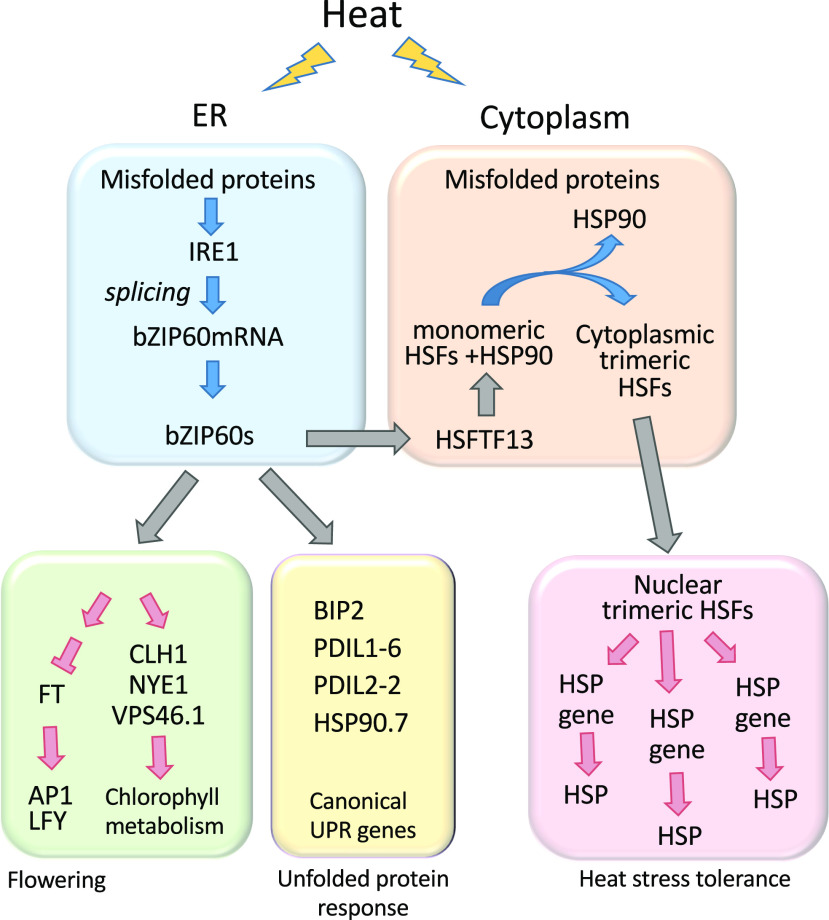
The transcription factor bZIP60 links a system that alerts plants to problems in protein folding to a system that protects plants from heat stress.
Abstract
The unfolded protein response (UPR) and the heat shock response (HSR) are two evolutionarily conserved systems that protect plants from heat stress. The UPR and HSR occur in different cellular compartments and both responses are elicited by misfolded proteins that accumulate under adverse environmental conditions such as heat stress. While the UPR and HSR appear to operate independently, we have found a link between them in maize (Zea mays) involving the production of the BASIC LEUCINE ZIPPER60 (bZIP60) transcription factor, a pivotal response of the UPR to heat stress. Surprisingly, a mutant (bzip60-2) knocking down bZIP60 expression blunted the HSR at elevated temperatures and prevented the normal upregulation of a group of heat shock protein genes in response to elevated temperature. The expression of a key HEAT SHOCK FACTOR TRANSCRIPTION FACTOR13 (HSFTF13, a HEAT SHOCK FACTOR A6B [HSFA6B] family member) was compromised in bzip60-2, and the HSFTF13 promoter was shown to be a target of bZIP60 in maize protoplasts. In addition, the upregulation by heat of genes involved in chlorophyll catabolism and chloroplast protein turnover were subdued in bzip60-2, and these genes were also found to be targets of bZIP60. Thus, the UPR, an endoplasmic-reticulum–associated response, quite unexpectedly contributes to the nuclear/cytoplasmic HSR in maize.
INTRODUCTION
Global temperatures are rising as a consequence of increasing concentrations of greenhouse gases in the atmosphere (www.epa.gov/climate-research). In a business-as-usual model, global temperature is predicted to increase 2.6°C to 4.8°C by the end of century according to the Intergovernmental Panel on Climate Change, 5th Assessment Report (http://www.climatechange2013.org). Given future warming scenarios, crop productivity will suffer (Bita and Gerats, 2013; Challinor et al., 2014; Zhao et al., 2017). In general, plant productivity increases with temperature to an optimum, and beyond that point yields decline (Tigchelaar et al., 2018). Increasing temperatures in the ambient temperature range accelerate time to flowering and increase stem elongation rates, a process referred to as “thermomorphogenesis.”
In response to higher temperatures, plants activate the cytoplasmic heat shock response (HSR) in which heat shock transcription factors (HSFs) upregulate the expression of heat shock protein (HSP) genes (Qu et al., 2013; Ohama et al., 2017). HSPs act as chaperones to maintain the function of plant proteins in response to heat stress (for example, see Jacob et al., 2017). In unstressed conditions HSFs are repressed by their interaction with HSP70 chaperones (Shi et al., 1998). HSFs are activated under elevated temperature conditions because heat leads to the accumulation of misfolded proteins that recruit chaperones away from HSFs in accordance with the chaperone titration model (Voellmy and Boellmann, 2007; Zheng et al., 2016). Liberated HSFs undergo trimerization, promoting their ability to bind to HSR elements (HSEs) and activate HSP genes (Morimoto, 1993). HSF responses to elevated temperature in plants are complex because of the large size of the HSF families, numbering 25 members in maize (Zea mays; Lin et al., 2011).
The unfolded protein response (UPR) is also activated by heat stress in another cellular compartment, the endoplasmic reticulum (ER). Although the UPR and the HSR occur in different cellular compartments, they both can be elicited by the accumulation of misfolded proteins, a condition in the ER referred to as ER stress. Adverse environmental conditions, such as heat stress, can perturb protein folding, an error-prone process, inducing ER stress and, thereby, eliciting the UPR. The UPR upregulates the expression of a canonical set of genes involved in ER protein import, folding, quality control, and export. The upregulation of the UPR genes is brought about by the activation of stress-transducing transcription factors in plants (Howell, 2013). One such factor is bZIP60, whose mRNA is spliced by an ER membrane-associated RNA splicing factor, INOSITOL REQUIRING ENZYME1 (IRE1; Deng et al., 2011; Nagashima et al., 2011). The splice endows bZIP60 with nuclear targeting capabilities. In addition, IRE1 activates autophagy during ER stress, resulting in degradation of ER fragments containing misfolded proteins and thus helping to alleviate the ER stress (Liu et al., 2012).
We are interested in determining whether the UPR and the HSR communicate with each other in response to heat stress and the extent to which the UPR contributes to heat stress tolerance in maize. It had been shown in Arabidopsis (Arabidopsis thaliana) and in maize seedlings that heat shock activates the splicing of bZIP60 mRNA (Deng et al., 2011; Li et al., 2012). In this study, we have assessed whether diurnal temperature changes similar to field conditions, rather than heat shock, result in the splicing of bZIP60 mRNA, which in turn elicits the UPR. We find that a myriad of genes is upregulated by heat, including some of the canonical UPR genes. Most importantly, we found that bZIP60 expression contributes to the upregulation of a constellation of HSP genes, indicating a critical link between the UPR in the ER and the nuclear/cytoplasmic heat shock system.
RESULTS
Effects of Heat on Maize Growth
To assess the effects of heat stress on maize, we used a controlled environment facility called the Enviratron to simulate field conditions (Bao et al., 2019). The Enviratron consists of eight growth chambers in which plants can be subjected to different environmental conditions (Supplemental Figures 1A and 1B). For our experiments, maize plants (inbred line W22) were subjected to conditions simulating normal diurnal rhythms of light and temperature, with increasing maximal daily temperature (MDT). Maize plants were grown continuously under four different temperature regimes with simulated morning temperatures ramped up over 6 h to a MDT of 31°C, 33°C, 35°C, or 37°C and simulated evening/night time temperatures ramped down over 8 h to 10°C below the MDT (Figure 1A). Light intensities in a 16-h/8-h photoperiod cycle were ramped up to 1,200 μmol/m2/s (measured 60 cm from the light source) to simulate dawn and down over 2 h to simulate dusk. Plant performance was assessed at different stages of growth, mid-vegetative (V4 and V5; 20 and 27 d after germination [DAG]) and reproductive stages (V13 to R1; see Methods for an explanation of vegetative stages; He et al., 2010). Plants grown at 33°C MDT produced the greatest shoot biomass (dry weight), while shoots on plants grown at 37°C MDT were somewhat reduced in growth (Supplemental Figures 2A and 2B).
Figure 1.
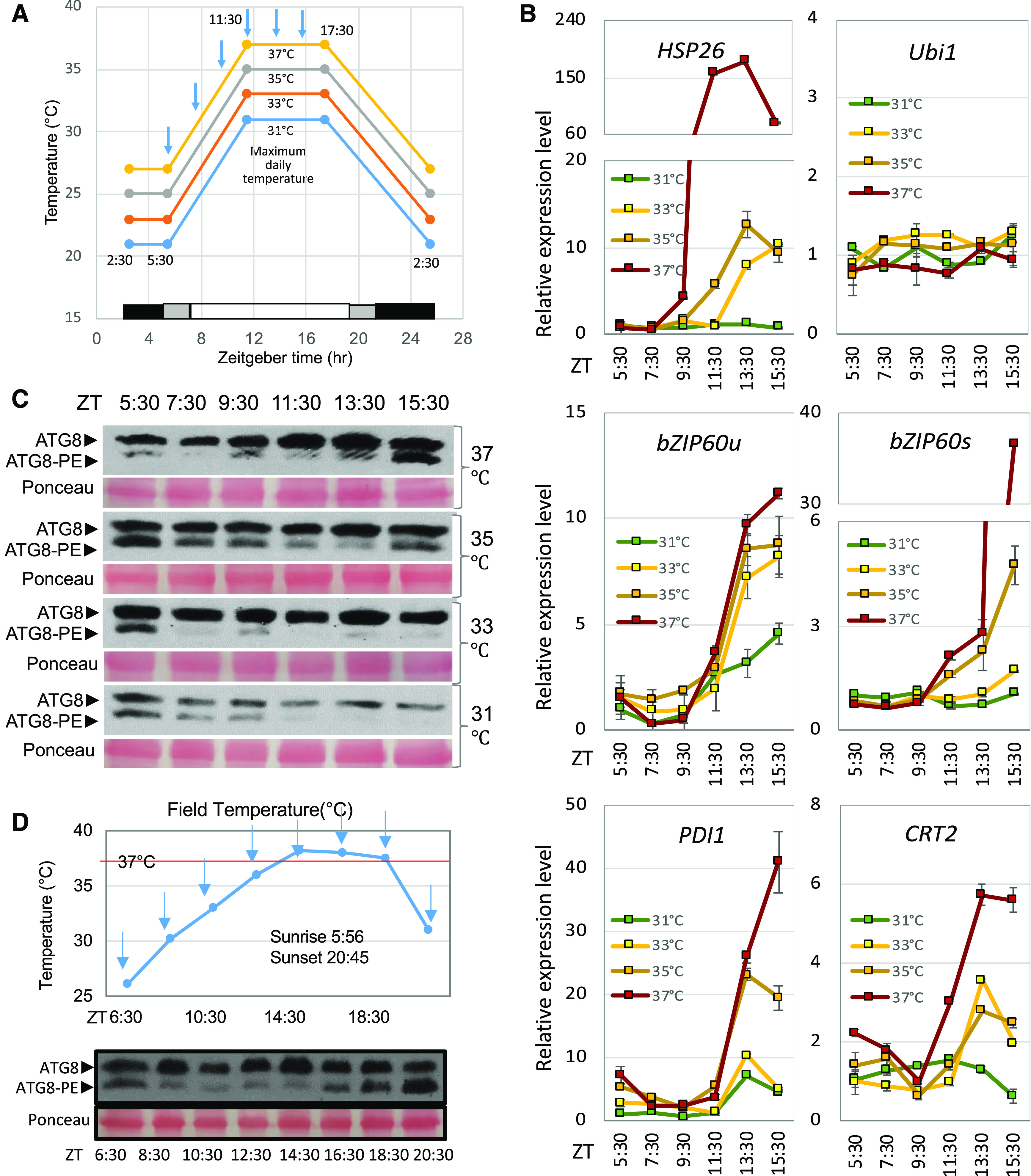
Responses to Diurnal Temperature Cycles in Maize.
(A) Maize plants (W22 and bzip60-2) were subjected to photoperiod and temperature cycles in which MDTs reached 31°C, 33°C, 35°C, or 37°C in the different growth chambers of the Enviratron. Morning and evening temperatures were ramped up or down over 6 and 8 h, respectively, while light intensities were ramped up or down over 2 h. V4 plants were sampled every 2 h for RT-qPCR analysis (blue arrows). V4 and V5 plants were sampled for RNA-seq analysis at ZT 11:30.
(B) RT-qPCR analysis of gene expression in W22 plants as the temperature was ramped up during the virtual morning to the MDT. RNA was extracted from leaf samples and gene expression patterns were assessed for HSP26 (Zm00001d028408), Ubi1 (Zm00001d015327), bZIP60 unspliced and spliced forms (bZIP60u and bZIP60s, Zm00001d046718), PDI1 (Zm00001d049099), and CRT2 (Zm00001d005460). The values are the means ± sd of three biological repeats.
(C) Lipidation of ATG8 to assess the induction of autophagy. Proteins were extracted from leaf samples every 2 h during the virtual morning. Shown is an immunoblot of proteins subjected to electrophoresis on polyacrylamide/urea gels, probed with anti-ATG8 antibody. The positions of free ATG8 and ATG8-PE are indicated as well as Ponceau-stained RUBISCO bands as a loading control.
(D) ATG8 lipidation and bZIP60 mRNA splicing were assessed in a field experiment on July 19, 2019. Top, air temperature was measured at a height above ground at which leaf samples were taken. The first fully expanded leaf on different plants at developmental stage V9 was sampled in triplicate every 2 h (blue arrows) over a 14-h period. Proteins were extracted from the samples, and ATG8 lipidation was assessed as in (C). One replicate is shown with Ponceau-stained proteins as a loading control. All three replicates showed the same pattern, although only one replicate is displayed here.
Effects of Heat on Gene Expression
To evaluate how different MDTs affect the program of gene expression, RNA was extracted from small strips of leaf lamina excised from the first fully expanded leaf of V4 and V5 plants (at 20 and 27 DAG, respectively). Plants at the V4 stage were sampled in triplicate every 2 h during the virtual morning (Figure 1A), and the extracted RNA was analyzed by RT-qPCR. Plants responded to the slow rise in temperature by upregulating HSR genes, exemplified by HSP26 (Zm00001d028408), which first reached a peak at Zeitgeber time (ZT) 11:30 in plants subjected to the highest MDT (37°C; Figure 1B). bZIP60 was also upregulated at elevated temperatures, as seen by the accumulation of its unspliced RNA (bZIP60u), which became apparent around ZT 11:30 at 33°C MDT and above. The spliced form of bZIP60 mRNA (bZIP60s) reflects the activation of IRE1, the ER membrane-associated RNA splicing factor (Deng et al., 2011; Nagashima et al., 2011). There was an increase in bZIP60s accumulation around ZT 13:30, ∼2 h after the temperature reached its MDT of 35°C or 37°C. Some of the canonical UPR genes, such as those encoding PROTEIN DISULFIDE ISOMERASE1 (PDI1, Zm00001d049099) and CALRETICULIN2 (CRT2, Zm00001d005460), were also upregulated with increasing MDT (Figure 1B).
Effects of Heat on Autophagy
ER stress and heat shock induce autophagy in plants (Liu and Bassham, 2013; Pu and Bassham, 2013; Yang et al., 2015; Bao et al., 2018). Therefore, we wanted to know if autophagy would be induced by higher MDTs and, if so, would autophagy follow the same diurnal temperature patterns as other UPR indicators. A method used as a proxy for autophagy activity is the lipidation of autophagy-related protein8 (ATG8; Ichimura et al., 2000; Chung et al., 2009; Srivastava et al., 2018). The lipidated and non-lipidated forms of ATG8 can be separated on urea gels in which the lipidated form (ATG8-phosphatidylethanolamine [ATG8-PE]) migrates more rapidly than the non-lipidated form (Figure 1C). We have validated that the more rapidly migrating form represents ATG8-PE by demonstrating that this form localizes to membranes when extracts are separated into soluble and membrane fractions, whereas the upper band representing non-lipidated ATG8 is soluble. Further, ATG8-PE is lost from membrane fractions when the extracts are treated with phospholipase D (Supplemental Figure 3A), upon which only non-lipidated ATG8 can be detected. A lipidated form of ATG8, ATG8-PE, accumulated to high levels in the virtual midafternoon (∼ZT 15:30), particularly at the higher MDT, 35°C and 37°C (Figure 1C). As expected, ATG8-PE levels were also high as plants were just coming out of the virtual night (ZT 5:30), because autophagy is induced by dark treatment (Supplemental Figure 3B; Izumi et al., 2013; Wang et al., 2013). Note that the levels of ATG8-PE at ZT 5:30 in the sample from plants at 37°C MDT were not as high as at other MDTs; however, this appeared to be a somewhat anomalous reading and was not reproduced in three other runs. We wanted to know whether the diurnal pattern of bZIP60 splicing and autophagy induction observed in the Enviratron are reproduced in maize under field conditions. On a rare summer day in Iowa when temperatures were predicted to reach 38°C and the skies remained cloudless all day, we sampled the first fully expanded leaf every 2 h on different plants at developmental stage V9. Air temperature was recorded at a height above ground at which leaf samples were taken in triplicate (Figure 1D). RNA and proteins were extracted from the leaf samples and assessed for bZIP60 splicing and ATG8 lipidation. bZIP60s began to appear at ∼14:30 as the temperature reached its highest level for the day, 38°C (Supplemental Figure 4A). Levels of bZIP60s began to fall off as the temperatures in the late afternoon and early evening declined. Autophagy indicated by ATG8 lipidation was at a high level at sunrise (after a dark night) in the 6:30 sample, as expected (Figure 1E; Supplemental Figure 4B). ATG8-PE levels began to rise at ∼16:30 at the time when the levels of UPR marker, bZIP60s, peaked. The ATG8-PE levels continued to rise until the last sample at 20:30. Thus, the daily patterns of UPR and autophagy in the field resembled and substantiated the patterns obtained in the Enviratron.
Role of bZIP60 in Heat Tolerance
Because we observed a strong bZIP60 mRNA splicing response at the MDTs, we investigated whether bZIP60 confers heat stress tolerance in maize. The maize genome contains a single bZIP60 homolog (Zm00001d046718) and to determine its function in maize, we analyzed lines with Mu transposon insertions in bZIP60 (Settles et al., 2007). Three different Mu insertion lines were available, which we call bzip60-1, -2, and -3 with insertions in the 5′-UTR and 3′-UTR of the gene (Figure 2A). Two of the lines, bzip60-1 and bzip60-2 (both lines 1 and 2) with insertions in the 5′-UTR, have reduced expression of bZIP60 (Figure 2B). These two lines were sensitive to heat shock at 42°C, but bzip60-3, with an insertion in the 3′-UTR, was not (Figure 2C). We carried forward with one of the two lines of bzip60-2 for further testing and demonstrated that the heat-sensitive phenotype in bzip60-2 cosegregated with the mutant gene in a BC1 F1-segregating population (Figure 2D).
Figure 2.
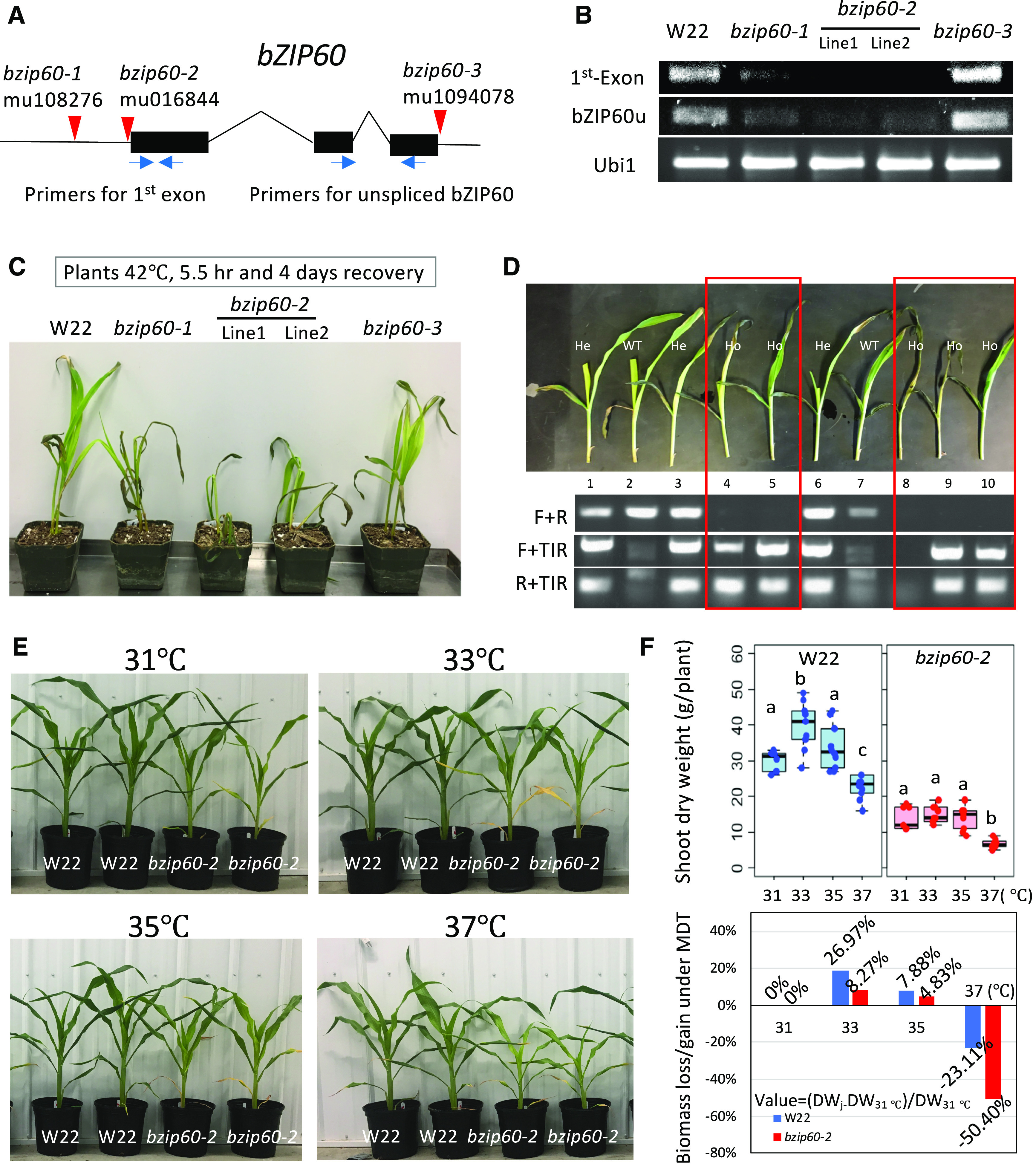
Characterization of bzip60 Mutants.
(A) Map of bZIP60 gene locus (Zm00001d046718, version 4, B73; Zm00004b032994, version 4b.1, W22) showing Mu insertion sites (red triangles) in the 5′- and 3′-UTRs. Primers are shown for the first exon and for the unspliced form of bZIP60 mRNA, bZIP60u (blue arrows).
(B) RT-qPCR analysis of the relative abundance of bZIP60 transcripts in the various bzip60 mutants and in the nonmutant parent line, W22. RNA transcript signals were amplified from the first exon and for the unspliced form of bZIP60 mRNA (bZIP60u). Note that two sublines (lines 1 and 2) were available for the line bzip60-2.
(C) Growth of bzip60 mutant seedlings and the parent line, W22, after a heat shock treatment (42°C, 5.5 h, and recovery for 4 d).
(D) Segregation of the heat-sensitive phenotype in a F2 population from a backcross of bzip60-2 to W22 (top). Genotypes (bottom) are aligned to the seedling images (top). Ho, homozygous for the mutant trait; He, heterozygous.
(E) Growth of bzip60-2 and W22 seedlings subjected to photoperiod and temperature cycles in which MDTs were set for 31°C, 33°C, 35°C, or 37°C in the different growth chambers of the Enviratron.
(F) Top, box plots of shoot biomass (dry weight) of plants at 28 DAG shown in (E).. Dots indicate individual plant measurements. The dark black line is the median, the boxes are the first and third quartile, and the whiskers are the minimum and maximum values in the Supplemental File. Different letters indicate significant differences between groups (P < 0.05, one-way ANOVA for each representation, W22 or bzip60-2), in at least five biological replicates. Bottom, Sshoot biomass gain or loss at the higher MDT was compared with shoot biomass at 31°C. For the sample at MDT, gain or loss = (DWj − DW31°C)/DW31°C. DW, dry weight.
We compared the performance of bzip60-2 to the W22 parent subjected to different MDTs, and for bzip60-2, the F2s from two back-cross generations (BC2F2) were tested. For each temperature regime, there were 12 plants for each genotype split between two chambers in the Enviratron set for the same temperature conditions, i.e., six bzip60-2 and six W22 plants in each of two chambers with the same temperature conditions. Plants were harvested, dried, and weighed to determine biomass at about V6 (28 DAG). The stature of the bzip60-2 plants grown at 31°C or 33°C MDT was similar to that for W22 plants (Figure 2E); however, the leaves and stalks were more slender and the biomass (dry weight) of bzip60-2 was consistently less than W22 (Figure 2F, upper representations). Nonetheless, the growth of bzip60-2 at 37°C was much reduced compared with the growth of W22 at 37°C (Figure 2F, lower representation). This clearly indicates that bzip60-2 has reduced vegetative growth and that the defect confers temperature sensitivity to the mutant plants.
RNA-Sequencing Analysis
Using RNA-sequencing (RNA-seq) analysis, we analyzed at greater depth the effects of temperature on gene expression in W22 and bzip60-2 V4 plants (20 DAG) and V5 plants (27 DAG) in the Enviratron as the temperature reached its MDT. Principal component analysis of the sequencing data showed that there was consistency between reps, but gene expression differences between conditions (different MDTs) and between genotypes (Supplemental Figure 5). From the RNA-seq data, thousands of differentially expressed genes (DEGs) were identified in response to increased MDTs at the V4 and V5 stages. At the V4 (20 DAG) stage in W22, there were increasing numbers of DEGs with increased MDT (Figure 3A). Except for the comparison between 33°C and 31°C MDT, there was a similar number of genes up- and downregulated when comparing 35°C and 37°C MDT to the control plants at 31°C MDT at V4 (Figure 3A; Supplemental Figure 6A). However, in W22 at the V5 (27 DAG) stage, there were far more upregulated than downregulated genes at 33°C and 35°C compared with 31°C MDT (Figure 3C; Supplemental Figure 6B). In bzip60-2, there were substantial numbers of DEGs at V4 (20 DAG), but far fewer at V5 (27 DAG; Figures 3B and 3D; Supplemental Figures 6A and 6B). Downregulated genes outnumbered upregulated ones in the mutant when comparing 33°C to 31°C at stage V4 and comparing 37°C to 31°C at V5.
Figure 3.
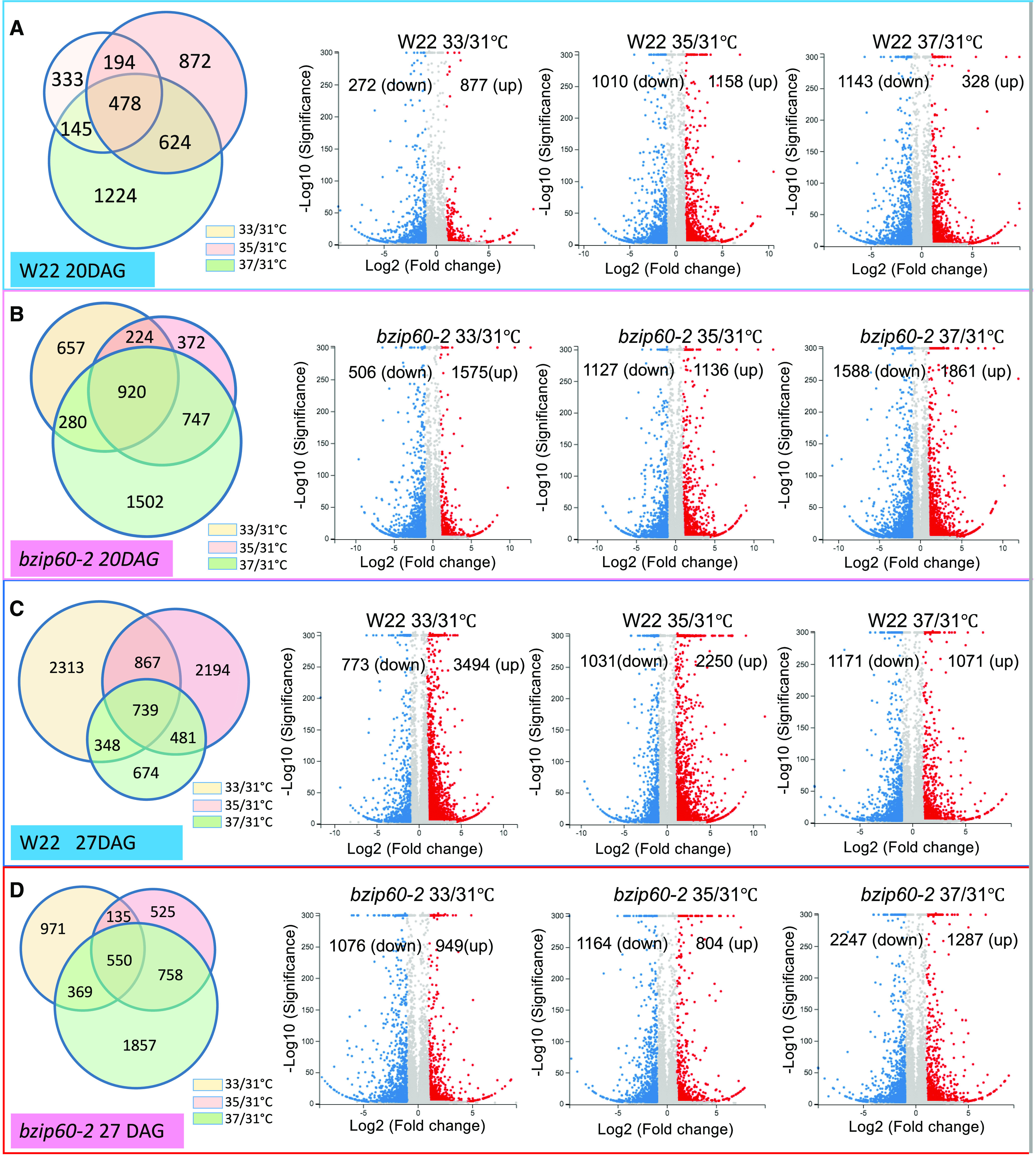
DEGs in Comparing Responses to Different MDTs.
Proportional Venn diagrams show the abundance of DEGs in comparing gene expression levels at 33°C, 35°C, or 37°C MDT to gene expression at 31°C MDT. Volcano plots indicate the number of DEGs that were downregulated or upregulated in comparing the responses at the different MDTs to 31°C MDTs. DEGs were identified with Q value ≤ 0.05 and absolute log2-fold value (for high MDT, compare with 31°C) >1.
(A) Response of W22 (developmental stage V4, 20 DAG) at different MDTs.
(B) Response of bzip60-2 (developmental stage V4, 20 DAG) at different MDTs.
(C) Response of W22 (developmental stage V5, 27 DAG) at different MDTs.
(D) Response of bzip60-2 (developmental stage V5, 27 DAG) at different MDTs.
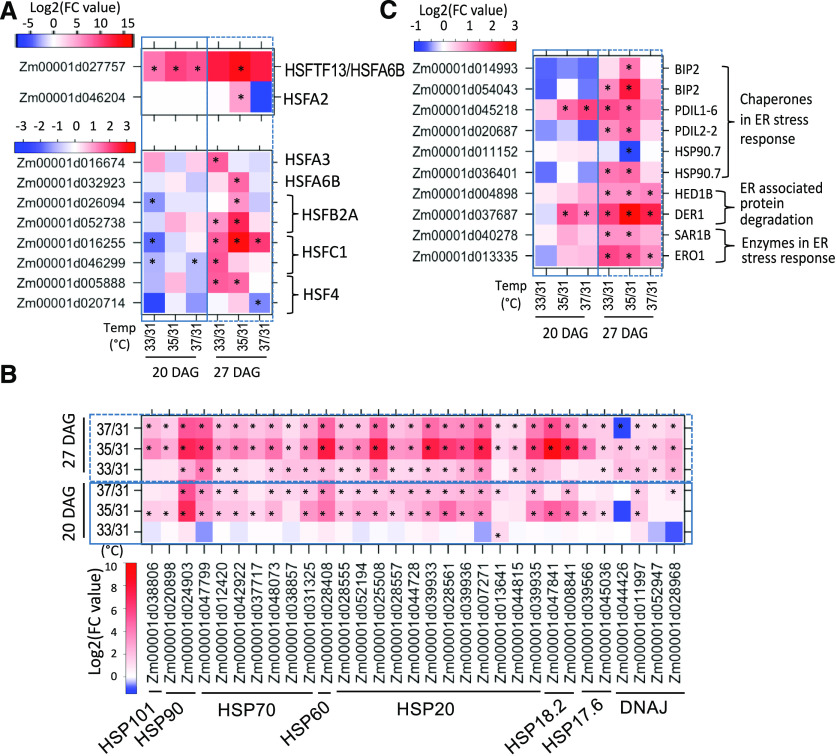
Analysis of the RNA-seq data revealed that HSR genes were the most responsive to the temperature regimes. HSP genes are under the control of HSFs, and nine HSFs were upregulated at elevated temperature at 27 DAG (Figure 4A; Supplemental Data Sets 1 and 2). One HSF (HSFTF13, HSFA6B, Zm00001d027757) in particular was highly induced with elevated MDTs at both V4 and V5 (20 and 27 DAG). As a group, the HSP genes were upregulated at 35°C and 37°C MDT and more so at developmental stage V5 (27 DAG; Figure 4B; Supplemental Data Sets 1 and 2). However, at stage V5, HSP gene upregulation was somewhat lower at 37°C than at 35°C MDT, suggesting that the longer growth at higher temperature (37°C MDT) may have impacted the upregulation of HSP gene expression.
Figure 4.
Changes in Expression of HSR Genes at Various MDTs.
Heat maps show the changes in expression of HSR genes at different MDTs compared with 31°C MDT. Data were obtained at two different developmental stages, V4 (20 DAG) and V5 (27 DAG). Colors represent log2-fold change in the means from triplicate samples comparing relative expression at 33°C to 37°C MDT to 31°C MDT. * indicates significant differencece when compared to the level at 31°C MDT (Q value ≤ 0.05 and absolute log2-fold value [level at the higher MDT, compared to the level at 31°C] >1).
(A) HSFs.
(B) HSPs.
(C) Canonical UPR genes.
Because bZIP60 mRNA splicing was induced by heat, we wanted to know if other canonical UPR genes than CRT2 and PDI1 were also regulated. A limited number of the DEGs in the different temperature regimes at V5 (27 DAG) were canonical UPR genes (Figure 4C; Supplemental Data Sets 1 and 2). However, a number of canonical UPR genes, such as SEC61 (β-subunit), ENDOPLASMIC RETICULUM DNAJ HOMOLOG 3a (ERdJ3a), STROMAL CELL DERIVED FACTOR2, and others that had been shown previously to be induced in maize seedling roots by tunicamycin (TM) treatment, were not induced by elevated MDT (Srivastava et al., 2018).
Effects of bzip60-2 on Gene Expression
We also used the RNA-seq analysis described above to identify DEGs in the comparison between bzip60-2 and the parent line, W22, at the different MDTs. This includes the differences between bzip60-2 and W22 at normal or unstressed temperatures, 31°C and 33°C, and stressed temperature conditions, 35°C and 37°C (Supplemental Figure 7A; Supplemental Data Sets 3 and 4). There were more upregulated than downregulated DEGs in bzip60-2 compared with W22, the parental line, at the V4 stage (20 DAG) at all the four MDTs (Supplemental Figure 7B). This trend was less obvious at the V5 stage (27 DAG) except at 31°C MDT, at which temperature conditions the upregulated genes in bzip60-2 vastly outnumbered the downregulated genes (Supplemental Figure 7C). Gene ontology (GO) analysis of genes upregulated in bzip60-2 compared with W22 at normal temperature conditions, 31°C, showed no specific enrichment of genes with common functions (Supplemental Figure 8). However, the genes downregulated in bzip60-2 in comparison to W22 were enriched for developmental and metabolic functions. This may help to explain why bzip60-2 plants have less biomass compared with W22 even under normal temperature conditions (31°C MDT).
The DEGs in the comparison between bzip60-2 and W22 can be classified into two different categories based on their expression patterns. Group-I genes differed in their expression between two lines but not in response to increased MDTs, and group-II differed between the two lines and also in response to increased MDTs (Supplemental Figure 9A). As shown in the comparison of bzip60-2 and W22 at normal temperature conditions (31°C), the highly DEGs in group-I were involved in post-embryonic development, vesicle-mediated transport, protein degradation, and transcriptional regulation processes (Supplemental Figures 9B to 9D).
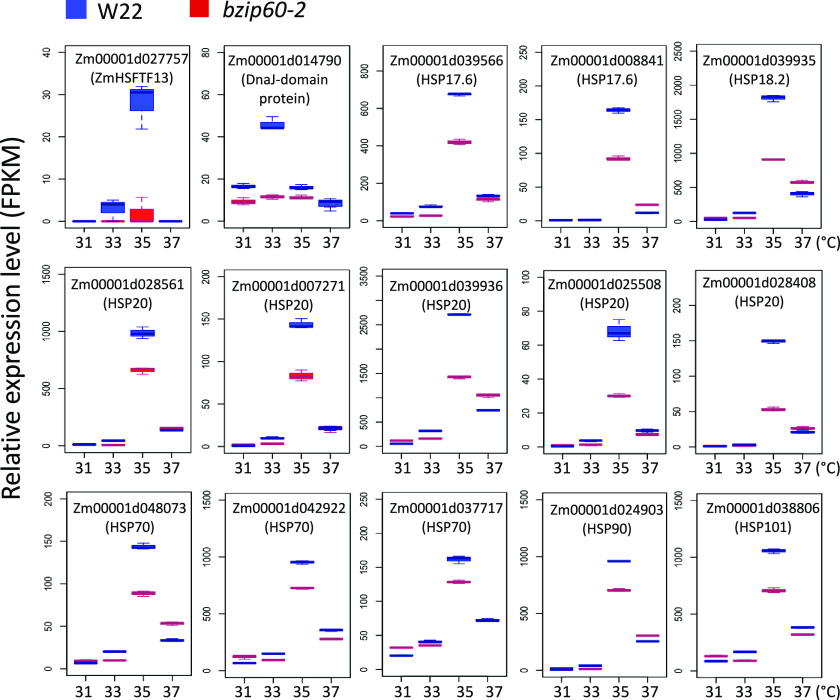
The group-II DEGs were further partitioned into six clusters using a k-means clustering algorithm (Supplemental Figure 10A). Cluster-1 DEGs were expressed lower in bzip60-2 than W22, but were upregulated in both genotypes at elevated MDTs (Supplemental Figure 10B). In contrast, cluster-3 DEGs were expressed higher in bzip60-2 but were repressed by elevated MDTs. We found quite unexpectedly that many of the HSP genes were group-II DEGs belonging to cluster 1, which were expressed lower in bzip60-2 than W22 but were induced in response to elevated temperature. The HSP genes were upregulated at elevated MDT in W22, but less so in bzip60-2, especially at 35°C in V5 (27 DAG) plants (Figure 5; Supplemental Data Sets 5 and 6). In searching for an explanation for this effect, we turned to the type-A heat shock factor, HSFTF13 (Zm00001d027757, HSFTF13, HSFA6B) described above, which was upregulated at elevated MDT. We found that HSFTF13 expression was elevated in the parent line, W22, but not in the mutant, bzip60-2 (Figure 6A, top left). HSFTF13 in W22 was upregulated at higher temperatures, particularly at 35°C, but the expression of the gene in bzip60-2 was undetectable at any temperature. This gene was the only HSF not upregulated in bzip60-2 at elevated MDT.
Figure 5.
Effect of bzip60-2 on the Expression of HSP Genes at Various MDTs.
Box plots show the relative expression levels (Fragments Per Kilobase of transcript per Million mapped reads) of HSP genes and a DnaJ gene (all Group-II genes) in bzip60-2 and W22 at different MDTs at the V5 stage (27 DAG). See legend in Figure 2F for an explanation of box-plot features.
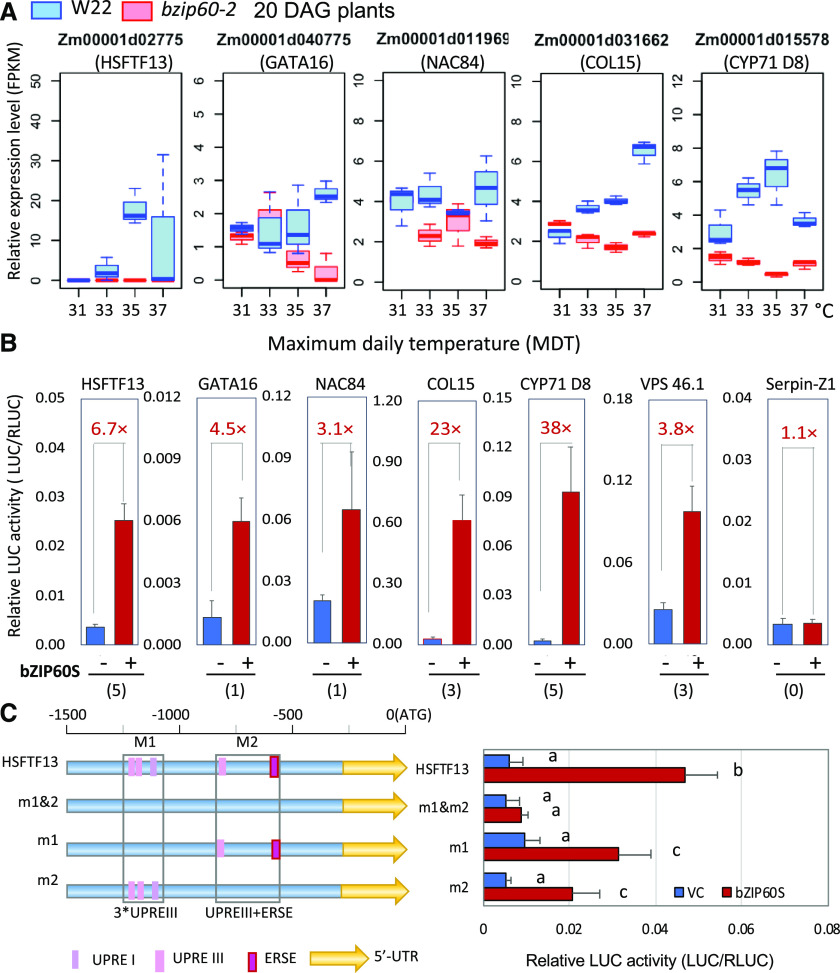
Figure 6.
Impact of bzip60-2 on the Expression of Various Genes at Different MDTs.
(A) Box plots showing the relative expression of various genes downregulated in bzip60-2 compared with W22 at different MDTs and at developmental stage V4 (20 DAG). See legend in Figure 2F for an explanation of box-plot features.
(B) Coexpression in maize mesophyll protoplasts of 35S:bZIP60s and various promoters linked to luciferase. Promoter activity is expressed as the relative activity of firefly luciferase versus Renilla luciferase. Values in red indicate the fold increase in expression plus or minus bZIP60s. Numbers in parentheses indicate the number of UPRE- or ERSE-like sequences in the promoters, which average ∼1.0 to 1.5 kb in size. Serpin-Z1 (Zm00001d013737) without obvious UPRE or ERSE elements in its promoter served as a negative control.
(C) Activity of the HSFTF13 promoter and various promoter mutants in maize mesophyll protoplasts. Here, 1.5 kb of the HSFTF13 promoter was linked to firefly luciferase, and UPR promoter elements in the distal region (M1) containing two UPRE-III elements and a UPRE-I element were mutated as described in “Methods.” The proximal region (M2) containing a UPRE-III element and bZIP17/28 binding site were also mutated. The constructs were cotransfected with 35S:bZIP60s in maize protoplasts as above and assessed for luciferase activities. Promoter activity is expressed as the relative activity of firefly luciferase versus Renilla luciferase. VC, empty vector control used for bZIP60s overexpression. Values are the means of four replicates ± sd. Different letters indicate significant differences between comparisons (P < 0.05, one-way ANOVA).
Because HSFTF13 was not upregulated in bzip60-2, we wanted to know if it is a target of bZIP60. We examined the promoter of HSFTF13 and found two groups of UPR response elements within 1.5 kb of the start of translation (Supplemental Figure 11). The distal group was composed of UPR response elements (UPREs)-IIIa and -IIIb (TCATCG/CGATGA) and a UPRE-I (TGACTG/CAGTCA) element (Iwata and Koizumi, 2005; Liu and Howell, 2010; Sun et al., 2013). It has been demonstrated in Arabidopsis that UPRE-III is a binding site for AtbZIP60 (Sun et al., 2013). The more proximal group is composed of a UPRE-IIIc and a bZIP17/28 binding site (CACGT/ACGTG; Plant PAN2.0; http://plantpan2.itps.ncku.edu.tw/TFBSinfo.php?matrix=TFmatrixID_0189). To determine whether bZIP60 activates the expression of HSFTF13, we coexpressed in maize protoplasts bZIP60s (bZIP60 spliced form) driven by the 35S promoter with a construct containing the HSFTF13 promoter linked to the luciferase gene. We found that luciferase expression was elevated nearly 7-fold by the expression of bZIP60s (Figure 6B, left). To determine if the UPR promoter elements are responsible for the direct activation, we mutated the UPRE-IIIa, UPRE-IIIb, and UPRE-I as mutational group M1, and UPRE-IIIc and the bZIP17/28 binding site as mutational group M2 (Figure 6C, left). Mutations in the two UPRE-IIIs and UPRE-1 element in the M1 group reduced the promoter activity in the protoplast assay and mutations in the UPRE-III and a bZIP17/28 binding site of the M2 group knocked down the activity further (Figure 6C, right). Mutations in both groups (M1 and M2) reduced the promoter activity down to background levels. We concluded that bZIP60s, indeed, activates the expression of HSFTF13 and that the activation depends on UPR cis elements in the promoter of the HSFTF13 gene. Therefore, we attribute the failure to fully upregulate the constellation of HSP genes in bzip60-2 in response to heat to the underexpression of HSFTF13.
Many of the genes downregulated in bzip60-2 in comparison to W22 were not canonical genes. Some of these genes are normally heat-induced, but others are not. Nonetheless, some of the genes not upregulated in bzip60-2 in comparison to W22 include the transcription factors GATA TRANSCRIPTION FACTOR16, NAC DOMAIN TRANSCRIPTION FACTOR84, CONSTANS-LIKE15, and CYTOCHROME P450 71D8 (Figure 6A). We wanted to find out whether these genes were also targeted by bZIP60, and, as before, we constructed promoter-luciferase gene fusions and tested them for expression in maize protoplasts cotransfected with CAMV35Sprom:bZIP60s. Each of the tested promoters contained UPR promoter elements and were activated by the expression of bZIP60 (Figure 6B), indicating that a number of noncanonical UPR genes depend on bZIP60 for expression. SERPIN-Z1, which does not have UPRE or ER stress response (ERSE) elements in its promoter, was used as a negative control and was not upregulated by bZIP60s.
Effects of Heat on Chlorophyll
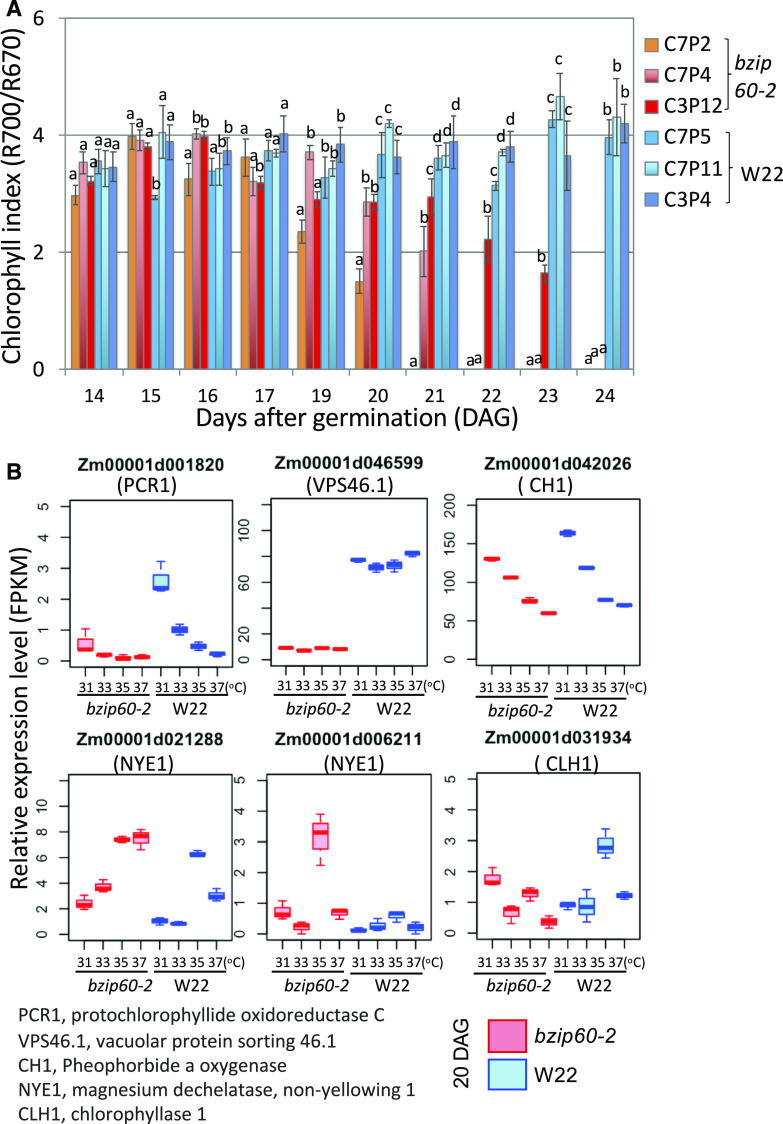
One of the more obvious signs of heat stress was accelerated senescence and loss of greenness in bzip60-2 with time at elevated MDT. We monitored chlorophyll indices robotically by hyperspectral reflectance (Supplemental Figure 12). Chlorophyll was estimated from the red edge in the spectra using the relationship R700/R670 (McMurtrey et al., 1994; Haboudane et al., 2008). We observed a general decline in chlorophyll index in bzip60-2 plants at 37°C MDT, although the decline occurred at different rates among the different bzip60-2 plants (Figure 7A). The chlorophyll content began to decline rapidly around 19 DAG in several of the bzip60-2 plants. These plants died after 24 DAG, and although several other bzip60-2 plants escaped, their earliest leaves tended to bleach and die.
Figure 7.
Effects of MDT and bzip60-2 on Chlorophyll Indices in Maize Leaves.
(A) Chlorophyll indices for the first fully expanded leaves on individual W22 and bzip60-2 plants recorded over a period of 10 d at 37°C MDT. C7P2, C7P4, and C3P12 are individual plants homozygous for bzip60-2. C7P5, C7P11, and C3P4 are individual W22 plants. Chlorophyll indices were determined from hyperspectral images taken robotically at the same virtual time of day for all plants. Different letters indicate significant differences between comparisons (P < 0.05, one-way ANOVA, for individual plants grown under the same conditions). The means of three readings per leaf are shown and the error bars represent SEMs.
(B) Box plots for the expression levels of various genes encoding chloroplast-targeted proteins in W22 and bzip60-2 at various MDTs. Roles of the genes shown here are represented in the chlorophyll metabolism pathway in Supplemental Figure 13. See legend in Figure 2F for an explanation of box-plot features.
In assessing the expression profiles of genes involved in chlorophyll synthesis and degradation at the V4 stage, we observed a general decline with increasing temperature in genes involved in chlorophyll synthesis, such as genes encoding protochlorophyllide oxidoreductase C, PCR1, and pheophorbide a oxygenase, CH1 (Figure 7B; Supplemental Figure 13). The expression of another gene involved in removing damaged proteins from the chloroplast, VACUOLAR SORTING PROTEIN46 (VPS46, also known as SUPERNUMERARY ALEURONE1), was unaffected by different MDTs, but was highly dependent on bZIP60 at all temperatures (Figure 7B). The dependence of this gene on bZIP60 function is noteworthy because the gene is involved in phagophore maturation and the efficient delivery of autophagic plastid bodies to the vacuole (Spitzer et al., 2015). Contrary to the downregulation of chlorophyll synthesis genes, some of the chlorophyll degradation genes such as CHLOROPHYLLASE1 and NON YELLOWING1 (NYE1), which convert chlorophyllide a to chlorophyll a and chlorophyll a to pheophytin a, respectively, are upregulated in bzip60-2 at various MDTs (Figure 7B). There is no simple explanation for the upregulation of these genes in bzip60-2. We suggest that they must be indirect targets of bZIP60 regulation because there is no precedence for bZIP60 acting as a repressor.
However, to determine whether bZIP60 activates the expression of VPS46, the promoter was linked to luciferase reporters, as described before, and coexpressed in maize protoplasts with a bZIP60 expression construct (Figure 6B, second representation from the right). The VPS46 promoter, which has three UPR promoter elements, was activated by the expression of bZIP60 nearly 4-fold, indicating that, indeed, VPS46 is also a likely target of bZIP60. In summary, the loss in greenness with increasing MDT in bzip60-2 corresponds with a decline in expression of some chlorophyll synthesis genes and an increase in expression of a gene likely involved in autophagic turnover of chloroplast components.
Response to Heat at Late Vegetative and Reproductive Stages
To assess the effects of the UPR at later vegetative and reproductive stages of growth, W22 and bzip60-2 plants were subjected to the same conditions as described for V4 to V5 plants, except they were allowed to grow to reproductive stage. We measured internode lengths on stalks of mature plants (Figure 8A) and found that during mid stages of growth from approximately five to nine internodes, the internodes for both W22 and bzip60-2 progressively shortened with increasing MDT (Figure 8B). However, internodes 10 to 14 elongated more at later stages in plants at optimal MDT (33°C to 35°C), and the differences between W22 and bzip60-2 internode lengths were most apparent at these higher MDTs (Figure 8C). Thus, for both W22 and bzip60-2 it appears that late-stage internodes elongate more at optimal MDTs than at higher or lower MDTs. In terms of late stages, we also compared plant height and shoot biomass at V13 (Figure 8D). At these stages, it is very clear that the bzip60-2 mutation penalizes vegetative growth—the mutant plants were shorter and had lower shoot fresh weight, particularly at the higher MDT. We also compared flowering time between W22 and bzip60-2 and used tassel emergence as a measure for this trait. We found that bzip60-2 flowered earlier than the parent line, W22, especially at higher temperature. It is likely that the extra stress at higher temperature imposed by a deficit in bZIP60 activity hampered vegetative growth and resulted in early flowering.
Figure 8.
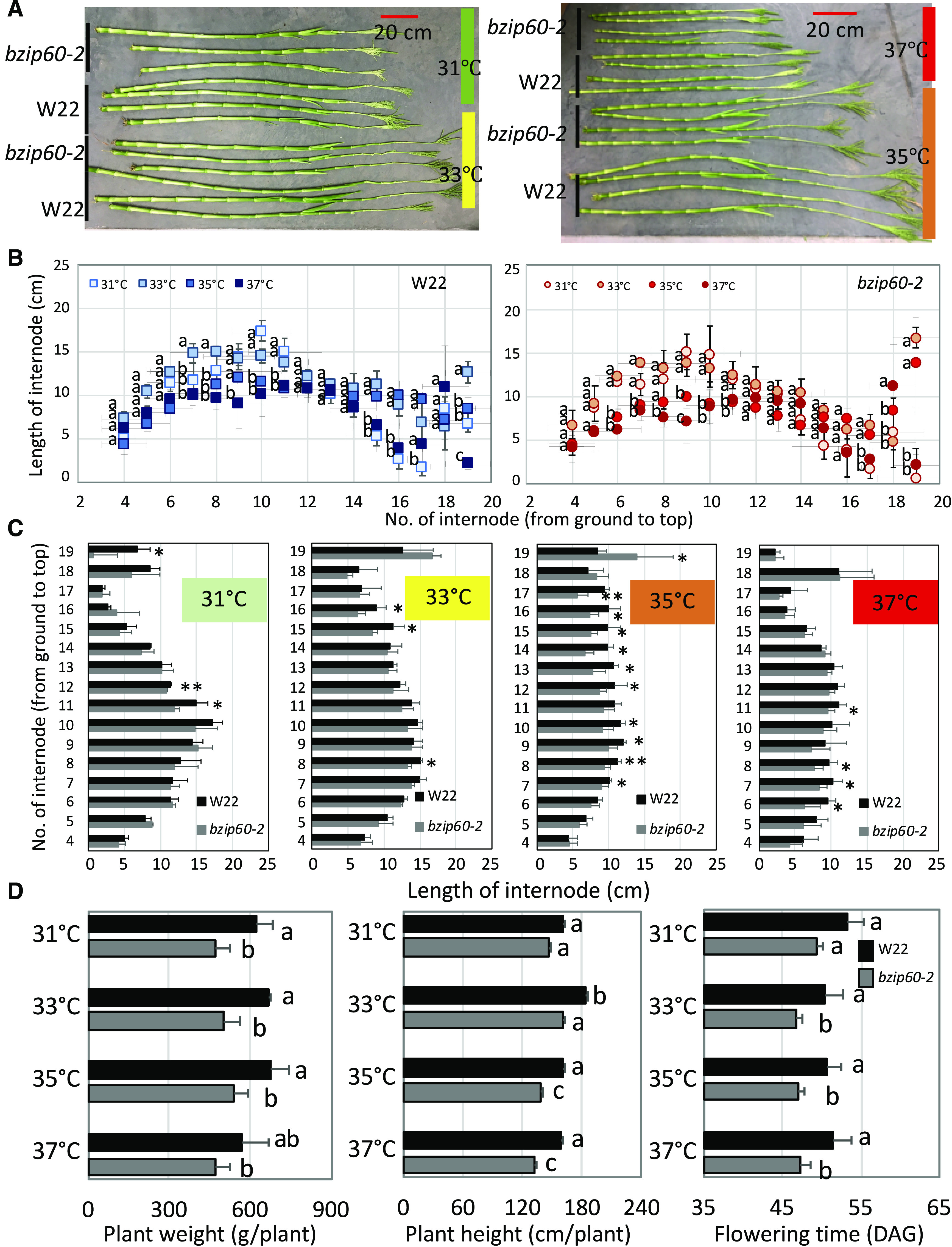
Growth of bzip60-2 Plants at Different MDT to Reproductive Stage.
(A) Stalks of W22 and bzip60-2 plants grown at different MDTs.
(B) Internode lengths of stalks of W22 and bzip60-2 plants grown at different MDTs. Different letters indicate significant differences between comparisons (P < 0.05, one-way ANOVA). For W22 (left) or bzip60-2 (right), data at 33°C, 35°C, or 37°C MDT compared with 31°C MDT.
(C) Comparison of internode lengths of stalks of W22 and bzip60-2 plants grown at different MDTs. Values are the means of three replicates ± sd. *Statistical significance for P < 0.05; **statistical significance for P < 0.01, using a t test.
(D) Plant fresh weight, plant height, and flowering time (days after germination to tassel emergence) for W22 and bzip60-2 at V13 stage (tassel emergence stage). Different letters indicate significant differences between comparisons (P < 0.05, two-way ANOVA). Data from plants of W22 and bzip60-2 at different MDTs were compared.
DISCUSSION
Plants have multiple systems to protect themselves from heat stress. Heat interferes with the proper folding of proteins during synthesis, leading to the accumulation of misfolded proteins. Both the HSR system in the cytoplasm and the UPR in the ER perceive the presence of misfolded proteins and act to mitigate the damage brought about by heat stress. In this study, we found a possible link between the UPR in the ER and the cytoplasmic HSR (Figure 9). The UPR and HSR are usually thought to be quite independent of each other. Both respond to heat, but are in different cellular compartments, and so the question is whether there is crosstalk between the two systems. The question has been addressed in yeast (Saccharomyces cerevisiae) where it has been found that the HSR helps UPR-deficient cells to survive ER stress (Liu, 2008). Mutant yeast cells deficient in the UPR (ire1D) are unable to survive the effects of treatment with TM or synthesis of a mutant carboxypeptidase Y (CPY*), a chronically misfolded protein. However, they can be rescued by introducing a constitutively active Hsf1 gene or by incubating the cells at 37°C to induce a mild HSR. Thus, the HSR can aid the UPR in dealing with misfolded proteins. But the real question is whether the UPR can activate the HSR. Liu (2008) explored that issue using a reporter gene in which a promoter bearing an HSE was linked to lacZ. They found that overexpression of CPY* was unable to upregulate the expression of lacZ in wild-type yeast cells. However, when IRE1 was knocked out in an ire1D mutant, lacZ expression could be activated either by CPY* overexpression or by treatment with TM. However, the expression was low and unable to rescue the impaired yeast. Thus, ER stress is limitedly able to activate the HSR, but not through the UPR. The limits of the relationship between the UPR and the HSR is also borne out by recognizing that there are only a few genes commonly upregulated by the two responses (Liu, 2008). Only nine of the 392 genes upregulated by the UPR are also upregulated by the HSR. One of these is KAR2, but none of these genes are ones that would regulate the HSR. In summary, the HSR provides yeast with protection against ER stress; however, the UPR system does not appear to reciprocate and help the HSR system to provide stress protection.
Figure 9.
Model Depicting the Role of the UPR and bZIP60 in HSRs in Maize.
The HSRs include the upregulation of HSFTF13 by bZIP60 and the post-translational activation of HSFTF13 and other HSFs by heat. Also shown is the upregulation by bZIP60 of a limited set of canonical UPR genes and the altered regulation by bZIP60 of genes involved in flowering and chlorophyll metabolism.
In our system in maize, heat activation of IRE1 leads to the splicing of bZIP60 mRNA, which encodes a nuclear targeted form of bZIP60. bZIP60 activates the expression of a type-A HSF, HSFTF13, which in turn upregulates the expression of HSP genes. We recognize that HSFTF13 is but one of 25 HSF genes in maize B73, of which 15 are group-A HSFs, which activate the expression of HSP genes (Lin et al., 2011). Despite the numbers of HSFs, HSFTF13 in Arabidopsis plays a pivotal role in the response to abscisic acid and in thermotolerance (Huang et al., 2016). Furthermore, in our system, the knockdown of bZIP60 in bzip60-2 impacts the expression of HSFTF13, resulting in plants that are more sensitive to elevated temperature.
We wanted to learn whether these systems are operative during the normal diurnal temperature cycles to which maize plants are subjected on hot summer days. We were surprised to see the activation of the HSR when the MDT in diurnal temperature cycles reached 35°C to 37°C. It would not be unreasonable to think that the HSR is only activated by heat shock, i.e., a stepwise increase to a higher temperature, such as 40°C, needed to activate these genes in maize (Cooper and Ho, 1983). In the diurnal temperature cycles to which we subjected maize plants, the temperatures were slowly ramped up over a 6-h period. Nonetheless, bZIP60 mRNA splicing and the HSR were activated as the temperatures reached their MDT. One might predict that ramping up the temperature would acclimate plants to the elevated temperature and would not upregulate HSR genes. Queitsch et al. (2000) showed in Arabidopsis that mild preconditioning treatments lead to the acquisition of thermal tolerance and protect plants from lethal damage at high temperatures. Initially we thought that the gradual ramping up of temperature in our system might be sufficient preconditioning to suppress the HSR; but this was not the case.
bZIP60 mRNA splicing serves as a biomarker for the UPR during the HSR, and we expected that the activated form of this transcription factor would upregulate the expression of a constellation of canonical UPR genes as has been demonstrated when Arabidopsis plants are treated with UPR stress agents, such as TM or dithiothreitol (Martínez and Chrispeels, 2003; Iwata and Koizumi, 2005; Iwata et al., 2008). We observed bZIP60 mRNA splicing in response to increasing daily temperature in our system; however, only a handful of the canonical UPR genes were upregulated under the elevated temperature conditions. We reported in another study in Arabidopsis that heat induced the upregulation of another canonical UPR gene, ERdJ3a, but the upregulation did not depend on the UPR (Howell, 2017), as it occurred in the background of a nearly null UPR mutant (bzip60-1 bzip28-1). The promoter of the ERdJ3a gene in Arabidopsis is decorated with a number of HSEs as well as UPREs, suggesting that the gene may respond to either heat or ER stress signals. Thus, the upregulation of a canonical UPR by heat does not necessarily mean that the response is an ER stress response (Howell, 2017).
Other investigators have described genome-wide responses to heat stress in plants with some pointing out that many transcription factors are upregulated in the response (Rizhsky et al., 2004; Swindell et al., 2007; Barah et al., 2013). Other investigators have also observed a dearth of canonical UPR genes upregulated in response to heat stress. Sugio et al. (2009) compared gene expression profiles after treatment of Arabidopsis with heat (37°C), the proline analog L-azetidine-2-carboxylic acid (AZC) and TM. They found only modest overlap between the gene expression profile comparing heat with AZC, even though both should lead to an accumulation of misfolded proteins. They found even fewer heat-shock–upregulated genes (17/955) overlapped with the TM-induced genes, and few of the AZC-upregulated genes (43/464) overlapped with the TM-induced genes. Thus, they surmised that Arabidopsis deploys a different combination of genes depending on the stress and the cellular compartment responding to the stress.
One of the genes that was unexpectedly driven by bZIP60 was VPS46 or SUPERNUMERARY ALEURONE1 (a homolog of CHARGED MULTIVESICULAR BODY PROTEIN [CHMP1A] in mammalian cells). VPS46 is required for autophagic degradation of plastid proteins in Arabidopsis (Spitzer et al., 2015). Therefore, this gene probably plays a role in quality control for the chloroplast, helping to remove proteins in the chloroplast misfolded by the effects of heat. We demonstrated that autophagy is induced at the higher MDT, and under these conditions, autophagy is likely to be adaptive, maintaining the viability of the chloroplasts. We observed in bzip60-2 at higher MDT the loss of chlorophyll possibly resulting from the destruction of chloroplasts. In addition, at higher MDT we observed a downregulation in genes involved in chlorophyll synthesis and an upregulation in genes involved in chloroplast turnover (Figure 9).
In conclusion, when we simulated the rising temperature during a warm summer day in the Enviratron, we found that the HSR in W22 maize was activated, and even more so at higher MDT. The rising temperatures also activated IRE1, leading to the splicing of bZIP60 mRNA, a reliable biomarker for the UPR (Figure 9). However, only a few of the canonical UPR genes were upregulated in response to the heat, while other usual targets of bZIP60 were not. Other noncanonical UPR genes were upregulated by heat stress—some dependent on the functionality of bZIP60. A bZIP60 knockdown mutant was found to be more sensitive to heat stress and underwent earlier senescence at higher MDT. The upregulation of an important HSF in response to heat stress was dependent on bZIP60 as were a number of HSPs. The failure to launch a full-fledged HSR in bzip60-2 is a likely cause for explaining the mutant’s susceptibility to heat.
METHODS
Plant Material
Maize (Zea mays) W22 and Mu insertion mutants in bZIP60 (Zm00001d046718) from the uniform Mu insertion lines were used in this study (McCarty et al., 2005). Mu mutants (bzip60-1, bzip60-2, and bzip60-3) were kindly provided by the Maize Stock Centre (http://maizecoop.cropsci.uiuc.edu) and were backcrossed and self-pollinated for two generations.
Plant Growth Conditions
Seeds of maize W22 and bzip60 mutants were germinated in soil in small pots with 13-h light/11-h dark at 26°C. For experiments in the Enviratron, 7-d–old plants were transplanted in 9-inch pots and randomly placed into 12 set positions within each chamber. The eight growth chambers in the Enviratron were used to simulate four different environmental conditions, i.e., with duplicate chambers for each condition. The performance of plants under these conditions was monitored by a robot, equipped with various sensors, that travels from chamber to chamber (Bao et al., 2019). The temperature and light cycles were offset by 40 min from chamber to chamber to allow the robot to enter the different chambers at the same time of virtual day. The daytime temperatures were ramped up over 6 h to an MDT of 31°C, 33°C, 35°C, or 37°C. Nighttime temperatures were ramped down over 8 h to 10°C below the MDT. Light intensities in a 16-h/8-h photoperiod cycle were also ramped up to 1,200 µmol/m2/s (measured 60 cm from the LED light source) or down over 2-h periods to simulate dawn and dusk. Water potential in the soil (Sunshine SB300 or MM900 Universal potting soil) was maintained at 50% (v/v) volumetric water content and relative humidity was held at 60%. Plant performance was assessed at three different stages of vegetative growth, V4, V5, and V13 to R1 stages (He et al., 2010), with the most extensive analysis performed at the mid-vegetative stages (V4 and V5). The stages are defined by the leaf numbers visible in the collars. For example, the V4 stage has leaf 4 visible in the collar. (The leaf collar is the light-colored band located at the base of an exposed leaf blade, where the leaf blade attaches to the plant stem.) The middle part of the first (newest) fully expanded leaf from plants at the V4 (20 DAG) and V5 (27 DAG) stages was collected for RNA-seq analysis. The V4-stage plants were sampled in triplicate every 2 h during the virtual morning for RNA and ATG8 lipidation analysis. Leaves were flash-frozen and stored at −80°C for RNA and protein extraction. Plants were harvested, and shoot biomass (dry weight) was measured at 28 DAG.
For the field experiment, W22 plants were grown at the Iowa State University Curtiss Research Farm under well-watered conditions and the first fully expanded leaf was sampled every 2 h on different plants at developmental stage V9. The weather conditions for conducting the experiment were ideal with clear skies and temperatures predicted to reach ∼38°C. Temperature readings were taken at the height above ground from which the leaf samples were taken. RNA and protein were extracted from the leaf samples and assessed for bZIP60 splicing and ATG8 lipidation.
RNA-Seq and RT-qPCR Analyses
RNA was extracted from small samples (0.1 g) of leaf lamina (avoiding the midrib) obtained from the middle of the first fully expanded leaves. The samples were taken in triplicate, one sample from each of three different plants, in the two chambers set for the same conditions. The samples obtained from the plants in the two different chambers were taken at the same time of virtual day, because the temperature and light cycles are offset to allow for sampling at the same time of day. The samples were separately ground to a powder in liquid nitrogen, and RNA was isolated using a Plant RNeasy Mini Kit and treated with DNase (Qiagen; www.qiagen.com/us/) according to the manufacturer’s instructions. RNA quality and quantity were determined by the UV absorbance spectra, gel electrophoresis, and model no. 2000 BioAnalyzer (Agilent; http://www.agilent.com).
For RT-PCR analysis, 500 ng total RNA was used for the cDNA synthesis (iScript cDNA Synthesis kit [Bio-Rad]; www.bio-rad.com), which in turn was utilized as template for RT-PCR analyses. Primers used in this study are listed in Supplemental Dataset 7. RT-qPCR was performed with StepOnePlus (Applied Biosystems; https://www.biosciences.ie/applied-biosystems) using a SYBR Green RT-PCR Kit Taq Universal SYBR Green Supermix (Bio-Rad; www.bio-rad.com) according to the manufacturer’s instructions. Relative gene expression levels were calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001) and employing maize ubiquitin1 (Ubi1) as a standard. The expression level of genes at ZT 5:30 in the chamber set for 31°C MDT was established as basal level (onefold). Three biological replicates were used for gene expression analysis.
For RNA-seq analysis, poly-A RNA was isolated from the purified RNA using oligo(dT)-attached magnetic beads and fragmented at elevated temperature with NEBNext RNA fragmentation buffer (New England BioLabs). cDNA library construction, sequencing, and primary bioinformatics analysis were performed by BGI Tech Solutions (Beijing Genomic Institute [BGI]; www.genomics.org.cn) as follows: First-strand cDNA was generated using random hexamer-primed reverse transcription, followed by second-strand cDNA synthesis. cDNA ends were repaired by A-Tailing followed by adding RNA Index Adapters. The cDNA fragments were amplified by PCR, and the products purified by Ampure XP Beads (Beckman Coulter). The products were validated for quality control using a model no. 2100 Bioanalyzer (Agilent Technologies). The double-stranded PCR products were heat-denatured and circularized using splint oligos (Diegelman and Kool, 2000). The single-strand circular DNAs were amplified with phi29 (Blanco et al., 1989) to make DNA nanoballs (Porreca, 2010). DNA nanoballs were loaded into the patterned nanoarray and single-end 50 base reads were generated on a model no. BGIseq500 platform (BGI).
Sequencing data were filtered with SOAPnuke (v1.5.2; github.com/BGI-flexlab/SOAPnuke) by removing reads (1) containing sequencing adapters, (2) in which >20% of the bases have a quality score <5, or (3) in which 5% or more of the bases are unknown (represented by N). Clean reads were stored in FastQ format (https://support.illumina.com/bulletins/2016/04/fastq-files-explained.html) and mapped to the reference maize B73 genome using the program HISAT2 (v2.0.4; http://www.ccb.jhu.edu/software/hisat/index.shtml). The software BowTie2 (v2.2.5; http://bowtie-bio.sourceforge.net/bowtie2/index.shtml" "http://bowtiebio.sourceforge.net/%20Bowtie2%20/index.shtml) was used to align the clean reads to the reference coding gene set plants.ensembl.org/info/website/ftp/index.html), and gene expression levels were calculated by the software RSEM (v1.2.12; github.com/deweylab/RSEM). Differential expression analysis was performed using the software DESeq2 (v1.4.5; www.bioconductor.org/packages/release/bioc/html/DESeq2.html) using a Q value ≤ 0.05. GO (http://www.geneontology.org/) enrichment analysis of annotated DEGs was performed by the program Phyper (https://en.wikipedia.org/wiki/Hypergeometric_distribution). The significance of the GO terms was corrected by log2ratio ≥ 1 and Q value with a threshold (Q value ≤ 0.05) by Bonferroni (Abdi, 2007).
ATG8 Lipidation Analysis
Leaves were harvested and immediately frozen in liquid nitrogen. ATG8 lipidation was measured as described by Chung et al. (2009) with minor modifications. Leaves were ground in liquid nitrogen and the powder was suspended in lysis buffer (50 mM of Tris-HCl at pH 8.0, 150 mM of NaCl, 1 mM of phenylmethanesulfonyl fluoride, 10 mM of iodoacetamide, and 1× Roche cOmplete mini protease inhibitor cocktail). The crude extract was filtered through one layer of Miracloth (cat no. 475855; EMD Millipore) and then centrifuged at 2,000g, 4°C for 5 min. Fifty-microgram protein samples were subjected to SDS-PAGE using 15% polyacrylamide gels with 6 M of urea in the resolving gel and analyzed by immunoblot using anti-ATG8 antibody (Agrisera; cat. no. AS142769).
Transient Assay Using Maize Protoplasts
Transient expression assays were performed to test the ability of bZIP60 to drive the expression of target genes. bZIP60s (the spliced form of bZIP60) was amplified from cDNAs produced from maize W22. bZIP60s was sequenced and inserted into plasmid pAN578 with a 35S promoter to drive the expression of bZIP60s. The promoters of candidate target genes (generally ∼1.5-kb upstream DNA sequences) were amplified from W22 DNA and fused to the firefly luciferase gene in plasmid pGreen II 8000 (SnapGene; www.snapgene.com/resources/plasmid_files/plant_vectors/). Primers used are listed in Supplemental Dataset 7. Transient expression assays were performed with maize mesophyll protoplasts as described by Sheen (2001), except W22 inbred lines were used as a source for protoplasts. Luciferase activity measurements were performed according to manufacturer’s instructions (E1910; Promega; www.promega.com).
The promoter of the HSFTF13 gene (Zm00001d027757) linked to luciferase was analyzed in greater detail in the maize protoplast system. The following mutations were made in the UPR promoter elements: UPRE-IIIa (CGATGA→GTACTT), UPRE-IIIb (CGATGA→GACTCG), UPRE-I (TGACTG→ACTGAC), UPRE-IIIc (CGATGA→GTACTT), and bZIP17/28 binding site (GCACGTGATG→CTAGGCGACT).
Statistical Analysis
Statistical analyses were conducted using the R package (https://www.r-project.org). One-way ANOVA and post hoc Tukey’s Honestly Significant Difference test were used for the multiple comparisons of the biomass of W22 and bzip60-2 under different MDTs, for the analysis of chlorophyll indices for individual plants grown under the same conditions and for the activity of the HSFTF13 promoter and various promoter mutants in maize mesophyll protoplasts. Two-way ANOVA and post hoc Tukey’s Honestly Significant Difference test were used for the multiple comparisons of biomass between W22 and bzip60-2 under different MDTs. P < 0.05 was considered significant between comparisons. For the analyses of internode length, a t test was used to compare the difference between bzip60-2 and W22 under the same growth conditions. The asterisk (*) represents statistical significance for P < 0.05 and the double asterisks (**) represent the statistical significance for P < 0.01 using a t test. For the RNA-seq analysis, three biological replicates were used. Differential expression analysis was performed using DESeq2 (v1.4.5, www.bioconductor.org/packages/release/bioc/html/ DESeq2.html), and DEGs were identified with Q value ≤ 0.05 and absolute log2-fold value >1. The Supplemental File provides results of the statistical analyses shown in the figures.
Accession Numbers
Sequence data from this article can be found in NCBI’s Gene Expression Omnibus database under record number GSE154373 and in the EnsemblPlants database under the following accession numbers (using v.4 nomenclature): bZIP60 (Zm00001d046718), HSP26 (Zm00001d028408), CRT2 (Zm00001d005460), PDI1 (Zm00001d049099), HSFTF13 (Zm00001d027757), CONSTANS-LIKE15 (Zm00001d031662), GATA TRANSCRIPTION FACTOR16 (Zm00001d040775), NAC DOMAIN TRANSCRIPTION FACTOR84 (Zm00001d011969), bZIP91 (Zm00001d007042), bZIP34 (Zm00001d038189), PCR1 (Zm00001d001820), CH1 (Zm00001d042026), NYE1 (Zm00001d021288), NYE1 (Zm00001d006211), CHLOROPHYLLASE1 (Zm00001d031934), VPS46 (Zm00001d046599), Ubi1 (Zm00001d015327), DNAJ domain protein (Zm00001d014790), HSP17.6 (Zm00001d039566), HSP17.6 (Zm00001d008841), HSP18.2 (Zm00001d039935), HSP20 (Zm00001d028561), HSP20 (Zm00001d072711), HSP20 (Zm00001d039936), HSP20 (Zm00001d025508), HSP70 (Zm00001d048073), HSP70 (Zm00001d042922), HSP70 (Zm00001d037717), HSP90 (Zm00001d024903), and HSP101 Zm00001d038806.
Supplemental Data
Supplemental Figure 1. The Enviratron facility for monitoring plant performance under different environmental conditions. Supports Figure 1.
Supplemental Figure 2. Growth of W22 plants at different MDTs. Supports Figure 1.
Supplemental Figure 3. Validation of the ATG8 lipidation assay. Supports Figure 1.
Supplemental Figure 4. Induction of autophagy under field conditions. Supports Figure 1.
Supplemental Figure 5. Principal component analysis of data from the RNA-seq analysis. Supports Figure 3.
Supplemental Figure 6. DEGs in the comparison between different MDTs. Supports Figure 3.
Supplemental Figure 7. DEGs in the comparison between bzip60-2 and W22. Supports Figure 4.
Supplemental Figure 8. GO terms for DEGs in the comparison of bzip60-2 to W22. Supports Figure 4.
Supplemental Figure 9. Different groups of DEGs based on differences in genotypes and MDTs. Supports Figure 4.
Supplemental Figure 10. Group-II DEGs with differences between genotypes and MDTs. Supports Figure 4.
Supplemental Figure 11. Map of the upstream region of HSFTF13 (HSFA6B). Supports Figure 6.
Supplemental Figure 12. Hyperspectral image to determine leaf chlorophyll index. Supports Figure 7.
Supplemental Figure 13. Chlorophyll biosynthesis and degradation pathway. Supports Figure 7.
Supplemental Data Set 1. Key DEGs identified in maize W22 in response to the increased MDTs.
Supplemental Data Set 2. All the DEGs identified in maize W22 in response to the increased MDTs.
Supplemental Data Set 3. Relative expression level of key genes used to generate the box plot.
Supplemental Data Set 4. Relative gene expression level (Fragments Per Kilobase of transcript per Million mapped reads) identified in this article.
Supplemental Data Set 5. Key DEGs identified between bzip60-2 and W22 at different MDTs.
Supplemental Data Set 6. All the DEGs identified between bzip60-2 and W22 at different MDTs.
Supplemental Data Set 7. Primers used in this article.
Supplemental File. Statistical analysis of the data in the figures.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
The authors thank Baomei Wang and Juren Zhang (Shandong University) for help with the RNA-seq analysis. The authors also thank the Enviratron team, particularly Scott Zarecor, Yin Bao, and Antony Chapman for making the Enviratron runs possible, and Patrick Schnable for providing field space. This work was supported by the National Science Foundation Plant Genome Research Program (grant IOS–1444339) and by Iowa State University’s Plant Sciences Institute Research Scholar Awards (to S.H.H.).
AUTHOR CONTRIBUTIONS
Z.X.L., D.C.B., and S.H.H. designed the research; Z.X.L., J.T., and R.S. performed the research; Z.X.L., D.C.B., and S.H.H. analyzed the data; Z.X.L., D.C.B., and S.H.H. wrote the article.
Footnotes
Articles can be viewed without a subscription.
References
- Abdi H.(2007). The Bonferroni and Sidák corrections for multiple comparisons In The Encyclopedia of Measurement and Statistics., Volume 1 (Thousand Oaks, CA: Sage; ), pp. 1–9. [Google Scholar]
- Bao Y., Pu Y., Yu X., Gregory B.D., Srivastava R., Howell S.H., Bassham D.C.(2018). IRE1B degrades RNAs encoding proteins that interfere with the induction of autophagy by ER stress in Arabidopsis thaliana. Autophagy 14: 1562–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y., et al. (2019). Assessing plant performance in the Enviratron. Plant Methods 15: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barah P., Jayavelu N.D., Mundy J., Bones A.M.(2013). Genome scale transcriptional response diversity among ten ecotypes of Arabidopsis thaliana during heat stress. Front. Plant Sci. 4: 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bita C.E., Gerats T.(2013). Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 4: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Bernad A., Lázaro J.M., Martín G., Garmendia C., Salas M.(1989). Highly efficient DNA synthesis by the phage phi 29 DNA polymerase. Symmetrical mode of DNA replication. J. Biol. Chem. 264: 8935–8940. [PubMed] [Google Scholar]
- Challinor A.J., Watson J., Lobell D.B., Howden S.M., Smith D.R., Chhetri N.(2014). A meta-analysis of crop yield under climate change and adaptation. Nat. Clim. Chang. 4: 287–291. [Google Scholar]
- Chung T., Suttangkakul A., Vierstra R.D.(2009). The ATG autophagic conjugation system in maize: ATG transcripts and abundance of the ATG8-lipid adduct are regulated by development and nutrient availability. Plant Physiol. 149: 220–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P., Ho T.H.(1983). Heat shock proteins in maize. Plant Physiol. 71: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Humbert S., Liu J.X., Srivastava R., Rothstein S.J., Howell S.H.(2011). Heat induces the splicing by IRE1 of a mRNA encoding a transcription factor involved in the unfolded protein response in Arabidopsis. Proc. Natl. Acad. Sci. USA 108: 7247–7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegelman A.M., Kool E.T.(2000). Chemical and enzymatic methods for preparing circular single-strand DNAs. Curr. Protoc. Nucl. Acid Chem. Chapter 5: Unit 5.2: 5.2.1-5.2.27. [DOI] [PubMed] [Google Scholar]
- Haboudane D., Tremblay N., Miller J.R., Vigneault P. (2008). Estimation of plant chlorophyll using hyperspectral observations and radiative transfer models: Spectral indices sensitivity and crop-type effects. In: IEEE International Geoscience and Remote Sensing Symposium. Boston MA: IEEE.
- He Q., Berg A., Li Y., Vallejos C.E., Wu R.(2010). Mapping genes for plant structure, development and evolution: Functional mapping meets ontology. Trends Genet. 26: 39–46. [DOI] [PubMed] [Google Scholar]
- Howell S.H.(2013). Endoplasmic reticulum stress responses in plants. Annu. Rev. Plant Biol. 64: 477–499. [DOI] [PubMed] [Google Scholar]
- Howell S.H.(2017). When is the unfolded protein response not the unfolded protein response? Plant Sci. 260: 139–143. [DOI] [PubMed] [Google Scholar]
- Huang Y.C., Niu C.Y., Yang C.R., Jinn T.L.(2016). The heat stress factor HSFA6b connects ABA signaling and ABA-mediated heat responses. Plant Physiol. 172: 1182–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y., Kirisako T., Takao T., Satomi Y., Shimonishi Y., Ishihara N., Mizushima N., Tanida I., Kominami E., Ohsumi M., Noda T., Ohsumi Y.(2000). A ubiquitin-like system mediates protein lipidation. Nature 408: 488–492. [DOI] [PubMed] [Google Scholar]
- Iwata Y., Fedoroff N.V., Koizumi N.(2008). Arabidopsis bZIP60 is a proteolysis-activated transcription factor involved in the endoplasmic reticulum stress response. Plant Cell 20: 3107–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y., Koizumi N.(2005). An Arabidopsis transcription factor, AtbZIP60, regulates the endoplasmic reticulum stress response in a manner unique to plants. Proc. Natl. Acad. Sci. USA 102: 5280–5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi M., Hidema J., Makino A., Ishida H.(2013). Autophagy contributes to nighttime energy availability for growth in Arabidopsis. Plant Physiol. 161: 1682–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P., Hirt H., Bendahmane A.(2017). The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 15: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Humbert S., Howell S.H.(2012). ZmbZIP60 mRNA is spliced in maize in response to ER stress. BMC Res. Notes 5: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.X., Jiang H.Y., Chu Z.X., Tang X.L., Zhu S.W., Cheng B.J.(2011). Genome-wide identification, classification, and analysis of heat shock transcription factor family in maize. BMC Genomics 12: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.X., Howell S.H.(2010). bZIP28 and NF-Y transcription factors are activated by ER stress and assemble into a transcriptional complex to regulate stress response genes in Arabidopsis. Plant Cell 22: 782–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.(2008). Heat shock response relieves ER stress. EMBO J. 27: 1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Bassham D.C.(2013). Degradation of the endoplasmic reticulum by autophagy in plants. Autophagy 9: 622–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Burgos J.S., Deng Y., Srivastava R., Howell S.H., Bassham D.C.(2012). Degradation of the endoplasmic reticulum by autophagy during endoplasmic reticulum stress in Arabidopsis. Plant Cell 24: 4635–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D.(2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Martínez I.M., Chrispeels M.J.(2003). Genomic analysis of the unfolded protein response in Arabidopsis shows its connection to important cellular processes. Plant Cell 15: 561–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty D.R., et al. (2005). Steady-state transposon mutagenesis in inbred maize. Plant J. 44: 52–61. [DOI] [PubMed] [Google Scholar]
- McMurtrey J.E. III, Chappelle E.W., Kim M.S., Meisinger J.J., Corp L.A.(1994). Distinguishing nitrogen-fertilization levels in-field corn (Zea mays L.) with actively induced fluorescence and passive reflectance measurements. Remote Sens. Environ. 47: 36–44. [Google Scholar]
- Morimoto R.I.(1993). Cells in stress: Transcriptional activation of heat shock genes. Science 259: 1409–1410. [DOI] [PubMed] [Google Scholar]
- Nagashima Y., Mishiba K., Suzuki E., Shimada Y., Iwata Y., Koizumi N.(2011). Arabidopsis IRE1 catalyses unconventional splicing of bZIP60 mRNA to produce the active transcription factor. Sci. Rep. 1: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohama N., Sato H., Shinozaki K., Yamaguchi-Shinozaki K.(2017). Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 22: 53–65. [DOI] [PubMed] [Google Scholar]
- Porreca G.J.(2010). Genome sequencing on nanoballs. Nat. Biotechnol. 28: 43–44. [DOI] [PubMed] [Google Scholar]
- Pu Y., Bassham D.C.(2013). Links between ER stress and autophagy in plants. Plant Signal. Behav. 8: e24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu A.L., Ding Y.F., Jiang Q., Zhu C.(2013). Molecular mechanisms of the plant heat stress response. Biochem. Biophys. Res. Commun. 432: 203–207. [DOI] [PubMed] [Google Scholar]
- Queitsch C., Hong S.W., Vierling E., Lindquist S.(2000). Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 12: 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky L., Liang H., Shuman J., Shulaev V., Davletova S., Mittler R.(2004). When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 134: 1683–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settles A.M., et al. (2007). Sequence-indexed mutations in maize using the UniformMu transposon-tagging population. BMC Genomics 8: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J.(2001). Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol. 127: 1466–1475. [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Mosser D.D., Morimoto R.I.(1998). Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 12: 654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer C., Li F., Buono R., Roschzttardtz H., Chung T., Zhang M., Osteryoung K.W., Vierstra R.D., Otegui M.S.(2015). The endosomal protein CHARGED MULTIVESICULAR BODY PROTEIN1 regulates the autophagic turnover of plastids in Arabidopsis. Plant Cell 27: 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R., et al. (2018). Response to persistent ER stress in plants: A multiphasic process that transitions cells from prosurvival activities to cell death. Plant Cell 30: 1220–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio A., Dreos R., Aparicio F., Maule A.J.(2009). The cytosolic protein response as a subcomponent of the wider heat shock response in Arabidopsis. Plant Cell 21: 642–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Yang Z.T., Song Z.T., Wang M.J., Sun L., Lu S.J., Liu J.X.(2013). The plant-specific transcription factor gene NAC103 is induced by bZIP60 through a new cis-regulatory element to modulate the unfolded protein response in Arabidopsis. Plant J. 76: 274–286. [DOI] [PubMed] [Google Scholar]
- Swindell W.R., Huebner M., Weber A.P.(2007). Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genomics 8: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigchelaar M., Battisti D.S., Naylor R.L., Ray D.K.(2018). Future warming increases probability of globally synchronized maize production shocks. Proc. Natl. Acad. Sci. USA 115: 6644–6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voellmy R., Boellmann F.(2007). Chaperone regulation of the heat shock protein response. Adv. Exp. Med. Biol. 594: 89–99. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yu B., Zhao J., Guo J., Li Y., Han S., Huang L., Du Y., Hong Y., Tang D., Liu Y.(2013). Autophagy contributes to leaf starch degradation. Plant Cell 25: 1383–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Srivastava R., Howell S.H., Bassham D.C.(2015). Activation of autophagy by unfolded proteins during endoplasmic reticulum stress. Plant J. 85: 83–95. [DOI] [PubMed] [Google Scholar]
- Zhao C., et al. (2017). Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 114: 9326–9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Krakowiak J., Patel N., Beyzavi A., Ezike J., Khalil A.S., Pincus D.(2016). Dynamic control of Hsf1 during heat shock by a chaperone switch and phosphorylation. eLife 5: e18638. [DOI] [PMC free article] [PubMed] [Google Scholar]