Abstract
Biomanufacturing of tissues/organs in vitro is our big dream, driven by two needs: organ transplantation and accurate tissue models. Over the last decades, 3D bioprinting has been widely applied in the construction of many tissues/organs such as skins, vessels, hearts, etc., which can not only lay a foundation for the grand goal of organ replacement, but also be served as in vitro models committed to pharmacokinetics, drug screening and so on. As organs are so complicated, many bioprinting methods are exploited to figure out the challenges of different applications. So the question is how to choose the suitable bioprinting method? Herein, we systematically review the evolution, process and classification of 3D bioprinting with an emphasis on the fundamental printing principles and commercialized bioprinters. We summarize and classify extrusion-based, droplet-based, and photocuring-based bioprinting methods and give some advices for applications. Among them, coaxial and multi-material bioprinting are highlighted and basic principles of designing bioinks are also discussed.
Keywords: 3D bioprinting, Extrusion-based bioprinting, Droplet-based bioprinting, Photocuring-based bioprinting, Bioink
1. Introduction
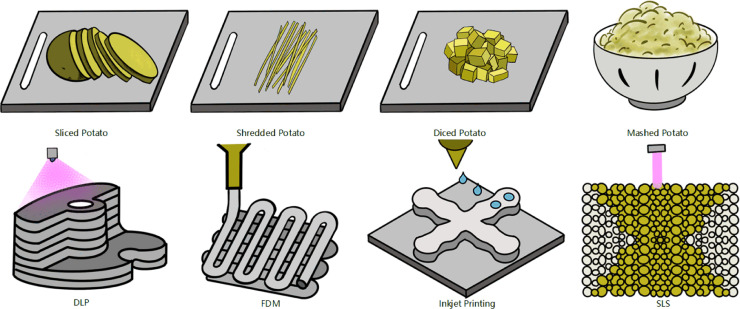
Three-dimensional (3D) printing, also known as additive manufacturing or rapid prototyping, whereby products are built on a layer-by-layer basis through a series of cross-sectional slices. It is like the inverse process of cutting potato into sliced, shredded, diced and mashed potato, while 3D printing assembles them to integrity. These four types of assembling potato correspondingly represent four typical processes of 3D printing: digital light processing (DLP) using planar projection, fused deposition modeling (FDM) using filaments, inkjet printing using micro spheres and selective laser sintering (SLS) using powders to be sintered (Fig. 1).
Fig. 1.
Four types of cutting potato corresponding to four typical 3D printing processes.
3D bioprinting which is a cross-science closely related to medical science, biology, mechanical engineering and material science, can be divided into two concepts: broadly speaking, 3D printing related to direct biomedical field can be regarded as 3D bioprinting; narrowly speaking, 3D bioprinting can be defined as the process of manipulating cell-laden bioinks to fabricate living structures. In the broad sense, bioprinting can be roughly classified into four levels. Level one is to manufacture structures without biocompatibility requirements, such as 3D printed products used in surgical path planning; level two is to create non-degradable products that are required to be biocompatible, such as titanium alloy joints and silicone prostheses for defect repair; level three is to fabricate biocompatible and degradable products, such as active ceramic bone and biodegradable vascular stent; level four which is the same concept of bioprinting in the narrow sense, is to manipulate living cells to build biomimetic 3D tissues, such as cell models used for drug screening and mechanism research, liver units, skin, blood vessels [1]. The concept of 3D bioprinting we discuss in this article is in the fourth level, which can also be known as cell printing.
Biomanufacturing of tissues/organs in vitro has long been a big dream pursued by humans, which is driven by two needs: organ transplantation and accurate tissue models. Firstly, there is a huge shortage of organs for transplantation. In 2016, there were 160 000 organ transplant recipients, but only 16000 organ donors in United States [2]. At present, utilizing 3D bioprinting to solve the shortage of organ transplantation is far too optimistic, because of the complexity of human organs which reflected in not only the biologically unrevealed mechanism of organ growth, but also the reproduction of delicate structure manufacturing. Secondly, traditional methods like two-dimensional (2D) cell culture or animal experiments applied for drug screening and medical mechanism studies, have a lot of flaws. Microenvironment in vivo is far more complicated than 2D cell culture, in which 2D models might lead to opposite results in some cases. And there is, after all, a huge difference of internal environment between animals and humans. These factors make the need of more accurate in vitro models becoming more and more urgent, and that's what 3D bioprinting is good at. Thanks to the capability of achieving spatio-temporal directional manipulation of various cells, 3D bioprinting has become the most ideal means to construct living 3D cell-laden structures in vitro. There is no doubt that 3D bioprinting will play an increasingly important role in the construction of in vitro organ models within a predictable period of time.
This paper begins with the evolution, process and classification of bioprinting. Fundamental principles and typical bioprinters of each bioprinting method are elaborated. Subsequently, combining with the experience of our research group, detailed discussions of applications including organoids biofabrication and drug researches for each bioprinting approach are provided. Afterwards, we make comparison among the different bioprinting approaches, in which selection of printing methods aiming at various applications is discussed. Evaluation standards of bioinks and several typical kinds are finally represented. There is no one perfect bioprinting approach, or bioink for all, the key is how to choose the most suitable printing method/bioink for different application scenarios. This review is not intended to be exhaustive in nature, but we choose specific applications which in our opinion, offer the greatest advance in their respective fields, and most promise for forthcoming work of a significant nature.
2. Evolution, process and classification of 3D bioprinting
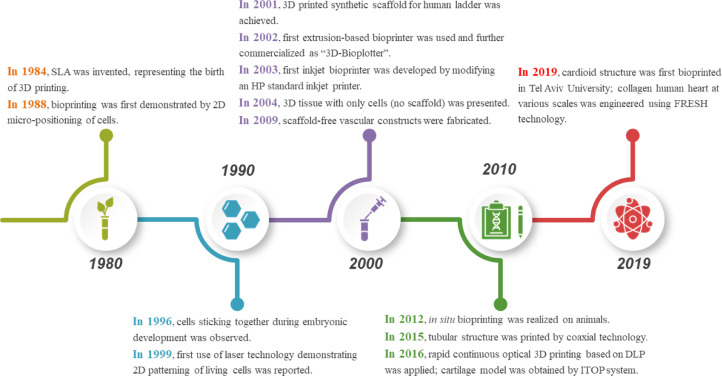
At present, it is not quite realistic to achieve 3D bioprinting of fully functional organs for transplantation. However, it is a fact cannot be denied that bioprinting techniques have evolved significantly. Decades ago, several pioneers, such as Vladimir Mironov, Gabor Forgacs and Thomas Boland, saw the natural combining of technologies including cell patterning and others, such as commercial inkjet printing, for building living structures that perhaps could serve in human organ transplantation one day [3], [4], [5]. A timeline for the evolution of bioprinting technology up to state-of-the-art is illustrated in Fig. 2.
Fig. 2.
A brief history of bioprinting.
In 1984, Charles Hull invented stereolithography (SLA) for printing 3D objects from digital data, symbolizing the birth of 3D printing. Bioprinting was first demonstrated in 1988 while Klebe using a standard Hewlett-Packard (HP) inkjet printer to deposit cells by cytoscribing technology [6]. In 1996, Forgacs and co-workers drew a conclusion that apparent tissue surface tension was the macroscopic manifestation of molecular adhesion between cells and provided a quantitative measure for tissue cohesion [7]. In 1999, Odde and Renn first utilized laser assisted bioprinting to deposit living cells for developing analogs with complex anatomy [8]. In 2001, direct printing of a scaffold in the shape of a bladder and seeding of human cells took place [9]. In 2002, the first extrusion-based bioprinting technology was reported by Landers et al., which was later commercialized as “3D-Bioplotter” [10]. Wilson and Boland developed the first inkjet bioprinter in 2003 by modifying an HP standard inkjet printer [11]. A year after, their team implemented cell-loaded bioprinting with a commercial SLA printer [12]. In the same year, 3D tissue with only cells (no scaffold) was developed. In 2006, electrohydrodynamic jetting was applied to deposit living cells [13]. Scaffold-free vascular tissue was engineered through bioprinting by Norotte et al. in 2009 [14]. In 2012, in situ bioprinting was attempted by Skardal et al. on mouse models [15]. The following years saw the introduction of many new bioprinting products, such as articular cartilage and artificial liver in 2012, tissue integration with circulatory system in 2014 and so on [16,17]. In 2015, coaxial technology was adopted by Gao et al. for fabrication of tubular structure [18]. In 2016, Pyo et al. applied rapid continuous optical 3D printing based on DLP [19]. In the same year, cartilage model was manufactured by Anthony Atala's research group using integrated tissue-organ printer (ITOP) [20]. In 2019, Noor et al. succeeded in manufacturing a perfusable scale-down heart [21]. And a few months later, bioprinting of collagen human hearts at various scales based on freeform reversible embedding of suspended hydrogels (FRESH) technology was achieved by Lee et al. [22].
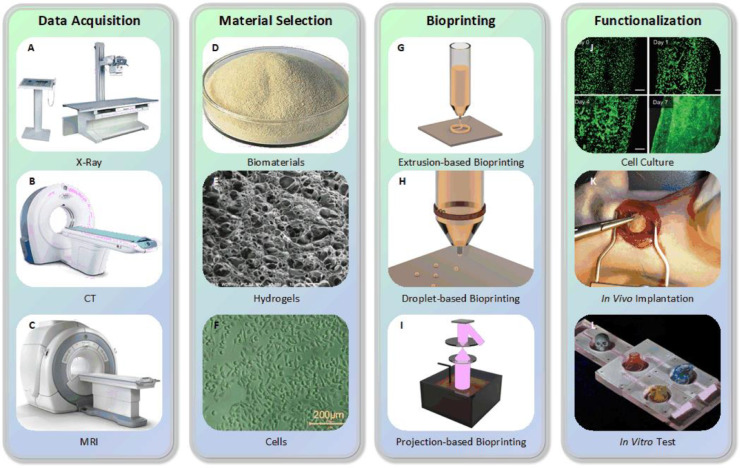
The process of 3D bioprinting can be classified into four steps (Fig. 3):
-
a.
Data acquisition. 3D models can be obtained by using X-ray, computed tomography (CT), magnetic resonance imaging (MRI), etc. techniques to scan and reconstruct, or directly using computer aided design (CAD) software to establish. 3D models would then be divided into 2D horizontal slices (with customizable size and orientation) by specific software. These data would be further processed into particles or filaments according to different bioprinting approaches.
-
b.
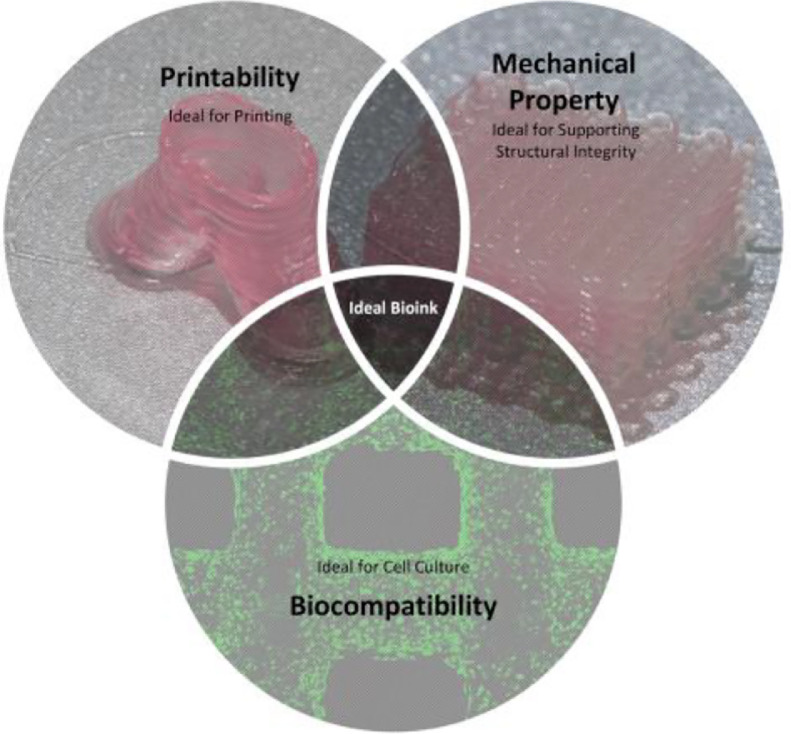
Material selection. Materials including cells, growth factors, hydrogels, etc. should be chosen carefully according to the requirement of printed structures and approaches. Strictly speaking, the combination of these biomaterials is called bioinks, while they could also be simply regarded as cell-laden hydrogels in most cases. The selection of bioinks is crucial to guarantee biocompatibility, printability and mechanical property, which would be further discussed in the last part of this review.
-
c.
Bioprinting. Before bioprinting, appropriate configuration of printing parameters needs to be confirmed. And observation during printing process is necessary to make adjustment when encounters any problems.
-
d.
Functionalization. After printing, to make dispersed cells forming connections and generating some functions of natural tissue/organ through physical and chemical stimulation is the target.
Fig. 3.
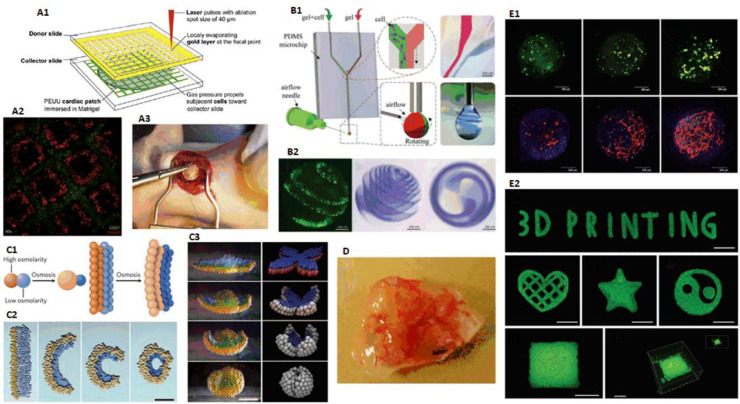
3D bioprinting process (A) X-ray machine, (B) CT machine, (C) MRI machine, (D) Alginate, (E) Scanning electron microscope (SEM) image of GelMA (reproduced with permission from [23], Copyright 2018 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim), (F) Image of human umbilical vein endothelial cells (HUVECs) (reproduced with permission from [24], Copyright 2018 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim), (G) Principle of extrusion-based bioprinting, (H) Principle of piezoelectric inkjet bioprinting, (I) Principle of digital light processing (DLP), (J) Culture of endothelial progenitor cell-laden blood vessel (reproduced with permission from [25], Copyright 2017 Gao et al.), (K) In vivo implantation of cardiac patches fabricated by laser induced forward transfer (LIFT) in rats (reproduced with permission from [26], Copyright 2011 Elsevier), (L) Biochip used for in vitro testing, adapted from unpublished work of our research team.
According to different prototyping principles and printing materials, 3D bioprinting is mainly based on three central approaches: extrusion-based, droplet-based and photocuring-based bioprinting. Extrusion-based bioprinting extrudes bioinks to form continuous filaments for building constructs; droplet-based bioprinting produces discrete droplets to stack into structures; and photocuring-based bioprinting takes advantage of photo-curing materials, to solidify and stack layer-by-layer to achieve 3D models.
3. Extrusion-based bioprinting
Extrusion-based bioprinting (also called direct ink writing), which derived from inkjet printing, is the most widely used approach of 3D bioprinting because of its versatility and affordability. Instead of single droplet, extrusion-based bioprinting produces ongoing filaments through continuous extrusion force. This approach can be used for printing a wide range of viscosities of biomaterials and different concentrations of cells [10]. For this reason, researchers prefer extrusion-based bioprinting to build tissue structures with sufficient mechanical property [27], [28], [29], [30]. In addition, coaxial and multi-material bioprinting can also be perfectly compatible with extrusion-based bioprinting for various kinds of applications.
3.1. Principles
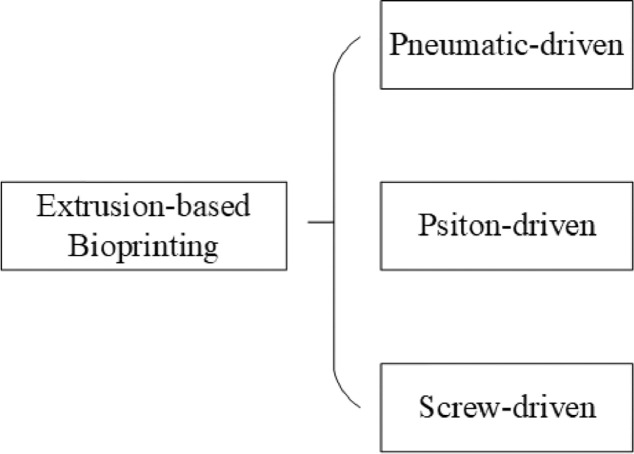
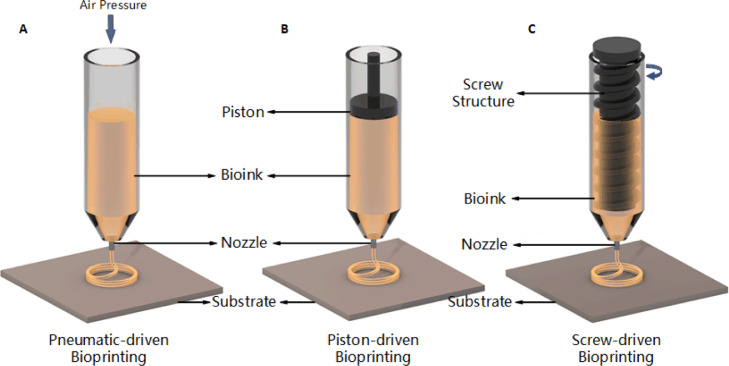
Theoretically, extrusion-based bioprinting extrudes bioink (usually from a syringe) through a nozzle by means of mechanical or pneumatic driven, to form continuous micro filaments which are subsequently deposited on them receiving substrate and finally stacked into desired structures. Substrate can be solid (e.g., culture dish), liquid (e.g., growth medium) or material derived from gel. The path of nozzle is usually generated by software according to digital models after configuration. Parameters such as temperature, nozzle diameter, extrusion pressure, movement speed, extrusion speed, path interval, etc. would influence the final bioprinted structures. According to the different actuating modes of liquid dispensing system, common extrusion-based bioprinting can be classified into pneumatic, piston and screw-driven (Fig. 4).
Fig. 4.
Classification of extrusion-based bioprinting.
3.1.1. Pneumatic-driven extrusion
Pneumatic-driven extrusion system utilizes compressed air to realize liquid dispensing. Usually it includes a syringe loaded with bioink, which is connected to an air pump through adapter and pipes (Fig. 5A). Hydrogels with shear-thinning property works sufficiently with pneumatic-driven system on account of its maintaining filament status after extrusion. Pneumatic-driven system requires sterilization of air from the air pump. Thus, using a filter on the airway would be ideal to minimize contamination of the bioprinted constructs. Besides, smooth extrusion needs to be guaranteed as far as possible, which means extra liquid or gel-based medium needs to be added when encounters with semi-solid or solid state bioink to optimize its viscosity.
Fig. 5.
Principles of extrusion-based bioprinting.
3.1.2. Piston-driven extrusion
It is generally acknowledged that mechanical driven liquid dispensing system is more suitable for extrusion of high viscosity biomaterials, such as synthetic or natural high-molecular polymers. Among them, piston-driven extrusion is quite common, and related devices such as micro-infusion pumps are easily acquired on the market. The piston in this system is connected to a motor through a guide screw. When motor starts, rotational motion of the guide screw transfers to linear motion of the piston, which pushes bioink out of the nozzle to form filaments (Fig. 5B).
3.1.3. Screw-driven extrusion
As another type of mechanical driven liquid dispensing system, screw-driven devices provide more volumetric control, and contribute to the extrusion of biomaterials with higher viscosities. The principle of screw-driven system is similar with piston-driven one except that a screw which is connected to the motor, is directly used for extrusion instead of the piston (Fig. 5C). Nevertheless, screw-driven devices not only provide more pressure, but also might damage the cell loaded in bioink in the meantime. Hence, a caution design of the screw parts is necessary.
Researches that combining piston-driven and screw-driven have also been reported. Visser et al. first printed polycaprolactone (PCL) by means of screw-driven, then printed hydrogel on PCL by means of piston-driven [31]. In short, compared to pneumatic-driven method, piston and screw-driven methods provide higher resolution and better printability with semi-solid or solid state biomaterials (e.g., cell aggregates) [27]. Whereas, devices using these two methods are volumetric limited, cleaning and disinfection complicated (especially for screw-driven devices), and cost more.
With appropriate bioinks, extrusion-based bioprinting is a reliable tool to fabricate biomaterials, especially for hydrogels with shear-thinning and rapid crosslinking properties. Nozzle diameter, bioink viscosity, nozzle movement speed, bioink extrusion speed, extrusion pressure, surface properties of substrate and so on would affect final bioprinted formation. For the reason of diversity, economy and capacity of printing porous structures, extrusion-based bioprinting is widely used by researchers all over the world.
3.2. Bioprinters
Since extrusion-base bioprinting is the most convenient affordable and common approach, there are numerous commercial bioprinters based on extrusion method on the market.
In our opinion, scaffolds printing based on FDM with cells transplanting afterwards is not truly bioprinting technology. Hence, 3D Bioplotter® which was capable of cell-laden bioprinting, could be defined as the first commercial 3D bioprinter in the world [32]. It was invented by a research group of University of Freiburg and commercialized by EnvisionTEC (which was founded in 1999 and regrouped into EnvisionTEC GmbH [33] in 2002) soon afterwards [10]. It can not only print cell-laden hydrogels such as gelatin, fibrin, alginate, agarose, etc. but also utilize hard polymers, inorganic ceramic materials such as PCL, hydroxyapatite (HA), tricalcium phosphate (TCP) particles to fabricate non-bioabsorbable scaffolds [34].
Another notable bioprinter was NovoGen MMX Bioprinter™ invented by Organovo (which was founded in Delaware, USA, 2007) in 2009 [35]. This compact device which could be put in a standard clean bench, had two nozzles to extrude cells, hydrogels, scaffolds or supporting matrix respectively. This device was first used to bioprint tissue spheroids along with a support structure made of agarose hydrogel. Tissue spheroids fused together and further matured into a tissue-like organization after printing process, and agarose was removed afterwards [14,36]. For now, this company no longer sell bioprinters any more, instead it transformed to a platform providing technical services including in vitro test, disease models and safety test.
In addition, there are several bioprinters enjoy high reputation in extrusion-based bioprinting: 3DDiscovery from RegenHU in Swiss, FABION from 3D Bioprinting Solutions in Russian, BIO V1 from REGEMAT3D in Spanish, INKREDIBLE from CELLINK in Swedish, BIOBOTTM and BIOASSEMBLYBOTⓇ from Advanced SOLUTIONS in American, BioScaffolder fromGeSim in German , 3DS Alpha and Omega Bioprinter from 3DYNAMIC SYSTEMS in British, ROKIT INVIVO from ROKIT in Korean, SYN^ from BIO3D in Singapore, Bioarchitect from Regenovo in China, etc.
Our research group has been working on bioprinting for years. We commercialized several 3D bioprinters in cooperation with Suzhou Intelligent Manufacturing Research Institute, such as extrusion-based bioprinter EFL-BP6601 and high-precision printer EFL-BP5800 (Fig. 6). The advantages of our products are functional modularization (independent pneumatic control module, temperature control module can be added) and portability (suitable for standard clean bench). BP6601 is user-friendly supported with multi-level schemes and personalized services. It can not only contribute to the researchers in the early stage to build a platform for bioprinting, but also meet higher needs of researchers in the advanced stage in the area of regeneration and repair, drug screening, tumor model, personalized medicine and so on. BP5800 can realize printing filaments with a diameter of 3 µm (compared to printing resolution of 100–200 µm for conventional 3D printer), which is suitable for efficient fabrication of high-precision bio-scaffolds, while high resolution leads to greatly improved biocompatibility.
Fig. 6.
(A) Extrusion-based bioprinter EFL-BP6601; (B) High-precision printer EFL-BP5800 (3 µm resolution).
3.3. Applications
The applications for bioprinting can be divided into four categories: cytobiology, drug research, tumor model and regenerative medicine. Cytobiology covers fabrication single cell or multicellular combination, including research on basic issues about cell growth, intercellular relationship, transgenosis. Drug research means pharmacokinetics, drug screening, ancillary drug development. Tumor model is mainly about setting up various kinds of tumor pathological models, researching tumorigenesis mechanism, targeted therapy and so on. Regenerative medicine which is more related with bioprinting, includes manufacturing of artificial tissue and organ, fabrication of neural tissue, cardioid, liver and so on, vascularization of scale-up tissue and cell therapy. These applications cover 3D bioprinting in both broad and narrow sense as we mentioned before. We will focus on applications on cell-laden extrusion-based bioprinting. In this section, after illustrating organoid tissues, drug research, disease model, we will emphasize on multi-material bioprinting and coaxial bioprinting which are quite hot and widely used in recent years.
3.3.1. Skin
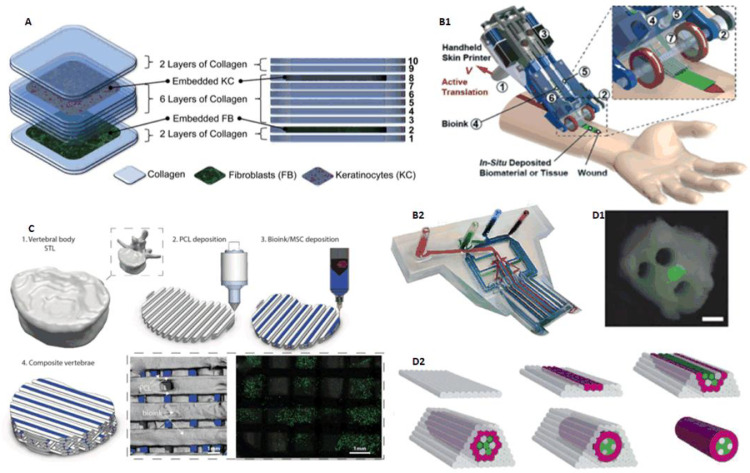
In 2009, Lee et al. utilized pneumatic extrusion assisted by micro valve control to build a four-nozzles bioprinter. Using this bioprinter, 10 layers of collagen hydrogel precursor in layer-by-layer fashion was printed, in which fibroblasts was printed in the second layer and keratinocytes was printed in the eighth layer separately, to realize multi-layered engineered tissue composites to mimic natural skin layers (Fig. 7A). Besides, in order to testify printability on non-planar surfaces for potential applications including skin wound repair, highly viable proliferation of cells was observed on a poly(dimethylsiloxane) (PDMS) mold with 3D surface contours as target substrate [37]. In 2014, Lee et al. from another research group used a similar bioprinting device except for deploying 8 nozzles, to bioprint keratinocytes, fibroblasts and collagen for representing the epidermis, dermis and dermal matrix of natural skin respectively. Histology and immunofluorescence characterization demonstrated that 3D printed skin tissue was morphologically and biologically representative of in vivo human skin tissue. 3D bioprinting offers several advantages in terms of shape and form retention, flexibility, reproducibility and high culture throughput. This study can also serve as a model for studying the pathophysiology of skin diseases [38].
Fig. 7.
(A) Alternately bioprinted fibroblasts and keratinocytes mimicking natural skin structures (reproduced with permission from [37], Copyright 2008 Elsevier); (B) Handheld 3D skin bioprinting device combining microfluidic technique (reproduced with permission from [43], Copyright 2018 the Royal Society of Chemistry); (C) Multi-nozzle bioprinting bone tissue (reproduced with permission from [46], Copyright 2016 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim); (D) Scaffold-free construction of neural grafts (reproduced with permission from [50], Copyright 2013 IOP Publishing Ltd).
In 2009, Kim et al. used a cryogenic refrigeration system to fabricate highly porous (>95%) collagen scaffolds composed of perpendicular collagen strands in successive layers. After subzero treatment (at −76 °C) and solidification, a keratinocyte/fibroblast co-culture on the printed scaffolds was introduced to show proliferation, migration and differentiation of cells [39]. Due to the relatively poor mechanical strength of the scaffolds, the same research group implemented coaxial bioprinting to build collagen–alginate scaffolds in 2011, which increased modulus about seven times. In this study, mice in vivo test was employed to prove good granulation tissue formation and rapid vascularization [40].
In 2012, after verifying the immunomodulatory and high proliferation properties (such as delivery of secreted trophic factors, inducing endothelial cell migration in vitro) of amniotic fluid-derived stem (AFS) cells, Skardal et al. used AFS cells-laden fibrin-collagen hydrogel as bioink, to bioprint on mice full-thickness skin wounds in the manner of thrombin/bioink alternately in situ, to demonstrate its capacity of treatment for large-scale wounds and burns [15].
In 2012, Leng et al. developed a device based on microfluidic technology to bioprint so called mosaic hydrogels. This device consisted of a ten-layer microfluidic device with 7 on-chip reservoirs included, a syringe pump, an annular gear pump and a collection drum. Researchers sandwiched one layer of cell-laden hydrogel into 10 layers of biopolymer hydrogel; and then by controlling on/off status of the 7 reservoirs, hydrogel sheets with desired pattern could be printed (including the word “TORONTO”) [41]. This device was applied into skin bioprinting in 2013. Using the microfluidic device, researchers achieved precise spatio-temporal control over cell location and seeding, to bioprint fibroblasts-laden hydrogel into wound dressings which were subsequently implanted into murine wound models. The results showed improved wound healing and keratinization were observed [42]. In 2018, Hakimi et al. from the same research group further optimized this device into a handheld skin printer which could be applied in rapid repairing deep wound. Just like a tape dispenser, this bioprinter fabricate skin cell-laden sheets onto severe skin wound (Fig. 7B). By embedding dermal and epidermal cells into different crosslinkable hydrogels containing alginate or fibrin mixed with collagen and HA, they were able to produce skin cell-laden sheets with controllable thickness, width, and composition. In situ bioprinting in murine and porcine excisional wound models illustrated the compatibility of this method with compliant wound surfaces [43].
3.3.2. Bone/cartilage
In the area of bone tissue application, using 3D bioprinting techniques to fabricate scaffolds is a common way. In 2015, Yao et al. used FDM technology to print PCL-HA scaffolds based on CT 3D reconstructional data. The biological related load capacity of these scaffolds was further tested [44]. In 2015, Pati et al. utilized muiti-nozzle method to print scaffolds made from a composite of PCL, poly(lactic-co-glycolic acid) (PLGA), β-TCP and mineralized extracellular matrix (ECM) laid by human nasal inferior turbinate tissue-derived mesenchymal stromal cells (hTMSCs). These ECM-ornamented scaffolds exhibit both osteoinductive and osteoconductive properties as revealed by their ability to increase mineralized tissue formation ectopically, and to increase bone regeneration orthotopically, respectively [45]. Since we are focusing on cell-laden bioprinting in this paper, examples concerning scaffolds without cell printing would no longer be mentioned.
In 2016, Daly et al. offered a method for bone tissue: bioprinting a developmental bone tissue precursor in vitro, then using this engineered rudiment as a template for subsequent vascularization and osteogenesis in vivo. Researchers used gamma-irradiated alginate incorporating Arg-Gly-Asp (RGD) adhesion peptides (which supported stronger chondrogenic differentiation), carrying adult mesenchymal stem cells (MSCs) as bioink, with printed PCL fibers (which contributed to mechanical property reinforcement) by means of multi-nozzle, to fabricate desired structure. The PCL fibers and bioink are printed by turns (Fig. 7C). After 12 weeks in vivo, this composite vertebral structure showed significantly higher levels of bone formation. It's worth mentioning that by controlling the placement of the bioink within every second PCL fiber spacing it was possible to introduce a network of interconnected bioink-free channels within the PCL construct, leading to better nutrient transportation [46].
In 2015, Kesti et al. used bioink consisted of gellan, alginate and a clinical product called BioCartilage (cartilage extracellular matrix particles), to bioprint cartilage grafts. This bioink was proven to support proliferation of chondrocytes and strong deposition of cartilage matrix proteins (in the presence of transforming growth factor beta-3) through MRI and histological evaluation after 8 weeks in vitro. Besides, co-extrusion of a cation-loaded transient support polymer was introduced to promote physical gelation for stabilizing overhanging structures [47].
In 2013, Kundu et al. applied an approach like Daly et al. bioprinting bone tissue [46], using a multi-head deposition system (MHDS), to print PCL and chondrocyte cell-encapsulated alginate hydrogel layer-by-layer by turns to obtain chondrocyte cell-laden scaffolds. 4 weeks mice experiment revealed enhanced cartilage tissue and type II collagen fibril formation in this hybrid scaffold [48].
In 2014, Lee et al. also utilized multi-nozzle extrusion device in the area of ear reconstruction. Researchers bioprinted PCL and cell-laden hydrogel by turns to fabricate the auricular cartilage and fat tissue of ear with poly(ethylene glycol) (PEG) as a sacrificial layer to support main structure. chondrocytes and adipocytes differentiated from adipose-derived stromal cells were encapsulated in hydrogel to dispense into the cartilage and fat regions, respectively [49].
In 2016, Kang et al. presented an integrated tissue–organ printer (ITOP) based on extrusion technology. Researchers printed cell-laden hydrogels together with biodegradable polymers (leaving microchannels into the tissue constructs to facilitate diffusion of nutrients) in integrated patterns and anchored on sacrificial hydrogels (e.g., Pluronic F-127). Mandible and calvarial bone, cartilage and skeletal muscle were reconstructed in this approach [20].
3.3.3. Nerve
In 2013, Owens et al. reported an approach to fabricate nerve grafts composed exclusively of cells and cell secreted material. Researchers used mouse bone marrow stem cells (BMSC) and Schwann cells (SCs) as bioink to form 0.5 mm diameter multicellular cylinders through extrusion. As shown in Fig. 7D, the structure was supported by an array of agarose rods (gray), which hold the conduit in place and would be removed after 7 d while the discrete bioink cylinders self-assembled. The outer ring was made of bioink composed completely of BMSC (red), and cylinders comprised of 90% BMSC and 10% SC (green) were alternated with agarose rods, which brought multiple lumina inside the grafts. The regenerative capacity of the grafts were then assessed which showed that this would be a promising approach of nerve graft fabrication and as a consequence to nerve regeneration [50].
3.3.4. Muscle/tendon
In 2015, Merceron et al. employed a 3D bioprinter with four cartridges for deposition of four different components to fabricate an integrated muscle–tendon unit (MTU) construct. Thermoplastic polyurethane (PU) was co-printed with C2C12 cell-laden hydrogel-based bioink for elasticity and muscle development on one side, while PCL was co-printed with NIH/3T3 cell-laden hydrogel-based bioink for stiffness and tendon development on the other. This study demonstrates the capacity of bioprinting integrated tissue constructs with region-specific biological and mechanical characteristics [51].
3.3.5. Adipose tissue
Artificial adipose tissue structure can be used for soft tissue reconstruction in plastic surgery and repair surgery. In 2015, Pati et al. employed decellularized adipose tissue (DAT) matrix encapsulating human adipose tissue-derived mesenchymal stem cells (hASCs) as bioink to bioprint flexible dome-shaped structures with engineered porosity within a PCL framework through a multi-nozzle device. Mice implantation experiment showed that the structure did not induce chronic inflammation or cytotoxicity post-implantation, but supported positive tissue infiltration, constructive tissue remodeling, and adipose tissue formation [52].
3.3.6. Blood vessel/vascularization
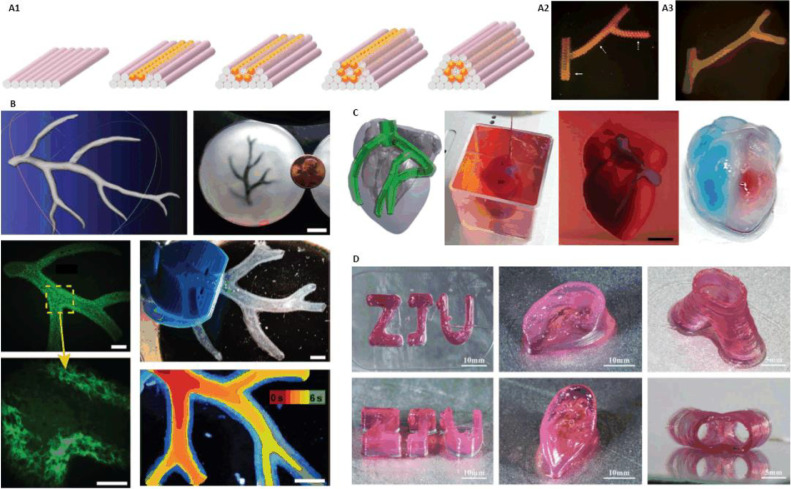
In 2009, Norotte et al. used human umbilical vein smooth muscle cells (HUVSMCs), human skin fibroblasts (HSFs) and porcine aortic smooth muscle cells (PASMCs) to build scaffold-free small-diameter multi-layered tubular vascular grafts. Cells were aggregated into discrete units, either multicellular spheroids or cylinders of controllable diameter (300–500 mm), which were printed layer-by-layer with agarose rods, used here as a molding template. The post-printing fusion of the discrete units resulted in single- and double-layered small diameter vascular tubes while agarose was removed (Fig. 8A). This method provided a way to engineer vessels of distinct shapes and hierarchical trees with distinct diameters [14].
Fig. 8.
(A) Vascular structures fabrication using agarose as sacrificial material (reproduced with permission from [14], Copyright 2009 Elsevier); (B) Vascular network printed in suspended hydrogel (reproduced with permission from [54], Copyright 2015 Hinton et al.); (C) 3D bioprinting whole heart containing major blood vessels (reproduced with permission from [21], Copyright 2019 Noor et al.); (D) Nanoclay and GelMA hybrid bioprinting complex structures (reproduced with permission from [64], Copyright 2019 IOP Publishing Ltd.).
In 2010, Skardal et al. fabricated bioartificial vessel-like grafts using Fab@Home printing system. Two kinds of TetraPEGs were converted to tetra-acrylate derivatives (TetraPAcs) which were used in turn to co-crosslink thiolated hyaluronic acid and gelatin derivatives into extrudable hydrogels for printing tissue constructs. Based on this, researchers applied NIH3T3 cells-laden hydrogel to build tubular tissue structure which maintained viability in culture for up to 4 weeks [53].
In 2015, Hinton et al. developed an approach termed FRESH on a MakerBot Replicator modified with a custom syringe-based extruder. Based on this technique, structures were built by embedding the printed hydrogel within a secondary hydrogel that served as a temporary, thermoreversible and biocompatible support. The support bath was composed of gelatin microparticles that acted like a Bingham plastic during the print process, behaving as a rigid body at low shear stresses but flowing as a viscous fluid at higher shear stress. This method facilitated bioprinting of hydrated materials with an elastic modulus <500 kPa including alginate, collagen and fibrin, to fabricate complex biological structures, such as an arterial tree (Fig. 8B) [54].
In fact, as a special category of extrusion-based bioprinting, coaxial bioprinting is very widely used in the field of vessels fabrication and vascularization. We will focus on coaxial bioprinting in the subsequent section.
3.3.7. Organoid
In 2013, Billiet et al. chose VA-086 as photo-initiator, gelatin methacryloyl (GelMA) encapsulating hepatocarcinoma cell line (HepG2) as bioink, to build 3D constructions. Mechanically stable cell-laden GelMA scaffolds displaying a maintained expression of liver specific functions with high cell viability (> 97%) was printed [55].
In 2016, Lee et al. utilized the multi-nozzle device mentioned before, using PCL framework and cell-laden collagen alternately printing method to build 3D construct. Since nonparenchymal cells could not survive when cultured alone in vitro, hepatocytes (HCs), human umbilical vein endothelial cells (HUVECs) and human lung fibroblasts (HLFs) were encapsulated in collagen bioink. Heterotypic interaction among HCs and nonparenchymal cells was proven to increase the survivability and functionality of HCs within the collagen gel by the result of vascular formation and functional abilities of HCs (i.e. albumin secretion and urea synthesis). This technology showed possibility for creating heterotypic cellular interaction within a structure for liver tissue engineering [56].
In 2015, for the first time, Horvath et al. demonstrated a technique of fabricating human air-blood tissue barrier analogue. Researchers used a multi-nozzle bioprinter to print a thin layer of ECM on porous membranes, and subsequently a layer of EA.hy926 endothelial cells was printed on Matrigel; then a second ECM layer is printed on it 2 d after. 3D organization, viability, proliferation and barrier quality of cells were assessed after they were cultivated for additional 3 days. This would be an excellent to engineer lung models for high-throughput screening of safety assessment and drug efficacy testing [57].
Valvular heart disease is a serious public health problem which can be treated by artificial valve replacement most commonly. However, it is inadequate for growing children or young adults. In 2012, Duan et al. fabricated living alginate/gelatin hydrogel valve conduits with anatomical architecture and direct incorporation of dual cell types including aortic root sinus smooth muscle cells (SMC) and aortic valve leaflet interstitial cells (VIC). This study provided a way to build anatomically complex, heterogeneously encapsulated aortic valve hydrogel conduits with 3D bioprinting [58].
Recently, a report of world's first “complete” heart bioprinting caught everyone's eye. In 2019, Noor et al. from Tel Aviv University adopted cells from patients’ omental tissue to be reprogrammed into pluripotent stem cells, and differentiated to cardiomyocytes and endothelial cells. These two types of cells are combined with hydrogels to form bioink to fabricate parenchymal cardiac tissue and blood vessels respectively, which demonstrated the ability to bioprint customized vascularized patches. Furthermore, a cellularized human heart with a natural architecture at the size of a rabbit heart (height: 20 mm, diameter: 14 mm) was printed which was perfusable but could not functionally pump blood (Fig. 8C). This study demonstrated the potential of engineering personalized tissues and organs, or for drug screening in an appropriate anatomical structure and patient-specific biochemical microenvironment [21]. A few months later, Lee et al. from Carnegie Mellon University succeeded in bioprinting components of human heart at various scales, from capillaries to the full organ, employing an optimized technique based on FRESH we discussed above [22,54]. Nonetheless, we believe these studies are not as that creative as reported by the media. Technically, bioprinting artificial heart structures with micro channels inside, and realizing cardiomyocytes beating at the same rate in a small area is not that hard. What is truly difficult is to accomplish internal functional blood vessels and large areas of cardiac tissue with sufficient toughness and elasticity. With the goal of cardioid organ replacement, leaving complicated cell sources aside, in vitro heart structure needs to meet two points: 1. Complex functional vascular network inside the structure is needed because of the intense energy exchange of heart beats; 2. Large areas of cardiac tissue with sufficient toughness and elasticity should be able to beat at the same rate, or pumping blood cannot be achieved. Structurally and morphologically, human organ is much more complicated than we can image right now. Many mysteries remain to be solved in the mechanism of organ growth and development which is also the biggest problem of organ 3D printing and replacement. There is still a long way for 3D bioprinting from structural similarity to functional realization.
3.3.8. Drug research
In fact, several papers we illustrated above could be counted into the application of drug research, such as Horvath et al. printed human air-blood tissue barrier analogue [57], Noor et al. printed patient-specific cellularized heart structure [21].
One of main goals of tissue engineering is to establish an in vitro pharmacokinetic model for drug screening and toxicologic study, to realistically and reliably predict human response to drug effects and potential toxic risks. In 2008, Chang et al. set up an in vitro 3D microfluidic, microanalytical, microorgan (3DM) device for simulation of the physiological human response to drug administrations and toxic chemical exposure. Integrated with a microfluidic platform and extrusion-based bioprinting technique, this study achieved reproducible fabrication of tissue constructs and 3D organ chambers with maintenance of structural integrity, enhancement of cell viability with control of cellular-level differentiation and tissue level function, meaning applied bioprinting 3D cell-encapsulated alginate-based tissue engineered constructs within tissue chambers to form a pharmacokinetic model [59]. Soon afterwards in 2010, the same research group developed an extrusion-based bioprinter to use alginate encapsulating HepG2 liver cells as bioink, to build a liver drug testing platform. In this system, drug metabolic capacity of liver was observed through optimally simulating in vivo 3D microenvironment, liver architecture or shear-mediated microfluidic perfusion flows [60].
In 2011, Snyder et al. expanded the system above, to set up a portable ground model for the study of drug conversion and radiation protection of living liver tissue analogs. Researchers used two cell types: HepG2 and human mammary epithelial (M10) in the analogs to study pro-drug conversion in a dual tissue microfluidic chip and resultant radioprotection to liver, which would contribute to understand the response of the multi-cellular biological system for long-term manned space exploration, disease models and biosensors [61].
3.3.9. Multi-material bioprinting
At present, 3D bioprinting concerning multiple materials which means two and more types of materials to build a structure in collaboration, becomes more and more widely used. The multi-material here could be Daly et al. adopted no-cell-laden PCL polymer with cell-laden bioink to print bone tissue precursor in turn [46]; it could be Lee et al. used different bioinks encapsulating keratinocytes, fibroblasts and collagen to print different parts of skin tissue [38]; it could be to utilize different material properties to form composite bioink for overcoming the limitation of different printing technologies and post tissue culture; it could also be to make bioink carrying different cell suspensions for co-printing in order to meet the requirements of cell viability and tissue functionalization. Although extrusion-based multi-material bioprinting has been widely used, droplet-based and photocuring-based bioprinting related to multi-material have been frequently reported nonetheless.
In 2014, Levato et al. proposed a method combining bioprinting with microcarrier technology. After comparing six different compositions of bioinks, advantages of hybrid bioink called GelMA-GG MC-MSCs, which contained GelMA, gellan and mesenchymal stromal cell (MSC)-laden polylactic acid microcarriers, were illustrated. Researchers applied this bioink in fabricating bilayered cartilage structure, showing potential of this microcarrier-based bioprinting approach in bone and osteochondral construction [62].
In 2018, Ying et al. presented an approach to prepare aqueous two-phase emulsion bioink, which could realize rapid engineering of porous cell-laden tissue constructs. This bioink consisted of two immiscible aqueous phases of cell-laden GelMA hydrogel and poly(ethylene oxide) (PEO). Through photo-crosslinking GelMA phase, rapid prototyping of hydrogel was achieved by extrusion-based bioprinting or digital micromirror device-based stereolithographic. PEO phase was removed by immersing in PBS for 24 h after printing, accomplishing printed cell-laden structure with interconnecting pores. This method provided a robust and versatile platform to engineer porous-structured tissue constructs which could be applied in tissue engineering, regenerative medicine, drug development, and personalized therapeutics [63].
In 2019, Gao et al. from our research group designed a nanoclay/GelMA hybrid bioprinting strategy, by which favorable printability of GelMA was achieved with excellent biocompatibility (Fig. 8D). This method offered an easy way to print complex scaffolds with good shape fidelity and biological performance, would provide potential applications for the customized therapy of tissue defects [64].
3.3.10. Coaxial bioprinting
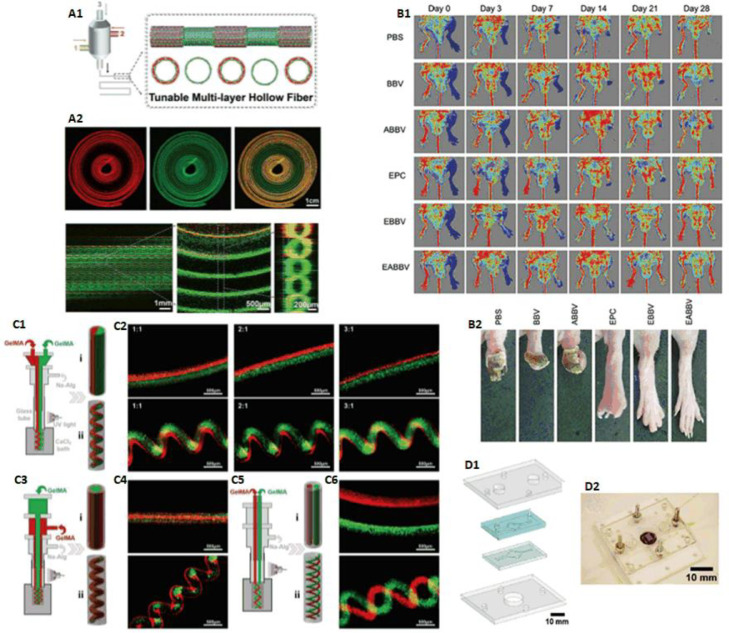
Coaxial bioprinting is a quite widely used extrusion-based printing approach, especially in the field of blood vessel/vascularization, which is not contradictory with the classification of extrusion-based bioprinting we discussed above. The classification of extrusion-based bioprinting into pneumatic-, piston- and screw-driven is based on the different driving modes of liquid dispensing system, while coaxial bioprinting named after the modality of nozzles, which can be pneumatic- or piston-driven as well. The greatest advantage of coaxial bioprinting is the capability of controllable construction of internal and external hierarchical structures. As we all know, hydrogels with excellent biocompatibility usually have insufficient mechanical strength, which can be solved by core-shell structures via coaxial bioprinting: core material ensures biocompatibility, shell material provides mechanical strength or vice versa. Besides, coaxial printing combined with sacrificial materials makes the printing of tubular structures more convenient.
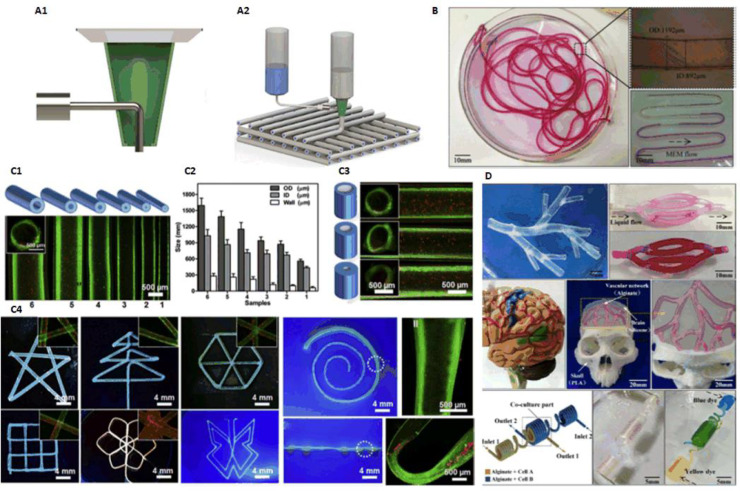
Different materials can be extruded through the inner and outer nozzles in coaxial mode, which was first used in electrostatic spinning to fabricate coaxial heterogeneous fiber structure. In 2015, Gao et al. from our research group presented an approach to fabricate hollow calcium alginate filaments by using a coaxial nozzle. High strength cell-laden hydrogel 3D structures with built-in microchannels were fabricated by controlling the crosslinking time to realize fusion of adjacent hollow filaments. The capability of perfusable filaments were also verified (Fig. 9B) [18]. Based on this study, they built 3D hydrogel-based vascular structures with multilevel fluidic channels (macro-channel for mechanical stimulation and microchannel for nutrient delivery and chemical stimulation) in 2017. A vascular circulation flow system, a cerebral artery surgery simulator, and a cell coculture model were fabricated to demonstrate potential tissue engineering applications of this printing method (Fig. 9D) [65].
Fig. 9.
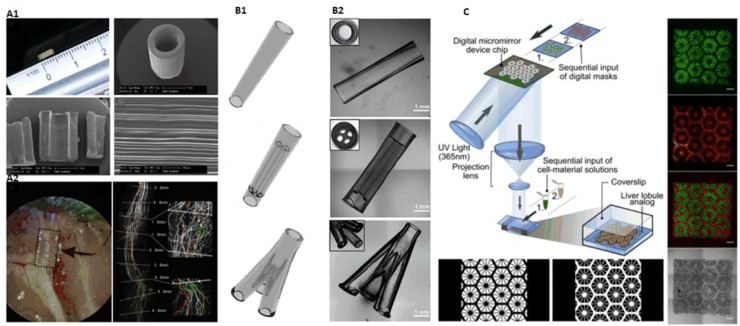
(A) Typical coaxial nozzle configuration (reproduced with permission from [67], Copyright 2016 IOP Publishing Ltd.); (B) Perfusable hollow vascular structure (reproduced with permission from [18], Copyright 2015 Elsevier); (C) 2D patterns built by perfusable tubes (reproduced with permission from [66], Copyright 2016 Elsevier); (D) Vessel-like structures with multilevel fluidic channels (reproduced with permission from [65], Copyright 2017 American Chemical Society).
In 2016, Jia et al. used GelMA, alginate, 4-arm poly(-ethylene glycol)-tetra-acrylate (PEGTA) as bioink, to deposit perfusable vascular structures with highly ordered arrangements by coaxial bioprinting approach (Fig. 9C) [66]. In 2016, Akkineni et al. using a typical coaxial nozzle (Fig. 9A), took alginate and poly(vinyl alcohol) (PVA) as shell material, soft biopolymer hydrogels including alginate, chitosan, gellan gum, gelatin and collagen as core, to fabricate structures, which could be used as 3D scaffolds with favorable mechanical properties in the construction of tissue models [67].
In 2016, Zhang et al. proposed a method based on coaxial printing and dual-step crosslinking: bioink encapsulating endothelial cells, which would form a layer of confluent endothelium, was used to build microfibrous hydrogel scaffolds, then cardiomyocytes were seeded on it to generate aligned myocardium capable of spontaneous and synchronous contraction. Researchers further embedded the bioprinted organoids into a microfluidic perfusion bioreactor composed of poly(methyl methacrylate) (PMMA) and PDMS to complete the endothelialized-myocardium-on-a-chip platform for cardiovascular toxicity evaluation (Fig. 10D) [68].
Fig. 10.
(A) Three-channel coaxial nozzles constructing tubular structure with switchable single/double layers (reproduced with permission from [69], Copyright 2018 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim); (B) Mice experiment of bioprinted vascular structures using Pluronic as sacrificial material (reproduced with permission from [25], Copyright 2017 Gao et al.); (C) Coaxial printing of double-layered, multi-layered, helical GelMA microfibers (reproduced with permission from [23], Copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim); (D) Coaxial printing to build microfluidic bioreactor (reproduced with permission from [68], Copyright 2016 Elsevier).
In 2017, Gao et al. introduced an approach to fabricate bio-blood-vessel (BBV) by coaxial bioprinting for the treatment of several ischemic diseases. Sacrificial material Pluronic F-127 was used as core material, and hybrid bioink, which consisted of endothelial progenitor cells (EPCs), atorvastatin-loaded PLGA microspheres (APMS), alginate, was used as shell, to bioprint BBV. The effect of the bioprinted structures was further demonstrated in mice experiments (Fig. 10B) [25].
In 2018, Pi et al. developed a multichannel coaxial extrusion system (MCCES) for building circumferentially multilayered tubular tissues. Perfusable cannular constructs can be continuously tuned up from monolayer to triple layers at regular intervals across the length of a bioprinted tube (Fig. 10A). In addition, human urothelial cells, human bladder SMCs were used to bioprint cannular urothelial tissue constructs, and human umbilical vein endothelial cells, human SMCs were used to bioprint vascular tissue constructs as well [69].
In 2018, Shao et al. from our research group proposed an approach for high-throughput bioprinting of heterogeneous hydrogel fibers, to fabricate blood vessel models. Coaxial bioprinting was applied to produce morphology-controllable GelMA microfibers encapsulated in calcium alginate. By adjusting the flow rates, GelMA microfibers with straight, wavy, and helical morphologies could be obtained (Fig. 10C). Using these microfibers, mini tissues containing human umbilical cord vein endothelial cells were built, in which cells could gradually migrate and connect to form lumen resembling blood vessels [23].
4. Droplet-based bioprinting
Other than extrusion-based bioprinting using continuous filaments as basic unit, droplet-based bioprinting regards independent and discrete droplets as basic unit which leads to relatively high resolution compared to extrusion-based bioprinting in a general way. Due to its simplicity and capability of precise control of biologics including cells, growth factors, genes, medicines, biomaterials, etc., droplet-based bioprinting has many applications in tissue engineering, regenerative medicine, transplantation, clinical, pharmacy, high-throughput screening and cancer research.
4.1. Principles
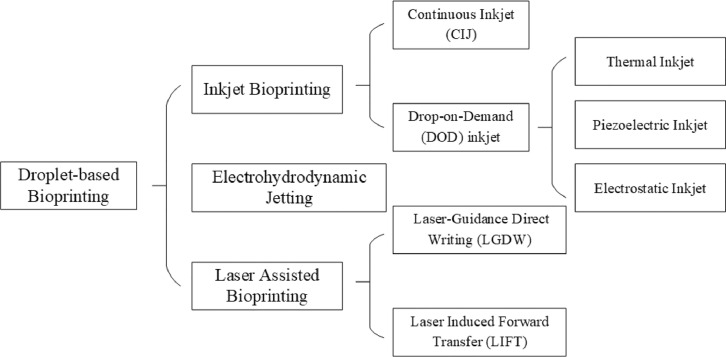
According to different droplets forming principles, droplet-based bioprinting can be divided into inkjet bioprinting, electrohydrodynamic jetting (EHDJ) and laser assisted bioprinting (LAB). Moreover, inkjet bioprinting can be subdivided into continuous inkjet (CIJ) printing and drop-on-demand (DOD) inkjet printing, while laser assisted bioprinting into laser guidance direct writing (LGDW) and laser induced forward transfer (LIFT) as well (Fig. 11).
Fig. 11.
Classification of droplet-based bioprinting.
4.1.1. Inkjet bioprinting
Inkjet bioprinting is regarded to be the first bioprinting technology [70], when Elmqvist of Siemens patented the first practical inkjet device in 1951. Later in 1960s, Sweet from Stanford University developed CIJ printing system. In 1970s, Zoltan, Kyser and Sears invented DOD inkjet printing system, which was licensed into the first commercial printer, the Siemens PT-80, in 1977 [71]. Klebe used a commercially available Hewlett-Packard (HP) thermal DOD inkjet printer to deposit a bioink solution comprising collagen and fibronectin in 1988, which was the first time using inkjet technology in bioprinting [6].
The process of inkjet printing can be considered as two steps: 1. the formation of discrete droplets which are directed to a desired location of substrate; 2. the interaction between droplets and substrate. As we mentioned above, there are two methods to form droplets: CIJ leverages a natural phenomenon called Rayleigh-plateau instability, which exhibits the natural tendency for a stream of liquid to undergo a morphological transformation to a train of discrete drops. The ink applied in CIJ is usually electrically conductive so that it can be guided by electric or magnetic fields. During the printing process, redundant droplets would be recirculated. DOD inkjet, in contrast, produces a droplet when required and droplet deposition is achieved by displacing the nozzle above the desired location before a droplet is ejected. Generally speaking, CIJ possesses higher drop generating frequencies, while DOD inkjet works at lower frequencies but achieves smaller drop volumes which lead to higher printing resolution. However, CIJ in biological applications has several issues of sterility with ink recirculation and waste if the unwanted ink was discarded. Therefore, most of prior researches based on inkjet technology utilized DOD approach [72].
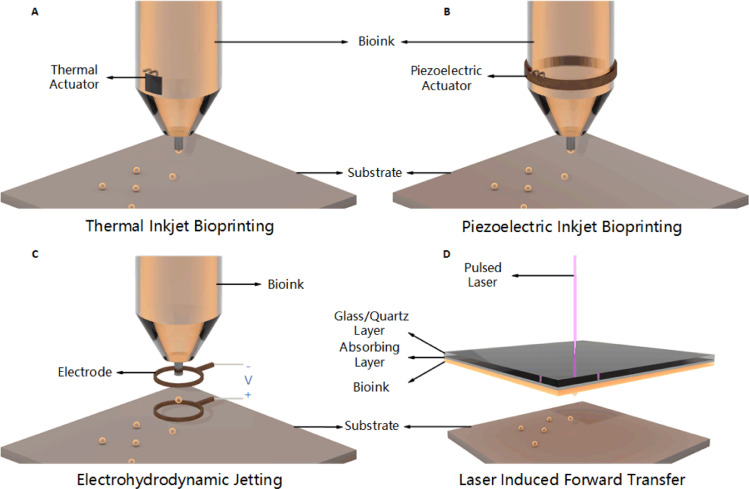
DOD inkjet bioprinting can be further divided into thermal, piezoelectric, electrostatic, etc. according to different droplet motivation mechanisms. Thermal inkjet bioprinting utilizes controllable impulsive voltage to locally heat the thermal actuator (e.g., film resistor) to high temperature (typically 200–300 °C), which leads to partial vaporization, forming a small bubble, thereby generating pressure pulse for bioink to overcome the surface tension to be ejected out of the nozzle orifice as the bubble collapses (Fig. 12A). After ejection, bioink in the nozzle is supplemented under the co-influence of thermal actuator cooling down and nozzle capillary siphoning. Despite the high temperatures capable of denaturizing hydrogel material, the heating time is sufficiently short that the heating has no detrimental effect on the stability of biocomponents [73]. Thermal inkjet bioprinting is widely used in various kinds of biologics including protein, cells, etc. Piezoelectric inkjet bioprinting, on the other hand, implements a piezoelectric actuator to form droplets. A rapid and reversible deformation of piezoelectric crystal produces when impulsive voltage applied, which causes a sudden change in volume of chamber, resulting in the propagation of acoustic waves, supplying pressure pulse for bioink needed to exceed the surface tension at the nozzle orifice (Fig. 12B) [74]. Similarly, electrostatic inkjet bioprinting exploits instantaneously volume increase to achieve ejection, by which impulse voltage is applied to a platen and a motor, leading to a bend on platen for extrusion of bioink [75]. The prototype of this technique came from a commercial electrostatic-driven inkjet nozzle [76]. As a matter of fact, there are many other approaches of inkjet bioprinting based on volume change for bioink jetting, we will not enumerate here.
Fig. 12.
Principles of droplet-based bioprinting.
No matter what types of DOD inkjet bioprinting, the rheological and surface tension properties of bioink dominate its printability. The requirements of bioink viscosity differ from different printing systems, with a threshold of 30 mPa/s nevertheless [77], [78], [79]. In addition, factors including nozzle diameter, distance between nozzle and substrate, impulse frequency of current/voltage, temperature gradient (for thermal inkjet), piezoelectric deformation property of actuator (for piezoelectric inkjet), etc. would finally affect droplet size and printing resolution [80].
4.1.2. Electrohydrodynamic jetting
Inkjet bioprinting produces droplets by extruding bioink through nozzles. In this case, a drastic pressure is employed when using a small diameter nozzle, which would occasionally affect cells viability. On the contrary, EHDJ exploits an electric field, which would obviously avoid excessive pressure on bioink [81]. The working principle of EHDJ is illustrated in Fig. 12C. First, fill the metallic nozzle with bioink to form a spherical meniscus at the tip of the nozzle because of surface tension; then a high voltage is applied between nozzle and substrate to produce an electric field, leading to the accumulation of mobile ions at the meniscus. As a result, electrostatic repulsions between the ions deform the meniscus into Taylor cone. Droplets are ejected when the electrostatic stresses overcome the surface tension under a sufficiently high voltage [81], [82], [83], [84], [85]. According to the voltage changing from low to high, the modalities of bioink would appear to be several conditions: micro-dripping, intermittent jetting, Tayler jetting, unsteady status, breakdown, etc. Most bioprinters based on EHDJ employ appropriate voltage for producing independent, discrete droplets. Studies have shown that factors including electric field intensity, cell concentration, bioink composition, etc. applied in EHDJ would affect the long-term cell viability post-printing [86]. Variation of voltage would affect the size of droplets (usually higher voltage leads to smaller droplets size) [86,87], and composition and concentration of bioink would affect the material transmission between cells. Size of droplets and the concentration of cells also affect the number of cells per drop. When the droplet size is more than 400 µm, the effect of material propagation is greatly reduced [88]. In general, EHDJ avoids excessive pressure that would be harmful to cells, and is particularly suitable for printing bioink with small orifice, high weight/volume ratio, or high cell concentration [13].
4.1.3. Laser assisted bioprinting
LAB a category of non-contacting, nozzle-free printing process to precisely deposit bio materials onto a substrate, including LGDW, LIFT, AFA-LIFT, biological laser processing (BioLP), matrix-assisted pulsed laser evaporation direct writing (MAPLE-DW), etc. Among them, AFA-LIFT, BioLP, MAPLE-DW technologies are optimized from LIFT for different application scenarios.
In 1999, Odde and Renn utilized laser-induced optical forces to deposit living cells patterned in 2D, which is called laser guidance direct writing [8,89]. The principle of LGDW is that a weakly focused laser beam (e.g., tunable diode laser beam with wavelength of 800 nm [90]) is directed towards a cell suspension, optically traps cells and guides them onto a substrate. A gradient force is generated when cells interacting with the light by which pulls the cells to the center of the light and guides them transferring onto a substrate (e.g., a coverslip) along the axial direction. The key parameter of this technology is the different refractive indices of the cells and surrounding fluid. Hence, very few compatible materials and biologics can be applied in LGDW, resulting in almost none studies have been reported in recent years.
LIFT was first used to transfer metals [91,92], it is now used for depositing biomaterials such as polypeptide, DNA, cells [93], [94], [95], [96]. As shown in Fig. 12D, a bioprinter based on LIFT usually has three main components: a pulse laser source, a ribbon structure coated with bioink and a receiving substrate. The ribbon structure often contains a laser absorbing intermediate layer (commonly made of gold or titanium, located between glass/quartz layer and bioink layer), depending on the optical properties and laser wavelength of bioink. The pulsed laser source is usually a nanosecond laser with ultraviolet or near-ultraviolet wavelength; the ribbon structure is basically a glass/quartz layer, which is transparent to the wavelength of laser radiation, with a layer of thermosensitive bioink (cells in the bioink either stick to the biopolymer or are evenly encapsulated in hydrogel) on it; the receiving substrate, which is coated with biopolymer or cell medium to ensure the adhesion and subsequent growth of the transferred cells, is located below the bioink coating and is used to receive droplets that are ejected at high speed [80]. The principle of LIFT is that focused pulsed laser is directed at the absorbing layer of the ribbon structure during printing, resulting in local evaporation and the formation of high-pressure bubbles, propelling the cell-containing material towards the receiving substrate. Physical explanation is that this area is in vibrational state with high energy after receiving the laser energy, the process of it transiting to ground state would locally release heat which causes the evaporation of bioink (or intermediate layer), resulting injetting. Ablation, plasma generation, thermoacoustic phenomena may accompany during this process, therefore the parameters of laser radiation need to be precisely controlled.
Several other methods of laser assisted bioprinting such as AFA-LIFT, BioLP, MAPLE-DW, come from LIFT. The first two use high voltage pulsed laser and metal/metal-oxide film as intermediate layer, while the latter one uses Low pressure pulsed laser and sacrificial hydrogels (e.g., Matrigel) as intermediate layer. Hopp et al. first utilized AFA-LIFT in cell printing in 2005, where its absorbing layer is thicker (100 nm) compared to traditional LIFT [97]. BioLP was first applied by Barron et al. in 2004 to map multiple biological material patterns at high speed and high reproducibility [98]. In addition to the thick absorption layer similar to AFA-LIFT (75–100 nm), it also includes movable receiving platform and CCD camera for localization of cell printing. MAPLE-DW was first applied by Wu et al. in 2001 in the area of bioprinting [99]. They used low-power pulsed laser (ultraviolet or near-ultraviolet) and matrix embedded transferring layer in order to promote the absorption of laser and energy transfer.
The biggest advantage of LAB lies in its ability to print patterns from biomaterials at high speed and high precision (micron scale) [100], and the combination of CAD/CAM system in the process of printing can further improve the printing accuracy (single cell or close to single cell) [101]. Such high precision makes it possible to use cell arrays from tissue engineering combining cells and corresponding biological factors to simulate the anisotropy and complexity of tissues [102]. LAB is also able to precisely bioprint different components of tissues (especially cells) and reproduce their spatial structure [89,103], providing a way to reproduce natural tissues and organs with precise structure and function [104]. Moreover, precise co-culture, such as complex cell structures, can also be achieved by controlling the spatial distribution of different cell types through LAB. In addition, LAB, as a kind of technology with non-contacting and nozzle-free, innately avoids problems such as nozzle clogging, non-reproducible because of solution viscosity, cross-infection, or receiving substrate damage. It produces smaller droplets (from nL to fL) and has higher precision, compared to inkjet bioprinting; its bioink has a higher concentration of cells which reduces the maturation time, compared to bioprinting technology with nozzles [105]. Other studies have shown that LAB is also beneficial to the construction of multi-layer cell structure [106], and more suitable for in situ and in vivo bioprinting [107].
4.2. Bioprinters
Compared to the development of commercial extrusion-based bioprinters, there are not many droplet-based bioprinters available on the market.
The first inkjet bioprinter was developed by Thomas Boland from Clemson University in 2003 by modifying an HP standard inkjet printer [11]. Related patents went to Organovo in USA, which has not commercialized any inkjet bioprinters yet [33]. Most of the bioprinters based on inkjet technology are homemade or modified standard inkjet printers in labs. For instance, Nishiyama et al. from University of Toyama built an electrostatic inkjet bioprinter in 2009 by combining stepper motors and EPSON's Sea-Jet™ nozzles, which succeeded in fabricating hollow cylinder structure [75]. Except for the Sea-Jet™ nozzles, DMP-2800 from Fujifilm Dimatrix, Xaar-126 piezoelectric inkjet print heads, etc. have also been reported to bioprinting a variety of cells and other biologics [108,109]. LabJet-Bio from Microjet in Japan (founded in 1997) is a piezoelectric inkjet dispensing system with high precision. It can be applied in bioprinting of protein, anti-body, enzyme, cells and reagents, producing bio-chips and bio-sensors, circuit design with nanometal ink, drug screening and manufacturing testing of cell sheets. French company Poetis used to develop commercial bioprinters based on LIFT technology. But now this company no longer sells its printing devices because its business model transferred to provide services such as bioprinting human skin for large cosmetic, chemical and pharmaceutical companies [33].
Other than inkjet, EHDJ, or LAB bioprinting technologies, Cyfuse Biomedical (founded in 2010) located in Tokyo, Japan, developed a bioprinting system Regenova based on a method called “Kenzan” in 2012, which could accomplish using only cells to bioprint structures. Using the company's own printing software, this system prints cell spheres into a thin needle array for culture, placing each sphere in a specific order and allowing the cells to fuse autonomously without the need of collagen or hydrogels [110]. This device has been able to 3D print blood vessels 2–3 mm in diameter, as well as nerves, liver tissue for drug screening and testing, cartilage and subchondral bone. In addition, there are other commercial droplet-based bioprinters including Autodrop Compact from Microdrop Technologies in German, jetlab 4 from MicroFab Technologies in USA, CellJet from Digilab in USA, etc.
4.3. Applications
4.3.1. Skin
In 2012, Sofokleous et al. designed a portable handheld multi-needle device based on EHDJ. Driven by a high voltage electric field, and adjusting the spray gun's angle according to reference angle (RA), this device could spray PLGA or poly(methylsilsesquioxane) (PMSQ) solution into multifunctional particles and fibers at the size of sub-micrometer to micrometer [111]. Although cell-laden bioink was not involved in this study, the enlightenment of this study is that, due to the flexible and safe characteristics of this portable device, researchers could try adding drugs, growth factors, coagulation factors or other substances into PLGA solution which would be applied clinically such as wound dressing and skin repair.
In 2013, based on the previous work [112,113], Michael et al. utilized the property that LIFT could precisely place different cell types in 3D, bioprinted fibroblasts and keratinocytes on top of a stabilizing matrix (Matriderm), to get a skin substitute, which was further tested in nude mice. After 11 d of bioprinting, the graft adhered well to the tissue around the skin wound, and proliferation and differentiation of the cells in the graft was observed (Fig. 13A). The effect of LAB technology in skin burn treatment was verified [114].
Fig. 13.
(A) Simulation cell proliferation state of skin by LIFT (reproduced with permission from [114], Copyright 2013 Michael et al.); (B) Inkjet printing zigzag tubular structure (reproduced with permission from [123], Copyright 2012 Wiley Periodicals, Inc.); (C) Inkjet printing horizontally and vertically branched vascular structures (reproduced with permission from [125], Copyright 2014 Wiley Periodicals, Inc.); (D) Cell-laden Y-shaped tubular structures using MAPLE-DW technique (reproduced with permission from [128], Copyright 2015 IOP Publishing Ltd.).
4.3.2. Bone/cartilage
In 2007, De Coppi et al. produced bone-like structures using inkjet bioprinters and implanted them into mice after in vitro culture. Highly mineralized tissue with similar density to endogenous bone tissue were observed [115]. In 2010, using a high-throughput laser bioprinting workstation they designed before [104], Keriquel et al. conducted mice calvaria repair experiment. Researchers adopted nano-hydroxyapatite (n-HA) for in situ bioprinting, filling critical size bone defects around 3 mm in diameter. The preliminary results demonstrated that in vivo bioprinting is possible for bone repair [107].
In 2012, Cui et al. employed a modified HP Deskjet 500 thermal inkjet printer, combining with simultaneous photopolymerization, to bioprint poly(ethylene glycol) dimethacrylate (PEGDMA) with human chondrocytes to repair defects in osteochondral plugs in layer-by-layer mode. The study verified the capability of inkjet bioprinting for controlling placement of individual cells, preserving cell viability, maintaining chondrogenic phenotype, and demonstrating integration with host tissue as well [116]. In 2012, Xu et al. exploited a hybrid inkjet printing/electrospinning system, to fabricate a five-layer tissue construct of 1 mm thickness, in the manner of two nozzles alternately bioprinting (electrospinning of PCL fibers while inkjet printing of rabbit elastic chondrocytes suspended in a fibrin–collagen hydrogel). The study demonstrated that fabricated constructs formed cartilage-like tissues both in vitro and in vivo, and hybrid scaffolds provided enhanced mechanical properties at the same time [117]. In 2015, a human ear structure was bioprinted by Markstedt et al. based on electromagnetic jet technology with a micro valve. Researchers mixed nanofibrillated cellulose (NFC) and alginate carrying human nasoseptal chondrocytes (hNC) as bioink. The ability of this method to bioprint complex structures was proved [118].
4.3.3. Nerve
In 2006, Eagles et al. demonstrated that EHDJ could deposit neurons at micron scale without significant disturbance to neurons, cells could survive and differentiate after printing [119]. In 2014, Lorber et al. verified the feasibility of piezoelectric inkjet bioprinting adult rat retinal ganglion cells (RGC) and glial cells. Experiments showed that this printing method had no significant effect on the survival of RGC/glial cells and the growth of RGC neurites, and the glial cells retained their growth-promoting properties after printing [120].
4.3.4. Blood vessel/vascularization
In 2006, Boland et al. demonstrated many layers of cells and hydrogels could be used to build desired structures by thermal inkjet bioprinters [121]. Cui and Boland further extended this method in 2009, when they employed human microvascular endothelial cells (HMVEC) and fibrin as bioink to fabricate microvascular structures. Micron-sized fibrin channels were precisely built by thermal inkjet bioprinter, during which cells aligned themselves inside the channels and proliferated to form confluent linings. This method proved the capability of thermal inkjet technique for simultaneous cell and scaffold printing applied in preparation of microvascular structures [122].
In 2012, using a piezoelectric inkjet bioprinter, Xu et al. fabricated fibroblast-laden zigzag cellular tubes in calcium chloride solution in a scaffold-free manner (Fig. 13B). Calcium chloride solution was used as both crosslinker and support material to provide buoyancy [123]. In his subsequent research in 2014, Xu further discussed the feasibility of combining vertical and horizontal bioprinting to efficiently and effectively manufacture complex tubular structures with vertical and horizontal branching characteristics [124]. In the same research group's follow-up study in 2015, Christensen et al. used the same device, using sodium alginate and mouse fibroblasts-laden bioink, to successfully print vessels with horizontal and vertical branches (Fig. 13C) [125].
In 2010, Wu and Ringeisen applied BioLP technology, using HUVEC and HUVSMC to fabricate branch/stem structures. This study discussed the effect of co-culture structures on cell growth and functionalization, which further indicated the possibility to direct the formation and growth of lumen and lumen network by BioLP [126]. In a subsequent study in 2011, Pirlo et al. from the same research group, further improved the stability of branches by printing another HUVSMCs layer on top of the HUVEC layer to form multi-layered structures [127].
In 2015, Xiong et al. adopted mouse fibroblasts-laden alginate solution as bioink to bioprint straight and Y-shaped fibroblast tubes in the manner of MAPLE-DW (Fig. 13D) post-printing cell viabilities immediately after printing as well as after 24 h incubation were above 60% for both structures [128].
4.3.5. Organoid
In 2011, Gaebel et al. applied LIFT technique and prepared a cardiac patch seeded with HUVEC and human MSC (hMSC) in a defined pattern for cardiac regeneration. Through rat experiments, this cardiac patch was proved to enhance angiogenesis in the border zone of infarction and preserve cardiac functions after acute myocardial infarction (Fig. 14A). This study provided a way of cardiac patches based on LIFT technology for the treatment of myocardial infarction [26].
Fig. 14.
(A) Implantation of cardiac patches fabricated by LIFT in rats (reproduced with permission from [26], Copyright 2011 Elsevier); (B) EHDJ constructing microspheres with complex structures (reproduced with permission from [130], Copyright 2018 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim); (C) Self-folding droplet networks simulating tissue structures (reproduced with permission from [131], Copyright 2013 Springer Nature Limited); (D) Inkjet printing vascularized tissue structure (reproduced with permission from [129], Copyright 2012 Elsevier); (E) EHDJ manufacturing cell-laden microspheres and patterns (reproduced with permission from [132], Copyright 2018 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim).
In 2013, Xu et al. developed a method for fabricating complex and heterogeneous 3D constructs with multiple cell types. Researchers used a modified thermal inkjet bioprinter, and separately mixed human AFS cells (hAFSCs), canine SMCs (dSMCs), bovine aortic endothelial cells (bECs) with calcium chloride, loaded into separate ink cartridges. These cells were then bioprinted layer-by-layer in a chamber filled with sodium alginate-collagen composite. Due to ionic crosslinking, bioink containing cells were then immobilized in designed areas within the gel. Complex 3D multi-cell hybrid constructs were established after repeating this printing process for several cycles (Fig. 14D). Subsequent experiments showed favorable cells viability, proliferation rates, phenotypic expression and physiological functions within the heterogeneous constructs, which expressed considerable functionality and vascularization in vivo [129].
In 2018, Zhao et al. from our research group realized the bioprinting of multicellular heterogeneous structures in microspheres in the manner of EHDJ, with a precision up to single-cell resolution. A microfluidic nozzle was applied to improve the capability of intricate cell encapsulation with heterotypic contact. Complex structures, such as rose, Tai chi pattern and single cell line can be easily achieved in spheroids (Fig. 14B). We further proved that the structure of spiral-based spheroids was a practical tool for building functional organoids in vitro by embedding multiple cells into the spheroid, contributing novel biomimetic asymmetrical prototypes for basic medical research and regenerative medicine [130].
4.3.6. Drug research/tumor model
In 2013, Durmus et al. developed a special droplet-based bioprinting method: tissue-mimicking printed networks of droplets separated by lipid bilayers, which could be functionalized with membrane proteins, were able to spontaneously fold and transmit electrical currents along predefined paths. Self-folding, stimuli-responsive droplets obtained in this way might be further applied with microfluidics in locally capturing, isolating and releasing rare cells for diagnostic applications (e.g., circulating tumor cells for cancer detection or CD4 cells for HIV monitoring) (Fig. 14C) [131].
In 2018, Xie et al. from our research group proposed a method for manufacturing microspheres in quantity based on EHDJ. We presented three typical applications in order to show the wide application prospect of the printed microspheres. (1) Cellular encapsulation: BMSCs maintained the capability of spreading in microspheres after undergoing the electro-assisted printing process; (2) Drug controlled release: microspheres containing dextran and bovine serum albumin (BSA) showed good controlled release effect; (3) 3D bioprinting: using cell-loaded microspheres as forming units, 3D structures could be printed directly by controlling orderly movement of the nozzle (Fig. 14E) [132].
5. Photocuring-based bioprinting
Photocuring-based bioprinting, literally, is an approach of bioprinting that utilizes photopolymerization characteristic of photosensitive polymers under precisely controlled lighting. Compared to other approaches of bioprinting, it usually has significant improvement on printing resolution and printing speed. Besides, it has a congenital advantage of no worrying about nozzle plugging, or shear stress affecting cell viability.
5.1. Principles
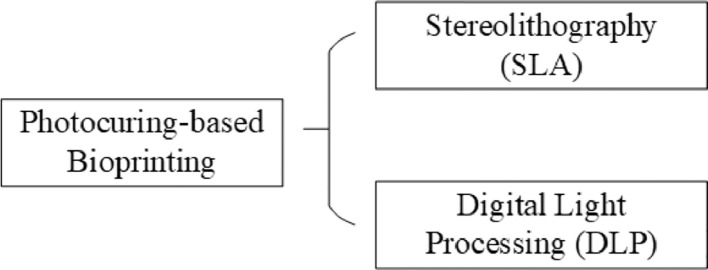
The most common use of photocuring-based bioprinting is to manufacture cell-free scaffolds, for cells to be seeded post-printing. Currently, however, cell-laden photocuring-based bioprinting has also been reported. According to different light scanning modes, photocuring-based bioprinting can further be classified into stereolithography (SLA), and digital light processing (DLP) (Fig. 15).
Fig. 15.
Classification of photocuring-based bioprinting.
5.1.1. Stereolithography
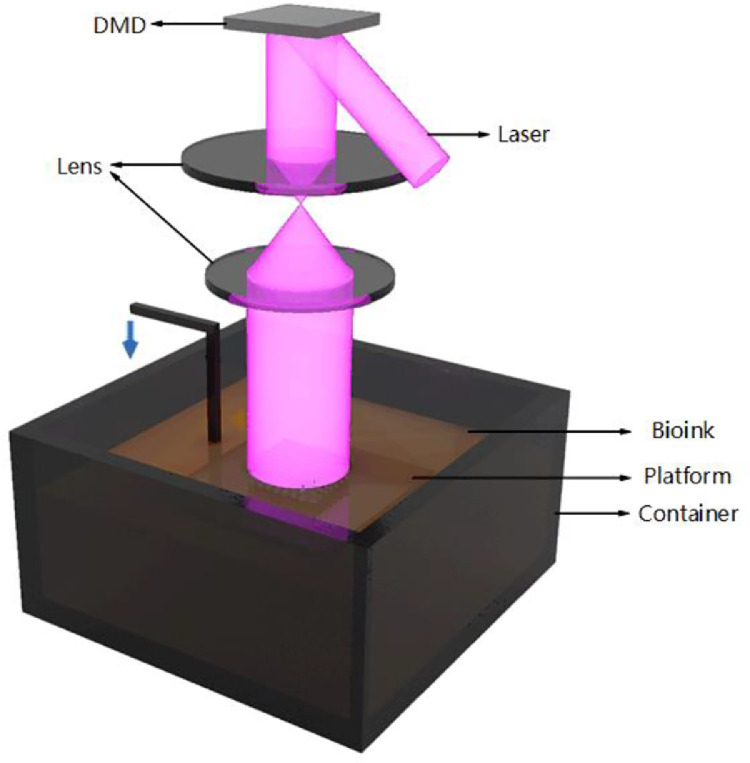
SLA is the first commercial 3D printing technology, which was invented by Charles W. Hull in 1984. He created 3D structures by selectively scanning a vat of light-sensitive material with ultraviolet light, allowing it to be cured layer by layer. In the bioprinting area, SLA is often applied to print precise tissue scaffolds with controllable geometry and porous structure due to its high resolution. It was not until 2004 that Boland's team at Clemson University implemented cell-loaded SLA printing with a commercial SLA printer (SLA-250, 3D Systems, Valencia, CA) [12]. After that, more research groups further optimized SLA technology, which led to its further expansion in the field of bioprinting. For now, however, SLA technology has been applied more in scaffold printing than cell-loaded bioprinting.
For a standard SLA bioprinter, bioink is filled in a tank with a platform that moves up and down. When printing the first layer, the platform shifts to the surface of the bioink solution and the liquid solidifies upon exposure to the UV laser. Theoretically, it is the energy provided by the laser that leads to the formation of covalent bonds between adjacent polymer chains in bioink solution. For each layer, laser scan through a 2D pattern. Instead of focusing directly on the ink solution, the laser reflects from a mirror for reaching appropriate point by moving in X and Y directions. By submerging the platform in the vat of liquid and moving it up/down a distance equal to the layer height, the newly cured ink solution is firmly adhered to the previous layer, this process repeats until the final 3D construct is completed [133,134]. In most cases, excess of bioink needs to be cleaned and further photo-curing is required post-printing. Factors such as laser power, scanning speed, exposure time, laser spot size, laser wavelength, etc. would determine the precision of SLA [134].
5.1.2. Digital light processing
Differs from SLA, DLP solidifies a complete layer at once instead of point-by-point. Fig. 16 shows a typical bottom-to-top DLP bioprinter, which means printing the bottom layer first, each new layer is above the previous one. It consists of three components: a container filled with bioink including photocurable hydrogel or photosensitive resin which can solidify exposed to laser of a specific wavelength (usually UV light), photoinitiator, cells, etc.; a lifting platform insures lowering a certain height (equals to the thickness of one layer) after one layer is finishing exposed, making it a new layer for photocuring; and a imaging system above the container.
Fig. 16.
Principle of DLP bioprinting.
The reason for photocuring one complete layer at once during the printing process is the use of a dynamic mask. Simply put, the mask carries a design pattern through which light passes and transmits the pattern to the receiving substrate. Layering software is used to slice the 3D digital model according to a certain thickness. Each layer is converted into a bitmap file, which is subsequently input into the dynamic mask. According to the graph displayed on the dynamic mask, each layer is exposed to solidify the surface of bioink. Liquid crystal display (LCD), spatial light modulator (SLM), digital micromirror device (DMD), etc. have been used as dynamic mask in DLP printing. In 1997, LCD was proposed as dynamic mask, which held several defects including low conversion speed (about 20 ms), large pixel size (low resolution), low filling rate, low optical density of refraction element (off mode), high light absorption (on mode). These defects limited the improvement of photocuring performance and resolution of plane projection. Nevertheless, DLP technology based on DMD dynamic mask in recent years has shown better performance and application prospect. The DMD offers better performance in terms of optical fill factor (85% with DMD vs. 64% with LCD) and light transmission (71% with DMD vs. 21% with LCD) [135]. Based on the bioprinting principle, it is apparent that compared with extrusion-, droplet-based bioprinting or SLA technologies, DLP has a considerable advantage in printing speed, since no matter how complex the structure is, the printing time of each layer does not increase. Besides, unlike the artificial interfaces formation between droplets in inkjet bioprinting, or adjacent fibers in extrusion-based one, DLP technology can fabricate 3D structures much smoother, which as a result, leads to greatly improved structural integrity and mechanical property.
5.2. Bioprinters
Most commercial photocuring-based printers (SLA or DLP) can be modified into bioprinters using a blend of biocompatible resins as bioink, which is the approach adopted by most photocuring-based research teams worldwide. It is worth mentioning that professor Shaochen Chen's team at the University of California San Diego has long been using the dynamic optical projection stereolithography (DOPsL) system in researches. Due to the wide application, it has received high attention from the academia and is a representative group applying DLP technology in bioprinting area.
Our team has commercialized a photocuring-based photocurable bioprinter, EFL-BP8600 (Fig. 17). After multiple levels of optimization, BP8600 is exclusively designed for high-precision printing of hydrogels especially for GelMA hydrogels. Multi-material, multi-cell bioprinting can be easily realized supported by several functional modules (e.g., computer-free module, material supplementary module, etc.).
Fig. 17.
DLP bioprinter EFL-BP8600.
5.3. Applications
5.3.1. Cartilage
In 2018, Zhu et al. reported a technology to fabricate cell-laden cartilage tissue based on stereolithography. Researchers used bioink consisted of MSCs (which could differentiate into chondrocytes), GelMA, polyethylene glycol diacrylate (PEGDA) (which could greatly improve printing resolution and mechanical properties), photoinitiator, and transforming growth factor beta 1 (TGF-β1) embedded nanospheres fabricated via a core–shell electro-spraying technique. Experiments shown that cells and microspheres were evenly distributed in the bioprinted cartilage constructs with well-defined architecture; and the TGF-β1 embedded in nanospheres maintained a sustained release up to 21 d, improving chondrogenic differentiation of encapsulated MSCs. This study provided a promising strategy for cartilage regeneration applying photocuring-based bioprinting [136].
5.3.2. Nerve
In 2015, Pateman et al. designed a µSLA-based setup with ∼50 mm printing resolution to build nerve guidance conduits (NGCs) which could be used for peripheral nerve repair. Researchers used PEG resin to fabricate NGCs with dimensions of 1 mm internal diameter, 5 mm length, 250 mm wall thickness. In vivo mouse experiments were conducted to verify its feasibility (Fig. 18A). While PEG was not typically conducive for cellular attachment, researchers observed that the photocurable form of PEG used herein was permissive for neuronal growth and experimental differentiation in vitro [137].
Fig. 18.
(A) NGCs built by µSLA and rats experiment (reproduced with permission from [137], Copyright 2015 Pateman et al.); (B) NGCs fabricated by DLP (reproduced with permission from [138], Copyright 2018 Elsevier); (C) Hydrogel-based liver structure (reproduced with permission from [141], Copyright 2016 National Academy of Sciences).
In 2018, Zhu et al. utilized a rapid continuous 3D printing platform based on photocuring to build NGCs (Fig. 18B). In vivo implantation of NGCs with microchannels into complete sciatic nerve transections of mouse models demonstrated the effective directional guidance of regenerating sciatic nerves via branching into microchannels and extending toward the distal end of injury site. Histological staining and immunostaining further confirmed the progressive directional nerve regeneration and branching behavior across the entire length [138].
5.3.3. Spinal cord
In 2019, Koffler et al. used a microscale continuous projection printing (µCPP) method to manufacture complex central nervous system (CNS) implants, to promote nerve growth and repair nerve connections in spinal cord injury sites. Bioprinted structures were implanted at the site of severe spinal cord injury in rats, with complete regeneration of new spinal cord tissue observed after several months, and functional movement of rat hindlimbs was significantly improved. While the axons would diffuse and regenerate in any direction, researchers printed personalized 3D scaffolds containing dozens of 200-micron pores, aligning the regenerated axons of one end of spinal cord injury with the other, which directed the growth of neural stem cells and axons into parallel linear arrays. It is worth mentioning that this method generated a printing resolution of 1 µm; and only 1.6 s was required to print a 2-mm scaffold, which was nearly a thousand times of speed compared to traditional bioprinting approaches. Scaffolds made of PEGDA-GelMA hydrogels provided considerable mechanical strength ensuring the channels and solid core in the scaffolds were not damaged or deformed during animal experiments. For biocompatibility, reactive cell layers and granulated tissues formed around PEGDA-GelMA scaffolds were significantly weakened, and astrocyte reaction at the injured site could be reconstructed, so that host astrocytes aligned with the growth axis of host axonal fascicles instead of blocking the it, which solved the inflammatory reaction of scaffolds implantation. This method could be extended for human spinal cord sizes and lesion geometries, providing a precise approach for regeneration of the central nervous system [139].
5.3.4. Blood vessel/vascularization
In 2017, Zhu et al. used a rapid bioprinting method called microscale continuous optical bioprinting (µCOB) to create prevascularized tissues. Multiple cell types mimicking the native vascular cell composition were encapsulated directly into hydrogels with precisely controlled distribution without the need of sacrificial materials or perfusion. In vitro experiments showed that endothelial cells spontaneously formed lumen-like structures due to their region-controlled biomaterial properties. In vivo implantation demonstrated the survival and progressive formation of the endothelial network in the prevascularized tissue, while bioprinted endothelial network with functional blood vessels featuring red blood cells anastomosed with host circulation [140]. As we discussed above, only by solving the problem of vascularization can printing of tissue and organs of large scale be truly realized.
5.3.5. Organoid
In 2016, Ma et al. used the same technique as the previous part, to fabricate a co-culture 3D biomimetic liver model including three types of cells: human induced pluripotent stem cells (hiPSCs), HUVECs and adipose-derived stem cells (ADSCs), while GelMA hydrogel was used as matrix material. Researchers encapsulated different types of cells in complementary patterns, mimicking the hepatic lobule structure by photopolymerization of the hydrogel matrices. Two patterns resembling the anatomical structures of hepatocytes and supporting cells were designed, which were adjusted to have approximated human liver lobules dimensions. A two-step photocuring strategy providing an effective and flexible method to construct in vitro liver model, which could improve the structure and function of hiPSC-derived hepatic progenitor cells (hiPSC-HPCs), was further proposed (Fig. 18C). This study could be applied for early personalized drug screening and in vitro liver pathophysiology research [141].
5.3.6. Clinical applications
In 2014, Gou et al. presented a liver-inspired 3D detoxification device bioprinted by using hydrogels with functional polydiacetylene nanoparticles. The nanoparticles could attract, capture and sense toxins, which was further improved by forming a modified liver lobule microstructure. This work provided a proof-of-concept of detoxification by a 3D-printed biomimetic nanocomposite construct in hydrogel, and might lead to a deeper level of clinical application [142].
6. Comparison among 3D bioprinting approaches
3D bioprinting has the advantages of multi-cell spatial directional control and controllable deposition of different cell densities, which makes it the most ideal means to construct in vitro organ models. As we discussed above, various bioprinting methods are invented to work out the challenges of different applications, and different bioprinting approaches also possess respective advantages and disadvantages.
Extrusion-based bioprinting is the most widely used bioprinting method at present. Nearly 30 000 3D printers are sold worldwide each year [143], and academic institutions are increasingly purchasing and applying extrusion-based technique in tissue and organ engineering research. Industrial-grade bioprinters are often considerably more expensive, but have better resolution, speed, spatial controllability and more flexibility in the material they can print [74]. For extrusion-based bioprinting, researchers usually take advantages of the temperature-sensitive crosslinking, shear thinning, etc. characteristics of biological materials. For example, some biomaterials flow at room temperature but solidify at about 0 °C, which ensures they can be extruded from the nozzle at room temperature and solidify to desired structures at low temperatures. And shear thinning property of non-Newtonian fluid means that the viscosity decreases with the increase of shear stress, which also ensures the smooth extrusion of biomaterials. The greatest advantage of extrusion-based technology is its printability of a very wide range of biocompatible materials (such as cell aggregates, cell-laden hydrogels, microcarriers, decellularization matrix components, etc.) covering a wide range of fluid properties. Materials with viscosities ranging from 30 to 6 × 107 mPa/s have been shown to be compatible with extrusion-based bioprinters [143], with higher-viscosity materials often providing structural support for the printed construct and lower-viscosity materials providing a suitable environment for maintaining cell viability and function [74]. Wide range of viscosities available with extrusion technology means that extrusion-based bioprinting can use high cell density bioinks, while achieving physiological cell density in artificial tissues has long been a major goal for bioprinting. In addition, to set up extrusion-based bioprinters is quite simple, commercial plotters or desktop 3D printers can easily be converted into extrusion-based bioprinters, this printing method is also suitable for low-cost customized services.
Compared with other printing approaches, extrusion-based bioprinting also has some disadvantages. First of all, extrusion printing accuracy is generally limited to 100 µm [58], which is lower than other biological approaches [144]. Secondly, the selection of bioink also needs to meet conditions including gelation, curing and shear thinning properties. In addition, due to the inevitable shear force during the extrusion process will affect the cell survival rate, cell viability of extrusion-based bioprinting is lower than that of inkjet printing, which is more significant when the cell density is high. Depending on the pressure and the diameter of the nozzle needle, the cell activity of extruded bio-printing ranged from 40% to 86% [145,146].
For now, the most commonly used droplet-based bioprinting are DOD inkjet bioprinting and laser assisted bioprinting. Since commercial inkjet printers are fairly affordable and can be easily converted to bioprinting devices, inkjet bioprinting is the lowest-cost bioprinting technology. Except for low cost, high precision, fast speed and compatibility with a variety of biological materials, inkjet bioprinters can also be equipped with multiple nozzles to meet the needs of printing different cells, biological materials or growth factors at the same time, which not only greatly increases the printing speed, but also realizes different functions.
However, viscosities limitation of bioink for inkjet bioprinting narrows the range of suitable biomaterials. Due to the small driving pressure, inkjet bioprinting is unable to print high viscosity materials or cells with high concentration, while material with low viscosity reduces the structural strength of the printing molding, leading to not satisfied to the requirements of subsequent in vitro culture and transplantation. It is difficult to achieve physiological cell density bioprinting with inkjet technology at present. In order to prevent nozzle clogging and reduce shear stress, inkjet printing often chooses low cell concentration (less than 10 million cells/ml) to facilitate the formation of droplets [79]. Besides, there may be mechanical or thermal damage to cells during inkjet printing process [73]. These shortcomings limit the wide application of inkjet printing technology.
Laser assisted bioprinting also has many advantages over other printing methods. Because of its nozzle-free printing mode, the problem of cell/biomaterials blocking the nozzles in either extrusion-based or inkjet bioprinting does not exist for laser assisted bioprinting. Meanwhile, laser assisted bioprinting can avoid direct contact between bioink and devices. This non-contacting manufacturing method will not cause mechanical damage to cells. Biomaterials with higher viscosity (1–300 mPa/s [97,112,147]) can be bioprinted using this method, leading to a wider range of materials selection than inkjet bioprinting. Under the action of laser with pulse repetition rate of up to 5000 Hz, it can print bioink with a cell density of up to 108/ml at the speed of 1600 mm/s and the precision of each droplet containing a single cell [148].
Despite all these advantages, laser assisted bioprinting has several obvious drawbacks. Firstly, the cost of laser assisted bioprinters is relatively high, there is a lack of commercial printing devices; and hydrogel materials suitable for laser assisted bioprinting are not abundant. Secondly, as described in the LIFT principle, it is time-consuming to coat bioink on the laser absorbing layer of the ribbon structure; this can be especially cumbersome facing printing multiple cell types/materials. In addition, when the printing device contains a metal laser absorption layer, the evaporation of the layer might cause the metal residue to appear in the final bioprinted structures [74].
The low mechanical strength of hydrogels, and the fragility of cells are considered in cell-laden bioprinting, while the commonly used extrusion-based bioprinting usually produce filaments with diameter larger than 100 µm, resulting in lower accuracy of 3D structures and repeatability not guaranteed. Compared to it, DLP is an approach based on surface projection with much higher printing resolution. In addition, the uncured liquid bioink can also provide a good support for the printed structure and avoid the collapse deformation of hydrogel during the printing process. There is also no disturbance of nozzle plugging and shear stress affecting cell viability. In our opinion, photocuring-based bioprinting will play an increasingly important role in cell-laden bioprinting, which is expected to replace extrusion-based bioprinting as the most mainstream bioprinting technology in the future.
7. Bioink
Developing appropriate bioinks has always been one of the most significant aspects for 3D bioprinting. Diverse properties of different tissues exist between extracellular substances and in cellular compartment, not to mention anisotropic structures inside each tissue, which makes developing one universal bioink for general use is unlikely to achieve [149]. Besides, the accumulation of functional and theoretical knowledge of tissues and organs is quite limited, leading to an urgent need for research and development of bioink.
The performance of bioinks can be evaluated by several main factors: printability, biocompatibility and mechanical property. Printability is to assess the formability of bioink, where tunable material viscosities, rapid conversion from sol state to gel state, and a wide range of printing parameters are required. Biocompatibility is to measure the ability of biomimicry, requiring bioink to be as similar as possible to the microenvironment of the printed cells in the body, where the cells proliferate, extend, differentiate and eventually communicate with each other. Mechanical property requires that the gelled bioink be strong enough to support subsequent culture and implantation processes. Bioprinted structures usually need to be cultured in vitro, during which nutrients perfusion and degradation might occur, requiring considerable strength to support. Insufficient mechanical property would also cause implant failure.
Therefore, bioink selection necessitates compromise among printability, biocompatibility and mechanical property (Fig. 19). Rational design of bioinks considering bioprinting process, cell growth and proliferation, and structural integrity requirements, can be adjusted according to the actual cell types and printing resolution needs. However, as a matter of fact, these three requirements of bioinks are kind of intrinsically contradictory in terms of mechanism. For example, higher viscosity of bioink contributes to a better printability, while on the contrary leads to a poorer biocompatibility. Hence, it is a crucial step in bioprinting to select appropriate bioink to meet specific needs targeting at different applications.
Fig. 19.
Evaluation for bioinks performance (adapted with permission from [64], Copyright 2019 IOP Publishing Ltd.).
ECM is mainly composed of three categories: structural proteins (e.g. collagen, elastin), specialized proteins (e.g. fibrin), and proteoglycans. One ideal bioink would undoubtedly be able to approach the natural ECM, and it also needs to be adjusted for matching different types of cells. In principle, to add substances which cells need to grow in body as far as possible is needed. For example, when bioprinting chondrocytes, the addition of HA, which is a common component in cartilage, can obviously facilitate the later cultivation and functionalization.
Typical bioinks applied in bioprinting includes hydrogels, decellularized matrix components, microcarriers, tissue spheroids and strands, cell pellet, and/or some advanced bioinks such as multi-material, interpenetrating network, nanocomposite, and supramolecular bioink, etc [150,151]. Because of the outstanding capability of providing a viable microenvironment for cell adhesion, growth and proliferation, hydrogels are deemed as one of the most excellent biomaterials in bioink. Naturally/synthetically derived hydrogels including alginate, fibrinogen, gelatin, collagen, silk fibroin, chitosan, agarose, Pluronics, HA, GelMA, PEG, PEO, etc., have been found countless applications in bioprinting. They are either ion-sensitive, photosensitive, thermosensitive, enzyme-sensitive or pH-responsive so that they can be easily gelled to form constructs before, during and/or after bioprinting [152].
Alginate is perhaps the most commonly used bioink at present, which has good formability and mechanical properties with simple ionic crosslinking mechanism. The main disadvantage of alginate is its chemical structure being unfriendly to cells adhesion, leading to relatively weak biocompatibility, affecting the transformation of cells into tissues post-printing. Hence, mixture of natural polymers such as gelatin, GelMA, collagen, fibrinogen, etc. with alginate is a quite popular method [66,[153], [154], [155]. By contrast, collagen-based bioink has improved biocompatibility due to its derivation from animals, but its disadvantages include slow forming speed, poor mechanical properties, and therefore the need for subsequent modification or blending with other materials. Among them, GelMA has drawn a lot of attention in recent years, due to its excellent cell compatibility and mechanical properties [156]. Our research team has also been applying GelMA as bioink widely, and it is now commercialized (EFL-GM series) integrated with our bioprinters for researchers with background in biology, medicine, mechanical engineering or materials engineering.
HA, also known as hyaluronan, can also be modified to create bioink with good printability for cell-laden bioprinting like gelatin. The tunable characteristics and mechanical properties of HA-based hydrogels make them quite competitive [157,158]. Fibrin is one type of hydrogel formed by the enzymatic reaction between thrombin and fibrinogen, which has been applied in the field of wound healing and skin grafts fabrication [15,159]. Chitosan, which is a linear polysaccharide molecule obtained from deacetylation of chitin, has a wide range of applications in cartilage regeneration, hemostatic and antibacterial devices, sponge scaffolds formation, wound dressings fabrication, etc [160]. In addition, different from cell-laden hydrogels directly used in fabrication of constructs, some bioinks plays a role as supporting materials or sacrificial components such as agarose, Pluronics or gelatin [20,25,161]. In general, researches on bioinks for 3D bioprinting has developed rapidly, and new compositions or modified bioinks has been continuously developed to achieve the most balanced position among printability, biocompatibility and mechanical property for satisfying applications in tissue engineering and regenerative medicine.
8. Outlook and conclusion
From proof-of-concept prints to complex multi-material tissue-like structure, 3D bioprinting which combines biomanufacturing with additional manufacturing, has achieved enormous progress over the recent years. Nevertheless, certain limitations related to bioprinting techniques, cellular sources, biomaterials selection, etc. still exist, which need to be overcome in future researches.
3D bioprinting technologies still have a lot of room for improvement. Tissues and organs are made of organizational structures with different sizes, different rigidities and different cells. Blood vessels, for example, which scale from 20 µm capillaries to 2.5 cm arteries, usually consist of three layers of cellular structures containing different types of cells (fibroblasts, smooth muscle cells and endothelial cells for each layer), with different cellular functions. The complexity of tissues and organs brings great difficulty to accurate bioprinting, to solve which, multi-scale, multi-material and multi-cell bioprinting is becoming a priority for future development. The low precision compared to natural tissues/organs is one of the major drawbacks of current bioprinting techniques. Most structures of tissues/organs are more delicate than current bioprinting devices can reach. Another common disadvantage of bioprinting techniques is the low bioprinting speed of complex scale-up structures, especially when it comes to multi-material alternately biofabrication. As we mentioned above, DLP is a method based on surface projection with much higher printing resolution and speed than other bioprinting approaches because of its intrinsic principle. In addition, in vitro tissue models, which is the key application of 3D bioprinting, need to be standardized not only in sizes, but also in biological and mechanical properties, while DLP possesses excellent uniformity and reproducibility compared to other methods. Although it cannot reach the versatility of extrusion-based bioprinting for now and only a few studies concerning multi-materials have been reported [162], DLP is still a promising bioprinting technology applied in the field of in vitro modeling in the near future.
The development and application of bioinks is a key point of bioprinting. Most human tissues/organs have complex combinations of ECM components with specific biological or mechanical influences [74]. It is unlikely that one single bioink can set up a “synthetic” microenvironment to mimic the actual in vivo situation, which leads to multi-material bioprinting increasingly important recently. And many published works still use a limited range of biomaterials at present. Printability, biocompatibility and mechanical property are the fundamental considerations to choose appropriate bioinks, as we discussed. There is no doubt that the development and stable synthesis of novel bioinks which can balance these three aspects, is the basis of bioprinting application.
Vascularization is the foundation of living bioprinted structures. Same as the challenge in the field of tissue engineering and regenerative medicine, ensuring of sufficient vascularization in biofabricated structures is a key factor for 3D bioprinting. The effective construction of multi-scale perfused vascular network, and subsequently promotion of its vascularization through mechanical or chemical stimulation, is the basis for biofabrication scale-up tissues. Although there are several ways to build vascular network, but each method has its limitations. Inkjet bioprinting and LAB have a high printing resolution, but it is hard to construct complex blood vessels network. Extrusion-based bioprinting based on sacrificial layer needs secondary forming which holds a complicated process. We believe that coaxial bioprinting, which holds advantages of feasibility for multi-scale fluidic channels and capability of perfused culture, is a good way to solve the vascularization issue of scale-up tissues, and it might further lay a solid foundation for tissues/organs transplantation.
Achieving functionalization is the foremost goal for 3D bioprinting. Besides, most of the researches for now are still focused on the printing process and mechanism, which is oriented by the idea of manufacturing, while functionalization is the core factor of 3D bioprinting leading it from basic research to practical application. To realize functionalization, bioinks need to possess good biocompatibility and mechanical property to meet the requirement of nutrient perfusion and implantation. Also, the construction of microenvironment mimicking in vivo scenarios, including mechanical and chemical stimulation such as perfusion culture and growth factors, is also crucial to the functionalization of bioprinted structures.
In addition to the challenges related to bioprinting techniques, bioink, vascularization and functionalization mentioned above, issues such as cell sources, bioreactor construction and even ethical problems also require considerable attention. The fully clinical translation of 3D bioprinting might take a long time until biofabricated tissues such as cartilage or skin, to be applied in transplantation of damaged tissues.
In this text, evolution, process, classification, typical bioprinters, bioinks and most significantly, representative researches of 3D bioprinting, as well as its advantage and disadvantage among different approaches are discussed. While several challenges remain to be addressed in achieving full clinical translation of bioprinting, there have been some very promising developments in the past few years. Construction of skin, bone, cartilage, vessels, organoids, etc. using 3D bioprinting technologies shows a lot of possibilities, and the combination of bioprinting and pharmaceutical sciences points out a new direction for future researches. It is anticipated that 3D bioprinting will continue to evolve and find its way from structurally similarity into functional realization.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
This work was sponsored by the National Natural Science Foundation of China (No. U1609207).
References
- 1.He Y., Gao Q., Liu A., Sun M., Fu J. 3D bioprinting: from structure to function. J Zhejiang Univ (Eng Sci) 2019;53(3):1–12. [Google Scholar]
- 2.Dong H., Fang Y., Wang D., Zhang H., Lei L., Luo Y. Current situation and thinking of organ donation at home and abroad. J Nurs. 2017;24(11):23–26. [Google Scholar]
- 3.Mironov V., Boland T., Trusk T., Forgacs G., Markwald R.R. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21(4):157–161. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 4.Boland T., Mironov V., Gutowska A., Roth E.A., Markwald R.R. Cell and organ printing 2: fusion of cell aggregates in three‐dimensional gels. Anat Rec Part A. 2003;272(2):497–502. doi: 10.1002/ar.a.10059. [DOI] [PubMed] [Google Scholar]
- 5.Mironov V. Printing technology to produce living tissue. Exp Opin Biol Ther. 2003;3(5):701–704. doi: 10.1517/14712598.3.5.701. [DOI] [PubMed] [Google Scholar]
- 6.Klebe R.J. Cytoscribing: a method for micropositioning cells and the construction of two-and three-dimensional synthetic tissues. Exp Cell Res. 1988;179(2):362–373. doi: 10.1016/0014-4827(88)90275-3. [DOI] [PubMed] [Google Scholar]
- 7.Foty R.A., Pfleger C.M., Forgacs G., Steinberg M.S. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development. 1996;122(5):1611–1620. doi: 10.1242/dev.122.5.1611. [DOI] [PubMed] [Google Scholar]
- 8.Odde D.J., Renn M.J. Laser-guided direct writing for applications in biotechnology. Trends Biotechnol. 1999;17(10):385–389. doi: 10.1016/s0167-7799(99)01355-4. [DOI] [PubMed] [Google Scholar]
- 9.Karzyński K., Kosowska K., Ambrożkiewicz F., Berman A., Cichoń J., Klak M. Use of 3D bioprinting in biomedical engineering for clinical application. Med Stud. 2018;34(1):93–97. [Google Scholar]
- 10.Landers R., Hubner U., Schmelzeisen R., Mulhaupta R. Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering. Biomaterials. 2002;23(23):4437–4447. doi: 10.1016/s0142-9612(02)00139-4. [DOI] [PubMed] [Google Scholar]
- 11.Wilson W.C., Jr., Boland T. Cell and organ printing 1: protein and cell printers. Anat Rec Part A. 2003;272(2):491–496. doi: 10.1002/ar.a.10057. [DOI] [PubMed] [Google Scholar]
- 12.Dhariwala B., Hunt E., Boland T. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng. 2004;10(9–10):1316–1322. doi: 10.1089/ten.2004.10.1316. [DOI] [PubMed] [Google Scholar]
- 13.Jayasinghe S.N., Qureshi A.N., Eagles P.A.M. Electrohydrodynamic jet processing: an advanced electric-field-driven jetting phenomenon for processing living cells. Small. 2006;2(2) doi: 10.1002/smll.200500291. :216–9. [DOI] [PubMed] [Google Scholar]
- 14.Norotte C., Marga F.S., Niklason L.E., Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30(30) doi: 10.1016/j.biomaterials.2009.06.034. :5910–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skardal A., Mack D., Kapetanovic E., Atala A., Jackson J.D., Yoo J. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cell Transl Med. 2012;1(11):792–802. doi: 10.5966/sctm.2012-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan B. State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Ann Biomed Eng. 2017;45(1):195–209. doi: 10.1007/s10439-016-1607-5. [DOI] [PubMed] [Google Scholar]
- 17.Dababneh A.B., Ozbolat I.T. Bioprinting technology: a current state-of-the-art review. J Manuf Sci Eng. 2014;136(6) [Google Scholar]
- 18.Gao Q., He Y., Fu J.Z., Liu A., Ma L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials. 2015;61:203–215. doi: 10.1016/j.biomaterials.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 19.Pyo S.H., Wang P., Hwang H.H., Zhu W., Warner J., Chen S. Continuous optical 3D printing of green aliphatic polyurethanes. ACS Appl Mater Interfaces. 2017;9(1):836–844. doi: 10.1021/acsami.6b12500. [DOI] [PubMed] [Google Scholar]
- 20.Kang H.W., Lee S.J., Ko I.K., Kengla C., Yoo J.J., Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34(3) doi: 10.1038/nbt.3413. :312–9. [DOI] [PubMed] [Google Scholar]
- 21.Noor N., Shapira A., Edri R., Gal I., Wertheim L., Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv Sci. 2019;6(11) doi: 10.1002/advs.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee A., Hudson A., Shiwarski D., Tashman J., Hinton T., Yerneni S. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365(6452):482–487. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- 23.Shao L., Gao Q., Zhao H., Xie C., Fu J., Liu Z. Fiber-based mini tissue with morphology-controllable gelma microfibers. Small. 2018;14(44) doi: 10.1002/smll.201802187. [DOI] [PubMed] [Google Scholar]
- 24.Nie J., Gao Q., Wang Y., Zeng J., Zhao H., Sun Y. Vessel-on-a-chip with hydrogel-based microfluidics. Small. 2018;14(45) doi: 10.1002/smll.201802368. [DOI] [PubMed] [Google Scholar]
- 25.Gao G., Lee J.H., Jang J., Lee D.H., Kong J.-.S., Kim B.S. Tissue engineered bio-blood-vessels constructed using a tissue-specific bioink and 3D coaxial cell printing technique: a novel therapy for ischemic disease. Adv Funct Mater. 2017;27(33):1700798. [Google Scholar]
- 26.Gaebel R., Ma N., Liu J., Guan J., Koch L., Klopsch C. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials. 2011;32(35):9218–9230. doi: 10.1016/j.biomaterials.2011.08.071. [DOI] [PubMed] [Google Scholar]
- 27.Ozbolat I.T., Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 28.Colosi C., Shin S.R., Manoharan V., Massa S., Costantini M., Barbetta A. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv Mater. 2016;28(4):677–684. doi: 10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trachtenberg J.E., Placone J.K., Smith B.T., Piard C.M., Santoro M., Scott D.W. Extrusion-based 3D printing of poly(propylene fumarate) in a full-factorial design. ACS Biomater Sci Eng. 2016;2(10):1771–1780. doi: 10.1021/acsbiomaterials.6b00026. [DOI] [PubMed] [Google Scholar]
- 30.Faulkner-Jones A., Fyfe C., Cornelissen D.J., Gardner J., King J., Courtney A. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication. 2015;7(4) doi: 10.1088/1758-5090/7/4/044102. [DOI] [PubMed] [Google Scholar]
- 31.Visser J., Peters B., Burger T.J., Boomstra J., Dhert W.J., Melchels F.P. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication. 2013;5(3) doi: 10.1088/1758-5082/5/3/035007. [DOI] [PubMed] [Google Scholar]
- 32.Ovsianikov A., Yoo J., Mironov V. Springer International Publishing; 2018. 3D printing and biofabrication. [Google Scholar]
- 33.Chua C.K., Leong K.F. World Scientific Publishing Co.; 2017. 3D printing and additive manufacturing: Principles and applications: The 5th edition of rapid prototyping: Principles and applications. [Google Scholar]
- 34.Sheshadri P., Shirwaiker R.A. Characterization of material–process–structure interactions in the 3D bioplotting of polycaprolactone. 3D Print Addit Manuf. 2015;2(1):20–31. [Google Scholar]
- 35.Chua C.K., Yeong W.Y. World Scientific Publishing Company; 2014. Bioprinting: principles and applications. [Google Scholar]
- 36.Ozbolat I.T., Moncal K.K., Gudapati H. Evaluation of bioprinter technologies. Addit Manuf. 2017;13:179–200. [Google Scholar]
- 37.Lee W., Debasitis J.C., Lee V.K., Lee J.H., Fischer K., Edminster K. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials. 2009;30(8):1587–1595. doi: 10.1016/j.biomaterials.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Lee V., Singh G., Trasatti J.P., Bjornsson C., Xu X., Tran T.N. Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C-Methods. 2014;20(6):473–484. doi: 10.1089/ten.tec.2013.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim G., Ahn S., Yoon H., Kim Y., Chun W. A cryogenic direct-plotting system for fabrication of 3D collagen scaffolds for tissue engineering. J Mater Chem. 2009;19(46):8817–8823. [Google Scholar]
- 40.Kim G., Ahn S., Kim Y., Cho Y., Chun W. Coaxial structured collagen–alginate scaffolds: fabrication, physical properties, and biomedical application for skin tissue regeneration. J Mater Chem. 2011;21(17):6165–6172. [Google Scholar]
- 41.Leng L., McAllister A., Zhang B., Radisic M., Gunther A. Mosaic hydrogels: one-step formation of multiscale soft materials. Adv Mater. 2012;24(27):3650–3658. doi: 10.1002/adma.201201442. [DOI] [PubMed] [Google Scholar]
- 42.Leng L., Amini-Nik S., Ba Q., Jeschke M., Günther A. Skin printer: microfluidic approach for skin regeneration and wound dressing. Proceedings of the 2013 international conference on miniaturized systems for chemistry and life sciences, MicroTAS, 17; Curran Associates, Freiburg, DE; 2013. pp. 1833–1835. [Google Scholar]
- 43.Hakimi N., Cheng R., Leng L., Sotoudehfar M., Ba P.Q., Bakhtyar N. Handheld skin printer: in situ formation of planar biomaterials and tissues. Lab Chip. 2018;18(10):1440–1451. doi: 10.1039/c7lc01236e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao Q., Wei B., Guo Y., Jin C., Du X., Yan C. Design, construction and mechanical testing of digital 3D anatomical data-based pcl-ha bone tissue engineering scaffold. J Mater Sci Mater Med. 2015;26(1):5360. doi: 10.1007/s10856-014-5360-8. [DOI] [PubMed] [Google Scholar]
- 45.Pati F., Song T.H., Rijal G., Jang J., Kim S.W., Cho D.W. Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials. 2015;37:230–241. doi: 10.1016/j.biomaterials.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 46.Daly A.C., Cunniffe G.M., Sathy B.N., Jeon O., Alsberg E., Kelly D.J. 3D bioprinting of developmentally inspired templates for whole bone organ engineering. Adv Healthc Mater. 2016;5(18):2353–2362. doi: 10.1002/adhm.201600182. [DOI] [PubMed] [Google Scholar]
- 47.Kesti M., Eberhardt C., Pagliccia G., Kenkel D., Grande D., Boss A. Bioprinting complex cartilaginous structures with clinically compliant biomaterials. Adv Funct Mater. 2015;25(48):7406–7417. [Google Scholar]
- 48.Kundu J., Shim J.H., Jang J., Kim S.W., Cho D.W. An additive manufacturing-based pcl-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med. 2015;9(11):1286–1297. doi: 10.1002/term.1682. [DOI] [PubMed] [Google Scholar]
- 49.Lee J.S., Hong J.M., Jung J.W., Shim J.H., Oh J.H., Cho D.W. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication. 2014;6(2) doi: 10.1088/1758-5082/6/2/024103. [DOI] [PubMed] [Google Scholar]
- 50.Owens C.M., Marga F., Forgacs G., Heesch C.M. Biofabrication and testing of a fully cellular nerve graft. Biofabrication. 2013;5(4) doi: 10.1088/1758-5082/5/4/045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Merceron T.K., Burt M., Seol Y.J., Kang H.W., Lee S.J., Yoo J.J. A 3D bioprinted complex structure for engineering the muscle-tendon unit. Biofabrication. 2015;7(3) doi: 10.1088/1758-5090/7/3/035003. [DOI] [PubMed] [Google Scholar]
- 52.Pati F., Ha D.H., Jang J., Han H.H., Rhie J.W., Cho D.W. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials. 2015;62:164–175. doi: 10.1016/j.biomaterials.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 53.Skardal A., Zhang J., Prestwich G.D. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials. 2010;31(24):6173–6181. doi: 10.1016/j.biomaterials.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 54.Hinton T.J., Jallerat Q., Palchesko R.N., Park J.H., Grodzicki M.S., Shue H.J. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1(9) doi: 10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Billiet T., Gevaert E., De Schryver T., Cornelissen M., Dubruel P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014;35(1):49–62. doi: 10.1016/j.biomaterials.2013.09.078. [DOI] [PubMed] [Google Scholar]
- 56.Lee J.W., Choi Y.J., Yong W.J., Pati F., Shim J.H., Kang K.S. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication. 2016;8(1) doi: 10.1088/1758-5090/8/1/015007. [DOI] [PubMed] [Google Scholar]
- 57.Horvath L., Umehara Y., Jud C., Blank F., Petri-Fink A., Rothen-Rutishauser B. Engineering an in vitro air-blood barrier by 3D bioprinting. Sci Rep. 2015;5:7974. doi: 10.1038/srep07974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duan B., Hockaday L.A., Kang K.H., Butcher J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A. 2013;101(5):1255–1264. doi: 10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang R., Nam J., Sun W. Direct cell writing of 3D microorgan for in vitro pharmacokinetic model. Tissue Eng Part C-Methods. 2008;14(2):157–166. doi: 10.1089/ten.tec.2007.0392. [DOI] [PubMed] [Google Scholar]
- 60.Chang R., Emami K., Wu H., Sun W. Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication. 2010;2(4) doi: 10.1088/1758-5082/2/4/045004. [DOI] [PubMed] [Google Scholar]
- 61.Snyder J.E., Hamid Q., Wang C., Chang R., Emami K., Wu H. Bioprinting cell-laden matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication. 2011;3(3) doi: 10.1088/1758-5082/3/3/034112. [DOI] [PubMed] [Google Scholar]
- 62.Levato R., Visser J., Planell J.A., Engel E., Malda J., Mateos-Timoneda M.A. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication. 2014;6(3) doi: 10.1088/1758-5082/6/3/035020. [DOI] [PubMed] [Google Scholar]
- 63.Ying G.L., Jiang N., Maharjan S., Yin Y.X., Chai R.R., Cao X. Aqueous two-phase emulsion bioink-enabled 3D bioprinting of porous hydrogels. Adv Mater. 2018;30(50) doi: 10.1002/adma.201805460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao Q., Niu X., Shao L., Zhou L., Lin Z., Sun A. 3D printing of complex gelma-based scaffolds with nanoclay. Biofabrication. 2019;11(3) doi: 10.1088/1758-5090/ab0cf6. [DOI] [PubMed] [Google Scholar]
- 65.Gao Q., Liu Z., Lin Z., Qiu J., Liu Y., Liu A. 3D bioprinting of vessel-like structures with multilevel fluidic channels. ACS Biomater Sci Eng. 2017;3(3):399–408. doi: 10.1021/acsbiomaterials.6b00643. [DOI] [PubMed] [Google Scholar]
- 66.Jia W., Gungor-Ozkerim P.S., Zhang Y.S., Yue K., Zhu K., Liu W. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016;106:58–68. doi: 10.1016/j.biomaterials.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akkineni A.R., Ahlfeld T., Lode A., Gelinsky M. A versatile method for combining different biopolymers in a core/shell fashion by 3D plotting to achieve mechanically robust constructs. Biofabrication. 2016;8(4) doi: 10.1088/1758-5090/8/4/045001. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y.S., Arneri A., Bersini S., Shin S.R., Zhu K., Goli-Malekabadi Z. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59. doi: 10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pi Q., Maharjan S., Yan X., Liu X., Singh B., van Genderen A.M. Digitally tunable microfluidic bioprinting of multilayered cannular tissues. Adv Mater. 2018;30(43) doi: 10.1002/adma.201706913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tuan R.S., Boland G., Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthrit Res Ther. 2003;5(1):32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ozbolat I.T. Elsevier; 2016. 3D bioprinting: fundamentals, principles and applications. [Google Scholar]
- 72.Saunders R.E., Derby B. Inkjet printing biomaterials for tissue engineering: bioprinting. Int Mater Rev. 2014;59(8):430–448. [Google Scholar]
- 73.Cui X.F., Dean D., Ruggeri Z.M., Boland T. Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol Bioeng. 2010;106(6):963–969. doi: 10.1002/bit.22762. [DOI] [PubMed] [Google Scholar]
- 74.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 75.Nishiyama Y., Nakamura M., Henmi C., Yamaguchi K., Mochizuki S., Nakagawa H. Development of a three-dimensional bioprinter: construction of cell supporting structures using hydrogel and state-of-the-art inkjet technology. J Biomech Eng. 2009;131(3) doi: 10.1115/1.3002759. [DOI] [PubMed] [Google Scholar]
- 76.Kamisuki S., Hagata T., Tezuka C., Nose Y., Fujii M., Atobe M. A low power, small, electrostatically driven driven commercial inkjet head. Proceedings of the eleventh annual international workshop on micro electro mechanical systems, MEMS 98; IEEE; 1998. [Google Scholar]
- 77.Reis N., Ainsley C., Derby B. Ink-jet delivery of particle suspensions by piezoelectric droplet ejectors. J Appl Phys. 2005;97(9):094903. [Google Scholar]
- 78.Seerden K.A.M., Reis N., Evans J.R.G., Grant P.S., Halloran J.W., Derby B. Ink-jet printing of wax-based alumina suspensions. J Am Ceram Soc. 2001;84(11):2514–2520. [Google Scholar]
- 79.Saunders R.E., Gough J.E., Derby B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials. 2008;29(2):193–203. doi: 10.1016/j.biomaterials.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 80.Zhang L.G., Fisher J.P., Leong K.W. Academic Press; 2015. 3D bioprinting and nanotechnology in tissue engineering and regenerative medicine. [Google Scholar]
- 81.Onses M.S., Sutanto E., Ferreira P.M., Alleyne A.G., Rogers J.A. Mechanisms, capabilities, and applications of high-resolution electrohydrodynamic jet printing. Small. 2015;11(34):4237–4266. doi: 10.1002/smll.201500593. [DOI] [PubMed] [Google Scholar]
- 82.Gasperini L., Maniglio D., Motta A., Migliaresi C. An electrohydrodynamic bioprinter for alginate hydrogels containing living cells. Tissue Eng Part C-Methods. 2015;21(2):123–132. doi: 10.1089/ten.TEC.2014.0149. [DOI] [PubMed] [Google Scholar]
- 83.Poellmann M.J., Barton K.L., Mishra S., Johnson A.J.W. Patterned hydrogel substrates for cell culture with electrohydrodynamic jet printing. Macromol Biosci. 2011;11(9):1164–1168. doi: 10.1002/mabi.201100004. [DOI] [PubMed] [Google Scholar]
- 84.Sutanto E., Shigeta K., Kim Y.K., Graf P.G., Hoelzle D.J., Barton K.L. A multimaterial electrohydrodynamic jet (e-jet) printing system. J Micromech Microeng. 2012;22(4) [Google Scholar]
- 85.Hayati I., Bailey A.I., Tadros T.F. Mechanism of stable jet formation in electrohydrodynamic atomization. Nature. 1986;319(6048):41–43. [Google Scholar]
- 86.Workman V.L., Tezera L.B., Elkington P.T., Jayasinghe S.N. Controlled generation of microspheres incorporating extracellular matrix fibrils for three-dimensional cell culture. Adv Funct Mater. 2014;24(18):2648–2657. doi: 10.1002/adfm.201303891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gasperini L., Maniglio D., Migliaresi C. Microencapsulation of cells in alginate through an electrohydrodynamic process. J Bioact Compat Polym. 2013;28(5):413–425. [Google Scholar]
- 88.Gudapati H., Dey M., Ozbolat I. A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials. 2016:10220–10242. doi: 10.1016/j.biomaterials.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 89.Odde D.J., Renn M.J. Laser-guided direct writing of living cells. Biotechnol Bioeng. 2000;67(3) doi: 10.1002/(sici)1097-0290(20000205)67:3<312::aid-bit7>3.0.co;2-f. :312–8. [DOI] [PubMed] [Google Scholar]
- 90.Nahmias Y., Schwartz R.E., Verfaillie C.M., Odde D.J. Laser-guided direct writing for three-dimensional tissue engineering. Biotechnol Bioeng. 2005;92(2):129–136. doi: 10.1002/bit.20585. [DOI] [PubMed] [Google Scholar]
- 91.Breckenfeld E., Kim H., Auyeung R.C.Y., Pique A. Laser-induced forward transfer of ag nanopaste. JOVE-J Vis Exp. 2016;(109):e53728. doi: 10.3791/53728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Skardal A., Atala A. Biomaterials for integration with 3-D bioprinting. Ann Biomed Eng. 2015;43(3):730–746. doi: 10.1007/s10439-014-1207-1. [DOI] [PubMed] [Google Scholar]
- 93.Chrisey D.B. Materials processing: the power of direct writing. Science. 2000;289(5481):879–881. doi: 10.1126/science.289.5481.879. [DOI] [PubMed] [Google Scholar]
- 94.Colina M., Serra P., Fernandez-Pradas J.M., Sevilla L., Morenza J.L. DNA deposition through laser induced forward transfer. Biosens Bioelectron. 2005;20(8):1638–1642. doi: 10.1016/j.bios.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 95.Dinca V., Kasotakis E., Catherine J., Mourka A., Ranella A., Ovsianikov A. Directed three-dimensional patterning of self-assembled peptide fibrils. Nano Lett. 2008;8(2):538–543. doi: 10.1021/nl072798r. [DOI] [PubMed] [Google Scholar]
- 96.Ringeisen B.R., Kim H., Barron J.A., Krizman D.B., Chrisey D.B., Jackman S. Laser printing of pluripotent embryonal carcinoma cells. Tissue Eng. 2004;10(3–4):483–491. doi: 10.1089/107632704323061843. [DOI] [PubMed] [Google Scholar]
- 97.Hopp B., Smausz T., Kresz N., Barna N., Bor Z., Kolozsvari L. Survival and proliferative ability of various living cell types after laser-induced forward transfer. Tissue Eng. 2005;11(11–12):1817–1823. doi: 10.1089/ten.2005.11.1817. [DOI] [PubMed] [Google Scholar]
- 98.Barron J.A., Wu P., Ladouceur H.D., Ringeisen B.R. Biological laser printing: a novel technique for creating heterogeneous 3-dimensional cell patterns. Biomed Microdevices. 2004;6(2):139–147. doi: 10.1023/b:bmmd.0000031751.67267.9f. [DOI] [PubMed] [Google Scholar]
- 99.Wu P.K., Ringeisen B.R., Callahan J., Brooks M., Bubb D.M., Wu H.D. The deposition, structure, pattern deposition, and activity of biomaterial thin-films by matrix-assisted pulsed-laser evaporation (maple) and maple direct write. Thin Solid Films. 2001;398:607–614. [Google Scholar]
- 100.Serra P., Fernandez-Pradas J.M., Colina M., Duocastella M., Dominguez J., Morenza J.L. Laser-induced forward transfer: a direct-writing technique for biosensors preparation. J Laser Micro/Nanoeng. 2006;1(3):236–242. [Google Scholar]
- 101.Barron J.A., Ringeisen B.R., Kim H.S., Spargo B.J., Chrisey D.B. Application of laser printing to mammalian cells. Thin Solid Films. 2004;453:383–387. [Google Scholar]
- 102.Barron J.A., Krizman D.B., Ringeisen B.R. Laser printing of single cells: statistical analysis, cell viability, and stress. Ann Biomed Eng. 2005;33(2):121–130. doi: 10.1007/s10439-005-8971-x. [DOI] [PubMed] [Google Scholar]
- 103.Guillemot F., Souquet A., Catros S., Guillotin B. Laser-assisted cell printing: principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomedicine. 2010;5(3):507–515. doi: 10.2217/nnm.10.14. [DOI] [PubMed] [Google Scholar]
- 104.Guillemot F., Souquet A., Catros S., Guillotin B., Lopez J., Faucon M. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 2010;6(7):2494–2500. doi: 10.1016/j.actbio.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 105.Koch L., Gruene M., Unger C., Chichkov B. Laser assisted cell printing. Curr Pharm Biotechnol. 2013;14(1):91–97. [PubMed] [Google Scholar]
- 106.Barron J.A., Spargo B.J., Ringeisen B.R. Biological laser printing of three dimensional cellular structures. Appl Phys A-Mater. 2004;79(4–6):1027–1030. [Google Scholar]
- 107.Keriquel V., Guillemot F., Arnault I., Guillotin B., Miraux S., Amedee J. In vivo bioprinting for computer- and robotic-assisted medical intervention: preliminary study in mice. Biofabrication. 2010;2(1) doi: 10.1088/1758-5082/2/1/014101. [DOI] [PubMed] [Google Scholar]
- 108.Choi W.S., Ha D., Park S., Kim T. Synthetic multicellular cell-to-cell communication in inkjet printed bacterial cell systems. Biomaterials. 2011;32(10):2500–2507. doi: 10.1016/j.biomaterials.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 109.Ferris C.J., Gilmore K.J., Beirne S., McCallum D., Wallace G.G., in het Panhuis M. Bio-ink for on-demand printing of living cells. Biomater Sci. 2013;1(2):224–230. doi: 10.1039/c2bm00114d. [DOI] [PubMed] [Google Scholar]
- 110.Yanagi Y., Nakayama K., Taguchi T., Enosawa S., Tamura T., Yoshimaru K. In vivo and ex vivo methods of growing a liver bud through tissue connection. Sci Rep. 2017;7(1):14085. doi: 10.1038/s41598-017-14542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sofokleous P., Stride E., Bonfield W., Edirisinghe M. Design, construction and performance of a portable handheld electrohydrodynamic multi-needle spray gun for biomedical applications. Mat Sci Eng C. 2013;33(1):213–223. doi: 10.1016/j.msec.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 112.Koch L., Kuhn S., Sorg H., Gruene M., Schlie S., Gaebel R. Laser printing of skin cells and human stem cells. Tissue Eng Part C-Methods. 2010;16(5):847–854. doi: 10.1089/ten.TEC.2009.0397. [DOI] [PubMed] [Google Scholar]
- 113.Koch L., Deiwick A., Schlie S., Michael S., Gruene M., Coger V. Skin tissue generation by laser cell printing. Biotechnol Bioeng. 2012;109(7):1855–1863. doi: 10.1002/bit.24455. [DOI] [PubMed] [Google Scholar]
- 114.Michael S., Sorg H., Peck C.T., Koch L., Deiwick A., Chichkov B. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS One. 2013;8(3):e57741. doi: 10.1371/journal.pone.0057741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.De Coppi P., Bartsch G., Jr., Siddiqui M.M., Xu T., Santos C.C., Perin L. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25(1):100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 116.Cui X., Breitenkamp K., Finn M.G., Lotz M., D'Lima D.D. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A. 2012;18(11–12):1304–1312. doi: 10.1089/ten.tea.2011.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu T., Binder K.W., Albanna M.Z., Dice D., Zhao W., Yoo J.J. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2013;5(1) doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 118.Markstedt K., Mantas A., Tournier I., Martinez Avila H., Hagg D., Gatenholm P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015;16(5):1489–1496. doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 119.Eagles P.A., Qureshi A.N., Jayasinghe S.N. Electrohydrodynamic jetting of mouse neuronal cells. Biochem J. 2006;394(2):375–378. doi: 10.1042/BJ20051838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lorber B., Hsiao W.K., Hutchings I.M., Martin K.R. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication. 2014;6(1) doi: 10.1088/1758-5082/6/1/015001. [DOI] [PubMed] [Google Scholar]
- 121.Boland T., Tao X., Damon B.J., Manley B., Kesari P., Jalota S. Drop-on-demand printing of cells and materials for designer tissue constructs. Mat Sci Eng C. 2007;27(3):372–376. [Google Scholar]
- 122.Cui X., Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30(31):6221–6227. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 123.Xu C., Chai W., Huang Y., Markwald R.R. Scaffold-free inkjet printing of three-dimensional zigzag cellular tubes. Biotechnol Bioeng. 2012;109(12):3152–3160. doi: 10.1002/bit.24591. [DOI] [PubMed] [Google Scholar]
- 124.Xu C., Zhang Z., Christensen K., Huang Y., Fu J., Markwald R.R. Freeform vertical and horizontal fabrication of alginate-based vascular-like tubular constructs using inkjetting. J Manuf Sci Eng. 2014;136(6):061020. [Google Scholar]
- 125.Christensen K., Xu C., Chai W., Zhang Z., Fu J., Huang Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol Bioeng. 2015;112(5):1047–1055. doi: 10.1002/bit.25501. [DOI] [PubMed] [Google Scholar]
- 126.Wu P.K., Ringeisen B.R. Development of human umbilical vein endothelial cell (huvec) and human umbilical vein smooth muscle cell (huvsmc) branch/stem structures on hydrogel layers via biological laser printing (biolp) Biofabrication. 2010;2(1) doi: 10.1088/1758-5082/2/1/014111. [DOI] [PubMed] [Google Scholar]
- 127.Pirlo R.K., Wu P., Liu J., Ringeisen B. Plga/hydrogel biopapers as a stackable substrate for printing huvec networks via biolp. Biotechnol Bioeng. 2012;109(1):262–273. doi: 10.1002/bit.23295. [DOI] [PubMed] [Google Scholar]
- 128.Xiong R., Zhang Z., Chai W., Huang Y., Chrisey D.B. Freeform drop-on-demand laser printing of 3D alginate and cellular constructs. Biofabrication. 2015;7(4) doi: 10.1088/1758-5090/7/4/045011. [DOI] [PubMed] [Google Scholar]
- 129.Xu T., Zhao W., Zhu J.M., Albanna M.Z., Yoo J.J., Atala A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials. 2013;34(1):130–139. doi: 10.1016/j.biomaterials.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 130.Zhao H., Chen Y., Shao L., Xie M., Nie J., Qiu J. Airflow-assisted 3D bioprinting of human heterogeneous microspheroidal organoids with microfluidic nozzle. Small. 2018;14(39) doi: 10.1002/smll.201802630. [DOI] [PubMed] [Google Scholar]
- 131.Durmus N.G., Tasoglu S., Demirci U. Functional droplet networks. Nat Mater. 2013;12(6):478–479. doi: 10.1038/nmat3665. [DOI] [PubMed] [Google Scholar]
- 132.Xie M., Gao Q., Zhao H., Nie J., Fu Z., Wang H. Electro-assisted bioprinting of low-concentration gelma microdroplets. Small. 2019;15(4) doi: 10.1002/smll.201804216. [DOI] [PubMed] [Google Scholar]
- 133.Billiet T., Vandenhaute M., Schelfhout J., Van Vlierberghe S., Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012;33(26):6020–6041. doi: 10.1016/j.biomaterials.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 134.Melchels F.P., Feijen J., Grijpma D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31(24):6121–6130. doi: 10.1016/j.biomaterials.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 135.Lu Y., Chen S. Projection printing of 3-dimensional tissue scaffolds. In: Liebschner M.A.K., editor. Computer-aided tissue engineering. Humana Press; New York: 2012. pp. 289–302. [Google Scholar]
- 136.Zhu W., Cui H., Boualam B., Masood F., Flynn E., Rao R.D. 3D bioprinting mesenchymal stem cell-laden construct with core-shell nanospheres for cartilage tissue engineering. Nanotechnology. 2018;29(18) doi: 10.1088/1361-6528/aaafa1. [DOI] [PubMed] [Google Scholar]
- 137.Pateman C.J., Harding A.J., Glen A., Taylor C.S., Christmas C.R., Robinson P.P. Nerve guides manufactured from photocurable polymers to aid peripheral nerve repair. Biomaterials. 2015;49:77–89. doi: 10.1016/j.biomaterials.2015.01.055. [DOI] [PubMed] [Google Scholar]
- 138.Zhu W., Tringale K.R., Woller S.A., You S., Johnson S., Shen H. Rapid continuous 3D printing of customizable peripheral nerve guidance conduits. Mater Today. 2018;21(9):951–959. doi: 10.1016/j.mattod.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Koffler J., Zhu W., Qu X., Platoshyn O., Dulin J.N., Brock J. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med. 2019;25(2):263–269. doi: 10.1038/s41591-018-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhu W., Qu X., Zhu J., Ma X., Patel S., Liu J. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials. 2017;124:106–115. doi: 10.1016/j.biomaterials.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ma X., Qu X., Zhu W., Li Y.S., Yuan S., Zhang H. Deterministically patterned biomimetic human ipsc-derived hepatic model via rapid 3D bioprinting. Proc Nat Acad Sci. 2016;113(8):2206–2211. doi: 10.1073/pnas.1524510113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gou M., Qu X., Zhu W., Xiang M., Yang J., Zhang K. Bio-inspired detoxification using 3D-printed hydrogel nanocomposites. Nat Commun. 2014;5:3774. doi: 10.1038/ncomms4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jones N. Science in three dimensions: the print revolution. Nature. 2012;487(7405):22–23. doi: 10.1038/487022a. [DOI] [PubMed] [Google Scholar]
- 144.Chang C.C., Boland E.D., Williams S.K., Hoying J.B. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res B. 2011;98(1):160–170. doi: 10.1002/jbm.b.31831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Smith C.M., Stone A.L., Parkhill R.L., Stewart R.L., Simpkins M.W., Kachurin A.M. Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue Eng. 2004;10(9–10):1566–1576. doi: 10.1089/ten.2004.10.1566. [DOI] [PubMed] [Google Scholar]
- 146.Chang R., Nam J., Sun W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng Part A. 2008;14(1):41–48. doi: 10.1089/ten.a.2007.0004. [DOI] [PubMed] [Google Scholar]
- 147.Gruene M., Deiwick A., Koch L., Schlie S., Unger C., Hofmann N. Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng Part C-Me. 2011;17(1):79–87. doi: 10.1089/ten.TEC.2010.0359. [DOI] [PubMed] [Google Scholar]
- 148.Guillotin B., Souquet A., Catros S., Duocastella M., Pippenger B., Bellance S. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 2010;31(28):7250–7256. doi: 10.1016/j.biomaterials.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 149.Gao G., Huang Y., Schilling A.F., Hubbell K., Cui X. Organ bioprinting: are we there yet? Adv Healthc Mater. 2018;7(1):1701018. doi: 10.1002/adhm.201701018. [DOI] [PubMed] [Google Scholar]
- 150.Hospodiuk M., Dey M., Sosnoski D., Ozbolat I.T. The bioink: a comprehensive review on bioprintable materials. Biotechnol Adv. 2017;35(2):217–239. doi: 10.1016/j.biotechadv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 151.Zhang Z., Jin Y., Yin J., Xu C., Xiong R., Christensen K. Evaluation of bioink printability for bioprinting applications. Appl Phys Rev. 2018;5(4):041304. [Google Scholar]
- 152.Heinrich M.A., Liu W., Jimenez A., Yang J., Akpek A., Liu X. 3D bioprinting: from benches to translational applications. Small. 2019;15(23) doi: 10.1002/smll.201805510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pan T., Song W., Cao X., Wang Y. 3D bioplotting of gelatin/alginate scaffolds for tissue engineering: influence of crosslinking degree and pore architecture on physicochemical properties. J Mater Sci Technol. 2016;32(9):889–900. [Google Scholar]
- 154.Gao T., Gillispie G.J., Copus J.S., Pr A.K., Seol Y.J., Atala A. Optimization of gelatin-alginate composite bioink printability using rheological parameters: a systematic approach. Biofabrication. 2018;10(3) doi: 10.1088/1758-5090/aacdc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang X., Lu Z., Wu H., Li W., Zheng L., Zhao J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl. 2018;83:195–201. doi: 10.1016/j.msec.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 156.Yue K., Trujillo-de Santiago G., Alvarez M.M., Tamayol A., Annabi N., Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (gelma) hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ouyang L., Highley C.B., Rodell C.B., Sun W., Burdick J.A. 3D printing of shear-thinning hyaluronic acid hydrogels with secondary cross-linking. ACS Biomater Sci Eng. 2016;2(10):1743–1751. doi: 10.1021/acsbiomaterials.6b00158. [DOI] [PubMed] [Google Scholar]
- 158.Law N., Doney B., Glover H., Qin Y., Aman Z.M., Sercombe T.B. Characterisation of hyaluronic acid methylcellulose hydrogels for 3D bioprinting. J Mech Behav Biomed Mater. 2018;77:389–399. doi: 10.1016/j.jmbbm.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 159.Yanez M., Rincon J., Dones A., De Maria C., Gonzales R., Boland T. In vivo assessment of printed microvasculature in a bilayer skin graft to treat full-thickness wounds. Tissue Eng Part A. 2015;21(1–2):224–233. doi: 10.1089/ten.tea.2013.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Croisier F., Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur Polym J. 2013;49(4):780–792. [Google Scholar]
- 161.Lee W., Lee V., Polio S., Keegan P., Lee J.H., Fischer K. On-demand three-dimensional freeform fabrication of multi-layered hydrogel scaffold with fluidic channels. Biotechnol Bioeng. 2010;105(6):1178–1186. doi: 10.1002/bit.22613. [DOI] [PubMed] [Google Scholar]
- 162.Miri A.K., Nieto D., Iglesias L., Goodarzi Hosseinabadi H., Maharjan S., Ruiz‐Esparza G.U. Microfluidics‐enabled multimaterial maskless stereolithographic bioprinting. Adv Mater. 2018;30(27) doi: 10.1002/adma.201800242. [DOI] [PMC free article] [PubMed] [Google Scholar]