Key Points
Question
What are the associations between neighborhood poverty, child cognitive performance, and brain structure after accounting for household-level poverty?
Findings
This cross-sectional study of 11 875 children aged 9 and 10 years found an association between neighborhood poverty, prefrontal and hippocampal volume, and performance on cognitive tasks. These results remained even after controlling for individual household income.
Meaning
The findings of this study provide evidence that the broader neighborhood context uniquely contributes to prefrontal and hippocampal development and cognitive performance and should be considered in studies of early life poverty and adversity.
This cross-sectional study evaluates whether neighborhood poverty is associated with cognitive function and prefrontal and hippocampal brain structure in ways that are dissociable from household socioeconomic status.
Abstract
Importance
The association between poverty and unfavorable cognitive outcomes is robust, but most research has focused on individual household socioeconomic status (SES). There is increasing evidence that neighborhood context explains unique variance not accounted for by household SES.
Objective
To evaluate whether neighborhood poverty (NP) is associated with cognitive function and prefrontal and hippocampal brain structure in ways that are dissociable from household SES.
Design, Setting, and Participants
This cross-sectional study used a baseline sample of the ongoing longitudinal Adolescent Brain Cognitive Development (ABCD) Study. The ABCD Study will follow participants for assessments each year for 10 years. Data were collected at 21 US sites, mostly within urban and suburban areas, between September 2019 and October 2018. School-based recruitment was used to create a participant sample reflecting the US population. Data analysis was conducted from March to June 2019.
Main Outcomes and Measures
NP and household SES were included as factors potentially associated with National Institutes of Health Toolbox Cognitive Battery subtests and hippocampal and prefrontal (dorsolateral prefrontal cortex [DLPFC], dorsomedial PFC [DMPFC], superior frontal gyrus [SFG]) volumes. Independent variables were first considered individually and then together in mixed-effects models with age, sex, and intracranial volume as covariates. Structural equation modeling (SEM) was used to assess shared variance in NP to brain structure and cognitive task associations. The tested hypotheses were formulated after data collection.
Results
A total of 11 875 children aged 9 and 10 years (5678 [47.8%] girls) were analyzed. Greater NP was associated with lower scores across all cognitive domains (eg, total composite: β = −0.18; 95% CI, −0.21 to −0.15; P < .001) and with decreased brain volume in the DLPFC (eg, right DLPFC: β = −0.09; 95% CI, −0.12 to −0.07; P < .001), DMPFC (eg, right DMPC: β = −0.07; 95% CI, −0.09 to −0.05; P < .001), SFG (eg, right SFG: β = −0.05; 95% CI, −0.08 to −0.03; P < .001), and right hippocampus (β = −0.04; 95% CI, −0.06 to −0.01; P = .01), even when accounting for household income. Greater household income was associated with higher scores across all cognitive domains (eg, total composite: β = 0.30; 95% CI, 0.28 to 0.33; P < .001) and larger volume in all prefrontal and hippocampal brain regions (eg, right hippocampus: β = 0.04; 95% CI, 0.02 to 0.07; P < .001) even when accounting for NP. The SEM model was a good fit across all cognitive domains, with prefrontal regions being associated with NP relations to language (picture vocabulary: estimate [SE], –0.03 [0.01]; P < .001; oral reading: estimate [SE], –0.02 [0.01]; P < .001), episodic memory (picture sequence: estimate [SE], –0.02 [0.01]; P = .008), and working memory (dimensional card sort: estimate [SE], –0.02 [0.01]; P = .001; flanker inhibitory control: estimate [SE], –0.01 [0.01]; P = .01; list sorting: estimate [SE], –0.03 [0.01]; P < .001) and hippocampal regions being associated with NP associations with language (picture vocabulary: estimate [SE], –0.01 [0.004]; P < .001) and episodic memory (picture sequence: estimate [SE], –0.01 [0.004]; P < 0.001).
Conclusions and Relevance
In this study, NP accounted for unique variance in cognitive function and prefrontal and right hippocampal brain volume. These findings demonstrate the importance of including broader environmental influences when conceptualizing early life adversity.
Introduction
Early poverty has been consistently associated with cognitive function deficits and lower school and standardized test performance.1 Furthermore, there is evidence that developmental differences in brain maturation may mediate the association between socioeconomic status (SES) and cognitive function and educational outcomes. Much of this research has centered on household SES. Less is known about the unique association of broader neighborhood SES environments with cognitive outcomes and brain maturation in children after accounting for individual household SES. Current models indexing poverty and early life adversity do not commonly include neighborhood-level measures, despite growing evidence that consideration of the neighborhood context is important for gaining a comprehensive understanding of mechanisms associated with cognitive and educational outcomes in children. As such, the goal of the current study was to examine whether neighborhood poverty (NP) was associated with cognitive function and brain volume in regions thought to be critical for a range of cognitive functions, even after accounting for household SES.
Numerous studies have demonstrated that children from households with lower SES score significantly lower on memory, language, and cognitive control tasks,2 score lower on intelligence tests and tests that measure academic achievement,3 and are more likely to fail courses, drop out of school, and be put into special education classes.4 A potential pathway by which impoverished environments could influence such outcomes is via maturation of brain structures important for cognitive development. For example, the association between household poverty and reduced hippocampal volume in children has been a robust finding in the literature.5,6,7,8,9 There are high concentrations of glucocorticoid receptors in the hippocampus, a region that has been robustly implicated in episodic memory and memory consolidation,10,11,12 which makes this region vulnerable to chronic stress responses via the hypothalamic-pituitary-adrenal (HPA) axis.13,14 When stress is chronic, glucocorticoid release can be maladaptive, resulting in desensitization of receptors and damage to surrounding tissue.15 Thus, it is has been hypothesized that chronic stressors associated with household poverty may be contributing to disruptions in hippocampal development, although there are other possible pathways, such as emotional or material deprivation, disruptions in parent-child relationship, nutrition, and exposure to toxins, that may also contribute to this relation.16,17,18,19 Lower SES has also been implicated in impaired maturation of the prefrontal cortex, whose protracted development may make it especially vulnerable to chronically stressful environments.1,20,21 Chronic activation of the HPA axis might similarly affect tissue volume and region function via glucocorticoid receptors. Reduced prefrontal volume and activity have been found in individuals from lower SES households.22 Previous literature has demonstrated that reduced volume and activity in prefrontal regions, like the dorsolateral prefrontal cortex (DLPFC), dorsal medial PFC (DMPFC), and superior frontal gyrus (SFG), are associated with lower performance on tasks indexing cognitive function.23,24,25,26
Although household-level SES is clearly important in understanding child development, some research suggests that the neighborhood context may also be important, particularly in geographic locations where structural and/or explicit racism may limit neighborhoods available to individuals who belong to specific racial and minority groups regardless of their household SES (eg, redlining practices).27 In a longitudinal study,28 individuals with higher neighborhood disadvantage were at greater risk of coronary heart disease, controlling for individual SES and education. Accumulation of neighborhood disadvantage between the ages of 16 and 43 years was associated with increased allostatic load (ie, the accumulation of chronic endocrine responses to stressful life events) in adulthood, even after accounting for personal adverse living experiences.29 Furthermore, children living in areas with large amounts of local violence had lower vocabulary and reading scores on an IQ test, even after accounting for household SES.30 Similarly, children living in neighborhoods containing more academically educated professionals had higher academic achievement and higher scores on vocabulary and reading assessments. This association was not fully explained by household income and other individual family factors.31
Given the literature outlined above, the goals of the current study were to test the following hypotheses: (1) NP accounts for variance in cognitive performance and prefrontal and hippocampal volumes in children, even when accounting for household SES variables, and (2) NP associations with volumes of prefrontal and hippocampal regions share variance with NP associations with cognitive function in school age children, providing evidence for the plausibility of the hypothesis that brain volume mediates the association of NP with cognitive function.
Methods
Participants
The sample for this study consisted of baseline data from 11 875 children recruited as part of the Adolescent Brain Cognitive Development (ABCD) Study, with recruitment across 21 sites designed to mirror the demographic characteristics of the United States.32 Data were collected between September 2019 and October 2018, using school-based recruitment to create a sample reflecting the US population. Race and ethnicity are highly confounded with both household income and NP, as they are in the US population, reflecting ongoing structural racism. Thus, we did not include race and ethnicity as covariates in the analyses presented below. eAppendix 1 in the Supplement contains analyses showing that most key findings hold when including race/ethnicity as a covariate. Data from ABCD Release 2.0.1 were used for the current study. Informed written consent for child and parent was obtained from parents, and child participants separately completed a written assent. This work was reviewed and approved by the Washington University human participants committee. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Participants who had missing data on some variables were not removed from the sample data set, as multilevel models will allow estimation with missing cases as more waves of data are collected. The sample sizes used for each analysis appear in eAppendix 2 in the Supplement. Analyses using the GGally and finalfit packages indicated that missingness of key variables (household income and NP) did suggest some association with missingness of other demographic variables (eg, race/ethnicity, sex) but did not indicate that missingness of outcome variables was based on certain levels of NP and household income (eAppendix 3 in the Supplement).
Measures
NP
Parents or guardians completed a residential history questionnaire, in which they provided the participant’s current home address. The participant’s primary home address was used to generate Area Deprivation Index (ADI) values,33 which were factor analyzed and used to create an aggregate measure of standardized NP (median, −0.22; range, −1.49 to 3.91). The final NP aggregate consisted of 9 of 17 ADI values, given that some variables with lower factor loadings seemed to reflect geographical differences in cost of living across sites that were less indicative of objective disadvantage (eg, median mortgage or rent costs) or less modern disadvantage indices (eg, percentage of homes without a telephone). Higher scores on NP indicate increased neighborhood disadvantage (eg, greater percentage of families living in poverty, increased unemployment, lower percentage of educational attainment at the neighborhood level). More information regarding calculation and distribution appears in eAppendix 4 in the Supplement; results obtained using the ADI sum score appear in eAppendix 5 in the Supplement.
Household SES
Household SES was measured using both household income and the Parent-Reported Financial Adversity Questionnaire (PRFQ). Household income (median, 8; range, 1-10) was the combined income of the primary caretaker and any additional household members.34 The PRFQ asked questions designed to determine whether families generally have enough money to pay for basic life expenses, such as food and health care.35 Household income and PRFQ scores were included as separate indicators of household SES because household income is a more objective measure of SES, while PRFQ indexes self-reported finances that may better account for the association of income level with area cost-of-living.
National Institutes of Health Toolbox Cognitive Battery
The National Institutes of Health Toolbox Cognitive Battery (NIHTB-CB) was administered to each participant.36 The NIHTB-CB is composed of tasks assessing 7 cognitive domains, as follows: picture vocabulary as a measure of verbal ability; flanker inhibitory control and dimensional change card sort as measures of attention and executive functioning; list sorting as a measure of working memory; pattern comparison for processing speed; picture sequencing for episodic memory; and oral reading recognition as a measure of reading ability.36 Scores used in the current study were age-corrected and z scored.
Imaging Procedure and Brain Segmentation
As previously described,37,38 participants were scanned using similar sequences on either a 3T Siemens, Phillips, or General Electric scanner with a 32-channel head coil. A 3-dimensional T1-weighted image (1-mm voxel resolution) was acquired as participants viewed the child-appropriate movie of their choice.37 Motion detection and correction software were used in real-time at the Siemens and GE sites.39,40
Based on previous literature, the a priori regions of interest were the hippocampus and 3 regions in the prefrontal cortex (ie, DLPFC, DMPFC, and SFG). FreeSurfer version 5.3.0 was used for cortical surface reconstruction and subcortical brain segmentation from the aseg atlas for hippocampal and Desikan atlas for superior frontal regions.38 eAppendix 6 in the Supplement describes quality control methods. DLPFC and DMPFC were parceled into genetically based subdivisions.41 Participant scans that were rated as unusable were not included in the released data set. As an additional follow-up to ensure that our results did not reflect T1 quality, we reran all analyses using only those children with a 0 on the artifact score (eAppendix 7 in the Supplement).
Statistical Analysis
Mixed-Effects Models
Data analysis was conducted from March to June 2019. Mixed-effects models were computed using the lmer function within the lme4 package in R version 3.6.2. (R Project for Statistical Computing).42 Calculation of intraclass correlation coefficient revealed that site did not account for a significant amount of variance in these models (<0.01 for cognitive outcomes and approximately 0.04 for brain outcomes). A more parsimonious model that included only a random effect of family was used for the current analyses. NP and household SES variables (household income and PRFQ) were first considered in separate models (models 1 and 2) and then were included together (model 3) to assess shared vs unique variance. Age, sex, and intracranial volume (for hippocampal and prefrontal analyses) were included as covariates. All variables were standardized for ease of comparison. Estimates were chosen to optimize the restricted maximum likelihood criterion. We performed t tests to look at each variable using the Sattherwaithe degrees of freedom method via the lmerTest package.43 Multiple comparisons were corrected using false discovery rate.44 Statistical significance was set at P < .05, and all tests were 2-tailed.
Structural Equation Models
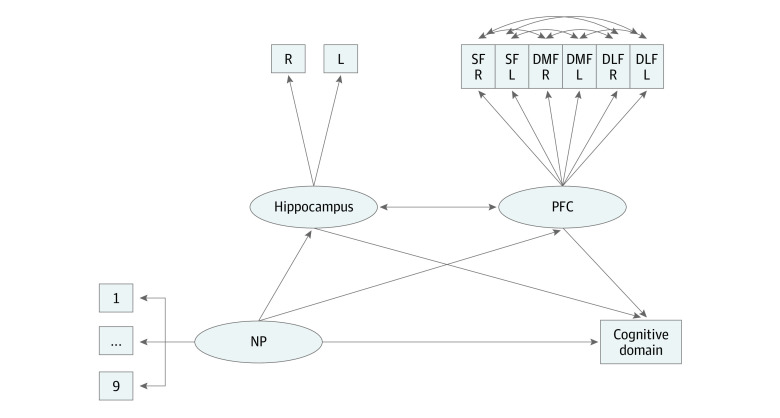
Causal mediation cannot be determined with cross-sectional data. Thus, we used the lavaan package in R version 3.6.3 (R Project for Statistical Computing)45 to conduct structural equation models (SEMs) of indirect associations to examine the plausibility of whether brain structure could mediate the association between NP and cognitive function by assessing the shared variance in NP-to-brain measures and NP-to–NIHTB-CB measures (Figure). First, an exploratory factor analysis (EFA) was performed (psych package in R) for brain regions and NIHTB-CB scores to reduce data dimensionality. The EFA suggested 2 brain factors (prefrontal and hippocampal) but indicated that the NIHTB-CB tests should each be considered individually (eAppendix 8 in the Supplement). Second, indirect-effects models were created, which set NP, prefrontal, and hippocampal factors as free to vary and included household income, age, sex, and intracranial volume as covariates. Fit indices (comparative fit index [CFI], root mean square error of approximation [RMSEA], and standardized root mean square residual [SRMR]) assessed whether the indirect-effects model fit the data well. Models were confirmed using a subset of the data that included only 1 child per family (ie, children with no siblings; n = 9988) to rule out family dependency confounds.
Figure. Schematic of SEM With Neighborhood Poverty and Brain Region Associations With Cognitive Performance.
DLF indicates dorsolateral prefrontal cortex; DMF, dorsomedial prefrontal cortex; L, left; NP, neighborhood poverty; R, right; SF, superior frontal gyrus.
Results
In this sample of 11 875 children, 5678 (47.8%) were girls. All participants were aged 9 or 10 years. Table 1 presents sex and race/ethnicity proportions. Household income and PRFQ were negatively correlated (r = −0.42; P < .001). NP was negatively correlated with household income (r = −0.56; P < .001) and positively correlated with PRFQ (r = 0.27; P < .001).
Table 1. Demographic Percentages in Current Sample.
| Demographic variable | Children, No. (%) (N = 11 875) |
|---|---|
| Sex | |
| Female | 5678 (47.8) |
| Male | 6184 (52.1) |
| NA | 13 (0.1) |
| Racea | |
| White | 8803 (74.1) |
| Black | 2515 (21.2) |
| Asian | 822 (6.9) |
| Native American or Alaskan | 411 (3.5) |
| Native Hawaiian or Pacific Islander | 37 (0.3) |
| Other race | 799 (6.7) |
| Did not know or did not disclose | 163 (1.3) |
| Ethnicity | |
| Hispanic or Latinx | 2407 (20.3) |
Counts for race exceed total sample count (n = 11 875) because parents were permitted to endorse multiple racial categories to describe child’s racial identity.
Association of NP With NIHTB-CB Scores
eAppendix 9 in the Supplement presents results using unstandardized variables. As shown in Table 2, without NP in the models (model 1), higher household income was associated with higher scores across all measures of the NIHTB-CB (eg, total composite: β = 0.38; 95% CI, 0.36 to 0.40; P < .001). Lower PRFQ was associated with higher scores on picture vocabulary, oral reading, dimensional card sort, and picture sequencing tasks. Without household income or PRFQ in the models (model 2), greater NP was also significantly associated with lower scores across all measures of the NIHTB-CB (eg, total composite: β = −0.41; 95% CI, −0.44 to −0.39; P < .001). Importantly, when household income, PRFQ, and NP were in the same model (model 3), both NP and household income continued to be independently associated with each of the scores (total composite, NP: β = −0.18; 95% CI, −0.21 to −0.15; P < .001; total composite, household income: β = 0.30; 95% CI, 0.28 to 0.33; P < .001), although PRFQ did not. Examination of unstandardized variables (eAppendix 9 in the Supplement) indicated that for every unit increase in NP, children scored 3.22 points lower on the NIHTB-CB composite, even when accounting for household SES.
Table 2. Model Output for NIHTB-CB Measures.
| NIHTB-CB measures | Neighborhood poverty | Household income | PRFQ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CIs) | t | P value | β (95% CIs) | t | P value | β (95% CIs) | t | P value | |
| Model 1a | |||||||||
| Picture vocabulary | NA | NA | NA | 0.36 (0.34 to 0.38) | 34.82 | <.001 | –0.04 (–0.06 to –0.02) | –2.63 | .01 |
| Oral reading | NA | NA | NA | 0.28 (0.26 to 0.3) | 25.89 | <.001 | –0.04 (–0.06 to –0.02) | –3.52 | <.001 |
| Dimensional card sort | NA | NA | NA | 0.18 (0.16 to 0.2) | 16.7 | <.001 | –0.03 (–0.06 to –0.01) | –3.08 | <.01 |
| Flanker inhibitory control | NA | NA | NA | 0.16 (0.14 to 0.19) | 15.18 | <.001 | 0.00 (–0.02 to 0.02) | –0.2 | .84 |
| List sorting | NA | NA | NA | 0.28 (0.26 to 0.31) | 26.73 | <.001 | –0.02 (–0.04 to 0) | –1.62 | .11 |
| Pattern comparison | NA | NA | NA | 0.11 (0.09 to 0.13) | 9.93 | <.001 | –0.02 (–0.04 to 0) | –1.95 | .05 |
| Picture sequencing | NA | NA | NA | 0.18 (0.16 to 0.2) | 16.75 | <.001 | –0.03 (–0.05 to –0.01) | –2.76 | .01 |
| Total composite | NA | NA | NA | 0.38 (0.36 to 0.4) | 36.91 | <.001 | –0.05 (–0.07 to –0.03) | –4.35 | <.001 |
| Model 2b | |||||||||
| Picture vocabulary | –0.4 (–0.42 to –0.38) | –35.85 | <.001 | NA | NA | NA | NA | NA | NA |
| Oral reading | –0.27 (–0.29 to –0.24) | –23.3 | <.001 | NA | NA | NA | NA | NA | NA |
| Dimensional card sort | –0.22 (–0.25 to –0.2) | –19.65 | <.001 | NA | NA | NA | NA | NA | NA |
| Flanker inhibitory control | –0.19 (–0.21 to –0.16) | –16.26 | <.001 | NA | NA | NA | NA | NA | NA |
| List sorting | –0.3 (–0.32 to –0.27) | –26.11 | <.001 | NA | NA | NA | NA | NA | NA |
| Pattern comparison | –0.14 (–0.17 to –0.12) | –12.71 | <.001 | NA | NA | NA | NA | NA | NA |
| Picture sequencing | –0.19 (–0.21 to –0.17) | –16.66 | <.001 | NA | NA | NA | NA | NA | NA |
| Total composite | –0.41 (–0.44 to –0.39) | –37.05 | <.001 | NA | NA | NA | NA | NA | NA |
| Model 3c | |||||||||
| Picture vocabulary | –0.18 (–0.21 to –0.15) | –12.43 | <.001 | 0.28 (0.25 to 0.3) | 21.98 | <.001 | –0.04 (–0.06 to –0.02) | –3.63 | .11 |
| Oral reading | –0.07 (–0.1 to –0.04) | –4.58 | <.001 | 0.25 (0.22 to 0.27) | 18.72 | <.001 | –0.04 (–0.06 to –0.02) | –3.44 | .11 |
| Dimensional card sort | –0.12 (–0.15 to –0.09) | –7.72 | <.001 | 0.13 (0.1 to 0.15) | 9.63 | <.001 | –0.03 (–0.05 to 0.01) | –2.76 | .21 |
| Flanker inhibitory control | –0.1 (–0.13 to –0.07) | –6.32 | <.001 | 0.12 (0.1 to 0.15) | 9.19 | <.001 | NA | NA | NA |
| List sorting | –0.13 (–0.16 to –0.1) | –8.62 | <.001 | 0.23 (0.2 to 0.25) | 17.4 | <.001 | NA | NA | NA |
| Pattern comparison | –0.09 (–0.12 to –0.06) | –6.16 | <.001 | 0.07 (0.04 to 0.09) | 5.03 | <.001 | NA | NA | NA |
| Picture sequencing | –0.07 (–0.1 to –0.04) | –4.84 | <.001 | 0.14 (0.12 to 0.17) | 10.8 | <.001 | –0.03 (–0.05 to –0.01) | –2.36 | .12 |
| Total composite | –0.18 (–0.21 to –0.15) | –12.44 | <.001 | 0.3 (0.28 to 0.33) | 23.92 | <.001 | –0.04 (–0.06 to –0.2) | –3.71 | .12 |
Abbreviation: NA, not applicable; NIHTB-CB, National Institutes of Health Toolbox–Cognitive Measures; PRFQ, Parent-Reported Financial Adversity Questionnaire.
Model 1 included household income and PRFQ as factors with age and sex included as covariates.
Model 2 included neighborhood poverty as a factor with age and sex included as covariates.
Model 3 included household income, PRFQ, and neighborhood poverty as factors with age and sex included as covariates.
Association of NP With Hippocampal Volume
eAppendix 9 in the Supplement presents results using unstandardized variables. As shown in Table 3, when NP was not in the models (model 1), higher household income was significantly associated with increased volume in bilateral hippocampus (eg, right hippocampus: β = 0.06; 95% CI, 0.05 to 0.08; P < .001). Lower PRFQ scores were significantly associated with increased volume in right hippocampus (β = –0.02; 95% CI, –0.04 to –0.01; P = .01). In model 2, higher NP was associated with decreased volume in both right and left hippocampus (right: β = –0.08; 95% CI, –0.10 to –0.06; P < .001; left: β = –0.06; 95% CI, –0.08 to –0.05; P = .04). With both household SES variables and NP included (model 3), both NP and household income continued to be independently associated with right hippocampal volume (NP: β = –0.04; 95% CI, –0.06 to –0.01; P = .01; household income: β = 0.04; 95% CI, 0.02 to 0.07; P < .001), while PRFQ was not. For left hippocampal volume, household income was significantly associated (β = 0.06; 95% CI, 0.04 to 0.08; P < .001), but NP was not (β = –0.02; 95% CI, –0.04 to –0.01; P = .21).
Table 3. Model Output for Brain Regions of Interest.
| Brain ROIs | Neighborhood poverty | Household income | PRFQ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CIs) | t | P value | β (95% CIs) | t | P value | β (95% CIs) | t | P value | |
| Model 1a | |||||||||
| Right hippocampus | NA | NA | NA | 0.06 (0.05 to 0.08) | 7.16 | <.001 | –0.02 (–0.04 to –0.01) | –2.63 | .01 |
| Left hippocampus | NA | NA | NA | 0.07 (0.05 to 0.09) | 7.73 | <.001 | –0.01 (–0.03 to 0.01) | –1.32 | .19 |
| Right SFG | NA | NA | NA | 0.10 (0.08 to 0.12) | 11.35 | <.001 | –0.02 (–0.03 to 0) | –1.78 | .09 |
| Left SFG | NA | NA | NA | 0.09 (0.07 to 0.10) | 10.22 | <.001 | –0.01 (–0.03 to 0) | –1.73 | .09 |
| Right DLPFC | NA | NA | NA | 0.12 (0.10 to 0.13) | 14.58 | <.001 | –0.02 (–0.03 to 0) | –2.35 | .03 |
| Left DLPFC | NA | NA | NA | 0.11 (0.10 to 0.13) | 14.39 | <.001 | –0.01 (–0.03 to 0) | –1.93 | .07 |
| Right DMPFC | NA | NA | NA | 0.10 (0.08 to 0.12) | 12.56 | <.001 | –0.02 (–0.04 to –0.01) | –2.68 | .01 |
| Left DMPFC | NA | NA | NA | 0.10 (0.08 to 0.11) | 12.25 | <.001 | –0.02 (–0.03 to 0) | –2.5 | .01 |
| Model 2b | |||||||||
| Right hippocampus | –0.08 (–0.10 to –0.06) | –8.35 | <.001 | NA | NA | NA | NA | NA | NA |
| Left hippocampus | –0.06 (–0.08 to –0.05) | –6.77 | .04 | NA | NA | NA | NA | NA | NA |
| Right SFG | –0.11 (–0.12 to –0.09) | –11.5 | <.001 | NA | NA | NA | NA | NA | NA |
| Left SFG | –0.10 (–0.12 to –0.08) | –10.97 | <.001 | NA | NA | NA | NA | NA | NA |
| Right DLPFC | –0.15 (–0.17 to –0.13) | –17.5 | <.001 | NA | NA | NA | NA | NA | NA |
| Left DLPFC | –0.13 (–0.15 to –0.12) | –16.75 | <.001 | NA | NA | NA | NA | NA | NA |
| Right DMPFC | –0.12 (–0.13 to –0.1) | –14.21 | <.001 | NA | NA | NA | NA | NA | NA |
| Left DMPFC | –0.11 (–0.12 to –0.09) | –13.98 | <.001 | NA | NA | NA | NA | NA | NA |
| Model 3c | |||||||||
| Right hippocampus | –0.04 (–0.06 to –0.01) | –2.89 | .01 | 0.04 (0.02 to 0.07) | 4.14 | <.001 | –0.02 (–0.04 to –0.01) | –2.6 | .05 |
| Left hippocampus | –0.02 (–0.04 to –0.01) | –1.29 | .21 | 0.06 (0.04 to 0.08) | 5.55 | <.001 | NA | NA | NA |
| Right SFG | –0.05 (–0.08 to –0.03) | –4.24 | <.001 | 0.08 (0.06 to 0.1) | 7.4 | <.001 | NA | NA | NA |
| Left SFG | –0.05 (–0.07 to –0.03) | –4.29 | <.001 | 0.07 (0.05 to 0.09) | 6.43 | <.001 | NA | NA | NA |
| Right DLPFC | –0.09 (–0.12 to –0.07) | –8.43 | <.001 | 0.08 (0.06 to 0.1) | 7.83 | <.001 | –0.01 (–0.03 to 0) | –1.68 | .09 |
| Left DLPFC | –0.08 (–0.1 to –0.06) | –7.7 | <.001 | 0.07 (0.06 to 0.09) | 8.06 | <.001 | NA | NA | NA |
| Right DMPFC | –0.07 (–0.09 to –0.05) | –6.08 | <.001 | 0.07 (0.05 to 0.09) | 7.54 | <.001 | –0.01 (–0.03 to 0) | –1.72 | .12 |
| Left DMPFC | –0.06 (–0.08 to –0.04) | –5.88 | <.001 | 0.07 (0.05 to 0.08) | 7.25 | <.001 | –0.01 (–0.03 to 0) | –1.69 | .12 |
Abbreviations: DLPFC, dorsal lateral prefrontal cortex; DMPFC, dorsal medial prefrontal cortex; NA, not applicable; PRFQ, Parent-Reported Financial Adversity Questionnaire; ROI, region of interest; SFG, superior frontal gyrus.
Model 1 included household income and PRFQ as factors with age, sex, and intracranial volume included as covariates.
Model 2 included neighborhood poverty as a factor, with age, sex, and intracranial volume included as covariates.
Model 3 included household income, PRFQ, and neighborhood poverty as factors with age, sex, and intracranial volume included as covariates.
Associations With Prefrontal Volumes
As shown in Table 3, when considered alone (model 1), greater household income was associated with increased volume in right and left SFG (right: β = 0.10; 95% CI, 0.08 to 0.12; P < .001; left: β = 0.09; 95% CI, 0.07 to 0.10; P < .001), DLPFC (eg, right: β = 0.12; 95% CI, 0.10 to 0.13; P < .001) and DMPFC (eg, right: β = 0.10; 95% CI, 0.08 to 0.12; P < .001). Lower PRFQ was also associated with increased volume in right DLPFC and both right and left DMPFC. When considered alone (model 2), higher NP was associated with decreased volume in both right and left SFG (right: β = –0.11; 95% CI, –0.12 to –0.09; P < .001; left: β = –0.10; 95% CI, –0.12 to –0.08; P < .001), DLPFC (eg, right: β = –0.15; 95% CI, –0.17 to –0.13; P < .001), and DMPFC (eg, right: β = –0.12; 95% CI, –0.13 to –0.10; P < .001. When included together, NP and household income were each significantly independently associated with volume in right and left hemispheres of each of the prefrontal regions. Greater NP was associated with volume in the DLPFC (eg, right DLPFC: β = −0.09; 95% CI, −0.12 to −0.07; P < .001), DMPFC (eg, right DMPC: β = −0.07; 95% CI, −0.09 to −0.05; P < .001), and SFG (eg, right SFG: β = −0.05; 95% CI, −0.08 to −0.03; P < .001). Follow-up exploratory analyses were conducted to determine whether NP’s association with brain volume extended to other prefrontal regions.
SEM Analyses
For each cognitive task, the model (which included both prefrontal and hippocampal regions simultaneously) was supported as a good fit for the databased on CFI, RMSEA, and SRMR indices (Table 4). Both the prefrontal and hippocampal factors were significantly associated with NP associations with picture vocabulary (prefrontal: estimate [SE], –0.03 [0.01]; P < .001; hippocampal: estimate [SE], –0.01 [0.004]; P < .001), oral reading (prefrontal: estimate [SE], –0.02 [0.01]; P < .001; hippocampal: estimate [SE], –0.01 [0.004]; P < .001), and picture sequence (prefrontal: estimate [SE], –0.01 [0.004]; P = .008; hippocampal: estimate [SE], –0.01 [0.004]; P < 0.01) tasks (Table 4). However, only the prefrontal factor was significantly associated with NP associations with the dimensional card sort (estimate [SE], –0.02 [0.01]; P = .001), flanker inhibitory control (estimate [SE], –0.01 [0.01]; P = .01), and list sorting (estimate [SE], –0.03 [0.01]; P < .001) tasks.
Table 4. Model Output for SEM Analyses Investigating Plausibility of Mediationa.
| Factor | Path a, NP and brain factors | Path b, brain factors and cognitive tasks | Path a × b, indirect effect | Path c, NP and cognitive tasks with brain factors in model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | z | P value | Estimate (SE) | z | P value | Estimate (SE) | z | P value | Estimate (SE) | z | P value | |
| Picture vocabulary | ||||||||||||
| NP | NA | NA | NA | NA | NA | NA | NA | NA | NA | –0.15 (0.02) | –10.26 | <.001 |
| Prefrontal factor | –0.23 (0.01) | –21.27 | <.001 | 0.15 (0.02) | 7.41 | <.001 | –0.03 (0.01) | –7.02 | <.001 | NA | NA | NA |
| Hippocampal factor | –0.19 (0.01) | –16.15 | <.001 | 0.07 (0.02) | 3.87 | <.001 | –0.01 (0.004) | –3.77 | <.001 | NA | NA | NA |
| Oral reading | ||||||||||||
| NP | NA | NA | NA | NA | NA | NA | NA | NA | NA | –0.06 (0.02) | –3.61 | <.001 |
| Prefrontal Factor | –0.23 (0.01) | –21.27 | <.001 | 0.08 (0.02) | 3.82 | <.001 | –0.02 (0.01) | –3.75 | <.001 | NA | NA | NA |
| Hippocampal factor | –0.19 (0.01) | –16.15 | <.001 | 0.07 (0.02) | 3.68 | <.001 | –0.01 (0.004) | –3.52 | <.001 | NA | NA | NA |
| Dimensional card sort | ||||||||||||
| NP | NA | NA | NA | NA | NA | NA | NA | NA | –0.1 (0.02) | –6.75 | <.001 | |
| Prefrontal factor | –0.23 (0.01) | –21.27 | <.001 | 0.07 (0.02) | 3.33 | <.01 | –0.02 (0.01) | –3.27 | .001 | NA | NA | NA |
| Hippocampal factor | –0.19 (0.01) | –16.15 | <.001 | 0.04 (0.02) | 1.9 | .06 | –0.01 (0.004) | –1.89 | .06 | NA | NA | NA |
| Flanker inhibitory control | ||||||||||||
| NP | NA | NA | NA | NA | NA | NA | NA | NA | NA | –0.08 (0.02) | –4.63 | <.001 |
| Prefrontal factor | –0.23 (0.01) | –21.27 | <.001 | 0.06 (0.02) | 2.64 | .01 | –0.01 (0.01) | –2.62 | .01 | NA | NA | NA |
| Hippocampal factor | –0.19 (0.01) | –16.15 | <.001 | 0.04 (0.02) | 1.85 | .06 | –0.01 (0.004) | –1.84 | .07 | NA | NA | NA |
| List sorting | ||||||||||||
| NP | NA | NA | NA | NA | NA | NA | NA | NA | NA | –0.12 (0.02) | –7.63 | <.001 |
| Prefrontal factor | –0.23 (0.01) | –21.27 | <.001 | 0.12 (0.02) | 5.73 | <.001 | –0.03 (0.01) | –5.56 | <.001 | NA | NA | NA |
| Hippocampal factor | –0.19 (0.01) | –16.15 | <.001 | 0.02 (0.02) | 0.92 | .36 | –0.01 (0.004) | –0.92 | .36 | NA | NA | NA |
| Pattern comparison | ||||||||||||
| NP | NA | NA | NA | NA | NA | NA | NA | NA | NA | –0.07 (0.02) | –4.5 | <.001 |
| Prefrontal factor | –0.23 (0.01) | –21.27 | <.001 | 0.04 (0.02) | 1.83 | .07 | –0.01 (0.01) | –1.82 | .07 | NA | NA | NA |
| Hippocampal factor | –0.19 (0.01) | –16.15 | <.001 | 0.02 (0.02) | 1.16 | .25 | –0.01 (0.004) | –1.15 | .25 | NA | NA | NA |
| Picture sequence | ||||||||||||
| NP | NA | NA | NA | NA | NA | NA | NA | NA | NA | –0.07 (0.02) | –4.32 | <.001 |
| Prefrontal factor | –0.23 (0.01) | –21.27 | <.001 | 0.06 (0.02) | 2.97 | .008 | –0.02 (0.01) | –2.93 | <.001 | NA | NA | NA |
| Hippocampal factor | –0.19 (0.01) | –16.15 | <.001 | 0.07 (0.02) | 3.49 | <.001 | –0.01 (0.004) | –3.42 | <.001 | NA | NA | NA |
Abbreviations: NA, not applicable; NP, neighborhood poverty.
Covariates included: age, sex, household income, and intracranial volume. Fit indices suggest that models were a good fit for the data: Prefrontal models (CFI = 0.936, RMSEA = 0.087, SRMR = 0.071); Hippocampal models (CFI = 0.940, RMSEA = 0.084, SRMR = 0.053). NP was modeled as the predictor with prefrontal and hippocampal factors as mediators, with separate cognitive tasks as the outcome (7 models total).
Discussion
Consistent with our hypothesis, the results of this study indicated that NP was significantly associated with a range of cognitive function domains as well as bilateral prefrontal and right hippocampal volumes, even after accounting for individual household income and PRFQ. Furthermore, the SEMs provided evidence that it is plausible that variation in prefrontal and hippocampal brain volume may mediate the association between NP and cognitive outcomes, with the relative contributions of prefrontal vs hippocampal volumes varying across cognitive domains. The effect sizes for associations with household SES were generally stronger than those for NP; therefore, we are not arguing that NP is more important than household SES. However, the fact that NP accounted for variance in addition to household SES supports the idea that consideration of the neighborhood context is important in conceptualizing the complex environment of the developing brain.
Higher levels of NP were independently and significantly associated with lower scores on all cognitive domains. Standardized effect sizes were conventionally small but nonetheless meaningful in indicating the need to assess the ways in which neighborhood characteristics may contribute to brain structure and cognitive development, as even small differences could build over time into larger differences in functional outcomes. These findings are consistent with previous literature demonstrating poorer school and cognitive performance among children raised in impoverished environments1,3,4,46 and provide further support for the importance of consideration of the neighborhood context in understanding child outcomes. A key question is what aspects of NP are critical for explaining cognitive function among children that are not fully accounted for by individual household function. A possibility is that the neighborhood context might be associated with school environment and/or funding, and the development of a range of cognitive processes are likely sensitive to school context and resources. Future research should include indices of local school context to investigate this hypothesis further. Importantly, the size and nature of the sample (mirroring the US child population) suggest that these findings are likely to be generalizable to broader normative populations.
Consistent with prior literature,6,7,8,9,10,11,12 higher household income was associated with increased volumes in all hippocampal and prefrontal brain regions. Additionally, higher NP was significantly and independently associated with reduced brain volume in DLPFC, DMPFC, SFG, and right hippocampal regions. Interestingly, the effect sizes for both NP and household SES variables’ association with brain volume were smaller than those for the cognitive measures. It is possible that the bigger effect sizes for cognitive function reflect a strong association between NP and school funding and quality, which may have a stronger association with cognitive function than brain metrics. In contrast, our NP measure may not be as strongly associated with other potential correlates, which may be more directly related to brain development. For example, individuals living in poverty are more likely to be exposed to lead and air pollutants in childhood.47,48,49 As such, it will be important to look at lead (and other toxin) exposure to determine whether it might be associated with brain volume in ways not captured by the current measure of NP. Additionally, NP was not independently associated with orbitofrontal cortex volume, suggesting that some specificity in NP relations to prefrontal regions (eAppendix 10 in the Supplement).
The SEM results provided evidence that prefrontal and hippocampal brain volumes explained variance in the association between NP and cognitive task performance, even when controlling for household income. Interestingly, the relative associations of prefrontal cortex and hippocampal volumes varied across cognitive measures. Both prefrontal and hippocampal volumes were significantly associated with the associations between NP and tasks indexing language ability and crystallized intelligence.50,51 The hippocampus is important for the consolidation of long-term information, and the role of the prefrontal cortex in language processing and production has been well-established.52,53,54 Similarly, both prefrontal and hippocampal volumes were significantly associated with the association between NP and episodic memory. This is consistent with a large body of literature identifying the hippocampus as critical for consolidation of episodic memories with prefrontal regions providing organizational support for encoding.11,12,55 In contrast, only prefrontal volumes were significantly associated with the association between NP and executive function and working memory tasks. This finding is consistent with literature suggesting that prefrontal regions support the top-down processing of stimuli, allowing for flexible and nonautomatic behavioral responses.56 These results provide evidence supporting the plausibility of a mediation, which can be assessed in subsequent studies as additional waves of data are collected.
Limitations
This study has limitations. All of the neighborhood variables were focused on SES. The inclusion of other neighborhood characteristics that may not directly index SES (physical or social factors such as number of grocery stores, green space, amount of litter, air pollution)47,48,49 might further elucidate associations between environmental context and various outcomes.57 Another limitation is that the current data set is cross-sectional, which means that direction of association cannot be determined. As additional waves of ABCD data accrue, the models tested here should be extended longitudinally to make a more compelling case for mediation and direction of effect.
Conclusions
This study found evidence for independent associations of household and neighborhood environment with brain and cognitive outcomes in preadolescent children. The study also provided evidence consistent with a pathway wherein variation in prefrontal and hippocampal volume partially explains the association between NP and scores on cognitive tests. The differential prefrontal and hippocampal associations are consistent with what would be expected for the different cognitive domains. These results provide evidence that the inclusion of neighborhood variables is a warranted addition in models of how early lived environments are associated with brain maturation and cognitive outcomes, which may inform the types of interventions offered to children from disadvantaged backgrounds.
eAppendix 1. Distributions of NP and Household Income in Black vs White Participants and Model Results Including Race/Ethnicity as a Covariate
eAppendix 2. Sample Sizes Used for Each MLM Analysis
eAppendix 3. Missingness Within the Study Sample and Comparison of Missing Values Among Demographic and Outcome Variables
eAppendix 4. Distribution of NP and Household Income in Overall Sample and Additional Information on Measure of NP, PRFQ, and Household Income
eAppendix 5. MLM Results Tables With ADI Weighted Sum as Independent Variable
eAppendix 6. FreeSurfer QC Methods
eAppendix 7. ML Model 3 Results Using Only Those Participants With a 0 on the Artifact Score
eAppendix 8. NIH Toolbox-CB and Brain ROI EFA
eAppendix 9. MLM Unstandardized Results Tables
eAppendix 10. MLM Results Looking at Orbital Frontal Regions as the Outcome Variable
References
- 1.Hair NL, Hanson JL, Wolfe BL, Pollak SD. Association of child poverty, brain development, and academic achievement. JAMA Pediatr. 2015;169(9):822-829. doi: 10.1001/jamapediatrics.2015.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farah MJ, Shera DM, Savage JH, et al. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110(1):166-174. doi: 10.1016/j.brainres.2006.06.072 [DOI] [PubMed] [Google Scholar]
- 3.Brooks-Gunn J, Duncan GJ. The effects of poverty on children. Future Child. 1997;7(2):55-71. doi: 10.2307/1602387 [DOI] [PubMed] [Google Scholar]
- 4.McLoyd VC. Socioeconomic disadvantage and child development. Am Psychol. 1998;53(2):185-204. doi: 10.1037/0003-066X.53.2.185 [DOI] [PubMed] [Google Scholar]
- 5.Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between income and the hippocampus. PLoS One. 2011;6(5):e18712. doi: 10.1371/journal.pone.0018712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luby J, Belden A, Botteron K, et al. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167(12):1135-1142. doi: 10.1001/jamapediatrics.2013.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ. Childhood socioeconomic status and adult brain size: childhood socioeconomic status influences adult hippocampal size. Ann Neurol. 2012;71(5):653-660. doi: 10.1002/ana.22631 [DOI] [PubMed] [Google Scholar]
- 8.Luby JL, Barch DM, Belden A, et al. Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc Natl Acad Sci U S A. 2012;109(8):2854-2859. doi: 10.1073/pnas.1118003109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noble KG, Grieve SM, Korgaonkar MS, et al. Hippocampal volume varies with educational attainment across the life-span. Front Hum Neurosci. 2012;6:307. doi: 10.3389/fnhum.2012.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7(2):217-227. doi: 10.1016/S0959-4388(97)80010-4 [DOI] [PubMed] [Google Scholar]
- 11.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625-641. doi: 10.1016/S0896-6273(02)00830-9 [DOI] [PubMed] [Google Scholar]
- 12.Tulving E, Markowitsch HJ. Episodic and declarative memory: role of the hippocampus. Hippocampus. 1998;8(3):198-204. doi: [DOI] [PubMed] [Google Scholar]
- 13.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79(1):16-29. doi: 10.1016/j.neuron.2013.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41(1):3-23. doi: 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54(3):200-207. doi: 10.1016/S0006-3223(03)00177-X [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev. 2014;47:578-591. doi: 10.1016/j.neubiorev.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amso D, Salhi C, Badre D. The relationship between cognitive enrichment and cognitive control: a systematic investigation of environmental influences on development through socioeconomic status. Dev Psychobiol. 2019;61(2):159-178. doi: 10.1002/dev.21794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cecil KM, Brubaker CJ, Adler CM, et al. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008;5(5):e112. doi: 10.1371/journal.pmed.0050112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyaradi A, Oddy WH, Hickling S, Li J, Foster JK. The relationship between nutrition in infancy and cognitive performance during adolescence. Front Nutr. 2015;2:2. doi: 10.3389/fnut.2015.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanson JL, Chung MK, Avants BB, et al. Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. J Neurosci. 2012;32(23):7917-7925. doi: 10.1523/JNEUROSCI.0307-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl). 2011;214(1):55-70. doi: 10.1007/s00213-010-2009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noble KG, Houston SM, Brito NH, et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18(5):773-778. doi: 10.1038/nn.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen ML, Sheridan MA, Sambrook KA, Meltzoff AN, McLaughlin KA. Socioeconomic disparities in academic achievement: a multi-modal investigation of neural mechanisms in children and adolescents. Neuroimage. 2018;173:298-310. doi: 10.1016/j.neuroimage.2018.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaked D, Katzel LI, Seliger SL, et al. Dorsolateral prefrontal cortex volume as a mediator between socioeconomic status and executive function. Neuropsychology. 2018;32(8):985-995. doi: 10.1037/neu0000484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Zhou M, Chen T, et al. Grit and the brain: spontaneous activity of the dorsomedial prefrontal cortex mediates the relationship between the trait grit and academic performance. Soc Cogn Affect Neurosci. 2017;12(3):452-460. doi: 10.1093/scan/nsw145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vuontela V, Steenari MR, Aronen ET, Korvenoja A, Aronen HJ, Carlson S. Brain activation and deactivation during location and color working memory tasks in 11-13-year-old children. Brain Cogn. 2009;69(1):56-64. doi: 10.1016/j.bandc.2008.05.010 [DOI] [PubMed] [Google Scholar]
- 27.Pattillo M. The color of law: a forgotten history of how our government segregated America. Contemp Sociol A J Rev. 2018;47(5):624-625. doi: 10.1177/0094306118792220ll [DOI] [Google Scholar]
- 28.Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health. 2001;91(11):1783-1789. doi: 10.2105/AJPH.91.11.1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gustafsson PE, San Sebastian M, Janlert U, Theorell T, Westerlund H, Hammarström A. Life-course accumulation of neighborhood disadvantage and allostatic load: empirical integration of three social determinants of health frameworks. Am J Public Health. 2014;104(5):904-910. doi: 10.2105/AJPH.2013.301707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharkey P. The acute effect of local homicides on children’s cognitive performance. Proc Natl Acad Sci U S A. 2010;107(26):11733-11738. doi: 10.1073/pnas.1000690107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dupere V, Leventhal T, Crosnoe R, Dion E. Understanding the positive role of neighborhood socioeconomic advantage in achievement: the contribution of the home, child care, and school environments. Dev Psychol. 2010;46(5):1227-1244. doi: 10.1037/a0020211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Compton WM, Dowling GJ, Garavan H. Ensuring the best use of data: the Adolescent Brain Cognitive Development Study. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2019.2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh GK. Area deprivation and widening inequalities in US mortality, 1969-1998. Am J Public Health. 2003;93(7):1137-1143. doi: 10.2105/AJPH.93.7.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barch DM, Gotlib IH, Bilder RM, et al. Common measures for National Institute of Mental Health funded research. Biol Psychiatry. 2016;79(12):e91-e96. doi: 10.1016/j.biopsych.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diemer MA, Mistry RS, Wadsworth ME, López I, Reimers F. Best practices in conceptualizing and measuring social class in psychological research. Anal Soc Issues Public Policy. 2013;13(1):77-113. doi: 10.1111/asap.12001 [DOI] [Google Scholar]
- 36.Luciana M, Bjork JM, Nagel BJ, et al. Adolescent neurocognitive development and impacts of substance use: overview of the Adolescent Brain Cognitive Development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci. 2018;32:67-79. doi: 10.1016/j.dcn.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casey BJ, Cannonier T, Conley MI, et al. ; ABCD Imaging Acquisition Workgroup . The Adolescent Brain Cognitive Development (ABCD) study: imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43-54. doi: 10.1016/j.dcn.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagler DJ Jr, Hatton S, Cornejo MD, et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage. 2019;202:116091. doi: 10.1016/j.neuroimage.2019.116091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White N, Roddey C, Shankaranarayanan A, et al. PROMO: real-time prospective motion correction in MRI using image-based tracking. Magn Reson Med. 2010;63(1):91-105. doi: 10.1002/mrm.22176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJW. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med. 2012;68(2):389-399. doi: 10.1002/mrm.23228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen CH, Gutierrez ED, Thompson W, et al. Hierarchical genetic organization of human cortical surface area. Science. 2012;335(6076):1634-1636. doi: 10.1126/science.1215330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1). doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 43.Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest: tests for random and fixed effects for linear mixed effect models. J Stat Softw. 2017;82(13). doi: 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- 44.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 45.Rosseel Y. Lavaan: an R package for structural equation modeling. J Stat Softw. 2012;48(2). doi: 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- 46.Farah MJ, Betancourt L, Shera DM, et al. Environmental stimulation, parental nurturance and cognitive development in humans. Dev Sci. 2008;11(5):793-801. doi: 10.1111/j.1467-7687.2008.00688.x [DOI] [PubMed] [Google Scholar]
- 47.Needleman HL, Bellinger D. The health effects of low level exposure to lead. Annu Rev Public Health. 1991;12:111-140. doi: 10.1146/annurev.pu.12.050191.000551 [DOI] [PubMed] [Google Scholar]
- 48.Huang PC, Su PH, Chen HY, et al. Childhood blood lead levels and intellectual development after ban of leaded gasoline in Taiwan: a 9-year prospective study. Environ Int. 2012;40:88-96. doi: 10.1016/j.envint.2011.10.011 [DOI] [PubMed] [Google Scholar]
- 49.Nevin R. Understanding international crime trends: the legacy of preschool lead exposure. Environ Res. 2007;104(3):315-336. doi: 10.1016/j.envres.2007.02.008 [DOI] [PubMed] [Google Scholar]
- 50.Weintraub S, Bauer PJ, Zelazo PD, et al. I. NIH Toolbox Cognition Battery (CB): introduction and pediatric data. Monogr Soc Res Child Dev. 2013;78(4):1-15. doi: 10.1111/mono.12031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80(11)(suppl 3):S54-S64. doi: 10.1212/WNL.0b013e3182872ded [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexander MP, Benson DF, Stuss DT. Frontal lobes and language. Brain Lang. 1989;37(4):656-691. doi: 10.1016/0093-934X(89)90118-1 [DOI] [PubMed] [Google Scholar]
- 53.Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17(1):353-362. doi: 10.1523/JNEUROSCI.17-01-00353.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gabrieli JDE, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci U S A. 1998;95(3):906-913. doi: 10.1073/pnas.95.3.906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fletcher PC, Shallice T, Dolan RJ. The functional roles of prefrontal cortex in episodic memory. I. Encoding. Brain. 1998;121(Pt 7):1239-1248. doi: 10.1093/brain/121.7.1239 [DOI] [PubMed] [Google Scholar]
- 56.Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135-168. doi: 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125-145. doi: 10.1111/j.1749-6632.2009.05333.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Distributions of NP and Household Income in Black vs White Participants and Model Results Including Race/Ethnicity as a Covariate
eAppendix 2. Sample Sizes Used for Each MLM Analysis
eAppendix 3. Missingness Within the Study Sample and Comparison of Missing Values Among Demographic and Outcome Variables
eAppendix 4. Distribution of NP and Household Income in Overall Sample and Additional Information on Measure of NP, PRFQ, and Household Income
eAppendix 5. MLM Results Tables With ADI Weighted Sum as Independent Variable
eAppendix 6. FreeSurfer QC Methods
eAppendix 7. ML Model 3 Results Using Only Those Participants With a 0 on the Artifact Score
eAppendix 8. NIH Toolbox-CB and Brain ROI EFA
eAppendix 9. MLM Unstandardized Results Tables
eAppendix 10. MLM Results Looking at Orbital Frontal Regions as the Outcome Variable