Abstract
Objective
To assess whether exposure to high temperatures in pregnancy is associated with increased risk for preterm birth, low birth weight, and stillbirth.
Design
Systematic review and random effects meta-analysis.
Data sources
Medline and Web of Science searched up to September 2018, updated in August 2019.
Eligibility criteria for selecting studies
Clinical studies on associations between high environmental temperatures, and preterm birth, birth weight, and stillbirths.
Results
14 880 records and 175 full text articles were screened. 70 studies were included, set in 27 countries, seven of which were countries with low or middle income. In 40 of 47 studies, preterm births were more common at higher than lower temperatures. Exposures were classified as heatwaves, 1°C increments, and temperature threshold cutoff points. In random effects meta-analysis, odds of a preterm birth rose 1.05-fold (95% confidence interval 1.03 to 1.07) per 1°C increase in temperature and 1.16-fold (1.10 to 1.23) during heatwaves. Higher temperature was associated with reduced birth weight in 18 of 28 studies, with considerable statistical heterogeneity. Eight studies on stillbirths all showed associations between temperature and stillbirth, with stillbirths increasing 1.05-fold (1.01 to 1.08) per 1°C rise in temperature. Associations between temperature and outcomes were largest among women in lower socioeconomic groups and at age extremes. The multiple temperature metrics and lag analyses limited comparison between studies and settings.
Conclusions
Although summary effect sizes are relatively small, heat exposures are common and the outcomes are important determinants of population health. Linkages between socioeconomic status and study outcomes suggest that risks might be largest in low and middle income countries. Temperature rises with global warming could have major implications for child health.
Systematic review registration
PROSPERO CRD 42019140136 and CRD 42018118113.
Introduction
The frequency and intensity of heatwaves and other extreme weather events is increasing rapidly owing to climate change and is set to escalate in the coming decades.1 Heatwaves and rising mean temperatures both present major health threats, especially for populations with limited physiological ability or socioeconomic means to respond or adapt to high temperatures.2
Pregnant women have only recently been included among the groups most vulnerable to heat stress,3 4 and it will take time for that recognition to translate into action. Studies in 2011 and 2015, for example, showed that the large majority of heatwave response plans in European Union countries had not identified pregnant women as a high risk group.5 6 The public also appears largely unaware of the risks of heat exposure during pregnancy.7
Several factors could account for the relative neglect of maternal and newborn health in research and policies on the impacts of, and adaptation to, climate change. Perhaps most notably, challenges with the measurement of exposures and adverse outcomes in maternal and newborn health make it difficult to attribute a single cause, such as heat exposure, to an outcome. This complexity is heightened by the lag times between heat exposure in pregnancy and adverse outcomes, which can be several months with congenital anomalies, for example, as these anomalies might become apparent only at birth. Additionally, lag times can even be decades, with outcomes that manifest in adulthood.8 9 10 Much of the research on climate change and health in low and middle income countries has centred on infectious diseases such as malaria, and in high income countries, the focus has been on heat related mortality among groups such as elderly people or those with chronic conditions.
Pregnancy raises the vulnerability of women to environmental hazards, including exogenous heat. The physiological and anatomical changes that occur during pregnancy pose particular challenges to thermoregulation.11 Internal heat production rises with fetal and placental metabolism, and with increased body mass and the resulting physical strain.12 Equally, pregnancy could bring social vulnerabilities to the fore, especially in low and middle income countries, where women often continue to perform household chores during pregnancy (eg, fetching wood and water, and subsistence farming). Exposure to high temperatures in agricultural and other outdoor work, could occur before the pregnancy is recognised,13 14 and, even late in pregnancy, poorer women might work beyond their heat tolerance limits to avoid losing pay.15
Systematically documenting the association of heat exposure with maternal and newborn health is important for quantifying the overall burden of climate related conditions, especially as adverse birth outcomes, such as prematurity, contribute many person years lost to these estimates.16 Measuring disease burden, in turn, is key to increasing the resources allocated to adaptation services for pregnant women, for tracking progress over time, and for supporting arguments for mitigation of climate change. Moreover, in order to design interventions to avoid the effect of heat on health, we need a clearer understanding of the different pathways from exposure to outcomes, and how these vary across settings and between population groups. Previous systematic reviews have summed the evidence for associations between exposure to high temperatures and maternal and newborn health outcomes.17 18 19 20 21 22 We update those reviews, with a focus on temperature connections with preterm birth, birth weight, and stillbirth. In particular, we examine the extent to which varying heat exposures (including heatwaves, increments per 1°C, and temperature threshold effects) affect these outcomes.
Methods
The review forms part of a larger systematic mapping survey of the impacts of high temperatures and adaptation interventions on health.23 In the mapping review, we identified heat exposure studies, defined as articles that assessed the effect on health of exposure to high temperatures, but did not include an intervention. We also identified heat adaptation studies, which assessed the outcomes of interventions aiming to counter the impacts of heat exposure.
In brief, in September 2018 we searched Medline (PubMed), Science Citation Index Expanded, Social Sciences Citation Index, and Arts and Humanities Citation Index (supplementary file 1).23 The search identified studies on exposure to high temperatures and not those that examined the impacts of low temperatures. Screening of titles and abstracts was done independently in duplicate, with any differences reconciled by a third reviewer or the principal investigator (MFC).
All studies that were classified as heat exposure studies or heat adaptation studies were then screened to identify papers that reported the association between heat in pregnancy and the review outcomes (preterm birth, birth weight, and stillbirth). An additional search using simplified search terms was done on Medline (PubMed) in September 2019 to locate articles published since the date of the initial search (supplementary file 1).24 Reference lists of included articles were also screened.
We included studies only on humans, published in Chinese, English, German, or Italian, and studies on heat related to weather and not heat sources such as saunas and hot baths. No date restrictions were applied. All study designs were eligible except systematic reviews, and studies that modelled the potential or hypothetical associations between heat exposure and the review outcomes. EPPI-Reviewer version 4 software25 provided a platform for management of articles, screening of titles, abstracts, and full text articles, and for data extraction. No contact was made with authors to obtain additional information.
Preterm birth was defined as a live birth before 37 completed weeks of gestation,26 but we also included studies reporting on the duration of gestation as a continuous variable. We incorporated studies that reported either birth weight in live births as a binary variable, low birth weight (weight <2500 g),26 or as a continuous variable. Studies that assessed temperature associations with death of a fetus after more than 20 weeks’ gestation were included in the stillbirth analyses.26
Data extraction
Eligible articles were extracted independently in duplicate and all reconciled by the principal investigator. Data were extracted on the following study characteristics: study location, dates when outcomes were measured, study design, and the way in which the temperature metrics had been used (supplementary tables 1a-c). Data extracted on outcomes included assessments of different lag periods and results in population subgroups (supplementary tables 2a-c). We did not extract data on the impacts of humidity, air pollution, or low temperatures (except in assessments of the shape of associations). Finally, single reviewers completed the critical appraisal checklist for analytical cross sectional studies developed by the Joanna Briggs Institute to assess the risk of bias (supplementary table 1d).27 Studies that were scored “yes” on six or fewer of the eight itemswere considered at high risk of bias.28
Data synthesis
We describe patterns in study characteristics over time and between study settings. We paid specific attention to analysing studies in low and middle income countries, and outcomes in population subgroups. We attempted to identify particular critical gestational periods or windows when exposure to heat might have had the largest impacts.
We classified temperature metrics into four groups. Firstly, heatwaves defined as two or more days with temperatures above a predefined threshold. Where multiple significant outcomes were reported, we used the effect size of a two day heatwave, with temperatures at or above the 90th centile. Secondly, changes in frequency of the outcome per 1°C rise in temperature, where we used effect estimates of lags on individual days (or date of birth) or of periods up to four weeks. The last two groups consisted of temperatures dichotomised as above and below a threshold level to assess the impacts of temperature on the whole of pregnancy or a full trimester (the third group); or in shorter periods, such as individual days or up to four weeks (fourth group).
In the third and fourth groups of temperature metrics, owing to considerable diversity in the way in which time and lag variables were used, we report the average combined effect (odds ratio) of the occurrence of the outcome at high versus low temperatures and are unable to provide specific temperature cutoff points for these. The high threshold level signals temperatures above the 75th, 90th, and 99th centile, for example, depending on individual studies. Fourteen studies did not report outcomes in any of these four categories and were not included in forest plots or summary measures.29 30 31 32 33 34 35 36 37 38 39 40 41 42 43
In studies that reported several significant outcomes, we selected the measure with the highest effect size. Given that the lag or temperature threshold at which impacts are largest is unknown and will probably vary between populations, the highest estimate might be the true highest level, rather than the result of multiple testing. As studies had assessed outcomes in a range of ways, we used the available statistics to convert effect measures to a common measure, allowing us to calculate an average combined effect, where possible. We attempted to explain statistical heterogeneity, including using meta-regression, to identify effect modification. We used random effects meta-analysis to describe the average exposure effect of temperature, with the summary statistic presented as an odds ratio.44 The decision on whether meta-analysis was appropriate was based on statistical heterogeneity as measured by the I2 statistic, and the direction and magnitude of the effect sizes. In instances where there were major outlier studies, we present the meta-analysis measures with and without the outlier studies and propose possible explanations for variations between studies. If meta-analysis was deemed inappropriate, we summarise the effect estimates, providing information on the magnitude and range of effects (median and interquartile range).28 Data syntheses do not assume that associations between absolute temperature levels and the outcomes are the same for different areas, but rather that the setting specific relative changes in temperature are sufficiently similar to combine into a synthesis measure (eg, comparing the preterm birth rate when the temperature is above and below the 90th centile in different settings). Low quality studies were included in the summary measures as their exclusion had little or no impact on the findings.
For analyses of differential impacts by socioeconomic status, we classified women with low education levels as having a low socioeconomic status if income data were missing. We aimed to assess changes in heat sensitivity over time, by comparing outcomes of studies conducted in the same area over time, or in studies that had stratified their findings by time period. The climate in each study site was classified into Köppen-Geiger climate zones, and studies ordered by latitude in the forest plot figures.45
Patient and public involvement
Patients were not involved in the development of the research question or its outcome measures, conduct of the research, or preparation of the manuscript owing to funding constraints and as the research involved review of published data.
Results
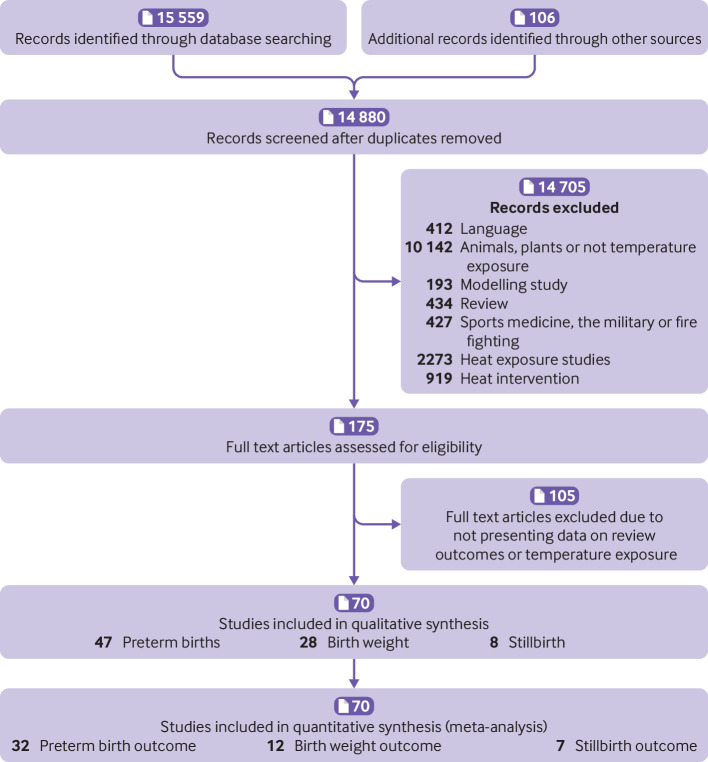
The search in the mapping review identified 14 880 non-duplicate articles (PRISMA flowchart, fig 1). None of the 919 studies coded as heat intervention studies included data on pregnant women and were all excluded. Overall, 2273 articles were classified as heat exposure studies, and 175 of these selected for full text screening (7.7%). In total, 70 studies were eligible for this review (13 presented data on more than one review outcome). Most studies covered impacts of heat exposure on preterm birth (n=47), 28 presented data on birth weight, and eight on stillbirth (supplementary table 1a-c). Most studies were time series analyses using population registries or birth records from facilities (n=58), 10 used case crossover methods, and two employed a case-control design.
Fig 1.
PRISMA flow diagram
The number of studies per year increased markedly over time, from only two studies before the year 2000, to 22 from 2000 to 2015, and to 46 thereafter (66% of the 70 eligible studies). The studies covered 24 countries, seven of which were low and middle income countries, consisting of none in lower income countries, one in lower middle income countries, and six in upper middle income countries). Most studies were set in North America (20), the European Union (17), or Australia and New Zealand (n=7). Two studies were done in sub-Saharan Africa. Six articles reported studies in China, all published since 2016. Articles were published a median of six years after the birth of the infants, and only three studies reported data on births that had occurred since 2015 (4%).
Temperature and lag windows were classified in a number of ways. In total, 33 (70%) of 47 papers on preterm birth were classified as high quality, as were 14 (50%) of 28 studies on birth weight, and seven (87.5%)of eight on stillbirths (supplementary table 1d). Weaknesses in research quality pertained mostly to the lack of a detailed description of the study population and setting (23 (33%) of 70 studies) and use of inappropriate analytical methods (18 (26%) of 70 studies). In most cases the study inclusion criteria and outcomes were well defined (65 (93%) and 66 (94%) of 70 studies, respectively).
Preterm births analysis
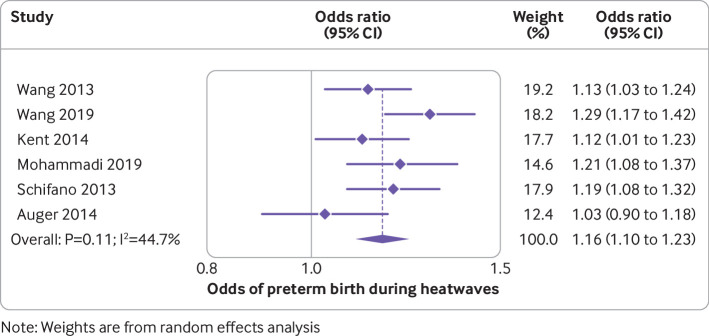
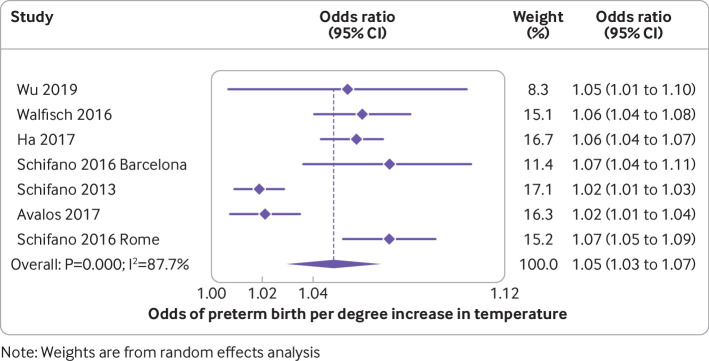
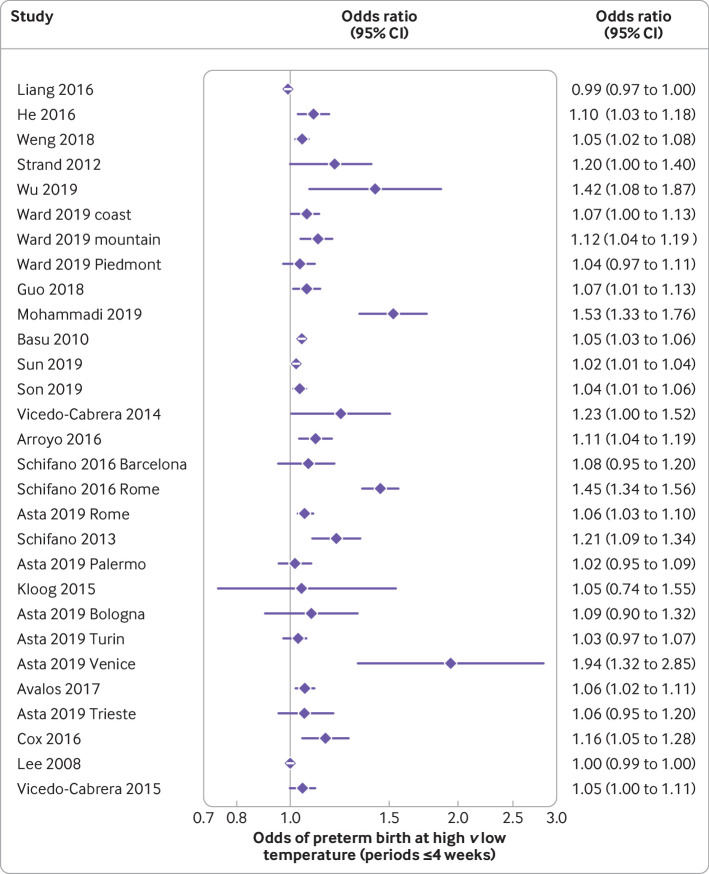
The median preterm birth rate of the included studies was 5.6% (interquartile range 5.0-7.9; range 2.6-15.5; supplementary table 2a). Of the 47 studies on preterm births, 40 documented an association between high temperatures and preterm birth. In meta-analysis of six studies, the odds of a preterm birth during a heatwave were 1.16-fold higher than on non-heatwave days (95% confidence interval 1.10 to 1.23; I2=44.7%; table 1, fig 2). Although there was considerable heterogeneity in estimates of the odds of preterm birth for each 1°C increase in temperature (I2=87.7%), all estimates showed significant effects in the same direction. Meta-analysis of this outcome showed an average odds of a preterm birth increased by 1.05 for each 1°C increase in temperature (95% confidence interval 1.03 to 1.07; fig 3). The summary measure of associations between exposure to high temperatures over a trimester or all of pregnancy was odds ratio 1.14 (95% confidence interval 1.11 to 1.16; I2=88.2%; supplementary fig 1). An analysis that excluded a study in Shenzhen, China that reported a protective effect of high temperatures,46 reduced the heterogeneity, but the overall estimate was similar. The authors of that study ascribed their findings to the high use of air conditioning in the study area, which had reduced exposure to high temperatures. The median odds ratio for preterm birth after exposure to high temperatures over short periods (<4 weeks) was 1.07 (interquartile range 1.05-1.16; supplementary fig 2). This value was slightly larger than the estimate in a meta-analysis involving 19 studies that excluded the Shenzhen study, and another in London where women had been exposed only to mild temperatures (odds ratio 1.0; 95% confidence interval 0.99 to 1.0; fig 4).46 47 The odds ratio in the meta-analysis involving all 21 studies was 1.01, but the finding here was dominated by the London study, which accounted for 59.9% of the weighting of the overall estimate.
Table 1.
Meta-analysis results by outcome and temperature metric
| No of studies | Synthesis method | No of studies in summary measure | Average effect size (OR (95% CI)) |
I2 (%) | |
|---|---|---|---|---|---|
| Preterm birth: | |||||
| Odds of preterm birth during heatwaves | 6 | Meta-analysis | 6 | 1.16 (1.10 to 1.23) | 44.7 |
| Odds of preterm birth per 1°C temperature increase | 6 | Meta-analysis | 6 | 1.05 (1.03 to 1.07); 5% increase per 1°C rise (3% to 7%) | 87.7 |
| Odds of preterm birth during high versus low temperatures (exposure over a trimester or all of pregnancy) | 9 | Meta-analysis | 9 | 1.14 (1.11 to 1.16) | 88.2 |
| 8* | 1.15 (1.13 to 1.18) | 65.2 | |||
| Odds of preterm birth during high versus low temperatures with exposure period <4 weeks | 21 | Meta-analysis | 21 | 1.01 (1.01 to 1.02) | 89.8 |
| 19† | 1.05 (1.04 to 1.05) | 83.6 | |||
| 21 | Summary of effect estimates | 21 | Median OR=1.07 (IQR 1.05-1.16; range 0.99-1.94) | — | |
| Birth weight: | |||||
| Odds of low birth weight during high versus low temperatures (exposure over a trimester or all of pregnancy) | 9 | Summary of effect estimates | 8‡ | Median OR 1.09 (IQR 1.04-1.47; range 1.01-2.49) | — |
| Changes in birth weight at high versus low temperatures | 6 | Summary of effect estimates | 4§ | Median difference −25.5 g (range −39.4 to −15.0) | — |
| Stillbirth: | |||||
| Odds of stillbirth during heatwaves | 1 | — | 1 | 1.46 (1.09 to 1.96) | — |
| Odds of stillbirth per 1°C temperature increase | 3 | Meta-analysis | 3 | 1.05 (1.01 to 1.08) | 81.3 |
| Odds of stillbirth during high versus low temperatures (exposure over a trimester or all of pregnancy) | 2 | Meta-analysis | 2 | 3.39 (2.33 to 4.96) | 27.8 |
| Odds of stillbirth during high versus low temperatures with exposure period <1 week | 4 | Meta-analysis | 4 | 1.24 (1.12 to 1.36) | 53.1 |
Fig 2.
Odds of preterm birth during heatwaves. Study details are given in supplementary table 2a
Fig 3.
Odds of preterm birth per degree increase in temperature. Study details are given in supplementary table 2a
Fig 4.
Odds of preterm birth at high versus low temperature (periods less than or equal to four weeks). Study details are given in supplementary table 2a
Most studies reported dose-response associations, where rates of preterm birth rose progressively with increasing levels of temperature or with longer durations of heat exposure (supplementary table 2a). Positive associations were detected in all lag windows, including five with heat exposure in the month of conception,51 52 53 54 55 or preconception53 (supplementary fig 7a). In low and middle income countries, five studies documented heat associations with preterm birth in the first and second trimester, while only three detected these effects in the last week of pregnancy. Conversely, in the European Union and Central Asia region only one study documented heat vulnerability in the first trimester, and eight did so in the last week of pregnancy, mostly at lag zero to three days before childbirth. Similarly, in North America only two studies noted associations in the first or second trimester, whereas six did so in the final week of pregnancy.
Six studies reported null findings, three of which were of low quality.35 42 56 One study set in Brisbane, Australia found no association between heat exposure and gestation length,57 but, in a separate analysis, did detect an association when the outcome was dichotomised as preterm birth.55 Another study in Canada did not detect an association between temperature and preterm birth, but reported that during heatwaves, early term births at 37-39 weeks were considerably more common than births above 40 weeks.58 As mentioned above, a study in London, United Kingdom, where maximum daily temperatures were below 25°C on almost all days, had null findings.47 One study in Shenzhen, China reported an apparent protective effect of heat exposure, which could be due to high air conditioning use in the study area, as noted above.46 Similarly, a study in northern California noted the effect sizes were lowest in the areas that had the highest use of air conditioning.59
Few studies reported the shape of association, though, of note, five studies, all in humid subtropical areas, detected U shaped associations, with the base covering a range from 18 to 25°C60 61 62 63 64 (supplementary table 2a). Three studies found that the size of diurnal fluctuations in temperature or the level of temperature during the night were more predictive of preterm births than levels during the day,37 65 66 and another study found stronger associations in areas that had high night time temperatures.67
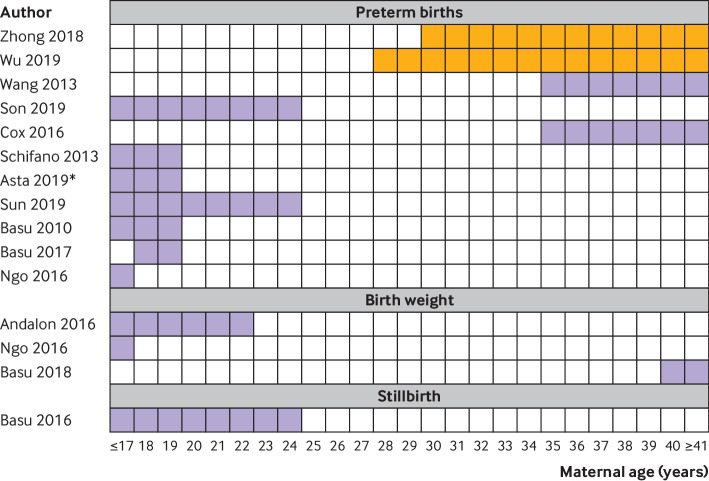
A study in Israel disaggregated findings by induced and spontaneous preterm births, and noted associations only with spontaneous births (induced births incidence rate ratio 0.99, 95% confidence interval 0.97 to 1.0).68 In the 11 studies on preterm birth that stratified findings by age group, the highest risks were noted in women under 25 (seven studies)64 69 70 71 72 73 74 or over 35 (two studies; fig 5).75 76 In two studies that did not fit this pattern, age had been dichotomised, making it difficult to determine specific risk bands.65 66 Associations between exposure to heat and preterm birth were strongest in low socioeconomic groups in all six studies that reported this composite variable (supplementary fig 8a).35 56 70 71 72 74 In one example, a study in South Korea, women with both low education levels and low socioeconomic status had a 1.1-fold increased hazard ratio of preterm birth for each quartile increase in temperature, considerably higher than that in other women in the study.73 In four studies in the USA, black or Hispanic women had two or more fold higher effect sizes than white women (fig 6).35 69 70 77 In one study where risks of preterm birth associated with heat were higher in whites than in other races, the differences in effect sizes were negligible.74 Associations between heat and preterm birth were highest in indigenous women in a study in the coastal region of Australia,75 but in non-indigenous women in the hot central region.78 Greater impacts of heat on female than male fetuses were noted in six of nine studies (supplementary fig 8b). Three studies found that associations between temperature and preterm birth were higher in women with chronic conditions such as diabetes or depression.64 69 72
Fig 5.
Analysis of associations between temperature and outcome by age of women during pregnancy. Purple shading indicates period of highest risk in studies that analysed with multiple age categories. Orange shading indicates studies with age as a binary variable, limiting the assessment of risk period. Data on whether differences between subgroups were significant were not provided for most comparisons. Only studies that showed a significant association between temperature and the outcome are included. *Only in Rome, one of six sites in the study. Study details are given in supplementary tables 2a, 2b, and 2c
Fig 6.
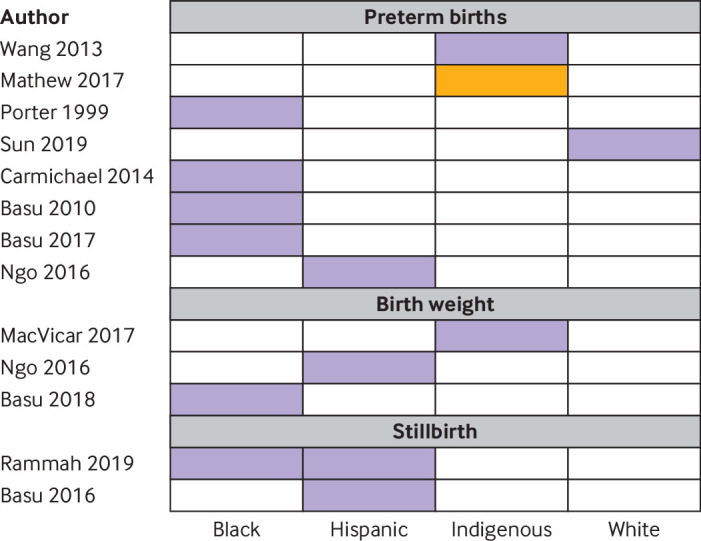
Analysis of associations between temperature and outcome by race of mother. Purple shading indicates subgroups with the highest effect estimates. Orange shading indicates those at lowest risk. Study details are given in supplementary tables 2a, 2b, and 2c
Overall, there was little evidence available on whether relationship between temperature and preterm birth had changed over time. A study in Brisbane, Australia, the only one that stratified findings by time period, found that the hazard ratio of preterm birth was lower in 2013 than in 1994 for the same temperature exposure.61
Birth weight
The median rate of low birth weights in the included studies was 3.0% (interquartile range 1.8-6.4; supplementary table 2b). Of the 16 studies that provided data on the association of temperature with low birth weight, 10 reported that risk increased at higher temperatures, and only one reported the converse (five had null findings). The median of the observed effects of high temperatures on odds of low birth weight was 1.09 (interquartile range 1.04-1.47; table 1; supplementary figs 2 and 3). No meta-analysis was done on any of the outcomes for birth weight given the marked variation in magnitude and direction of effect. We also did not present a summary measure of changes in birth weight for each degree increase in temperature given the high levels of methodological diversity between these studies.
Of the 19 studies reporting birth weight as a continuous variable, 12 noted decreases in birth weight at higher temperatures, including two where the direction of effect varied by trimester (supplementary figure 7b),79 80 three studies had non-significant findings, and four noted that weight increased at higher temperatures. Generally, the impacts of temperature on weight were small, with most studies reporting changes of under 10 g per change in degree, or under 20 g when comparing high and low temperatures (supplementary table 2b, supplementary fig 4). Changes in birth weight were especially small in studies in low and middle income countries.48 81 Notably, in the lag measures, high temperatures in the last four weeks of pregnancy appeared to have little impact on birth weight, and were even associated with an increase in birth weight in two studies (supplementary fig 7b).48 80 Among studies that showed an association between reduced weight and high temperatures, the largest impacts were in women aged less than or equal to 22 or above 4029 71 79; who were black,79 indigenous,48 or Hispanic 71; and of low socioeconomic status29 71 79 (fig 5, fig 6, supplementary fig 8a).
Stillbirth
The median stillbirth rate was 6.2 per 1000 births (interquartile range 4.4-6.4; supplementary table 2c). All eight included studies detected an increase in stillbirths at higher temperatures (table 1). In most cases, associations between temperature and stillbirth were most pronounced in the last week or month of pregnancy (supplementary fig 7c). Stillbirths increased 1.46-fold (95% confidence interval 1.09 to 1.96) in the one study in Brisbane, Australia that reported this outcome.55 In meta-analysis, stillbirths increased by 1.05 (95% confidence interval 1.01 to 1.08) per 1°C rise in temperature, by 1.24-fold (1.12 to 1.36) at lags measured on individual days in the last week of pregnancy,62 82 and by 3.39-fold (2.33 to 4.96) when temperature effects were examined over a trimester or the whole pregnancy period61 82 (supplementary figs 5 and 6). In subgroup analyses, point estimates of associations between heat and stillbirth were higher in term than in preterm stillbirths,82 in black and Hispanic women than white women,83 in younger women,84 male fetuses, and had reduced over time61 (fig 5, fig 6, and supplementary fig 8b). Only one study provided information on multiple pregnancies, reporting associations similar to those with singleton pregnancies.82 None of the eight studies were done in low and middle income countries.
Discussion
Principal findings
The systematic review findings—in particular, the size and relative consistency of associations and dose-response patterns, appear to support the hypothesis that heat exposure increases the likelihood of adverse pregnancy outcomes. Association with preterm births and stillbirths in some analyses shows these outcomes rising by about 1.05-fold per 1°C increase in temperature, and the odds of a preterm birth to be 1.16-fold higher during heatwaves.
Associations of heat with preterm birth and stillbirth appear to be stronger and more consistent than those with birth weight. For example, only 18 of 28 studies which assessed birth weight found an association, compared with 40 of 47 preterm birth studies. This is even more remarkable as measurement of gestation is subject to a range of errors, which are probably non-differential, biasing findings to the null, whereas one might expect data on birth weight to be of higher quality. In other studies the associations of heat exposure with other measures of newborn anthropometry, such as birth length, also appear small,29 50 81 and no association was noted between temperature and ultrasound measurements of fetal anthropometry in an Australian study.85 Taken together, evidence for heat sensitivity on birth weight is limited and most studies reported only minor changes. It is important to note, however, that even apparently minor decrements in birth weight could have a major impact on public health as exposure to high temperatures is common and escalating.
We were unable to discern any clear windows of vulnerability during pregnancy, except that heat exposure in the final weeks of pregnancy appears most important for preterm births and stillbirths. Some evidence suggested that pregnant women in low and middle income countries were vulnerable to heat exposure throughout pregnancy, whereas vulnerability among women in high income countries was largely confined to the last weeks of pregnancy. Any effects on birth weight seem to occur early in pregnancy.
It is also difficult to isolate which dimension(s) of heat mediate the impacts noted. The evidence was strongest and most consistent for heatwaves, although the largest effect sizes were from measures of the cumulative dose of heat over the whole of pregnancy. Three studies reported sizable impacts of diurnal fluctuations in temperature.37 65 66 Clearly, the complexity of thermoregulation in pregnancy and the interactions between temperature and other meteorological variables, make it difficult to isolate which aspects of heat are responsible for each adverse pregnancy outcome. A better understanding of these complexities and how they vary by climate zone could inform the design of adaption services. For example, several studies pointed to a threshold temperature of 18-25°C, above which rates of preterm births escalate and interventions might be needed. This cutoff point might also explain why the London study had null findings as the temperature was below 25°C on almost all days in that study.47
The review was not designed to assess associations between low temperature and birth outcomes, though these were documented in several studies, most of which reported U shaped associations between temperature and outcomes.46 51 61 62 64 79 86 87 Links between exposures to cold and other outcomes, such as mortality, are well documented. Little attention is paid to these links when climate change is discussed, even though the changes in climate which are occurring encompass both increases in temperatures and periods of low temperature extremes.88
Several health conditions in newborns, other than those assessed here, have been linked to heat exposure, including small for gestational age and congenital anomalies.17 18 19 20 21 22 89 Sensitivity to heat might also vary by sex of the fetus. A study in Japan, which noted higher spontaneous abortions among male than female fetuses after a period of heat exposure,90 is consistent with the study in our review. We found that higher temperatures were associated with stillbirths in male fetuses.84 The preponderance of female newborns in studies on preterm birth in the review could be explained by this differential sensitivity. Heat exposure is implicated in many conditions in pregnant women, with evidence pointing to heat effects on pre-eclampsia, prolonged labour, and antepartum or postpartum haemorrhage.32 91 92 During heatwaves, pregnant women might also have higher rates of admission to hospital emergency departments and increased cardiovascular events, such as stroke and myocardial infarction.93 94 Some studies have also linked emotional stress and sleep disturbance in pregnancy to hot weather.95 96 Group B streptococcus colonisation of the vagina and cervix of pregnant women could increase at higher ambient temperatures,97 raising risks for newborn sepsis. Lastly, it is important to note that in utero exposure to heat has been linked with a range of childhood and adult conditions, and even long term economic prospects.8 9 10 98 99 Each of these topics warrants closer study.
Associations between temperature and birth outcomes appear especially pronounced among women in low socioeconomic groups. This suggests that pregnant women in low and middle income countries could be at particular risk from heat exposure. Women in low and middle income countries already have high rates of preterm birth (10.4% of births in Asia are preterm and 12.0% in sub-Saharan Africa),100 about twice the rates of the studies in this review. Preterm birth and low birth weight are both key risks for neonatal mortality, which accounts for almost half of all child deaths under five.101 Heat related risks might be especially high among subgroups of pregnant women in low and middle income countries who have reduced physiological ability to respond to high temperatures, including women with multiple pregnancy, obesity, malaria, HIV infection, or mental health and other chronic conditions.69 70 Many of the protections that are afforded to these groups in high income countries are unavailable in low and middle income countries. As the world’s temperature increases, risks for pregnant women in low and middle income countries become even more concerning as their ability to reduce heat exposure through space cooling, for example, could be severely constrained.
Future intervention and methodological implications
The review highlights the need for research to identify and study interventions to reduce problems due to heat among pregnant women (no such studies were located in the mapping review which preceded this review). During pregnancy, a package of home based services could be provided by community health workers whose work already largely centres on pregnant women and children. Many of the WHO recommended interventions for health promotion in pregnant women could be adapted for this purpose.102 Heat reduction in labour wards in low and middle income countries might include optimising natural ventilation, making fans and cold potable water available, and integrating heat counselling within the services provided by health workers, HIV counsellors, and birth companions. These interventions warrant further investigation. It is important to closely monitor maternal hydration when temperatures are high. In the Paris heatwave of 2003, for example, oligohydramnios, possibly caused by maternal dehydration, was diagnosed in 17.5% of term pregnancies, compared with 4.4% in the same week in the previous year,103 consistent with a similar study in the USA.104 Rates of dehydration and heat stress might be high in women who give birth in facilities that have little heat resistance, where temperatures can be as much as 4°C higher than outdoors.105 106 Lastly, cash transfers and other interventions which build resilience could assist pregnant women to access adaptive interventions such as cool, clean water, or reduce their need to earn income in the final weeks of pregnancy.107
Several observations on research methodology should be mentioned. Standardising temperature metrics, lag durations, and subpopulation analyses in future studies would enable direct comparison between studies, identification of windows of vulnerability, and more robust estimates of overall size of associations. Standardisation could also reduce selective reporting of significant findings. Few studies examined whether temperature effects varied across subpopulations—critical evidence that would inform the targeting of specific groups of pregnant women. Many of these limitations could be overcome by an individual participant data meta-analysis that combined raw data from several studies in a single analysis. Additionally, the application of data science methodologies, such as machine learning, could also offer new opportunities to advance knowledge on this topic, and the association of heat exposure with health more generally.
Strengths and limitations
The review included more studies than previous reviews and covered three outcomes, allowing comparisons among these outcomes and a more comprehensive assessment of heat sensitivity in pregnancy. The review has several limitations, however. Differences in the ways that temperature and lag measures were used meant that we had to develop decision rules for classifying temperature metrics and other variables. Additionally, about a third of studies were of low quality, limiting analysis. Moreover, publication bias, multiple testing, and selective reporting of positive associations (eg, at different lag times) might have been common, as with all observational research.
More generally, considerable challenges exist in interpreting studies that examine impacts of environmental exposures on birth outcomes, especially spontaneous abortion or stillbirth. For example, severely damaged embryos or major birth defects from an environmental exposure could result in spontaneous abortion, often before pregnancy recognition.108 Heat related early pregnancy loss might be difficult to detect, though studies of assisted reproduction provide one way of doing so. In one such study in Qatar, for example, spontaneous abortions in hotter months were double those in cooler months.109 Importantly, potential confounding by air pollution could occur, and future reviews need to explicitly examine this problem. Furthermore, adaptation measures such as air conditioning use, and heat action planning might attenuate the strength of associations between heat exposures and adverse outcomes.6 This possibly explains the inconsistent findings of the study in Shenzhen, China46 and the very low point estimates in a study in Seoul, South Korea.73 Null findings might be interpreted as the absence of an association, rather than as the effectiveness of adaptation measures. Given these complexities, we presented some meta-analyses that excluded those studies which could have been influenced by high levels of adaptation. Major challenges exist in interpreting these findings, however, and future reviews might consider stratifying analyses by use of air conditioning, for example.
Conclusions
The studies included could be subject to a range of measurement biases, but this review suggests that exposures to high temperature might be associated with birth outcomes, with evidence most consistent and effect sizes largest for preterm birth and stillbirth.
Overall, the review highlights the need to identify interventions targeting heat related conditions in pregnant women, especially in women at the age extremes and in lower socioeconomic groups, and to determine their effectiveness. Pregnant women merit a place alongside the groups typically considered as at high risk for heat related conditions. Estimates of the global burden of disease from heat effects on newborns could be modelled from the evidence collated in this review. These estimates are key for securing funding for reducing heat exposure for pregnant women, for demonstrating the health risks of climate change more generally, and for supporting efforts to curtail greenhouse gas emissions. Given increases in the frequency and intensity of heatwaves, the number of pregnant women exposed to these conditions worldwide, and the significant individual and societal burdens associated with preterm birth and stillbirth, research and policy initiatives to deal with these connections are a high priority.
What is already known on this topic
Physiological and anatomical changes in pregnancy compromise a woman’s ability to thermoregulate, potentially raising risks of adverse pregnancy outcomes
Increases in global temperatures heighten concerns about heat impacts on health, especially in vulnerable groups with limited physiological or socioeconomic ability to respond
Previous systematic reviews have summed evidence of associations between exposure to high temperatures and pregnancy outcomes, but have included few studies and have not assessed variations across population groups and by type of heat exposure
What this study adds
This systematic review collates evidence that exposure to high temperature is associated with an increase in adverse pregnancy outcomes, especially preterm birth and stillbirth, and among women in lower socioeconomic groups
The study shows the potential effects on health of continued increases in mean global temperatures and of the frequency of heatwaves
Web extra.
Extra material supplied by authors
Web appendix: Supplementary material
Climate Change and Heat-Health Study Group: Britt Nakstad (professor, Institute of Clinical Medicine, Centre of Global Health, University of Oslo, Oslo, Norway; Department of Paediatrics and Adolescent Health, University of Botswana, Gaborone, Botswana), Caradee Y Wright (extraordinary lecturer, Department of Geography, Geoinformatics and Meteorology, Faculty of Natural and Agricultural Sciences, University of Pretoria, South Africa; senior specialist scientist, Environment and Health Research Unit, South African Medical Research Council, Pretoria, South Africa), Chloe Harvey (independent consultant, Bangkok, Thailand), Chongying Wang (associate professor, Department of Social Psychology, Zhou Enlai School of Government, Nankai University, Tianjin, China), Dilara Durusu (masters student, Maastricht University, Maastricht, Netherlands), Fiona Scorgie (senior lecturer, Wits Reproductive Health and HIV Institute, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa), Helen Rees (executive director, Wits Reproductive Health and HIV Institute, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa), Lois Harden (associate professor, Department of Physiology, University of the Witwatersrand, Johannesburg, South Africa), Nathalie Roos (obstetrician and gynaecologist, Karolinska Institutet, Department of Medicine, Solna, Clinical Epidemiology Division, Stockholm, Sweden), Olof Stephansson (associate professor, Karolinska Institutet, Department of Medicine, Solna, Clinical Epidemiology Division, Stockholm, Sweden), Stanley M F Luchters (professor, Department of Population Health, Aga Khan University, Nairobi, Kenya; International Centre for Reproductive Health, Department of Public Health and Primary Care, Ghent University, Ghent, Belgium; Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, VIC, Australia; Burnet Institute, Melbourne, VIC, Australia), Thomas Roux (doctoral student, School of Public Health, Physiotherapy and Sports Science, University College Dublin, Dublin, Ireland).
Contributors: MFC conceived and coordinated the study and wrote the first draft. MDP, AA, MH, AM, CPS, BW, MR, RH, and MB screened titles, abstracts, and full text articles, and assisted with data extraction from included papers. SH provided senior inputs to data analysis and conceptualisation of the article. All members of the Climate Change and Heat-Health Study Group provided key inputs to the article, including screening of articles, data extraction, and input on specific specialist topic areas. All authors reviewed the various drafts of the article and cleared it for publication. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. MFC and SMFL serves as guarantors.
Funding: WRHI opportunities fund, and the Global Change Institute, University of the Witwatersrand, South Africa. This work was supported by the Natural Environment Research Council (NERC), the Research Council of Norway (RCN), and The Swedish Research Council for Health, Working Life and Welfare (Forte) in collaboration with the Swedish Research Council (Vetenskapsrådet); coordinated through a Belmont Forum partnership. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from NERC, RCN, and Forte in collaboration with Vetenskapsrådet, coordinated through a Belmont Forum partnership, for the submitted work. MFC and FS hold investments in the fossil fuel industry through their pension funds. The University of the Witwatersrand holds investments in the fossil fuel industry through their endowments and other financial reserves. AM is a member of the Global Change Institute, University of the Witwatersrand, where part of the administrative support is funded by Exxaro, which has interests in coal mining and renewable energy. MR has a pension fund with Irish Life, which includes investments in fossil fuel companies.
Ethical approval: Not required as a systematic review of published data.
Data sharing: No additional data available.
The lead author and manuscript’s guarantor (MFC) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: It will not be possible to send the results to research participants given that this study is a systematic review. We plan to disseminate the findings to the public through a range of media channels, and to facilitate the inclusion of its results in reports on the health impacts of rises in temperature.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Collaborators: Climate Change and Heat-Health Study Group, Britt Nakstad, Caradee Y Wright, Chloe Harvey, Chongying Wang, Dilara Durusu, Fiona Scorgie, Helen Rees, Lois Harden, Nathalie Roos, Olof Stephansson, Stanley M F Luchters, and Thomas Roux
References
- 1. Hoegh-Guldberg O, Jacob D, Taylor M, et al. The human imperative of stabilizing global climate change at 1.5°C. Science 2019;365:eaaw6974. 10.1126/science.aaw6974 [DOI] [PubMed] [Google Scholar]
- 2.Intergovernmental Panel on Climate Change. Special report on global warming of 1.5°C. https://www.ipcc.ch/sr15/. 2018.
- 3.United Nations. Framework Convention on Climate Change. Subsidiary Body for Scientific and Technological Advice. 46th session. Nairobi work programme on impacts, vulnerability and adaptation to climate change. Human health and adaptation: understanding climate impacts on health and opportunities for action. 2017. https://unfccc.int/sites/default/files/resource/docs/2017/sbsta/eng/02.pdf [Google Scholar]
- 4.USAID. Heat waves and human health: emerging evidence and experience to inform risk management in a warming world. 2019. https://www.climatelinks.org/sites/default/files/asset/document/2019_USAID-ATLAS_Heat-Waves-and-Human-Health.pdf.
- 5. Lowe D, Ebi KL, Forsberg B. Heatwave early warning systems and adaptation advice to reduce human health consequences of heatwaves. Int J Environ Res Public Health 2011;8:4623-48. 10.3390/ijerph8124623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de’ Donato FK, Leone M, Scortichini M, et al. Changes in the effect of heat on mortality in the last 20 years in nine European cities. Results from the PHASE Project. Int J Environ Res Public Health 2015;12:15567-83. 10.3390/ijerph121215006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gil Cuesta J, van Loenhout JA, Colaço MD, Guha-Sapir D. General population knowledge about extreme heat: a cross-sectional survey in Lisbon and Madrid. Int J Environ Res Public Health 2017;14:E122. 10.3390/ijerph14020122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duchoslav J. Prenatal temperature shocks reduce cooperation: evidence from public goods games in Uganda. Front Behav Neurosci 2017;11:249. 10.3389/fnbeh.2017.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ho JY. Early-life environmental exposures and height, hypertension, and cardiovascular risk factors among older adults in India. Biodemography Soc Biol 2015;61:121-46. 10.1080/19485565.2015.1045580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Isen A, Rossin-Slater M, Walker R. Relationship between season of birth, temperature exposure, and later life wellbeing. Proc Natl Acad Sci U S A 2017;114:13447-52. 10.1073/pnas.1702436114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laburn H. How does the fetus cope with thermal challenges? Physiology (Bethesda) 1996;11 10.1152/physiologyonline.1996.11.2.96 [DOI] [Google Scholar]
- 12. Wells JC. Thermal environment and human birth weight. J Theor Biol 2002;214:413-25. 10.1006/jtbi.2001.2465 [DOI] [PubMed] [Google Scholar]
- 13. Judge CM, Chasan-Taber L, Gensburg L, Nasca PC, Marshall EG. Physical exposures during pregnancy and congenital cardiovascular malformations. Paediatr Perinat Epidemiol 2004;18:352-60. 10.1111/j.1365-3016.2004.00586.x [DOI] [PubMed] [Google Scholar]
- 14. Suarez L, Felkner M, Hendricks K. The effect of fever, febrile illnesses, and heat exposures on the risk of neural tube defects in a Texas-Mexico border population. Birth Defects Res A Clin Mol Teratol 2004;70:815-9. 10.1002/bdra.20077 [DOI] [PubMed] [Google Scholar]
- 15. Flocks J, Vi Thien Mac V, Runkle J, Tovar-Aguilar JA, Economos J, McCauley LA. Female farmworkers’ perceptions of heat-related illness and pregnancy health. J Agromedicine 2013;18:350-8. 10.1080/1059924X.2013.826607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. GBD 2017 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1859-922. 10.1016/S0140-6736(18)32335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carolan-Olah M, Frankowska D. High environmental temperature and preterm birth: a review of the evidence. Midwifery 2014;30:50-9. 10.1016/j.midw.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 18. Kuehn L, McCormick S. Heat exposure and maternal health in the face of climate change. Int J Environ Res Public Health 2017;14:E853. 10.3390/ijerph14080853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poursafa P, Keikha M, Kelishadi R. Systematic review on adverse birth outcomes of climate change. J Res Med Sci 2015;20:397-402. [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y, Yu C, Wang L. Temperature exposure during pregnancy and birth outcomes: An updated systematic review of epidemiological evidence. Environ Pollut 2017;225:700-12. 10.1016/j.envpol.2017.02.066. [DOI] [PubMed] [Google Scholar]
- 21. Strand LB, Barnett AG, Tong S. The influence of season and ambient temperature on birth outcomes: a review of the epidemiological literature. Environ Res 2011;111:451-62. 10.1016/j.envres.2011.01.023 [DOI] [PubMed] [Google Scholar]
- 22. Beltran AJ, Wu J, Laurent O. Associations of meteorology with adverse pregnancy outcomes: a systematic review of preeclampsia, preterm birth and birth weight. Int J Environ Res Public Health 2013;11:91-172. 10.3390/ijerph110100091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edgar AM, Wernecke B, Erasmus B, et al. The impacts of heat on health and effectiveness of interventions to reduce these impacts: a systematic review in the era of climate change. 2018. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018118113.
- 24.Areal A, Kline A, Manyuchi AE, et al. The impact of exposure to high temperatures on preterm births: a systematic review. PROSPERO CRD42019140136. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019140136. 2019.
- 25.Thomas J, Brunton J, Graziosi S. EPPI-Reviewer 4: software for research synthesis. EPPI-Centre Software. Social Science Research Unit, UCL Institute of Education. 2018. https://eppi.ioe.ac.uk/cms/er4/Features/tabid/3396/Default.aspx [Google Scholar]
- 26.World Health Organization. International statistical classification of diseases and related health problems, 10th revision. 2016. https://icd.who.int/browse10/Content/statichtml/ICD10Volume2_en_2010.pdf
- 27.Joanna Briggs Institute Critical Appraisal tools. JBI systematic reviews: checklist for analytical cross sectional studies. 2017. https://joannabriggs.org/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Analytical_Cross_Sectional_Studies2017_0.pdf
- 28.McKenzie JE, Brennan SE. Chapter 12: Synthesizing and presenting findings using other methods. Cochrane Handbook for Systematic Reviews of Interventions Version 6 2019. https://training.cochrane.org/handbook/current/chapter-12 [Google Scholar]
- 29. Andalon M, Azevedo JP, Rodriguez-Castelan C, et al. Weather shocks and health at birth in Colombia. World Dev 2016;82:69-82. 10.1016/j.worlddev.2016.01.015 [DOI] [Google Scholar]
- 30. Carmichael SL, Cullen MR, Mayo JA, et al. Population-level correlates of preterm delivery among black and white women in the U.S. PLoS One 2014;9:e94153. 10.1371/journal.pone.0094153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lajinian S, Hudson S, Applewhite L, Feldman J, Minkoff HL. An association between the heat-humidity index and preterm labor and delivery: a preliminary analysis. Am J Public Health 1997;87:1205-7. 10.2105/AJPH.87.7.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cil G, Cameron TA. Potential climate change health risks from increases in heat waves: abnormal birth outcomes and adverse maternal health conditions. Risk Anal 2017;37:2066-79. 10.1111/risa.12767 [DOI] [PubMed] [Google Scholar]
- 33. Muresan D, Staicu A, Zaharie G, Marginean C, Rotar IC. The influence of seasonality and weather changes on premature birth incidence. Clujul Med 2017;90:273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ngo NS, Horton RM. Climate change and fetal health: The impacts of exposure to extreme temperatures in New York City. Environ Res 2016;144(Pt A):158-64. 10.1016/j.envres.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 35. Porter KR, Thomas SD, Whitman S. The relation of gestation length to short-term heat stress. Am J Public Health 1999;89:1090-2. 10.2105/AJPH.89.7.1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tustin K, Gross J, Hayne H. Maternal exposure to first-trimester sunshine is associated with increased birth weight in human infants. Dev Psychobiol 2004;45:221-30. 10.1002/dev.20030. [DOI] [PubMed] [Google Scholar]
- 37. Yackerson N, Piura B, Sheiner E. The influence of meteorological factors on the emergence of preterm delivery and preterm premature rupture of membrane. J Perinatol 2008;28:707-11. 10.1038/jp.2008.69. [DOI] [PubMed] [Google Scholar]
- 38. Yu X, Feric Z, Cordero JF, Meeker JD, Alshawabkeh A. Potential influence of temperature and precipitation on preterm birth rate in Puerto Rico. Sci Rep 2018;8:16106. 10.1038/s41598-018-34179-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wells JC, Cole TJ. Birth weight and environmental heat load: a between-population analysis. Am J Phys Anthropol 2002;119:276-82. 10.1002/ajpa.10137 [DOI] [PubMed] [Google Scholar]
- 40. Chodick G, Shalev V, Goren I, Inskip PD. Seasonality in birth weight in Israel: new evidence suggests several global patterns and different etiologies. Ann Epidemiol 2007;17:440-6. 10.1016/j.annepidem.2006.10.013 [DOI] [PubMed] [Google Scholar]
- 41. Elter K, Ay E, Uyar E, Kavak ZN. Exposure to low outdoor temperature in the midtrimester is associated with low birth weight. Aust N Z J Obstet Gynaecol 2004;44:553-7. 10.1111/j.1479-828X.2004.00314.x [DOI] [PubMed] [Google Scholar]
- 42. Wolf J, Armstrong B. The association of season and temperature with adverse pregnancy outcome in two German states, a time-series analysis. PLoS One 2012;7:e40228. 10.1371/journal.pone.0040228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Strand LB, Barnett AG, Tong S. Maternal exposure to ambient temperature and the risks of preterm birth and stillbirth in Brisbane, Australia. Am J Epidemiol 2012;175:99-107. 10.1093/aje/kwr404 [DOI] [PubMed] [Google Scholar]
- 44.Deeks JJ, Higgins JPT, Altman DG, et al. Chapter 10: Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions, version 6, 2019. https://training.cochrane.org/handbook/current/chapter-10 [Google Scholar]
- 45.Geiger R. [Classification of climates after W. Köppen]. Landolt-Börnstein – Zahlenwerte und Funktionen aus Physik, Chemie, Astronomie, Geophysik und Technik, alte Serie. Springer, 1954:603-7. [Google Scholar]
- 46. Liang Z, Lin Y, Ma Y, et al. The association between ambient temperature and preterm birth in Shenzhen, China: a distributed lag non-linear time series analysis. Environ Health 2016;15:84. 10.1186/s12940-016-0166-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee SJ, Hajat S, Steer PJ, Filippi V. A time-series analysis of any short-term effects of meteorological and air pollution factors on preterm births in London, UK. Environ Res 2008;106:185-94. 10.1016/j.envres.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 48. MacVicar S, Berrang-Ford L, Harper S, Huang Y, Namanya Bambaiha D, Yang S. Whether weather matters: evidence of association between in utero meteorological exposures and foetal growth among indigenous and non-indigenous mothers in rural Uganda. PLoS One 2017;12:e0179010. 10.1371/journal.pone.0179010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Madsen C, Gehring U, Walker SE, et al. Ambient air pollution exposure, residential mobility and term birth weight in Oslo, Norway. Environ Res 2010;110:363-71. 10.1016/j.envres.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 50. Bruckner TA, Modin B, Vågerö D. Cold ambient temperature in utero and birth outcomes in Uppsala, Sweden, 1915-1929. Ann Epidemiol 2014;24:116-21. 10.1016/j.annepidem.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 51. Zheng X, Zhang W, Lu C, Norbäck D, Deng Q. An epidemiological assessment of the effect of ambient temperature on the incidence of preterm births: identifying windows of susceptibility during pregnancy. J Therm Biol 2018;74:201-7. 10.1016/j.jtherbio.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 52. Giorgis-Allemand L, Pedersen M, Bernard C, et al. The influence of meteorological factors and atmospheric pollutants on the risk of preterm birth. Am J Epidemiol 2017;185:247-58. 10.1093/aje/kww141 [DOI] [PubMed] [Google Scholar]
- 53. Guo T, Wang Y, Zhang H, et al. The association between ambient temperature and the risk of preterm birth in China. Sci Total Environ 2018;613-614:439-46. 10.1016/j.scitotenv.2017.09.104 [DOI] [PubMed] [Google Scholar]
- 54. Ha S, Liu D, Zhu Y, Kim SS, Sherman S, Mendola P. Ambient temperature and early delivery of singleton pregnancies. Environ Health Perspect 2017;125:453-9. 10.1289/EHP97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang J, Tong S, Williams G, Pan X. Exposure to heat wave during pregnancy and adverse birth outcomes: an exploration of susceptible windows. Epidemiology 2019;30(Suppl 1):S115-21. 10.1097/EDE.0000000000000995 [DOI] [PubMed] [Google Scholar]
- 56. Tustin K, Gross J, Hayne H. Maternal exposure to first-trimester sunshine is associated with increased birth weight in human infants. Dev Psychobiol 2004;45:221-30. 10.1002/dev.20030 [DOI] [PubMed] [Google Scholar]
- 57. Li S, Wang J, Xu Z, et al. Exploring associations of maternal exposure to ambient temperature with duration of gestation and birth weight: a prospective study. BMC Pregnancy Childbirth 2018;18:513. 10.1186/s12884-018-2100-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Auger N, Naimi AI, Smargiassi A, Lo E, Kosatsky T. Extreme heat and risk of early delivery among preterm and term pregnancies. Epidemiology 2014;25:344-50. 10.1097/EDE.0000000000000074 [DOI] [PubMed] [Google Scholar]
- 59. Avalos LA, Chen H, Li DK, Basu R. The impact of high apparent temperature on spontaneous preterm delivery: a case-crossover study. Environ Health 2017;16:5. 10.1186/s12940-017-0209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. He JR, Liu Y, Xia XY, et al. Ambient temperature and the risk of preterm birth in Guangzhou, China (2001-2011). Environ Health Perspect 2016;124:1100-6. 10.1289/ehp.1509778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li S, Chen G, Jaakkola JJK, Williams G, Guo Y. Temporal change in the impacts of ambient temperature on preterm birth and stillbirth: Brisbane, 1994-2013. Sci Total Environ 2018;634:579-85. 10.1016/j.scitotenv.2018.03.385 [DOI] [PubMed] [Google Scholar]
- 62. Weng YH, Yang CY, Chiu YW. Adverse neonatal outcomes in relation to ambient temperatures at birth: a nationwide survey in Taiwan. Arch Environ Occup Health 2018;73:48-55. 10.1080/19338244.2017.1299084 [DOI] [PubMed] [Google Scholar]
- 63. Zheng X, Zhang W, Lu C, Norbäck D, Deng Q. An epidemiological assessment of the effect of ambient temperature on the incidence of preterm births: identifying windows of susceptibility during pregnancy. J Therm Biol 2018;74:201-7. 10.1016/j.jtherbio.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 64. Asta F, Michelozzi P, Cestari L, et al. [Effects of high temperature and air pollution on the risk of preterm births. Analysis in six Italian cities, 2001-2010]. Epidemiol Prev 2019;43:152-60. 10.19191/EP19.2-3.P152.054. [DOI] [PubMed] [Google Scholar]
- 65. Zhong Q, Lu C, Zhang W, Zheng X, Deng Q. Preterm birth and ambient temperature: strong association during night-time and warm seasons. J Therm Biol 2018;78:381-90. 10.1016/j.jtherbio.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 66. Wu M, Song L, Zheng X, et al. Prenatal exposure of diurnal temperature range and preterm birth: findings from a birth cohort study in China. Sci Total Environ 2019;656:1102-7. 10.1016/j.scitotenv.2018.11.305 [DOI] [PubMed] [Google Scholar]
- 67. Ward A, Clark J, McLeod J, Woodul R, Moser H, Konrad C. The impact of heat exposure on reduced gestational age in pregnant women in North Carolina, 2011-2015. Int J Biometeorol 2019;63:1611-20. 10.1007/s00484-019-01773-3 [DOI] [PubMed] [Google Scholar]
- 68. Walfisch A, Kabakov E, Friger M, Sheiner E. Trends, seasonality and effect of ambient temperature on preterm delivery. J Matern Fetal Neonatal Med 2017;30:2483-7. 10.1080/14767058.2016.1253063 [DOI] [PubMed] [Google Scholar]
- 69. Basu R, Chen H, Li DK, Avalos LA. The impact of maternal factors on the association between temperature and preterm delivery. Environ Res 2017;154:109-14. 10.1016/j.envres.2016.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Basu R, Malig B, Ostro B. High ambient temperature and the risk of preterm delivery. Am J Epidemiol 2010;172:1108-17. 10.1093/aje/kwq170 [DOI] [PubMed] [Google Scholar]
- 71. Ngo NS, Horton RM. Climate change and fetal health: the impacts of exposure to extreme temperatures in New York City. Environ Res 2016;144(Pt A):158-64. 10.1016/j.envres.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 72. Schifano P, Lallo A, Asta F, De Sario M, Davoli M, Michelozzi P. Effect of ambient temperature and air pollutants on the risk of preterm birth, Rome 2001-2010. Environ Int 2013;61:77-87. 10.1016/j.envint.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 73. Son JY, Lee JT, Lane KJ, Bell ML. Impacts of high temperature on adverse birth outcomes in Seoul, Korea: disparities by individual- and community-level characteristics. Environ Res 2019;168:460-6. 10.1016/j.envres.2018.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sun S, Weinberger KR, Spangler KR, Eliot MN, Braun JM, Wellenius GA. Ambient temperature and preterm birth: a retrospective study of 32 million US singleton births. Environ Int 2019;126:7-13. 10.1016/j.envint.2019.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang J, Williams G, Guo Y, Pan X, Tong S. Maternal exposure to heatwave and preterm birth in Brisbane, Australia. BJOG 2013;120:1631-41. 10.1111/1471-0528.12397 [DOI] [PubMed] [Google Scholar]
- 76. Cox B, Vicedo-Cabrera AM, Gasparrini A, et al. Ambient temperature as a trigger of preterm delivery in a temperate climate. J Epidemiol Community Health 2016;70:1191-9. 10.1136/jech-2015-206384 [DOI] [PubMed] [Google Scholar]
- 77. Carmichael SL, Cullen MR, Mayo JA, et al. Population-level correlates of preterm delivery among black and white women in the US. PLoS One 2014;9:e94153. 10.1371/journal.pone.0094153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mathew S, Mathur D, Chang AB, et al. Examining the effects of ambient temperature on pre-term birth in Central Australia. Int J Environ Res Public Health 2017;14:147. 10.3390/ijerph14020147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Basu R, Rau R, Pearson D, Malig B. Temperature and term low birth weight in California. Am J Epidemiol 2018;187:2306-14. 10.1093/aje/kwy116 [DOI] [PubMed] [Google Scholar]
- 80. Lawlor DA, Leon DA, Davey Smith G. The association of ambient outdoor temperature throughout pregnancy and offspring birthweight: findings from the Aberdeen children of the 1950s cohort. BJOG 2005;112:647-57. 10.1111/j.1471-0528.2004.00488.x [DOI] [PubMed] [Google Scholar]
- 81. Rashid H, Kagami M, Ferdous F, et al. Temperature during pregnancy influences the fetal growth and birth size. Trop Med Health 2016;45:1. 10.1186/s41182-016-0041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Auger N, Fraser WD, Smargiassi A, Bilodeau-Bertrand M, Kosatsky T. Elevated outdoor temperatures and risk of stillbirth. Int J Epidemiol 2017;46:200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rammah A, Whitworth KW, Han I, Chan W, Hess JW, Symanski E. Temperature, placental abruption and stillbirth. Environ Int 2019;131:105067. 10.1016/j.envint.2019.105067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Basu R, Sarovar V, Malig BJ. Association between high ambient temperature and risk of stillbirth in California. Am J Epidemiol 2016;183:894-901. 10.1093/aje/kwv295 [DOI] [PubMed] [Google Scholar]
- 85. Hansen CA, Barnett AG, Pritchard G. The effect of ambient air pollution during early pregnancy on fetal ultrasonic measurements during mid-pregnancy. Environ Health Perspect 2008;116:362-9. 10.1289/ehp.10720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kloog I, Novack L, Erez O, Just AC, Raz R. Associations between ambient air temperature, low birth weight and small for gestational age in term neonates in southern Israel. Environ Health 2018;17:76. 10.1186/s12940-018-0420-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sun S, Spangler KR, Weinberger KR, Yanosky JD, Braun JM, Wellenius GA. Ambient temperature and markers of fetal growth: a retrospective observational study of 29 million US singleton births. Environ Health Perspect 2019;127:67005. 10.1289/EHP4648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Krstić G. Apparent temperature and air pollution vs. elderly population mortality in Metro Vancouver. PLoS One 2011;6:e25101. 10.1371/journal.pone.0025101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yackerson N, Piura B, Sheiner E. The influence of meteorological factors on the emergence of preterm delivery and preterm premature rupture of membrane. J Perinatol 2008;28:707-11. 10.1038/jp.2008.69 [DOI] [PubMed] [Google Scholar]
- 90. Fukuda M, Fukuda K, Shimizu T, Nobunaga M, Mamsen LS, Yding Andersen C. Climate change is associated with male:female ratios of fetal deaths and newborn infants in Japan. Fertil Steril 2014;102:1364-1370.e2. 10.1016/j.fertnstert.2014.07.1213 [DOI] [PubMed] [Google Scholar]
- 91. Tam WH, Sahota DS, Lau TK, Li CY, Fung TY. Seasonal variation in pre-eclamptic rate and its association with the ambient temperature and humidity in early pregnancy. Gynecol Obstet Invest 2008;66:22-6. 10.1159/000114252 [DOI] [PubMed] [Google Scholar]
- 92. Tran TC, Boumendil A, Bussieres L, et al. Are meteorological conditions within the first trimester of pregnancy associated with the risk of severe pre-eclampsia? Paediatr Perinat Epidemiol 2015;29:261-70. 10.1111/ppe.12196 [DOI] [PubMed] [Google Scholar]
- 93. Davis RE, Novicoff WM. The impact of heat waves on emergency department admissions in Charlottesville, Virginia, USA. Int J Environ Res Public Health 2018;15:16. 10.3390/ijerph15071436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ha S, Nguyen K, Liu D, et al. Ambient temperature and risk of cardiovascular events at labor and delivery: a case-crossover study. Environ Res 2017;159:622-8. 10.1016/j.envres.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lin Y, Hu W, Xu J, et al. Association between temperature and maternal stress during pregnancy. Environ Res 2017;158:421-30. 10.1016/j.envres.2017.06.034 [DOI] [PubMed] [Google Scholar]
- 96. Okun ML, Roberts JM, Marsland AL, Hall M. How disturbed sleep may be a risk factor for adverse pregnancy outcomes. Obstet Gynecol Surv 2009;64:273-80. 10.1097/OGX.0b013e318195160e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dadvand P, Basagana X, Figueras F, Sunyer J, Nieuwenhuijsen MJ. Climate and group B streptococci colonisation during pregnancy: present implications and future concerns. BJOG 2011;118:1396-400. 10.1111/j.1471-0528.2011.03044.x [DOI] [PubMed] [Google Scholar]
- 98.Adhvaryu A, Fenske J, Kala N, et al. Fetal origins of mental health: evidence from Africa. CSAE Working Paper WPS/2015-15. 2015. http://www.csae.ox.ac.uk/materials/papers/csae-wps-2015-15.pdf.
- 99. Miao Y, Shen YM, Lu C, Zeng J, Deng Q. Maternal exposure to ambient air temperature during pregnancy and early childhood pneumonia. J Therm Biol 2017;69:288-93. 10.1016/j.jtherbio.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 100. Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019;7:e37-46. 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.United Nations Inter-agency Group for Child Mortality Estimation (UN IGME). Levels & trends in child mortality: report 2018. https://www.unicef.org/publications/index_103264.html. 2018.
- 102.World Health Organization. WHO recommendations on health promotion interventions for maternal and newborn health. https://www.who.int/maternal_child_adolescent/documents/health-promotion-interventions/en/. 2015. [PubMed]
- 103. Luton D, Alran S, Fourchotte V, Sibony O, Oury JF. Paris heat wave and oligohydramnios. Am J Obstet Gynecol 2004;191:2103-5. 10.1016/j.ajog.2004.05.090 [DOI] [PubMed] [Google Scholar]
- 104. Sciscione AC, Costigan KA, Johnson TR. Increase in ambient temperature may explain decrease in amniotic fluid index. Am J Perinatol 1997;14:249-51. 10.1055/s-2007-994137 [DOI] [PubMed] [Google Scholar]
- 105. Wright CY, Street RA, Cele N, et al. Indoor temperatures in patient waiting rooms in eight rural primary health care centers in northern South Africa and the related potential risks to human health and wellbeing. Int J Environ Res Public Health 2017;14:11. 10.3390/ijerph14010043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gough KV, Yankson PWK, Wilby RL, et al. Vulnerability to extreme weather events in cities: implications for infrastructure and livelihoods. J Br Acad 2019;7(s2):155-81. 10.5871/jba/007s2.155. [DOI] [Google Scholar]
- 107. Chersich MF, Luchters S, Blaauw D, et al. Safeguarding maternal and child health in South Africa by starting the child support grant before birth: design lessons from pregnancy support programmes in 27 countries. S Afr Med J 2016;106:1192-210. 10.7196/SAMJ.2017.v106i12.12011 [DOI] [PubMed] [Google Scholar]
- 108. Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Lippincott Williams and Wilkins, 2008. [Google Scholar]
- 109. Ali J, Al-Natsha SD, Shahata MAM, et al. Effect of high environmental temperature on fertilization, zygote cleavage, embryo quality, pregnancy and abortion in human assisted reproduction cycles. Med Sci Res 1999;27:565-7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material