NIGT1.2 plays an important role in modulating low-Pi-dependent uptake of phosphate and nitrate, tending towards maintenance of the phosphorus to nitrogen balance in plants during Pi starvation.
Abstract
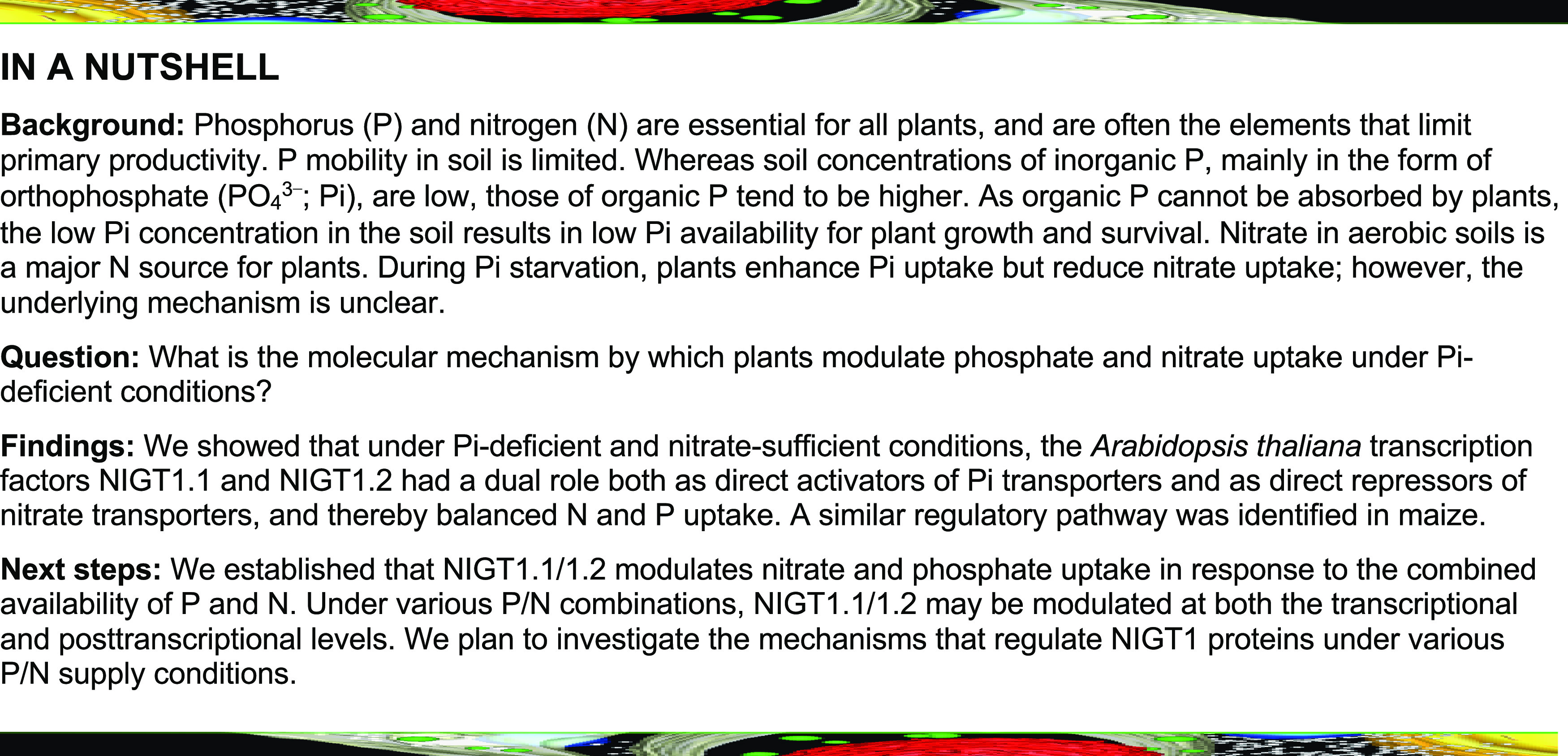
Phosphorus and nitrogen are essential macronutrients for plant growth and crop production. During phosphate (Pi) starvation, plants enhanced Pi but reduced nitrate (NO3−) uptake capacity, and the mechanism is unclear. Here, we show that a GARP-type transcription factor NITRATE-INDUCIBLE, GARP-TYPE TRANSCRIPTIOANL REPRESSOR1.2 (NIGT1.2) coordinately modulates Pi and NO3− uptake in response to Pi starvation. Overexpression of NIGT1.2 increased Pi uptake capacity but decreased NO3− uptake capacity in Arabidopsis (Arabidopsis thaliana). Furthermore, the nigt1.1 nigt1.2 double mutant displayed reduced Pi uptake but enhanced NO3− uptake under low-Pi stress. During Pi starvation, NIGT1.2 directly up-regulated the transcription of the Pi transporter genes PHOSPHATE TRANSPORTER1;1 (PHT1;1) and PHOSPHATE TRANSPORTER1;4 (PHT1;4) and down-regulated expression of NO3− transporter gene NITRATE TRANSPORTER1.1 (NRT1.1) by binding to cis-elements in their promoters. Further genetic assays demonstrated that PHT1;1, PHT1;4, and NRT1.1 were genetically epistatic to NIGT1.2. We also identified similar regulatory pathway in maize (Zea mays). These data demonstrate that the transcription factor NIGT1.2 plays a central role in modulating low-Pi-dependent uptake of Pi and NO3−, tending toward maintenance of the phosphorus to nitrogen balance in plants during Pi starvation.
Introduction
Phosphorus (P) is a macronutrient that is essential for plant growth and crop production and is an important component of the fertilizers used to sustain modern agriculture. Approximately 50 million tons of P fertilizer is required annually for crop production worldwide, but crops assimilate no more than 30% of P fertilizer (Good and Beatty, 2011; López-Arredondo et al., 2014). Phosphate (Pi) is the least available nutrient in fertilizer because it is highly immobile in soil and easily bound to oxides and hydroxides of Fe3+ and Al3+ or converted to organic matter by microorganisms (Marschner and Rimmington, 1988; Raghothama, 1999; López-Arredondo et al., 2014). As a result, ∼70% of cultivated land worldwide is deficient in plant-available Pi (Hinsinger, 2001; Kirkby and Johnston, 2008; López-Arredondo et al., 2014; Nguyen et al., 2015).
To maintain their growth under low-Pi stress, plants have evolved various strategies that overcome limited Pi availability. During Pi starvation, plants increase Pi uptake through alteration of root architecture and function (Péret et al., 2011; Liang et al., 2014; López-Arredondo et al., 2014), increases in phosphatase activity (López-Arredondo et al., 2014), and secretion of organic acids (Liang et al., 2014; López-Arredondo et al., 2014).
Nitrogen (N) is another macronutrient essential for plant growth, and nitrate in aerobic soils is a major N source for plants (Liu et al., 2017). Pi and NO3− acquisition in plants are interacting processes (Kant et al., 2011). Transcriptional profiling of Arabidopsis (Arabidopsis thaliana) and maize (Zea mays) have shown that P-deprived plants undergo substantial changes in the expression of many genes involved in nitrogenmetabolism, reduction, and uptake (Wu et al., 2003; Morcuende et al., 2007; Schlüter et al., 2013). During Pi starvation, NO3− uptake is reduced in various plant species, such as tobacco (Nicotiana tabacum; Rufty et al., 1990), barley (Hordeum vulgare; Rufty et al., 1991), soybean (Glycine max; Rufty et al., 1993), maize (de Magalhaes et al., 1998), bean (Phaseolus vulgaris; Gniazdowska et al., 1999), tomato (Solanum lycopersicum; de Groot et al., 2003), and lupin (Lupinus luteus; Kleinert et al., 2014). Pi starvation also decreases the activity of nitrate reductase in bean (Phaseolus vulgaris; Gniazdowska and Rychter, 2000) and N fixation in Sesbania rostrata (Aono et al., 2001) and lupin (Kleinert et al., 2014). However, the interaction and balance between NO3− and Pi in plants during Pi starvation has not been well studied.
Arabidopsis NITRATE-INDUCIBLE, GARP-TYPE TRANSCRIPTIONAL REPRESSOR1.2 (NIGT1.2), also named HYPERSENSITIVITY TO LOW PHOSPHATE-ELICITED PRIMARY ROOT SHORTENING1 HOMOLOG2 (HHO2), is a myb-related transcription factor and is a homolog to NIGT1 primitively identified in rice (Oryza sativa; Sawaki et al., 2013). Arabidopsis NIGT1.2/HHO2 can modulate the growth of primary and lateral roots in response to Pi starvation (Nagarajan et al., 2016). Recently, four NIGT1s have been reported to be negative regulators in the Arabidopsis response to nitrogen starvation and directly repress transcription of high-affinity NO3− transporter genes NITRATE TRANSPORTER2.1 (NRT2.1) and NITRATE TRANSPORTER2.4 (NRT2.4; Kiba et al., 2018; Maeda et al., 2018). The NIGT1 genes are transcriptionally regulated by the transcription factor PHOSPHATE STARVATION RESPONSE 1 (PHR1; Maeda et al., 2018), a master regulator of the Arabidopsis Pi-starvation response (Bustos et al., 2010).
In this study, we established that Arabidopsis NIGT1.2 coordinately modulated Pi and NO3− uptake during Pi starvation. The transcription of NIGT1.2 was induced by low-Pi stress and NO3− in Arabidopsis. During Pi starvation, Arabidopsis NIGT1.2 bound to the promoters of the Pi transporter genes PHT1;1 and PHT1;4, which increased Pi uptake, and also to the promoter of the NO3− transporter gene NRT1.1, which repressed NO3− acquisition. Pi deficiency also reduced NO3− uptake capacity in maize, where ZmNIGT1.2, the maize homolog of AtNIGT1.2, modulated low-Pi-dependent NO3− acquisition by down-regulating the maize NO3− transporter gene ZmNPF2. These findings reveal that Pi deficiency results in the antagonistic acquisition of Pi and NO3− through a process modulated by the transcription factor NIGT1.2.
Results
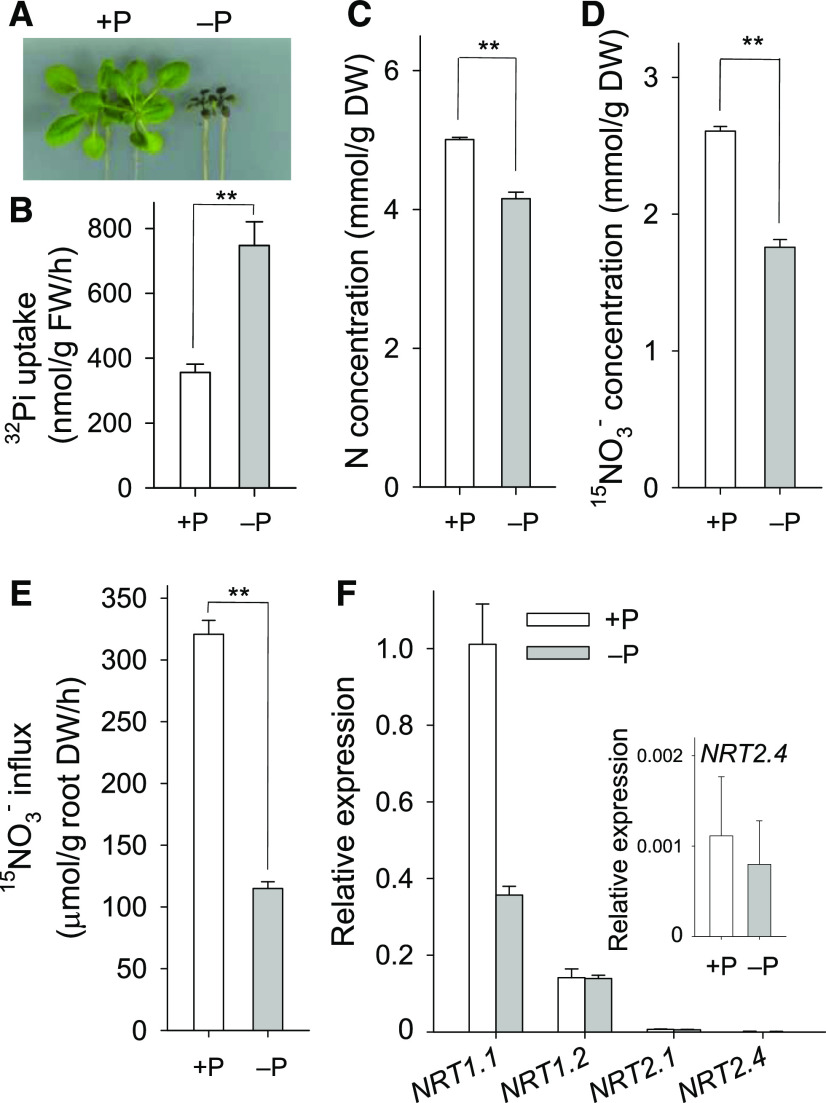
Pi Deficiency Enhances Pi and Represses NO3− Uptake in Arabidopsis
The Pi concentration in the soil solution is typically 10 μM or less, and plants often suffer Pi deficiency (Raghothama, 1999). A previous report showed that Pi and NO3− had an antagonistic interaction (Kant et al., 2011), and we wondered whether there was an interaction between Pi and NO3− uptake under low-Pi stress. When 7-d-old Arabidopsis seedlings were transferred to hydroponic medium with (+P) or without (−P) Pi for 14 d, the growth of Arabidopsis was impaired by Pi deficiency (Figure 1A), and the Pi uptake rate was significantly enhanced during Pi starvation (Figure 1B), similar to previous reports (Devaiah et al., 2007). Measurement of N-related physiological parameters showed that N concentration was lower in Arabidopsis seedlings grown under Pi-deficient conditions than in those grown under Pi-sufficient conditions (Figure 1C) and that Pi deficiency reduced Arabidopsis nitrate concentration (Figure 1D) and nitrate uptake (Figure 1E).
Figure 1.
Pi Deficiency Enhances Arabidopsis Phosphate Uptake and Represses Nitrate Uptake.
(A) Phenotypic comparison of Arabidopsis wild-type plants grown in hydroponic solution with (+P) or without (−P) Pi for 14 d.
(B) Pi (32P) uptake capacity was measured in 7-d-old Arabidopsis plants transferred to +P or −P hydroponic solution for 3 d. Data are shown as mean ± se (n = 3).
(C) N concentration of 7-d-old Arabidopsis transferred to +P or −P hydroponic solution for 14 d. Data are shown as mean ± se (n = 3).
(D) Nitrate concentration of 7-d-old Arabidopsis transferred to +P or −P hydroponic solution with 5 mM NO3− (5 atom% 15N) for 14 d. Data are shown as mean ± se (n = 3).
(E) Nitrate influx was measured in 7-d-old Arabidopsis transferred to MS (+P, 1.25 mM Pi) or low-Pi (−P, 10 μM Pi) medium for another 7 d. Data are shown as mean ± se (n = 4).
(F) RT-qPCR analysis of NRT1s and NRT2s during Pi starvation. Seven-day-old wild-type Arabidopsis seedlings were transferred to MS medium (+P, 1.25 mM Pi) or low-Pi (−P, 10 μM Pi) medium for 5 d, and then roots were harvested at the indicated time points for RNA extraction. Data are shown as mean ± se (n = 3). Asterisks in (B), (C), (D), and (E) indicate significant differences compared with wild-type plants (#) by Student’s t test: *P < 0.05; **P < 0.01.
The molecular mechanism of NO3− uptake in Arabidopsis is well characterized (Wang et al., 2012, 2018). Arabidopsis has two NO3− uptake systems: a high-affinity system and a low-affinity system. Two NO3− transporters, NRT1.2 (a low-affinity NO3− transporter) and NRT1.1 (a dual-affinity NO3− transporter), are involved in NO3− uptake under nitrate-sufficient conditions (Tsay et al., 1993; Huang et al., 1999; Liu et al., 1999; Wang et al., 2012). The NRT2 family members, such as NRT2.1 and NRT2.4, participate in high-affinity NO3− uptake (Wang et al., 2018). Measuring the transcripts of NRT1s and NRT2s under low-Pi stress indicated that NRT1.1 transcription was significantly repressed by low-Pi stress, whereas the transcripts of NRT1.2, NRT2.1, and NRT2.4 were not modulated by low-Pi stress (Figure 1F), suggesting that Pi deficiency represses Arabidopsis NO3− uptake via down-regulation of NRT1.1.
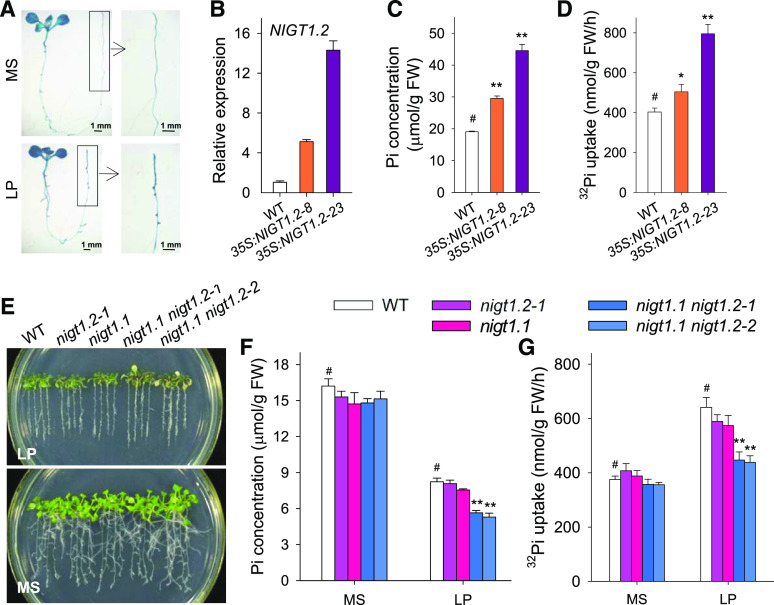
NIGT1.2 Positively Modulates Pi Uptake during Pi Starvation
A previous report demonstrated that Arabidopsis PHT1;1 and PHT1;4 are the main Pi transporters participating in Pi uptake in roots (Shin et al., 2004). During Pi starvation, the transcription of PHT1;1 and PHT1;4 was elevated (Shin et al., 2004), and the expression of NRT1.1 was repressed (Figure 1F). To screen for the transcription factor that directly modulates expression of PHT1;1 and/or NRT1.1, we conducted a yeast one-hybrid assay using the promoters of PHT1;1 and NRT1.1 with a high-throughput Arabidopsis transcription factor screening system (Ou et al., 2011). At1g68670, also named HHO2 (Nagarajan et al., 2016) or NIGT1.2 (Kiba et al., 2018; Maeda et al., 2018), was isolated using both the PHT1;1 promoter and NRT1.1 promoter, suggesting that NIGT1.2 directly modulates the expression of PHT1;1 and NRT1.1. As a transcription factor, the NIGT1.2 protein was exclusively localized in the nucleus (Supplemental Figure 1A; Kiba et al., 2018). Transcription of NIGT1.2 was induced by Pi deficiency and NO3− treatment (Supplemental Figure 1B), similar to a previous reportby Kiba et al. (2018). A β-glucuronidase (GUS) staining assay further confirmed that the transcription of NIGT1.2 was elevated under low-Pi stress (Figure 2A).
Figure 2.
NIGT1.2 Positively Modulates Pi Acquisition in Arabidopsis during Pi Starvation.
(A) GUS staining assay of the ProNIGT1.2:GUS transgenic line. Seven-day-old ProNIGT1.2:GUS seedlings were transferred to MS or low-Pi (LP) medium for 5 d and then stained for GUS.
(B) Analysis of NIGT1.2 expression using RT-qPCR in 10-d-old NIGT1.2-overexpressing and wild-type plants. Data are shown as mean ± se (n = 3).
(C) Measurement of Pi concentration. Seven-day-old seedlings were transferred to MS medium for 5 d and then harvested for Pi concentration measurement. Data are shown as mean ± se (n = 3).
(D) 32Pi uptake capacity was measured in 10-d-old seedlings germinated and grown on MS medium. Data are shown as mean ± se (n = 3).
(E) Phenotypic comparison of the nigt1.2, nigt1.1, nigt1.1 nigt1.2 double mutants, and wild-type seedlings during Pi starvation. Seven-day-old seedlings were transferred to LP or MS medium for another 7 d, and then photographs were taken.
(F) Pi concentration measurement in 7-d-old seedlings grown on MS or LP medium for 5 d. Data are shown as mean ± se (n = 4).
(G) 32Pi uptake capacity was measured in 7-d-old seedlings grown on MS or LP medium for 3 d. Data are shown as mean ± se (n = 4). Asterisks in (C), (D), (F), and (G) indicate significant differences compared with wild-type plants (#) by Student’s t test: *P < 0.05; **P < 0.01.
Given that NIGT1.2 was induced during Pi starvation (Figures 2A; Supplemental Figure 1B; Kiba et al., 2018; Maeda et al., 2018), we decided to generate NIGT1.2-overexpressing Arabidopsis lines for further assessment. Two NIGT1.2-overexpressing lines, 35S:NIGT1.2-8 and 35S:NIGT1.2-23, were selected for further study, because they showed gradated increases in NIGT1.2 expression (Figure 2B). Seedlings from both lines showed higher Pi concentration than wild-type seedlings, consistent with their NIGT1.2 expression levels (Figure 2C), suggesting that overexpression of NIGT1.2 increased Arabidopsis Pi concentration. Next, we analyzed the Pi uptake rate further by transferring 10-d-old seedlings into a Pi uptake solution containing 500 μM Pi supplemented with 32P orthophosphate and measuring the Pi uptake over a 6-h period. The NIGT1.2-overexpressing lines displayed a substantial increase in Pi uptake capacity compared with wild-type plants, and the increment was closely related to the NIGT1.2 expression level (Figure 2D). These data indicate that overexpression of NIGT1.2 enhances the Pi absorption in Arabidopsis.
To further confirm the function of NIGT1.2, we obtained three T-DNA insertion mutants of NIGT1.2 from the Arabidopsis Biological Resource Center (http://abrc.osu.edu), which we named nigt1.2-1, nigt1.2-2, and nigt1.2-3 (Supplemental Figure 2A). RT-qPCR analysis showed that transcription of NIGT1.2 was knocked down in three nigt1.2 mutants (Supplemental Figure 2B), whereas the Pi concentrations of the nigt1.2 mutants were similar to that of wild-type plants under both Pi-sufficient and Pi-deficient conditions (Supplemental Figure 2C).
The NIGT1.1/HHO3 was a homolog of NIGT1.2 in the Arabidopsis genome (Medici et al., 2015; Maeda et al., 2018), and the NIGT1.1 was also induced by low-Pi stress and NO3− (Supplemental Figure 3; Kiba et al., 2018; Maeda et al., 2018). We generated a nigt1.1 mutant and nigt1.1 nigt1.2 double mutant using CRISPR/Cas9 technology (Supplemental Figure 4). The nigt1.1 mutant displayed a similar phenotype as the nigt1.2 mutant and wild-type plants under low-Pi stress (Figure 2E) and showed a Pi concentration and Pi uptake rate similar to those of wild-type plants (Figures 2F and 2G). Interestingly, the nigt1.1 nigt1.2 double mutants displayed a low-Pi sensitive phenotype and significantly reduced Pi concentrations and Pi uptake capacity compared with wild-type plants under Pi-deficient conditions, and there were no obvious differences in Pi concentration or uptake between nigt1.1 nigt1.2 double mutants and wild-type plants when grown under Pi-sufficient conditions (Figures 2F and 2G). These data demonstrate that NIGT1.2 positively modulates Pi uptake during Pi starvation.
NIGT1.2 Directly Up-Regulates Pi Transporter Genes during Pi Starvation
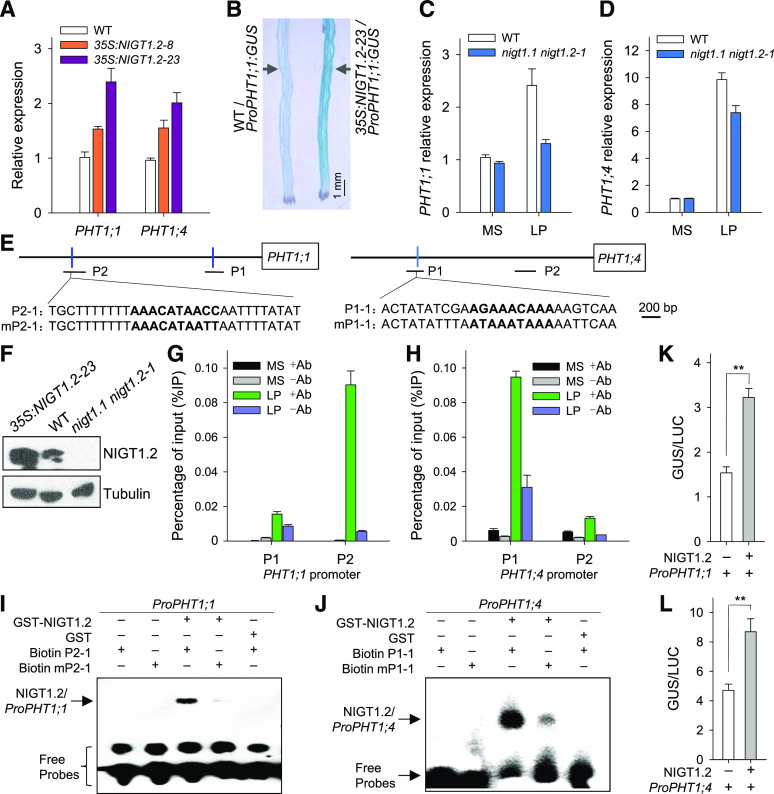
NIGT1.2 modulated Pi uptake in response to environmental Pi supply (Figure 2), and the PHT1;1 and PHT1;4 are the main transporters for Pi uptake in roots in both low- and high-Pi environments (Shin et al., 2004). We hypothesized that NIGT1.2 modulates Pi uptake by directly regulating expression of PHT1;1 and/or PHT1;4. RT-qPCR analysis showed that the transcription of both PHT1;1 and PHT1;4 was increased in the NIGT1.2-overexpressing lines, and the increments in the levels of PHT1;1 and PHT1;4 were consistent with the NIGT1.2 expression levels in NIGT1.2-overexpressing lines (Figure 3A). In addition, when we crossed the ProPHT1;1:GUS transgenic line (Wang et al., 2014) with the wild-type plants and NIGT1.2-overexpressing line (35S:NIGT1.2-23), GUS staining was enhanced in the NIGT1.2-overexpressing line (Figure 3B), indicating that NIGT1.2 positively regulated transcription of PHT1;1. We also tested the expression of PHT1;1 and PHT1;4 in the nigt1.1 nigt1.2 double mutant and found that under Pi-sufficient conditions, there was no detectable difference between the nigt1.1 nigt1.2 double mutant and the wild-type plants. In contrast, under low-Pi stress, transcription of both PHT1;1 and PHT1;4 was significantly enhanced, and these low-Pi-dependent enhancements were repressed in the nigt1.1 nigt1.2 double mutant relative to wild-type plants (Figures 3C and 3D), suggesting that NIGT1.2 positively modulates PHT1;1 and PHT1;4 transcription under low-Pi stress.
Figure 3.
NIGT1.2 Directly Modulates Transcription of PHT1;1 and PHT1;4 in Response to Low-Pi Stress.
(A) RT-qPCR analysis of PHT1;1 and PHT1;4 in the roots of NIGT1.2-overexpressing lines and wild-type seedlings germinated and grown on MS medium for 10 d. Data are shown as mean ± se (n = 3).
(B) GUS staining showing expression pattern of PHT1;1 in roots of the NIGT1.2-overexpressing line and wild-type plants germinated and grown on MS medium for 7 d.
(C) and (D) RT-qPCR analysis of PHT1;1 (C) and PHT1;4 (D) in roots of 7-d-old nigt1.1 nigt1.2 double mutant and wild-type seedlings grown on MS or LP medium for 5 d. Data are shown as mean ± se (n = 3).
(E) Schematic representation of PHT1;1 and PHT1;4 promoter regions showing the relative positions of cis-regulatory elements (blue line). P1 and P2 indicate PCR fragments for ChIP-qPCR assay, and P2-1, mP2-1, P1-1, and mP1-1 are EMSA probes.
(F) Immunoblot analysis of NIGT1.2 protein using anti-NIGT1.2 antibody in NIGT1.2-overexpressing (35S:NIGT1.2-23), nigt1.1 nigt1.2 double mutant, and wild-type plants grown on MS medium. Tubulin was used as the loading control.
(G) and (H) ChIP-qPCR assay of NIGT1.2 binding to promoters of PHT1;1 (G) and PHT1;4 (H) in vivo. Seven-day-old wild-type seedlings were grown on MS or LP medium for 5 d, and then roots were harvested for ChIP-qPCR assay using anti-NIGT1.2 antibody. Data are shown as mean ± se (n = 3).
(I) and (J) EMSA of recombinant NIGT1.2 binding to promoters of PHT1;1 (I) and PHT1;4 (J) in vitro.
(K) and (L) Transient overexpression of NIGT1.2 fused to ProPHT1;1:GUS (K) or ProPHT1;4:GUS (L) in N. benthamiana leaves. Data are shown as mean ± se (n = 5). Asterisks in (K) and (L) indicate significant differences compared with wild-type plants (#) by Student’s t test: *P < 0.05; **P < 0.01.
Previous reports indicate that the transcription factor HYPERSENSITIVITY TO LOW PI-ELICITED PRIMARY ROOT SHORTENING1 (HRS1), a homolog of NIGT1.2, can bind to the up-regulatory elements AGANNNAAA and AAACNNAACC (Medici et al., 2015). The cis-element analysis showed that the promoters of PHT1;1 and PHT1;4 contained two (AGANNNAAA and AAACNNAACC) and one (AGANNNAAA) of the up-regulatory cis-motifs, respectively (Figure 3E). We next conducted a chromatin immunoprecipitation (ChIP) assay to confirm that NIGT1.2 bound to the PHT1;1 and PHT1;4 promoters in plants. Seven-day-old wild-type seedlings were transferred to Murashige and Skoog (MS) or low Pi (LP, with 10 μM Pi) medium for 5 d, and then the roots were harvested for ChIP assay using anti-NIGT1.2 antibody (Figure 3F). Chromatin immunoprecipitated with anti-NIGT1.2 antibody was mainly enriched in the P2 fragment of the PHT1;1 promoter in plants grown under low-Pi stress, and no NIGT1.2 enrichment at either P1 or P2 of the PHT1;1 promoter was detected under Pi-sufficient conditions (Figure 3G). NIGT1.2 also bound to the P1 fragment of the PHT1;4 promoter during Pi starvation, but it did not bind to the PHT1;4 promoter at all under Pi-sufficient conditions (Figure 3H). These data indicate that NIGT1.2 bound to the promoters of PHT1;1 and PHT1;4 under Pi-deficient conditions, which is consistent with the expression changes of PHT1;1 and PHT1;4 in the nigt1.1 nigt1.2 double mutant during Pi starvation.
We next conducted an electrophoretic mobility shift assay (EMSA) to test whether NIGT1.2 bound to cis-elements within the PHT1;1 and PHT1;4 promoters. The NIGT1.2 recombinant protein produced an up-shift of the P2-1 probe on the P2 fragment of the PHT1;1 promoter, and when the cis-element in the P2-1 probe was mutated from AAACATAACC to AAACATAATT, this up-shift was almost abolished (Figure 3I). The NIGT1.2 recombinant protein also bound to the P1-1 probe of the PHT1;4 promoter (Figure 3J), and when the cis-element AGAAACAAA in the P1-1 of the PHT1;4 promoter was mutated, the signal of the up-shifted NIGT1.2-mP1-1 complex was repressed (Figure 3J). These data demonstrate that NIGT1.2 can bind to promoters of PHT1;1 and PHT1;4 in vitro and in vivo.
To further test the function of NIGT1.2 in the regulation of PHT1;1 and PHT1;4 expression, we performed a transient expression experiment in Nicotiana benthamiana leaves and found that NIGT1.2 up-regulated the activities of the PHT1;1 and PHT1;4 promoters (Figures 3K and 3L).
NIGT1.2 Negatively Modulates NO3− Uptake during Pi Starvation
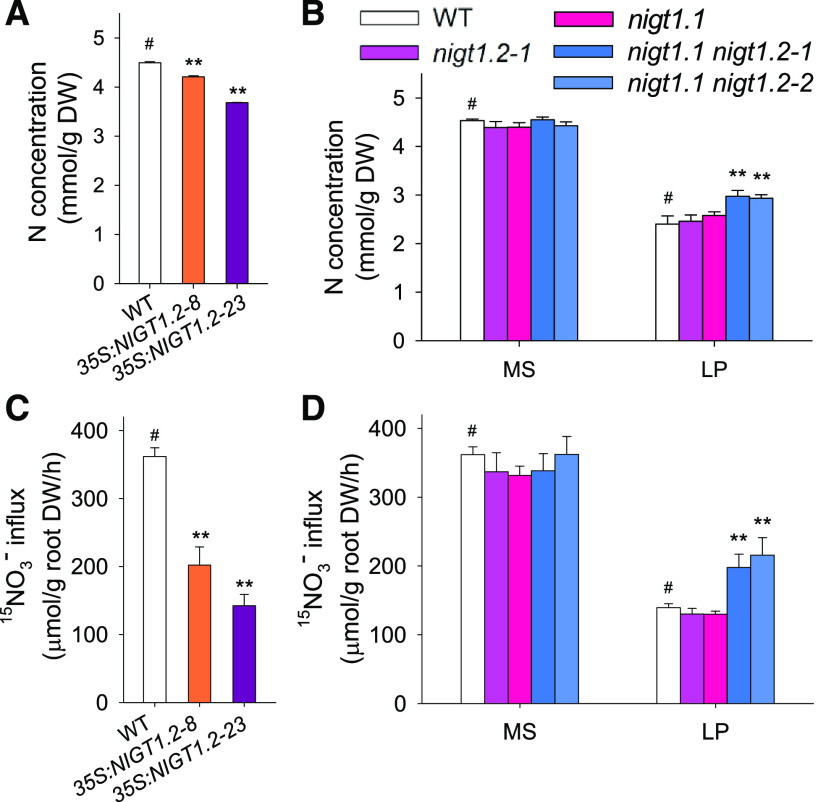
We further measured N-related physiological parameters in the NIGT1.2-overexpressing lines and nigt1.1 nigt1.2 double mutants under different Pi conditions. The N concentration was lower in NIGT1.2-overexpressing lines than in wild-type plants (Figure 4A). Moreover, the 35S:NIGT1.2-23 line, which had a higher transcript level of NIGT1.2 than 35S:NIGT1.2-8 (Figure 2B), also showed lower N concentration (Figure 4A). The nigt1.1 nigt1.2 double mutants displayed no obvious difference in N concentration as compared with wild-type plants when grown under Pi-sufficient conditions but showed a significantly increased N concentration under Pi-deficient conditions (Figure 4B). The N concentration of the nigt1.2-1 or nigt1.1 mutant was similar to that of wild-type plants under both Pi-sufficient and Pi-deficient conditions (Figure 4B).
Figure 4.
NIGT1.2 Reduces Arabidopsis Nitrate Influx under Low-Pi Stress.
(A) N concentration of 7-d-old NIGT1.2-overexpressing lines transferred to MS medium for another 7 d. Data are shown as mean ± se (n = 3).
(B) N concentration of 7-d-old nigt1.2-1, nigt1.1, and nigt1.1 nigt1.2 double mutants transferred to MS or LP medium for 7 d. Data are shown as mean ± se (n = 3).
(C) 15NO3− influx of 7-d-old NIGT1.2-overexpressing lines and wild-type plants transferred to MS medium for another 7 d. Data are shown as mean ± se (n = 4).
(D) 15NO3− influx of 7-d-old nigt1.2-1, nigt1.1, nigt1.1 nigt1.2 double mutant and wild-type plants transferred to MS or LP medium for 7 d. Data are shown as mean ± se (n = 3). Asterisks in (A), (B), (C), and (D) indicate significant differences compared with wild-type plants (#) by Student’s t test: *P < 0.05; **P < 0.01.
Next, we measured the NO3− influx. The NIGT1.2-overexpressing lines displayed decreased NO3− influx compared with wild-type plants under Pi-sufficient conditions, and this decrease of NO3− influx was negatively dependent on NIGT1.2 expression level (Figure 4C). The nigt1.1 nigt1.2 double mutant showed no difference in NO3− influx from wild-type plants under Pi-sufficient conditions but significantly elevated NO3− influx under Pi-deficient conditions (Figure 4D). These data demonstrate that NIGT1.2 inhibits NO3− influx under low-Pi stress.
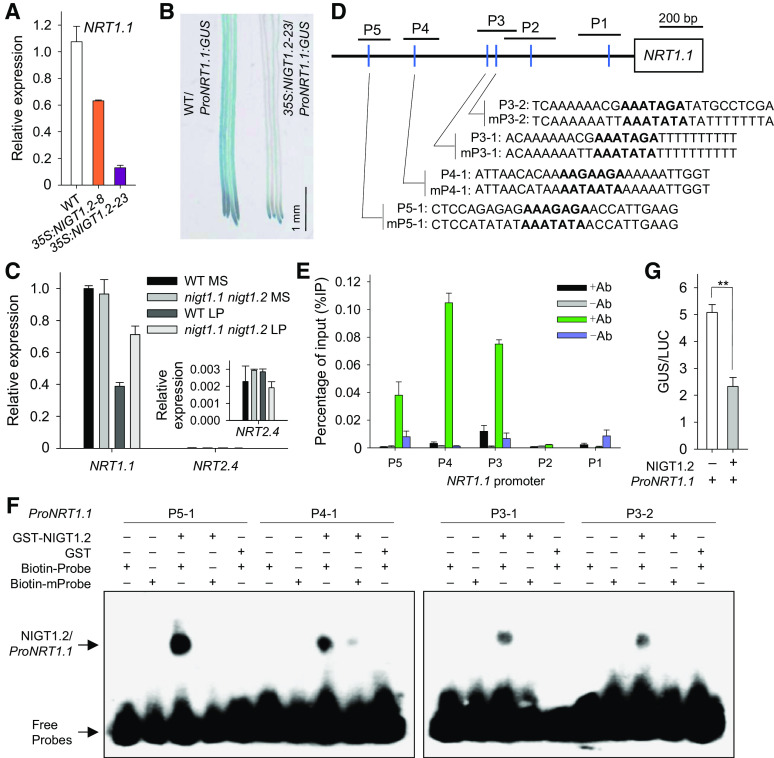
NIGT1.2 Directly Down-Regulates NRT1.1 during Pi Starvation
We then measured NRT1.1 expression in the NIGT1.2-overexpressing lines and the nigt1.1 nigt1.2 double mutant. The transcription of NRT1.1 was repressed in the NIGT1.2-overexpressing lines, and the degree of repression of NRT1.1 was consistent with the NIGT1.2 expression level (Figure 5A). We also crossed the ProNRT1.1:GUS transgenic line (Krouk et al., 2010) with wild-type plants and 35S:NIGT1.2-23, and GUS staining results showed that NIGT1.2 repressed the activity of the NRT1.1 promoter (Figure 5B). There was no difference in NRT1.1 expression between nigt1.1 nigt1.2 double mutant and wild-type plants under Pi-sufficient conditions (Figure 5C). During Pi starvation, however, the transcription of NRT1.1 was repressed significantly in the wild-type plants but much less in the nigt1.1 nigt1.2 double mutant (Figure 5C), suggesting that NIGT1.2 down-regulated transcription of NRT1.1 under low-Pi stress. There was no difference in NRT2.4 expression between nigt1.1 nigt1.2 double mutant and wild-type plants under Pi-sufficient or Pi-deficient conditions (Figure 5C).
Figure 5.
NIGT1.2 Directly Down-Regulates NRT1.1 Expression in Response to Low-Pi Stress.
(A) RT-qPCR analysis of NRT1.1 expression in roots of NIGT1.2-overexpressing lines and wild-type seedlings germinated and grown on MS medium for 10 d. Data are shown as mean ± se (n = 3).
(B) GUS staining showing the expression pattern of NRT1.1 in roots of NIGT1.2-overexpressing and wild-type plants germinated and grown on MS medium for 7 d.
(C) RT-qPCR analysis of NRT1.1 and NRT2.4 expression in roots of 7-d-old nigt1.1 nigt1.2 double mutant and wild-type seedlings grown on MS or LP medium for 5 d. Data are shown as mean ± se (n = 3).
(D) Schematic representation of NRT1.1 promoter showing relative position of cis-element (blue line). P1 to P5 indicate PCR fragments for ChIP-qPCR, and P3-1 to P5-1 are EMSA probes.
(E) ChIP-qPCR assay of NIGT1.2 binding to NRT1.1 promoter in vivo. Seven-day-old wild-type seedlings were transferred to MS or LP medium for 5 d, and then roots were harvested for ChIP-qPCR assay using anti-NIGT1.2 antibody. Data are shown as mean ± se (n = 3).
(F) EMSA of recombinant NIGT1.2 binding to NRT1.1 promoter in vitro.
(G) Transient overexpression of NIGT1.2 fused to ProNRT1.1:GUS in N. benthamiana leaves. Data are shown as mean ± se (n = 5). Asterisks indicate significant differences compared with wild-type plants (#) by Student’s t test: **P < 0.01.
The transcription factor HRS1, the homolog of NIGT1.2, also binds to the core down-regulatory cis-element AGA (Medici et al., 2015). There were six down-regulatory cis-elements within the NRT1.1 promoter (Figure 5D), and we hypothesized that NIGT1.2 down-regulated NRT1.1 expression by binding to its promoter. We therefore conducted a ChIP assay on the roots of 7-d-old wild-type seedlings grown with or without low-Pi stress. In samples from seedlings grown under Pi-sufficient conditions, no NIGT1.2 enrichment was detected at the NRT1.1 promoter fragments, P1 to P5, which contain one or two down-regulatory cis-regulatory elements; in contrast, in samples from Pi-deficient seedlings, the chromatin immunoprecipitated with anti-NIGT1.2 antibody was clearly enriched at the P5, P4, and P3 fragments of the NRT1.1 promoter (Figure 5E), indicating that NIGT1.2 can bind to the NRT1.1 promoter in vivo under low-Pi stress. We conducted the EMSA experiment to determine whether NIGT1.2 bound to down-regulatory cis-elements in these three fragments. The recombinant NIGT1.2 protein produced up-shifts for the P5-1, P4-1, P3-1, and P3-2 probes, each of which contains a cis-element, and when these cis-elements were mutated, the up-shift was virtually abolished (Figure 5F). These data demonstrate that NIGT1.2 binds to the NRT1.1 promoter in vitro and in vivo.
To further confirm that NIGT1.2 directly modulated NRT1.1 expression, we coexpressed ProNRT1.1:GUS with 35S:NIGT1.2 in N. benthamiana leaves. The NIGT1.2 significantly repressed NRT1.1 promoter activity, compared with that in plants expressing ProNRT1.1:GUS alone (Figure 5G).
Epistatic Relationship between NIGT1.2 and PHT1s or NRT1.1
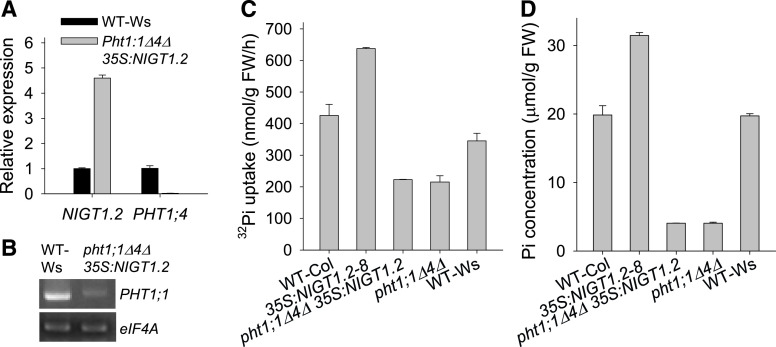
The NIGT1.2-overexpressing lines showed increased Pi uptake compared with wild-type plants (Figure 2), and NIGT1.2 positively regulated the transcription of PHT1;1 and PHT1;4 (Figure 3). The pht1;1Δ4Δ double mutant displayed a 75% reduction in Pi uptake capacity relative to wild-type plants, and the pht1;1Δ4Δ double mutant was in the Wassilewskija ecotype (Shin et al., 2004). To test the epistatic relationship between NIGT1.2 and PHT1;1/PHT1;4, we generated the pht1;1Δ4Δ 35S:NIGT1.2 line by overexpressing NIGT1.2 in the pht1;1Δ4Δ double mutant (Figures 6A and 6B). The NIGT1.2-overexpressing line (35S:NIGT1.2-8) had an increased Pi uptake capacity and Pi concentration relative to wild-type plants of the ecotype Columbia (Col), whereas the pht1;1Δ4Δ 35S:NIGT1.2 transgenic line displayed a reduced Pi uptake capacity and Pi concentration, similar to the pht1;1Δ4Δ double mutant (Figures 6C and 6D), indicating thatPHT1;1 and PHT1;4 were genetically epistatic to NIGT1.2.
Figure 6.
The pht1:1Δ4Δ 35S:NIGT1.2 Transgenic Line Shows a Similar Pi Uptake Capacity to the pht1:1Δ4Δ Double Mutant.
(A) RT-qPCR analysis of NIGT1.2 and PHT1;4 in the pht1:1Δ4Δ 35S:NIGT1.2 transgenic line. Data are shown as mean ± se (n = 3).
(B) RT-PCR analysis of PHT1;1 expression in the pht1:1Δ4Δ 35S:NIGT1.2 transgenic line.
(C) 32Pi uptake capacity of genotypes germinated and grown on MS medium for 10 d. Data are shown as mean ± se (n = 4).
(D) Pi concentration of 7-d-old genotypes transferred to MS medium for 5 d. Data are shown as mean ± se (n = 5).
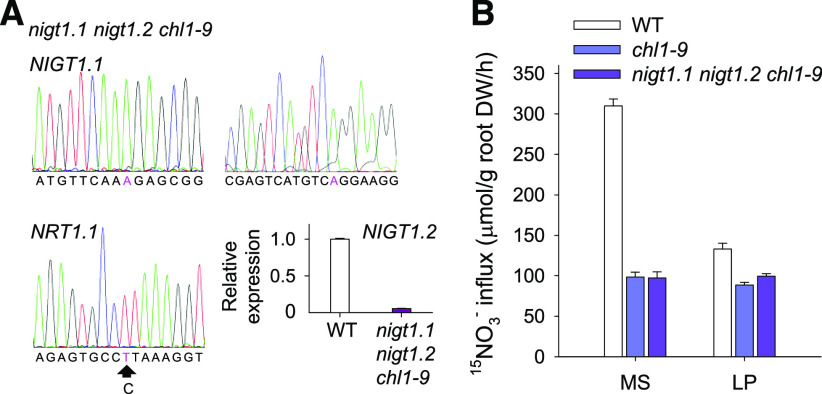
The molecular and biochemical assays showed that NIGT1.1/1.2 down-regulated NRT1.1 expression under low-Pi stress, and the nigt1.1 nigt1.2 double mutants displayed increased NO3− uptake rates and elevated expression of NRT1.1 relative to wild-type plants during Pi starvation (Figures 4 and 5). Then we generated the nigt1.1 nigt1.2 chl1-9 triple mutant (Figure 7A) by crossing the nigt1.1 nigt1.2 double mutant with chl1-9 mutant, which had a point mutation in NRT1.1 resulting from Leu replacing Pro492 and was defective in both high- and low-affinity NO3− uptake (Ho et al., 2009). The NO3− uptake of wild-type plants was significantly reduced under low-Pi stress compared with that under Pi-sufficient conditions, whereas the chl1-9 mutant showed a slightly reduced NO3− uptake under Pi-deficient conditions relative to that under Pi-sufficient conditions (Figure 7B), indicating that the NRT1.1 was a core NO3− transporter in response to Pi starvation. Disruption of NRT1.1 in the nigt1.1 nigt1.2 double mutant resulted in a decreased NO3− uptake, similar to the chl1-9 mutant, under Pi-sufficient or Pi-deficient conditions (Figure 7B). These data suggest that NIGT1.1/1.2 modulate low-Pi-dependent NO3− uptake by down-regulating NRT1.1 expression.
Figure 7.
The nigt1.1 nigt1.2 chl1-9 Triple Mutant Shows a Similar Nitrate Influx to the chl1-9 Mutant.
(A) The mutation of the nigt1.1 nigt1.2 chl1-9 triple mutant. The mutations in NIGT1.1 and NRT1.1 were evaluated by sequencing, and the mutant sites are indicated by pink letters. The expression of NIGT1.2 in the nigt1.1 nigt1.2 chl1-9 triple mutant was analyzed by RT-qPCR.
(B) 15NO3− influx of 7-d-old seedlings transferred to MS or LP medium for 7 d. Data are shown as mean ± se (n = 4).
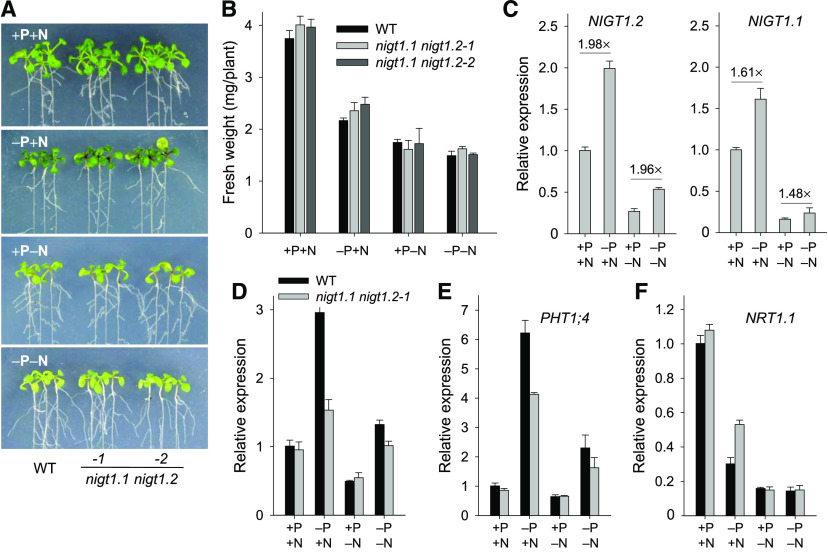
We also tested the phenotypes of the nigt1.1 nigt1.2 double mutant under N and P combinatorial conditions. As shown in Figure 8A, the nigt1.1 nigt1.2 double mutants were sensitive to low-Pi stress, with anthocyanin accumulation in leaves, compared with wild-type seedlings under −P+N conditions; when grown under +P−N conditions, the nigt1.1 nigt1.2 double mutants showed N-deficient phenotypes with yellow leaves, similar to wild-type seedlings; and when grown under −P−N conditions, both the nigt1.1 nigt1.2 double mutants and wild-type seedlings displayed N-deficient phenotypes, but not the Pi-deficient phenotypes. The biomasses of nigt1.1 nigt1.2 double mutants were similar to those of wild-type plants under N and P combinatorial conditions (Figure 8B). Further, we tested the transcription of NIGT1.1 and NIGT1.2 under N and P combinatorial conditions. The RT-qPCR results showed that the expression of NIGT1.1 and NIGT1.2 was induced by low-Pi stress (−P+N versus +P+N; −P−N versus +P−N), and these low-Pi inductions of NIGT1.1 and NIGT1.2 were independent of NO3− provision (Figure 8C). The transcription of PHT1;1 and PHT1;4 was increased under Pi-deficient conditions (−P+N versus +P+N; −P−N versus +P−N), and this increase was repressed in the nigt1.1 nigt1.2 double mutant, typically under −P+N conditions (Figures 8D and 8E). NRT1.1 expression was decreased in wild-type plants under −P+N conditions relative to that under +P+N conditions, and this decrease was weakened in the nigt1.1 nigt1.2 double mutants (Figure 8F). Interestingly, the repression of NRT1.1 by low-Pi stress was abolished in the nigt1.1 nigt1.2 double mutants and wild-type plants under −P−N conditions relative to +P−N conditions (Figure 8F), indicating that the transcriptional regulation of NRT1.1 was NO3− dependent.
Figure 8.
NIGT1.1 and NIGT1.2 Modulate the Transcription of NRT1.1 and PHT1s in Response to a Combination of P and N Availability.
(A) Phenotypes of the nigt1.1 nigt1.2 double mutants under N and P combinatorial conditions. Seven-day-old seedlings were transferred to MS medium (+P+N), without phosphate (−P+N), without nitrate (+P−N), or without phosphate and nitrate (−P−N) for 5 d, and then photographs were taken. The NH4+ in each medium was replaced with 0.5 mM ammonium succinate.
(B) Biomass analysis of the nigt1.1 nigt1.2 double mutants and wild-type plants under N and P combinatorial conditions. Data are shown as mean ± se (n = 3).
(C) RT-qPCR analysis of NIGT1.2 and NIGT1.1 in roots of 7-d-old wild-type Arabidopsis plants transferred to media with P/N combinations for 5 d. Data are shown as mean ± se (n = 3).
(D) to (F) RT-qPCR analysis of PHT1;1 (D), PHT1;4 (E), and NRT1.1 (F) in roots of 7-d-old nigt1.1 nigt1.2 double mutant and wild-type Arabidopsis plants transferred to media with various P/N combinations for 5 d. Data are shown as mean ± se (n = 3).
Identification of NIGT1.2-Modulated Genes under Low-Pi Stress
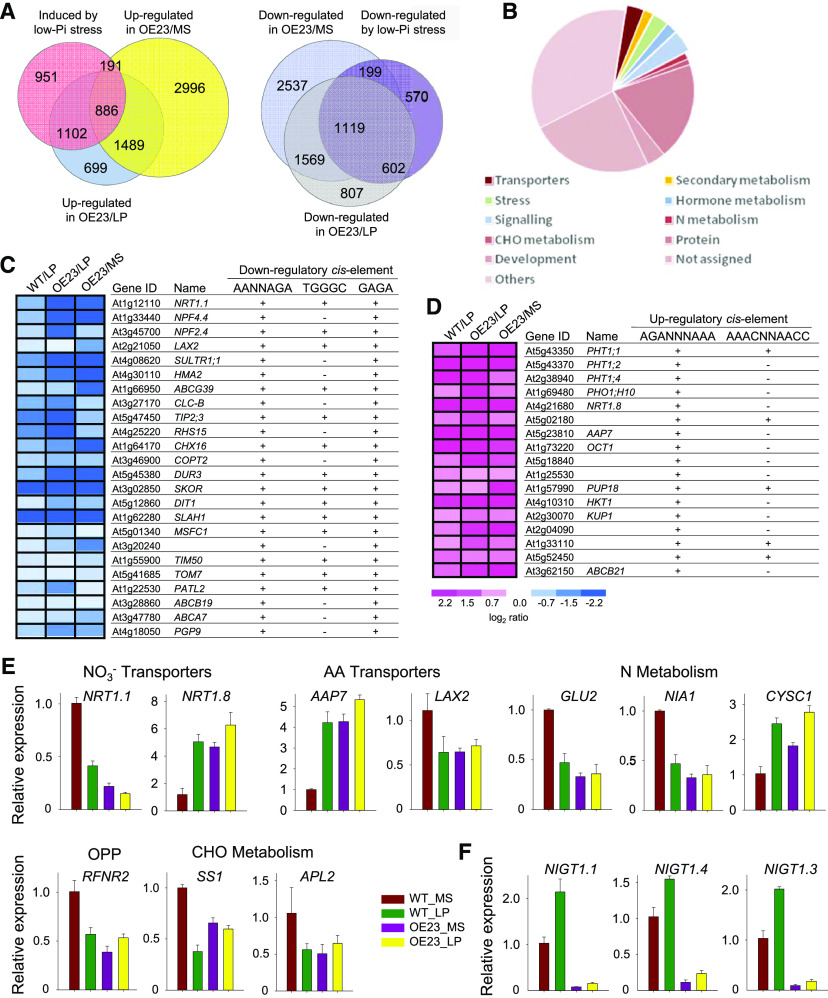
To investigate the genome-wide transcriptional control exerted by NIGT1.2 during Pi starvation, we conducted an RNA sequencing experiment using roots from 7-d-old plants of wild-type Arabidopsis and a NIGT1.2-overexpressing line (OE23) grown on either standard MS medium or LP medium for 5 d. We identified differentially expressed genes (DEGs) relating to low-Pi stress, i.e., with expression that differed between the Pi-sufficient (MS) and Pi-deficient (LP) groups of wild-type plants, based on P ≤ 0.05. We identified DEGs modulated by NIGT1.2, i.e., between the NIGT1.2-overexpressing line (OE23) and the wild-type plants, under Pi-sufficient conditions based on P ≤ 0.05. In wild-type roots, we identified 3130 genes that were up-regulated and 2490 that were down-regulated during Pi starvation, and among these, 2179 (69.6%) up-regulated and 1920 (77.1%) down-regulated genes were modulated by constitutive overexpression of NIGT1.2 (Figure 9A).
Figure 9.
Identification of Target Genes of NIGT1.2.
(A) Venn diagram showing overlaps between genes induced by low-Pi stress and genes modulated by NIGT1.2. Seven-day-old NIGT1.2-overexpressing seedlings (OE23) and wild-type seedlings were transferred to MS or LP medium for 5 d, and then the roots were harvested for RNA-seq analysis.
(B) MapMan functional categories for low-Pi- and NIGT1.2-regulated genes.
(C) and (D) Hierarchical clustering analysis of transporter genes that were regulated by low-Pi stress and NIGT1.2 simultaneously.
(E) RT-qPCR analysis of some N-related genes encoding various categories of proteins, arbitrarily selected from among the NIGT1.2-regulated genes, in the roots of 7-d-old OE23 and wild-type seedlings grown on MS or LP medium for 5 d. Data are shown as mean ± se (n = 3).
(F) RT-qPCR analysis of the three NIGT1.2 homologs NIGT1.1, NIGT1.4, and NIGT1.3 in the roots of 7-d-old OE23 and wild-type seedlings transferred to MS or LP medium for 5 d. Data are shown as mean ± se (n = 3).
Among the DEGs modulated by NIGT1.2 under low-Pi stress, NIGT1.2 modulated genes related to diverse key cellular and metabolic functions (Figure 9B). Remarkably, 41 transporter genes were modulated by low-Pi stress via NIGT1.2, of which 24 were down-regulated, and 17 were up-regulated (Figures 9C and 9D). These 41 transporter genes encoded Pi transporters, NO3− transporters, amino acid transporters, calcium transporters, sugar transporters, and peptide transporters, among others (Figures 9C and 9D), suggesting that NIGT1.2 modulates transmembrane transport during Pi starvation. Further promoter sequence analysis showed that all 24 down-regulated transporter genes contained two or three down-regulatory cis-elements in their ∼1500-bp promoters (Figure 9C), and all 17 up-regulated transporter genes had one or both up-regulatory cis-elements in their ∼1500-bp promoters (Figure 9D), suggesting that NIGT1.2 might directly regulate the transcription of these transporter genes.
Interestingly, the genes modulated by the Pi starvation response and NIGT1.2 were enriched for functional classes of genes involving NO3− and amino acid transporters, nitrogen metabolism, carbohydrate (CHO)-metabolism and signaling (Figure 9B). Further analysis of the RT-qPCR analysis results showed that N-related genes encoding proteins from several major functional classes, including NO3− and amino acid transporters, amino acid and nitrogen metabolism, oxidative pentose-phosphate pathway, and CHO metabolism, were transcriptionally regulated by low-Pi stress and NIGT1.2 (Figure 9E), suggesting that NIGT1.2 plays important roles in regulating low-Pi-coupled NO3− responses. The transcription of homologs of NIGT1.2, NIGT1.1, HRS1/NIGT1.4, and NIGT1.3 was induced by low-Pi stress (Figure 9F), similar to what has previously been reported by Kiba et al. (2018) and Maeda et al. (2018). Moreover, the expression of all three of these genes was clearly repressed in the NIGT1.2-overexpressing line (Figure 9F).
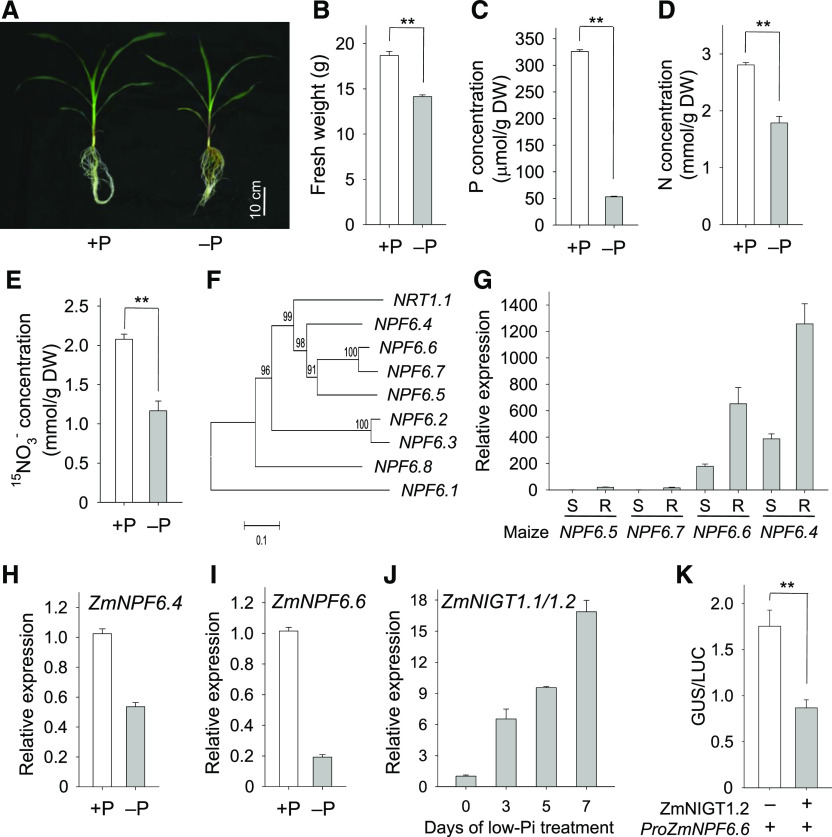
Pi Deficiency Represses NO3− Uptake in Maize
Maize (Zea mays) is an important crop predominantly cultivated in soils where NO3− is often the primary source of nitrogen available for growth (Wen et al., 2017). When 9-d-old plants of the maize hybrid line B73 were transferred to hydroponic medium with (+P) or without (–P) Pi for 11 d, their growth was repressed by Pi deficiency (Figures 10A and 10B). The P concentration was much lower in maize grown under Pi-deficient as compared with Pi-sufficient conditions (Figure 10C). Interestingly, the N concentration was also significantly lower in maize grown under Pi starvation as compared with Pi-sufficient conditions (Figure 10D), similar to what we observed in Arabidopsis (Figure 1). NO3− influx in maize was also reduced under Pi-deficient conditions (Figure 10E), suggesting that Pi deficiency decreased NO3− uptake in maize.
Figure 10.
Pi Deficiency Represses Maize Nitrate Influx.
(A) Phenotypic comparison of maize inbred B73 grown under Pi-sufficient and Pi-deficient conditions. Nine-day-old maize B73 plants were transferred to one-eighth modified Hoagland nutrient solution with (+P) or without Pi (−P) for 11 d.
(B) to (D) Biomass (B), phosphorus concentration (C), and nitrogen concentration (D) of 9-d-old maize B73 transferred to +P or −P solution for 11 d. Data are shown as mean ± se (n = 5).
(E) Nitrate concentration of 9-d-old maize B73 transferred to +P or −P solution with 5 mM NO3− (5 atom% 15N) nitrate for 11 d. Data are shown as mean ± se (n = 5).
(F) Neighbor-joining tree analysis was conducted using MEGA6.
(G) RT-qPCR analysis of maize nitrate transporter gene NPFs in shoots (S) and roots (R). Data are shown as mean ± se (n = 4).
(H) and (I) RT-qPCR analysis of ZmNPF6.4 (H) and ZmNPF6.6 (I) in roots of 9-d-old maize B73 seedlings transferred to +P or −P solution for 5 d.
(J) RT-qPCR analysis of ZmNIGT1.1/1.2 in roots of maize B73 during Pi starvation. Data are shown as mean ± se (n = 4).
(K) Transient expression of ZmNIGT1.2 fused to ProNPF2:GUS in N. benthamiana leaves. Data are shown as mean ± se (n = 5). Asterisks in (B) to (E) and (K) indicate significant differences compared with wild-type plants (#) by Student’s t test: **P < 0.01.
There are 79 members of the nitrate transporter/peptide transporter family (NRT1/PTR family, or NPF family) in maize B73, and eight members belong to NPF6 subfamily, named ZmNPF6.1 to ZmNPF6.8 (Figure 10F; Léran et al., 2014). Two NPF6 genes, ZmNPF6.4 and ZmNPF6.6, showed relatively high expression in roots (Figure 10G; maizeGDB, www.maizegdb.org). Their protein products ZmNPF6.4 and ZmNPF6.6, both homologs of Arabidopsis NRT1.1 (Figure 10F; Supplemental Data Set 1), function as NO3− transport proteins (Wen et al., 2017). ZmNPF6.4 displays a low-affinity NO3− transport activity in oocytes, and ZmNPF6.6 transports NO3− across a broad NO3− concentration range, including high- and low-affinity ranges (Wen et al., 2017). Because NO3− uptake was reduced in maize during Pi starvation (Figure 10E; de Magalhaes et al., 1998), we tested the transcription of ZmNPF6.4 and ZmNPF6.6 under Pi-deficient conditions. The RT-qPCR results showed that the transcription of both ZmNPF6.4 and ZmNPF6.6 was repressed in maize during Pi starvation (Figures 10H and 10I). There were several down-regulatory cis-elements (AANNAGA) in the ZmNPF6.4 and ZmNPF6.6 promoters, suggesting that ZmNPF6.4 and ZmNPF6.6 were transcriptionally regulated by the maize homolog of transcription factor AtNIGT1.2.
The maize genome contains two homologs of AtNIGT1.2, which we named ZmNIGT1.1 (Zm00001d023402) and ZmNIGT1.2 (Zm00001d023411), and these two ZmNIGT1 genes had 94.8% identity of coding sequence. The transcription of both genes was induced in maize roots during Pi starvation (Figure 10J). Further, ZmNIGT1.2 repressed the activity of the ZmNPF6.6 promoter during transient expression in N. benthamiana (Figure 10K), suggesting that ZmNIGT1.2 down-regulated the transcription of ZmNPF6.6.
Discussion
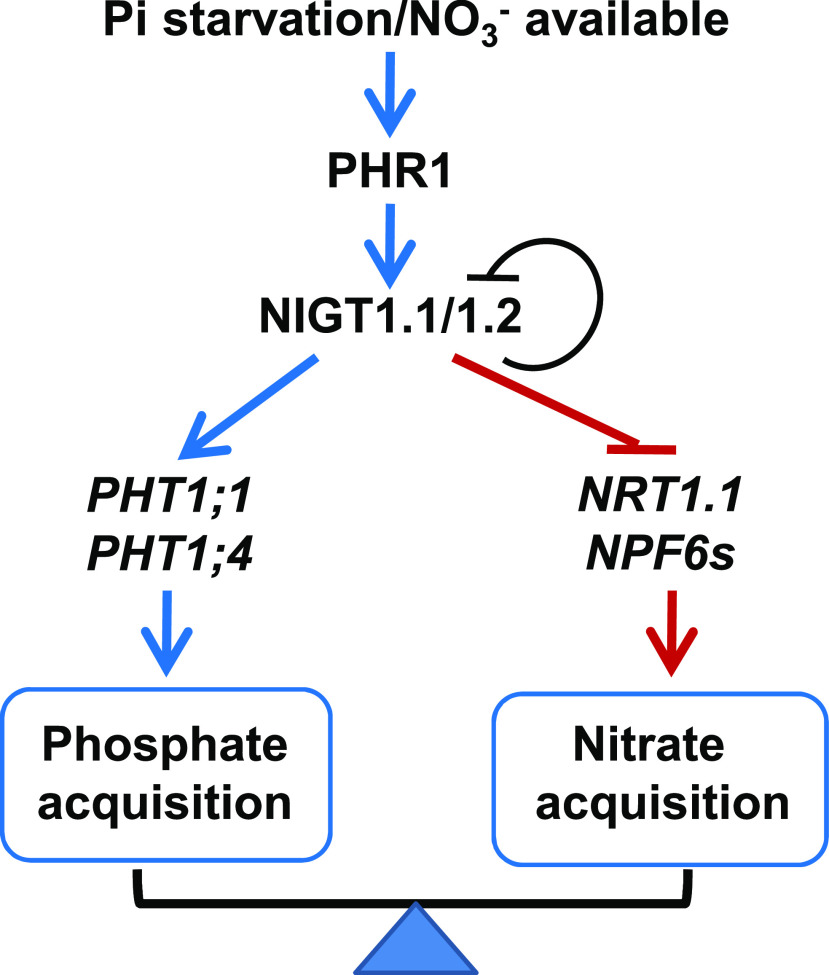
In this study, we uncovered a NIGT1.2- and NIGT1.1-dependent regulatory pathway mediating the antagonistic cross talk between Pi and NO3− uptake in plants under low-Pi stress. These findings allow us to propose a working model for low-Pi-stress-related Pi and NO3− uptake modulation by these two transcription factors (Figure 11).
Figure 11.
A Model of NIGT1.1/1.2-PHT1s/NRTs-Regulatory Pathway in Plant Regulating Phosphate and Nitrate Uptake.
Under Pi-deficient and NO3–-sufficient conditions, NIGT1.1 and NIGT1.2 have a dual role both as direct activators of Pi transporters and as direct repressors of NO3− transporters to balance N and P uptake.
As a transcription factor, NIGT1.2 was localized in the nucleus, and its transcription was induced during Pi starvation (Supplemental Figure 1; Kiba et al., 2018), which was modulated by the core transcription factor PHR1 (Bustos et al., 2010; Maeda et al., 2018). Overexpression of NIGT1.2 enhanced the Pi uptake capacity (Figure 2) but reduced NO3− uptake capacity (Figure 4) in Arabidopsis, and the nigt1.1 nigt1.2 double mutants displayed decreased Pi but increased NO3− uptake capacity under low-Pi stress (Figures 2 and 4), demonstrating that NIGT1.2 modulated the uptake of Pi and NO3− during Pi starvation. In Arabidopsis, PHT1;1 and PHT1;4 are the main Pi transporters functioning in Pi uptake under both Pi-sufficient and Pi-deficient conditions (Shin et al., 2004), and NRT1.1 functions as an important dual-affinity NO3− transporter involved in multiple phases of NO3− uptake (Liu et al., 1999; Wang et al., 2012). Pi starvation induced PHT1;1 and PHT1;4 transcription (Figures 3C and 3D; Shin et al., 2004) but repressed NRT1.1 expression (Figure 1F). EMSA and ChIP analysis demonstrated that during low-Pi stress, NIGT1.2 directly bound to the promoters of PHT1;1, PHT1;4, and NRT1.1, and RT-qPCR results showed that under low-Pi conditions, NIGT1.2 up-regulated the transcription of PHT1;1 and PHT1;4 but down-regulated that of NRT1.1 (Figures 3 and 5), indicating that NIGT1.2 modulated Pi-dependent antagonistic absorption of Pi and NO3− by directly regulating the transcription of PHT1;1, PHT1;4, and NRT1.1.
Kiba et al. (2018) found that besides the N starvation-responsive genes, NIGT1s also modulated the expression of SPX DOMAIN genes (SPXs) and PHOSPHATE2 (PHO2), which encode main regulators in response to Pi starvation (Liu et al., 2012; Puga et al., 2014). The transcription of SPX1, SPX4, and PHO2 was elevated in the NIGT1.2ox line under N sufficiency and decreased in the NIGT1.2ox under N starvation conditions, compared with wild-type Arabidopsis (Kiba et al., 2018). We then measured the transcription of SPXs and PHO2 in the nigt1.1 nigt1.2 double mutant under Pi starvation conditions. The expression of SPX1and SPX4 in the nigt1.1 nigt1.2 double mutant was similar to that in wild-type plants under either Pi-sufficient or Pi-deficient conditions (Supplemental Figures 5A and 5B). The PHO2 expression was slightly reduced in the nigt1.1 nigt1.2 double mutant relative to that in wild-type plants under Pi-sufficient conditions and similar between the nigt1.1 nigt1.2 double mutant and wild-type under Pi-deficient conditions (Supplemental Figure 5C). These data suggest that NIGT1.1/1.2 modulate the transcription of SPXs and PHO2 in response to N starvation, and to a lesser extent in response to P starvation.
In addition to our work in Arabidopsis, we also tested NO3− uptake in maize under low-Pi stress. N concentration and NO3− influx were reduced in maize under Pi-deficient conditions (Figure 10), as previously reported by de Magalhaes et al. (1998). ZmNPF6.4 and ZmNPF6.6, two homologs of ArabidopsisNRT1.1, showed NO3− transport activities under nitrate-sufficient conditions (Wen et al., 2017), and their transcription was repressed during Pi starvation (Figures 10H and 10I), indicating that Pi deficiency repressed maize NO3− absorption by repressing the transcription of NO3− transporter genes. ZmNIGT1.1 and ZmNIGT1.2, two homologs of AtNIGT1.2, were transcriptionally induced during Pi starvation and in turn repressed the promoter activity of ZmNPF2 in N. benthamiana assays (Figures 10J and 10K). These data indicate that the Pi-dependent NIGT1.1/1.2-NRT/NPF regulatory pathway exists in crop plants as well as in the model plant Arabidopsis.
Previous reports showed that NIGT1 proteins function as transcriptional repressors of the NRT2.1 and NRT2.4 promoters (Kiba et al., 2018; Maeda et al., 2018). In this work, NIGT1.2 acted as both an activator and repressor (Figures 3 and 5), similar to proteins such as HRS1/NIGT1.4, NLP7, WRKY6, and WRKY42 (Robatzek and Somssich, 2002; Chen et al., 2009b; Castrillo et al., 2013; Marchive et al., 2013; Medici et al., 2015; Su et al., 2015). According to previous reports, the function of a transcription factor as an activator or a repressor presumably was determined by the structure of the promoter, the binding sites, the flanking sequences, and its interplay regulator (Blauwkamp et al., 2008; Medici et al., 2015). NIGT1.2 can bind to two types of cis-motifs, AGANNNAAA and AAACNNAACC, in the promoters of PHT1;1 and PHT1;4, which are up-regulated by NIGT1.2; AANNAGA, TGGGA, and GAGA in the promoter of NRT1.1, which is down-regulated by NIGT1.2 (Figures 3 and 5), similar to HRS1/NIGT1.4 (Medici et al. 2015). Furthermore, the binding of NIGT1.2 to the PHT1;4 promoter was sequence specific and displayed different requirements for the sequences flanking this motif (Figures 3E and 3J). These data suggest that the NIGT1 proteins, such as NIGT1.2 and NIGT1.4, function as transcriptional repressors or activators, at least partially through binding to different cis-motifs.
Besides modulating Pi and NO3− uptake, NIGT1.2 also modulated the transcription of N-response genes, including genes related to amino acid metabolism, amino acid transport, and N metabolism, and those encoding oxidative pentose-phosphate pathway, CHO, and N-response transcription factors (Figure 9). Notably, these genes were transcriptionally modulated during Pi starvation, and most of their promoters contained cis-regulatory elements for NIGT1.2 binding (Figure 9), suggesting that NIGT1.2 modulated N-related cellular metabolism under low-Pi stress.
NIGT1.2 transcription was also up-regulated by NO3− (Supplemental Figure 1B; Medici et al., 2015; Kiba et al., 2018; Maeda et al., 2018), and NIGT1.2 overexpression increased Pi uptake but repressed NO3– uptake (Figures 2 and 4), indicating that NIGT1.2 participates in Pi and NO3− influx under N-sufficient conditions. NIGT1.2 directly activated the transcription of both PHT1;1 and PHT1;4 (Figure 3), which encode two main Pi transporters involved in root Pi transport under both Pi-sufficient and Pi-deficient conditions (Shin et al., 2004). NIGT1.2 directly down-regulated the transcription of NRT1.1 (Figure 5), which encodes an important dual-affinity NO3− transporter that functions under both N-sufficient and N-deficient conditions (Tsay et al., 1993; Liu et al., 1999; Liu and Tsay, 2003; Wang et al., 2012, 2018) and transcriptionally down-regulated NRT2.1 (Maeda et al., 2018) and NRT2.4 (Kiba et al., 2018), which encode two typical high-affinity NO3− transporters that function mainly under N-deficient conditions (Filleur et al., 2001; Orsel et al., 2004; Li et al., 2007; Kotur et al., 2012; Wang et al., 2012, 2018). NIGT1.2 (also named HHO2) also modulated the number and length of lateral roots irrespective of the Pi availability (Nagarajan et al., 2016), which may influence the Pi and NO3− uptake. Then, NIGT1.1 and NIGT1.2 may play important roles in increasing Pi and NO3− uptake under conditions of fluctuating NO3− and Pi availability.
Recently, Medici et al. (2019) and Hu et al. (2019) demonstrated that nitrogen, mainly NO3−, triggers the phosphate starvation response (PSR). Nitrate triggers phosphate starvation-induced gene expression through the SPX4-PHR2 module (Hu et al., 2019), PSR strongly depends on N provision, and N provision modulates PHR1accumulation and turnover (Medici et al., 2019). NIGT1.2 expression was induced by NO3− (Supplemental Figure 1; Kiba et al., 2018) and was modulated by the transcription factor PHR1 (Bustos et al., 2010; Maeda et al., 2018), suggesting that the transcription factor NIGT1.2 participates in the nitrate-dependent PSR response. Ueda and Yanagisawa (2019) also indicated that the NIGT1s, including the NIGT1.1 and NIGT1.2, participate in integrating NO3− and Pi signals from various environment factors and triggering appropriate responses. Our work provides insight into NIGT1.1- and NIGT1.2-modulated low-Pi-stress-related phosphate and nitrate uptake.
METHODS
Plant Materials
The Col-0 ecotype was used as wild-type Arabidopsis (Arabidopsis thaliana) in this study. The T-DNA insertion lines GK_122B12, Salk_137632, and Salk_070096 (referred to as the nigt1.2-1, nigt1.2-2, and nigt1.2-3 mutants) were ordered from the Arabidopsis Biological Resource Center (http://www.arabidopsis.org/abrc). The ProPHT1;1:GUS (Wang et al., 2014) and ProNRT1.1:GUS (Krouk et al., 2010) line were described previously. To construct 35S:NIGT1.2, the coding sequence of NIGT1.2 was amplified by PCR using gene-specific primers (Supplemental Table) and cloned into the pCXSN vector (Chen et al., 2009a). A pair of small guide RNA targets (M1, ATAATGATGATGTTCAAGAGCGG; M2, AGCTATCGAGTCATGTCGGAAGG) in NIGT1.1 was cloned into the pHEE2A-TRI vector (Wang et al., 2015), and transformed into wild-type Arabidopsis and nigt1.2-1 to generate the nigt1.1 mutant and nigt1.1 nigt1.2 double mutant, respectively. All constructs were transformed into plants via floral dip transformation (Clough and Bent, 1998).
Plant Growth Conditions
Arabidopsis seeds were stratified at 4°C for 72 h and then germinated and grown on MS medium at 22°C with a 16-h-fluorescent daily light period (light intensity 100μmol/m2s). The LP medium was made by modifying MS medium to contain only 10 μM Pi, as well as agar instead of agarose (Promega). For Arabidopsis hydroponic culture, the seedlings were grown in one-fourth MS nutrient solution without supplement of Suc. For maize (Zea mays) hydroponic culture, 9-d-old maize seedlings without endosperm were transferred to one-eighth Hoagland nutrient solution with or without 250 μM KH2PO4, and grown at 28°C with a 14-h-fluorescent daily light period (light intensity 350 μmol/m2s).
Physiological Measurements
The Pi concentration and Pi uptake measurements for Arabidopsis and maize seedlings were quantified as previously described by Su et al. (2015). The nitrogen and 15NO3− concentration in Arabidopsis and maize were modified from previously described by Hu et al. (2015). For measurements of nitrogen and 15NO3− concentration in Arabidopsis, the 7-d-old seedlings grown on MS were transferred to modified LP or MS medium containing 5 mM NO3− (5 atom% 15N) and the NH4NO3 was replaced by ammonium succinate. For nitrogen and 15NO3− concentration in maize, 9-d-old maize B73 plants were transferred to +P or −P solution with 5 mM NO3− (5 atom% 15N) for 11 d. Whole seedlings were dried at 80°C for 3 d and analyzed using isotope ratio mass spectrometry (EA-DELTAplus XP).
For NO3− influx assay, 7-d-old Arabidopsis seedlings were grown on LP or MS medium for another 7 d. The plants were washed with 0.1 mM CaSO4 for 1 min, and then transferred to uptake solution for 5 min. The uptake solution was MS solution containing 20 mM NO3− (99 atom% 15N) and 0.5 mM ammonium succinate, without NH4NO3 and agar. Roots were washed with 0.1 mM CaSO4 for 1 min and dried at 80°C for 3 d and then analyzed using isotope ratio mass spectrometry (EA-DELTAplus XP). Influx of 15NO3− was calculated from the total N and 15N concentration in roots.
GUS Staining and Subcellular Localization
A GUS staining assay was performed as previously described by Chen et al. (2009b). For the subcellular localization experiment, NIGT1.2 fused to GFP was cloned into a modified pCAMBIA1300:GFP vector, resulting in a NIGT1.2-GFP construct. The NIGT1.2-GFP construct and GFP alone were each transformed into leaves of Nicotiana benthamiana through transient expression assays as previously described by Chen et al. (2009b). GFP fluorescence in the transformed leaves was imaged using a confocal laser scanning microscope (LSM510, Carl Zeiss).
Transient Expression Assay in N. benthamiana
The transient expression assay was performed as previously described by Chen et al. (2009b). The ProPHT1;1:GUS construct was previously described by Wang et al. (2014). To construct ProNRT1.1 and ProZmNPF2, the ∼1.5 kb of the promoter regions of NRT1.1 and ZmNPF2 were cloned into the pCAMBIA1381 vector. To construct 35S:ZmNIGT1.2, the coding sequence of ZmNIGT1.2 was cloned into the pCXSN vector. The primer sequences used are listed in the Supplemental Table. Super:LUC was added as an internal control in each infiltration sample. The GUS and LUC activities were measured in each infiltrated sample, and the GUS/LUC ratio was used to quantify the promoter activity.
RT-qPCR Analysis
RT-qPCR analysis was conducted as previously described by Huang et al. (2016). Relative quantitative results were calculated by normalization to Actin2/8 in Arabidopsis and to Ubiquitin (ZmUBQ; GenBank accession number: BT018032) in maize. Each experiment was performed in biological triplicate. The primers used are listed in the Supplemental Table.
RNA Sequencing Analysis
Arabidopsis plants were grown as described above, with pooled roots from 120 seedlings used for each of three independently grown and harvested biological replicates. For each biological replicate, 3 μg of rRNA-depleted RNA was used for cDNA conversion and an Illumina sequencing library was generated using the RNA library prep kit for Illumina (NEB). After sequencing, Illumina adapter sequences were aligned to the Arabidopsis genome TAIR10 using TopHat2 (Kim et al., 2013), and differential expression analysis was performed using DESeq2 (Love et al., 2014). The raw data were submitted to the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/sra/PRJNA625449; no. PRJNA625449). DEGs were determined for wild type_MS versus wild type_LP and wild type_MS versus OE23_MS with a P ≤ 0.05 cut-off. The function of enriched genes was analyzed using the Classification SuperViewer Tool on the BAR website (http://bar.utoronto.ca/ntools/cgi-bin/ntools_classification_superviewer.cgi) with the MapMan classification source option. A heatmap was generated with Gene Cluster 3.0 (de Hoon et al., 2004) and visualized with Java Treeview (Saldanha, 2004).
Protein Expression and Antibody Generation
The coding sequence of NIGT1.2 was cloned into the pGEX-4T-1 vector to produce the GST-NIGT1.2 vector, and then GST-NIGT1.2 was introduced into and expressed in Escherichia coli strain BL21. The E. coli cells were induced with 0.2 mM isopropyl-D-1-thiogalactopyranoside overnight at 18°C and collected by centrifugation. The polyclonal antibody against NIGT1.2 was generated by inoculating mice.
ChIP-qPCR and EMSA
For ChIP-qPCR, 7-d-old wild-type Arabidopsis seedlings were transferred to MS or LP medium for 5 d, and then the roots were harvested for the ChIP experiment. The ChIP experiment was performed using anti-NIGT1.2 antibody as previously described by Chen et al. (2009b) and Huang et al. (2016). EMSA was conducted as previously described by Huang et al. (2016). The primers used are listed in the Supplemental Table.
Phylogenetic Analysis
The Arabidopsis NRT1.1 and maize NPF sequences were retrieved from the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov) database. For the phylogenetic analysis, the amino acid sequences were aligned in ClustalX (version 2.0.11) with default parameters. Bootstrap values were obtained based on 900 replicates. Evolutionary analysis was conducted in MEGA6 software.
Statistical Analysis
Data are shown as mean ± se of one representative experiment. Student’s t test was used to compare significance between treatment or genotypes. P value was shown as P < 0.05 or P < 0.01 to indicate significant difference.
Accession Numbers
Sequence data from this article can be found in the EMBL/GenBank data libraries under the following accession numbers: NIGT1.1 (AT1G25550), NIGT1.2 (AT1G68670), PHT1;1 (AT5G43350), PHT1;4 (AT2G38940), NRT1.1 (AT1G12110), NRT1.2 (AT1G69850), NRT2.1 (AT1G08090), NRT2.4 (AT5G60770), ZmNIGT1.1 (Zm00001d023411), ZmNIGT1.2 (Zm00001d023402), ZmNPF6.4 (Zm00001d024587), and ZmNPF6.6 (Zm00001d029932).
Supplemental Data
Supplemental Figure 1. NIGT1.2 expression pattern.
Supplemental Figure 2. Identification of nigt1.2 mutants and Pi concentration measurement.
Supplemental Figure 3. Analysis of NIGT1.1 expression using RT-qPCR.
Supplemental Figure 4. Generation of the nigt1.1 and nigt1.1 nigt1.2 double mutant using CRISPR/Cas9 technology.
Supplemental Figure 5. Analysis of SPXs and PHO2 expression using RT-qPCR.
Supplemental Table. Primer sequences used in this study.
Supplemental Data Set 1. Alignments used to generate the phylogeny presented in Figure 10F.
Supplemental Data Set 2. Student’s t test tables.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Alain Gojon for providing the ProNRT1.1:GUS line. This work was supported by the Ministry of Agriculture of China for transgenic research (grant 2016ZX08009002), the National Key Research and Development Program of China (grant 2016YFD0100707), the National Natural Science Foundation of China (grants 31970273, 31670245, and 31921001), the Chinese Universities Scientific Fund (grant 2020TC153), and the Beijing Outstanding University Discipline Program.
AUTHOR CONTRIBUTIONS
Y.-F.C. designed the project; X.W., H.-F.W., Y.C., and M.-M.S. performed the experiments; Y.-F.C. and X.W. analyzed the data and wrote the article.
References
- Aono T., Kanada N., Ijima A., Oyaizu H.(2001). The response of the phosphate uptake system and the organic acid exudation system to phosphate starvation in Sesbania rostrata. Plant Cell Physiol. 42: 1253–1264. [DOI] [PubMed] [Google Scholar]
- Blauwkamp T.A., Chang M.V., Cadigan K.M.(2008). Novel TCF-binding sites specify transcriptional repression by Wnt signalling. EMBO J. 27: 1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos R., Castrillo G., Linhares F., Puga M.I., Rubio V., Pérez-Pérez J., Solano R., Leyva A., Paz-Ares J.(2010). A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet. 6: e1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo G., et al. (2013). WRKY6 transcription factor restricts arsenate uptake and transposon activation in Arabidopsis. Plant Cell 25: 2944–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Songkumarn P., Liu J., Wang G.L.(2009a). A versatile zero background T-vector system for gene cloning and functional genomics. Plant Physiol. 150: 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.F., Li L.Q., Xu Q., Kong Y.H., Wang H., Wu W.H.(2009b). The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell 21: 3554–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F.(1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- de Groot C.C., Marcelis L.F.M., van den Boogaard R., Kaiser W.M., Lambers H.(2003). Interaction of nitrogen and phosphorus nutrition in determining growth. Plant Soil 248: 257–268. [Google Scholar]
- de Hoon M.J., Imoto S., Nolan J., Miyano S.(2004). Open source clustering software. Bioinformatics 20: 1453–1454. [DOI] [PubMed] [Google Scholar]
- de Magalhaes J.V., Alves V.M.C., de Novais R.F.(1998). Nitrate uptake by corn under increasing periods of phosphorus starvation. J. Plant Nutr. 21: 1753–1763. [Google Scholar]
- Devaiah B.N., Karthikeyan A.S., Raghothama K.G.(2007). WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol. 143: 1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filleur S., Dorbe M.F., Cerezo M., Orsel M., Granier F., Gojon A., Daniel-Vedele F.(2001). An arabidopsis T-DNA mutant affected in Nrt2 genes is impaired in nitrate uptake. FEBS Lett. 489: 220–224. [DOI] [PubMed] [Google Scholar]
- Gniazdowska A., Krawczak A., Mikulska M., Rychter A.M.(1999). Low phosphate nutrition alters bean plants’ ability to assimilate and translocate nitrate. J. Plant Nutr. 22: 551–563. [Google Scholar]
- Gniazdowska A., Rychter A.M.(2000). Nitrate uptake by bean (Phaseolus vulgaris L.) roots under phosphate deficiency. Plant Soil 226: 79–85. [Google Scholar]
- Good A.G., Beatty P.H.(2011). Fertilizing nature: a tragedy of excess in the commons. PLoS Biol. 9: e1001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsinger P.(2001). Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 237: 173–195. [Google Scholar]
- Ho C.H., Lin S.H., Hu H.C., Tsay Y.F.(2009). CHL1 functions as a nitrate sensor in plants. Cell 138: 1184–1194. [DOI] [PubMed] [Google Scholar]
- Hu B., et al. (2019). Nitrate-NRT1.1B-SPX4 cascade integrates nitrogen and phosphorus signalling networks in plants. Nat. Plants 5: 401–413. [DOI] [PubMed] [Google Scholar]
- Hu B., et al. (2015). Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 47: 834–838. [DOI] [PubMed] [Google Scholar]
- Huang N.C., Liu K.H., Lo H.J., Tsay Y.F.(1999). Cloning and functional characterization of an Arabidopsis nitrate transporter gene that encodes a constitutive component of low-affinity uptake. Plant Cell 11: 1381–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Feng C.Z., Ye Q., Wu W.H., Chen Y.F.(2016). Arabidopsis WRKY6 transcription factor acts as a positive regulator of abscisic acid signaling during seed germination and early seedling development. PLoS Genet. 12: e1005833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant S., Peng M., Rothstein S.J.(2011). Genetic regulation by NLA and microRNA827 for maintaining nitrate-dependent phosphate homeostasis in arabidopsis. PLoS Genet. 7: e1002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba T., et al. (2018). Repression of nitrogen starvation responses by members of the Arabidopsis GARP-type transcription factor NIGT1/HRS1 subfamily. Plant Cell 30: 925–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., Salzberg S.L.(2013). TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby E., Johnston A.E.(2008). Soil and fertilizer phosphorus in relation to crop nutrition In The Ecophysiology of Plant-Phosphorus Interactions, White P.J., and Hammond J.P., eds (Dordrecht, the Netherlands: Springer; ), pp. 177–223. [Google Scholar]
- Kleinert A., Venter M., Kossmann J., Valentine A.(2014). The reallocation of carbon in P deficient lupins affects biological nitrogen fixation. J. Plant Physiol. 171: 1619–1624. [DOI] [PubMed] [Google Scholar]
- Kotur Z., Mackenzie N., Ramesh S., Tyerman S.D., Kaiser B.N., Glass A.D.(2012). Nitrate transport capacity of the Arabidopsis thaliana NRT2 family members and their interactions with AtNAR2.1. New Phytol. 194: 724–731. [DOI] [PubMed] [Google Scholar]
- Krouk G., et al. (2010). Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 18: 927–937. [DOI] [PubMed] [Google Scholar]
- Léran S., et al. (2014). A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends Plant Sci. 19: 5–9. [DOI] [PubMed] [Google Scholar]
- Li W., Wang Y., Okamoto M., Crawford N.M., Siddiqi M.Y., Glass A.D.(2007). Dissection of the AtNRT2.1:AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol. 143: 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., Wang J., Zhao J., Tian J., Liao H.(2014). Control of phosphate homeostasis through gene regulation in crops. Curr. Opin. Plant Biol. 21: 59–66. [DOI] [PubMed] [Google Scholar]
- Liu K.H., Huang C.Y., Tsay Y.F.(1999). CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell 11: 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K.H., et al. (2017). Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nature 545: 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K.H., Tsay Y.F.(2003). Switching between the two action modes of the dual-affinity nitrate transporter CHL1 by phosphorylation. EMBO J. 22: 1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.Y., Huang T.K., Tseng C.Y., Lai Y.S., Lin S.I., Lin W.Y., Chen J.W., Chiou T.J.(2012). PHO2-dependent degradation of PHO1 modulates phosphate homeostasis in Arabidopsis. Plant Cell 24: 2168–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arredondo D.L., Leyva-González M.A., González-Morales S.I., López-Bucio J., Herrera-Estrella L.(2014). Phosphate nutrition: improving low-phosphate tolerance in crops. Annu. Rev. Plant Biol. 65: 95–123. [DOI] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S.(2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y., Konishi M., Kiba T., Sakuraba Y., Sawaki N., Kurai T., Ueda Y., Sakakibara H., Yanagisawa S.(2018). A NIGT1-centred transcriptional cascade regulates nitrate signalling and incorporates phosphorus starvation signals in Arabidopsis. Nat. Commun. 9: 1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchive C., Roudier F., Castaings L., Bréhaut V., Blondet E., Colot V., Meyer C., Krapp A.(2013). Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nat. Commun. 4: 1713. [DOI] [PubMed] [Google Scholar]
- Marschner H., Rimmington G.(1988). Mineral nutrition of higher plants. Plant Cell Environ. 11: 147–148. [Google Scholar]
- Medici A., Marshall-Colon A., Ronzier E., Szponarski W., Wang R., Gojon A., Crawford N.M., Ruffel S., Coruzzi G.M., Krouk G.(2015). AtNIGT1/HRS1 integrates nitrate and phosphate signals at the Arabidopsis root tip. Nat. Commun. 6: 6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici A., et al. (2019). Identification of molecular integrators shows that nitrogen actively controls the phosphate starvation response in plants. Plant Cell 31: 1171–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcuende R., Bari R., Gibon Y., Zheng W., Pant B.D., Bläsing O., Usadel B., Czechowski T., Udvardi M.K., Stitt M., Scheible W.R.(2007). Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ. 30: 85–112. [DOI] [PubMed] [Google Scholar]
- Nagarajan V.K., Satheesh V., Poling M.D., Raghothama K.G., Jain A.(2016). Arabidopsis MYB-Related HHO2 exerts a regulatory influence on a subset of root traits and genes governing phosphate homeostasis. Plant Cell Physiol. 57: 1142–1152. [DOI] [PubMed] [Google Scholar]
- Nguyen G.N., Rothstein S.J., Spangenberg G., Kant S.(2015). Role of microRNAs involved in plant response to nitrogen and phosphorous limiting conditions. Front Plant Sci 6: 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsel M., Eulenburg K., Krapp A., Daniel-Vedele F.(2004). Disruption of the nitrate transporter genes AtNRT2.1 and AtNRT2.2 restricts growth at low external nitrate concentration. Planta 219: 714–721. [DOI] [PubMed] [Google Scholar]
- Ou B., Yin K.Q., Liu S.N., Yang Y., Gu T., Wing Hui J.M., Zhang L., Miao J., Kondou Y., Matsui M., Gu H.Y., Qu L.J.(2011). A high-throughput screening system for Arabidopsis transcription factors and its application to Med25-dependent transcriptional regulation. Mol. Plant 4: 546–555. [DOI] [PubMed] [Google Scholar]
- Péret B., Clément M., Nussaume L., Desnos T.(2011). Root developmental adaptation to phosphate starvation: better safe than sorry. Trends Plant Sci. 16: 442–450. [DOI] [PubMed] [Google Scholar]
- Puga M.I., et al. (2014). SPX1 is a phosphate-dependent inhibitor of Phosphate Starvation Response 1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 111: 14947–14952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghothama K.G.(1999). Phosphate acquisition. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50: 665–693. [DOI] [PubMed] [Google Scholar]
- Robatzek S., Somssich I.E.(2002). Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev. 16: 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T.W. Jr., Israel D.W., Volk R.J., Qiu J., Sa T.(1993). Phosphate regulation of nitrate assimilation in soybean. J. Exp. Bot. 44: 879–891. [Google Scholar]
- Rufty T.W. Jr., Siddiqi M.Y., Glass A.D.M., Ruth T.J.(1991). Altered 13NO3- influx in phosphorus limited plants. Plant Sci. 76: 43–48. [Google Scholar]
- Rufty T.W., Mackown C.T., Israel D.W.(1990). Phosphorus stress effects on assimilation of nitrate. Plant Physiol. 94: 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha A.J.(2004). Java Treeview—extensible visualization of microarray data. Bioinformatics 20: 3246–3248. [DOI] [PubMed] [Google Scholar]
- Sawaki N., Tsujimoto R., Shigyo M., Konishi M., Toki S., Fujiwara T., Yanagisawa S.(2013). A nitrate-inducible GARP family gene encodes an auto-repressible transcriptional repressor in rice. Plant Cell Physiol. 54: 506–517. [DOI] [PubMed] [Google Scholar]
- Schlüter U., Colmsee C., Scholz U., Bräutigam A., Weber A.P., Zellerhoff N., Bucher M., Fahnenstich H., Sonnewald U.(2013). Adaptation of maize source leaf metabolism to stress related disturbances in carbon, nitrogen and phosphorus balance. BMC Genomics 14: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H., Shin H.S., Dewbre G.R., Harrison M.J.(2004). Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 39: 629–642. [DOI] [PubMed] [Google Scholar]
- Su T., Xu Q., Zhang F.C., Chen Y., Li L.Q., Wu W.H., Chen Y.F.(2015). WRKY42 modulates phosphate homeostasis through regulating phosphate translocation and acquisition in Arabidopsis. Plant Physiol. 167: 1579–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay Y.F., Schroeder J.I., Feldmann K.A., Crawford N.M.(1993). The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 72: 705–713. [DOI] [PubMed] [Google Scholar]
- Ueda Y., Yanagisawa S.(2019). Perception, transduction, and integration of nitrogen and phosphorus nutritional signals in the transcriptional regulatory network in plants. J. Exp. Bot. 70: 3709–3717. [DOI] [PubMed] [Google Scholar]
- Wang H., Xu Q., Kong Y.H., Chen Y., Duan J.Y., Wu W.H., Chen Y.F.(2014). Arabidopsis WRKY45 transcription factor activates PHOSPHATE TRANSPORTER1;1 expression in response to phosphate starvation. Plant Physiol. 164: 2020–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.Y., Cheng Y.H., Chen K.E., Tsay Y.F.(2018). Nitrate transport, signaling, and use efficiency. Annu. Rev. Plant Biol. 69: 85–122. [DOI] [PubMed] [Google Scholar]
- Wang Y.Y., Hsu P.K., Tsay Y.F.(2012). Uptake, allocation and signaling of nitrate. Trends Plant Sci. 17: 458–467. [DOI] [PubMed] [Google Scholar]
- Wang Z.P., Xing H.L., Dong L., Zhang H.Y., Han C.Y., Wang X.C., Chen Q.J.(2015). Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 16: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z., Tyerman S.D., Dechorgnat J., Ovchinnikova E., Dhugga K.S., Kaiser B.N.(2017). Maize NPF6 proteins are homologs of Arabidopsis CHL1 that are selective for both nitrate and chloride. Plant Cell 29: 2581–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Ma L., Hou X., Wang M., Wu Y., Liu F., Deng X.W.(2003). Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol. 132: 1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]