Abstract
Aims/Introduction
The association between diabetes and periodontal disease is considered to be bidirectional. However, there is still controversy surrounding the relationship between periodontal disease and type 1 diabetes. We investigated whether insulin improves periodontitis without any local treatments for periodontitis under type 1 diabetes conditions using the ligature‐induced experimental periodontitis model.
Materials and Methods
Type 1 diabetic rats were induced by streptozotocin injection. Experimental periodontitis was induced by ligature in normal and diabetic rats. Half of the diabetic rats were treated with insulin. Two weeks after the ligature, periodontitis was evaluated.
Results
Insulin treatment significantly improved inflammatory cell infiltration and inflammatory cytokine gene expression, leading to suppression of alveolar bone loss, in the periodontitis of diabetic rats. Insulin also suppressed the periodontitis‐increased nitric oxide synthase‐positive cells in periodontal tissue of the diabetic rats. Even without induction of periodontitis, diabetic rats showed decreased gingival blood flow and an increased number of nitric oxide synthase‐positive cells in the gingiva and alveolar bone loss compared with normal rats, all of which were ameliorated by insulin treatment. We further confirmed that insulin directly suppressed lipopolysaccharide‐induced inflammatory cytokine expressions in THP‐1 cells.
Conclusions
There were abnormalities of periodontal tissue even without the induction of periodontitis in streptozotocin‐induced diabetic rats. Insulin treatment significantly ameliorated periodontitis without local periodontitis treatment in diabetic rats. These data suggest the therapeutic impacts of insulin on periodontitis in type 1 diabetes.
Keywords: Insulin, Periodontitis, Type 1 diabetes
There was a slight increase in the numbers of inflammatory cells in the gingiva on the periodontitis side of normal rats and on the control side of diabetic rats. The inflammatory cells were markedly increased on the periodontitis side of diabetic rats. Insulin treatment decreased the inflammatory cells in the periodontitis gingiva of diabetic rats.

Introduction
Periodontitis is a chronic inflammation whereby pathogens in periodontal pockets destroy the gingiva, and subsequently, the alveolar bone is absorbed 1 . The induction and propagation of periodontitis are through a dental plaque, which interacts with the immune defenses of the host, leading to inflammation 1 , 2 , 3 . In diabetes patients, periodontal disease is considered to be one of the diabetic complications because of the high prevalence and severity 4 , 5 . The involvement of several biological abnormalities based on diabetes, such as immune response deficiency, vascular and bone abnormalities, and metabolic disorders, are considered 6 , 7 . As the interaction between diabetes and periodontal disease is considered to be bidirectional 8 , the prevention of periodontal disease is crucial not only for oral health, but also for the maintenance of good glycemic control in diabetes patients.
Epidemiological and clinical evidence for an association between periodontitis and diabetes is accumulating 9 . However, there is controversy regarding the relationship between periodontal disease and type 1 diabetes. Several epidemiological studies showed that type 1 diabetes patients with poor glycemic control have been demonstrated to have aggravated periodontal disease compared with patients with good glycemic control 10 , 11 . In contrast, a meta‐analysis and recent cohort study did not show a significant difference of the periodontal status in non‐diabetic controls and type 1 diabetes patients 12 , 13 . A systematic review showed a lack of sufficient evidence for the relationship between periodontitis and glycemic control in type 1 diabetes patients 9 , 14 .
Ligature‐induced experimental periodontitis progresses in a similar manner as human periodontal disease 15 . In the present study, we investigated whether the administration of insulin in the absence of local periodontal therapy improved ligature‐induced experimental periodontitis in streptozotocin (STZ)‐induced type 1 diabetic rats. We also confirmed that insulin directly suppressed the expression of inflammatory cytokines in a human monocyte/macrophage cell line, THP‐1 cells.
Methods
Animals and induction of diabetes
Male Sprague–Dawley rats (aged 5 weeks) were provided by Chubu Kagakushizai (Nagoya, Japan). Six‐week‐old Sprague–Dawley rats were weighed after the overnight fasting and STZ (60 mg/kg; Sigma‐Aldrich, St. Louis, MO, USA) was injected for the induction of diabetes. Rats with plasma glucose concentrations of >15 mmol/L were selected as the diabetic rats at 1 week after the STZ‐injection. The experimental animal groups were as follows: normal rats, diabetic rats and diabetic rats with insulin treatment (n = 20 in each group). All rats were induced with experimental periodontitis on one side of the maxillary second molar (M2), as described below. During the experiments, one normal rat and two diabetic rats died and were excluded from the experiments. All experimental protocols were carried out according to the Regulations for Animal Experiments in Aichi Gakuin University, and were approved by the Institutional Animal Care and Use Committees of Aichi Gakuin University (AGUD‐043).
Induction of periodontitis
Two weeks after the administration of STZ, a nylon ligature was tied around the M2 to induce periodontitis, as previously described 16 . The M2 on the other side remained without any treatment as the control side (Figure 1a).
Figure 1.
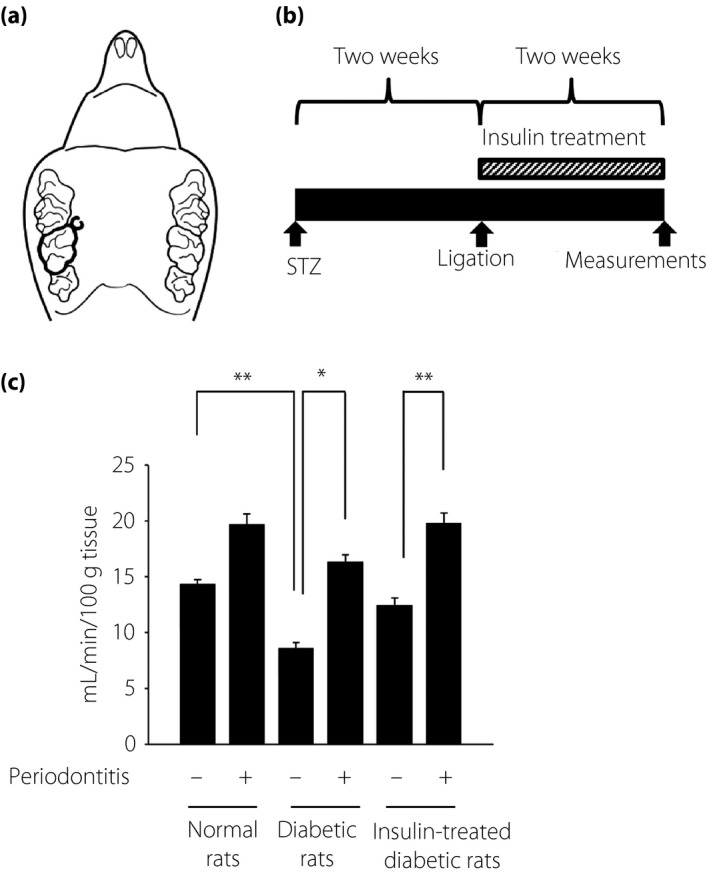
(a) Ligature‐induced periodontitis. Nylon thread was ligated around the unilateral maxillary second molar. The other side was untreated as the control. (b) Streptozotocin (STZ) was intraperitoneally injected to induce diabetes. Two weeks after STZ injection, experimental periodontitis was induced by ligation. Half of the diabetic rats received insulin treatment for 2 weeks. At 2 weeks post‐surgery, physiological and pathological assessments were carried out. (c) Gingival blood flow of the buccal side above the maxillary second molar was measured using a laser Doppler blood flow meter. The results are expressed as the mean ± standard error of the mean (n = 9). *P < 0.05, ** P < 0.01.
Insulin treatment
Half of the diabetic rats were treated with insulin. Insulin treatment was carried out by the insertion of insulin pellets (LinShin Canada Co., Toronto, ON, Canada; n = 20). We cut a small lesion in the back skin and inserted an insulin pellet that was left in for 2 weeks (Figure 1b). The effects of insulin were confirmed by reductions in blood glucose.
Blood flow in the gingival tissue
The blood flow in the gingival tissue was measured using a laser Doppler blood flow meter (FLO‐N1; Omega Wave Inc., Tokyo, Japan). The rats (n = 9 in each group) were anesthetized by the intraperitoneal injection of a combination of medetomidine, midazolam and butorphanol. Rats were placed on a heated pad to keep a constant rectal temperature of 37°C. Blood flow on the buccal side of the gum of the M2 was measured.
Tissue collection
Two weeks after the ligation, rats were killed by an overdose of pentobarbital (1.5 mg/kg). For messenger ribonucleic acid (mRNA) and protein analyses, gingival tissues were obtained and snap‐frozen in liquid nitrogen and kept at −80 °C until use. For immunohistological and micro‐computed tomography (CT) analyses, maxillary bones with gingiva on both sides were fixed in a 10% formalin solution.
Gene expression of gingiva
Total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), and complementary deoxyribonucleic acid was synthesized using ReverTra Ace (Toyobo, Osaka, Japan). Primers and probes were purchased from Taqman Gene Expression Assays (Applied Biosystems, Foster City, CA, USA). Real‐time quantitative polymerase chain reaction was carried out and measured by the ABI Prism 7000 (Applied Biosystems; n = 8 in each group). Relative quantity was calculated by the ΔΔCt method using β2 microglobulin as the endogenous control 17 .
Histological evaluation
Fixed tissues were decalcified in 10% ethylenediaminetetraacetic acid for 5 weeks, dehydrated using a graded ethanol series and then embedded in paraffin. Specimens were sectioned (4‐µm thick) and stained with hematoxylin–eosin (n = 6 in each group). For immunohistological staining, anti‐inducible nitric oxide synthase (iNOS) antibody (Bioss Antibodies Inc., Woburn, MA, USA) was used for the first antibody and subsequently stained using Simplestain rat system (Nichirei, Tokyo, Japan; n = 6 in each group).
Micro‐CT imaging
Maxillae were scanned by micro‐CT (R_mCT; Rigaku Corporation, Tokyo, Japan; n = 6 in each group). The distance from the mesial buccal cement–enamel junction to the alveolar bone crest of the second molar was measured as a marker of bone height. The percentage of bone resorption was calculated as the distance from the mesial buccal cement–enamel junction to the alveolar bone crest/root length × 100.
Lipopolysaccharide‐stimulated inflammatory response in THP‐1 cells
A human monocyte/macrophage cell line, THP‐1 cells, was purchased from American Type Culture Collection (Manassas, VA, USA). After the THP‐1 cells were starved without serum for 24 h, human insulin (Sigma‐Aldrich) was added at the concentration of 10 or 100 nmol/L 30 min before lipopolysaccharide (LPS) from Escherichia coli (Sigma‐Aldrich) stimulation. LPS (100 ng/mL) was added and cells were collected 4 h later for total RNA extraction using RNeasy (Qiagen, Valencia, CA, USA).
Statistical analysis
All group values were expressed as the mean ± standard error of the mean. Statistical analyses were made by two‐way anova followed by the Bonferroni correction for multiple comparisons. Differences were considered significant at the P < 0.05 level.
Results
Bodyweights, blood glucose and glycated hemoglobin
Diabetic rats showed significant weight loss, hyperglycemia and higher glycated hemoglobin compared with the normal rats (P < 0.001; Table 1). Insulin treatment of the diabetic rats significantly increased the bodyweight, and decreased blood glucose and glycated hemoglobin levels.
Table 1.
Characteristics of normal, diabetic and insulin‐treated diabetic rats
| Bodyweight (g) | Blood glucose (mmol/L) | HbA1c (%) | |
|---|---|---|---|
| Normal rats | 323.8 ± 7.3** | 6.4 ± 0.2** | 4.0 ± 0.1** |
| Diabetic rats | 192.8 ± 22.0 | 30.9 ± 1.8 | 7.1 ± 0.2 |
| Insulin‐treated diabetic rats | 295.8 ± 18.5* | 7.6 ± 0.6** | 5.2 ± 0.2** |
P < 0.01,
P < 0.001 vs diabetic rats. HbA1c, glycated hemoglobin.
Gingival blood flow
On the control side, gingival blood flow in the diabetic rats was significantly lower compared with that in the normal rats (P < 0.01), which was ameliorated by insulin treatment (the control side of normal rats: 14.3 ± 0.4 mL/min/100 g, the control side of the diabetic rats: 8.6 ± 0.5 mL/min/100 g, the control side of the insulin‐treated diabetic rats: 12.4 ± 0.5 mL/min/100 g; Figure 1c). The induction of periodontitis led to an increase in gingival blood flow. In the diabetic rats, the gingival blood flow of the periodontitis side was significantly increased by 1.9‐fold from the control side. Insulin treatment tended to decrease the increased rate of blood flow by periodontitis in the diabetic rats (1.5‐fold increase); however, the increase is still significant (P < 0.01).
mRNA expressions of the inflammatory cytokines in the gingiva
A small increase in the mRNA expression of tumor necrosis factor (TNF)‐α and iNOS on the periodontitis side were shown in the normal rats, but they were not significant (Figure 2). In the diabetic rats, periodontitis induced significant increases in both TNF‐α and iNOS mRNA expression compared with the expression on the control side (P < 0.001). The increase in TNF‐α and iNOS mRNA expression induced by periodontitis was significantly greater in the diabetic rats compared with those in normal rats. Insulin treatment significantly suppressed the periodontitis‐induced increase in TNF‐α and iNOS mRNA expression in the diabetic rats (P < 0.01 and P < 0.001, respectively).
Figure 2.
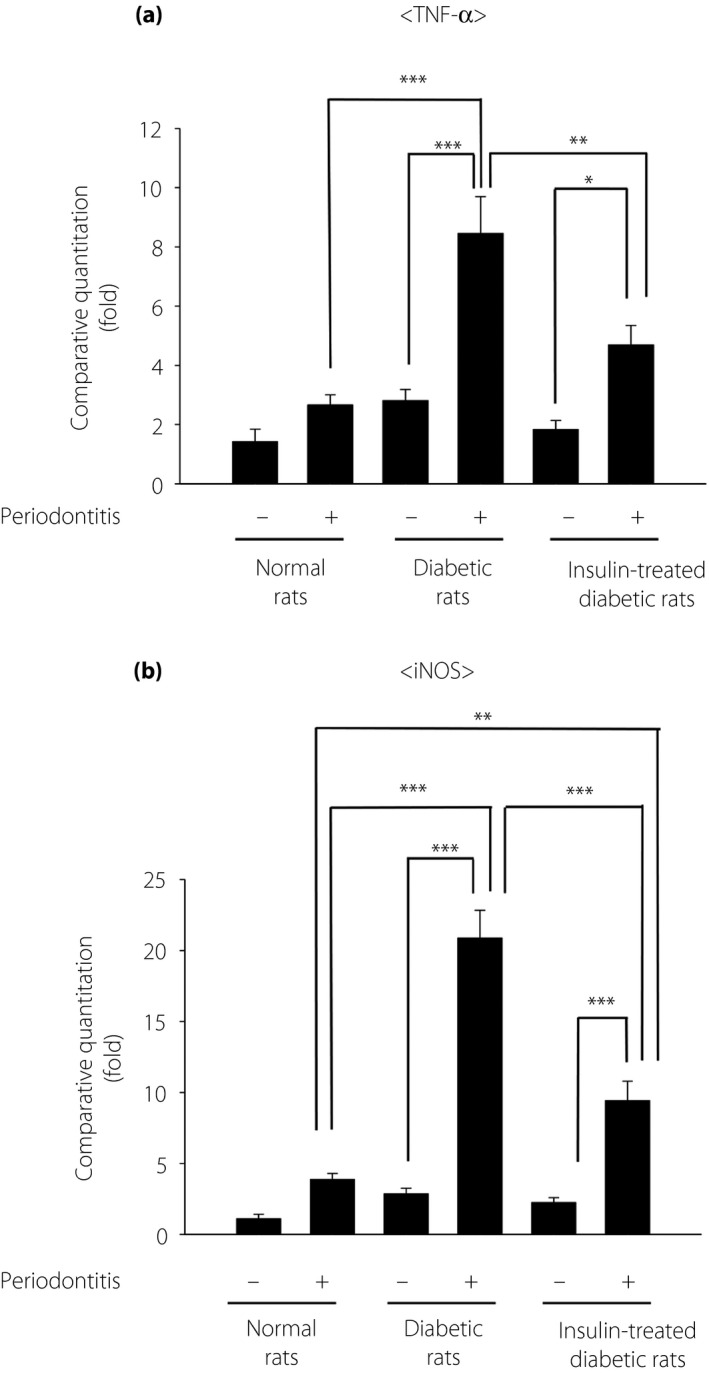
Gingival gene expression of (a) tumor necrosis factor (TNF)‐α and (b) inducible nitric oxide synthase (iNOS) in the gingiva. The results are expressed as the mean ± standard error of the mean (n = 8). *P < 0.05, ** P < 0.01, ***P < 0.001.
Periodontitis‐induced bone resorption
The micro‐CT images showed periodontitis‐induced alveolar bone resorption (Figure 3a,b). The most severe bone resorption was seen on the periodontitis side of the alveolar bone of diabetic rats. Quantitative analyses showed that the bone resorption was slightly, but significantly, higher on the periodontitis side of normal rats, and on the control side of diabetic rats compared with that on the control side of normal rats (P < 0.01 and P < 0.05, respectively). Insulin significantly decreased periodontitis‐induced alveolar bone resorption by 64% in diabetic rats (P < 0.001).
Figure 3.
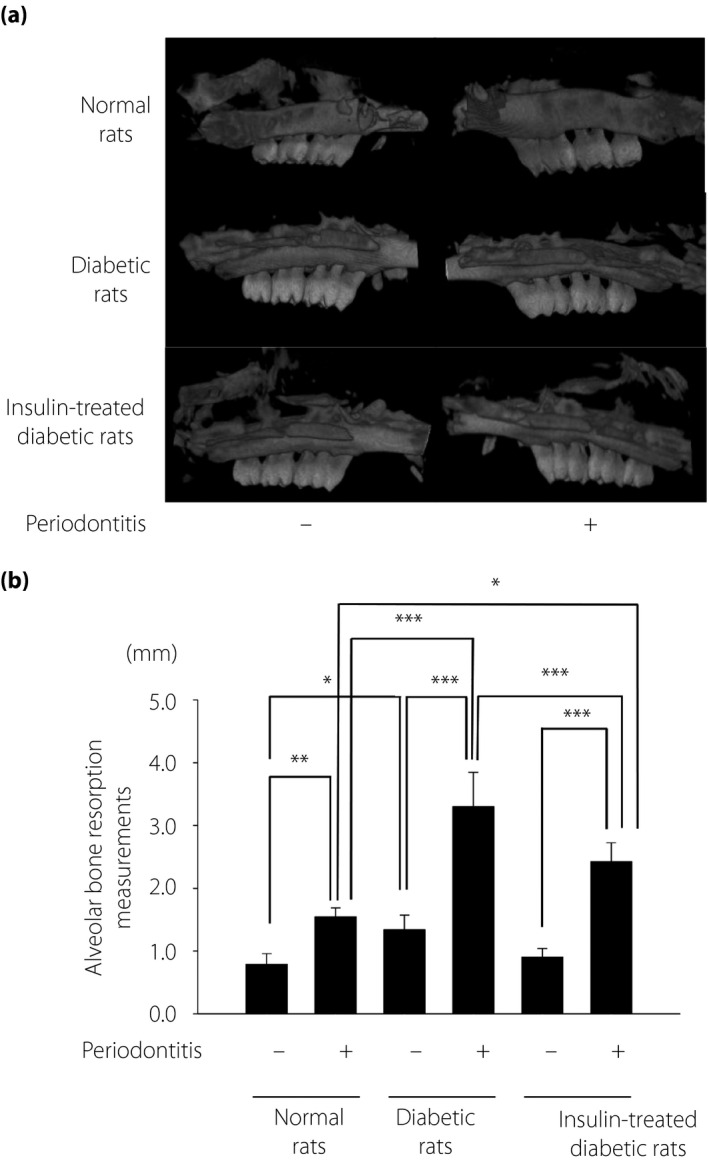
Micro‐computed tomography images of the maxillae 2 weeks after the induction of periodontitis. (a) Representative micro‐computed tomography image of the control and the periodontitis sides in the normal and diabetic rats. (b) Alveolar bone absorption was measured as the distance from the mesial buccal cement–enamel junction to the alveolar bone crest of the second molar. The results are expressed as the mean ± standard error of the mean (n = 6). *P < 0.05, ** P < 0.01, ***P < 0.001.
Histological evaluation of periodontal tissue
There was a slight increase in the numbers of inflammatory cells in the gingiva of the periodontitis side of normal rats and on the control side of the diabetic rats (Figure 4). In contrast, periodontitis severely increased the inflammatory cells in the gingiva of the diabetic rats. Insulin treatment decreased the inflammatory cells in the periodontitis gingiva of the diabetic rats.
Figure 4.
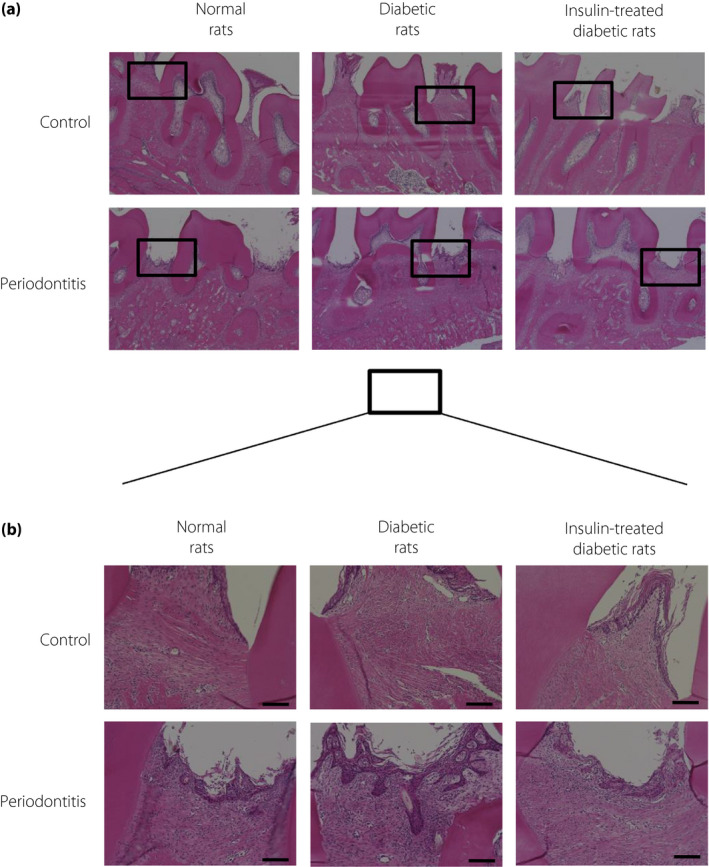
(a) Representative hematoxylin–eosin staining of gingiva on the control and the periodontitis sides of normal and diabetic rats. (b) High‐power representative photographs of hematoxylin–eosin staining of gingiva. Insulin treatment decreased the inflammatory cells in the periodontitis gingiva of the diabetic rats. Bar, 100 µm.
iNOS‐positive cells in periodontal tissue
Periodontitis increased the number of iNOS‐positive cells in the gingiva of normal rats (P < 0.001; Figure 5). Even on the control side of the diabetic rats, the number of iNOS‐positive cells was higher than that on the control side of the normal rats (P < 0.001). Periodontitis induced the highest number of iNOS‐positive cells in diabetic rats. Insulin treatment significantly suppressed the number of iNOS‐positive cells in diabetic rats (P < 0.001).
Figure 5.
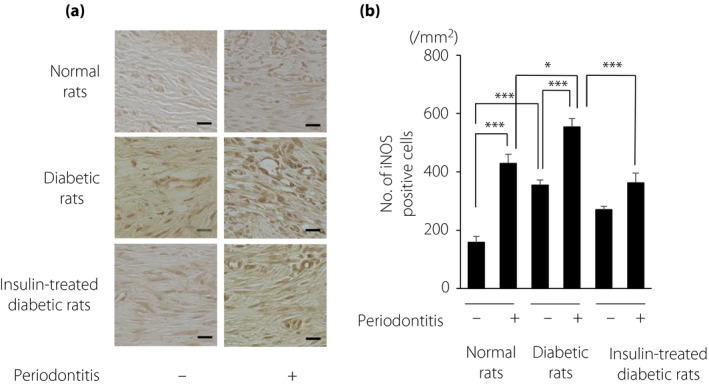
(a) Immunohistological staining of inducible nitric oxide synthase (iNOS) in the gingiva. Bar, 20 µm. (b) Quantitative analyses of iNOS‐positive cells in the gingiva. The results are expressed as the mean ± standard error of the mean (n = 6). *P < 0.05, *** P < 0.001.
Anti‐inflammatory effects of insulin
To explore the direct anti‐inflammatory effects of insulin, we investigated the effects of insulin on LPS‐induced inflammatory cytokine production in THP‐1 cells. LPS significantly increased the gene expression of the inflammatory cytokines, TNF‐α and iNOS. Pretreatment with insulin significantly inhibited LPS‐induced TNF‐α and iNOS expression (P < 0.001). Insulin doses of 10 and 100 nmol/L suppressed LPS‐stimulated TNF‐α expression by 52.7 and 61.3%, and iNOS expression by 39.7 and 46.5%, respectively (Figure 6).
Figure 6.
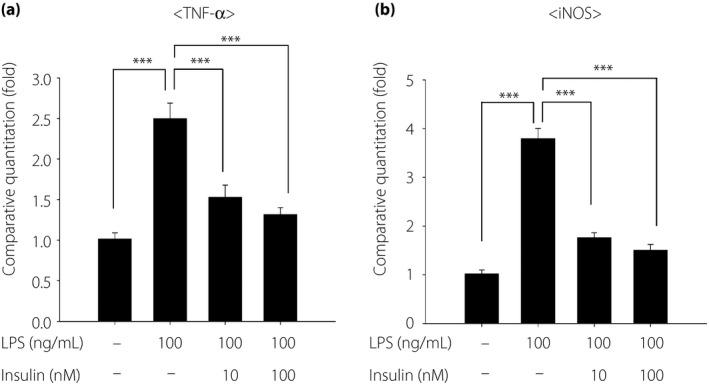
Inhibitory effects of insulin on lipopolysaccharide (LPS)‐induced inflammatory gene expressions. The messenger ribonucleic acid gene expression of (a) tumor necrosis factor (TNF)‐α and (b) inducible nitric oxide synthase (iNOS) were determined by real‐time quantitative polymerase chain reaction. Insulin was added 30 min before LPS stimulation. Then, the cells were stimulated with LPS for 4 h. Results are expressed as the mean ± standard error of the mean (n = 6). ***P < 0.001.
Discussion
The induction of periodontitis by ligation induced severe inflammation and alveolar bone loss in STZ‐induced type 1 diabetic rats. In the present study, we have provided direct evidence that insulin treatment improves periodontitis under diabetic conditions without local treatment of periodontitis.
The ligature model of periodontitis was widely used to initiate periodontal disease in experimental animals 18 , 19 , 20 . Plaque accumulation around the ligature induces acute and chronic inflammation in the gingiva, subsequently leading to alveolar bone loss. The change of periodontitis occurs more acutely than human periodontitis. However, the inflammation and bone less in the ligature model are dependent on ligature accumulation of bacteria, which is similar to human periodontitis. In the present study, ligature‐induced plaque accumulation induced more severe periodontitis accompanied with inflammatory cell infiltration and increases in TNF‐α and iNOS mRNA expression in diabetic rats compared with the normal condition; these findings are consistent with previous animal and clinical investigations 21 , 22 , 23 .
In type 2 diabetes, there is a consensus that periodontitis is significantly associated with poorer glycemic control 24 , 25 . However, there is still controversy as to whether lowering blood glucose improves periodontal disease in type 1 diabetes patients 9 , 14 . In the present study, we showed that insulin treatment improved periodontitis in type 1 diabetes model rats without any local treatment of periodontitis, suggesting the pivotal role of insulin and glycemic control on the treatment of periodontitis in type 1 diabetes.
Impairment in the microcirculation in the gingiva was observed on the control side of diabetic rats, which was consistent with our previous investigation 16 . The impairment of microcirculation was a crucial pathophysiological disorder in diabetes. Microvascular dysfunction is the common pathogenesis of diabetic microvascular complications, such as retinopathy, nephropathy and neuropathy 26 , 27 , 28 . The present results might suggest that the gingiva under the diabetic condition might share similar biological abnormalities and microvascular dysfunction with other diabetic complications.
In addition to gingival blood flow, several abnormalities were shown on the control side of the periodontal tissue in diabetic rats. The number of iNOS‐positive cells in the gingiva and alveolar bone loss was significantly increased on the control side of the gingiva in diabetic rats compared with that in normal rats. These results might account for the high morbidity of periodontitis in the type 1 diabetes condition.
Clinical studies found a positive correlation between the gingival blood flow and the severity of gingival inflammation 29 , 30 . Inflammation increases and activates iNOS, which subsequently produces excess nitric oxide. In a rat model of periodontitis, the selective iNOS inhibitor, mercaptoethylguanidine, ameliorated alveolar bone loss 31 . We previously showed that iNOS gene expression and nitrotyrosine levels, a footprint of nitrosative stress, in the gingiva were increased not only by periodontitis, but also by the diabetic condition 16 . Once periodontitis was induced in the diabetic rats, iNOS gene/protein expressions and gingival blood flow were markedly increased, both of which were suppressed by insulin treatment.
An in vitro study showed that insulin had immunosuppressive effects on LPS‐stimulated inflammatory cytokine expressions in THP‐1 cells. Zhu et al. 32 showed that the anti‐inflammatory effects of insulin were dose‐dependent, but did not depend on glycemic control, by adjusting the dosage ratio of glucose and insulin in rats. There is a possibility that insulin treatment might have a direct immunosuppressive effect on periodontitis besides the glucose‐lowering effect. Furthermore, Maekawa et al. 33 showed the inhibition of osteoblast differentiation and enhanced osteoclast activation with the greater alveolar bone absorption in periodontitis of STZ‐induced diabetic mice. In addition, previous studies showed that insulin increased osteoblast differentiation and osteoclastogenesis 34 , 35 . Further study is required to show the effects beyond glucose‐lowering of insulin on periodontitis in type 1 diabetes.
In summary, the gingiva in STZ‐induced diabetic rats showed similar biological abnormalities and microvascular dysfunction with other diabetic complications. Insulin treatment improved basal gingival blood flow, and suppressed the periodontitis‐induced inflammation of the gingiva and the alveolar bone absorption in STZ‐induced diabetic rats. As the effects of insulin could be observed without local periodontal therapy, these results suggest the pivotal role of insulin in diabetes‐associated exacerbation of periodontitis in type 1 diabetes.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This research was supported in part by a Grant‐in‐Aid for Scientific Research (16K20683) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), and in part by the “Strategic Research AGU‐Platform Formation (2008–2012)” Project for Private Universities: matching fund subsidy from the MEXT of Japan.
J Diabetes Investig. 2020
References
- 1. Papapanou PN, Sanz M, Buduneli N, et al Periodontitis: consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. J Periodontol 2018; 89(Suppl 1): S173–S182. [DOI] [PubMed] [Google Scholar]
- 2. Slots J. Periodontitis: facts, fallacies and the future. Periodontol 2000; 2017: 7–23. [DOI] [PubMed] [Google Scholar]
- 3. Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers 2017; 3: 17038. [DOI] [PubMed] [Google Scholar]
- 4. Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care 1993; 16: 329–334. [PubMed] [Google Scholar]
- 5. Graziani F, Gennai S, Solini A, et al A systematic review and meta‐analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes: an update of the EFP‐AAP review. J Clin Periodontol 2018; 45: 167–187. [DOI] [PubMed] [Google Scholar]
- 6. Kitada M, Zhang Z, Mima A, et al Molecular mechanisms of diabetic vascular complications. J Diabetes Investig 2010; 1: 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ni Y, Fan D. Diabetes mellitus is a risk factor for low bone mass‐related fractures: a meta‐analysis of cohort studies. Medicine 2017; 96: e8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chapple IL, Genco R. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol 2013; 84: S106–S112. [DOI] [PubMed] [Google Scholar]
- 9. Sanz M, Ceriello A, Buysschaert M, et al Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol 2018; 45: 138–149. [DOI] [PubMed] [Google Scholar]
- 10. Firatli E. The relationship between clinical periodontal status and insulin‐dependent diabetes mellitus. Results after 5 years. J Periodontol 1997; 68: 136–140. [DOI] [PubMed] [Google Scholar]
- 11. Demmer RT, Holtfreter B, Desvarieux M, et al The influence of type 1 and type 2 diabetes on periodontal disease progression: prospective results from the Study of Health in Pomerania (SHIP). Diabetes Care 2012; 35: 2036–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chávarry N, Vettore M, Sansone C, et al The relationship between diabetes mellitus and destructive periodontal disease: a meta‐analysis. Oral Health Prev Dent 2009; 7: 107–127. [PubMed] [Google Scholar]
- 13. Roy M, Gastaldi G, Courvoisier DS, et al Periodontal health in a cohort of subjects with type 1 diabetes mellitus. Clin Exp Dent Res 2019; 5: 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borgnakke WS, Ylostalo PV, Taylor GW, et al Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Clin Periodontol 2013; 40(Suppl 14): S135–S152. [DOI] [PubMed] [Google Scholar]
- 15. Rovin S, Costich ER, Gordon HA. The influence of bacteria and irritation in the initiation of periodontal disease in germfree and conventional rats. J Periodontal Res 1966; 1: 193–204. [DOI] [PubMed] [Google Scholar]
- 16. Nishikawa T, Naruse K, Kobayashi Y, et al Involvement of nitrosative stress in experimental periodontitis in diabetic rats. J Clin Periodontol 2012; 39: 342–349. [DOI] [PubMed] [Google Scholar]
- 17. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 18. Abe T, Hajishengallis G. Optimization of the ligature‐induced periodontitis model in mice. J Immunol Methods 2013; 394: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marchesan J, Girnary MS, Jing L, et al An experimental murine model to study periodontitis. Nat Protoc 2018; 13: 2247–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Molon RS, Park CH, Jin Q, et al Characterization of ligature‐induced experimental periodontitis. Microsc Res Tech 2018; 81: 1412–1421. [DOI] [PubMed] [Google Scholar]
- 21. Naguib G, Al‐Mashat H, Desta T, et al Diabetes prolongs the inflammatory response to a bacterial stimulus through cytokine dysregulation. J Invest Dermatol 2004; 123: 87–92. [DOI] [PubMed] [Google Scholar]
- 22. Pan Z, Guzeldemir E, Toygar HU, et al Nitric oxide synthase in gingival tissues of patients with chronic periodontitis and with and without diabetes. J Periodontol 2010; 81: 109–120. [DOI] [PubMed] [Google Scholar]
- 23. Morohoshi M, Fujisawa K, Uchimura I, et al Glucose‐dependent interleukin 6 and tumor necrosis factor production by human peripheral blood monocytes in vitro . Diabetes 1996; 45: 954–959. [DOI] [PubMed] [Google Scholar]
- 24. Taylor GW, Burt BA, Becker MP, et al Severe periodontitis and risk for poor glycemic control in patients with non‐insulin‐dependent diabetes mellitus. J Periodontol 1996; 67: 1085–1093. [DOI] [PubMed] [Google Scholar]
- 25. Chang JF, Yeh JC, Chiu YL, et al Periodontal pocket depth, hyperglycemia, and progression of chronic kidney disease: a population‐based longitudinal study. Am J Med 2017; 130: 61–69.e1. [DOI] [PubMed] [Google Scholar]
- 26. Malik R, Newrick P, Sharma A, et al Microangiopathy in human diabetic neuropathy: relationship between capillary abnormalities and the severity of neuropathy. Diabetologia 1989; 32: 92–102. [DOI] [PubMed] [Google Scholar]
- 27. Yasuda H, Dyck PJ. Abnormalities of endoneurial microvessels and sural nerve pathology in diabetic neuropathy. Neurology 1987; 37: 20. [DOI] [PubMed] [Google Scholar]
- 28. Kim AY, Chu Z, Shahidzadeh A, et al Quantifying microvascular density and morphology in diabetic retinopathy using spectral‐domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci 2016; 57: 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hinrichs JE, Jarzembinski C, Hardie N, et al Intrasulcular laser Doppler readings before and after root planing. J Clin Periodontol 1995; 22: 817–823. [DOI] [PubMed] [Google Scholar]
- 30. Gleissner C, Kempski O, Peylo S, et al Local gingival blood flow at healthy and inflamed sites measured by laser Doppler flowmetry. J Periodontol 2006; 77: 1762–1771. [DOI] [PubMed] [Google Scholar]
- 31. Lohinai Z, Benedek P, Feher E, et al Protective effects of mercaptoethylguanidine, a selective inhibitor of inducible nitric oxide synthase, in ligature‐induced periodontitis in the rat. Br J Pharmacol 1998; 123: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhu Z, Hu T, Wang Z, et al Anti‐inflammatory and organ protective effect of insulin in scalded MODS rats without controlling hyperglycemia. Am J Emerg Med 2018; 36: 202–207. [DOI] [PubMed] [Google Scholar]
- 33. Maekawa S, Katagiri S, Takeuchi Y, et al Bone metabolic microarray analysis of ligature‐induced periodontitis in streptozotocin‐induced diabetic mice. J Periodontal Res 2017; 52: 233–245. [DOI] [PubMed] [Google Scholar]
- 34. Bilotta FL, Arcidiacono B, Messineo S, et al Insulin and osteocalcin: further evidence for a mutual cross‐talk. Endocrine 2018; 59: 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oh JH, Lee JY, Joung SH, et al Insulin enhances RANKL‐induced osteoclastogenesis via ERK1/2 activation and induction of NFATc1 and Atp6v0d2. Cell Signal 2015; 27: 2325–2331. [DOI] [PubMed] [Google Scholar]


