 43: 1426–1434. [DOI] [PubMed] [Google Scholar]
- 74. Fritsche A, Stefan N, Haring H, et al Avoidance of hypoglycemia restores hypoglycemia awareness by increasing beta‐adrenergic sensitivity in type 1 diabetes. Ann Intern Med 2001; 134(9 Pt 1): 729–736. [DOI] [PubMed] [Google Scholar]
- 75. Cox D, Gonder‐Frederick L, Polonsky W, et al A multicenter evaluation of blood glucose awareness training‐II. Diabetes Care 1995; 18: 523–528. [DOI] [PubMed] [Google Scholar]
- 76. Hopkins D, Lawrence I, Mansell P, et al Improved biomedical and psychological outcomes 1 year after Structured Education In Flexible insulin therapy for people with type 1 diabetes: The U.K. DAFNE experience. Diabetes Care 2012; 35: 1638–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. de Zoysa N, Rogers H, Stadler M, et al A Psychoeducational program to restore hypoglycemia awareness: The DAFNE‐HART Pilot Study. Diabetes Care 2014; 37: 863–866. [DOI] [PubMed] [Google Scholar]
- 78. Spallone V, Ziegler D, Freeman R, et al Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 2011; 27: 639–653. [DOI] [PubMed] [Google Scholar]
- 79. Pop‐Busui R, Braffett BH, Zinman B, et al Cardiovascular autonomic neuropathy and cardiovascular outcomes in the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabetes Care 2017; 40: 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. O'Brien IA, McFadden JP, Corrall RJ. The influence of autonomic neuropathy on mortality in insulin‐dependent diabetes. Q J Med 1991; 79: 495–502. [PubMed] [Google Scholar]
- 81. Soedamah‐Muthu SS, Chaturvedi N, Witte DR, et al Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care 2008; 31: 1360–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pop‐Busui R, Evans GW, Gerstein HC, et al Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010; 33: 1578–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Maser RE, Mitchell BD, Vinik AI, et al The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta‐analysis. Diabetes Care 2003; 26: 1895–1901. [DOI] [PubMed] [Google Scholar]
- 84. Beijers HJ, Ferreira I, Bravenboer B, et al Microalbuminuria and cardiovascular autonomic dysfunction are independently associated with cardiovascular mortality: evidence for distinct pathways: the Hoorn Study. Diabetes Care 2009; 32: 1698–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pop‐Busui R, Low PA, Waberski BH, et al Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation 2009; 119: 2886–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nathan DM, Genuth S, Lachin J, et al The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 87. Pop‐Busui R. What do we know and we do not know about cardiovascular autonomic neuropathy in diabetes. J Cardiovasc Transl Res 2012; 5: 463–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hoeldtke RD, Boden G. Epinephrine secretion, hypoglycemia unawareness, and diabetic autonomic neuropathy. Ann Intern Med 1994; 120: 512–517. [DOI] [PubMed] [Google Scholar]
- 89. Meyer C, Grossmann R, Mitrakou A, et al Effects of autonomic neuropathy on counterregulation and awareness of hypoglycemia in type 1 diabetic patients. Diabetes Care 1998; 21: 1960–1966. [DOI] [PubMed] [Google Scholar]
- 90. Bottini P, Boschetti E, Pampanelli S, et al Contribution of autonomic neuropathy to reduced plasma adrenaline responses to hypoglycemia in IDDM: evidence for a nonselective defect. Diabetes 1997; 46: 814–823. [DOI] [PubMed] [Google Scholar]
- 91. Hepburn DA, Patrick AW, Eadington DW, et al Unawareness of hypoglycaemia in insulin‐treated diabetic patients: prevalence and relationship to autonomic neuropathy. Diabet Med 1990; 7: 711–717. [DOI] [PubMed] [Google Scholar]
- 92. Ryder RE, Owens DR, Hayes TM, et al Unawareness of hypoglycaemia and inadequate hypoglycaemic counterregulation: no causal relation with diabetic autonomic neuropathy. BMJ 1990; 301: 783–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Olsen SE, Bjorgaas MR, Asvold BO, et al Impaired awareness of hypoglycemia in adults with type 1 diabetes is not associated with autonomic dysfunction or peripheral neuropathy. Diabetes Care 2016; 39: 426–433. [DOI] [PubMed] [Google Scholar]
- 94. Jaiswal M, McKeon K, Comment N, et al Association between impaired cardiovascular autonomic function and hypoglycemia in patients with type 1 diabetes. Diabetes Care 2014; 37: 2616–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kennedy FP, Go VL, Cryer PE, et al Subnormal pancreatic polypeptide and epinephrine responses to insulin‐induced hypoglycemia identify patients with insulin‐dependent diabetes mellitus predisposed to develop overt autonomic neuropathy. Ann Intern Med 1988; 108: 54–58. [DOI] [PubMed] [Google Scholar]
- 96. Koivikko ML, Salmela PI, Airaksinen KE, et al Effects of sustained insulin‐induced hypoglycemia on cardiovascular autonomic regulation in type 1 diabetes. Diabetes 2005; 54: 744–750. [DOI] [PubMed] [Google Scholar]
- 97. Rao AD, Bonyhay I, Dankwa J, et al Baroreflex sensitivity impairment during hypoglycemia: implications for cardiovascular control. Diabetes 2016; 65: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Adler GK, Bonyhay I, Failing H, et al Antecedent hypoglycemia impairs autonomic cardiovascular function: implications for rigorous glycemic control. Diabetes 2009; 58: 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lee SP, Yeoh L, Harris ND, et al Influence of autonomic neuropathy on QTc interval lengthening during hypoglycemia in type 1 diabetes. Diabetes 2004; 53: 1535–1542. [DOI] [PubMed] [Google Scholar]
- 100. Limberg JK, Farni KE, Taylor JL, et al Autonomic control during acute hypoglycemia in type 1 diabetes mellitus. Clin Auton Res 2014; 24: 275–283. [DOI] [PubMed] [Google Scholar]
- 101. Ang L, Dillon B, Mizokami‐Stout K, et al Cardiovascular autonomic neuropathy: A silent killer with long reach. Auton Neurosci 2020; 225: 102646. [DOI] [PubMed] [Google Scholar]
- 102. Mohseni S, Hildebrand C. Hypoglycaemic neuropathy in BB/Wor rats treated with insulin implants: electron microscopic observations. Acta Neuropathol 1998; 96: 151–156. [DOI] [PubMed] [Google Scholar]
- 103. Potter CG, Sharma AK, Farber MO, et al Hypoglycemic neuropathy in experimental diabetes. J Neurol Sci 1988; 88(1–3): 293–301. [DOI] [PubMed] [Google Scholar]
- 104. Bernardi L, Rosengard‐Barlund M, Sandelin A, et al Short‐term oxygen administration restores blunted baroreflex sensitivity in patients with type 1 diabetes. Diabetologia 2011; 54: 2164–2173. [DOI] [PubMed] [Google Scholar]
- 105. Esposito P, Mereu R, De Barbieri G, et al Trained breathing‐induced oxygenation acutely reverses cardiovascular autonomic dysfunction in patients with type 2 diabetes and renal disease. Acta Diabetol 2016; 53: 217–226. [DOI] [PubMed] [Google Scholar]
- 106. Burger AJ, Weinrauch LA, D'Elia JA, et al Effect of glycemic control on heart rate variability in type I diabetic patients with cardiac autonomic neuropathy. Am J Cardiol 1999; 84: 687–691. [DOI] [PubMed] [Google Scholar]
- 107. Mohseni S. Hypoglycemic neuropathy. Acta Neuropathol 2001; 102: 413–421. [DOI] [PubMed] [Google Scholar]
- 108. Jensen VF, Molck AM, Bogh IB, et al Effect of insulin‐induced hypoglycaemia on the peripheral nervous system: focus on adaptive mechanisms, pathogenesis and histopathological changes. J Neuroendocrinol 2014; 26: 482–496. [DOI] [PubMed] [Google Scholar]
- 109. Gibbons CH, Freeman R. Treatment‐induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain 2014; 138: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gibbons CH, Freeman R. Treatment‐induced diabetic neuropathy: a reversible painful autonomic neuropathy. Ann Neurol 2010; 67: 534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bolli GB, De Feo P, De Cosmo S, et al A reliable and reproducible test for adequate glucose counterregulation in type I diabetes mellitus. Diabetes 1984; 33: 732–737. [DOI] [PubMed] [Google Scholar]
- 112. Deary IJ, Hepburn DA, MacLeod KM, et al Partitioning the symptoms of hypoglycaemia using multi‐sample confirmatory factor analysis. Diabetologia 1993; 36: 771–777. [DOI] [PubMed] [Google Scholar]
- 113. Towler DA, Havlin CE, Craft S, et al Mechanism of awareness of hypoglycemia: perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes 1993; 42: 1791–1798. [DOI] [PubMed] [Google Scholar]
- 114. Janssen MM, Snoek FJ, Heine RJ. Assessing impaired hypoglycemia awareness in type 1 diabetes: agreement of self‐report but not of field study data with the autonomic symptom threshold during experimental hypoglycemia. Diabetes Care 2000; 23: 529–532. [DOI] [PubMed] [Google Scholar]
- 115. Zenz S, Mader JK, Regittnig W, et al Impact of C‐Peptide Status on the Response of Glucagon and Endogenous Glucose Production to Induced Hypoglycemia in T1DM. J Clin Endocrinol Metab 2018; 103: 1408–1417. [DOI] [PubMed] [Google Scholar]
- 116. Hwang JJ, Parikh L, Lacadie C, et al Hypoglycemia unawareness in type 1 diabetes suppresses brain responses to hypoglycemia. J Clin Invest 2018; 128: 1485–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hirsch IB, Boyle PJ, Craft S, et al Higher glycemic thresholds for symptoms during beta‐adrenergic blockade in IDDM. Diabetes 1991; 40: 1177–1186. [DOI] [PubMed] [Google Scholar]
- 118. Hjemdahl P. Inter‐laboratory comparison of plasma catecholamine determinations using several different assays. Acta Physiol Scand Suppl 1984; 527: 43–54. [PubMed] [Google Scholar]
- 119. Speight J, Barendse SM, Singh H, et al Characterizing problematic hypoglycaemia: iterative design and preliminary psychometric validation of the Hypoglycaemia Awareness Questionnaire (HypoA‐Q). Diabet Med 2016; 33: 376–385. [DOI] [PubMed] [Google Scholar]
- 120. Graveling AJ, Frier BM. Impaired awareness of hypoglycaemia: a review. Diabetes Metab 2010; 36(Suppl 3): S64–S74. [DOI] [PubMed] [Google Scholar]
- 121. Pop‐Busui R, Boulton AJ, Feldman EL, et al Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017; 40: 136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Low PA, Benrud‐Larson LM, Sletten DM, et al Autonomic symptoms and diabetic neuropathy: a population‐based study. Diabetes Care 2004; 27: 2942–2947. [DOI] [PubMed] [Google Scholar]
- 123. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology . Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology 1996; 46:1470. [DOI] [PubMed] [Google Scholar]
- 124. Ang L, Cowdin N, Mizokami‐Stout K, et al Update on the management of diabetic neuropathy. Diabetes Spectr 2018; 31: 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996; 93: 1043–1065. [PubMed] [Google Scholar]
- 126. Pop‐Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care 2010; 33: 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bernardi L, Spallone V, Stevens M, et al Methods of investigation for cardiac autonomic dysfunction in human research studies. Diabetes Metab Res Rev 2011; 27: 654–664. [DOI] [PubMed] [Google Scholar]
- 128. Ziegler D, Keller J, Maier C, et al Diabetic neuropathy. Exp Clin Endocrinol Diabetes 2014; 122: 406–415. [DOI] [PubMed] [Google Scholar]
- 129. McGill JB, Ahmann A. Continuous glucose monitoring with multiple daily insulin treatment: outcome studies. Diabetes Technol Ther 2017; 19(S3): S3–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ly TT, Hewitt J, Davey RJ, et al Improving epinephrine responses in hypoglycemia unawareness with real‐time continuous glucose monitoring in adolescents with type 1 diabetes. Diabetes Care 2011; 34: 50–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Little SA, Leelarathna L, Walkinshaw E, et al Recovery of hypoglycemia awareness in long‐standing type 1 diabetes: a multicenter 2 x 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self‐monitoring (HypoCOMPaSS). Diabetes Care 2014; 37: 2114–2122. [DOI] [PubMed] [Google Scholar]
- 132. Leelarathna L, Little SA, Walkinshaw E, et al Restoration of self‐awareness of hypoglycemia in adults with long‐standing type 1 diabetes: hyperinsulinemic‐hypoglycemic clamp substudy results from the HypoCOMPaSS trial. Diabetes Care 2013; 36: 4063–4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. van Beers CA, DeVries JH, Kleijer SJ, et al Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open‐label, crossover trial. Lancet Diabetes Endocrinol 2016; 4: 893–902. [DOI] [PubMed] [Google Scholar]
- 134. Rickels MR, Peleckis AJ, Dalton‐Bakes C, et al Continuous glucose monitoring for hypoglycemia avoidance and glucose counterregulation in long‐standing type 1 diabetes. J Clin Endocrinol Metab 2018; 103: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Heinemann L, Freckmann G, Ehrmann D, et al Real‐time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet 2018; 391(10128): 1367–1377. [DOI] [PubMed] [Google Scholar]
- 136. Mancini G, Berioli MG, Santi E, et al Glucose monitoring: a review of the literature with a special focus on type 1 diabetes. Nutrients 2018; 10: 992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bolinder J, Antuna R, Geelhoed‐Duijvestijn P, et al Novel glucose‐sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non‐masked, randomised controlled trial. Lancet 2016; 388(10057): 2254–2263. [DOI] [PubMed] [Google Scholar]
- 138. Charleer S, De Block C, Van Huffel L, et al Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): a prospective observational real‐world cohort study. Diabetes Care 2020; 43: 389–397. [DOI] [PubMed] [Google Scholar]
- 139. Haak T, Hanaire H, Ajjan R, et al Flash glucose‐sensing technology as a replacement for blood glucose monitoring for the management of insulin‐treated type 2 diabetes: a multicenter. Open‐Label Randomized Controlled Trial. Diabetes Ther 2017; 8: 55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kroeger J, Fasching P, Hanaire H. 99‐LB: Meta‐analysis of three real‐world, chart review studies to determine the effectiveness of FreeStyle Libre Flash Glucose Monitoring System on HbA1c in adults with type 2 diabetes. Diabetes 2019; 68(Supplement 1): 99‐LB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Reddy M, Jugnee N, El Laboudi A, et al A randomized controlled pilot study of continuous glucose monitoring and flash glucose monitoring in people with Type 1 diabetes and impaired awareness of hypoglycaemia. Diabet Med 2018; 35: 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Fokkert MJ, van Dijk PR, Edens MA, et al Performance of the FreeStyle Libre Flash glucose monitoring system in patients with type 1 and 2 diabetes mellitus. BMJ Open Diabetes Res Care 2017; 5: e000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sato T, Oshima H, Nakata K, et al Accuracy of flash glucose monitoring in insulin‐treated patients with type 2 diabetes. J Diabetes Investig 2019; 10: 846–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Lin YK, Hung M, Sharma A, et al Impaired awareness of hypoglycemia continues to be a risk factor for severe hypoglycemia despite the use of continuous glucose monitoring system in type 1 diabetes. Endocr Pract 2019; 25: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Cook AJ, DuBose SN, Foster N, et al Cognitions associated with hypoglycemia awareness status and severe hypoglycemia experience in adults with type 1 diabetes. Diabetes Care 2019; 42: 1854–1864. [DOI] [PubMed] [Google Scholar]
- 146. Lin YK, Groat D, Chan O, et al Associations between the time in hypoglycemia and hypoglycemia awareness status in type 1 diabetes patients using continuous glucose monitoring systems. Diabetes Technol Ther 2020. 10.1089/dia.2020.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Battelino T, Danne T, Bergenstal RM, et al Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019; 42: 1593–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Shah VN, DuBose SN, Li Z, et al Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab 2019; 104: 4356–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Barnard‐Kelly KD, Polonsky WH. Development of a novel tool to support engagement with continuous glucose monitoring systems and optimize outcomes. J Diabetes Sci Technol 2020; 14: 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Lin YK, Groat D, Chan O, et al Alarm settings of continuous glucose monitoring systems and associations to glucose outcomes in type 1 diabetes. J Endocr Soc 2020; 4: bvz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Puhr S, Derdzinski M, Parker AS, et al Real‐world hypoglycemia avoidance with a predictive low glucose alert does not depend on frequent screen views. J Diabetes Sci Technol 2020; 14: 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]


