Abstract
Aegle marmelos (L.) is a seasonal fruit that contains significant amounts of bioactives like, phenolic acids (gallic acids, 2,3-dihydroxy benzoic acid, chlorogenic acid, p-coumaric acid, vanillic acid), flavonoid (rutin), organic acids (oxalic acid, tartaric acid, malic acid, lactic acid, acetic acid, citric acid, propionic acid, succinic acid, fumaric acid), vitamin C, vitamin B group (thiamine, niacin, pyridoxine, pantothenic acid, biotin, cobalamins, riboflavin), tocopherols (α-tocopherol, β-tocopherol, γ-tocopherol, δ-tocopherol), carotenes (α-carotene, β-carotene, γ-carotene, δ-carotene) and also rich in essential minerals (potassium, calcium, phosphorus, sodium, iron, copper, manganese). This study provides a comprehensive composition analysis (determined using RP-HPLC and Energy Dispersive X-Ray Fluorescence (EDXRF) Spectroscopy). In vitro medicinal activities (antioxidant activity, anti-inflammatory activity, anti-diabetic activity) are quantified for different bael samples. The study also investigates the changes of these bioactive components with freeze, sun, hot air, and microwave drying. The study gives a proper vision to preserve the nutraceutically rich pulp by converting it into fruit leather.
Keywords: Food science, Natural product chemistry, Microwave drying, Freeze drying, Hot air drying, Sun drying, Polyphenols, Vitamins, Organic acids, In vitro nutraceutical Activities, Multivariate analysis, EDXRF analysis
Food science; Natural product chemistry; Microwave drying; Freeze drying; Hot air drying; Sun drying; Polyphenols; Vitamins; Organic acids; In vitro nutraceutical Activities; Multivariate analysis; EDXRF analysis
1. Introduction
Bael (Aegle marmelos L.) is the only species within the monotypitonus Aegle of Ructaceae family, native to the dry (tropical and subtropical regions) forests of hilly and plain areas of South Asian countries like Thailand, Pakistan, Bangladesh, Srilanka, India, and Malaysia (Sharma and Dubey, 2013; Neeraj and Johar, 2017; Sarkar et al., 2020a, Sarkar et al., 2020b, Sarkar et al., 2020c). This fruit is rich in health-promoting bioactive compounds such as polyphenols, flavonoids, carotenes, vitamins, and organic acids. It also contains essential minerals like potassium, calcium, phosphorus, sodium, iron, copper, and manganese in significant amounts (Manandhar et al., 2018,Ranganna, 1986). In this study, the proper quantitative amounts of most of the above-mentioned components were unveiled using reverse-phase high performance liquid chromatography (RP-HPLC). Energy dispersive X-ray fluorescence (EDXRF) analysis was also performed for the identification and quantification of the minerals.
The bioactive compounds (marmelosin, luvangetin, aurapten, psoralen, marmelide, tannin, riboflavin, aegeline, β-carotene, lupeol, eugenol) of Aegle marmelos fruit show multiple biological activities like, antihelminthic, antibacterial, antiulcer, antispasmodic, artemicide, anti-diarrhea, astringent, antiproliferative, anticancer, anti-diabetic, anti-inflammatory and antioxidant activities (Lim, 2012; Sarkar et al., 2020d). Reactive oxygen species (ROS) is a harmful group of components which get generated during the aerobic metabolic processes in human body cells and these radicals lead towards many types of health-related diseases like stroke, cardiovascular disease, arthritis, asthma, retinal damage, neuro degeneration, diabetes, chronic obstructive pulmonary disease and dermatitis (La Vecchia et al., 1998). Bioactive compounds (mainly polyphenols) show protective effects against cell oxidation by scavenging these ROS (Kaur and Kapoor, 2008). In inflammatory disorder proteinases lead the protein denaturation, here we tried to evaluate the ability of fruit extract to inhibit both the proteinases and protein denaturation. While for inducing diabetic disorder, α-amylase plays a major role by raising the sugar level in the body, here we studied the inhibitory activities of the extracts to prevent α-amylase to form simple sugars from starch. Previously many authors reported the in-vivo activities of Aegle marmelos (L.) against these diseases (Manandhar et al., 2018).
In spite of being rich in these beneficiary components and activities, it is still underutilized because of its unavailability throughout the year. The ripe fruits are available only from March to June (Hazra et al., 2019; Baliga et al., 2013). After harvesting, the shelf life of bael fruit is normally two weeks at room temperature (27–32 °C). The shelf life can be enhanced to twelve weeks by preserving it at 9 °C (85–90% relative humidity), but it is highly sensitive to spoilage (chilling injury) below 9 °C (Pal et al., 2017; Roy et al., 2011). The possible solution we found is to convert it into fruit leather which is one of the prominent methods, mainly used to preserve fully ripe unconsumed or excess fruit pulp for a long period of time without adding any preservative, and can be consumed as snacks or, dessert for a long. Bael leather can be utilized in the fruit bar industry, fruit cake industry, and importantly as ayurvedic medicine. The traditional way of making leather is to dry the pulp in the sun, which is one of the easiest and cheapest methods. However, the main problems associated with this are uncertain climatic conditions, microorganisms, dust, dirt and insect infections that produce an inferior quality product in terms of hygiene (Uribe et al., 2019). Therefore, it is better to focus on other possible alternatives, mainly industrial methods like, microwave drying, hot air drying, and freeze drying which gives better yields in terms of production rate and quality of the leather. In this study, the quality of fresh and differently dried Aegle marmelos (L.) fruit leathers in terms of nutraceutical components, compositional changes, and medicinal activities was evaluated.
2. Materials and methods
Ripe Aegle marmelos (L.) fruits were purchased from the Kole market of Kolkata, West Bengal, India. Sound fresh ripe bael fruits of uniform size were randomly chosen depending on their visual appearance. Generally, the shelf life of bael fruit is 3–4 days if not broken, although pulping was done immediately after brought to the laboratory.
2.1. Chemical used
The solvents (ethanol; methanol; acetonitrile; water) and standards (chlorogenic; p-coumaric; ferulic; sinapic; 2,3-dihydroxybenzoic; gallic; vanillic acids; quercetin; apigenin; myricetin; rutin; kaempferol; oxalic acid; tartaric acid; malic acid; lactic acid; acetic acid; citric acid; propionic acid; succinic acid; fumaric acid; L-ascorbic acid; thiamine; riboflavin; niacin; pantothenic acid; pyridoxine; biotin; cobalamins; α-tocopherol; β-tocopherol; γ-tocopherol; δ-tocopherol; α-carotene; β-carotene; γ-carotene and δ-carotene) used were of chromatography grade. Other reagents (Folin-Ciocalteus's phenol reagent; sodium carbonate; sodium nitrite; aluminium chloride; sodium hydroxide; di sodium phosphate; mono sodium phosphate; trifluoroacetic acid; potassium ferricyanide; ferric chloride; potassium persulphate; trolox; tris hydrochloride; pyrogallol; ethylenediaminetetraacetic acid; bovine serum albumin; corn starch; 3,5-dinitrosalicylic acid; 2,4,6-tris(2-pyridyl)-s-triazine, 2,2-diphenyl-2-picrylhydrazyl; α-amylase; 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonicacid); phosphoric acid), phosphate-buffered saline and hydrogen peroxide solution were of analytical grade. All the chemicals were purchased Merck and Sigma-Aldrich, India. C18 columns were bought from the Waters (India) Private Limited.
2.2. Sample preparation
After breaking the fruit rind, only the pulp of the fruit pulp was extracted using a muslin cloth. Bael flesh was poured onto glass Petri dishes which were previously oiled with glycerol and a uniform puree load of 0.5 g/cm2 was maintained for all the samples (Diamante et al., 2014). A total of twelve Petri dishes were prepared by counting three samples for each drying technique. The drying operation was conducted following our previous work (Sarkar et al., 2020a, Sarkar et al., 2020b, Sarkar et al., 2020c).
2.3. Drying
2.3.1. Freeze drying
Samples were frozen at -50 °C for 12 h to solidify, in a deep freezer (New Brunswick Scientific, England; Model no: C340-86) and further dried in a laboratory-scale freeze dryer (FDU1200, EYELA, Japan) by maintaining -40 °C and 0.1 mBar.
2.3.2. Sun drying
Samples were kept in sunlight under open-air conditions. The pulp was sun dried for two days, six hours each (9 a.m.–3 p.m.). The overall temperature throughout the time period was 33 ± 2 °C.
2.3.3. Hot-air drying
Samples were dried in a laboratory hot air dryer (Concepts International, Kolkata, India) by maintaining a temperature of 60 °C and an air flow rate of 1 m/s for consecutive six hours to get a final moisture content of 14–17%.
2.3.4. Microwave drying
Microwave oven (Samsung, Combi CE1031LAT, Mumbai, India) drying was carried out at 100 W for successive 20 min to get the expected moisture content.
All the drying processes were performed until the leathers reached a moisture content between 14-17% (fresh weight basis). Pulp, microwave, sun, hot, and freeze dried leathers were coded as BP, BM, BS, BH, and BF, respectively. All the analyses were completed immediately after sample preparation to avoid any deterioration.
2.4. Extraction preparation
2.4.1. Ultrasonic-assisted extraction
1.00 g of each sample (BP, BM, BS, BH, and BF) were extracted with 30.00 ml of the ethanol-water mixture (1:1) using ultra-sonication (Trans-O-Sonic, Mumbai) for an hour and filtered with 0.45 μm Millipore filter. The final volume of the filtrate was 25.00 ml, so the dose becomes 1.00 gm/25.00 ml or, 40 mg/ml. This filtrate was used for the overall quantification of polyphenols, flavonoids, determination of medicinal values (antioxidant activity, anti-inflammatory activity, anti-diabetic activity), also in HPLC analysis of phenolic acids, flavonoids, and organic acids.
2.5. Total polyphenols
TPC test was performed using the protocol mentioned by Ainsworth and Gillespie (2007), while the Baba and Malik (2015) method was followed to determine TFC content. The results were reported on a dry basis.
2.6. HPLC analysis
2.6.1. Phenolic acids and flavonoid
The ethanolic extract was injected into an RP-HPLC system (Alliance 2695 HPLC system, Waters Corporation, Massachusetts, USA) for quantification of phenolic acids and flavonoids. The separation was achieved with a C18 column of 4.6 mm (internal diameter) and 5 μm pore size. Two solvents, solvent A (0.5% aqueous H3PO4 solution) and solvent B (9:1 methanol-water solution) were used as eluents at a flow rate of 1 ml/min in the following order: 100% A for the first 8 min, 7:3 A-B in the interval of 8–15 min, 1:1 A-B for 15–20 min, 2:3 A-B for 20–25 min, 3:7 A-B for 25–35 min and 100 % B for the last 5 min by maintaining 38 °C temperature. Quantification was carried out with a dual lambda (λ) absorbance UV detector 2487 at 280 nm and for data processing, Empower 2 software (Waters Corporation) was used (Sarkar et al., 2020a, Sarkar et al., 2020b, Sarkar et al., 2020c). Phenolic acids (chlorogenic, p-coumaric, ferulic, sinapic, 2,3-dihydroxybenzoic, gallic, vanillic acids) and flavonoids (quercetin, apigenin, myricetin, rutin, kaempferol) were used as standards.
2.6.2. Organic acids and vitamin C (ascorbic acid)
The quantification of the organic acids and vitamin C was carried out using an RP-HPLC system (Alliance 2695 HPLC system, Waters Corporation, Massachusetts, USA). We followed Sami et al. (2014), to extract vitamin C using metaphosphoric acid (0.3 M) and acetic acid (1.4 M). For quantification, extracts were injected in the system and eluted with 10 mM KH2PO4 solution (pH 2.5). The separation was done using a C18 column of 4.6 mm internal diameter (3.5 μm pore size) at a flow rate of 0.5 ml/min and chromatogram was observed at 214 nm (Hu et al., 2020). In total nine organic acids were quantified namely, oxalic acid, tartaric acid, malic acid, lactic acid, acetic acid, citric acid, propionic acid, succinic acid, fumaric acid, and L-ascorbic acid.
2.6.3. Vitamin B group
For sample preparation, 2.00 g sample was taken in 25 ml of 0.1 NH2SO4 solution and incubated at 121 °C for 30 min. The mixture was then cooled and the pH was fixed to 4.5 using 2.5 M sodium acetate solution and 50 mg α-amylase enzyme was mixed. The mixture was incubated overnight at 35 °C. Then, the preparation was filtered with a Whatman No. 4 filter and the filtrate was diluted up to 50.00 ml of distilled water and filtered again with a 0.45 μm Millipore filter (AOAC International, 1990). 10 μl filtrate was infused into the RP-HPLC system (Alliance 2695 HPLC system, Waters Corporation, Massachusetts, USA). The separation was achieved by a C18 column (internal diameter: 4.6 mm; pore size: 3.5μm) using a mobile phase of 0.5% aqueous H3PO4/30% aqueous acetonitrile solution at a flow rate of 0.5 ml/min (gradient elution) by maintaining a temperature of 35 °C and quantification was at 254 nm Sarkar et al., 2020e. In total 7 standards were used, thiamine (vitamin B1), riboflavin (vitamin B2), niacin (vitamin B3), pantothenic acid (vitamin B5), pyridoxine (vitamin B6), biotin (vitamin B7), and cobalamins (vitamin B12).
2.6.4. Tocopherols and carotenes
To determine tocopherols and carotene content, 0.10 g of pyrogallic acid, 1.00 g sample, 3 ml (50%) KOH, and 7 ml ethanol were added, agitated, and heated up using a water bath at 50 °C for 40 min. To neutralize the preparation double-distilled water was used, further anhydrous sodium sulfate (Na2SO4) was used to dehydrate it. The preparation was concentrated to 5 ml (approx.) with a water bath maintaining 50 °C, followed by dilution to 10.00 ml by using methanol and filtered with 0.45 μm Millipore filter (Sami et al., 2014). The filtrate was injected into the RP-HPLC system. The preparation was eluted using 100% methanol through a C18 column of 4.6 mm internal diameter at a flow rate of 0.5 ml/min by maintaining 35 °C and absorbance was recorded at 292 nm and 450 nm respectively for tocopherols and carotenes. In total 8 standards were used α, β, γ, and δ forms for both tocopherols and carotenes.
2.7. Energy Dispersive X-Ray fluorescence (EDXRF) analysis
For elemental analysis using an energy-dissipative X-ray system (Quantax, Bruker Nano GmbH, Germany) differently dried samples (BM, BS, BH, and BF) were powdered, and pellets were formed of 50 mm × 50 mm x 20 mm. All spectra were processed to quantify potassium (K), calcium (Ca), phosphorus (P), sodium (Na), iron (Fe), copper (Cu), manganese (Mn). The machine was operated at a voltage of 10 kV and an X-flash 5010 detector (silicon drift detector) was used for secondary electrons at a high vacuum.
2.8. Antioxidant activity
DPPH and ABTS assays were performed as mentioned in Kasote et al. (2019), while to measure hydrogen peroxide (H2O2) and super-oxide radical (O2-) scavenging activity we followed Prathapan et al. (2012). To assess FRAP, Benzie and Strain (1996) method was followed. All medicinal activities were reported in percentage (using Eq. (1)) by recording the absorbance of the test solution with respect to a null control, using only the pure solvent replaced by the extract (Rajan et al., 2011; Pati et al., 2020),
| (1) |
For, all the assessment ethanolic extracts were used.
2.9. Anti-inflammatory activity
2.9.1. Protein denaturation using bovine-serum albumin (BSA) and egg-albumin (EA)
To prepare 5.00 ml of the reaction mixture, 0.20 ml of egg-albumin was diluted with 2.80 ml phosphate buffer saline of pH 6.4 and at the end, 2.00 ml ethanolic extract of 40 mg/ml concentration was added in the preparation. The preparations were taken in screw-capped test tubes and incubated at 37 °C for 15 min, followed by heating at70 °C for 5 min in a water bath. The absorbance of the cooled preparation was evaluated in a UV-Vis spectrophotometer at 660 nm against ethanol as blank (Chandra et al., 2012). The same test was repeated by taking BSA instead of egg albumin and the percentage of inhibition of protein denaturation was calculated using Eq. (2).
| (2) |
2.9.2. Anti-proteinase activity
The test was executed as mentioned by Sakat et al., (2010), with some customization (Gunathilake et al., 2018). To prepare the test solution, 0.12 mg trypsin, 2 ml of 0.02 MTris-HCl buffer (pH7.4),0.04 ml ethanolic extract, and 1.96 ml methanol were poured into a screw-capped test tube. The mixture was incubated at 37 °C for 5 min and further 2.00 ml of 0.8% (w/v) casein solution was mixed. The preparation was incubated again for another 20 min. To stop the reaction 4 ml of perchloric acid solution (70%) was poured into the preparation. The centrifugation was performed to obtain a clear supernatant out of the cloudy suspension and the absorbance was evaluated at 210 nm against a blank of ethanol. Here also buffer was taken instead of the extract for control. Eq. (3) was used for calculating the anti-proteinase activity for all the samples.
| (3) |
2.10. Anti-diabetic activity
A procedure according to Abirami et al. (2014) was performed to determine the anti-diabetic activity with some modifications. To prepare the test solution, 1.00 ml sample extract (40 mg/ml), 1 ml of 0.02 M sodium phosphate buffer, and 1 mg/ml α-amylase solution were poured into a screw-capped test tube and incubated at 30 °C. Further, it was mixed with 1 ml 1% aqueous starch and kept at 37 °C for 60 min. The reaction was paused with 1 ml of 3,5-dinitro salicylic acid. The mixture was then boiled in a water bath for 5 min at 90 °C and immediately cooled to 30 °C (room temperature) using an ice bath. Later, the preparation was diluted to 8.00 ml using water (distilled) and absorbance was evaluated using a UV-vis spectrophotometer at 540 nm against ethanol as blank. The control was prepared by replacing the sample extract with the same volume of buffer. The results were calculated using Eq. (4) and expressed in percentage.
| (4) |
2.11. Calculation to convert data in dry weight basis
The moisture content of all samples was recorded following the AOAC method (AOAC International 2000). For the actual comparison between raw pulp and dried leather samples, all the values were calculated and reported based on dry weight using the following Eq. 5 and Eq. 6,
| (5) |
| [6] |
2.12. Statistical and multivariate analysis
All evaluations were carried out in triplicates and presented as mean ± standard deviation. Total values were calculated using “∑mean ± sqrt∑(s.d.2)”. The Shapiro-Wilk test was performed for normal distribution testing. One-way ANOVA was conducted at 95% confidence interval using SPSS 14.0 while PCA (principal component analysis) and correlation analysis were carried out with R Studio software version 3.4.4 (2018-03-15) software. The cluster analysis (hierarchical clustering heat map) was performed using MATLAB 2018a.
3. Results and discussion
3.1. Total polyphenols (TPC and TFC)
The phenolic compounds that exist in fruits have low potency, which is responsible for various beneficial activities like antioxidant and anti-inflammatory activities. These phenolic functional groups share their electron pair or hydrogen with the ROS (reactive oxygen species) to scavenge them (Baba and Malik, 2015). These phytochemicals mainly exist in polymeric forms bound to the cell wall, during different drying operations the heat flux generated, which may incur the breakdown of the polymeric linkage and leading to the development of the antioxidant of lower molecular weight (Lee et al., 2017).
The TPC content in Aegle marmelos (L.) pulp varies from 1.02 g GAE/100 ml to 8.73 g GAE/100 g as reported by Charoensiddhi and Anprung (2008) and Panda et al. (2013). We also found a similar value of 2.3 ± 0.03 g GAE/100 g for BP. Due to drying processes, the TPC value varied significantly, with a maximum in BM and minimum for the BS (Table 1). The amount increased only by 9.11% for BM while for BS, BH, and BF the retentions were 75.91%, 70.44%, 98.3%, respectively. However, the differences in the case of BP, BM, and BF were insignificant (p = 0.09). While an insignificant change was observed for the BS and BH also (p = 0.48).
Table 1.
Polyphenol content of all the samples (BP: fruit pulp; BM: microwave dried sample; BS: sun dried sample, BH: hot air dried sample; BF: freeze dried sample).
| BP | BM | BS | BH | BF | |
|---|---|---|---|---|---|
| Total Polyphenols | |||||
| TPC (GAE mg/g) | 23.04 ± 0.33a | 25.14 ± 1.15a | 17.49 ± 1.15b | 16.23 ± 1.16b | 22.65 ± 0.17a |
| TFC (CE mg/g) | 11.63 ± 0.44c | 16.27 ± 0.66ab | 14.51 ± 0.30b | 9.74 ± 0.35d | 18.17 ± 0.33a |
|
Phenolic acids (mg/100g) | |||||
| Gallic acid (GA) | 617.17 ± 2.58a | 605.55 ± 3.45b | 592.15 ± 3.33cd | 580.27 ± 4.13d | 595.57 ± 1.29c |
| 2,3-dihydroxy benzoic acid (DHBA) | nd | 22.45 ± 0.19b | 20.17 ± 0.42c | 35.94 ± 0.18a | 10.35 ± 0.24d |
| Chlorogenic acid (CGA) | 0.38 ± 0.00e | 56.31 ± 0.26a | 46.57 ± 0.53c | 49.65 ± 0.43b | 30.97 ± 0.55d |
| p-Coumaric acid (p-CA) | 233.54 ± 1.32e | 337.77 ± 2.01b | 361.42 ± 1.49a | 305.95 ± 1.13c | 243.07 ± 1.02d |
| Vanillic acid (VA) | 69.98 ± 0.2d | 71.45 ± 0.87c | 102.40 ± 0.93a | 97.50 ± 0.84b | 52.80 ± 0.76e |
|
Flavonoid (mg/100g) | |||||
| Rutin | 32.25 ± 0.17e | 59.90 ± 0.05a | 56.25 ± 0.19b | 43.40 ± 0.11c | 40.50 ± 0.02d |
All values are reported on dry basis. The amounts are provided in the mean ± standard deviation (s.d.) form, after performing at least triplicate experiments. The superscript letters a, b, c, d and e shown in the table represent the significant differences (p < 0.05) for the same parameters of the different samples. GAE mg/gm = Gallic Acid Equivalent mg/gm; CE mg/gm = Catechin Equivalent mg/gm; nd = not detected.
During the microwave drying the polar molecules present in the cellular matrix vibrate spontaneously by the microwave radiation, this causes rapid temperature rise with the generation of vigorous compressive forces which consecutively distort the cell wall and allows more polyphenols to be accessible. The hastier heating phenomenon in the microwave drying causes the deactivation of polyphenol oxidases more quickly than other drying processes, so the degradation of enzymatic polyphenol was reduced. And even for the freeze-drying, the enzyme gets inactivated at low temperature, so the maximum retention was observed. Valadez-Carmona et al. (2017), found a similar trend, highest in microwave dried cacao pod husks followed by freeze dried and hot air dried sample. Previously, Hazra et al. (2019) also observed an insignificant change after freeze drying of bael (Aegle marmelos L.).
In our study, 1.16 ± 0.04 g CE/100 g TFC value was observed for the BP, which is similar to the value reported by Charoensiddhi and Anprung (2008) studied for Thai Aegle marmelos (L.) fruit. We observed an increase in TFC values by 39.89%, 24.76%, and 56.23% for BM, BS, and BF, respectively. While depletion in the amount was observed for the BH by 16.25%; an insignificant change was observed for BM, BS (p = 0.06), while for BM, BF (p = 0.08). Saifullah et al. (2019), reported the highest TFC in freeze dried lemon myrtle leaves compared to other drying (hot air, microwave, and sun). Whereas Nguyen and Le (2018) found, microwave drying yield higher TFC value than hot air drying for carrot peel.
3.2. HPLC analysis
3.2.1. Phenolic acids and flavonoid
Phenolic acids are nearly 1/3 of the dietary phenols (depending upon type, variety, and nature of foodstuffs), exist in both free and bound forms in various parts of plants and having antibacterial, antiviral, anti-inflammatory, anti-carcinogenic and vasodilator actions (Robbins, 2003; Duthie et al., 2000; Shahidi and Naczk, 1995). In plants, bound-phenolics mainly exist by forming ester or acetyl bonds (Zadernowski et al., 2009). Phenolic acids mainly comprise of two subgroups, hydroxycinnamic acids and hydroxybenzoic acids (Saifullah et al. (2019)).
In total five phenolic acids, two from the hydroxycinnamate subclass (chlorogenic and p-coumaric acids) and three from the hydroxybenzoic (gallic, 2,3-dihydroxybenzoic and vanillic acid) were detected in BS, BM, BH, BF; while only 2,3-dihydroxybenzoic acid was not detected in BP. Gallic acid (GA) is the predominant phenolic acid for all the samples (Table 1). Gallic acid was observed maximum in BP (617.17 ± 2.58 mg/100 g), while the same was decreased by 1.86%, 4.02%, 5.99%, and 3.40% for BM, BS, BH, and BF, respectively. All the drying treatments significantly (p = 3.34 × 10−5) affected the GA content, though the GA content varied insignificantly for BS, BH (p = 0.11) and BS, BF (p = 0.07). Saifullah et al. (2019) found a similar trend of lowering GA in the case of freeze, sun, and hot air drying of Backhousia citriodora leaves. Gasecka et al., (2020) observed a higher GA content for fresh Hericium erinaceus, than the oven dried sample. Despite being absent in pulp, the 2,3-dihydroxybenzoic acid came into existence after undergoing the drying processes and detected maximum in BH (35.94 ± 0.18 mg/100 g). This was may be due to the conversion of chorismic acid into DHBA with a rise in temperature (Gibson, 1964). Chlorogenic acid (CGA) was present in the second-lowest amount in the pulp among the detected phenolic acids, it also increased significantly with all the drying procedures (p = 4.40 × 10−22) and measured highest in BM (56.31 ± 0.26 mg/100 g). Slatnar et al. (2011) also reported the enhancement in CGA content due to sun and oven-drying. The existence of CGA in bound/esterified form in the fresh pulp may be the reason behind its low detection, while the drying conditions may allow the better release (Wildermuth et al., 2016). Both for p-coumaric acid (p-CA) and vanillic acid (VA) the amount was significantly lower in BP than the differently dried products. p-CA content increased by 44.63%, 54.76%, 31.01%, 4.08% for BM, BS, BH, and BF, respectively. In the case of VA also, the values increased by 2.1%, 46.33%, 39.33% for BM, BS, and BH, respectively. But, for BF it was decreased by 24.55%.
Rutin is the glycoside composed of quercetin and disaccharide rutinose. It is also known as vitamin P and is widely distributed in vegetables, fruits, and medicinal herbs (Hosseinzadeh and Nassiri-Asl, 2014). Manandhar et al. (2018) reported that rutin is the major flavonoid present in Aegle marmelos (L.). During HPLC analysis, only rutin was detected among the other given flavonoids. It was found maximum for BM (59.90 ± 0.05 mg/100 g) and a minimum for BP (32.25 ± 0.17 mg/100 g). The rutin content was significantly increased by 87.18%, 75.78%, 35.62%, and 26.56% after microwave, sun, hot air, and freeze-drying, respectively. Previously some studies also reported that in the case of freeze and sun drying the amount of rutin content increased for black grape and figs respectively (Çoklar and Akbulut, 2017; Kamiloglu and Capanoglu, 2015). Kamiloglu and Capanoglu. (2015) suggested the higher detection in sun dried sample is mainly due to the cell wall breakdown and/or release from sequestration.
3.2.2. Organic acids
Quantification of the organic acids found in fruits is considered to be a crucial parameter for the assessment of their quality since these acids not only contribute to the flavor but also provide stability and nutrition (Walker and Famiani, 2018). In total nine organic acids were quantified namely, oxalic acid (OA), tartaric acid (TA), malic acid (MA), lactic acid (LA), acetic acid (AA), citric acid (CA), propionic acid (PA), succinic acid (SA) and fumaric acid (FA) in all the bael samples. Previously, Yadav et al. (2011) quantified only three organic acids (tartaric, malic acid, and oxalic acid) for the bael pulp, in the range of 40–210 mg/100 g (0.04–0.21%), whereas in our study, the amount ranged within 38.66–265.33 mg/100 g DB, for the same (Table 2).
Table 2.
Organic acid profile and ascorbic acid content of all the samples (BP: fruit pulp; BM: microwave dried sample; BS: sun dried sample, BH: hot air dried sample; BF: freeze dried sample).
| Organic Acids (mg/100 g) | BP | BM | BS | BH | BF |
|---|---|---|---|---|---|
| Oxalic Acid (OA) | 38.66 ± 0.06c | 80.34 ± 0.88a | 48.07 ± 0.57b | 29.47 ± 0.37d | 29.47 ± 0.32d |
| Tartaric Acid (TA) | 265.33 ± 2.08a | 201.86 ± 0.58b | 93.51 ± 1.20d | 138.10 ± 0.58c | 91.46 ± 0.57e |
| Malic Acid(MA) | 108.11 ± 0.69b | 186.81 ± 0.46a | 88.89 ± 0.52c | 60.01 ± 0.12e | 63.10 ± 0.49d |
| Lactic Acid(LA) | 765.48 ± 2.66b | 794.14 ± 2.03a | 721.12 ± 1.22c | 372.32 ± 0.65d | 76.88 ± 1.20e |
| Acetic Acid(AA) | 728.99 ± 0.29b | 261.01 ± 0.52c | 584.50 ± 2.73a | 215.75 ± 0.30d | 41.63 ± 0.22e |
| Citric Acid (CA) | 542.45 ± 1.45a | 395.36 ± 0.88b | 17.59 ± 0.15e | 271.62 ± 0.82c | 50.63 ± 0.11d |
| Propionic acid (PA) | 104.21 ± 0.58a | 103.82 ± 0.31a | 37.62 ± 0.37c | 53.79 ± 0.29b | 54.80 ± 0.47b |
| Succinic Acid(SA) | 8.69 ± 0.11a | 1.19 ± 0.08c | 0.57 ± 0.02d | 2.65 ± 0.12b | 8.55 ± 0.17a |
| Fumaric Acid(FA) | 394.89 ± 1.76b | 517.28 ± 0.83a | 19.55 ± 0.23e | 57.08 ± 0.17c | 38.48 ± 0.38d |
| Ascorbic Acid (Asc-A) | 8.40 ± 0.01a | 4.81 ± 0.00c | 3.74 ± 0.00e | 4.27 ± 0.00d | 6.00 ± 0.01b |
All values are reported on dry basis. The amounts are provided in the mean ± standard deviation (s.d.) form, after performing at least triplicate experiments. The superscript letters a, b, c, d and e shown in the table represent the significant differences (p < 0.05) for the same parameters of the different samples.
It was observed that lactic acid (765.48 ± 2.66 mg/100 g) was the predominant one followed by acetic acid (728.99 ± 0.29 mg/100 g), citric acid (542.45 ± 1.45 mg/100 g), and fumaric acid (394.89 ± 1.76 mg/100 g) for BP. While oxalic acid (38.66 ± 0.06 mg/100 g) and succinic acid (8.69 ± 0.11 mg/100 g) contributed in a lesser amount in the profile. For lactic acid, the trend followed BM > BP > BS > BH > BF, and for acetic acid, it was BP > BM > BS > BH > BF. Gaseckaet. al. (2019) also observed a similar trend for lactic acid and acetic acid in the case of fresh, natural convective and oven-dried Hericiumerinaceus (Bull.). Li et al. (2015) also found higher acetic acid content in microwave dried Pleurotuseryngii than the hot air-dried sample. In most of the cases, the drying procedures significantly affected the organic acid content. Both for oxalic acid (p = 0.99) and propionic acid (p = 0.13), an insignificant change was noticed between hot air and freeze dried samples.
Most of the previous studies reported the effect of drying on the amount of citric, malic, and succinic acids for edible mushrooms (Pleurotus eryngii; Stropharia rugosoannulata; Leccinum scabrum (Bull.) Gray; Hericium erinaceus (Bull.) Pears), Figs (Ficus carica L.), Jujubes (Ziziphus jujuba Mill.), lemon slices (Slatnar et al., 2011; Hu et al., 2020; Gasecka et al., 2020; Li et al., 2015; Gao et al., 2012; Ding et al., 2017). For citric acid, it was found that after drying there was a retention of 72.93%, 3.24%, 50.07%, and 7.67% for BM, BS, BH, and BF, respectively. Gao et al. (2012) observed a similar trend for fresh, freeze, sun, and oven-dried jujube. In the case of malic acid, retentions of 82.22%, 55.5%, 58.36% were observed for BS, BH, and BF, while the same for BM increased by 72.79%. This is in agreement with the observations reported by Calín-Sánchez et al. (2013) and Gasecka et al. (2020) for vacuum microwave, convective, and freeze dried pomegranate and hot air-dried Hericium erinaceus (Bull.), respectively. Likewise, citric acid, the amount for succinic acid decreased with processing by 86.31%, 93.45%, 69.51%, and 1.62% for BM, BS, BH, and BF respectively. An insignificant difference was also observed between BP and BF for the succinic acid content (p = 0.55). This is in line with the findings of Gao et al. (2012) for fresh, sun, and oven-dried jujube.
During drying the depletion of organic acids was found, which can be explained by decarboxylation and/or dehydration with the evaporation of carboxylic acids (Chu and Clydesdale, 1976). While in some of the cases the content surprisingly increased probably due to the formation of organic acid from heat-accelerated reactions between different sugars and nitrogen-free carboxylic acids (Delgado et al., 2018).
3.2.3. Vitamin C (ascorbic acid)
L- ascorbic acid (AscA) is a water-soluble nutrient which not only prevents diseases like scurvy but also serves as a biological antioxidant. But, the main problem with ascorbic acid is that it easily degrades, depending on variables such as light, temperature, and the presence of oxygen (Santos and Silva, 2009). Ascorbic acid primarily goes through chemical degradation involving oxidation to form dehydro-ascorbic acid, followed by hydrolysis to 2,3-diketogulonic acid and further polymerization to nutritionally inactivated products (Chang et al., 2006).
The amount of ascorbic acid was observed to be the highest for BP (8.40 ± 0.01 mg/100 g) and due to different drying methods, a significant decrement (p = 4.99 × 10−4) in the content was observed. Here, high aerial oxidation and long drying time may cause higher degradation for BH and BS, only 50.86%, and 44.57% retentions were observed. Also the application of higher temperature speeds up the ascorbic acid oxidation specifically for hot air drying. Maharaj and Sanket (1996) observed higher content of ascorbic acid in case of forced convective drying compared to natural convective drying for Dasheen leaves. Minimal destruction was observed for BF, which may be due to low-temperature processing followed by BM, owing to higher dehydration rate and shorter drying time (Qing-guo et al., 2006). For BF and BM, the retentions were 71.44% and 57.28%, respectively. Chang et al. (2006) found better retention of vitamin C for freeze-dried sample compared to vacuum assisted microwave drying, whereas air drying stood last for carrot slices. Due to the heat-labile characteristics of ascorbic acid, it was expected to have a higher variation between BP, BF, and other dried leathers. Though BH showed high retention (50.83%) of vitamin C, but the result is in agreement within the range of 44.28–63.85% previously reported by Chang et al. (2006) and Chan et al. (1997) for hot air dried tomatoes (I-Tien-Hung variety) and seaweed, respectively. Uribe et al. (2019) also reported an increase by 11.05% of vitamin C in the convective dried brown alga. It can be concluded that the retention of vitamin C is also dependent on sample characteristics.
3.2.4. Vitamin B group
Vitamin B group encompasses a class of water-soluble compounds and consists of 8 major groups which are thiamine (vit B1), niacin (vit B3), pyridoxine (vit B6), pantothenic acid (vit B5), biotin (vit B7), cobalamins (vit B12), riboflavin (vit B2), and folate (vit B9). Most of these get deteriorated rapidly in UV-light, oxygen, and high temperatures. Riboflavin (vit B2) and pantothenic acid (vit B5) get deteriorated at a fast pace under higher temperatures while thiamine (B1), folate (B9) are extremely sensitive to both, oxygenated and high-temperature conditions.
In total 7 standards were used and all were detected for A. marmelos pulp. In our samples, vitamin B12 was the predominant one, followed by vitamin B1 and then vitamin B2.
Vitamin B12 is one of the essential vitamins for the transition of homocysteine to methionine. Without cobalamins the transition procedure becomes incompetent and homocysteine amount increases. The higher levels of homocysteine can be harmful to the blood vessels, enhancing the possibilities of cardiovascular diseases (He et. al., 2004; Ishihara et al., 2008). We aimed to have found the best drying method to minimize the vitamin B complex destruction. The levels of vitamin B12 content in samples ranged from 0.11 to 1.65 mg/100 g DW, where the maximum loss was measured for BH (Table 3). Due to drying the degradation was significant (p = 7.35 × 10−15) and the amount decreased by 75.12%, 86.75%, 92.94%, 88.53 % respectively for BM, BS, BH, and BF. Similar result for microwave drying was observed by Tian et al. (2015), however, for hot air drying the result was different. Watanabe et al. (1998) also studied the effect of microwave drying on several foods (milk, beef, pork) and reported that microwave heating causes an appreciable degradation (30–40%) of the vitamin B12 molecules.
Table 3.
Vitamin B profile of all the samples (BP: fruit pulp; BM: microwave dried sample; BS: sun dried sample, BH: hot air dried sample; BF: freeze dried sample).
| Vitamin B group (μg/100g) | BP | BM | BS | BH | BF |
|---|---|---|---|---|---|
| Thiamine (vit B1) | 346.29 ± 12.80a | 97.41 ± 0.01d | 140.21 ± 0.10c | 375.48 ± 13.30a | 274.32 ± 0.13b |
| Riboflavin (vit B2) | 337.68 ± 15.27a | 272.76 ± 3.60b | 224.63 ± 5.03b | 329.72 ± 20.81a | 336.59 ± 16.33a |
| Niacin (vit B3) | 145.33 ± 0.29a | 35.95 ± 0.03b | 26.85 ± 0.04c | 22.67 ± 0.43d | 12.76 ± 0.04e |
| Pantothenic acid (vit B5) | 42.46 ± 2.43b | 7.05 ± 0.01c | 45.73 ± 0.56ab | 46.81 ± 0.55a | 1.76 ± 0.01d |
| Pyridoxine (vit B6) | 97.25 ± 0.06a | 30.16 ± 0.01d | 64.66 ± 0.05b | 49.75 ± 0.05c | 13.08 ± 0.01e |
| Biotin (vit B7) | 179.13 ± 3.87d | 106.52 ± 0.09e | 191.56 ± 4.16c | 236.56 ± 0.50b | 419.68 ± 0.13a |
| Cobalamins (vit B12) | 1650.75 ± 50.40a | 410.60 ± 0.06b | 213.11 ± 4.89c | 116.97 ± 0.41e | 194.35 ± 4.58d |
| Total value | 2798.89 ± 54.38 | 960.45 ± 3.60 | 906.75 ± 8.17 | 1177.96 ± 24.71 | 1252.54 ± 16.96 |
All values are reported on dry basis. The amounts are provided in the mean ± standard deviation (s.d.) form, after performing at least triplicate experiments. Total value calculated using “∑mean ± sqrt∑(s.d.2)”. The superscript letters a, b, c, d and e shown in the table represent the significant differences (p < 0.05) for the same parameters of the different samples.
Thiamine (vit B1) works as a coenzyme in different decarboxylation reactions (Schnellbaecher et al., 2019). Vitamin B1 content was greater for BH (375.48 ± 13.30 μg/100 g) than for BP (346.29 ± 12.80 μg/100 g), however, the change was statistically insignificant (p = 0.06). It was found lowest for microwave drying (97.41 ± 0.01 μg/100 g), a similar observation was previously reported by Alajaji and El-Adawy (2006) for chickpea. A significant overall reduction in amount was observed due to the drying methods (p = 4.25 × 10−12). The amount of thiamine decreased by 71.87%, 59.51%, and 20.78% for BM, BS, and BF, respectively. Previously, a similar trend between untreated bulgur, hot air, and sun dried bulgur was also observed (Kadakal et al., 2007). This may be due to higher aerial oxidation and the presence of light (Dwivedi and Arnold, 1973).
Riboflavin (vit B2) is one of the essential components required in the enzymatically controlled metabolism of nutrients like lipids, carbohydrates, and amino acids (Choe et al., 2005; Cardoso et al., 2012; Combs, 2012). The amount of vitamin B2 was maximum for BP (337.68 ± 15.27 μg/100 g) and minimum for BS (272.76 ± 3.60 μg/100 g). Here, the amounts for BP, BH, and BF were statistically insignificant (p = 0.83) but, for BM and BS the change was significant (p = 1.76 × 10−4), and the amount reduced by 19.22% and 33.47%, respectively. A similar trend was observed by Kadakal et al. (2007) for hot air and sun dried bulgur. Alajaji and El-Adawy (2006) also observed a similar trend between raw and microwave dried chickpea.
Other complexes such as niacin, pantothenic acid, pyridoxine, and biotin cover about 15–40% of the total amount. For niacin and pyridoxine the change due to drying was significant, while an insignificant change was observed for BP and BS regarding the pantothenic acid (p = 0.09). For biotin, the processing caused an undefined enhancement in amount by 134.28%, 32.06%, and 6.93% for BF, BH, and BS, respectively.
The amount of total vitamin B profile was the lowest for sun drying (906.75 ± 8.17 μg/100 g) and the highest for fresh pulp (2798.89 ± 54.38 μg/100 g), after processing the maximum retention was observed for freeze dried leather.
3.2.5. Carotenes
Carotenes are health-beneficial pigments and account for the color-diversity among the variety of fruits. The prominent carotenes for most of the fruits are α and β -carotene, which account for the yellow and orange color, respectively (Khoo et al., 2011).
There was no published study which compared the α-, β-, γ- and δ-carotene content of fresh Aegle marmelos (L.) pulp and it's differently dried leathers treated until the present observation. The changes in the amount of these carotenes with different drying are provided in Table 4. From the data, we can observe that the presence of α-carotene is highest in the pulp, which decreased by 97.48%, 67.44%, 68.41% for the BS, BH, and BF, respectively. Followed by γ- and β-, while δ-carotene is the lowest. We found 51.67 ± 1.67 μg/100 g β-carotene in the pulp, which is in accordance with the study done by Panda et al. (2013) for Aegle marmelos (L.) fruit must. It was also observed that the values increased in BS (151.58 ± 0.34 μg/100 g), BF (153.43 ± 0.67 μg/100 g), and BH (55.38 ± 0.16 μg/100 g) by 1.93 folds, 1.97 folds, and 7.37%, respectively. Gao et al. (2012) also found a similar enhancement in the values between fresh, freeze, sun, and hot air dried jujube fruit. For, α-carotene the content was insignificantly altered for BH and BF, while a similar observation was there for β-, γ- and δ-carotene during sun and freeze drying. The overall carotene content was observed to be the highest in the pulp, while the overall carotene retention was the highest in BH followed by BF, BS, and BM. Previously, Bechoff et al. (2009) also did a similar study, they observed higher retention of pro-vitamin A for hot air dried sweet potato than for sun drying.
Table 4.
Carotene profile (Provitamin A) of all the samples (BP: fruit pulp; BM: microwave dried sample; BS: sun dried sample, BH: hot air dried sample; BF: freeze dried sample) in dry basis.
| Carotenes (μg/100g) | BP | BM | BS | BH | BF |
|---|---|---|---|---|---|
| α-carotene (alpha C) | 1698.22 ± 33.33a | nd | 42.76 ± 1.15c | 552.8 ± 3.17b | 536.38 ± 6.02b |
| β-carotene (beta C) | 51.67 ± 1.67b | nd | 151.58 ± 0.34a | 55.38 ± 0.16b | 153.43 ± 0.67a |
| γ-carotene (gamma C) | 84.05 ± 0.90b | 31.95 ± 0.67c | 22.37 ± 1.33d | 476.17 ± 0.30a | 18.43 ± 0.49d |
| δ-carotene (delta C) | nd | nd | 45.03 ± 0.55a | nd | 43.74 ± 0.33a |
| Total value | 1833.94 ± 33.38 | 31.95 ± 0.67 | 261.74 ± 1.87 | 1084.35 ± 3.18 | 751.98 ± 6.08 |
All values are reported on dry basis. The amounts are provided in the mean ± standard deviation (s.d.) form, after performing at least triplicate experiments. The superscript letters a, b, c, d and e shown in the table represent the significant differences (p < 0.05) for the same parameters of the different samples. nd = not detected.
3.2.6. Tocopherols
Tocopherols are important lipophilic antioxidants, which naturally occur in four isomeric forms (δ-, γ-, α- and β-tocopherols). During the biosynthesis of tocopherols, γ-tocopherol methyltransferase catalyzes the conversion of γ- and δ-tocopherols respectively to α- and β-tocopherols (Fritsche et al., 2017). In this study, we tried to evaluate the effects of different drying techniques on each tocopherol.
The tocopherols composition of Aegle marmelos (L.) pulp and differently dried leathers are presented in Table 5. From the Table 5, it can be observed that the pulp contains only γ- and δ-tocopherols and their amount decreased significantly with the microwave, sun, hot and freeze drying by 98.82%, 98.8%, 68.64%, 97.82% and 84.69%, 96.82%, 86.04%, 98.56% respectively. γ-tocopherol insignificantly varied for BS and BM (p = 0.62). A significant variation was observed for all the other bael samples. The maximum retention of δ-tocopherol was observed in BM (15.18%), while the same for γ-tocopherol was observed in BH (31.69%). The relative higher content of the tocopherol in BH and BM might be due to the fact that the occurrence of the tocopherol within the lipid segment of the samples; which by virtue shielded the thermal destruction of tocopherol (Uribe et al., 2018, 2019; Van Hoed et al., 2009). The higher value of standard deviation for BH may have resulted from the uneven distribution of lipid segment and less uniform exposure to degrading drying conditions. A similar observation was also observed by Uribe et al. (2019) for α-tocopherol. While being absent in BP, α-tocopherol was found for BM (0.07 ± 0.00 mg/100 g), BS (0.13 ± 0.00 mg/100 g), whereas presence of β-tocopherol was detected in BM (0.48 ± 0.01 mg/100 g), BS (0.47 ± 0.00 mg/100 g) and BH (0.50 ± 0.01 mg/100 g).
Table 5.
Tocopherol (vitamin E) profile of all the samples (BP: fruit pulp; BM: microwave dried sample; BS: sun dried sample, BH: hot air dried sample; BF: freeze dried sample).
| Tocopherols (mg/100g) | BP | BM | BS | BH | BF |
|---|---|---|---|---|---|
| α-tocopherol (alpha T) | nd | 0.07 ± 0.00b | 0.13 ± 0.00a | nd | nd |
| β-tocopherol (beta T) | nd | 0.48 ± 0.01a | 0.47 ± 0.00a | 0.50 ± 0.01a | nd |
| γ-tocopherol (gamma T) | 27.50 ± 0.11d | 0.32 ± 0.00c | 0.32 ± 0.00c | 8.71 ± 0.23a | 0.57 ± 0.01b |
| δ-tocopherol (delta T) | 11.12 ± 0.05a | 1.70 ± 0.01c | 0.35 ± 0.00d | 1.55 ± 0.01b | 0.15 ± 0.00e |
All values are reported on dry basis. The amounts are provided in the mean ± standard deviation (s.d.) form, after performing at least triplicate experiments. The superscript letters a, b, c, d and e shown in the table represent the significant differences (p < 0.05) for the same parameters of the different samples. nd = not detected.
Some earlier studies also reported the enhancement of α-tocopherol along with temperature till 60 °C in convective drying (Laoretani et al., 2014; Al Juhaimi et al., 2018). This may be due to the stability of γ-tocopherol methyltransferase enzyme which converts γ- and δ-tocopherols into α- and β-tocopherols at ambient and/or higher temperature (<50 °C) (Shigeoka et al., 1992). While in BF, the change in the amount of α- and β-tocopherol was insignificant with respect to BP, which may be explained as, the optimum temperature for γ-tocopherol methyltransferase is about 40 °C, at a lower temperature the enzyme may be deactivated (Gálvez-Valdivieso et al., 2011) as well as the reaction kinetics may be impacted in lower temperature. However, the actual reaction mechanism is yet to be revealed. As some of the earlier studies reported opposite trends also for α-tocopherol in jujube fruit and Moringa oleifera leaves drying (Gao et al., 2012; Saini et al., 2014).
3.3. Mineral analysis
Minerals are mainly divided into macro-minerals (major minerals) and micro-minerals (trace minerals). For sample preparation of EDXRF, the sample needs to be dried either by freeze drying as reported by Allegretta et al. (2019) or, need to be dried in an oven (Turhan et al., 2010) for reduction of the moisture to such a level that pellet formation is possible. In this study, as the moisture content of all the samples was in the vicinity of 15%, pellet formation was feasible. Here we observed the effect of different drying techniques (BM, BS, BH, and BF) on the result of EDXRF. In total seven minerals were quantified for the dried samples, four macro-minerals (K, Ca, P, and Na) and three micro-minerals (Fe, Cu, and Mn). The amounts of the individual minerals for the samples are listed in Table 6. It was observed that BS (7460.18 ± 7.43 mg/kg) consists of the highest amount of the total mineral content, while for BH (7439.07 ± 26.86 mg/kg) the amount was the lowest. Similar to Uribe et al. (2019) we also observed an insignificant change for macro-minerals, which was maybe due to the existence of the same amount of solid content in every sample. In the case of Fe, Cu and Mn we found a statistically significant difference between BS, BH (p = 0.2); BH, BF (p = 0.4); and BS, BF (p = 0.3), respectively. Though the differences were statistically significant, these may not have much influence on the nutritional characteristics of bael samples. Previously, some studies (Sajib et al., 2014; Islam et al., 2013; Baliga et al., 2011) observed the mineral content (mg/100 g) of Aegle marmelos (L.) pulp, which varied from 78-600 for K, 30–85 for Ca, 31.8 for P, 6.65–11.9 for Na, 0.6–1.93 for Fe, 0.02–0.21 for Cu, and 0.23 for Mn. We also observed similar results with a little variation that may be due to differences in cultivar, soil, harvesting time, and conditions. Though it is the first kind of approach to compare mineral profiles of differently dried food products, it is an established fact that for mineral composition and quantification purposes EDXRF produces results in line with that can be achieved by ICP-AES process (Feng et al., 2020; McCarthy et al., 2019; Hannaker et al., 1984).
Table 6.
Mineral contents of all the dried samples (BM: microwave dried sample; BS: sun dried sample, BH: hot air dried sample; BF: freeze dried sample).
| Minerals (mg/kg) | BM | BS | BH | BF |
|---|---|---|---|---|
| Potassium (K) | 6343.80 ± 3.38a | 6344.13 ± 3.66a | 6337.13 ± 14.53a | 6337.13 ± 8.82a |
| Calcium (Ca) | 553.10 ± 1.33a | 548.77 ± 0.33a | 556.77 ± 4.17a | 563.44 ± 6.50a |
| Phosphorus (P) | 280.15 ± 1.15a | 281.15 ± 2.08a | 278.48 ± 8.57a | 283.15 ± 2.64a |
| Sodium (Na) | 109.79 ± 3.17a | 110.36 ± 2.90a | 106.72 ± 1.70a | 111.16 ± 2.88a |
| Iron (Fe) | 13.89 ± 1.45ab | 17.22 ± 0.57a | 13.56 ± 0.88b | 16.56 ± 2.72ab |
| Copper (Cu) | 10.45 ± 0.48ab | 9.62 ± 0.41ab | 5.41 ± 2.16b | 13.08 ± 1.59a |
| Manganese (Mn) | 148.97 ± 2.90ab | 149.64 ± 5.33a | 140.98 ± 20.27ab | 124.31 ± 5.77b |
| Total value | 7460.18 ± 5.95 | 7460.18 ± 7.43 | 7439.07 ± 26.86 | 7448.84 ± 13.36 |
All values are reported on dry basis. The amounts are provided in the mean ± standard deviation (s.d.) form, after performing at least triplicate experiments. Total value calculated using “∑mean ± sqrt∑(s.d.2)”. The superscript letters a, b, c and d shown in the table represent the significant differences (p < 0.05) for the same parameters of the different samples.
3.4. Antioxidant activity
ABTS, FRAP, and H2O2 assay were used to measure the ability of antioxidants to quench hydrophilic ABTS●+, Fe3+, and H2O2 (Cano et al., 2002; Jimenez-Alvarez et al., 2008). For lipophilic radical scavenging activity DPPH assay was performed (Kasote et al., 2019,Sánchez-Ria ñ o et al., 2019). During the ABTS assay, an ABTS●+ of blue-green chromosphere was produced by oxidation due to the addition of potassium persulfate. Here, activity was measured in terms of the amount of decolorized radical and was the highest for BM (86.42 ± 0.26%). The lowest activity was observed for BH (79.65 ± 0.1%) and an insignificant change was observed among the other two dried (BF and BS) samples (p = 0.07).
In the case of FRAP, ferric ion (Fe3+) reduced to the ferrous ion (Fe2+) form by accepting an electron, donated by antioxidant under acidic conditions, which with TPTZ (2,4,6-tris(2-pyridyl)-s-triazine) forms a complex to give an intense blue color and shows a strong absorption maximum at 593 nm. Similar to the ABTS assay, in the FRAP assay, the highest reducing power was observed for BM (96.18 ± 0.34%), but here the lowest activity was for BF (89.91 ± 0.42%). Here, the changes for BP and BH were insignificant (p = 0.06). Previously, a similar trend for both FRAP and ABTS assay of differently dried (freeze, sun, microwave, and hot air) oyster mushroom was reported by Piskov et al. (2020). But, exceptionally in case of the ABTS assay, a reverse trend was observed between BH and BF.
The DPPH assay has been done by considering that the antioxidant is a hydrogen donor, owing to the fact that the purple-colored DPPH• (α,α-diphenyl-β-picryl hydrazine) is reduced to yellow colored DPPH-H upon the reaction with a hydrogen atom. Here the ability of different extracts of fresh and differently dried samples to donate hydrogen was measured. Among them, BM revealed the highest activity (64.81 ± 0.28%), while the BS showed the lowest activity (37.13 ± 0.25%). Different drying procedures caused a significant change in the activity (p = 8.21 × 10−21). A similar trend was reported previously, by Nguyen and Le (2018) for carrot peel. Wijewardana et al. (2016) studied DPPH assay for freeze, sun, and air oven-dried Aegle marmelos (L.) pulp and found a similar trend but, the amounts were different, this is maybe due to different variety and different breeding conditions.
Unlike above radicals, which are extraneous matter to biological systems, hydrogen peroxide and superoxide are reactive oxygen species (ROS) that get generated during different biochemical reactions in the living organisms. These ROS are moderately reactive, but they are precursors of other highly reactive radicals which damage cells and generate more new reactive species.
Superoxide (O2●-) is also the precursor of other oxidizing agents, which includes oxidizing radicals, peroxynitrite, oxidized halogens, and singlet oxygen such as hypochlorous acid, which may cause considerable harmful effect on biological systems, so it is important to study the activity against this (Babior, 1997). The highest superoxide radical scavenging activity was shown by BF (83.2 ± 0.33%), whereas the lowest activity was observed for BH (74.8 ± 0.19%). However, the difference between BH and BS was insignificant (p = 0.53). Thi and Hwang (2016) also observed a similar trend for freeze, sun, and oven drying for black chokeberries.
For, the H2O2 scavenging assay polyphenols of the extracts may donate electrons to neutralize H2O2 into water. We observed that BP (64.47 ± 0.35%) contained significantly higher (p = 84 × 10−6) H2O2 activity than BM, though BM possessed higher H2O2 scavenging activity than BF but the difference was statistically insignificant (p = 0.06). Similarly, BH (59.34 ± 0.39%) and BS (59.46 ± 0.18%) treatment impart statistically insignificant (p = 0.83) differences for the H2O2 activity. A similar trend for H2O2 activity was reported by Novaković et al. (2011) among fresh, air dried, and freeze dried raspberry.
Prathapan et al. (2012) reported DPPH and superoxide scavenging activity for baelpulp, however, the content was mentioned as an IC50 value. Rajan et al. (2011) reported DPPH, ABTS, Reducing Power, Super Oxide radical and H2O2 scavenging activity for different doses (between 20-100 μg/ml) of Aegle marmelos (L.) extract, for our samples the activities were greater due to use of higher dose (40 mg/ml).
3.5. Anti-inflammatory activity
This investigation was carried out to observe the ability of differently dried and fresh Aegle marmelos (L.) pulp extracts to prevent the thermal protein denaturation and coagulation, to evaluate its anti-inflammatory activity. The denatured protein expressed as antigens, which are associated with Type III hypersensitive reaction causes diseases like, serum sickness, glomerulo-nephritis, rheumatoid arthritis, and systemic lupus erythematosus (Williams et al., 2008). According to the study by Gell and Benacerraf (1959), it can be concluded that the heat-denatured proteins are potent as native proteins to stimulate delayed hypersensitivity and it was already confirmed by Gilman et al. (1990) that the conventional Non-Steroidal Anti-Inflammatory Drugs (NSAID) also work by preventing the denaturation of proteins. Thusit can be concluded that the anti-denaturation assay is an appropriate in vitro procedure to assess the anti-inflammatory activity. Drying methods significantly affected the BSA (p = 9.41 × 10−13) and egg-albumin (p = 2.62 × 10−10) inhibitory activity. The order of BSA and egg-albumin heat-induced protein denaturation inhibitory activity was as follows: BS < BH < BP < BF < BM. With comparison to the fresh pulp, the BSA and egg-albumin denaturation inhibition activity increased by 6.77%, 10.03% and 2.67%, 6.29% for BM and BF respectively, whereas the same was decreased with respect to the BP by 18.68%, 14.49%, and 9.2%, 5.29% for BS and BH respectively. Sharma et al. (2006); Rahman and Parvin (2014) reported Aegle marmelos (L.) possessed the invitro and invivo anti-inflammatory activity. We have found a positive correlation by 0.89, 0.85, and 0.86 respectively with TPC for BSA, egg-albumin denaturation inhibitory activity, and anti-proteinase. The review article of Zhu et al. (2018) also seconds our observation which mentioned multiple studies that reported anti-inflammatory activities of polyphenols against various in vitro models.
Proteinases are mainly linked with arthritic reactions, which are present in the lysosomal granules of neutrophils. The proteinases from leukocytes play a major role in promoting tissue damage during inflammation and it was suggested that the extract may prevent these lysosomal elements such as bactericidal enzymes and proteinases from resisting further damage (Chou, 1997). To inhibit these serine proteinases it is essential to have hydroxyl groups at the ortho and para position of the benzene ring attached to the benzopyrone in flavonoids. It is proposed that these hydroxyl groups may form the hydrogen bridges with the amino acid residues located near or, at the active site on the trypsin molecule (Brinkworth et al., 1992). Drying methods significantly affect (p = 5.82 × 10−11) the anti-proteinase activity. The anti-proteinase activity was increased for BM and BF by 2.66% and 1.64% respectively, while the same was decreased by 2.46% and 5.89% for BH and BS respectively than BP (Table 7). Behera et al., (2012), reported invivo anti-proteinase activity of unripe bael on albino rats.
Table 7.
Antioxidant activities, anti-diabetic activity, anti-inflammatory activities of all the samples (BP: fruit pulp; BM: microwave dried sample; BS: sun dried sample, BH: hot air dried sample; BF: freeze dried sample).
| Medicinal Activities (%) | BP | BM | BS | BH | BF |
|---|---|---|---|---|---|
| Anti-diabetic activity | |||||
| α-amylase inhibitory (AD) | 74.59 ± 0.57c | 78.85 ± 1.54a | 72.38 ± 0.88e | 73.93 ± 0.75d | 78.7 ± 0.71b |
|
Anti-inflammatory activity | |||||
| Bovine-serum denaturation inhibitory (BS) | 39.66 ± 1.88c | 42.34 ± 0.97a | 32.25 ± 1.20e | 36.01 ± 0.67d | 40.72 ± 0.80b |
| Egg-albumin denaturation inhibitory (EA) | 57.42 ± 0.98c | 63.18 ± 0.32a | 49.1 ± 0.39e | 54.38 ± 1.18d | 61.03 ± 0.74b |
| Anti-proteinase activity (AP) | 80.19 ± 1.45c | 82.32 ± 0.76a | 75.47 ± 0.95e | 78.22 ± 2.30d | 81.5 ± 0.84b |
|
Anti-oxidant activity | |||||
| DPPH scavenging activity (DPPH) | 58.95 ± 0.33c | 64.81 ± 0.28a | 37.13 ± 0.25e | 52.95 ± 0.29d | 62.95 ± 0.3b |
| FRAP assay (FRAP) | 93.81 ± 0.49b | 96.18 ± 0.34a | 90.29 ± 0.42c | 94.44 ± 0.4b | 89.91 ± 0.42d |
| H2O2 scavenging activity (H2O2) | 64.47 ± 0.35a | 61.78 ± 0.29b | 59.46 ± 0.18c | 59.34 ± 0.39c | 60.68 ± 0.3b |
| Super oxide scavenging activity (SO) | 79.21 ± 0.25c | 82.49 ± 0.42b | 75.9 ± 0.27d | 74.8 ± 0.19d | 83.2 ± 0.33a |
| ABTS assay (ABTS) | 83.91 ± 0.17c | 86.42 ± 0.26a | 84.38 ± 0.21b | 79.65 ± 0.1d | 83.5 ± 0.14b |
All values are reported on dry basis. The amounts are provided in the mean ± standard deviation (s.d.) form, after performing at least triplicate experiments. The superscript letters a, b, c, d and e shown in the table represent the significant differences (p < 0.05) for the same parameters of the different samples.
3.6. Anti-diabetic activity
It is estimated that the number of diabetic patients will be around 439 million by 2030, out of which 300 million are expecting type-2 diabetes mellitus (T2DM) (Shaw et al., 2010). The awareness of the issue has led to a tendency to screen natural sources for anti-diabetic activity. Starch contains amylase and amylopectin, where, á-amylase splits the á-1,4-glycosidic linkages in amylose to yield maltose and glucose (American Diabetes Association, 2014,Zhou et al., 2013) and also hydrolyses amylopectin and glycogen to produce a mixture of branched and unbranched oligosaccharides (Smith and Morton, 2010), which further in small intestine breaks down into monosaccharides. á-amylase inhibitors (also known as, starch blockers) prevent or reduce down the absorption of starch into the body, by reducing or, inhibiting the activities of á-amylases. Flavonols like quercetin and flavones have an experimentally substantiated effect on á-amylase inhibition activity. Both flavonols and flavones contain a C=O group at the 4th carbon atom in the pyrone ring. Therefore the benzopyrone and benzene rings are involved in a pi-electron conjugation system through p-orbital sharing. Hence these two sub-classes of flavonoid groups deliberately provide amended binding property along with á-amylase. Both the benzene rings possess hydroxyl groups in the ortho and meta position, which is the favourable position for a reaction with catalytic enzymes (Lo Piparo et al., 2008). Bael is recognized for decades due to its anti-diabetic property (Prathapan et al., 2012). BM and BF showed significantly higher anti-diabetic activity than BP with 5.71% and 5.51% greater activity with respect to fresh pulp respectively, whereas the same property was significantly lower for BH and BS; 0.89% and 2.96% decrease in anti-diabetic activity pertaining to the fresh pulp, was observed for hot-air and sun drying respectively (Table 7). Zhu et al. (2012) observed higher anti-diabetic activity for freeze dried bitter melon than hot-air dried sample. This activity is positively correlated by 0.79 and 0.7 with TPC and TFC.
3.7. Multivariate analysis
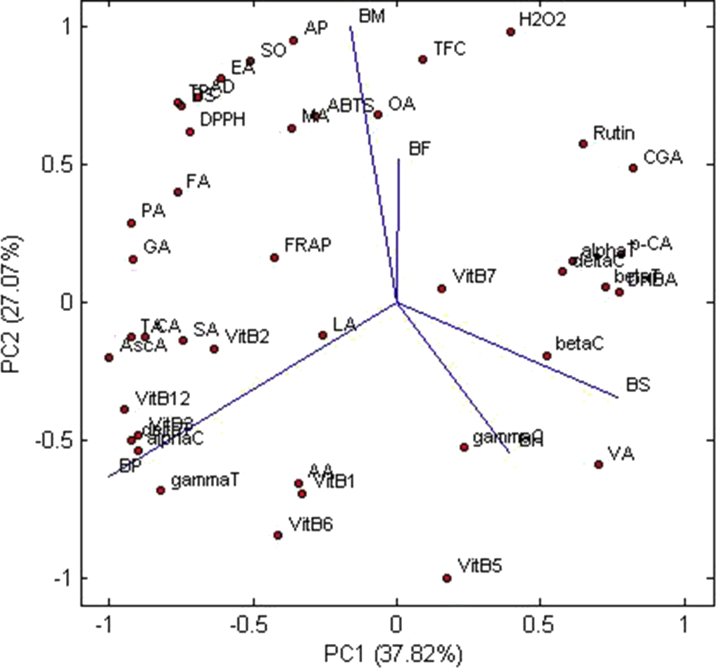
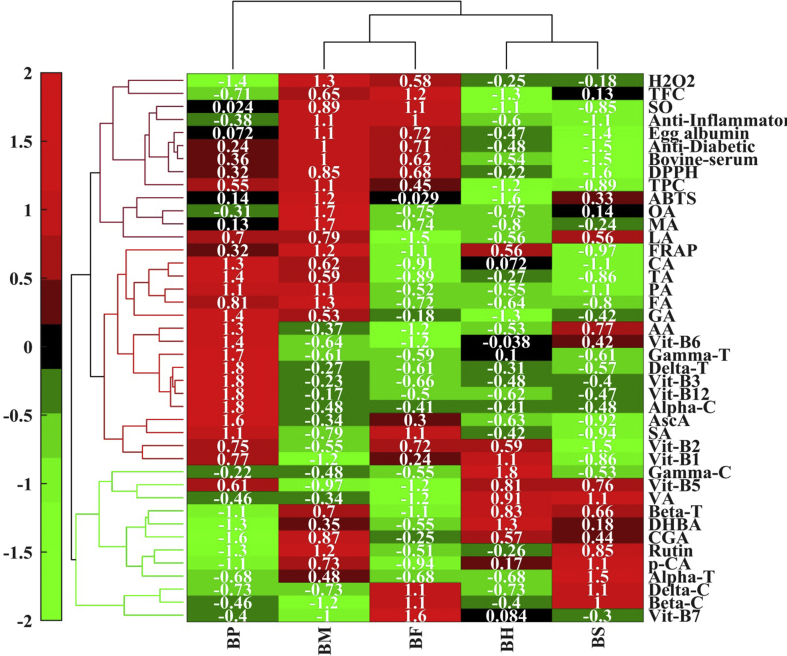
Multivariate analysis is performed if, it is not feasible to proceed with a simple visual assessment to determine the differences between different samples. We used PCA, HCA heat map, and correlation for altogether 43 factors, including bioactive compounds and medicinal activities to study the relative variability among the different Aegle marmelos (L.)samples. The results of PCA shows that 94.05% of the total variability comprised of the first three components, PC1 (37.82%), PC2 (27.07%), and PC3 (22.29%). From Figure 1, it can be observed that BS, BH, and BF exist on the positive side of the PC1 while BP and BM contributed to the negative side. BM and BF loading showed similar positive PC2 scores, but the BP, BH, and BS were located differently from the other two samples in the negative quadrant of PC2. The Figure 1 also suggested that the most of the factors like vitamin B2 (B2), succinic acid (SA), tartaric acid (TA), citric acid (CA), ascorbic acid (Asc A), vitamin B12 (B12), vitamin B3 (B3), δ-tocopherol (delta T) and α-carotene (alpha C), went along with the BP, followed by BM where, anti-inflammatory activity, super-oxide activity (SO), bovine-serum activity, egg-albumin activity, anti-diabetic activity, TPC, DPPH formed a cluster and malic acid (MA), ABTS, oxalic acid (OA), TFC are overlapped by both BM and BF. For the BS, vanillic acid (VA), dihydroxy benzoic acid (DHBA), protocatechuic acid (p-CA), α-tocopherol (alpha T), β-tocopherol (beta T), β-carotene (beta C), δ-carotene (delta C) formed a cluster separately and only vitamin C went with the BH. Even from the dendrogram of the factors shown in Figure 2, three main clusters were observed. Based on the characteristics of the investigated factors, the dendrogram of the different samples clearly depicted that BP stands differently, while BF and BM shared a common cluster. For both BH and BS, higher destruction of some of factors was observed due to prolonged processing time, so they were clustered together. The heat map provided a correlation between the samples and factors, which provide a clear summary of the interrelation within the effects of drying and compositional aspects of bael.
Figure 1.
Principal component analysis of all the samples (BP: fruit pulp; BM: microwave dried sample; BS: sun dried sample, BH: hot air dried sample; BF: freeze dried sample) based on total polyphenols (TPC: Total polyphenol content; TFC: Total flavonoid content), phenolic acids (GA: Gallic acid; DHBA: 2, 3-dihydroxy benzoic acid; CGA: Chlorogenic acid; p-CA: p-Coumaric acid; VA: Vanillic acid), vitamin B group (Vit B1: Thiamine(vitamin B1); Vit B3: Niacin(vitamin B3); Vit B6: Pyridoxine(vitamin B6); Vit B5: Pantothenic acid(vitamin B5); Vit B7: Biotin(vitamin B7); Vit B12: Cobalamins(vitamin B12); Vit B2: Riboflavin(vitamin B2)), tocopherols (delta-T: δ Tocopherol; gamma-T: γ Tocopherol; beta-T: β Tocopherol; alpha-T: α Tocopherol), orgainc acids and vitamin C (OA: Oxalic acid; TA: Tartaric acid; MA: Malic acid; LA: Lactic acid; AA: Acetic acid; CA: Citric acid; PA: Propionic acid; SA: Succinic acid; FA: Fumaric acid; Asc-A: Ascorbic acid), carotenes (alpha-C: α Carotene; beta-C: β Carotene; gamma-C: γ Carotene; delta-C: δ Carotene), anti-oxidant activities (DPPH: DPPH scavenging activity; FRAP: FRAP assay; H2O2: H202 scavenging activity; SO: Super oxide scavenging activity; ABTS: ABTS assay, anti-diabetic activity (AD) and anti-inflammatory activity (EA: egg-albumin denaturation inhibitory activity, BS: bovine-serum denaturation inhibitory activity, AP: anti-proteinase activity.
Figure 2.
Hierarchical cluster-heat map analysis of all the samples(BP: fruit pulp; BM: microwave dried sample; BS: sun dried sample, BH: hot air dried sample; BF: freeze dried sample) based on total polyphenols (TPC: Total polyphenol content; TFC: Total flavonoid content), phenolic acids (GA: Gallic acid; DHBA: 2, 3-dihydroxy benzoic acid; CGA: Chlorogenic acid; p-CA: p-Coumaric acid; VA: Vanillic acid), vitamin B group (Vit B1: Thiamine(vitamin B1); Vit B3: Niacin(vitamin B3); Vit B6: Pyridoxine(vitamin B6); Vit B5: Pantothenic acid(vitamin B5); Vit B7: Biotin(vitamin B7); Vit B12: Cobalamins(vitamin B12); Vit B2: Riboflavin(vitamin B2)), tocopherols (delta-T: δ Tocopherol; gamma-T: γ Tocopherol; beta-T: β Tocopherol; alpha-T: α Tocopherol), orgainc acids and vitamin C (OA: Oxalic acid; TA: Tartaric acid; MA: Malic acid; LA: Lactic acid; AA: Acetic acid; CA: Citric acid; PA: Propionic acid; SA: Succinic acid; FA: Fumaric acid; Asc-A: Ascorbic acid), carotenes (alpha-C: α Carotene; beta-C: β Carotene; gamma-C: γ Carotene; delta-C: δ Carotene), anti-oxidant activities (DPPH: DPPH scavenging activity; FRAP: FRAP assay; H2O2: H202 scavenging activity; SO: Super oxide scavenging activity; ABTS: ABTS assay), anti-diabetic activity (AD) and anti-inflammatory activity (EA: egg-albumin denaturation inhibitory activity, BS: bovine-serum denaturation inhibitory activity, AP: anti-proteinase activity).
4. Conclusion
From the results it can be observed that different components get affected differently depending on the mechanism of the drying like, in BM bound components (mainly polyphenols) get released, so the total content increased with respect to BP and some were destroyed in the lesser amount due lower time of operation. For, BS some enzymes get optimal working conditions, which affected the compounds differently (polyphenols get reduced, whereas tocopherols increased), while surprisingly BH showed enhancements of vitamin (vitamin B1 and B5) and carotene (γ-carotene) content. Maximum retention for most of the components was observed in low-temperature treatment (BF). The best result was found in BM, with respect to the medicinal activities. The study also shows that the mineral content doesn't change along with different drying using EDXRF analysis.
This comprehensive study gives a detailed discussion on the effects of drying on different components (in total of 50 factors for each drying technique) and shows the diversity in the behaviour of the factors. It also reveals the proper quantitative composition of bael, many of them not previously revealed. It also reflects light upon the traditional preservation method, which can be used to overproduced pulpy crops.
Declarations
Author contribution statement
Sudipta Kumar Hazra: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Tanmay Sarkar: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Molla Salauddin: Performed the experiments; Wrote the paper.
Hassan I. Sheikh: Performed the experiments; Analyzed and interpreted the data.
Siddhartha Pati: Analyzed and interpreted the data; Wrote the paper.
Runu Chakraborty: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank Dr. Dipankar Mondal (Associate Professor (Scientist E), Institute of Nano Science and Technology) for his guidance to perform EDXRF analysis. We also acknowledge Dr. Ramdhan Majhi (MDLC facility, Indian Institute of Chemical Biology) for his continuous support and guidance in HPLC analysis.
Contributor Information
Siddhartha Pati, Email: patisiddhartha@gmail.com.
Runu Chakraborty, Email: crunu@hotmail.com.
References
- Abirami A., Nagarani G., Siddhuraju P. In Vitro antioxidant, anti-diabetic, cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrushystrixand C.maxima fruits. Food Sci. Hum. Wellness. 2014;3:16–25. [Google Scholar]
- Ainsworth E., Gillespie K. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007;2:875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- Al Juhaimi F., Özcan M.M., Uslu N., Ghafoor K. The effect of drying temperatures on antioxidant activity, phenolic compounds, fatty acid composition and tocopherol contents in citrus seed and oils. J. Food Sci. Technol. 2018;55(1):190–197. doi: 10.1007/s13197-017-2895-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alajaji S., El-Adawy T. Nutritional composition of chickpea (Cicerarietinum L.) as affected by microwave cooking and other traditional cooking methods. J. Food Compos. Anal. 2006;19:806–812. [Google Scholar]
- Allegretta I., Gattullo C.E., Renna M., Paradiso V.M., Terzano R. Rapid multi-element characterization of microgreens via total-reflection X-rayfluorescence (TXRF) spectrometry. Food Chem. 2019;296:86–93. doi: 10.1016/j.foodchem.2019.05.187. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(1):81–90. [Google Scholar]
- AOAC International . fifteenth ed. 1990. Official Methods of Analysis. Arlington, Va, USA. [Google Scholar]
- AOAC International . seventeenth ed. 2000. Official Methods of Analysis of the Association of the Official Analytical Chemists. Washington, DC, USA. [Google Scholar]
- Baba S.A., Malik S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of ArisaemajacquemontiiBlume. J. Taibah Univ. Sci. 2015;9(4):449–454. [Google Scholar]
- Babior B.M. Superoxide: a two-edged sword. Braz. J. Med. Biol. Res. 1997;30(2):141–155. doi: 10.1590/s0100-879x1997000200001. [DOI] [PubMed] [Google Scholar]
- Baliga M.S., Bhat H., Joseph N., Fazal F. Phytochemistry and medicinal uses of theBael fruit (Aeglemarmelos Correa): a concise review. Food Res. Int. 2011;44:1768–1775. [Google Scholar]
- Baliga M.S., Mane P.P., Joseph N., Jimmy R. Gastrointestinal Disorders, Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease. Academic Press; 2013. Chapter 20 - review on the protective effects of the indigenous Indian medicinal plant, bael (Aeglemarmelos correa) pp. 313–324. [Google Scholar]
- Bechoff A., Dufour D., Dhuique-Mayer C., Marouzé C., Reynes M., Westby A. Effect of hot air, solar and sun drying treatments on provitamin A retention in orange-fleshed sweet potato. J. Food Eng. 2009;92(2):164–171. [Google Scholar]
- Behera J.P., Mohanty B., Ramani Y.R., Rath B., Pradhan S. Effect of aqueous extract of Aeglemarmelos unripe fruit on inflammatory bowel disease. Indian J. Pharmacol. 2012;44(5):614–618. doi: 10.4103/0253-7613.100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brinkworth R.I., Stoermer M.J., Fairlie D.P. Flavones are inhibitors of HIV-1 proteinase. Biochem. Biophys. Res. Commun. 1992;188(2):631–637. doi: 10.1016/0006-291x(92)91103-w. [DOI] [PubMed] [Google Scholar]
- Calín-Sánchez Á., Figiel A., Hernández F. Chemical composition, antioxidant capacity, and sensory quality of pomegranate (punicagranatum L.)Arils and rind as affected by drying method. Food Bioprocess Technol. 2013;6:1644–1654. [Google Scholar]
- Cano A., Alcaraz O., Acosta M., Arnao M.B. On-line antioxidant activity determination: comparison of hydrophilic and lipophilic antioxidant activity using the ABTS•+ assay. Redox Rep. 2002;7(2):103–109. doi: 10.1179/135100002125000334. [DOI] [PubMed] [Google Scholar]
- Cardoso D.R., Libardi S.H., Skibsted L.H. Riboflavin as a photosensitizer. Effects on human health and food quality. Food Funct. 2012;3(5):487–502. doi: 10.1039/c2fo10246c. [DOI] [PubMed] [Google Scholar]
- Chan J.C.C., Cheung P.C.K., Ang P.O. Comparative studies on the effect of three drying methods on the nutritional composition of seaweed Sargassumhemiphyllum (Turn.) C. Ag. J. Agric. Food Chem. 1997;45(8):3056–3059. [Google Scholar]
- Chandra S., Dey P., Bhattacharya S. Preliminary in vitro assessment of anti-inflammatory property of Mikaniascandens flower extract. J. Adv. Pharm. Educ. Res. 2012;2(1):25–31. [Google Scholar]
- Chang C., Lin H., Chang C., Liu Y. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. J. Food Eng. 2006;77:478–485. [Google Scholar]
- Charoensiddhi S., Anprung P. Bioactive compounds and volatile compounds of Thai bael fruit (AegleMarmelos (L.)Correa) as a valuable source for functional food ingredients. Int. Food Res. J. 2008;15 [Google Scholar]
- Choe E., Huang R., Min D.B. Chemical reactions and stability of riboflavin in foods. J. Food Sci. 2005;70(1):R28–R36. [Google Scholar]
- Chou C.T. The anti-inflammatory effect of an extract of Tripterygiumwilfordii hook F on adjuvant-induced paw oedema in rats and inflammatory mediators release. Phytother Res. 1997;11:152–154. [Google Scholar]
- Chu N.T., Clydesdale F.M. Decomposition of organic acids during processing and storage. J. Milk Food Technol. 1976;39:477–480. [Google Scholar]
- Çoklar H., Akbulut M. Effect of sun, oven and freeze-drying on anthocyanins, phenolic compounds and antioxidant activity of black grape (Ekşikara) (Vitisvinifera L.) S. Afr. J. Enol. Vitic. 2017;38(2):264–272. [Google Scholar]
- Combs G.F. Elsevier Academic Press; San Diego: 2012. The Vitamins: Fundamental Aspects in Nutrition and Health. [Google Scholar]
- Delgado T., Ramalhosa E., Pereira J.A., Casal S. Organic acid profile of chestnut (Castaneasativa Mill.) as affected by hot air convective drying. Int. J. Food Prop. 2018;21(1):557–565. [Google Scholar]
- Diamante L.M., Bai X., Busch J. Fruit leathers: method of preparation and effect of different conditions on qualities. Int. J. Food Sci. 2014;2014:1–12. doi: 10.1155/2014/139890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Wang R., Zhang J., Li G., Zhang J., Ou S., Shan Y. Effect of drying temperature on the sugars, organic acids, limonoids, phenolics, and antioxidant capacities of lemon slices. Food SciBiotechnol. 2017;26:1523–1533. doi: 10.1007/s10068-017-0221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie G.G., Duthie S.J., Kyle J.A.M. Plant polyphenols in cancer and heart disease. J. Agric. Food Chem. 2000;56:1084–1090. [Google Scholar]
- Dwivedi B.K., Arnold R.G. Chemistry of thiamine degradation in food products and model systems: a review. J. Agric. Food Chem. 1973;21(1):54–60. doi: 10.1021/jf60185a004. [DOI] [PubMed] [Google Scholar]
- Feng X., Zhang H., Yu P. X-ray fluorescence application in food, feed, and agricultural science: a critical review. Crit. Rev. Food Sci. Nutr. 2020;60:1–12. doi: 10.1080/10408398.2020.1776677. [DOI] [PubMed] [Google Scholar]
- Fritsche S., Wang X., Jung C. Recent advances in our understanding of tocopherol biosynthesis in plants: an overview of key genes, functions, and breeding of vitamin E improved crops. Antioxidants (Basel, Switzerland) 2017;6(4):99. doi: 10.3390/antiox6040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez-Valdivieso G., Cardeñosa R., Vera J.M., Pineda M., Aguilar M. γ-Tocopherolmethyltransferase from the green alga Chlamydomonasreinhardtii: functional characterization and expression analysis. Physiol. Plantarum. 2011;143(4):316–328. doi: 10.1111/j.1399-3054.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- Gao Q., Wu C., Wang M., Xu B., Du L. Effect of drying of jujubes (Ziziphusjujuba Mill.) on the contents of sugars, organic acids, α-tocopherol, β-carotene, and phenolic compounds. J. Agric. Food Chem. 2012;60(38):9642–9648. doi: 10.1021/jf3026524. [DOI] [PubMed] [Google Scholar]
- Gasecka M., Siwulski M., Magdziak Z., Budzyńska S., Stuper K., Niedzielski P., Mleczek M. The effect of drying temperature on bioactive compounds and antioxidant activity of Leccinumscabrum (Bull.) Gray and Hericiumerinaceus (Bull.) Pers. J. Food Sci. Technol. 2020;57:513–525. doi: 10.1007/s13197-019-04081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gell P.G., Benacerraf B. Studies on hypersensitivity. II. Delayed hypersensitivity to denatured proteins in Guinea pigs. Immunology. 1959;2(1):64–70. [PMC free article] [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem. J. 1964;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A.G., Rall T., Nies A., Taylor P. Insel PA: analgesic-antipyretics and antiinflammatory agents: DrugEmployed in the treatment of rheumatoid arthritis and gout. InGoodman Gilman’s Pharmacol. Basis Therapeut. 1990:638–681. [Google Scholar]
- Gunathilake K.D.P.P., Ranaweera K.K.D.S., Rupasinghe H.P.V. Influence of boiling, steaming and frying of selected leafy vegetables on the in vitro anti-inflammation associated biological activities. Plants. 2018;7(22) doi: 10.3390/plants7010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannaker P., Haukka M., Sen S.K. Comparative study of ICP-AES and XRF analysis of major and minor constituents on geological materials. Chem. Geol. 1984;42:319–324. [Google Scholar]
- Hazra S.K., Salauddin M., Sarkar T., Roy A., Chakraborty R. Effect of freeze drying on antioxidant properties of bael fruit (Aglemarmelos) Biotechnol. Biol. Sci. 2019:426–430. [Google Scholar]
- He K., Merchant A., Rimm E.B., Rosner B.A., Stampfer M.J., Willett W.C., Ascherio A. Folate, vitamin B6, and B12 intakes in relation to risk of stroke among men. Stroke. 2004;35:169–174. doi: 10.1161/01.STR.0000106762.55994.86. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H., Nassiri-Asl M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J. Endocrinol. Invest. 2014;37:783–788. doi: 10.1007/s40618-014-0096-3. [DOI] [PubMed] [Google Scholar]
- Hu S., Feng X., Huang W., Ibrahim S., Liu Y. Effects of drying methods on non-volatile taste components of Strophariarugoso-annulatamushrooms. LWT. 2020;127:109428. [Google Scholar]
- Ishihara J., Iso H., Inoue M., Iwasaki M., Okada K., Kita Y., Kokubo Y., Okayama A., Tsugane S. Intake of folate, vitamin B6, and vitamin B12 and the risk of CHD: the Japan public health center-based prospective study cohort I. J. Am. Coll. Nutr. 2008;27:127–136. doi: 10.1080/07315724.2008.10719684. [DOI] [PubMed] [Google Scholar]
- Islam M.M., Shams B., Siraj S., Hasan M.K., Masum S.M., Chowdhury J.U. Comparative study of minerals content in green and ripe bael (wood apple) powder. Int. J. Basic Appl. Sci. 2013;11:133–136. [Google Scholar]
- Jimenez-Alvarez D., Giuffrida F., Vanrobaeys F., Golay P.A., Cotting C., Lardeau A., Keely B.J. High-throughput methods to assess lipophilic and hydrophilic antioxidant capacity of food extracts in vitro. J. Agric. Food Chem. 2008;56(10):3470–3477. doi: 10.1021/jf703723s. [DOI] [PubMed] [Google Scholar]
- Kadakal Ç., Ekinci R., Yapar A. The effect of cooking and drying on the watersoluble vitamins content of bulgur. Food Sci. Technol. Int. 2007;13:349–354. [Google Scholar]
- Kamiloglu S., Capanoglu E. Polyphenol content in figs (Ficuscarica L.): effect of sun-drying. Int. J. Food Prop. 2015;18(3):521–535. [Google Scholar]
- Kasote D.M., Jayaprakasha G.K., Patil B.S. Leaf disc assays for rapid measurement of antioxidant activity. Sci. Rep. 2019;9:1884. doi: 10.1038/s41598-018-38036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C., Kapoor H.C. Antioxidants in fruits and vegetables - the millennium’s health. Int. J. Food Sci. Technol. 2008;36(7):703–725. [Google Scholar]
- Khoo H., Prasad K.N., Kong K., Jiang Y., Ismail A. Carotenoids and their isomers: color pigments in fruits and vegetables. Molecules. 2011;16:1710–1738. doi: 10.3390/molecules16021710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Vecchia C., Decarli A., Pagano R. Vegetable consumption and risk of chronic disease. Epidemiology. 1998;9:208–210. [PubMed] [Google Scholar]
- Laoretani D., Fernández M., Crapiste G., Nolasco S. Effect of drying operating conditions on Canola oil tocopherol content. Antioxidants (Basel, Switzerland) 2014;3(2):190–199. doi: 10.3390/antiox3020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.T, Lin W.C, Yu B., Lee T.T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals - A review. Asian-Australas. J. Anim. sci. 2017:299–308. doi: 10.5713/ajas.16.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Feng T., Zhou F., Zhou S., Liu Y., Li W., Ye R., Yang Y. Effects of drying methods on the tasty compounds of Pleurotuseryngii. Food Chem. 2015;166:358–364. doi: 10.1016/j.foodchem.2014.06.049. [DOI] [PubMed] [Google Scholar]
- Lim T.K. 2012. Aeglemarmelos, Edible Medicinal and Non-medicinal Plants; pp. 594–618. [Google Scholar]
- Lo Piparo E., Scheib H., Frei N. Flavonoids for controlling starch digestion: structural requirements for inhibiting human alpha-amylase. J. Med. Chem. 2008;51(12):3555–3561. doi: 10.1021/jm800115x. [DOI] [PubMed] [Google Scholar]
- Maharaj V., Sankat C.K. Quality changes in dehydrated dasheen leaves: effects of blanching pre-treatments and drying conditions. Food Res. Int. 1996;29(5–6):563–568. [Google Scholar]
- Manandhar B., Paudel K.R., Sharma B., Karki R. Phytochemical profile and pharmacological activity of Aeglemarmelos Linn. J. Integr. Med. 2018 doi: 10.1016/j.joim.2018.04.007. [DOI] [PubMed] [Google Scholar]
- McCarthy W.P., Daly K., Fenelon A., O’Connor C., McCarthy N.A., Hogan S.A., Tobin J.T., O’Callaghan T.F. Energydispersive X-ray fluorescence spectrometry as a tool for the rapid determination of the five major minerals (Na, Mg, K, P and Ca) in skim milk powder. Int. J. Dairy Technol. 2019;73:459–467. [Google Scholar]
- Neeraj B.V., Johar V. Bael (Aeglemarmelos) extraordinary species of India: a review. Int. J. Curr. Microbiol. Appl. Sci. 2017;6(3):1870–1887. [Google Scholar]
- Nguyen V.T., Le M.D. Influence of various drying conditions on phytochemical compounds and antioxidant activity of carrot peel. Beverages. 2018;4(4):80. [Google Scholar]
- Novaković M., Stevanović S., Gorjanović S., Jovanovic P., Tešević V., Janković M., Sužnjević D. Changes of hydrogen peroxide and radical-scavenging activity of raspberry during osmotic, convective, and freeze-drying. J. Food Sci. 2011;76(4):C663–C668. doi: 10.1111/j.1750-3841.2011.02144.x. [DOI] [PubMed] [Google Scholar]
- Pal P., Singh S.V., Singh S.K., Schandra S., Chataurvedi O.P. Studies on the storage life of bael (Aeglemarmelos correa) Int. J. Agric. Invent. 2017;2(1):28–32. [Google Scholar]
- Panda S.K., Sahu U., Behera S., Ray R. Bio-processing of bael [Aeglemarmelos L.] fruits into wine with antioxidants. Food Biosci. 2013;5:34–41. [Google Scholar]
- Pati S., Chatterji A., Dash B.P., Raveen B.N., Sarkar T., Shahimi S., Atan H.E., Binti T.S.B.M., Jena P., Mohanta Y.K., Acharya D. Structural Characterization and Antioxidant Potential of Chitosan by γ-Irradiation from the Carapace of Horseshoe Crab. Polymers. 2020;12(10) doi: 10.3390/polym12102361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskov S., Timchenko L., Grimm W.D., Rzhepakovsky I., Avanesyan S., Sizonenko M., Kurchenko V. Effects of various drying methods on some physico-chemical properties and the antioxidant profile and ACE inhibition activity of oyster mushrooms (PleurotusOstreatus) Foods. 2020;9(2):160. doi: 10.3390/foods9020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prathapan A., Krishna M.S., Nisha V.M., Sundaresan A., Raghu K.G. Polyphenol rich fruit pulp of Aeglemarmelos (L.) Correa exhibits nutraceutical properties to down regulate diabetic complications—an in vitro study. Food Res. Int. 2012;48(2):690–695. [Google Scholar]
- Qing-guo Hu., Min Z., Mujumdar A.S., Wei-hua D., Jin-cai S. Effects of different drying methods on the quality changes of granular Edamame. Dry. Technol. 2006;24(8):1025–1032. [Google Scholar]
- Rahman S., Parvin R. Therapeutic potential of Aeglemarmelos (L.)- an overview. Asian Pac. J. Trop Dis. 2014;4(1):71–77. [Google Scholar]
- Rajan S., Gokila M., Jency P., Brindha P., Sujatha R.K. Antioxidant and Phytochemical properties of AegleMarmelos fruit pulp. Int. J. Curr. Pharm. Res. 2011;3:65–70. [Google Scholar]
- Ranganna S. Tata McGraw-Hill Book Co; New Delhi: 1986. Handbook of Analysis and Quality Control for Fruit and Vegetable Products. [Google Scholar]
- Robbins R.J. Phenolic acids in foods: an overview of analytical methodology. J. Agric. Food Chem. 2003;51:2866–2887. doi: 10.1021/jf026182t. [DOI] [PubMed] [Google Scholar]
- Roy S.K., Saran S., Kitinoja L. Woodhead Publishing; 2011. 9 - Bael (Aeglemarmelos (L.) Corr. Serr.),Postharvest Biology and Technology of Tropical and Subtropical Fruits; pp. 186–216. [Google Scholar]
- Saifullah M., McCullum R., McCluskey A., Vuong Q. Effects of different drying methods on extractable phenolic compounds and antioxidant properties from lemon myrtle dried leaves. Heliyon. 2019;5(12) doi: 10.1016/j.heliyon.2019.e03044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini R.K., Shetty N., Prakash M., Giridhar P. Effect of dehydration methods on retention of carotenoids, tocopherols, ascorbic acid and antioxidant activity in Moringaoleifera leaves and preparation of a RTE product. J. Food Sci. Technol. 2014;51:2176–2182. doi: 10.1007/s13197-014-1264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajib M., Hoque M., Yeasmin S., Khatun H. Minerals and heavy metals concentration in selected tropical fruits of Bangladesh. Int. Food Res. J. 2014;21:1731–1736. [Google Scholar]
- Sakat S., Juvekar A.R., Gambhire M.N. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int. J. Pharm. Pharm. Sci. 2010;2(1):146–155. [Google Scholar]
- Sami R., Li Y., Qi B., Wang S., Zhang Q., Han F., Ma Y., Jing J., Jiang L. HPLC analysis of water-soluble vitamins (B2, B3, B6, B12, and C) and fat-soluble vitamins (E, K, D, A, and β-carotene) of Okra (Abelmoschusesculentus) J. Chem. 2014;2014:1–6. [Google Scholar]
- Sánchez-Riaño A.M., Solanilla-Duque J.F., Méndez-Arteaga J.J., Váquiro-Herrera H.A. Bioactive potential of Colombian feijoa in physiological ripening stage. J. Saudi Soc. Agric. Sci. 2019 [Google Scholar]
- Santos P.H.S., Silva M.A. Kinetics of L-ascorbic acid degradation in pineapple drying under ethanolic atmosphere. Dry. Technol. 2009;27(9):947–954. [Google Scholar]
- Sarkar T., Salauddin M., Hazra S.K., Chakraborty R. A novel data science application approach for classification of nutritional composition, instrumental colour, texture and sensory analysis of bael fruit (Aeglemarmelos (L) correa) Int. J. Intell. Networks. 2020;1:59–66. [Google Scholar]
- Sarkar T., Salauddin M., Hazra S.K., Chakraborty R. Effect of cutting edge drying technology on the physicochemical and bioactive components of mango (Langra variety) leather. J. Agric. Food Res. 2020:100074. [Google Scholar]
- Sarkar T., Salauddin M., Hazra S.K., Chakraborty R. Artificial neural network modelling approach of drying kinetics evolution for hot air oven, microwave, microwave convective and freeze dried pineapple. SN Appl. Sci. 2020;2:1621. [Google Scholar]
- Sarkar T., Salauddin M., Chakraborty R. In-depth pharmacological and nutritional properties of bael (Aegle marmelos): A critical review. J. Agric. Food Res. 2020;2:1–23. [Google Scholar]
- Sarkar T., Salauddin M., Hazra S.K., Chakraborty R. The impact of raw and differently dried pineapple (Ananas comosus) fortification on the vitamins, organic acid and carotene profile of dairy rasgulla (sweetened cheese ball) Heliyon. 2020;6(10) doi: 10.1016/j.heliyon.2020.e05233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnellbaecher A., Binder D., Bellmaine S., Zimmer A. Vitamins in cell culture media: stability and stabilization strategies. Biotechnol. Bioeng. 2019;116(6):1537–1555. doi: 10.1002/bit.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi F., Naczk M. Technomic Pub. Co.; USA: 1995. Food phenolics.Sources, Chemistry, Effects and Applications, Lancaster. [Google Scholar]
- Sharma N., Dubey W. History and taxonomy of Aeglemarmelos: a review. Int. J. Pure Appl. Biosci. 2013;1:7–13. [Google Scholar]
- Sharma P.C., Bhatia V., Bansal N., Sharma A. A review on Bael tree. Nat. Product. Radiance. 2006;6(2):171–178. [Google Scholar]
- Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of theprevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Shigeoka S., Ishiko H., Nakano Y., Mitsunaga T. Isolation and properties of γ-tocopherolmethyltransferase in Euglena gracilis. Biochimicaet Biophysica Acta (BBA) - Lipids Lipid Metabol. 1992;1128(2–3):220–226. doi: 10.1016/0005-2760(92)90311-i. [DOI] [PubMed] [Google Scholar]
- Slatnar A., Klancar U., Stampar F., Veberic R. Effect of drying of figs (Ficuscarica L.) on the contents of sugars, organic acids, and phenolic compounds. J. Agric. Food Chem. 2011;59(21):11696–11702. doi: 10.1021/jf202707y. [DOI] [PubMed] [Google Scholar]
- Smith M.E., Morton D.G. second ed. Churchill Livingstone; 2010. 8 - DIGESTION and ABSORPTION, the Digestive System; pp. 129–152. [Google Scholar]
- Thi N.D., Hwang E. Effects of drying methods on contents of bioactive compounds and antioxidant activities of black chokeberries (Aroniamelanocarpa) Food Sci. Biotechnol. 2016;25:55–61. doi: 10.1007/s10068-016-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Zhao Y., Huang J., Zeng H., Zheng B. Effects of different drying methods on the product quality and volatile compounds of whole shiitake mushrooms. Food Chem. 2015;197(A) doi: 10.1016/j.foodchem.2015.11.029. [DOI] [PubMed] [Google Scholar]
- Turhan S., Zararslz A., Karabacak H. Determination of element levels in selected wild mushroom species in Turkey using non-destructive analytical techniques. Int. J. Food Prop. 2010;13(4):723–731. [Google Scholar]
- Uribe E., Pardo-Orellana C.M., Vega-Gálvez A., Ah-Hen K.S., Pastén A., García V., Aubourg S.P. 2019. Effect of Drying Methods on Bioactive Compounds, Nutritional, Antioxidant, and Antidiabetic Potential of Brown Alga Durvillaeaantarctica, Drying Technology. [Google Scholar]
- Uribe E., Vega-Galvez A., Vargas N., Pasten A., Rodrıguez K., Ah-Hen K.S. Phytochemical components and amino acid profile of Brown seaweed Durvillaeaantarctica as affected by air drying temperature. J. Food Sci. Technol. 2018;55:4792–4801. doi: 10.1007/s13197-018-3412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoed V., de Clercq N., Echim C., Adnsjelkovic M., Leber E., De Wettinck K., Verhé R. Berry seeds: a source of speciality oils with high content of bioactives and nutritional value. J. Food Lipids. 2009;16:33–49. [Google Scholar]
- Valadez-Carmona L., Plazola-Jacinto C.P., Hernández-Ortega M., Hernández-Navarro M.D., Villarreal F., Necoechea-Mondragón H., Ortiz-Moreno A., Ceballos-Reyes G. , Effects of microwaves, hot air and freeze-drying on the phenolic compounds, antioxidant capacity, enzyme activity and microstructure of cacao pod husks (Theobroma cacao L.) Innov. Food Sci. Emerg. Technol. 2017;41:378–386. [Google Scholar]
- Walker R., Famiani F. In: Warrington I., editor. Vol. 45. John Wiley & Sons, Inc.; 2018. pp. 371–430. (“Chapter 8.Organic Acids in Fruits: Metabolism, Functions and Contents” in Horticultural Reviews). [Google Scholar]
- Watanabe F., Abe K., Fujita T., Goto M., Hiemori M., Nakano Y. Effects of microwave heating on the loss of vitamin B12 in foods. J. Agric. Food Chem. 1998;46(1):206–210. doi: 10.1021/jf970670x. [DOI] [PubMed] [Google Scholar]
- Wijewardana R., Nawarathne S., Wickramasinghe I., Gunawardane C., Wasala W., Thilakarathne B. Retention of physicochemical and antioxidant properties of dehydrated bael (AegleMarmelos) and palmyra (BorassusFlabellifer) fruit powders. Procedia Food Sci. 2016;6 [Google Scholar]
- Wildermuth S.R., Young E.E., Were L.M. Chlorogenic acid oxidation and its reaction with sunflower proteins to form green-colored complexes. Compr. Rev. Food Sci. Food Saf. 2016 doi: 10.1111/1541-4337.12213. [DOI] [PubMed] [Google Scholar]
- Williams L., O'Connar A., Latore L., Dennis O., Ringer S., Whittaker J., Conrad J., Vogler B., Rosner H., Kraus W. The in vitro anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (Immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals, in the early stages of the drug discovery process. W. Indian Med. J. 2008;57:327–331. [PubMed] [Google Scholar]
- Yadav N., Singh P., Mehrotra R. Determination of SomeEthnomedicinally important constituents of Aeglemarmelos fruit during different stages of ripening. Chin. J. Nat. Med. 2011;9(3):204–209. [Google Scholar]
- Zadernowski R., Czaplicki S., Naczk M. Phenolic acid profiles of mangosteen fruits (Garciniamangostana) Food Chem. 2009;112:685–689. [Google Scholar]
- Zhou K., Slavin M., Lutterodt H., Whent M., Eskin N.A.M., Yu L. third ed. Academic Press; 2013. Chapter 1 - Cereals and Legumes, Biochemistry of Foods; pp. 3–48. [Google Scholar]
- Zhu F., Du B., Xu B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: a review. Crit. Rev. Food Sci. Nutr. 2018;58(8):1260–1270. doi: 10.1080/10408398.2016.1251390. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Dong Y., Qian X., Cui F., Guo Q., Zhou X., Wang Y., Zhang Y., Xiong Z. Effect of superfine grinding on antidiabetic activity of bitter melon powder. Int. J. Mol. Sci. 2012;13(11):14203–14218. doi: 10.3390/ijms131114203. [DOI] [PMC free article] [PubMed] [Google Scholar]