Abstract
Aims/Introduction
Pregnant women with gestational diabetes mellitus (GDM) have been reported to have higher serum triglyceride (TG) levels during the entire gestational period. However, whether TGs contribute to the incidence of GDM remains unclear. This study aimed to evaluate whether higher serum TG level during early first trimester is associated with GDM.
Materials and Methods
A prospective single‐center cohort study was carried out among pregnant women (n = 2,949) who received regular antenatal care in Fu Xing Hospital, Capital Medical University, Beijing, China. GDM was diagnosed between 24 and 28 gestational weeks. Serum TG levels were measured during gestational weeks 6–8 (TG0) and 16–18 (TG1). TG elevation was the difference between TG1 and TG0.
Results
In total, 581 pregnant women developed GDM. A 13.1, 18.5 and 28.8% incidence of GDM was observed in women with low, referent and high TG0 levels, respectively. Among women with prepregnancy body mass index <24 kg/m2 and prepregnancy body mass index ≥24 kg/m2, those with high TG0 levels had 2.4‐ and 2.3‐fold increased odds of developing GDM, respectively, compared with pregnant women with low TG0 levels. A positive dose–response relationship was observed between continuous TG0 and TG elevation, and the odds of GDM; a positive association was observed between TG elevation and the odds of developing GDM in pregnant women with intermediate to high TG0 levels.
Conclusion
High TG level during the early first trimester, and TG elevation from the first to early second trimester are associated with GDM development, which persists even after adjusting for confounders.
Keywords: Gestational diabetes mellitus, Pregnancy, Triglyceride
This is the first study to evaluate the elevations in triglyceride from the early first trimester (weeks 6–8) to the early second trimester (weeks 16–18) in such a large sample. We observed the association between triglyceride and the odds of developing gestational diabetes mellitus, and confirmed this association in all the stratified prepregnancy body mass index groups. Based on our results, we recommend specialized management of pregnant women with high triglyceride levels during 6–8 weeks of gestation, which could help prevent high‐risk patients from developing gestational diabetes mellitus.
![]()
Introduction
Gestational diabetes mellitus (GDM) is a condition of glucose intolerance that occurs during pregnancy. The reported incidence of GDM varies from 3 to 25%, depending on the criteria and population studied 1 , 2 , 3 . In China, the incidence of GDM was 17%, when diagnosed according to the criterion issued by the International Association of Diabetes and Pregnancy Study Groups 4 . Pregnant women with GDM were reported to have higher risks of cesarean section, vaginal laceration, pre‐eclampsia or gestational hypertension, macrosomia, neonatal hypoglycemia, preterm delivery and other adverse pregnancy outcomes 5 . Higher fasting glucose level, prepregnancy overweight/obesity, prepregnancy body mass index (pre‐BMI) and family history of diabetes mellitus have previously been suggested as the risk factors for GDM 6 . The serum triglyceride (TG) level of pregnant women was reported to be associated with the incidence of GDM in both retrospective and prospective studies 7 , 8 . Dyslipidemia during gestation and its effects on pregnancy outcomes have attracted much attention in the past few years 9 , 10 .
To satisfy the nutritional demands of the developing fetus, maternal lipid metabolism, especially plasma TG level, is altered dramatically during pregnancy 11 . Researchers found that after an initial decrease during the early first trimester (5–8 weeks) 9 , 12 , 13 , the TG level increases linearly throughout pregnancy 14 . It was reported that TG levels in the second and third trimesters among pregnant women with GDM were higher than the corresponding levels among pregnant women without GDM 10 , 15 , 16 , 17 . Recently, a meta‐analysis confirmed that pregnant women with GDM continue to have high TG levels throughout pregnancy 18 . Some studies showed that higher maternal TG levels in early pregnancy contributed to the development of subsequent GDM 6 , 9 , 13 , 19 , 20 , 21 ; however, some other studies were unable to confirm these findings 10 , 14 , 16 , 17 . It was also reported that plasma TG concentrations did not differ between pregnant women with and without GDM 22 , 23 . Whether the increase of TG level is a risk factor for GDM was controversial. As the TG level increased with gestational age, the time of measurement of TG was important. We hypothesized that TG levels of pregnant women during gestational weeks 6–8 were independently associated with the development of GDM. To testify this hypothesis, pregnant women in the early first trimester (gestational weeks 6–8) were recruited to form a prospective cohort and were followed up until their GDM was diagnosed.
Methods
Patients
This prospective cohort study was carried out from July 2016 to June 2017. Pregnant women who attended regular antenatal care at and opted to give birth in the obstetric department of Fu Xing Hospital, Capital Medical University, Beijing, China, were recruited. This study was carried out according to the guidelines laid down in the Declaration of Helsinki, and it was approved by the ethics board of the Capital Medical University (approval number 2012sy29). Written informed consent was obtained from all participants. Women between 6 and 8 weeks’ gestation, aged >18 years, those with a singleton pregnancy, and those who made regular prenatal visits and opted to deliver in Fu Xing Hospital were recruited. Women with prepregnancy cardiovascular disease, chronic hypertension, prepregnancy diabetes, thyroid disorder, those taking medications known to affect glycemic and lipid metabolism, and those with twin pregnancy were excluded.
All participants were asked to complete a questionnaire with the help of clinicians. The demographic information, and data regarding pre‐pregnancy bodyweight (self‐reported), gravidity, parity, date of the last menstrual period, medical history, reproductive history, smoking status and alcohol use, and a family history of diabetes and hypertension were included in the questionnaire. During the follow‐up period, the bodyweight and blood pressure of the participants were measured, and any complications were recorded at each antenatal visit. Gestational age was calculated based on the last menstrual period and the first trimester ultrasound.
Pre‐BMI was calculated as the prepregnancy bodyweight (kg) divided by the square of the height (meters). Based on the recommendations of the China Obesity Task Force of the Chinese Ministry of Health, the pregnant women were divided into four groups, namely underweight (pre‐BMI <18.5 kg/m2), normal weight and overweight (pre‐BMI 18.5–23.9 and 24.0–27.9 kg/m2), and obese (pre‐BMI ≥28 kg/m2) 24 .
Biochemical analysis
Fasting (>8 h) venous blood samples were collected from all participants by trained nurses during gestational weeks 6–8 and 16–18. The samples were collected in a 3.5‐mL vacutainer (BD, Plymouth, UK). Gray vacuum blood vessels containing fluoride were used for detection of blood glucose levels, and SST II Advance Tubes containing inert separation glue were used for detection of lipid levels. Plasma samples were processed immediately after collection by centrifugation (351 g for 10 min at 4°C); 1‐mL aliquots were obtained and stored at −80°C until being assayed at the biochemical laboratory of Fu Xing Hospital. Both TG and glucose concentrations were measured using a Hitachi type 7180 automatic biochemical analyzer (Hitachi High‐Tech Science Systems Corporation, Tokyo, Japan) and monitored by a well‐trained examiner. The intra‐assay and interassay coefficients of variation in this analysis were ≤5 and ≤10%, respectively. TG elevation was calculated as the difference between TG1 (TG level at gestational weeks 16–18) and TG0 (TG level at gestational weeks 6–8).
Diagnosis of GDM
The International Association of Diabetes and Pregnancy Study Groups criteria were used for the diagnosis of GDM. The one‐step oral glucose tolerance test (75 g, 2 h) was carried out at 24–28 weeks of gestation. GDM was diagnosed when one or more glucose indices met or exceeded the following cut‐offs: fasting ≥5.1 mmol/L; 1 h ≥10.0 mmol/L; and 2 h ≥8.5 mmol/L, according to the International Association of Diabetes and Pregnancy Study Groups criteria.
Statistical analysis
The Kolmogorov–Smirnov (D test) was used to assess the normality of the data. Descriptive statistics included means and standard deviations for continuous variables as they are normally distributed, and numbers and percentages for categorical variables. Group comparisons were carried out using the χ2‐test or analysis of variance, as appropriate. Student’s t‐test was used to compare two continuous variables, one‐way anova was used to compare three continuous variables, and the χ2‐test was used to compare categorical variables. Multivariable logistic regression analysis was carried out to determine independent risk factors for the development of GDM. Furthermore, the relationship between TG and GDM in stratified pre‐BMI groups was examined. To estimate the relative association between GDM, and varying concentrations of TG and TG elevation, each participant was categorized according to Tukey’s hinges percentile analysis (<25th, 25–75th and ≥75th) of TG0 level and TG elevation. Restricted cubic spline regression analysis was used to analyze the dose–response relationship between continuous TG0 and TG elevation, and the odds of GDM. A P‐value <0.05 was considered statistically significant. SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA) was used for all analyses.
Results
In total, 3,028 women were initially recruited, out of which 15 withdrew and 64 were excluded because of missing results for TG level during 24–28 weeks of gestation. Finally, a total of 2,949 women were included, out of whom 581 had GDM and 2,368 did not have GDM (Table 1). Almost all women were of Han nationality. Women with GDM were older and had higher pre‐BMI, baseline fasting serum blood glucose (FBG), and TG0 levels, and TG elevation (P < 0.01).
Table 1.
Clinical and biochemical characteristics of pregnant women with and without gestational diabetes mellitus
| Characteristics | Control group (n = 2,368) | GDM (n = 581) | P‐value |
|---|---|---|---|
| Age (years) | 30.6 ± 3.8 | 31.9 ± 3.9 | <0.01 |
| Height (m) | 1.6 ± 0.5 | 1.6 ± 0.5 | 0.28 |
| Weight (kg) | 57.8 ± 8.8 | 62.0 ± 10.6 | <0.01 |
| Pre‐BMI (kg/m2) | 21.8 ± 3.0 | 23.3 ± 3.6 | <0.01 |
| Weight gain (kg) | 1.2 ± 2.4 | 1.3 ± 2.4 | 0.19 |
| Parity | 1.3 ± 0.5 | 1.4 ± 0.5 | 0.07 |
| FBG (mmol/L) | 4.7 ± 0.4 | 4.9 ± 0.5 | <0.01 |
| TG0 (mmol/L) | 1.0 ± 0.4 | 1.2 ± 0.6 | <0.01 |
| TG1 (mmol/L) | 1.7 ± 0.6 | 2.0 ± 0.7 | <0.01 |
| TG elevation (mmol/L) | 0.7 ± 0.5 | 0.8 ± 0.5 | <0.01 |
FBG, fasting blood glucose during gestational weeks 6–8; GDM, gestational diabetes mellitus; pre‐BMI, prepregnancy body mass index; TG elevation, change in triglyceride level from gestational weeks 6–8 to week 16–18; TG0, triglyceride level during gestational weeks 6–8; TG1, triglyceride level during gestational weeks 16–18; weight gain, change of weight from preconception to gestational weeks 16–18.
To analyze the relationship between TG0 and the incidence of GDM, the pregnant women were divided into three groups according to their TG0 levels, namely the lowest (<25th, TG0 <0.7 mmol/L), intermediate (25–75th, TG0 0.7–1.2 mmol/L) and highest percentile (≥75th, TG0 ≥1.2 mmol/L). The results showed that the incidence of GDM increased with the increase of TG0 levels; the incidence was 13.1, 18.5 and 28.8% in the lowest, intermediate and highest percentile of TG0 groups, respectively (P < 0.01; Figure 1).
Figure 1.
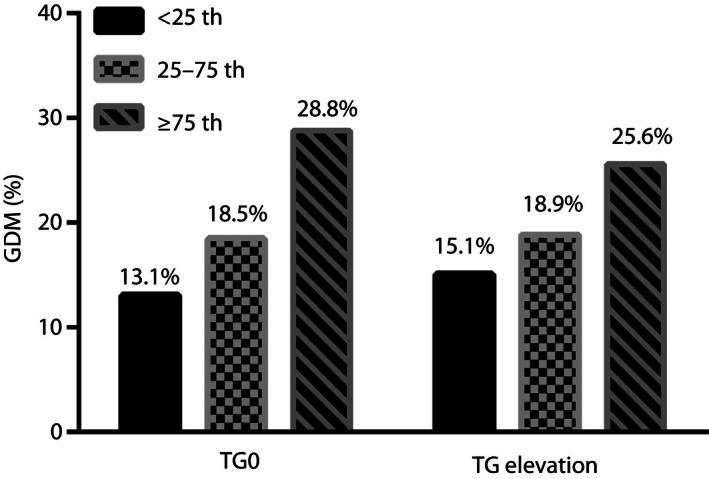
Incidence of gestational diabetes mellitus (GDM) according to Tukey’s hinges percentile analysis of triglyceride level in gestational weeks 6–8 (TG0) level and change in triglyceride level from gestational weeks 6–8 to weeks 16–18 (TG elevation). TG0 was divided into <0.7, 0.7–1.2 and ≥1.2 mmol/L groups, and TG elevation was divided into <0.4, 0.4–0.9 and ≥0.9 mmol/L groups according to Tukey’s hinges percentile analysis (<25, 25–75 and ≥75%).
TG elevation was calculated to assess the speed of TG elevation during the first to second trimester of gestation. Pregnant women were also divided into three groups based on the TG elevation (<0.4, 0.4–0.9 and ≥0.9 mmol/L), according to Tukey’s hinges percentile analysis (<25th, 25–75th and ≥75th), to evaluate the association between increased speed of TG elevation and the odds of developing GDM. As shown in Figure 1, the incidence of GDM increased with increase in TG elevation, to 15.1% in the lowest TG elevation group, 18.9% in the intermediate TG elevation group and 25.7% in the highest TG elevation group (P < 0.01).
Forward stepwise logistic regression was used, and the results showed that both TG0 and TG elevation were associated with higher odds of developing GDM after adjusting for age, pre‐BMI, FBG and a family history of diabetes mellitus (Figure 2). Compared with the lowest quartile (<25th), pregnant women with intermediate (25–75th percentile; adjusted odds ration [aOR] 1.38, 95% confidence interval [CI] 1.06–1.80) and highest (aOR 2.24, 95% CI 1.66–3.04) TG0 levels had increased odds of developing GDM. Elevation in TG was also associated with GDM. The aOR was 1.48 (95% CI 1.14–1.94) and 1.88 (95% CI 1.42–2.50) for pregnant women with intermediate and highest TG elevation, respectively.
Figure 2.
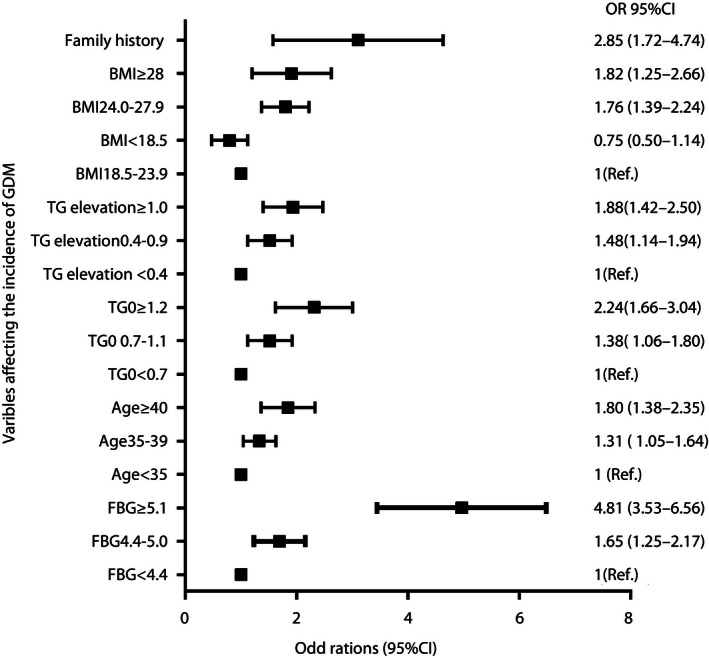
Odds ratios of fasting blood glucose in gestational weeks 6–8 (FBG), age, triglyceride level in gestational weeks 6–8 (TG0), change in triglyceride level from gestational weeks 6–8 weeks to 16–18 (TG elevation), prepregnancy body mass index (pre‐BMI) and family history as categorical variables associated with the occurrence of gestational diabetes mellitus (GDM). CI, confidence interval; Ref., reference.
Table 2 shows the results of a binary logistic regression analysis of the odds of developing GDM stratified by pre‐BMI according to the Chinese criterion. The odds of developing GDM increased with the increase of TG, both in women with normal bodyweight and those in the overweight and obesity categories. Compared with women with the lowest TG0 levels (<0.7 mmol/L), those with the highest TG0 values had 2.4‐fold (95% CI 1.65–3.36) increased odds of developing GDM in the pre‐BMI <24 kg/m2 (normal group), and a 2.3‐fold (95% CI 1.25–4.31) increased odds of developing GDM in the pre‐BMI ≥24 kg/m2 (overweight/obesity categories) groups. Similarly, 1.9‐fold increased odds of developing of GDM were observed in women with the highest TG elevation (≥0.9 mmol/L), both in normal bodyweight and overweight/obesity categories, as compared with their peers with the lowest TG elevation (<0.4 mmol/L).
Table 2.
Binary logistic regression analysis of risk of gestational diabetes mellitus stratified by prepregnancy body mass index according to the Chinese criterion
| Variable | Pre‐BMI <24 kg/m2 (n = 2,280) | Pre‐BMI ≥24 kg/m2 (n = 669) | ||||||
|---|---|---|---|---|---|---|---|---|
| B | Wald | OR (95% CI) | P‐value | B | Wald | OR (95% CI) | P‐value | |
| TG0 (mmol/L) | 22.87 | <0.01 | 11.27 | <0.01 | ||||
| <0.7 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 0.7–1.1 | 0.35 | 5.41 | 1.42 (1.06–1.91) | <0.01 | 0.29 | 0.89 | 1.34 (0.73–2.47) | 0.37 |
| ≥1.2 | 0.86 | 22.39 | 2.35 (1.65–3.36) | <0.01 | 0.84 | 7.16 | 2.32 (1.25–4.31) | <0.01 |
| TG elevation (mmol/L) | 14.17 | 0.01 | 6.97 | 0.03 | ||||
| <0.4 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 0.4–0.9 | 0.42 | 6.70 | 1.52 (1.11–2.08) | <0.01 | 0.38 | 2.32 | 1.47 (0.90–2.41) | 0.13 |
| ≥1.0 | 0.65 | 14.09 | 1.92 (1.37–2.70) | <0.01 | 0.68 | 6.83 | 1.98 (1.19–3.30) | <0.01 |
CI, confidence interval; OR, odds ratio; Pre‐BMI, prepregnancy body mass index; TG elevation, change in triglyceride level from gestational weeks 6–8 to weeks 16–18; TG0, triglyceride level during gestational weeks 6–8.
Table 3 shows the effect of TG elevation on the incidence of GDM on three different ranks of TG0 levels. In the intermediate TG0 (0.7–1.2 mmol/L) group, after adjustment for confounding factors including age, FBG, family history of diabetes and TG0, both women with intermediate TG elevation (0.4–0.9 mmol/L) and high TG elevation (≥0.9 mmol/L) had 1.8‐fold increased odds of developing GDM, compared with their counterparts with the lowest TG elevation (<0.4 mmol/L). Meanwhile, in the high TG0 (≥1.2 mmol/L) group, increased odds of developing GDM were observed in women with the highest TG elevation (≥0.9 mmol/L), aOR 2.03 (95% CI 1.31–3.14), compared with women with the lowest TG elevation. However, in the low TG0 (<0.7 mmol/L) group, after adjustment for age, FBG, family history of diabetes mellitus and TG0, TG elevation showed no association with the odds of developing GDM.
Table 3.
Binary logistic regression analysis of risk of gestational diabetes mellitus stratified by triglyceride level during gestational weeks 6–8
| Variable (mmol/L) | TG0 <0.7 mmol/L (n = 725) | 0.7 ≥ TG0 < 1.2 mmol/L (n = 1,503) | TG0 ≥1.2 mmol/L (n = 721) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Wald | OR (95% CI) | P‐value | B | Wald | OR (95% CI) | P‐value | B | Wald | OR (95% CI) | P‐value | |
| TG elevation | 4.54 | 0.10 | 8.41 | 0.02 | 10.51 | <0.01 | ||||||
| <0.4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| 0.4–0.9 | −0.64 | 2.89 | 0.53 (0.25–1.10) | 0.09 | 0.59 | 7.72 | 1.80 (1.19–2.72) | <0.01 | 0.22 | 0.98 | 1.24 (0.81–1.92) | 0.32 |
| ≥1.0 | −0.50 | 3.65 | 0.61 (0.37–1.01) | 0.06 | 0.60 | 6.69 | 1.82 (1.16–2.86) | <0.01 | 0.71 | 10.07 | 2.03 (1.31–3.14) | <0.01 |
CI, confidence interval; OR, odds ratio; TG elevation, change in triglyceride level from gestational weeks 6–8 to weeks 16–18; TG0, triglyceride level during gestational weeks 6–8.
Using TG0 equal to 1.0 mmol/L or TG elevation equal to 0.5 mmol/L groups as the reference, restricted cubic spline regression helped to analyze the dose–response relationship between continuous TG0 and TG elevation, and the odds of developing GDM (Figure 3), the ratios of which were estimated by logistic regression modeling, after adjusting for maternal age, pre‐BMI and family history of diabetes mellitus. Based on the TG0 and TG elevation outcome association trajectory, a significant non‐linear dose–response relationship was observed between GDM and the continuous TG0 and TG elevation (Figure 3).
Figure 3.
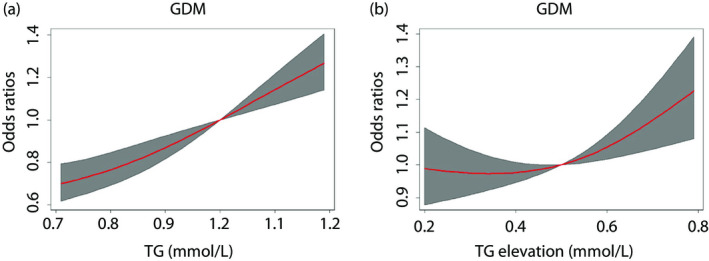
Odds of developing gestational diabetes mellitus (GDM) according to TG0, triglyceride level during gestational weeks 6–8 (TG0) and change in triglyceride level from gestational weeks 6–8 to weeks 16–18 (TG elevation) as continuous variables. Gray areas are 95% confidence intervals. Odds ratios were estimated using logistic regression modeling, after adjusting for maternal age, prepregnancy body mass index (pre‐BMI) and a family history of diabetes mellitus.
Discussion
The present study showed that TG level in the early first trimester was associated with the development of GDM in pregnant women with normal bodyweight, as well as in those with overweight/obesity, adding new evidence to the association between TG and GDM, which was consistent with the previous literature 6 . High TG elevation from the first to early second trimester predicted high odds of developing GDM, especially in pregnant women with a high TG level during early pregnancy, which was independent of the influence of FBG, maternal pre‐BMI, age or a family history of diabetes mellitus.
The results showed that patients with GDM had a higher TG level from gestational weeks 6–8, which afforded high odds of developing GDM after adjusting for confounders. It was reported that pregnant women with GDM had significantly higher preconception TG levels 25 , indicating that high serum TG levels in the first trimester should be considered as a risk factor for GDM. Lipid abnormalities serve as an inducing factor for insulin resistance 26 , because the excess TG storage, particularly in skeletal muscles, might contribute to increasing insulin resistance 27 , which is the main pathophysiology of type II diabetes mellitus and GDM. As there is little impact of gestational hormonal changes on TG metabolism during gestational weeks 5–8, pre‐existing insulin resistance and high TG level might be the main causes of high TG levels during this period. Therefore, TG levels should be monitored as early as possible during pregnancy. Furthermore, Nolan et al. 14 found a stronger association between TG levels measured early in gestation (weeks 5–8) and GDM in Asian women, but not in Australian/Western European women. The present study carried out in the Chinese population re‐testified the association between early TG and the odds of developing GDM. These results speculate that ethnicity might be a confounding factor.
The risk factors for GDM, including maternal age, pre‐BMI and a family history of diabetes, are well known. Besides these traditional risk factors, among Chinese women 28 , a history of spontaneous abortion was also reported as a risk factor for GDM. A recent review 29 suggested the association between hypothyroidism, polycystic ovary syndrome and the odds of developing GDM. In the present study, maternal age, pre‐BMI and a family history of diabetes mellitus were considered as confounders when the risk of TG for GDM was analyzed using logistic regression analysis. Although maternal age and pre‐BMI might be related to an increase in TG levels, which, in turn, might be the consequence of higher pre‐BMI, we put these factors together to calculate the aORs after the collinearity test.
Higher TG level was often observed before and during gestation in obese pregnant women 30 . To eliminate the effect of pre‐BMI on GDM, we stratified the pre‐BMI into pre‐BMI <24 kg/m2 and pre‐BMI ≥24 kg/m2 groups according to the Chinese criterion, and testified the association between TG and GDM in both groups. Although Maitland et al. 31 found no significant difference of TG levels measured in the early second trimester between pregnant women with and without GDM, most of the studies showed a positive association between TG and GDM after adjusting for confounding factors, including pre‐BMI. 10 , 13 , 20 . Based on these results, high TG levels in the early first trimester (gestational weeks 6–8) should be considered as an independent risk factor for GDM in all pregnant women, irrespective of their bodyweight.
Furthermore, as observed in the present study, higher TG elevation was a risk factor for GDM after adjusting for confounders. Although Ryckman et al. 18 showed that TG was elevated, with significant difference in the levels between women with and without GDM from the early trimester to the second trimester when GDM was traditionally diagnosed during gestational weeks 24–28, to the best of our knowledge, few studies have evaluated the association between early TG elevation and the odds of developing GDM. Shen et al. 21 found that high TG elevation before 28 weeks of gestation was associated with higher odds of developing GDM. In the current study, the significant difference in TG elevation from gestational weeks 6–8 to gestational weeks 16–18 was much earlier than that observed in previous studies. Shen et al. 21 showed that TG elevation during gestation was associated with bodyweight gain in the second trimester. The discovery of hyperlipidemia in early pregnancy might provide more opportunities for reducing the odds of developing GDM by reducing TG elevation through lifestyle interventions.
In pregnant women with low TG0 level (<0.7 mmol/L), high TG elevation was not associated with the development of GDM. As high TG level was a protective factor for small‐for‐gestational‐age infants 15 , women with very low TG level at the beginning of gestation might require TG elevation to satisfy the development of the growing fetus. The present study shows that serum TG level and TG elevation should be addressed to reduce the likelihood of a diagnosis of GDM.
In summary, high TG level and TG elevation should be recognized as early as in the first trimester. Early measurement of TG levels and provision of individualized nutritional guidance from the first to the early second trimester of pregnancy are recommended for the prevention of GDM.
The present study had some limitations. The diet records of all participants were unavailable. As TG level is affected greatly by nutrition, the impact of the energy and the composition of diet on TG level should have been analyzed. Another limitation of the study was the self‐reported pre‐BMI data. In Beijing, all women utilize community health services when trying to become pregnant, and learn how to measure their own weight and height. They visit the Obstetrics Clinic only after they become pregnant. Although we were unable to check the pre‐BMI of the participants in person, the self‐reported data, under the supervision of community care workers, were generally reliable.
To the best of our knowledge, this is the first study to evaluate the elevations in TG from the early first trimester (weeks 6–8) to the early second trimester (weeks 16–18) in such a large sample. We observed the association between TG and the odds of developing GDM, and confirmed this association in all the stratified pre‐BMI groups. However, it is important to consider that the study population included only Chinese women. It is possible that the association between early‐pregnancy TG levels and the odds of developing GDM varies between different ethnic groups, being particularly strong in Chinese women. Based on the present results, we recommend specialized management of pregnant women with high TG levels during the first 6–8 weeks of gestation, which could help prevent high‐risk patients from developing GDM.
Disclosure
There authors declare no conflict of interest.
Acknowledgments
We thank all the pregnant women who participated in this study. We thank the Editor and the reviewers for their suggestions and comments, which helped to improve the quality of this manuscript. This project was supported by Beijing Natural Science Foundation Program and Scientific Research Key Program of Beijing Municipal Commission of Education (KZ201710025018), a combination of the basic and clinical fund from Capital Medical University Fund (17JL87), and by The National Key Research and Development Program of China (2016YFC1000105). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
J Diabetes Investig. 2020
References
- 1. Listed N. National Institutes of Health consensus development conference statement: diagnosing gestational diabetes mellitus, March 4–6, 2013. Obstet Gynecol 2013; 122: 358–369. [DOI] [PubMed] [Google Scholar]
- 2. Farrar D, Fairley L, Santorelli G, et al Association between hyperglycaemia and adverse perinatal outcomes in south Asian and white British women: analysis of data from the Born in Bradford cohort. Lancet Diabetes Endocrinol 2015; 3: 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farrar D, Simmonds M, Griffin S, et al The identification and treatment of women with hyperglycaemia in pregnancy: an analysis of individual participant data, systematic reviews, meta‐analyses and an economic evaluation. Health Technol Assess 2016; 20: 1–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu WW, Yang HX, Wei YM, et al Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in china. Diabetes Care 2013; 36: 586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Denney JM, Quinn KH. Gestational diabetes: underpinning principles, surveillance, and management. Obstet Gynecol Clin North Am 2018; 45: 299–314. [DOI] [PubMed] [Google Scholar]
- 6. Chen W, Zhu W, Wei Y, et al The predictive effects of early pregnancy lipid profiles and fasting glucose on the risk of gestational diabetes mellitus stratified by body mass index. J Diabetes Res 2016; 2016: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang C, Zhu W, Wei Y, et al The associations between early pregnancy lipid profiles and pregnancy outcomes. J Perinatol 2017; 37: 127–133. [DOI] [PubMed] [Google Scholar]
- 8. Pazhohan A, Rezaee MM, Pazhohan N. Association of first‐trimester maternal lipid profiles and triglyceride‐glucose index with the risk of gestational diabetes mellitus and large for gestational age newborn. J Matern Fetal Neonatal Med 2019; 32: 1167–1175. [DOI] [PubMed] [Google Scholar]
- 9. Wiznitzer A, Mayer A, Novack V, et al Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: a population‐based study. Am J Obstet Gynecol 2009; 201: 482.e1–482.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sanchez‐Vera I, Bonet B, Viana M, et al Changes in plasma lipids and increased low‐density lipoprotein susceptibility to oxidation in pregnancies complicated by gestational diabetes: consequences of obesity. Metabolism 2007; 56: 1527–1533. [DOI] [PubMed] [Google Scholar]
- 11. Herrera E, Ortega‐Senovilla H. Disturbances in lipid metabolism in diabetic pregnancy ‐ Are these the cause of the problem? Best Pract Res Clin Endocrinol Metab 2010; 24: 515. [DOI] [PubMed] [Google Scholar]
- 12. Mankuta D, Elami‐Suzin M, Elhayani A, et al Lipid profile in consecutive pregnancies. Lipids Health Dis 2010; 9: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bao W, Dar S, Zhu Y, et al Plasma concentrations of lipids during pregnancy and the risk of gestational diabetes mellitus: a longitudinal study. J Diabetes 2018; 10: 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nolan CJ, Riley SF, Sheedy MT, et al Maternal serum triglyceride, glucose tolerance, and neonatal birth weight ratio in pregnancy. Diabetes Care 1995; 18: 1550. [DOI] [PubMed] [Google Scholar]
- 15. Jin WY, Lin SL, Hou RL, et al Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population‐based study from China. BMC Pregnancy Childbirth 2016; 16: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Montelongo A, Lasunción MA, Pallardo LF, et al Longitudinal study of plasma lipoproteins and hormones during pregnancy in normal and diabetic women. Diabetes 1992; 41: 1651. [DOI] [PubMed] [Google Scholar]
- 17. Paradisi G, Ianniello F, Tomei C, et al Longitudinal changes of adiponectin, carbohydrate and lipid metabolism in pregnant women at high risk for gestational diabetes. Gynecol Endocrinol 2010; 26: 539–545. [DOI] [PubMed] [Google Scholar]
- 18. Ryckman KK, Spracklen CN, Smith CJ, et al Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta‐analysis. BJOG 2015; 122: 643–651. [DOI] [PubMed] [Google Scholar]
- 19. Enquobahrie DA, Williams MA, Qiu C, et al Early pregnancy lipid concentrations and the risk of gestational diabetes mellitus. Diabetes Res Clinical Pract 2005; 70: 134–142. [DOI] [PubMed] [Google Scholar]
- 20. Li G, Kong L, Zhang L, et al Early pregnancy maternal lipid profiles and the risk of gestational diabetes mellitus stratified for body mass index. Reprod Sci 2015; 22: 712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shen H, Liu X, Chen Y, et al Associations of lipid levels during gestation with hypertensive disorders of pregnancy and gestational diabetes mellitus: a prospective longitudinal cohort study. BMJ Open 2016; 6: e013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rizzo M, Berneis K, Altinova AE, et al Atherogenic lipoprotein phenotype and LDL size and subclasses in women with gestational diabetes. Diabet Med 2008; 25: 1406–1411. [DOI] [PubMed] [Google Scholar]
- 23. Emet T, Ustuner I, Guven SG, et al Plasma lipids and lipoproteins during pregnancy and related pregnancy outcomes. Arch Gynecol Obstet 2013; 288: 49–55. [DOI] [PubMed] [Google Scholar]
- 24. Zhou B. Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Chinese Journal of Epidemiology 2002; 23: 5–10. [PubMed] [Google Scholar]
- 25. Baumfeld Y, Novack L, Wiznitzer A, et al Pre‐Conception dyslipidemia is associated with development of preeclampsia and gestational diabetes mellitus. PLoS ONE 2015; 10: e0139164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galbo T, Shulman GI. Lipid‐induced hepatic insulin resistance. Aging 2013; 5: 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kelley D, Goodpaster B. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care 2001; 24: 933. [DOI] [PubMed] [Google Scholar]
- 28. Yang H, Wei Y, Gao X, et al Risk factors for gestational diabetes mellitus in Chinese women—a prospective study of 16, 286 pregnant women in China. Diabet Med 2009; 26: 1099–1104. [DOI] [PubMed] [Google Scholar]
- 29. Giannakou K, Evangelou E, Yiallouros P, et al Risk factors for gestational diabetes: an umbrella review of meta‐analyses of observational studies. PLoS ONE 2019; 14: e0215372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alvarez JJ, Montelongo A, Iglesias A, et al Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. J Lipid Res 1996; 37: 299–308. [PubMed] [Google Scholar]
- 31. Maitland RA, Seed PT, Briley AL, et al Prediction of gestational diabetes in obese pregnant women from the UK Pregnancies Better Eating and Activity (UPBEAT) pilot trial. Diabet Med 2014; 31: 963–970. [DOI] [PubMed] [Google Scholar]


