Abstract
Aims/Introduction
Obesity and metabolic syndrome are well‐known to be associated with multiple chronic diseases. Currently, high‐dose liraglutide has been used for weight control in non‐diabetic patients. Considering incretin‐based therapy is more effective in Asian populations, the effect of low‐dose liraglutide in weight control among these non‐diabetic groups has not been well evaluated. Our study aimed to evaluate the efficacy of low‐dose liraglutide in weight control among Taiwan patients.
Materials and Methods
From July 2017 to December 2018, 46 non‐diabetic patients with metabolic syndrome were included. They had received low‐dose liraglutide at 0.6 or 1.2 mg per day for weight reduction for 12 weeks. After then, changes in bodyweight, waist and metabolic factors were examined. Overt bodyweight reduction was defined as a decrease of >5% within 12 weeks.
Results
With 12 weeks of medication use, both groups showed statistical weight reduction. Higher doses of liraglutide had better efficacy, and 44.4% of patients in the liraglutide 1.2 mg group reached overt weight reduction, whereas just 32.1% in the 0.6 mg group had achieved this. Young age was found to be a predictor factor for a positive finding (odds ratio 0.941, P = 0.037). Early responders with decreased bodyweight of >4.2% within the first 4 weeks indicated a better chance to achieve measurable weight reduction.
Conclusions
Low‐dose liraglutide still has high efficacy in weight reduction in Taiwanese people, especially for those of younger age.
Keywords: Low‐dose liraglutide, Weight reduction, Taiwan
Low‐dose liraglutide and weight reduction

Introduction
Obesity and metabolic syndrome are well‐known as chronic diseases, and are associated with various diseases, including diabetes mellitus, cardiovascular disease and even cancer 1 , 2 , 3 . Obesity and metabolic syndrome could also increase 30% of the overall mortality rate by each 5 kg/m2 of the body mass index (BMI) 4 . The higher the BMI, the more the reduction in median survival. In patients with BMI 30–35 kg/m2, median survival is reduced by 2–4 years, whereas in patients with BMI 40–45 kg/m2, the reduction in survival is up to 8–10 years. Furthermore, obesity, metabolic syndrome and the associated diseases cause a huge economic burden for our National Health Insurance. Although we already know the harmful effects of obesity and metabolic syndrome in human health, the occurrence and severity of obesity and metabolic syndrome have still increased gradually. In Taiwan, the prevalence of morbid obesity (defined as BMI >39 kg/m2) has increased from 0.4 to 1.4%, and the prevalence of obesity (defined as BMI >27 kg/m2) has increased from 11.8 to 22.0% in the past 20 years 5 . Therefore, how to effectively control the occurrence and severity of obesity is an urgent national health issue.
Incretin‐based therapies have been widely used for type 2 diabetes control in recent years. They provide a glucose‐dependent insulinotropic effect with very low risk of hypoglycemia. Compare with white people, diabetes mellitus in Asian people is more likely as a result of β‐cell dysfunction. Diabetes mellitus in Asia also shows less adiposity and less insulin resistance 6 . By improving the function of β‐cells, incretin‐based therapies have been found to be more effective in Asian groups 7 .
Liraglutide, a glucagon‐like peptide‐1 receptor agonist, has been used for glycemic control in type 2 diabetes patients for years. Other than sound function in plasma glucose control and cardiac protection 8 , liraglutide has also shown an extra benefit in weight control by reducing appetite and partially by delaying gastric emptying 9 . Furthermore, high‐dose liraglutide of 3.0 mg per day could also help non‐diabetic patients with weight management 10 , 11 . Currently, high‐dose liraglutide is indicated for weight management in the USA and European Union.
Compared with the regular dosage of liraglutide for diabetes mellitus control in the USA and the European Union, relatively low doses of liraglutide of 0.3 mg per day as the starting dose and 1.8 mg per day as the maximum dose were selected in Japan, but remained at high efficacy levels in plasma glucose control 12 , 13 . Meanwhile, the efficacy of low‐dose liraglutide in weight reduction, especially in non‐diabetic patients, is still unclear.
In the present study, we aimed to evaluate the efficacy of low‐dose liraglutide in weight management among non‐diabetic patients. We also evaluated the improvement of biochemistry factors related to metabolic syndrome after 12 weeks of low‐dose liraglutide use. This is the first study to evaluate the efficacy of low‐dose liraglutide in weight control among non‐diabetic obesity patients in Taiwan.
Methods
We retrospectively reviewed non‐diabetic obese patients with metabolic syndrome who received low‐dose liraglutide for weight control at Chang Gung Memorial Hospital, Taoyuan City, Taiwan, between July 2017 and December 2018. They had received nutrition and exercise consultation in regular clinic follow up. All patients were aged >18 years with stable bodyweight. Exclusion criteria were type 1 or type 2 diabetes mellitus, endocrine diseases that cause obvious bodyweight change, history of exposure to medications that induced bodyweight change in the recent 3 months and family history of medullary thyroid carcinoma. The study was approved by the institutional review board of Chang Gung Memorial Hospital No. 201900085A3.
Metabolic syndrome was defined as more than three of the metabolic abnormalities mentioned below: waist >90 cm in men and 80 cm in women; systolic blood pressure >130 mmHg or diastolic blood pressure >85 mmHg; fasting plasma glucose level >100 mg/dL; high‐density lipoprotein <40 mg/dL in men and 50 mg/dL in women; and triglycerides >150 mg/dL. Obesity was defined as patients with BMI >27 kg/m2. The definition of metabolic syndrome and obesity were selected according to the Health Promotion Administration, Ministry of Health and Welfare, Taiwan.
All patients started medication therapy with liraglutide 0.6 mg per day and were rapidly titrated to 1.2 mg per day if there was no adequate suppression of appetite within the first week. After this, all patients continued to use a fixed dose (0.6 or 1.2 mg per day, respectively) to complete 12 weeks of therapy. In total, 46 patients were included in this study (Table 1), and were grouped according to the dosage of liraglutide (0.6 or 1.2 mg per day, respectively). Metabolic factors including bodyweight, waist, BMI, fasting plasma glucose level, glycated hemoglobin (HbA1c), serum insulin level, homeostatic model assessment for insulin resistance (HOMA‐IR), serum cholesterol profile, alanine aminotransferase and uric acid were followed at baseline and at 12 weeks of medication use. To evaluate the efficacy of liraglutide, we also corrected the dosage of medication according to body surface area 14 . Overt body change was defined as losing >5% of baseline bodyweight within 12 weeks according to the Food and Drug Administration’s guideline for weight loss medications 15 . The primary end‐point was to evaluate the efficacy of low‐dose liraglutide for weight control, with the secondary end‐point evaluating the improvement of aforementioned metabolic at 12 weeks of low‐dose liraglutide.
Table 1.
Demographic characteristics of non‐diabetic patients with metabolic syndromes and obesity using various dosages of liraglutide
| Liraglutide 0.6 mg (n = 28) | Liraglutide 1.2 mg (n = 18) | P‐value | |
|---|---|---|---|
| Sex (male), n (%) | 13 (46.4) | 11 (61.1) | 0.331 |
| Age (years) | 41.5 (29.0–47.0) | 35.5 (26.8–47) | 0.232 |
| Weight (kg) | 92.2 (80.7–109.8) | 94.5 (83.0–117.2) | 0.597 |
| BMI (kg/m2) | 34.5 (31.0–38.6) | 36.0 (31.2–40.1) | 0.512 |
| Waist circumference (cm) | 106.0 (101.5–130.0) | 105.0 (100.0–123.5) | 0.627 |
| Blood pressure (mmHg) | |||
| Systolic | 145.0 (129.8–163.3) | 140.0 (136.0–160.0) | 0.829 |
| Diastolic | 89.0 (83.8–93.8) | 93.0 (75.0–102.0) | 0.626 |
| Fasting glucose (mg/dL) | 88.0 (85.0–89.0) | 88.0 (82.0–95.0) | 0.871 |
| Glycated hemoglobin (%) | 6.1 (5.9–6.6) | 5.9 (5.6–6.2) | 0.108 |
| Insulin (μU/mL) | 16.2 (10.7–20.5) | 16.3 (12.3–26.0) | 0.773 |
| HOMA‐IR | 3.5 (2.4–4.5) | 3.9 (2.5–5.9) | 0.528 |
| Insulin : glucose ratio | 0.18 (0.11–0.24) | 0.18 (0.14–0.29) | 0.792 |
| Cholesterol (mg/dL) | |||
| Total | 176.0 (160.0–227.0) | 185.5 (169.8–208) | 0.954 |
| HDL cholesterol | 40.0 (36.8–48) | 39.0 (33.8–47.5) | 0.519 |
| LDL cholesterol | 122.5 (96.0;164.0) | 114.0 (103.0;128.0) | 0.426 |
| VLDL cholesterol | 27.0 (19.25–34.25) | 28.5 (21.75–45.0) | 0.314 |
| Non‐HDL cholesterol | 138.0 (118.8–188.5) | 140.0 (128.0–166.3) | 1.000 |
| Triglycerides (mg/dL) | 134.0 (92.0–186.0) | 145.0 (120.0–277.3) | 0.211 |
| Cr (mg/dL) | 0.80 (0.56–0.98) | 0.74 (0.61–0.91) | 0.902 |
| eGFR (mL/min/1.73 m2) | 99.0 (80.5–125.0) | 103.0 (89.5–121.0) | 0.663 |
| ALT (mg/dL) | 50.0 (24.0–80.0) | 47.0 (28.5–97.5) | 0.664 |
| HsCRP (mg/dL) | 6.67 (2.39–11.59) | 5.75 (3.12–10.86) | 0.059 |
| Uric acid (mg/dL) | 7.5 (6.3–8.7) | 7.2 (6.2–9.0) | 0.747 |
| Liraglutide dose per body surface (mg/m2) | 0.29 (0.26–0.31) | 0.57 (0.50–0.62) | <0.001* |
Values are presented as medians and interquartile ranges for continuous variables or n (%) for categorical variables. The Mann–Whitney U‐test was used for continuous variables; the χ2‐test was used for categorical variables.
P‐value < 0.05. ALT, alanine aminotransferase; BMI, body mass index; Cr, creatinine; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; HOMA‐IR, Homeostatic Model Assessment for Insulin Resistance; HsCRP, high sensitivity C‐reactive protein; LDL, low‐density lipoprotein; VLDL, very low‐density lipoprotein.
Statistical analysis
Demographic and clinical characteristics of the patients are presented as the percentage for categorical variables, and medians and interquartile ranges for continuous variables. The χ2‐test for categorical variables and the Mann–Whitney U‐test for continuous variables were used to examine the difference between the two groups of patients. The Wilcoxon signed‐rank test was used to evaluate the improvement of metabolic factors at 12 weeks of low‐dose liraglutide use. Predictive factors associated with overt weight loss at week 12 were examined by the binary logistic regression test. The receiver operating characteristic (ROC) curve and Youden’s index were carried out to determine the most predictive bodyweight change in those who showed bodyweight loss in the first 4 weeks. All data were analyzed by using the Statistical Package for Social Sciences (SPSS version 19; SPSS Inc., Chicago, IL, USA).
Results
Of the total 46 patients receiving low‐dose liraglutide, 28 patients received 0.6 mg liraglutide per day and 18 patients received 1.2 mg liraglutide per day (Table 1). The percentage of male patents was 52.2%, and total patients median age was 38.5 years. The median BMI of all patients was 35.2 kg/m2. A total of 27 patients suffered from hyperglycemia, and 32 patients suffered from dyslipidemia. All metabolic factors, including sex, age, bodyweight, waist, BMI, fasting plasma glucose level, HbA1c, serum insulin level, HOMA‐IR, serum cholesterol profile, alanine aminotransferase and uric acid showed no significant difference between the two groups. After correcting with body surface area, patients with liraglutide 0.6 mg had a statistically significant low dosage of medication use compared with patients with liraglutide 1.2 mg (0.29 vs 0.57 mg/m2, P < 0.001).
At 12 weeks of low‐dose liraglutide use, the two groups of patients both showed statistical significance in weight reduction (Table 2; Figure 1). Patients with liraglutide 0.6 mg showed relatively high effectiveness in weight reduction, but this was statistically insignificant compared with patients with liraglutide 1.2 mg (−6.4 vs −5.6%, P = 0.770). Patients with liraglutide 0.6 mg showed less overt weight reduction compared with patients with liraglutide 1.2 mg, but remained statistically insignificant (32.1 vs 44.4%, P = 0.474).
Table 2.
Changes in bodyweight and metabolic factors between baseline and week 12
| Liraglutide 0.6 mg (n = 28) | Liraglutide 1.2 mg (n = 18) | Liraglutide 0.6 mg vs liraglutide 1.2 mg | |||
|---|---|---|---|---|---|
| Change in 12 weeks | P‐value | Change in 12 weeks | P‐value | ||
| Change in bodyweight | |||||
| Percentage of bodyweight (%) | −6.4 | −5.6 | 0.710 | ||
| Kilograms of bodyweight | −7.0 | <0.001* | −6.0 | <0.001* | 0.830 |
| Loss of >5% of bodyweight, n (%) | 9 (32.1) | 8 (44.4) | 0.474 | ||
| Loss of >10% of bodyweight, n (%) | 4 (14.8) | 4 (22.2) | 0.488 | ||
| BMI (kg/m2) | −1.8 | <0.001* | −2.0 | 0.002* | 0.599 |
| Waist circumference (cm) | −4.2 | 0.000* | −6.4 | 0.008* | 0.326 |
| Fasting glucose (mg/dL) | 0.9 | 0.794 | −8.2 | 0.286 | 0.043** |
| Glycated hemoglobin (%) | −0.5 | 0.005* | −0.3 | 0.017* | 0.089 |
| Insulin (μU/mL) | −1.3 | 0.463 | −5.4 | 0.237 | 0.568 |
| HOMA‐IR | −0.2 | 0.463 | −1.7 | 0.128 | 0.253 |
| Insulin : glucose ratio | −0.02 | 0.500 | −0.02 | 0.893 | 0.854 |
| Cholesterol (mg/dL) | |||||
| Total | −5.6 | 0.538 | −26.0 | 0.289 | 0.506 |
| HDL cholesterol | 0.8 | 0.569 | −0.6 | 0.553 | 0.674 |
| LDL cholesterol | −2.4 | 0.485 | 2.2 | 0.875 | 0.710 |
| VLDL cholesterol | −1.0 | 0.182 | −1.3 | 0.735 | 0.463 |
| Non‐HDL cholesterol | −13.3 | 0.102 | 5.2 | 0.859 | 0.350 |
| Triglycerides (mg/dL) | −9.8 | 0.170 | −60.3 | 0.754 | 0.147 |
| ALT (mg/dL) | −25.2 | 0.001* | −25.8 | 0.136 | 0.388 |
| HsCRP (mg/dL) | −2.34 | 0.093 | −5.90 | 0.674 | 0.345 |
| Uric acid (mg/dL) | −1.4 | 0.002* | −0.4 | 0.041* | 0.147 |
Values are presented as the mean or n (%). The Mann–Whitney U‐test was used for continuous variables; the χ2‐test was used for categorical variables; the Wilcoxon signed‐rank test was used to compare bodyweight and metabolic factor between baseline and week 12.
P value <0.05 when comparing baseline metabolic factors with baseline.
P value <0.05 when comparing metabolic factors between liraglutide 0.6 mg and liraglutide 1.2 mg. ALT, alanine aminotransferase; BMI, body mass index; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic model assessment for insulin resistance; HsCRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; VLDL, very low‐density lipoprotein.
Figure 1.
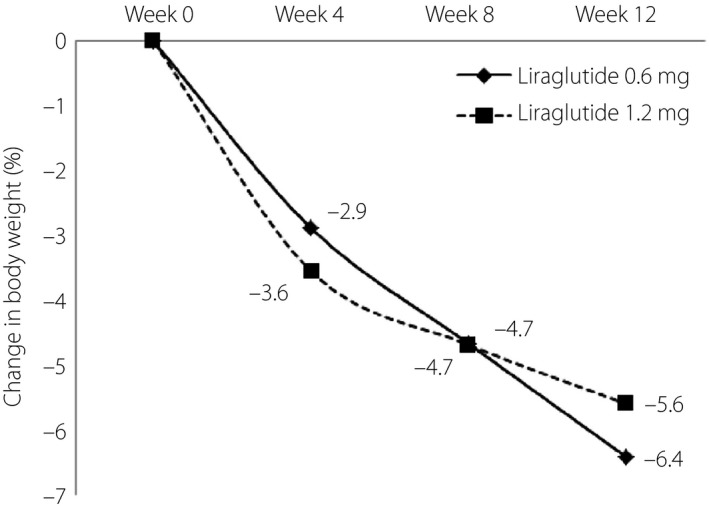
Liraglutide and weight reduction. The line graphs show the mean bodyweight change from baseline within 12 weeks of liraglutide use. Both liraglutide 0.6 and 1.2 mg per day showed statistical significant in weight reduction. An early response with rapid weight reduction in the first 4 weeks is observed.
Accompanying the reduction in bodyweight, waist circumference also showed a statistically significant reduction compared with baseline (−4.2 vs −6.7 cm). Liraglutide 0.6 mg showed a tendency for less effective waist circumference reduction compared with liraglutide 1.2 mg, but was still statistically insignificant (P = 0.326).
Patients receiving 12 weeks of low‐dose liraglutide showed an improvement in plasma glucose status. HbA1c showed a statistically significant improvement compared with baseline (−0.5 vs −0.3%). Fasting glucose, serum insulin level, HOMA‐IR, insulin and glucose ratio showed numerical improvement compared with baseline; furthermore, there were also improvements in the metabolic factors mentioned above. Patients showed improved alanine aminotransferase (−25.2 vs −25.8 mg/dL), high‐sensitivity C‐reactive protein (HsCRP; −2.34 vs −5.90 mg/dL), uric acid (−1.4 vs −0.4 mg/dL) and serum cholesterol profiles.
After evaluating the predictive factors for overt weight reduction, only age showed a significant association with weight reduction (odds ratio 0.941, 95% confidence interval 0.888–0.996, P = 0.037; Table 3). To detect the optimal cut‐off points to predict overt weight reduction in the first 4 weeks, ROC curve analysis and Youden’s index were applied. Decreased bodyweight with 4.2% from baseline showed a sensitivity of 73.3% and a specificity of 88.9%. The area under the ROC curve was 0.894 with 95% CI 0.797–0.991; P‐value < 0.001; and standard error 0.049 (Figure 2).
Table 3.
Predictor factors associated with overt weight loss at week 12
| Odds ratio (95% confidence interval) | P‐value | |
|---|---|---|
| Dosage of liraglutide | 1.689 (0.498–5.732) | 0.401 |
| Sex | 3.400 (0.947–12.210) | 0.061 |
| Age | 0.941 (0.888–0.996) | 0.037* |
| Weight | 1.020 (0.995–1.045) | 0.111 |
| BMI | 1.100 (0.994–1.217) | 0.065 |
| Waist circumference | 1.030 (0.986–1.076) | 0.190 |
| Fasting glucose | 0.964 (0.902–1.031) | 0.289 |
| Glycated hemoglobin | 0.609 (0.127–2.913) | 0.535 |
| Insulin | 0.990 (0.916–1.069) | 0.790 |
| HOMA‐IR | 0.944 (0.673–1.324) | 0.737 |
| Insulin : glucose ratio | 0.151 (0.000–190.953) | 0.604 |
| Total cholesterol | 0.992 (0.978–1.007) | 0.301 |
| HDL cholesterol | 0.984 (0.920–1.051) | 0.625 |
| LDL cholesterol | 0.989 (0.970–1.009) | 0.266 |
| VLDL cholesterol | 0.987 (0.938–1.039) | 0.621 |
| Non‐HDL cholesterol | 0.994 (0.981–1.007) | 0.368 |
| Triglycerides | 1.000 (0.996–1.004) | 0.875 |
| ALT | 1.008 (0.994–1.023) | 0.262 |
| HsCRP | 0.999 (0.944–1.058) | 0.974 |
| Uric acid | 1.085 (0.785–1.499) | 0.620 |
| Liraglutide dose per body surface | 1.397 (0.026, 76.429) | 0.870 |
The binary logistic regression test was used for odds ratio evaluation.
P value <0.05. ALT, alanine aminotransferase; BMI, body mass index; HDL, high‐density lipoprotein; HOMA‐IR, Homeostatic Model Assessment for Insulin Resistance; HsCRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; VLDL, very low‐density lipoprotein.
Figure 2.
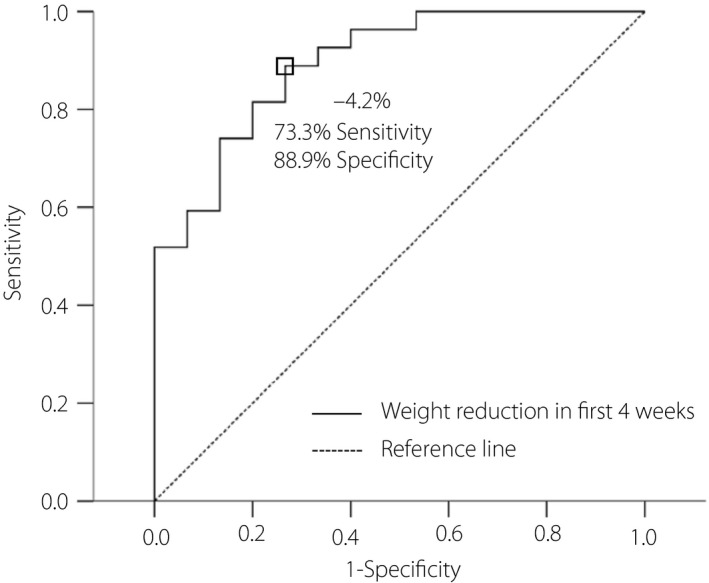
Receiver operating characteristic curve analysis was carried out by SPSS to determine the best cut‐off point to predict overt weight reduction in the first 4 weeks. The best discrimination point to predict overt weight reduction in the 4 weeks is determined by Youden’s index. Decreased bodyweight of 4.2% from baseline showed a sensitivity of 73.3% and a specificity of 88.9%. The area under the receiver operating characteristic curve was 0.894, with 95% confidence interval 0.797–0.991; P‐value < 0.001; standard error 0.049.
Discussion
Weight reduction by diet control and exercise is considered as first‐line therapy for metabolic syndrome 16 , 17 . For patients with hyperglycemia, lifestyle modification could decrease the relative risk of diabetes by 40–70% through improvement of insulin sensitivity and β‐cell function 18 . Furthermore, every 1‐kg decrease in bodyweight would reduce the risk of diabetes by 16% 19 . Even in patients already diagnosed as type 2 diabetes mellitus, adequate weight reduction could achieve remission of diabetes 20 . In a previous study, almost 50% of patients achieved diabetes remission by 12 months of diet control and related adequate weight reduction. Persistent remission from diabetes was observed at 24 months and was highly associated with sustained weight reduction 21 .
In the present study, patients had significant weight reduction with low‐dose liraglutide (−6.4% for liraglutide 0.6 mg and −5.6% for liraglutide 1.2 mg, respectively). A higher dosage of liraglutide showed better performance in weight control. Patients with liraglutide 1.2 mg per day had a higher chance of achieving overt weight reduction (32.1% for liraglutide 0.6 mg and 44.4% for liraglutide 1.2 mg). This finding is consistent with a previous study, where a higher dose of liraglutide had better efficacy in weight control. With liraglutide 3.0 mg per day for 56 weeks, up to 63.2% of patients had overt weight reduction 11 . A similar result was also observed after correcting the dosage of liraglutide in regard to body surface area. Patients with a higher dose of liraglutide per body surface area showed a higher chance of achieving overt weigh reduction (32.1% for 0.29 mg/m2 and 44.4% for 0.57 mg/m2). The same tendency was observed in a previous study, where high dose per body surface had better performance in weight reduction (52.1% for 0.57 mg/m2, 53.3% for 0.85 mg/m2, 60.8% for 1.12 mg/m2 and 76.1% for 1.41 mg/m2, respectively) 22 . However, the general population in Taiwan has relatively lower BMI compared with people in the USA 23 . As a result, the present study better reflects the real obese ethnic group in Taiwan; furthermore, the present results showed that low‐dose liraglutide has high efficacy in Taiwanese patients. This finding suggests that liraglutide should be administered with a more flexible dosage when used in Taiwan for weight control.
In additional to a good reduction in bodyweight, insulin resistance also numerically improved in the present patients. After 12 weeks of low‐dose liraglutide use, patients had significantly decreased HbA1c and improved serum insulin levels, HOMA‐IR, and insulin : glucose ratio. Obesity is one of the most important causes of insulin resistance, and induces generalized chronic inflammation 24 , 25 . In obese patients, adipose tissue becomes overnutritive and is under persistent metabolic stress. In this status, the accumulation of macrophages in adipose tissue is induced by multiple cytokines, adipokines and free fatty acids released by adipocytes. Adipose tissue could further act as an endocrine organ and induce insulin resistance not only in local tissue, but also in liver and muscles. Furthermore, obesity could also induce insulin resistance directly by the endoplasmic reticulum stress pathway 26 . Activation of c‐Jun N‐terminal kinase in adipose tissue, muscle and liver by obesity would influence serine phosphorylation of insulin receptor substrate‐1 and induced generalized insulin resistance. By control of obesity, insulin resistance could effectively be decreased and further prevent the occurrence of diabetes.
Metabolic syndrome is one of the most important risk factors of cardiovascular disease 27 , 28 and can cause cardiovascular disease through various mechanisms, including atherogenic dyslipidemia with elevated triglycerides, increased low‐density lipoprotein and low high‐density lipoprotein level, elevated blood pressure and glucose, prothrombotic status, and pro‐inflammatory status 29 . Because of its complex mechanisms, although weight control has been known to significantly improve the risk and outcome of cardiovascular disease 30 , 31 , 32 , more monitoring factors other than BMI are required to better reflect the outcome 33 . Clinically, we chose HsCRP as an observation factor, because it can represent a complex inflammatory response throughout the whole body, as caused by metabolic syndrome 34 . In the present study, after 12 weeks of low‐dose liraglutide, we not only observed a significant weight loss, but also saw numerical improvements in HsCRP and lipid profile. In addition, we also found an improvement in serum uric acid. Increased serum uric acid is also recognized as a risk factor of cardiovascular disease as a result of its association with hypertension, oxidative stress, chronic inflammation, endothelial dysfunction and carotid atherosclerosis 35 , 36 , 37 . This improvement in uric acid might not only come from differences in diet and lifestyle, but also from the effect of glucagon‐like peptide‐1 agonist by increasing absolute urinary excretion of uric acid through inhibiting Na+/H+ exchanger type 3 in the renal proximal tubules 38 . Overall, good improvement in all metabolic factors related to metabolic syndrome indicates that low‐dose liraglutide not only has a relatively high efficacy in weight reduction, but also a benefit in reducing the risk of cardiovascular disease.
In addition to the benefits caused by weight reduction, liraglutide has been proven to have direct protective effects in multiple organs, including the brain, pancreatic β‐cell function, liver and heart. Glucagon‐like peptide‐1 receptor in cerebral cortex 39 seems to have a protective effect from cerebral ischemia, Parkinson’s disease and even psychotic diseases through an anti‐apoptotic effect, neuronal renewal, anti‐oxidative effect, anti‐inflammatory effect, and limitation of β‐amyloid and neurofibrillary tangles accumulation 40 , 41 , 42 . The use of liraglutide could also improve liver function and histological features in patients with non‐alcoholic steatohepatitis 43 . Furthermore, liraglutide therapy could decrease the expression of β1‐adrenoreceptor expression in the heart and change the myocardial energy source from fatty acid to glucose 44 , resulting in improvement of cardiac function 45 . Low‐dose liraglutide is a safe, multiple beneficial and economical weight loss therapy.
In a previous study, for the use of liraglutide 3.0 mg per day, early response with weight loss of >4% at 16 weeks was the best predictive factor of weight loss for >5% at 56 weeks 46 . The present study found an even faster trend that weight loss of >4.2% was the best predictive factor of weight loss >5% after 12 weeks (sensitivity of 73.3% and specificity of 88.9%; area under ROC curve of 0.894). This finding suggests that clinicians can have faster assessments when using liraglutide for weight control. The present study also found that younger patients had a better chance of achieving overt weight reduction (odds ratio 0.941, 95% CI 0.888–0.996, P = 0.037). Lean body mass declines gradually as aging occurs 47 , with lean body mass gradually increasing until 30–40 years‐of‐age, and then decreases gradually after the late 40s 48 . Younger patients tend to have a better metabolic rate and daily performance, which contribute to a better chance achieving overt weight reduction. Currently, liraglutide has been approved for use in children with type 2 diabetes since 2019 49 . An increasing number of studies have shown that in childhood or even adolescence, liraglutide shows a good response in serum glucose and bodyweight 50 , 51 . Early use of liraglutide for weight control is safe and could reduce metabolic syndrome‐related chronic illness.
There were several limitations to the present study. First of all, after 12 weeks of liraglutide, patients had improved metabolic factors, including insulin resistance, waist line size, liver function and HsCRP, which indicated decreased risk in diabetes and cardiovascular disease. However, further examination including body composition measured by dual‐energy X‐ray densitometry and abdominal ultrasound examination might better represent the improvement in metabolic syndrome. Second, the sample size and follow‐up duration was relatively small. In the present study, although there was statistical insignificance between 0.6 and 1.2 mg in weight reduction (P = 0.770), patients taking liraglutide 0.6 mg showed less overt weight reduction compared with patients taking liraglutide 1.2 mg, but remained statistically insignificant (32.1 vs 44.4%, P = 0.474). An expanded sample size and prolonged follow‐up period might allow the difference between the two groups to become more statistically significant.
In conclusion, low dosage of liraglutide has relatively high efficacy in weight control among Taiwan populations, especially for those of younger age. For clinical use, an early response with weight reduction of >4.2% from baseline within the first 4 weeks is a predictive factor for a successful result.
Disclosure
The authors declare no conflict of interest.
J Diabetes Investig. 2020
References
- 1. Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol 2002; 3: 565–574. [DOI] [PubMed] [Google Scholar]
- 2. Isomaa B, Almgren P, Tuomi T, et al Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001; 24: 683–689. [DOI] [PubMed] [Google Scholar]
- 3. Haffner SM, Valdez RA, Hazuda HP, et al Prospective analysis of the insulin‐resistance syndrome (syndrome X). Diabetes 1992; 41: 715–722. [DOI] [PubMed] [Google Scholar]
- 4. Prospective Studies C, Whitlock G, Lewington S, et al Body‐mass index and cause‐specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373: 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang HC, Yang HC, Chang HY, et al Morbid obesity in Taiwan: prevalence, trends, associated social demographics, and lifestyle factors. PLoS One 2017; 12: e0169577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yabe D, Seino Y, Fukushima M, et al beta cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep 2015; 15: 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seino Y, Kuwata H, Yabe D. Incretin‐based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig 2016; 7(Suppl 1): 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marso SP, Daniels GH, Brown‐Frandsen K, et al Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Can J, Sloth B, Jensen CB, et al Effects of the once‐daily GLP‐1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non‐diabetic adults. Int J Obes 2014; 38: 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. le Roux CW, Astrup A, Fujioka K, et al 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double‐blind trial. Lancet 2017; 389: 1399–1409. [DOI] [PubMed] [Google Scholar]
- 11. Pi‐Sunyer X, Astrup A, Fujioka K, et al A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015; 373: 11–22. [DOI] [PubMed] [Google Scholar]
- 12. Ito D, Iuchi T, Kurihara S, et al Efficacy and clinical characteristics of liraglutide in japanese patients with type 2 diabetes. J Clin Med Res 2015; 7: 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seino Y, Kaneko S, Fukuda S, et al Combination therapy with liraglutide and insulin in Japanese patients with type 2 diabetes: a 36‐week, randomized, double‐blind, parallel‐group trial. J Diabetes Investig 2016; 7: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mosteller RD. Simplified calculation of body‐surface area. N Engl J Med 1987; 317: 1098. [DOI] [PubMed] [Google Scholar]
- 15. U.S. Department of Health and Human Services. Food and Drug Administration CfDEaRC . Guidance for Industry: Developing Products for Weight Management (Draft Guidance), 2007.
- 16. Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am 2014; 43: 1–23. [DOI] [PubMed] [Google Scholar]
- 17. Knowler WC, Barrett‐Connor E, Fowler SE, et al Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tabak AG, Herder C, Rathmann W, et al Prediabetes: a high‐risk state for diabetes development. Lancet 2012; 379: 2279–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamman RF, Wing RR, Edelstein SL, et al Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006; 29: 2102–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lean MEJ, Leslie WS, Barnes AC, et al Primary care‐led weight management for remission of type 2 diabetes (DiRECT): an open‐label, cluster‐randomised trial. Lancet 2018; 391: 541–551. [DOI] [PubMed] [Google Scholar]
- 21. Lean MEJ, Leslie WS, Barnes AC, et al Durability of a primary care‐led weight‐management intervention for remission of type 2 diabetes: 2‐year results of the DiRECT open‐label, cluster‐randomised trial. Lancet Diabetes Endocrinol 2019; 7: 344–355. [DOI] [PubMed] [Google Scholar]
- 22. Astrup A, Rossner S, Van Gaal L, et al Effects of liraglutide in the treatment of obesity: a randomised, double‐blind, placebo‐controlled study. Lancet 2009; 374: 1606–1616. [DOI] [PubMed] [Google Scholar]
- 23. Yeh CJ, Chang HY, Pan WH. Time trend of obesity, the metabolic syndrome and related dietary pattern in Taiwan: from NAHSIT 1993–1996 to NAHSIT 2005–2008. Asia Pac J Clin Nutr 2011; 20: 292–300. [PubMed] [Google Scholar]
- 24. de Luca C, Olefsky JM. Stressed out about obesity and insulin resistance. Nat Med 2006; 12: 41–42; discussion 2. [DOI] [PubMed] [Google Scholar]
- 25. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Investig 2005; 115: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ozcan U, Cao Q, Yilmaz E, et al Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004; 306: 457–461. [DOI] [PubMed] [Google Scholar]
- 27. Yusuf S, Joseph P, Rangarajan S, et al Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high‐income, middle‐income, and low‐income countries (PURE): a prospective cohort study. Lancet 2019; 395: 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mottillo S, Filion KB, Genest J, et al The metabolic syndrome and cardiovascular risk a systematic review and meta‐analysis. J Am Coll Cardiol 2010; 56: 1113–1132. [DOI] [PubMed] [Google Scholar]
- 29. Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 2004; 89: 2595–2600. [DOI] [PubMed] [Google Scholar]
- 30. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 2009; 53: 1925–1932. [DOI] [PubMed] [Google Scholar]
- 31. Sierra‐Johnson J, Romero‐Corral A, Somers VK, et al Prognostic importance of weight loss in patients with coronary heart disease regardless of initial body mass index. Eur J Cardiovasc Prev Rehabil 2008; 15: 336–340. [DOI] [PubMed] [Google Scholar]
- 32. Zhao Y, Yu BY, Liu Y, et al Weight reduction and cardiovascular benefits: protocol for a systematic review and meta‐analysis. Medicine 2018; 97: e13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xing Z, Tang L, Chen J, et al Association of predicted lean body mass and fat mass with cardiovascular events in patients with type 2 diabetes mellitus. CMAJ 2019; 191: E1042–E1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, et al C‐reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta‐analysis. Lancet 2010; 375: 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008; 359: 1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gagliardi AC, Miname MH, Santos RD. Uric acid: a marker of increased cardiovascular risk. Atherosclerosis 2009; 202: 11–17. [DOI] [PubMed] [Google Scholar]
- 37. Ishizaka N, Ishizaka Y, Toda E, et al Association between serum uric acid, metabolic syndrome, and carotid atherosclerosis in Japanese individuals. Arterioscler Thromb Vasc Biol 2005; 25: 1038–1044. [DOI] [PubMed] [Google Scholar]
- 38. Tonneijck L, Muskiet MHA, Smits MM, et al Effect of immediate and prolonged GLP‐1 receptor agonist administration on uric acid and kidney clearance: post‐hoc analyses of four clinical trials. Diabetes Obes Metab 2018; 20: 1235–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Farr OM, Sofopoulos M, Tsoukas MA, et al GLP‐1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP‐1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo‐controlled trial. Diabetologia 2016; 59: 954–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wicinski M, Socha M, Malinowski B, et al Liraglutide and its neuroprotective properties‐focus on possible biochemical mechanisms in Alzheimer's disease and cerebral ischemic events. Int J Mol Sci 2019; 20: 1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu W, Jalewa J, Sharma M, et al Neuroprotective effects of lixisenatide and liraglutide in the 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine mouse model of Parkinson's disease. Neuroscience 2015; 303: 42–50. [DOI] [PubMed] [Google Scholar]
- 42. Dixit TS, Sharma AN, Lucot JB, et al Antipsychotic‐like effect of GLP‐1 agonist liraglutide but not DPP‐IV inhibitor sitagliptin in mouse model for psychosis. Physiol Behav 2013; 115: 38–41. [DOI] [PubMed] [Google Scholar]
- 43. Eguchi Y, Kitajima Y, Hyogo H, et al Pilot study of liraglutide effects in non‐alcoholic steatohepatitis and non‐alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN‐J). Hepatol Res 2015; 45: 269–278. [DOI] [PubMed] [Google Scholar]
- 44. Hansen J, Brock B, Botker HE, et al Impact of glucagon‐like peptide‐1 on myocardial glucose metabolism revisited. Rev Endocr Metab Disord 2014; 15: 219–231. [DOI] [PubMed] [Google Scholar]
- 45. Sassoon DJ, Tune JD, Mather KJ, et al Glucagon‐like peptide 1 receptor activation augments cardiac output and improves cardiac efficiency in obese swine after myocardial infarction. Diabetes 2017; 66: 2230–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fujioka K, O'Neil PM, Davies M, et al Early Weight loss with liraglutide 3.0 mg predicts 1‐year weight loss and is associated with improvements in clinical markers. Obesity 2016; 24: 2278–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chumlea WC, Guo SS, Kuczmarski RJ, et al Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord 2002; 26: 1596–609. [DOI] [PubMed] [Google Scholar]
- 48. Junno JA, Niskanen M, Maijanen H, et al The effect of age and body composition on body mass estimation of males using the stature/bi‐iliac method. J Hum Evol 2018; 115: 122–129. [DOI] [PubMed] [Google Scholar]
- 49. Bacha F. FDA approval of GLP‐1 receptor agonist (liraglutide) for use in children. Lancet Child Adolescent Health 2019; 3: 595–597. [DOI] [PubMed] [Google Scholar]
- 50. Tamborlane WV, Barrientos‐Perez M, Fainberg U, et al Liraglutide in children and adolescents with type 2 diabetes. N Engl J Med 2019; 381: 637–646. [DOI] [PubMed] [Google Scholar]
- 51. Mastrandrea LD, Witten L, Carlsson Petri KC, et al Liraglutide effects in a paediatric (7–11 y) population with obesity: a randomized, double‐blind, placebo‐controlled, short‐term trial to assess safety, tolerability, pharmacokinetics, and pharmacodynamics. Pediatr Obes 2019; 14: e12495. [DOI] [PMC free article] [PubMed] [Google Scholar]


