Abstract
Whether low-dose ionizing radiation can cause cancer is a critical and long-debated question in radiation protection. Since the Biological Effects of Ionizing Radiation report by the National Academies in 2006, new publications from large, well-powered epidemiological studies of low doses have reported positive dose-response relationships. It has been suggested, however, that biases could explain these findings. We conducted a systematic review of epidemiological studies with mean doses less than 100 mGy published 2006–2017. We required individualized doses and dose-response estimates with confidence intervals. We identified 26 eligible studies (eight environmental, four medical, and 14 occupational), including 91 000 solid cancers and 13 000 leukemias. Mean doses ranged from 0.1 to 82 mGy. The excess relative risk at 100 mGy was positive for 16 of 22 solid cancer studies and 17 of 20 leukemia studies. The aim of this monograph was to systematically review the potential biases in these studies (including dose uncertainty, confounding, and outcome misclassification) and to assess whether the subset of minimally biased studies provides evidence for cancer risks from low-dose radiation. Here, we describe the framework for the systematic bias review and provide an overview of the eligible studies.
Whether low doses of ionizing radiation (<100 mGy) can cause cancer is the most critical and long-debated question for radiation protection standards (1, 2). Currently, the key sources of low-dose radiation exposure to the general population are diagnostic medical exposures like computed tomograph (CT) scans and natural background radiation (3). There are also about 20 million workers in the world who are exposed due to their occupation, including medical workers, aircrew, and nuclear workers (3).
The last major US review of the experimental and epidemiological evidence for cancer risks from low-dose exposures was conducted by the National Academies Biological Effects of Ionizing Radiation (BEIR) VII committee in 2006 (2). The committee concluded that “the available scientific evidence is consistent with a linear dose-response relationship between ionizing radiation and the development of cancer in humans (2).” This conclusion has been questioned, however, because it was largely based on animal and mechanistic studies combined with epidemiological data from higher dose exposures (>100 mGy) rather than direct data from populations exposed to doses less than 100 mGy. The authors of the BEIR VII report highlighted the difficulty in providing direct human evidence, because the risks are likely to be small and very large studies with minimal potential biases are needed to detect them.
Since the BEIR VII report a number of new publications from large, well-powered epidemiological studies have reported positive dose-response relationships, supporting excess cancer risks from low-dose and low–dose rate radiation exposure (4). These studies maximized statistical power to detect small excess risks by focusing on the most radiosensitive populations and outcomes (eg, leukemia after childhood exposure), combining individual-level data from several studies (pooling) or using large-scale electronic record linkage. Nevertheless, it has been suggested that biases such as confounding and dose error could explain the positive findings.
The aim of this monograph was to systematically assess the epidemiological evidence for excess cancer risks from low-dose radiation using the novel approach of conducting a systematic bias assessment. This is in contrast with the traditional approach of a systematic review when the focus is usually on evaluating study quality, which does not necessarily correlate with bias [see, eg, the recent National Council on Radiation Protection (NCRP) commentary (5)]. Such reviews also rarely consider the impact of potential bias on parameter estimates with respect to direction or magnitude of the bias.
We reviewed studies published since the BEIR VII report in 2006 (2), including studies of environmental, medical, and occupational radiation; childhood and adulthood exposures from acute or chronic exposures; and outcomes including the most radiogenic cancers (leukemia, breast, and thyroid cancer) as well as all solid cancers. Our findings will have important implications for radiation protection standards, which currently rely on the linear no-threshold assumption because of the lack of direct evidence for cancer risks from low-dose exposures. In addition, our framework can serve as a model for reviewing epidemiological evidence for other exposures where there is controversy about the potential impact of biases.
Here we describe the framework, the criteria for including studies in our review, and the search criteria and give a brief overview of each eligible study and summarize the main study findings.
Framework
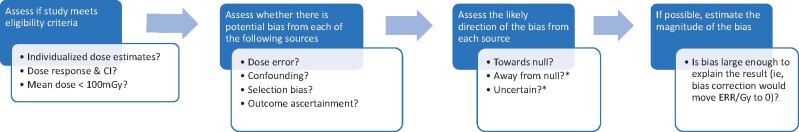
The general framework for our systematic bias evaluation is shown in Figure 1. After selecting the eligible studies using the criteria described below, we assessed for each study whether there was potential bias in the dose-response risk estimate from dose error, confounding or selection bias, or outcome ascertainment bias. Where we identified evidence of potential bias from any of these sources, we assessed the direction, that is, whether bias was likely to be towards or away from the null, or if the direction was uncertain. For assessment of our primary aim of whether there was an increased risk of cancer from low-dose ionizing radiation, it was most critical to ascertain whether there was bias away from the null. For the combined bias assessment, we assessed whether there was any source of bias that was likely away from the null or of uncertain direction. Positive studies with bias in the positive direction or bias of uncertain direction were excluded in the final assessment. The statistical and epidemiological methodologies for these evaluations are described in detail in the relevant manuscripts in this monograph on 1) dosimetry (6), 2) confounding or selection bias (7), and 3) outcome ascertainment (8). The relevant data abstracted from the publications are also summarized in each of those manuscripts along with a more traditional review of the relevant study methodology. The summary manuscript describes the combined bias assessment and summary statistical evaluations, including a sign test for assessment of whether the median excess relative risk (ERR) equals zero and assessed the impact of excluding positive studies with potential bias away from the null (9). We also conducted a meta-analysis to quantify the ERR and assess consistency across studies. In the manuscript on interpretation, we discuss other issues that affect the interpretation of study findings, including the biological rationale, study power, and model misspecification (10).
Figure 1.
Framework for the systematic bias assessment. *If aim is to assess whether there is evidence of an effect then priority is to identify biases away from null. CI = confidence interval; ERR = excess relative risk.
To illustrate how our approach differs from a traditional systematic review, we consider the example of potential confounding by smoking. Typically, if a study of all solid cancers and radiation has not controlled for smoking, then it would be considered a low-quality study because of the potential for confounding by smoking and possibly excluded from the systematic review. We take the evaluation a step further by performing an individualized bias assessment by assessing whether there is evidence that the radiation dose is likely associated with smoking in that study population, which is a necessary condition for confounding. Even if smoking data were not available for the entire cohort, this can be assessed if there are substudies (eg, nested case-control studies) that had collected smoking data or by proxy outcomes (eg, is radiation dose related to chronic obstructive pulmonary disease). If no relevant information is available, then we used theoretical assessments based on empirical knowledge of the strength of the relationship between the confounder and the disease to calculate the prevalence of the confounder that would be required to completely explain the observed association (7).
Standard epidemiological theory is used to assess the direction of the bias. For example, if smoking and radiation dose are positively correlated and smoking is a risk factor for all solid cancers, then smoking will be a positive confounder of the radiation dose-all solid cancer association and bias the estimate of association away from the null. There are a few circumstances where it is not possible to determine the direction of the bias because the necessary data are not available for that or a similar study population. For most studies, it was not feasible to estimate the magnitude of the potential bias because insufficient data were available in the publications. However, for a few examples of the most informative studies with minimum data requirements, we were able to conduct theoretical calculations to assess whether it was feasible for the bias to completely explain the result, for example, move the risk estimate to 0. See, for example, smoking and solid cancers for the International Nuclear Workers Study (INWORKS) study in the confounding article (7).
Study Eligibility
We conducted a systematic literature review of epidemiological studies published since the BEIR VII report in 2006 (2) and before December 31, 2017. Studies were eligible for inclusion if they were epidemiological studies of human populations exposed to low-dose radiation (mean dose < 100 mGy), predominantly low-linear energy transfer radiation exposure. We used mean dose for the eligibility because it was available for all of the studies but also abstracted the dose range and the percentage of the study participants that were exposed to doses of 100+ mGy where available. When not available, we estimated this percentage assuming a log-normal distribution. We required individualized dose estimates for the study participants and that the publications provided risk estimates and confidence intervals (CIs) for the dose response for cumulative radiation dose. The search strategy and results are described in detail in the Appendix.
Summary of Study Findings
Most studies reported results from models in which the relative risk is assumed to be a linear function of dose and reported as an ERR per unit dose. We abstracted the ERR at 100 mGy and confidence intervals from each eligible study for all cancers (or site-specific cancers where all cancers were not available) and leukemia, which is the most radiosensitive cancer, but excluding chronic lymphocytic leukemia (CLL) if possible, which is less radiogenic (11). We converted risk estimates (and confidence intervals) from Gray or milligray to 100 mGy where necessary. For the subset of studies that fitted log-linear rather than linear relative risk models, we first estimated the relative risk at 100 mGy and then subtracted 1. The assumptions underlying the combined effect of multiple exposures are different in these two models, but at low doses the log-linear estimates are reasonable approximations to the linear estimates. Further details are provided in (10). Most of the studies of populations who received whole-body exposure reported results for all solid cancers and for leukemia separately. We have focused our review on these results rather than site-specific solid cancer analyses, which may lack power and are at risk from multiple testing. For nonuniform exposures, the results are generally for specific cancer sites that were highly exposed and/or highly radiosensitive, for example, brain tumors after pediatric CT scans and breast cancer after medical occupational exposures. In describing the study findings, we defined statistical significance as a two-sided test for trend of P less than .05.
Overview of Eligible Studies
We identified 26 eligible studies, including eight studies of environmental (12–19), four medical (20–23), and 14 studies of occupational exposure (24–37) (Table 1). Overall, the studies included 3.6 million individuals (although 2 million of these came from the Swiss population-based background study) of whom approximately 91 000 developed solid cancers and 13 000 leukemias. There is some overlap between studies because the UK National Registry of Radiation Workers (NRRW) (26), French, and US workers are also included in the INWORKS cohort (34, 39), although the endpoints differed for the UK NRRW (cancer incidence vs cancer mortality as used by INWORKS). Mean doses ranged from 0.1 mSv from the Three Mile Island accident (13) to 82 mSv in Chornobyl liquidators (30). Most of the study participants were exposed to doses less than 100 mGy; only five studies had more than 10% of the participants with doses of 100+ mGy. Two studies included adults and children (17, 19), seven of the studies focused on childhood exposure (12, 15, 16, 18, 21–23), and the majority evaluated adulthood exposure because they were occupational studies.
Table 1.
Description of eligible studies
| Study | Reference, year (citation) | Age at exposure | Mean dosea | Dose range | 100+ mSv/mGy, % | Solid cancers | Leukemia | Cohort size |
|---|---|---|---|---|---|---|---|---|
| Environmental | ||||||||
| Chernobyl residents (leukemia) | Davis, 2006 (12) | Childhood | 6 mGyb | 0–265 mGy | 20% >5 mGy | — | 421 | — |
| Three Mile Island | Han, 2011 (13) | Adulthood | 0.1 mSv | 0–0.8 mSv | 0% | 1643 | 55 | 21 494 |
| Chinese background | Tao, 2012 (14) | Adulthood | 66 mGy | 0–125+ mGy | 11% | 941 | 15 | 31 604 |
| GB background | Kendall, 2013 (15) | Childhood | 4 mSv | 0–31 mSv | 0% | 18 389 | 9058 | — |
| Swiss background | Spycher, 2015 (16) | Childhood | 9 mSv | 0–49 mSv | 0% | 1252 | 530 | 2 093 660 |
| Techa river | Davis, 2015 (17) | All ages | 60 mGy | 0–960 mGy | 11%d | 1993 | — | 17 435 |
| Finnish background | Nikkila, 2016 (18) | Childhood | 2 mSvc | 0–12 mSv | 0% | — | 1093 | — |
| Taiwanese residents | Hsieh, 2017 (19) | All ages | 48 mSv | 0–2363 mSv | 9% | 236 | 11 | 6242 |
| Medical | ||||||||
| Canadian cardiac imaging | Eisenberg, 2011 (20) | Adulthood | 20 mSvd | 0–30+ mSv | 1%d | 12 020 | — | 82 861 |
| French pediatric CT (brain/leukemia) | Journy, 2016 (21) | Childhood | 23/9 mGye | 0–100+ mGy | 2%/1%e | 15 | 12 | 58 620 |
| UK pediatric CT (brain/leukemia) | Berrington, 2016 (22) | Childhood | 43/12 mGye | 0–400+ mGy | 6%/1%e | 112 | 70 | 178 604 |
| PIRATES (low dose) (thyroid) | Lubin, 2017 (23) | Childhood | 30 mGy | 0–200 mGy | 10% | 394 | — | 107 594 |
| Occupational | ||||||||
| Korean workers | Ahn, 2008 (24) | Adulthood | 6 mSv | 0–50+ mSv | <1%d | 247 | 9 | 79 679 |
| Chernobyl liquidators (leukemia) | Kesminiene, 2008 (25) | Adulthood | 51 mGy | 0–500+ mGy | 14% | — | 19 | — |
| UKNRRW | Muirhead, 2009 (26) | Adulthood | 25 mSv | 0–600+ mSv | 6% | 10 855 | 362 | 174 541 |
| Korean nuclear workers | Jeong, 2010 (27) | Adulthood | 20 mSv | 0–480 mSv | 5% | 203 | — | 16 236 |
| Rocketdyne workers | Boice, 2011 (28) | Adulthood | 14 mSv | 0–1000 mSv | 3% | 648 | 25 | 46 970 |
| Japanese workers | Akiba, 2012 (29) | Adulthood | 12 mSv | 0–<450 mSv | 3% | 2636 | 80 | 200 583 |
| Ukrainian Chernobyl liquidators (leukemia) | Zablotska, 2013 (30) | Adulthood | 82 mGy | 0–2600 mGy | 22% | — | 52 | — |
| Canadian nuclear workers | Zablotska, 2014 (31) | Adulthood | 22 mSv | 0–679 mSv | 3% | 437 | 21 | 45 316 |
| German nuclear workers | Merzenich, 2014 (32) | Adulthood | 30 mSv | 0–100+ mSv | 9% | 119 | 7 | 8746 |
| US nuclear workers | Schubauer-Berigan, 2015 (33) | Adulthood | 20 mSv | 0–700 mSv | 5% | 10 877 | 369 | 119 195 |
| INWORKS | Richardson, 2015 (34) | Adulthood | 21/16 mGyg | 0–1332 mGy | 3%/3%d,g | 17 957 | 531 | 308 297 |
| US atomic veterans | Caldwell, 2016 (35) | Adulthood | 9 mGy | 0–580 mGy | <1%d | 1922 | na | 114 270 |
| USRT (breast, skin, and brain cancers) | Preston, 2016 (36) | Adulthood | 37/56/12 mGyf | 0–1735 mGy | 8%/14%/<1%f | 1922/3615/193f | — | 110 000 |
| French nuclear workers | Leuraud, 2017 (37) | Adulthood | 26 mSv | 0–669 mSv | 3%d | 2536 | 57 | 59 004 |
See Daniels et al. (2020) (6) for details of the dose metrics. C-C = case-control; na = not available; INWORKS = International Nuclear Workers Study.
Dose in the controls.
Median dose.
Assuming log-normal distribution.
Brain/red bone marrow dose.
Breast/skin/brain cancers.
Solid cancers/leukemia.
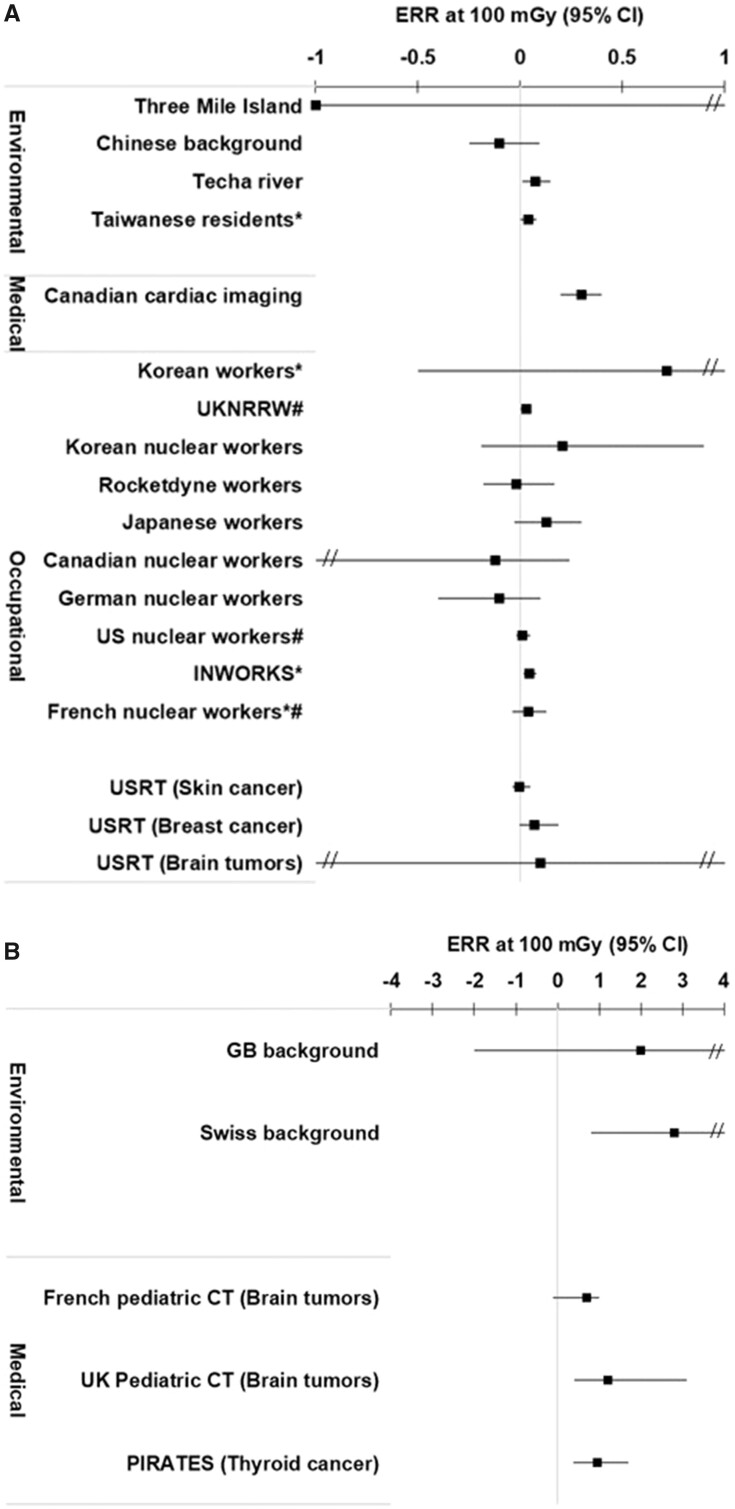
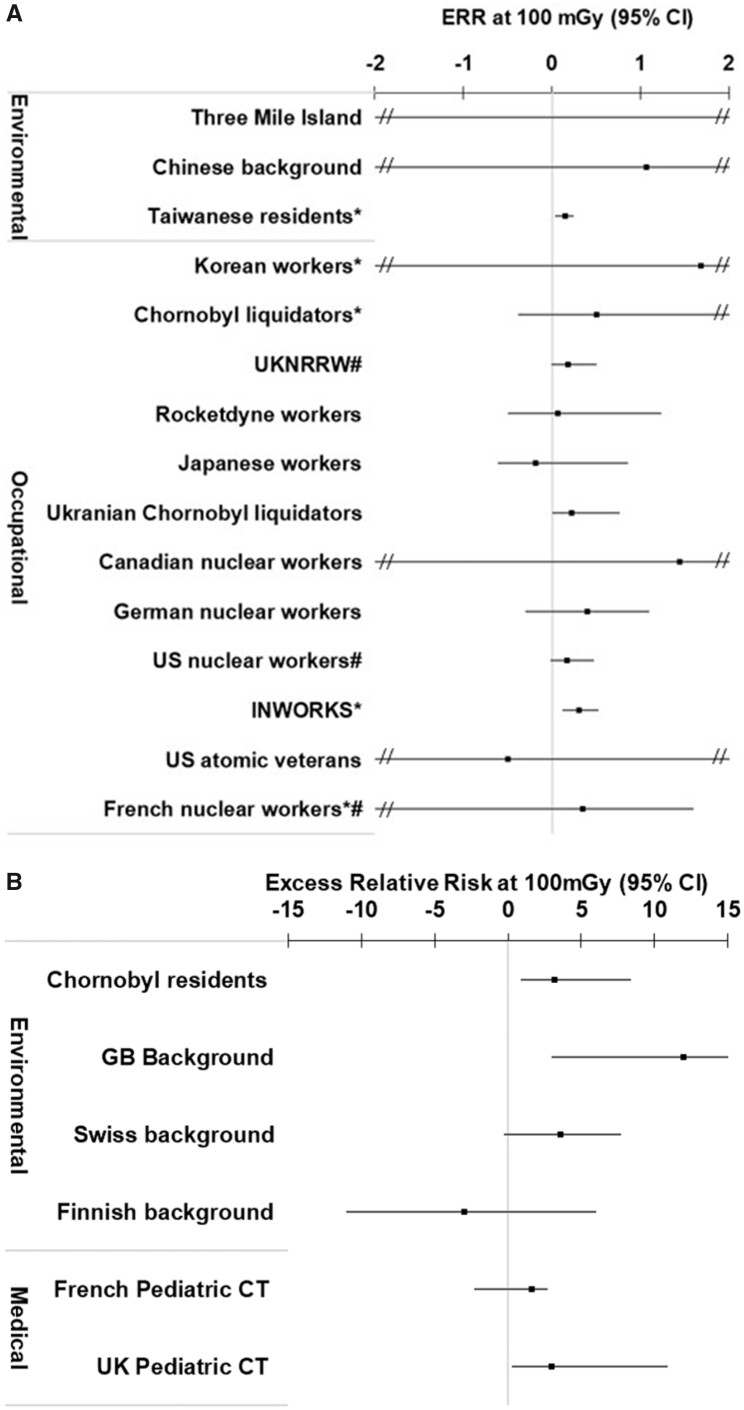
The ERR at 100 mGy for all cancers (or the site-specific solid cancer) was positive (ie, >0) for 16 of the 22 independent studies that evaluated this endpoint (Figure 2, A and B; Appendix Table A5). For leukemia, the ERR at 100 mGy was positive for 17 of the 20 independent studies with available data (Figure 3, A and B; Appendix Table A6). All of the studies reported dose-response risk estimates with 95% confidence intervals with the exception of the Taiwanese (19), INWORKS (34, 39), and Korean and French nuclear workers (37), which reported 90% confidence intervals. For context, when assessing the magnitude of the risk estimates, the ERR at 100 mGy in the Life Span Study following an acute exposure at age 30 years and attained age 70 years would be in the range of 0.01 to 0.05 for solid cancers (38), and for childhood exposure it is 4.5 for leukemia and 2.2 for brain tumors (40).
Figure 2.
A) Excess relative risk (ERR) per 100 mGy and 95% confidence intervals (CI) for all solid cancers (or site-specific solid cancers) following exposure in adulthood or at any age (Techa and Taiwanese residents) for the eligible studies. *90% confidence intervals. #Cohorts included in INWORKS. B) ERR per 100 mGy and 95% confidence intervals for all cancers (or site-specific solid cancers) following exposure in childhood for the eligible studies.
Figure 3.
A) Excess relative risk (ERR) at 100 mGy (and 95% confidence intervals [CIs]) for leukemia following exposure in adulthood or any ages (Taiwanese residents) for the eligible studies. *90% confidence intervals. #Cohorts included in INWORKS. ERR for Three Mile Island = 19. B) ERR at 100 mGy (and 95% confidence intervals) for leukemia following childhood exposure for the eligible studies.
Environmental Radiation Exposure
Four of the eight eligible studies in this category were of natural background radiation exposure (14–16, 18), and four were of populations exposed accidentally (12, 13, 17, 19). The studies are described in order of year of publication.
Chernobyl Residents Childhood Leukemia Case-Control Study (Davis et al., 2006) (12)
This population-based case-control study of 421 cases of acute leukemia in children exposed under age 6 years to fallout from the Chernobyl accident was conducted in Ukraine, Belarus, and Russia (12). Two controls were selected from each case from polyclinics in the same residential area and matched on birth year, sex, and residence at the time of the accident. Dose estimates were based on questionnaire data from in-person interviews with the parents, with a mean dose of 6 mGy and maximum of 265 mGy in the controls. The ERR at 100 mGy was positive and statistically significant.
Three Mile Island Accident (Han et al., 2011) (13)
This is a cohort based on a registry of 21 494 white adults who lived within 5 miles of the Three Mile Island nuclear plant on the date of the accident in 1979 (13). Dose estimation was based on residential location and the amount of time each person stayed in the 5-mile area during the 10 days following the accident. Mean dose was 0.1 mSv with a maximum of approximately 0.8 mSv. Cancer incidence was ascertained between 1982 and 1995 because follow-up of the cohort was completed in 1996. The ERR at 100 mGy for all cancers (n = 1643) was negative (but statistically nonsignificant) and was positive (but statistically nonsignificant) for leukemia (n = 55).
Chinese Background Radiation (Tao et al., 2012) (14)
This is a cohort of 31 604 adults of Guangdong Province in China, an area known for high background radiation from sources including thorium (14). During the study period of 1979 to 1998, the mean cumulative dose was 66 mGy, and 11% had doses of 100+ mGy (estimated as a person-years weighted average). Cancer mortality (n = 956) was ascertained using active follow-up methods. The estimated ERR at 100 mGy was statistically nonsignificantly negative for cancer mortality (excluding leukemia) and was statistically nonsignificantly positive for leukemia (n = 15).
Great Britain Background Radiation (Kendall et al., 2013) (15)
This matched case-control study included 9058 cases of childhood leukemia and 18 389 cases of other childhood cancers matched to 36 793 controls diagnosed in Great Britain between 1980 and 2006 (15). The cumulative mean red bone marrow dose from residential gamma and radon exposures was 4 mSv with a maximum of 31 mSv. Risks were estimated for gamma and radon doses separately, and on average radon contributed about only 10% of the total dose. There was a statistically significant positive dose-response relationship for leukemia and red bone marrow dose but no clear evidence of a relationship between other childhood cancers and background radiation exposure.
Swiss Background Radiation (Spycher et al., 2015) (16)
A cohort of 2 million children was constructed using census data from 1990 and 2000 linked to the Swiss Childhood Cancer Registry (16). The census data were used to geocode residence, and the mean cumulative radiation dose was 9 mSv with a maximum of 49 mSv. During the follow-up period to 2008, there were 530 childhood leukemias and 1252 other childhood cancers diagnosed. There was a statistically significant dose-response relationship for childhood cancers (excluding leukemia) and background radiation and a positive but statistically nonsignificant dose-response relationship for leukemia.
Techa River (Davis et al., 2015) (17)
Radioactive material was released into the Techa river by the Mayak nuclear weapons facility between 1949 and 1956. A cohort of 17 435 adults and children who were residents of the local villages was constructed who received external radiation exposure from gamma rays due to contamination of the river shoreline and internal exposure from consumption of contaminated water, milk, and food. The mean stomach dose was 60 mGy with a maximum of nearly 1 Gy, and 11% of patients had doses of 100+ mGy. There were 1993 solid cancers ascertained up to 2007 and evidence of a statistically significant positive dose-response relationship (17). The mean red bone marrow dose was above our threshold of 100 mGy for this population, and therefore the separate analysis of leukemia was ineligible.
Finnish Background Radiation (Nikkila et al., 2016) (18)
A Finnish study of childhood leukemia (n = 1093) and matched controls (n = 3279) used the Population Register to collect complete residential histories from birth (18). The estimated median cumulative red bone marrow dose from a combination of natural background radiation and fallout from Chernobyl was 2 mSv with a maximum of 12 mSv. The ERR at 100 mSv was negative but statistically nonsignificant.
Taiwanese Residents (Hseih et al.) (19)
This cohort of 6242 adults and children was accidentally exposed to chronic gamma irradiation from contaminated steel used to reinforce their apartment buildings (19). The mean cumulative dose was 48 mSv, but exposures were as high as 2 Sv and 9% received doses of 100+ mSv. The exposures occurred between 1983 and 1992 when the contamination was discovered. Follow-up for cancer incidence (n = 236 solid cancers and 11 leukemias) has been reported through 2012. There was a positive dose response for all cancers (excluding leukemia) with an ERR at 100 mSv of 0.04 (90% CI = 0.0 to 0.08) and for leukemia of 0.15 (90% CI = 0.03 to 0.24).
Medical Radiation Exposures
Canadian Cardiac Imaging (Eisenberg et al., 2011) (20)
A hospital discharge database was used to ascertain a cohort of 82 861 patients who had an acute myocardial infarction (and no history of cancer) between 1996 and 2006 (20). Doses from cardiac imaging and therapeutic procedures were estimated for each patient with a mean dose of 20 mSv, a maximum of 30+ mSv, and only 1% with doses of 100+ mSv. Incident cancer diagnoses were ascertained using the same hospital databases. There was a statistically significant dose response for all cancers (n = 12 020) with an ERR at 100 mSv of 0.3 (95% CI = 0.2 to 0.4).
French Pediatric CT Study (Journy et al., 2016) (21)
This cohort of children (n = 58 620) who had a CT scan before age 10 years between 2000 and 2010 was linked to the French childhood cancer registry, which captures diagnoses up to age 15 years (21). During the follow-up period, 12 leukemias and 15 brain tumors were diagnosed. Mean doses were 9 mGy to the red bone marrow and 23 mGy to the brain and maximum doses of 100+ mGy, although only 2% received doses of 100+ mGy (41). After exclusion of children with cancer-predisposing conditions, there was a statistically nonsignificant positive dose response for leukemia and brain tumors in relation to cumulative organ doses from the CT scans.
UK Pediatric CT (Berrington et al., 2016) (22)
This is a cohort of approximately 178 604 children and young adults (age <22 years) who underwent CT scans in hospitals in the United Kingdom between 1985 and 2002. Cancer incidence was obtained by record linkage to the national cancer registry, and with follow-up to 2008 there were 70 cases of leukemia or myelodysplastic syndrome and 112 brain tumors diagnosed after exclusion of cases with cancer-predisposing conditions or unreported brain tumors (22). The mean red bone marrow dose was 12 mGy and mean brain dose was 43 mGy, with a maximum of more than 400 mGy for children who underwent multiple head CT scans. In total, 6% of children received doses of 100+ mGy to the brain and 1% with 100+ mGy to the red bone marrow. There was a statistically significant dose-response relationship for brain tumors in relation to cumulative brain dose and leukemia in relation to red bone marrow dose from the CT scans.
PIRATES Thyroid Cancer Pooling Study (Lubin et al., 2017) (23)
This pooled analysis of nine cohorts of 107 594 children included eight cohorts of medical exposures (including treatment for benign and malignant diseases) and the Japanese atomic bomb survivors. For this monograph, we considered the results from the analysis that was restricted to children who received less than 200 mGy to the thyroid, with a mean dose of 30 mGy and 10% with doses of 100+ mGy (23). This analysis included 394 incident thyroid cancers, of which 137 were from the Life Span Study of Japanese atomic bomb survivors and 186 from the Israeli study of children treated with radiation for tinea capitis. Therefore, in our assessment of the potential biases that could have affected this pooling project, we focused on the issues related to these two studies because they contributed 82% of the cases to the pooled analysis. There was a statistically significant linear dose-response relationship when doses were restricted to less than 200 mGy, which was still statistically significant and not materially altered when doses were restricted to less than 100 mGy.
Occupational Exposures
Korean Radiation Workers (Ahn et al., 2008) (24)
This cohort included 79 679 workers from nuclear power, medical, research, and other facilities who were under radiation surveillance and first exposed between 1984 and 2004 (24). The mean dose was 6 mSv with less than 1% with doses of 100+ mSv. Follow-up was from 1992 (the period when cause of death was first available in the Korean registry) until 2004, and there were 247 cancer deaths excluding leukemia and nine leukemia deaths during this period. There was a statistically nonsignificant positive dose-response relationship for all cancer deaths and for the small number of leukemia deaths.
Chernobyl Liquidators Leukemia Case-Control Study (Kesminiene et al., 2008) (25)
A case-control study of clean-up workers from Belarus, Russia, and the Baltic states was conducted by interviewing 19 eligible workers who developed (non-CLL) leukemia and 83 controls (25). The cases were ascertained from population-based cancer registries in each country. Red bone marrow doses were reconstructed from the interviews, and the mean dose was approximately 40 mGy with a maximum of 500+ mGy and 14% with doses 100+ mGy. The dose response was positive but statistically nonsignificant.
UK National Registry of Radiation Workers (Muirhead et al., 2009) (26)
This cohort of 174 541 workers with dose records includes individuals from the nuclear power, research, medical, and defense industries (26). The mean occupational exposure was 25 mSv, the maximum was 600+ mSv, and 6% had doses of 100+ mSv. The cohort was linked with UK cancer registration data, and with follow-up to 2002 there were 10 855 incident cancers (excluding leukemia) and 362 leukemias. There was a statistically significant positive dose-response relationship for all cancers combined, and for leukemia it was positive but statistically nonsignificant.
Korean Nuclear Workers (Jeong et al., 2010) (27)
This is a subcohort of nuclear workers within the larger Korean workers cohort described above who completed a questionnaire and clinical check-up between 1992 and 2005 (27). Because there were few female employees the study focused on 16 236 males, of which 8429 were radiation workers (they were issued with a dosimeter) and the remainder were classified as nonradiation workers. The cohort was linked to the national cancer registry, and 203 incident cancers were ascertained up to 2005. The mean cumulative dose among the radiation workers was 20 mSv, the maximum was 480 mSv, and 5% had doses of 100+ mSv. There was a positive but statistically nonsignificant dose-response relationship for all incident cancers.
Rocketdyne Workers (Boice et al., 2011) (28)
A cohort of workers employed at US nuclear research facilities between 1948 and 1999 included 41 169 workers involved in rocket testing and nonradiation activities and 5801 involved in radiation activities (including 2232 who received internal monitoring) (28). The mean external dose in the radiation workers was 14 mSv and the maximum was 1 Sv, but only 3% received doses of 100+ mSv. Linkage to the national death index through 2008 identified 648 cancer deaths (excluding leukemia) and 25 leukemia deaths among radiation workers. There was a positive but statistically nonsignificant dose-response relationship for leukemia and negative non-statistically significant for all cancers.
Japanese Radiation Workers (Akiba and Mizumo, 2012) (29)
This cohort is the third phase of the study and is comprised of 200 583 workers in the Radiation Dose Registration Center with mortality information from the national death registry from 1991 to 2002 (29). The mean occupational dose was 12 mSv with a maximum of 450 mSv, but only 3% with doses of 100+ mSv (42). There were 2636 cancer deaths excluding leukemia and 80 from leukemia during the follow-up period. There was a positive but statistically nonsignificant dose-response relationship for total cancer mortality and a statistically nonsignificant negative dose-response relationship for leukemia and occupational radiation exposure.
Ukrainian Chernobyl Liquidators Leukemia Case-Control Study (Zablotska et al., 2013) (30)
A nested case-control study of 52 cases of (non-CLL) leukemia diagnosed between 1986 and 2006 and 863 controls was conducted from a cohort of Ukrainian clean-up workers (30). Occupational radiation exposure was reconstructed using questionnaire data. In the controls, the estimated mean red bone marrow dose was 82 mGy, the maximum was 2.5+ Gy, and 22% received doses of 100+ mGy. There was a statistically significant positive dose-response relationship with estimated red bone marrow dose.
Canadian Nuclear Workers (Zablotska et al., 2014) (31)
This cohort of nuclear workers was created by linking employment records with the Canadian National Dose Registry and included 45 316 participants employed between 1956 and 1994 (31). The mean cumulative occupational dose was 22 mSv, the maximum was 679 mSv, and 3% had doses of 100+ mSv. Because of evidence of incomplete dose records for workers employed before 1965, the “best estimates” of radiation risk in the most recent analysis excluded these earliest workers. In the 42 228 workers employed after 1964, there were 437 solid cancer deaths and 13 leukemia deaths during the follow-up period (1956–1994). There was a statistically nonsignificant negative dose-response relationship for all solid cancer mortality and statistically nonsignificant increased risk of leukemia mortality with occupational dose.
German Nuclear Workers (Merzenich et al., 2014) (32)
The cohort was comprised of 8746 male workers from 17 nuclear power plants in West Germany who were employed in 1991 (when medical examinations were initiated) or started employment before 2009 (32). Follow-up for cancer mortality was via local population registries, and by 2009 there were 126 cancer deaths ascertained, including seven deaths from leukemia. The mean cumulative dose was 30 mSv and 9% had doses of 100+ mSv. There was a statistically nonsignificant negative dose response for all solid cancer mortality and a statistically nonsignificant positive dose response for leukemia mortality and occupational dose.
US Nuclear Workers (Schubauer-Berigan et al., 2015) (33)
This is a pooled study of five cohorts of 119 195 workers from US nuclear weapons facilities and one naval shipyard who started radiation work as early as 1944 and were followed-up to 2005 (33). The mean dose was 20 mSv although some received up to 700 mSv. Only 5% had doses of 100+ mSv, and only 1.9% had confirmed internal exposures. There were 10 877 cancer deaths excluding leukemia and 369 leukemia deaths during the follow-up period. There was a statistically nonsignificant positive dose-response relationship for leukemia mortality and for all cancer mortality (excluding leukemia).
International Nuclear Workers Study (Richardson et al., 2015) (34)
The INWORKS comprises data from the US and French nuclear workers studies combined with the UK NRRW to form a cohort of 308 297 workers (34, 39). The mean colon dose was 21 mGy vs 16 mGy to the red bone marrow with a maximum of 1+ Gy and 3% with doses of 100+ mGy. Overall, there were 17 957 solid cancer deaths and 531 leukemia deaths during the follow-up. There were statistically significant positive dose-response relationships for all solid cancer and leukemia mortality in relation to occupational radiation exposure.
US Atomic Veterans (Caldwell et al., 2016) (35)
Leukemia mortality patterns were examined in groups of US atomic weapons test participants from the Eight Series Cohort (n = 114 270) followed through 2010 (22). Main analyses focused on categorical dose in participants within a single test series; however, the authors briefly described findings from a model estimating the linear ERR of leukemia mortality in the full cohort. Cumulative red bone marrow dose was reported for a 1% random sample of the full cohort, with mean of 3 mGy, maximum of 500 mGy, and less than 1% with doses greater than 100 mGy (43). Case numbers were not provided for the dose-response analysis, which was negative but not statistically significant.
United States Radiologic Technologists (Preston et al., 2016) (36)
US radiologic technologists who were certified for at least 2 years between 1926 and 1982 were sent a series of questionnaires about their work history, lifestyle, and self-reported cancer diagnoses. A series of site-specific cancer analyses has been conducted for the most radiosensitive cancer sites. In the cohort of approximately 110 000 workers, there were 1922 breast cancer diagnoses, 3615 skin cancers, and 193 brain tumor deaths (36, 44, 45). The mean cumulative breast dose was 37 mGy, mean skin dose was 56 mGy, and mean brain dose was 12 mGy with maximum doses over 1 Gy in those who worked during the earliest periods. Although 14% received skin doses of 100+ mGy, 8% had breast doses of 100+ mGy, and less than 1% had brain doses of 100+ mGy. Overall, there was a positive, statistically nonsignificant dose-response relationship for breast cancer and occupational radiation exposure but no clear relationship for brain tumor deaths or skin cancers.
French Nuclear Workers (Leuraud et al., 2017) (37)
Workers from French nuclear facilities who were employed between 1950 and 2004 were followed from 1968, when the national death registry was established (37). In the 59 004 workers, there were 2536 deaths from solid cancer and 57 from leukemia by 2004. The mean dose was 26 mSv, the maximum was 669 mSv, and only 3% had doses of 100+ mSv. There were positive but statistically nonsignificant dose-response relationships for all solid cancer and leukemia mortality in relation to occupational dose.
Ineligible Studies
When considering epidemiological studies published since 2006, there were 14 radiation dose-response studies excluded for failing just one criterion (Table 2) (46–59). These included seven studies with a mean dose greater than 100 mGy (46–52), five studies ineligible because they only published risk estimates for categories of dose rather than a dose response (53–57), and two background radiation studies excluded because dose rate rather than cumulative dose was assessed (58, 59). Five of the six studies excluded because the mean dose exceeded 100 mGy reported statistically significant (P < .1) positive dose-response relationships. In these studies, the estimated percentage of patients with doses of 100+ mGy was also higher than in all our eligible studies at 35–70%. Six of the seven remaining studies were mostly null; the exception was the French biology researchers who found a statistically significant dose response for all solid cancers (Ptrend = .03) but did not report the dose-response coefficient (55).
Table 2.
Descriptive characteristics of the ineligible studies and reason for exclusion (studies with an internal dose-response analysis that were ineligible for only one reason).
| Population | Reference, year (citation) | Reason for exclusion | Findings for solid cancers and leukemia (or primary cancer site) |
|---|---|---|---|
| Breast cancer in US scoliosis | Ronckers, 2008 (46) | Mean dose = 120 mGy | Borderline statistically significant positive dose-response relationship for breast cancer (Ptrend = .06) |
| Kerala background | Nair, 2009 (47) | Mean dose = 161 mGy | Statistically nonsignificant negative dose response for all solid cancers (excluding leukemia) (Ptrend > .5) |
| Chornobyl clean-up workers | Kesminiene, 2012 (48) | Mean dose = 100–200 mGy | Statistically significant positive dose-response for thyroid cancer |
| Techa river | Krestinina, 2013 (49) | Mean dose = 410 mGy | Statistically significant positive dose response for all leukemias (Ptrend < .001) |
| Mayak workers | Solkinokov, 2015 (50) | Mean dose = 354 mGy | Statistically significant positive dose response for all solid cancers (excluding lung, liver, and bone cancers) (Ptrend = .01) |
| Chornobyl clean-up workers | Kascheev, 2015 (51) | Mean dose = 132 mGy | Statistically significant positive dose response for total cancer incidence (Ptrend = .034) and mortality (Ptrend = .05) |
| Chinese medical workers | Sun, 2016 (52) | Mean badge dose = 250 mGy | Statistically significant positive dose response for all solid cancer incidence (Ptrend = .002) |
| US shipyard workers | Matanoski, 2008 (53) | Categorical risk estimates | Statistically nonsignificant increased risk of leukemia in highest dose category. No trend tests presented. |
| Australian nuclear test | Gun, 2008 (54) | Categorical risk estimates | No increased risk of solid cancer or leukemia across dose categories (Ptrend > .05) |
| French biology researchers | Guseva, 2008 (55) | Categorical risk estimates | Statistically significant increasing trend for all cancer deaths across dose categories (P = .03 5-year lag) |
| Finnish reindeer herders | Kurttio, 2010 (56) | Categorical risk estimates | No overall increased risk of cancer across dose categories (Ptrend = .28) but significant increased risk with dose for exposure age <15 y (Ptrend = .003) |
| Childhood x-rays | Hammer, 2009 (57) | Categorical risk estimates | No increased risk of solid cancer (Ptrend = .32) or leukemia (Ptrend = .26) across dose categories |
| French background (Geocap case-control) | Demoury, 2017 (58) | Risk for dose rate not cumulative dose | Acute leukemia not related to background radiation exposure (Ptrend > .05) |
| German background | Spix, 2017 (59) | Risk for dose rate not cumulative dose | Statistically nonsignificant positive increased risk of lymphoid leukemia (Ptrend = .54) |
Discussion
We identified a large body of epidemiological data published in the period 2006–2017 that assessed the evidence of cancer risks following low-dose radiation exposures. The majority of the 26 eligible studies (mean dose of <100 mGy) reported positive dose-response relationships for solid cancer risks and/or leukemia. In this first article, we described our general framework for the systematic bias evaluation that is described in detail in the subsequent manuscripts in the monograph.
Major international and national organizations routinely review the epidemiological studies of cancer risks from ionizing radiation exposure, including the BEIR VII reports (2), the United Nations Scientific Committe on the Effects of Atomic Radiation (1), the UK Advisory Group on Ionising Radiation committees (60), and the recent NCRP commentary (5). These reviews all follow the traditional model of summarizing the findings of each study accompanied by a brief description of the strengths and weaknesses and potential biases. They have universally concluded there is ample evidence that ionizing radiation is a carcinogen and that most types of cancer can be caused by radiation exposure. The inclusion criteria for our review are broadly similar to those used by many of these organizations, with a focus on studies that have evaluated the dose-response relationship, which is one of the Bradford Hill viewpoints on causality (61). A key difference in eligibility criteria is that our review is the first, to our knowledge, to focus on the low-dose human studies, defined as a mean dose less than 100 mGy. We used mean dose for the eligibility because it was the only statistic that was available for all the studies. However, because doses were generally log-normally distributed, this criterion also ensured that most of the study participants had doses less than 100 mGy. Although there were only four studies where all patients were exposed to less than 100 mGy, only five of the eligible studies had more than 10% of patients exposed to 100+ mGy with a maximum of 22%, meaning that the majority of the study patients were exposed to low doses. It is unclear why only 12 of the 26 studies we included here were also assessed in the recent NCRP commentary on the linear nonthreshold model (5). The most important difference with these previous reviews is our systematic bias analysis to review the studies using statistical and epidemiological methods to systematically assess the evidence for bias. Further discussion of our findings and comparison with the results and methods from previous reviews of the epidemiological evidence will be presented in the summary manuscript (9). We also discuss other potential sources of bias, including reporting and publication bias, discuss the strengths and limitations of our bias assessment approach, and provide recommendations for additional data that could facilitate future systematic bias evaluations.
Notes
Affiliations of authors: Division of Cancer Epidemiology & Genetics, Radiation Epidemiology Branch, Bethesda, MD, USA (ABdG, EG, MSL, MPL, JHL); National Institute for Occupational Safety and Health, Cincinnati, OH, USA (RDD); Barcelona Institute for Global Health (ISGlobal), Barcelona, Catalonia, Spain (EC, IT-C); Universitat Pompeu Fabra (UPF), Barcelona, Catalonia, Spain (EC, IT-C); CIBER Epidemiología y Salud Pública (CIBERESP), Madrid, Spain (EC, IT-C); Radiation Effects Research Foundation, Hiroshima, Japan (HMC); Department of Epidemiology and Biostatistics, Netherlands Cancer Institute, Amsterdam, the Netherlands (MH); Brandenburg Medical School Theodor Fontane, Institute of Biostatistics and Registry Research, Neuruppin, Germany (MH); Cancer Epidemiology Unit, NDPH, Oxford, UK (GK); IRSN, Paris, France (DL); Hirosoft International, Seattle (DLP); University of North Carolina, Chapel Hill, NC, USA (DBR); University of Southern California, Los Angeles, CA (DS); and International Agency for Research on Cancer, Lyon, France (MKS-B).
The authors have no conflicts of interest to report. The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The views expressed are those of the authors and do not necessarily represent the decisions, policy, or views of their respective institutions. The lead and corresponding authors had full access to the data in the study and final responsibility for the decision to submit for publication.
Funding
This work was supported in part by the Intramural Research Program, National Cancer Institute, National Institutes of Health, and the U.S. Department of Energy (grant no. DEHS0000091) (doe.gov).
Appendix: Literature Review
A structured literature review was conducted to identify original analytic epidemiologic research published since the BEIR VII report. Eligibility for synthesis was restricted to research that described quantitative analyses of a potential relationship between cumulative ionizing radiation exposure and cancer. These analyses stem from internal comparisons made in observational studies and report effect measures in terms of excess relative risk, relative risk attributable risk, or excess absolute risk. Eligible studies comprise research involving medical, occupational, and environmental exposure scenarios. Other inclusion criteria were:
The primary exposure was low linear energy transfer (low-LET) electromagnetic radiation (eg, studies of uranium miners, air crews, and residential radon exposures were excluded).
Doses were quantified and the average absorbed dose to the tissue of interest appeared less than or equal to 100 mGy.
The exposure was best characterized as low dose and low-dose rate (eg, radiotherapy studies were excluded).
When multiple studies of the same population were published, only the most informative study is included in the synthesis. Information from preceding studies was made available for use in support of synthesis. Ecological studies, clinical trials, reviews, meta-analyses, proceedings, abstracts, commentaries, correspondence, and news articles were excluded. Review methods followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines.
The search was conducted in two phases. First, a library scientist identified a set of potentially relevant studies based on general search criteria identified in the Population, Exposure, Comparator, and Outcome Statement (Table A1). Seven public domain databases were systematically searched for new research articles published between January 1, 2006 and April 19, 2018 (Table A2). There were no restrictions placed on age, sex, language, or geography. The search results were used to populate an Endnote X7.7.1 database. Duplicate records were removed using the EndNote “find duplicates” function with preference set to match on title, author, and year. This wide-sweeping search identified 5080 candidate articles for screening in the next phase (Table A3).
The second phase of the review was conducted by a health scientist. Using the publication database, titles and abstracts were reviewed to identify and exclude lingering duplicates and to screen out clearly ineligible studies. Remaining studies comprised the set selected for full article review by the study team. Full review resulted in additional exclusions to arrive a set of studies meeting all eligibility criteria (Table A4).
Table A1.
PECO statementa
| PECO element1 | Evidence stream | Articles or features included | Articles or features excluded |
|---|---|---|---|
| Population | Human |
|
|
| Exposure | Human |
|
|
| Comparator | Human |
|
|
| Outcome | Human |
|
|
| General considerations | |||
|
|
||
ERR = excess relative risk; LET = linear energy transfer; PECO = Population, Exposure, Comparator, and Outcome; RCT = randomized control trial; RR = relative risk.
Table A2.
Search strategya
| Database | Strategy | Run date |
|---|---|---|
|
|
April 19, 2018 |
|
|
April 19, 2018 |
|
|
April 20, 2018 |
|
|
April 20, 2018 |
|
|
April 20, 2018 |
|
|
April 20, 2018 |
|
|
April 20, 2018 |
CINAHL = Cumulative Index of Nursing and Allied Health Literature; ERR = excess relative risk; NTIS = National Technical Information Service.
Table A3.
Phase I: initial search by library scientist
| Database | Records |
|---|---|
| Medline | 4045 |
| Embase | 550 |
| CINAHL | 58 |
| NTIS | 9 |
| GreenFILE | 33 |
| Scopus | 376 |
| Agricultural and Environmental Science Database (ProQuest) | 903 |
| Total records | 5974 |
| Minus duplicatesa | −894 |
| Available records | 5080 |
Duplicates were identified using the Endnote automated “find duplicates” function with preference set to match on title, author, and year. CINAHL = Cumulative Index of Nursing and Allied Health Literature; NTIS = National Technical Information Service.
Table A4.
Phase II: screening and final review resultsa
| Review steps | Records remaining |
|---|---|
| Available records | 5080 |
| Subtract duplicates not identified by Endnote | −8 |
| Eligible for screening | 5072 |
| Subtract ineligible records based on title and abstract review | −4983 |
| Eligible for full article review | 89 |
| Subtract ineligible records based on full article review | −63 |
| Eligible studies | 26 |
CINAHL = Cumulative Index of Nursing and Allied Health Literature; NTIS = National Technical Information Service.
Table A5.
. Estimated ERR at 100 mGy and 95% confidence intervals for all solid cancers (or site-specific solid cancers) for eligible studies
| Study | Outcome | ERR at 100 mGy (95% CI) |
|---|---|---|
| Environmental | ||
| Three Mile Island | All cancers | −1 (−6 to 3) |
| Chinese background | All cancer excl. leukemia | −0.101 (−0.253 to 0.095) |
| GB background | All cancer excl. leukemia | 2 (−2.0 to 6.0) |
| Swiss background | All cancers | 2.8 (0.8 to 4.8) |
| Techa river | Solid cancers | 0.077 (0.013 to 0.150) |
| Taiwanese residents | All solid cancers | 0.04 (0 to 0.08)a |
| Medical | ||
| Canadian cardiac imaging | All cancers | 0.3 (0.2 to 0.4) |
| French pediatric CT (brain tumors) | Brain cancer | 0.7 (−0.1 to 1.0) |
| UK pediatric CT (brain tumors) | Brain cancer | 1.2 (0.4 to 3.1) |
| PIRATES (thyroid cancer) | Thyroid | 0.96 (0.37 to 1.70) |
| Occupational | ||
| Korean workers | All cancers | 0.72 (−0.5 to 2.1)a |
| UKNRRW | All cancers excl. leukemia | 0.03 (0.0 to 0.06) |
| Korean nuclear workers | All cancers excl. leukemia | 0.21 (−0.19 to 0.9) |
| Rocketdyne workers | All cancers excl. leukemia | −0.02 (−0.18 to 0.17) |
| Japanese workers | All cancers excl. leukemia | 0.13 (−0.03 to 0.30) |
| Canadian nuclear workersb | Solid cancers | −0.12 (<−0.15 to 0.24) |
| German nuclear workers | Solid cancers | −0.1 (−0.4 to 0.1) |
| US nuclear workers | All cancers excl. leukemia | 0.01 (−0.02 to 0.05) |
| INWORKS | Solid cancers | 0.047 (0.018 to 0.079)a |
| USRT (breast cancer) | Breast | 0.07 (−0.005 to 0.19) |
| USRT (brain cancer) | Brain | 0.1 (<−0.3 to 1.5) |
| USRT (skin cancer) | Skin | −0.001 (−0.04 to 0.05) |
| French nuclear workers | Solid cancers | 0.04 (−0.04 to 0.13)a |
90% CI. CI = confidence interval; CT = computed tomography; ERR = excess relative risk; INWORKS = International Nuclear Workers Study; UKNRRW = UK National Registry of Radiation Workers; USRT = US Radiologic Technologists.
bThe Canadian Study is restricted to the cohort excluding early AECL workers.
Table A6.
Estimated ERR at 100 mGy and 95% confidence intervals for leukemia for eligible studies
| Study | Outcome | ERR at 100 mGy (95% CI) |
|---|---|---|
| Environmental | ||
| Chernobyl residents | All leukemia | 3.2 (0.9 to 8.4) |
| Three Mile Island | All leukemia | 19 (−3 to 45) |
| Chinese background | All leukemia | 1.068 (<0 to inf) |
| GB background | All leukemia | 12 (3.0 to 22.0) |
| Swiss background | All leukemia | 3.6 (−0.3 to 7.7) |
| Finnish background | All leukemia | −3 (−11 to 6) |
| Taiwanese residents | Leukemia excl. CLL | 0.15 (0.03 to 0.24)a |
| Medical | ||
| French Pediatric CT | All leukemia | 1.6 (−2.3 to 2.7) |
| UK Pediatric CT | Leukemia or MDS | 3 (0.3 to 10.9) |
| Occupational | ||
| Korean workers | All leukemia | 1.68 (−3.4 to 14.9)a |
| Chornobyl liquidators | Leukemia excl. CLL | 0.5 (−0.38 to 5.70)a |
| UKNRRW | Leukemia excl. CLL | 0.18 (−0.006 to 0.50) |
| Rocketdyne workers | Leukemia excl. CLL | 0.06 (−0.50 to 1.23) |
| Japanese workers | All leukemia | −0.19 (−0.61 to 0.86) |
| Ukrainian Chornobyl liquidators | Leukemia excl. CLL | 0.221 (0.005 to 0.761) |
| Canadian nuclear workersb | Leukemia excl. CLL | 1.44 (<−0.15 to 14.6) |
| German nuclear workers | Leukemia excl. CLL | 0.4 (−0.3 to 1.1) |
| US nuclear workers | Leukemia excl. CLL | 0.17 (−0.02 to 0.47) |
| INWORKS | Leukemia excl. CLL | 0.3 (0.12 to 0.52)a |
| US atomic veterans | Leukemia excl. CLL | −0.5 (−14 to 4) |
| French nuclear workers | Leukemia excl. CLL | 0.35 (<0 to 1.6)a |
90% CI. ERR = excess relative risk; CLL = chronic lymphocytic leukemia; CT = computed tomograph; ERR = excess relative risk; INWORKS = International Nuclear Workers Study; UKNRRW = UK National Registry of Radiation Workers; USRT = US Radiologic Technologists.
bThe Canadian Study is restricted to the cohort excluding early AECL workers.
References
- 1.United Nations ScientificCommittee on the Effects of Atomic Radiation (UNSCEAR). Summary of Low-Dose Radiation Effects on Health. New York: United Nations; 2011. [Google Scholar]
- 2.National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation (BEIR VII) Phase 2. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 3.United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and effects of ionizing radiation New York: United Nations; 2008. [Google Scholar]
- 4.Kitahara CM, Linet MS, Rajaraman P, et al. A new era of low-dose radiation epidemiology. Curr Environ Health Rep. 2015;2(3):236–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCRP. Implications of Recent Epidemiologic Studies for the Linear-Nonthreshold Model and Radiation Protection. Bethesda, MD: National Council on Radiation Protection and Measurements; 2018.
- 6.Daniels RD, Kendall GM, Thierry-Chef I, et al. Strengths and Weaknesses of Dosimetry Used in Studies of Low-Dose Radiation Exposure and Cancer. JNCI Monographs. 2020; 2020(56):114--132. [DOI] [PMC free article] [PubMed]
- 7.Schubauer-Berigan MK, Berrington de González A, Cardis E, et al. Evaluation of Confounding and Selection Bias in Epidemiological Studies of Populations Exposed to Low-Dose, High-Energy Photon Radiation. JNCI Monographs. 2020; 2020(56):133--153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linet MS, Schubauer-Berigan MK, Berrington de Gonzales A. Outcome Assessment in Epidemiological Studies of Low-Dose Radiation Exposure and Cancer Risks: Sources, Level of Ascertainment, and Misclassification. JNCI Monographs. 2020; 2020(56):154--175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauptmann M, Robert D, Cardis E, et al. Epidemiological Studies of Low-Dose Ionizing Radiation and Cancer: Summary Bias Assessment and Meta-Analysis. JNCI Monographs. 2020; 2020(56):188--200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert ES, Little MP, Preston DL, et al. Issues in Interpreting Epidemiologic Studies of Populations Exposed to Low-Dose, High-Energy Photon Radiation. JNCI Monographs. 2020; 2020(56):176--187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UNSCEAR. United Nations Scientific Committee on the Effects of Atomic Radiation 2006 Report to the General Assembly with Scientific Annexes A and B. In: Effects of Ionizing Radiation; New York: United Nations; 2006.
- 12.Davis S, Day RW, Kopecky KJ, et al. Childhood leukaemia in Belarus, Russia, and Ukraine following the Chernobyl power station accident: results from an international collaborative population-based case-control study. Int J Epidemiol. 2006;35(2):386–396. [DOI] [PubMed] [Google Scholar]
- 13.Han YY, Youk AO, Sasser H, et al. Cancer incidence among residents of the Three Mile Island accident area: 1982-1995. Environ Res. 2011;111(8):1230–1235. [DOI] [PubMed] [Google Scholar]
- 14.Tao Z, Akiba S, Zha Y, et al. Cancer and non-cancer mortality among inhabitants in the high background radiation area of Yangjiang, China (1979-1998). Health Phys. 2012;102(2):173–181. [DOI] [PubMed] [Google Scholar]
- 15.Kendall GM, Little MP, Wakeford R, et al. A record-based case-control study of natural background radiation and the incidence of childhood leukaemia and other cancers in Great Britain during 1980-2006. Leukemia. 2013;27(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spycher BD, Lupatsch JE, Zwahlen M, et al., for the Swiss Pediatric Oncology Group. Background ionizing radiation and the risk of childhood cancer: a census-based nationwide cohort study. Environ Health Perspect. 2015;123(6):622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis FG, Yu KL, Preston D, et al. Solid cancer incidence in the Techa River incidence cohort: 1956-2007. Radiat Res. 2015;184(1):56–65. [DOI] [PubMed] [Google Scholar]
- 18.Nikkila A, Erme S, Arvela H, et al. Background radiation and childhood leukemia: a nationwide register-based case-control study. Int J Cancer. 2016;139(9):1975–1982. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh WH, Lin IF, Ho JC, et al. 30 years follow-up and increased risks of breast cancer and leukaemia after long-term low-dose-rate radiation exposure. Br J Cancer. 2017;117(12):1883–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenberg MJ, Afilalo J, Lawler PR, et al. Cancer risk related to low-dose ionizing radiation from cardiac imaging in patients after acute myocardial infarction. CMAJ. 2011;183(4):430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Journy N, Roue T, Cardis E, et al. Childhood CT scans and cancer risk: impact of predisposing factors for cancer on the risk estimates. J Radiol Prot. 2016;36(1):N1–N7. [DOI] [PubMed] [Google Scholar]
- 22.Berrington de Gonzalez A, Salotti JA, McHugh K, et al. Relationship between paediatric CT scans and subsequent risk of leukaemia and brain tumours: assessment of the impact of underlying conditions. Br J Cancer. 2016;114(4):388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubin JH, Adams MJ, Shore R, et al. Thyroid cancer following childhood low-dose radiation exposure: a pooled analysis of nine cohorts. J Clin Endocrinol Metab. 2017;102(7):2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn YS, Park RM, Koh DH. Cancer admission and mortality in workers exposed to ionizing radiation in Korea. J Occup Environ Med. 2008;50(7):791–803. [DOI] [PubMed] [Google Scholar]
- 25.Kesminiene A, Evrard AS, Ivanov VK, et al. Risk of hematological malignancies among Chernobyl liquidators. Radiat Res. 2008;170(6):721–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muirhead CR, O'Hagan JA, Haylock RG, et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer. 2009;100(1):206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong M, Jin YW, Yang KH, et al. Radiation exposure and cancer incidence in a cohort of nuclear power industry workers in the Republic of Korea, 1992-2005. Radiat Environ Biophys. 2010;49(1):47–55. [DOI] [PubMed] [Google Scholar]
- 28.Boice JD Jr, Cohen SS, Mumma MT, et al. Updated mortality analysis of radiation workers at Rocketdyne (Atomics International), 1948-2008. Radiat Res. 2011;176(2):244–258. [DOI] [PubMed] [Google Scholar]
- 29.Akiba S, Mizuno S. The third analysis of cancer mortality among Japanese nuclear workers, 1991-2002: estimation of excess relative risk per radiation dose. J Radiol Prot. 2012;32(1):73–83. [DOI] [PubMed] [Google Scholar]
- 30.Zablotska LB, Bazyka D, Lubin JH, et al. Radiation and the risk of chronic lymphocytic and other leukemias among Chornobyl cleanup workers. Environ Health Perspect. 2013;121(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zablotska LB, Lane RS, Thompson PA. A reanalysis of cancer mortality in Canadian nuclear workers (1956-1994) based on revised exposure and cohort data. Br J Cancer. 2014;110(1):214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merzenich H, Hammer GP, Troltzsch K, et al. Mortality risk in a historical cohort of nuclear power plant workers in Germany: results from a second follow-up. Radiat Environ Biophys. 2014;53(2):405–416. [DOI] [PubMed] [Google Scholar]
- 33.Schubauer-Berigan MK, Daniels RD, Bertke SJ, et al. Cancer mortality through 2005 among a pooled cohort of U.S. nuclear workers exposed to external ionizing radiation. Radiat Res. 2015;183(6):620–631. [DOI] [PubMed] [Google Scholar]
- 34.Richardson DB, Cardis E, Daniels RD, et al. Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ. 2015;351:h5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caldwell GG, Zack MM, Mumma MT, et al. Mortality among military participants at the 1957 PLUMBBOB nuclear weapons test series and from leukemia among participants at the SMOKY test. J Radiol Prot. 2016;36(3):474–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preston DL, Kitahara CM, Freedman DM, et al. Breast cancer risk and protracted low-to-moderate dose occupational radiation exposure in the US Radiologic Technologists Cohort, 1983-2008. Br J Cancer. 2016;115(9):1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leuraud K, Fournier L, Samson E, et al. Mortality in the French cohort of nuclear workers. Radioprotection. 2017;52(3):199–210. [Google Scholar]
- 38.Grant EJ, Brenner A, Sugiyama H, et al. Solid cancer incidence among the life span study of atomic bomb survivors: 1958-2009. Radiat Res. 2017;187(5):513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leuraud K, Richardson DB, Cardis E, et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol. 2015;2(7):e276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Journy N, Rehel JL, Ducou Le Pointe H, et al. Are the studies on cancer risk from CT scans biased by indication? Elements of answer from a large-scale cohort study in France. Br J Cancer. 2015;112(1):185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwasaki T, Murata M, Ohshima S, et al. Second analysis of mortality of nuclear industry workers in Japan, 1986-1997. Radiat Res. 2003;159(2):228–238. [DOI] [PubMed] [Google Scholar]
- 43.Beck HL, Till JE, Grogan HA, et al. Red bone marrow and male breast doses for a cohort of atomic veterans. Radiat Res. 2017;187(2):221–228. [DOI] [PubMed] [Google Scholar]
- 44.Kitahara CM, Linet MS, Balter S, et al. Occupational radiation exposure and deaths from malignant intracranial neoplasms of the brain and CNS in U.S. radiologic technologists, 1983-2012. Am J Roentgenol. 2017;208(6):1278–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee T, Sigurdson AJ, Preston DL, et al. Occupational ionising radiation and risk of basal cell carcinoma in US radiologic technologists (1983-2005). Occup Environ Med. 2015;72(12):862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ronckers CM, Doody MM, Lonstein JE, et al. Multiple diagnostic x-rays for spine deformities and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(3):605–613. [DOI] [PubMed] [Google Scholar]
- 47.Nair RR, Rajan B, Akiba S, et al. Background radiation and cancer incidence in Kerala, India-Karanagappally cohort study. Health Phys. 2009;96(1):55–66. [DOI] [PubMed] [Google Scholar]
- 48.Kesminiene A, Evrard AS, Ivanov VK, et al. Risk of thyroid cancer among Chernobyl liquidators. Radiat Res. 2012;178(5):425–436. [DOI] [PubMed] [Google Scholar]
- 49.Krestinina LY, Davis FG, Schonfeld S, et al. Leukaemia incidence in the Techa River Cohort: 1953-2007. Br J Cancer. 2013;109(11):2886–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sokolnikov M, Preston D, Gilbert E, et al. Radiation effects on mortality from solid cancers other than lung, liver, and bone cancer in the Mayak worker cohort: 1948-2008. PLoS One. 2015;10(2):e0117784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kashcheev VV, Chekin SY, Maksioutov MA, et al. Incidence and mortality of solid cancer among emergency workers of the Chernobyl accident: assessment of radiation risks for the follow-up period of 1992-2009. Radiat Environ Biophys. 2015;54(1):13–23. [DOI] [PubMed] [Google Scholar]
- 52.Sun Z, Inskip PD, Wang J, et al. Solid cancer incidence among Chinese medical diagnostic x-ray workers, 1950-1995: estimation of radiation-related risks. Int J Cancer. 2016;138(12):2875–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matanoski GM, Tonascia JA, Correa-Villasenor A, et al. Cancer risks and low-level radiation in U.S. shipyard workers. J Radiat Res. 2008;49(1):83–91. [DOI] [PubMed] [Google Scholar]
- 54.Gun RT, Parsons J, Crouch P, et al. Mortality and cancer incidence of Australian participants in the British nuclear tests in Australia. Occup Environ Med. 2008;65(12):843–848. [DOI] [PubMed] [Google Scholar]
- 55.Guseva Canu I, Rogel A, Samson E, et al. Cancer mortality risk among biology research workers in France: first results of two retrospective cohorts studies. Int Arch Occup Environ Health. 2008;81(6):777–785. [DOI] [PubMed] [Google Scholar]
- 56.Kurttio P, Pukkala E, Ilus T, et al. Radiation doses from global fallout and cancer incidence among reindeer herders and Sami in Northern Finland. Occup Environ Med. 2010;67(11):737–743. [DOI] [PubMed] [Google Scholar]
- 57.Hammer GP, Seidenbusch MC, Schneider K, et al. A cohort study of childhood cancer incidence after postnatal diagnostic X-ray exposure. Radiat Res. 2009;171(4):504–512. [DOI] [PubMed] [Google Scholar]
- 58.Demoury C, Marquant F, Ielsch G, et al. Residential exposure to natural background radiation and risk of childhood acute leukemia in France, 1990-2009. Environ Health Perspect. 2017;125(4):714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spix C, Grosche B, Bleher M, et al. Background gamma radiation and childhood cancer in Germany: an ecological study. Radiat Environ Biophys. 2017;56(2):127–138. [DOI] [PubMed] [Google Scholar]
- 60.Health Protection Agency. RCE-19: Risk of Solid Cancers Following Radiation Exposure - Estimates for the UK Population. Chilton, UK: Health Protection Agency; 2011. [Google Scholar]
- 61.Hill AB. The environment and disease: association or causation? 1965. J R Soc Med. 2015;108(1):32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]