Abstract
The unprecedented application of pesticides in Punjab, India during green revolution has lead to an environmental crisis due to the accumulation of persistent organic and pesticide pollutants in the environment and biota of this region. The present study aimed at estimating the abundance of pesticide contaminants in three biological matrices of 36 dogs suffering from malignant canine mammary tumor (mCMT) and 6 tumor free control dogs from Punjab, India. Presence of individual and total pesticides in canine biological samples, age and bodyweight of canine patients was assessed as a potential risk factor for mCMT using logistic regression analysis. Chi-square test was employed to determine tissue-specific accumulations of individual pesticides. Spearman's correlation coefficient was estimated to determine the association between the levels of total pesticides in different tissue matrices and with age and bodyweight of mCMT cases. Gas chromatography-ECD analysis of serum, mammary tissue and adjoining mammary adipose tissue revealed fourteen different pesticides including γ-HCH, α-HCH, dieldrin, aldrin, heptachlor, butachlor, p,p-DDT, o,p-DDT, p,p-DDD, p,p-DDE, L-cyhalothrin, permethrin, fipronil, and fenitrothion. Heptachlor, γ-HCH, aldrin and p,p-DDT were more frequently detected, whereas, p,p-DDE and o,p-DDT were the least common. Differential accumulation of pesticides in tissue matrices, particularly between serum and mammary tissue/adipose tissue was observed. We could not find any association between the total pesticide concentrations among serum, mammary tissue and mammary adipose tissue in mCMT cases. We found that the odds for individual pesticide for serum, mammary tissue and adipose tissue were associated with high uncertainties; however, the total pesticide concentration in mammary tissue was near non-significantly associated with higher risk of mCMT with low uncertainty. Statistically non-significant higher odds of CMT occurrence with increase in age was noticed No association between the concentration of total pesticides in different matrices and age and bodyweight of canine subjects was found.
Keywords: Cancer research, Environmental science, Toxicology, Veterinary medicine, Clinical research, Clinical toxicology, Environmental pollution, Environmental toxicology, Oncology, Pathology, Adipose, Pet dog, Breast cancer, Ecotoxicology, Organochlorine pesticides, Persistent organic pollutants
Cancer research; Environmental science; Toxicology; Veterinary medicine; Clinical research; Clinical toxicology; Environmental pollution; Environmental toxicology; Oncology; Pathology; Adipose; Pet dog; Breast cancer; Ecotoxicology; Organochlorine pesticides; Persistent organic pollutants.
1. Introduction
Persistent organic pollutants (POPs) are a class of toxic organic chemicals having high environmental persistence due to the extremely low rate of natural degradation. POPs disseminate to distant places through natural means and, therefore, can affect living organisms far away from their actual site of production and release, even to regions where they have never been used [1]. Being lipophilic and resistant to photolytic, chemical and biological degradation, they tend to bioaccumulate in the environment and the fatty tissues of animals and humans [2]. The most addressed and reviewed POPs includes a group of 12 highly toxic and persistent organic compounds, popularly known as ‘Dirty Dozen’ [3]. Among these 12 POPs, eight (aldrin, chlordane, DDT, dieldrin, endrin, heptachlor, mirex and toxaphene) belongs to the pesticide group.
Pesticides are intended for controlling the pest population and have played a crucial role in controlling insect-borne diseases and in enhancing agricultural productivity. However, due to the indiscriminate application over the past few decades, pesticides, particularly organochlorines (OCs), have become ubiquitous and a serious threat to the environment. Considering the long-term hazards of OCs, application of various OCs has been banned in the majority of the developed countries. However, some countries are still using OCs as a primary means to control pests. Pesticide exposure to humans/animals occurs mainly through contaminated food and water, and high lipid solubility and inefficient metabolism further lead to bioaccumulation in animal and human tissues [4]. Besides, the acquisition of OCs by ingestion of animal products, especially fatty animal products such as milk, meat and fish containing pesticide residues further tend to biomagnify the body burden [5], rendering humans and their companions more susceptible for acute and chronic toxic effects of pesticides (Fossi et al., 1999). Additionally, ingestion of indoor dust is also considered as an important exposure pathway in humans as well as companion animals [6, 7].
Accumulation of POPs and OCs in adipose tissues has been reported worldwide and is a global health concern. Such a build-up of pesticide residues in body tissues has been implicated in cancers, birth defects, endocrine disruption, immunological, behavioural, neurological and reproductive discrepancies in human and animal species [8, 9]. Among various speculated POPs/OCs induced ailments, human breast cancer (BC) is one of the most controversial and also amongst the most extensively studied ones. With a share of 25.1% of all cancers, BC is the most common cancer in women throughout the world [10]. Despite remarkable progress in the field of early diagnosis and adjuvant therapy, morbidity and mortality due to BC continue to rise, [11]. Likewise, its counterpart in canines, i.e. canine mammary tumor (CMT) represent nearly 50% of all neoplasms that afflict female dogs [12] and of these, 41–53% are malignant [13]. As in humans, dogs develop spontaneous tumours and old age, hormonal therapy, obesity in early life and dietary factors increase the risk of mammary tumors in dogs [14, 15]. Also, the frequency and response to spontaneous tumors in canines often parallels human neoplasms [16, 17, 18]. Although canine and human mammary tumors are among the most important neoplastic conditions leading to high mortality in respective species, yet their etiology remains largely unknown.
Profound attempts have been made to shed light on the role of pesticides in the causation of mammary tumors, however, the results have been largely equivocal. Although a direct link between pesticides and BC occurrence has not been established, a few studies have supported an association between the level of pesticide residues in tissues and occurrence of BC. Varying levels of pesticide residues in serum and mammary adipose tissue of BC patients have been reported [19, 20]. Injections of certain pesticides induced the development of mammary cancer in male mice, which are rather naturally resistant to mammary cancer [21]. Besides, prenatal and neonatal exposure to pesticide residues, particularly dieldrin, have been associated with an increased incidence of mammary tumors in rats [22]. Additionally, in vitro studies conducted in breast cancer cell lines have also reported accelerated growth and proliferation of breast epithelial cells in response to higher levels of pesticide residues [23].
Dogs share the same indoor environment and food as humans and hence may be exposed to a similar carcinogenic load. Therefore, the emphasis has been laid in the recent past to validate companion animals as a model for human exposure to environmental contaminants. Pesticides have been reported in healthy dogs [24, 25] as well as CMT affiliated dogs [26, 27, 28] from different parts of the world, including Japan, France, Italy, Pakistan and Brazil. Majority of the above studies were restricted to mere detection of pesticide residues in canine tissues without undermining the role of pesticide in CMT occurrence. A pioneering work by Severe and co-workers (2015) demonstrated a wide range of POPs in the serum and adipose tissue of CMT cases; however, the primary target tissue i.e. neoplastic mammary tissue was not analysed for POPs burden [28]. In absence of information on the status of pesticide load in Indian dogs, the present study aimed at detecting pesticides in the serum, mammary tissue and mammary adipose tissue of canines suffering from spontaneous malignant mammary neoplasia from Punjab, one of the largest pesticides consuming state of India. Furthermore, any possible role of pesticides in the induction of CMT was explored by comparing the results with tumor-free dogs as control subjects.
2. Material and methods
2.1. Ethical considerations
All the activities were carried out as per the guidelines of the Institute Ethics Committee for Animal Welfare and all applicable institutional guidelines for the care and welfare of animals were followed. The study does not involve any animal experimentation and included only clinical cases as a source of samples.
2.2. Animal characteristics and clinical findings
The present study included 36 spontaneous malignant CMT (hereafter, referred as mCMT) cases presented to the Small Animal Clinics of Guru AngadDev Veterinary and Animal Sciences University, Ludhiana, Punjab, India, from December 2011 to April 2013. During the study period, a total of 28,220 cases were presented to the Small Animal Clinics. All tumor suspected cases were examined clinically and a tentative diagnosis was made based on the history and clinical examination. Seventy-one cases were histologically confirmed as tumor cases and forty-one of them were diagnosed as mCMT based on the histopathological examination. Among 41 cases, 36 cases which underwent surgical tumor resection were included in the study. Among the total canine cases presented to the Small Animal Clinics during the study period, samples from 6 tumor-free female dogs were also taken which served as control. The tumor-free status of animals was confirmed through clinical examination and abdominal ultrasound and radiography.
A general examination of mCMT cases was carried out and a detailed history along with various physiological parameters of mCMT cases was recorded. Age, breed, body weight, rectal temperature, food, confinement status and presence of any respiratory distress were recorded through a questionnaire. As pregnancy, parturition and lactation have been shown to influence the release of pesticide residues from the body, information on the history of parturition and lactation was also recorded. The mCMT cases were examined critically and clinical findings such as glands affected with tumor, the consistency/texture of tumor mass, presence of haemorrhages on tumor surfaces, signs of inflammation and ulceration were recorded.
2.3. Sample collection and histopathology
Blood samples were collected before surgery from overnight fasted animals in gel vacutainers without anticoagulant. The vacutainers were kept undisturbed at room temperature for 30–60 min for clot formation. Serum was harvested by centrifugation at 3000 RPM for 15 min and stored in multiple small aliquots of 1–2 ml each at -20 °C until analysis [29]. Lack of owner's consent restricted the collection of blood from 33 cases out of the total 36 cases.
For detection of pesticide residues in canine mammary tissue and adjacent mammary adipose tissue (hereafter referred as adipose tissue), samples were collected immediately after the excisional surgery from multiple sites depending upon the size, encapsulation and availability of adjoining adipose tissue. Collected samples were placed in self-sealing polyethene bags and were stored at -20 °C within half an hour of collection until further processing. Lack of associated adipose tissue and small size of tumor further constraint the sample collection from all the cases and mammary adipose tissue and neoplastic mammary tissue could be collected in 31 and 35 cases, respectively.
Samples from 6 tumor-free female dogs that were brought to the Small Animal Clinics due to some critical condition, such as renal failure and hind limb paralysis, were collected similarly as from dogs with mCMT. Serum samples were collected before death/euthanasia of animals whereas mammary tissue and adjacent adipose tissues were collected immediately following the death with the consent of the respective animal owners. The control sample size was severely restricted due to the consensual rationale of the animal owners to provide the samples considering the critical state of the animals.
For histopathological characterization, the representative tissue samples were collected from multiple sites and immediately preserved in 10% neutral buffered formalin. The samples were dehydrated in ascending grades of alcohol and were cleared in acetone and benzene. The paraffin-embedded tissues were sectioned and stained with routine H & E technique [30]. The stained slides were examined microscopically for the tumor classification, tumor grading and presence of angiogenesis, cyst formation, lymphatic emboli and necrosis.
2.4. Detection of pesticide residues
2.4.1. Reagents and chemicals
All the reagents used were of gas chromatographic grade (Merck, Germany). All the pesticide standards used were purchased from Sigma-Aldrich and were of analytical grade. Standard stock solutions of individual pesticides (100 μg/mL) were prepared in acetone: hexane (1:1, v/v) and stored at 4 °C in glass vials. The internal standard, fenvalerate (10 μg/mL), was prepared in methanol. Spiking solutions for individual pesticides were prepared following dilution of stock solution in acetone.
2.4.2. Sample preparation
Pesticide residues in serum were extracted using the method described previously [31] with slight modifications. Briefly, 1 mL serum samples were equilibrated at room temperature in a centrifuge tube. Serum proteins were denatured using an equal volume of 1% acetic acid. The analytes were extracted using liquid-liquid extraction with hexane: dichloromethane (9:1 v/v, 3.0 mL). The tubes were vortexed for 1 min and centrifuged at 1800 rpm for 2 min. The top organic layer was drawn into another clean centrifuge tube (15 mL) and the extraction was repeated twice. The pooled organic phase was concentrated to 0.5 mL for further purification. Lipids and other interferences were eliminated through column chromatography clean-up using florisil. Florisil (1 gm) was packed into a glass column in between two layers of anhydrous sodium sulfate (1 g). The column was pre-washed with 8 mL of hexane and the concentrated sample extract was added to the column. The column was rinsed twice with 0.5 mL of hexane. For recovering residues, elution was carried out with 8 mL hexane: dichloromethane (1:9, v/v). The resultant eluate was concentrated to 1–2 mL and stored in labelled glass stopper vials for analysis in GC.
For extraction of pesticide residues in mammary tissue and adipose tissue, 1 gm of mammary tissue/adipose tissue was minced with 3–5 gm of activated (500 °C for 2 h) anhydrous sodium sulfate using a pestle mortar until the samples became moisture-free. Dehydrated samples were continuously mixed with 20 mL of hexane: acetone (1:1, v/v) solution for 2 min using a magnetic stirrer in a corked conical flask. A pause of 15 min was given and the process was repeated twice. Samples were left undisturbed for 6 h and thereafter filtered with Whatman filter paper. The filtrate was concentrated to dryness and again reconstituted with 4 mL of hexane. Four mL of acetonitrile saturated with hexane was mixed with the filtrate through rigorous vortexing and the solution was left for layer separation and, thereafter, the lower fraction was collected. The process was repeated twice and the pooled lower fraction was concentrated to 1–2 mL. As for serum samples, cleanup to remove lipid and other interferences were achieved through florisil. Considering the sensitivity of some OCs to concentrated acids, florisil was preferred over sulfuric acid treatment and acid silica for purification of concentrated sample extract. For recovering residues, elution was carried out with 15 mL of hexane: acetone (1:1) solution. The resultant eluate was concentrated to 2–3 mL and stored in labelled glass stopper vials for further analysis in GC. Procedural blanks were prepared with the exclusion of clinical samples.
2.4.3. Apparatus and GC analysis
The cleaned-up extracts were analysed using PerkinElmer® Clarus® 500 Gas Chromatograph (Perkin Elmer, Massachusetts, U.S.) equipped with Ni63 electron capture detector (ECD). Separation of analytes was achieved on fused silica capillary columns coated with a 5% diphenyl and 95% dimethylpolysiloxane as stationary phase (length-30 m, id-0.32 mm, film thickness- 0.25μm, Elite-5, Perkin Elmer, Massachusetts, U.S.). GC operational conditions were used as described previously with slight modifications [31]. Highly purified nitrogen at a flow rate of 14 mm/min and pressure 117.6 kPa was used as carrier gas. Temperature conditions were kept as follows: injector temperature: 270 °C; oven temperature: 170 °C and detector temperature: 310 °C.
Two microliters of the cleaned-up extract were injected into GC through the manual injection port. Winacds 6.2 software was used for integration and computation of signals. The pesticides were identified and quantified by analysis of the retention time and peak of the sampled chromatographs with those of standards ran under the same operating conditions. The runs were made in batches of five samples and to evaluate potential contamination (inter-samples or with standards) and to normalise base value (which may be influenced by pesticide persistence, notably in solvents, recipients and equipment), procedural blanks were run after each batch. After every 10 samples, a spiked standard blank was run for quality assurance. The mean recoveries of fortified serum, mammary tissue and mammary adipose tissue were >80% which is in accordance with the acceptable recovery range of 70–120% [32]. The estimated concentrations of pesticide residues in specimens were not corrected for recovery value. The detection limit of the method ranged from 0.5-5.5 ng/ml, 4.4–18 ng/g and 0.4–12 ng/g for serum, mammary adipose tissue and mammary tissue, respectively.
2.5. Statistical analysis
The Concentrations of pesticide residues were expressed as means ± standard deviation. Pearson's chi-square (χ2) test was used to analyze the differential accumulation of pesticides in biological samples of mCMT cases. χ2 test was further employed to examine the numeration data on presence/absence of pesticides in mCMT and control group. Correlation between concentration of total pesticides in the different tissue matrices (n = 28; concurrent samples were available) and age and body weight of mCMT cases was estimated using Spearman's correlation (ρ) test (since the data was not normally distributed). χ2 test and ρ test were conducted using SPSS statistical packages (Version 20.0). The bioaccumulation of pesticide residues in mCMT versus control group was also assessed by point-biserial correlation using R package ‘ltm’, version 1.1–1 [33]. The cases and controls were not matched in this study; therefore, unconditional multivariable logistic regression analysis was performed to assess the association of individual pesticide as well as the total concentration of all pesticides with the incidence of mCMT cases. The predictor variables considered for logistic regression analysis were concentrations of the individual as well as total pesticides, age and body weight. Parturition and breed of canine patients did not fir the model due to incomplete data and low frequencies, respectively, and thus were excluded from the multiple logistic regression analysis. Two separate models of logistic regression analysis were considered for each of serum, mammary tissue and adipose tissue-one with concentrations of individual pesticides, age and body weight and another one with total pesticide concentration, age and body weight (to avoid the problem of multicollinearity, as total pesticide concentration was derived by summing up the concentrations of individual pesticides). R package ‘rms’ version 6.0–1 was used for logistic regression [34]. These analyses were performed in the R programming environment with R version 4.0.2 [35].
3. Results
3.1. Characteristics of mCMT cases and control animals
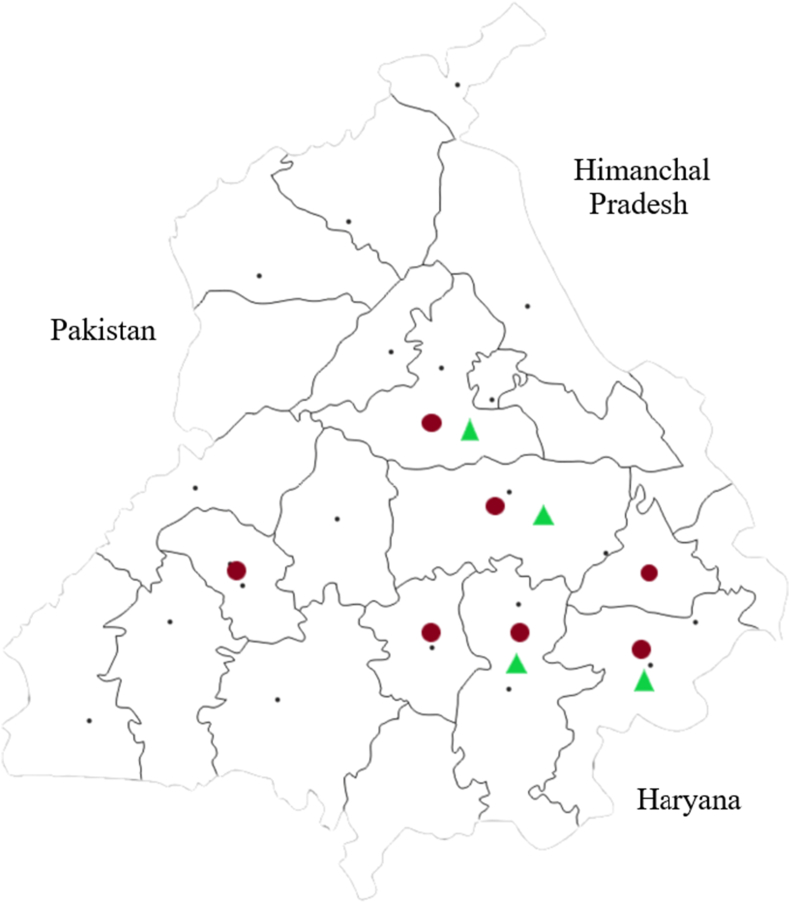
The age of canines ranged from 2.5 to 15 years with the majority of mCMT cases lying in the age group of >4–8 yrs, followed by > 8–12 yrs. Although ovariohysterectomy was performed in only 8.3% (3/36) cases, despite this, half of the population (16/32) was never bred and had no history of parturition/lactation. No significant differences in the mean age (p = 0.506) and body weight (p = 0.79) could be observed among the mCMT cases and control group. Around 85% (36/42) of the dogs included in the study were exclusively fed with the homemade human food and the rest of the animals (all belonging to the mCMT group) were occasionally supplemented with commercial dog food. The mCMT cases represented different regions of Punjab (Figure 1) including Ludhiana (n = 20), Jalandhar (n = 6), Patiala (n = 4), Kotkapura (n = 2), Sangrur (n = 2), Barnala (n = 1), and Fatehgarh Sahib (n = 1). The control animals represented Ludhiana (n = 3), Jalandhar (n = 1), Patiala (n = 1) and Sangrur (n = 1). Eighty-one per cent of the animals (34/42) included in the study and residing in the urban areas were held within the house premises with limited access to the outdoor environment. Rest of the animals (all mongrels) had access to the outdoor premises for at least a few hours each day. Different attributes of mCMT cases and control animals including age, breed, body weight, and parturition status are briefed in Table 1 and detailed in Supplementary Table 1.
Figure 1.
Map of Punjab, India showing the inhabitant regions of canines suffering from canine mammary tumor (circle) and their tumor-free counterparts (triangle) sampled for detection of pesticide residues. The site marked represents the district of sampled canines and not the actual residentiary within the district.
Table 1.
Physiological attributes of CMT and control cases.
| Characteristics |
CMT cases (n = 36) |
Control cases (n = 6) |
|---|---|---|
| Age (yrs) | ||
| Mean ± SD | 8.18 ± 3.10 | 7.5 ± 2.05 |
| −4 yrs | 3 | 1 |
| >4–8 yrs | 16 | 3 |
| >8–12 yrs | 13 | 2 |
| >12 yrs | 4 | |
| Bodyweight (kg) | ||
| Mean ± SD | 23.75 ± 12.02 | 22.23 ± 11.96 |
| Breeds | ||
| Labrador | 8 | 1 |
| Spitz | 8 | 2 |
| German Shepherd | 6 | 2 |
| Mongrel | 8 | 1 |
| Dachshund | 3 | 0 |
| Cocker Spaniel | 2 | 0 |
| Great Dane | 1 | 0 |
| Ovariohysterectomy | ||
| Yes | 3 | -- |
| No | 33 | |
| Parturition and lactation |
(n = 32) | (n = 4) |
| Zero | 16 | 2 |
| Once | 5 | 1 |
| Twice | 5 | 1 |
| Three times | 2 | |
| Four times | 3 | |
| Five times | 1 | |
CMT = canine mammary tumor; SD = standard deviation; n = number of cases.
3.2. Clinical manifestations and histopathological findings
The clinical and histopathological attributes of malignant CMT in the present study are depicted in Table 2. The classification and grading of mCMT were done as per the guideline for canine mammary gland tumor [36].
Table 2.
Clinical and histopathological attributes of mCMT cases.
| Clinical and gross findings |
Histopathological findings |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Percentage∗ (n) | Characteristic | Percentage∗ (n) | ||||||
| Mammary gland involved | Tumor classification | ||||||||
| Thoracic | Cranial | 11 (4) | Carcinoma | Simple | 17 (6) | ||||
| Caudal | 14 (5) | Complex | 36 (13) | ||||||
| Abdominal | Cranial | 19 (7) | Sarcoma | 3 (1) | |||||
| Caudal | 39 (14) | Carcinosarcoma | 39 (14) | ||||||
| Inguinal | 80 (29) | Others | 5 (2) | ||||||
| Consistency/texture of tumor mass | Tumor grading∗∗ (n = 19) | ||||||||
| Soft | 39 (14) | Grade I | 31.5 (6) | ||||||
| Hard | 61 (22) | Grade II | 31.5 (6) | ||||||
| External hemorrhage | Grade III | 37 (7) | |||||||
| Present | 28 (10) | Angiogenesis | |||||||
| Absent | 72 (26) | Present | 97 (35) | ||||||
| Inflammatory signs | Absent | 3 (1) | |||||||
| Present | 36 (13) | Lymphatic emboli | |||||||
| Absent | 64 (23) | Present | 19 (7) | ||||||
| Ulceration | Absent | 81 (29) | |||||||
| Present | 17 (6) | Cyst formation | |||||||
| Absent | 83 (30) | Present | 22 (8) | ||||||
| Tumor size | Absent | 78 (28) | |||||||
| ≤3 cm | 14 (5) | Necrosis | |||||||
| 3–5 cm | 17 (6) | Present | 69 (25) | ||||||
| ≥5 cm | 69 (25) | Absent | 31 (11) | ||||||
Rounded up to the nearest tenth.
Only for carcinoma cases as per the guidelines of Misdorp 2002.
3.3. Detection of pesticide residues in canine tissues
Fourteen different pesticides including γ-HCH, α-HCH, dieldrin, aldrin, heptachlor, butachlor, p,p-DDT, o,p-DDT, p,p-DDD, p,p-DDE, L-cyhalothrin, permethrin, fipronil, and fenitrothion could be detected in the serum, neoplastic mammary tissue and adjoining adipose tissue of mCMT affiliated dogs. γ-HCH was the most frequently detected pesticide in the present study with the detection frequency of 80.55% [29] in mCMT cases. Heptachlor was detected in 69.4% cases and was second most commonly detected pesticide. Aldrin and p,p-DDT were among other frequently detected pesticides with detection frequency in 66.6% and 58.3% cases, respectively. Besides this, fenitrothion, α-HCH and L-cyhalothrin were also detected in 66.6%, 50%, 41.6% and 38.8% cases, respectively. p,p-DDE and o,p-DDT were the least frequently detected pesticides and could be detected in only one mammary adipose tissue and serum, respectively. Although, pesticides could be detected in the samples collected from tumor-free dogs, however, the detection frequency and the concentration of the majority of pesticide residues were lower as compared to their tumor affiliated counterparts. The mean concentrations and range of individual pesticides are detailed in Table 3 and the pesticide burden of the individual canine patient is detailed in Supplementary Table 1.
Table 3.
Levels of different pesticide residues in CMT affiliated dogs and tumor free dogs.
| Pesticides | Serum (μg/ml) |
Mammary adipose tissue (μg/g) |
Mammary tissue (μg/g) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CMT cases (n = 33) |
Tumor free dogs (n = 6) |
CMT cases (n = 31) |
Tumor free dogs (n = 6) |
CMT cases (n = 35) |
Tumor free dogs (n = 6) |
|||||||
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| p,p DDT | 0.051 ± 0.289 | 0–1.686 | ND | - | 0.986 ± 1.665 | 0–6.537 | 0.078 ± 0.174 | 0–0.466 | 0.574 ± 1.256 | 0–4.687 | 0.053 ± 0.119 | 0–0.319 |
| o,p DDT | 0.015 ± 0.082 | 0–0.4795 | ND | - | ND | - | ND | - | ND | - | ND | - |
| p,p DDD | 0.040 ± 0.126 | 0–0.483 | ND | - | 0.033 ± 0.183 | 0–1.021 | ND | - | 0.331 ± 1.333 | 0–6.316 | ND | - |
| α HCH | 0.005 ± 0.011 | 0–0.0463 | 0.075 ± 0.167 | 0–0.447 | 0.012 ± 0.049 | 0–0.247 | ND | - | 0.024 ± 0.061 | 0–0.299 | ND | - |
| γ HCH | 0.303 ± 0.607 | 0–3.022 | 0.091 ± 0.157 | 0–0.429 | 0.304 ± 0.457 | 0–2.133 | ND | - | 0.248 ± 0.290 | 0–1.168 | 0.130 ± 0.196 | 0–0.509 |
| Butachlor | 0.291 ± 0.794 | 0–3.1156 | ND | - | 0.171 ± 0.952 | 0–5.302 | 1.022 ± 2.285 | 0–6.13 | 0.539 ± 1.049 | 0–4.363 | ND | - |
| L-cyhalothrin | 0.199 ± 0.476 | 0–2.109 | ND | - | ND | - | ND | - | 0.044 ± 0.130 | 0–0.504 | ND | - |
| Heptachlor | 0.606 ± 1.201 | 0–3.342 | 0.080 ± 0.179 | 0–0.480 | 0.292 ± 0.641 | 0–2.137 | ND | - | 0.226 ± 0.473 | 0–1.635 | ND | - |
| Dieldrin | 0 | 0.006 | ND | - | 0.011 ± 0.032 | 0–0.147 | ND | - | 0.011 ± 0.027 | 0–0.107 | ND | - |
| Fenitrothion | ND | - | ND | - | 1.395 ± 1.885 | 0–5.182 | ND | - | 0.331 ± 0.709 | 0–2.282 | ND | - |
| Aldrin | 0.078 ± 0.365 | 0–2.101 | 0.651 ± 1.280 | 0–3.494 | 0.337 ± 0.552 | 0–1.674 | ND | - | 0.303 ± 0.465 | 0–1.504 | ND | - |
| Fipronil | ND | - | ND | - | 0.323 ± 1.138 | 0–5.774 | ND | - | 0.376 ± 1.386 | 0–7.272 | ND | - |
| Permethrin | 0.060 ± 0.240 | 0–1.122 | 0.167 ± 0.373 | 0–0.999 | 0.003 ± 0.018 | 0.098 | ND | - | 0.070 ± 0.247 | 0–1.222 | ND | - |
| p,p DDE | ND | - | ND | - | 0.055 ± 0.304 | 0–1.692 | ND | - | ND | - | ND | - |
ND = Not Detected; CMT = canine mammary tumor; n = number of cases; SD = standard deviation.
3.4. Tissue-specific accumulation of pesticides
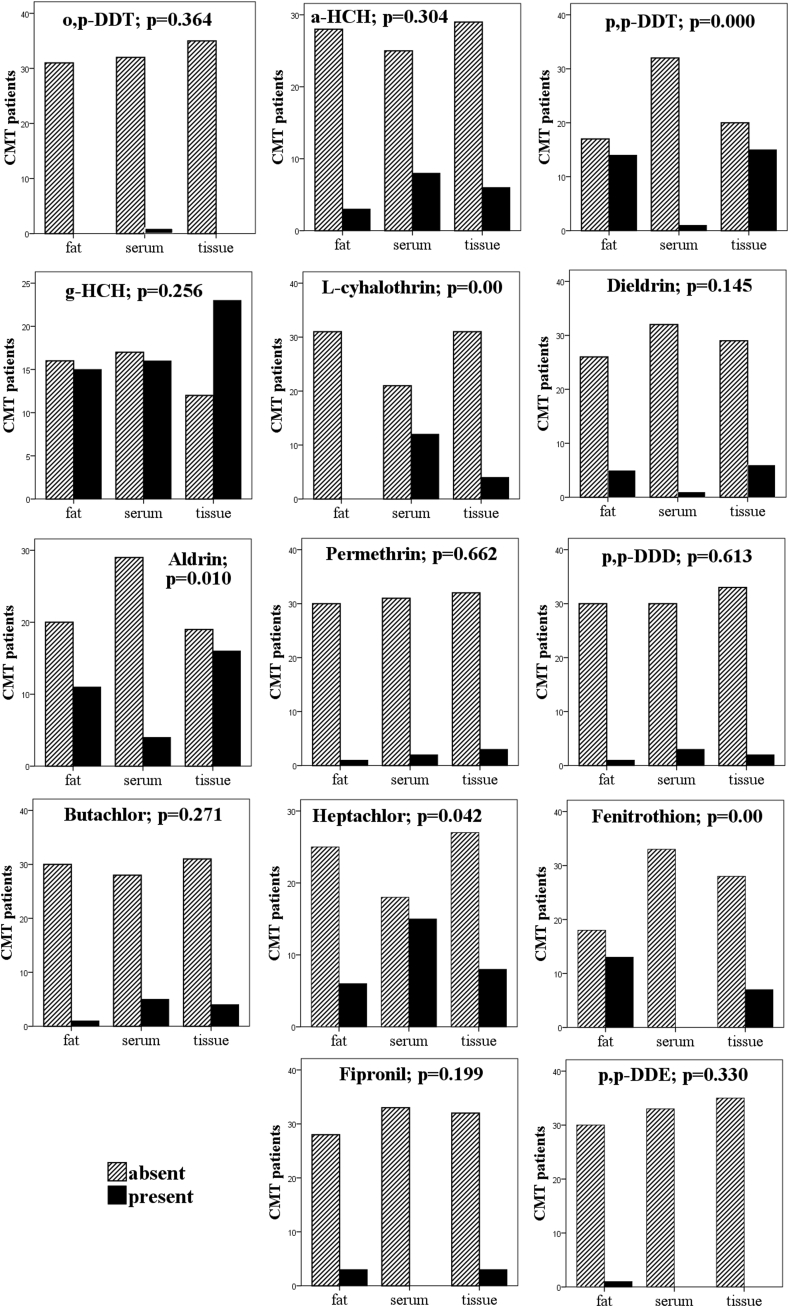
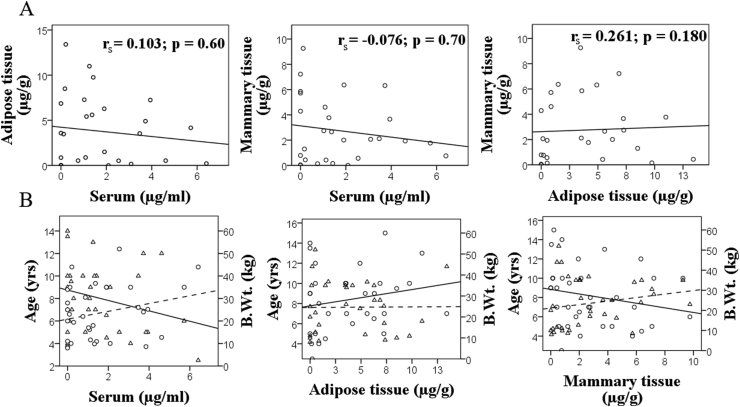
Chi-square analysis revealed a differential accumulation of pesticides in tissue samples collected from mCMT cases (Figure 2). Fenitrothion (p < 0.00), p,p-DDT (p < 0.00) and aldrin (p = 0.010) were detected in significantly higher proportions of mammary tissue and mammary adipose tissue samples as compared to serum, whereas, L-cyhalothrin (p < 0.00) and heptachlor (p = 0.042) were detected in significantly higher number of serum samples than in adipose or mammary tissue (Figure 2). Furthermore, some of the pesticides were absent in specific tissue samples, namely, fipronil, dieldrin, and fenitrothion in serum; L-cyhalothrin, o,p-DDT and permethrin in adipose tissue; o,p-DDT and p,p-DDE in mammary tissue. Dieldrin, γ-HCH, α-HCH, butachlor, permethrin, p,p-DDD, p,p-DDE, fipronil, and o,p-DDT did not show any significant differences in a predilection for specific tissue types (Figure 2). Furthermore, no significant relationship between the concentration of total pesticides in serum, mammary tissue and mammary adipose tissue-derived from mCMT cases was found (Figure 3A).
Figure 2.
Differential accumulation of individual pesticide in various tissue matrices of canines suffering from malignant canine mammary tumor.
Figure 3.
Correlation plots for total pesticides among (A) different biological matrices and (B) with age and bodyweight of malignant canine mammary tumor cases. Straight and dotted lines represent line of best fit for age and bodyweight respectively; circles and triangles shows the representative values for age and bodyweight, respectively.
3.5. Comparison of pesticides among mCMT and control group
In mammary tissues, aldrin was detected in 45.7% of mCMT cases which is significantly higher (p = 0.034) as compared to tumor-free subjects where aldrin couldn't be detected. Similarly, γ-HCH (48.4% versus 0%; p = 0.027) and fenitrothion (41.9% versus 0%; p = 0.049) were also detected with significantly higher frequency in mammary adipose tissue among mCMT affiliated dogs as compared to tumor-free dogs (Table 4). Contrary to this, no significant differences in the detection frequency of individual pesticides were noted in the serum samples among the two groups. Additionally, point-biserial correlation analysis also validates that the concentration of total pesticides have a greater tendency to bio-accumulate in the mammary tissue (rpb = 0.36) and adipose tissue (rpb = 0.30) of mCMT cases as compared to the control group. Except for butachlor in the adipose tissue, all the other pesticides were detected preferentially in the mammary tissue and adipose tissue of mCMT cases (Supplementary Table 2). High rrb values further substantiate that certain pesticides are more likely to be detected in the mammary tissue and adipose tissue of mCMT cases than the control group.
Table 4.
Percent positivity of different pesticide residues in mCMT and tumor-free animals.
| Pesticides | Serum |
Mammary Adipose tissue |
Mammary Tissue |
||||||
|---|---|---|---|---|---|---|---|---|---|
| % detection in tumor free dogs | % detection in CMT cases | Sig. (p) | % detection in tumor free dogs | % detection in CMT cases | p value | % detection in tumor free dogs | % detection in CMT cases | Sig. (p) | |
| p,p-DDT | 0 | 3 | 0.666 | 16.7 | 45.2 | 0.193 | 16.7 | 42.9 | 0.224 |
| o,p-DDT | 0 | 3 | 0.666 | 0 | 0 | - | 0 | 0 | - |
| Aldrin | 33.3 | 12.1 | 0.185 | 0 | 35.5 | 0.082 | 0 | 45.7 | 0.034∗ |
| Dieldrin | 0 | 3 | 0.666 | 0 | 16.1 | 0.290 | 0 | 17.1 | 0.272 |
| Heptachlor | 16.7 | 45.5 | 0.187 | 0 | 19.4 | 0.239 | 0 | 22.9 | 0.192 |
| Butachlor | 0 | 15.2 | 0.307 | 16.7 | 3.2 | 0.183 | 0 | 11.4 | 0.383 |
| L-cyhalothrin | 0 | 36.4 | 0.076 | 0 | 0 | - | 0 | 11.4 | 0.383 |
| α-HCH | 16.7 | 24.2 | 0.685 | 0 | 9.7 | 0.427 | 0 | 17.1 | 0.272 |
| γ-HCH | 50 | 48.5 | 0.946 | 0 | 48.4 | 0.027∗ | 33.3 | 65.7 | 0.133 |
| Permethrin | 16.7 | 6.1 | 0.370 | 0 | 3.2 | 0.656 | 0 | 8.6 | 0.456 |
| p,p-DDD | 0 | 9.1 | 0.442 | 0 | 3.2 | 0.656 | 0 | 5.7 | 0.548 |
| p,p-DDE | 0 | 0 | - | 0 | 3.2 | 0.656 | 0 | 0 | - |
| Fipronil | 0 | 0 | - | 0 | 9.7 | 0.427 | 0 | 8.6 | 0.456 |
| Fenitrothion | 0 | 0 | - | 0 | 41.9 | 0.049∗ | 0 | 20 | 0.229 |
Significant difference at 5% level of significance; CMT = canine mammary tumor.
3.6. Pesticides as potential risk factor for mCMT
The concentration of individual and total pesticides and two potential risk factors for mCMT (age and bodyweight of canine patients) were analysed through a multiple logistic regression model. We found that the odds for individual pesticide for serum, mammary tissue and adipose tissue were associated with high uncertainties (standard errors) and cannot be relied upon (Table 5). Standard errors were high due to two reasons: one, the sample size in this study was small; and two, the individual pesticides were not detected in all the mCMT cases, further reducing the effective sample size. More importantly, total pesticide concentration in mammary tissue was near-significantly associated with a higher risk of mCMT with low uncertainty (Table 6). Moreover, the total pesticide concentration in mammary tissue had a more pronounced effect than its concentration in the adipose tissue and serum in the probable occurrence of mCMT (Table 6).
Table 5.
Logistic regression analysis of mCMT and individual pesticides in different biological matrices of canines.
| Mammary tissue |
Adipose tissue |
Serum |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimates of log odds | S.E. | P values | Estimates of log odds | S.E. | P values | Estimates of log odds | S.E. | P values | |
| p,p-DDT | 6.532 | 6.06 | 0.28 | -2.30 | 4.03 | 0.57 | -8.30 | 386.33 | 0.98 |
| o,p-DDT | 1.162 | 29.72 | 0.97 | - | - | - | 26.88 | 1341.83 | 0.98 |
| α-HCH | -395.182 | 1921.48 | 0.84 | -314.46 | 1963.88 | 0.87 | -8.92 | 16.77 | 0.59 |
| γ-HCH | 3.682 | 11.26 | 0.74 | 69.71 | 281.43 | 0.80 | 1.55 | 3.92 | 0.69 |
| Butachlor | 5.12 | 51.35 | 0.92 | -2.47 | 443.60 | 0.99 | -1.73 | 94.08 | 0.98 |
| L-cyhalothrin | -93.94 | 842.33 | 0.91 | - | - | - | 114.52 | 521.71 | 0.83 |
| Heptachlor | 52.12 | 133.62 | 0.70 | -17.96 | 1116.93 | 0.99 | 1.64 | 2.06 | 0.42 |
| Dieldrin | 502.71 | 3317.60 | 0.88 | 121.59 | 3800.28 | 0.97 | 1619.96 | 116590.16 | 0.99 |
| Fenitrothion | 10.64 | 55.33 | 0.85 | 7.61 | 34.61 | 0.82 | - | - | - |
| Aldrin | 53.07 | 226.09 | 0.81 | 18.47 | 98.68 | 0.85 | -2.36 | 5.56 | 0.67 |
| Fipronil | -4.38 | 86.47 | 0.96 | -6.40 | 857.46 | 0.99 | - | - | - |
| Permethrin | 16.48 | 340.94 | 0.96 | 140.57 | 3932.62 | 0.97 | -2.26 | 1.98 | 0.25 |
| p,p-DDD | - | - | - | -36.50 | 403.48 | 0.93 | 34.18 | 831.28 | 0.97 |
| Age (yrs) | 1.57 | 0.98 | 0.11 | 0.40 | 0.29 | 0.17 | -0.03 | 0.22 | 0.89 |
| B.Wt. (kg) | -0.26 | 0.20 | 0.20 | -0.08 | 0.08 | 0.33 | 0.02 | 0.07 | 0.74 |
Table 6.
Logistic regression analysis of mCMT and a pool of total pesticide in different biological matrices of canines.
| Estimates of log odds |
S.E. |
P values |
|
|---|---|---|---|
| Serum | |||
| Total pesticide concentration | 0.26 | 0.32 | 0.42 |
| Age (yrs) | 0.12 | 0.18 | 0.50 |
| B.Wt. (kg) | 0.005 | 0.04 | 0.91 |
| Adipose tissue | |||
| Total pesticide concentration | 0.33 | 0.22 | 0.14 |
| Age (yrs) | 0.08 | 0.17 | 0.62 |
| B.Wt. (kg) | 0.01 | 0.04 | 0.82 |
| Mammary tissue | |||
| Total pesticide concentration | 4.99 | 0.90 | 0.08 |
| Age (yrs) | 0.54 | 0.33 | 0.10 |
| B.Wt. (kg) | -0.01 | 0.05 | 0.88 |
Although statistically non-significant, higher odds of mCMT occurrence with increase in age were noticed (Table 6). Furthermore, we observed that the odds of mCMT occurrence decreases with increased in the bodyweight of canines (Table 6). No association between the concentration of total pesticides in different matrices and age (rs = 0.157, p = 0.398 for adipose tissue; rs = -0.228, p = 0.202 for serum; rs = -0.265, p = 0.124 for mammary tissue) and bodyweight (rs = 0.046, p = 0.805 for adipose tissue; rs = 0.241, p = 0.176 for serum; rs = 0.275, p = 0.110 for mammary tissue) of canine subjects was found (Figure 3B).
4. Discussion
Pesticide-exposed humans are considered to be at higher risk of developing mammary neoplasia [37, 38]. On the other hand, several studies have dissociated pesticides from BC risk [39, 40]. Surprisingly, the role that pesticides may play in the aetiology of CMT has not been explored yet. To this end, the present study was envisaged for the detection of pesticide residues in mCMT cases and to elucidate the role of pesticides in mCMT as an etiological risk factor.
In the present study, the gas chromatic analysis revealed 14 different pesticide residues with detection of HCH isomers and DDT congeners in the majority of the canine cases. Other pesticides of major concern detected with high frequency in the present study include aldrin, heptachlor and fenitrothion. Nath et al. (1998) examined organochlorine pesticides in adipose tissue of goat, sheep and ox, and, detected DDT congeners and HCH isomers with mean concentrations quite similar to that of the present study [41]. In the recent past, Bedi and co-workers also reported POPs, predominantly DDT and HCH in the bovine milk, fish tissue and human's breast milk sampled from the nearby areas where the present study was conducted [42, 43, 44]. Additionally, comparable to the present study, heptachlor, cyhalothrin, butachlor and fipronil were also reported as common contaminants [44]. Previous studies have shown p,p-DDE as the predominant DDT metabolite in human and farm animal tissues [45, 46], however, the present study could find p,p-DDE in only 3% of serum samples with p,p-DDT, as the major contaminant. This discrepancy may be attributed either to the recent DDT exposures or to the differential metabolism and lesser generation of p,p-DDE in canines as suggested previously [24, 47]. Severe et al (2015) have reported that dogs are incapable of storing any isomers or metabolites of DDT pesticide in all its known forms (o,p’-DDE, p,p’-DDE, o,p’-DDD, p,p’-DDD, o,p’-DDT, p,p’-DDT), whereas p,p’-DDE is the major OCP contributor in humans [28]. This was further validated by Ruiz-Suarez et al. (2016) who compared the levels of organic pollutants in the serum of humans and dogs residing in the same area and could detect p,p-DDE in only 2.3% dogs as against 100% detection in humans [48]. Ban on technical HCH in 1997 with permission to use γ-HCH until March 2015 may be the reason for the predominance of γ-HCH in the present over other HCH isomers. Results with similar pesticides residue profile in CMT cases have been reported from several countries [26, 27, 28]. To the best of our knowledge, this is the first investigation on the detection of pesticides in mCMT cases from India.
. Different biological matrices have been used to assess the lifelong body burden of pesticides, including serum, breast tissue, adipose tissues from various anatomical sites, breast milk, umbilical serum, etc. [49, 50, 51, 52, 53, 54]. Equivocal results have been reported regarding the use of single or multiple biological media for pesticide detection. Also, contradictory reports are available about the association between levels of pesticide residues in serum, breast tissue and adipose tissue. We failed to find any association between the levels of total pesticides in serum and mammary tissue/mammary adipose tissue of mCMT cases. Interestingly, we found tissue-specific accumulation of certain individual pesticides. In support of our study, Archibeque-Engle et al. (1997) reported no association between serum and adipose tissue concentrations and concluded that serum concentrations of PCBs and OCPs are poor predictors of adipose tissue concentrations. Similarly, Pauwels et al. (2000) could detect only 50% of pesticides in serum samples against adipose tissue [55]. No relationship between organochlorine pesticides in human serum and adipose tissue was also reported by Waliszewski et al. (2004). On the other hand, multiple studies have suggested a linear correlation between levels of selective pesticides in the serum and other biological media and reported serum as a proxy for other biological media concentrations [49, 50, 52, 54].
Bioaccumulation of pesticides in different tissue compartments depends on several factors such as chemical nature of pesticide (lipid solubility); mode, dose and duration of exposure, the biological half-life of the compound and the metabolic profile of the tissue concerned. Organochlorine pesticides are highly lipid-soluble and tend to sequester and biomagnify in the adipose tissue, as reflected by significantly higher detection of aldrin, fenitrothion and p,p-DDT in the mammary adipose tissue in the present study. Besides, serum levels are regulated by acute of recent exposures, pathophysiological state of lipolysis [56, 57]. Information on the bioaccumulation of pesticide residues in canine tissues is meagre and to our knowledge, this is the first study to incorporate three different biological matrices of canine patients for estimating the relationship between pesticide concentrations across the three media. Nonetheless, the metabolism and kinetics of pesticide residues in canine mammary tissue and mammary adipose tissue remains largely unexplored and may differ significantly from humans [25, 28]. Under such conditions and considering the differential tissue accumulation profile of pesticides in mCMT cases, we suggest including multiple biological matrices for assessing lifelong pesticide exposures in canine patients.
Growing evidence shows that pesticide exposure is associated with breast cancer risk [58, 59, 60, 61]. Besides xenoestrogenic activity [62], other mechanisms of pesticide mediated dysregulations have been reported [63]. Besides, contradictory results have also been reported which dissociate pesticides form breast cancer [64, 65]. Lack of consensual findings in different investigations may be attributed to various factors including variation in age, ethnicity, dietary pattern, geographical location, agricultural practices, historical and current level of POPs exposure, biological specimens used for analysis, etc. Despite extensive research in humans, literature about the role of pesticides in the causation of mCMT is almost non-existing. We compared the pesticide detection frequency among mCMT and control group and found that γ-HCH, aldrin and fenitrothion were detected preferentially in the mammary tissue/adipose tissue of mCMT cases. These pesticides have been linked with various human cancers, including breast cancer [66, 67, 68, 69, 70]. However, when concentrations of individual pesticides were analyzed as a potential risk factor for mCMT using multivariable logistic regression, high variability expressed as standard deviations were observed, making it impractical to make a precise conclusion. More importantly, higher odds (β value = 4.99) were noted with total pesticide concentrations in mammary tissue, but with marginally non-significance (most probably due to small control sample size), suggesting that exposure of canine mammary tissue to a pool of pesticides may presumably act as a risk for mCMT. Moreover, a mixture of pesticides may lead to a synergistic effect and the combined effects may be of much higher magnitude than those expected from the exposure of a single pesticide [71]. Boada et al. (2012) reported that a mixture of endocrine-disrupting compounds, rather than a single compound, acts as a determining risk for breast cancer due to the complex interactions with endogenous factors. Notably, under such circumstances, pesticides with low levels and without any significant differences among the CMT cases and tumor-free dogs may also critically affect the proliferative outcome. Furthermore, because the latency period of breast cancer may vary up to 15 years [72], the concentration at the time of pesticide detection may not truly reflect the pesticide exposure at the time of initiation of CMT.
Age is considered as a confounding factor in the accumulation of pesticide residues in humans [49, 50, 73, 74]. Some studies, however, reported no association between pesticide accumulation and age of human subjects [75, 76]. No such information is available for canines, particularly regarding the mCMT. We couldn't find any correlation between the concentration of total pesticides in canine tissues and the age and bodyweight of mCMT cases. With age advancement, the exposure time also increases which leads to pesticide biomagnifications. However, several endogenous and exogenous factors, such as placental transfer and lactation eliminate pesticides from the body [77, 78]. Thus, the equilibrium between the pesticide acquisition and release defines the overall pesticide body burden and doesn't rely solely on age.
5. Conclusion
Our data, which was severely incapacitated by the small sample size of control animals, prevent us from asserting the role of individual pesticides in mCMT induction and should be an important lesson as well as consideration for future studies. However, more importantly, our study demonstrated that pesticides belonging to different classes are present in the tissues of companion dogs in Punjab, India which is significant from the human health perspective. Differential tissue accumulation profile of pesticides in canines was delineated and a mixture of pesticide residues was hypothesized as a probable risk for mCMT when present in mammary tissues.
Declarations
Author contribution statement
Siddharth Gautam: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Indrasen Chauhan, Kuldip Gupta: Analyzed and interpreted the data.
Naresh Kumar Sood: Conceived and designed the experiments.
Chitra Joshi: Analyzed and interpreted the data; Wrote the paper.
Kamalpreet Kaur Gill: Performed the experiments.
Rajdeep Kaur: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to the Vice-chancellor and Director of Research, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana for providing the necessary facilities for the research.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Manahan S. seventh ed. ed. Lewis Publishers/CRC Press LLC; London: 2000. [Google Scholar]
- 2.Ashraf M.A. Persistent organic pollutants (POPs): a global issue, a global challenge. Environ. Sci. Pollut. Res. Int. 2017;24:4223–4227. doi: 10.1007/s11356-015-5225-9. [DOI] [PubMed] [Google Scholar]
- 3.UNEP . United Nations Environmental Programme; Vienna, Austria: 2004. Stockholm Convention on Persistent Organic Compounds. [Google Scholar]
- 4.El-Shahawi M.S., Hamza A., Bashammakh A.S., Al-Saggaf W.T. An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta. 2010;80:1587–1597. doi: 10.1016/j.talanta.2009.09.055. [DOI] [PubMed] [Google Scholar]
- 5.Verner M.A., Sonneborn D., Lancz K., Muckle G., Ayotte P., Dewailly E., Kocan A., Palkovicova L., Trnovec T., Haddad S., Hertz-Picciotto I., Eggesbo M. Toxicokinetic modeling of persistent organic pollutant levels in blood from birth to 45 months of age in longitudinal birth cohort studies. Environ. Health Perspect. 2013;121:131–137. doi: 10.1289/ehp.1205552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dirtu A.C., Ali N., Van den Eede N., Neels H., Covaci A. Country specific comparison for profile of chlorinated, brominated and phosphate organic contaminants in indoor dust. Case study for Eastern Romania, 2010. Environ. Int. 2012;49:1–8. doi: 10.1016/j.envint.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Guo W., Park J.S., Wang Y., Gardner S., Baek C., Petreas M., Hooper K. High polybrominated diphenyl ether levels in California house cats: house dust a primary source? Environ. Toxicol. Chem. 2012;31:301–306. doi: 10.1002/etc.1700. [DOI] [PubMed] [Google Scholar]
- 8.Mnif W., Hassine A.I., Bouaziz A., Bartegi A., Thomas O., Roig B. Effect of endocrine disruptor pesticides: a review. Int. J. Environ. Res. Publ. Health. 2011;8:2265–2303. doi: 10.3390/ijerph8062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanborn M., Kerr K.J., Sanin L.H., Cole D.C., Bassil K.L., Vakil C. Non-cancer health effects of pesticides: systematic review and implications for family doctors. Canad. Family Phys. Medecin de Famille Canadien. 2007;53:1712–1720. [PMC free article] [PubMed] [Google Scholar]
- 10.Ghoncheh M., Pournamdar Z., Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac. J. Cancer Prev. APJCP: Asian Pac. J. Cancer Prev. APJCP. 2016;17:43–46. doi: 10.7314/apjcp.2016.17.s3.43. [DOI] [PubMed] [Google Scholar]
- 11.Raica M., Jung I., Cimpean A.M., Suciu C., Muresan A.M. From conventional pathologic diagnosis to the molecular classification of breast carcinoma: are we ready for the change? Rom. J. Morphol. Embryol. 2009;50:5–13. [PubMed] [Google Scholar]
- 12.Egenvall A., Bonnett B.N., Ohagen P., Olson P., Hedhammar A., von Euler H. Incidence of and survival after mammary tumors in a population of over 80,000 insured female dogs in Sweden from 1995 to 2002. Prev. Vet. Med. 2005;69:109–127. doi: 10.1016/j.prevetmed.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Misdorp W. Tumors of the mammary gland. In: Meuten D.J., editor. Tumors in Domestic Animals. fourth ed. Iowa State Press; Iowa: 2002. pp. 575–606. [Google Scholar]
- 14.Salas Y., Marquez A., Diaz D., Romero L. Epidemiological study of mammary tumors in female dogs diagnosed during the period 2002-2012: a growing animal health problem. PloS One. 2015;10 doi: 10.1371/journal.pone.0127381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Queiroga F.L., Raposo T., Carvalho M.I., Prada J., Pires I. Canine mammary tumours as a model to study human breast cancer: most recent findings. In Vivo. 2011;25:455–465. [PubMed] [Google Scholar]
- 16.Abdelmegeed S.M., Mohammed S. Canine mammary tumors as a model for human disease. Oncol. Lett. 2018;15:8195–8205. doi: 10.3892/ol.2018.8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiffman J.D., Breen M. Comparative oncology: what dogs and other species can teach us about humans with cancer. Phil. Trans. Roy. Soc. Lond. B Biol. Sci. 2015;370 doi: 10.1098/rstb.2014.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raposo T.P., Arias-Pulido H., Chaher N., Fiering S.N., Argyle D.J., Prada J., Pires I., Queiroga F.L. Comparative aspects of canine and human inflammatory breast cancer. Semin. Oncol. 2017;44:288–300. doi: 10.1053/j.seminoncol.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Arrebola J.P., Fernandez-Rodriguez M., Artacho-Cordon F., Garde C., Perez-Carrascosa F., Linares I., Tovar I., Gonzalez-Alzaga B., Exposito J., Torne P., Fernandez M.F., Olea N. Associations of persistent organic pollutants in serum and adipose tissue with breast cancer prognostic markers. Sci. Total Environ. 2016;566–567:41–49. doi: 10.1016/j.scitotenv.2016.04.188. [DOI] [PubMed] [Google Scholar]
- 20.Enan E., Matsumura F. Activation of c-Neu tyrosine kinase by o,p'-DDT and beta-HCH in cell-free and intact cell preparations from MCF-7 human breast cancer cells. J. Biochem. Mol. Toxicol. 1998;12:83–92. doi: 10.1002/(sici)1099-0461(1998)12:2<83::aid-jbt3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 21.Davis D.L., Bradlow H.L., Wolff M., Woodruff T., Hoel D.G., Anton-Culver H. Medical hypothesis: xenoestrogens as preventable causes of breast cancer. Environ. Health Perspect. 1993;101:372–377. doi: 10.1289/ehp.93101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong P.S., Matsumura F. Promotion of breast cancer by beta-hexachlorocyclohexane in MCF10AT1 cells and MMTV-neu mice. BMC Canc. 2007;7:130. doi: 10.1186/1471-2407-7-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calaf G.M., Echiburu-Chau C., Roy D. Organophosphorous pesticides and estrogen induce transformation of breast cells affecting p53 and c-Ha-ras genes. Int. J. Oncol. 2009;35:1061–1068. doi: 10.3892/ijo_00000421. [DOI] [PubMed] [Google Scholar]
- 24.Ali N., Malik R.N., Mehdi T., Eqani S.A., Javeed A., Neels H., Covaci A. Organohalogenated contaminants (OHCs) in the serum and hair of pet cats and dogs: biosentinels of indoor pollution. Sci. Total Environ. 2013;449:29–36. doi: 10.1016/j.scitotenv.2013.01.037. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz-Suarez N., Camacho M., Boada L.D., Henriquez-Hernandez L.A., Rial C., Valeron P.F., Zumbado M., Gonzalez M.A., Luzardo O.P. The assessment of daily dietary intake reveals the existence of a different pattern of bioaccumulation of chlorinated pollutants between domestic dogs and cats. Sci. Total Environ. 2015;530–531:45–52. doi: 10.1016/j.scitotenv.2015.05.070. [DOI] [PubMed] [Google Scholar]
- 26.Andrade F.H., Figueiroa F.C., Bersano P.R., Bissacot D.Z., Rocha N.S. Malignant mammary tumor in female dogs: environmental contaminants. Diagn. Pathol. 2010;5:45. doi: 10.1186/1746-1596-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colodel M.M., Ferreira I., Martins V.M.V., Almeida A.A., Lopes M.D., Rocha N.S. Spontaneous mammary carcinomas in female dogs: association between immunohistochemical degrees of neoplasia aggressiveness and residual pyrethroids. Open J. Vet. Med. 2012;2:207–215. [Google Scholar]
- 28.Severe S., Marchand P., Guiffard I., Morio F., Venisseau A., Veyrand B., Le Bizec B., Antignac J.P., Abadie J. Pollutants in pet dogs: a model for environmental links to breast cancer. SpringerPlus. 2015;4:27. doi: 10.1186/s40064-015-0790-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muddasir B., Dhar J.K., Shafiqa A., Qureshi S., Tariq J., Gupta B. Pesticide residues in blood serum samples from inhabitants of 'Dal Lake' Hamlets in Jammu and Kashmir, India (2008-2010) J. Environ. Sci. Toxicol. Food Technol. 2012;1:26–31. [Google Scholar]
- 30.Bancroft J.D., Gamble M. sixth ed. Churchill Livingstone, Elsevier; China: 2008. Theory and Practice of Histological Techniques. [Google Scholar]
- 31.Gill U.S., Schwartz H.M., Wheatley B. Development of a method for the analysis of PCB congeners and organochlorine pesticides in blood/serum. Chemosphere. 1996;32:1055–1076. doi: 10.1016/0045-6535(96)00025-2. [DOI] [PubMed] [Google Scholar]
- 32.AOAC . 1999. Guidelines for Single-Laboratory Validation of Analytical Methods for Trace Level Concentrations of Organic Chemicals. [Google Scholar]
- 33.Rizopoulos D. ltm: an R package for latent variable modeling and item response theory analyses. J. Stat. Software. 2006;17:1–25. [Google Scholar]
- 34.Harrell F.E., Jr. 2020. rms: regression modeling strategies. R package version 6.0-1. [Google Scholar]
- 35.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 36.Misdorp W. Tumors of the mammary gland. In: Meuten D.J., editor. Tumors in Domestic Animals. fourth ed. Iowa State Press; Iowa: 2002. pp. 575–606. [Google Scholar]
- 37.Arrebola J.P., Belhassen H., Artacho-Cordon F., Ghali R., Ghorbel H., Boussen H., Perez-Carrascosa F.M., Exposito J., Hedhili A., Olea N. Risk of female breast cancer and serum concentrations of organochlorine pesticides and polychlorinated biphenyls: a case-control study in Tunisia. Sci. Total Environ. 2015;520:106–113. doi: 10.1016/j.scitotenv.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 38.Fenga C. Occupational exposure and risk of breast cancer. Biomed. Rep. 2016;4:282–292. doi: 10.3892/br.2016.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pavuk M., Cerhan J.R., Lynch C.F., Kocan A., Petrik J., Chovancova J. Case-control study of PCBs, other organochlorines and breast cancer in Eastern Slovakia. J. Expo. Anal. Environ. Epidemiol. 2003;13:267–275. doi: 10.1038/sj.jea.7500277. [DOI] [PubMed] [Google Scholar]
- 40.Niehoff N.M., Nichols H.B., White A.J., Parks C.G., D'Aloisio A.A., Sandler D.P. Childhood and adolescent pesticide exposure and breast cancer risk. Epidemiology. 2016;27:326–333. doi: 10.1097/EDE.0000000000000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nath S.B., Unnikrishnan V., Gayathri V., Chitra P.S., Preeja C.N., Murthy M.K.R. Organochlorine pesticide residues in animal tissues and their excretion through milk. J. Food Sci. Technol. 1998;35:547–548. [Google Scholar]
- 42.Bawa P., Bedi J.S., Gill J.P.S., Aulakh R.S., Kumar A., Arora K. Persistent organic pollutants residues in human breast milk from Bathinda and Ludhiana districts of Punjab, India. Arch. Environ. Contam. Toxicol. 2018;75(4):512–520. doi: 10.1007/s00244-018-0512-3. [DOI] [PubMed] [Google Scholar]
- 43.Bedi J.S., Singh V., Gupta A., Gill J.P.S., Aulakh R.S. Persistent organic pollutants (POPs) in fresh water farm fish species from Punjab (India) and evaluation of their dietary intake for human risk assessment. Hum. Ecol. Risk Assess. 2017:1–14. [Google Scholar]
- 44.Bedi J.S., Gill J.P., Aulakh R.S., Kaur P. Pesticide residues in bovine milk in Punjab, India: spatial variation and risk assessment to human health. Arch. Environ. Contam. Toxicol. 2015;69:230–240. doi: 10.1007/s00244-015-0163-6. [DOI] [PubMed] [Google Scholar]
- 45.Hardell L., van Bavel B., Lindstrom G., Bjornfoth H., Orgum P., Carlberg M., Sorensen C.S., Graflund M. Adipose tissue concentrations of p,p'-DDE and the risk for endometrial cancer. Gynecol. Oncol. 2004;95:706–711. doi: 10.1016/j.ygyno.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 46.National Children's Study Placenta C., Nanes J.A., Xia Y., Dassanayake R., Jones R.M., Li A., Stodgell C.J., Walker C., Szabo S., Leuthner S., Durkin M.S., Moye J., Miller R.K. Selected persistent organic pollutants in human placental tissue from the United States. Chemosphere. 2014;106:20–27. doi: 10.1016/j.chemosphere.2013.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Storelli M.M., Storelli A., Barone G., Franchini D. Accumulation of polychlorinated biphenyls and organochlorine pesticide in pet cats and dogs: assessment of toxicological status. Sci. Total Environ. 2009;408:64–68. doi: 10.1016/j.scitotenv.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 48.Ruiz-Suárez N., Rial C., Boada L.D., Henríquez-Hernández L.A., Valeron P.F., Camacho M., Zumbado M., González M.A., Lara P., Luzardo O.P. Are pet dogs good sentinels of human exposure to environmental polycyclic aromatic hydrocarbons, organochlorine pesticides and polychlorinated biphenyls? J. Appl. Anim. Res. 2016;44:135–145. [Google Scholar]
- 49.Artacho-Cordon F., Fernandez-Rodriguez M., Garde C., Salamanca E., Iribarne-Duran L.M., Torne P., Exposito J., Papay-Ramirez L., Fernandez M.F., Olea N., Arrebola J.P. Serum and adipose tissue as matrices for assessment of exposure to persistent organic pollutants in breast cancer patients. Environ. Res. 2015;142:633–643. doi: 10.1016/j.envres.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 50.Rusiecki J.A., Matthews A., Sturgeon S., Sinha R., Pellizzari E., Zheng T., Baris D. A correlation study of organochlorine levels in serum, breast adipose tissue, and gluteal adipose tissue among breast cancer cases in India. Canc. Epidemiol. Biomark. Prevent.: Publ. Am. Assoc. Canc. Res.- Cosponsored by the American Society of Preventive Oncology. 2005;14:1113–1124. doi: 10.1158/1055-9965.EPI-04-0356. [DOI] [PubMed] [Google Scholar]
- 51.Waliszewski S.M., Carvajal O., Infanzon R.M., Trujillo P., Hart M.M. Copartition ratios of persistent organochlorine pesticides between human adipose tissue and blood serum lipids. Bull. Environ. Contam. Toxicol. 2004;73:732–738. doi: 10.1007/s00128-004-0487-9. [DOI] [PubMed] [Google Scholar]
- 52.Whitcomb B.W., Schisterman E.F., Buck G.M., Weiner J.M., Greizerstein H., Kostyniak P.J. Relative concentrations of organochlorines in adipose tissue and serum among reproductive age women. Environ. Toxicol. Pharmacol. 2005;19:203–213. doi: 10.1016/j.etap.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 53.Archibeque-Engle S.L., Tessari J.D., Winn D.T., Keefe T.J., Nett T.M., Zheng T. Comparison of organochlorine pesticide and polychlorinated biphenyl residues in human breast adipose tissue and serum. J. Toxicol. Environ. Health. 1997;52:285–293. doi: 10.1080/00984109708984065. [DOI] [PubMed] [Google Scholar]
- 54.Sharaf N.E., Elserougy S.M., Hussein A.S.E.-D.A., Abou-Arab A., Ahmed S.B., Abdel-Hamid E. Organochlorine pesticides in breast milk and other tissues of some Egyptian mothers. Am.-Eurasian J. Agric. Environ. Sci. 2008;4:434–442. [Google Scholar]
- 55.Pauwels A., Covaci A., Weyler J., Delbeke L., Dhont M., De Sutter P., D'Hooghe T., Schepens P.J. Comparison of persistent organic pollutant residues in serum and adipose tissue in a female population in Belgium, 1996-1998. Arch. Environ. Contam. Toxicol. 2000;39:265–270. doi: 10.1007/s002440010104. [DOI] [PubMed] [Google Scholar]
- 56.Imbeault P., Chevrier J., Dewailly E., Ayotte P., Despres J.P., Tremblay A., Mauriege P. Increase in plasma pollutant levels in response to weight loss in humans is related to in vitro subcutaneous adipocyte basal lipolysis. Int. J. Obes. Relat. Metab. Disord.: J. Int. Assoc. Study Obes. 2001;25:1585–1591. doi: 10.1038/sj.ijo.0801817. [DOI] [PubMed] [Google Scholar]
- 57.Kim M.J., Marchand P., Henegar C., Antignac J.P., Alili R., Poitou C., Bouillot J.L., Basdevant A., Le Bizec B., Barouki R., Clement K. Fate and complex pathogenic effects of dioxins and polychlorinated biphenyls in obese subjects before and after drastic weight loss. Environ. Health Perspect. 2011;119:377–383. doi: 10.1289/ehp.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Demers A., Ayotte P., Brisson J., Dodin S., Robert J., Dewailly E. Risk and aggressiveness of breast cancer in relation to plasma organochlorine concentrations. Canc. Epidemiol. Biomarkers Prev.: Publ. Am. Assoc. Canc. Res. - Cosponsored by the American Society of Preventive Oncology. 2000;9:161–166. [PubMed] [Google Scholar]
- 59.Gatto N.M., Longnecker M.P., Press M.F., Sullivan-Halley J., McKean-Cowdin R., Bernstein L. Serum organochlorines and breast cancer: a case-control study among African-American women. Canc. Causes Contr.: CCC. 2007;18:29–39. doi: 10.1007/s10552-006-0070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang M., Zhao M., Zhou S., Chen K., Zhang C., Liu W. Assessing the underlying breast cancer risk of Chinese females contributed by dietary intake of residual DDT from agricultural soils. Environ. Int. 2014;73:208–215. doi: 10.1016/j.envint.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Waliszewski S.M., Bermudez M.T., Infanzon R.M., Silva C.S., Carvajal O., Trujillo P., Gomez Arroyo S., Villalobos Pietrini R., Saldana V.A., Melo G., Esquivel S., Castro F., Ocampo H., Torres J., Hayward-Jones P.M. Persistent organochlorine pesticide levels in breast adipose tissue in women with malignant and benign breast tumors. Bull. Environ. Contam. Toxicol. 2005;75:752–759. doi: 10.1007/s00128-005-0815-8. [DOI] [PubMed] [Google Scholar]
- 62.Pestana D., Teixeira D., Faria A., Domingues V., Monteiro R., Calhau C. Effects of environmental organochlorine pesticides on human breast cancer: putative involvement on invasive cell ability. Environ. Toxicol. 2015;30:168–176. doi: 10.1002/tox.21882. [DOI] [PubMed] [Google Scholar]
- 63.Yanez L., Borja-Aburto V.H., Rojas E., de la Fuente H., Gonzalez-Amaro R., Gomez H., Jongitud A.A., Diaz-Barriga F. DDT induces DNA damage in blood cells. Studies in vitro and in women chronically exposed to this insecticide. Environ. Res. 2004;94:18–24. doi: 10.1016/s0013-9351(03)00047-1. [DOI] [PubMed] [Google Scholar]
- 64.Boada L.D., Zumbado M., Henriquez-Hernandez L.A., Almeida-Gonzalez M., Alvarez-Leon E.E., Serra-Majem L., Luzardo O.P. Complex organochlorine pesticide mixtures as determinant factor for breast cancer risk: a population-based case-control study in the Canary Islands (Spain) Environ. Health: Glob. Access Sci. Source. 2012;11:28. doi: 10.1186/1476-069X-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohn B.A., La Merrill M., Krigbaum N.Y., Yeh G., Park J.S., Zimmermann L., Cirillo P.M. DDT exposure in utero and breast cancer. J. Clin. Endocrinol. Metabol. 2015;100:2865–2872. doi: 10.1210/jc.2015-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andersen H.R., Vinggaard A.M., Rasmussen T.H., Gjermandsen I.M., Bonefeld-Jorgensen E.C. Effects of currently used pesticides in assays for estrogenicity, androgenicity, and aromatase activity in vitro. Toxicol. Appl. Pharmacol. 2002;179:1–12. doi: 10.1006/taap.2001.9347. [DOI] [PubMed] [Google Scholar]
- 67.Goto S., Asada S., Fushiwaki Y., Mori Y., Tanaka N., Umeda M., Nakajima D., Takeda K. Tumor-promoting activity and mutagenicity of 5 termiticide compounds. J. UOEH. 2004;26:423–430. doi: 10.7888/juoeh.26.423. [DOI] [PubMed] [Google Scholar]
- 68.Hoyer A.P., Jorgensen T., Grandjean P., Hartvig H.B. Repeated measurements of organochlorine exposure and breast cancer risk (Denmark) Canc. Causes Contr.: CCC. 2000;11:177–184. doi: 10.1023/a:1008926219539. [DOI] [PubMed] [Google Scholar]
- 69.Loomis D., Guyton K., Grosse Y., El Ghissasi F., Bouvard V., Benbrahim-Tallaa L., Guha N., Mattock H., Straif K., International Agency for Research on Cancer Monograph Working Group ILF Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. Lancet Oncol. 2015;16:891–892. doi: 10.1016/S1470-2045(15)00081-9. [DOI] [PubMed] [Google Scholar]
- 70.Ociepa-Zawal M., Rubis B., Wawrzynczak D., Wachowiak R., Trzeciak W.H. Accumulation of environmental estrogens in adipose tissue of breast cancer patients. J. Environ. Sci. Health - Part A Toxic/Hazard. Subst. Environ. Eng. 2010;45:305–312. doi: 10.1080/10934520903468038. [DOI] [PubMed] [Google Scholar]
- 71.Soto A.M., Chung K.L., Sonnenschein C. The pesticides endosulfan, toxaphene, and dieldrin have estrogenic effects on human estrogen-sensitive cells. Environ. Health Perspect. 1994;102:380–383. doi: 10.1289/ehp.94102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petralia S.A., Vena J.E., Freudenheim J.L., Dosemeci M., Michalek A., Goldberg M.S., Brasure J., Graham S. Risk of premenopausal breast cancer in association with occupational exposure to polycyclic aromatic hydrocarbons and benzene. Scand. J. Work. Environ. Health. 1999;25:215–221. doi: 10.5271/sjweh.426. [DOI] [PubMed] [Google Scholar]
- 73.Bagga D., Anders K.H., Wang H.J., Roberts E., Glaspy J.A. Organochlorine pesticide content of breast adipose tissue from women with breast cancer and control subjects. J. Natl. Cancer Inst. 2000;92:750–753. doi: 10.1093/jnci/92.9.750. [DOI] [PubMed] [Google Scholar]
- 74.He T.T., Zuo A.J., Wang J.G., Zhao P. Organochlorine pesticides accumulation and breast cancer: a hospital-based case-control study. Tumour Biol.: J. Int. Soc. Oncodev. Biol. Med. 2017;39 doi: 10.1177/1010428317699114. 1010428317699114. [DOI] [PubMed] [Google Scholar]
- 75.Moon H.B., Lee D.H., Lee Y.S., Choi M., Choi H.G., Kannan K. Polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in adipose tissues of Korean women. Arch. Environ. Contam. Toxicol. 2012;62:176–184. doi: 10.1007/s00244-011-9679-6. [DOI] [PubMed] [Google Scholar]
- 76.Sanghi R., Pillai M.K., Jayalekshmi T.R., Nair A. Organochlorine and organophosphorus pesticide residues in breast milk from Bhopal, Madhya Pradesh, India. Hum. Exp. Toxicol. 2003;22:73–76. doi: 10.1191/0960327103ht321oa. [DOI] [PubMed] [Google Scholar]
- 77.Eik Anda E., Nieboer E., Dudarev A.A., Sandanger T.M., Odland J.O. Intra- and intercompartmental associations between levels of organochlorines in maternal plasma, cord plasma and breast milk, and lead and cadmium in whole blood, for indigenous peoples of Chukotka, Russia. J. Environ. Monit.: JEM. 2007;9:884–893. doi: 10.1039/b706717h. [DOI] [PubMed] [Google Scholar]
- 78.Lopez-Espinosa M.J., Murcia M., Iniguez C., Vizcaino E., Llop S., Vioque J., Grimalt J.O., Rebagliato M., Ballester F. Prenatal exposure to organochlorine compounds and birth size. Pediatrics. 2011;128:e127–134. doi: 10.1542/peds.2010-1951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.