Abstract
Aims/Introduction
To evaluate gestational weight gain (GWG) in the first and second trimester as a risk factor for gestational diabetes mellitus (GDM) among women pregnant with singletons.
Materials and Methods
This was a cohort study of women with singleton pregnancies who delivered between 1 January 2013 and 31 October 2014 in a Chinese hospital. We collected data from medical records from the first antenatal visit to delivery. All pregnant women were subjected to an oral glucose tolerance test for diagnosis of GDM during the second trimester. GWG in the first and second trimester was calculated by subtracting the prepregnancy weight from weight within 4 weeks of the oral glucose tolerance test. We categorized GWG into insufficient, appropriate and excessive according to the Institute of Medicine guidelines and population quantiles. Univariable and multivariable analyses were used to determine the association between GWG and GDM risk.
Results
Of 10,422 pregnant women, we identified 8,356 eligible women with 1,622 (19.4%) diagnosed with GDM. Univariable analysis showed that GWG that exceeded the Institute of Medicine recommendation might be associated with risk of GDM (P < 0.05), but this association was not observed by multivariable analysis (adjusted odds ratio 1.07, [95% confidence interval 0.94–1.21]). Univariable and multivariable analyses both showed that GWG exceeding the 90th and 95th quantiles of included women, respectively, were at increased risk for GDM (adjusted odds ratio >P90 vs P10–P90 adjusted odds ratio 1.31, [95% confidence interval 1.12–1.52]; >P95 vs P5‐P95 adjusted odds ratio 1.45 [95% confidence interval 1.16–1.81]).
Conclusions
Excessive GWG in the first and second trimester might be a risk factor for GDM, which highlights the importance of appropriate weight gain during pregnancy.
Keywords: China, Gestational diabetes mellitus, Gestational weight gain
To determine whether excessive gestational weight gain in the first and second trimester is a risk factor for developing gestational diabetes (GDM) among women pregnant with singletons, we carried out this cohort study based on repeated measures data of 10,422 pregnant women in China. All pregnant women were diagnosed with GDM by the 75g oral glucose tolerance test, and gestational weight gain was categorized into insufficient, appropriate and excessive according to the Institute of Medicine guidelines and population quantiles, respectively. Our study found that excessive gestational weight gain in the first and second trimester might be a risk factor for developing GDM, highlighting the importance of maintaining appropriate weight gain during pregnancy to prevent GDM.

Introduction
Gestational diabetes mellitus (GDM), one of the most common metabolic disorders during pregnancy, is defined as glucose intolerance first developed or diagnosed in the second and third trimester of pregnancy. The prevalence of GDM has increased over the past few decades along with increased prevalence of obesity, increased maternal age and more sensitive diagnostic criteria for GDM 1 . Given that the prevalence of GDM might be as high as 21% in Asian countries 1 , and GDM can contribute to severe adverse outcomes, such as pre‐eclampsia, cesarean delivery, shoulder dystocia, macrosomia, neonatal hypoglycemia 2 , 3 and increased risk for development of subsequent type 2 diabetes mellitus and obesity for both mother and child 4 , 5 , prevention of GDM during prenatal care is of great importance.
Weight management is an important consideration during pregnancy. Being overweight or obese before pregnancy is a risk factor for GDM 6 , but the effect of gestational weight gain (GWG) on GDM has not been clarified. Few studies have focused on the association between GWG and GDM, and these studies reached conflicting conclusions. Some studies showed that excessive GWG might increase the risk for developing GDM 7 , 8 , whereas others failed to reach this conclusion 6 , 9 , 10 . Some of these studies used GWG at delivery 6 , 9 , rather than GWG before diagnosis of GDM, which might have confounded the results. Furthermore, as GWG 11 and maternal metabolism changes vary across gestational trimesters 12 , some studies have evaluated whether excessive GWG during the first or second trimester is a predictor for developing GDM 7 , 13 , 14 , 15 , 16 .
In 2009, the Institute of Medicine (IOM) guideline recommended the appropriate GWG stratified by the prepregnancy body mass index (BMI) 17 . However, the IOM did not indicate whether GWG was associated with the risk for developing GDM. Therefore, we carried out this cohort study based on repeated measures data of pregnant women in Southwest China to determine whether GWG before diagnosis of GDM was associated with increased risk for developing GDM.
Methods
Study setting
This was a retrospective cohort study carried out at the West China Women and Children’s Hospital of Sichuan University, which is a referral hospital located in southwest China, and carried out >7,000 deliveries each year. Pregnant woman set up a specific electronic file at the first antenatal visit before the 15th gestational week, then had regular follow‐up visits every 2–4 weeks until delivery. Maternal weight was measured at each visit. All pregnant women were required to routinely receive a 75‐g oral glucose tolerance test (OGTT) during the gestational 24–28th weeks for diagnosis of GDM. The study was approved by the Medical Ethics Committee of West China Second Hospital, Sichuan University (No. 2016‐028). The data in our study are available on request.
Eligibility criteria
We included women pregnant with singletons who delivered between 1 January 2013 and 31 October 2014 at the target hospital, and had registered at the first prenatal visit before 15 gestational weeks.
We excluded individuals with diabetes mellitus before the first prenatal visit, based on self‐report and confirmation by clinicians. We also excluded patients who did not undergo the OGTT within 2 weeks of the 24–28th gestational week target range (e.g., <22nd gestational week or >30th gestational week). Patients who did not have gestational weight measured within 4 weeks before OGTT were also excluded. Finally, we also excluded obese women (BMI >30 kg/m2), because the number of individuals in this category was too small to provide a representative sample.
Data collection
Data were collected using a predefined and pilot‐tested case report form by research staff who had been trained in the study procedures and administration of case report forms. They extracted the necessary data from medical charts and electronic medical records, and double‐entered all of the data into a research database. Data quality was checked for consistency, and information not recorded in medical records was regarded as missing.
Data in our study included maternal demographics (e.g., maternal age, years of education, place of residence), history of diseases (e.g., GDM), history of gestation and birth (e.g., gravidity, parity, in vitro fertilization), maternal anthropometry (height, prepregnancy weight, maternal weight measured at each prenatal visit) and gestational comorbidities.
Gestational comorbidities included cardiovascular diseases (e.g., congenital heart disease, rheumatic heart disease, cardiomyopathy, hypertensive heart disease, pericarditis, arrhythmia, chronic hypertension), viral hepatitis (e.g., surface antigen of the hepatitis B virus‐positive), endocrine diseases (e.g., hyperthyroidism, subclinical hypothyroidism, hypothyroidism, thyroiditis, diabetes), hematological diseases (e.g., iron deficiency anemia, megaloblastic anemia, aplastic anemia, thalassemia), respiratory diseases (e.g., thoracic deformity, bronchial asthma, pulmonary tuberculosis), urinary diseases (e.g., acute urinary tract infection, chronic glomerulonephritis, nephrotic syndrome, chronic renal insufficiency), immune diseases (e.g., systemic lupus erythematosus, antiphospholipid antibody syndrome, Sjogren’s syndrome), neuropsychiatric diseases (e.g., epilepsy, depression, schizophrenia, anxiety disorder) and reproductive diseases (e.g., fibroids, adenomyosis, syphilis, AIDS, gonorrhea, ovarian cyst, polycystic ovary syndrome, colpomycosis, endometrial polyp).
Diagnosis of GDM
According to the Guideline for Diagnosis of GDM issued by the Obstetrics Group of Chinese Medical Association in 2014 18 , we diagnosed GDM using the 75‐g OGTT test. If any value of fasting blood glucose, or at 1 or 2 h after OGTT, was higher than 5.1, 10.0 or 8.5 mmol/L, respectively, then GDM was diagnosed.
Determination of gestational weight gain
Gestational weight gain (GWG) was determined as maternal weight gain before diagnosis of GDM, which was the latest measurement within 4 weeks before the OGTT test. Self‐reported prepregnancy weight, confirmed by doctors at the first prenatal visit, was subtracted from the value taken before the OGTT test to obtain GWG.
We used two classification methods to categorize GWG into insufficient, appropriate and excessive, stratified by prepregnancy BMI (underweight <18.5 kg/m2, normal 18.5–24.9 kg/m2 and overweight 25.0–29.9 kg/m2) 19 . According to the USA IOM recommendations 17 , we calculated the appropriate range of GWG for the gestational week at which maternal weight was measured (Table 1). In addition, we determined the appropriate GWG ranges based on the percentiles of GWG among the whole population (Table 2).
Table 1.
Gestational weight gain recommended by the Institute of Medicine guideline
| Prepregnancy BMI (WHO) | First trimester GWG range | Second & third trimester rate of GWG | Range of GWG before OGTT † | |
|---|---|---|---|---|
| Underweight | <18.5 kg/m2 | 0.5–2.0 kg | 0.51 (0.44–0.58) kg/week | 0.5 + (w‐13) × 0.44–2.0 + (w‐13) × 0.58 kg |
| Normal | 18.5–24.9 kg/m2 | 0.5–2.0 kg | 0.42 (0.35–0.50) kg/week | 0.5 + (w‐13) × 0.35–2.0 + (w‐13) × 0.50 kg |
| Overweight | 25.0–29.9 kg/m2 | 0.5–2.0 kg | 0.28 (0.23–0.33) kg/week | 0.5 + (w‐13) × 0.23–2.0 + (w‐13) × 0.33 kg |
Gestational week during which gestational weight was recorded during the prenatal care visit nearest within 4 weeks before oral glucose tolerance test (OGTT). BMI, body mass index; GWG, gestational weight gain; w, the gestational week during which gestational weight was measured for calculating GWG before OGTT; WHO, World Health Organization.
Table 2.
Quantiles of gestational weight gain of included pregnant women
| Prepregnancy BMI (WHO) | n | Quantiles of GWG before OGTT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| P5 | P10 | P25 | P50 | P75 | P90 | P95 | |||
| Underweight | <18.5 kg/m2 | 1,287 | 2.8 kg | 2.5 kg | 5.0 kg | 6.0 kg | 8.0 kg | 10.0 kg | 11.0 kg |
| Normal | 18.5–24.9 kg/m2 | 6,437 | 2.0 kg | 3.0 kg | 4.5 kg | 6.0 kg | 8.0 kg | 9.0 kg | 10.5 kg |
| Overweight | 25.0–29.9 kg/m2 | 632 | 1.0 kg | 2.0 kg | 3.5 kg | 5.0 kg | 7.0 kg | 9.0 kg | 10.0 kg |
GWG, gestational weight gain; OGTT, oral glucose tolerance test; WHO, World Health Organization.
Statistical analysis
We evaluated maternal demographics, gestational characteristics and comorbidities across underweight, normal and overweight pregnant women by frequency (percentage), and compared the distribution differences using the χ2‐test or Fisher’s exact test.
We carried out univariable analysis to determine whether GWG before OGTT stratified by IOM, quantiles of GWG (cut‐off values: P10 & P90; P5 & P95), prepregnancy BMI (underweight, normal, overweight), maternal age (19–24 years, 25–29 years, 30–34 years, 35–46 years), years of education (≥17, 13–16, 10–12, <10), place of residence (urban/rural), gravidity (1/>1), parity (primipara/multipara), GDM history (yes/no), in vitro fertilization (IVF; yes/no), cardiovascular diseases (yes/no), viral hepatitis (yes/no), endocrine diseases (yes/no), hematological diseases (yes/no), respiratory diseases (yes/no), urinary diseases (yes/no), immune diseases (yes/no), neuropsychiatric diseases (yes/no) and reproductive diseases (yes/no) were associated with the risk for developing GDM.
We then generated three multivariable logistic regression models to examine the association between GWG during the first and second trimester and the risk for developing GDM, after adjusting for potential confounders that were meaningful in clinical practice and statistically significant in the univariable analysis with P < 0.05. Model 1 evaluated GWG before OGTT stratified by IOM recommendations, and adjusted for prepregnancy BMI, maternal age, gravidity, parity, GDM history, in vitro fertilization, viral hepatitis and hematological diseases. Model 2 evaluated the 10th and 90th quantiles of GWG compared with those of the entire population, and adjusted for the same confounders as model 1. Model 3 evaluated the 5th and 95th quantiles of GWG compared with those of the entire population, and adjusted for the same confounders as model 1 and model 2. The adjusted odds ratio (aOR) and 95% confidence interval (CI) of the three models are shown in Table 5.
To further clarify the association between excessive GWG during the first and second trimester and the risk for developing GDM among women in different prepregnancy BMI categories, we also carried out multivariable logistic regression models among three subgroups (underweight, normal weight and overweight) respectively, adjusted for the same confounders as model 1–3, except prepregnancy BMI. The aOR and 95%CI of the three models in each subgroup are shown in Tables S1–S3. Subsequently, to verify whether interaction effects exist between GWG during the first and second trimester and prepregnancy BMI on the risk of developing GDM, we carried out a test for interaction by adding the interaction item in the multivariable logistic model among the whole population.
All statistical analyses were carried out using R software version 3.6.3 (R Development Core Team, Vienna, Austrian). A two‐tailed P‐value of <0.05 was considered statistically significant for all analyses.
Results
We collected data from 10,422 pregnant women who delivered between 1 January 2013 and 31 October 2014 in our hospital. After excluding 404 multiple gestations and 360 women with missing OGTT values, 9,680 women who were pregnant with singletons with gestation periods >28 weeks remained. Among these, 1,274 women were excluded according to the eligibility criteria and 50 women were obese. Finally, data from 8,356 eligible pregnant women were analyzed. (Figure 1).
Figure 1.
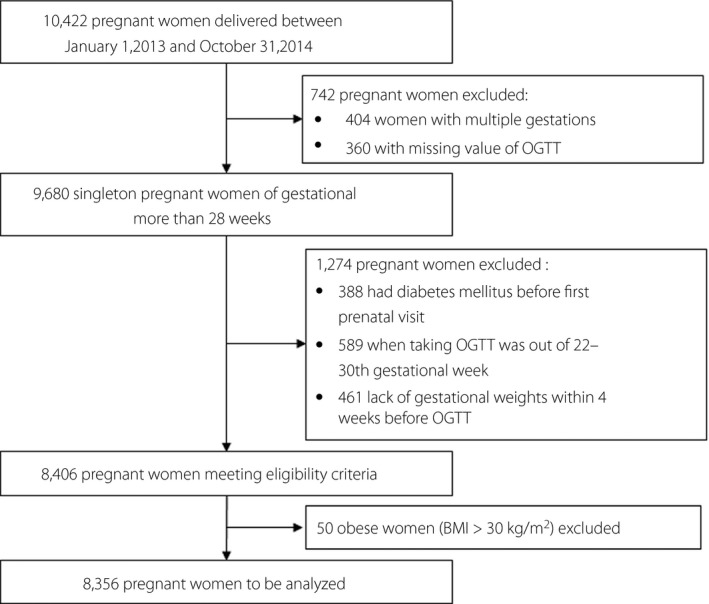
Flow chart of participants included in the present study. BMI, body mass index; OGTT, oral glucose tolerance test.
Of the 8,356 women included in the study, the average maternal age was 30.3 ± 3.9 years, and women aged >35 years accounted for 14.3% of the included participants. A total of 89.6% were educated for ≥13 years, 87.8% lived in urban settings, 56.0% had gravidity greater than one, 83.4% were primipara, 0.4% had a history of GDM and 3.3% received IVF. In addition, 3.2% of the participants had cardiovascular diseases, 4.6% had viral hepatitis, 10.4% had endocrine diseases, 3.5% had hematological diseases, 0.2% had respiratory diseases, 0.3% had urinary diseases, 0.5% had immune diseases, 0.2% had neuropsychiatric diseases and 7.6% had reproductive diseases. There were statistically significant differences in maternal age, years of education, gravidity, parity, use of IVF, cardiovascular diseases and reproductive diseases among the prepregnancy categories. (Table 3).
Table 3.
Characteristics of included pregnant women by prepregnancy body mass index categories
| Characteristics at baseline | Overall (n = 8,356) | Underweight (n = 1,287) | Normal (n = 6,437) | Overweight (n = 632) | P‐value |
|---|---|---|---|---|---|
| Maternal age (years) | |||||
| 19–24 | 383 (4.58) | 113 (8.78) | 255 (3.96) | 15 (2.37) | <0.001 |
| 25–29 | 3,400 (40.69) | 666 (51.75) | 2,554 (39.68) | 180 (28.48) | |
| 30–34 | 3,377 (40.41) | 419 (32.56) | 2,661 (41.34) | 297 (46.99) | |
| 35–46 | 1,196 (14.31) | 89 (6.92) | 967 (15.02) | 140 (22.15) | |
| Education years | |||||
| ≥17 | 1,242 (14.86) | 168 (13.05) | 1,014 (15.75) | 60 (9.49) | <0.001 |
| 13–16 | 6,248 (74.77) | 1,002 (77.86) | 4,786 (74.35) | 460 (72.78) | |
| 10–12 | 593 (7.10) | 89 (6.92) | 436 (6.77) | 68 (10.76) | |
| <10 | 273 (3.27) | 28 (2.18) | 201 (3.12) | 44 (6.96) | |
| Residence | |||||
| Urban | 7,339 (87.83) | 1,143 (88.81) | 5,656 (87.87) | 540 (85.44) | 0.10 |
| Rural | 1,017 (12.17) | 144 (11.19) | 781 (12.13) | 92 (14.56) | |
| Gravidity | |||||
| 1 | 3,675 (43.98) | 669 (51.98) | 2,790 (43.34) | 216 (34.18) | <0.001 |
| >1 | 4,681 (56.02) | 618 (48.02) | 3,647 (56.66) | 416 (65.82) | |
| Parity | |||||
| Primipara | 6,971 (83.43) | 1,153 (89.59) | 5,336 (82.90) | 482 (76.27) | <0.001 |
| Multipara | 1,385 (16.57) | 134 (10.41) | 1,101 (17.10) | 150 (23.73) | |
| GDM history | |||||
| No | 8,327 (99.65) | 1,282 (99.61) | 6,417 (99.69) | 628 (99.37) | 0.29† |
| Yes | 29 (0.35) | 5 (0.39) | 20 (0.31) | 4 (0.63) | |
| In vitro fertilization | |||||
| No | 8,078 (96.67) | 1,273 (98.91) | 6,213 (96.52) | 592 (93.67) | <0.001 |
| Yes | 278 (3.33) | 14 (1.09) | 224 (3.48) | 40 (6.33) | |
| Cardiovascular diseases | |||||
| No | 8,092 (96.84) | 1,249 (97.05) | 6,242 (96.97) | 601 (95.09) | 0.03 |
| Yes | 264 (3.16) | 38 (2.95) | 195 (3.03) | 31 (4.91) | |
| Viral hepatitis | |||||
| No | 7,969 (95.37) | 1,239 (96.27) | 6,129 (95.22) | 601 (95.09) | 0.24 |
| Yes | 387 (4.63) | 48 (3.73) | 308 (4.78) | 31 (4.91) | |
| Endocrine diseases | |||||
| No | 7,488 (89.61) | 1,145 (88.97) | 5,785 (89.87) | 555 (87.82) | 0.20 |
| Yes | 871 (10.42) | 142 (11.03) | 652 (10.13) | 77 (12.18) | |
| Hematological diseases | |||||
| No | 8,062 (96.48) | 1,245 (96.74) | 6,199 (96.30) | 618 (97.78) | 0.13 |
| Yes | 294 (3.52) | 42 (3.26) | 238 (3.70) | 14 (2.22) | |
| Respiratory diseases | |||||
| No | 8,340 (99.81) | 1,286 (99.92) | 6,422 (99.77) | 632 (100) | 0.38† |
| Yes | 16 (0.19) | 1 (0.08) | 15 (0.23) | 0 (0.00) | |
| Urinary diseases | |||||
| No | 8,334 (99.74) | 1,285 (99.84) | 6,417 (99.69) | 632 (100) | 0.40† |
| Yes | 22 (0.26) | 2 (0.16) | 20 (0.31) | 0 (0.00) | |
| Immune diseases | |||||
| No | 8,318 (99.55) | 1,278 (99.30) | 6,413 (99.63) | 627 (99.21) | 0.09† |
| Yes | 38 (0.45) | 9 (0.70) | 24 (0.37) | 5 (0.79) | |
| Neuropsychiatric diseases | |||||
| No | 8,336 (99.76) | 1,285 (99.84) | 6,419 (99.72) | 632 (100) | 0.51† |
| Yes | 20 (0.24) | 2 (0.16) | 18 (0.28) | 0 (0.00) | |
| Reproductive diseases | |||||
| No | 7,722 (92.41) | 1,232 (95.73) | 5,927 (92.08) | 563 (89.08) | <0.001 |
| Yes | 634 (7.59) | 55 (4.27) | 510 (7.92) | 69 (10.92) | |
Data are shown by frequency (percentage). †Fisher’s exact test. BMI, body mass index; GDM, gestational diabetes mellitus. P value in bold indicates statistical significance.
We identified 1,622 (19.4%) pregnant women diagnosed with GDM. The average prepregnancy BMI of the included participants was 21.0 ± 2.5 kg/m2, 1,287 (15.4%) were classified as underweight prepregnancy, 6,437 (77.0%) were classified as normal weight and 632 (7.6%) were classified as overweight. According to the IOM recommendations, 2,610 (31.2%) pregnant women had excessive GWG before OGTT, and 1,141 (13.7%) women had GWG exceeding the 90th percentile of GWG for the whole population.
Univariable analysis showed that excessive GWG according to the IOM or GWG greater than the 90th or 95th percentile of that among the entire population were associated with an increased risk of GDM (P < 0.05). In addition, prepregnancy BMI, maternal age, gravidity, GDM history, use of IVF, viral hepatitis and hematological diseases were potential confounders (P < 0.05; Table 4).
Table 4.
Univariable analysis of association between gestational weight gain, prepregnancy body mass index, maternal characteristics and risk of gestational diabetes mellitus
| Characteristics |
Overall (n = 8,356) |
GDM (n = 1,622) |
Non‐GDM (n = 6,734) |
P‐value |
|---|---|---|---|---|
| GWG before OGTT (kg) | ||||
| Appropriate (IOM) | 4,044 (48.40) | 772 (47.60) | 3,272 (48.59) | 0.012 |
| Insufficient (IOM) | 1,702 (20.37) | 299 (18.43) | 1,403 (20.83) | |
| Excessive (IOM) | 2,610 (31.24) | 551 (33.97) | 2,059 (30.58) | |
| Quantiles of GWG | ||||
| Appropriate (P10–P90) | 6,569 (78.61) | 1,257 (77.50) | 5,312 (78.88) | 0.008 |
| Insufficient (<P10) | 646 (7.73) | 109 (6.72) | 537 (7.97) | |
| Excessive (>P90) | 1,141 (13.65) | 256 (15.78) | 885 (13.14) | |
| Quantiles of GWG | ||||
| Appropriate (P5–P95) | 7,600 (90.95) | 1,464 (90.26) | 6,136 (91.12) | 0.004 |
| Insufficient (<P5) | 285 (3.41) | 43 (2.65) | 242 (3.59) | |
| Excessive (>P95) | 471 (5.64) | 115 (7.09) | 356 (5.29) | |
| Prepregnancy BMI (WHO) | ||||
| Underweight | 1,287 (15.40) | 166 (10.23) | 1,121 (16.65) | <0.001 |
| Normal | 6,437 (77.03) | 1,231 (75.89) | 5,206 (77.31) | |
| Overweight | 632 (7.56) | 225 (13.87) | 407 (6.04) | |
| Maternal age (years) | ||||
| 19–24 | 383 (4.58) | 31 (1.91) | 352 (5.23) | <0.001 |
| 25–29 | 3,400 (40.69) | 492 (30.33) | 2,908 (43.18) | |
| 30–34 | 3,377 (40.41) | 752 (46.36) | 2,625 (38.98) | |
| 35–46 | 1,196 (14.31) | 347 (21.39) | 849 (12.61) | |
| Education years | ||||
| ≥17 | 1,242 (14.86) | 235 (14.49) | 1,007 (14.95) | 0.13 |
| 13–16 | 6,248 (74.77) | 1,193 (73.55) | 5,055 (75.07) | |
| 10–12 | 593 (7.10) | 135 (8.32) | 458 (6.80) | |
| <10 | 273 (3.27) | 59 (3.64) | 214 (3.18) | |
| Residence | ||||
| Urban | 7,339 (87.83) | 1,419 (87.48) | 5,920 (87.91) | 0.67 |
| Rural | 1,017 (12.17) | 203 (12.52) | 814 (12.09) | |
| Gravidity | ||||
| 1 | 3,675 (43.98) | 619 (38.16) | 3,056 (45.38) | <0.001 |
| >1 | 4,681 (56.02) | 1,003 (61.84) | 3,678 (54.62) | |
| Parity | ||||
| Primipara | 6,971 (83.43) | 1,311 (80.83) | 5,660 (84.05) | 0.002 |
| Multipara | 1,385 (16.57) | 311 (19.17) | 1,074 (15.95) | |
| GDM history | ||||
| No | 8,327 (99.65) | 1,601 (98.71) | 6,726 (99.88) | <0.001 |
| Yes | 29 (0.35) | 21 (1.29) | 8 (0.12) | |
| In vitro fertilization | ||||
| No | 8,078 (96.67) | 1,539 (94.88) | 6,539 (97.10) | <0.001 |
| Yes | 278 (3.33) | 83 (5.12) | 195 (2.90) | |
| Cardiovascular diseases | ||||
| No | 8,092 (96.84) | 1,573 (96.98) | 6,519 (96.81) | 0.78 |
| Yes | 264 (3.16) | 49 (3.02) | 215 (3.19) | |
| Viral hepatitis | ||||
| No | 7,969 (95.37) | 1,526 (94.08) | 6,443 (95.68) | 0.007 |
| Yes | 387 (4.63) | 96 (5.92) | 291 (4.32) | |
| Endocrine diseases | ||||
| No | 7,488 (89.61) | 1,435 (88.47) | 6,053 (89.89) | 0.06 |
| Yes | 871 (10.42) | 190 (11.71) | 681 (10.11) | |
| Hematological diseases | ||||
| No | 8,062 (96.48) | 1,580 (97.41) | 6,482 (96.26) | 0.029 |
| Yes | 294 (3.52) | 42 (2.59) | 252 (3.74) | |
| Respiratory diseases | ||||
| No | 8,340 (99.81) | 1,619 (99.82) | 6,721 (99.81) | 1.00† |
| Yes | 16 (0.19) | 3 (0.18) | 13 (0.19) | |
| Urinary diseases | ||||
| No | 8,334 (99.74) | 1,617 (99.69) | 6,717 (99.75) | 0.60† |
| Yes | 22 (0.26) | 5 (0.31) | 17 (0.25) | |
| Immune diseases | ||||
| No | 8,318 (99.55) | 1,619 (99.82) | 6,699 (99.48) | 0.11 |
| Yes | 38 (0.45) | 3 (0.18) | 35 (0.52) | |
| Neuropsychiatric diseases | ||||
| No | 8,336 (99.76) | 1,620 (99.88) | 6,716 (99.73) | 0.40† |
| Yes | 20 (0.24) | 2 (0.12) | 18 (0.27) | |
| Reproductive diseases | ||||
| No | 7,722 (92.41) | 1,484 (91.49) | 6,238 (92.63) | 0.13 |
| Yes | 634 (7.59) | 138 (8.51) | 496 (7.37) | |
Data are shown by frequency (percentage). †Fisher’s exact test. BMI, body mass index; GDM, gestational diabetes mellitus; GWG, gestational weight gain; IOM, Institute of Medicine; OGTT, oral glucose tolerance test; WHO, World Health Organization. P value in bold indicates statistical significance.
After univariable analysis, multivariable logistic regression models adjusted for potential confounders were generated. Model 1 showed that GWG before OGTT stratified by IOM was not associated with the risk for developing GDM (aOR 1.07, 95% CI 0.94–1.21), adjusted by prepregnancy BMI, maternal age, gravidity, parity, GDM history, in vitro fertilization, viral hepatitis and hematological diseases. Model 2 showed that GWG greater than the 90th quantile of that of the entire population was associated with an increased risk for developing GDM (aOR1.31, 95% CI 1.12–1.52), adjusted by the same confounders as in model 1. Model 3 showed that GWG greater than the 95th quantile of that of the entire population was also associated with increased risk for developing GDM (aOR 1.45, 95% CI 1.16–1.81), adjusted by the same confounders as in model 1 and model 2 (Table 5).
Table 5.
Multivariable logistic regression of gestational weight gain and risk for gestational diabetes mellitus
| Variables in model | Model 1 † | Model 2 ‡ | Model 3 § |
|---|---|---|---|
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| GWG before OGTT (kg) | |||
| Appropriate (IOM) | 1.00 | — | — |
| Insufficient (IOM) | 0.90 (0.78–1.05) | — | — |
| Excessive (IOM) | 1.07 (0.94–1.21) | — | — |
| Quantiles of GWG | |||
| Appropriate (P10‐P90) | — | 1.00 | — |
| Insufficient (<P10) | — | 0.87 (0.70–1.08) | — |
| Excessive (>P90) | — | 1.31 (1.12–1.52) | — |
| Quantiles of GWG | |||
| Appropriate (P5‐P95) | — | — | 1.00 |
| Insufficient (<P5) | — | — | 0.77 (0.55–1.07) |
| Excessive (>P95) | — | — | 1.45 (1.16–1.81) |
| Pre‐pregnancy BMI | |||
| Underweight (<18.5 kg/m2) | 0.72 (0.60–0.86) | 0.72 (0.60–0.86) | 0.71 (0.59–0.85) |
| Normal (18.5–24.9 kg/m2) | 1.00 | 1.00 | 1.00 |
| Overweight (≥25.0 kg/m2) | 2.13 (1.78–2.55) | 2.17 (1.82–2.60) | 2.14 (1.79–2.56) |
| Maternal age (years) | |||
| 19–24 | 1.00 | 1.00 | 1.00 |
| 25–29 | 1.83 (1.27–2.73) | 1.84 (1.28–2.75) | 1.84 (1.27–2.75) |
| 30–34 | 2.90 (2.01–4.32) | 2.94 (2.04–4.38) | 2.93 (2.03–4.37) |
| 35–46 | 3.92 (2.67–5.93) | 3.98 (2.71–6.02) | 3.95 (2.70–5.99) |
| Gravidity | |||
| 1 | 1.00 | 1.00 | 1.00 |
| >1 | 1.14 (1.01–1.29) | 1.14 (1.01–1.29) | 1.15 (1.02–1.30) |
| Parity | |||
| Primipara | 1.00 | 1.00 | 1.00 |
| Multipara | 0.88 (0.75–1.03) | 0.88 (0.75–1.03) | 0.87 (0.74–1.02) |
| GDM history | |||
| No | 1.00 | 1.00 | 1.00 |
| Yes | 10.09 (4.54–24.73) | 10.30 (4.63–25.22) | 10.17 (4.58–24.89) |
| In vitro fertilization | |||
| No | 1.00 | 1.00 | 1.00 |
| Yes | 1.29 (0.98–1.69) | 1.29 (0.98–1.69) | 1.29 (0.98–1.69) |
| Viral hepatitis | |||
| No | 1.00 | 1.00 | 1.00 |
| Yes | 1.34 (1.05–1.70) | 1.35 (1.05–1.71) | 1.34 (1.05–1.71) |
| Hematological diseases | |||
| No | 1.00 | 1.00 | 1.00 |
| Yes | 0.73 (0.51–1.005) | 0.73 (0.51–1.01) | 0.72 (0.51–0.999) |
OR, adjusted odds ratio; CI, confidence interval. OR (95% CI) in bold indicates statistical significance.
Model 1 evaluated gestational weight gain (GWG) before oral glucose tolerance test (OGTT) stratified by Institute of Medicine (IOM) recommendations, adjusted by pre‐pregnancy body mass index (BMI), maternal age, gravidity, parity, gestational diabetes mellitus (GDM) history, in vitro fertilization, viral hepatitis and hematological diseases.
Model 2 evaluated GWG before OGTT stratified by the 10th and 90th quantiles of that of the included population, adjusted by the same confounders as in model 1.
Model 3 evaluated GWG before OGTT stratified by the 5th and 95th quantiles of that of the included population, adjusted by the same confounders as in model 1 and model 2.
In addition, the subgroup analysis concluded a similar significant association among women with prepregnant underweight and normal weight, showing GWG more than the 90th quantile of that of the entire population was associated with increased risk of GDM (aOR 1.67, 95% CI 1.02–2.66 underweight; aOR 1.31, 95% CI 1.10–1.55 normal weight); whereas among prepregnant normal weight women, GWG more than the 95th quantile was also associated with increased risk of GDM (aOR 1.60, 95% CI 1.24–2.06). However, among prepregnant overweight women, we found no association between excessive GWG and risk of GDM (Tables S1–S3). Furthermore, there existed no statistical significance regarding the interaction effect between GWG during the first and second trimester (according to IOM, P10–P90, P5–P95, respectively) and prepregnancy BMI on the risk of developing GDM (test for interaction: P = 0.65 for IOM, 0.41 for P10–P90, 0.07 for P5–P95, respectively).
Discussion
The present study found that excessive GWG greater than the 90th or 95th quantile of that of the entire population among Chinese women pregnant with singletons without diabetes mellitus increased the risk for developing GDM. Our study highlighted the importance of appropriate weight gain during gestation to prevent GDM. However, according to the IOM recommendation, we found no association between excessive GWG and the risk for developing GDM.
Because of the high prevalence of GDM, and the related short‐ and long‐term adverse effects on the mother and child, 20 , 21 GDM is an issue that must be addressed, and requires further scientific research. Previous research has shown that there are a large number of risk factors associated with GDM, such as advanced maternal age, prepregnancy overweight or obesity, family history of diabetes, history of GDM or giving birth to a large baby, and number of pregnancies and births. 22 However, the effect of GWG during the first and/or second trimester on the risk for developing GDM has not been clarified.
GDM, characterized by insulin resistance and reduced insulin secretion 23 , might be associated with excessive GWG. As shown in previous studies, pregnant women with excessive GWG, especially those who were prepregnancy overweight, were more likely to accumulate adipose tissue in visceral depots rather than in subcutaneous depots, such as in the hips and thighs, which is associated with a greater risk for adverse outcomes 24 , 25 . Accumulation of fat in visceral depots could result in an increased risk for developing insulin resistance and subsequent exhaustion of pancreatic β‐cells, leading to inadequate insulin secretion and GDM 1 , 14 .
The present findings that excessive GWG was associated with an increased risk for developing GDM among women with singletons were consistent with the findings of previous studies 7 , 8 , 16 , However, some studies reached different conclusions 6 , 9 , 10 . Compared with other representative studies 14 , 15 , 16 , 26 , there were differences in methodological characteristics. First, the populations in developed countries were different from Chinese women in terms of ethnicity and anthropometry 27 , 28 , 29 . For example, American women are generally taller than Chinese women, and more likely to be overweight 15 , 28 . Second, other studies evaluated GWG during the first or second trimester 14 , 15 , 16 , 26 , and the method for calculating GWG might have been different from ours in other studies 30 . For example, some studies subtracted self‐reported prepregnancy weight from maternal weight at delivery 6 , 9 . Third, we considered potential confounders in our multivariable analysis, including prepregnancy BMI, maternal age, gravidity, parity, history of GDM, use of IVF 31 , viral hepatitis 32 and hematological diseases 33 . These were important and prevalent factors among Chinese women, but were rarely considered in other studies 1 , 7 , 14 , 15 , 16 , 26 .
According to the IOM guideline, we found no association between excessive GWG and the risk for developing GDM in our multivariable analysis. This might have been because the IOM recommendations were developed based on white and black populations 17 . However, there are obvious differences in anthropometry, dietary intake, culture, and lifestyles between the USA and Chinese populations 28 , 34 , 35 . As reported in our previous study, women who are prepregnancy normal weight or overweight tended to gain more weight than the IOM recommendation 11 . Another study that included women pregnant with singletons in Shanghai of China also concluded that the IOM recommendation was likely not optimal for the Chinese population 36 .
It is worth noting that, in subgroup analysis among pregnant women with different prepregnancy BMIs, apparent subgroup differences would occur regarding the association of excessive GWG in the first and second trimester with the risk of developing GDM, which can probably be attributed to chance and the limited sample size among these subgroups 37 . In addition, we found no interaction effects between GWG during the first and second trimester and prepregnancy BMI acting on the risk of developing GDM, accordingly, our conclusions from multivariable analysis among the whole population should be more credible.
The present study had several strengths. First, this cohort study was based on repeatedly measured data in a real‐world clinical practice, verified excessive GWG might be a risk factor for GDM, providing further evidence regarding GWG and GDM among Chinese women. Second, we used two classification methods for GWG; that is, the total GWG before OGTT was categorized by the IOM recommendations, and by multiple quantiles of GWG among the whole population, which provided a more comprehensive evaluation of the association between GWG and GDM. Third, our study was rigorous with regard to the calculation of GWG, determination of GDM, use of multivariable analysis adjusted for other potential confounders and proper interpretation of subgroup analysis.
The present study also had some limitations. First, this was a single‐center study and did not include obese women, which might limit the generalizability of the conclusions. Second, our data were collected from medical records and the prepregnancy weights were self‐reported. Although weights were measured at the first antenatal visit, self‐reports might have been subject to recall bias 38 .
In summary, excessive GWG in the first and second trimester might be a risk factor for developing GDM, which highlights the importance of appropriate weight gain during pregnancy. However, well‐designed, multicenter, prospective studies are required to further determine the appropriate GWG for pregnant Chinese women.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 Multivariable logistic regression of gestational weight gain and risk for gestational diabetes mellitus among prepregnant underweight women (n = 1,287).
Table S2 Multivariable logistic regression of gestational weight gain and risk for gestational diabetes mellitus among prepregnant normal weight women (n = 6,437).
Table S3 Multivariable logistic regression of gestational weight gain and risk for gestational diabetes mellitus among prepregnant overweight women (n = 632).
Acknowledgments
This study was funded by the National Key Research and Development Program of Reproductive Health & Major Birth Defects Control and Prevention (2016YFC1000406), the National Natural Science Foundation of China (71704122, 71974138), and the National Science and Technology Major Project (2018ZX10302206‐004‐005).
J Diabetes Investig. 2020
References
- 1. Chiefari E, Arcidiacono B, Foti D, et al Gestational diabetes mellitus: an updated overview. J Endocrinol Invest 2017; 40: 899–909. [DOI] [PubMed] [Google Scholar]
- 2. Gare DJ. Hyperglycemia and adverse pregnancy outcomes — NEJM. N Engl J Med 2008; 5: 1991–2002. [DOI] [PubMed] [Google Scholar]
- 3. Bellamy L, Casas JP, Hingorani AD, et al Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta‐analysis. Lancet 2009; 373: 1773–1779. [DOI] [PubMed] [Google Scholar]
- 4. Law KP, Zhang H. The pathogenesis and pathophysiology of gestational diabetes mellitus: deductions from a three‐part longitudinal metabolomics study in China. Clin Chim Acta 2017; 468: 60–70. [DOI] [PubMed] [Google Scholar]
- 5. Mahzari MM, Alwadi FA, Alhussain BM, et al Development of type 2 diabetes mellitus after gestational diabetes in a cohort in KSA: prevalence and risk factors. J Taibah Univ Med Sci 2018; 13: 582–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hung TH, Hsieh TT. Pregestational body mass index, gestational weight gain, and risks for adverse pregnancy outcomes among Taiwanese women: a retrospective cohort study. Taiwan J Obstet Gynecol 2016; 55: 575–581. [DOI] [PubMed] [Google Scholar]
- 7. Liang Y, Li DT, Chen MX, et al Associations of pre‐pregnancy body mass index and gestational weight gain with gestational diabetes mellitus: a cohort study in southwest China. J Sichuan Univ Med Sci 2019; 50: 83–87. (Chinese). [PubMed] [Google Scholar]
- 8. Arora P, Tamber Aeri B. Gestational weight gain among healthy pregnant women from Asia in comparison with Institute of Medicine (IOM) Guidelines‐2009: a systematic review. J Pregnancy 2019; 2019: 3849596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li C, Liu Y, Zhang W. Joint and independent associations of gestational weight gain and pre‐pregnancy Body Mass Index with outcomes of pregnancy in Chinese women: a retrospective cohort study. PLoS ONE 2015; 10: e0136850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cosson E, Cussac‐Pillegand C, Benbara A, et al Pregnancy adverse outcomes related to pregravid body mass index and gestational weight gain, according to the presence or not of gestational diabetes mellitus: a retrospective observational study. Diabetes Metab 2016; 42: 38–46. [DOI] [PubMed] [Google Scholar]
- 11. Tan J, Ren Y, Qi Y, et al The pattern of gestational weight gains among Chinese women: a repeated measure analysis. Sci Rep 2018; 8: 15865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schiavone M, Putoto G, Laterza F, et al Gestational diabetes: an overview with attention for developing countries. Endocr Regul 2016; 50: 62–71. [DOI] [PubMed] [Google Scholar]
- 13. Cho EH, Hur J, Lee KJ. Early gestational weight gain rate and adverse pregnancy outcomes in Korean women. PLoS ONE 2015; 10: e0140376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hedderson MM, Gunderson EP, Ferrara A, et al Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol 2010; 115: 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. MacDonald SC, Bodnar LM, Himes KP, et al Patterns of gestational weight gain in early pregnancy and risk of gestational diabetes mellitus. Epidemiology 2017; 28: 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhong C, Li X, Chen R, et al Greater early and mid‐pregnancy gestational weight gain are associated with increased risk of gestational diabetes mellitus: a prospective cohort study. Clin Nutr ESPEN 2017; 22: 48–53. [DOI] [PubMed] [Google Scholar]
- 17. Voerman E, Santos S, Inskip H, et al Association of gestational weight gain with adverse maternal and infant outcomes. JAMA 2019; 321: 1702–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Obstetrics Group of Chinese Medical Association . Guideline for diagnosis and treatment of gestational diabetes mellitus (2014). Zhonghua Fu Chan Ke Za Zhi 2014; 49: 561–571. (Chinese).25354853 [Google Scholar]
- 19. WHO . Obesity and overweight. The World Health Organization; Available from: https://wwwwhoint/en/news‐room/fact‐sheets/detail/obesity‐and‐overweight 2018. [Google Scholar]
- 20. Sugiyama M, Cash H, Roseveare C, et al Assessment of gestational diabetes and associated risk factors and outcomes in the Pacific Island Nation of Palau. Matern Child Health J 2017; 21: 1961–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jeppesen C, Maindal H, Kristensen J, et al National study of the prevalence of gestational diabetes mellitus among Danish women from 2004 to 2012. Scand J Public Health 2017; 45: 811–817. [DOI] [PubMed] [Google Scholar]
- 22. Karacam Z, CelIk D. The prevalence and risk factors of gestational diabetes mellitus in Turkey: a systematic review and meta‐analysis. J Matern Fetal Neonatal Med 2019; 1–11. [DOI] [PubMed] [Google Scholar]
- 23. Reilly BM. Count your blessings. N Engl J Med 2019; 380: 1690–1693. [DOI] [PubMed] [Google Scholar]
- 24. Ehrenberg HM, Huston‐Presley L, Catalano PM. The influence of obesity and gestational diabetes mellitus on accretion and the distribution of adipose tissue in pregnancy. Am J Obstet Gynecol 2003; 189: 944–948. [DOI] [PubMed] [Google Scholar]
- 25. Kinoshita T, Itoh M. Longitudinal variance of fat mass deposition during pregnancy evaluated by ultrasonography: the ratio of visceral fat to subcutaneous fat in the abdomen. Gynecol Obstet Invest 2006; 61: 115–118. [DOI] [PubMed] [Google Scholar]
- 26. Hantoushzadeh S, Sheikh M, Bosaghzadeh Z, et al The impact of gestational weight gain in different trimesters of pregnancy on glucose challenge test and gestational diabetes. Postgrad Med J 2016; 92: 520–524. [DOI] [PubMed] [Google Scholar]
- 27. Chakkalakal RJ, Gebretsadik T, Jagasia S, et al Variation in the relationship between gestational diabetes diagnosis and total gestational weight gain by race/ethnicity. Diabetes Res Clin Pract 2015; 108: e14–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lagiou P, Hsieh CC, Trichopoulos D, et al Birthweight differences between USA and China and their relevance to breast cancer aetiology. Int J Epidemiol 2003; 32: 193–198. [DOI] [PubMed] [Google Scholar]
- 29. Pu J, Zhao B, Wang EJ, et al Racial/ethnic differences in gestational diabetes prevalence and contribution of common risk factors. Paediatr Perinat Epidemiol 2015; 29: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amorim AR, Linne Y, Kac G, et al Assessment of weight changes during and after pregnancy: practical approaches. Mater Child Nutr 2008; 4: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wahlberg A. The birth and routinization of IVF in China. Reprod Biomed Soc 2016; 2: 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol 2013; 28(Suppl 1): 7–10. [DOI] [PubMed] [Google Scholar]
- 33. Guolin H, Xin S, Jing T, et al Survey of prevalence of iron deficiency and iron deficiency anemia in pregnant women in urban areas of China. Chin J Obstet Gynecol 2018; 53: 761–767. [DOI] [PubMed] [Google Scholar]
- 34. Zhou BF, Stamler J, Dennis B, et al Nutrient intakes of middle‐aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP study. J Hum Hypertens 1990s; 17: 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lau Y. Traditional Chinese pregnancy restrictions, health‐related quality of life and perceived stress among pregnant women in Macao, China. Asian Nurs Res (Korean Soc Nurs Sci) 2012; 6: 27–34. [DOI] [PubMed] [Google Scholar]
- 36. Jiang X, Liu M, Song Y, et al The Institute of Medicine recommendation for gestational weight gain is probably not optimal among non‐American pregnant women: a retrospective study from China. J Matern Fetal Neonatal Med 2019; 32: 1353–1358. [DOI] [PubMed] [Google Scholar]
- 37. Xin Sun, Ioannidis John PA, Thomas Agoritsas, et al How to use a subgroup analysis: users' guide to the medical literature. JAMA 2014; 311: 405–411. [DOI] [PubMed] [Google Scholar]
- 38. Harris HE, Ellison GT. Practical approaches for estimating prepregnant body weight. J Nurse Midwife 1998; 43: 97–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Multivariable logistic regression of gestational weight gain and risk for gestational diabetes mellitus among prepregnant underweight women (n = 1,287).
Table S2 Multivariable logistic regression of gestational weight gain and risk for gestational diabetes mellitus among prepregnant normal weight women (n = 6,437).
Table S3 Multivariable logistic regression of gestational weight gain and risk for gestational diabetes mellitus among prepregnant overweight women (n = 632).


