Abstract
Background:
Hair cortisol is a measure of chronic or repeated hypothalamic-pituitary-adrenal axis activation in response to physical or psychological stressors. Hair cortisol has been successfully used as a measure of chronic stress in adults and children; however, its use as a valid measure in preterm infants has been limited by challenges in measuring cortisol in the low mass samples collectable from these infants.
Objectives:
The purpose of this report is to present a novel protocol for the measurement of hair cortisol in very low mass hair samples.
Methods:
Small changes were made to previously published protocols. After washing and pulverizing the hair samples, a double methanol cortisol extraction was performed. Samples were spiked with a known quantity of cortisol and analyzed in duplicate using an enzyme-linked immunosorbent assay.
Results:
Hair cortisol was detectable in samples weighing between 0.4 and 10.9mg. The mean cortisol level was 23.74 pg/mg hair (SD = 26.38).
Discussion:
With small changes to previously published laboratory protocols, cortisol is quantifiable in low mass hair samples from preterm infants. This technical advance is an important step towards quantifying the stress experiences of hospitalized preterm infants.
Keywords: cortisol, preterm infant, stress
Preterm infants experience numerous stressors during their hospitalization in the neonatal intensive care unit (NICU; Cruz et al., 2016). Stress exposure in these infants is associated with abnormal brain development (Brummelte et al., 2012; Smith et al., 2011) and impaired long-term neurodevelopment (Valeri et al., 2015; Vinall et al., 2014). While counts of stressful experiences provide an objective measure of the quantity of stress exposures (Newnham et al., 2009), subjective experiences and chronic stress responses are more challenging to measure in this nonverbal population.
Hair cortisol (HC) is often used as a measure of chronic stress responses in studies of adults and children (Vives et al., 2015). Studies have revealed that exposure to childhood adversity is predictive of higher HC in young children (Karlen et al., 2015; Palmer et al., 2013), and that high HC levels are associated with poorer socioemotional functioning (Palmer et al., 2013). Published protocols to measure HC generally include a washing step, a method for breaking down the hair’s protein matrix and increasing the surface area for subsequent cortisol extraction, one or more methanol extraction steps, and the analysis of HC by either immunoassay or liquid chromatography-tandem mass spectrometry (Russell et al., 2015). While HC can be accurately measured in hair samples greater than 10mg (Greff et al., 2019), Hoffman et al. (2017) developed a protocol to measure cortisol in low mass samples, defined as samples weighing as little as 2.5mg, from preterm and term-born neonates. However, due, in part, to the methodological challenges of measuring cortisol in low mass samples of hair, only a few researchers have measured hair cortisol in preterm infants (Hoffman et al., 2017; Hollanders et al., 2017; Yamada et al., 2007). HC is not yet a widely used measure in this patient population; however, once validated, HC could be useful to quantify the stress experience of hospitalized preterm infants.
Objectives
The ability to measure HC in very low mass hair samples may represent an important advance in our ability to quantify the stress experience of hospitalized preterm infants. We have been unable to detect cortisol in low mass hair samples using the protocol published by Hoffman et al. (2017) in our lab (unpublished data). The purpose of this paper is to report the development of a novel protocol to measure HC in low mass hair samples from preterm infants. Additionally, as an exploratory objective, we examined associations between HC and select demographic and clinical variables.
Methods
Sample
Participants in this study were a subset of those enrolled in a larger nonexperimental study to examine the relationships among stress exposure in the NICU and neurodevelopmental outcomes (author redacted). In consideration of cultural values related to the cutting of hair (Wright et al., 2018), parents of infants enrolled in the larger study were permitted to opt out of hair collection during the informed consent process. Infants enrolled in the primary study were born 28–31 weeks postmenstrual age (PMA) and admitted to one of four NICUs in a large Midwest metropolitan area. Infants were included in the study if they were: (a) born within the specified age range; (b) less than two weeks old at the time of enrollment; (c) born to mothers who were English-speaking and able to provide informed consent; and (d) born at a delivery hospital with one of the four included NICUs or were transferred into a participating NICU before the second day of life. Infants were excluded if they: (a) had admission blood cultures positive for sepsis; (b) had congenital anomalies with known effects for neurodevelopment; (c) were born to mothers with confirmed chorioamnionitis; (d) had signs of neonatal abstinence syndrome or were born to mothers with a known history of opioid drug abuse; (e) were born small for gestational age; (f) were diagnosed with a neurologic abnormality; or (g) born to mothers with oligohydramnios. Infants from the primary study were included in the current study if their mothers provided informed consent for hair collection. There were no additional inclusion or exclusion criteria. Approval for the study was provided by the institutional review boards of the clinical and academic sites. Mothers of enrolled infants provided written informed consent prior to any study procedures.
Demographic and Clinical Data
Demographic and clinical data were collected from the electronic health record and included PMA at birth, birthweight, infant sex and race, prenatal exposures to medications and tobacco, postnatal exposures to medications, and medical treatments. Illness acuity was measured using the Neonatal Medical Index (NMI; Korner et al., 1993). Infants were assigned an NMI score between 1 (lowest acuity) and 5 (highest acuity) based on birthweight, level of respiratory support required, and extent of clinical complications.
Hair Cortisol Analysis
We collected hair samples at approximately 35 weeks PMA for the measurement of HC. Using surgical clippers, a member of the research team shaved 1–2 centimeters of hair from the base of the infant’s hairline across the nape of the neck and placed the sample in a preweighed, round-end, microcentrifuge tube. After hair collection, the tubes were reweighed to determine the mass of hair collected. Hair samples were stored at room temperature in a dark environment and processed within 16 months of collection. HC is stable in hair samples stored at room temperature indefinitely if the samples are protected from ultraviolet light (Greff et al., 2019).
We followed the washing and processing steps of the hair cortisol analysis as previously described (Greff et al., 2019; Hoffman et al., 2017). To wash the hair, we pipetted high performance liquid chromatography (HPLC)-grade isopropanol (250μL) into each microcentrifuge tube. Tubes were repeatedly inverted and incubated on a shaking table for 5 minutes. We decanted the isopropanol and repeated the washing step. We expect that little to no cortisol was extracted from the hair during this standard washing step and tested this assumption using adult hair samples. In eight samples from adult participants with detectable levels of HC following pulverization and methanol extraction, cortisol was not detectable in the isopropanol used for washing (data not shown).
Following the isopropanol wash, the open tubes containing infant hair were evaporated under a stream of air for 48 hours at room temperature to ensure complete isopropanol evaporation. To fragment the hair’s protein matrix and increase the surface area for cortisol extraction, we cut hair samples into 2–4mm pieces using fine tipped scissors and subsequently pulverized these samples for 5 minutes in a Retsch 400 ball mill (Verder Scientific, Newton, PA) with two 5mm chrome steel balls. This process resulted in powdered hair samples.
To increase the cortisol yield, we used repeated methanol extractions (Palmer et al., 2013; Slominski et al., 2015). We incubated powdered hair samples in 550μL HPLC-grade methanol for 24 hours at room temperature on a shaking table to provide constant agitation and, subsequently, centrifuged the tubes at 5000 × g for 5 minutes to pellet the powdered hair. Taking care not to disturb the pellet, we withdrew 500μL of the methanol supernatant and placed it in a clean microcentrifuge tube. We repeated the methanol extraction with an additional 550μL methanol and combined the methanol supernatants from the two extractions (1mL total). From previous studies in our lab, we have found that methanol extractions beyond the two included in this protocol do not increase the cortisol yield (data not shown). We allowed the methanol to evaporate at room temperature overnight and reconstituted the cortisol samples from the extracted methanol in 55μL of cortisol immunoassay diluent buffer (Salimetrics, State College, PA), a phosphate buffer.
We analyzed samples in duplicate and quantified the cortisol levels in the reconstituted samples using a high sensitivity, salivary cortisol enzyme immunoassay kit (Salimetric, State College, PA). We quantified HC according the manufacturer’s instructions with the exception of a “spike” step to increase cortisol levels in the samples within the detectable range of the assay. Thus, after pipetting 25μL of standards and samples into the appropriate wells of the 96-well assay plate, we added 25μL of the 0.333ug/dL standard to all samples, resulting in a 1:2 dilution of samples. The remainder of the manufacturer’s protocol was unchanged. We analyzed the assay plate in a Powerwave plate reader (BioTek, Winooski, VT) at 450nm and subtracted background values from all assay wells. In the calculations, we subtracted the 0.333ug/dL standard reading from the sample readings. Samples that resulted in a negative number were considered nondetectable. We converted cortisol levels from ug/dL, as measured by the assay, to pg/mg—based on the mass of hair collected and analyzed using the following formula:
where A = μg/dl from assay output; B = weight (in mg) of collected hair; C = vol. (in ml) of methanol added to the powdered hair; D = vol. (in ml) of methanol recovered from the extract and subsequently dried down; E = vol. (in ml) of assay buffer used to reconstitute the dried extract; 10,000 accounts for changes in metrics; 2 accounts for the dilution factor after addition of the spike; and F = final value of hair cortisol concentration in pg/mg.
Statistical Analyses
To explore the associations between HC and select demographic and clinical variables, we used Spearman correlations for continuous variables, Wilcoxon Rank Sum for binary variables, and Kruskal-Wallis test of ranks for categorical variables.
Results
Of the 73 preterm infants enrolled in the nonexperimental study, mothers of 36 infants (49%) consented to hair collection. At 35 weeks PMA, we were unable to collect hair from seven of these infants due to insufficient hair quantity (Figure). Of the 29 hair samples analyzed, cortisol was detectable in 25 (86%). Table 1 presents the sample characteristics for the infants providing hair samples with detectable cortisol. The average coefficient of variation for duplicates in the assay was 1.3%, and the R2 value for the standard curve from which HC in the samples were determined was 0.994. We were able to detect cortisol in hair samples weighing as little as 0.4mg, and the mean cortisol level was 23.74 pg/mg hair (SD = 26.38 pg/mg hair).
Figure 1.
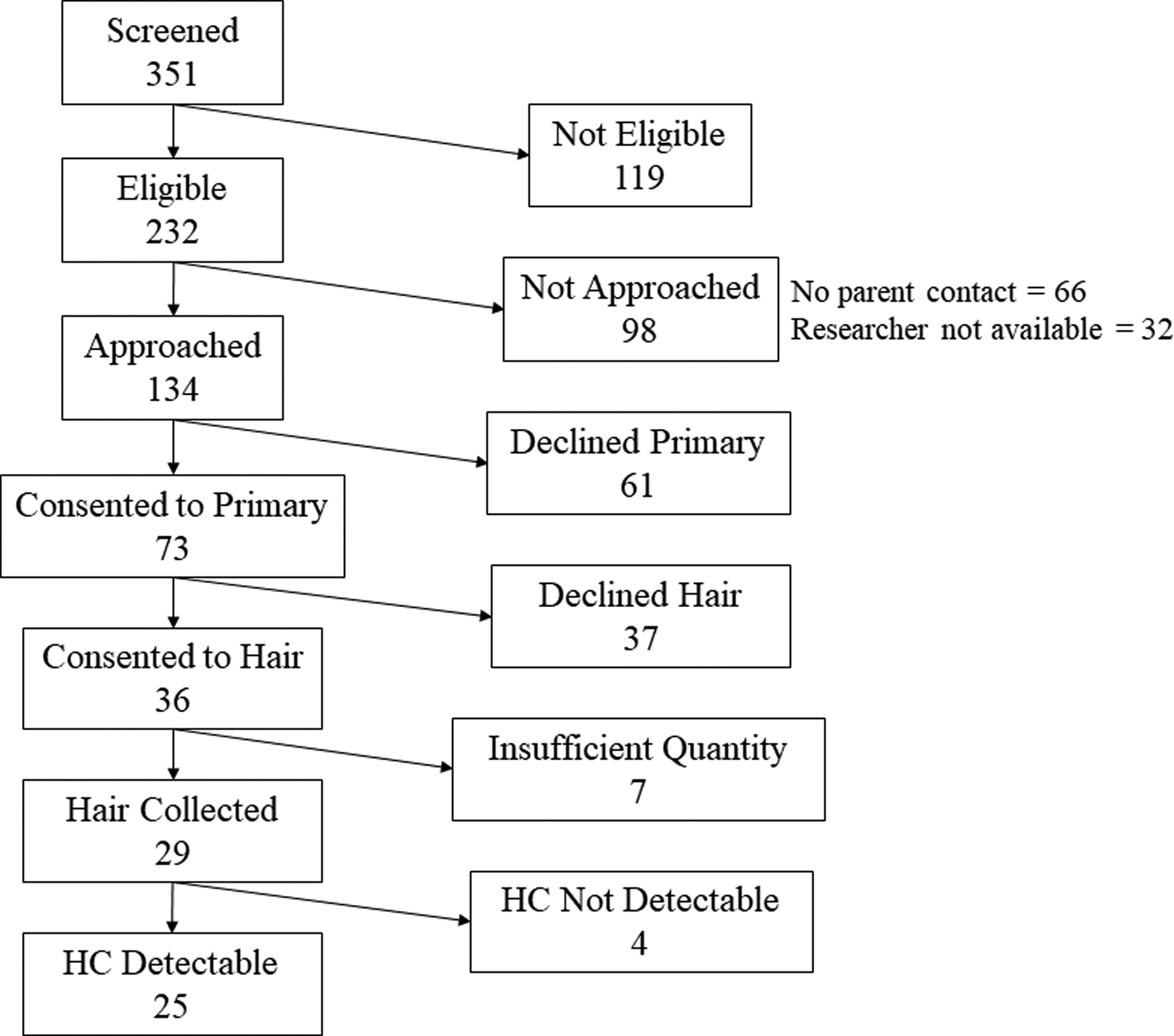
Participants included in the current study. Note. HC, hair cortisol.
Table 1.
Sample Characteristics (n = 25) for Infants Providing Hair Samples with Detectable Cortisol
| n (%) | ||
|---|---|---|
| Male | 16 (64) | |
| White race | 22 (88) | |
| Maternal perinatal steroids | 20 (80) | |
| Mean (SD)/Median1 | Min – Max | |
| PMA (weeks) | 30.1 (1.1) | 28.1 – 31.7 |
| Birthweight (g) | 1540 (387) | 770 – 2331 |
| PMA at collection (weeks) | 35.8 (1) | 34.6 – 38.1 |
| Hair mass collected (mg) | 3.3 (2.9) | 0.4 – 10.9 |
| Hair cortisol (pg/mg hair) | 23.7 (26.4) | 2.1 – 124.9 |
| Neonatal Medical Index | 51 | 2 – 5 |
Note. PMA = post-menstrual age; g = grams; mg = milligrams; pg = picograms
In the exploratory analysis, we found few associations between HC and other demographic and clinical variables. Only postnatal exposure to caffeine and prenatal exposure to maternally administered antibiotics were significantly associated with HC (Table 2).
Table 2.
Associations between Hair Cortisol and Demographic and Clinical Variables
| Statistic (ρ)1 | p-value | |
|---|---|---|
| PMA | 0.14 | 0.5 |
| Birthweight | 0.024 | 0.91 |
| PMA at collection | −0.023 | 0.91 |
| Postnatal age at collection | −0.0081 | 0.97 |
| Statistic (z)2 | p-value | |
| Infant sex (male/female) | −0.28 | 0.78 |
| Infant race (White/Black) | 0.42 | 0.68 |
| Infant antibiotics (yes/no) | 1.8 | 0.071 |
| Infant caffeine (yes/no) | 2.4 | 0.019 |
| Infant steroids (yes/no) | −1.42 | 0.16 |
| Maternal antibiotics (yes/no) | 2.8 | 0.0046 |
| Maternal tobacco use (yes/no) | −0.95 | 0.34 |
| Statistic (χ2)3 | p-value | |
| Neonatal Medical Illness (clinical acuity) | 0.58 | 0.9 |
| Maternal steroid doses | 1.13 | 0.77 |
Note.
Spearman correlations (ρ) for continuous variables;
Wilcoxon Rank Sum (z) for binary variables;
Kruskal-Wallis test (χ2) for categorical variables.]
Discussion
By performing a double methanol extraction and spiking samples with a known standard quantity of cortisol, we were able to detect cortisol in the majority (86%) of low mass hair samples. While the accuracy and precision of this measure have not been confirmed, this protocol provides an initial first step in our ability to quantify chronic or repeated HPA axis activation in preterm infants. Researchers have used multiple methanol extractions to increase cortisol yield in previous studies of HC (Palmer et al., 2013; Slominski et al., 2015); however, this is the first study, to our knowledge, that has added a spike step. The purpose of the spike step was to increase the measurable cortisol level in the sample assay wells. When the optical density values were interpolated, the sample values were measurable in the middle of the standard curve, ensuring that the low mass differences of the samples were within the detection threshold of the assay. Using this technique, we found that a standard, commercially available, enzyme-linked immunoassay (Salimetrics, State College, PA) detected cortisol in samples between 0.4–10.9mg.
There is significant value in objectively measuring stress responses in preterm infants, as pain-related stress exposure is associated with long-term HPA axis functioning (Brummelte et al., 2015; Grunau et al., 2013; Provenzi et al., 2016) and neurodevelopment (Valeri et al., 2015; Vinall et al., 2014). Measuring stress exposure through checklists of stressors (Cong et al., 2017; Newnham et al., 2009) or counts of skin-breaking procedures (Brummelte et al., 2015; Grunau et al., 2013) has proven feasible in previous studies. Though, these measures are not able to account for differences in the developmental regulation of the HPA axis that occurs in preterm infants (Bolt et al., 2002) or differences in perceived stress among individuals or within individual preterm infants—which may change as they age (Bembich et al., 2016). Moreover, HC offers a noninvasive measure that, unlike plasma or salivary cortisol, is not affected by the possible discomfort associated with collection (Greff et al., 2019).
The use of HC as a measure of chronic stress responses in preterm infants requires thoughtful planning and consideration of potentially significant confounders. Fetal hair undergoes its first catagen (destruction)/telogen (hair loss) cycle between 24 and 28 weeks PMA; therefore, HC measured in hair collected during neonatal period from preterm infants likely represents HPA axis responses after the 28th week of pregnancy (Gareri & Koren, 2010). Neonatal and maternal postpartum HC are correlated (Hollanders et al., 2017). Thus, HC in preterm infants may partially reflect maternal stress responses during gestation. In addition, the HPA axis undergoes significant developmental maturation during fetal life and infancy, which may affect HC levels (Bolt et al., 2002; Lewis & Ramsay, 1995; Tollenaar et al., 2010). To avoid confounding due to differences in HPA axis maturation, HC levels should only be compared in infants when hair is collected at the same approximate PMA from all study participants.
There are no published normal reference ranges for HC in preterm infants. The mean HC in our sample of preterm infants (M = 23.74 pg/mg, SD = 26.38 pg/mg) is much lower than that measured by Hoffman et al. (2017) in their study of neonates (M = 281.8 pg/mg, SD = 141.6 pg/mg). However, 88% of neonates in the study conducted by Hoffman et al. (2017) were born at term, which could account for the discrepancy in HC levels. Other researchers report neonatal HC levels between 51–1294 pg/mg for infants born 33.9–42.1 weeks PMA (M = 39.5 weeks, SD = 1.8 weeks) using liquid chromatography-tandem mass spectrometry to quantify cortisol (Hollanders et al., 2017). Hoffman et al. (2017) found a significant positive correlation between PMA and HC. There may be a quadratic relationship between HC and age; children and older adults appear to have higher levels of HC than middle-aged adults (Dettenborn et al., 2012). Among children and infants born at term, higher HC levels are associated with younger age (Dettenborn et al., 2012; Karlen et al., 2013; Tollenaar et al., 2010). Therefore, comparisons of our results with results from studies of older infants, adolescents, and adults are not meaningful. Additional studies of HC in preterm infants are needed to establish normal reference ranges.
Limitations and Suggestions for Future Research
We have presented a feasible method for the measurement of HC in low mass hair samples from preterm infants. This protocol requires additional testing to ensure accuracy and precision of the method. Future studies should measure HC in samples of different masses from the same source to ensure that HC levels can be accurately replicated in very low mass samples. We were unable to test the accuracy of our protocol due to the limited amount of hair in our samples that was available for analysis. We were also unable to use incremental spike levels on the same hair samples due to the low mass of samples obtainable from preterm infants. Future studies testing the accuracy of this protocol could include multiple incremental levels. Moreover, the addition of a spike can introduce error into the final HC measures attributable to differences in spike recovery. In this study, the spike was added to each sample well of the 96-well ELISA plate, and HC was averaged across duplicates. To minimize the effect of differences in spike recovery, samples should be analyzed at least in duplicate. Finally, future studies should test the convergent validity of HC in preterm infants using objective measures of stress exposure such as stressor checklists (Newnham et al., 2009) or 24 hour urinary cortisol, which has previously demonstrated a moderate correlation with HC in adults (Sauvé et al., 2007).
Conclusion
HC is an innovative measure of chronic stress responses and has been a useful measure in studies of stress and health in adults and children. The ability to measure HC in very low mass samples of hair from preterm infants is an important advance in our ability to describe the stress experiences of hospitalized preterm infants. Building on previously published methods for the measurement of HC, we have presented a simple, feasible method for the measurement of HC in low mass samples.
Acknowledgement:
Funding for this study was provided by the National Institute of Nursing Research of the National Institutes of Health under award numbers F31NR017321 (Nist, PI) and T32NR014225 (Pickler & Melnyk, MPI; Nist, Fellow); Association of Women’s Health, Obstetric, and Neonatal Nurses and Kimberly-Clark; National Association of Neonatal Nurses; Midwest Nursing Research Society; Sigma Theta Tau International; Rockefeller University Heilbrunn Family Center for Research Nursing through the generosity of the Heilbrunn Family; and The Ohio State University Alumni Grants for Graduate Research and Scholarship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
Ethical Conduct of Research: This study was approved by the Nationwide Children’s Hospital (# IRB17–00554) and The Ohio State University (# 2017N0030) Institutional Review Boards.
References
- Bembich S, Marrazzo F, Barini A, Ravalico P, Cont G, & Demarini S (2016). The cortical response to a noxious procedure changes over time in preterm infants. Pain, 157, 1979–1987. 10.1097/j.pain.0000000000000605 [DOI] [PubMed] [Google Scholar]
- Bolt RJ, van Weissenbruch MM, Lafeber HN, & Delemarre-van de Waal HA (2002). Development of the hypothalamic-pituitary-adrenal axis in the fetus and preterm infant. Journal of Pediatric Endocrinology and Metabolism, 15, 759–770. 10.1515/JPEM.2002.15.6.759 [DOI] [PubMed] [Google Scholar]
- Brummelte S, Chau CMY, Cepeda IL, Degenhardt A, Weinberg J, Synnes AR, & Grunau RE (2015). Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology, 51, 151–163. 10.1016/j.psyneuen.2014.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, Gover A, Synnes AR, & Miller SP (2012). Procedural pain and brain development in premature newborns. Annals of Neurology, 71, 385–396. 10.1002/ana.22267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong X, Wu J, Vittner D, Xu W, Hussain N, Galvin S, Fitzsimons M, McGrath JM, & Henderson WA (2017). The impact of cumulative pain/stress on neurobehavioral development of preterm infants in the NICU. Early Human Development, 108, 9–16. 10.1016/j.earlhumdev.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MD, Fernandes AM, & Oliveira CR (2016). Epidemiology of painful procedures performed in neonates: A systematic review of observational studies. European Journal of Pain, 20, 489–498. 10.1002/ejp.757 [DOI] [PubMed] [Google Scholar]
- Dettenborn L, Tietze A, Kirschbaum C, & Stalder T (2012). The assessment of cortisol in human hair: Associations with sociodemographic variables and potential confounders. Stress, 15, 578–588. 10.3109/10253890.2012.654479 [DOI] [PubMed] [Google Scholar]
- Gareri J, & Koren G (2010). Prenatal hair development: Implications for drug exposure determination. Forensic Science International, 196, 27–31. 10.1016/j.forsciint.2009.12.024 [DOI] [PubMed] [Google Scholar]
- Greff MJE, Levine JM, Abuzgaia AM, Elzagallaai AA, Rieder MJ, & van Uum SHM (2019). Hair cortisol analysis: An update on methodological considerations and clinical applications. Clinical Biochemistry, 63, 1–9. 10.1016/j.clinbiochem.2018.09.010 [DOI] [PubMed] [Google Scholar]
- Grunau RE, Cepeda IL, Chau CMY, Brummelte S, Weinberg J, Lavoie PM, Ladd M, Hirschfeld AF, Russell E, Koren G, van Uum S, Brant R, & Turvey SE (2013). Neonatal pain-related stress and NFKBIA genotype are associated with altered cortisol levels in preterm boys at school age. PloS One, 8, e73926 10.1371/journal.pone.0073926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MC, D’Anna-Hernandez K, Benitez P, Ross RG, & Laudenslager ML (2017). Cortisol during human fetal life: Characterization of a method for processing small quantities of newborn hair from 26 to 42 weeks gestation. Developmental Psychobiology, 59, 123–127. 10.1002/dev.21433 [DOI] [PubMed] [Google Scholar]
- Hollanders JJ, van der Voorn B, Kieviet N, Dolman KM, de Rijke YB, van den Akker ELT, Rotteveel J, Honig A, & Finken MJJ (2017). Interpretation of glucocorticoids in neonatal hair: A reflection of intrauterine glucocorticoid regulation? Endocrine Connections, 6, 692–699. 10.1530/ec-17-0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlen J, Frostell A, Theodorsson E, Faresjö T, & Ludvigsson J (2013). Maternal influence on child HPA axis: A prospective study of cortisol levels in hair. Pediatrics, 132, e1333–e1340. 10.1542/peds.2013-1178 [DOI] [PubMed] [Google Scholar]
- Karlén J, Ludvigsson J, Hedmark M, Faresjö Å, Theodorsson E, & Faresjö T (2015). Early psychosocial exposures, hair cortisol levels, and disease risk. Pediatrics, 135, e1450–e1457. 10.1542/peds.2014-2561 [DOI] [PubMed] [Google Scholar]
- Korner AF, Stevenson DK, Kraemer HC, Spiker D, Scott DT, Constantinou J, & Dimiceli S (1993). Prediction of the development of low birth weight preterm infants by a new neonatal medical index. Journal of Developmental and Behavioral Pediatrics, 14, 106–111. 10.1097/00004703-199304000-00005 [DOI] [PubMed] [Google Scholar]
- Lewis M, & Ramsay D (1995). Stability and change in cortisol and behavioral response to stress during the first 18 months of life. Developmental Psychobiology, 28, 419–428. 10.1002/dev.420280804 [DOI] [PubMed] [Google Scholar]
- Newnham CA, Inder TE, & Milgrom J (2009). Measuring preterm cumulative stressors within the NICU: The Neonatal Infant Stressor Scale. Early Human Development, 85, 549–555. 10.1016/j.earlhumdev.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Palmer FB, Anand KJS, Graff JC, Murphy LE, Qu Y, Völgyi E, Rovnaghi CR, Moore A, Tran QT, & Tylavsky FA (2013). Early adversity, socioemotional development, and stress in urban 1-year-old children. Journal of Pediatrics, 163, 1733–1739.e1. 10.1016/j.jpeds.2013.08.030 [DOI] [PubMed] [Google Scholar]
- Provenzi L, Giusti L, Fumagalli M, Tasca H, Ciceri F, Menozzi G, Mosca F, Morandi F, Borgatti R, & Montirosso R (2016). Pain-related stress in the neonatal intensive care unit and salivary cortisol reactivity to socio-emotional stress in 3-month-old very preterm infants. Psychoneuroendocrinology, 72, 161–165. 10.1016/j.psyneuen.2016.07.010 [DOI] [PubMed] [Google Scholar]
- Russell E, Kirschbaum C, Laudenslager ML, Stalder T, de Rijke Y, van Rossum EF, Van Uum S, & Koren G (2015). Toward standardization of hair cortisol measurement: Results of the first international interlaboratory round robin. Therapeutic Drug Monitoring, 37, 71–75. 10.1097/ftd.0000000000000148 [DOI] [PubMed] [Google Scholar]
- Sauvé B, Koren G, Walsh G, Tokmakejian S, & Van Uum SHM (2007). Measurement of cortisol in human hair as a biomarker of systemic exposure. Clinical and Investigative Medicine, 30, E183–E191. 10.25011/cim.v30i5.2894 [DOI] [PubMed] [Google Scholar]
- Slominski R, Rovnaghi CR, & Anand KJ (2015). Methodological considerations for hair cortisol measurements in children. Therapeutic Drug Monitoring, 37, 812–820. 10.1097/ftd.0000000000000209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GC, Gutovich J, Smyser C, Pineda R, Newnham C, Tjoeng TH, Vavasseur C, Wallendorf M, Neil J, & Inder T (2011). Neonatal intensive care unit stress is associated with brain development in preterm infants. Annals of Neurology, 70, 541–549. 10.1002/ana.22545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollenaar MS, Jansen J, Beijers R, Riksen-Walraven JM, & de Weerth C (2010). Cortisol in the first year of life: Normative values and intra-individual variability. Early Human Development, 86, 13–16. 10.1016/j.earlhumdev.2009.12.003 [DOI] [PubMed] [Google Scholar]
- Valeri BO, Holsti L, & Linhares MB (2015). Neonatal pain and developmental outcomes in children born preterm: A systematic review. Clinical Journal of Pain, 31, 355–362. 10.1097/ajp.0000000000000114 [DOI] [PubMed] [Google Scholar]
- Vinall J, Miller SP, Bjornson BH, Fitzpatrick KPV, Poskitt KJ, Brant R, Synnes AR, Cepeda IL, & Grunau RE (2014). Invasive procedures in preterm children: Brain and cognitive development at school age. Pediatrics, 133, 412–421. 10.1542/peds.2013-1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives AH, De Angel V, Papadopoulos A, Strawbridge R, Wise T, Young AH, Arnone D, & Cleare AJ (2015). The relationship between cortisol, stress and psychiatric illness: New insights using hair analysis. Journal of Psychiatric Research, 70, 38–49. 10.1016/j.jpsychires.2015.08.007 [DOI] [PubMed] [Google Scholar]
- Wright KD, Ford JL, Perazzo J, Jones LM, Mahari S, Sullenbarger BA, & Laudenslager ML (2018). Collecting hair samples for hair cortisol analysis in African Americans. JoVE (Journal of Visualized Experiments), 136, e57288 10.3791/57288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, Taddio A, Newman C, & Koren G (2007). Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology, 92, 42–49. 10.1159/000100085 [DOI] [PubMed] [Google Scholar]


