Abstract
Objectives
The early infection dynamics of patients with SARS-CoV-2 are not well understood. We aimed to investigate and characterize associations between clinical, laboratory, and imaging features of asymptomatic and pre-symptomatic patients with SARS-CoV-2.
Methods
Seventy-four patients with RT-PCR-proven SARS-CoV-2 infection were asymptomatic at presentation. All were retrospectively identified from 825 patients with chest CT scans and positive RT-PCR following exposure or travel risks in outbreak settings in Japan and China. CTs were obtained for every patient within a day of admission and were reviewed for infiltrate subtypes and percent with assistance from a deep learning tool. Correlations of clinical, laboratory, and imaging features were analyzed and comparisons were performed using univariate and multivariate logistic regression.
Results
Forty-eight of 74 (65%) initially asymptomatic patients had CT infiltrates that pre-dated symptom onset by 3.8 days. The most common CT infiltrates were ground glass opacities (45/48; 94%) and consolidation (22/48; 46%). Patient body temperature (p < 0.01), CRP (p < 0.01), and KL-6 (p = 0.02) were associated with the presence of CT infiltrates. Infiltrate volume (p = 0.01), percent lung involvement (p = 0.01), and consolidation (p = 0.043) were associated with subsequent development of symptoms.
Conclusions
COVID-19 CT infiltrates pre-dated symptoms in two-thirds of patients. Body temperature elevation and laboratory evaluations may identify asymptomatic patients with SARS-CoV-2 CT infiltrates at presentation, and the characteristics of CT infiltrates could help identify asymptomatic SARS-CoV-2 patients who subsequently develop symptoms. The role of chest CT in COVID-19 may be illuminated by a better understanding of CT infiltrates in patients with early disease or SARS-CoV-2 exposure.
Key Points
• Forty-eight of 74 (65%) pre-selected asymptomatic patients with SARS-CoV-2 had abnormal chest CT findings.
• CT infiltrates pre-dated symptom onset by 3.8 days (range 1–5).
• KL-6, CRP, and elevated body temperature identified patients with CT infiltrates. Higher infiltrate volume, percent lung involvement, and pulmonary consolidation identified patients who developed symptoms.
Keywords: SARS-CoV, Asymptomatic infections, Pneumonia, Virus shedding
Introduction
The high rate of human-to-human transmission of SARS-CoV-2 [1] is related in part to marked pre-symptomatic transmission [2], when infectiousness peaks. Moreover, the vast majority of infections still remain undocumented [3]. Meanwhile, containment and mitigation heavily rely upon strict compliance with isolation, which depends upon a diagnosis or self-identification via symptoms [4–7]. The asymptomatic population remains an underestimated risk during this epidemic [8–10], and currently sparse data are available to understand the transmission dynamics of this population. Furthermore, asymptomatic patients infected with SARS-CoV-2 shed similar viral burdens [8, 11].
Characterization of this asymptomatic population with early chest CT may clarify transmission dynamics. A better understanding of chest CT in an asymptomatic population with infection may inform prognostic modeling or elucidate potential roles for CT in targeted cohorts with exposure or travel history, as an epidemiologic tool to help contain or mitigate outbreaks. Herein, chest CT was analyzed in initially asymptomatic patients who were reverse-transcriptase polymerase chain reaction (RT-PCR) positive for SARS-CoV-2. CT was also correlated to clinical and laboratory features and subsequent symptoms, to characterize infection patterns of SARS-CoV-2 virus in outbreak settings.
Materials and methods
Patient cohort
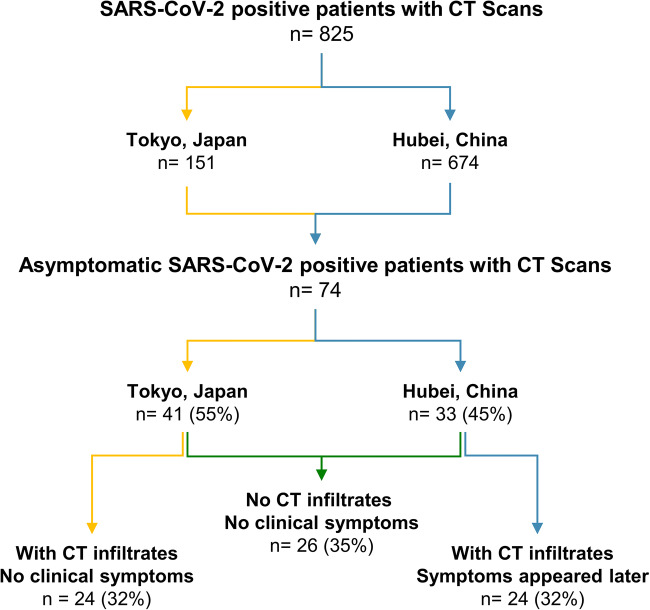
Local Research Board approvals were obtained for this retrospective study based on the regulations at the affiliated sites. Clinical, laboratory, and chest CT features were reviewed from a multinational database of 825 patients who all had laboratory-confirmed SARS-CoV-2 and also underwent baseline chest CT upon presentation. A subset of 74 patients was identified with both a history of exposure to COVID-19 and no symptoms at initial presentation. The 825 total patients included 151 patients from Tokyo, Japan (including 103 passengers and crew from the Diamond Princess cruise ship and 48 community-acquired infections), and 674 patients evaluated in Hubei Province, China (all seen between January 21st and April 1st, 2020). Screening with chest CT was conducted either because of an exposure history of contact with patients with proven or suspected COVID-19 or because of high exposure risk due to travel to high prevalence regions (outbreak zones in Hubei or on cruise ship). Asymptomatic patients had positive RT-PCR for SARS-CoV-2, but no clinical symptoms (defined at the time as dyspnea, cough, and fever). No patients required intensive care unit hospitalization, intubation, or died during follow-up.
Clinical and laboratory features
Clinical features and laboratory measurements, including RT-PCR on the day of admission, can be found in Table 1. Patients in the Tokyo cohort also had immunoglobulins (IgG, IgA, IgM) and the pulmonary inflammation marker, Krebs von den Lungen 6 (KL-6).
Table 1.
Comparison of clinical, CT findings, and laboratory data for asymptomatic patients with and without COVID-19 related CT findings
| Patients with CT infiltrates (n = 48) | Patients without CT infiltrates (n = 26) | p value | ||||
|---|---|---|---|---|---|---|
| Number | % | Number | % | |||
| Clinical features | Gender (F) | 31 | 65 | 12 | 46 | 0.198 |
| Smoking habits: current/never/ex | 15/30/3 | 31/63/6 | 6/18/2 | 23/69/8 | 0.741 | |
| Significant past medical history | 12 | 25 | 9 | 35 | 0.635 | |
| Contact with SARS-CoV-2 infected patient | 35 | 73 | 10 | 38 | 0.008* | |
| Avg | SD | Avg | SD | |||
| Age (years) | 54 | 17 | 53 | 21 | 0.954 | |
| Body height (cm) | 162 | 7 | 162 | 8 | 1.000 | |
| Body weight (kg) | 64 | 11 | 63 | 10 | 0.502 | |
| BMI | 24.5 | 3.5 | 23.9 | 3.4 | 0.476 | |
| Body temperature (°C) | 37.0 | 0.7 | 36.6 | 0.3 | 4.12 × 10-4* | |
| Respiratory rate (breaths/min) | 16 | 2 | 16 | 2 | 0.722 | |
| Systolic BP (mmHg) | 137 | 24 | 136 | 22 | 0.964 | |
| Diastolic BP (mmHg) | 92 | 15 | 86 | 10 | 0.038* | |
| Heart rate (beats/min) | 77 | 18 | 76 | 14 | 0.798 | |
| SpO2 (%) | 98 | 1 | 97 | 3.4 | 0.216 | |
| RT-PCR positive period (days) | 10.2 | 3.7 | 11.6 | 5.3 | 0.616 | |
| Laboratory features | BUN (mmol/L) | 4.34 | 1.57 | 5.19 | 1.67 | 0.064 |
| Creatinine (μmol/L) | 71 | 19 | 80 | 22 | 0.141 | |
| AST (IU/L) | 29 | 20 | 25 | 8 | 0.831 | |
| ALT (IU/L) | 28 | 28 | 28 | 23 | 0.738 | |
| T-Bil (μmol/L) | 5.84 | 6.48 | 2.28 | 4.06 | 0.280 | |
| γGTP (IU/L) | 45 | 49 | 32 | 20 | 0.653 | |
| Amylase (IU/L) | 79 | 28 | 85 | 32 | 0.464 | |
| LDH (mmol/L) | 210 | 65 | 179 | 25 | 0.187 | |
| Albumin (g/L) | 41 | 4 | 41 | 10 | 0.218 | |
| CRP (mg/L) | 10.8 | 15.8 | 1.7 | 2.0 | 0.001* | |
| UA (μmol/L) | 305 | 78 | 297 | 56 | 0.735 | |
| PCT (ng/mL) | 0.18 | 0.22 | 0.23 | 0.16 | 0.337 | |
| IgG (mg/L)† | 1338 | 327 | 1400 | 325 | 0.586 | |
| IgA (mg/L)† | 271 | 135 | 300 | 131 | 0.517 | |
| IgM (mg/L)† | 88 | 39 | 84 | 43 | 0.419 | |
| KL-6 (U/mL)† | 337 | 173 | 227 | 71 | 0.021* | |
| RBC (× 1012/L) | 5 | 1 | 5 | 1 | 0.260 | |
| Hemoglobin (g/L) | 141 | 17 | 140 | 20 | 0.610 | |
| WBC (× 109/L) | 5.37 | 1.76 | 6.59 | 1.98 | 0.018* | |
| Platelet (× 109/L) | 224 | 71 | 240 | 70 | 0.385 | |
| Neutrophil (%) | 62.9 | 11.0 | 64.5 | 8.9 | 0.507 | |
| Lymphocyte (%) | 28.3 | 9.3 | 26.7 | 8.2 | 0.458 | |
| Monocyte (%) | 7.1 | 3.0 | 6.3 | 2.3 | 0.340 | |
| Eosinophil (%) | 1.4 | 1.7 | 1.8 | 1.7 | 0.065 | |
| APTT (time) | 29 | 5 | 30 | 4 | 0.215 | |
| APTT (std) | 28 | 0 | 28 | 0 | 0.284 | |
| PT (%) | 105 | 16 | 97 | 7 | 0.095 | |
| PT (INR) | 0.97 | 0.08 | 1.01 | 0.05 | 0.020* | |
Abbreviations: CT, computed tomography; γGTP, gamma-glutamyl transferase; T-Bil, total bilirubin; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; SpO2, capillary hemoglobin oxygen saturation; BUN, blood urea nitrogen; CRP, C-reactive protein; UA, uric acid; PCT, procalcitonin; Ig, immunoglobulin; HU, Hounsfield units; LDH, lactate dehydrogenase; PT, prothrombin time; PT (INR), prothrombin time calculated as international normalized ratio; RBC, red blood cell count; WBC, white blood cell count; Avg, average; SD, standard deviation
*p < 0.05
†Values were available for Tokyo, Japan, cohort only
CT features
Chest CTs were obtained within one day of admission. CTs were retrospectively reviewed by radiologists blinded to clinical and laboratory information. Three radiologists manually annotated and segmented lung infiltrates (www.itksnap.org) and 3 other radiologists reviewed CTs independently for the presence of infiltrates, location, types of infiltrate (ground glass, consolidation, intralobular septal lines [“crazy paving”]), atelectasis, reticulation, mosaic attenuation, number of distinct infiltrates, peripheral or central location of the infiltrates, presence of effusion, bronchial wall thickening, tree-in-bud nodules, and preexisting lung disease (including emphysema, bronchiectasis, and fibrosis). After independent characterization of CT images by each radiologist, discrepancies were present in 12 patients which were sorted out by consensus among the 3 radiologists. Volume and average attenuation of the infiltrate, healthy lung, and whole lung were extracted.
Deep learning–based lung segmentation
All chest CTs underwent automated whole lung segmentation using artificial intelligence (AI) via a custom deep neural network model. The lung segmentation model was trained using a previously described AH-Net architecture [12]. The extent of lung involvement was calculated from infiltrate volume (segmented manually) divided by the overall lung volumes (segmented by model). The quality of the lung segmentation was jointly and non-independently rated on a scale of 1–5 (5 = near perfect to 1 = highly inaccurate) by 3 radiologists who came to consensus.
Statistical analysis
Clinical, laboratory, and CT imaging characteristics were analyzed using averages and standard deviations (avg ± SD) or total numbers and percentages (#; %). All features were examined for collinearity using a Pearson correlation analysis, with |r|> 0.5 considered to be a moderate or better correlation. To compare groups, an unpaired Student t test or a Mann-Whitney U test was performed for parametric and nonparametric data respectively. Individual feature performance was evaluated by receiver operating characteristic (ROC) curve, area under the curve (AUC) with standard error (SE), sensitivity (Se), and specificity (Sp). The optimal cutoff point was determined by minimizing the distance between ROC plot and point (0.1).
A preliminary and limited assessment on the feasibility of multivariate logistic lasso regression analysis was performed to identify independently significant features. All features were initially considered for inclusion in the model. Among multiple models, the model with the highest AUC, Se, Sp, and odds ratio (OR) was chosen. The 95% confidence interval (CI) was also reported for logistic regression analyses. Statistical analyses were performed in R (version 3.6.3).
Results
Patient cohorts
Seventy-four patients were initially asymptomatic with RT-PCR positive for SARS-CoV-2. This included 41 patients from Tokyo, Japan, and 33 patients from Hubei, China. The combined population showed an average age of 54 ± 18 years old with females being more represented (43/74; 58%). Based on the epidemiologic history and tracing, 44/74 patients (59%) reported contact with a SARS-CoV-2-infected patient. The observation period for the total asymptomatic study population was 12.1 ± 3.1 days.
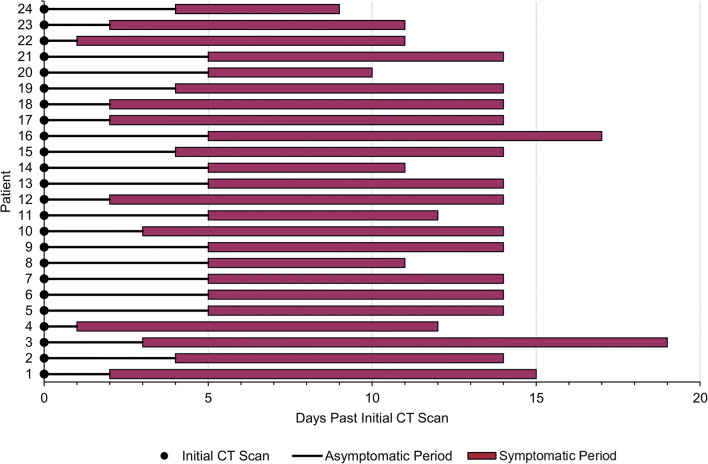
The RT-PCR positive study population was separated into three groups based on CT findings and symptoms (Fig. 1). A majority of patients (48/74; 65%) presented with infiltrates on chest CT. Twenty-four of 74 patients (32%) had CT infiltrates, but did not develop clinical symptoms. Twenty-four of 74 patients (32%) had CT infiltrates and then developed clinical symptoms 1–5 days (3.8 ± 1.5 days) after the CT scan. For patients who developed symptoms, the average duration of symptoms was 9.7 ± 2.6 days with an average observation period of 13.9 ± 1.8 days (Fig. 2). Twenty-six of 74 patients (35%) had no CT infiltrates and no clinical symptoms. No patients in this group without CT infiltrates went on to develop symptoms.
Fig. 1.
Patient selection flow chart
Fig. 2.
Modified swimmer plot of pre-symptomatic patients. Description of patients with CT infiltrates who went on to develop symptoms 1–5 days after initial scan
Characterization of CT infiltrates in asymptomatic patients
A total of 48 asymptomatic patients had COVID-19-related CT infiltrates (Table 2). Twenty-eight of 48 patients (58%) presented bilateral involvement. Only left or right lobes were affected for 12/48 (25%) and 8/48 (17%) of patients, respectively. In the majority of cases, infiltrates were diffuse (23/48; 48%) and involved only inferior lobes in 17/48 (35%) and only superior lobes in 7/48 (15%). All but one CT positive patient presented with a peripheral predominance of these infiltrates.
Table 2.
Characterization of the asymptomatic population with CT findings
| CT finding | Total | % of 48 |
|---|---|---|
| Patients with CT infiltrates | 48 | 100 |
| Localization | ||
| Bilateral | 28 | 58 |
| Left | 12 | 25 |
| Right | 8 | 17 |
| Diffuse | 23 | 48 |
| Inferior | 17 | 35 |
| Superior | 7 | 15 |
| Peripheral | 47 | 94 |
| Central | 0 | 0 |
| Both peripheral and central | 1 | 2 |
| Infiltrate type | ||
| Ground glass opacity | 45 | 94 |
| Consolidation | 22 | 46 |
| Crazy paving | 13 | 27 |
| Bronchial sign | 1 | 2 |
| Tree in bud | 1 | 2 |
| Pleural effusion | 0 | 0 |
| Preexisting lung disease | 10 | 21 |
| Avg | SD | |
| Average number of infiltrates | 3.9 | ±3.0 |
| Lung volume | ||
| Infiltrate volume (cm3) | 111 | 114 |
| Whole lung volume (cm3) | 4233 | 1144 |
| Percent lung involvement (%) | 2.9 | 3.8 |
| Lung attenuation | ||
| Infiltrate attenuation (HU) | - 610 | 94 |
| Whole lung attenuation (HU) | - 830 | 42 |
Abbreviations: CT, computed tomography; HU, Hounsfield unit, Avg, average; SD, standard deviation
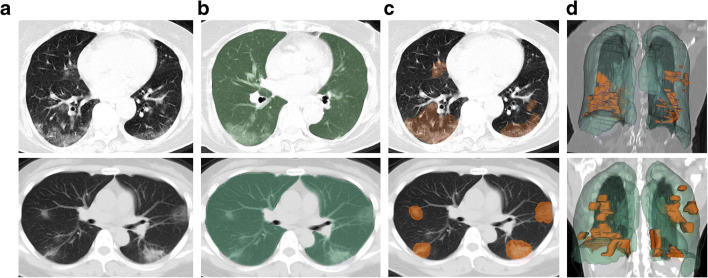
The most frequently represented imaging feature was ground glass opacity (45/48; 94%), followed by consolidation (22/48; 46%) and intralobular septal lines (13/48; 27%). Ten of 48 patients (21%) had preexisting lung diseases. Figure 3 shows two representative cases of an asymptomatic and pre-symptomatic patient with CT infiltrates.
Fig. 3.
Representative chest CT images of two asymptomatic patients. Top—CT scan of a 78-year-old female who never developed COVID-19 symptoms and remained asymptomatic during course of RT-PCR positivity, despite bilateral ground glass opacities on CT. Bottom—CT scan of a 41-year-old female who developed symptoms 5 days after CT. Highlighting the higher attenuation of infiltrates, as consolidations. a Axial chest CT slice with typical infiltrates of COVID-19 pneumonia. b Deep learning–derived whole lung segmentation (green) superimposed over axial chest CT slice. c Superimposed segmented infiltrates (orange) over axial chest CT slice. d Anterior view of 3D volumes of whole lung (green) and infiltrate (orange) segmentations over a coronal chest CT slice
Radiologists’ qualitative rating of the adequacy of the deep learning–based lung segmentation resulted in an average of 4.66, and 90.4% of segmentations achieved a score of 4 or 5 (out of 5). None reported major errors.
Based on manual segmentations, the average volume of the lung infiltrates was 111 ± 144 cm3 per patient. The average attenuation of the infiltrates was - 610 ± 94 HU, while non-diseased lung had an average attenuation of - 830 ± 42 HU.
Correlation of clinical and laboratory data to CT infiltrates
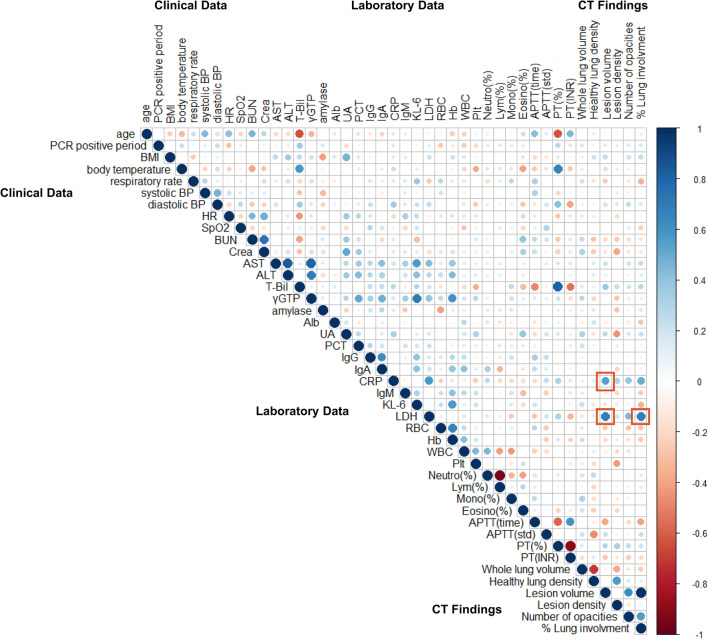
Collinearity between clinical data, laboratory data, and CT infiltrates was assessed for all asymptomatic patients and displayed as a correlation diagram heat map (Fig. 4). Specific to the CT infiltrates, a positive correlation was found between LDH and both infiltrate volume (r = 0.68, p = 2.19 × 10-9) and percentage lung involvement (r = 0.67, p = 2.7 × 10-7). CRP was positively correlated to infiltrate volume (r = 0.54, p = 5.83 × 10-6).
Fig. 4.
Correlation diagram heat map that examines collinearity between clinical, laboratory, and CT findings of asymptomatic patients. Color indicates the value of the correlation coefficient (r). The circle size and color intensity are proportional to the correlation coefficient (r), with positive correlations (r > 0) shown in red and negative correlations (r < 0) shown in blue. A red box highlights a strong positive correlation between LDH and CRP with infiltrate volume and LDH with percent lung involvement
Comparison of asymptomatic patients with and without CT infiltrates
Forty-eight asymptomatic patients had COVID-19-related CT infiltrates and 26 had no CT infiltrates (Table 1). When comparing the groups, a higher proportion of patients with infiltrates reported contact with a SARS-CoV-2-infected patient (73% versus 38%, p = 0.008) compared to those without CT infiltrates, whose did not report contact, or who may have had only travel exposure to high prevalence locations. Patients with CT infiltrates also had a slightly elevated body temperature (37 ± 0.7 versus 36.6 ± 0.3 °C, p = 4.12 × 10-4) and diastolic blood pressure (92 ± 15 versus 86 ± 10 mmHg, p = 0.038) compared to those with no CT infiltrates (Table 1).
Analysis of laboratory data showed that patients with CT infiltrates had higher CRP (10.8 ± 15.8 versus 1.7 ± 2.0 mg/L, p = 0.001) and KL-6 (337 ± 173 versus 227 ± 71 U/mL, p = 0.021) and lower white blood count (5.4 ± 1.8 versus 6.6 ± 2.0x109/L, p = 0.018) and PT (INR) (0.97 ± .08 versus 1.01 ± .05, p = 0.020) compared to patients with no CT infiltrates.
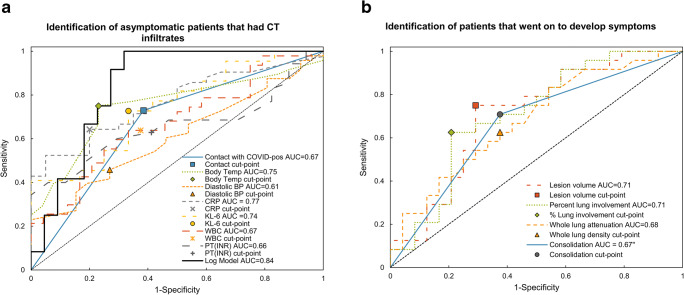
From the univariate ROC analysis, body temperature (AUC = 0.75, Se = 0.75, Sp = 0.77), CRP (AUC = 0.77, Se = 0.64, Sp = 0.80), and KL-6 (AUC = 0.75, Se = 0.73, Sp = 0.67) were best at distinguishing asymptomatic patients who had CT infiltrates compared to those asymptomatic patients without CT infiltrates (Fig. 5). Table 3 gives ROC analysis and optimal cut points for all parameters found to have p < 0.05.
Fig. 5.
ROC analysis of variables to differentiate (a) asymptomatic patients what had CT infiltrates vs. those who did not, and (b) pre-symptomatic patients who went on to develop symptoms vs. those who remained asymptomatic
Table 3.
ROC analysis results for independent variables for differentiation of asymptomatic patients with and without CT findings and between patients who developed symptoms later and those who remained asymptomatic.
| Variable | AUC (upper and lower limit) | SE AUC | Se | Sp | Optimal cut point |
|---|---|---|---|---|---|
| Patients with and without CT infiltrates | |||||
| Contact with SARS-CoV-2 positive patient (positive) | 0.67 (0.56–0.79) | 0.058 | 0.73 | 0.62 | N/A binary |
| Body temperature | 0.75 (0.64–0.86) | 0.058 | 0.75 | 0.77 | 36.7 °C |
| Diastolic blood pressure | 0.61 (0.48–0.74) | 0.066 | 0.46 | 0.73 | 92 mmHg |
| CRP | 0.77 (0.65–0.88) | 0.060 | 0.64 | 0.80 | 2.85 mg/L |
| KL-6 | 0.75 (0.58–0.91) | 0.085 | 0.73 | 0.67 | 216 U/mL |
| WBC | 0.63 (0.54–0.80) | 0.066 | 0.64 | 0.67 | 5.63 × 109/L |
| PT (INR) | 0.66 (0.51–0.80) | 0.075 | 0.60 | 0.71 | 0.99 |
| Patients who went on to develop symptoms vs. those who remained asymptomatic | |||||
| Infiltrate volume | 0.71 (0.56–0.86) | 0.078 | 0.75 | 0.71 | 59.1 cm3 |
| Percent lung involvement | 0.71 (0.56–0.86) | 0.077 | 0.63 | 0.79 | 1.96% |
| Whole lung attenuation | 0.68 (0.53–0.83) | 0.078 | 0.63 | 0.63 | - 840 HU |
| Consolidation (present) | 0.67 (0.53–0.80) | 0.069 | 0.71 | 0.63 | N/A binary |
Abbreviations: AUC, area under curve; SE, standard error; Se, sensitivity; Sp, specificity; CT, computed tomography; CRP, C-reactive protein; PT (INR), prothrombin time calculated as international normalized ratio; WBC, white blood cell count; HU, Hounsfield unit
From the limited multivariate logistic regression analysis, KL-6 and CRP were independently associated with the presence of COVID-19-related CT infiltrates in asymptomatic patients. The multivariate logistic regression model that included both KL-6 and CRP demonstrated an AUC of 0.84 (SE = 0.068, Se = 0.92, Sp = 0.73, Fig. 5) with an optimal cutoff point of 0.25. Model parameters are given in Table 4.
Table 4.
Model parameters from the limited multivariate logistic regression analysis. The two models predicts the classification in the first group (1)
| Multivariate logistic regression model | Estimate | SE | z value | Pr (>) |
|---|---|---|---|---|
| Model 1: Patients with CT infiltrates (1) vs. patients without CT infiltrate | ||||
| Intercept | - 3.606 | 1.576 | 2.288 | 0.022 |
| KL-6 | 0.0127 | 0.005 | 2.317 | 0.021 |
| CRP | 0.269 | 0.147 | 1.824 | 0.068 |
| Model 2: Patients with CT infiltrates who developed symptoms (1) vs. patients without CT infiltrate nor clinical symptom | ||||
| Intercept | - 0.636 | 0.412 | - 1.543 | 0.123 |
| Presence of consolidation | 1.398 | 0.616 | 2.270 | 0.023 |
Abbreviations: SE, standard error; CRP, C-reactive protein; CT, computed tomography
Comparison of patients with CT infiltrates who remained asymptomatic versus those who subsequently developed symptoms
Among the 48 asymptomatic patients with CT infiltrates, 24/48 (50%) went on to develop symptoms 1 to 5 days after their initial CT scan (3.8 ± 1.5 days) (Fig. 2). Twenty-four of 48 patients (50%) never developed symptoms during follow-up (average follow-up 50 days). As expected, the observation period for the asymptomatic patients that never developed symptoms was slightly less than those that developed symptoms (11.1 ± 3.3 versus 13.9 ± 1.8 days).
A comparison of CT infiltrates found that patients who subsequently developed symptoms had a larger infiltrate volume (146 ± 171 versus 76 ± 103 cm3, p = 0.014), higher percent lung involvement (3.7 ± 4.5 versus 2.0 ± 2.7 %, p = 0.013), a lower whole lung attenuation (- 817 ± 47 versus - 805 ± 182 HU, p = 0.030), and a higher prevalence of consolidation (63% versus 29%, p = 0.043) when compared to patients who did not develop clinical symptoms during follow-up (Table 5).
Table 5.
Comparison of CT features between asymptomatic patients with CT findings who never developed symptoms and those who went on to develop symptoms 1–5 days after the original CT scan
| Patients with CT infiltrates and no clinical symptoms (n = 24) | Patients with CT infiltrates and symptoms appeared later (n = 24) | p value | |||
| Avg | SD | Avg | SD | ||
| Lung volume | |||||
| Whole lung volume (cm3) | 4231 | 1067 | 4235 | 1239 | 0.798 |
| Healthy lung volume (cm3) | 4156 | 1092 | 4097 | 1264 | 0.976 |
| Infiltrate volume (cm3) | 76 | 103 | 146 | 171 | 0.014* |
| Percent lung involvement (%) | 2.0 | 2.7 | 3.7 | 4.5 | 0.013* |
| Lung attenuation | |||||
| Whole lung attenuation (HU) | - 805 | 182 | - 817 | 47 | 0.030* |
| Healthy lung attenuation (HU) | - 846 | 30 | - 825 | 47 | 0.089 |
| Infiltrate attenuation (HU) | - 633 | 84 | - 587 | 99 | 0.085 |
| Localization | Number | % | Number | % | |
| Bilateral | 12 | 50 | 16 | 67 | 0.277 |
| Left | 6 | 25 | 6 | 25 | |
| Right | 6 | 25 | 2 | 8 | |
| Peripheral | 24 | 100 | 23 | 96 | 1.000 |
| Central | 0 | 0 | 1 | 4 | |
| Main lobe | |||||
| Diffuse | 8 | 33 | 15 | 63 | 0.120 |
| Inferior | 11 | 46 | 6 | 25 | |
| Superior | 5 | 21 | 2 | 8 | |
| Infiltrate type | |||||
| Consolidation | 7 | 29 | 15 | 63 | 0.043* |
| Ground glass opacity | 23 | 96 | 22 | 92 | 1.000 |
| Crazy paving | 10 | 42 | 3 | 13 | 0.051 |
| Preexisting lung disease | 5 | 21 | 1 | 4 | 0.190 |
| Avg | SD | Avg | SD | ||
| Number of infiltrates | 3.4 | 2.9 | 4.4 | 3.0 | 0.288 |
Abbreviations: Avg, average; SD, standard deviation; HU, Hounsfield unit
*p < 0.05
From univariate ROC analysis, segmentation volume (AUC = 0.71, Se = 0.75, Sp = 0.71) and percent lung involvement (AUC = 0.71, Se = 0.63, Sp = 0.79) were best able to distinguish pre-symptomatic patients from those who remained asymptomatic (Table 3, Fig. 5).
From this limited multivariate analysis, a higher prevalence of consolidation (OR = 4.05, 95%CI = 1.25–14.27) was independently associated with subsequent development of symptoms among the initially asymptomatic patients with CT infiltrates (AUC = 0.67, SE = 0.069, Se = 0.71, Sp = 0.63) with an optimal cut point probability of 0.67; however, the data is limited and likely overfit in the multivariate analysis. The model parameters are given in Table 4.
Discussion
The role of chest CT in asymptomatic and pre-symptomatic patients with SARS-CoV-2 remains ill-defined. Seventy-four such patients are presented here from 2 different outbreak locations during the COVID-19 pandemic. Dampening the impact of SARS-CoV-2 may depend upon better insight into incompletely understood transmission dynamics. Pre-symptomatic transmission is common [2], and a better understanding could lead to more informed containment and mitigation. CT provides a non-invasive and repeatable informational window into lung infection dynamics in this viral pandemic enigma. Limited reports with small numbers show that CT infiltrates may be present in asymptomatic patients [13–16]. However, pre-test patient selection bias and prevalence likely determine this frequency.
Asymptomatic patients with travel or exposure histories may have CT infiltrates, possibly related to SARS-CoV-2 prevalence in high-risk populations. Many passengers with SARS-CoV-2 from the Diamond Princess Cruise ship were asymptomatic, with 44/82 (54%) of asymptomatic patients having CT abnormalities [17, 18]. However, as with this study, a potential overlap of patients with previously published studies is not possible to evaluate due to requisite de-identification and anonymization processes. This Tokyo cohort differs from previous independent studies in that limited multivariate analysis with continuous lab variables was used here. This study focused on the timing of symptom onset versus CT, and also utilized AI segmentation tools, with additional cohorts from China [19]. During the outbreak in Hubei Province, CT was utilized upfront, alongside RT-PCR, not only at initial symptomatic presentation but also in asymptomatic patients suspected of having COVID-19 after exposure to infected persons or travel to high prevalence or contaminated environments [20]. It is paramount to containment strategies to better understand these asymptomatically but infected patients who may silently spread the virus if they remain undiagnosed and un-isolated. Given that CT has detected COVID-19 in some asymptomatic patients before RT-PCR detection of infection [21], a combination of RT-PCR tests and CT may be applied to optimally identify asymptomatically but infected patients and their contacts [22].
The proportion of asymptomatic but infected people remains poorly defined [23], but recent estimates showed that up to 80% of COVID-19 patients have mild or asymptomatic [24]. In one study, about 3 in 4 patients with COVID-19 stated they had no known exposure to symptomatic people or potential high-risk environments [1]. Twenty-nine of 33 (88%) of screened SARS-CoV-2-positive pregnant women presenting for delivery in New York City were asymptomatic [25]. These asymptomatic carriers may be responsible for a majority of virus transmission [2, 3, 17].
The transmission dynamics in 94 COVID-19 patients showed the highest viral load, viral shedding, and infectiousness near or before the time of symptom onset, near the end of the incubation period [2]. Although influenza has pre-symptomatic viral replication and infectivity, SARS-CoV-2 infectivity may peak earlier, which complicates detection and isolation strategies [26]. This leaves the exact duration of pre-symptomatic transmission unknown [8]. However, given the mean 5–6-day incubation period for SARS-CoV-2 (range 2–14 days) and that CT was able to detect infection 3.8 ± 1.5 days (1–5 days) before symptoms developed in the present study, the silent progression may be capable of being captured [19].
Additionally, half of 16 patients with COVID-19 presented positive RT-PCR after symptom resolution and may then continue to shed viral RNA [23]. Seroconversion typically occurs after 6 to 12 days, regardless of RT-PCR status [27], while the median duration of virus shedding by RNA was 20 days in survivors (ranging 8–37 days) [28]. CT is a widely available tool that could be applied in a targeted but limited fashion, to shed light on the critical issue of asymptomatic transmission, or to better define prevalence in specific cohorts. Still, it remains clear that the presence of subclinical CT infiltrates in COVID-19 before, after, or irrespective of symptoms remains an enigma.
Several radiology and thoracic professional organizations have recommended against using CT or do not include the role of CT for screening, diagnosis, or contact tracing of COVID-19 [29–31]. Although CT might be useful alongside RT-PCR in the acute setting of early COVID-19 [21], it may suffer from low negative predictive value and specificity, in a low prevalence setting, despite its high sensitivity [32]. Some guidelines recommend that CT be reserved for hospitalized symptomatic patients with specific clinical indications [29], which discourages the use of CT in asymptomatic populations to assist with contact tracing. An accurate definition of prevalence including asymptomatic patients could theoretically better inform policy based on epidemiology models to predict early isolation, contact tracing, sentinel surveillance, or back to work strategies. CT testing carries a low risk of infection for technologists and other healthcare personnel involved in the scans [20]. 3340 CT scans for suspected COVID-19 were performed in one location without a single staff infection reported [33]. Chest CT can also be effective with ultralow radiation doses [34].
CT infiltrates in pre-selected asymptomatic patients with SARS-CoV-2 infection in high prevalence settings are not uncommon. This occurred in nearly two-thirds of initially asymptomatic patients in this study with exposure history and positive RT-PCR for SARS-CoV-2. CRP and KL-6 levels identified which exposed asymptomatic patients with SARS-CoV-2 had CT infiltrates on presentation. This association is not surprising for markers of inflammation (CRP) and interstitial lung disease or acute respiratory distress syndrome (KL-6). With much more data, it is possible that an elevated yet normal body temperature could also add to predictive models, given the limited association uncovered here in asymptomatic patients with vs without infiltrates. The early identification of patients with specific CT infiltrates, such as consolidation, was able to predict subsequent development of symptoms. This early prognosticator becomes even more important as therapies develop such as Remdesivir, which may be more effective when administered early [35]. Asymptomatic convalescence may also find clandestine CT infiltrates typical for COVID-19 in the presence of negative throat RT-PCR, but positive induced sputum RT-PCR [36].
A major limitation of this study is the small sample size, which limits the ability to implement statistical validation techniques for the multivariate analysis. The multivariate model is likely overfit, which reduces the validity of dependent conclusions. The present findings are merely a demonstration of future work that could include more model data or independent validation and testing. An additional limitation of this study is the retrospective nature, and the selection of RT-PCR positive patients. This limits the ability to further understand the role of CT in the timeframe prior to availability of RT-PCR test results (or with false-negative results in early disease). Our study evaluated patients from 2 different sites with heterogeneous clinical protocols, which inevitably presents selection bias and heterogeneity of data, with some missing laboratory data. Furthermore, asymptomatic patients with CT infiltrate came from one cohort, while patients with subsequent symptom onset and CT infiltrates came from another geographic cohort. This cohort difference may introduce other unknown biases related to preselection, CT timing, or other viral exposure differences. However, heterogeneity in general may also enhance generalizability. Independent validation is requisite.
Without defining the exact prevalence within specific outbreak populations, the transmission patterns will remain ill-defined, but 50% of transmissions might be occurring in the pre-symptomatic phase [37]. CT may facilitate characterization in the asymptomatic but exposed populations who are actively shedding. CT scan was able to detect infections at a very early stage of disease, during early incubation, 3.8 days before symptom onset. Although potentially valuable as an addition to RT-PCR in certain settings, the exact role of CT for asymptomatic patients needs to be more clearly defined, especially given its near-immediate availability and turn-around time.
Acknowledgments
The authors would like to thank the COVID-19 team of the Self-Defense Forces Central Hospital, Tokyo, and the Xiangyang NO. 1 People’s Hospital Affiliated to Hubei University of Medicine Xiangyang, Hubei, China, for enabling the research. BW is Principal Investigator on the following CRADA’s (Cooperative Research & Development Agreements) between NIH and related commercial partners that involve artificial intelligence and deep learning: Philips Image Guided Therapy (CRADA), Philips Research (CRADA), Philips (CRADA), Siemens (CRADA), and NVIDIA (CRADA). Licensed Patents/Royalties: No patents related to this specific work. The content of this publication does not necessarily reflect the views or policies of the National Institutes of Health, the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations
- AI
Artificial intelligence
- CI
Confidence interval
- COVID-19
Coronavirus Disease 2019
- CRP
C-reactive protein
- KL-6
Krebs von den Lungen 6
- LDH
Lactate dehydrogenase
- RT-PCR
Reverse-transcriptase polymerase chain reaction
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- SE
Standard error
- Se
Sensitivity
- Sp
Specificity
Funding
Work supported by the NIH Intramural Targeted Anti-COVID-19 (ITAC) Program, funded by the National Institute of Allergy and Infectious Diseases.
This work was supported by the Center for Interventional Oncology and the Intramural Research Program of the National Institutes of Health (NIH) by intramural NIH Grants Z01 1ZID BC011242 and CL040015.
This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. 75N91019D00024, Task Order No. 75N91019F00129.
MB is a recipient of the 2019 Alain Rahmouni SFR-CERF research grant provided by the French Society of Radiology together with the French Academic College of Radiology.
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is Bradford J. Wood.
Conflict of interest
NIH and Bradford Wood receive royalties for licensed patents from Philips. NVIDIA and Philips may own intellectual property in the field.
Nicole Varble is an employee of Philips Research.
Dong Yang, Ziyue Xu, Daguang Xu, Mona Flores are employees of NVIDIA corp.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
This work is not under consideration elsewhere for publication; however, the Diamond Princess has a broad scope of publications, none of which exactly match these findings or analyses. Please find these selected references:
1. Inui S, Fujikawa A, Jitsu M et al (2020) Chest CT Findings in cases from the cruise ship “Diamond Princess” with coronavirus disease 2019 (COVID-19). Radiology: Cardiothoracic Imaging. DOI:10.1148/ryct.2020200110
2. Mizumoto K, Kagaya K, Zarebski A, Chowell G (2020) Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill 25:2000180
3. Tabata S, Imai K, Kawano S et al (2020) Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: a retrospective analysis. Lancet Infect Dis. DOI:10.1016/S1473-3099(20)30482-5
Methodology
• retrospective
• observational
• multicenter study
Footnotes
The original online version of this article was revised: The last sentence of section “Characterization of CT infiltrates in asymptomatic patients” was worded incorrectly. The correct wording is: “The average attenuation of the infiltrates was - 610 ± 94 HU, while nondiseased lung had an average attenuation of - 830 ± 42 HU.”
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nicole Varble and Maxime Blain contributed equally to this work.
Change history
12/8/2020
A Correction to this paper has been published: 10.1007/s00330-020-07552-8
References
- 1.Li Q, Guan X, Wu P, et al. Early Transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He X, Lau EHY, Wu P et al (2020) Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 10.1038/s41591-020-0869-5 [DOI] [PubMed]
- 3.Li R, Pei S, Chen B et al (2020) Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2). Science. 10.1126/science.abb3221 [DOI] [PMC free article] [PubMed]
- 4.Ye F, Xu S, Rong Z et al (2020) Delivery of infection from asymptomatic carriers of COVID-19 in a familial cluster. Int J Infect Dis. 10.1016/j.ijid.2020.03.042 [DOI] [PMC free article] [PubMed]
- 5.Pan X, Chen D, Xia Y, et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20:410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao ZQ, Wan R, He LY, Hu YC, Chen W (2020) The enlightenment from two cases of asymptomatic infection with SARS-CoV-2: is it safe after 14 days of isolation? Int J Infect Dis. 10.1016/j.ijid.2020.03.041 [DOI] [PMC free article] [PubMed]
- 7.Li Y, Hu Y, Yu Y et al (2020) Positive result of Sars-Cov-2 in faeces and sputum from discharged patient with COVID-19 in Yiwu, China. J Med Virol. 10.1002/jmv.25905 [DOI] [PMC free article] [PubMed]
- 8.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi M, Yokoe DS, Havlir DV (2020) Asymptomatic transmission, the Achilles’ heel of current strategies to control Covid-19. N Engl J Med. 10.1056/NEJMe2009758 [DOI] [PMC free article] [PubMed]
- 10.Arons MM, Hatfield KM, Reddy SC et al (2020) Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 10.1056/NEJMoa2008457 [DOI] [PMC free article] [PubMed]
- 11.Lee YL, Liao CH, Liu PY et al (2020) Dynamics of anti-SARS-Cov-2 IgM and IgG antibodies among COVID-19 patients. J Infect. 10.1016/j.jinf.2020.04.019 [DOI] [PMC free article] [PubMed]
- 12.Liu S, Xu D, Zhou SK, et al. 3D anisotropic hybrid network: transferring convolutional features from 2D images to 3D anisotropic volumes International Conference on Medical Image Computing and Computer Assisted Intervention. Cham: Springer; 2018. pp. 851–858. [Google Scholar]
- 13.Zhou X, Li Y, Li T, Zhang W (2020) Follow-up of the asymptomatic patients with SARS-CoV-2 infection. Clin Microbiol Infect. 10.1016/j.cmi.2020.03.024 [DOI] [PMC free article] [PubMed]
- 14.Hu Z, Song C, Xu C et al (2020) Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 10.1007/s11427-020-1661-4 [DOI] [PMC free article] [PubMed]
- 15.An P, Song P, Wang Y, Liu B (2020) Asymptomatic patients with novel Coronavirus disease (COVID-19). Balkan Med J. 10.4274/balkanmedj.galenos.2020.2020.4.20 [DOI] [PMC free article] [PubMed]
- 16.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inui S, Fujikawa A, Jitsu M et al (2020) Chest CT findings in cases from the cruise ship “Diamond Princess” with coronavirus disease 2019 (COVID-19). Radiology Cardiothoracic Imaging. 10.1148/ryct.2020200110 [DOI] [PMC free article] [PubMed]
- 19.Tabata S, Imai K, Kawano S et al (2020) Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: a retrospective analysis. Lancet Infect Dis. 10.1016/S1473-3099(20)30482-5 [DOI] [PMC free article] [PubMed]
- 20.Xue H, Jin Z (2020) The appropriate position of radiology in COVID-19 diagnosis and treatment—current status and opinion from China. Chin J Acad Radiol. 10.1007/s42058-020-00030-6 [DOI] [PMC free article] [PubMed]
- 21.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 Cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X, Fu B, Chen L, Feng Y (2020) Serological tests facilitate identification of asymptomatic SARS-CoV-2 infection in Wuhan, China. J Med Virol. 10.1002/jmv.25904 [DOI] [PMC free article] [PubMed]
- 23.Chang D, Mo G, Yuan X et al (2020) Time kinetics of viral clearance and resolution of symptoms in novel coronavirus infection. Am J Respir Crit Care Med 201:1150–1152 [DOI] [PMC free article] [PubMed]
- 24.European Centre for Disease Prevention and Control (2020) Rapid risk assessment: coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK – seventh update. European Center for Disease Prevention and Control. Available via https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-coronavirus-disease-2019-covid-19-pandemic#no-link. Accessed 7 May 2020
- 25.Sutton D, Fuchs K, D’Alton M, Goffman D (2020) Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 10.1056/NEJMc2009316 [DOI] [PMC free article] [PubMed]
- 26.Lau LL, Cowling BJ, Fang VJ, et al. Viral shedding and clinical illness in naturally acquired influenza virus infections. J Infect Dis. 2010;201:1509–1516. doi: 10.1086/652241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfel R, Corman VM, Guggemos W et al (2020) Virological assessment of hospitalized patients with COVID-2019. Nature. 10.1038/s41586-020-2196-x [DOI] [PubMed]
- 28.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.(2020) ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. American College of Radiology. Available via https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed 7 May 2020
- 30.Nair A, Rodrigues JCL, Hare S, et al. A British Society of Thoracic Imaging statement: considerations in designing local imaging diagnostic algorithms for the COVID-19 pandemic. Clin Radiol. 2020;75:329–334. doi: 10.1016/j.crad.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sethuraman N, Jeremiah SS, Ryo A (2020) Interpreting diagnostic tests for SARS-CoV-2. JAMA. 10.1001/jama.2020.8259 [DOI] [PubMed]
- 32.Wen Z, Chi Y, Zhang L, et al. Coronavirus disease 2019: initial detection on chest CT in a retrospective multicenter study of 103 Chinese subjects. Radiology Cardiothoracic Imaging. 2020;2:e200092. doi: 10.1148/ryct.2020200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Z, Zhao S, Li Z et al (2020) The battle against coronavirus disease 2019 (COVID-19): emergency management and infection control in a radiology department. J Am Coll Radiol. 10.1016/j.jacr.2020.03.011 [DOI] [PMC free article] [PubMed]
- 34.Dangis A, Gieraerts C, Bruecker YD, et al. Accuracy and reproducibility of low-dose submillisievert chest CT for the diagnosis of COVID-19. Radiology Cardiothoracic Imaging. 2020;2:e200196. doi: 10.1148/ryct.2020200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.NIAID (2020) NIH clinical trial shows remdesivir accelerates recovery from advanced COVID-19. National Institute of Allergy and Infectious Diseases. Available via https://www.niaid.nih.gov/news-events/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19. Accessed 7 May 2020
- 36.Han H, Luo Q, Mo F, Long L, Zheng W (2020) SARS-CoV-2 RNA more readily detected in induced sputum than in throat swabs of convalescent COVID-19 patients. Lancet Infect Dis. 10.1016/s1473-3099(20)30174-2 [DOI] [PMC free article] [PubMed]
- 37.Sun K, Wang W, Gao L et al (2020) Transmission heterogeneities, kinetics, and controllability of SARS-CoV-2. Preprint available at https://www.medrxiv.org/content/medrxiv/early/2020/08/13/2020.08.09.20171132.full.pdf. Accessed 18 Oct 2020 [DOI] [PMC free article] [PubMed]