Abstract
All physical connections between sister chromatids must be broken before cells can divide, and eukaryotic cells have evolved multiple ways in which to process branchpoints connecting DNA molecules separated both spatially and temporally. A single DNA link between chromatids has the potential to disrupt cell cycle progression and genome integrity, so it is highly likely that cells require a nuclease that can process remaining unresolved and hemi-resolved DNA junctions and other branched species at the very late stages of mitosis. We argue that ANKLE1 probably serves this function in human cells (LEM-3 in Caenorhabditis elegans). LEM-3 has previously been shown to be located at the cell mid-body, and we show here that human ANKLE1 is a nuclease that cleaves a range of branched DNA species. It thus has the substrate selectivity consistent with an enzyme required to process a variety of unresolved and hemi-resolved branchpoints in DNA. Our results suggest that ANKLE1 acts as a catch-all enzyme of last resort that allows faithful chromosome segregation and cell division to occur.
Keywords: chromosome segregation, helical junction resolution, ultrafine DNA bridges, LEM-3
Abbreviation: GST, glutathione S-transferase
Graphical abstract
Highlights
-
•
Human ANKLE1 is a nuclease that cleaves branched DNA.
-
•
ANKLE1 cleaves a variety of branched DNA species, including four-way junctions, flaps and Y-structures.
-
•
The activity of ANKLE1 is consistent with a catch-all nuclease to sever links between chromosomes prior to cell division.
-
•
ANKLE1 is probably the enzyme of last resort processing links between chromosomes.
Introduction
To ensure faithful genome maintenance, chromatids must be precisely segregated to daughter cells. This requires the removal of all physical connections between sister chromatids before cells divide. These include intermediates of DNA recombination, such as four-way (Holliday) junctions, intertwined chromatids, and loci remaining unreplicated at the point when cells reach the metaphase-anaphase transition. Cytologically, DNA connections between segregating chromatids take the form of chromatin bridges [1., 2., 3.], or ultrafine bridges [4,5]; the latter do not stain with DAPI but are associated with BLM and PICH helicases. DNA bridges form in each cell cycle, their number being increased under DNA damaging conditions, or when DNA replication is challenged [6]. Failure to process DNA bridges impedes the segregation of chromatids and leads to genome instability via chromosome breakage, or cleavage furrow regression during cytokinesis resulting in binucleated cells and polyploidy.
To ensure that all connections are removed before cells divide, cells have evolved highly redundant mechanisms that remove all bridges. In eukaryotes, four-way helical (Holliday) junctions are processed during S-phase by dissolution, using the combined activities of the Blooms helicase and topoisomerase IIIα [7., 8., 9.]. If junctions persist, they are resolved by two major junction-cleavage activities using structure-specific nucleases. The SLX4-SLX1-MUS81-EME1 hetero-tetramer acts in the nucleus and is part of a larger complex of nucleases organized by the SLX4 scaffold protein [10., 11., 12., 13.]. Within this complex, SLX1 and MUS81 are structure-specific nucleases. Remaining junctions are processed by the cytoplasmic GEN1 (Yen1 in budding yeast) nuclease that acts during late M-phase and anaphase [14., 15., 16.]. GEN1 function as a homodimer, resolving junctions by two sequential cleavage reactions [17., 18., 19.]. The properties of GEN1 are very similar to junction-resolving enzymes of lower organisms and phage [20].
There is considerable redundancy between junction dissolution and resolution activities, and genetic interaction between the corresponding mutants have been reported. The loss of a single activity is generally well tolerated, but when cells become defective in multiple activities then they become very sensitive to DNA damage [21., 22., 23.] and aberrant chromosomes are formed [23., 24., 25.]. It has been shown in human cells that the combinations of SLX4 and either BLM or GEN1 are synthetic lethal [26], underlining the vital importance of having a functional junction resolution activity.
Despite this considerable redundancy, a single DNA link between chromatids has the potential to disrupt cell cycle progression and genome integrity, and it is likely that cells require a nuclease that can process remaining unresolved and hemi-resolved DNA junctions and other branched species at the very late stages of mitosis, and a contender for this activity is LEM-3/ANKLE1. LEM-3 was discovered in a genetic screen for embryonic lethality in Caenorhabditis elegans following ionizing irradiation [27]. The protein sequence has a predicted GIY–YIG nuclease domain, and was shown to exhibit nucleolytic activity on supercoiled DNA. ANKLE1 is the corresponding ortholog in humans and other metazoans (Figure S1) [28]. Several lines of evidence now suggest that LEM-3/ANKLE1 might be responsible for processing DNA bridges during anaphase. First, LEM-3 has been found to interact genetically with MUS81, SLX1 and SLX 4 with embryonic lethality resulting in lem-3 double mutants, all single mutants being viable [3,29]. Second, LEM-3 is excluded from the nucleus and accumulates at the mid-body the structure where the two daughter cells finally abscise from each other, starting from the early stages of cytokinesis, and genetic evidence indicate that such localization is important for LEM-3 activity in vivo [30]. Finally, chromosomal bridges persist in lem-3 mutants upon treatment with various DNA damaging agents, and under conditions where DNA replication or chromosome decondensation is compromised [30]. Thus, LEM-3/ANKLE1 exhibits many of the properties that would be expected for a nuclease whose role is to process persistent helical junctions that would otherwise lead to the regression of the cytokinetic furrow and ensuing polyploidization and aneuploidy.
What is currently missing from this analysis is a knowledge of the substrate specificity for either LEM-3 or ANKLE1. We have therefore expressed human ANKLE1 in insect cells and studied its nuclease activity on a wide variety of branched DNA species. We find that ANKLE1 is a nuclease that is specific for branched DNA, cleaving a wide range of helical junctions, flap structures and splayed Y-junctions. Thus, ANKLE1 has exactly the type of substrate specificity that would be expected for an enzyme that is located at the cell mid-body whose role is to act as a catch-all final defense to process DNA junctions that have evaded all the other levels of enzymatic attack. Our results suggest that ANKLE1 (and most probably LEM-3) acts as the enzyme of last resort allowing for faithful chromosome segregation and cell division.
Results
Expression of human ANKLE1 in insect cells
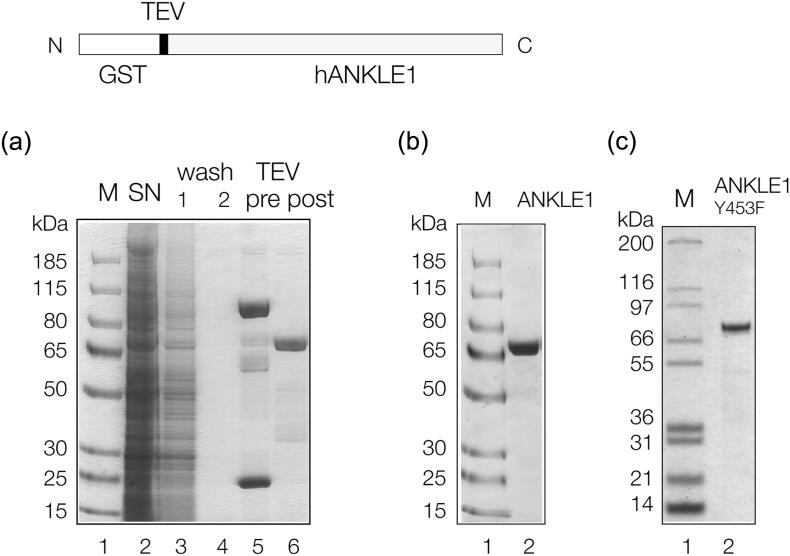
A synthetic cDNA encoding human ANKLE1 was constructed with codons optimized for expression in insect cells, carrying an N-terminal fusion with glutathione S-transferase (GST) and an intervening TEV protease cleavage site (Figure 1). The gene was incorporated into the baculovirus genome in Escherichia coli DH10EMBacY by Tn7 transposition [31], and the resulting DNA was transfected into Sf9 insect cells. In order to obtain generation 2 recombinant virus (V2), two subsequent infections were carried out. Optimal production of hANKLE1 was achieved by infecting 1.0 × 106 cells/ml of freshly diluted Sf9 cells with 1:500 (v/v) V2. GST-tagged hANKLE1 was purified using affinity chromatography on glutathione Sepharose 4B, followed by TEV cleavage on the column to remove the GST tag. The resulting non-fusion hANKLE1 was further purified by gel filtration after which the hANKLE1 migrated as a single species at the expected size on polyacrylamide gel electrophoresis in the presence of SDS (Figure 1). The protein was confirmed as human ANKLE1 by fragmentation-mass spectrometric analysis of trypsin-digested protein (Figure S2). Two isoforms of hANKLE1 were investigated with or without an N-terminal extension of 54 amino acids (Figure S1). Both forms (with and without an N-terminal GST tag) were found to have similar nuclease activity on branched DNA (Figure S3). The majority of experiments here were performed using the 615-amino-acid form with the GST tag removed.
Figure 1.
Expression and purification of human ANKLE1 in insect cells. Top: Schematic of GST-hANKLE1 fusion protein. The fusion can be cleaved by TEV protease to release hANKLE1. (a) Denaturing gel electrophoresis of expressed hANKLE1. Sf9 insect cells were lysed and centrifuged. The soluble fraction was incubated with glutathione Sepharose 4B for 1 h, allowed to settle and the supernatant taken (track 2). The resin was treated with TEV protease. Track 1: protein markers, molecular masses are indicated left; track 2: proteins not binding to the glutathione resin in cytoplasmic extract; tracks 3 and 4: sequential washes of the glutathione resin; track 5: proteins binding to the glutathione resin in cytoplasmic extract, including GST-hANKLE1, endogenous insect GST, and some proteins whose sizes are smaller than GST-hANKLE1 (these could include incomplete translation products); track 6: hANKLE1 released from the resin after cleavage with TEV protease. (b) Denaturing gel electrophoresis of unfused hANKLE1 after gel filtration. Track 1: protein markers, molecular masses are indicated left; track 2. purified hANKLE1. (c) Denaturing gel electrophoresis of unfused hANKLE1 Y453F. Track 1: protein markers, molecular masses are indicated left; track 2: purified hANKLE1 Y453F.
Human ANKLE1 is a nuclease with specificity for branched DNA
We first explored whether or not human ANKLE1 expressed and purified from insect cells exhibits nuclease activity on branched DNA species. The sequences of the DNA substrates were all derived from a well-characterized four-way DNA junction 3 [32] (the provenance of all the junctions is explained in Figure S4), each with a radioactive-[5′-32P] label on the same x strand. We found that a variety of three- and four-way DNA junctions, flap and needle (a duplex with a single-stranded overhang at the 3′ end) structures were cleaved by hANKLE1 (Figure 2). We observed that the enzyme was especially active in the presence of Mn2+ ions (Figure S5), and optimal conditions for cleavage were found to be 20 mM cacodylate (pH 6.5), 50 mM KCl, 2 mM MnCl2, 0.1 mg/ml BSA. 5 nM DNA substrate were incubated with 330 nM hANKLE1, i.e. experiments were performed under probable single-turnover conditions.
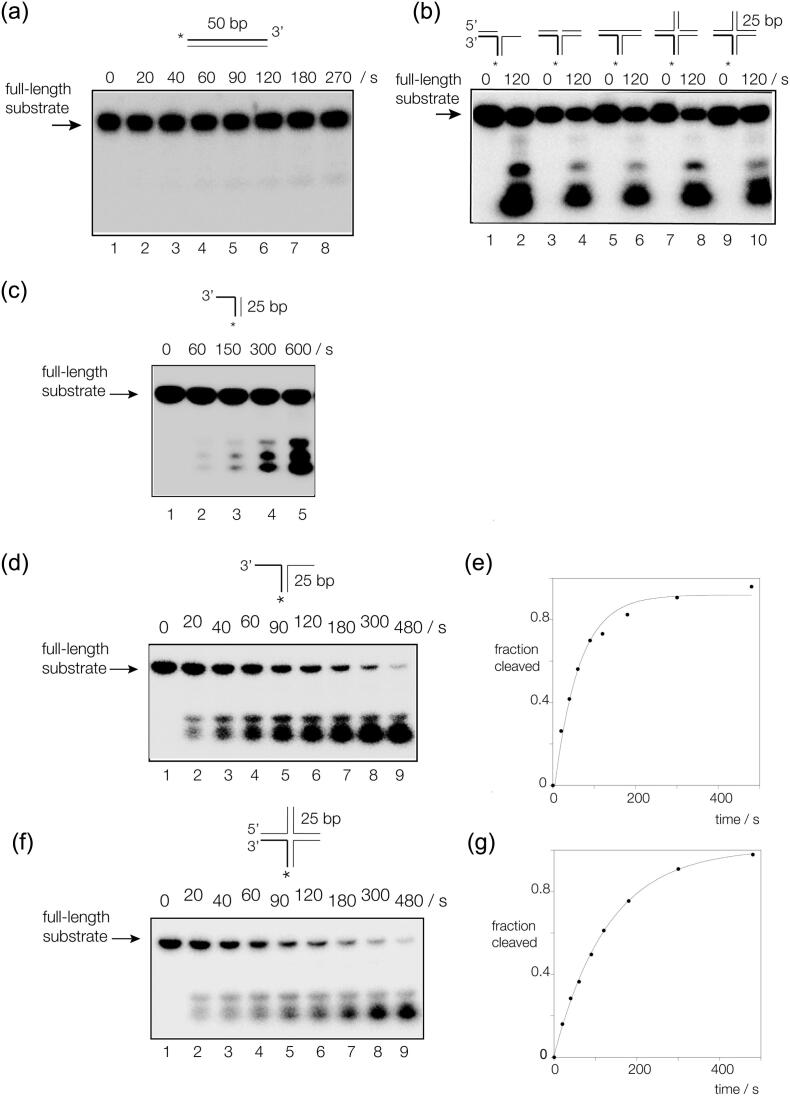
Figure 2.
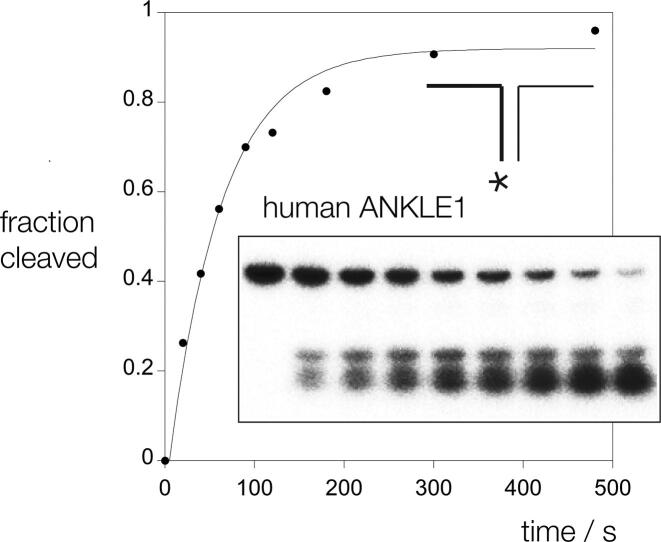
Human ANKLE1 selectively cleaves a variety of branched DNA species. A series of DNA species were formed by hybridization of component strands, all containing the same radioactively [5′-32P]-labeled x strand indicated by an asterisk. The provenance of these species is shown in Figure S4 and their sequences in Table S1. DNA was incubated with hANKLE1 under single turnover conditions in 20 mM cacodylate (pH 6.5), 2 mM MnCl2, 50 mM KCl, 0.1 mg/ml BSA at 37 °C. After the indicated times of incubation, the reaction was terminated and the substrate and products were separated by denaturing gel electrophoresis. With the exception of the duplex, all arm lengths are 25 bp. (a) Duplex DNA. The 50 bp DNA was incubated with hANKLE1 for 270 s, with aliquots removed at the indicated times. (b) Five different branched DNA species were incubated with hANKLE1 for 0 and 120 s, and cleavage analyzed by denaturing gel electrophoresis. The species were: tracks 1 and 2, a 5′ flap structure; tracks 3 and 4, a three-way junction with a nick at the point of stand exchange; tracks 9 and 10, tracks 5 and 6, an intact three-way junction; tracks 7 and 8, a four-way junction with a nick at the point of stand exchange; an intact four-way junction. (c) Cleavage of a needle DNA structure by human ANKLE1. This simple structure is cleaved by hANKLE1, but at a rate that is significantly slower than that of the splayed Y junction. (d and e) A splayed YX-junction. The DNA was incubated with hANKLE1 for 480 s, with aliquots removed at the indicated times. Note that the substrate is almost fully cleaved by the end of the incubation. Part (e) shows a plot of reaction progress as a function of time. The data (points) were fitted to a single exponential function (line). (f and g) A four-way (Holliday) junction. The DNA was incubated with hANKLE1 for 480 s, with aliquots removed at the indicated times. The junction is also almost fully cleaved by the end of the incubation. Part (g) shows a plot of reaction progress as a function of time. The data (points) were fitted to a single exponential function (line).
hANKLE1 exhibits low activity on duplex DNA (Figure 2(a)) or single-stranded DNA (data not shown), but is active on a wide variety of branched DNA (Figure 2(b) and (c), Figure S4). Time courses of incubation of a splayed YX junction (i.e. a duplex with non-complementary single-stranded extensions) and a 4H four-way junction show that both are cleaved to completion. Fitting reaction progress to single exponential functions gives rates of kobs = 0.016 and 0.008 s−1 for the Y-junction and four-way junctions respectively (Figure 2(e) and (g)), and the splayed YX junction is cleaved slightly more efficiently than the other substrates. We observe that a splayed YH junction (this is derived from the top half of junction 3, see Figure S4) is cleaved to completion (Figure S6), yet is totally unrelated to the splayed YX junction in terms of sequence. Indeed, all four splayed Y junctions that can be derived from the four-way junction 3 are cleaved by hANKLE1 (Figure S7). It is clear that in each case the cleavage is restricted to the double-stranded section and the single-stranded part is not cleaved. The sites of cleavage in the double-stranded arms are not uniformly distributed, reflecting some sequence dependence of cleavage rate, and TG is cleaved with the greatest efficiency. The YX junction is cleaved ~ 2.5 times faster than the other three junctions, likely reflecting the number of preferred TG sequences within the X arm.
We conclude that human ANKLE1 is primarily a structure-selective nuclease of broad specificity capable of cleaving a wide variety of branched DNA species.
ANKLE1 cleaves double-stranded DNA close to branchpoints
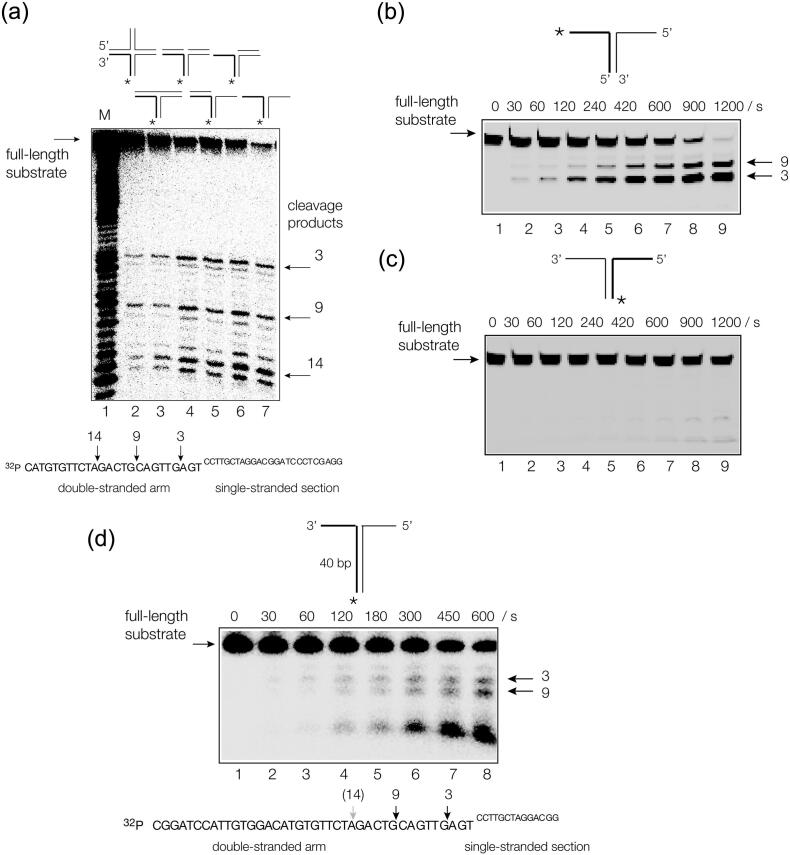
We next mapped ANKLE1 cleavage sites in the different branched DNA substrates at the nucleotide level (Figure 3(a)). These are four-way (4H) and three-way (3H) helical junctions, a three-way junction with a single-strand break at the point of strand exchange, 5′ and 3′ flaps and a splayed YX junction comprising a duplex with non-complementary single stranded sections at one end. Each species can be derived from the four-way junction 3 (see Figure S4), and include a common strand (the x strand) that was radioactively [5′-32P]-labeled. This strand is cleaved at the same sites in each branched DNA species by ANKLE1. All the cleavage occurs 5′ to the point of strand exchange; in double-stranded DNA in each case, including the splayed Y-junction. Major cleavages are observed 3, 9 and 14 nucleotides 5′ to the point of strand exchange; thus, these sites have been designated by these numbers. Each site conforms to the sequence TR ↓ (R = purine), indicating a degree of sequence selectivity in cleavage. However, the primary selectivity is at the level of structure; these sites must be adjacent to a junction for optimal cleavage.
Figure 3.
Human ANKLE cleaves double-stranded DNA close to helical branchpoints. (a) Cleavage positions in a series of branched DNA species analyzed by denaturing gel electrophoresis at single-nucleotide resolution. The positions of cleavage are arrowed on the sequence of the radioactively [5′-32P]-labeled x strand shown below the autoradiograph. (b) Analysis of the cleavage of the splayed YX-junction with the x strand fluorescently labeled at its 3′ end. The DNA was incubated with hANKLE1 for 1200 s, with aliquots removed at the indicated times. This reveals that the major cleavage occurs at site 3, i.e. 3 nt from the point of strand exchange. (c) Analysis of the cleavage of the splayed YX-junction with the r strand fluorescently labeled at its 3′ end. The DNA was incubated with hANKLE1 for 1200 s, with aliquots removed at the indicated times. Note that the r strand is very weakly cleaved. (d) Cleavage of a splayed YX-junction with an extended double-stranded helical arm of 40 bp. Analysis of the cleavage of the splayed Y-junction with the x strand radioactively [5′-32P]-labeled. The DNA was incubated with hANKLE1 for 600 s, with aliquots removed at the indicated times.
The cleavages on the splayed YX junction were investigated in greater detail. First, the same x strand was fluorescently-labeled at its 3′ end by fluorescein. ANKLE1 cleaves the strand to completion, cleaving site 3 twice as fast as at site 9 (Figure 3(b)). In this experiment, cleavage at site 14 was not observed, indicating that it occurs subsequently to cleavage at sites 3 or 9. Second, we fluorescently 3′-labeled the other (r) strand of the junction. By comparison with the x strand there is very little cleavage on the r strand by ANKLE1 (Figure 3(c)). We constructed a version of the splayed YX junction in which the double-stranded section was extended to 40 bp in length (Figure 3(d)). Incubation with ANKLE1 lead to cleavage at a new site closer to the 5′ end of the double-stranded arm. We conclude that enzyme predominantly cleaves on DNA strand 5′ to a helical junction. The significant cleavage sites are the three and nine sites, but additional cleavages occur close to the 5′ end of the molecule. Having cleaved close to the junction, it is conceivable that the enzyme then moves further away from the junction to make additional cleavages. The more distant sites could be a result of having an open end and may not relevant to the normal function of ANKLE1. All observed cleavages are within double-stranded sections of DNA. We have tested this further by comparison of two needle structures (Figure S8). These differ only in the length of the lower strand, which is 3 nt shorter at the 5′ end for needle 2 compared to needle 1. This moves the junction between single- and double-stranded DNA by 3 nt. Site a is 3 nt from the junction of needle 1 and is cleaved efficiently (along with sites b and c). However, this site becomes the double-single strand junction in needle 2, whereupon it is no longer cleaved. Thus, the exact same DNA sequence is cleaved in needle 1 where it lies within the double-stranded DNA, but is not cleaved in needle 2 where is lies at the junction between the double- and single-stranded DNA. Other cleavage sites (sites b and c) are not affected as they remain within the double stranded DNA.
The GIG-YIG motif of human ANKLE1 is required for nuclease cleavage close to DNA branchpoints
ANKLE1 has a highly conserved GIY–YIG domain at its C-terminal end (Figure 4(a)). GIY–YIG domains function as the active centers of a number of nucleases [33], including R-Eco29kl [34], I-TevI [35], UvrC [36] and the junction-resolving enzyme SLX1 [37]. For ANKLE1, the sequence is FTY453 (31 amino acids) Y486VG. The tyrosine residues play an important role in the probable mechanism of phosphodiester bond hydrolysis, so we made a Y453F mutation that removes the phenolic hydroxyl of the tyrosine (Figure 1(c)). This atomic mutant of ANKLE1 was essentially inactive on the splayed Y-junction (Figure 4(b)), showing the importance of the GIY–YIG domain in the function of the nuclease. In confirmation of this, we have also found that a Y486F mutant was similarly inactive (Figure S9). We conclude that both tyrosine phenolic oxygen atoms of the GIY_YIG motif are essential for the cleavage of DNA junctions by human ANKLE1.
Figure 4.
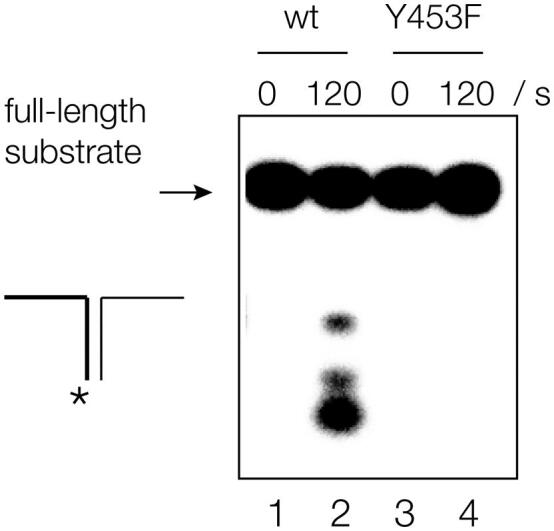
Cleavage of branched DNA by human ANKLE requires the GIY-YIG domain.
A mutant in the first tyrosine residue of the GIY-YIG domain (Y453F) was prepared. A splayed YX-junction radioactively [5′-32P]-labeled on the x strand was incubated with either wild-type hANKLE1 (tracks 1 and 2) or Y453F hANKLE1 (tracks 3 and 4) for either 0 or 120 s. Note that the DNA incubated with the mutant enzyme was uncleaved. Note that only a single atom of hANKLE1 has been changed by the mutation. A corresponding mutant in the other tyrosine (Y486F) was also inactive (Figure S9).
Discussion
These studies have shown that human ANKLE1 expressed in insect cells is a nuclease that is selective for branched DNA species. Helical junctions with and without single-strand breaks, flaps of various kinds and splayed Y-structures are all subject to hydrolysis of phosphodiester linkages at sites in double-stranded DNA close to the junction. The range of branched DNA substrates is wider than a conventional Holliday junction-resolving enzyme such as GEN1, consistent with a role in processing unresolved or partially resolved junctions that might result from a failure to complete processing of recombination intermediates. Resolving enzymes like GEN1 have evolved processes whereby the second strand cleavage event is significantly accelerated relative to the first [17,38,39], so increasing the probability of bilateral cleavage during the lifetime of the complex. However, if that fails for any reason then a hemi-resolved junction could result. A number of different branched species might result from aberrant processing of junctions, any of which might result in DNA bridging between chromatids. It is therefore probable that a nuclease with relatively wide substrate specificity would be required to clean up debris from these events.
Fluorescence microscopy has previously shown that the C. elegans LEM-3 that is orthologous with ANKLE1 (Figure S1) is located at the cell mid-body, where it likely contributes to the resolution of residual DNA bridges just before cells divide [30]. These bridges can be induced by ionizing radiation and mutagens such as hydroxyurea, by slowing DNA replication, by DNA de-condensation defects, as well as by inhibition of enzymes associated with Holliday junction dissolution or resolution such as MUS81, SLX1 and SLX4 [3]. While the exact nature and origins of DNA bridges are not fully understood at present, they are observed from the onset of anaphase, through telophase, up to the point where cells divide during cytokinesis and it is likely that they arise in part from failed or incomplete resolution of recombination intermediates, which must be processed before cell division can be completed. We have now shown that human ANKLE1 has broad-range structural selectivity for a range of branched DNA species. That together with the sub-cellular location of the closely-related LEM3 in C. elegans is fully consistent with a role at cytokinesis to process DNA branchpoints that have not been resolved at an earlier stage of the cell cycle. The broad specificity could lead to the cleavage of branched DNA structures that occur during DNA duplication, and overexpression of ANKLE1 in the nucleus was shown to trigger DNA damage [28]. This might explain why LEM-3 appears to be excluded from the nucleus [3]. Put briefly, ANKLE1 has the required specificity and the correct location to act as a catch-all “enzyme of last resort” to process a variety of junctions in residual DNA bridges in order to allow cell division to be completed.
Materials and Methods
Expression and purification of human ANKLE1
DNA with the human ANKLE1 coding sequence, codon optimized for baculovirus expression was synthesized by GeneArt Gene Synthesis (Thermo Fisher). The DNA was cloned into MultiBac expression vector pKL with an N-terminal GST tag. Active site mutants Y453F and Y486F were generated by site-directed mutagenesis (QuikChange, Agilent). DNA encoding wild-type or mutant hANKLE1 was integrated into baculovirus genome by the transformation into E. coli DH10EMBacY by Tn7 transposition. The recombinant bacmids, initial DNA (V0), generation 1 (V1) and 2 virus (V2) containing ANKLE1 were prepared according to protocols published by Berger et al. [31].
Insect Sf9 cells at a density of 106 cells/ml were infected with V2 virus in a 1:500 ratio (vol/vol) and incubated at 27 °C shaking at 150 rpm. The cells were harvested at day 3 of proliferation arrest, and the cell pellet was suspended and lysed in lysis buffer containing 20 mM Hepes (pH 7.5), 500 mM KCl, 10% glycerol, 1 mM DTT, 0.1% Triton-X and EDTA-free Protease Inhibitor Cocktail (cOmplete™, Roche). After centrifugation at 20,000g at 4 °C, the cytoplasmic extract was incubated with glutathione Sepharose® 4B (GE) for 1 h at 4 °C. The resin was washed with 20 mM Hepes (pH 7.5), 500 mM KCl, 10% glycerol, 1 mM DTT, 0.1% Triton-X. After washing, the resin was incubated with TEV Protease (NEB) for 3 h at room temperature. The cleaved hANKLE1 was then subject to gel filtration chromatography on Superdex® 200 (GE). hANKLE1 concentration was estimated spectrophotometrically by absorption at 280 nm using an extinction coefficient of 66,000 M−1 cm−1.
Characterization of expressed human ANKLE1 by fragmentation and mass spectrometry
A fragment of polyacrylamide gel containing the human ANKLE1 was destained and reacted with 10 mM DTT and 55 mM iodoacetamide to reduce disulfide bonds and alkylate free cysteines. The protein was then digested with 12.5 μg/ml trypsin in 20 mM ammonium bicarbonate at 30 °C for 16 h. The peptide mixture was dried and suspended to 50 μl 1% formic acid, and then separated by reversed-phase chromatography. Peptides were initially trapped on an Acclaim PepMap 100 (C18, 100 μM × 2 cm) and then separated on an Easy-Spray PepMap RSLC C18 column (75 μM × 50 cm) (Thermo Scientific). Samples were transferred to a Q Exactive plus mass spectrometer via an Easy-Spray source with temperature set at 50 °C and a source voltage of 2.0 kV. The precursor ion is selected by the quadrupole and isolated for fragmentation using higher energy collisional dissociation. The ions were transferred to an orbitrap to provide high resolution accurate mass data of the fragmented peptides. The peptides are identified by the data analysis software by reference to a protein database.
Oligonucleotide synthesis
Oligonucleotides were synthesized using β-cyanoethyl phosphoramidite chemistry [40,41]. Fully deprotected oligonucleotides were purified by gel electrophoresis on a 10% (w/v) polyacrylamide gel in 90 mM Tris·borate (pH 8.5), 2 mM EDTA (TBE buffer) containing 8M urea. Oligonucleotides were detected by UV shadowing and recovered by electroelution and ethanol precipitation. Concentrations were estimated by absorbance at 260 nm. Oligonucleotides labeled with fluorescein at their 3′-termini were synthesized using 6-fluorescein CPG columns (Glen Research 20-2961). Oligonucleotides were radioactively [5′-32P]-labeled using [γ-32P] ATP (PerkinElmer) using T4 polynucleotide kinase (Fermentas) for 30 min at 37 °C in in 50 mM Tris (pH 7.6), 10 mM MgCl2, 5 mM DTT, 0.1 mM spermidine. All DNA sequences used are tabulated in Table S1.
Preparation of DNA substrates
Substrates were hybridized by mixing 1 μM of one radioactively [5′-32P]-labeled strand with 1.5 μM of the other required strands (see Figure S4) and incubated for 2 min at 80 °C followed by slow cooling overnight. DNA substrates were purified by electrophoresis in 6% (29:1) polyacrylamide gel in TBE. The labeled DNA was recovered by electroelution in 0.5 × TBE. After ethanol precipitation, the DNA substrate concentration was measured spectrophotometrically by absorption at 260 nm and adjusted to 50 nM. Splayed Y junctions with a 3′-fluorescently-labeled strand were prepared by mixing equimolar quantities of labeled and unlabeled strands at 20 μM and hybridizing and purified as above.
Analysis of DNA cleavage by human ANKLE1
[5′-32P]-labeled DNA substrate (5 nM) was incubated with 330 nM hANKLE1 for 10 min at 20 °C in 20 mM cacodylate (pH 6.5), 50 mM KCl, 0.1 mg/ml BSA. These concentrations are expected to be close to single-turnover conditions. After a 3-min pre-incubation at 37 °C, the cleavage reaction was initiated by the addition of MnCl2 (unless stated otherwise) to a final concentration of 2 mM. Aliquots were taken at chosen times and the reaction terminated by addition of EDTA to a final concentration of 20 mM and 50% formamide. The aliquots were heated at 95 °C for 15 min and then loaded on to a 14% (19:1) polyacrylamide gel in TBE, 8 M urea. Radioactive DNA was detected by exposure to a storage phosphor screens, and visualized using a BAS-1500 phosphorimager (Fuji). Fluorescent DNA was visualized in the gel using a Typhoon FLA9000 imager using a 473 nm laser. Data were analyzed as the fraction of cleaved DNA (fc) versus time (t) and fitted by non-linear regression to:
| (1) |
where f0 is the fraction of DNA cleaved at the end of the reaction and k is the observed rate of cleavage.
Acknowledgments
Acknowledgments
We thank Drs Anne-Cécile Déclais and Tim Wilson for discussion, and Kenny Beattie, Samantha Kosto and Douglas Lamont for mass spectrometry data collection and analysis. We are grateful to Elisa Garcia-Wilson, Taciana Kasciukovic and Harinath Doodhi for technical assistance in baculovirus expression system and protein purification. We acknowledge Cancer Research UK (program grant A18604) and BBSCRC (BB/S002782/1) for financial support of our research in Dundee, and the Korean Institute for Basic Science (IBS-R022-A1-2019).
Credit author statement
A.D.J.F. performed nuclease cleavage experiments and measured kinetics. J.F.S. made expression constructs, expressed and purified human ANKLE1. A.K. contributed expertise on the expression of proteins in insect cells. D.M.J.L. and A.G. conceived and designed the project, wrote and revised the paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmb.2020.08.022.
Appendix A. Supplementary data
Supplementary material
References
- 1.Wyatt H.D., Sarbajna S., Matos J., West S.C. Coordinated actions of SLX1-SLX4 and MUS81-EME1 for Holliday junction resolution in human cells. Molec. cell. 2013;52:234–247. doi: 10.1016/j.molcel.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 2.Chan Y.W., Fugger K., West S.C. Unresolved recombination intermediates lead to ultra-fine anaphase bridges, chromosome breaks and aberrations. Nature Cell Biol. 2018;20:92–103. doi: 10.1038/s41556-017-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong Y., Velkova M., Silva N., Jagut M., Scheidt V., Labib K., Jantsch V., Gartner A. The conserved LEM-3/Ankle1 nuclease is involved in the combinatorial regulation of meiotic recombination repair and chromosome segregation in Caenorhabditis elegans. PLoS Genet. 2018;14 doi: 10.1371/journal.pgen.1007453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan K.L., North P.S., Hickson I.D. BLM is required for faithful chromosome segregation and its localization defines a class of ultrafine anaphase bridges. EMBO J. 2007;26:3397–3409. doi: 10.1038/sj.emboj.7601777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumann C., Korner R., Hofmann K., Nigg E.A. PICH, a centromere-associated SNF2 family ATPase, is regulated by Plk1 and required for the spindle checkpoint. Cell. 2007;128:101–114. doi: 10.1016/j.cell.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 6.Chan K.L., Palmai-Pallag T., Ying S., Hickson I.D. Replication stress induces sister-chromatid bridging at fragile site loci in mitosis. Nature Cell Biol. 2009;11:753–760. doi: 10.1038/ncb1882. [DOI] [PubMed] [Google Scholar]
- 7.Ira G., Malkova A., Liberi G., Foiani M., Haber J.E. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell. 2003;115:401–411. doi: 10.1016/s0092-8674(03)00886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu L., Hickson I.D. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- 9.Mimitou E.P., Symington L.S. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008;455:770–774. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munoz I.M., Hain K., Déclais A.-C., Gardiner M., Toh G.W., Sanchez-Pulido L., Heuckmann J., Toth R. Coordination of structure-specific nucleases by human SLX4/BTBD12 is essential for DNA repair. Molec. Cell. 2009;35:116–127. doi: 10.1016/j.molcel.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Fekairi S., Scaglione S., Chahwan C., Taylor E.R., Tissier A., Coulon S., Dong M.Q., Ruse C. Human SLX4 is a Holliday junction resolvase subunit that binds multiple DNA repair/recombination endonucleases. Cell. 2009;138:78–89. doi: 10.1016/j.cell.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svendsen J.M., Smogorzewska A., Sowa M.E., O'Connell B.C., Gygi S.P., Elledge S.J., Harper J.W. Mammalian BTBD12/SLX4 assembles a Holliday junction resolvase and is required for DNA repair. Cell. 2009;138:63–77. doi: 10.1016/j.cell.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen S.L., Bergstralh D.T., Kohl K.P., LaRocque J.R., Moore C.B., Sekelsky J. Drosophila MUS312 and the vertebrate ortholog BTBD12 interact with DNA structure-specific endonucleases in DNA repair and recombination. Molec. Cell. 2009;35:128–135. doi: 10.1016/j.molcel.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ip S.C., Rass U., Blanco M.G., Flynn H.R., Skehel J.M., West S.C. Identification of Holliday junction resolvases from humans and yeast. Nature. 2008;456:357–361. doi: 10.1038/nature07470. [DOI] [PubMed] [Google Scholar]
- 15.Bailly A.P., Freeman A., Hall J., Declais A.C., Alpi A., Lilley D.M., Ahmed S., Gartner A. The Caenorhabditis elegans homolog of Gen1/Yen1 resolvases links DNA damage signaling to DNA double-strand break repair. PLoS genetics. 2010;6 doi: 10.1371/journal.pgen.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rass U., Compton S.A., Matos J., Singleton M.R., Ip S.C., Blanco M.G., Griffith J.D., West S.C. Mechanism of Holliday junction resolution by the human GEN1 protein. Genes Dev. 2010;24:1559–1569. doi: 10.1101/gad.585310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman A.D.J., Liu Y., Declais A.C., Gartner A., Lilley D.M.J. GEN1 from a thermophilic fungus is functionally closely similar to non-eukaryotic junction-resolving enzymes. J. Molec. Biol. 2014;426:3946–3959. doi: 10.1016/j.jmb.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Freeman A.D.J., Declais A.C., Wilson T.J., Gartner A., Lilley D.M.J. Crystal structure of a eukaryotic GEN1 resolving enzyme bound to DNA. Cell Rep. 2015;13:2565–2575. doi: 10.1016/j.celrep.2015.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y., Freeman A.D.J., Declais A.C., Lilley D.M.J. A monovalent ion in the DNA binding interface of the eukaryotic junction-resolving enzyme GEN1. Nucleic Acids Res. 2018;46:11089–11098. doi: 10.1093/nar/gky863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Déclais A.C., Lilley D.M.J. New insight into the recognition of branched DNA structure by junction-resolving enzymes. Curr. Opin. Struct. Biol. 2008;18:86–95. doi: 10.1016/j.sbi.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Crossan G.P., van der Weyden L., Rosado I.V., Langevin F., Gaillard P.H., McIntyre R.E., Gallagher F., Kettunen M.I. Disruption of mouse Slx4, a regulator of structure-specific nucleases, phenocopies Fanconi anemia. Nat Genet. 2011;43:147–152. doi: 10.1038/ng.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castor D., Nair N., Déclais A.C., Lachaud C., Toth R., Macartney T.J., Lilley D.M.J., Arthur J.S. Cooperative control of Holliday junction resolution and DNA repair by the SLX1 and MUS81–EME1 nucleases. Molec. cell. 2013;52:221–233. doi: 10.1016/j.molcel.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y., Qian Y., Zhou G., Lv J., Yan Q., Dong X. Effect of GEN1 interference on the chemosensitivity of the breast cancer MCF-7 and SKBR3 cell lines. Oncol Lett. 2016;11:3597–3604. doi: 10.3892/ol.2016.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wechsler T., Newman S., West S.C. Aberrant chromosome morphology in human cells defective for Holliday junction resolution. Nature. 2011;471:642–646. doi: 10.1038/nature09790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarbajna S., Davies D., West S.C. Roles of SLX1–SLX4, MUS81–EME1, and GEN1 in avoiding genome instability and mitotic catastrophe. Gene Dev. 2014;28:1124–1136. doi: 10.1101/gad.238303.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garner E., Kim Y., Lach F.P., Kottemann M.C., Smogorzewska A. Human GEN1 and the SLX4-associated nucleases MUS81 and SLX1 are essential for the resolution of replication-induced Holliday junctions. Cell reports. 2013;5:207–215. doi: 10.1016/j.celrep.2013.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dittrich C.M., Kratz K., Sendoel A., Gruenbaum Y., Jiricny J., Hengartner M.O. LEM-3—a LEM domain containing nuclease involved in the DNA damage response in C. elegans. PloS one. 2012;7 doi: 10.1371/journal.pone.0024555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brachner A., Braun J., Ghodgaonkar M., Castor D., Zlopasa L., Ehrlich V., Jiricny J., Gotzmann J. The endonuclease Ankle1 requires its LEM and GIY–YIG motifs for DNA cleavage in vivo. J. Cell Sci. 2012;125:1048–1057. doi: 10.1242/jcs.098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braun J., Meixner A., Brachner A., Foisner R. The GIY–YIG type endonuclease ankyrin repeat and LEM domain-containing protein 1 (ANKLE1) is dispensable for mouse hematopoiesis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong Y., Sonneville R., Wang B., Scheidt V., Meier B., Woglar A., Demetriou S., Labib K. LEM-3 is a midbody-tethered DNA nuclease that resolves chromatin bridges during late mitosis. Nat. Commun. 2018;9:728. doi: 10.1038/s41467-018-03135-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzgerald D.J., Berger P., Schaffitzel C., Yamada K., Richmond T.J., Berger I. Protein complex expression by using multigene baculoviral vectors. Nat. Methods. 2006;3:1021–1132. doi: 10.1038/nmeth983. [DOI] [PubMed] [Google Scholar]
- 32.Duckett D.R., Murchie A.I.H., Diekmann S., von Kitzing E., Kemper B., Lilley D.M.J. The structure of the Holliday junction and its resolution. Cell. 1988;55:79–89. doi: 10.1016/0092-8674(88)90011-6. [DOI] [PubMed] [Google Scholar]
- 33.Dunin-Horkawicz S., Feder M., Bujnicki J.M. Phylogenomic analysis of the GIY–YIG nuclease superfamily. BMC Genomics. 2006;7:1–19. doi: 10.1186/1471-2164-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mak A.N., Lambert A.R., Stoddard B.L. Folding, DNA recognition, and function of GIY–YIG endonucleases: crystal structures of R.Eco29kI. Structure. 2010;18:1321–1331. doi: 10.1016/j.str.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Roey P., Meehan L., Kowalski J.C., Belfort M., Derbyshire V. Catalytic domain structure and hypothesis for function of GIY–YIG intron endonuclease I-TevI. Nat. Struct. Biol. 2002;9:806–811. doi: 10.1038/nsb853. [DOI] [PubMed] [Google Scholar]
- 36.Truglio J.J., Rhau B., Croteau D.L., Wang L., Skorvaga M., Karakas E., DellaVecchia M.J., Wang H. Structural insights into the first incision reaction during nucleotide excision repair. EMBO J. 2005;24:885–894. doi: 10.1038/sj.emboj.7600568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaur V., Wyatt H.D.M., Komorowska W., Szczepanowski R.H., de Sanctis D., Gorecka K.M., West S.C., Nowotny M. Structural and mechanistic analysis of the Slx1–Slx4 endonuclease. Cell Rep. 2015;10:1467–1476. doi: 10.1016/j.celrep.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fogg J.M., Schofield M.J., Déclais A.-C., Lilley D.M.J. Yeast resolving enzyme CCE1 makes sequential cleavages in DNA junctions within the lifetime of the complex. Biochemistry. 2000;39:4082–4089. doi: 10.1021/bi992785v. [DOI] [PubMed] [Google Scholar]
- 39.Fogg J.M., Lilley D.M.J. Ensuring productive resolution by the junction-resolving enzyme RuvC: large enhancement of the second-strand cleavage rate. Biochemistry. 2000;39:16125–16134. doi: 10.1021/bi001886m. [DOI] [PubMed] [Google Scholar]
- 40.Beaucage S.L., Caruthers M.H. Deoxynucleoside phosphoramidites - a new class of key intermediates for deoxypolynucleotide synthesis. Tetrahedron Lett. 1981;22:1859–1862. [Google Scholar]
- 41.Sinha N.D., Biernat J., McManus J., Koster H. Polymer support oligonucleotide synthesis XVIII: Use of b-cyanoethyl-N,N-dialkylamino/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984;12:4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material