Abstract
Nanocarriers (NCs) are promising tools to improve drug delivery across the blood–brain barrier (BBB) for more effective treatment of brain disorders, although there is a scarcity of clinical translation of brain-directed NCs. In order to drive the development of brain-oriented NCs toward clinical success, it is essential to understand the prerequisites for nanodelivery to be successful in brain treatment. In this Perspective, we present how pharmacokinetic/pharmacodynamic (PK/PD), formulation and nanotoxicity factors impact the therapeutic success of brain-specific nanodelivery. Properties including high loading efficiency, slow in vivo drug release, long systemic circulation, an increase in unbound brain-to-plasma concentration/exposure ratio (Kp,uu,brain), high drug potency, and minimal nanotoxicity are prerequisites that should preferably be combined to maximize the therapeutic potential of a brain-targeted NC. The PK of brain-directed NCs needs to be evaluated in a more therapeutically relevant manner, focusing on the released, unbound drug. It is more crucial to increase the Kp,uu,brain than to improve the ability of the NC to cross the BBB in its intact form. Brain-targeted NCs, which are mostly developed for treating brain tumors, including metastases, should aim to enhance drug delivery not just to tumor regions with disrupted BBB, but equally important to regions with intact BBB where the drugs themselves have problems reaching. This article provides critical insights into how a brain-targeted nanoformulation needs to be designed and optimized to achieve therapeutic success in the brain.
Keywords: nanocarrier, brain delivery, therapeutic success, PK/PD, formulation, nanotoxicity
Introduction
Chronic and acute central nervous system (CNS) disorders such as neurodegenerative diseases, neuroinflammation, primary and metastatic brain tumors, ischemic stroke, traumatic brain injury, etc., represent a growing medical problem globally.1,2 To date, it remains very challenging to achieve effective treatment for these diseases, owing to the presence of the blood–brain barrier (BBB), which efficiently regulates the transport of endogenous and exogenous molecules between blood and brain.3,4 Due to the tight junctions between the brain capillary endothelial cells and the extensively expressed efflux transporters, the BBB plays a pivotal role in protecting the CNS, preventing blood-derived toxic molecules from reaching the brain.5,6 However, this protective nature of the BBB also poses an enormous challenge to neurotherapeutic agents, limiting their access to the brain targets at effective concentrations.
The limited success in developing BBB-penetrating drugs has promoted the innovation of various strategies to improve brain drug delivery. Among these strategies, nanocarriers (NCs), e.g., liposome, nanoparticle, micelle, nanoemulsion, nanocrystal, and dendrimer, have emerged as promising approaches that have received increasing research attention from both academia and pharmaceutical industry.7,8 Although many nanoformulations for non-CNS therapies are widely used in clinical practice, clinical development of brain-directed nanoformulations is considerably lagging behind with no clinically approved CNS nanomedicines to date.2,9 Furthermore, ongoing clinical trials of NCs specifically for CNS indications only account for 4% of the total numbers of trials of NCs (data extracted in August 2020) (Table 1). Together, these facts imply that current nanodelivery approaches may be inefficient in surmounting the BBB to an extent that significantly improves the therapeutic index compared to the drug itself. In order to drive the clinical translation, there is a strong need for a better understanding of nanodelivery to the brain, in particular of what the prerequisites are for nanodelivery to achieve clinical success in brain treatment, and how a nanoformulation should be properly designed and optimized.
Table 1. Numbers of Ongoing Clinical Trials of Therapeutic and Diagnostic Nanomedicinesa.
| nos. of
ongoing clinical trials |
||
|---|---|---|
| type of NC | for all indications | for CNS indications |
| liposome | 498 | 11 |
| nanoparticle | 141 | 11 |
| dendrimer | 1 | 0 |
| nanocrystal | 7 | 5 |
| nanoemulsion | 6 | 0 |
| micelle | 8 | 0 |
| sum | 661 | 27 |
All data (not yet recruiting, recruiting, active, and enrolling by invitation cases) were extracted from ClinicalTrials.gov in August 2020.
Another problem limiting the clinical applicability of brain-directed NCs is the lack of in vivo assessments in general. From all of the publications related to NC-mediated brain delivery in PubMed, in vivo evaluations were only involved in less than one-third of the articles (Table 2). When it comes to evaluating the performance of an NC in vivo, assessing pharmacodynamics (PD) (e.g., measuring brain tumor growth) is preferred in most of the studies as an ultimate proof of successful delivery. However, PD measurements are unable to provide any quantitative and direct evidence of how much an NC improves drug delivery to the brain. The absence of a PD effect after NC administration does not necessarily reflect the lack of improvement in brain delivery. Instead, the NC may have increased the delivery, but not to an extent sufficient for a therapeutic concentration to be reached in the brain. Although pharmacokinetics (PK) and biodistribution studies of NCs are sometimes performed together with PD measurements, total drug concentrations in plasma and brain are often measured, which fails to provide any information on released, therapeutically, and toxicologically relevant entities. To date, quantitative assessments on how NCs may affect the released unbound drug remain extremely scarce, which limits the translational potential of brain-specific nanodelivery.10−14 In fact, without the PK of unbound drug in plasma and brain, it is extremely difficult to evaluate the PK/PD relationships and the therapeutic index of an NC.
Table 2. Number of Published Articles (Excluding Reviews) in PubMed Searched with Certain Keywords (Data Extracted in August 2020).
| nos. of publications with or without the additional
keyword “in vivo” |
||
|---|---|---|
| searched keywords | without “in vivo” | with “in vivo” |
| “liposome” and “brain” | 3078 | 603 |
| “nanoparticle” and “brain” | 6115 | 2014 |
| “dendrimer” and “brain” | 275 | 100 |
| “micelle” and “brain” | 644 | 165 |
| “nanoemulsion” and “brain” | 136 | 57 |
| sum | 10 248 | 2939 |
In this Perspective, we first briefly recapitulate the nanodelivery systems suitable for CNS drug delivery before systematically discussing the factors contributing to the in vivo therapeutic success of nanodelivery to the brain (Figure 1). We also discuss the necessity of performing in vivo quantitative studies for NCs, the mechanisms by which NCs interact with the BBB, and whether NCs should aim to improve drug delivery across disease-influenced BBB or healthy BBB. With these aspects discussed, this Perspective aims to provide critical insights on what needs to be considered for clinical success of treating devastating brain diseases and how the properties of a nanoformulation should be optimized in order to better design and develop NC-based brain treatments.
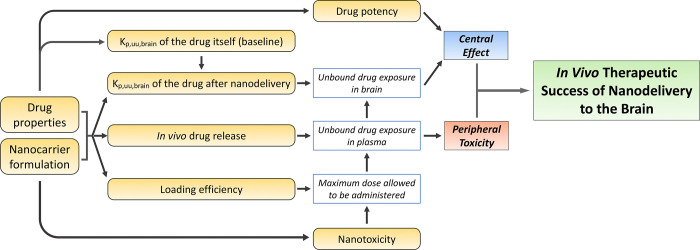
Figure 1.
Schematic representation of factors contributing to the in vivo therapeutic success of nanodelivery to the brain. The NC formulation in conjunction with drug properties could impact loading efficiency, in vivo drug release, and Kp,uu,brain of the drug. Whether or not nanotoxicity occurs is dependent on the NC formulation used. Drug-specific properties like Kp,uu,brain and potency are important. The Kp,uu,brain of the drug itself will determine whether and how much the brain delivery can be improved by nanodelivery. Both loading efficiency and nanotoxicity have an impact on the maximum dose allowed to be administered, which will further influence unbound drug exposure in plasma and brain. In vivo drug release will affect unbound drug exposure in plasma. The Kp,uu,brain of the drug after nanodelivery will influence how high the unbound brain exposure could be. Unbound brain exposure, together with drug potency, will determine the drug effect in the CNS. Drug-induced peripheral side effects are associated with unbound drug exposure in plasma. It is the central effect and peripheral side effect combined that determine the therapeutic success of nanodelivery to the brain.
Current State-of-the-Art for Brain-Directed Nanodelivery
Today, no nanoformulations that specifically aim at increasing drug delivery across the BBB are available on the market. However, there are many clinically approved nanomedicines (nontargeted) mainly for treating non-CNS diseases, especially various cancers.9,15,16 It remains unknown whether or not these nanoformulations are also capable of improving brain delivery compared to the unformulated drug.
NCs that may be clinically useful for brain drug delivery today mainly include liposomes, albumin nanoparticles (NPs), and polymeric NPs. Liposomes have been widely used in clinical practice mainly for non-CNS indications since the first liposomal formulation was approved in 1995 (Doxil). Liposomes feature excellent safety profiles and the ability to encapsulate both hydrophilic and lipophilic therapeutic agents, including both small molecules and large biologics without the need to modify the compounds.2,17 Currently, there are only a limited number of clinical trials in which liposomal formulations are investigated for treating brain diseases. In the majority of these trials, marketed nontargeted liposomal formulations are used either alone or in combination with other drugs. Several examples include liposomal irinotecan (Onivyde) for brain metastases (ClinicalTrails.gov: NCT03328884), liposomal cytarabine (DepoCyt) together with rituximab and methotrexate for CNS prophylaxis of lymphoma (ClinicalTrails.gov: NCT00945724), and liposomal amphotericin B (AmBisome) for cryptococcal meningitis (ClinicalTrails.gov: NCT03945448). The only brain-targeted liposomal formulation that has been tested in clinical research is glutathione PEGylated liposomal doxorubicin (2B3–101) using glutathione (GSH) as a BBB-targeting ligand. 2B3–101 has completed a Phase I/IIa trial in patients with gliomas or brain metastases (ClinicalTrails.gov: NCT01386580) and is currently being investigated in a Phase II trial for treating breast cancer with leptomeningeal metastases (ClinicalTrails.gov: NCT01818713). In preclinical studies, a variety of BBB-targeting ligands including antibodies, peptides, proteins and small molecules have been investigated in combination with liposomes for improved brain delivery.17,18 The enhanced pharmacological effects in vivo have been often shown as proof of delivery in these studies.
Albumin NPs have also been extensively used in the clinic with Abraxane (nanoparticles albumin-bound paclitaxel) approved in 2005 by the FDA for cancer treatments.2,19 Currently, a new nanoformulation, nanoparticle albumin-bound rapamycin (ABI-009), is being studied in multiple clinical trials for treating different CNS disorders, including high-grade glioma and glioblastoma, Leigh or Leigh-like syndrome, and surgically refractory epilepsy (ClinicalTrails.gov: NCT03463265, NCT03747328, and NCT03646240). To improve drug delivery across the BBB, albumin nanoparticles have been tested in many preclinical studies, either without a BBB-targeting ligand20 or with a ligand like transferrin,21 apolipoprotein (Apo) A-I, B-100, and E,22,23 cell-penetrating peptide,24 or antitransferrin/insulin receptor antibodies.25,26 The improved brain delivery in these studies was shown based on in vivo brain distribution, pharmacological evaluation (e.g., antitumor efficacy), or visualization techniques like transmission electron microscopy.
Polymeric NPs are the most studied NCs in preclinical research. However, their clinical translation remains slow with only limited investigations in clinical trials, none of them focusing on brain delivery. The commonly used polymers are biodegradable and biocompatible including poly(butyl cyanoacrylate) PBCA, poly(lactic-co-glycolic acid) PLGA, and chitosan.2 As summarized in several reviews, various moieties like cell-penetrating peptides, Apo E, angiopep-2, transferrin, and antitransferrin receptor antibody have been tested as BBB-targeting ligands conjugated on polymeric NPs, and brain-targeting effects have been shown from in vivo studies.19,27,28 However, nanotoxicity remains a huge issue for polymeric NPs, potentially limiting their clinical translation.19,29 When applying polymeric NPs for brain delivery, it is worth noting that nanotoxicity may lead to (temporary) BBB opening and potentially even result in neurotoxicity if intact NPs cross the BBB.27,30
There are also some other types of NCs involved in clinical studies. For example, gold nanocrystals (CNM-Au8) are currently being evaluated in multiple Phase II trials for the treatment of different CNS disorders such as multiple sclerosis, Parkinson’s diseases, and amyotrophic lateral sclerosis (ClinicalTrails.gov: NCT03993171, NCT03815916, NCT03843710, NCT04098406, and NCT03536559). Another novel nanoformulation is bacterially derived nanocells encapsulating doxorubicin with tumor-targeting bispecific antibodies (EGFR(V)-EDV-Dox), which is being investigated in a Phase I trial for glioblastoma multiforme (ClinicalTrails.gov: NCT02766699).
What Factors Could Impact the Therapeutic Success of Nanodelivery to the Brain
Multiple factors could determine the in vivo therapeutic success of NC-mediated brain delivery through their influence on the maximum dose administered, unbound drug exposure in brain or plasma, central effect, and/or peripheral toxicity (Figure 1). These factors can be divided into three categories: PK/PD factors, NC formulation, and nanotoxicity.
PK/PD Factors
The Unbound Brain-to-Plasma Exposure Ratio (Kp,uu,brain)
The most therapeutically relevant measurement of brain exposure is based on unbound drug concentrations. One way of evaluating these concentrations is to estimate the partitioning coefficient of the unbound drug across the BBB (Kp,uu,brain).31,32 This parameter describes the ratio of target site exposure associated with a central effect to off-target site exposure (unbound plasma concentrations) related to a peripheral side effect. Kp,uu,brain is the most important parameter in CNS drug discovery to evaluate drug candidates for brain action and can be used to estimate the dose needed for central action. Briefly, a Kp,uu,brain around unity suggests predominant passive diffusion or similar efflux and influx transport at the BBB. If Kp,uu,brain is below unity, active efflux is more efficient than active influx, while a Kp,uu,brain higher than unity indicates that active influx dominates the transport at the BBB.32,33
Kp,uu,brain is also a critical parameter to investigate and optimize when developing NC-based brain treatments.34 By comparing the Kp,uu,brain values of a drug with or without nanoencapsulation, the ability of nanodelivery to influence drug transport across the BBB could be quantitatively evaluated, without being confounded by other in vivo processes of the NC. The more the Kp,uu,brain can be increased, the more therapeutically effective and less peripherally toxic the nanodelivery would be.
The Kp,uu,brain of the drug payload itself plays a key role in the therapeutic success of nanodelivery to the brain. For drugs with active efflux at the BBB (Kp,uu,brain < 1), NCs could potentially increase their Kp,uu,brain if the right formulation is chosen.10,12 However, depending on how low the Kp,uu,brain is for the drug itself, the magnitude of Kp,uu,brain increase by nanodelivery required for therapeutic success may be different. For example, for a drug with Kp,uu,brain of 0.1, a 10-fold increase in Kp,uu,brain by nanodelivery would be adequate to elicit brain effect if the required therapeutic concentration in the brain is similar to the unbound plasma concentration. However, for a drug with Kp,uu,brain of 0.01, a 100-fold increase in Kp,uu,brain would be required from the NC if the therapeutically relevant concentration is at the same level as the unbound plasma concentration. In general, given similar potency and unbound plasma exposure, drugs with more efficient efflux at the BBB would pose a greater challenge for nanodelivery and require a higher increase in Kp,uu,brain to achieve therapeutic success.
For drugs that already show active uptake at the BBB (Kp,uu,brain > 1), NC encapsulation will very likely not further increase their brain uptake, but rather reduce the Kp,uu,brain and, therefore, therapeutic performance. This is exemplified by two recent studies showing that encapsulation in PEGylated liposomes and lipid core nanocapsules significantly decreased the Kp,uu,brain of diphenhydramine and quetiapine.11,35
Potency
The therapeutic potency and Kp,uu,brain of a CNS drug combined determine whether the drug will be pharmacologically effective in the CNS without being toxic in the periphery. Given similar Kp,uu,brain values, drugs with higher therapeutic potency can more easily elicit brain effect since the required therapeutic concentration is lower compared to less potent drugs. Some highly potent CNS drugs, like risperidone and paliperidone, can still exert their effect in the brain even if they penetrate the BBB to a limited extent.36
From our previous studies, the increase in Kp,uu,brain resulting from nanodelivery was found to be maximally 15-fold for methotrexate.10,12−14 Although 15-fold represents a large improvement, it is not guaranteed that nanodelivery can increase the Kp,uu,brain to the same or even larger magnitude for any given drug. Therefore, high therapeutic potency is a prerequisite for successful nanodelivery to the brain, as it will increase the possibility of attaining therapeutic concentrations in the CNS, even if the NC would not drastically improve Kp,uu,brain. A good example to show how drug potency limits the therapeutic success of nanodelivery is an earlier study on DAMGO, a low potent opioid peptide.13 Although the Kp,uu,brain of DAMGO was doubled from 0.05 to 0.1 when delivered with glutathione PEGylated liposomes, the unbound brain concentration of DAMGO was still below the therapeutic level, although the maximally possible NC dose was administered.
Low toxic potency in the periphery is also a prerequisite for successful nanodelivery to the brain, especially when the NC is not able to substantially increase Kp,uu,brain. This is because, with lower toxic potency in the periphery, the maximum tolerated drug dose will be higher. As a result, the NC can be given at a higher drug dose to achieve desired therapeutic concentrations in the brain.
Half-life
A favorable feature of NC encapsulation is the possibility of prolonging plasma half-life by, e.g., coating the NC with a hydrophilic molecule like polyethylene glycol (PEG). A longer half-life is achieved by the slow release from the NC, as well as by minimal systemic elimination of the intact NC. After administration of a nanoformulation, the half-life of the released, unbound drug is extended with broader and flatter PK profiles, with a decreased peak concentration (Cmax) but a similar area under the curve (AUC) compared to the unformulated drug. Given that the central effect of the drug is AUC-driven and the peripheral toxicity is Cmax-driven, the prolonged half-life by nanoencapsulation was proven to increase the therapeutic index by reducing peripheral side effects.34 If the PD effect is driven by the unbound drug concentration in the brain, a prolonged drug half-life will allow brain action to last longer compared to the unformulated drug.
The ability of NCs to protect payloads from degradation in plasma and prolong circulation time could be particularly important for biologic payloads like peptides and small interfering RNAs (siRNAs). After systemic administration in free form, these macromolecules often undergo rapid elimination or degradation in blood circulation, exhibiting unfavorable PK profiles with plasma half-lives of just a few minutes, which greatly limits their therapeutic potential in the CNS.37,38 Formulating these biologics in NCs has been proven to be effective in solving their stability issue in vivo. For example, encapsulation in liposomes dramatically increased the half-life of DAMGO (6.9 h vs 9.2 min of free DAMGO).39 A similar finding was also shown for siRNA when formulated in PEGylated liposomes, with extended half-life compared to unformulated siRNA.40 In fact, we have previously found that a CNS drug with a shorter half-life in itself will benefit more therapeutically from NC encapsulation.34 Therefore, for CNS-acting peptides and siRNAs with extremely short circulation times, nanodelivery holds the potential to tremendously improve their therapeutic performance. Another issue is whether nanoencapsulation will also improve uptake across the BBB, which would further improve the gain of the formulation. However, according to our simulations, in this case, a significant improvement is the protection from degradation and prolonged half-life in plasma.34
NC Formulation
In the current nanodelivery field, too much attention is paid to designing innovative NC formulations and characterizing their in vitro properties like size, charge, morphology, in vitro release, and cellular uptake, which are, of course, important to evaluate. However, all of these in vitro characterizations are of less value if not connected with in vivo assessments. In fact, the NC formulation in conjunction with the drug properties could simultaneously impact multiple in vitro and in vivo properties, including loading efficiency, in vivo drug release, and Kp,uu,brain, which ultimately determines the opportunity of achieving therapeutic success.
The composition of an NC (e.g., containing different phospholipids) and the type of NC (e.g., liposomes vs nanoparticles), together with the drug properties, will determine the drug loading efficiency. For instance, the loading efficiency of methotrexate was lower in PEG liposomes with hydrogenated soy phosphatidylcholine (HSPC) than in egg-yolk phosphatidylcholine (EYPC) counterparts.12 While liposomes can obtain a loading efficiency of more than 90% when using a remote loading method,41 polymeric NPs normally allow approximately 10% of the drug to be encapsulated.19 The loading efficiency of diphenhydramine in PEG-EYPC liposomes is much lower than that of methotrexate in the same formulation.11,12 An NC formulation with higher loading efficiency would meet the required therapeutic concentration/exposure in the brain more easily, as the maximum drug dose allowed to be given is higher with the same volume administered. As exemplified from the above-mentioned DAMGO case, high loading efficiency of an NC is particularly important when delivering drugs with low potency to the CNS. Improving the loading efficiency solely is, however, inadequate for improving the therapeutic index and has to be combined with additional changes in NC properties (release rate or Kp,uu,brain) to obtain this goal.
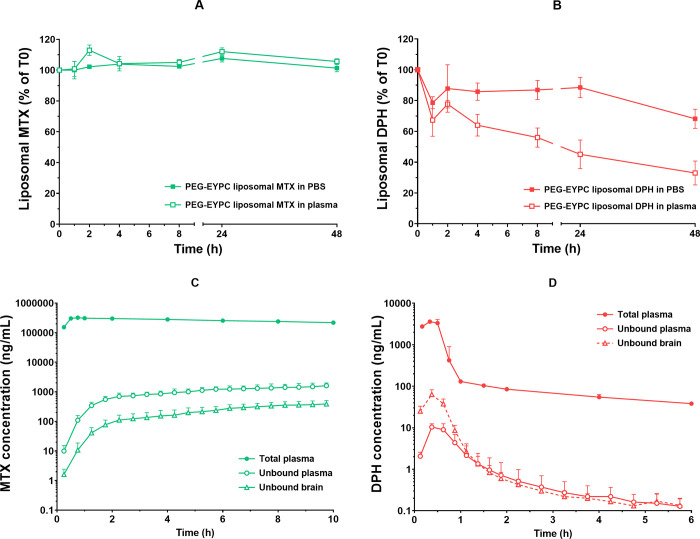
The in vivo drug release properties will naturally be different depending on the NC formulation as well as the payload drug. To illustrate, methotrexate was released faster from EYPC-based than from HSPC-based liposomal formulations, reflected by significantly higher unbound-to-total plasma concentration ratios of methotrexate from PEG-EYPC compared to PEG-HSPC formulations.10,12 Furthermore, when encapsulating in PEG-EYPC liposomes, diphenhydramine was released much faster compared to methotrexate based on both in vitro and in vivo findings (Figure 2).11,12 In a simulation study, the in vivo drug release was found to be strongly associated with therapeutic performance due to its influence on peripheral side effects.34
Figure 2.
Different in vitro and in vivo release of PEG-EYPC liposomal formulation encapsulating methotrexate or diphenhydramine. After incubation in phosphate-buffered saline (PBS) and rat plasma at 37 °C up to 48 h, (A) PEG-EYPC liposomal methotrexate had excellent stability in vitro with minimal drug release. (B) Instability of PEG-EYPC liposomal diphenhydramine was found with faster drug release in plasma than in PBS. The concentration–time profiles of unbound drug concentration in brain interstitial fluid (open triangles) and plasma (open circles) and total drug concentration in plasma (filled circles) after 30 min intravenous infusion of (C) PEG-EYPC liposomal methotrexate or (D) PEG-EYPC liposomal diphenhydramine. In line with the in vitro findings, PEG-EYPC liposomal methotrexate was notably stable in systemic circulation with a long half-life and sustainable drug release, reflected by PK profiles of total and unbound drug in plasma. A very different biphasic PK profile of total diphenhydramine in plasma was observed after PEG-EYPC liposomal diphenhydramine was administered. The fast decline in the early period indicates a fast diphenhydramine release from the liposomes early after administration, which correlates with the in vitro results (redrawn with permission from the publishers11,12).
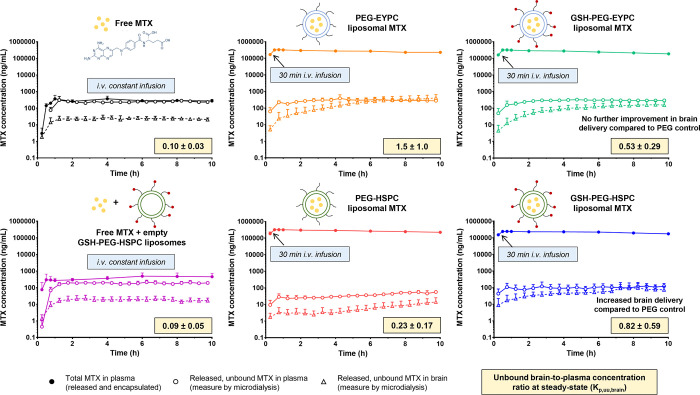
Likely, the most important factor for improving the therapeutic index is how the NC formulation is capable of increasing the Kp,uu,brain of a drug, as this will give a distribution advantage and improve the central effect without necessarily influencing peripheral toxicity. As an example of in vivo differences between NC formulations, it was found that, while PEG-EYPC liposomes substantially increased the Kp,uu,brain of methotrexate, formulations based on HSPC did not affect the Kp,uu,brain at all.12,14 Furthermore, glutathione (GSH), as a BBB-anchoring ligand conjugated to PEG liposomes of methotrexate, showed a brain-targeting effect only when it was combined with the HSPC-based but not the EYPC-based formulation (Figure 3).12
Figure 3.
Unbound brain-to-plasma concentration ratios at steady state (Kp,uu,brain) and observed concentration–time profiles for the unbound drug concentration in brain interstitial fluid (open triangles) and plasma (open circles), and total drug concentration in plasma (filled circles) after intravenous administration of free methotrexate, free methotrexate + empty liposomes, and different liposomal formulations10 (with permission from the publisher).
Based on our experience, the in vivo performance of NC formulations are very difficult to predict from in vitro experiments. Therefore, aiming to maximize the therapeutic potential in the brain, the NC formulation should be carefully optimized to possess several favorable features (ideally combined) including high loading efficiency, slow in vivo release rate, and large enhancement in Kp,uu,brain.
As formulation and drug properties combined decide in vitro and in vivo properties of an NC, it is unrealistic to expect that one nanoformulation would universally be suitable to deliver any given drug to the brain. Depending on the drug to be encapsulated, the NC formulation needs to be specifically designed and optimized. In the current nanodelivery field, a common approach to test and visualize whether an NC can improve brain delivery is in vivo fluorescence imaging. This approach involves loading an NC with a fluorescent dye. After administration of a dye-loaded NC, fluorescence intensity is detected in the whole body of a living small animal or brain sections by a sensitive camera.42,43 However, this method is problematic since improved brain delivery of the dye does not necessarily guarantee a similar improvement of the actual drug that the NC aims to deliver.44 It was also shown that the brain distribution of different fluorescent dyes varied when delivered with the same NPs.44 Therefore, it is crucial to view the NC and drug to be delivered as an integrated system, analyzing the actual drug, not a drug surrogate.
Nanotoxicity
In vivo safety concerns about nanomaterials like polymers, especially after repeated administration of NCs, remain a key factor limiting NCs’ human use.45,46 The potential toxicities associated with the constituted nanomaterials (so-called nanotoxicity) include acute and/or chronic peripheral immunogenicity, (temporary) BBB disruption, and even neurotoxicity. If any nanotoxicity occurs, there would be dose limits for the nanomaterials. Consequently, the NC may not be administered at the required drug dose to reach therapeutic exposure in the brain.
To minimize potential nanotoxicity, it is important to choose the proper types of NC and safe nanomaterials. Liposomes have better safety features compared to other types of NCs and are normally nontoxic in both CNS and periphery, as they are composed of biocompatible lipids.47 From a functional perspective, liposomes do not seem to influence the BBB integrity as coadministering empty liposomes with unformulated drugs did not impact their Kp,uu,brain compared to administering unformulated drugs alone.10,12,14 In order to produce polymeric NPs with acceptable safety profiles, it is essential to use biocompatible and biodegradable polymers like PLGA, although the potential toxicity, particularly long-term toxicity, of polymeric NPs remains elusive. As combining NCs with BBB-targeting ligands may be required to enable CNS-targeted delivery, it is also critical to ensure that these ligands are not immunogenic and will not lead to BBB disruption or neurotoxicity. In this regard, endogenous molecules with known safety and compatibility properties like glutathione and transferrin may be better choices as targeting ligands compared with exogenous moieties like antitransferrin receptor antibodies or synthesized cell-penetrating peptides.
Increasing the loading efficiency and Kp,uu,brain of a brain-directed NC may also lower the risk of nanotoxicity. In both cases, the NC can be administered at a lower excipient dose and thereby possibly decrease the risk of toxicity.2
How to Evaluate the In Vivo Performance/Success of Nanodelivery to the Brain
Currently, there are still methodological issues regarding how to properly evaluate the performance/success of nanodelivery to the brain in vivo. PD measurements like nociceptive tests, behavior tests, tumor growth, and survival rate can be used as the ultimate proof of whether brain drug delivery benefits from nanoencapsulation. However, for any brain-targeted NC developed toward clinical application, evaluating PD solely is not optimal and has to be combined with PK assessments in order to accurately describe the PK/PD relationships for both effectiveness and safety.
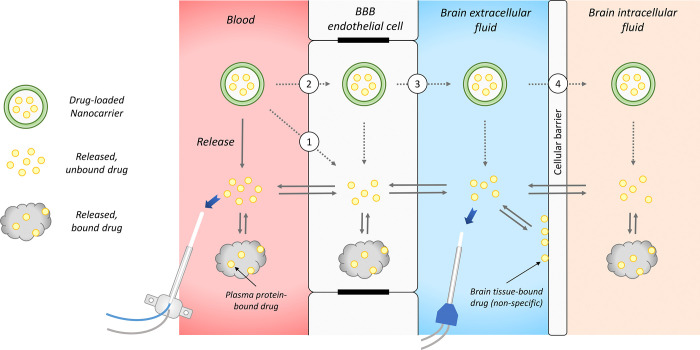
When it comes to evaluating PK of brain-targeted NCs, most of the studies focus on determining total drug concentrations (encapsulated plus released) in plasma and whole brain tissue.48 However, this is insufficient if the purpose is to provide information on possible improvements in brain delivery. After the administration of an NC, there are three drug entities in plasma: NC-encapsulated drug, released plasma protein-bound drug, and released drug in the unbound form (Figure 2). If the NC can cross the BBB in intact form, there would be three similar entities in the brain interstitial fluid (ISF) as well (Figure 4). Measuring only the total drug is obviously not able to differentiate the NC-encapsulated drug (normally with very high concentration) from the released, unbound drug being the therapeutically/toxicologically relevant moiety.
Figure 4.
Potential in vivo “fate” of brain-directed NCs and the critical role of microdialysis in evaluating the in vivo performance of nanodelivery to the brain. After administration of an NC in blood, the drug payload will release from the NC. Once the drug is released, it will behave based on its own properties, being transported across the BBB and cellular barrier and also binding to plasma protein, brain cellular membrane, and intracellular components. NCs may contribute to improved brain drug delivery through several proposed mechanisms: (1) NCs interact and fuse with the BBB endothelial cell membrane and then release the drug to the endothelial cells. (2) NCs are endocytosed into BBB endothelial cells, followed by drug release within the endothelial cells. (3) NCs are transcytosed across the BBB, before releasing the drug in brain extracellular fluid. (4) Transcytosed NCs are further internalized into brain cells, after which the drug is released intracellularly. Microdialysis separates the released, unbound drug from the drug remaining in the NC, enabling continuous quantifying therapeutically and toxicologically relevant drug entities over time, as described by the blue arrows.
Another limitation associated with analyzing whole brain tissue is that only one terminal brain samples can be taken from one individual. As a result, the time aspects of brain delivery cannot be examined without substantially increasing the use of animals. Furthermore, the contamination of NC-associated drug in the brain tissue either from the residue blood or from NC bound to endothelial cells (if the residual blood is completely removed through perfusion) may confound the quantification of the drug that has actually entered the brain.
Microdialysis is a valuable and probably the best tool for PK evaluation of nanodelivery to the brain, as long as the delivered drug is microdialysable and the study design is proper.10−14,35,49 The unique feature of a microdialysis probe is that it has a semipermeable membrane, thus allowing only the unbound drug concentrations to be measured continuously. Therefore, microdialysis is able to separate the released, biologically active entity from the encapsulated drug and the released, protein-bound drug as the biologically inactive entities. By combining microdialysis with regular blood sampling, processes like in vivo drug release and drug transport across the BBB can be quantitatively and separately assessed over time.
The major limitation of microdialysis is that it cannot be applied to lipophilic drugs, as these drugs tend to stick to microdialysis tubings and probes and therefore compromise the reliability of the measurements.50 Therefore, when trying to quantitatively evaluate the nanodelivery of lipophilic drugs to the brain, other techniques are needed.
The ultrafiltration method with a stable isotope tracer can be useful in evaluating unbound drug concentrations in plasma after administration of a nanoformulation, irrespective of the lipophilicity of the drug payload.51,52 However, the usefulness of ultrafiltration in assessing unbound drug levels in whole brain tissue is limited. This is because the required homogenization of brain tissue prior to ultrafiltration may destroy the intact NCs that potentially enter brain parenchyma and release the encapsulated drug, thereby leading to an overestimation of unbound drug concentrations in the brain.
Cerebral open flow microperfusion (cOFM), as a novel in vivo technique for continuous sampling of brain ISF, can be useful in measuring brain drug concentrations after administration of nanoformulations. As cOFM allows unfiltered and nondialyzed sampling in brain ISF without certain cutoff, it overcomes the limitations of microdialysis and can be theoretically used to study all substances regardless of their lipophilicity.53,54 However, since cOFM samples are unfiltered, they include both unbound drug and NC-encapsulated drug, if intact NCs cross the BBB. They need to be further differentiated using, i.e., ultrafiltration, in order to determine drug concentrations in each entity.55 Therefore, cOFM, if combined with other separation techniques, would provide similar information on unbound drug concentrations in the brain as microdialysis, and would also offer additional mechanistic insights on whether intact NC could cross the BBB by potentially analyzing the separated NC-encapsulated drug entity. Overall, despite the complexity of analytical procedures, cOFM sampling combined with ultrafiltration might potentially be applied to quantitatively and mechanistically evaluate nanodelivery of lipophilic drugs to the brain. However, this combination is not yet tested.
In general, the crucial role of microdialysis in separating the released, unbound drug concentrations from the NC-encapsulated drug over time is irreplaceable, as there are yet no other techniques proven to achieve this goal.
Kp,uu,brain Increase More Therapeutically Important than NC Transcytosis
The possible mechanisms by which NCs could improve drug delivery across the BBB have been summarized in several excellent reviews.27,45,56 The major mechanisms proposed include (Figure 4): (1) NCs interact with the BBB endothelial cell membrane, followed by membrane fluidization with the NC, thereby facilitating drug penetration into the endothelial cells and then the brain; (2) NCs are endocytosed into the endothelial cells, after which the drug is released within the cells and delivered into the brain; (3) NCs are transcytosed in intact form across the endothelial cells, before releasing the drug in brain ISF; (4) NCs are internalized into brain cells and release the drug intracellularly. NCs may influence drug transport across the BBB in a more complex manner than expected, involving multiple above-mentioned mechanisms simultaneously. However, based on current methods/models, it remains very challenging to explore whether the actual mechanism involves any or several of the four proposed ones. Although in vitro cellular models may be useful for a mechanistic understanding of how NCs facilitate drug delivery at the BBB, it is difficult to confirm the mechanisms based on in vivo models.2 Fluorescence or electron microscopy may serve as useful tools to analyze in vivo samples, visualizing if NCs are within endothelial cells or they have crossed the BBB.23,57
It is our opinion that NCs do not necessarily have to cross the BBB in the intact form in order to improve brain delivery and therapeutic effect. This is exemplified by earlier studies where nontargeted PEG liposomes that are considered to be incapable of penetrating the BBB by themselves could drastically increase the brain uptake (Kp,uu,brain) of methotrexate.10,12 For a drug that has an intracellular site of action in the brain, an increased Kp,uu,brain by nanodelivery can also help elicit higher intracellular concentrations and thereby PD effects. This is because the poor BBB penetration, rather than limited intracellular distribution, is often the major reason for unsuccessful treatment.36 Once enough drug is delivered into the brain ISF, it will be more likely to exert the PD effect intracellularly, since many drugs have intracellular-to-extracellular concentration ratio values around unity.36,58
From a safety perspective, it may even be preferable that an NC could improve the Kp,uu,brain of the drug payload without entering the brain in its intact form, as this will reduce the risk of neurotoxicity associated with the nanomaterial. In the current field of nanodelivery to the brain, a biocompatible way of thinking is generally lacking. There are many studies in which nanotherapeutics were directly injected into the brain (mainly tumor) through, i.e., convection-enhanced delivery to circumvent the BBB or were given intravenously combined with BBB opening techniques (e.g., focused ultrasound plus microbubbles), aiming at facilitating NC accumulation in the brain parenchyma.59−62 However, although increased brain accumulation of intact NC may elevate unbound drug concentration at the site of action, nanomaterial-induced neurotoxicity remains a huge concern, which may ultimately limit the applicability of any nanodelivery involving BBB bypassing or disruption.
Therefore, when developing an NC-based brain delivery system, more focus should be put on investigating how much an NC could increase the uptake across the BBB, rather than if the NC could in itself enter the brain.
NCs Should Aim to Improve Drug Delivery Across Not Just the Tumor-Affected BBB
Currently, NCs have been mainly developed to deliver oncologic drugs, normally poor BBB-penetrants, to the CNS for the treatment of brain tumors. It is well-known that various pathological conditions, including brain tumors, can disrupt the integrity and function of the BBB.63−65 However, the BBB disruption in primary tumors like glioblastoma multiforme or brain metastases is heterogeneous depending on tumor region and individual tumor.66,67 Brain primary and metastatic tumors are highly infiltrative and, therefore, need to be treated as whole brain diseases. Therapeutic levels of chemotherapeutic drugs may be successfully delivered to the tumor core, where the BBB is disrupted.68,69 However, at the tumor rim as well as in regions where the tumors just start to grow, the BBB mostly remains intact.70,71 As a result, the treatment at these regions can be ineffective, since anticancer drugs normally have poor penetration across the intact BBB. Ultimately, the failure to effectively deliver oncologic drugs to all regions where brain tumor cells are present will become a major reason for unsuccessful treatment.66 Thus, when delivering antitumor drugs with NCs for treating brain cancers including metastases, it is equally important to improve drug delivery to the tumor regions with a BBB disruption as well as to the regions with a healthy BBB. Therefore, in preclinical evaluations, it is crucial to show the ability of NCs to enhance the delivery of an anticancer agent also to a healthy brain.
Conclusions and Outlook
For a brain-targeted NC, the prerequisites for successful brain treatment while having minimal peripheral toxicity include high loading efficiency, slow in vivo drug release, long systemic circulation, a large increase in Kp,uu,brain, high drug potency, and minimal nanotoxicity. These properties should preferably be combined in one nanoformulation in order to maximize the therapeutic performance in the CNS. The therapeutic potential of a brain-directed NC can be determined by multiple factors including the improvement in Kp,uu,brain by nanodelivery, NC-driven modulation of drug half-life, the potency of the drug payload, in vivo drug release properties, loading efficiency of the NC, NC formulation (affects all above-mentioned factors), and drug- or nanomaterial-induced toxicity.
From therapeutic and safety perspectives, it is more critical to elevate Kp,uu,brain for a brain-targeted NC than to enhance the BBB-crossing of the NC in intact form. This is not only because Kp,uu,brain is the parameter directly and quantitatively linked to CNS therapeutic effect versus peripheral toxicity (drug-induced) but also because the intact NC transcytosed into the brain will increase the risk of neurotoxicity. When developing an NC-based treatment for brain tumors, it is crucial to show that the NC is capable of improving drug delivery not just across the tumor-affected BBB but equally important across the healthy BBB to ensure effective treatments of all tumor sites.
It is our opinion that scientists from the nanoformulation, PK/PD, and toxicology fields should work collaboratively, understanding the prerequisites for nanodelivery to the brain and how to properly design and optimize a brain-directed nanoformulation. With all of this combined, we believe that the clinical success of nanomedicine-based CNS treatments will be achieved in the future.
The authors declare no competing financial interest.
References
- Vieira D. B.; Gamarra L. F. Getting into the brain: liposome-based strategies for effective drug delivery across the blood–brain barrier. Int. J. Nanomed. 2016, 11, 5381–5414. 10.2147/IJN.S117210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard P. J.; Visser C. C.; Boer M.; Appeldoorn C. C.; Rip J.. Blood-to-brain drug delivery using nanocarriers. In Drug delivery to the brain; Hammarlund-Udenaes M., de Lange E. C., Thorne R. G., Eds.; Springer: New York, 2014; pp 433–54. [Google Scholar]
- Abbott N. J. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J. Inherited Metab. Dis. 2013, 36 (3), 437–49. 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- Abbott N. J.; Patabendige A. A.; Dolman D. E.; Yusof S. R.; Begley D. J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37 (1), 13–25. 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Abbott N. J.; Ronnback L.; Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7 (1), 41–53. 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- de Lange E. C.; Hammarlund-Udenaes M. Translational aspects of blood–brain barrier transport and central nervous system effects of drugs: from discovery to patients. Clin. Pharmacol. Ther. 2015, 97 (4), 380–94. 10.1002/cpt.76. [DOI] [PubMed] [Google Scholar]
- Karim R.; Palazzo C.; Evrard B.; Piel G. Nanocarriers for the treatment of glioblastoma multiforme: Current state-of-the-art. J. Controlled Release 2016, 227, 23–37. 10.1016/j.jconrel.2016.02.026. [DOI] [PubMed] [Google Scholar]
- Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know?. Adv. Drug Delivery Rev. 2014, 71, 2–14. 10.1016/j.addr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Choi Y. H.; Han H. K. Nanomedicines: current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Invest. 2018, 48 (1), 43–60. 10.1007/s40005-017-0370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.; Gaillard P. J.; de Lange E. C. M.; Hammarlund-Udenaes M. Targeted brain delivery of methotrexate by glutathione PEGylated liposomes: How can the formulation make a difference?. Eur. J. Pharm. Biopharm. 2019, 139, 197–204. 10.1016/j.ejpb.2019.04.004. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Gaillard P. J.; Rip J.; de Lange E. C. M.; Hammarlund-Udenaes M. In Vivo Quantitative Understanding of PEGylated Liposome’s Influence on Brain Delivery of Diphenhydramine. Mol. Pharmaceutics 2018, 15 (12), 5493–5500. 10.1021/acs.molpharmaceut.8b00611. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Rip J.; Gaillard P. J.; de Lange E. C. M.; Hammarlund-Udenaes M. The Impact of Liposomal Formulations on the Release and Brain Delivery of Methotrexate: An In Vivo Microdialysis Study. J. Pharm. Sci. 2017, 106 (9), 2606–2613. 10.1016/j.xphs.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Lindqvist A.; Rip J.; Gaillard P. J.; Bjorkman S.; Hammarlund-Udenaes M. Enhanced brain delivery of the opioid peptide DAMGO in glutathione pegylated liposomes: a microdialysis study. Mol. Pharmaceutics 2013, 10 (5), 1533–41. 10.1021/mp300272a. [DOI] [PubMed] [Google Scholar]
- Lindqvist A.; Rip J.; van Kregten J.; Gaillard P. J.; Hammarlund-Udenaes M. In vivo Functional Evaluation of Increased Brain Delivery of the Opioid Peptide DAMGO by Glutathione-PEGylated Liposomes. Pharm. Res. 2016, 33 (1), 177–185. 10.1007/s11095-015-1774-3. [DOI] [PubMed] [Google Scholar]
- Weissig V.; Pettinger T. K.; Murdock N. Nanopharmaceuticals (part 1): products on the market. Int. J. Nanomed. 2014, 9, 4357–73. 10.2147/IJN.S46900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmo A. C.; Mitragotri S. Nanoparticles in the clinic. Bioeng Transl Med. 2016, 1 (1), 10–29. 10.1002/btm2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rip J. Liposome technologies and drug delivery to the CNS. Drug Discovery Today: Technol. 2016, 20, 53–58. 10.1016/j.ddtec.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Lai F.; Fadda A. M.; Sinico C. Liposomes for brain delivery. Expert Opin. Drug Delivery 2013, 10 (7), 1003–22. 10.1517/17425247.2013.766714. [DOI] [PubMed] [Google Scholar]
- Costantino L.; Boraschi D. Is there a clinical future for polymeric nanoparticles as brain-targeting drug delivery agents?. Drug Discovery Today 2012, 17 (7–8), 367–78. 10.1016/j.drudis.2011.10.028. [DOI] [PubMed] [Google Scholar]
- Wan X.; Zheng X.; Pang X.; Pang Z.; Zhao J.; Zhang Z.; Jiang T.; Xu W.; Zhang Q.; Jiang X. Lapatinib-loaded human serum albumin nanoparticles for the prevention and treatment of triple-negative breast cancer metastasis to the brain. Oncotarget 2016, 7 (23), 34038–51. 10.18632/oncotarget.8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra V.; Mahor S.; Rawat A.; Gupta P. N.; Dubey P.; Khatri K.; Vyas S. P. Targeted brain delivery of AZT via transferrin anchored pegylated albumin nanoparticles. J. Drug Target 2006, 14 (1), 45–53. 10.1080/10611860600612953. [DOI] [PubMed] [Google Scholar]
- Kreuter J.; Hekmatara T.; Dreis S.; Vogel T.; Gelperina S.; Langer K. Covalent attachment of apolipoprotein A-I and apolipoprotein B-100 to albumin nanoparticles enables drug transport into the brain. J. Controlled Release 2007, 118 (1), 54–8. 10.1016/j.jconrel.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Zensi A.; Begley D.; Pontikis C.; Legros C.; Mihoreanu L.; Wagner S.; Buchel C.; von Briesen H.; Kreuter J. Albumin nanoparticles targeted with Apo E enter the CNS by transcytosis and are delivered to neurones. J. Controlled Release 2009, 137 (1), 78–86. 10.1016/j.jconrel.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Lin T.; Zhao P.; Jiang Y.; Tang Y.; Jin H.; Pan Z.; He H.; Yang V. C.; Huang Y. Blood-Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano 2016, 10 (11), 9999–10012. 10.1021/acsnano.6b04268. [DOI] [PubMed] [Google Scholar]
- Ulbrich K.; Hekmatara T.; Herbert E.; Kreuter J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71 (2), 251–6. 10.1016/j.ejpb.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Ulbrich K.; Knobloch T.; Kreuter J. Targeting the insulin receptor: nanoparticles for drug delivery across the blood–brain barrier (BBB). J. Drug Targeting 2011, 19 (2), 125–132. 10.3109/10611861003734001. [DOI] [PubMed] [Google Scholar]
- Masserini M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013, 2013, 1–18. 10.1155/2013/238428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzoni E.; Cesaretti A.; Polchi A.; Di Michele A.; Tancini B.; Emiliani C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10 (1), 4. 10.3390/jfb10010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean T.; Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Delivery Rev. 2010, 62 (1), 3–11. 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Rempe R.; Cramer S.; Huwel S.; Galla H. J. Transport of Poly(n-butylcyano-acrylate) nanoparticles across the blood–brain barrier in vitro and their influence on barrier integrity. Biochem. Biophys. Res. Commun. 2011, 406 (1), 64–9. 10.1016/j.bbrc.2011.01.110. [DOI] [PubMed] [Google Scholar]
- Gupta A.; Chatelain P.; Massingham R.; Jonsson E. N.; Hammarlund-Udenaes M. Brain distribution of cetirizine enantiomers: comparison of three different tissue-to-plasma partition coefficients: K(p), K(p,u), and K(p,uu). Drug Metab. Dispos. 2006, 34 (2), 318–23. 10.1124/dmd.105.007211. [DOI] [PubMed] [Google Scholar]
- Hammarlund-Udenaes M.; Friden M.; Syvanen S.; Gupta A. On the rate and extent of drug delivery to the brain. Pharm. Res. 2008, 25 (8), 1737–50. 10.1007/s11095-007-9502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund-Udenaes M.Pharmacokinetic concepts in brain drug delivery. In Drug delivery to the brain; Hammarlund-Udenaes M., de Lange E. C., Thorne R. G., Eds.; Springer: New York, 2014; pp 127–61. [Google Scholar]
- Hu Y.; Hammarlund-Udenaes M.; Friden M. Understanding the Influence of Nanocarrier-Mediated Brain Delivery on Therapeutic Performance Through Pharmacokinetic-Pharmacodynamic Modeling. J. Pharm. Sci. 2019, 108 (10), 3425–3433. 10.1016/j.xphs.2019.05.029. [DOI] [PubMed] [Google Scholar]
- Carreno F.; Paese K.; Silva C. M.; Guterres S. S.; Dalla Costa T. Pharmacokinetic Investigation of Quetiapine Transport across Blood-Brain Barrier Mediated by Lipid Core Nanocapsules Using Brain Microdialysis in Rats. Mol. Pharmaceutics 2016, 13 (4), 1289–97. 10.1021/acs.molpharmaceut.5b00875. [DOI] [PubMed] [Google Scholar]
- Loryan I.; Sinha V.; Mackie C.; Van Peer A.; Drinkenburg W.; Vermeulen A.; Morrison D.; Monshouwer M.; Heald D.; Hammarlund-Udenaes M. Mechanistic understanding of brain drug disposition to optimize the selection of potential neurotherapeutics in drug discovery. Pharm. Res. 2014, 31 (8), 2203–19. 10.1007/s11095-014-1319-1. [DOI] [PubMed] [Google Scholar]
- Gebauer M.; Skerra A. Prospects of PASylation(R) for the design of protein and peptide therapeutics with extended half-life and enhanced action. Bioorg. Med. Chem. 2018, 26 (10), 2882–2887. 10.1016/j.bmc.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Soutschek J.; Akinc A.; Bramlage B.; Charisse K.; Constien R.; Donoghue M.; Elbashir S.; Geick A.; Hadwiger P.; Harborth J.; John M.; Kesavan V.; Lavine G.; Pandey R. K.; Racie T.; Rajeev K. G.; Rohl I.; Toudjarska I.; Wang G.; Wuschko S.; Bumcrot D.; Koteliansky V.; Limmer S.; Manoharan M.; Vornlocher H. P. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 2004, 432 (7014), 173–8. 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- Lindqvist A.; Rip J.; Gaillard P. J.; Bjorkman S.; Hammarlund-Udenaes M. Enhanced brain delivery of the opioid peptide DAMGO in glutathione pegylated liposomes: a microdialysis study. Mol. Pharmaceutics 2013, 10 (5), 1533–41. 10.1021/mp300272a. [DOI] [PubMed] [Google Scholar]
- Sakurai Y.; Hatakeyama H.; Sato Y.; Hyodo M.; Akita H.; Harashima H. Gene silencing via RNAi and siRNA quantification in tumor tissue using MEND, a liposomal siRNA delivery system. Mol. Ther. 2013, 21 (6), 1195–203. 10.1038/mt.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W.; Lionberger R.; Yu L. X. In vitro and in vivo characterizations of PEGylated liposomal doxorubicin. Bioanalysis 2011, 3 (3), 333–44. 10.4155/bio.10.204. [DOI] [PubMed] [Google Scholar]
- van Rooy I.; Cakir-Tascioglu S.; Hennink W. E.; Storm G.; Schiffelers R. M.; Mastrobattista E. In Vivo Methods to Study Uptake of Nanoparticles into the Brain. Pharm. Res. 2011, 28 (3), 456–471. 10.1007/s11095-010-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaydess A.; Duysen E.; Li Y.; Gilman V.; Kabanov A.; Lockridge O.; Bronich T. Visualization of exogenous delivery of nanoformulated butyrylcholinesterase to the central nervous system. Chem.-Biol. Interact. 2010, 187 (1–3), 295–8. 10.1016/j.cbi.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R. L.; Householder K. T.; Chung E. P.; Prakapenka A. V.; DiPerna D. M.; Sirianni R. W. A critical evaluation of drug delivery from ligand modified nanoparticles: Confounding small molecule distribution and efficacy in the central nervous system. J. Controlled Release 2015, 220, 89–97. 10.1016/j.jconrel.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlieghe P.; Khrestchatisky M. Medicinal chemistry based approaches and nanotechnology-based systems to improve CNS drug targeting and delivery. Med. Res. Rev. 2013, 33 (3), 457–516. 10.1002/med.21252. [DOI] [PubMed] [Google Scholar]
- Yang H. Nanoparticle-mediated brain-specific drug delivery, imaging, and diagnosis. Pharm. Res. 2010, 27 (9), 1759–71. 10.1007/s11095-010-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse J.; El-Aneed A. Properties, engineering and applications of lipid-based nanoparticle drug-delivery systems: current research and advances. Nanomedicine (London, U. K.) 2010, 5 (8), 1237–60. 10.2217/nnm.10.107. [DOI] [PubMed] [Google Scholar]
- Stern S. T.; Martinez M. N.; Stevens D. M. When Is It Important to Measure Unbound Drug in Evaluating Nanomedicine Pharmacokinetics?. Drug Metab. Dispos. 2016, 44 (12), 1934–1939. 10.1124/dmd.116.073148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund-Udenaes M. Intracerebral microdialysis in blood–brain barrier drug research with focus on nanodelivery. Drug Discovery Today: Technol. 2016, 20, 13–18. 10.1016/j.ddtec.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Chaurasia C. S.; Muller M.; Bashaw E. D.; Benfeldt E.; Bolinder J.; Bullock R.; Bungay P. M.; DeLange E. C.; Derendorf H.; Elmquist W. F.; Hammarlund-Udenaes M.; Joukhadar C.; Kellogg D. L. Jr.; Lunte C. E.; Nordstrom C. H.; Rollema H.; Sawchuk R. J.; Cheung B. W.; Shah V. P.; Stahle L.; Ungerstedt U.; Welty D. F.; Yeo H. AAPS-FDA workshop white paper: microdialysis principles, application and regulatory perspectives. Pharm. Res. 2007, 24 (5), 1014–25. 10.1007/s11095-006-9206-z. [DOI] [PubMed] [Google Scholar]
- Skoczen S.; McNeil S. E.; Stern S. T. Stable isotope method to measure drug release from nanomedicines. J. Controlled Release 2015, 220, 169–174. 10.1016/j.jconrel.2015.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoczen S. L.; Stern S. T. Improved Ultrafiltration Method to Measure Drug Release from Nanomedicines Utilizing a Stable Isotope Tracer. Methods Mol. Biol. 2018, 1682, 223–239. 10.1007/978-1-4939-7352-1_19. [DOI] [PubMed] [Google Scholar]
- Birngruber T.; Sinner F. Cerebral open flow microperfusion (cOFM) an innovative interface to brain tissue. Drug Discovery Today: Technol. 2016, 20, 19–25. 10.1016/j.ddtec.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Hummer J.; Altendorfer-Kroath T.; Birngruber T. Cerebral Open Flow Microperfusion to Monitor Drug Transport Across the Blood-Brain Barrier. Curr. Protoc Pharmacol 2019, 85 (1), e60. 10.1002/cpph.60. [DOI] [PubMed] [Google Scholar]
- Birngruber T.; Raml R.; Gladdines W.; Gatschelhofer C.; Gander E.; Ghosh A.; Kroath T.; Gaillard P. J.; Pieber T. R.; Sinner F. Enhanced doxorubicin delivery to the brain administered through glutathione PEGylated liposomal doxorubicin (2B3–101) as compared with generic Caelyx,((R))/Doxil((R))--a cerebral open flow microperfusion pilot study. J. Pharm. Sci. 2014, 103 (7), 1945–1948. 10.1002/jps.23994. [DOI] [PubMed] [Google Scholar]
- Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv. Drug Delivery Rev. 2001, 47 (1), 65–81. 10.1016/S0169-409X(00)00122-8. [DOI] [PubMed] [Google Scholar]
- Weiss C. K.; Kohnle M. V.; Landfester K.; Hauk T.; Fischer D.; Schmitz-Wienke J.; Mailander V. The first step into the brain: uptake of NIO-PBCA nanoparticles by endothelial cells in vitro and in vivo, and direct evidence for their blood–brain barrier permeation. ChemMedChem 2008, 3 (9), 1395–403. 10.1002/cmdc.200800130. [DOI] [PubMed] [Google Scholar]
- Friden M.; Bergstrom F.; Wan H.; Rehngren M.; Ahlin G.; Hammarlund-Udenaes M.; Bredberg U. Measurement of unbound drug exposure in brain: modeling of pH partitioning explains diverging results between the brain slice and brain homogenate methods. Drug Metab. Dispos. 2011, 39 (3), 353–62. 10.1124/dmd.110.035998. [DOI] [PubMed] [Google Scholar]
- Umlauf B. J.; Shusta E. V. Exploiting BBB disruption for the delivery of nanocarriers to the diseased CNS. Curr. Opin. Biotechnol. 2019, 60, 146–152. 10.1016/j.copbio.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y. E.; Bu T.; Saltzman W. M. Nanomaterials for convection-enhanced delivery of agents to treat brain tumors. Curr. Opin Biomed Eng. 2017, 4, 1–12. 10.1016/j.cobme.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.clinicaltrials.gov/ct2/show/NCT01906385.
- https://www.clinicaltrials.gov/ct2/show/NCT03566199.
- Liebner S.; Dijkhuizen R. M.; Reiss Y.; Plate K. H.; Agalliu D.; Constantin G. Functional morphology of the blood–brain barrier in health and disease. Acta Neuropathol. 2018, 135 (3), 311–336. 10.1007/s00401-018-1815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M. D.; Sagare A. P.; Zlokovic B. V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14 (3), 133–150. 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M. D.; Zhao Z.; Montagne A.; Nelson A. R.; Zlokovic B. V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99 (1), 21–78. 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkaria J. N.; Hu L. S.; Parney I. F.; Pafundi D. H.; Brinkmann D. H.; Laack N. N.; Giannini C.; Burns T. C.; Kizilbash S. H.; Laramy J. K.; Swanson K. R.; Kaufmann T. J.; Brown P. D.; Agar N. Y. R.; Galanis E.; Buckner J. C.; Elmquist W. F. Is the blood–brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol 2018, 20 (2), 184–191. 10.1093/neuonc/nox175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskar K. S.; Rudraraju V.; Mittapalli R. K.; Samala R.; Thorsheim H. R.; Lockman J.; Gril B.; Hua E.; Palmieri D.; Polli J. W.; Castellino S.; Rubin S. D.; Lockman P. R.; Steeg P. S.; Smith Q. R. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm. Res. 2012, 29 (3), 770–81. 10.1007/s11095-011-0601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail D. F.; Joyce J. A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell 2017, 31 (3), 326–341. 10.1016/j.ccell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tellingen O.; Yetkin-Arik B.; de Gooijer M. C.; Wesseling P.; Wurdinger T.; de Vries H. E. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates 2015, 19, 1–12. 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Hambardzumyan D.; Bergers G. Glioblastoma: Defining Tumor Niches. Trends Cancer 2015, 1 (4), 252–265. 10.1016/j.trecan.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolburg H.; Noell S.; Fallier-Becker P.; Mack A. F.; Wolburg-Buchholz K. The disturbed blood–brain barrier in human glioblastoma. Mol. Aspects Med. 2012, 33 (5–6), 579–89. 10.1016/j.mam.2012.02.003. [DOI] [PubMed] [Google Scholar]