Abstract
Vaccines therapeutics manipulate host's immune system and have broad potential for cancer prevention and treatment. However, due to poor immunogenicity and limited safety, fewer cancer vaccines have been successful in clinical trials. Over the past decades, nanotechnology has been exploited to deliver cancer vaccines, eliciting long-lasting and effective immune responses. Compared to traditional vaccines, cancer vaccines delivered by nanomaterials can be tuned towards desired immune profiles by (1) optimizing the physicochemical properties of the nanomaterial carriers, (2) modifying the nanomaterials with targeting molecules, or (3) co-encapsulating with immunostimulators. In order to develop vaccines with desired immunogenicity, a thorough understanding of parameters that affect immune responses is required. Herein, we discussed the effects of physicochemical properties on antigen presentation and immune response, including but not limited to size, particle rigidity, intrinsic immunogenicity. Furthermore, we provided a detailed overview of recent preclinical and clinical advances in nanotechnology for cancer vaccines, and considerations for future directions in advancing the vaccine platform to widespread anti-cancer applications.
Keywords: Cancer, Vaccines, Nanotechnology, Antigens, Peptide, mRNA
Graphical abstract
Cancer vaccines delivered by nanomaterials can be tuned towards desired immune profiles by (1) optimizing the physicochemical properties of the nanomaterial carriers, (2) modifying the nanomaterials with targeting molecules, or (3) co-encapsulating with immunostimulators.
1. Introduction
Cancer is one of the leading causes of death around the world. In 2018 alone, about 609 640 people are dying from cancer in the United States, which is equivalent to that almost 1700 deaths occur every day [1]. Over the last few decades, significant progress has been made in treating cancers. Traditional approaches including surgery, chemotherapy and radiotherapy have dramatically improved the survival of cancer bearing patients. However, recurrence and multi-drug resistance still remain insurmountable obstacles that limit the successful eradication and long-term treatment of cancers [2,3].
Inherent immune system plays a vital role in the recognition and eradication of tumor cells, based on which, immunotherapies have been used to treat cancers [4]. Since 2011, FDA has approved six immunotherapeutic drugs to treat advanced metastatic melanoma, hematologic malignancy, non-small cell lung cancer, and etc., with obvious survival benefits [5], [6], [7], [8], [9]. For example, the 1-year survival of stage IV melanoma patients has been increased to over 80% as compared to 30% before 2010 [10]. Among all immunotherapeutic drugs under clinical investigation, checkpoint inhibitor based immunotherapies (i.e., cytotoxic T lymphocyte-associated molecule-4 (CTLA-4), programmed cell death receptor-1 (PD-1) and programmed cell death ligand-1 (PD-L1)) have dramatically revoluted cancer treatments and gained rapid clinical successes [11]. Another immunotherapeutic strategy which has recently gained considerable attention in clinic is the chimeric antigen receptor T cell (CAR-T) therapy. Despite a risk of cytokine release syndrome, CAR-T therapy showed promising elimination of B-cell lymphoma [12].
Vaccine strategies are another alternative for treating cancers, which was developed due to the successful experience in the field of vaccinology and infectious diseases. The term of cancer vaccine refers to a vaccine that prevents either infections with cancer-causing viruses or the development of cancer in certain high risk individuals (known as prophylactic cancer vaccine) and treats existing cancer (known as therapeutic cancer vaccine) [13]. The first therapeutic cancer vaccine product, sipuleucel-T (Provenge), for prostate cancer, was approved in the US in 2010 [14]. Recent clinical results indicate that cancer vaccines have shown the ability to decrease cancer recurrence and increase overall survival. The clinical benefit of therapeutic cancer vaccines has therefore been established [15]. Cancer vaccination comprises an array of approaches that seek to generate, amplify, or skew (or a combination thereof) antitumor immunity [16].
To develop stronger cancer vaccines, a clear understanding of the mode of action of cancer vaccines is required. Intradermal, subcutaneous and intramuscular administration make cancer vaccines exposure to APCs. APCs uptake vaccines and present the antigens on major histocompatibility complex (MHC) class I or II. Through the afferent lymph, APCs travel into the lymph nodes, where they prime and activate T cells. And then the activated effector T cells migrate through the efferent lymph to blood and infiltrate the tumor bed, where T cells recognize and kill targeted cancer cells [15,17]. Macrophages, B cells and dendritic cells (DCs) can all function as APCs to promote antigen-specific immune cell activation, among which DCs are the most potent APC population [18,19]. The presentation of antigen mediated by DCs is thought to occur though two major routes. In the first pathway, endogenous antigenic proteins are degraded by proteasomes in cytosol of DCs. Their derived peptide fragments are presented by MHC-I molecules, which engender activation of antigen-specific CD8+ cytotoxic T lymphocytes (CTLs) which are the main effector cells against tumor cells [20]. In contrast, exogenous antigenic proteins are taken up by DCs via endocytosis by means of vesicles known as endosomes before fusing with lysosomes where these molecules are degraded to peptide fragments. Then these peptide fragments are presented on MHC-II molecules, generating activation of CD4+ T lymphocytes which assist CTLs activation, function, and survival [21], [22], [23], [24]. Therefore, Delivery of cancer vaccines in a platform that can activate CD4+ T cells and CTLs is important for effective antitumor immunotherapy.
Successful cancer vaccines must meet the following requirements: (1) a selection of the right tumor associated antigen is essential to stimulate effective T cells, otherwise antigens are not drivers of oncogenesis and activated T cell clones may be weak. Some antigens that have been identified and tested in clinical trials will be discussed below. (2) Achieving sufficient antigen concentration in DCs and the activation of DCs are important for cancer vaccine, otherwise, tolerance ensues [25]. So vaccines need a rational vaccine design to carry targeting ligand and immunostimulatory reagents. (3) CTLs are a major force to kill all tumour cell types. However, CD4+ T cells are needed for the expansion of CTLs and the generation of CD8+ memory cells [26]. It is important for cancer vaccines to cause durable CD4+ and CD8+ responses. In DCs, internalized exogenous antigens can be presented to CD8+ T cells through cross-presentation [27]. (4) Cancer vaccines cannot be expected to act as a monotherapy [15]. The negative influence of the tumor microenvironment and other immunosuppressive factors limits the success of cancer vaccines [18]. It will be a perfect strategy that combine cancer vaccines with chemotherapy, inhibitors of immunosuppression and other therapy forms. The application of nanotechnology in vaccines can satisfy these requirements. Nanoparticles as cancer vaccines offer unique advantages over the traditional cancer vaccines, including: (1) protection of vaccines from degradation; (2) targeting DCs with the use of ligands; (3) co-delivery vaccines and adjuvants or other agents to enhance antitumor response; (4) enhancing cross-presentation to induce CTLs; (5) the ability to control release and distribution. In this review, we discussed recent progress on nanotechnology for cancer vaccine delivery and design considerations for nanoparticles (NPs) as cancer vaccine carriers.
2. Commonly used antigens and vaccines for anti-cancer therapy
Cancer vaccines can specifically recognize different types of tumor associated antigens (TAAs), including overexpressed antigens, oncofetal antigens, cancer-testis (CT) antigens and mutated antigens [16,28]. Unfortunately, poor immune response can be caused by central tolerance and peripheral immune tolerance which result from expression of these self-antigens in the thymus and normal cells, respectively [28,29]. Therefore, the selection of TAAs as vaccine targets is crucial, which directly affects the potency of immune response and the subsequent anti-cancer efficacy.
2.1. Identified tumor associated antigens
In order to elicit a tumor-specific immune response, great efforts have been made to identify cancer antigens using tumor-reactive T cells [30]. Identified immunogenic tumor antigens include Trp1/Trp2 (melanoma); carcinoembryonic antigen (CEA; colorectal cancer and others); prostate specific antigen (PSA; prostate cancer); Her2/neu (breast cancer); MUC1 (lung cancer) and so on [31]. These antigens have shown efficacy as targets in vitro and have been tested in clinical trials, while ideal, defined, shared targets have not been identified for many tumor types [32].
2.2. Whole tumor cells or tumor lysates
Tumors which vary in physiological location, TAA repertoire, vascularization, surrounding stroma, and other properties are not homogenous tissues that can be effectively treated with a single antigen epitope vaccination tactic [32]. Considering the large number of potential tumor antigens for each individual cancer, vaccination with whole tumor cells which carry the full array of tumor specific mutations and all of the patient's MHC class I and II molecules has been seen as the optimal strategy. Autologous and allogeneic tumor cells can be used as tumor cell vaccines. In addition, tumor lysate has also been identified as a cancer vaccine candidate since it can generate therapeutic anti-tumor immune responses as a source of TAAs [33]. To further improve immune response, tumor lysates are usually preloaded into the major APCs, DCs, before vaccination. DC-loaded and presented tumor antigens directly activate effector T or B cells in the germinal center, yielding improved clinical outcomes [32,[34], [35], [36]. Although a vast majority of different TAAs can be presented and targeted simultaneously using whole tumor or tumor lysate vaccines, however, the clinical application of this group of vaccine is severely limited by limited identification of TAAs, the potential tumorigenesis-related toxicity issues, as well as insufficient concentration of each antigen. The threshold level of antigen is needed for recognition by T cells, so Low concentration of antigen can lead to T cell tolerance [37]. Moreover, tumor lysates are able to provide immunoregulatory cytokines such as IL-10 and TGF-β, inducing a tolerogenic transformation [36].
2.3. DNA vaccines
DNA vaccines are closed circular DNA plasmids designed to encode different antigens as well as various other immunomodulatory molecules of interest to manipulate the resulting antitumor immune responses [38,39]. With many advantages such as simplicity, ease of manufacturing and safety, DNA vaccines have the potential to induce tumor-specific immune responses according to preclinical and clinical evidence accumulated during the last two decades [40], [41], [42]. A DNA vaccine encoding tumor associated antigen prostatic acid phosphatase (pTVG-HP) has been combined with GM-CSF (as an adjuvant) and tested in the phase II clinical trial to treat prostate cancer. Meanwhile, several other DNA vaccines against prostate cancer are being evaluated in phase I trials [38]. Aside from prostate cancer, DNA vaccines against bladder carcinoma, breast carcinoma, medullary thyroid cancer, nasopharyngeal cancer and other cancers are also widely studied in the clinical research [43]. Despite the above-mentioned promising clinical trials, the efficacy of naked DNA vaccines is still limited by low transfection rates in vivo, raising the need for ideal delivery system [44]. While non-viral particulate delivery systems have showed increased antitumor immunogenicity with encouraging results [45], [46], [47], virus vectors are still the major delivery vehicles with intrinsic capability to invade cells and to deliver manipulated genetic payloads in the clinical investigations [48].
2.4. Subunit peptide vaccine
The most common vaccination strategy is subunit peptide vaccine. Subunit vaccines either synthesized or purified from pathogens are easy to manufacture and safe to administer. Sipuleucel-T has been approved by the US Food and Drug Administration (FDA) as the first standard peptide vaccine for prostate tumors and a growing number of subunit peptide vaccines have been in the application for cancer treatment such as colorectal cancer, lung cancer, pancreatic cancer, gastric cancer and breast cancer in clinical trials [49]. However, their major drawbacks are weak immunogenicity and short-term immune responses [50], [51], [52]. Synthetic long peptides (SLPs) that is long enough to include multiple MHC class I and II epitopes can alleviate the problem [16,53]. SLPs have achieved clinical efficacy in patients with premalignant disease induced by high-risk HPV [54]. Nevertheless, peptides alone are poorly immunogenic and require the assistance of adjuvants [55]. To this end, there has been a growing focus on therapeutic strategies based on nanotechnology to enhance the potency of subunit vaccines by protection of antigen from degradation and controlling the pharmacokinetic and profile as adjuvants [14,56]
2.5. mRNA vaccine
mRNA vaccines, synthesized by in vitro transcription using a bacteriophage RNA polymerase and template DNA that encodes the antigen(s) of interest, express proteins in the cytoplasm to stimulate an immune response after internalized by host cells [57]. mRNA has recently attained focus as a promising vaccine approach. Several mRNA-based vaccines have, thus, entered clinical development [58]. mRNA vaccines have several advantages over conventional vaccines and DNA-based vaccines. mRNA vaccines enable delivery of high numbers of patient-specific antigens and costimulatory signals, show no risk of infection or insertional mutagenesis and have the potential for rapid, inexpensive and scalable manufacturing [57,59]. However, their instability and inefficiency in vivo delivery need to be addressed. The most attractive strategy to solve the above-mentioned problems is to encapsulate mRNA in biocompatible nanomaterial systems, which will be extensively described in the later chapters [60].
2.6. Personalized vaccine and neoantigen
The advent of powerful genomic sequencing technology provides the possibility of personalized vaccines targeting defined neoantigens [61], [62], [63]. Neoantigens, which are generated by somatic genetic mutations in cancer cells, can be recognized as “non-self” antigens by the immune system [64]. Personalized cancer vaccines targeting neoantigens limit the likelihood of tolerance as well as normal tissue toxicity and improve antitumor immune response compared with common cancer vaccines [65]. At present, there are mainly two kinds of personalized vaccine, RNA vaccine and synthetic long peptide vaccine. Personalized RNA mutanome vaccines developed by Sahin et al. and personalized peptide vaccines developed by Ott and his colleagues have already shown positive phase I clinical results, which is breakthrough for personalized vaccines [66,67]. For targeted delivery of personalized peptides and RNAs into DCs, liposomes can be a good choice owing to their safety, biocompatibility, manufacturability and the ability to protect personalized vaccines from degradation.
3. Effect of physicochemical properties on the immune response and nanocarriers design
Effective delivery system facilitates the encapsulated antigens to target APCs, inducing activation of Teff cells, while decreases systemic toxicity. Since different types of antigens (i.e., peptide, cell lysate, mRNA) often have their distinct physical properties, the selection of an appropriate delivery system thus becomes crucial for inducing a desired immune response. For examples, delivery systems (e.g. virus particles) for DNA vaccine must be designed to allow their efficient internalization into cell nucleus for subsequent transcription and antigen expression. Whereas, subunit peptides are often suffered from low immunogenicity and fast clearance; therefore, nano- or micro- sized delivery systems are used to improve the retention of peptide vaccines, along with the inclusion of adjuvants to enhance their immunogenicity. Furthermore, to design ideal nanocarriers with low toxicity and stronger antitumor immune response, a clear understanding of the interactions between delivery system and APCs is required.
In the current section, physicochemical properties of the delivery vehicles that may potentially affect the types of delivered antigens, the interaction with APCs and the immune response are discussed. The physicochemical properties can be summarized as follow: particle size, surface charge, particle rigidity, targeting ligand and intrinsic immunogenicity.
3.1. Particle size
The particle size of NPs may be decisive for their intracellular fate which influences the distribution, cellular uptake mechanism and the type of immune response.
Following subcutaneous, intradermal or intramuscular administration, the majority of small particles (< 20 nm) will preferably drain to blood capillaries and subsequently be eliminated; particles between 20 and 100 nm will drain into the lymphatic nodes (LNs) where they will be taken up by APCs; larger NPs (> 100 nm) will most likely stay at the injection sites until they are captured and transported to LNs by APCs [68,69]. In addition, size can affect the cellular uptake mechanism. Particles of 20-200 nm are generally taken up via endocytosis while particles with a dimension greater than 500 nm are preferentially taken up via phagocytosis [70]. Particles with size < 150 nm, in particular 50–80 nm, are generally internalized by cells via classic receptor-mediated endocytosis (clathrin-dependent or caveolae-dependent) [71]. The particle size of NPs is likely to influence their distribution and cellular uptake mechanism, thus affecting the induced immune response. Studies showed that smaller particles induced Th2 immune responses after administration while larger particles promote IFN-γ and typical Th1 responses [72,73]. Therefore, particle size is one of the key roles in regulating vaccine functions.
3.2. Surface charge
The surface charge of NPs is another important consideration for designing cancer vaccines. Dimethyldiacylammonium (DDA) and trehalose dibehenate (TDB) compose DDA:TDB, also known as CAF01, which is being evaluated in clinical trials, has been widely used as cationic liposome vaccine carrier material [74], [75], [76]. Henriksen-Lacey et al. compared the biodistribution and immunogenicity of neutral liposomes composed of distearoyl-glycero-phosphatidylcholine (DSPC) with TDB against DDA:TDB liposomes. Injection of cationic DDA:TDB liposomes loaded with antigens 85B-ESAT-6 leads to a deposition of the liposome at the site of injection (SOI) with 46% of the original dose remaining 14 d post injection. In contrast, DSPC:TDB liposomes in SOI was undetectable at Day 1 post injection. This result suggested that cationic charge is an important factor for the retention of the liposomal component at the SOI [74]. One possible explanation is that the aggregation of cationic liposomes results in a depot-effect where liposomes and antigens are retained in the SOI for an extended period of time [77]. Of note, the observation that DDA:TDB liposomes induced a more prolonged antigen presentation compared to DSPC:TDB liposomes suggests that the immune response induced by DSPC:TDB is lower than that induced by DDA:TDB. An explanation is that DDA can serve as an adjuvant to activate the pro-inflammatory signals. Indeed, Henriksen-Lacey et al. have demonstrated that DDA induced secretion of several pro-inflammatory cytokines and chemokines, facilitating consistent influx of inflammatory monocytes, macrophages and activated NK cells, all of which substantially improved both innate and adaptive immune response [74]. In addition, the cell membranes of APCs are negatively charged and they attract positively charged NPs due to the electrostatic interaction, and thus cationic NPs can lead to enhanced uptake and presentation of antigen [78,79].
Despite prolonged antigen response, functionalization of nanoparticles with cationic moieties also disrupts the integrity of the cell's plasma and vesicular membranes, leading to a dose-dependent cellular toxicity [80], so appropriate dosing levels should be finely tuned when using cationic NPs for vaccine delivery [14,81].
3.3. Particle rigidity
Particle rigidity can affect the lymphatic transportation and immune cell uptake of antigens, and thus influence the immune response. To study this, Christensen et al. compared the immune responses of DDA which forms solid ordered phase liposomes with its structural analog dimethyldioleoylammonium (DODA) that forms fluid disordered phase liposomes. Their study suggested that DDA-based rigid particles, in contrast to the soft DODA-based liposomes, were more difficult to be transported through narrow lymphtic vessels, and thus trapped in the local injection site to form a vaccine depot that continuous attracted APCs, facilitating immune cell activation. Therefore, the immune response induced by the rigid DDA-based liposomes was 2 magnitudes higher than that obtained with the fluid DODA-based liposomes [82]. The similar observation was also pointed out by Merkel et al. in their recent studies [83]. Besides forming a local immune depot, rigid NPs have been shown with an enhanced immune cell uptake. Sun et al. synthesized NPs of varying rigidity to show “hard” NPs enter immune cells more easily than flexible (“soft”) [84]. Whereas, Anselmo et al. showed that soft NPs exhibited significantly reduced cellular uptake in macrophages, endothelial cells and cancer cells [85]. Therefore, preferential immune cell uptake of rigid particles are likely to further enhance antigen presentation and immune activation. Despite all these hypothesis and observations, more in depth mechanistic studies that examine the effect of particle rigidity on the immune response are still extremely valuable and highly required in vaccine design.
3.4. Targeting ligand
Tumor antigens have to be engulfed and processed by DCs, which are initiators of adaptive immunity [86]. In addition, as long as antigens remain outside the lymphatic tissues, they will be ignored by the immune systems [70]. Therefore, cancer vaccines need a rational targeting design that achieves concentrated antigen delivery to lymphatic tissues, in particular, APCs (e.g. DCs, macrophages and B cells).
NPs co-delivering tumor antigens and other stimulatory molecules can also be modified with targeting moieties through molecular recognition (i.e., ligand-receptor interaction or antibody-antigen recognition) on their surfaces to achieve efficient DC-specific delivery. It has been shown that mannose receptor (MR), a member of calcium-dependent C-type lectin receptor (CLR) family, primarily expressed on APCs [87], [88], [89]. Wang et al. encapsulated ovalbumin (OVA) antigen with 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) cationic liposomes (LP) or DOTAP-PEG-mannose liposomes (LP-Man) to generate depot or lymphatic-targeted liposome vaccines, respectively. The result of in vivo imaging showed that LP mostly accumulated near the injection site, whereas LP-Man not only effectively accumulated in draining lymph nodes (LNs) and the spleen, but also enhanced the uptake by resident antigen-presenting cells [90]. In a similar study, Jiang et al. showed that the targeted galactosylated liposomes effectively facilitated antigen uptake by DCs as compared to that mediated by unmodified liposomes both in vitro and in vivo [91]. Moreover, receptors such as DEC-205, DC-SIGN, Fc receptor, CD40, or CD11c are expressed on DCs and often used as targets [92]. It should be noted that distinct subsets of DCs are specialized for different effects on the immune system. While the top-performing DC subset for antigen presentation along with the optimal targeting receptor for antigen uptake are still remained as overhanging questions, recent studies have suggested that combinatorial targeting of multiple DC subsets may significantly enhance the efficacy of DC targeting and antigen presentation [93].
Additionally, it is worth noting that antigens carried via NPs can enter DCs via multiple endocytosis pathways, not only depending the presence of specific targeting ligands but also their particle size, surface charge and particle rigidity discussed above. Therefore, the key to successful targeting DCs using NPs is to select the appropriate ligand against distinct DC surface receptor, as well as the appropriate modulating of their physicochemical properties to improve the potency of cancer vaccines.
3.5. Co-delivery with immunostimulatory reagents
A modern strategy for designing novel vaccines is the subunit vaccines which are highly purified and thus show reduced immunogenicity due to the absence of exogenous immune-activating components [94]. Therefore, it is necessary to add adjuvants to provide the innate immunopotentiation and to direct the desired adaptive immune response. Adjuvants have several functions to augment immune responses: (1) to activate innate immunity, (2) to steer the immune response toward Th1 or Th2 immunity, and (3) to enhance cross-presentation [95]. Toll-like receptors (TLRs) agonists have been widely investigated as adjuvants for cancer vaccines [96,97]. TLRs are regarded as pivotal pattern recognition receptors (PRRs) of innate immunity, which recognize pathogens through sensing pathogen-associated molecular patterns (PAMPs) [98]. TLRs are also crucial for induction of adaptive immune responses as they are potent DC activators, augment T-cell responses, enhance cross-presentation and downregulate suppressive effects of Tregs [70,96,99]. TLR3 is expressed in endosomes, where it can sense RNA virus infection by recognizing intracellular dsRNA [100]. Poly(inosinic-polycytidylic acid) (Poly I:C), a synthetic TLR3 ligand, has been reported as vaccine adjuvants in cancer clinical trials [96]. Han et al. developed a poly-lactic-co-glycolic acid (PLGA)-NP system encapsulating both OVA and poly I:C and this system increased DC maturation, enhanced antigen cross-presentation in DCs and induced cytotoxic T cell responses, which led to potent antitumor efficacy in EG.7 and TC-1 tumor bearing mice [101]. TLR4, a founding member of the TLR family, recognizes lipopolysaccharide (LPS) of Gram-negative bacteria that can cause septic shock [100]. Monophosphoryl lipid A (MPLA), the TLR4 agonist, has shown an excellent safety and efficacy profile in clinical as an adjuvant [96]. Boks et al. explored liposomes containing the glycan LeX for specific DC-SIGN targeting, and an adjuvant and a tumor antigen. They discovered that incorporation of MPLA into glycoliposomes significantly induced DC maturation and production of pro-inflammatory cytokines and enhanced antigen cross-presentation of the melanoma tumour antigen gp100280–288 peptide to CD8+ T cells compared to nonglycosylated MPLA liposomes [102]. Atefeh Razazan and his colleagues also discovered co-formulation of MPLA and tumour antigens not only induces high antitumor immunity but also enhances therapeutic efficacy [103]. TLR9, another endosomal TLR, recognizes unmethylated 2a-deoxyribo(cytidine-phosphateguanosine) (CpG) DNA motifs that are frequently present in bacteria and viruses [100]. Yoshizaki et al. loaded CpG-DNA into pH-sensitive polymer-modified liposomes and these liposomes exhibited strong antitumor effects compared with conventional pH-sensitive polymer-modified liposomes [104]. Similarly, Mansourian et al. encapsulated p5 HER-2/neu derived peptide (p5) in DOTAP–cholesterol–dioleoylphosphatidylethanolamine (DOPE) liposomes co-administered with CpG-ODN. DOTAP–cholesterol–DOPE liposomes + CpGODN can induce both CD8+ and CD4+ responses that significantly inhibit the tumor progression [105]. Multiple other TLR agonists have also been considered as adjuvants [98]. Moreover, it has been reported that triggering multiple TLRs could activate synergetic immune response upon a combinational agonist engagement [106,107]. For examples, both Silva et al. [108] and Fox et al. [109] have demonstrated that combining CpG and poly I:C into polyester NPs significantly improved Th1 response and facilitated cancer treatment.
4. Nanotechnology for subunit peptide delivery
Subunit peptide is the most common vaccination strategies, however, their anticancer response are limited due to weak immunogenicity and short-term immune responses; therefore, optimal delivery systems that can overcome these obstacles are greatly needed. Table 1 provides a summary of recent research on NPs as subunit peptide and mRNA cancer vaccines carriers.
Table 1.
A summary of NPs as subunit peptide and mRNA cancer vaccines carriers.
| Forms | Formulations | Antigen used | Tumor model | Injection route | Preclinical or clinical | Ref. |
|---|---|---|---|---|---|---|
| Subunit peptide vaccine | Lipid NPs | Synthetic long peptides | None | Intradermally | Preclinical | [111] |
| OVA | E.G7-OVA tumor | Subcutaneously | Preclinical | [20,112] | ||
| Trp2 peptide | Melanoma | Subcutaneously | Preclinical | [113] | ||
| Polymeric NPs | OVA | E.G7-OVA | Subcutaneously | Preclinical | [114] | |
| TRP2 | Melanoma | Subcutaneously | Preclinical | [115] | ||
| OVA | Subcutaneously | Preclinical | [116] | |||
| OVA/Gp100/Trp1/ Trp2Obsl1Kif18b/Def8/ Reps1/Adpgk/Dpagt1 | Melanoma/colon cancer | Subcutaneously | Preclinical | [117] | ||
| Protein conjugate | E7 peptide/Trp2 | TC-1 tumors/ melanomas | Subcutaneously | Preclinical | [118] | |
| Gold | OVA | EG7-OVA | Subcutaneously | Preclinical | [119] | |
| SIINFEKL peptide | None | Intradermally | Preclinical | [120] | ||
| Aluminum | OVA | Melanoma | Subcutaneously | Preclinical | [121] | |
| Miconeedles | OVA | Melanoma | Intradermally | Preclinical | [122] | |
| OVA | Intradermally | Preclinical | [123] | |||
| mRNA vaccine | Lipid NPs | MART-1 | Melanoma | Intravenously | Preclinical | [124] |
| gp100 and TRP2 | Melanoma | Subcutaneously | Preclinical | [125] | ||
| NY-ESO-1, MAGE-A3, tyrosinase and TPTE | Melanoma | Intravenously | Clinical | [126] | ||
| OVA | None | Intravenously | Preclinical | [127] |
4.1. Liposomes and lipid NPs
Among the particulate drug delivery systems used for antigen delivery, liposomes were the first marketed system. The success clinical translation of liposomal vaccines and the increasing interest in their development can be attributed to key advantages such as safety and versatility. Marketed liposome-based drugs such as DoxiIⓇ (Johnson & Johnson) and AmBisomeⓇ (Gilead Sciences) and vaccine preparations like EpaxalⓇ and InflexalⓇ (Crucell, Leiden, The Netherlands) show that liposomes are safe and well tolerated [110], [111], [112], [113]. They have the capability of entrapping both lipophilic and hydrophilic agents, in the lipid membrane and in the aqueous core, respectively [114]. This versatility allows antigens of all types to be incorporated in liposome formulations [115]. The versatility is also reflected in active targeting with the use of ligands and combination of immunomodulators, such as TLR agonists or other PRR agonists to enhance immunological response. Liposomes as carriers of peptide vaccine have attracted wide attention. Varypataki et al. encapsulated a synthetic long peptide (SLP) harboring the model OVA peptide with poly I:C in cationic liposomes consisting of DOTAP and 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC). Results showed that the liposomal formulation efficiently delivered the SLP to DCs in vitro and induced a functional CD8+ T cell immune response in vivo to the CTL epitope present in the SLP at least 25 fold increase over the poly I:C-adjuvanted soluble SLP [116]. Eiji Yuba and his colleagues developed pH-sensitive fusogenic DOPE/egg yolk phosphatidylcholine (EYPC) liposomes using two types of pH-sensitive poly(glycidol) derivatives with either a linear (MGlu-LPG) or hyperbranched (MGlu-HPG) structure. Results revealed that these polymer-modified liposomes can introduce fluorescently tagged antigen (FITC-OVA) into cytosol of DCs efficiently, inducing DC maturation and efficient antigen presentation largely through MHC-I molecules, resulting extensive shrinkage of tumor burden [20]. To meet the requirements of biosafety and biodegradation from clinical application, Eiji Yuba and his colleagues used pH-sensitive dextran derivatives (MGlu-attached dextran: MGlu-Dex) to prepare pH-sensitive liposomes. MGlu–Dex modified liposomes also exhibited pH-sensitive significant destabilization in the weakly acidic pH region and delivered their contents efficiently into the cytosol [117].
Lipid NP is a pivotal biocompatible and biodegradable drug delivery and formulation platform like liposomes [118]. Xu et al. developed lipid–calcium–phosphate (LCP) NPs that consist of a calcium phosphate core and an asymmetrical lipid bilayer used as a Trp2 peptide vaccine delivery system for melanoma treatment. Two phosphor-serine residues were added to the N-terminal of the peptide, increasing the efficiency of p-Trp2 peptide to co-precipitate with calcium phosphate. CpG ODN was also encapsulated in LCPs modified with mannose. Compared with free Trp2 peptide/CpG, vaccination with LCP elicited a strong antigen-specific CTL response, resulting in superior inhibition of tumor growth in both B16F10 subcutaneous and lung metastasis models [119].
4.2. Inorganic particles
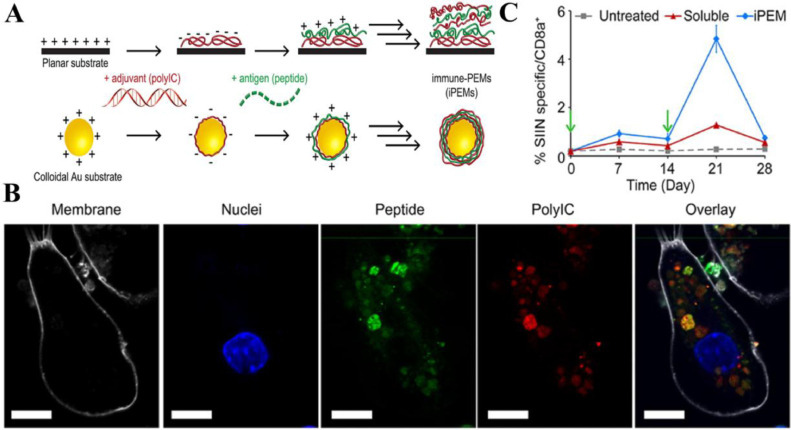
Targeting TAAs to solid tumors using inorganic particles has been an area of sustained interest. Gold nanoparticles (GNPs) have garnered particular interest as nanomaterials for vaccines delivery carriers since GNPs are nontoxic, immunologically inert and can be synthesized with well-controlled properties (e.g., size and shape) [120], [121], [122], [123]. Because of facile synthesis with tunable size, Kang et al. explored the effect of OVA-carrying GNPs with three different diameters (10, 22, and 33 nm) on the efficiency of delivery to draining LNs and induction of CD8+ T-cell responses. Results revealed that 22- and 33-nm OVA-GNPs were superior to 10-nm OVA-GNPs in both LN delivery and induction of CD8+ T-cell responses [124]. Zhang et al. developed a new vaccine platform based on GNPs. Immune polyelectrolyte multilayers (iPEM) (including anionic poly I:C and cationic antigen peptides) were self-assembled on gold nanoparticle templates (iPEM-GNPs) via the “layer-by-layer” strategy without solvents and other structural components, which promoted high levels of antigen-specific CD8+ T cells (Fig. 1) [125].
Fig. 1.
iPEM assembled on gold nanoparticle templates promoted antigen-specific T Cell response. (A) The anionic adjuvant and cationic antigen peptides were self-assembled on gold nanoparticle templates via electrostatic interactions. (B) Confocal microscopy images demonstrated high levels of peptide and poly I:C located within DCs following a 3 h incubation with iPEM-GNPs. White: cell membrane; blue: nucleus; Green: peptide; Red: polyI:C; scale bars are 10 µm. (C) Mice were immunized with simple mixtures of soluble antigen and adjuvant or iPEM-GNPs on day 0 then boosted on day 14. Frequency of CD8+ T cells in peripheral blood over 28 days demonstrated immunization with iPEM-GNPs promoted efficient primary and secondary CD8+ T cell responses in mice. Reproduced with permission from [120]. Copyright 2015 American Chemical Society.
Aluminum-containing adjuvants are widely used and approved by the United States FDA for human use. The traditional aluminum hydroxide forms particulate of 1–20 µm in an aqueous solution with weakly or moderately potentiating antigen-specific antibody responses. Using OVA and Bacillus anthracis protective antigen protein as model antigens, Li et al. synthesized aluminum hydroxide NPs of 112 nm and showed that antigens adsorbed on the aluminum hydroxide NPs induced a stronger antigen-specific antibody response than on microparticles and local inflammation induced by NPs in the injection sites was milder than that induced by microparticles. Aluminum hydroxide NPs represents an effective and safe approach to improve its adjuvanticity [126].
Other inorganic NPs (such as iron oxide magnetic NPs and upconversion NPs) have been shown to be potential delivery vehicles for antigens/adjuvants for tracking and vaccination [127], [128], [129]. One additional important consideration is that inorganic particles may accumulate in the body, resulting long-term toxicity.
4.3. Albumin peptide conjugates or NPs
Due to ready availability, ease of chemical modification and good biocompatibility, albumin-based drug delivery systems (DDSs) have captured massive attentions in the recent decades. Both human serum albumin (HSA) and bovine serum albumin (BSA) have been utilized to prepare albumin NPs, through methods such as desolvation, emulsification, self-assembly, thermal gelation [130,131]. Several albumin-based therapeutic agents have been approved by FDA [130]. Liu and his colleagues noticed that compounds which bind avidly to serum albumin can be delivered to draining LNs. Utilizing this property, they designed LN-targeting molecular vaccines via the synthesis of amphiphiles (amph-vaccines) comprised of an antigen (or adjuvant) cargo linked to a lipophilic albumin-binding tail. Upon ex-vivo binding with albumin, this novel vaccines exhibited 30-fold increases in T-cell priming, leading to enhanced anti-tumor efficacy and reduced systemic toxicity as compared to the parent compounds [132]. In another study, Zhu et al. developed albumin/vaccine nanocomplexes in situ utilizing the exogenous vaccine components (CpG and SIINFEKL) self-assembled with the endogenous albumin. This nanocomplexes significantly improved the accumulation of antigens and CpG in lymph nodes, which was ∼100-fold higher than benchmark incomplete Freund's adjuvant (IFA). Consequently, the nanocomplexes induced ∼10-fold more frequent peripheral antigen specific CTLs response than IFA-emulsifying vaccines [133]. To understand the mechanisms of albumin-binding carriers boosting the vaccine mediated CTL response, Wang et al. demonstrated that albumin not only facilitated the accumulation of antigens in draining LNs, but also enhanced internalization of antigens to APCs and improved the stability of antigens in acidic intracellular organelles [134].
4.4. Polymer NPs
Benefiting from the development of biodegradable polymers which represents a revolution in medicine spanning over 50 years, biotechnological advancements have made great progress in drug delivery [135]. Polymers can be from natural origin, as chitosan, gamma polyglutamic acid (γ-PGA), hyaluronic acid or synthesized, as polyethyleneimine, polylactic acid, Polypropylene sulfide, acrylic acid based polymers, and PLGA. The advantages of polymers (such as biodegradability, low toxicity and biocompatibility) have highlighted polymeric NPs as an ideal delivery strategy. Because of stability, targeted and controlled release and the ability to entrap and/or adsorb both hydrophilic and hydrophobic molecules, polymeric NPs are attractive platforms in cancer vaccines. In fact, numerous polymeric NPs such as chitosan, γ-PGA, PLGA and acrylic acid based polymers have been shown to enhance antitumor immune response [136], [137], [138], [139]. In addition, Luo et al. developed a synthetic polymeric nanoparticle (PC7A NP) which was prepared following a solvent evaporation method using synthetic methacrylate monomers and PEG-b-PR block copolymers. The minimalist nanovaccine comprised a simple physical mixture of an antigen and PC7A NP, which enhances antigen delivery and cross-presentation and stimulates the STING pathway to boost antitumor immunity. Furthermore, synergy with anti- programmed cell death protein 1 (PD-1) antibody showed great results with 100% survival over 60 d in a TC-1 tumour model [140].
4.5. Miconeedles
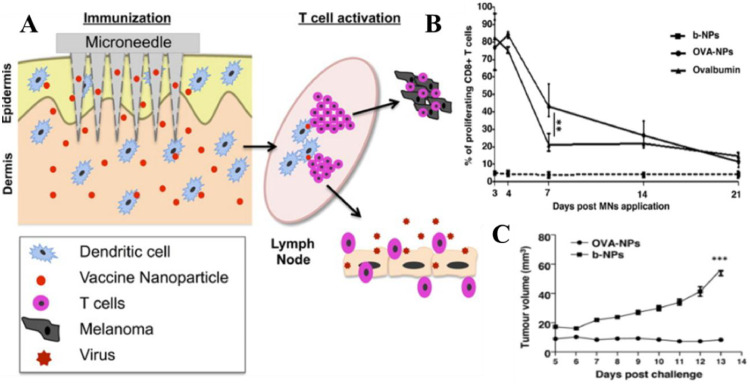
Appropriate vaccine administration is the key element to ensure powerful immune response [141]. Most vaccines are injected via the subcutaneous (SC) or intramuscular (IM) routes using a hypodermic needle. This approach is low-cost, rapid and direct, but inevitably associated with numerous unwanted disadvantages such as low patient compliance, safe needle disposal, potential contamination and need for trained personnel for administration [142]. In addition, antigen delivery by hypodermic injection will bypass the skin's immune cells leading to poor vaccination because subcutaneous fat and muscle tissue contain relatively few DCs [141]. In contrast, microneedles (MNs), needle-like structures, can be simple for patients to apply for transdermal delivery of biomacromolecules across the stratum corneum barrier. Because the dermis and the epidermis are densely populated by different subsets of DCs, delivery of antigen by microneedle arrays (MAs) has the potential for greater immunogenicity. MAs designed for vaccine delivery are mostly dissolving ones which are usually fabricated with biocompatible materials [143,144]. Antigen and/or adjuvant can be encapsulated into multifunctional particles (such as liposomes and polymeric particles) to form the multifunctional particle-constructed MAs (MPMAs), which can achieve targeting delivery and controlled release [144]. Zaric et al. showed that antigen (OVA)-encapsulated PLGA NPs can be successfully delivered to skin layers by dissolving MAs. This delivery system facilitates the specific targeting of skin DCs which were able to deliver NPs to cutaneous draining lymph nodes via the afferent lymphatics, leading to potent activation of antigenspecific IFN-γ secreting effector CD4+ and CD8+ T cells and attenuated tumor growth (Fig. 2) [145]. Zhao and his colleagues did a similar study. OVA- and platycodin (PD)-loaded liposomes (OVA-PD-Lipos) were incorporated into dissolving MAs. This system was chemically stable and showed a significantly enhanced immune response [146]. Overall, the dissolving MAs- based system is an attractive approach to improve efficacy of subunit vaccine delivery.
Fig. 2.
MAs with OVA-NPs induce efficient antitumor responses. (A) Schematic of the minimalist design of the MAs with OVA-NPs. (B) Microneedle immunization with OVA-NPs significantly increased proliferation of OVA-specific CD8+ T cells compared to soluble OVA and b-NPs (blank NPs). (C) None of the mice immunized with MNs loaded with OVA-NPs developed significant tumor growth, while mice immunized with b-NP displayed significant tumor growth. Reproduced with permission from [122]. Copyright 2013 American Chemical Society.
5. Nanotechnology for mRNA cancer vaccine delivery
Clinical trials with direct administration of synthetic mRNAs encoding tumor antigens demonstrated safety and induction of tumor-specific immune responses [147]. However, naked mRNAs generate weak immune response, so delivery system for better immune responses and minimal toxicity is vital and challenging. Delivery system must meet the following requirements: (1) protect mRNA from degradation by endonucleases; (2) target APCs; (3) induce endosomal escape before degradation [148].
5.1. Lipid NPs
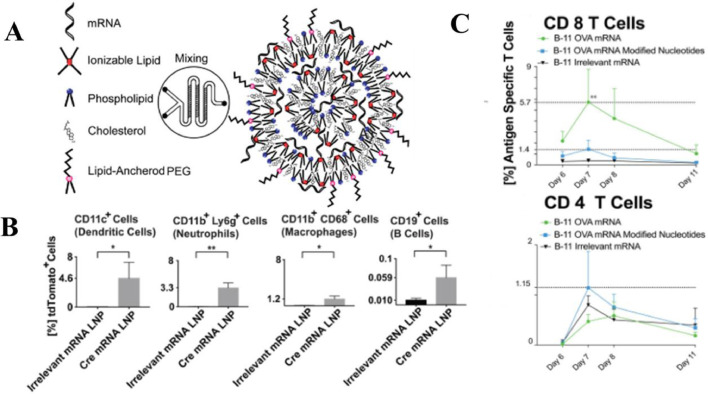
Lipid NPs are highly versatile as their characteristics can be altered to increase delivery efficiency and tailor the immune response and they have been successfully used to deliver mRNA cancer vaccines [149], [150], [151], [152]. Oberli et al. developed lipid NPs containing mRNA coding for the tumor-associated antigens gp100 and TRP2. In this formulation, the ionizable lipid is positively charged at low pH may also help with cellular uptake and endosomal escape and the PEGylated lipid hinders NPs aggregation. The optimized formulation achieved efficient transfection in different immune cellpopulations (including dendritic cells, macrophages, neutrophils, and B cells) and antigen presentation on MHC-I, leading to a strong CD 8 T cell activation (Fig. 3) [148]. The administration route of mRNA lipid NPs might have a significant influence on the strength of the ensuing immune response. Via intravenous administration, RNA-lipoplexes (RNA-LPX) developed by Kranz et al. can target DCs precisely and effectively in vivo. This system is based on well-known lipid carriers such as DOTMA, DOTAP, DOPE and cholesterol, without the need for functionalization of particles with molecular ligands, solely with appropriate negative net charge. RNA-LPX mediates its efficient uptake and expression of the encoded antigen by DC populations and macrophages in various lymphoid compartments and induces strong effector and memory T-cell responses leading to rejection of progressive tumors. In addition, RNA-LPX has been in clinical phase I trial [153]. Broos et al. also developed systemic delivery of lipid mRNA particles (LMPs) which are uptake by various APCs and result in Strong Antigen-specifc T-cell Responses [154].
Fig. 3.
Lipid NPs for mRNA vaccine delivery against cancer. (A) The lipid nanoparticles were prepared by microfluidic device via mixing the aqueous phase containing the mRNA and the ethanol phase containing the lipophilic compounds. (B) Ai14D reporter mice were immunized with lipid NPs containing mRNA coding for Cre-recombinase. Quantification of the percentage of transfected cells indicated that mRNA lipid NPs could transfect different APCs. (C) The percentage of OVA specific CD8 T cells induced by mRNA lipid NPs was significantly higher than the other two groups. Reproduced with permission from [125]. Copyright 2016 American Chemical Society.
5.2. Polymer or dendrimer NPs
Lipid-polymer hybrid NPs (LPNs) have emerged as an interesting vaccine delivery system which combines the advantages of polymeric NPs with physical stability and liposomes with biomimetic characteristics. Su et al. developed biodegradable core-shell structured NPs with a pH-responsive poly(β-amino-ester) (PBAE) core enveloped by a phospholipid bilayer shell to deliver mRNA vaccines. This system mediated cytosolic delivery of mRNA with low cytotoxicity and intranasal administration of these particles greatly improved the expression of reporter protein luciferase [155].
Dendrimers are highly branched polymers. Their architecture comprises with three distinct domains: a central core, repetitive branching units, and terminal groups, which provide modifiable surface functionalities [156]. Among numerous classes of dendrimers, polyamidoamine (PAMAM) and polypropyleneimine (PPI) based dendrimers are undeniably the most employed vectors which have gained tremendous attention [157]. Chahal et al. synthesized new alkylated dendrimer by reacting PAMAM G1 dendrimer with 2-tridecyl-oxirane to form NPs encapsulating mRNA together with lipid-anchored PEG. This system successfully induced antibody production and antigen-specific CTLs [158]. There are few studies about polymer or dendrimer NPs as mRNA cancer vaccine carriers, and this potential needs further research.
6. Summary and future perspective
Cancer vaccines are required to be safe, effective and affordable, which benefit greatly from NPs due to their safety, controlled release, targeting to DCs and improved antigen uptake as well as enhanced immunogenicity. Here we reviewed the considerations when designing NPs as cancer vaccine delivery systems. The physicochemical properties such as size, zeta potential, particle rigidity, targeting ligand and intrinsic immunogenicity have been shown to play crucial roles in the modulation of antigen distribution, cellular uptake mechanism, antigen presentation and the type and strength of immune response. So it is important to take these characteristics into account to induce maximal antitumor responses. In addition, we discussed recent progress on nanotechnology for cancer vaccine delivery, we envision this review will serve as a guidance for future cancer vaccine design.
Despite this, tumor progression is a complicated balance between anti-tumor response induced by the host immune system and the suppressive immune microenvironment [86]. Therefore, the combination of cancer vaccines, together with treatments that restrict the cancer microenvironment would further improve the immunotherapeutic outcome, and have been extensively evaluated both preclinically and clinically [15]. One of such approaches is the combination of cancer vaccines with checkpoint inhibitor blockades. It is well known that ligation of PD-1 with PD-L1 leads to T cell dysfunction, exhaustion and tolerance [159]. Blocking these inhibitory pathways should result in amplification of the T cell-mediated immune response, latter of which considered as the main effector of cancer vaccines. Strategies to improve effective T cells infiltration while block the functions of suppressive immune cells within tumor microenvironment would be another candidate for combining with cancer vaccine. Several literature research and clinical studies have suggested that over 70% of cancers (especially solid tumors) are poorly infiltrated by CD8+ T cells, which are the leading causes of therapeutic failure, not only for cancer vaccines, but also for checkpoint blockades and CAR-T therapies [160]. Small kinase inhibitors, antibodies or RNAi that modulate the suppressive immune environment (i.e., siRNA against STAT3, small molecules against CXCR4, tyrosine kinase inhibitor against vascular endothelial growth factor) have been showed to significantly improved tumor regression and survival [161], [162], [163], [164]. Another viable option for combination treatment is the addition of chemotherapeutic agents. Chemotherapeutic agents (such as cisplatin [165], docetaxel [166], doxorubicin [167]) have immunomodulatory properties that can enhance vaccine-mediated antitumor immune responses. The mechanisms of synergy depend on the type of drugs and the specific vaccine employed, as well as the dosing schedule of each modality [168]. The versatility of nanocarriers may help combination therapy achieve their full potential. It is worth noting that the timing of combination and the selection of more powerful cancer vaccines are critical parameters that can profoundly affect the clinical antitumor effect.
In conclusion, NPs are good candidates for delivering cancer vaccines due to their safety and versatility. The design considerations discussed in the review provide guidance for improving cancer vaccine potency. However, due to the extensive suppressive immune microenvironment, cancer vaccines alone are difficult to prevent disease recurrence, which requires further tuning of the suppressive tumor microenvironment to improve T cell penetration and activation in situ. Therefore, we envision that the combination therapy together with smart NPs design would be the way to go in the future development of cancer vaccines.
Conflicts of interest
The authors have no competing interests.
Acknowledgments
This work was supported by Shandong Province major scientific and technological innovation projects: the key technology of advanced pharmaceutics and delivery system (2018CXGC1411).
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Parhi P., Mohanty C., Sahoo S.K. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today. 2012;17(17–18):1044–1052. doi: 10.1016/j.drudis.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Saraswathy M., Gong S. Different strategies to overcome multidrug resistance in cancer. Biotechnol Adv. 2013;31(8):1397–1407. doi: 10.1016/j.biotechadv.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Ascierto P.A., Daniele B., Hammers H., Hirsh V., Kim J., Licitra L. Perspectives in immunotherapy: meeting report from the “immunotherapy bridge”, napoli, november 30th 2016. J Transl Med. 2017;15(1):205. doi: 10.1186/s12967-017-1309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P., Wagner K., Wolchok J.D., Allison J.P. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11(11):805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer J.R., Govindan R., Anders R.A., Antonia S.J., Sagorsky S., Davies M.J. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC) J Immunother Cancer. 2018;6(1):75. doi: 10.1186/s40425-018-0382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietrich B., Srinivas S. Urothelial carcinoma: the evolving landscape of immunotherapy for patients with advanced disease. Res Rep Urol. 2018;10:7–16. doi: 10.2147/RRU.S125635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rini B.I., McDermott D.F., Hammers H., Bro W., Bukowski R.M., Faba B. Society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of renal cell carcinoma. J Immunother Cancer. 2016;4:81. doi: 10.1186/s40425-016-0180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan R.J., Atkins M.B., Kirkwood J.M., Agarwala S.S., Clark J.I., Ernstoff M.S. An update on the society for immunotherapy of cancer consensus statement on tumor immunotherapy for the treatment of cutaneous melanoma: version 2.0. J Immunother Cancer. 2018;6(1):44. doi: 10.1186/s40425-018-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ascierto P.A., Agarwala S.S., Ciliberto G., Demaria S., Dummer R., Duong C.P.M. Future perspectives in melanoma research “melanoma bridge”, napoli, November 30th–3rd December 2016. J Transl Med. 2017;15(1):236. doi: 10.1186/s12967-017-1341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karaki S., Anson M., Tran T., Giusti D., Blanc C., Oudard S. Is there still room for cancer vaccines at the era of checkpoint inhibitors. Vaccines (Basel) 2016;4(4) doi: 10.3390/vaccines4040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z., Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark Res. 2018;6:4. doi: 10.1186/s40364-018-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolhassani A., Safaiyan S., Rafati S. Improvement of different vaccine delivery systems for cancer therapy. Mol Cancer. 2011;10:3. doi: 10.1186/1476-4598-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia Y., Omri A., Krishnan L., McCluskie M.J. Potential applications of nanoparticles in cancer immunotherapy. Hum Vacc Immunother. 2017;13(1):63–74. doi: 10.1080/21645515.2016.1245251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melief C.J., van Hall T., Arens R., Ossendorp F., van der Burg S.H. Therapeutic cancer vaccines. J Clin Investig. 2015;125(9):3401–3412. doi: 10.1172/JCI80009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butterfield L.H. Cancer vaccines. BMJ. 2015;350:h988. doi: 10.1136/bmj.h988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen D.S., Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Schlom J. Therapeutic cancer vaccines: Current status and moving forward. J Natl Cancer Inst. 2012;104(8):599–613. doi: 10.1093/jnci/djs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon J.R., Ma Y., Churchman L., Gordon S.A., Dawicki W. Regulatory dendritic cells for immunotherapy in immunologic diseases. Front Immunol. 2014;5:7. doi: 10.3389/fimmu.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuba E., Harada A., Sakanishi Y., Watarai S., Kono K. A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials. 2013;34(12):3042–3052. doi: 10.1016/j.biomaterials.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 21.Antony P.A., Piccirillo C.A., Akpinarli A., Finkelstein S.E., Speiss P.J., Surman D.R. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring t regulatory cells. J Immunol. 2005;174(5):2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung K., Hayashi R., Lafond-Walker A., Lowenstein C., Pardoll D., Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188(12):2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toes R.E., Ossendorp F., Offringa R., Melief C.J. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189(5):753–756. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marzo A.L., Kinnear B.F., Lake R.A., Frelinger J.J., Collins E.J., Robinson B.W. Tumor-specific CD4+ T cells have a major “post-licensing” role in ctl mediated anti-tumor immunity. J Immunol. 2000;165(11):6047–6055. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- 25.Palucka K., Ueno H., Fay J., Banchereau J. Dendritic cells and immunity against cancer. J Intern Med. 2011;269(1):64–73. doi: 10.1111/j.1365-2796.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barinov A., Galgano A., Krenn G., Tanchot C., Vasseur F., Rocha B. CD4/CD8/dendritic cell complexes in the spleen: CD8+ T cells can directly bind CD4+ T cells and modulate their response. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0180644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alloatti A., Kotsias F., Magalhaes J.G., Amigorena S. Dendritic cell maturation and cross‐presentation: timing matters! Immunol Rev. 2016;272(1):97–108. doi: 10.1111/imr.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Burg S.H., Arens R., Ossendorp F., van Hall T., Melief C.J. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16(4):219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 29.Tagliamonte M., Petrizzo A., Tornesello M.L., Buonaguro F.M., Buonaguro L. Antigen-specific vaccines for cancer treatment. Hum Vacc Immunother. 2014;10(11):3332–3346. doi: 10.4161/21645515.2014.973317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang R.F., Wang H.Y. Immune targets and neoantigens for cancer immunotherapy and precision medicine. Cell Res. 2017;27(1):11–37. doi: 10.1038/cr.2016.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melero I., Gaudernack G., Gerritsen W., Huber C., Parmiani G., Scholl S. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11(9):509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 32.Butterfield L.H. Dendritic cells in cancer immunotherapy clinical trials: are we making progress? Front Immunol. 2013;4:454. doi: 10.3389/fimmu.2013.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joshi V.B., Geary S.M., Gross B.P., Wongrakpanich A., Norian L.A., Salem A.K. Tumor lysate-loaded biodegradable microparticles as cancer vaccines. Exp Rev Vacc. 2014;13(1):9–15. doi: 10.1586/14760584.2014.851606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koido S. Dendritic-tumor fusion cell-based cancer vaccines. Int J Mol Sci. 2016;17(6):868. doi: 10.3390/ijms17060828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rainone V., Martelli C., Ottobrini L., Biasin M., Texido G., Degrassi A. Immunological characterization of whole tumour lysate-loaded dendritic cells for cancer immunotherapy. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0146622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez F.E., Gleisner A., Falcon-Beas F., Osorio F., Lopez M.N., Salazar-Onfray F. Tumor cell lysates as immunogenic sources for cancer vaccine design. Hum Vacc Immunother. 2014;10(11):3261–3269. doi: 10.4161/21645515.2014.982996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coulie P.G., Van den Eynde B.J., van der Bruggen P., Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14(2):135–146. doi: 10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- 38.Zahm C.D., Colluru V.T., McNeel D.G. DNA vaccines for prostate cancer. Pharmacol Ther. 2017;174:27–42. doi: 10.1016/j.pharmthera.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang B., Jeang J., Yang A., Wu T.C., Hung C.F. DNA vaccine for cancer immunotherapy. Hum Vacc Immunother. 2014;10(11):3153–3164. doi: 10.4161/21645515.2014.980686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rinaldi M., Signori E., Rosati P., Cannelli G., Parrella P., Iannace E. Feasibilty of in utero DNA vaccination following naked gene transfer into pig fetal muscle: Transgene expression, immunity and safety. Vaccine. 2006;24(21):4586–4591. doi: 10.1016/j.vaccine.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 41.Fioretti D., Iurescia S., Fazio V.M., Rinaldi M. DNA vaccines: Developing new strategies against cancer. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/174378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colluru V.T., Johnson L.E., Olson B.M., McNeel D.G. Preclinical and clinical development of DNA vaccines for prostate cancer. Urol Oncol. 2016;34(4):193–204. doi: 10.1016/j.urolonc.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pol J., Bloy N., Obrist F., Eggermont A., Galon J., Herve Fridman W. Trial watch: DNA vaccines for cancer therapy. Oncoimmunology. 2014;3(1):e28185. doi: 10.4161/onci.28185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Senovilla L., Vacchelli E., Garcia P., Eggermont A., Fridman W.H., Galon J. Trial watch. OncoImmunology. 2014;2(4):e23803. doi: 10.4161/onci.23803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fotoran W.L., Santangelo R., de Miranda B.N.M., Irvine D.J., Wunderlich G. DNA-loaded cationic liposomes efficiently function as a vaccine against malarial proteins. Mol Ther Methods Clin Dev. 2017;7:1–10. doi: 10.1016/j.omtm.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garu A., Moku G., Gulla S.K., Chaudhuri A. Genetic immunization with in vivo dendritic cell-targeting liposomal DNA vaccine carrier induces long-lasting antitumor immune response. Mol Ther. 2016;24(2):385–397. doi: 10.1038/mt.2015.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srinivas R., Garu A., Moku G., Agawane S.B., Chaudhuri A. A long-lasting dendritic cell DNA vaccination system using lysinylated amphiphiles with mannose-mimicking head-groups. Biomaterials. 2012;33(26):6220–6229. doi: 10.1016/j.biomaterials.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Hardee C.L., Arevalo-Soliz L.M., Hornstein B.D., Zechiedrich L. Advances in non-viral DNA vectors for gene therapy. Genes (Basel) 2017;8(2):65. doi: 10.3390/genes8020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao Y.F., Jie M.M., Li B.S., Hu C.J., Xie R., Tang B. Peptide-based treatment: a promising cancer therapy. J Immunol Res. 2015;2015 doi: 10.1155/2015/761820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahdev P., Ochyl L.J., Moon J.J. Biomaterials for nanoparticle vaccine delivery systems. Pharm Res. 2014;31(10):2563–2582. doi: 10.1007/s11095-014-1419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perrie Y., Crofts F., Devitt A., Griffiths H.R., Kastner E., Nadella V. Designing liposomal adjuvants for the next generation of vaccines. Adv Drug Deliv Rev. 2016;99(Pt A):85–96. doi: 10.1016/j.addr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Azmi F., Ahmad Fuaad A.A.H., Skwarczynski M., Toth I. Recent progress in adjuvant discovery for peptide-based subunit vaccines. Hum Vacc Immunother. 2013;10(3):778–796. doi: 10.4161/hv.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosalia R.A., Quakkelaar E.D., Redeker A., Khan S., Camps M., Drijfhout J.W. Dendritic cells process synthetic long peptides better than whole protein, improving antigen presentation and t-cell activation. Eur J Immunol. 2013;43(10):2554–2565. doi: 10.1002/eji.201343324. [DOI] [PubMed] [Google Scholar]
- 54.Melief C.J., van der Burg S.H. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8(5):351–360. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 55.Varypataki E.M., Silva A.L., Barnier-Quer C., Collin N., Ossendorp F., Jiskoot W. Synthetic long peptide-based vaccine formulations for induction of cell mediated immunity: A comparative study of cationic liposomes and PLGA nanoparticles. J Control Release. 2016;226:98–106. doi: 10.1016/j.jconrel.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 56.Bolhassani A., Javanzad S., Saleh T., Hashemi M., Aghasadeghi M.R., Sadat S.M. Polymeric nanoparticles: Potent vectors for vaccine delivery targeting cancer and infectious diseases. Hum Vaccin Immunother. 2014;10(2):321–332. doi: 10.4161/hv.26796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McNamara M.A., Nair S.K., Holl E.K. RNA-based vaccines in cancer immunotherapy. J Immunol Res. 2015;2015 doi: 10.1155/2015/794528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sahin U., Kariko K., Tureci O. mRNA-based therapeutics-developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 59.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phua K.K., Nair S.K., Leong K.W. Messenger RNA (mRNA) nanoparticle tumour vaccination. Nanoscale. 2014;6(14):7715–7729. doi: 10.1039/c4nr01346h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gubin M.M., Zhang X., Schuster H., Caron E., Ward J.P., Noguchi T. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patch A.M., Christie E.L., Etemadmoghadam D., Garsed D.W., George J., Fereday S. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521(7553):489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 63.Waddell N., Pajic M., Patch A.M., Chang D.K., Kassahn K.S., Bailey P. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kiyotani K., Chan H.T., Nakamura Y. Immunopharmacogenomics towards personalized cancer immunotherapy targeting neoantigens. Cancer Sci. 2018;109(3):542–549. doi: 10.1111/cas.13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu Y.C., Robbins P.F. Cancer immunotherapy targeting neoantigens. Semin Immunol. 2016;28(1):22–27. doi: 10.1016/j.smim.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sahin U., Derhovanessian E., Miller M., Kloke B.P., Simon P., Lower M. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 67.Ott P.A., Hu Z., Keskin D.B., Shukla S.A., Sun J., Bozym D.J. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Correia-Pinto J.F., Csaba N., Alonso M.J. Vaccine delivery carriers: insights and future perspectives. Int J Pharm. 2013;440(1):27–38. doi: 10.1016/j.ijpharm.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 69.Fan Y., Moon J.J. Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy. Vaccines. 2015;3(3):662–685. doi: 10.3390/vaccines3030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saleh T., Shojaosadati S.A. Multifunctional nanoparticles for cancer immunotherapy. Hum Vacc Immunother. 2016;12(7):1863–1875. doi: 10.1080/21645515.2016.1147635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conniot J., Silva J.M., Fernandes J.G., Silva L.C., Gaspar R., Brocchini S. Cancer immunotherapy: nanodelivery approaches for immune cell targeting and tracking. Front Chem. 2014;2:105. doi: 10.3389/fchem.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mann J.F., Shakir E., Carter K.C., Mullen A.B., Alexander J., Ferro V.A. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine. 2009;27(27):3643–3649. doi: 10.1016/j.vaccine.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 73.Badiee A., Khamesipour A., Samiei A., Soroush D., Shargh V.H., Kheiri M.T. The role of liposome size on the type of immune response induced in BALB/c mice against leishmaniasis: Rgp63 as a model antigen. Exp Parasitol. 2012;132(4):403–409. doi: 10.1016/j.exppara.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 74.Henriksen-Lacey M., Christensen D., Bramwell V.W., Lindenstrom T., Agger E.M., Andersen P. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a cmi response. J Control Release. 2010;145(2):102–108. doi: 10.1016/j.jconrel.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 75.Henriksen-Lacey M., Devitt A., Perrie Y. The vesicle size of DDA:TDB liposomal adjuvants plays a role in the cell-mediated immune response but has no significant effect on antibody production. J Control Release. 2011;154(2):131–137. doi: 10.1016/j.jconrel.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 76.Tian M., Zhou Z., Tan S., Fan X., Li L., Ullah N. Formulation in DDA-MPLA-TDB liposome enhances the immunogenicity and protective efficacy of a DNA vaccine against mycobacterium tuberculosis infection. Front Immunol. 2018;9:310. doi: 10.3389/fimmu.2018.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Henriksen-Lacey M., Bramwell V.W., Christensen D., Agger E.M., Andersen P., Perrie Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J Control Release. 2010;142(2):180–186. doi: 10.1016/j.jconrel.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 78.Foged C., Brodin B., Frokjaer S., Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298(2):315–322. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 79.Korsholm K.S., Agger E.M., Foged C., Christensen D., Dietrich J., Andersen C.S. The adjuvant mechanism of cationic dimethyldioctadecylammonium liposomes. Immunology. 2007;121(2):216–226. doi: 10.1111/j.1365-2567.2007.02560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McConnell K.I., Shamsudeen S., Meraz I.M., Mahadevan T.S., Ziemys A., Rees P. Reduced cationic nanoparticle cytotoxicity based on serum masking of surface potential. J Biomed Nanotechnol. 2016;12(1):154–164. doi: 10.1166/jbn.2016.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krishnamachari Y., Geary S.M., Lemke C.D., Salem A.K. Nanoparticle delivery systems in cancer vaccines. Pharm Res. 2011;28(2):215–236. doi: 10.1007/s11095-010-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Christensen D., Henriksen-Lacey M., Kamath A.T., Lindenstrom T., Korsholm K.S., Christensen J.P. A cationic vaccine adjuvant based on a saturated quaternary ammonium lipid have different in vivo distribution kinetics and display a distinct CD4 T cell-inducing capacity compared to its unsaturated analog. J Control Release. 2012;160(3):468–476. doi: 10.1016/j.jconrel.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 83.Merkel T.J., Jones S.W., Herlihy K.P., Kersey F.R., Shields A.R., Napier M. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc Natl Acad Sci USA. 2011;108(2):586–591. doi: 10.1073/pnas.1010013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun J., Zhang L., Wang J., Feng Q., Liu D., Yin Q. Tunable rigidity of (polymeric core)-(lipid shell) nanoparticles for regulated cellular uptake. Adv Mater. 2015;27(8):1402–1407. doi: 10.1002/adma.201404788. [DOI] [PubMed] [Google Scholar]
- 85.Anselmo A.C., Zhang M., Kumar S., Vogus D.R., Menegatti S., Helgeson M.E. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano. 2015;9(3):3169–3177. doi: 10.1021/acsnano.5b00147. [DOI] [PubMed] [Google Scholar]
- 86.Chandrasekaran S., King M.R. Microenvironment of tumor-draining lymph nodes: opportunities for liposome-based targeted therapy. Int J Mol Sci. 2014;15(11):20209–20239. doi: 10.3390/ijms151120209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kojima N., Biao L., Nakayama T., Ishii M., Ikehara Y., Tsujimura K. Oligomannose-coated liposomes as a therapeutic antigen-delivery and an adjuvant vehicle for induction of in vivo tumor immunity. J Control Release. 2008;129(1):26–32. doi: 10.1016/j.jconrel.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 88.Sheng K.C., Kalkanidis M., Pouniotis D.S., Esparon S., Tang C.K., Apostolopoulos V. Delivery of antigen using a novel mannosylated dendrimer potentiates immunogenicity in vitro and in vivo. Eur J Immunol. 2008;38(2):424–436. doi: 10.1002/eji.200737578. [DOI] [PubMed] [Google Scholar]
- 89.Kojima N., Ishii M., Kawauchi Y., Takagi H. Oligomannose-coated liposome as a novel adjuvant for the induction of cellular immune responses to control disease status. Biomed Res Int. 2013;2013 doi: 10.1155/2013/562924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang C., Liu P., Zhuang Y., Li P., Jiang B., Pan H. Lymphatic-targeted cationic liposomes: a robust vaccine adjuvant for promoting long-term immunological memory. Vaccine. 2014;32(42):5475–5483. doi: 10.1016/j.vaccine.2014.07.081. [DOI] [PubMed] [Google Scholar]
- 91.Jiang P.L., Lin H.J., Wang H.W., Tsai W.Y., Lin S.F., Chien M.Y. Galactosylated liposome as a dendritic cell-targeted mucosal vaccine for inducing protective anti-tumor immunity. Acta Biomater. 2015;11:356–367. doi: 10.1016/j.actbio.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 92.Joshi M.D., Unger W.J., Storm G., van Kooyk Y., Mastrobattista E. Targeting tumor antigens to dendritic cells using particulate carriers. J Control Release. 2012;161(1):25–37. doi: 10.1016/j.jconrel.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 93.Sehgal K., Dhodapkar K.M., Dhodapkar M.V. Targeting human dendritic cells in situ to improve vaccines. Immunol Lett. 2014;162(1 Pt A):59–67. doi: 10.1016/j.imlet.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tandrup Schmidt S., Foged C., Korsholm K.S., Rades T., Christensen D. Liposome-based adjuvants for subunit vaccines: formulation strategies for subunit antigens and immunostimulators. Pharmaceutics. 2016;8(1):7. doi: 10.3390/pharmaceutics8010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Obeid J., Hu Y., Slingluff C.L., Jr. Vaccines, adjuvants, and dendritic cell activators-current status and future challenges. Semin Oncol. 2015;42(4):549–561. doi: 10.1053/j.seminoncol.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Adams S. Toll-like receptor agonists in cancer therapy. Immunotherapy. 2009;1(6):949–964. doi: 10.2217/imt.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Temizoz B., Kuroda E., Ishii K.J. Vaccine adjuvants as potential cancer immunotherapeutics. Int Immunol. 2016;28(7):329–338. doi: 10.1093/intimm/dxw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shi M., Chen X., Ye K., Yao Y., Li Y. Application potential of toll-like receptors in cancer immunotherapy: systematic review. Medicine. 2016;95(25):e3951. doi: 10.1097/MD.0000000000003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khan S., Bijker M.S., Weterings J.J., Tanke H.J., Adema G.J., van Hall T. Distinct uptake mechanisms but similar intracellular processing of two different toll-like receptor ligand-peptide conjugates in dendritic cells. J Biol Chem. 2007;282(29):21145–21159. doi: 10.1074/jbc.M701705200. [DOI] [PubMed] [Google Scholar]
- 100.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 101.Han H.D., Byeon Y., Kang T.H., Jung I.D., Lee J.W., Shin B.C. Toll-like receptor 3-induced immune response by poly(d,l-lactide-co-glycolide) nanoparticles for dendritic cell-based cancer immunotherapy. Int J Nanomed. 2016;11:5729–5742. doi: 10.2147/IJN.S109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boks M.A., Ambrosini M., Bruijns S.C., Kalay H., van Bloois L., Storm G. MPLA incorporation into DC-targeting glycoliposomes favours anti-tumour T cell responses. J Control Release. 2015;216:37–46. doi: 10.1016/j.jconrel.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 103.Razazan A., Behravan J., Arab A., Barati N., Arabi L., Gholizadeh Z. Conjugated nanoliposome with the HER2/neu-derived peptide gp2 as an effective vaccine against breast cancer in mice xenograft model. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0185099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoshizaki Y., Yuba E., Sakaguchi N., Koiwai K., Harada A., Kono K. pH-sensitive polymer-modified liposome-based immunity-inducing system: effects of inclusion of cationic lipid and CpG-DNA. Biomaterials. 2017;141:272–283. doi: 10.1016/j.biomaterials.2017.07.001. :272–83. [DOI] [PubMed] [Google Scholar]
- 105.Mansourian M., Badiee A., Jalali S.A., Shariat S., Yazdani M., Amin M. Effective induction of anti-tumor immunity using p5 HER-2/neu derived peptide encapsulated in fusogenic DOTAP cationic liposomes co-administrated with CpG-ODN. Immunol Lett. 2014;162(1 Pt A):87–93. doi: 10.1016/j.imlet.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 106.Napolitani G., Rinaldi A., Bertoni F., Sallusto F., Lanzavecchia A. Selected toll-like receptor agonist combinations synergistically trigger a t helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6(8):769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tan R.S., Ho B., Leung B.P., Ding J.L. TLR cross-talk confers specificity to innate immunity. Int Rev Immunol. 2014;33(6):443–453. doi: 10.3109/08830185.2014.921164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Silva J.M., Zupancic E., Vandermeulen G., Oliveira V.G., Salgado A., Videira M. In vivo delivery of peptides and toll-like receptor ligands by mannose-functionalized polymeric nanoparticles induces prophylactic and therapeutic anti-tumor immune responses in a melanoma model. J Control Release. 2015;198:91–103. doi: 10.1016/j.jconrel.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 109.Fox C.B., Sivananthan S.J., Duthie M.S., Vergara J., Guderian J.A., Moon E. A nanoliposome delivery system to synergistically trigger tlr4 and tlr7. J Nanobiotechnol. 2014;12:17. doi: 10.1186/1477-3155-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tollemar J., Klingspor L., Ringdén O. Liposomal amphotericin B (Ambisome) for fungal infections in immunocompromised adults and children. Clin Microbiol Infect. 2001;7:68–79. doi: 10.1111/j.1469-0691.2001.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 111.Herzog C., Hartmann K., Kunzi V., Kursteiner O., Mischler R., Lazar H. Eleven years of inflexal v-a virosomal adjuvanted influenza vaccine. Vaccine. 2009;27(33):4381–4387. doi: 10.1016/j.vaccine.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 112.Gaillard P.J., Appeldoorn C.C., Dorland R., van Kregten J., Manca F., Vugts D.J. Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2b3-101) PLoS One. 2014;9(1):e82331. doi: 10.1371/journal.pone.0082331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lim J., Song Y.J., Park W.S., Sohn H., Lee M.S., Shin D.H. The immunogenicity of a single dose of hepatitis a virus vaccines (Havrix(R) and Epaxal(R)) in korean young adults. Yonsei Med J. 2014;55(1):126–131. doi: 10.3349/ymj.2014.55.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bozzuto G., Molinari A. Liposomes as nanomedical devices. Int J Nanomed. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watson D.S., Endsley A.N., Huang L. Design considerations for liposomal vaccines: Influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012;30(13):2256–2272. doi: 10.1016/j.vaccine.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Varypataki E.M., van der Maaden K., Bouwstra J., Ossendorp F., Jiskoot W. Cationic liposomes loaded with a synthetic long peptide and poly(I:C): a defined adjuvanted vaccine for induction of antigen-specific T cell cytotoxicity. AAPS J. 2015;17(1):216–226. doi: 10.1208/s12248-014-9686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yuba E., Tajima N., Yoshizaki Y., Harada A., Hayashi H., Kono K. Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy. Biomaterials. 2014;35(9):3091–3101. doi: 10.1016/j.biomaterials.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 118.Kraft J.C., Freeling J.P., Wang Z., Ho R.J. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J Pharm Sci. 2014;103(1):29–52. doi: 10.1002/jps.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu Z., Ramishetti S., Tseng Y.C., Guo S., Wang Y., Huang L. Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic t-lymphocyte response against melanoma and its lung metastasis. J Control Release. 2013;172(1):259–265. doi: 10.1016/j.jconrel.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 120.Khlebtsov N., Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev. 2011;40(3):1647–1671. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 121.Parveen S., Misra R., Sahoo S.K. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine. 2012;8(2):147–166. doi: 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 122.Almeida J.P.M., Lin A.Y., Figueroa E.R., Foster A.E., Drezek R.A. In vivo gold nanoparticle delivery of peptide vaccine induces anti-tumor immune response in prophylactic and therapeutic tumor models. Small. 2015;11(12):1453–1459. doi: 10.1002/smll.201402179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tao Y., Ju E., Li Z., Ren J., Qu X. Engineered CpG-antigen conjugates protected gold nanoclusters as smart self-vaccines for enhanced immune response and cell imaging. Adv Funct Mater. 2014;24(7):1004–1010. [Google Scholar]
- 124.Kang S., Ahn S., Lee J., Kim J.Y., Choi M., Gujrati V. Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic t-lymphocyte responses. J Control Release. 2017;256:56–67. doi: 10.1016/j.jconrel.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 125.Zhang P., Chiu Y.C., Tostanoski L.H., Jewell C.M. Polyelectrolyte multilayers assembled entirely from immune signals on gold nanoparticle templates promote antigen-specific t cell response. ACS Nano. 2015;9(6):6465–6477. doi: 10.1021/acsnano.5b02153. [DOI] [PubMed] [Google Scholar]
- 126.Li X., Aldayel A.M., Cui Z. Aluminum hydroxide nanoparticles show a stronger vaccine adjuvant activity than traditional aluminum hydroxide microparticles. J Control Release. 2014;173:148–157. doi: 10.1016/j.jconrel.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xiang J., Xu L., Gong H., Zhu W., Wang C., Xu J. Antigen–loaded upconversion nanoparticles for dendritic cell stimulation, tracking, and vaccination in dendritic cell-based immunotherapy. ACS Nano. 2015;9(6):6401–6411. doi: 10.1021/acsnano.5b02014. [DOI] [PubMed] [Google Scholar]
- 128.Sungsuwan S., Yin Z., Huang X. Lipopeptide-coated iron oxide nanoparticles as potential glycoconjugate-based synthetic anticancer vaccines. ACS Appl Mater Interfaces. 2015;7(31):17535–17544. doi: 10.1021/acsami.5b05497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ruiz-de-Angulo A., Zabaleta A., Gomez-Vallejo V., Llop J., Mareque-Rivas J.C. Microdosed lipid-coated (67)Ga-magnetite enhances antigen-specific immunity by image tracked delivery of antigen and CpG to lymph nodes. ACS Nano. 2016;10(1):1602–1618. doi: 10.1021/acsnano.5b07253. [DOI] [PubMed] [Google Scholar]
- 130.Karimi M., Bahrami S., Ravari S.B., Zangabad P.S., Mirshekari H., Bozorgomid M. Albumin nanostructures as advanced drug delivery systems. Expert Opin Drug Deliv. 2016;13(11):1609–1623. doi: 10.1080/17425247.2016.1193149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.An F.F., Zhang X.H. Strategies for preparing albumin-based nanoparticles for multifunctional bioimaging and drug delivery. Theranostics. 2017;7(15):3667–3689. doi: 10.7150/thno.19365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu H., Moynihan K.D., Zheng Y., Szeto G.L., Li A.V., Huang B. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507(7493):519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhu G., Lynn G.M., Jacobson O., Chen K., Liu Y., Zhang H. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat Commun. 2017;8(1):1954. doi: 10.1038/s41467-017-02191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang P., Zhao P., Dong S., Xu T., He X., Chen M. An albumin-binding polypeptide both targets cytotoxic T lymphocyte vaccines to lymph nodes and boosts vaccine presentation by dendritic cells. Theranostics. 2018;8(1):223–236. doi: 10.7150/thno.21691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kamaly N., Yameen B., Wu J., Farokhzad O.C. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem Rev. 2016;116(4):2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koppolu B., Zaharoff D.A. The effect of antigen encapsulation in chitosan particles on uptake, activation and presentation by antigen presenting cells. Biomaterials. 2013;34(9):2359–2369. doi: 10.1016/j.biomaterials.2012.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kurosaki T., Kitahara T., Nakamura T., Nishida K., Fumoto S., Kodama Y. Development of effective cancer vaccine using targeting system of antigen protein to apcs. Pharm Res. 2012;29(2):483–489. doi: 10.1007/s11095-011-0571-x. [DOI] [PubMed] [Google Scholar]
- 138.Zhang Z., Tongchusak S., Mizukami Y., Kang Y.J., Ioji T., Touma M. Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials. 2011;32(14):3666–3678. doi: 10.1016/j.biomaterials.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 139.Wilson J.T., Keller S., Manganiello M.J., Cheng C., Lee C.C., Opara C. pH-responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano. 2013;7(5):3912–3925. doi: 10.1021/nn305466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Luo M., Wang H., Wang Z., Cai H., Lu Z., Li Y. A sting-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2017;12(7):648–654. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Al-Zahrani S., Zaric M., McCrudden C., Scott C., Kissenpfennig A., Donnelly R.F. Microneedle-mediated vaccine delivery: Harnessing cutaneous immunobiology to improve efficacy. Exp Opin Drug Deliv. 2012;9(5):541–550. doi: 10.1517/17425247.2012.676038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim Y.C., Park J.H., Prausnitz M.R. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64(14):1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hong X., Wei L., Wu F., Wu Z., Chen L., Liu Z. Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine. Drug Des Devel Ther. 2013;7:945–952. doi: 10.2147/DDDT.S44401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang X., Wang N., Li N., Zhen Y., Wang T. Multifunctional particle-constituted microneedle arrays as cutaneous or mucosal vaccine adjuvant-delivery systems. Hum Vacc Immunother. 2016;12(8):2075–2089. doi: 10.1080/21645515.2016.1158368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zaric M., Lyubomska O., Touzelet O., Poux C., Al-Zahrani S., Fay F. Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-d,l-lactide-co-glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano. 2013;7(3):2042–2055. doi: 10.1021/nn304235j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhao J.H., Zhang Q.B., Liu B., Piao X.H., Yan Y.L., Hu X.G. Enhanced immunization via dissolving microneedle array-based delivery system incorporating subunit vaccine and saponin adjuvant. Int J Nanomed. 2017;12:4763–4772. doi: 10.2147/IJN.S132456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Midoux P., Pichon C. Lipid-based mRNA vaccine delivery systems. Exp Rev Vacc. 2015;14(2):221–234. doi: 10.1586/14760584.2015.986104. [DOI] [PubMed] [Google Scholar]
- 148.Oberli M.A., Reichmuth A.M., Dorkin J.R., Mitchell M.J., Fenton O.S., Jaklenec A. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17(3):1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hess P.R., Boczkowski D., Nair S.K., Snyder D., Gilboa E. Vaccination with mrnas encoding tumor-associated antigens and granulocyte-macrophage colony-stimulating factor efficiently primes ctl responses, but is insufficient to overcome tolerance to a model tumor/self antigen. Cancer Immunol Immunother. 2006;55(6):672–683. doi: 10.1007/s00262-005-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mockey M., Bourseau E., Chandrashekhar V., Chaudhuri A., Lafosse S., Le Cam E. mRNA-based cancer vaccine: prevention of b16 melanoma progression and metastasis by systemic injection of mart1 mRNA histidylated lipopolyplexes. Cancer Gene Ther. 2007;14(9):802–814. doi: 10.1038/sj.cgt.7701072. [DOI] [PubMed] [Google Scholar]
- 151.Perche F., Benvegnu T., Berchel M., Lebegue L., Pichon C., Jaffres P.A. Enhancement of dendritic cells transfection in vivo and of vaccination against b16f10 melanoma with mannosylated histidylated lipopolyplexes loaded with tumor antigen messenger RNA. Nanomedicine. 2011;7(4):445–453. doi: 10.1016/j.nano.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 152.Pollard C., Rejman J., De Haes W., Verrier B., Van Gulck E., Naessens T. Type i ifn counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Mol Ther. 2013;21(1):251–259. doi: 10.1038/mt.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534(7607):396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 154.Broos K., Van der Jeught K., Puttemans J., Goyvaerts C., Heirman C., Dewitte H. Particle-mediated intravenous delivery of antigen mRNA results in strong antigen-specific t-cell responses despite the induction of type i interferon. Mol Ther Nucl Acids. 2016;5(6):e326. doi: 10.1038/mtna.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Su X., Fricke J., Kavanagh D.G., Irvine D.J. In vitro and in vivo mRNA delivery using lipid-enveloped pH-responsive polymer nanoparticles. Mol Pharm. 2011;8(3):774–787. doi: 10.1021/mp100390w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Palmerston Mendes L., Pan J., Torchilin V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules. 2017;22(9):1401. doi: 10.3390/molecules22091401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kesharwani P., Iyer A.K. Recent advances in dendrimer-based nanovectors for tumor-targeted drug and gene delivery. Drug Discov Today. 2015;20(5):536–547. doi: 10.1016/j.drudis.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chahal J.S., Khan O.F., Cooper C.L., McPartlan J.S., Tsosie J.K., Tilley L.D. Dendrimer-RNA nanoparticles generate protective immunity against lethal ebola, h1n1 influenza, and toxoplasma gondii challenges with a single dose. Proc Natl Acad Sci USA. 2016;113(29):E4133–E4142. doi: 10.1073/pnas.1600299113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu-Monette Z.Y., Zhang M., Li J., Young K.H. PD-1/PD-L1 blockade: have we found the key to unleash the antitumor immune response? Front Immunol. 2017;8:1597. doi: 10.3389/fimmu.2017.01597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Teng M.W., Ngiow S.F., Ribas A., Smyth M.J. Classifying cancers based on t-cell infiltration and PD-L1. Cancer Res. 2015;75(11):2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Geiger J.L., Grandis J.R., Bauman J.E. The STAT3 pathway as a therapeutic target in head and neck cancer: barriers and innovations. Oral Oncol. 2016;56:84–92. doi: 10.1016/j.oraloncology.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wurth R., Bajetto A., Harrison J.K., Barbieri F., Florio T. CXCL12 modulation of CXCR4 and CXCR7 activity in human glioblastoma stem-like cells and regulation of the tumor microenvironment. Front Cell Neurosci. 2014;8:144. doi: 10.3389/fncel.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tsukamoto H., Fujieda K., Senju S., Ikeda T., Oshiumi H., Nishimura Y. Immune-suppressive effects of interleukin-6 on T-cell-mediated anti-tumor immunity. Cancer Sci. 2018;109(3):523–530. doi: 10.1111/cas.13433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Du Four S., Maenhout S.K., De Pierre K., Renmans D., Niclou S.P., Thielemans K. Axitinib increases the infiltration of immune cells and reduces the suppressive capacity of monocytic mdscs in an intracranial mouse melanoma model. Oncoimmunology. 2015;4(4) doi: 10.1080/2162402X.2014.998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tseng C.W., Hung C.F., Alvarez R.D., Trimble C., Huh W.K., Kim D. Pretreatment with cisplatin enhances E7-specific CD8+ T-cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res. 2008;14(10):3185–3192. doi: 10.1158/1078-0432.CCR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Garnett C.T., Schlom J., Hodge J.W. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14(11):3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shurin G.V., Tourkova I.L., Kaneno R., Shurin M.R. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009;183(1):137–144. doi: 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hodge J.W., Ardiani A., Farsaci B., Kwilas A.R., Gameiro S.R. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin Oncol. 2012;39(3):323–339. doi: 10.1053/j.seminoncol.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]