Abstract
Background
Patients with schizophrenia spectrum disorders (SSD) tend to lack insight, which is linked to poor outcomes. The effect size of previous treatments on insight changes in SSD has been small. Metacognitive interventions may improve insight in SSD, although this remains unproved.
Methods
We carried out a systematic review and meta-analysis of randomized controlled trials (RCTs) to examine the effects of metacognitive interventions designed for SSD, namely Metacognitive Training (MCT) and Metacognitive Reflection and Insight Therapy (MERIT), on changes in cognitive and clinical insight at post-treatment and at follow-up.
Results
Twelve RCTs, including 10 MCT RCTs (n = 717 participants) and two MERIT trials (n = 90), were selected, totalling N = 807 participants. Regarding cognitive insight six RCTs (n = 443) highlighted a medium effect of MCT on self-reflectiveness at post-treatment, d = 0.46, p < 0.01, and at follow-up, d = 0.30, p < 0.01. There was a small effect of MCT on self-certainty at post-treatment, d = −0.23, p = 0.03, but not at follow-up. MCT was superior to controls on an overall Composite Index of cognitive insight at post-treatment, d = 1.11, p < 0.01, and at follow-up, d = 0.86, p = 0.03, although we found evidence of heterogeneity. Of five MCT trials on clinical insight (n = 244 participants), which could not be meta-analysed, four of them favoured MCT compared v. control. The two MERIT trials reported conflicting results.
Conclusions
Metacognitive interventions, particularly Metacognitive Training, appear to improve insight in patients with SSD, especially cognitive insight shortly after treatment. Further long-term RCTs are needed to establish whether these metacognitive interventions-related insight changes are sustained over a longer time period and result in better outcomes.
Key words: Insight, outcomes, metacognitive interventions, schizophrenia spectrum disorders
Introduction
Every year up to two million people across the world receive a first diagnosis of schizophrenia (Jongsma et al., 2018), which remains associated with poor long-term clinical and social outcomes (Morgan et al., 2014), including higher mortality rates than the general population (Hjorthøj, Stürup, McGrath, & Nordentoft, 2017).
In line with this, insight has been consistently linked with outcome in psychosis – greater insight, better outcomes (Lincoln, Lüllmann, & Rief, 2007; Lysaker, Pattison, Leonhardt, Phelps, & Vohs, 2018). Impaired clinical insight (insight thereafter) can be considered as a cardinal feature of psychotic disorders (Carpenter, Strauss, & Bartko, 1973), especially schizophrenia (Amador et al., 1994). The multidimensional model of insight proposed by David (1990), which included: (i) awareness of having a mental illness, (ii) the ability to recall previous psychotic experiences as abnormal and (iii) treatment compliance, has received much support from research over the last three decades (Amador & David, 2004; David, 2019; Lysaker et al., 2018). However, the evidence of that previous treatments targeting insight are effective in schizophrenia spectrum disorders (SSD) is limited, which may have been due to not addressing what appears to underlie poor insight in SSD, namely metacognitive deficits (Lysaker et al., 2018a; Nair, Palmer, Aleman, & David, 2014).
Thus, in 2004 Henry and Ghaemi conducted a systematic review of 13 previous randomized and non-randomized studies (Henry & Ghaemi, 2004) on different types of intervention, such as psychoeducation (PSE), psychoanalytically oriented therapies, cognitive-behavioural therapy (CBT), video recorded self-observation and antipsychotics, for changing insight. These standard treatments targeting the classic symptoms of schizophrenia failed to improve insight, which was a secondary outcome measure in most of the included studies. In 2013 the results from 19 randomized controlled trials (RCTs) on insight, none of which tested metacognitive interventions, were meta-analysed (Pijnenborg, van Donkersgoed, David, & Aleman, 2013). Although the overall effect size, d = 0.34, was medium and significant, which suggested that insight could be improved following integrative treatments, further subanalyses for specific interventions, namely CBT, PSE and adherence therapy, yielded smaller non-significant effects. Hence, no evidence-based intervention could be recommended for enhancing insight in SSD.
However, as alluded to above, the metacognitive basis of insight, which is supported by recent meta-analytic literature (Lysaker et al., 2018; Nair et al., 2014), may shed some light on this, that is, metacognitively oriented interventions may improve insight. Metacognition may be defined as ‘knowledge and cognition about cognitive phenomena’ (Flavell, 1979, p. 906) or ‘the ability to think of one's and others’ thinking' (Wells & Purdon, 1999). Patients with SSD have been demonstrated to have poorer metacognitive performance than the general population (Beck, Baruch, Balter, Steer, & Warman, 2004; Dimaggio & Lysaker, 2010), which has been associated with impaired insight in SSD (Lysaker et al., 2018; Nair et al., 2014). Cognitive insight is a core metacognitive domain which refers to the person's ability to evaluate and correct his/her own distorted beliefs and misinterpretations (self-reflectiveness) and the tendency to overconfidence in one's conclusions (self-certainty) (Beck et al., 2004). Clinical insight therefore represents a different, albeit related, construct from cognitive insight. Moreover, the association between the two has been recently shown to be weaker than previously thought (Van Camp, Sabbe, & Oldenburg, 2017). Nevertheless, interventions targeting metacognitive skills, including cognitive insight, should improve clinical insight too, provided clinical insight has a metacognitive basis (Lysaker et al., 2018; Nair et al., 2014), which forms the context for this systematic review and meta-analysis.
Two metacognitive interventions were designed for patients with SSD, namely Metacognitive Training (MCT) (Moritz & Woodward, 2007) and Metacognition Reflection and Insight Therapy (MERIT) (Lysaker & Klion, 2018). In addition, so-called Metacognitive Therapy was developed by Wells and colleagues (Fisher & Wells, 2009), which targets the dysfunctional metacognitive belief about thinking, thus contributing to symptom improvement in a range of mental disorders, such as depression, anxiety and obsessive-compulsive disorder, although more recently it has also been applied to psychosis. By focusing on different topics such as attributional style, jumping to conclusions, changing beliefs, empathy, memory, depression and self-esteem and stigma, MCT seeks ‘to sow the seeds of doubt’ (Moritz et al., 2014a) regarding cognitive biases related to delusional ideas. MCT is usually delivered by trained staff to a group of 8–10 patients as 8 weekly, or twice a week, 45–60-minute sessions, although individual MCT is available (Moritz et al., 2014a). The MCT manual can be accessed at no cost at http://www.uke.de/mkt. MERIT is an integrated form of psychotherapy which promotes recovery from psychosis by helping patients to make more constructive narratives about themselves and others so they can set up their own recovery goals through metacognitive tasks more focused on reflection than on cognitive biases (Lysaker & Klion, 2018). MERIT takes the form of 24 face-to-face individual 50-minute sessions. This noted, theoretical differences between these three metacognitive interventions can become rather blurred in the clinical setting. Moreover, other non-metacognitive interventions, such as cognitive-behavioural therapy, may actually involve metacognitive processes, although this remains subject to further debate (Moritz & Lysaker, 2018a). Nevertheless, since insight in SSD was demonstrated to have a metacognitive basis (Lysaker et al., 2018; Nair et al., 2014), both MCT and MERIT should contribute to improving insight.
One systematic review (Moritz et al., 2014a) and four meta-analyses (Eichner & Berna, 2016; Jiang, Zhang, Zhu, Li, & Li, 2015; Liu, Tang, Hung, Tsai, & Lin, 2018; Philipp et al., 2019) demonstrated MCT to improve positive psychotic symptoms, although a 2016 meta-analysis failed to replicate this (van Oosterhout et al., 2016a). In a later reply letter from the meta-analysis' authors (van Oosterhout et al., 2016b) the inclusion of four additional studies (Aghotor, Pfueller, Moritz, Weisbrod, & Roesch-Ely, 2010; Gawęda, Krężołek, Olbryś, Turska, & Kokoszka, 2015; Moritz et al., 2011a; So et al., 2015) in the meta-analysis (van Oosterhout et al., 2016a) made the effects of MCT on positive symptoms (g = 0.32) and delusions (g = 0.31), although not on data gathering bias, become significant (van Oosterthout et al., 2016b). No systematic reviews or meta-analyses of MERIT trials have been conducted to date. Most importantly, whether these metacognitive interventions may contribute to gaining insight remains unknown (David, 2019) since conflicting results from previous RCTs reporting insight data, which are detailed below, have not been subject to systematic review or meta-analysis to date.
We carried out a new systematic review and a meta-analysis of previous RCTs to investigate the effect of MCT and MERIT on clinical and cognitive insight (as co-primary outcome measures) in patients with SSD. We hypothesized: (i) that MCT and MERIT will improve clinical and cognitive insight compared with the control intervention; (ii) that the effect size of MCT and MERIT on clinical and cognitive insight changes at follow-up will be larger than immediately after treatment, thus replicating previous findings of longer-term ‘sleeper’ effects of MCT on delusions (Moritz et al., 2014b).
Methods
This systematic review and meta-analysis complied with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, Altman, & PRISMA Group, 2009).
Search strategy
We conducted a literature search in PubMed, PsycInfo and Embase of articles that reported the effects of MCT and MERIT on clinical and/or cognitive insight in samples of patients with SSD from January 2007, when MCT became available (MERIT was developed in 2014), to June 2019. The search strategy used Medical Subjects Headings (MeSH) terms and keywords (‘metacog*’, ‘MCT’, ‘MERIT,’ ‘insight*,’ ‘awareness*,’ and ‘consciousness*’, ‘schizophr*,’ ‘psychos*,’ ‘psychot*,’ ‘illness*,’), including cross-referencing. The only initial search limitation was by English language. References from selected articles were cross-reviewed and selected if they met the following criteria and were indexed into PubMed, PsycInfo or Embase.
Selection criteria
All the abstracts from the initial search were independently screened by JDLM and OA against the following selection criteria. Any doubt about meeting/not meeting the selection criteria was resolved by reviewing the full article:
-
•
Sample size of more than 10 patients;
-
•
Age: 16–64 years;
-
•
Diagnosis: ‘Schizophrenia spectrum disorders’ (SSD) encompassing schizophrenia, schizoaffective disorder, delusional disorder and psychotic disorder Not Otherwise Specified, according to either International Statistical Classification of Diseases (ICD), 10th Revision (World Health Organization, 1993), or Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) and Fourth Edition Text Revision (DSM-IV-TR) definitions (American Psychiatric Association, 2000); or first-episode psychosis (FEP) according to the DSM-IV-TR criteria (American Psychiatric Association, 2000). In terms of diagnosis as a selection criterion, we decided to use a broader category, such as SSD, in order to include all the trials testing MCT or MERIT in samples of patients with any non-affective psychotic disorder, thus aiming to answer the research question formulated above. Although the inclusion of a tiny proportion of patients with bipolar disorder (or affective psychosis) cannot be ruled out, particularly in first episode or early onset psychosis trials (e.g. Ochoa et al., 2017), based on the aims of the included studies and reported samples characteristics this seems very unlikely.
-
•Outcome measures:
- o Clinical Insight had to be assessed before and after treatment with a validated instrument which, based on the search, included the Insight and Treatment Attitudes Questionnaire (ITAQ) (McEvoy et al., 1989), the Schedule for Assessment of Insight, expanded version (SAI-E) (Kemp & David, 1997), the Scale to assess Unawareness of Mental Disorders (SUMD) (Amador et al., 1993), the Birchwood Insight Scale (BIS) (Birchwood et al., 1994), the general item 12 of the Positive and Negative Syndrome Scale (PANSS) (Kay, Fiszbein, & Opler, 1987) and a unidimensional insight questionnaire (Kokoszka, Telichowska-Leśna, & Radzio, 2008) used in one trial (Gawęda et al., 2015);
- o Cognitive Insight had to be measured before and after treatment with the Beck Cognitive Insight Scale (BCIS) (Beck et al., 2004);
-
•
Clinical setting: In-, out-patients and mixed samples were included;
-
•
In case of replication studies by the same group, only the latest one with the largest sample size was considered;
-
•
Only RCT were included regardless of the masking (double-, single-blind or unblind).
Statistical analysis
Individual effect sizes (Cohen's d) for control and intervention conditions of each RCT were calculated with reported means and standard deviations using the effect size calculation software developed by Wilson (2010). Subsequently, the effect size of the control condition was subtracted from the effect size of the intervention, resulting in one effect size for each study (Morris & DeShon, 2002).
Meta-analyses were performed with the program Review Manager 5.0, developed by the Cochrane Collaboration. Effect sizes were weighted by their standard error. Based on the reported means and standard deviations of insight scores before- and after-treatment (Lipsey & Wilson, 2001), between-group differences in cognitive and clinical insight were calculated at three timepoints (at baseline, at post-treatment and at follow-up) using a random-effects model (Borenstein, Hedges, Higgins, & Rothstein, 2010), which was chosen to account for the influence of the differences in interventions received across the study groups. Because of the variance in the type of interventions, a random-effects model was used. The overall effect size was calculated and represented in a forest plot together with the effect size of each study.
We adjusted for the presence of any publication bias using the Duval and Tweedie ‘trim-and-fill’ method (Duval & Tweedie, 2000). Heterogeneity was measured with the Q statistic yielding a chi-square and p-value and the I2 statistic with scores above 50% and 75% indicating moderate and high heterogeneity, respectively (Higgins, Thompson, Deeks, & Altman, 2003). The summary statistics were illustrated with forest and funnel plots. Forest plots provide an indication of heterogeneity between studies (Phan, Xie, Di Eusanio, & Yan, 2014); while the funnel plot is utilized to detect and illustrate publication bias (Duval & Tweedie, 2000b). Statistical significance was set at p ⩽ 0.05.
Results
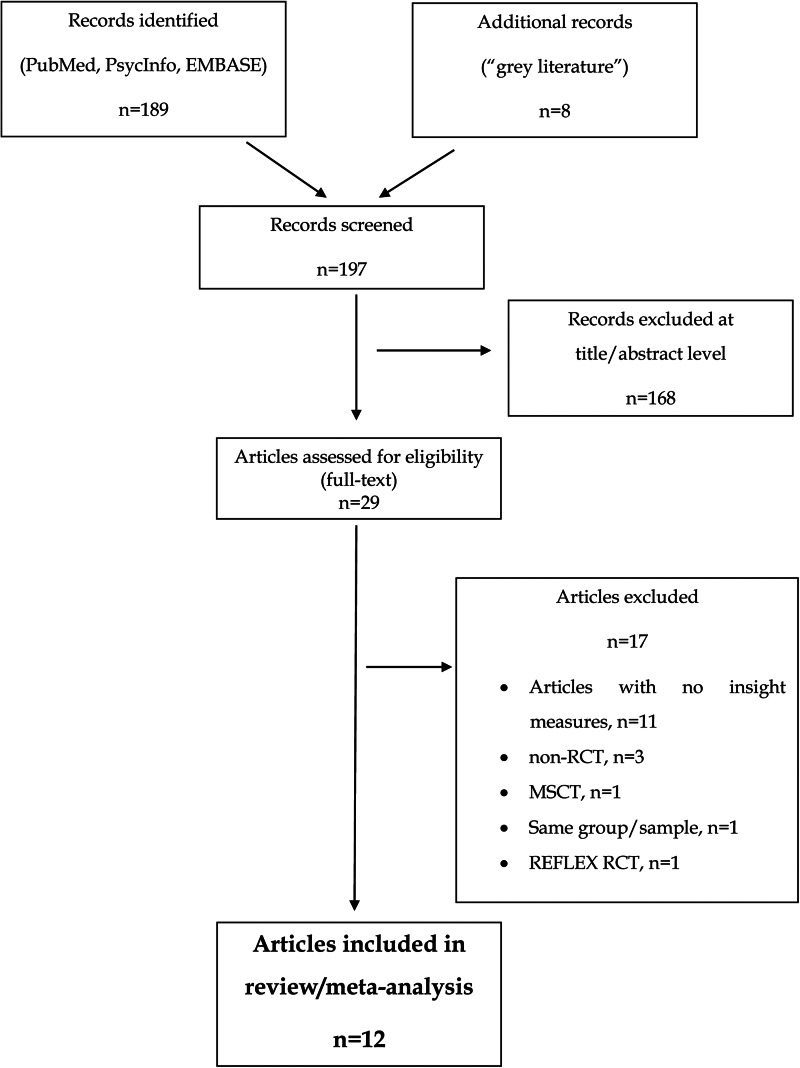
The initial search yielded 189 references. Eight more records were retrieved from the grey literature, although none of them fulfilled the above selection criteria. Hence, 197 records were screened at an abstract level. In total, 168 of these articles were excluded based on our selection criteria. Twenty-nine articles were assessed for eligibility so the full text was reviewed. Seventeen articles were excluded as follows: 11 articles did not report on insight measures (Aghotor et al., 2010; Buonocore et al., 2015; Erawati, Keliat, Helena, & Hamid, 2014; Kumar et al., 2015; Moritz et al., 2011a, 2011b, 2013, 2014b; Naughton et al., 2012; Ross, Freeman, Dunn, & Garety, 2011; So et al., 2015) and 3 articles did not have a RCT design (Balzan, Delfabbro, Galletly, & Woodward, 2014; Favrod, Maire, Bardy, Pernier, & Bonsack, 2011; Ussorio et al., 2016). One study used an overlapping sample (Moritz et al., 2018b) and 2 trials tested other metacognitive interventions such as ‘metacognition and social cognition training’ (MSCT) (Rocha & Queirós, 2013) and the so-called REFLEX (Pijnenborg et al., 2019), both of which are briefly described below. Hence, 12 articles fulfilled the selection criteria, so were included in the systematic review and meta-analysis, which totalled n = 807 participants. Of these, n = 713 individuals (83.3%) received the allocated interventions and completed the post-treatment assessment, at which attrition rates ranged from 0% (Balzan, Mattiske, Delfabbro, Liu, & Galletly, 2019; Kuokkanen, Lappalainen, Repo-Tiihonen, & Tiihonen, 2014) to 27% (Ochoa et al., 2017). Seven studies followed-up participants over 6 months (Andreou et al., 2017; Balzan et al., 2019; de Jong et al., 2019; Favrod et al., 2014; Kuokkanen et al., 2014; Ochoa et al., 2017; van Oosterhout et al., 2014). Attrition rates over the 6-month follow-up period ranged from 1% (Kuokkanen et al., 2014) to 41.4% (de Jong et al., 2019). The flow chart in Fig. 1 shows the studies selection process and Table 1 provides full details of included studies.
Fig. 1.
Flow chart of the studies selection process.
Table 1.
Description of the selected studies
| Study | Ni | NPT | Attrition rate PT (%) | Follow-up | NFU | Attrition rate FU (%) | Diagnosis | Intervention | Control | Blind | Insight scale | Insight as outcome | Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Favrod et al. (2014) | E = 26 C = 26 T = 52 |
E = 24 C = 24 T = 48 |
E = 7.7 C = 7.7 T = 7.7 |
6 months | E = 24 C = 23 T = 47 |
E = 7.7 C = 11.5 T = 9.6 |
SSD, ICD-10 | G-MCT | TAU | Yes | SUMD | Secondary | Delusion awareness increased at post-treatment and at follow-up at a significantly larger effect size in MCT than in controls. |
| Briki et al. (2014) | E = 35 C = 33 T = 68 |
E = 25 C = 25 T = 50 |
E = 28.6 C = 24.2 T = 26.5 |
n/a | n/a | n/a | SSD, DSM-IV | G-MCT | SR | Yes | SUMD | Primary | Awareness of, and attribution of, hallucinations affected by group MCT at a non-significant level. |
| Kuokkanen et al. (2014) | E = 10 C = 10 T = 20 |
E = 10 C = 10 T = 20 |
E = 0 C = 0 T = 0 |
6 months | E = 8 C = 10 T = 18 |
E = 20.0 C = 0 T = 10.0 |
Schizophr ICD-10 |
G-MCT | TAU | Yes | PANSS | Secondary | No significant pre-post-treatment between-group differences in clinical insight. |
| Lam et al. (2015) | E = 38 C = 39 T = 77 |
E = 36 C = 38 T = 74 |
E = 5 C = 2.5 T = 3.8 |
n/a | n/a | n/a | SSD, DSM-IV | G-MCT | TAU | No | BCIS | Primary | Increased SR and CI |
| Andreou et al. (2017) | E = 46 C = 46 T = 92 |
E = 44 C = 35 T = 79 |
E = 4.3 C = 23.9 T = 14.1 |
6 months | E = 37 C = 23 T = 60 |
E = 19.6 C = 50.0 T = 34.8 |
SSD, DSM-IV | I-MCT | CogPack | Yes | BCIS | Secondary | Increased SR post-treatment and at follow-up |
| Ochoa et al. (2017) | E = 65 C = 57 T = 122 |
E = 48 C = 41 T = 89 |
E = 26.1 C = 28.1 T = 27.0 |
6 months | E = 41 C = 40 T = 81 |
E = 36.9 C = 29.8 T = 33.6 |
SSD, DSM-IV-TR, <5 years | G-MCT | PSE | Yes | BCIS | Secondary | Decreased SC post-treatment, not at follow-up. Increased CI post-treatment and at follow-up |
| Balzan et al. (2019) | E = 27 C = 27 T = 54 |
E = 27 C = 27 T = 54 |
E = 0 C = 0 T = 0 |
6 months | E = 26 C = 26 T = 52 |
E = 3.8 C = 3.8 T = 3.8 |
SSD, MINI | I-MCT | CR | No | SAI BCIS |
Secondary | Increased clinical insight in MCT at post-treatment Increased clinical insight in controls at post-treatment, with decrease at follow-up. Decreased SC in MCT at post-treatment. Decreased SC in controls at follow-up. |
| van Oosterhout et al. (2014) | E = 75 C = 79 T = 154 |
E = 58 C = 70 T = 128 |
E = 22.7 C = 11.4 T = 16.9 |
6 months | E = 51 C = 60 T = 111 |
E = 32.0 C = 24.0 T = 27.9 |
Schizo DSM-IV |
G-MCT | TAU | Yes | BCIS | Secondary | No between-group differences in cognitive insight |
| Ahuir et al. (2018) | E = 14 C = 14 T = 28 |
n/a | n/a | n/a | n/a | n/a | Psychotic disorder, DSM-IV <3 years of duration | G-MCT | PSE | Cross-over | BCIS | Secondary | No between-group differences in cognitive insight |
| Gawęda et al. (2015) | E = 26 C = 24 T = 50 |
E = 23 C = 21 T = 44 |
E = 11.5 C = 12.5 T = 12.0 |
n/a | n/a | n/a | Schizophr ICD-10 |
G-MCT | TAU | Yes | Kokozska | Secondary | Increase in illness insight after MCT þ TAU as compared to the TAU condition |
| Vohs et al. (2018) | E = 10 C = 10 T = 20 |
E = 8 C = 10 T = 18 |
E = 20.0 C = 0 T = 10.0 |
n/a | n/a | n/a | SSD, DSM-IV | MERIT | TAU | Yes | PANSS SUMD |
Improved insight at post-treatment | |
| de Jong et al. (2019) | E = 35 C = 35 T = 70 |
E = 24 C = 26 T = 50 |
E = 31.4 C = 25.7 T = 28.6 |
6 months | E = 18 C = 23 T = 41 |
E = 48.6 C = 34.3 T = 41.4 |
SSD, DSM-IV-TR | MERIT | TAU | No | BCIS | No improvement in cognitive insight. |
Ni, initial sample size; NPT, sample size at post-treatment; PT, post-treatment; FU, follow-up; SSD, schizophrenia spectrum disorder; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, fourth edition; DMS-IV-TR, Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revised; E, experimental; C, Control; G-MCT, Group metacognitive training; I-MCT, individual metacognitive training; TAU, treatment as usual; BCIS, Beck Cognitive Insight Scale (Beck et al., 2004); SR, Self-Reflectiveness; SC, self-certainty; CI, Composite Index; MINI, Mini International Neuropsychiatric Interview (Sheehan et al., 1998); SAI, Schedule for Assessment of Insight (Kemp and David, 1997); PANSS, The Positive and Negative Syndrome Scale (Kay et al., 1987); SUMD, Scale to assess Unawareness of Mental Disorder (Amador et al., 1993); MERIT, Metacognitive Reflection and Insight Therapy; ICD −10, 10th revision of the International Statistical Classification of Diseases (ICD).
Ten studies used MCT in samples of patients with SSD (total N = 717 participants). These were divided into six articles on cognitive insight, which were meta-analysed, and five articles with non-meta-analysable data on clinical insight, including one MCT trial reporting clinical and cognitive insight results (Balzan et al., 2019). Owing to the use of different insight assessment scales (in some studies insight was measured in a unidimensional manner, while in others multidimensional scales were used) and the lack of consistent quantitative data needed, a meta-analysis of clinical insight RCTs was not feasible. As an alternative, we conducted a narrative review of the findings from the selected studies.
MCT and cognitive insight
Six studies reported on the effects of MCT on cognitive insight, that is, BCIS scores (Ahuir et al., 2018; Andreou et al., 2017; Balzan et al., 2019; Lam et al., 2015; Ochoa et al., 2017; van Oosterhout et al., 2014). Full details are provided in Table 1. Sample sizes ranged from n = 28 (Ahuir et al., 2018) to n = 154 (van Oosterhout et al., 2014), totalling N = 443 participants. Details of the meta-analytic results of these six studies, including heterogeneity and publication bias, are provided in Table TS1 of the online Supplementary Material. A visual inspection of the funnel plots (online supplementary Figures FS1, FS2, FS3, FS4, FS5, FS6, FS7, FS8 and FS9) revealed some asymmetry so we adjusted for potential missing studies, although this did not alter the results below.
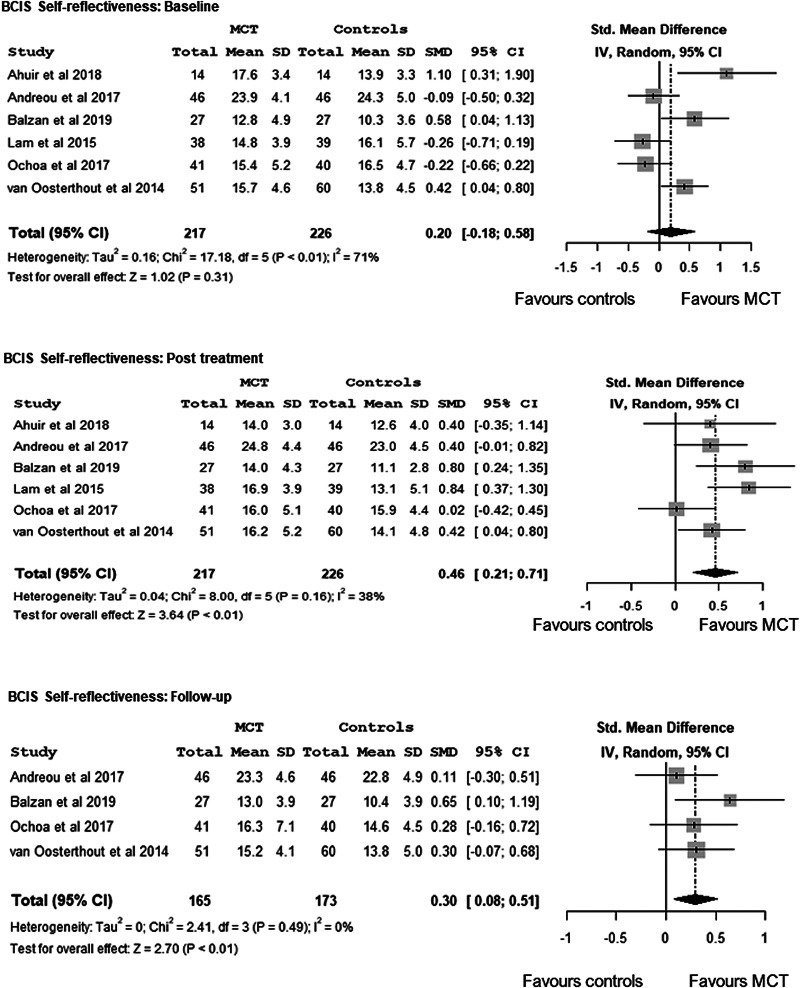
The overall effect on self-reflectiveness (higher self-reflectiveness score, greater cognitive insight), which was not observed at baseline, d = 0.20, 95% CI −0.18 to 0.58, Z = 1.02, p = 0.31, favoured MCT compared with controls both at post-treatment, d = 0.46, 95% CI 0.21–0.71, Z = 3.64, p < 0.01, and, at a lesser level, at follow-up, d = 0.30, 95% CI 0.08–0.51, Z = 2.70, p < 0.01. There was no evidence of heterogeneity at post-treatment, I2 = 38%, p = 0.16, or at follow-up, I2 = 0%, p = 0.49 (Fig. 2).
Fig. 2.
Meta-analysis of the effects of Metacognitive Training on cognitive insight - BCIS-Self-Reflectiveness -: at baseline, at post-treatment and at a follow-up.
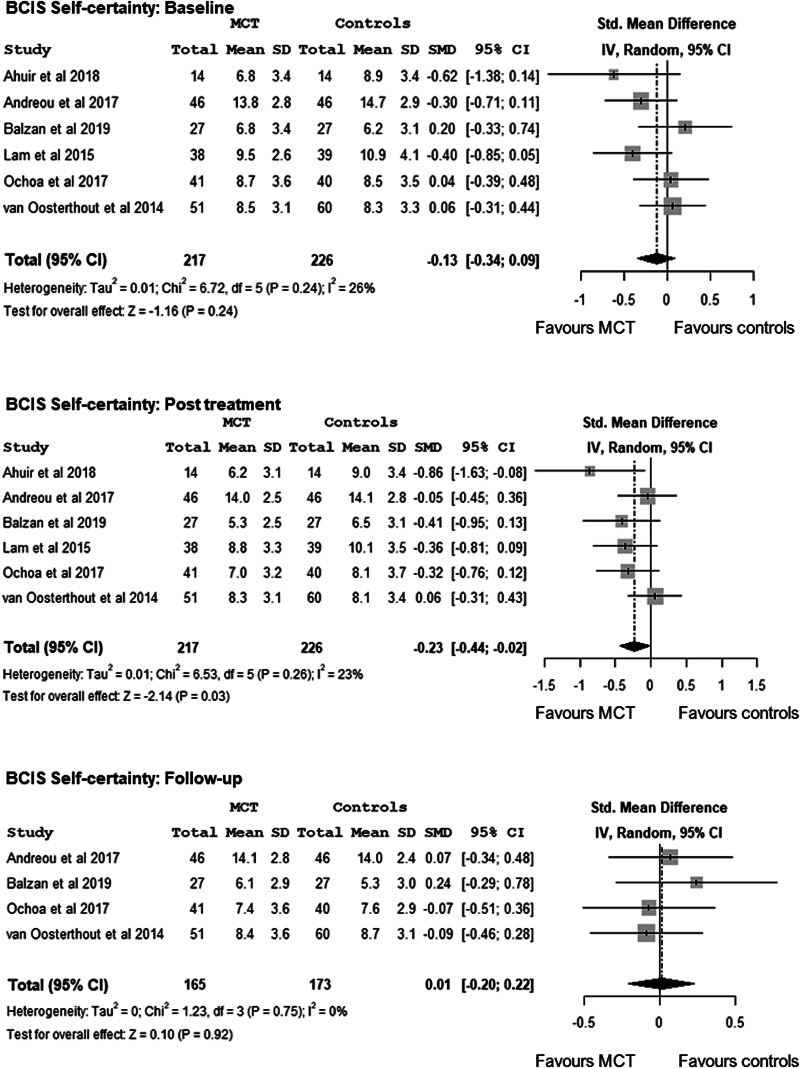
In terms of self-certainty (lower self-certainty score indicating greater cognitive insight), while baseline between-group differences were non-significant, d = −0.13, 95% CI −0.34 to 0.09, Z = −1.16, p = 0.24, meta-analysis showed MCT to be superior to the control condition at post-treatment at a significant small effect size, d = −0.23, 95% CI −0.44 to −0.02, Z = −2.14, p = 0.03, but not at follow-up, d = 0.01, 95% CI −0.20 to 0.22, Z = 0.10, p = 0.92. No evidence of heterogeneity was found at post-treatment, I2 = 23%, p = 0.26 or at follow-up, I2 = 0%, p = 0.75 (Fig. 3).
Fig. 3.
Meta-analysis of the effects of Metacognitive Training on cognitive insight - BCIS-Self-Certainty -: at baseline, at post-treatment and at follow-up.
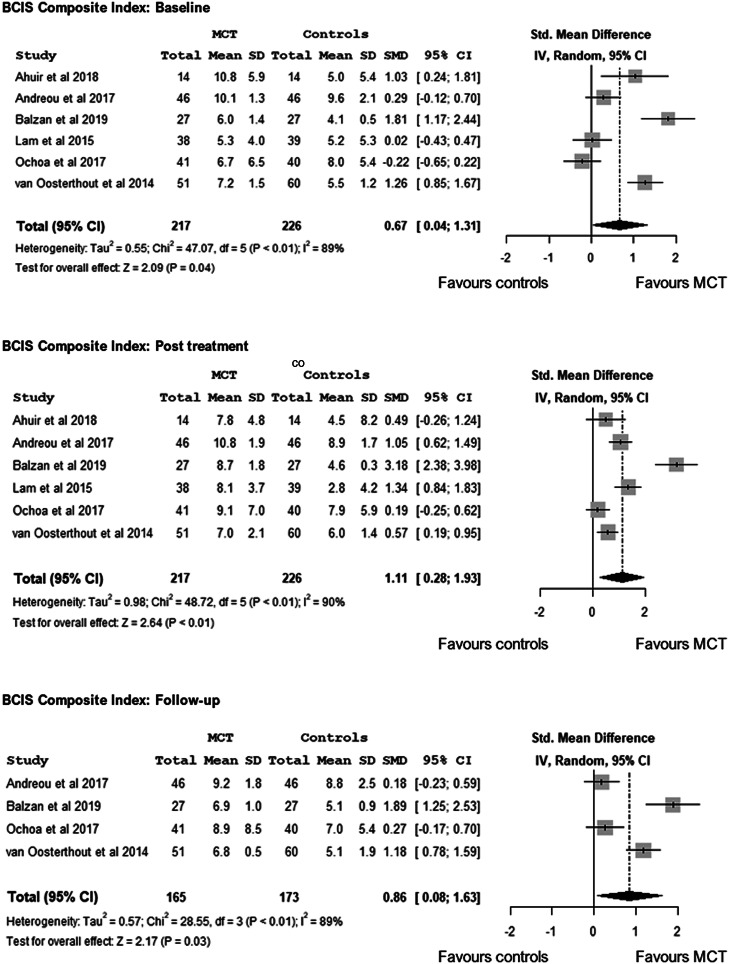
Although only three studies reported on Composite Index (higher Composite Index score, greater insight) (Ahuir et al., 2018; Lam et al., 2015; Ochoa et al., 2017), based on the self-reflectiveness and self-certainty scores, we calculated the Composite Index for the remaining three studies (Andreou et al., 2017; Balzan et al., 2019; van Oosterhout et al., 2014). There were baseline differences between treatment-arms favouring MCT v. controls, d = 0.67, 95% CI 0.04–1.31, Z = 2.09, p = 0.04. Post-treatment meta-analysis revealed a large effect, d = 1.11, 95% CI 0.28–1.93, Z = 2.64, p < 0.01, and evidence of heterogeneity, I2 = 90.0%, p < 0.01. Based on four follow-up trials (Andreou et al., 2017; Balzan et al., 2019; Ochoa et al., 2017; van Oosterhout et al., 2014), the meta-analysis favoured MCT over controls, d = 0.86, 95% CI 0.08–1.63, Z = 2.17, p = 0.03, although we found evidence of heterogeneity, I2 = 89.0%, p < 0.01 (Fig. 4).
Fig. 4.
Meta-analysis of the effects of Metacognitive Training on cognitive insight - BCISComposite Index -: at baseline, at post-treatment and at follow-up.
MCT and clinical insight
Five RCTs (N = 244) reported on the effects of MCT on clinical insight (Balzan et al., 2019; Briki et al., 2014; Favrod et al., 2014; Gawęda et al., 2015; Kuokkanen et al., 2014), including one RCT on both cognitive and clinical insight (Balzan et al., 2019). Out of these five trials, four of them favoured MCT.
First, from a multidimensional approach to insight measured with the SUMD, a 6-month follow-up RCT from France found that group MCT [compared with treatment as usual (TAU)] was effective in helping patients gain insight. Specifically, awareness of delusions improved in the MCT group (compared with controls) both at post-treatment and at 6-month follow-up with a significant medium-large effect size, d = 0.51 and d = 0.56, respectively, although differences in the attribution of delusions were non-significant (Favrod et al., 2014). In line with this, the results from a trial with a sample of n = 68 SSD patients (Briki et al., 2014) favoured group MCT compared to a control condition of supportive therapy in terms of awareness of hallucinations with small effect size, F(1.46,3.75), p = 0.058, r = −0.25, although this was not replicated by other SUMD scores. A trial in which insight was assessed unidimensionally with the Kokozska et al.'s insight questionnaire (Kokoszka et al., 2008) in n = 52 SSD outpatients revealed a large effect size increase in insight, d = 0.98, in the MCT group compared with TAU (Gawęda et al., 2015).
More recently, individual MCT was compared with an active control intervention, namely cognitive remediation, in a cohort of n = 56 schizophrenia patients, who were followed-up over 6 months (Balzan et al., 2019). Total insight scores, which were measured with the SAI, increased significantly from baseline to post-treatment in both treatment arms, with a larger effect size for MCT, d = 0.70, compared with controls, d = 0.29. From post-treatment to follow-up, total insight scores decreased in both groups, although this was only significant in the control group. The Group × Time interaction for total insight scores based on the ANOVA model, although statistically significant, p = 0.009, yielded a small effect size, ŋ2 = 0.07 (Balzan et al., 2019).
However, a 6-month follow-up trial testing group MCT against TAU in n = 20 forensic patients with schizophrenia failed to find between-group differences in terms of clinical insight gain evaluated with the single PANSS item (Kuokkanen et al., 2014).
Metacognitive reflection and insight therapy
The first MERIT trial (Vohs et al., 2018) recruited n = 20 early psychosis patients who were randomized to either MERIT or TAU. Insight was assessed with the SUMD before and after treatment. There were between-group differences in clinical insight improvement favouring MERIT at a medium effect size, F(1,16) = 11.5, p < 0.001, pη2 = 0.4. A larger 6-month follow-up trial (n = 70), however, failed to replicate this positive effect of MERIT on cognitive insight compared with TAU (de Jong et al., 2019).
Discussion
Main findings
We carried out a systematic review and meta-analysis of RCTs testing the effects of two metacognitive interventions designed for patients with SSD, namely MCT and MERIT, on insight changes, from which two main conclusions can be drawn. First, in line with our first hypothesis, MCT was found to improve cognitive insight, particularly self-reflectiveness, which was also supported by meta-analytic results, and two clinical insight dimensions, such as illness awareness and awareness of some psychotic phenomena, including delusions and hallucinations. However, somewhat in contrast to our expectations (hypothesis i), this was not replicated by the two MERIT trials. Second, our results showed that the effects of MCT and MERIT on insight were larger at post-treatment than at follow-up, which conflicted our hypothesis ii. We also noted relevant between-study methodological differences, which did not permit us to apply meta-analytic techniques to the clinical insight trials.
Metacognitive interventions and cognitive insight
Cognitive insight measured by a 15-item self-rated scale (BCIS) has two factors, namely self-reflectiveness and self-certainty (Beck et al., 2004). Patients with psychotic disorders were reported to have lower self-reflectiveness and higher self-confidence than the general population (Beck et al., 2004). Hence, interventions aim to increase self-reflectiveness and to decrease self-certainty. In this regard, all the selected trials reporting self-reflectiveness data found MCT to be superior to a control condition, according to our meta-analysis. Hence, it seems that MCT does improve patient's ability to reflect on his/her own thoughts and to correct interpretations based on contradictory evidence and/or external feedback, particularly shortly after treatment. Longer-term trials are needed to establish whether this cognitive insight improvement is maintained over time. As alluded to above, SSD patients have a tendency to overconfidence in their beliefs, which is known as self-certainty. Our meta-analysis revealed MCT to reduce patients' self-certainty after treatment, while this effect was not observed at follow-up. In keeping with this, a reanalysis (Köther et al., 2012) of data from an included RCT (Moritz et al., 2013) revealed MCT (when compared with cognitive remediation-CogPack®) to reduce the amount of overconfidence ratings (which ranged from 1, i.e. ‘100% sure’, to 4, i.e. ‘100% uncertain’) of mental state perceptions assessed with the Reading the Mind in the Eyes test (Baron-Cohen, Wheelwright, Hill, Raste, & Plumb, 2001). Consistent with this, the three selected trials using an overall measure of cognitive insight, the BCIS Composite Index score, favoured MCT over TAU (Lam et al., 2015) or PSE (Ahuir et al., 2018; Ochoa et al., 2017).
Only one trial reported on both cognitive and clinical insight (Balzan et al., 2019), which linked MCT-related cognitive insight improvement with better clinical insight. Of note, controls received an active intervention, namely cognitive remediation. Hence, this trial, although awaiting replication studies, showed MCT to be superior to cognitive remediation in increasing cognitive and clinical insight levels.
In summary, our meta-analytic results on cognitive insight appear to provide sufficient evidence to recommend MCT as a cognitive insight improving intervention for patients with SSD, particularly in terms of increasing self-reflectiveness, while we could not observe such a relevant effect on self-certainty, particularly in the long-term. Two non-mutually exclusive explanations may have contributed to this finding. First, while MCT does seem to enhance patient's ability to reflect on his/her own thoughts, particularly in terms of receiving and accepting external feedback, this intervention may fail to make patients become less (over)-confident in their own conclusions. In other words, MCT may be more helpful in improving cognitive insight based on external feedback (i.e. others-based cognitive insight), while confidence in one's thoughts, which is, of course, a more internal process, may be less prone to such an intervention (i.e. oneself-based cognitive insight). Second, it could be argued that the impact of MCT on self-certainty, which was supported by a significant small effect size at post-treatment but not at follow-up, may require maintenance MCT sessions. In this regard, a MCT-based smartphone application has recently become available (https://clinical-neuropsychology.de/app_en), which showed promising results in terms of feasibility and efficacy for depressive symptoms (Lüdtke, Pult, Schröder, Moritz, & Bücker, 2018). In addition, the extent to which this cognitive insight improvement results in better clinical insight and outcomes in the longer-term, which is what really matters since SSD tend to be chronic, remains to be established.
Our results, however, went against our second hypothesis, that is, we failed to find larger effects of MCT on insight in the longer-term than immediately after treatment. This said, the RCT behind this postulation (Moritz et al., 2014b) revealed the so-called ‘sleeper’ effects of MCT on delusions at 3 years, while the longest follow-up period of the trials included in our review was 6 months. Hence, sustained effects of MCT on insight in the (very) long-term cannot be ruled out despite our negative results. On the other hand, the only RCT testing the effect of MERIT on cognitive insight (de Jong et al., 2019) failed to find this in comparison to TAU, which warrants further investigation. As discussed further below, MERIT seems to improve patient's ability to make narrative judgements about concepts such as mental illness and recovery, that is, MERIT may be more helpful in tackling clinical insight rather than cognitive insight.
Metacognitive interventions and clinical insight
We identified five MCT trials on clinical insight (Balzan et al., 2019; Briki et al., 2014; Favrod et al., 2014; Gawęda et al., 2015; Kuokkanen et al., 2014), four of which (Balzan et al., 2019; Briki et al., 2014; Favrod et al., 2014; Gawęda et al., 2015) favoured MCT. Of note, three of these trials (Balzan et al., 2019; Briki et al., 2014; Favrod et al., 2014) measured insight with multidimensional scales, consistently with the multidimensional model of insight (Amador & David, 2004; David, 1990; Lysaker & Klion, 2018). MCT may thus affect clinical insight dimensions differently. For instance, one trial found MCT to improve illness awareness in comparison to cognitive remediation after treatment, although no significant between-group differences were observed at 6-month follow-up (Balzan et al., 2019). This raises issues about the extent to which the gain in awareness of having an illness was an actual response to MCT or the consequence of psychotic symptoms remission. Also, this trial (Balzan et al., 2019) used individual MCT. Hence, participants, who consented to an intensive face-to-face intervention, may have had higher baseline insight levels than those who refused to take part in the trial, thus introducing a selection bias. Indeed, the two trials testing individual MCT (Andreou et al., 2017; Balzan et al., 2019) reported much higher BCIS scores than the other studies, all of which used group MCT. Nevertheless, future trials should properly investigate differences in outcomes, including insight, between both MCT approaches.
The other two trials using a multidimensional insight scale, such as the SUMD, reported MCT to increase insight into psychotic symptoms, such as delusions (Favrod et al., 2014) and hallucinations (Briki et al., 2014). Also, this positive impact of MCT on clinical insight was replicated by another trial (Gawęda et al., 2015) in terms of illness awareness gain, although MCT was compared with TAU and outcomes were only measured after treatment by means of a less widely used insight scale (Kokoszka et al., 2008). On the other hand, one small (n = 20) trial (Kuokkanen et al., 2014) failed to replicate this, which may have been due to insufficient statistical power and/or measuring insight in a unidimensional manner through the PANSS item. Another potentially unpowered (n = 20) RCT failed to show that MERIT improved clinical insight (Vohs et al., 2018). However, given the lack of follow-up data potential longer-term benefits from MERIT in terms of clinical insight gain cannot be ruled out.
In summary, four out of five trials found MCT to positively affect clinical insight in comparison to TAU (Favrod et al., 2014; Gawęda et al., 2015) or some control interventions, such as cognitive remediation (Balzan et al., 2019) or supportive therapy (Briki et al., 2014). However, at 6-month follow-up, only one trial reported improvements in delusion awareness as a result of receiving MCT (Favrod et al., 2014). These intensive metacognitive interventions, particularly individual MCT (Balzan et al., 2019) and MERIT (Vohs et al., 2018), appear to make patients gain awareness of mental illness and symptoms. However, further trials are warranted to establish whether delivering these interventions as maintenance treatment may contribute to sustaining this insight improvement in the long-term.
Overall drop-out rates were very low, ranging from 0% (Balzan et al., 2019; Kuokkanen et al., 2014) to 27% (Ochoa et al., 2017) at post-treatment and from 1% (Kuokkanen et al., 2014) to 41.4% (de Jong et al., 2019) at 6-month follow-up. This may reflect the high levels of participants' satisfaction with MCT, in line with previous studies [e.g. (Moritz et al., 2014b)], including a meta-analysis (Eichner & Berna, 2016). In addition, the high attendance rates may be the result of a therapeutic effect of MCT on attendees. However, a potential selection bias cannot be ruled out since participants tend to be more compliant than those who refuse to enrol research studies, particularly RCTs.
Other non-included metacognitive interventions
As shown in Fig. 1, we identified n = 17 studies which, although reporting on metacognition-based interventions and insight, did not meet our selection criteria (Aghotor et al., 2010; Balzan et al., 2014; Buonocore et al., 2015; Erawati et al., 2014; Favrod et al., 2011; Kumar et al., 2015; Moritz et al., 2011a, 2011b, 2013, 2014b; 2018; Naughton et al., 2012; Pijnenborg et al., 2019; Rocha & Queirós, 2013; Ross et al., 2011; So et al., 2015; Ussorio et al., 2016). Overall, these trials support the use of metacognitive interventions for enhancing insight. Of note, a metacognition-based intervention called REFLEX was showed to improve insight-related aspects such as internalized stigma (Pijnenborg et al., 2019).
Limitations
We searched three major databases, such as PubMed, PsycInfo and EMBASE, and we retrieved n = 8 papers from the grey literature. However, the search was limited to English language papers and the selection criteria may have been too restrictive, particularly regarding insight scales. Hence, this review may not include all the metacognitive interventions-based trials reporting insight data, especially those in the very grey literature outside PubMed, PsycInfo and EMBASE. Also, the clinical insight studies' results could not be meta-analysed for reasons given above. In addition, it should be noted that the MCT modules on self-esteem and stigma were not available in the original manual of MCT, although the selected trials did not specify this. As a result, we could not investigate whether these two additional modules may have a larger effect on insight compared with the original MCT manual. With regard to the cognitive insight studies, two issues need to be considered. First, the BCIS is a self-report, hence ratings may have been affected by the degree of cooperation and motivation, which tends to be low in subjects with limited insight; and some caution is therefore needed when interpreting BCIS results. Second, cognitive insight was a secondary outcome in most of the included studies, which may also explain why the composite index was only reported in three selected trials. Finally, this was a systematic review and meta-analysis of RCTs, most of which were part of larger research projects, which were not focused on insight. As a result, the comprehensiveness and duration of the studies assessments may have contributed to increasing insight themselves, in addition to the potential selection bias due to being a participant in a RCT, that is, giving consent to such a lengthy protocol, which included two or three face-to-face interviews.
Clinical relevance and implications for future research
This systematic review therefore adds to the field, namely treatments for lack of insight in SSD, which is of major clinical relevance given the association of insight with outcomes. In particular, one previous systematic review (Henry & Ghaemi, 2004) and a meta-analysis (Pijnenborg et al., 2013) reported small effects of previous treatments for insight in psychosis, although metacognitive interventions were unavailable at that time. Hence, our systematic review and meta-analysis would provide support for delivering metacognitive interventions, particularly MCT, in clinical settings managing psychosis patients to improve insight.
Future trials should include clinical and social outcomes measures other than psychotic symptoms, such as readmissions, suicidal behaviour and psychosocial functioning, including self-appraisal of quality of life. Larger sample sizes and longer follow-up periods are required to examine rare outcomes. Multidimensional insight scales should be used since the impact of metacognitive interventions on individual insight dimensions may differ, which may also change over time. Future trials should also compare individual with group interventions, including cost-effectiveness investigations. With regard to the control group, instead of TAU, in which case between-group differences in insight changes may be attributed to the effect of attending a weekly group rather than to the metacognitive intervention, controls should receive an active intervention such as PSE (Ochoa et al., 2017) or supportive therapy (Briki et al., 2014). Indeed, this is the question that clinicians intend to answer, i.e., ‘Which intervention should I put my patient on (for improving insight)?’, while no intervention (i.e. TAU) does not tend to be considered an option. In line with this, only five out of the twelve selected trials used an active control intervention (see Table 1 for details), which may limit, to some degree, the apparent benefits from MCT and MERIT to enhance insight.
Acknowledgements
We are very grateful to our colleagues from the Department of Psychiatry of Hospital Universitario Fundación Jiménez Díaz, who are too numerous to mention by name, for their helpful comments on earlier versions of this manuscript. J.D.L.M. acknowledges funding support from the Universidad Autónoma de Madrid and European Union-European Commission via the 2019–2021 Intertalentum Project & Marie Skłodowska-Curie Actions Grant (GA 713366). O.A. is further funded by the National Institute for Health Research (NIHR) (NIHR Post-Doctoral Fellowship - PDF-2018-11-ST2-020). The views expressed in this publication are those of the authors and not necessarily those of the Spanish National Health Service, the United Kingdom National Health Service (NHS), the National Institute for Health Research or the Department of Health and Social Care.
Conflict of interest
J.D.L.M. has lectured for Janssen and Otsuka Pharmaceuticals and A.S.D. has received honoraria from Janssen and Roche Pharmaceuticals. The other authors have no conflict of interest to declare.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291720003384.
click here to view supplementary material
References
- Aghotor, J., Pfueller, U., Moritz, S., Weisbrod, M., & Roesch-Ely, D. (2010). Metacognitive training for patients with schizophrenia (MCT): Feasibility and preliminary evidence for its efficacy. Journal of Behavior Therapy and Experimental Psychiatry, 41(3), 207–211. doi: 10.1016/j.jbtep.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Ahuir, M., Cabezas, Á, Miñano, M. J., Algora, M. J., Estrada, F., Solé, M., … Labad, J. (2018). Improvement in cognitive biases after group psychoeducation and metacognitive training in recent-onset psychosis: A randomized crossover clinical trial. Psychiatry Research, 270, 720–723. doi: 10.1016/j.psychres.2018.10.066. [DOI] [PubMed] [Google Scholar]
- Amador, X.F., & David, A. S. (Eds.). (2004). Insight and psychosis (2nd ed.). New York: Oxford University Press. [Google Scholar]
- Amador, X. F., Flaum, M., Andreasen, N. C., Strauss, D. H., Yale, S. A., Clark, S. C., & Gorman, J. M. (1994). Awareness of illness in schizophrenia and schizoaffective and mood disorders. Archives of General Psychiatry, 51(10), 826–836. doi: 10.1001/archpsyc.1994.03950100074007. [DOI] [PubMed] [Google Scholar]
- Amador, X. F., Strauss, D. H., Yale, S. A., Flaum, M. M., Endicott, J., & Gorman, J. M. (1993). Assessment of insight in psychosis. The American Journal of Psychiatry, 150(6), 873–879. doi: 10.1176/ajp.150.6.873. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (Eds.). (2000). Diagnostic and statistical manual of mental disorders: DSM-IV-TR (4th ed., text revision). Washington, DC: Author. [Google Scholar]
- Andreou, C., Wittekind, C. E., Fieker, M., Heitz, U., Veckenstedt, R., Bohn, F., & Moritz, S. (2017). Individualized metacognitive therapy for delusions: A randomized controlled rater-blind study. Journal of Behavior Therapy and Experimental Psychiatry, 56, 144–151. doi: 10.1016/j.jbtep.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Balzan, R. P., Delfabbro, P. H., Galletly, C. A., & Woodward, T. S. (2014). Metacognitive training for patients with schizophrenia: Preliminary evidence for a targeted, single-module programme. The Australian and New Zealand Journal of Psychiatry, 48(12), 1126–1136. doi: 10.1177/0004867413508451. [DOI] [PubMed] [Google Scholar]
- Balzan, R. P., Mattiske, J. K., Delfabbro, P., Liu, D., & Galletly, C. (2019). Individualized metacognitive training (MCT + ) reduces delusional symptoms in psychosis: A randomized clinical trial. Schizophrenia Bulletin, 45(1), 27–36. doi: 10.1093/schbul/sby152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen, S., Wheelwright, S., Hill, J., Raste, Y., & Plumb, I. (2001). The “reading the mind in the eyes” test revised version: A study with normal adults, and adults with asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry, 42, 241–251. doi: 10.1111/1469-7610.00715. [DOI] [PubMed] [Google Scholar]
- Beck, A. T., Baruch, E., Balter, J. M., Steer, R. A., & Warman, D. M. (2004). A new instrument for measuring insight: The Beck Cognitive Insight Scale. Schizophrenia Research, 68(2–3), 319–329. doi: 10.1016/S0920-9964(03)00189-0. [DOI] [PubMed] [Google Scholar]
- Birchwood, M., Smith, J., Drury, V., Healy, J., Macmillan, F., & Slade, M. (1994). A self-report Insight Scale for psychosis: Reliability, validity and sensitivity to change. Acta Psychiatrica Scandinavica, 89(1), 62–67. doi: 10.1111/j.1600-0447.1994.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Borenstein, M., Hedges, L. V., Higgins, J. P. T., & Rothstein, H. R. (2010). A basic introduction to fixed-effect and random-effects models for meta-analysis. Research Synthesis Methods, 1(2), 97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- Briki, M., Monnin, J., Haffen, E., Sechter, D., Favrod, J., Netillard, C., … Vandel, P. (2014). Metacognitive training for schizophrenia: A multicentre randomised controlled trial. Schizophrenia Research, 157(1–3), 99–106. doi: 10.1016/j.schres.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Buonocore, M., Bosia, M., Riccaboni, R., Bechi, M., Spangaro, M., Piantanida, M., … Cavallaro, R. (2015). Combined neurocognitive and metacognitive rehabilitation in schizophrenia: Effects on bias against disconfirmatory evidence. European Psychiatry, 30(5), 615–621. doi: 10.1016/j.eurpsy.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Carpenter, W. T., Strauss, J. S., & Bartko, J. J. (1973). Flexible system for the diagnosis of schizophrenia: Report from the WHO International Pilot Study of Schizophrenia. Science (New York, N.Y.), 182(4118), 1275–1278. doi: 10.1126/science.182.4118.1275. [DOI] [PubMed] [Google Scholar]
- David, A. S. (1990). Insight and psychosis. The British Journal of Psychiatry, 156, 798–808. doi: 10.1192/bjp.156.6.798. [DOI] [PubMed] [Google Scholar]
- David, A. S. (2019). Insight and psychosis: The next 30 years. The British Journal of Psychiatry, 18, 1–3. doi: 10.1192/bjp.2019.217. [DOI] [PubMed] [Google Scholar]
- de Jong, S., van Donkersgoed, R. J. M., Timmerman, M. E., Aan Het Rot, M., Wunderink, L., Arends, J., … Pijnenborg, G. H. M. (2019). Metacognitive reflection and insight therapy (MERIT) for patients with schizophrenia. Psychological Medicine, 49(2), 303–313. doi: 10.1017/S0033291718000855. [DOI] [PubMed] [Google Scholar]
- Dimaggio, G., & Lysaker, P. H. (2010). Metacognition and severe adult mental disorders: From research to treatment. London: Routledge. [Google Scholar]
- Duval, S., & Tweedie, R. (2000a). A nonparametric ‘Trim and Fill’ method of accounting for publication bias in meta-analysis. Journal of the American Statistical Association, 95(449), 89–98. doi: 10.1080/01621459.2000.10473905. [DOI] [Google Scholar]
- Duval, S., & Tweedie, R. (2000b). Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56(2), 455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Eichner, C., & Berna, F. (2016). Acceptance and efficacy of metacognitive training (MCT) on positive symptoms and delusions in patients with schizophrenia: A meta-analysis taking into account important moderators. Schizophrenia Bulletin, 42(4), 952–962. doi: 10.1093/schbul/sbv225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erawati, E., Keliat, B. A., Helena, N., & Hamid, A. (2014). The influence of metacognitive training on delusion severity and metacognitive ability in schizophrenia. Journal of Psychiatric and Mental Health Nursing, 21(9), 841–847. doi: 10.1111/jpm.12130. [DOI] [PubMed] [Google Scholar]
- Favrod, J., Maire, A., Bardy, S., Pernier, S., & Bonsack, C. (2011). Improving insight into delusions: A pilot study of metacognitive training for patients with schizophrenia. Journal of Advanced Nursing, 67(2), 401–407. doi: 10.1111/j.1365-2648.2010.05470.x. [DOI] [PubMed] [Google Scholar]
- Favrod, J., Rexhaj, S., Bardy, S., Ferrari, P., Hayoz, C., Moritz, S., … Bonsack, C. (2014). Sustained antipsychotic effect of metacognitive training in psychosis: A randomized-controlled study. European Psychiatry, 29(5), 275–281. doi: 10.1001/jamapsychiatry.2014.1038. [DOI] [PubMed] [Google Scholar]
- Fisher, P., & Wells, A. (2009). Metacognitive therapy. The CBT distinctive features series. Sussex, U.K: Routledge. [Google Scholar]
- Flavell, J. H. (1979). Metacognition and cognitive monitoring: A new area of cognitive-developmental inquiry. American Psychologist, 34(10), 906–911. doi: 10.1037/0003-066X.34.10.906. [DOI] [Google Scholar]
- Gawęda, Ł, Krężołek, M., Olbryś, J., Turska, A., & Kokoszka, A. (2015). Decreasing self-reported cognitive biases and increasing clinical insight through meta-cognitive training in patients with chronic schizophrenia. Journal of Behavior Therapy and Experimental Psychiatry, 48, 98–104. doi: 10.1016/j.jbtep.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Henry, C., & Ghaemi, S. N. (2004). Insight in psychosis: A systematic review of treatment interventions. Psychopathology, 37(4), 194–199. doi: 10.1159/000080131. [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. T., Thompson, S. G., Deeks, J. J., & Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorthøj, C., Stürup, A. E., McGrath, J. J., & Nordentoft, M. (2017). Years of potential life lost and life expectancy in schizophrenia: A systematic review and meta-analysis. The Lancet Psychiatry, 4(4), 295–301. doi: 10.1016/S2215-0366(17)30078-0. [DOI] [PubMed] [Google Scholar]
- Jiang, J., Zhang, L., Zhu, Z., Li, W., & Li, C. (2015). Metacognitive training for schizophrenia: A systematic review. Shanghai Archives of Psychiatry, 27(3), 149–157. doi: 10.11919/j.issn.1002-0829.215065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma, H. E., Gayer-Anderson, C., Lasalvia, A., Quattrone, D., Mulè, A., Szöke, A., ... European Network of National Schizophrenia Networks Studying Gene-Environment Interactions Work Package 2 (EU-GEI WP2) Group. (2018). Treated incidence of psychotic disorders in the multinational EU-GEI study. JAMA Psychiatry, 75(1), 36–46. doi: 10.1001/jamapsychiatry.2017.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay, S. R., Fiszbein, A., & Opler, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13(2), 261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kemp, R., & David, A.S. (1997). Insight and compliance In Blackwell B. (Ed.), Treatment compliance and the therapeutic alliance. The Netherlands: Harwood Academic Publishers. [Google Scholar]
- Kokoszka, A., Telichowska-Leśna, A., & Radzio, R. (2008). A questionnaire of insight into schizophrenia – ‘my thoughts and feelings’. Psychiatria Polska, 42(4), 491–502. Retrieved from: http://www.psychiatriapolska.pl/uploads/images/PP_4_2008/Kokoszka%20s491_Psychiatria%20Polska%204_2008.pdf. [PubMed] [Google Scholar]
- Köther, U., Veckenstedt, R., Vitzthum, F., Roesch-Ely, D., Pfueller, U., Scheu, F., & Moritz, S. (2012). “Don´t give me that look” – overconfidence in false mental state perception in schizophrenia. Psychiatry Research, 196(1), 1–8. doi: 10.1016/j.psychres.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Kumar, D., Rao, M. G., Raveendranathan, D., Venkatasubramanian, G., Varambally, S., & Gangadhar, B. N. (2015). Metacognitive training for delusion in treatment-resistant schizophrenia. Clinical Schizophrenia & Related Psychoses, 9(1), 40–43. doi: 10.3371/CSRP.KURA.031513. [DOI] [PubMed] [Google Scholar]
- Kuokkanen, R., Lappalainen, R., Repo-Tiihonen, E., & Tiihonen, J. (2014). Metacognitive group training for forensic and dangerous non-forensic patients with schizophrenia: A randomised controlled feasibility trial. Criminal Behaviour and Mental Health: CBMH, 24(5), 345–357. [DOI] [PubMed] [Google Scholar]
- Lam, K. C. K., Ho, C. P. S., Wa, J. C., Chan, S. M. Y., Yam, K. K. N., Yeung, O., … Balzan, R. P. (2015). Metacognitive training (MCT) for schizophrenia improves cognitive insight: A randomized controlled trial in a Chinese sample with schizophrenia spectrum disorders. Behaviour Research and Therapy, 64, 38–42. doi: 10.1016/j.brat.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Lincoln, T. M., Lüllmann, E., & Rief, W. (2007). Correlates and long-term consequences of poor insight in patients with schizophrenia. A systematic review. Schizophrenia Bulletin, 33(6), 1324–1342. doi: 10.1093/schbul/sbm002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsey, M. W., & Wilson, D. B. (2001). Practical meta-analysis. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Liu, Y.-C., Tang, C.-C., Hung, T.-T., Tsai, P.-C., & Lin, M.-F. (2018). The efficacy of metacognitive training for delusions in patients with schizophrenia: A meta-analysis of randomized controlled trials informs evidence-based practice. Worldviews on Evidence-Based Nursing, 15(2), 130–139. doi: 10.1111/wvn.12282. [DOI] [PubMed] [Google Scholar]
- Lüdtke, T., Pult, L. K., Schröder, J., Moritz, S., & Bücker, L. (2018). A randomized controlled trial on a smartphone self-help application (Be Good to Yourself) to reduce depressive symptoms. Psychiatry Research, 269, 753–762. doi: 10.1016/j.psychres.2018.08.113. [DOI] [PubMed] [Google Scholar]
- Lysaker, P. H., & Klion, R. E. (2018). Recovery, meaning-making, and severe mental illness: A comprehensive guide to metacognitive reflection and insight therapy (First edition). New York, NY: Routledge, Taylor & Francis Group. [Google Scholar]
- Lysaker, P. H., Pattison, M. L., Leonhardt, B. L., Phelps, S., & Vohs, J. L. (2018). Insight in schizophrenia spectrum disorders: Relationship with behavior, mood and perceived quality of life, underlying causes and emerging treatments. World Psychiatry, 17(1), 12–23. doi: 10.1002/wps.20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy, J. P., Freter, S., Everett, G., Geller, J. L., Appelbaum, P., Apperson, L. J., & Roth, L. (1989). Insight and the clinical outcome of schizophrenic patients. The Journal of Nervous and Mental Disease, 177(1), 48–51. doi: 10.1097/00005053-198901000-00008. [DOI] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, C., Lappin, J., Heslin, M., Donoghue, K., Lomas, B., Reininghaus, U., … Dazzan, P. (2014). Reappraising the long-term course and outcome of psychotic disorders: The AESOP-10 study. Psychological Medicine, 44(13), 2713–2726. doi: 10.1017/S0033291714000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz, S., Andreou, C., Schneider, B. C., Wittekind, C. E., Menon, M., Balzan, R. P., & Woodward, T. S. (2014a). Sowing the seeds of doubt: A narrative review on metacognitive training in schizophrenia. Clinical Psychology Review, 34(4), 358–366. doi: 10.1016/j.cpr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Moritz, S., Kerstan, A., Veckenstedt, R., Randjbar, S., Vitzthum, F., Schmidt, C., … Woodward, T. S. (2011a). Further evidence for the efficacy of a metacognitive group training in schizophrenia. Behaviour Research and Therapy, 49(3), 151–157. doi: 10.1016/j.brat.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Moritz, S., & Lysaker, P. H. (2018a). Metacognition – What did James H. Flavell really say and the implications for the conceptualization and design of metacognitive interventions. Schizophrenia Research, 201, 20–26. doi: 10.1016/j.schres.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Moritz, S., Mahlke, C. I., Westermann, S., Ruppelt, F., Lysaker, P. H., Bock, T., & Andreou, C. (2018b). Embracing psychosis: A cognitive insight intervention improves personal narratives and meaning-making in patients with schizophrenia. Schizophrenia Bulletin, 44(2), 307–316. doi: 10.1093/schbul/sbx072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz, S., Veckenstedt, R., Andreou, C., Bohn, F., Hottenrott, B., Leighton, L., … Roesch-Ely, D. (2014b). Sustained and “Sleeper” effects of group metacognitive training for schizophrenia: A randomized clinical trial. JAMA Psychiatry, 71(10), 1103. doi: 10.1001/jamapsychiatry.2014.1038. [DOI] [PubMed] [Google Scholar]
- Moritz, S., Veckenstedt, R., Bohn, F., Hottenrott, B., Scheu, F., Randjbar, S., … Roesch-Ely, D. (2013). Complementary group Metacognitive Training (MCT) reduces delusional ideation in schizophrenia. Schizophrenia Research, 151(1–3), 61–69. doi: 10.1016/j.schres.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Moritz, S., Veckenstedt, R., Randjbar, S., Vitzthum, F., & Woodward, T. S. (2011b). Antipsychotic treatment beyond antipsychotics: Metacognitive intervention for schizophrenia patients improves delusional symptoms. Psychological Medicine, 41(9), 1823–1832. [DOI] [PubMed] [Google Scholar]
- Moritz, S., & Woodward, T. S. (2007). Metacognitive training for schizophrenia patients (MCT): A pilot study on feasibility, treatment adherence, and subjective efficacy. German Journal of Psychiatry, 10(3), 69–78. Retrieved from: https://psycnet.apa.org/record/2008-12205-003. [Google Scholar]
- Morris, S. B., & DeShon, R. P. (2002). Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological Methods, 7(1), 105–125. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- Nair, A., Palmer, E. C., Aleman, A., & David, A. S. (2014). Relationship between cognition, clinical and cognitive insight in psychotic disorders: A review and meta-analysis. Schizophrenia Research, 152(1), 191–200. doi: 10.1016/j.schres.2013.11.033. [DOI] [PubMed] [Google Scholar]
- Naughton, M., Nulty, A., Abidin, Z., Davoren, M., O'Dwyer, S., & Kennedy, H. G. (2012). Effects of group metacognitive training (MCT) on mental capacity and functioning in patients with psychosis in a secure forensic psychiatric hospital: A prospective-cohort waiting list controlled study. BMC Research Notes, 5, 302. doi: 10.1186/1756-0500-5-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa, S., López-Carrilero, R., Barrigón, M. L., Pousa, E., Barajas, A., Lorente-Rovira, E., … Spanish Metacognition Study Group. (2017). Randomized control trial to assess the efficacy of metacognitive training compared with a psycho-educational group in people with a recent-onset psychosis. Psychological Medicine, 47(9), 1573–1584. doi: 10.1017/S0033291716003421. [DOI] [PubMed] [Google Scholar]
- Phan, K., Xie, A., Di Eusanio, M., & Yan, T. D. (2014). A meta-analysis of minimally invasive versus conventional sternotomy for aortic valve replacement. The Annals of Thoracic Surgery, 98(4), 1499–1511. doi: 10.1016/j.athoracsur.2014.05.060. [DOI] [PubMed] [Google Scholar]
- Philipp, R., Kriston, L., Lanio, J., Kühne, F., Härter, M., Moritz, S., & Meister, R. (2019). Effectiveness of metacognitive interventions for mental disorders in adults-A systematic review and meta-analysis (METACOG). Clinical Psychology & Psychotherapy, 26(2), 227–240. doi: 10.1002/cpp.2345. [DOI] [PubMed] [Google Scholar]
- Pijnenborg, G. H. M., de Vos, A. E., Timmerman, M. E., Van der Gaag, M., Sportel, B. E., Arends, J., … Aleman, A. (2019). Social cognitive group treatment for impaired insight in psychosis: A multicenter randomized controlled trial. Schizophrenia Research, 206, 362–369. doi: 10.1016/j.schres.2018.10.018. [DOI] [PubMed] [Google Scholar]
- Pijnenborg, G. H. M., van Donkersgoed, R. J. M., David, A. S., & Aleman, A. (2013). Changes in insight during treatment for psychotic disorders: A meta-analysis. Schizophrenia Research, 144(1–3), 109–117. doi: 10.1016/j.schres.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Rocha, N. B. F., & Queirós, C. (2013). Metacognitive and social cognition training (MSCT) in schizophrenia: A preliminary efficacy study. Schizophrenia Research, 150(1), 64–68. doi: 10.1016/j.schres.2013.07.057. [DOI] [PubMed] [Google Scholar]
- Ross, K., Freeman, D., Dunn, G., & Garety, P. (2011). A randomized experimental investigation of reasoning training for people with delusions. Schizophrenia Bulletin, 37(2), 324–333. doi: 10.1093/schbul/sbn165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan, D. V., Lecrubier, Y., Sheehan, K., Amorim, P., Janavs, J., Weiller, E., …Dunbar, G. C. (1998). The MINI International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview. Journal of Clinical Psychiatry, 59(20), 22–23. [PubMed] [Google Scholar]
- So, S. H.-W., Chan, A. P., Chong, C. S.-Y., Wong, M. H.-M., Lo, W. T.-L., Chung, D. W.-S., & Chan, S. S. (2015). Metacognitive training for delusions (MCTd): Effectiveness on data-gathering and belief flexibility in a Chinese sample. Frontiers in Psychology, 6, 730. doi: 10.3389/fpsyg.2015.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussorio, D., Giusti, L., Wittekind, C. E., Bianchini, V., Malavolta, M., Pollice, R., … Roncone, R. (2016). Metacognitive training for young subjects (MCT young version) in the early stages of psychosis: Is the duration of untreated psychosis a limiting factor? Psychology and Psychotherapy, 89(1), 50–65. doi: 10.1111/papt.12059. [DOI] [PubMed] [Google Scholar]
- Van Camp, L. S. C., Sabbe, B. G. C., & Oldenburg, J. F. E. (2017). Cognitive insight: A systematic review. Clinical Psychology Review, 55, 12–24. doi: 10.1016/j.cpr.2017.04.011. [DOI] [PubMed] [Google Scholar]
- van Oosterhout, B., Krabbendam, L., de Boer, K., Ferwerda, J., van der Helm, M., Stant, A. D., & van der Gaag, M. (2014). Metacognitive group training for schizophrenia spectrum patients with delusions: A randomized controlled trial. Psychological Medicine, 44(14), 3025–3035. doi: 10.1017/S0033291714000555. [DOI] [PubMed] [Google Scholar]
- van Oosterhout, B., Smit, F., Krabbendam, L., Castelein, S., Staring, A. B. P., & van der Gaag, M. (2016a). Metacognitive training for schizophrenia spectrum patients: A meta-analysis on outcome studies. Psychological Medicine, 46(1), 47–57. doi: 10.1017/S0033291715001105. [DOI] [PubMed] [Google Scholar]
- van Oosterhout, B., Smit, F., Krabbendam, L., Castelein, S., Staring, A. B. P., & van der Gaag, M. (2016b). Psychological Medicine, 46, 2003–2005. doi: 10.1017/S003329171600009X. [DOI] [PubMed] [Google Scholar]
- Vohs, J. L., Leonhardt, B. L., James, A. V., Francis, M. M., Breier, A., Mehdiyoun, N., … Lysaker, P. H. (2018). Metacognitive reflection and insight therapy for early psychosis: A preliminary study of a novel integrative psychotherapy. Schizophrenia Research, 195, 428–433. doi: 10.1016/j.schres.2017.10.041. [DOI] [PubMed] [Google Scholar]
- Wells, A., & Purdon, C. (1999). Metacognition and cognitive-behaviour therapy: A special issue. Clinical Psychology & Psychotherapy, 6(2), 71–72. doi: 10.1002/(SICI)1099-0879(199905)6:23.0.CO;2-G. [DOI] [Google Scholar]
- Wilson, D. B. (2010). Meta-analysis macros for SAS, SPSS, and Stata. Retrieved from: http://mason.gmu.edu/~dwilsonb/ma.html.
- World Health Organization. (Ed.). (1993). The ICD-10 classification of mental and behavioural disorders: Diagnostic criteria for research. Geneva: Author. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291720003384.
click here to view supplementary material