Abstract
Aims/Introduction
To carry out a cross‐sectional single‐center study in a Japanese hospital to determine the diagnostic value of handgrip measurement to detect locomotive syndrome (LS).
Materials and Methods
Consecutive outpatients underwent an LS risk test, which comprised a stand‐up test and a two‐step test, and a handgrip measurement, along with general diabetes‐related tests. We calculated the prevalence of LS, and evaluated the association between handgrip strength and LS.
Results
We enrolled 234 patients in this study. The prevalence of LS in the stand‐up and two‐step tests was 51.5 and 79.0%, respectively. The prevalence of LS in the stand‐up or two‐step tests increased with age both in men and women. Using the stand‐up and two‐step tests, 107 patients (46.7%) were diagnosed with LS. The area under the receiver operating characteristic curve, used to assess our identification of LS in terms of grip strength in men and women, showed 95% confidence intervals of 0.703 (0.563–0.813) and 0.698 (0.500–0.842), respectively. The odds ratios of grip strength for LS were 0.90 (95% confidence interval 0.83–0.97) and 0.87 (95% confidence interval 0.76–0.98) in men and women, respectively.
Conclusions
Our findings showed that handgrip measurement was useful in detecting LS, and LS should be considered when evaluating patients with type 2 diabetes mellitus.
Keywords: Sarcopenia, Skeletal muscle, Muscle strength
The prevalence of locomotive syndrome in patients with type 2 diabetes is approximately 50–70%. Grip strength is associated with locomotive syndrome in patients with type 2 diabetes. Handgrip measurement is useful for detecting locomotive syndrome.

Introduction
The Japanese Orthopedic Association proposed the term ‘locomotive syndrome’ (LS) to identify individuals with locomotive organ impairment 1 . Recent studies reported that up to 47 million people in Japan are estimated to have LS 2 , 3 , which results in motor function deterioration and musculoskeletal pathologies, including osteoporosis and sarcopenia 2 , 4 . LS is a known risk factor for cardiovascular disease, reduced quality of life and increased medical costs 5 . However, the extent of LS has not been determined in other countries 4 .
Type 2 diabetes mellitus is associated with an increase in physical disability. A loss of skeletal muscle mass or function as a result of polyneuropathy, microvascular pathophysiology, insulin resistance or chronic inflammation in patients with type 2 diabetes mellitus has previously been reported 6 , 7 , 8 . Type 2 diabetes mellitus has also been reported to be associated with sarcopenia 9 , 10 , 11 . Although LS appears more similar to dynapenia than to sarcopenia, the extent of this disease among patients in other countries remains to be confirmed 4 . Low muscular volume has been observed in some patients with LS; therefore, most patients with sarcopenia are likely to have LS 12 .
Elderly patients with type 2 diabetes mellitus, tested for sarcopenia, have been reported to have poorer handgrip strength 13 , 14 , 15 , and grip strength has been found to be associated with sarcopenia in patients with type 2 diabetes mellitus 16 .
The prevalence rate for LS in patients with type 2 diabetes mellitus is likely to be high, and a simple handgrip measurement test could be useful to test for LS; however, this method of patient assessment remains to be investigated. Previous studies, which were carried out in healthy individuals, reported that handgrip would reflect LS 17 , 18 , 19 , 20 , 21 . We aimed to investigate the prevalence of LS, and the diagnostic value of handgrip measurement for detecting LS in a population of Japanese patients with type 2 diabetes mellitus.
Methods
Study design
Currently, we are participating in a multicenter, multipurpose cohort study; namely, the KAMOGAWA‐DM cohort study (RBMR‐E‐466) in Kyoto Prefectural University of Medicine Hospital and in four other general hospitals 14 , 15 , 16 . As part of this cohort study, an LS risk test is being carried out at Kameoka Municipal Hospital (Kameoka City, Japan). This test involves a stand‐up test and a two‐step test, in addition to general type 2 diabetes mellitus‐related tests 1 . We calculated the prevalence of LS using these two tests, and evaluated the association between type 2 diabetes mellitus‐related tests and LS in patients with type 2 diabetes mellitus. Patients were diagnosed with LS according to the results of either the stand‐up test or the two‐step test. We then classified patients according to whether sarcopenia was confirmed or not, using cut‐off points for grip strength (26 kg for men, 18 kg for women), which have been associated with sarcopenia in elderly patients 13 , 14 . All study procedures were approved by our local Research Ethics Committee and were carried out in accordance with the Declaration of Helsinki, and informed consent was obtained from all patients.
Patients
From August 2018 to October 2018, we recruited 234 consecutive patients with type 2 diabetes mellitus aged between 23 and 89 years, who regularly attended a diabetes outpatient clinic at Kameoka Municipal Hospital, Kameoka, Japan. Inclusion criteria comprised patients with type 2 diabetes mellitus who were able to complete a stand‐up test, a two‐step test and a grip strength test, and who could be assessed using a bioimpedance test. Exclusion criteria comprised patients with an existing lower limb disability. Based on American Diabetes Association criteria 22 , type 2 diabetes mellitus was diagnosed as a fasting plasma glucose level of >126 mg/dL (7.0 mmol/L) or a casual plasma glucose level of >200 mg/dL (11.1 mmol/L).
Data collection
Blood samples taken in the morning were used for biochemical measurements. Glycated hemoglobin (HbA1c), serum lipid profile (low‐density lipoprotein cholesterol, triglycerides and high‐density lipoprotein cholesterol) and other biochemical data were determined according to standard laboratory measurements. HbA1c was expressed as a National Glycohemoglobin Standardization Program unit 23 . Patient data, including age, duration of type 2 diabetes mellitus, cigarette smoking status, alcohol consumption status and antidiabetes medication, were obtained at the time of the tests. Smoking status (current, past or never having smoked) was assessed during the interview. In total, two grip strength test measurements were carried out with the right hand and the left hand, using a handgrip dynamometer (TTM Smedley Dynamo Meter; Tsutsumi, Tokyo, Japan) and the maximum grip strength value was used for analysis 14 , 24 . A direct segmental multifrequency bioelectrical impedance analyzer (InBody 770®; InBody Japan Inc., Tokyo, Japan) was used to measure body composition, such as body mass index or skeletal muscle mass index (SMI). These devices have been validated and found to correlate well with the dual‐energy X‐ray absorptiometry method 25 , 26 , and have also been reported to be useful in analyzing the body composition of Japanese patients with type 2 diabetes mellitus 13 , 27 .
Stand‐up test
We used a stand‐up test to measure lower extremity muscle power 28 , 29 , 30 . The patients were requested to stand up, using one or both legs, from a seated position off a 10–40‐cm high (10‐cm increments) stool. In the first part of the test, the patient was requested to stand up from a 40‐cm high stool using one leg. When the patient was able to stand up and hold the position for >3 s, the test was deemed successful. After passing that test, the same patient then attempted to stand up on one leg from a 30‐cm high stool and, if unable to do so, to stand up using both legs. One study suggested that the stand‐up test might be associated with bone density and gait speed 31 . If a patient was unable to stand up from a 40‐cm high stool, they were classified with stage 1 LS. If a patient could not stand up from a 20‐cm stool using both legs, they were classified with stage 2 LS.
Two‐step test
A two‐step test has been shown to be useful to screen walking ability 28 , 29 . Here, a patient stood on a starting line and took two steps forward at maximum stride while trying to keep balance and not fall, and then used both feet to stop. The distance walked during the two steps was measured and divided by the height of the individual to obtain the two‐step value. This test was repeated twice, and the better result was used in the analysis. The two‐step value closely correlates with walking speed. Values <1.3 and 1.1 were rated as LS stages 1 and 2, respectively.
Statistical analysis
Statistical analyses were carried out using JMP version 10.0 software (SAS Institute Inc., Cary, NC, USA). All continuous variables are presented as medians and ranges or as absolute numbers. The difference between multiple groups was evaluated using the Kruskal–Wallis test for continuous variables, and a χ2‐test for categorical variables. We calculated odds ratios (ORs) and 95% confidence intervals (95% CIs) using a logistic regression model. The following known risk factors for LS were considered as covariates to adjust for the effects of various factors on LS: age per 1‐year aging; HbA1c per 1% increase; use of antidiabetes medication, which was defined as without (=0) or with (=1); sex was defined as female (=0) or male (=1); and grip strength per 1‐kg increase, using multivariable logistic regression analyses. Receiver operating characteristic (ROC) curves for grip strength to assess the ability to identify LS were constructed and compared with those for SMI. We selected a point on the ROC curve that represented the largest sum of sensitivity and specificity as the optimal cut‐off point for grip strength that was associated with LS. Statistically significant P‐values were defined as P < 0.05.
Results
The patients’ clinical characteristics (n = 117 [50%] men, n = 117 [50%] women) are shown in Table 1. The clinical characteristics of patients in the male and female subgroups according to LS stages are shown in Table 2.
Table 1.
Patient characteristics
| Men | Women | |
|---|---|---|
| n (%) | 117 (50%) | 117 (50%) |
| Age (years) | 67.0 (58.0–72.0) | 65.5 (53.3–72.8) |
| Diabetic retinopathy (−/+) | 108/8 | 105/12 |
| Diabetic nephropathy (−/+) | 90/27 | 91/26 |
| Diabetic neuropathy (−/+) | 105/12 | 100/17 |
| Cardiovascular disease (−/+) | 102/15 | 111/6 |
| Fracture(−/+) | 114/3 | 113/4 |
| Body mass index (kg/m2) | 23.8 (21.9–25.9) | 25.1 (21.5–28.0) |
| Skeletal mass index (kg/m2) | 7.55 (6.88–8.09) | 6.38 (5.75–7.01) |
| Systolic blood pressure (mmHg) | 134 (124–144) | 135 (125–141) |
| Diastolic blood pressure (mmHg) | 80.0 (73.0–86.0) | 79.5 (69.0–85.0) |
| Hemoglobin A1c (%) | 7.5 (6.3–8.7) | 7.2 (6.5–8.2) |
| Total cholesterol (mg/dL) | 185 (166–211) | 208 (182–230) |
| Triglycerides (mg/dL) | 113 (77–174) | 128 (81–161) |
| Creatinine (mg/dL) | 0.84 (0.74–1.05) | 0.63 (0.56–0.70) |
| Smoking (never/previous/current) | 67/31/19 | 105/9/3 |
| Drinking (never/social/everyday) | 110/3/4 | 112/3/2 |
| Antidiabetic drug (−/+) | 30/87 | 29/88 |
| Antihypertensive drug (−/+) | 67/50 | 67/50 |
| Antihyperlipidemic drug (−/+) | 93/24 | 81/36 |
| Drug for treatment of osteoporosis (−/+) | 115/2 | 113/4 |
| Grip strength (kg) | 37.0 (30.8–41.3) | 23.0 (19.0–27.0) |
| Loco‐check (non‐LS/positive LS) | 30/8 | 31/11 |
| Stand‐up test (non‐LS/LS stage 1/LS stage 2) | 61/41/14 | 51/40/24 |
| Two‐step test (non‐LS/LS stage 1/LS stage 2) | 33/81/1 | 15/94/5 |
For categorical variables, n (%) is presented. For continuous variables, median (interquartile range) is presented. LS, locomotive syndrome.
Table 2.
Characteristics of male and female patients according to the presence or absence of locomotive syndrome in the stand‐up test or two‐step test
| Locomotive syndrome | Stand‐up test | P | Two‐step test | P | ||||
|---|---|---|---|---|---|---|---|---|
| n | Stage 1 | Stage 2 | No | Stage 1 | Stage 2 | |||
| Male patients | ||||||||
| n | 61 | 41 | 13 | – | 33 | 81 | 1 | – |
| Age (years) | 63 (48–68) | 71 (64.5–75) | 74 (60–82) | <0.001 | 63 (48–68) | 69 (58.5–74) | 60 | 0.008 |
| Body mass index (kg/m2) | 24.1 (22.0–26.3) | 23.4 (21.8–25.4) | 24.0 (20.4–25.5) | 0.788 | 23.8 (21.1–27.3) | 23.5 (22.0–25.9) | 24.5 | 0.873 |
| Skeletal mass index (kg/m2) | 7.74 (7.01–8.07) | 7.20 (6.72–7.88) | 7.12 (6.23–8.56) | 0.056 | 7.74 (7.30–8.29) | 7.31 (6.79–7.99) | 7.12 | 0.110 |
| Grip strength (kg) | 39.5 (33.6–44.8) | 34.0 (29.3–40.0) | 28.3 (22.3–30.8) | <0.001 | 40.0 (34.3–44.8) | 34.0 (29.4–40.1) | 19.5 | 0.003 |
| Systolic blood pressure (mmHg) | 129 (121–142) | 135 (128–145) | 130 (126–146) | 0.462 | 129 (121–141) | 134 (125–145) | 126 | 0.512 |
| vDiastolic blood pressure (mmHg) | 80 (75–89) | 77 (69–86) | 77 (74–82) | 0.456 | 79 (73–85.5) | 79 (71–87) | 82 | 0.921 |
| Hemoglobin A1c (%) | 7.7 (6.9–9.0) | 7.4 (6.8–8.7) | 7.2 (6.7–8.2) | 0.651 | 7.5 (6.8–8.7) | 7.4 (6.8–8.8) | 7.3 | 0.960 |
| Total cholesterol (mg/dL) | 196.5 (171.5–216) | 181 (159–204) | 176 (149.5–200.8) | 0.091 | 183 (173.5–209) | 187 (163–213.5) | 154 | 0.487 |
| Triglycerides (mg/dL) | 127 (80.5–182.5) | 106 (77–165) | 106 (77–177.8) | 0.333 | 177 (77–166) | 113 (80–181) | 183 | 0.489 |
| Creatinine (mg/dL) | 0.88 (0.76–1.04) | 0.80 (0.68–1.07) | 0.94 (0.65–1.11) | 0.296 | 0.88 (0.78–1.05) | 0.83 (0.71–1.07) | 0.69 | 0.380 |
| Urinary albumin excretion (mg/g Cr) | 17.4 (7.33–40.3) | 19.8 (8.95–115.1) | 314.9 (17.1–983.2) | 0.024 | 11.5 (7.30–55.6) | 21.7 (9.30–112.8) | 66.8 | 0.178 |
| Smoking (never/previous/current) | 32/16/13 | 23/12/6 | 11/2/0 | 0.035 | 17/10/6 | 23/12/6 | 1/0/0 | 0.008 |
| Drinking (never/social/everyday) | 56/1/4 | 39/1/1 | 13/0/0 | 0.118 | 30/1/3 | 77/2/2 | 1/0/0 | 0.031 |
| Antidiabetic drug (−/+) | 23/38 | 5/36 | 2/11 | 0.073 | 10/23 | 20/61 | 0/1 | 0.007 |
| Antihypertensive drug (−/+) | 42/19 | 22/19 | 2/11 | 0.086 | 23/10 | 43/38 | 0/1 | 0.0283 |
| Antihyperlipidemic drug (−/+) | 49/12 | 33/8 | 9/4 | 0.007 | 26/7 | 65/16 | 0/1 | 0.027 |
| Drug for treatment of osteoporosis (−/+) | 60/1 | 40/1 | 13/0 | 0.028 | 33/0 | 79/2 | 1/0 | 0.070 |
| Diabetic retinopathy (−/+) | 57/4 | 37/3 | 13/0 | 0.173 | 31/2 | 76/5 | 1/0 | 0.047 |
| Diabetic nephropathy (−/+) | 51/10 | 32/9 | 6/7 | 0.083 | 30/3 | 59/22 | 1/0 | 0.067 |
| Diabetic neuropathy (−/+) | 56/5 | 35/6 | 12/1 | 0.015 | 32/1 | 70/11 | 1/0 | 0.047 |
| Cardiovascular disease (−/+) | 57/4 | 36/5 | 8/5 | 0.148 | 31/2 | 70/11 | 1/0 | 0.937 |
| Female patients | ||||||||
| n | 51 | 40 | 24 | – | 15 | 94 | 5 | – |
| Age (years) | 57 (49–68) | 68 (62–75) | 72 (64–80) | <0.001 | 61 (51–69) | 66 (53–74) | 66 (60–78) | 0.263 |
| Body mass index (kg/m2) | 25.0 (21.6–27.9) | 24.3 (21.1–27.5) | 26.7 (22.9–32.1) | 0.198 | 25.2 (23.0–26.8) | 24.8 (21.1–28.0) | 34.3 (24.1–35.7) | 0.163 |
| Skeletal mass index (kg/m2) | 6.48 (5.87–7.06) | 6.14 (5.62–6.99) | 6.61 (5.62–7.14) | 0.613 | 6.42 (5.87–6.88) | 6.34 (5.66–7.07) | 6.87 (5.93–8.15) | 0.507 |
| Grip strength (kg) | 26.5 (23.0–30.3) | 20.0 (18.0–24.0) | 20.0 (15.9–23.1) | <0.001 | 25.8 (19.8–30.0) | 22.5 (19.0–27.0) | 20.0 (18.5–22.3) | 0.167 |
| Systolic blood pressure (mmHg) | 133 (117–140) | 135 (128–147) | 137 (130–143) | 0.287 | 134 (120–149) | 134 (125–142) | 137 (132–147) | 0.600 |
| Diastolic blood pressure (mmHg) | 78 (72–86) | 80 (69–85) | 83 (70–87) | 0.759 | 79 (73–85.5) | 79 (71–87) | 82 | 0.188 |
| Hemoglobin A1c (%) | 6.9 (6.4–7.7) | 7.3 (6.5–8.6) | 7.2 (6.5–8.2) | 0.420 | 6.9 (6.3–7.4) | 7.2 (6.5–8.2) | 7.2 (6.5–8.7) | 0.558 |
| Total cholesterol (mg/dL) | 213 (195–237) | 200 (178–225) | 193 (177–216) | 0.041 | 207 (187–220) | 210 (182–231) | 178 (166–211) | 0.287 |
| Triglycerides (mg/dL) | 136 (85–171) | 118 (71–142) | 123 (87–152) | 0.149 | 142 (107–178) | 124 (76–155) | 111 (76–195) | 0.302 |
| Creatinine (mg/dL) | 0.63 (0.56–0.70) | 0.64 (0.57–0.70) | 0.66 (0.54–0.73) | 0.582 | 0.64 (0.57–0.68) | 0.63 (0.56–0.70) | 0.72 (0.48–0.84) | 0.760 |
| Urinary albumin excretion (mg/g Cr) | 14.0 (8.78–34.5) | 19.6 (13.4–63.3) | 41.2 (17.4–96.3) | 0.001 | 11.0 (8.1–23.6) | 20.8 (13–46.1) | 35.9 (18.1–497.2) | 0.036 |
| Smoking (never/previous/current) | 47/4/0 | 36/2/2 | 20/3/1 | 0.052 | 13/2/0 | 85/7/2 | 4/0/1 | 0.051 |
| Drinking (never/social/everyday) | 48/2/1 | 39/0/1 | 24/0/0 | 0.106 | 15/0/0 | 90/2/2 | 5/0/0 | 0.039 |
| Antidiabetic drug | 18/33 | 9/31 | 1/23 | 0.082 | 5/10 | 23/71 | 0/5 | 0.027 |
| Antihypertensive drug | 31/20 | 27/13 | 8/16 | 0.048 | 10/5 | 55/39 | 0/5 | 0.059 |
| Antihyperlipidemic drug | 36/15 | 30/10 | 14/10 | 0.014 | 8/7 | 72/22 | 0/5 | 0.115 |
| Drug for treatment of osteoporosis (−/+) | 48/3 | 40/0 | 23/1 | 0.104 | 14/1 | 91/3 | 5/0 | 0.021 |
| Diabetic retinopathy (−/+) | 48/3 | 36/4 | 19/5 | 0.055 | 12/3 | 87/7 | 3/2 | 0.097 |
| Diabetic nephropathy (−/+) | 43/8 | 30/10 | 16/8 | 0.044 | 14/1 | 72/22 | 2/3 | 0.049 |
| Diabetic neuropathy (−/+) | 44/7 | 31/9 | 23/1 | 0.048 | 12/3 | 80/14 | 5/0 | 0.020 |
| Cardiovascular disease (−/+) | 49/2 | 36/4 | 24/0 | 0.145 | 13/2 | 90/4 | 5/0 | 0.039 |
Data are expressed as the mean (standard deviation) or absolute number. The P‐value of the Kruskal–Wallis test for continuous variables and χ2‐test for categorical variables are shown.
The prevalence of LS in the stand‐up and two‐step tests was 51.5 and 79.0%, respectively (Figure 1). The prevalence of LS in the stand‐up test or in the two‐step test increased according to age in men and women (Figures 2,3). In both the stand‐up and two‐step tests, 107 patients (46.7%) were diagnosed with LS (Figure S1).
Figure 1.
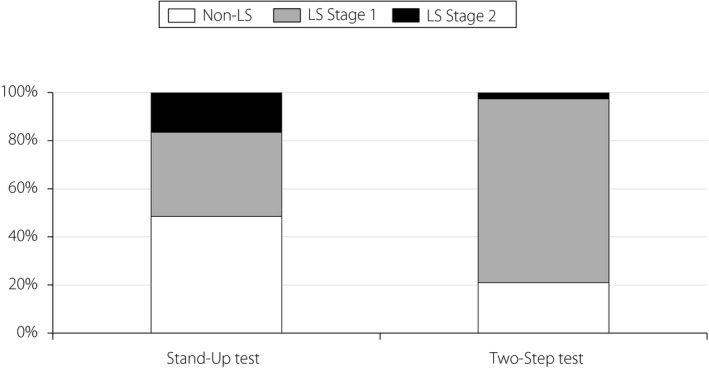
The prevalence of locomotive syndrome (LS) using the stand‐up test, and the two‐step test.
Figure 2.
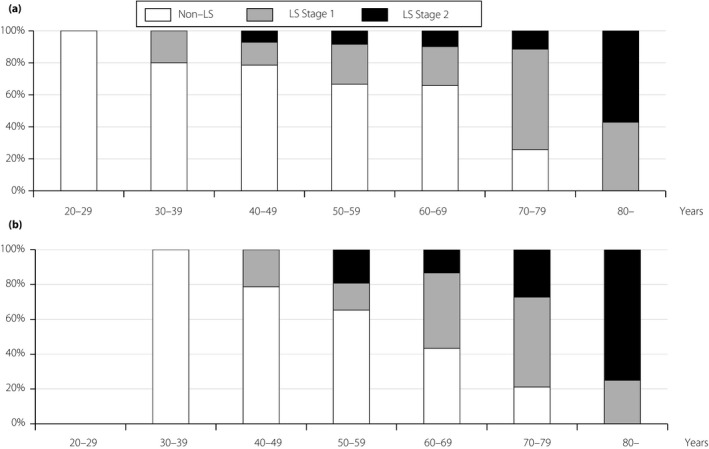
The prevalence of locomotive syndrome (LS) according to age using the stand‐up test. (a) Men. (b) Women.
Figure 3.
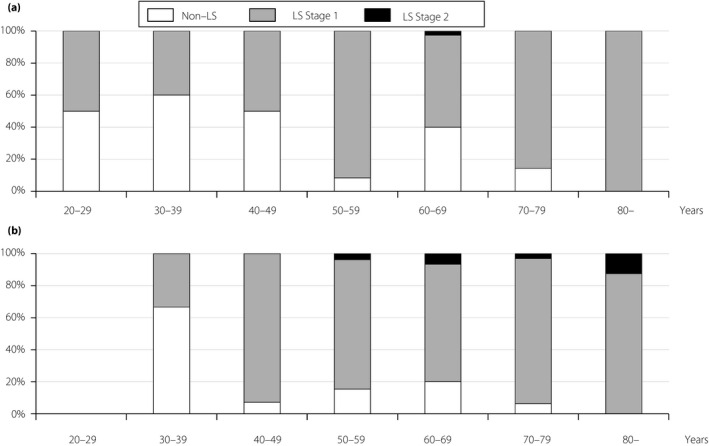
The prevalence of locomotive syndrome (LS) according to age using the two‐step test. (a) Men. (b) Women.
The area under the ROC curve (AUROC), used to assess the ability to identify LS curves for grip strength, showed a 95% CI of 0.703 (0.563–0.813) and a 95% CI of 0.698 (0.500–0.842) in men and women, respectively (Figure 4). The optimal cut‐off points (sensitivity and specificity) for grip strength associated with LS were 42.5 kg (0.865 and 0.474) and 24.5 kg (0.667 and 0.727) in men and women, respectively. The AUROC for LS using a grip strength test was found to be greater than the AUROC of the SMI in men (P = 0.02) and in women (P = 0.04; Figure S2). When we classified patients with or without LS using these cut‐off points for grip strength, the prevalence ratios (95% CI) were 1.57 (1.04–2.36) and 1.19 (1.01–1.40) in men and women, respectively.
Figure 4.
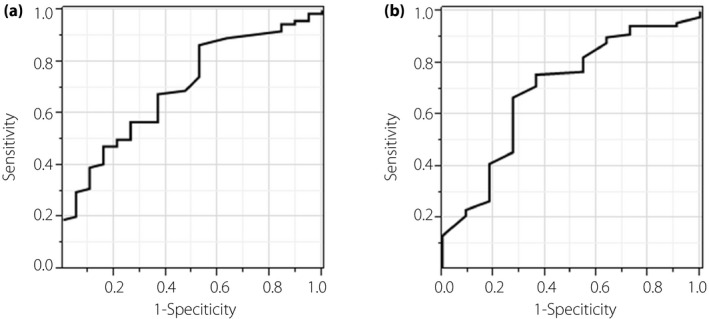
Area under the receiver operating characteristic curve of grip strength in locomotive syndrome. (a) Men. (b) Women.
Using logistic regression analyses, the ORs regarding grip strength for LS were 0.90 (95% CI 0.83–0.97) and 0.87 (95% CI 0.76–0.98) in men and women, respectively. Adjusted ORs concerning grip strength for LS were 0.91 (95% CI 0.83–0.99) and 0.92 (95% CI 0.80–1.05) in men and women, respectively (Table 3). Using logistic regression analyses, our findings regarding patients with type 2 diabetes mellitus and LS showed that ORs in relation to our HbA1c results with LS were 1.22 (95% CI 0.91–1.75) and 2.27 (95% CI 1.15–5.35) in male and female patients, respectively. Using logistic regression analyses in relation to the use of antidiabetes medication, the ORs for male and female patients with LS were 0.58 (95% CI 0.23–1.50) and 0.40 (95% CI 0.12–1.39), respectively. When we calculated ORs regarding handgrip strength for patients with LS whose HbA1c levels were ≤6.4%, using logistic regression analysis, our results for this group of patients were 0.91 (95% CI 0.54–1.38) and 0.86 (95% CI 0.70–1.01) in men and women, respectively. There was no association between LS and glycemic control, other diabetes parameters or the status of diabetes‐related complications.
Table 3.
Unadjusted odds ratios and multivariate adjusted odds of grip strength per 1‐kg increase for positive locomotive syndrome
| Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) | |
|---|---|---|
| Men | 0.90 (0.83–0.97) | 0.91 (0.83–0.99) |
| Women | 0.87 (0.76–0.98) | 0.92 (0.80–1.05) |
The adjusted model is adjusted for age per 1‐year increase, use of antidiabetic drug and hemoglobin A1c per 1% increase. Use of antidiabetic drug was defined as without (=0) or with (=1). CI, confidence interval.
In total, 64 (79.6%) male patients and 74 (89.1%) female patients had LS without sarcopenia. A total of 10 (10.8%) male patients and 12 (11.9%) female patients were found to have both LS and sarcopenia. There were no sarcopenia‐only patients (Figure S3).
In terms of the prevalence of LS in the whole cohort, the prevalence of LS in patients carrying out the stand‐up and two‐step tests was 53.1 and 78.9%, respectively (Figure S4). Using logistic regression analyses, the ORs for age per 1‐year aging and sex (female for male) regarding patients with LS were 1.03 (95% CI 1.01–1.06) and 2.51 (95% CI 1.22–5.42), respectively. When we calculated the ORs regarding handgrip strength in patients with LS aged ≤64 years, the ORs were 0.95 (95% CI 0.84–1.05) and 0.89 (95% CI 0.75–1.03) in men and women, respectively.
Discussion
The findings of the present study indicated an approximate 50% prevalence rate for LS in patients with type 2 diabetes mellitus.
Musculoskeletal ambulation disability syndrome complex is known to be an important complication among patients with type 2 diabetes mellitus. Compared with patients without type 2 diabetes mellitus, the risk of disability is 50–80% higher for people with type 2 diabetes mellitus 9 ; therefore, we estimated that the risk of LS would also be high. In the present study, 46.7% of patients were found to have LS, and the prevalence of LS increased according to age. These results have much in common with findings reported in a previous large regional residential cohort in Japan 2 , 5 , 32 .
The mechanism with which type 2 diabetes mellitus leads to LS remains unclear. Several possible mechanisms underlying the relationship between type 2 diabetes mellitus and the progression of movement disorders have been postulated in previous studies. Type 2 diabetes mellitus has been associated with a rapid loss of skeletal muscle strength and quality 33 , 34 , 35 , 36 . Diabetes‐related complications, such as microvascular disease or neuropathy, have been reported to exacerbate a loss of strength and quality in skeletal muscle 10 , 11 . Patients with type 2 diabetes mellitus also have an increased risk of fracture 37 , 38 ; therefore, a loss of skeletal muscle and a bone metabolism disorder might be associated with LS in patients with type 2 diabetes mellitus. The presence of systemic chronic inflammation, which is a multifactorial process, has also been shown to lead to movement disorders in patients with type 2 diabetes mellitus 33 , 39 , and this mechanism might influence the increased prevalence of LS among patients with type 2 diabetes mellitus. However, there was no positive association between LS and type 2 diabetes mellitus status or treatment. Furthermore, the association between handgrip strength and LS found among patients without type 2 diabetes mellitus or within the general population should be also considered. However, no comparisons with the general population were made in this cohort study.
One strength of the present study was that we used a procedure that was simple to carry out in a clinical setting. The stand‐up test has been shown to be a simple method of assessing patients’ lower extremity muscular strength 25 , 26 , 27 , 28 . The two‐step test can assess patients’ walking ability, including muscular strength, balance and flexibility of the lower extremities 25 , 26 . However, we might have underestimated the prevalence of a movement disorder in patients with type 2 diabetes mellitus, because we excluded patients who had pre‐existing lower limb disabilities. In such cases, other tests should be used to diagnose LS. Handgrip measurements are among the simplest clinical tests to carry out. Previously, in one part of our cohort study, grip strength was associated with sarcopenia in patients with type 2 diabetes mellitus 11 . In this study, grip strength was also associated with LS. Optimal cut‐off points for grip strength associated with LS were 42.5 kg in men and 24.5 kg in women. Furthermore, when other cut‐off points for grip strength have been selected in terms of an association with sarcopenia in older patients 13 , 14 , these cut‐off points for sensitivity and specificity were determined as 26 kg (0.137 and 0.000) or 35 kg (0.521 and 0.737) in men, and 18 kg (0.211 and 0.909) or 22 kg (0.511 and 0.727) in women, respectively. Measuring handgrip strength might be a clinically useful and easy test to carry out before stand‐up and two‐step tests to diagnose LS, when handgrip strength has been determined to be lower than these values. When patients were classified with or without sarcopenia, using cut‐off points for grip strength (men, 26 kg; women, 18 kg), no patients were classified as having sarcopenia only. These results are consistent with loss of muscle strength and sarcopenia leading to a decline in locomotor function.
However, in patients of both sexes, no significant association was found between LS and muscle mass, expressed using SMI (Table S1). The AUROC to assess the ability to identify the LS for SMI was 95% CI 0.597 (0.484–0.701) in men, and 95% CI 0.560 (0.420–0.692) in women. The optimal cut‐off points for sensitivity and specificity for SMI, which is associated with LS, were 7.3 kg/m2 (0.506 and 0.769) and 5.7 kg/m2 (0.275 and 0.726) in men and women, respectively. These results are similar to those of a previous study 14 , although there was no significant association found between LS and SMI.
Locomotive syndrome was easily diagnosed using stand‐up and two‐step tests without using a direct segmental multifrequency bioelectrical impedance analyzer, such as an InBody 770®. All patients with sarcopenia were classified as having LS among the patients with type 2 diabetes mellitus in the present study. When assessing a decline in locomotor function in patients with type 2 diabetes mellitus, handgrip measurements are recommended, as these are simple clinical tests that can be carried out during an initial assessment. Based on the results of the present study, if handgrip strength is found to be <42.5 kg in men and <24.5 kg in women, we recommend that patients carry out a stand‐up test and two‐step test to diagnose LS.
The handgrip test was useful for strength assessment, and was associated with a diagnosis of LS in the present study. However, this test cannot be used as a single assessment of balance and mobility. Gait speed is a predictor of long‐term survival and disability 40 . It can also be used as a simple clinical test for LS, although gait speed was not assessed simultaneously in this study. Reduced handgrip strength has also been associated with symptoms of depression 41 . Sleep quality should also be considered when evaluating LS. Further studies are required to determine whether there is an association between handgrip strength and LS, as well as symptoms of depression or sleep quality.
This study might be of limited relevance to the elderly population, because we included young people aged >20 years; however, it is important to note that LS can occur in patients aged >30 years. The clinical role of LS in young patients has not been evaluated in epidemiological studies. As this study involved young patients, the cut‐off points for LS might not be relevant to elderly patients, which significantly reduce the clinical value of this study. In assessing older populations, discrepancies in terms of high performance‐based but low self‐reported physical functioning levels has been associated with an increased risk of future falls in older adults aged 65–89 years 42 . Careful assessment is required regarding older people whose subjective perceptions of their physical functioning capacity is lower than those of similar age and sex, even if their actual physical functioning appears to be objectively high. The prevalence of LS might largely depend on age and sex, and its prevalence in the whole cohort needs to be explained more carefully. In terms of the prevalence of LS in the whole cohort, the prevalence of LS in patients carrying out the stand‐up and two‐step tests was shown in Figure S4. Using logistic regression analyses, age and sex were associated with LS. When we calculated the ORs regarding handgrip strength in patients with LS aged ≤64 years, there was not a significant association between handgrip strength and LS both in men and women.
The present study had several limitations. First, precise demonstrations of the cause–effect relationship between LS and type 2 diabetes mellitus parameters or the status of other diabetes‐related complications were not shown in our cross‐sectional data. Prospective studies are required to evaluate the association between LS and type 2 diabetes mellitus. Second, the study population comprised Japanese patients; therefore, whether these findings can be generalized to other ethnic groups is uncertain. Third, the present study only used a single baseline measurement of handgrip strength, which might have resulted in potential bias. Further studies are required to assess the relationship between average handgrip strength and LS. Finally, the findings might not be supported using the Youden method for cut‐off selection, as cut‐off points could be freely selected depending on the test application. Further validation studies should be undertaken to test the hypothesis.
In conclusion, the prevalence of LS in patients with type 2 diabetes mellitus was found to be approximately 50–70%. Greater attention is required to identify LS in patients with type 2 diabetes mellitus. Handgrip strength measurements are useful tests to detect LS in patients with type 2 diabetes mellitus.
Disclosure
NK, TO, NK, MH, YH and MF received grant and research support from AstraZeneca plc, Astellas Pharma Inc. Bristol‐Myers Squibb K.K., Daiichi Sankyo Co., Ltd., Eli Lilly Japan K.K., Kyowa Hakko Kirin Company Ltd., Kowa Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., MSD K.K., Mitsubishi Tanabe Pharma Corp., Novo Nordisk Pharma Ltd., Nippon Chemiphar Company Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Sanofi K.K., Taisho Toyama Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and TERUMO Co. The other authors declare no conflict of interest. The sponsors were not involved in the study design, nor in the collection, analysis, or interpretation of data, nor in the writing of this manuscript or in the decision to submit the article for publication. The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article. The authors declare that although they are affiliated with a department that is supported financially by a pharmaceutical company, the authors received no current funding for this study. Department affiliation does not alter the authors’ adherence to all journal policies on sharing data and materials.
Supporting information
Figure S1 | The prevalence of locomotive syndrome using the stand‐up test and the two‐step test.
Figure S2 | Area under the receiver operating characteristic curve for grip strength and skeletal muscle mass index in locomotive syndrome.
Figure S3 | The prevalence of locomotive syndrome and sarcopenia.
Figure S4 | The prevalence of locomotive syndrome using the two‐step test for the entire cohort.
Table S1 | Unadjusted odds ratios and multivariate adjusted odds of skeletal muscle mass index per 1‐kg/m2 increase for positive locomotive syndrome.
Acknowledgments
The authors thank all medical staff working in Kameoka Municipal Hospital, and we thank Editage for English language editing.
This study was funded by the Japan Osteoporosis Foundation.
J Diabetes Investig. 2020
References
- 1. Nakamura K. A “super‐aged” society and the “locomotive syndrome”. J Orthop Sci 2008; 13: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ishibashi H. Locomotive syndrome in Japan. Osteoporos Sarcopenia 2018; 4: 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoshimura N, Muraki S, Oka H, et al Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab 2009; 27: 620–628. [DOI] [PubMed] [Google Scholar]
- 4. Yoshimura N, Muraki S, Oka H, et al Association between new indices in the locomotive syndrome risk test and decline in mobility: third survey of the ROAD study. J Orthop Sci 2015; 20: 896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Otani K, Takegami M, Fukumori N, et al Locomotor dysfunction and risk of cardiovascular disease, quality of life, and medical costs: design of the Locomotive Syndrome and Health Outcome in Aizu Cohort Study (LOHAS) and baseline characteristics of the study population. J Orthop Sci 2012; 17: 261–271. [DOI] [PubMed] [Google Scholar]
- 6. Ogata N, Chikazu D, Kubota N, et al Insulin receptor substrate‐1 in osteoblast is indispensable for maintaining bone turnover. J Clin Investig 2000; 105: 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akune T, Kadowaki T, Kawaguchi H, et al PPAR g insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors find the latest version : PPAR γ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Investig 2004; 113: 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takayanagi H, Iizuka H, Juji T, et al Involvement of receptor activator of nuclear factor κB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum 2000; 43: 259–269. [DOI] [PubMed] [Google Scholar]
- 9. Wong E, Backholer K, Gearon E, et al Diabetes and risk of physical disability in adults: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2013; 1: 106–114. [DOI] [PubMed] [Google Scholar]
- 10. Umegaki H. Sarcopenia and frailty in older patients with diabetes mellitus. Geriatr Gerontol Int 2016; 16: 293–299. [DOI] [PubMed] [Google Scholar]
- 11. Fukuoka Y, Narita T, Fujita H, et al Importance of physical evaluation using skeletal muscle mass index and body fat percentage to prevent sarcopenia in elderly Japanese diabetes patients. J Diabetes Investig 2019; 10: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshimura N, Muraki S, Iidaka T, et al Prevalence and co‐existence of locomotive syndrome, sarcopenia, and frailty: the third survey of Research on Osteoarthritis/Osteoporosis Against Disability (ROAD) study. J Bone Miner Metab 2019; 37: 1058–1066. [DOI] [PubMed] [Google Scholar]
- 13. Shimizu Y, Sato S, Koyamatsu J, et al Handgrip strength and subclinical carotid atherosclerosis in relation to platelet levels among hypertensive elderly Japanese. Oncotarget 2017; 8: 69362–69369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaji A, Hashimoto Y, Kobayashi Y, et al Sarcopenia is associated with tongue pressure in older patients with type 2 diabetes: a cross‐sectional study of the KAMOGAWA‐DM cohort study. Geriatr Gerontol Int 2019; 19: 153–158. [DOI] [PubMed] [Google Scholar]
- 15. Sakai R, Hashimoto Y, Ushigome E, et al Understanding of antidiabetic medication is associated with blood glucose in patients with type 2 diabetes: at baseline date of the KAMOGAWA‐DM cohort study. J Diabetes Investig 2019; 10: 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okamura T, Hashimoto Y, Miki A, et al High brain natriuretic peptide is associated with sarcopenia in patients with type 2 diabetes: a cross‐sectional study of KAMOGAWA‐DM cohort study. Endocr J 2019; 66: 369–377. [DOI] [PubMed] [Google Scholar]
- 17. Yoshimura N, Oka H, Muraki S, et al Reference values for hand grip strength, muscle mass, walking time, and one‐leg standing time as indices for locomotive syndrome and associated disability: the second survey of the ROAD study. J Orthop Sci 2011; 16: 768–77. [DOI] [PubMed] [Google Scholar]
- 18. Muramoto A, Imagama S, Ito Z, et al Threshold values of physical performance tests for locomotive syndrome. J Orthop Sci 2013; 18: 618–626. [DOI] [PubMed] [Google Scholar]
- 19. Tajika T, Yamamoto A, Oya N, et al Association between dysfunction of upper extremity and locomotive syndrome in general population. J Orthop Sci 2017; 22: 144–148. [DOI] [PubMed] [Google Scholar]
- 20. Tanaka S, Ando K, Kobayashi K, et al Relationship between locomotive syndrome and body composition among community‐dwelling middle‐age and elderly individuals in Japan: the Yakumo study. Mod Rheumatol 2019; 29: 491–495. [DOI] [PubMed] [Google Scholar]
- 21. Kobayashi K, Imagama S, Ando K, et al Weakness of grip strength reflects future locomotive syndrome and progression of locomotive risk stage: a 10‐year longitudinal cohort study. Mod Rheumatol 2019; 24: 1–7. [DOI] [PubMed] [Google Scholar]
- 22. The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the DESCRIPTION OF DIABETES CATEGORIES OF GLUCOSE. Diabetes Care 2003; 26: 5–20. [Google Scholar]
- 23. Seino Y, Nanjo K, Tajima N, et al Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010; 1: 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen LK, Liu LK, Woo J, et al Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc 2014; 15: 95–101. [DOI] [PubMed] [Google Scholar]
- 25. Kim M, Shinkai S, Murayama H, et al Comparison of segmental multifrequency bioelectrical impedance analysis with dual‐energy X‐ray absorptiometry for the assessment of body composition in a community‐dwelling older population. Geriatr Gerontol Int 2015; 15: 1013–1022. [DOI] [PubMed] [Google Scholar]
- 26. Anderson LJ, Erceg DN, Schroeder ET. Utility of multifrequency bioelectrical impedance compared with dual‐energy x‐ray absorptiometry for assessment of total and regional body composition varies between men and women. Nutr Res 2012; 32: 479–485. [DOI] [PubMed] [Google Scholar]
- 27. Hashimoto Y, Osaka T, Fukuda T, et al The relationship between hepatic steatosis and skeletal muscle mass index in men with type 2 diabetes. Endocr J 2016; 63: 877–884. [DOI] [PubMed] [Google Scholar]
- 28. Nakamura K, Ogata T. Locomotive syndrome: definition and management. Clin Rev Bone Miner Metab 2016; 14: 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ogata T, Muranaga S, Ishibashi H, et al Development of a screening program to assess motor function in the adult population: a cross‐sectional observational study. J Orthop Sci 2015; 20: 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miyamoto R, Sawada SS, Gando Y, et al Stand‐up test overestimates the decline of locomotor function in taller people: a cross‐sectional analysis of data from the Kameda Health Study. J Phys Ther Sci 2019; 31: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ohsawa T, Shiozawa H, Saito K, et al Relation between the stand‐up test and gait speed, knee osteoarthritis, and osteoporosis using calcaneal quantitative ultrasound ‐ Cross‐sectional study. J Orthop Sci 2016; 21: 74–78. [DOI] [PubMed] [Google Scholar]
- 32. Yoshimura N, Muraki S, Nakamura K, et al Epidemiology of the locomotive syndrome: the research on osteoarthritis/osteoporosis against disability study 2005–2015. Mod Rheumatol 2017; 27: 1–7. [DOI] [PubMed] [Google Scholar]
- 33. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi‐system aging phenotypes: a systematic review and meta‐analysis. PLoS One 2009; 4: e4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalyani RR, Metter EJ, Egan J, et al Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care 2015; 38: 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoon JW, Ha YC, Kim KM, et al Hyperglycemia is associated with impaired muscle quality in older men with diabetes: the Korean Longitudinal Study on Health and Aging. Diabetes Metab J 2016; 40: 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mori H, Kuroda A, Araki M, et al Advanced glycation end‐products are a risk for muscle weakness in Japanese patients with type 1 diabetes. J Diabetes Investig 2017; 8: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamamoto M, Yamaguchi T, Yamauchi M, et al Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res 2009; 24: 702–709. [DOI] [PubMed] [Google Scholar]
- 38. Schwartz AV, Vittinghoff E, Bauer DC, et al Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA 2011; 305: 2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Volpato S, Maraldi C, Fellin R. Type 2 diabetes and risk for functional decline and disability in older persons. Curr Diabetes Rev 2010; 6: 134–143. [DOI] [PubMed] [Google Scholar]
- 40. Studenski S, Perera S, Patel K, et al Gait speed and survival in older adults. JAMA 2011; 305: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fukumori N, Yamamoto Y, Takegami M, et al Association between hand‐grip strength and depressive symptoms: Locomotive Syndrome and Health Outcomes in Aizu Cohort Study (LOHAS). Age Ageing 2015; 44: 592–598. [DOI] [PubMed] [Google Scholar]
- 42. Kamitani T, Yamamoto Y, Fukuma S, et al Association between the discrepancy in self‐reported and performance‐based physical functioning levels and risk of future falls among community‐dwelling older adults: the locomotive syndrome and health outcomes in Aizu Cohort Study (LOHAS). J Am Med Dir Assoc 2019; 20: 195–200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | The prevalence of locomotive syndrome using the stand‐up test and the two‐step test.
Figure S2 | Area under the receiver operating characteristic curve for grip strength and skeletal muscle mass index in locomotive syndrome.
Figure S3 | The prevalence of locomotive syndrome and sarcopenia.
Figure S4 | The prevalence of locomotive syndrome using the two‐step test for the entire cohort.
Table S1 | Unadjusted odds ratios and multivariate adjusted odds of skeletal muscle mass index per 1‐kg/m2 increase for positive locomotive syndrome.


