Key Points
Questions
Are there associations between receipt of payments from device manufacturers to physicians and selection of a manufacturers’ device when selecting an implantable cardioverter-defibrillator (ICD) or cardiac resynchronization therapy-defibrillator (CRT-D)?
Findings
In this cross-sectional study using data from 2016 through 2018, 145 900 patients received an ICD or CRT-D by 4435 physicians using devices from 4 device manufacturers, 94% of whom received payments from device manufacturers. Patients were substantially more likely to receive devices made by the manufacturer that provided the highest total payment to the physician who performed an ICD implant than each other individual manufacturer (absolute differences in proportional use from the expected prevalence range, 14.5%-30.6%).
Meaning
Patients more often received an implantable cardioverter-defibrillator or cardiac resynchronization therapy-defibrillator device made by the manufacturer that provided the largest payments to the physicians than to each of 3 other manufacturers individually.
Abstract
Importance
Little is known about the association between industry payments and medical device selection.
Objective
To examine the association between payments from device manufacturers to physicians and device selection for patients undergoing first-time implantation of a cardioverter-defibrillator (ICD) or cardiac resynchronization therapy-defibrillator (CRT-D).
Design, Setting, and Participants
In this cross-sectional study, patients who received a first-time ICD or CRT-D device from any of the 4 major manufacturers (January 1, 2016-December 31, 2018) were identified. The data from the National Cardiovascular Data Registry ICD Registry was linked with the Open Payments Program’s payment data. Patients were categorized into 4 groups (A, B, C, and D) corresponding to the manufacturer from which the physician who performed the implantation received the largest payment. For each patient group, the proportion of patients who received a device from the manufacturer that provided the largest payment to the physician who performed implantation was determined. Within each group, the absolute difference in proportional use of devices between the manufacturer that made the highest payment and the proportion of devices from the same manufacturer in the entire study cohort (expected prevalence) was calculated.
Exposures
Manufacturers’ payments to physicians who performed an ICD or CRT-D implantation.
Main Outcomes and Measures
The primary outcome of the study was the manufacturer of the device used for the implantation.
Results
Over a 3-year period, 145 900 patients (median age, 65 years; 29.6% women) received ICD or CRT-D devices from the 4 manufacturers implanted by 4435 physicians at 1763 facilities. Among these physicians, 4152 (94%) received payments from device manufacturers ranging from $2 to $323 559 with a median payment of $1211 (interquartile range, $390-$3702). Between 38.5% and 54.7% of patients received devices from the manufacturers that had provided physicians with the largest payments. Patients were substantially more likely to receive devices made by the manufacturer that provided the largest payment to the physician who performed implantation than they were from each other individual manufacturer. The absolute differences in proportional use from the expected prevalence were 22.4% (95% CI, 21.9%-22.9%) for manufacturer A; 14.5% (95% CI, 14.0%-15.0%) for manufacturer B; 18.8% (95% CI, 18.2%-19.4%) for manufacturer C; and 30.6% (95% CI, 30.0%-31.2%) for manufacturer D.
Conclusions and Relevance
In this cross-sectional study, a large proportion of ICD or CRT-D implantations were performed by physicians who received payments from device manufacturers. Patients were more likely to receive ICD or CRT-D devices from the manufacturer that provided the highest total payment to the physician who performed an ICD or CRT-D implantation than each other manufacturer individually.
This cross-sectional study examines the association between payments from device manufacturers to physicians and device selection for patients undergoing first-time implantation of a cardioverter-defibrillator (ICD) or cardiac resynchronization therapy-defibrillator (CRT-D).
Introduction
There are long-standing concerns about financial transactions between clinicians and the biomedical industry.1,2 To increase the transparency of industry-physician relationships, the US Congress enacted the Physician Payments Sunshine Act mandating that the biomedical industry and group-purchasing organizations report payments through the Open Payments Program.3,4,5 Information about eligible payments is publicly available through the Open Payments Program website.6
The fundamental concern about physician payments is that such payments may change how physicians select treatment options and that their choices may not necessarily be consistent with the best interests of the patient. Studies have reported that payments to physicians were associated with a greater likelihood of prescriptions of branded medications over generic medications.7,8 However, investigators have not explored whether associations exist between payments from device manufacturers and device selection. A study specific to such devices is warranted because the factors that influence device selection may differ from medications. Among existing manufacturers of implantable cardioverter-defibrillators (ICDs) or cardiac resynchronization therapy-defibrillators (CRT-Ds), there is no clear evidence of improved safety or efficacy for one manufacturer’s products over another’s.9,10,11,12,13 In this setting, physician payments from device manufacturers may influence device selection.
Furthermore, the association between payments and measures of quality of care have not been evaluated. Although industry payments to physicians would unlikely be correlated with quality of care directly, it is possible that the practice patterns of physicians who receive payments may differ from those of physicians who did not. The objective of this study was to examine the association between physician payments and device selection, quality of care, and clinical outcomes of patients undergoing first-time ICD implantation.
Methods
Data Sources
This is a cross-sectional study of the National Cardiovascular Data Registry’s (NCDR) ICD Registry and general payments data of the Open Payments Program from January 1, 2016, through December 31, 2018. The NCDR ICD Registry has been described previously.14,15 Briefly, the Centers for Medicaid & Medicare Services (CMS) issued the Coverage with Evidence Decision in 2005. This policy mandated hospitals to report data related to ICDs implanted for primary prevention in Medicare beneficiaries to a suitable national registry. However, 80% of the participating sites submit data on all ICD or CRT-D implantations representing 90% of implantations submitted to the registry.15,16 During the study period, 1808 hospitals submitted information about ICD implants.17 The data submitted to the ICD Registry goes through quality testing including auditing a sample of participating hospitals.18 The Human Investigation Committee of the Yale University School of Medicine approved the use of a limited data set from the ICD Registry for research purposes. Hospitals participating in the registry collect and submit the data to the registry for quality improvement.14,15 As such, informed consent was not required.
Details about the Open Payments Program have been reported previously.4,19,20,21 The Open Payments Program mandates that manufacturers and group provider organizations report individual payments worth $10 or aggregate payments of more than $100 per year. Industry payments to physicians are broadly categorized into 3 groups: general payments, research payments, and ownership payments. General payments—which include payments for travel and lodging, food and beverage, consulting, and speaking at dinner talks—raise more concerns because they often appear tied to marketing and promotional activities.22 Each transaction contains information about the associated manufacturer and product, value, and nature of payment. In this study, the analysis was limited to general payments data because prior studies have demonstrated that only 1% of physicians held ownership interest and 2% of total research payments were made to individual physicians.23,24
Study Design and Study Population
The study cohort consisted of patients who received a first-time ICD or CRT-D between January 1, 2016, and December 31, 2018. Patients who underwent generator change or lead-only procedures and patients with a prior permanent pacemaker, ICD, or a CRT-D were excluded. The study cohort was limited to patients who received either an ICD or a CRT-D from the 4 ICD manufacturers that represented the 99.8% of implants performed over the study period. The overarching objective of the study was not to focus on the practices of a specific manufacturer but rather to examine the association between industry payments and patterns of care including device selection. When the American College of Cardiology NCDR ICD Registry’s research and publications committee approved the study, it was with the understanding that the names of specific manufacturers would not be disclosed. As such, manufacturer-specific information, including names of 4 major manufacturers, was removed and the manufacturers were randomly labeled A, B, C, and D.
The Open Payments Program data were linked with implantations recorded in the ICD Registry using physician national provider identification numbers. The study population was stratified into 2 cohorts based on whether or not their physician had received payments. Patients whose physicians received payments were further grouped by the manufacturer that had made payments to physicians who performed the implantation: patient group A, B, C, or D. Patients whose physician received payments from more than 1 device manufacturer were placed in the group representing the manufacturer that provided the largest proportion of payments. For example, patients in patient group A had their implant performed by a physician who received the largest proportion of total payments from manufacturer A (Figure).
Figure. Flow Diagram of Study Cohort and Patient Groups by Manufacturer Payment.
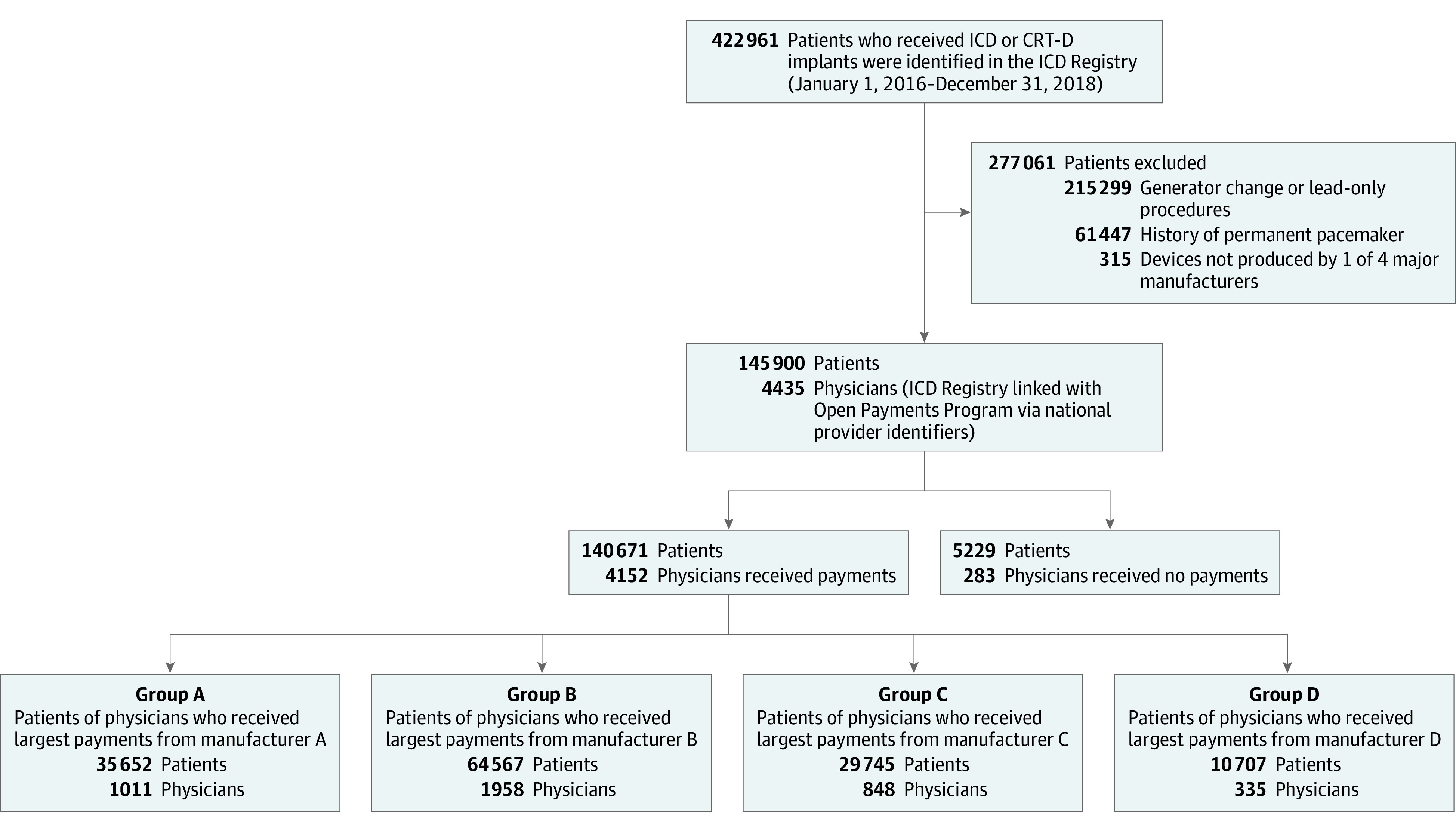
CRT-D indicates cardiac resynchronization therapy-defibrillator; ICD, implantable cardioverter-defibrillator.
Outcomes
The primary outcome of the study was the manufacturer of the device used for the implantation. Secondary outcomes were measures of quality of care: procedure-related complications, use of CRT-D among eligible patients, and use of guideline-recommended medications at discharge among eligible patients. Procedure-related complications included death, cardiac arrest, myocardial infraction, infection, pericardial tamponade, pneumothorax, stroke, hematoma, valve injury, peripheral embolus, and conduction block. Patients were considered eligible for CRT-D if they met criteria class I or IIa based on 2012 focused update to the 2008 American College of Cardiology guidelines.25 Guideline-recommended medications at discharge included the use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker for patients with an ejection fraction of less than 40% and β-blockers for patients with left ventricular dysfunction or history of myocardial infarction.26
Statistical Analysis
Analyses in this study were conducted at the patient level except the descriptive analysis of physician payments (Table 1), which was performed at the physician level using Open Payments Program data. Among patient groups, the total number of physicians in each group, annual total value of payments, and annual median value of payments (interquartile range [IQR]) per physician were analyzed. An annualized value of payments was calculated by averaging total payments made to physicians over 3 years. Furthermore, the percentage of physicians in 4 payment categories were calculated based on the annual total value of payments received ($1-<$100, $100-<$1000, $1000-<$10 000, and ≥$10 000). If a physician’s profile identification number did not appear in any year of the Open Payments Program data (2016-2018), it was assumed that the physician received no payments during that time.
Table 1. Payment Characteristics by 4 Patient Groups.
| Patient groupa | Total | ||||
|---|---|---|---|---|---|
| A | B | C | D | ||
| No. of physicians | 1011 | 1958 | 848 | 335 | 4152 |
| No. of patients | 35 652 | 64 567 | 29 745 | 10 707 | 140 671 |
| Total value of payments per year, $ | 6 425 843 | 7 585 202 | 4 621 655 | 1 773 774 | 20 406 474 |
| Payment per physician per year, $ | |||||
| Median (IQR) | 1329 (269-5311) | 1174 (435-3114) | 978 (299-2963) | 2228 (836-5615) | 1211 (390-3702) |
| Mean (SD) | 6356 (13 837) | 3874 (9489) | 5450 (16 939) | 5295 (10 260) | 4915 (12 535) |
| Range | 4-113 996 | 2-129 175 | 4-323 559 | 5-108 733 | 2-323 559 |
| Proportion of total value of payment received from individual manufacturers | |||||
| A | 71 | 11 | 12 | 11 | 26 |
| B | 15 | 74 | 17 | 14 | 43 |
| C | 10 | 12 | 68 | 8 | 22 |
| D | 4 | 3 | 3 | 67 | 9 |
| Proportion of physicians by different payment categories (%) | |||||
| $1-<$100 | 12 | 11 | 14 | 7 | 12 |
| $100-<1000 | 31 | 35 | 36 | 22 | 33 |
| $1000-<10 000 | 39 | 45 | 37 | 56 | 43 |
| $10 000-<25 000 | 11 | 6 | 7 | 12 | 8 |
| ≥$25 000 | 7 | 3 | 6 | 3 | 4 |
| Proportion of physicians receiving payments by ≥1 manufacturer | |||||
| 1 | 7 | 9 | 8 | 4 | 8 |
| 2 | 16 | 19 | 16 | 10 | 17 |
| 3 | 41 | 39 | 42 | 18 | 38 |
| 4 | 36 | 33 | 34 | 68 | 37 |
Abbreviation: IQR, Interquartile range.
Analysis for this table was performed at physician level.
Baseline demographic, clinical, and hospital characteristics across patient groups were examined for the entire population of patients who received an ICD or CRT-D between or among different patient groups using χ2, t, and Wilcoxon rank sum tests for categorical and continuous variables, where appropriate. Race and ethnicity were included because studies have identified race- and ethnic-based differences in receipt of ICD and CRT-D among eligible patients.27,28 The ICD Registry captures race and ethnicity using fixed categories. For participating hospitals, it was not known whether patients or hospital staff made the determination of race and ethnicity. For all patient groups, the proportion of patients who received an ICD or CRT-D from each of the 4 manufacturers was measured using a frequency table analysis. Two types of absolute difference in proportional use of specific device were calculated: first, the absolute difference between the proportion of devices from a manufacturer that provided the highest payments in a patient group and the proportion of devices from the same manufacturer in the entire study cohort (expected prevalence); and second, the absolute difference between the proportion of devices from the manufacturer that provided the highest payments in a patient group and the proportion of devices from the second commonly used manufacturer in the same patient group. Two types of absolute difference in proportional use were calculated by limiting to patients of physicians who received more than $25 000 annually.
To examine the association between payments and device selection, hierarchical logistic regression models were applied with adjustment for patient clustering among physicians and physician procedure volume. The models evaluated the likelihood of use of a device from each manufacturer across patient groups compared with use of that manufacturer’s device among patients of physicians who received no payments. Two sensitivity analyses were conducted: the first restricting the study cohort to patients of physicians who received 75% of total payments from 1 of the 4 manufacturers; the second excluding patients from hospitals in which devices from only 1 manufacturer were available (236 hospitals [13%]; 5446 total implants [4%]).
To evaluate the association between the total value of payments and device selection, each physician group was stratified by the total value of payments ($1-<$100, $100-<$1000, $1000-<$10 000, and ≥$10 000) from the corresponding manufacturer. The proportional use of that manufacturer’s device was calculated for each payment category. Hierarchical logistic regression models were developed to compare the likelihood of implanting a device made by the manufacturer across payment categories. These models were adjusted for clustering of patients among physicians and physician procedural volume.
For the analysis examining association between quality of care and physician payments, the study cohort was stratified into 4 categories by total value of payment (0-<$100, $100-<$1000, $1000-<$10 000, and ≥$10 000) received by the physician who performed the ICD or CRT-D implantation. Patient group 0 to <$100 included patients of physicians who did not receive payments and patients of physicians who received less than $100 per year. Using a χ2 test, the association between categories of the amount of payment received by a physician who performed an implantation and the quality of care received by patients was examined based on the frequency table analysis. Hierarchical logistic regression was used to examine the association between receipt of payments and quality of care both without adjustment and with adjustment for the relevant confounders, including patient demographics (eg, age, sex); medical history (eg, heart failure, atrial fibrillation); diagnostics (eg, most recent left ventricular ejection fraction [LVEF] %, or QRS duration), procedure information (eg, ICD indication, device type), as well as for the mix of patients among physicians.29
For the ICD Registry data, rate of missingness of variables in this study were rare (<1%) except for the following: LVEF (2.6%), QRS morphology (6.5%), and blood urea nitrogen (2.0%). Imputation of missing values was performed using the study group median for continuous variables and the most common value for categorical variables.30,31 Tests for statistical significance were 2-tailed and evaluated at a significance level of .05. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
During the study period (January 1, 2016-December 31, 2018), a total of 422 961 patients underwent ICD or CRT-D implantation procedures at 1808 hospitals. After excluding 215 299 patients who had undergone a generator change or a lead-only procedure, 61 447 patients who had permanent pacemakers, and 315 patients who received an ICD or CRT-D made by other manufacturers, the final study cohort consisted of 145 900 patients who had undergone first-time ICD or CRT-D implantation performed by 4435 physicians at 1763 hospitals (Figure).
A total of 140 671 patients (96.4%) underwent an ICD or CRT-D implantation performed by 4152 physicians (94%) who received payments. The remaining 5229 patients (3.6%) underwent an ICD or CRT-D implantation performed by 283 physicians (6%) who did not receive payments. Overall, 96% of patients underwent implantations at hospitals that had used ICD or CRT-D devices from more than 1 manufacturer.
Physician Characteristics
Of 4435 physicians in the study cohort, 4152 physicians (94%) received payments from device manufacturers for approximately $20.4 million per year (Table 1). Of those who received payments, 331 physicians (8%) received payments from a single manufacturer, whereas 3821 (92%) received payments from more than 1 manufacturer: 701 (17%) received payments from 2 manufacturers, 1597 (38%) received payments from 3 manufacturers, and 1523 (37%) from all 4 manufacturers. The largest proportion of physicians (43%) received total payments ranging between $1000 and $10 000 or less, while 4% of physicians received total payments exceeding $25 000.
Patients were stratified into 4 groups based on which manufacturer provided the largest total payment to the physician who performed the implantation (Table 1). Overall, the median total annual payments were $1211 per physician (interquartile range [IQR], $390-$3702; range, $2-$323 559). Physicians in group D received highest median payment of $2228 (IQR, $836-$5615) whereas those in group C received the lowest median payment of $978 (IQR, $299-$2963). Physicians received 67% to 74% of their total value of payments from the manufacturer that provided the highest value payment.
Patient Characteristics
Baseline characteristics of patients in the cohort are presented in Table 2. The median age was 65 years (IQR, 18 years); 29.6% were women; 55.4% had ischemic heart disease; and 75.3% had an LVEF of less than 35%. Patients received the following device types: single chamber ICD, 47.8%; dual chamber ICD, 28.4%; and CRT-D, 23.8%. A total of 78.3% of patients received ICD or CRT-D for primary prevention. Overall, 83% of patients received devices at private or community hospitals, and 53% of the patients received devices at teaching hospitals. There were no clinically important differences in patient characteristics among the patient groups.
Table 2. Patient and Hospital Characteristics for Patients Receiving Cardiac Resynchronization Therapy–Defibrillator or Implantable Cardioverter-Defibrillator Among All Patients.
| Characteristic | Patients of physicians who received payments (n = 140 671) | Patients of physicians who received no payments (n = 5229) | Overall (N = 145 900) | ||||
|---|---|---|---|---|---|---|---|
| Group A (n = 35 652) | Group B (n = 64 567) | Group C (n = 29 745) | Group D (n = 10 707) | ||||
| Patient characteristics | |||||||
| Demographics | |||||||
| Age, median (IQR), y | 65 (18) | 65 (18) | 65 (17) | 64 (19) | 64 (18) | 65 (18) | |
| Sex, % | |||||||
| Men | 70.1 | 70.4 | 70.4 | 70.2 | 71.2 | 70.4 | |
| Women | 29.9 | 29.6 | 29.6 | 29.8 | 28.8 | 29.6 | |
| Race/ethnicity, %a | |||||||
| Non-Hispanic White | 74.9 | 77.2 | 76.3 | 71.9 | 74.5 | 76.0 | |
| Non-Hispanic Black | 19.8 | 18.4 | 18.7 | 22.7 | 17.7 | 19.1 | |
| Hispanic | 1.1 | 0.8 | 0.7 | 0.9 | 1.2 | 0.9 | |
| Non-Hispanic Asian | 2.1 | 1.9 | 2.3 | 2.6 | 3.3 | 2.1 | |
| Other | 2.1 | 1.7 | 2.0 | 1.9 | 3.3 | 1.9 | |
| Insurance type, % | |||||||
| Medicare | 52.6 | 53.0 | 54.3 | 51.7 | 51.0 | 53.0 | |
| Private | 31.3 | 31.4 | 31.2 | 30.3 | 31.4 | 31.2 | |
| Medicaid | 10.0 | 10.1 | 9.4 | 12.4 | 12.0 | 10.2 | |
| Other | 6.1 | 5.6 | 5.1 | 5.6 | 5.6 | 5.6 | |
| Indications for an ICD placement, % | |||||||
| Heart failure | 86.0 | 85.7 | 85.8 | 86.0 | 83.9 | 85.8 | |
| Nonischemic dilated cardiomyopathy | 43.7 | 43.3 | 42.6 | 43.7 | 41.9 | 43.3 | |
| Atrial fibrillation | 25.8 | 25.5 | 26.3 | 25.6 | 25.3 | 25.8 | |
| LVEF <35% | 76.1 | 75.3 | 74.9 | 74.9 | 72.3 | 75.3 | |
| QRS duration ≥120 ms | 37.2 | 36.9 | 36.5 | 34.8 | 36.7 | 36.7 | |
| Indication type, % | |||||||
| Primary prevention | 79.2 | 77.8 | 78.5 | 78.6 | 75.9 | 78.3 | |
| Secondary prevention | 20.8 | 22.2 | 21.5 | 21.4 | 24.1 | 21.7 | |
| Selected comorbidities, % | |||||||
| Ischemic heart disease | 54.7 | 55.7 | 56.0 | 54.5 | 53.7 | 55.4 | |
| Diabetes | 38.6 | 38.6 | 38.8 | 38.4 | 37.1 | 38.6 | |
| Chronic lung disease | 17.7 | 19.2 | 18.1 | 18.6 | 18.3 | 18.5 | |
| Cerebrovascular disease | 13.1 | 13.4 | 13.7 | 13.8 | 13.0 | 13.4 | |
| Current dialysis | 3.4 | 3.3 | 3.4 | 4.3 | 3.2 | 3.4 | |
| Device type, % | |||||||
| Single-chamber ICD | 45.9 | 47.8 | 48.3 | 50.5 | 52.2 | 47.8 | |
| Dual-chamber ICD | 28.9 | 28.7 | 27.7 | 27.4 | 26.0 | 28.4 | |
| Cardiac resynchronization therapy–defibrillator | 25.1 | 23.5 | 24.0 | 22.0 | 21.9 | 23.8 | |
| Hospital characteristics | |||||||
| Hospital type, % | |||||||
| Private or community | 85.1 | 82.7 | 83.3 | 79.0 | 78.0 | 83.0 | |
| University | 12.2 | 14.9 | 15.2 | 19.8 | 20.0 | 14.9 | |
| Government | 2.7 | 2.4 | 1.5 | 1.1 | 2.0 | 2.2 | |
| Teaching hospital | 49.4 | 53.3 | 54.4 | 58.7 | 66.0 | 53.4 | |
Abbreviations: CRT-D, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter defibrillator; IQR, interquartile range; LVEF, left ventricular ejection fraction.
Race/ethnicity in the ICD Registry were reported based on the terminology from the US Census Bureau as non-Hispanic White, non-Hispanic Black, Hispanic, Non-Hispanic Asian, and other. Others constitute American Indian/Alaska Native and Native Hawaiian/Pacific Islander.
Association Between Physician Payments and Likelihood of Being Implanted With a Manufacturer’s Device
Between 38.5% and 54.7% of patients received ICD or CRT-D devices from manufacturers that compensated the largest payments to physicians (Table 3). The absolute difference between the proportion of devices from a manufacturer that provided the highest payments in a patient group and the proportion of devices from the same manufacturer in the entire study cohort (expected prevalence) was 22.4% (95% CI, 21.9%-22.9%) for manufacturer A; 14.5% (95% CI, 14.0%-15.0%) for manufacturer B; 18.8% (95% CI, 18.2%-19.4%) for manufacturer C; and 30.6% (95% CI, 30.0%-31.2%) for manufacturer D.
Table 3. Proportion of Patients in Each Patient Group Receiving an Implantable Cardioverter-Defibrillator or Cardiac Resynchronization Therapy-Defibrillator From 4 Manufacturers.
| Patient Group | No. of payments (n = 5229) | Overall (N = 145 900) | Absolute difference between highest proportion and the expected prevalence, % (95% CI)a | ||||
|---|---|---|---|---|---|---|---|
| A (n = 35 652) | B (n = 64 567) | C (n = 29 745) | D (n = 10 707) | ||||
| Proportion of devices implanted from a manufacturer, % | |||||||
| A | 45.4 | 15.8 | 15.6 | 16.0 | 15.3 | 23.0 | 22.4 (21.9-22.9) |
| B | 26.2 | 54.7 | 31.6 | 21.8 | 43.8 | 40.2 | 14.5 (14.0-15.0) |
| C | 22.1 | 24.5 | 47.7 | 23.8 | 32.4 | 28.9 | 18.8 (18.2-19.4) |
| D | 6.3 | 4.9 | 5.0 | 38.5 | 8.5 | 7.9 | 30.6 (30.0-31.2) |
| Absolute difference between highest proportion and second-highest proportion within patient group (95% CI)b | 19.2 (18.5-19.9) | 30.2 (29.7-30.7) | 16.1 (15.4-16.9) | 14.7 (13.5-15.9) | |||
The difference between the proportion of devices from a manufacturer that provided the highest payments in a patient group and the proportion of devices from the same manufacturer in the entire study cohort.
The difference between the proportion of devices from a manufacturer that provided the highest payments in a patient group and the proportion of devices from the second commonly used manufacturer in the same patient group.
Within the subgroup of patients of physicians who received more than $25 000 annually, 51.1% to 59.5% of patients received an ICD or CRT-D device from the manufacturers that provided the largest payment (eTable 1 in the Supplement).
Patients were substantially more likely to receive ICD or CRT-D devices made by the manufacturer that provided the highest total payment than were patients of physicians who received no payment: the odds ratios (ORs) were 6.07 (95% CI, 4.37-8.44) for manufacturer A; 2.06 (95% CI, 1.57-2.70) for manufacturer B; 2.62 (95% CI, 1.97-3.48) for manufacturer C; and 17.00 (95% CI, 10.99-26.29) for manufacturer D (all P < .001) (eTable 2 in the Supplement). Findings were similar when the study cohort was limited to patients of physicians who received more than 75% of payments from 1 manufacturer (eTable 3 in the Supplement).
In patient groups A and D, patients of physicians who received high-value payments (>$10 000 per year) from the manufacturer were more likely to receive a device from the same manufacturer than were patients of physicians who received small-value payments (<$100) (Table 4). In patient group B, patients of physicians who received high-value payments from manufacturer B were less likely to receive a device from manufacturer B compared with patients of physicians who received small-value payments. The odds of patients in group C receiving a device from manufacturer C did not differ across payment categories. Findings were similar when the study cohort was limited to patients of physicians who received more than 75% of total payments from 1 of the 4 manufacturers (eTable 4 in the Supplement) and after excluding hospitals in which devices from only 1 manufacturer were available (eTable 5 in the Supplement).
Table 4. Association Between Value of Payments and Likelihood of Receiving an Implantable Cardioverter Defibrillator or Cardiac Resynchronization Therapy–Defibrillator From Specific Device Manufacturers.
| Patient group | ||||
|---|---|---|---|---|
| A | B | C | D | |
| No. of patients | 35 652 | 64 567 | 29 745 | 10 707 |
| Total patients per payment category (%) | ||||
| Total value of payment per year | ||||
| $1 to <$100 | 3465 (9.7) | 5006 (7.8) | 2892 (9.7) | 379 (3.5) |
| $100 to <1000 | 9985 (28.0) | 28 669 (44.4) | 13 130 (44.1) | 2201 (20.6) |
| $1000 to <10 000 | 15 948 (44.7) | 26 954 (41.8) | 10 053 (33.8) | 6749 (63.0) |
| ≥$10 000 | 6254 (17.5) | 3938 (6.1) | 3670 (12.3) | 1378 (12.9) |
| Patients who received an ICD or CRT-D from the same manufacturer (%)a | ||||
| Total value of payment per year | ||||
| $1 to<$100 | 1245/3465 (35.9) | 2961/5006 (59.2) | 1301/2892 (45.0) | 82/379 (21.6) |
| $100 to <1000 | 4641/9985 (46.5) | 15 140/28 669 (52.8) | 5469/13 130 (41.7) | 866/2201 (39.4) |
| $1000 to <10 000 | 6986/15 948 (43.8) | 15 230/26 954 (56.5) | 5428/10 053 (54.0) | 2331/6749 (34.5) |
| ≥$10 000 | 3315/6254 (53.0) | 2017/3938 (51.2) | 2000/3670 (54.5) | 838/1378 (60.8) |
| Likelihood of receiving an ICD or CRT-D from the same manufacturera | ||||
| Total value of payment per year, OR (95% CI)b | ||||
| $1-<$100 | 1 [Reference]c | |||
| $100-<1000 | 1.65 (1.08-2.53) |
0.66 (0.49-0.89) |
0.90 (0.60-1.35) |
2.62 (0.99-6.91) |
| $1000-<10 000 | 1.24 (0.81-1.91) |
0.69 (0.51-0.93) |
1.48 (0.96-2.28) |
2.21 (0.87-5.62) |
| ≥$10 000 | 2.03 (1.21-3.43) |
0.38 (0.25-0.60) |
1.64 (0.93-2.91) |
6.57 (2.09-20.67) |
Abbreviation: CRT-D, cardiac resynchronization therapy–defibrillator; ICD, implantable cardioverter defibrillator; OR, odds ratio.
The same manufacturer indicates the manufacturer that provide highest payments to the physicians who performed ICD or CRT-D implantation.
Adjusted for the patients clustering among physicians and physician procedure volume (procedural volume reflects procedures reported in the registry and may not be reflective of total procedural volume for any given physician).
Reference was patients of physicians who received payments between $1 and<$100 in the corresponding patient group. Only physicians who received payments were included, so those who did not receive any payments were excluded.
Quality of Care
Overall rates of death or in-hospital complication were 1.0%; 84.6% of patients eligible for CRT-D therapy received a biventricular device and 85.3% of patients were prescribed guideline-recommended medications at discharge. Unadjusted rates of in-hospital procedural complications or death did not vary by presence or total value of physician payments. There were statistically significant differences in the use of CRT-D among eligible patients, with higher rates of CRT-D use among physicians receiving high-value payments (78.1% in $0-<$100 per year group vs 87.5% in ≥$10 000 per year). Use of guideline-recommended medications at discharge also varied modestly across payment groups (eTable 6 in the Supplement). For detailed individual measures of in-hospital procedural-related complications or death, see eTable 7 in the Supplement. In adjusted analyses, patients of physicians receiving high-value payments were more likely to receive CRT-Ds when indicated (>$10 000 per year: OR, 2.33; 95% CI, 1.89-2.87) and guideline recommended medications at discharge (>10 000 per year: OR, 1.19; 95% CI, 1.03-1.39) than patients of physicians receiving $0 to $100. However, no consistent differences were observed for risk of in-hospital complications or death (eTable 8 in the Supplement).
Discussion
In this analysis of the NCDR ICD Registry, 96.4% of patients underwent first-time ICD or CRT-D implantations performed by physicians who had financial relationships with device manufacturers. Patients were more likely to receive an ICD or CRT-D device from the manufacturer that provided the highest total payment to the physician who performed an ICD or CRT-D implantation than each other manufacturer individually. Physicians who received payments were more likely to implant a CRT-D when indicated.
The factors that guide device selection have not been well characterized but are likely complex, including patient, device, hospital, and physician considerations.9,10,11,12,27,28 Patients may express a preference for a device from a particular manufacturer based on experiences of family and friends. Furthermore, there are clinical scenarios that may dictate the use of a particular manufacturer’s device. For example, a patient requiring a subcutaneous ICD could only receive a device by 1 manufacturer. Similarly, differences in device functionality, such as compatibility with magnetic resonance imaging, lead characteristics, and battery life may influence clinical decision-making.9,32 In addition, hospital contracting could restrict device choice.33 However, in this study, devices from a single manufacturer were used in 236 hospitals (13%).
Nevertheless, it is likely that physician choice may be an important determinant of device selection. This study’s findings raise the possibility that payments from device manufacturers may influence the physician’s choice in selection of a manufacturer’s device. However, this may not be the only contributing factor. Physicians who perform ICD or CRT-D implantations may develop a preference for a specific line of products based on years of experience and familiarity. Working with a small number of devices may improve efficiency of clinical care, particularly in the outpatient setting. For example, it may be easier for staff to perform device interrogations and access remote monitoring data of device from 1 or 2 manufacturers.
Prior studies evaluating the association between physician payments from industry and practice patterns have largely focused on prescription medications.7,8,24 The current study builds on this work, providing insights into the association between industry payments to physicians and selection of medical devices. The strengths of associations between payments and device selection were higher with device selection in this study (OR range, 2.06-17.00) than associations reported in previous studies involving prescription patterns for medications (OR range, 1.18-2.18).7,8,24
The underlying reasons for differences in strength of association between payments and medication choice vs payments and device choice are unclear and may be attributable to differences in methods and data sources. Conversely, the observed variation may represent real differences in the potential effect of industry payments in diverse contexts. One difference is that there are relatively few options for ICDs compared with the much larger array of medications available for common conditions such as hypertension and dyslipidemia.7,8 The frequency of interactions between physicians and representatives of device manufacturers may differ from that of representatives from pharmaceutical companies. Representatives from device manufacturers are often present during implantation and may assist with device interrogation, remote monitoring, and reprogramming.
Although medical device manufacturers promote patient care by supporting training for physicians in the latest advancements in technology, sponsoring scientific sessions, and funding clinical research, there are concerns about possible undue bias introduced by physician-industry interactions. A recent study that explored physicians’ attitudes toward the Sunshine Act reported that most physicians were unaware or unconvinced that industry payments influenced their practice patterns.34 However, studies have consistently found that any kind of industry-physician interaction, even small-value payments, may be associated with measurable differences in prescribing patterns.8,24
To our knowledge, the current study is likely the first to evaluate the association between payments to physicians and quality of care. For 2 of the 3 measures of quality, such as prescription of guideline-recommended therapy or in-hospital complications or death, there was no consistent relationship observed with physician payments. However, use of CRT-D was higher among patients of physicians who received high-value payments. This finding may be clinically relevant because CRT-D may improve the survival of eligible patients.35 The data from this study suggest that the presence of payments from device manufacturers was not a reliable marker of in-hospital quality care. However, additional studies assessing the association between receipt of payments and longer-term outcomes may be warranted.
Limitations
This study has several limitations. First, due to the observational, cross-sectional nature of the data, this study could not ascertain whether implanting the devices preceded or followed a physician’s receipt of payments. Thus, it remains uncertain whether payments led to the selection of a particular device or rewarded specific behaviors, or, contrarily, were unrelated to physician practice patterns. Therefore, findings identify associations but no temporality or causality. Second, the current study cannot exclude the possibility of unmeasured confounders, including patients’ preferences, patients’ clinical factors that may warrant one device over another, and differences in devices, such as battery life, that might play a role in the selection of an implant.32 Third, relatively little is known about the comparative effectiveness of devices from various manufacturers used between 2016 and 2018.9,10,11,12,13 The present study does not provide evidence about the potential for differences in long-term outcomes associated with device selection. Fourth, due to the structure of Open Payments Program data, this study was unable to assess whether the strength of association between payments and device selection vary by different types of general payments, such as payments made for food and beverage or consulting fees. Fifth, only general payments made to physicians were evaluated in this study, and the analysis did not include payments related to research and ownership. However, as prior research estimated that only 1% of physicians held ownership interest and 2% of total research payments were made to individual physicians.23,24 Sixth, the study did not include all device implantations because the CMS mandates that implantations performed on Medicare beneficiaries for primary prevention be recorded in the ICD Registry. Nevertheless, 80% of hospitals report all implantations, including non-Medicare beneficiaries and those for secondary prevention.15,17 Seventh, inaccuracies and inconsistencies in the Open Payments Program data have been reported.19 Although CMS has improved the accuracy, consistency, and precision of the payment data, some of the payments included in the analysis may be inaccurate.36
Conclusions
In this cross-sectional study, a large proportion of ICD or CRT-D implantations were performed by physicians who received payments from device manufacturers. Patients were more likely to receive ICD or CRT-D devices from the manufacturer that provided the highest total payment to the physician who performed an ICD or CRT-D implantation than each other manufacturer individually.
eTable 1. The Proportion of Patients in Each Patient Group Implanted with Devices by Physicians Who Received Payments of >$25,000 per year
eTable 2. Association Between Receipt of Payment and Likelihood of Receiving Implantable Cardioverter-Defibrillator or Cardiac Resynchronization Therapy–Defibrillator from Specific Device Manufacturers (N=145,900)
eTable 3. Association Between Receipt of Payment and Likelihood of Receiving Implantable Cardioverter-Defibrillator or Cardiac Resynchronization Therapy–Defibrillator from Specific Device Manufacturers among Patients of Physicians Who Received More than 75% of Payments from 1 Manufacturer (N=61,460)
eTable 4. Association Between Value of Payments and Likelihood of Receiving Implantable Cardioverter-Defibrillator or Cardiac Resynchronization Therapy–Defibrillator from Specific Manufacturers (Among patients of physicians who received 75% or more of total payments from one of the four manufacturers)
eTable 5. Association Between Value of Payments and Receipt of Implantable Cardioverter-Defibrillator or Cardiac Resynchronization Therapy–Defibrillator from Specific Manufacturers (sensitivity analysis after excluding hospitals in which devices from only one manufacturer were available among patients of physicians who received payments)
eTable 6. Quality of Care by Physician Payments
eTable 7. In-hospital Complications by Physician Payments
eTable 8. Association Between Value of Physician Payments and Quality of Care
References
- 1.Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283(3):373-380. doi: 10.1001/jama.283.3.373 [DOI] [PubMed] [Google Scholar]
- 2.Campbell EG, Gruen RL, Mountford J, Miller LG, Cleary PD, Blumenthal D. A national survey of physician-industry relationships. N Engl J Med. 2007;356(17):1742-1750. doi: 10.1056/NEJMsa064508 [DOI] [PubMed] [Google Scholar]
- 3.Merino JG. Physician payment Sunshine Act. BMJ. 2013;347:f4828. doi: 10.1136/bmj.f4828 [DOI] [PubMed] [Google Scholar]
- 4.Agrawal S, Brennan N, Budetti P. The Sunshine Act—effects on physicians. N Engl J Med. 2013;368(22):2054-2057. doi: 10.1056/NEJMp1303523 [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal MB, Mello MM. Sunlight as disinfectant—new rules on disclosure of industry payments to physicians. N Engl J Med. 2013;368(22):2052-2054. doi: 10.1056/NEJMp1305090 [DOI] [PubMed] [Google Scholar]
- 6.The Facts About Open Payments Data https://openpaymentsdata.cms.gov/summary. Accessed May 10, 2020.
- 7.Yeh JS, Franklin JM, Avorn J, Landon J, Kesselheim AS. Association of industry payments to physicians with the prescribing of brand-name statins in Massachusetts. JAMA Intern Med. 2016;176(6):763-768. doi: 10.1001/jamainternmed.2016.1709 [DOI] [PubMed] [Google Scholar]
- 8.DeJong C, Aguilar T, Tseng C-W, Lin GA, Boscardin WJ, Dudley RA. Pharmaceutical industry-sponsored meals and physician prescribing patterns for Medicare beneficiaries. JAMA Intern Med. 2016;176(8):1114-1122. doi: 10.1001/jamainternmed.2016.2765 [DOI] [PubMed] [Google Scholar]
- 9.von Gunten S, Schaer BA, Yap SC, et al. Longevity of implantable cardioverter defibrillators: a comparison among manufacturers and over time. Europace. 2016;18(5):710-717. doi: 10.1093/europace/euv296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer DB, Hatfield LA, McGriff D, et al. Transvenous implantable cardioverter-defibrillator lead reliability: implications for postmarket surveillance. J Am Heart Assoc. 2015;4(6):e001672. doi: 10.1161/JAHA.114.001672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horlbeck FW, Mellert F, Kreuz J, Nickenig G, Schwab JO. Real-world data on the lifespan of implantable cardioverter-defibrillators depending on manufacturers and the amount of ventricular pacing. J Cardiovasc Electrophysiol. 2012;23(12):1336-1342. doi: 10.1111/j.1540-8167.2012.02408.x [DOI] [PubMed] [Google Scholar]
- 12.Landolina M, Curnis A, Morani G, et al. Longevity of implantable cardioverter-defibrillators for cardiac resynchronization therapy in current clinical practice: an analysis according to influencing factors, device generation, and manufacturer. Europace. 2015;17(8):1251-1258. doi: 10.1093/europace/euv109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross JS, Bates J, Parzynski CS, et al. Can machine learning complement traditional medical device surveillance? a case study of dual-chamber implantable cardioverter-defibrillators. Med Devices (Auckl). 2017;10:165-188. doi: 10.2147/MDER.S138158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kremers MS, Hammill SC, Berul CI, et al. The National ICD Registry Report: version 2.1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 2013;10(4):e59-e65. doi: 10.1016/j.hrthm.2013.01.035 [DOI] [PubMed] [Google Scholar]
- 15.Masoudi FM, Oetgen WJ. The NCDR ICD registry: a foundation for quality improvement. J Am Coll Cardiol. 2017;70(13):1673-1674. doi: 10.1016/j.jacc.2017.08.021 [DOI] [PubMed] [Google Scholar]
- 16.McClellan MB, Tunis SR. Medicare coverage of ICDs. N Engl J Med. 2005;352(3):222-224. doi: 10.1056/NEJMp048354 [DOI] [PubMed] [Google Scholar]
- 17.Masoudi FA, Ponirakis A, de Lemos JA, et al. Trends in US cardiovascular care: 2016 report from 4 ACC national cardiovascular data registries. J Am Coll Cardiol. 2017;69(11):1427-1450. doi: 10.1016/j.jacc.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 18.Messenger JC, Ho KK, Young CH, et al. ; NCDR Science and Quality Oversight Committee Data Quality Workgroup . The National Cardiovascular Data Registry (NCDR) data quality brief: the NCDR data quality program in 2012. J Am Coll Cardiol. 2012;60(16):1484-1488. doi: 10.1016/j.jacc.2012.07.020 [DOI] [PubMed] [Google Scholar]
- 19.Agrawal S, Brown D. The physician payments Sunshine Act—two years of the open payments program. N Engl J Med. 2016;374(10):906-909. doi: 10.1056/NEJMp1509103 [DOI] [PubMed] [Google Scholar]
- 20.Annapureddy A, Murugiah K, Minges KE, Chui PW, Desai N, Curtis JP. Industry payments to cardiologists. Circ Cardiovasc Qual Outcomes. 2018;11(12):e005016. doi: 10.1161/CIRCOUTCOMES.118.005016 [DOI] [PubMed] [Google Scholar]
- 21.Annapureddy A, Sengodan P, Mahajan S, et al. Distribution of industry payments among medical directors of catheterization and electrophysiology laboratories from the top 100 US hospitals. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2018.8775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Natures of payment. Centers for Medicare & Medicaid Services. Last modified November 12, 2019, Accessed May 10, 2020. https://www.cms.gov/OpenPayments/About/Natures-of-Payment
- 23.Tringale KR, Marshall D, Mackey TK, Connor M, Murphy JD, Hattangadi-Gluth JA. Types and distribution of payments from industry to physicians in 2015. JAMA. 2017;317(17):1774-1784. doi: 10.1001/jama.2017.3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleischman W, Agrawal S, King M, et al. Association between payments from manufacturers of pharmaceuticals to physicians and regional prescribing: cross sectional ecological study. BMJ. 2016;354:i4189-i4189. doi: 10.1136/bmj.i4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tracy CM, Epstein AE, Darbar D, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society . 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. [corrected]. Circulation. 2012;126(14):1784-1800. doi: 10.1161/CIR.0b013e3182618569 [DOI] [PubMed] [Google Scholar]
- 26.Hunt SA, Abraham WT, Chin MH, et al. ; American College of Cardiology Foundation; American Heart Association . 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53(15):e1-e90. doi: 10.1016/j.jacc.2008.11.013 [DOI] [PubMed] [Google Scholar]
- 27.Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298(13):1525-1532. doi: 10.1001/jama.298.13.1525 [DOI] [PubMed] [Google Scholar]
- 28.Farmer SA, Kirkpatrick JN, Heidenreich PA, Curtis JP, Wang Y, Groeneveld PW. Ethnic and racial disparities in cardiac resynchronization therapy. Heart Rhythm. 2009;6(3):325-331. doi: 10.1016/j.hrthm.2008.12.018 [DOI] [PubMed] [Google Scholar]
- 29.Dewland TA, Pellegrini CN, Wang Y, Marcus GM, Keung E, Varosy PD. Dual-chamber implantable cardioverter-defibrillator selection is associated with increased complication rates and mortality among patients enrolled in the NCDR implantable cardioverter-defibrillator registry. J Am Coll Cardiol. 2011;58(10):1007-1013. doi: 10.1016/j.jacc.2011.04.039 [DOI] [PubMed] [Google Scholar]
- 30.Betz JK, Katz DF, Peterson PN, et al. outcomes among older patients receiving implantable cardioverter-defibrillators for secondary prevention: from the NCDR ICD registry. J Am Coll Cardiol. 2017;69(3):265-274. doi: 10.1016/j.jacc.2016.10.062 [DOI] [PubMed] [Google Scholar]
- 31.Kawata H, Bao H, Curtis JP, et al. Cardiac resynchronization defibrillator therapy for nonspecific intraventricular conduction delay versus right bundle branch block. J Am Coll Cardiol. 2019;73(24):3082-3099. doi: 10.1016/j.jacc.2019.04.025 [DOI] [PubMed] [Google Scholar]
- 32.Zungsontiporn N, Loguidice M, Daniels J. Important parameters for implantable cardioverter defibrillator selection. Card Electrophysiol Clin. 2018;10(1):145-152. doi: 10.1016/j.ccep.2017.11.015 [DOI] [PubMed] [Google Scholar]
- 33.Lind KD. Understanding the market for implantable medical devices. AARP Public Policy Institute. Published August 2017. Accessed May 10, 2020. https://www.aarp.org/content/dam/aarp/ppi/2017/08/understanding-the-market-for-implantable-medical-devices.pdf
- 34.Chimonas S, DeVito NJ, Rothman DJ. Bringing transparency to medicine: exploring physicians’ views and experiences of the Sunshine Act. Am J Bioeth. 2017;17(6):4-18. doi: 10.1080/15265161.2017.1313334 [DOI] [PubMed] [Google Scholar]
- 35.Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64(10):1047-1058. doi: 10.1016/j.jacc.2014.06.1178 [DOI] [PubMed] [Google Scholar]
- 36.Open payments data: review of accuracy precision, and consistency in reporting. US Dept of Health and Human Services. Published August 3, 2018. Accessed May 10, 2020. https://oig.hhs.gov/oei/reports/oei-03-15-00220.asp
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. The Proportion of Patients in Each Patient Group Implanted with Devices by Physicians Who Received Payments of >$25,000 per year
eTable 2. Association Between Receipt of Payment and Likelihood of Receiving Implantable Cardioverter-Defibrillator or Cardiac Resynchronization Therapy–Defibrillator from Specific Device Manufacturers (N=145,900)
eTable 3. Association Between Receipt of Payment and Likelihood of Receiving Implantable Cardioverter-Defibrillator or Cardiac Resynchronization Therapy–Defibrillator from Specific Device Manufacturers among Patients of Physicians Who Received More than 75% of Payments from 1 Manufacturer (N=61,460)
eTable 4. Association Between Value of Payments and Likelihood of Receiving Implantable Cardioverter-Defibrillator or Cardiac Resynchronization Therapy–Defibrillator from Specific Manufacturers (Among patients of physicians who received 75% or more of total payments from one of the four manufacturers)
eTable 5. Association Between Value of Payments and Receipt of Implantable Cardioverter-Defibrillator or Cardiac Resynchronization Therapy–Defibrillator from Specific Manufacturers (sensitivity analysis after excluding hospitals in which devices from only one manufacturer were available among patients of physicians who received payments)
eTable 6. Quality of Care by Physician Payments
eTable 7. In-hospital Complications by Physician Payments
eTable 8. Association Between Value of Physician Payments and Quality of Care


