Abstract
Aims/Introduction
A low level of urine pH (U‐pH) has been reported to be associated with metabolic disorders. However, the relationship between the incidence of diabetes mellitus and U‐pH has not yet been fully addressed.
Materials and Methods
We investigated the relationship between U‐pH and the development of diabetes mellitus during a 10‐year period in a general population of individuals who received annual health examinations in 2006 (n = 28,990). After exclusion of individuals with missing data, and those with diabetes mellitus and/or chronic kidney disease at baseline, a total of 12,476 individuals (men/women: 8,027/4,449) who received health examinations at least once during the period from 2007 to 2016 were recruited. The recruited individuals were divided into four groups according to their U‐pH levels: groups of U‐pH ≤5.0, 5.5, 6.0 and ≥6.5.
Results
During a 10‐year period, 521 men (6.5%) and 132 women (3.0%) had new onset of diabetes mellitus. The cumulative incidence of diabetes mellitus was 7.5% (men/women: 9.3%/4.4%) per 100 person‐years. The hazard ratios (HRs) in the U‐pH ≤5.0 (HR 1.93) and U‐pH 5.5 groups (HR 1.46) were significantly higher than that in the U‐pH ≥6.5 group as a reference for men, but not for women. After adjustment of age, obesity, fasting glucose, smoking and alcohol drinking habits, family history of diabetes mellitus, and use of drugs for hypertension and dyslipidemia, HR in the U‐pH ≤5.0 group (HR 1.39) was significantly higher than that in the U‐pH ≥6.5 group for men, but not for women.
Conclusions
Low U‐pH predicts new onset of diabetes mellitus in a general population of men.
Keywords: Bicarbonate ions, Nonvolatile acid, Urine pH
We investigated the relationship between urine pH (U‐pH) and the development of diabetes mellitus during a 10‐year period in a general population of subjects who received annual health examinations (n = 12,476, men/women: 8,027/4,449). After adjustment of confounders, hazard ratio in the group of U‐pH ≤ 5.0 (HR: 1.39) was significantly higher than that in the group of U‐pH ≥ 6.5 in men but not in women. Low U‐pH predicts new onset of diabetes mellitus in a general population of male subjects.

Introduction
Diabetes mellitus is a critical issue, as it compromises healthy longevity by multiple complications, including atherosclerotic cardiovascular disease and diabetic nephropathy 1 , 2 . The prevalence of diabetes mellitus is continuously increasing worldwide as a result of changes in lifestyle, such as eating habits and exercise 3 . Therefore, it is important to identify individuals at high risk for new onset of diabetes mellitus and to carry out appropriate intervention, such as dietary advice and exercise encouragement, at a stage before the onset of diabetes mellitus 4 .
In the body, acidic substances are constantly produced by dietary components or cellular metabolism, and are excreted from the body through the lungs and kidneys to maintain homeostasis 5 . Volatile acids are loaded as CO2 by cellular aerobic metabolism and are excreted by pulmonary respiration 5 . The amount of volatile acids metabolized and excreted in the body is approximately 15,000 mEq/day 6 . In contrast, non‐volatile acids, such as sulfuric acid, phosphoric acid and lactic acid, are loaded by the diet and cell metabolism, and are excreted from the kidneys as urine 7 , 8 . The amount of non‐volatile acids excreted from the body is approximately 1 mEq/kg/day 9 , and acid loading in urine results in lowered urine pH (U‐pH). Therefore, U‐pH is an index of acid loading in the body.
It has been shown that U‐pH is inversely correlated with bodyweight and body mass index (BMI) 10 , 11 , 12 . A low level of U‐pH has also been reported to be associated with metabolic disorders, such as metabolic syndrome 13 , 14 , insulin resistance 15 , 16 , impaired glucose tolerance 17 , diabetes mellitus 18 and non‐alcoholic fatty liver disease 19 , 20 . In a longitudinal analysis, a low level of U‐pH was shown to be a risk factor for new onset of metabolic syndrome in a 5‐year period 21 . Furthermore, logistic regression analysis in a cohort study of 3,119 men showed that the risk of development of diabetes mellitus during a 5‐year period was significantly higher in men with U‐pH ≤5.0 than in men with U‐pH ≥ 6.5 (odds ratio 2.69) 22 . However, whether such a relationship between U‐pH and new onset of diabetes mellitus exists in women and in other large cohorts has not yet been fully investigated. Therefore, we investigated the relationship between basal levels of U‐pH and new onset of diabetes mellitus during a 10‐year period in a general population using a Cox proportional hazards analysis divided into groups by sex.
Methods
The present study was carried out as a project of the Broad‐range Organization for REnal, Arterial and cardiac studies by Sapporo Medical University Affiliates (BOREAS) investigators. The study was a retrospective analysis of data in a prospectively followed cohort designed as the BOREAS‐DM1 study. The study conformed to the principles outlined in the Declaration of Helsinki, and was carried out with the approval of the institutional ethical committee of Sapporo Medical University (Number: 29‐2‐64). Written informed consent was obtained from all of the participants.
Study participants and clinical end‐point
All of the individuals who received annual health examinations at Keijinkai Maruyama Clinic, Sapporo, Japan, in 2006 were enrolled in this registry (n = 28,990). A flow chart of the study participants is shown in Figure 1. Prespecified exclusion criteria were the absence of data for U‐pH, urinary protein, serum creatinine, fasting glucose and hemoglobin A1c (HbA1c) at baseline, and diagnosis of diabetes mellitus and/or chronic kidney disease at baseline. After exclusion, a total of 12,476 individuals (men/women: 8,027/4,449) who received health examinations at least once in the period from 2007 to 2016 were recruited in the present study. The clinical end‐point was the development of diabetes mellitus. The use of antidiabetic drugs during a 10‐year follow‐up period was checked in annual health checkups.
Figure 1.
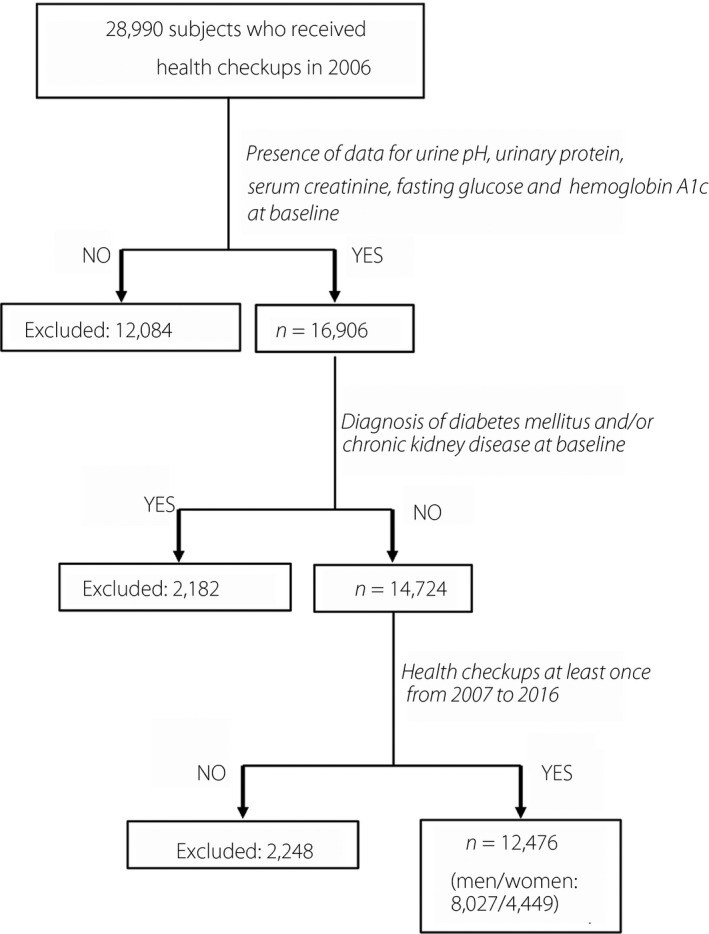
Flow chart of the selected study participants. Among 28,990 participants enrolled in 2006, a total of 12,476 participants (men/women: 8,027/4,449) were finally recruited for analyses in the present study.
Measurement
Medical examinations, blood pressure measurements, and samplings of urine and blood were carried out after an overnight fast. U‐pH and urinary protein were examined by the dipstick method. Body height and weight were measured in light clothing without shoes, and BMI was calculated as bodyweight in kilograms divided by height in meters squared. HbA1c level was presented as National Glycohemoglobin Standardization Program equivalent value. As an indicator of renal function, estimated glomerular filtration rate (eGFR) was calculated from data for serum creatinine, age and sex using the following equation: eGFR (mL/min/1.73 m2) = 194 × serum creatinine(−1.094) × age(−0.287) × 0.739 (if female) 23 . A self‐administered questionnaire survey was carried out to obtain information on smoking habit, alcohol drinking habit, family history of diabetes mellitus, and use of drugs for diabetes mellitus, hypertension and dyslipidemia. Obesity was defined as BMI ≥25 kg/m2, and chronic kidney disease was defined as eGFR <60 mL/min/1.73 m2 or positive for urinary protein. Diabetes mellitus was diagnosed in accordance with the guidelines of the American Diabetes Association 24 : fasting glucose ≥126 mg/dL, HbA1c ≥ 6.5% or self‐reported use of antidiabetic drugs.
Statistical analysis
Numeric variables are expressed as means ± standard deviation for parameters with normal distributions and as medians (interquartile ranges) for parameters with skewed distributions. The distribution of each parameter was tested for its normality using the Shapiro–Wilk W‐test. Clinical parameters were separated by men and women, and each sex group was divided into four subgroups according to baseline U‐pH levels: groups of U‐pH ≤5.0, 5.5, 6.0 and ≥6.5. Comparison between two groups was carried out with Student’s t‐test for parametric parameters, and with Mann–Whitney’s U‐test for non‐parametric parameters. Intergroup differences in percentages of demographic parameters were examined by the χ2‐test. One‐way analysis of variance was used for detecting significant differences between data in multiple groups. Intergroup differences in survival rates were examined by log–rank tests of Kaplan–Meier curves. The hazard ratio (HR) and 95% confidence interval (CI) for the development of diabetes mellitus as a clinical end‐point were calculated by using a Cox proportional hazards model with adjustment of confounders, including age, obesity, fasting glucose (≥100 mg/dL), smoking habit, alcohol drinking habit, family history of diabetes mellitus, and use of drugs for hypertension and dyslipidemia at baseline. Each parameter of liver function, including aspartate aminotransferase, alanine aminotransferase and γ‐glutamyl transpeptidase, was also incorporated as a covariate in additional Cox proportional hazards models. A P‐value of <0.05 was considered statistically significant. All data were analyzed by using EZR software (Saitama, Japan) 25 .
Results
Characteristics of the study participants
Characteristics of the recruited and excluded individuals are shown in Table S1. As individuals with diabetes mellitus and/or chronic kidney disease at baseline were excluded, the excluded individuals had significantly higher levels of fasting glucose and HbA1c, and lower levels of eGFR than did the recruited individuals. Demographic parameters and metabolic profiles of the recruited individuals are summarized in Table 1. Women had significantly lower BMI, lower blood pressure, lower levels of albumin, uric acid, fasting glucose, HbA1c, aspartate aminotransferase, alanine aminotransferase, γ‐glutamyl transpeptidase, total cholesterol, low‐density lipoprotein cholesterol and triglycerides, and higher levels of eGFR, high‐density lipoprotein cholesterol and U‐pH than did men. The frequencies of obesity and habits of smoking and alcohol drinking were significantly lower in women than in men.
Table 1.
Characteristics of the recruited participants
| Total | Men | Women | P | |
|---|---|---|---|---|
| n = 12,476 | n = 8,027 | n = 4,449 | ||
| Age (years) | 47 ± 9 | 48 ± 9 | 47 ± 9 | <0.001 |
| Body mass index | 23.1 ± 3.3 | 23.9 ± 3.0 | 21.6 ± 3.2 | <0.001 |
| Obesity | 3,780 (30.3) | 3,055 (38.1) | 725 (16.3) | <0.001 |
| Systolic blood pressure (mmHg) | 116 ± 16 | 119 ± 15 | 110 ± 16 | <0.001 |
| Diastolic blood pressure (mmHg) | 74 ± 11 | 77 ± 10 | 69 ± 10 | <0.001 |
| Smoking habit | 4,362 (35.8) | 3,532 (45.1) | 830 (19.1) | <0.001 |
| Alcohol drinking habit | 3,274 (26.2) | 2,742 (34.2) | 532 (12.0) | <0.001 |
| Family history of DM | 2,174 (17.4) | 1,270 (15.8) | 904 (20.3) | <0.001 |
| Medications | ||||
| Antihypertensive drugs | 714 (9.9) | 809 (10.1) | 299 (6.7) | <0.001 |
| Antidyslipidemic drugs | 1,108 (8.9) | 315 (3.9) | 171 (3.8) | 0.836 |
| Biochemical data | ||||
| Albumin (g/dL) | 4.3 ± 0.2 | 4.4 ± 0.2 | 4.3 ± 0.2 | <0.001 |
| eGFR (mL/min/1.73m2) | 85 ± 13 | 84 ± 12 | 87 ± 14 | <0.001 |
| Uric acid (mg/dL) | 5.4 ± 1.4 | 6.0 ± 1.2 | 4.3 ± 0.9 | <0.001 |
| Fasting glucose (mg/dL) | 89 ± 9 | 91 ± 9 | 86 ± 8 | <0.001 |
| HbA1c (%) | 5.2 ± 0.3 | 5.2 ± 0.3 | 5.1 ± 0.3 | <0.001 |
| AST (U/L) | 21 (18–26) | 22 (19–28) | 19 (16–22) | <0.001 |
| ALT (U/L) | 20 (15–30) | 25 (18–36) | 15 (12–20) | <0.001 |
| γGTP (U/L) | 30 (19–55) | 62 (27–72) | 18 (14–26) | <0.001 |
| Total cholesterol (mg/dL) | 204 ± 33 | 205 ± 33 | 203 ± 33 | 0.052 |
| LDL cholesterol (mg/dL) | 121 ± 30 | 123 ± 30 | 118 ± 30 | <0.001 |
| HDL cholesterol (mg/dL) | 61 ± 15 | 56 ± 14 | 69 ± 15 | <0.001 |
| Triglycerides (mg/dL) | 91 (63–135) | 109 (77–157) | 67 (50–93) | <0.001 |
| Urine pH | 5.9 ± 0.7 | 5.9 ± 0.7 | 6.0 ± 0.8 | <0.001 |
Variables are expressed as number (%), mean ± standard deviation or median (interquartile ranges).
Obesity was defined as body mass index ≥25. γGTP, gamma glutamyl transpeptidase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
The recruited participants were divided into four groups according to their U‐pH levels: groups of U‐pH ≤5.0, 5.5, 6.0 and ≥6.5. Basal characteristics of men and women in the four groups at baseline are shown in Table 2. There were significant differences in BMI, presence of obesity, and levels of fasting glucose and HbA1c in the four groups of men, but there were no significant differences in the four groups of women. Men in the low U‐pH groups tended to have higher BMI, higher frequency of obesity and higher level of fasting glucose than did men in the high U‐pH groups.
Table 2.
Characteristics of the participants divided by urine pH at baseline
| U‐pH ≤5.0 | U‐pH = 5.5 | U‐pH = 6.0 | U‐pH ≥6.5 | P | |
|---|---|---|---|---|---|
| n | |||||
| Men | 1,610 | 2,442 | 1,675 | 2,300 | – |
| Women | 987 | 1,171 | 814 | 1,477 | – |
| Age (years) | |||||
| Men | 50 ± 8 | 47 ± 9 | 47 ± 9 | 48 ± 10 | <0.001 |
| Women | 48 ± 9 | 46 ± 9 | 47 ± 10 | 48 ± 9 | <0.001 |
| Body mass index | |||||
| Men | 24.2 ± 3.1 | 23.8 ± 3.1 | 23.8 ± 3.0 | 23.6 ± 2.9 | <0.001 |
| Women | 21.5 ± 3.2 | 21.6 ± 3.3 | 21.6 ± 3.4 | 21.7 ± 3.1 | 0.568 |
| Obesity † | |||||
| Men | 713 (44.3) | 916 (37.5) | 630 (37.6) | 796 (34.6) | <0.001 |
| Women | 155 (15.7) | 203 (17.3) | 134 (16.5) | 233 (15.8) | 0.684 |
| Fasting glucose (mg/dL) | |||||
| Men | 92 ± 10 | 91 ± 9 | 91 ± 8 | 90 ± 8 | <0.001 |
| Women | 86 ± 8 | 86 ± 8 | 86 ± 8 | 85 ± 8 | 0.510 |
| HbA1c (%) | |||||
| Men | 5.3 ± 0.4 | 5.2 ± 0.3 | 5.2 ± 0.3 | 5.2 ± 0.3 | <0.001 |
| Women | 5.2 ± 0.3 | 5.1 ± 0.3 | 5.1 ± 0.3 | 5.2 ± 0.3 | 0.002 |
Variables are expressed as number (%) or mean ± standard deviation.
Obesity was defined as body mass index ≥25. HbA1c, hemoglobin A1c; U‐pH, urine pH.
Cumulative incidence for new onset of diabetes mellitus during the follow‐up period
The mean follow‐up period was 6.8 years (range 1–10 years), and follow‐up summation was 84,808 (men/women: 54,634/30,174) person‐years. Among the 12,476 recruited participants, 521 men (6.5%) and 132 women (3.0%) had new onset of diabetes mellitus during a 10‐year period. The cumulative incidence of new onset of diabetes mellitus was 7.5% (men/women: 9.3%/4.4%, 95% CI 7.0–8.1) per 100 person‐years.
Impact of baseline U‐pH on the clinical endpoint during a 10‐year follow‐up period
Kaplan–Meier survival curves showed that there was a significant intergroup difference in the cumulative incidence of new onset of diabetes mellitus for men, but not for women (Figure 2). Cox proportional hazards analysis for new onset of diabetes mellitus during a 10‐year follow‐up period showed that HRs in the U‐pH ≤5.0 (HR 1.93) and U‐pH 5.5 groups (HR 1.46) were significantly higher than that in the U‐pH ≥6.5 group as a reference for men (Table 3). After adjustment of age, obesity and fasting glucose (model 1), HRs in the U‐pH ≤5.0 (HR 1.52) and U‐pH 5.5 groups (HR: 1.33) were significantly higher than that in the U‐pH ≥6.5 group for men. After adjustment of age, obesity, fasting glucose, smoking and alcohol drinking habits, family history of diabetes mellitus, and the use of drugs for hypertension and dyslipidemia (model 2), HR in the U‐pH ≤5.0 group (HR 1.39) was still significantly higher than that in the U‐pH ≥6.5 group as a reference for men. When each parameter of liver function, including aspartate aminotransferase, alanine aminotransferase and γ‐glutamyl transpeptidase, was additionally incorporated as a covariate, HRs in the U‐pH ≤5.0 group were still significantly higher than those in the U‐pH ≥6.5 group as a reference for men (Table S2). In women, there was no significant difference between HRs of the four U‐pH groups (Table 4, Table S3).
Figure 2.
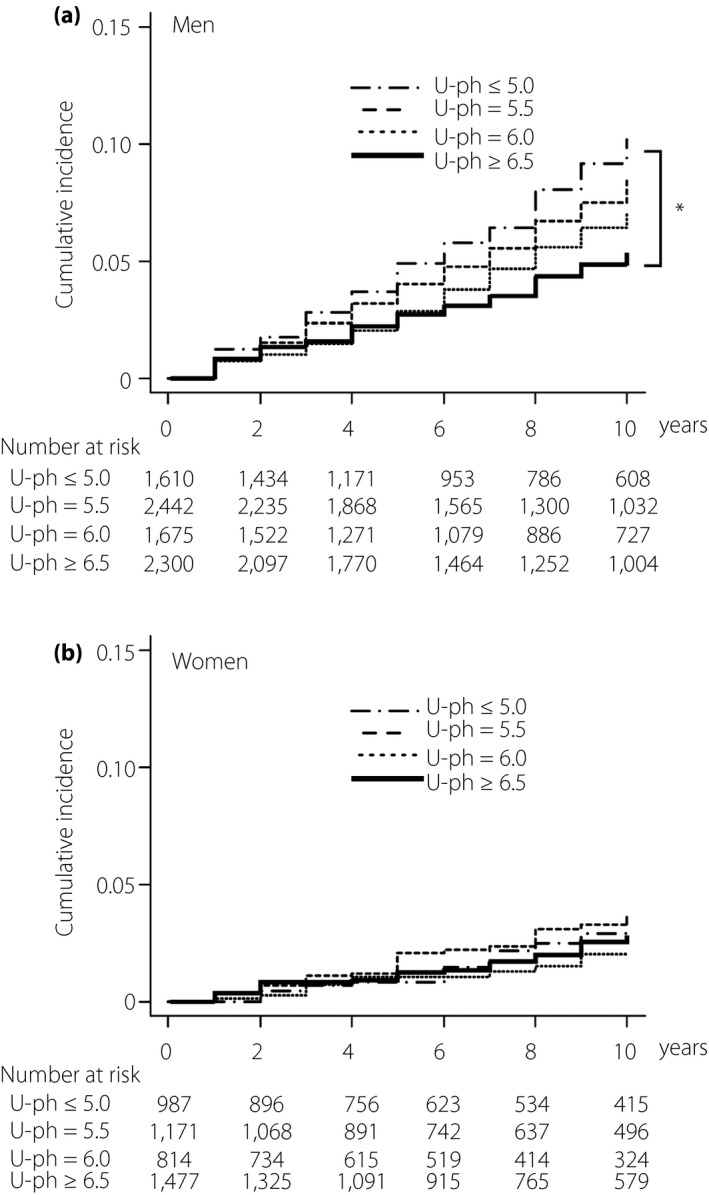
Cumulative incidence of diabetes mellitus in four groups of urine pH at baseline. (a) Kaplan–Meier survival curve analyses for cumulative incidence of diabetes mellitus in men (n = 8,027) with baseline urine pH (U‐pH) ≤5.0 (n = 1,610), 5.5 (n = 2,442), 6.0 (n = 1,675) and ≥6.5 (n = 2,300). (b) Kaplan–Meier survival curve analyses for cumulative incidence of diabetes mellitus in women (n = 4,449) with baseline urine pH (U‐pH) ≤5.0 (n = 987), 5.5 (n = 1,171), 6.0 (n = 814) and ≥6.5 (n = 1,477). *P < 0.001.
Table 3.
Cox proportional hazard analyses for new onset of diabetes mellitus in men
| Unadjusted | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| U‐pH ≤5.0 | 1.93 (1.54–2.52) | <0.001 | 1.52 (1.18–1.95) | <0.001 | 1.39 (1.07–1.79) | 0.001 |
| U‐pH 5.5 | 1.46 (1.15–1.85) | 0.001 | 1.33 (1.04–1.69) | 0.002 | 1.17 (0.91–1.50) | 0.197 |
| U‐pH 6.0 | 1.18 (0.89–1.55) | 0.231 | 1.09 (0.83–1.44) | 0.504 | 1.05 (0.79–1.39) | 0.711 |
| U‐pH ≥6.5 | Reference | – | Reference | – | Reference | – |
| Age (per 10 years) | – | – | 1.17 (1.06–1.30) | <0.001 | 1.18 (1.06–1.32) | <0.001 |
| Obesity † | – | – | 1.91 (1.60–2.29) | <0.001 | 1.76 (1.46–2.12) | <0.001 |
| Fasting glucose (≥100 mg/dL) | – | – | 9.7 (8.0–11.7) | <0.001 | 9.7 (8.0–11.8) | <0.001 |
| Smoking habit | – | – | – | – | 1.76 (1.46–2.11) | <0.001 |
| Alcohol drinking habit | – | – | – | – | 0.83 (0.68–1.05) | 0.140 |
| Family history of DM | – | – | – | – | 1.41 (1.15–1.73) | <0.001 |
| Antihypertensive drugs | – | – | – | – | 1.20 (0.93–1.54) | 0.145 |
| Antidyslipidemic drugs | – | – | – | – | 1.63 (1.18–2.25) | <0.001 |
| (AIC 8,960) | (AIC 8,195) | (AIC 7,788) | ||||
n = 8,027. †Obesity was defined as body mass index ≥25. AIC, Akaike's information criterion; CI, confidence interval; DM, diabetes mellitus; HR, hazard ratio; U‐pH, urine pH.
Table 4.
Cox proportional hazards analyses for new onset of diabetes mellitus in women
| Unadjusted | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| U‐pH ≤5.0 | 1.18 (0.73–1.90) | 0.481 | 1.03 (0.64–1.66) | 0.888 | 1.02 (0.62–1.67) | 0.930 |
| U‐pH 5.5 | 1.25 (0.80–1.95) | 0.322 | 1.11 (0.70–1.75) | 0.635 | 1.17 (0.74–1.86) | 0.494 |
| U‐pH 6.0 | 1.08 (0.64–1.81) | 0.762 | 0.99 (0.59–1.67) | 0.987 | 1.01 (0.60–1.72) | 0.952 |
| U‐pH ≥6.5 | Reference | – | Reference | – | Reference | – |
| Age (per 10 years) | – | – | 1.31 (1.08–1.58) | 0.004 | 1.35 (1.10–1.66) | 0.004 |
| Obesity † | – | – | 2.61 (1.82–3.73) | <0.001 | 2.61 (1.80–3.77) | <0.001 |
| Fasting glucose (≥100 mg/dL) | – | – | 13.3 (9.2–19.0) | <0.001 | 12.4 (8.5–17.9) | <0.001 |
| Smoking habit | – | – | – | – | 1.38 (0.89–2.15) | 0.142 |
| Alcohol drinking habit | – | – | – | – | 0.93 (0.54–1.60) | 0.797 |
| Family history of DM | – | – | – | – | 1.73 (1.18–2.54) | 0.004 |
| Antihypertensive drugs | – | – | – | – | 1.20 (0.72–2.00) | 0.464 |
| Antidyslipidemic drugs | – | – | – | – | 1.65 (0.92–9.75) | 0.091 |
| (AIC 2,104) | (AIC 1,871) | (AIC 1,802) | ||||
n = 4,449. †Obesity was defined as body mass index ≥25. AIC, Akaike's information criterion; CI, confidence interval; DM, diabetes mellitus; HR, hazard ratio; U‐pH, urine pH.
Discussion
The present study using a large number of participants (n = 12,476, men/women: 8,027/4,449) who received an annual health checkup showed that a low level of U‐pH was significantly associated with the risk of diabetes mellitus development during a 10‐year period in men who had no diabetes mellitus and/or chronic kidney disease at baseline. It has been reported that a low level of U‐pH is associated with several metabolic disorders 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 . A previous study showed an association between low U‐pH and new onset of diabetes mellitus in men 22 , similar to results of the present study. The risk of diabetes mellitus development in men with U‐pH ≤5.0 (odds ratio 2.69; participants with U‐pH ≥6.5 as a reference) in the previous study 22 seemed to be higher than that in the present study (HR 1.93 in the unadjusted model). In the previous study 22 , the cumulative incidence of diabetes mellitus was not analyzed, and only men (n = 3,119) were examined for a 5‐year period. In the present study, we included more than a twofold larger number of men and longer follow‐up period, and showed a significant association of low U‐pH with the risk of new‐onset diabetes mellitus in men after adjustment of age, obesity, fasting glucose, liver function, smoking and alcohol drinking habits, family history of diabetes mellitus, and the use of drugs for hypertension and dyslipidemia. In addition, we found a significant sex difference in the relationship between U‐pH and the risk of new‐onset diabetes mellitus: there was no significant association between U‐pH and new onset of diabetes mellitus during a 10‐year follow up in women.
In the body, non‐volatile acidic substances are constantly loaded by the diet and cell metabolism, and H+ is excreted from the kidneys with bicarbonate ions, titrated acids (such as phosphate) and ammonia produced from glutamine in the proximal tubule 5 , 7 , 26 , 27 . Therefore, net acid excretion 28 and dietary acid load 29 , 30 are associated with low U‐pH level. It has been reported that accumulation of non‐volatile acids is associated with metabolic abnormalities, including impaired glucose tolerance, insulin resistance and diabetes mellitus 12 , 31 , 32 , 33 , 34 . Insulin is directly involved in the excretion of H+ from the kidneys through the activation of Na+/H+ exchanger 3 26 , 27 , suggesting that hyperinsulinemia as a result of insulin resistance decreases U‐pH level. In addition, renal production of ammonia, which titrates the acid–base balance, is impaired in a condition of insulin resistance 13 , 14 , 15 , 16 , 35 , 36 . Furthermore, the ratio of urinary ammonium to total urinary acid excretion and net acid excretion by ammonium from the kidneys were shown to be reduced in patients with diabetes mellitus 18 . These findings suggest that metabolic disorders derived from insulin resistance cause acid accumulation in the body, resulting in a low U‐pH level. Conversely, low interstitial fluid pH attenuates the insulin signaling pathway and increases insulin resistance 37 , 38 , 39 , 40 . As U‐pH is positively correlated with body fluid pH 41 , U‐pH level would be a surrogate marker of insulin resistance in the body. In fact, we found that low U‐pH was associated with obesity and high fasting glucose in men who had no diabetes mellitus (Table 2).
In the present study, an association between low U‐pH and new onset of diabetes mellitus was found in men, but not in women. U‐pH has been reported to be higher in women than in men 42 , 43 , which was confirmed in the present study. The rate and amount of urine excretion of citrate, which has a basic property, were reported to be significantly higher in women than in men in a condition of hospitalization with a restricted diet 44 , suggesting that women are protected from lowering of U‐pH level. In fact, a cross‐sectional study using a large number of Korean participants (n = 22,297, men/women: 13,895/8,402) showed that low U‐pH was associated with obesity in men, but not in women 45 . Another cross‐sectional study using apparently healthy Japanese adults (n = 3,629, men/women: 2,311/1,318) also showed that low U‐pH was associated with both high (≥27 kg/m2) and low (<19 kg/m2) levels of BMI 46 . Taken together, the results suggest that differences in physique, citrate excretion rate and/or dietary preference are involved in the sex difference in the relationship between U‐pH and risk for diabetes mellitus.
Another possible reason for the sex difference in the association between low U‐pH and new onset of diabetes mellitus is the difference in estrogen levels. It has been reported that estrogen has favorable effects on glucose homeostasis, and protects against hyperglycemia by reducing hepatic glucose production and enhancing glucose transport in skeletal muscle 47 . Therefore, women, especially those before menopause, might be protected from the acid loading‐induced risk of diabetes mellitus.
The strong points of the present study were that the study participants were recruited from a large number of individuals in a general population, and that new onset of diabetes mellitus during a 10‐year period was examined. However, the present study had some limitations. First, as the present study recruited individuals who received an annual health checkup, the influence of self‐selection bias cannot be denied. Relatively, a more healthy population would be included in the recruited individuals, and the results might be underestimated. As the processing of missing data was based on the listwise method, selection bias was also considered to be a research limitation. Second, only Japanese individuals were recruited in the present study, and it is not clear whether the results apply to other races. Third, there was a possibility of type II error, especially in statistical analyses of women, because the number of women was much smaller than that of men. Fourth, a test paper method using an indicator for U‐pH was used instead of an electrode method because of its convenience and cost. The electrode method is thought to be more reliable, but recent studies have shown the accuracy of the test strip method 48 . Fifth, diet, medication and comorbidities including urinary infection, which might affect U‐pH, were not evaluated in the present study, although individuals with diabetes mellitus and/or chronic kidney disease at baseline were excluded. Finally, the relationship between change in U‐pH and onset of diabetes mellitus was not investigated in the present study, and this relationship needs to be examined in the future.
In conclusion, a low level of U‐pH is an independent predictor of the development of diabetes mellitus in men, but not in women. The pathophysiology underlying the association between acid loading in the body and diabetes mellitus development needs to be addressed in future basic and clinical studies.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 | Characteristics of the recruited and excluded individuals.
Table S2 | Cox proportional hazards analyses for new onset of diabetes mellitus including liver function as a covariate in men.
Table S3 | Cox proportional hazards analyses for new onset of diabetes mellitus including liver function as a covariate in women.
Acknowledgments
This study was supported by Grants for Education and Research 2018 and 2019 from Sapporo Medical University.
J Diabetes Investig. 2020
References
- 1. Partanen J, Niskanen L, Lehtinen J, et al Natural history of peripheral neuropathy in patients with non‐insulin‐dependent diabetes mellitus. N Engl J Med 1995; 333: 89–94. [DOI] [PubMed] [Google Scholar]
- 2. Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes 2015; 6: 1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017; 128: 40–50. [DOI] [PubMed] [Google Scholar]
- 4. Group IDFDA . Update of mortality attributable to diabetes for the IDF Diabetes Atlas: estimates for the year 2013. Diabetes Res Clin Pract 2015; 109: 461–465. [DOI] [PubMed] [Google Scholar]
- 5. Hamm LL, Nakhoul N, Hering‐Smith KS. Acid‐base homeostasis. Clin J Am Soc Nephrol 2015; 10: 2232–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poupin N, Calvez J, Lassale C, et al Impact of the diet on net endogenous acid production and acid‐base balance. Clin Nutr 2012; 31: 313–321. [DOI] [PubMed] [Google Scholar]
- 7. Curthoys NP, Moe OW. Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol 2014; 9: 1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lemann J Jr, Bushinsky DA, Hamm LL. Bone buffering of acid and base in humans. Am J Physiol Renal Physiol 2003; 285: F811–F832. [DOI] [PubMed] [Google Scholar]
- 9. Scialla JJ, Anderson CA. Dietary acid load: a novel nutritional target in chronic kidney disease? Adv Chronic Kidney Dis 2013; 20: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maalouf NM, Sakhaee K, Parks JH, et al Association of urinary pH with body weight in nephrolithiasis. Kidney Int 2004; 65: 1422–1425. [DOI] [PubMed] [Google Scholar]
- 11. Taylor EN, Curhan GC. Body size and 24‐hour urine composition. Am J Kidney Dis 2006; 48: 905–915. [DOI] [PubMed] [Google Scholar]
- 12. Cameron MA, Maalouf NM, Adams‐Huet B, et al Urine composition in type 2 diabetes: predisposition to uric acid nephrolithiasis. J Am Soc Nephrol 2006; 17: 1422–1428. [DOI] [PubMed] [Google Scholar]
- 13. Maalouf NM, Cameron MA, Moe OW, et al Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol 2007; 2: 883–888. [DOI] [PubMed] [Google Scholar]
- 14. Otsuki M, Kitamura T, Goya K, et al Association of urine acidification with visceral obesity and the metabolic syndrome. Endocr J 2011; 58: 363–367. [DOI] [PubMed] [Google Scholar]
- 15. Takahashi S, Inokuchi T, Kobayashi T, et al Relationship between insulin resistance and low urinary pH in patients with gout, and effects of PPARalpha agonists on urine pH. Horm Metab Res 2007; 39: 511–514. [DOI] [PubMed] [Google Scholar]
- 16. Shimodaira M, Okaniwa S, Nakayama T. Association of low urine pH with insulin resistance in non‐diabetic Japanese subjects. Exp Clin Endocrinol Diabetes 2018; 126): 357–361. [DOI] [PubMed] [Google Scholar]
- 17. Yoshida S, Miyake T, Yamamoto S, et al Relationship between urine pH and abnormal glucose tolerance in a community‐based study. J Diabetes Investig 2018; 9: 769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maalouf NM, Cameron MA, Moe OW, et al Metabolic basis for low urine pH in type 2 diabetes. Clin J Am Soc Nephrol 2010; 5: 1277–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyake T, Yoshida S, Yamamoto S, et al Low urine pH is associated with non‐alcoholic fatty liver disease: a community‐based cross‐sectional study. Intern Med 2018; 57: 2799–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okamura T, Hashimoto Y, Hamaguchi M, et al Low urine pH is a risk for non‐alcoholic fatty liver disease: a population‐based longitudinal study. Clin Res Hepatol Gastroenterol 2018; 42: 570–576. [DOI] [PubMed] [Google Scholar]
- 21. Hara S, Tsuji H, Ohmoto Y, et al High serum uric acid level and low urine pH as predictors of metabolic syndrome: a retrospective cohort study in a Japanese urban population. Metabolism 2012; 61: 281–288. [DOI] [PubMed] [Google Scholar]
- 22. Hashimoto Y, Hamaguchi M, Nakanishi N, et al Urinary pH is a predictor of diabetes in men; a population based large scale cohort study. Diabetes Res Clin Pract 2017; 130: 9–14. [DOI] [PubMed] [Google Scholar]
- 23. Matsuo S, Imai E, Horio M, et al Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 24. American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‐2019. Diabetes Care 2019; 42: S13–S28. [DOI] [PubMed] [Google Scholar]
- 25. Kanda Y. Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant 2013; 48: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klisic J, Hu MC, Nief V, et al Insulin activates Na(+)/H(+) exchanger 3: biphasic response and glucocorticoid dependence. Am J Physiol Renal Physiol 2002; 283: F532–F539. [DOI] [PubMed] [Google Scholar]
- 27. Fuster DG, Bobulescu IA, Zhang J, et al Characterization of the regulation of renal Na+/H+ exchanger NHE3 by insulin. Am J Physiol Renal Physiol 2007; 292: F577–F585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Remer T, Berkemeyer S, Rylander R, et al Muscularity and adiposity in addition to net acid excretion as predictors of 24‐h urinary pH in young adults and elderly. Eur J Clin Nutr 2007; 61: 605–609. [DOI] [PubMed] [Google Scholar]
- 29. Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc 1995; 95: 791–797. [DOI] [PubMed] [Google Scholar]
- 30. Welch AA, Mulligan A, Bingham SA, et al Urine pH is an indicator of dietary acid‐base load, fruit and vegetables and meat intakes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)‐Norfolk population study. Br J Nutr 2008; 99: 1335–1343. [DOI] [PubMed] [Google Scholar]
- 31. Farwell WR, Taylor EN. Serum bicarbonate, anion gap and insulin resistance in the National Health and Nutrition Examination Survey. Diabet Med 2008; 25: 798–804. [DOI] [PubMed] [Google Scholar]
- 32. Reaven GM, Hollenbeck C, Jeng CY, et al Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes 1988; 37: 1020–1024. [DOI] [PubMed] [Google Scholar]
- 33. Tai ES, Tan ML, Stevens RD, et al Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian‐Indian men. Diabetologia 2010; 53: 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fagherazzi G, Vilier A, Bonnet F, et al Dietary acid load and risk of type 2 diabetes: the E3N‐EPIC cohort study. Diabetologia 2014; 57: 313–320. [DOI] [PubMed] [Google Scholar]
- 35. Weiner ID, Verlander JW. Role of NH3 and NH4+ transporters in renal acid‐base transport. Am J Physiol Renal Physiol 2011; 300: F11–F23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palmer BF, Clegg DJ. Electrolyte and acid‐base disturbances in patients with diabetes mellitus. N Engl J Med 2015; 373: 548–559. [DOI] [PubMed] [Google Scholar]
- 37. Souto G, Donapetry C, Calvino J, et al Metabolic acidosis‐induced insulin resistance and cardiovascular risk. Metab Syndr Relat Disord 2011; 9: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aoi W, Hosogi S, Niisato N, et al Improvement of insulin resistance, blood pressure and interstitial pH in early developmental stage of insulin resistance in OLETF rats by intake of propolis extracts. Biochem Biophys Res Commun 2013; 432: 650–653. [DOI] [PubMed] [Google Scholar]
- 39. Aoi W, Marunaka Y. Importance of pH homeostasis in metabolic health and diseases: crucial role of membrane proton transport. Biomed Res Int 2014; 2014: 598986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marunaka Y. Roles of interstitial fluid pH in diabetes mellitus: glycolysis and mitochondrial function. World J Diabetes 2015; 6: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ring T, Nielsen S. Whole body acid‐base modeling revisited. Am J Physiol Renal Physiol 2017; 312: F647–F653. [DOI] [PubMed] [Google Scholar]
- 42. Curhan GC, Taylor EN. 24‐h uric acid excretion and the risk of kidney stones. Kidney Int 2008; 73: 489–496. [DOI] [PubMed] [Google Scholar]
- 43. Lieske JC, Turner ST, Edeh SN, et al Heritability of urinary traits that contribute to nephrolithiasis. Clin J Am Soc Nephrol 2014; 9: 943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Worcester EM, Bergsland KJ, Gillen DL, et al Mechanism for higher urine pH in normal women compared with men. Am J Physiol Renal Physiol 2018; 314: F623–F629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Song JH, Doo SW, Yang WJ, et al Influence of obesity on urinary pH with respect to sex in healthy Koreans. Urology 2011; 78: 1244–1247. [DOI] [PubMed] [Google Scholar]
- 46. Nakajima K, Oda E, Kanda E. Latent association between low urine pH and low body weight in an apparently healthy population. Scand J Clin Lab Invest 2016; 76: 58–63. [DOI] [PubMed] [Google Scholar]
- 47. Tramunt B, Smati S, Grandgeorge N, et al Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020; 63: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Desai RA, Assimos DG. Accuracy of urinary dipstick testing for pH manipulation therapy. J Endourol 2008; 22: 1367–1370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Characteristics of the recruited and excluded individuals.
Table S2 | Cox proportional hazards analyses for new onset of diabetes mellitus including liver function as a covariate in men.
Table S3 | Cox proportional hazards analyses for new onset of diabetes mellitus including liver function as a covariate in women.


