The reactive oxygen species wave propagates through vascular bundles during the systemic response of Arabidopsis (Arabidopsis thaliana) plants to excess light stress.
Abstract
Systemic signaling and systemic acquired acclimation (SAA) are essential for plant survival during episodes of environmental stress. Recent studies highlighted a key role for reactive oxygen species (ROS) signaling in mediating systemic responses and SAA during light stress in Arabidopsis (Arabidopsis thaliana). These studies further identified the RESPIRATORY BURST OXIDASE HOMOLOG D (RBOHD) protein as a key player in mediating rapid systemic ROS responses. Here, we report that tissue-specific expression of RBOHD in phloem or xylem parenchyma cells of the rbohD mutant restores systemic ROS signaling, systemic stress-response transcript expression, and SAA to a local treatment of light stress. We further demonstrate that RBOHD and RBOHF are both required for local and systemic ROS signaling at the vascular bundles of Arabidopsis. Taken together, our findings highlight a key role for RBOHD-driven ROS production at the vascular bundles of Arabidopsis in mediating light stress–induced systemic signaling and SAA. In addition, they suggest that the integration of ROS, calcium, electric, and hydraulic signals, during systemic signaling, occurs at the vascular bundles.
INTRODUCTION
Plants and other multicellular organisms are able to transmit different chemical and physical signals over long distances, sometimes traversing their entire length. These signals coordinate the response of the entire organism to different stresses, pathogens, and/or other stimuli and are thought to play a key role in the acclimation, defense, and adaptation of different organisms to their environment (Choi et al., 2017; Takahashi et al., 2018; Kollist et al., 2019; Wang et al., 2019; Fichman and Mittler, 2020). In plants, a plethora of chemical and physical signals are transmitted from a single leaf or root tip subjected to stress (i.e., a local tissue) to the entire plant (i.e., systemic tissues). These signals, collectively termed systemic signals, include different chemicals and volatiles, such as salicylic acid, abscisic acid (ABA), and methyl jasmonate, as well as small peptides, and physical signals such as electric signals and hydraulic waves (Galvez-Valdivieso et al., 2009; Szechyńska-Hebda et al., 2010; Devireddy et al., 2018; Nguyen et al., 2018; Toyota et al., 2018; Takahashi et al., 2018; Wang et al., 2019). In recent years, a role for two interlinked signal transduction molecules, that is, Ca2+ and reactive oxygen species (ROS), was identified in the systemic response of plants to different abiotic stimuli such as excess light stress or wounding. These two signals were further shown to propagate from cell to cell over long distances in plants and were termed the Ca2+ and ROS waves (Miller et al., 2009; Choi et al., 2014; Toyota et al., 2018; Zandalinas and Mittler, 2018; Fichman et al., 2019; Fichman and Mittler, 2020).
The ROS wave is essential for coordinating systemic metabolic responses that generate different metabolic signatures in different parts of the plant (Choudhury et al., 2018); for coordinating systemic transcriptomic responses that activate transcriptional networks essential for plant acclimation (Zandalinas et al., 2019); and for coordinating systemic physiological responses, such as changes in stomatal aperture (Devireddy et al., 2018, 2020). In addition, the ROS wave is required for successful plant acclimation to light and heat stresses (Suzuki et al., 2013; Devireddy et al., 2018; Zandalinas et al., 2019, 2020). The initiation, propagation, and maintenance of the ROS wave in response to abiotic stress is dependent on the plasma membrane–localized RESPIRATORY BURST OXIDASE HOMOLOG D (RBOHD; AT5G47910) protein (Miller et al., 2009; Zandalinas and Mittler, 2018; Fichman and Mittler, 2020). However, genetic evidence in the form of complementation studies, as well as cell biology studies elucidating the role of RBOHD in different plant tissues during systemic signaling, has thus far been lacking. RBOHD belongs to a family of 10 different proteins in Arabidopsis (Arabidopsis thaliana), able to produce superoxide radicals at the apoplast in response to a cytosolic calcium and/or phosphorylation signal (Suzuki et al., 2011; Kadota et al., 2015; Fichman and Mittler, 2020). The superoxide radicals generated at the apoplast by RBOH proteins are dismutated spontaneously, or via superoxide dismutases present in the apoplast, to generate H2O2 that can enter cells via aquaporins (Mittler et al., 2011; Gilroy et al., 2016; Choi et al., 2017; Mittler, 2017; Rodrigues et al., 2017; Clemente‐Moreno et al., 2019; Fichman and Mittler, 2020; García et al., 2020). Of the 10 RBOHs in Arabidopsis, RBOHD and RBOHF (AT1G64060) are the two primarily expressed in aboveground vegetative tissues (Torres et al., 2002; Kwak et al., 2003; Morales et al., 2016).
Based on their relative speed, as well as their physical and chemical properties, four different systemic signals are thought to copropagate, or at least interact with each other, during rapid systemic signaling in plants. These include the ROS, Ca2+, hydraulic, and electric waves (Mittler et al., 2011; Gilroy et al., 2016; Choi et al., 2017; Fichman and Mittler, 2020). Calcium waves and electrical signals are dependent on the function of the calcium-permeable glutamate receptor-like (GLR) GLR3.3 and GLR3.6 channels (Mousavi et al., 2013; Nguyen et al., 2018; Toyota et al., 2018; Wudick et al., 2018; Shao et al., 2020), and calcium and ROS waves were proposed to be linked through the function of RBOHD (Mittler et al., 2011; Evans et al., 2016). RBOHD was further shown to be required for the propagation of certain electric signals in response to light stress (Suzuki et al., 2013). Although it is unknown how hydraulic waves are linked to electric, ROS, and calcium waves, it was proposed that different mechanosensitive channels (Basu and Haswell, 2017) could sense hydraulic waves at systemic tissues and convert them into calcium signals (Choi et al., 2017; Kollist et al., 2019; Fichman and Mittler, 2020). Calcium signals can further influence ROS signals via the function of different calcium binding proteins and kinases/phosphatase molecular switches or by directly binding to the E and F helices (EF)-hand domains of RBOHD (Suzuki et al., 2011; Kadota et al., 2015; Fichman and Mittler, 2020). Although the Ca2+, hydraulic, and electric waves were recently shown to propagate through the vascular bundles of plants, utilizing plant tissues such as phloem, xylem parenchyma, and bundle sheath cells (Mousavi et al., 2013; Sade et al., 2014; Nguyen et al., 2018; Toyota et al., 2018; Shao et al., 2020), the cell types and tissues through which the ROS wave propagates in plants are currently unknown (Fichman and Mittler, 2020; Fichman et al., 2020).
RESULTS
Complementation of Systemic ROS Signaling in the rbohD Mutant with RBOHD Driven by Different Tissue-Specific Promoters
The RBOHD protein is expressed in almost all tissues of Arabidopsis, including epidermis, mesophyll, stomata, and vascular bundles (Hao et al., 2014; Morales et al., 2016). To determine in what tissues its expression is vital for systemic ROS signaling in response to a local treatment of excess high-light (HL) stress, we attempted to complement the rbohD mutant with different binary vectors driving the stable expression of RBOHD using its native promoter or different tissue-specific promoters (Figure 1; Supplemental Figures 1 and 2; Supplemental Table 1). The tissue-specific promoters chosen for our analysis were successfully used in previous studies to drive the expression of different proteins in their corresponding tissues (Funk et al., 2002; Hooker et al., 2002; Yoshimoto et al., 2003; Endo et al., 2007; Avci et al., 2008; Mustroph et al., 2009; Dhondt et al., 2010; Sawa and Kay, 2011; Cui et al., 2014; Liu et al., 2014; Matías-Hernández et al., 2016). In addition, to confirm that the RBOHD protein, expressed under the control of the different tissue-specific promoters (Supplemental Table 1), accumulates in these tissues, we generated a second set of transgenic plants in which the RBOHD protein was tagged with the green fluorescence protein (GFP) at its N terminus, and we determined that the GFP signal was present in the correct tissues associated with the different tissue-specific promoters (Supplemental Figure 1; not used in Figures 1 to 3). To image excess light stress–driven local and systemic changes in ROS levels, we used the whole-plant live ROS imaging technique we recently developed that is based on the oxidation of dichlorofluorescein (DCF), fumigated as an 2′,7′-dichlorofluorescein (H2DCFDA) dye, into plants prior to the stress treatment (Fichman et al., 2019; Zandalinas et al., 2020).
Figure 1.
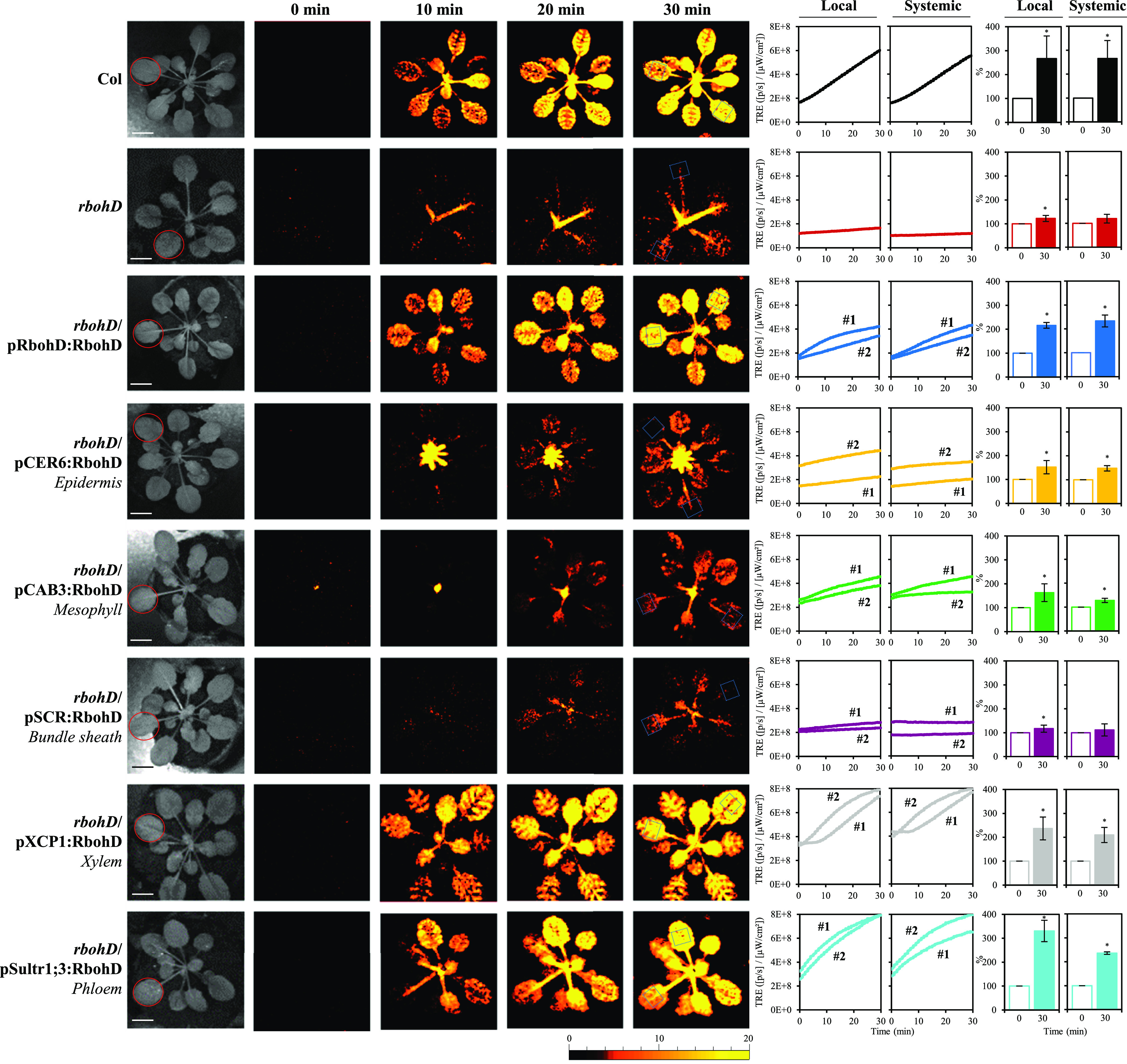
Complementation of Light Stress–Induced Local and Systemic ROS Signaling in the rbohD Mutant with RBOHD Driven by Different Tissue-Specific Promoters.
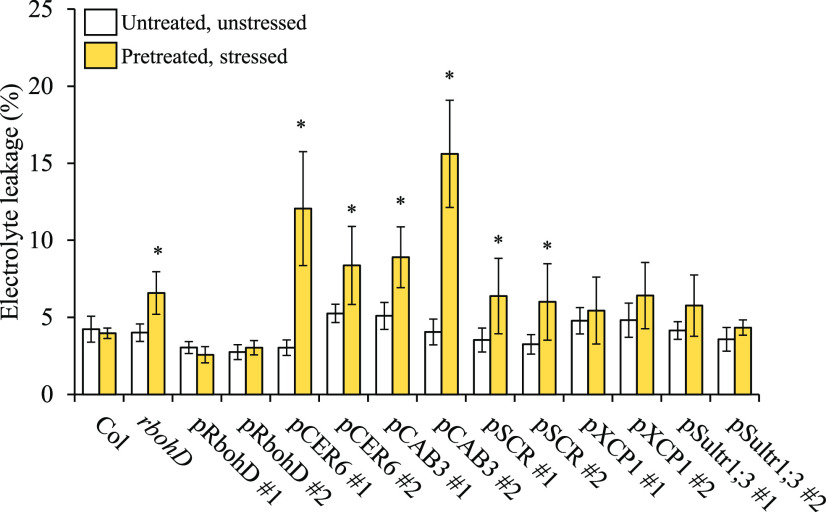
Representative time-lapse images of whole-plant ROS levels (indicated by DCF oxidation) in the wild type (Columbia-0 [Col]), rbohD and the different rbohD complemented Arabidopsis plants subjected to a 2-min local HL stress treatment (applied to the local leaf only), are shown on left; representative line graphs showing continuous measurements of ROS levels in local and systemic leaves of the wild type, rbohD, and two independent homozygous complemented lines (nos. 1 and 2), over the entire course of the experiment (0 to 30 min), are shown in the middle (ROIs for some of them are indicated with blue boxes); and statistical analysis of ROS levels in local and systemic leaves at 0 and 30 min is shown on right (conducted for 10 different plants each from two independent lines; Student’s t test, sd, n = 20, *P < 0.05). All experiments were repeated at least three times with similar results. Treated local leaves are indicated with a red circle. Bar = 1 cm. CAB, CHLOROPHYLL A/B BINDING PROTEIN; CER, ECERIFERUM; ROI, region of interest; SCR, SCARECROW; SULTR, SULFATE TRANSPORTER; TRE, total radiant efficiency; XCP, XYLEM CYSTEINE PEPTIDASE.
Figure 3.
Complementation of Light Stress–Induced SAA in the rbohD Mutant with RBOHD Driven by Different Tissue-Specific Promoters.
Light stress–induced systemic leaf cell injury (measured as electrolyte leakage) of the wild type (Columbia-0 [Col]), rbohD, and the different rbohD complemented Arabidopsis plants was determined. Systemic leaves were either untreated, nonpretreated, and unstressed (untreated, unstressed) or subjected to light stress following a pretreatment of one local leaf with light stress (pretreated, stressed). SAA is evident by a low level of electrolyte leakage in light-stressed systemic leaves (pretreated, stressed), which is similar to that of untreated and unstressed leaves (untreated, unstressed). Ten different plants each from two independent complemented lines for each construct (nos. 1 and 2) were subjected to light stress, and cell injury was measured in systemic leaves by electrolyte leakage. Student’s t test, sd, n = 20, *P < 0.05. CAB, CHLOROPHYLL A/B BINDING PROTEIN; CER, ECERIFERUM; SCR, SCARECROW; SULTR, SULFATE TRANSPORTER; XCP, XYLEM CYSTEINE PEPTIDASE.
As shown in Figure 1, and in agreement with our previous study of the rbohD mutant (Fichman et al., 2019), local and systemic ROS responses to a local treatment of HL stress (evident by increase in the accumulation of oxidized DCF), were strongly attenuated in the rbohD mutant. Complementation of RBOHD expression in the rbohD mutant, using the native RBOHD promoter, completely restored light stress–induced local and systemic ROS responses (evident by accumulation of oxidized DCF; Figure 1; Fichman et al., 2019). By contrast, complementation of RBOHD expression using an epidermis-specific promoter (CER6; Hooker et al., 2002; Mustroph et al., 2009) or mesophyll-specific promoter (CAB3; Endo et al., 2007; Matías-Hernández et al., 2016) only partially restored light stress–induced local and systemic ROS responses, whereas complementation of RBOHD expression using a bundle sheath–specific promoter (SCR; Dhondt et al., 2010; Cui et al., 2014) failed to restore light stress–driven ROS responses (Figure 1). Complementation of local and systemic ROS responses to the wild-type levels (evident by accumulation of oxidized DCF) was however achieved using a xylem parenchyma–specific promoter (XCP1; Funk et al., 2002; Avci et al., 2008) or phloem-specific promoter (Sultr1;3; Yoshimoto et al., 2003; Sawa and Kay, 2011; Figure 1; Supplemental Figure 2). The results presented in Figure 1 and Supplemental Figures 1 and 2 demonstrate that RBOHD expression in the xylem parenchyma or phloem is essential for systemic ROS signaling in response to a local treatment of excess light stress. Restoring whole-leaf systemic ROS responses via expression of RBOHD in such a limited number of cells of the rbohD mutant (evident by accumulation of oxidized DCF; Figure 1; Supplemental Figures 1 and 2) further suggests that, under the conditions studied in Figure 1, other ROS-producing mechanisms might be involved in generating ROS in systemic tissues. These could include RBOHF or other apoplastic oxidases and/or cytosolic, mitochondrial, peroxisomal, or chloroplastic ROS-producing mechanisms (Mittler, 2017; Kollist et al., 2019).
Complementation of Local and Systemic Zat12 Expression in the rbohD Mutant with RBOHD Driven by Different Tissue-Specific Promoters
To determine whether the complementation of systemic ROS signaling in the rbohD mutant also restored systemic transcript expression in response to excess light stress, we determined the expression of the ZAT12 gene (AT5G59820) in local and systemic tissues of the different control and complemented lines using the ZAT12 promoter:luciferase reporter. The zinc finger protein ZAT12 was initially identified as a central regulator essential for light stress responses and acclimation in Arabidopsis (Iida et al., 2000). Further studies determined that ZAT12 plays an important role in the response of plants to many other abiotic stresses (Davletova et al., 2005). As shown in Figure 2, compared to the wild type, the systemic expression of ZAT12 in response to local application of HL stress, detected under the same experimental conditions used to visualize the ROS wave in Figure 1, was suppressed in the rbohD mutant. By contrast, local expression of ZAT12 was not. This finding demonstrated that ZAT12 expression is triggered in local tissues independent of RBOHD. By contrast, the systemic expression of ZAT12 was dependent on RBOHD during systemic responses to light stress. Similar to our findings with systemic ROS signaling in the different RBOHD tissue-specific complementation lines (Figure 1; Supplemental Figure 2), light stress–induced systemic expression of Zat12 could be restored to the wild-type levels only upon expression of RBOHD in the rbohD mutant using its own native promoter or the xylem parenchyma– or phloem-specific promoters (Figure 2). The results presented in Figure 2 demonstrate that RBOHD expression in the xylem parenchyma or phloem is essential for systemic transcript expression of the key light stress–response gene ZAT12 during the systemic response of Arabidopsis to a local treatment of HL stress.
Figure 2.
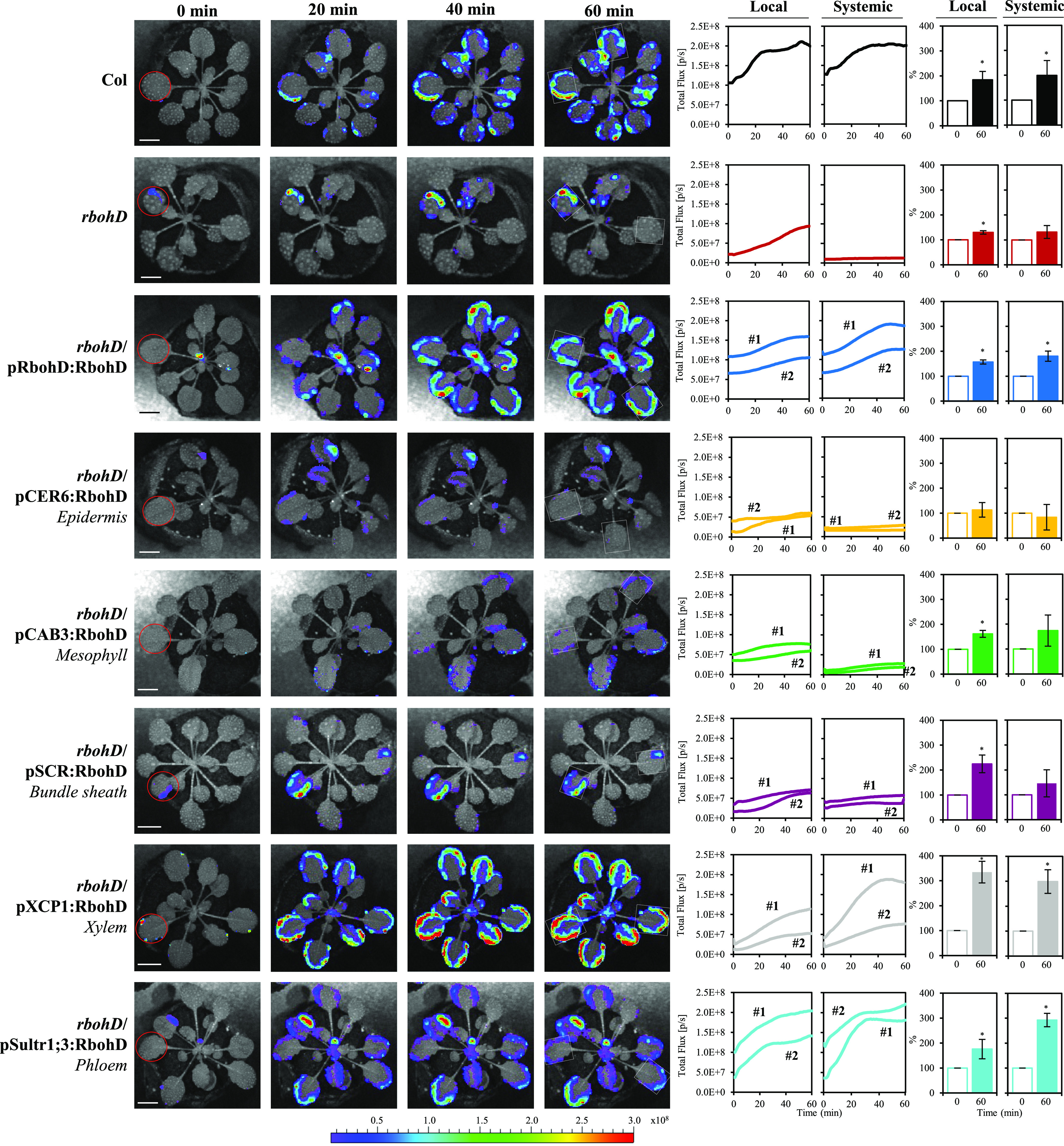
Complementation of Light Stress–Induced Local and Systemic ZAT12 Expression in the rbohD Mutant with RBOHD Driven by Different Tissue-Specific Promoters.
Representative time-lapse images of local and systemic ZAT12:luciferase activity in the wild type (Columbia-0 [Col]), rbohD, and the different ZAT12:luciferase-expressing double homozygous rbohD complemented Arabidopsis plants subjected to a 2-min local HL stress treatment (applied to the local leaf only) are shown on left; representative line graphs showing continuous measurements of ZAT12:luciferase activity in local and systemic leaves of the wild type, rbohD, and two independent homozygous complemented lines (nos. 1 and 2), over the entire course of the experiment (0 to 30 min), are shown in the middle (ROIs for some of them are indicated with white boxes); and statistical analysis of ZAT12:luciferase activity in local and systemic leaves at 0 and 30 min is shown on right (conducted for 10 different plants each from two independent lines; Student’s t test, sd, n = 20, *P < 0.05). All experiments were repeated at least three times with similar results. Treated local leaves are indicated with a red circle. Bar = 1 cm. CAB, CHLOROPHYLL A/B BINDING PROTEIN; CER, ECERIFERUM; ROI, region of interest; SCR, SCARECROW; SULTR, SULFATE TRANSPORTER; XCP, XYLEM CYSTEINE PEPTIDASE.
Complementation of SAA in the rbohD Mutant with RBOHD Driven by Different Tissue-Specific Promoters
The rbohD mutant is unable to acclimate its systemic tissues to light stress following a local treatment of HL stress applied to a single (local) leaf (Suzuki et al., 2013; Devireddy et al., 2018; Zandalinas et al., 2019). Because RBOHD expression in the xylem parenchyma or phloem cells restored systemic ROS signaling and systemic expression of ZAT12 in the rbohD mutant (Figures 1 and 2; Supplemental Figure 2), it could potentially restore SAA following local application of HL stress. We therefore tested all complemented lines described in Figure 1 for SAA following a local HL stress treatment. For this purpose, we used an SAA assay based on measuring excess light stress–induced systemic leaf injury in the presence or absence of a short light stress pretreatment applied to a single local leaf (Suzuki et al., 2013; Devireddy et al., 2018; Zandalinas et al., 2019, 2020). As shown in Figure 3, complementing the rbohD mutant with RBOHD expressed via its native promoter, or the xylem parenchyma- or phloem-specific promoters, restored HL stress–driven SAA to the rbohD mutant. By contrast, complementing RBOHD expression in the rbohD mutant using the epidermis-, mesophyll-, or bundle sheath–specific promoters failed to restore SAA to light stress (Figure 3). The results presented in Figures 1 to 3 reveal that expression of RBOHD at the xylem parenchyma or phloem cells of the rbohD mutant is sufficient to restore systemic ROS signaling, expression of the ZAT12 light stress–response regulator, and SAA, to the rbohD mutant. Taken together, these findings underscore a key role for RBOHD, present at the vascular tissues (phloem or xylem parenchyma) of Arabidopsis, in mediating the systemic response of plants to light stress.
Because the expression of RBOHD in such a limited subset of cells had such a dramatic effect on systemic ROS signaling, transcript expression and SAA (Figures 1 to 3; Supplemental Figure 2), other ROS-producing mechanisms might be required for mediating ROS accumulation in many of the other plant cells that do not express RBOHD in the rbohD mutant. Potential clues to the existence of such additional players in the systemic ROS signaling response of Arabidopsis to light stress include our current and previous findings that low levels of systemic ROS (evident by accumulation of oxidized DCF) can still be detected in the rbohD mutant (Figure 1; Fichman et al., 2019). As suggested above, one potential candidate for this function, based on its expression pattern and function (Torres et al., 2002; Kwak et al., 2003; Morales et al., 2016), is RBOHF.
RBOHD and RBOHF Are Both Required for Mediating Systemic ROS Signaling in Response to a Local HL Stress Treatment
To test whether RBOHF is also required for systemic ROS signaling during the response of Arabidopsis plants to light stress, we subjected the wild type, rbohD, rbohF, and rbohD rbohF double mutants (Torres et al., 2002; Kwak et al., 2003; Miller et al., 2009) to a local HL stress treatment and imaged local and systemic ROS levels (evident by accumulation of oxidized DCF) in these plants. As shown in Figure 4, rbohD, rbohF, and rbohD rbohF were all deficient in systemic ROS signaling in response to a local application of excess light stress. Interestingly, the low residual ROS levels, detected in the rbohD mutant (evident by accumulation of oxidized DCF; Figure 1; Fichman et al., 2019), can also be detected in the rbohF mutant (albeit weaker). By contrast, no residual ROS levels were detected in the rbohD rbohF double mutant (Figure 4). Fluorescence microscopy imaging of ROS levels (evident by DCF oxidation), conducted on petioles of local and systemic leaves of the different mutants following local application of light stress (Figure 5), revealed that low levels of ROS could be found at the vascular bundles of the rbohD or rbohF mutants at the local or systemic tissues in response to light stress. By contrast, ROS levels were undetected by fluorescence microscopy at the vascular bundles of local or systemic leaves of the rbohD rbohF double mutant (Figure 5). Although excess light-driven ROS was primarily detected at the vascular tissues of local and systemic leaves of the wild-type plants, in local leaves high ROS levels were also detected at other cell layers, such as the epidermis (Figure 5). While the detection of ROS at the epidermis was evident in the wild type and the rbohF mutant, similar levels were not detected in the rbohD or the rbohD rbohF double mutant. The findings presented in Figures 4 and 5 support a model in which RBOHD and RBOHF cooperate to drive local and systemic ROS signaling (evident by DCF oxidation) at the vascular bundles of Arabidopsis during light stress and indicate that RBOHD might also control ROS levels at the epidermis of local leaves. Because the lack of RBOHD was sufficient to block the spread of a systemic ROS wave response from the local to the systemic leaves, even when H2O2 was directly applied to the local leaf (Supplemental Figure 3A), the suppressed systemic response of the rbohD or the rbohD rbohF double mutants may not simply result from the lack of ROS responses at the local tissue. Systemic ROS signaling may therefore require RBOHD expression at the local, as well as systemic tissues (Suzuki et al., 2013). Future studies using the different tissue-specific promoters (Figures 1 to 3; Supplemental Figure 2) to drive RBOHF expression in different tissues of the rbohF mutant are likely to sharpen our understanding of RBOHF function in mediating the ROS wave. Nonetheless, such studies are not likely to alter the key findings of this study revealing that expression of RBOHD in phloem and xylem parenchyma cells is required for transmitting the ROS wave during the systemic response of Arabidopsis to light stress.
Figure 4.
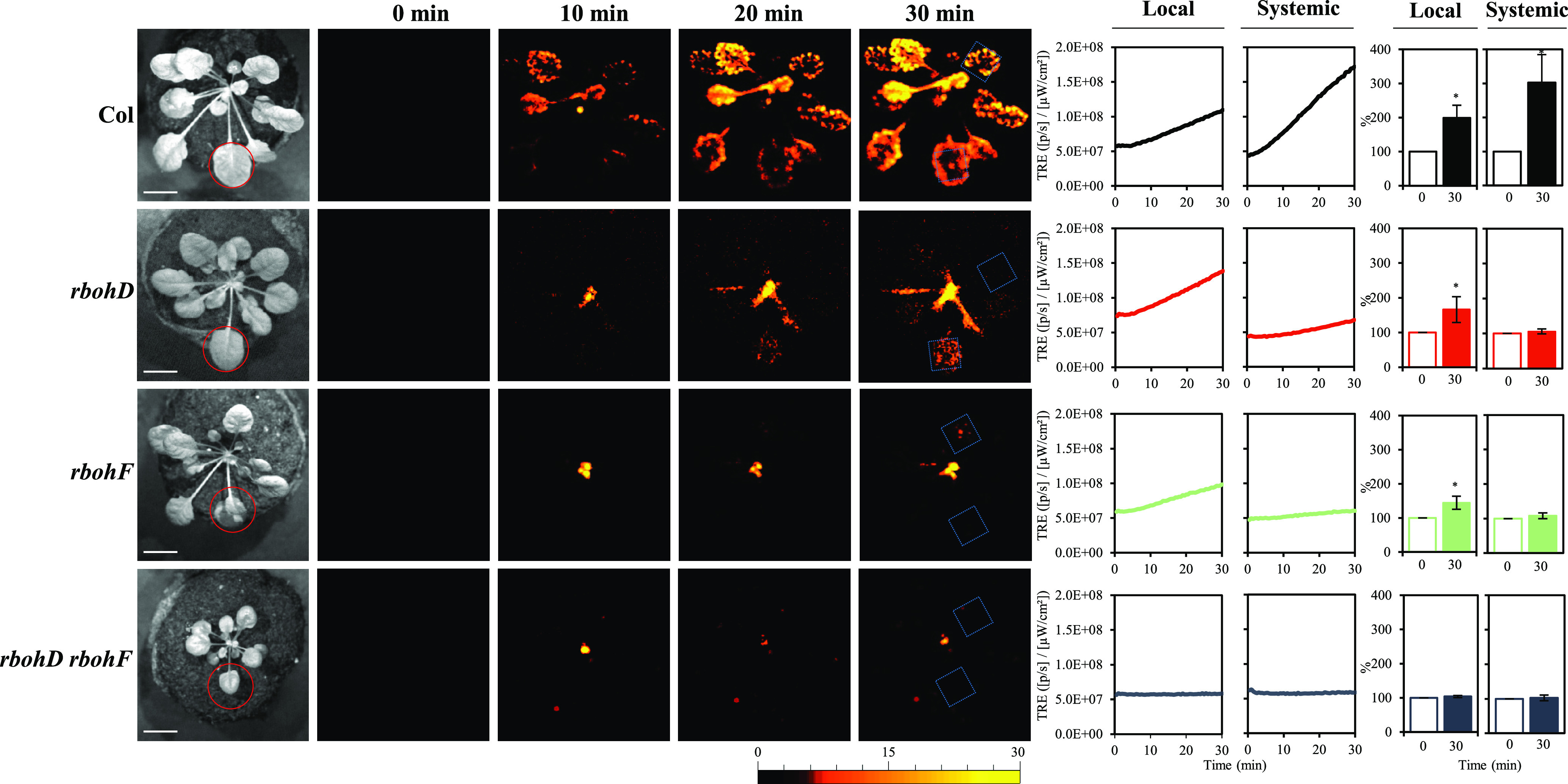
RBOHD and RBOHF Are Required for Mediating Systemic ROS Signaling in Response to a Local HL Stress Treatment.
Representative time-lapse images of whole-plant ROS levels (indicated by DCF oxidation) of the wild type (Columbia-0 [Col]), rbohD, rbohF, and rbohD robhF double mutant Arabidopsis plants subjected to a 2-min local HL stress treatment (applied to the local leaf only) are shown on left; representative line graphs showing continuous measurements of ROS levels in local and systemic leaves of the wild type, rbohD, rbohF, and rbohD robhF mutants, over the entire course of the experiment (0 to 30 min), are shown in the middle (ROIs for some of them are indicated with blue boxes); and statistical analysis of ROS levels in local and systemic leaves at 0 and 30 min is shown on right (conducted for 10 different plants from the wild type and each of the different mutants; Student’s t test, sd, n = 10, *P < 0.05). All experiments were repeated at least three times with similar results. Treated local leaves are indicated with a red circle. RBOHF, RESPIRATORY BURST OXIDASE HOMOLOG F; ROI, region of interest; TRE, total radiant efficiency.
Figure 5.
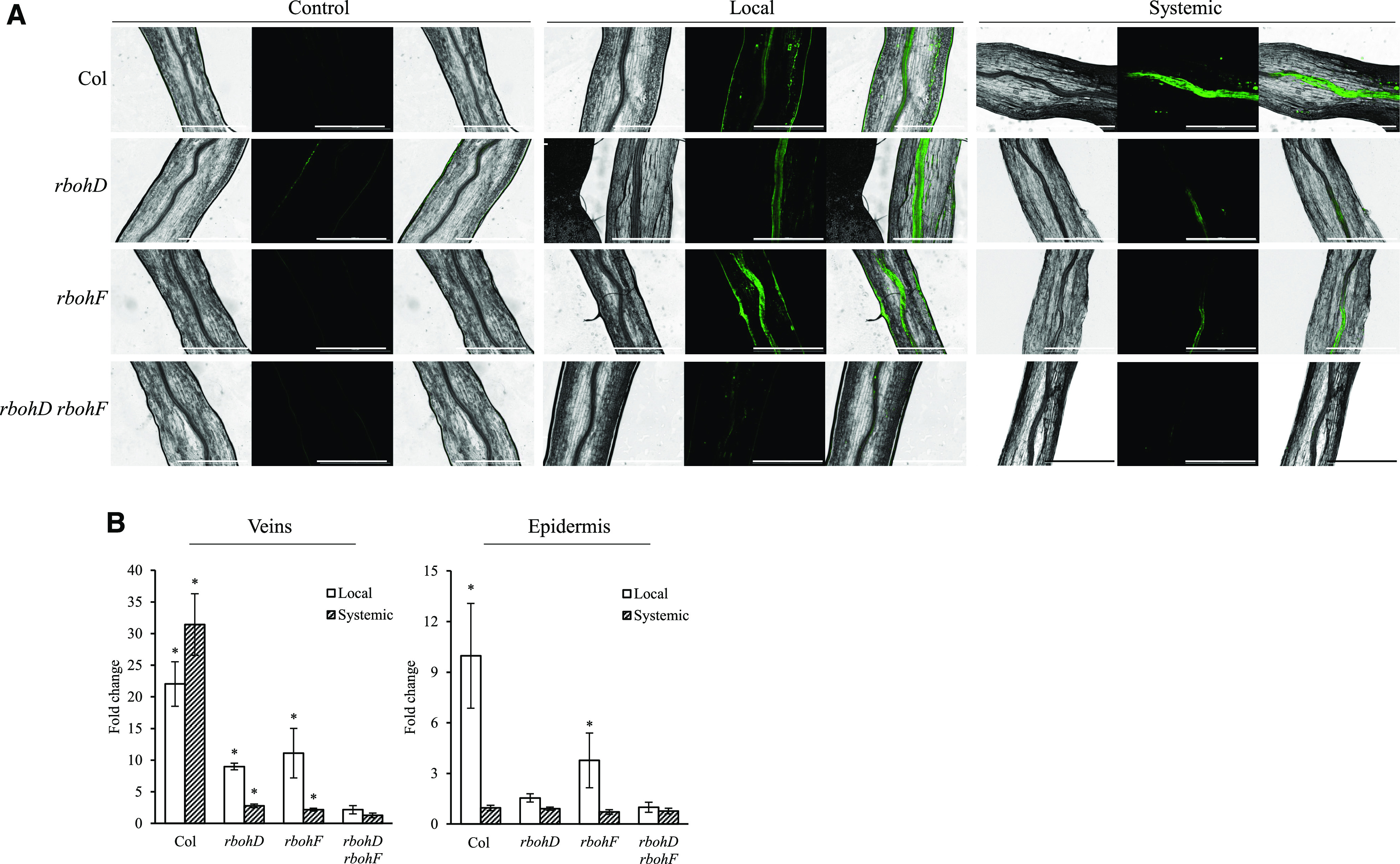
Detection of ROS in Petioles from Local and Systemic Leaves of the Wild-Type, rbohD, rbohF, and rbohD rbohF Double Mutant Plants Subjected to a 2-Min HL Stress Treatment (Applied to the Local Leaf Only).
(A) Representative fluorescence microscopy images of ROS detection in petioles of the wild type and the different mutants subjected to a 2-min local HL stress treatment. Bar = 1000 µm.
(B) Bar graph of fold change in fluorescent intensity in veins and epidermis of the wild type (Columbia-0 [Col]) and the different mutants subjected to a 2-min HL stress treatment (applied to the local leaf only; conducted for 10 different plants each from the wild type and the different rboh mutants; Student’s t test, sd, n = 10, *P < 0.05). RBOHF, RESPIRATORY BURST OXIDASE HOMOLOG F.
DISCUSSION
A perpetual cell-to-cell process of ROS-induced ROS production, that is, the ROS wave, propagates from a local tissue subjected to stress to the entire plant and is required for the induction of different acclimation transcripts, metabolites, and physiological responses in systemic tissues that results in SAA of systemic tissues to stress (Miller et al., 2009; Mittler et al., 2011; Choudhury et al., 2018; Devireddy et al., 2018, 2020; Kollist et al., 2019; Zandalinas et al., 2019, 2020; Fichman and Mittler, 2020). This process was originally found to depend on a single protein, RBOHD (Miller et al., 2009; Fichman et al., 2019). The ROS wave results in enhanced ROS production in almost all cells of the plant (Fichman et al., 2019), and this pattern is in agreement with the tissue-specific expression map of RBOHD (Hao et al., 2014; Morales et al., 2016), suggesting that RBOHD in all plant tissues is involved in ROS production during this process. However, our current findings that restoring RBOHD expression solely in the xylem parenchyma or phloem cells of the rbohD mutant is sufficient to restore systemic whole-plant ROS signaling (Figure 1; Supplemental Figure 2) strongly suggest that other ROS-producing pathways and mechanisms might be involved in this response. Indeed, as shown in Figures 4 and 5, RBOHF that is present in the rbohD mutant is also required for this response. Although the expression pattern of RBOHF is not identical to that of RBOHD, it still covers almost all aboveground plant tissues (Morales et al., 2016). It is therefore possible that, at least at the vascular bundles or veins of Arabidopsis, RBOHD and RBOHF cooperate in driving whole-plant ROS production during the systemic response to a local application of light stress. In the absence of one of them, ROS do not accumulate to high enough levels to trigger a whole-plant ROS signaling response (Figures 4 and 5), while in the presence of both, the ROS wave that propagates through the vascular bundles of plants can trigger a whole-plant ROS signaling response that results in the induction of systemic stress-response transcript accumulation and SAA (Figures 1 to 5). Of course, other ROS-producing mechanisms such as apoplastic oxidases and/or cytosolic, mitochondrial, peroxisomal, or chloroplastic ROS-producing mechanisms (Mittler, 2017; Kollist et al., 2019) could also participate in this process. RBOH-dependent ROS production in response to light stress was recently reported in bundle sheath strands of rice (Oryza sativa; Xiong et al., 2020), further supporting our findings that ROS-mediated signaling at the vascular bundles or veins of plants plays a key role in systemic responses to light stress (Figures 1 to 5).
The findings that restoring RBOHD expression in the xylem parenchyma or phloem is sufficient to restore whole-plant systemic ROS signaling further suggest that RBOHD function in these two cell types is complementary. Interestingly, the function of both GLR proteins GLR3.3 and GLR3.6 was previously shown to be required for the propagation of electric signals, the calcium wave, and potentially some ROS signal, but GLR3.3 is expressed in phloem cells, and GLR3.6 in xylem parenchyma cells (Mousavi et al., 2013; Nguyen et al., 2018; Toyota et al., 2018; Wang et al., 2019; Lew et al., 2020; Shao et al., 2020). Taken together, our results could suggest an interesting scenario in which RBOHD and/or RBOHF function is required for the coupling of the ROS, calcium, and electric waves (Choi et al., 2017; Fichman and Mittler, 2020) in at least one of these tissues, or both, functioning together with either GLR3.3 and/or GLR3.6 (Fichman et al., 2020). In support of a link between the ROS wave and electric signals are also our previous findings that the propagation of certain types of electric signals is suppressed in the rbohD mutant (Suzuki et al., 2013). Because hydraulic waves are also thought to propagate through the vascular bundles (Sade et al., 2014; Gilroy et al., 2016; Choi et al., 2017; Fichman and Mittler, 2020), our findings that RBOHD expression in xylem parenchyma or phloem is sufficient to restore whole-plant systemic ROS signaling could further suggest that the ROS, calcium, electric, and hydraulic waves are all integrated in these tissues.
A key role for bundle sheath cells in mediating excess light stress–induced systemic responses was previously reported in Arabidopsis, highlighting veins as important hubs for ABA and ROS signaling (Galvez-Valdivieso et al., 2009; Kangasjärvi et al., 2009; Fichman and Mittler, 2020). In support of this role, as well as the findings described in our current study (Figures 1 to 5), are our previous findings that systemic stomatal aperture closure responses occur faster the closer stomata are to leaf veins (Devireddy et al., 2020). The expression of RBOHD in vascular bundles may therefore drive ROS signaling in these cells, affecting the stomata closest to them first, during the systemic response to light stress (Devireddy et al., 2020). This spatial relationship could further be explained by an interaction between ABA signaling and ROS production occurring at the vascular bundles (Galvez-Valdivieso et al., 2009; Kangasjärvi et al., 2009; Devireddy et al., 2018, 2020). Interestingly, although bundle sheath cells were shown to play a key role in systemic ABA and ROS signaling in response to abiotic stress (Galvez-Valdivieso et al., 2009; Kangasjärvi et al., 2009), they were not found by our analysis to be essential for mediating the systemic ROS wave response (Figures 1 to 3). This finding could suggest that the role of bundle sheath cells in mediating systemic signaling is linked in Arabidopsis to that of phloem and/or xylem parenchyma cells and that these different cell layers could cooperate in the systemic response of plants to stress. Phloem and/or xylem parenchyma cells could therefore mediate the ROS wave, while bundle sheath cells could integrate it with ABA signaling.
Taken together, our findings reveal that in addition to Ca2+, electric, and hydraulic signals (Mousavi et al., 2013; Sade et al., 2014; Toyota et al., 2018; Shao et al., 2020), ROS signals are also mediated through the plant vascular system. The potential integration of all these rapid systemic signals (Gilroy et al., 2016; Choi et al., 2017; Fichman and Mittler, 2020; Fichman et al., 2020) could therefore occur at the vascular bundles of plants, underscoring the importance of these tissues in mediating plant acclimation to different abiotic stresses and other stimuli. Vascular bundles could therefore serve as the central rapid systemic signaling super highway of plants.
METHODS
Plant Material, Growth Conditions, and Constructs
Arabidopsis (Arabidopsis thaliana cv Columbia-0), rbohD, rbohF, and rbohD rbohF plants (confirmed transposon insertion mutants; Torres et al., 2002; Kwak et al., 2003; Miller et al., 2009) were grown in peat pellets (Jiffy-7, Jiffy; http://www.jiffygroup.com/) at 23°C under short-day growth conditions (10-h-light/14-h-dark cycle, 50 µmol m−2 s−1; Supplemental Figure 4). For complementing the rbohD mutant, promoter fragments representing between 579 to 3000 bp upstream to the 5′ of the start codon of CER6 (AT1G68530; Hooker et al., 2002; Mustroph et al., 2009), CAB3 (AT1G29910; Endo et al., 2007; Matías-Hernández et al., 2016), SCR (AT3G54220; Dhondt et al., 2010; Cui et al., 2014), XCP1 (AT4G35350; Funk et al., 2002; Avci et al., 2008), Sultr1;3 (AT1G22150; Yoshimoto et al., 2003; Sawa and Kay, 2011), and RBOHD (AT5G47910; Supplemental Table 1; Torres et al., 2002) were isolated by PCR from genomic DNA, sequenced, ligated upstream to the RBOHD gene, and cloned into pCAMBIA2301 vectors. Agrobacterium tumefaciens GV3101 was transformed with all constructs and used to generate transgenic homozygous rbohD plants. Transgenic homozygous rbohD plants expressing the luciferase reporter under the control of the Zat12 promoter were obtained as previously described by Miller et al. (2009) and complemented with the different vectors for RBOHD expression as described above. A third set of vectors for expressing RBOHD fused in frame to GFP (N-terminal fusion) under the control of the different promoters described above were also generated and characterized. Homozygous lines were obtained for all constructs described above, and two to three different homozygous lines were used for all GFP localization, ROS imaging, transcript expression analyses using luciferase imaging, and SAA, as described below. At least two different independent homozygous lines were used for each assay and at least 10 different individual plants from each line were analyzed and averaged (for a total of at least 20 different plants per time point per treatment).
Light Stress, ROS, Luciferase and GFP Imaging, and Data Analysis
To image whole-plant ROS levels, plants were fumigated with 50 μM H2DCFDA (excitation/emission, 495 nm/517 nm; Millipore-Sigma; Ortega-Villasante et al., 2018) in 50 mM phosphate buffer, pH 7.4, containing 0.01% (v/v) Silwet L-77 (LEHLE seeds) using a portable mini nebulizer (Punasi Direct) for 30 min as previously described by Fichman et al. (2019) and Zandalinas et al. (2020). As a control for dye penetration, plants were fumigated with 50 μM H2DCFDA for 30 min, followed by fumigation with H2O2 as previously described in Supplemental Figures 3B and 3C, and by Fichman et al. (2019) and Zandalinas et al. (2020). In addition, plants were fumigated with 50 μM H2DCFDA for 30 min and then with 10 mM ascorbic acid or 5 mM glutathione for 10 min and then with 1 mM H2O2 for 5 min (Supplemental Figures 3B and 3C). For luciferase imaging, plants were sprayed with 1 mM luciferin (sodium salt; GOLD BioTechnology) solution according to Miller et al. (2009). Following H2DCFDA or luciferin application, local leaves were exposed to a light intensity of 1700 µmol m−2 s−1 for 2 min using a ColdVision fiber optic light-emitting diode light source (Schott; Supplemental Figure 4) as described by Devireddy et al. (2018) and Zandalinas et al. (2019, 2020). Imaging ROS accumulation (evident by DCF oxidation) and luciferase activity in response to a local treatment of light stress was conducted with an IVIS Lumina S5 platform using Living Image 4.7.2 software (PerkinElmer) as described by Miller et al. (2009), Fichman et al. (2019) and Zandalinas et al. (2020). Because of the relatively young age of plants used, the activity of luciferase controlled by the ZAT12 promoter was mainly detected at the edge of leaves (Miller et al., 2009; Fichman et al., 2019). GFP localization and DCF imaging were also performed by Lionheart FX fluorescence (BioTek) and TCS SP8 (Leica) multiphoton confocal microscopes. All experiments were repeated at least three times each with the wild type, rbohD, and two to three different transgenic rbohD complemented lines.
Systemic Acquired Acclimation Assays
A single leaf was pretreated for 15 min with a light intensity of 1700 µmol m−2 s−1. Plants were then incubated for 45 min under controlled conditions. Following the recovery period, a systemic leaf was exposed to a light intensity of 1700 µmol m−2 s−1 for 45 min. Systemic leaves were then analyzed for electrolyte leakage as described by Suzuki et al. (2013), Devireddy et al. (2018), and Zandalinas et al. (2019, 2020). Control plants were untreated (Suzuki et al., 2013; Devireddy et al., 2018; Zandalinas et al., 2019, 2020).
Statistical Analyses
Results are presented as the mean ± sd. Statistical analyses were performed by two-tailed Student’s t test (asterisks denote statistical significance at P < 0.05 with respect to controls; Supplemental Table 2; Suzuki et al., 2013; Choudhury et al., 2018; Devireddy et al., 2018; Fichman et al., 2019; Zandalinas et al., 2019, 2020).
Supplemental Data
Supplemental Figure 1. Representative images of tissue-specific RBOHD-GFP fusion protein expression in the rbohD mutant using different tissue-specific promoters (supports Figures 1 to 3).
Supplemental Figure 2. Complementation of light stress-induced local and systemic ROS signaling in the rbohD mutant with RBOHD driven by the phloem-specific promoter pSUC2 (supports Figures 1 to 3).
Supplemental Figure 3. Controls for systemic ROS signaling in the rbohD mutant in response to a local application of H2O2, H2DCFDA dye penetration, and ROS signal quenching by two different antioxidants (supports Figures 1 and 4).
Supplemental Figure 4. Light spectrum and intensities used for growing plants under control conditions and during HL treatments.
Supplemental Table 1. Primers used to amplify each tissue-specific promoter by PCR from genomic DNA.
Supplemental Table 2. P-values of statistical analysis.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
This work was supported by funding from the National Science Foundation (grants MCB-1936590, IOS-1932639, and IOS-1353886) and the University of Missouri. We apologize to all authors of papers not mentioned in this article due to space limitations.
AUTHOR CONTRIBUTIONS
S.I.Z. and Y.F. performed experiments and analyzed the data. R.M. and S.I.Z. designed experiments, analyzed the data, and wrote the article.
Footnotes
Articles can be viewed without a subscription.
References
- Avci U., Earl Petzold H., Ismail I.O., Beers E.P., Haigler C.H.(2008). Cysteine proteases XCP1 and XCP2 aid micro-autolysis within the intact central vacuole during xylogenesis in Arabidopsis roots. Plant J. 56: 303–315. [DOI] [PubMed] [Google Scholar]
- Basu D., Haswell E.S.(2017). Plant mechanosensitive ion channels: An ocean of possibilities. Curr. Opin. Plant Biol. 40: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W.G., Miller G., Wallace I., Harper J., Mittler R., Gilroy S.(2017). Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. Plant J. 90: 698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W.G., Toyota M., Kim S.-H., Hilleary R., Gilroy S.(2014). Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc. Natl. Acad. Sci. USA 111: 6497–6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury F.K., Devireddy A.R., Azad R.K., Shulaev V., Mittler R.(2018). Local and systemic metabolic responses during light-induced rapid systemic signaling. Plant Physiol. 178: 1461–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente-Moreno M.J., Gago J., Díaz-Vivancos P., Bernal A., Miedes E., Bresta P., Liakopoulos G., Fernie A.R., Hernández J.A., Flexas J.(2019). The apoplastic antioxidant system and altered cell wall dynamics influence mesophyll conductance and the rate of photosynthesis. Plant J. 99: 1031–1046. [DOI] [PubMed] [Google Scholar]
- Cui H., Kong D., Liu X., Hao Y.(2014). SCARECROW, SCR-LIKE 23 and SHORT-ROOT control bundle sheath cell fate and function in Arabidopsis thaliana. Plant J. 78: 319–327. [DOI] [PubMed] [Google Scholar]
- Davletova S., Schlauch K., Coutu J., Mittler R.(2005). The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 139: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devireddy A.R., Arbogast J., Mittler R.(2020). Coordinated and rapid whole-plant systemic stomatal responses. New Phytol. 225: 21–25. [DOI] [PubMed] [Google Scholar]
- Devireddy A.R., Zandalinas S.I., Gómez-Cadenas A., Blumwald E., Mittler R.(2018). Coordinating the overall stomatal response of plants: Rapid leaf-to-leaf communication during light stress. Sci. Signal. 11: 518. [DOI] [PubMed] [Google Scholar]
- Dhondt S., Coppens F., De Winter F., Swarup K., Merks R.M.H., Inzé D., Bennett M.J., Beemster G.T.S.(2010). SHORT-ROOT and SCARECROW regulate leaf growth in Arabidopsis by stimulating S-phase progression of the cell cycle. Plant Physiol. 154: 1183–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Mochizuki N., Suzuki T., Nagatani A.(2007). CRYPTOCHROME2 in vascular bundles regulates flowering in Arabidopsis. Plant Cell 19: 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M.J., Choi W.-G., Gilroy S., Morris R.J.(2016). A ROS-assisted calcium wave dependent on the AtRBOHD NADPH oxidase and TPC1 cation channel propagates the systemic response to salt stress. Plant Physiol. 171: 1771–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichman Y., Mittler R.(2020). Rapid systemic signaling during abiotic and biotic stresses: Is the ROS wave master of all trades? Plant J. 102: 887–896. [DOI] [PubMed] [Google Scholar]
- Fichman Y., Miller G., Mittler R.(2019). Whole-plant live imaging of reactive oxygen species. Mol. Plant 12: 1203–1210. [DOI] [PubMed] [Google Scholar]
- Fichman Y., Zandalinas S.I., Mittler R.(2020). Untangling the ties that bind different systemic signals in plants. Sci. Signal. 13: eabb9505. [DOI] [PubMed] [Google Scholar]
- Funk V., Kositsup B., Zhao C., Beers E.P.(2002). The Arabidopsis xylem peptidase XCP1 is a tracheary element vacuolar protein that may be a papain ortholog. Plant Physiol. 128: 84–94. [PMC free article] [PubMed] [Google Scholar]
- Galvez-Valdivieso G., Fryer M.J., Lawson T., Slattery K., Truman W., Smirnoff N., Asami T., Davies W.J., Jones A.M., Baker N.R., Mullineaux P.M.(2009). The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. Plant Cell 21: 2143–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García G., Clemente-Moreno M.J., Díaz-Vivancos P., García M., Hernández J.A.(2020). The apoplastic and symplastic antioxidant system in onion: Response to long-term salt stress. Antioxidants 9: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S., Białasek M., Suzuki N., Górecka M., Devireddy A.R., Karpiński S., Mittler R.(2016). ROS, calcium, and electric signals: Key mediators of rapid systemic signaling in plants. Plant Physiol. 171: 1606–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao H., Fan L., Chen T., Li R., Li X., He Q., Botella M.A., Lin J.(2014). Clathrin and membrane microdomains cooperatively regulate RbohD dynamics and activity in Arabidopsis. Plant Cell 26: 1729–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker T.S., Millar A.A., Kunst L.(2002). Significance of the expression of the CER6 condensing enzyme for cuticular wax production in Arabidopsis. Plant Physiol. 129: 1568–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida A., Kazuoka T., Torikai S., Kikuchi H., Oeda K.(2000). A zinc finger protein RHL41 mediates the light acclimatization response in Arabidopsis. Plant J. 24: 191–203. [DOI] [PubMed] [Google Scholar]
- Kadota Y., Shirasu K., Zipfel C.(2015). Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol. 56: 1472–1480. [DOI] [PubMed] [Google Scholar]
- Kangasjärvi S., Nurmi M., Tikkanen M., Aro E.M.E., Kangasjarvi S., Nurmi M., Tikkanen M., Aro E.M.E.(2009). Cell-specific mechanisms and systemic signalling as emerging themes in light acclimation of C3 plants. Plant Cell Environ. 32: 1230–1240. [DOI] [PubMed] [Google Scholar]
- Kollist H., Zandalinas S.I., Sengupta S., Nuhkat M., Kangasjärvi J., Mittler R.(2019). Rapid responses to abiotic stress: Priming the landscape for the signal transduction network. Trends Plant Sci. 24: 25–37. [DOI] [PubMed] [Google Scholar]
- Kwak J.M., Mori I.C., Pei Z.M., Leonhardt N., Torres M.A., Dangl J.L., Bloom R.E., Bodde S., Jones J.D., Schroeder J.I.(2003). NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22: 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew T.T.S., et al. (2020). Real-time detection of wound-induced H2O2 signalling waves in plants with optical nanosensors. Nat. Plants 6: 404–415. [DOI] [PubMed] [Google Scholar]
- Liu L., Zhang J., Adrian J., Gissot L., Coupland G., Yu D., Turck F.(2014). Elevated levels of MYB30 in the phloem accelerate flowering in Arabidopsis through the regulation of FLOWERING LOCUS T. PLoS One 9: e89799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matías-Hernández L., Aguilar-Jaramillo A.E., Osnato M., Weinstain R., Shani E., Suárez-López P., Pelaz S.(2016). TEMPRANILLO reveals the mesophyll as crucial for epidermal trichome formation. Plant Physiol. 170: 1624–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Schlauch K., Tam R., Cortes D., Torres M.A., Shulaev V., Dangl J.L., Mittler R.(2009). The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2: ra45. [DOI] [PubMed] [Google Scholar]
- Mittler R.(2017). ROS are good. Trends Plant Sci. 22: 11–19. [DOI] [PubMed] [Google Scholar]
- Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V.B., Vandepoele K., Gollery M., Shulaev V., Van Breusegem F.(2011). ROS signaling: The new wave? Trends Plant Sci. 16: 300–309. [DOI] [PubMed] [Google Scholar]
- Morales J., Kadota Y., Zipfel C., Molina A., Torres M.A.(2016). The Arabidopsis NADPH oxidases RbohD and RbohF display differential expression patterns and contributions during plant immunity. J. Exp. Bot. 67: 1663–1676. [DOI] [PubMed] [Google Scholar]
- Mousavi S.A.R.R., Chauvin A., Pascaud F., Kellenberger S., Farmer E.E.(2013). GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500: 422–426. [DOI] [PubMed] [Google Scholar]
- Mustroph A., Zanetti M.E., Jang C.J.H., Holtan H.E., Repetti P.P., Galbraith D.W., Girke T., Bailey-Serres J.(2009). Profiling translatomes of discrete cell populations resolves altered cellular priorities during hypoxia in Arabidopsis. Proc. Natl. Acad. Sci. USA 106: 18843–18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C.T., Kurenda A., Stolz S., Chételat A., Farmer E.E.(2018). Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proc. Natl. Acad. Sci. USA 115: 10178–10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Villasante C., Burén S., Blázquez-Castro A., Barón-Sola Á., Hernández L.E.(2018). Fluorescent in vivo imaging of reactive oxygen species and redox potential in plants. Free Radic. Biol. Med. 122: 202–220. [DOI] [PubMed] [Google Scholar]
- Rodrigues O., Reshetnyak G., Grondin A., Saijo Y., Leonhardt N., Maurel C., Verdoucq L.(2017). Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc. Natl. Acad. Sci. USA 114: 9200–9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade N., Shatil-Cohen A., Attia Z., Maurel C., Boursiac Y., Kelly G., Granot D., Yaaran A., Lerner S., Moshelion M.(2014). The role of plasma membrane aquaporins in regulating the bundle sheath-mesophyll continuum and leaf hydraulics. Plant Physiol. 166: 1609–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa M., Kay S.A.(2011). GIGANTEA directly activates Flowering Locus T in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 108: 11698–11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Q., Gao Q., Lhamo D., Zhang H., Luan S.(2020). Two glutamate- and pH-regulated Ca2+ channels are required for systemic wound signaling in Arabidopsis. Sci. Signal. 13: eaba1453. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Miller G., Morales J., Shulaev V., Torres M.A., Mittler R.(2011). Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 14: 691–699. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Miller G., Salazar C., Mondal H.A., Shulaev E., Cortes D.F., Shuman J.L., Luo X., Shah J., Schlauch K., Shulaev V., Mittler R.(2013). Temporal-spatial interaction between reactive oxygen species and abscisic acid regulates rapid systemic acclimation in plants. Plant Cell 25: 3553–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szechyńska-Hebda M., Kruk J., Górecka M., Karpińska B., Karpiński S.(2010). Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell 22: 2201–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi F., Suzuki T., Osakabe Y., Betsuyaku S., Kondo Y., Dohmae N., Fukuda H., Yamaguchi-Shinozaki K., Shinozaki K.(2018). A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 556: 235–238. [DOI] [PubMed] [Google Scholar]
- Torres M.A., Dangl J.L., Jones J.D.G.(2002). Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 99: 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyota M., Spencer D., Sawai-Toyota S., Jiaqi W., Zhang T., Koo A.J., Howe G.A., Gilroy S.(2018). Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361: 1112–1115. [DOI] [PubMed] [Google Scholar]
- Wang G., et al. (2019). Systemic root-shoot signaling drives jasmonate-based root defense against nematodes. Curr. Biol. 29: 3430–3438. [DOI] [PubMed] [Google Scholar]
- Wudick M.M., Portes M.T., Michard E., Rosas-Santiago P., Lizzio M.A., Nunes C.O., Campos C., Santa Cruz Damineli D., Carvalho J.C., Lima P.T., Pantoja O., Feijó J.A.(2018). CORNICHON sorting and regulation of GLR channels underlie pollen tube Ca2+ homeostasis. Science 360: 533–536. [DOI] [PubMed] [Google Scholar]
- Xiong H., Hua L., Reyna-Llorens I., Shi Y., Chen K.-M., Smirnoff N., Kromdijk J., Hibberd J.(2020). The rice bundle sheath produces reactive oxygen species during high light stress via NADPH oxidase. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto N., Inoue E., Saito K., Yamaya T., Takahashi H.(2003). Phloem-localizing sulfate transporter, Sultr1;3, mediates re-distribution of sulfur from source to sink organs in Arabidopsis. Plant Physiol. 131: 1511–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas S.I., Fichman Y., Devireddy A.R., Sengupta S., Azad R.K., Mittler R.(2020). Systemic signaling during abiotic stress combination in plants. Proc. Natl. Acad. Sci. USA 117: 13810–13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandalinas S.I., Mittler R.(2018). ROS-induced ROS release in plant and animal cells. Free Radic. Biol. Med. 122: 21–27. [DOI] [PubMed] [Google Scholar]
- Zandalinas S.I., Sengupta S., Burks D., Azad R.K., Mittler R.(2019). Identification and characterization of a core set of ROS wave-associated transcripts involved in the systemic acquired acclimation response of Arabidopsis to excess light. Plant J. 98: 126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]