Abstract
Associational resistance theory predicts that insect herbivory decreases with increasing tree diversity in forest ecosystems. However, the generality of this effect and its underlying mechanisms are still debated, particularly since evidence has accumulated that climate may influence the direction and strength of the relationship between diversity and herbivory.
We quantified insect leaf herbivory and leaf chemical defences (phenolic compounds) of silver birch Betula pendula in pure and mixed plots with different tree species composition across 12 tree diversity experiments in different climates. We investigated whether the effects of neighbouring tree species diversity on insect herbivory in birch, that is, associational effects, were dependent on the climatic context, and whether neighbour-induced changes in birch chemical defences were involved in associational resistance to insect herbivory.
We showed that herbivory on birch decreased with tree species richness (i.e. associational resistance) in colder environments but that this relationship faded as mean annual temperature increased.
Birch leaf chemical defences increased with tree species richness but decreased with the phylogenetic distinctiveness of birch from its neighbours, particularly in warmer and more humid environments.
Herbivory was negatively correlated with leaf chemical defences, particularly when birch was associated with closely related species. The interactive effect of tree diversity and climate on herbivory was partially mediated by changes in leaf chemical defences.
Our findings confirm that tree species diversity can modify the leaf chemistry of a focal species, hence its quality for herbivores. They further stress that such neighbour-induced changes are dependent on climate and that tree diversity effects on insect herbivory are partially mediated by these neighbour-induced changes in chemical defences.
Keywords: associational resistance, Betula pendula, biodiversity, leaf phenolics, mixed forests, phylogenetic diversity, plant–insect interactions, TreeDivNet
1. Introduction
The influence of plant species diversity on plant–herbivore interactions is an old but still topical question for ecologists (Castagneyrol et al., 2019; Siemann et al., 1998). Ecological studies in forests have demonstrated that increased tree species diversity generally leads to lower amount of damage caused by insect herbivores, a phenomenon known as associational resistance (Barbosa et al., 2009; Castagneyrol et al., 2014; Iverson et al., 2014). But several other studies have also reported no change (Haase et al., 2015) or even increased insect herbivory when mixing tree species, that is, associational susceptibility (Castagneyrol et al., 2018; Schuldt et al., 2010). These inconsistent findings demonstrate the need for improved understanding of ecological processes underlying tree diversity effects on insect herbivory in forests.
Early attempts to explain associational resistance were mainly based on processes determined by host plant density (e.g. the resource concentration hypothesis; Hambäck et al., 2014), or mediated by natural enemies (e.g. the natural enemies hypothesis; Moreira et al., 2016) or by non-host volatile chemical compounds (e.g. the semiochemical diversity hypothesis; Jactel et al., 2011). Only recently, researchers have recognized that associational resistance could also result from changes in host traits, such as nutritional quality and production of anti-herbivores defences, induced by heterospecific neighbours (Castagneyrol et al., 2018; Glassmire et al., 2016; Moreira et al., 2014). Indeed, several reviews have shown that tree diversity can promote plant productivity (Jactel et al., 2018; Zhang et al., 2012), which, in turn, could result in reduced production of leaf chemical defences because of a trade-off between growth and defences (Endara & Coley, 2011; Herms & Mattson, 1992). Consistently, increased tree diversity was found to be associated with lower concentrations of leaf chemical defences, including polyphenols, tannins, glycosids and alkaloids (Castagneyrol et al., 2018; Muiruri et al., 2019; Rosado-Sánchez et al., 2018; Walter et al., 2012).
Assuming that tree species traits involved in interactions with herbivores or in resource acquisition are phylogenetically conserved, greater tree phylogenetic diversity should amplify trait dissimilarity between species (Srivastava et al., 2012) and hence, the magnitude of associational resistance mechanisms including the effect of tree diversity on defences. In particular, greater plant functional diversity is expected to (a) extend resource concentration effects to herbivore species with wider diet breadth (Castagneyrol et al., 2014), (b) foster host-finding disruption due to the greater complexity of the visual and chemical environments (Jactel et al., 2011) and (c) increase resource use complementarity between plant species resulting in higher growth and lower defence levels. Consistently, the degree of phylogenetic or functional dissimilarity between focal plant species and their heterospecific neighbours was found to affect associational effects on herbivory, with greater impacts than plant species richness per se in most cases (Castagneyrol et al., 2014; Dinnage, 2013; Schuldt et al., 2014; Yguel et al., 2011).
Until now, studies on associational resistance have largely overlooked possible interactions with abiotic factors (but see Kambach et al., 2016; Walter et al., 2012). Yet, climate has well-documented effects on herbivore activity, abundance and diversity, as well as on plant growth and the production of plant anti-herbivore defences, including leaf phenolics. This was demonstrated through both experiments (e.g. Bauerfeind & Fischer, 2013; Pineau et al., 2017) and observational studies along latitudinal and elevation gradients (Kozlov et al., 2015; Moreira, Castagneyrol, et al., 2018; Moreira, Galman, et al., 2018; Rodríguez-Castañeda, 2013; but see Anstett et al., 2016; Moles et al., 2011). Importantly, recent studies showed an interplay between tree diversity and climatic conditions, whereby climate could alter the effect of tree diversity on ecosystem processes (Castagneyrol et al., 2018; Jactel et al., 2019; Ratcliffe et al., 2017) and conversely, tree diversity could buffer the adverse effect of extreme climatic events on trees (Jactel et al., 2017). Being able to account for the effect of climate is therefore a major opportunity to strengthen our understanding of the variability in the magnitude and direction of associational effects in mixed forests.
Using a unique network of tree diversity experiments ranging from temperate to boreal biomes (TreeDivNet; Paquette et al., 2018), we quantified insect leaf herbivory and leaf chemical defences (phenolic compounds) in silver birch Betula pendula in plots with different tree species composition across 12 locations with different climates. First, we addressed the effects of tree species diversity on insect herbivory and leaf chemical defences in silver birch, asking which of tree species richness, phylogenetic diversity or their combination best explained both response variables. We hypothesized that tree diversity was associated with lower herbivory (associational resistance) and lower chemical defence levels (due to higher complementarity and growth-defence trade-off), and that these effects were stronger when considering phylogenetic distinctiveness of the focal species instead of species richness, considering that phylogenetic diversity accounts for niche differentiation. Second, we tested whether diversity–herbivory and diversity–defences relationships depended on climate. Since there is no consensus in the available literature, we had no particular directional hypothesis regarding the influence of climate. Finally, we tested whether climate and diversity effects on insect herbivory were mediated by changes in leaf chemical defences. Our study is one of the first to investigate defence-mediated associational effects on insect herbivory in relation with the climatic context. We aimed at building towards a more comprehensive understanding of the interactive effects of tree species diversity and climate on forest resistance to insect pests.
2. Materials and Methods
2.1. Natural history
The silver birch (Betula pendula Roth, Betulaceae) is a deciduous tree native to most of Europe (Beck et al., 2016) that tolerates an extremely wide range of climatic and edaphic conditions. In its native range, silver birch supports a large community of insect herbivores, especially lepidopteran and hymenopteran (i.e. sawflies) leaf chewers and miners (Beck et al., 2016; Zúbrik et al., 2013).
2.2. Plot and tree selection in TreeDivNet experiments
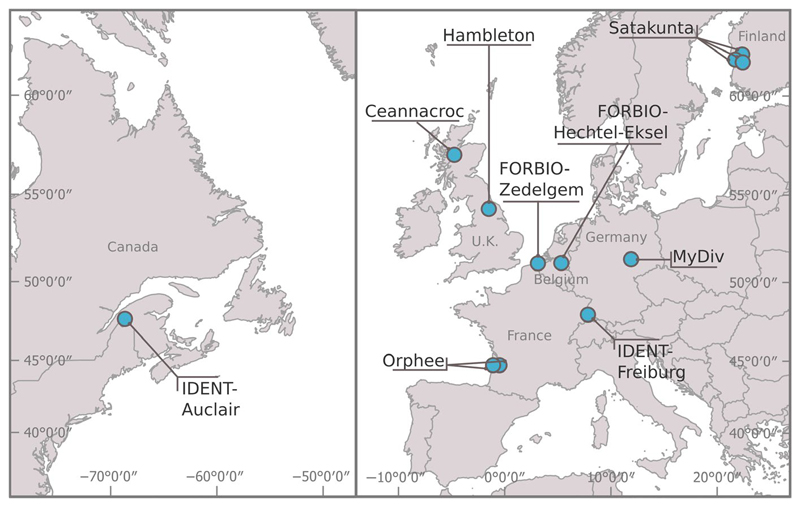
TreeDivNet consists of 27 long-term tree diversity experiments specifically designed to investigate the effects of tree species diversity on forest functioning (Grossman et al., 2018). Because the experiments are globally distributed, TreeDivNet is particularly well-suited to explore how tree diversity effects on herbivory vary with climate. We collected data from 12 sites belonging to six tree diversity experiments where silver birch was present (Figure 1; Table S1). These sites encompassed temperate and boreal biomes of the northern hemisphere and spanned over 17 decimal degrees in latitude, covering about half of the latitudinal span of silver birch (Beck et al., 2016). At each site, we selected silver birch monoculture plots and mixed species plots where silver birch was present. Tree species richness in those mixtures ranged from two to six species (including silver birch) and included broadleaved or coniferous species, or a mix of both. The species composition of mixture plots varied among sites. At certain sites, species composition types were replicated in two to three blocks. We randomly selected three to five birch trees in the core area of each experimental plot (i.e. avoiding border trees to limit edge effects). The final dataset was derived from 564 trees planted in 157 plots.
Figure 1. Map of the TreeDivNet experimental sites included in the study.
2.3. Leaf collection and damage assessment
In all, 50 leaves per birch tree were haphazardly sampled in mid-July 2017 (2014 for the three Finnish sites). We assessed insect leaf herbivory as the overall percentage of leaf area removed by three common feeding guilds of insect herbivores: chewers, miners and skeletonizers. We assigned each leaf to one of seven classes of damage: (a) 0% of leaf area removed, (b) 1%–5%, (c) 6%–15%, (d) 16%–25%, (e) 26%–50%, (f) 51%–75% and (g) 76%–100%. To reduce unconscious bias in insect herbivory assessment, we split leaves from each tree into two equal pools that were separately processed by two independent observers unaware of leaf origin. Then, we aggregated insect herbivory estimates at the tree level by averaging the median values of damage class of all leaves. In the case of the three Finnish sites (Satakunta areas 1, 2 and 3), the methodology differed slightly but was still consistent (see Muiruri et al., 2019). On average, insect herbivores damaged 3.91% (±2.60%) of leaf area (damages ranged from 0.36% to 13.03% of leaf area; Table S2). We are confident that we did not underestimate herbivory by overlooking missing leaves since leaves did not start falling at the sampling time. The observed levels of insect herbivory were low and comparable with those observed in other studies on silver birch (e.g. Castagneyrol et al., 2018; Kozlov et al., 2015; Muiruri et al., 2019).
2.4. Phylogenetic isolation
Given that tree species diversity effects on herbivores can be affected by phylogenetic dissimilarity between the tree species in the mixture, we used phylogenetic information to account for differences in tree species composition of mixed stands across the experiments (Srivastava et al., 2012). We used the phylomatic function from the brranching package in r (with tree R20120829; Chamberlain, 2018) to obtain an overall phylogenetic tree comprising the overall pool of tree species (Figure S2). Node ages down to family level were derived from Magallón et al. (2015). Genus node ages were approximated by dividing the length of the edge from the family node to the tip by two. The same was subsequently done for species nodes considering edge length from the genus node to the tip. For each plot, we pruned the overall phylogenetic tree to obtain a sub-tree corresponding to the pool of tree species present in the plot.
Many metrics have been developed to characterize phylogenetic diversity of a pool of species (Miller et al., 2017). Here, we computed Faith’s total phylogenetic diversity (PD, pd function in picante package; Kembel et al., 2010), mean pairwise phylogenetic distance (MPD, mpd function), mean phylogenetic distance between birch and associated species (β-MPD, comdist function) and birch evolutionary distinctiveness (ED, evol.distinct function; Redding & Mooers, 2006). PD and MPD are community-level phylogenetic diversity indices, whereas β-MPD and ED are species–species indices representing the phylogenetic isolation of silver birch from other tree species present in each plot. ED was eventually preferred to other phylogenetic diversity metrics because it was less correlated with species richness (Figure S1).
2.5. Climate data
We extracted mean annual temperature and total annual rainfall averaged over the 1979–2013 period (hereafter referred to as ‘temperature’ and ‘rainfall’, respectively) for each study site using the Climatologies at High resolution for the Earth’s Land Surface Areas dataset (CHELSA; Karger et al., 2017; Figure S4a). In the ORPHEE experiment, half of the plots were located in irrigated blocks sprinkled with 3 mm of water per night from May to October. An annual surplus of 552 mm was thus added to the rainfall amount obtained from the CHELSA database in these plots. To account for this additional irrigation treatment, data collected in the ORPHEE experiment were considered as data from two distinct sites (irrigated vs. non-irrigated). Overall, our network of tree diversity experiments covered a 17° latitudinal gradient and encompassed 10°C of variation in the mean annual temperature and 964 mm of variation in the annual rainfall.
2.6. Leaf phenolics
Leaf phenolics have been reported to confer resistance against insect herbivores in several tree species including birch (Forkner et al., 2004; Moreira, Galman, et al., 2018; Riipi et al., 2005) and therefore represent a suitable proxy for assessing leaf chemical defences (or leaf nutritional quality to herbivores). We quantified the concentration of phenolic compounds on a subsample of five birch leaves—with little (<5%) or no damage—per tree, following a procedure based on ultra-high-performance liquid chromatography (as in Moreira, Galman, et al., 2018; Visakorpi et al., 2019). Following drying (at 45°C during 72 hr) and grinding of leaves, we extracted phenolic compounds from 20 mg of powdered dry leaf tissue with 1 ml of 70% methanol in an ultrasonic bath for 15 min, followed by centrifugation (Moreira et al., 2014). We then transferred the extracts to chromatographic vials. Ultra-High-Performance Liquid-Chromatograph (UHPLC Nexera LC-30AD; Shimadzu) equipped with a Nexera SIL-30AC injector and one SPD-M20A UV/VIS photodiode array detector was used to perform the chromatographic analyses. The compound separation was carried out on a Kinetex™ 2.6 μm C18 82–102 Å, LC Column 100 × 4.6 mm, protected with a C18 guard cartridge. The flow rate was 0.4 ml/min and the oven temperature was set at 25°C. The mobile phase consisted of two solvents: water–formic acid (0.05%) (A) and acetonitrile–formic acid (0.05%) (B), starting with 5% B and using a gradient to obtain 30% B at 4 min, 60% B at 10 min, 80% B at 13 min and 100% B at 15 min. The injection volume was 3 μl. We identified four groups of phenolic compounds: flavonoids, ellagitannins and gallic acid derivates (hydrolysable tannins), proanthocyanidins (condensed tannins) and hydroxycinnamic acid (precursors to lignins). We quantified flavonoids as rutin equivalents, condensed tannins as catechin equivalents, hydrolysable tannins as gallic acid equivalents, and precursors to lignins as ferulic acid equivalents (Moreira, Castagneyrol, et al., 2018; Moreira, Galman, et al., 2018). Quantification of these phenolic compounds was achieved by external calibration using calibration curves at 0.25, 0.5, 1, 2 and 5 μg/ml. Total phenolic concentration was equal to 334.57 ± 107.48 mg/g of leaf dry matter on average and ranged from 13.51 to 775.08 mg/g (Table S3).
2.7. Statistical analyses
First, we used linear mixed models (LMM) to test for the effects of tree species richness and birch phylogenetic distinctiveness on insect herbivory and leaf phenolic concentration (two normally distributed response variables). To test whether tree species richness, phylogenetic distinctiveness of birch or the combination of both best predicted the response variables, we built three models for each response variable with (a) tree species richness, (b) birch evolutionary distinctiveness (ED) or (c) tree species richness and birch ED (main effects plus interaction) as predictors. We calculated the Akaike information criterion corrected for small sample size (AICc) of each model to identify the best model—with the lowest AICc—for a given response variable (Burnham & Anderson, 2002; Johnson & Omland, 2004). If the AICc difference between two models was less than two, they were considered equally likely. The best herbivory and phenolic models were used in the subsequent analyses.
Second, we used LMMs to test for the effects of climate on insect herbivory and leaf phenolic concentration. For each response variable, we used the full version of the best model(s) (with the lowest AICc) from the first step to which we added temperature and rainfall main effects, as well as all two-way and three-way interactions.
Third, we tested whether the variability in insect herbivory was accounted for by phenolic concentration. Specifically, we included leaf phenolic concentration as a covariate in the full model(s) from the second step. Two-way interactions involving leaf phenolic concentration and temperature, rainfall or tree diversity were also included in the model(s) to test for interactive effects. By comparing results of insect herbivory models without (second step) versus with leaf phenolics (third step), we tested whether the effects of tree diversity and climate on insect herbivory were mediated by changes in leaf chemical defences. That would be the case if a significant effect of tree species diversity and/or climate on insect herbivory became non-significant after including leaf phenolic concentration as a covariate.
In the last two steps, full models were simplified following a backward selection approach, which consisted of sequentially dropping the terms with the lowest impact on model fit, starting with the highest-order interactions. Model simplification was done using log-likelihood tests based on a χ 2 distribution with significance threshold set at α = 0.05.
In all models, we accounted for the hierarchical structure of data using Plot nested within Block, nested within Site, as a random factor (i.e. 1|Site/Block/Plot in R syntax). By doing so, we accounted for the hierarchical structure of the data, as well as potential variance in the response variables arising from uncontrolled factors such as site location within birch distribution range, local soil properties, species pool or tree planting density. Because of the nature of the TreeDivNet network, these factors were confounded with the Site. To further ensure that herbivory or defence patterns were not driven by uncontrolled factors at the site level, we regressed the residuals of the final models against latitude and climatic conditions (Figure S5). We found no particular pattern in the residuals suggesting that no ‘hidden treatments’ at site level (associated with their latitudinal position) might have biased our test of diversity and climate effects.
In all models, predictors were scaled and centred, which made it possible to compare the magnitude of the effects even when interaction terms were significant (Schielzeth, 2010). Collinearity among all predictors was found to be weak enough to limit inflation of the variance of estimated model parameters (variation inflation factors [VIFs] less than two). Model parameters were estimated by restricted likelihood estimation and the significance (α = 0.05) of the regression coefficients was tested with Student t tests and Satterthwaite’s approximation for degrees of freedom. We evaluated model fit by calculating the percentage of variance explained by fixed and by fixed plus random effects (Nakagawa & Schielzeth, 2013).
Concentrations of all types of phenolic compounds were positively correlated with each other (Figure S6), which made it inappropriate to use all phenolic types as predictors of insect herbivory in the same model (inflation of the variance of estimated model parameters). We therefore ran separate models for each type of phenolics. Concentrations of all types of phenolic compounds and concentrations of total phenolics covaried with climate and diversity predictors, that is, direction of effects were consistent across phenolic types (Table 1; Table S4). We additionally summarized the information on phenols using a principal component analysis (PCA) with the concentrations of the four individual phenolic compounds (Figure S7). The three first components of the PCA altogether explained 92% of the variance and the points were well homogeneously distributed. The first axis was associated with the concentrations of flavonoids and hydrolysable tannins, the second axis with the concentration of condensed tannins and the third with the concentration of lignin. Besides, PCA coordinates on the first three axes were all positively correlated with the concentration of total phenolics (Figure S8). Based on these elements, we choose to present the results for total phenolic concentration only in the main text.
Table 1.
Effects of tree species diversity on (a) insect herbivory and (b) leaf phenolic concentration. Comparison of models with species richness, birch evolutionary distinctiveness (ED) or both diversity metrics as predictors. Bold predictors have a significant effect. AICc*: best (lowest) AICc
| Predictors | Standardized estimate ± SD | df | t value | p value | AICc | Random intercept effects (variance ± SD) | |||
|---|---|---|---|---|---|---|---|---|---|
| Site | Block:Site | Plot:Block:Site | |||||||
| (a) Herbivory | |||||||||
| w/Sp. richness | 0.00 (0.57) | 2,322.47* | 3.10 ± 1.76 | 0.25 ± 0.50 | 0.46 ± 0.68 | ||||
| Intercept | 3.81 ± 0.53 | 11.28 | 7.24 | <0.001 | |||||
| Species richness | −0.03 ± 0.09 | 143.74 | −0.34 | 0.737 | |||||
| w/Birch ED | 0.00 (0.57) | 2,322.57* | 3.10 ± 1.76 | 0.25 ± 0.50 | 0.46 ± 0.68 | ||||
| Intercept | 3.81 ± 0.53 | 11.28 | 7.25 | <0.001 | |||||
| Birch ED | 0.01 ± 0.09 | 122.90 | 0.11 | 0.916 | |||||
| w/Sp. richness and Birch ED | 0.00 (0.57) | 2,328.52 | 3.07 ± 1.75 | 0.25 ± 0.50 | 0.45 ± 0.67 | ||||
| Intercept | 3.75 ± 0.53 | 11.42 | 7.12 | <0.001 | |||||
| Species richness | 0.01 ± 0.10 | 140.37 | 0.11 | 0.916 | |||||
| Birch ED | 0.16 ± 0.13 | 128.18 | 1.20 | 0.231 | |||||
| Sp. richness × Birch ED | 0.27 ± 0.17 | 135.56 | 1.56 | 0.121 | |||||
| (b) Phenolics | |||||||||
| w/Sp. richness | 0.00 (0.65) | 5,819.74 | 5,930 ± 77 | 0 ± 0 | 1663 ± 41 | ||||
| Intercept | 332.29 ± 23.67 | 10.09 | 14.04 | <0.001 | |||||
| Species richness | 4.35 ± 4.67 | 146.67 | 0.93 | 0.353 | |||||
| w/Birch ED | 0.02 (0.65) | 5,810.79 | 5,953 ± 77 | 0 ± 0 | 1,446 ± 38 | ||||
| Intercept | 331.80 ± 23.68 | 10.09 | 14.01 | ||||||
| Birch ED | −15.05 ± 4.71 | 126.40 | −3.19 | 0.002 | |||||
| w/Sp. richness and Birch ED | 0.03 (0.65) | 5,798.61* | 5,997 ± 77 | 0 ± 0 | 1,375 ± 37 | ||||
| Intercept | 332.00 ± 23.87 | 10.26 | 13.91 | <0.001 | |||||
| Species richness | 10.74 ± 4.90 | 141.50 | 2.19 | 0.030 | |||||
| Birch ED | −19.33 ± 6.87 | 147.95 | −2.82 | <0.006 | |||||
| Sp. richness × Birch ED | −0.43 ± 8.98 | 156.27 | −0.05 | 0.962 | |||||
All analyses were conducted in r (version 3.5.1; R Core Development Team, 2013) with the following packages: lmerTest (Kuznetsova et al., 2017), car (Fox & Weisberg, 2018) and MuMIn (Barton, 2018).
3. Results
3.1. Tree species diversity effects on insect herbivory and leaf phenolics
We found no significant effect of species richness or birch evolutionary distinctiveness (ED) per se on insect herbivory, and no interactive effect of the two diversity metrics either (Table 1a). The herbivory model with species richness and the herbivory model with birch ED had the lowest AICc values, not differing by more than two units (Table 1a). These two models were thus used in the subsequent analyses while the herbivory model with both diversity metrics, which had a higher AICc value (Table 1a), was eliminated.
We found a significant negative effect of birch ED on leaf phenolic such that birch leaves were less defended when birches were more phylogenetically distinct from their neighbours (Table 1b). In contrast, we found a significant and positive effect of tree species richness on leaf phenolic concentration. The phenolics model with birch ED only had a lower AICc value than the model with species richness only (Table 1b), but both of these models had higher AICc values than the model with both diversity variables included (Table 1b). Hence, the best phenolics model that was used in subsequent analyses was the model with both species richness and birch ED.
3.2. Effects of climate and tree diversity on insect herbivory
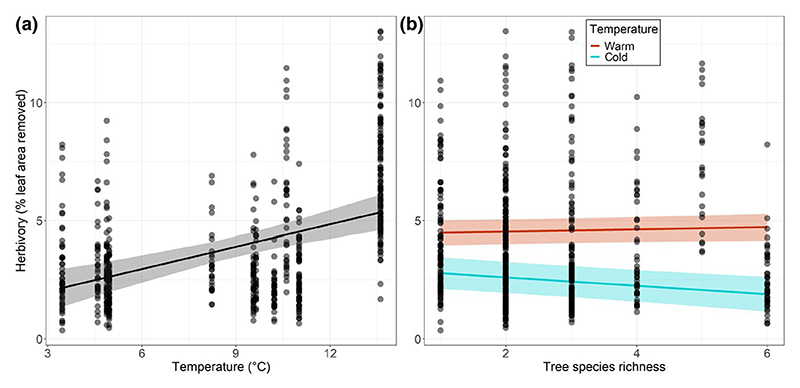
We found that insect herbivory on birch leaves significantly increased with increasing temperature (Figure 2a; Table 2a) both in the model with tree species richness and in the model with birch ED. Rainfall had no significant effect on insect herbivory.
Figure 2.
Relationships (a) between insect herbivory on silver birch leaves and mean annual temperature and (b) between insect herbivory and tree species richness for two contrasted temperature levels. The figure shows observed data (points) as well as model predictions (solid lines) and standard errors (shaded areas). In panel (a), species richness was set at a median value to compute predictions. ‘Warm’ and ‘cold’ temperature levels corresponded to 0.25 and 0.75 quartiles of the observed temperature range, respectively
Table 2.
Effects of tree diversity, temperature and rainfall on (a) insect herbivory and (b) leaf phenolic concentration. Predictors that were excluded from the final model during simplification are not shown. Bold predictors have a significant effect
| Predictors | Standardized estimate ± SD | df | t value | p value | Random intercept effects (variance ± SD) | |||
|---|---|---|---|---|---|---|---|---|
| Site | Block:Site | Plot:Block:Site | ||||||
| (a) Herbivory | ||||||||
| w/Sp. richness | 0.22 (0.59) | 1.95 ± 1.40 | 0.25 ± 0.50 | 0.41 ± 0.64 | ||||
| Intercept | 3.81 ± 0.43 | 10.35 | 8.93 | <0.001 | ||||
| Species richness | −0.05 ± 0.09 | 142.59 | −0.551 | 0.582 | ||||
| Temperature | 1.21 ± 0.46 | 10.26 | 2.64 | 0.024 | ||||
| Sp. richness × Temperature | 0.19 ± 0.09 | 135.27 | 2.14 | 0.034 | ||||
| w/Birch ED | 0.21 (0.59) | 1.91 ± 1.38 | 0.25 ± 0.50 | 0.45 ± 0.67 | ||||
| Intercept | 3.82 ± 0.42 | 10.34 | 9.05 | <0.001 | ||||
| Temperature | 1.21 ± 0.45 | 10.24 | 2.68 | 0.023 | ||||
| (b) Phenolics | ||||||||
| w/Sp. richness and Birch ED | 0.46 (0.67) | 1586.60 ± 39.82 | 0.00 ± 0.00 | 993.60 ± 31.52 | ||||
| Intercept | 327.30 ± 13.30 | 6.89 | 24.62 | <0.001 | ||||
| Species richness | 11.47 ± 4.39 | 148.33 | 2.61 | 0.010 | ||||
| Birch ED | −13.37 ± 5.16 | 140.89 | −2.59 | 0.011 | ||||
| Temperature | 53.06 ± 14.32 | 6.82 | 3.71 | 0.008 | ||||
| Rainfall | −31.14 ± 13.33 | 6.83 | −2.34 | 0.053 | ||||
| Birch ED × Temperature | −8.84 ± 4.89 | 124.77 | −1.81 | 0.073 | ||||
| Birch ED × Rainfall | −5.15 ± 4.75 | 131.52 | −1.09 | 0.280 | ||||
| Temp. × Rainfall | 29.79 ± 12.72 | 6.72 | 2.34 | 0.053 | ||||
| Birch ED × Temp. × Rainfall | −13.22 ± 4.15 | 121.02 | −3.19 | 0.002 | ||||
The effect of tree species richness on insect herbivory was contingent upon temperature (significant species richness × temperature interaction; Table 2a). In particular, insect herbivory decreased with increasing tree species richness at low temperatures but was not affected by tree species richness at higher temperatures (Figure 2b). In the final simplified model, tree species richness and temperature collectively explained 22% of the variability in insect herbivory (; ). By contrast, there was no significant effect of the interaction between birch ED and temperature or rainfall (Table 2a). Independent effects of birch ED and temperature collectively explained 21% of the variability in insect herbivory (; ).
3.3. Effects of climate and tree diversity on leaf phenolic concentration
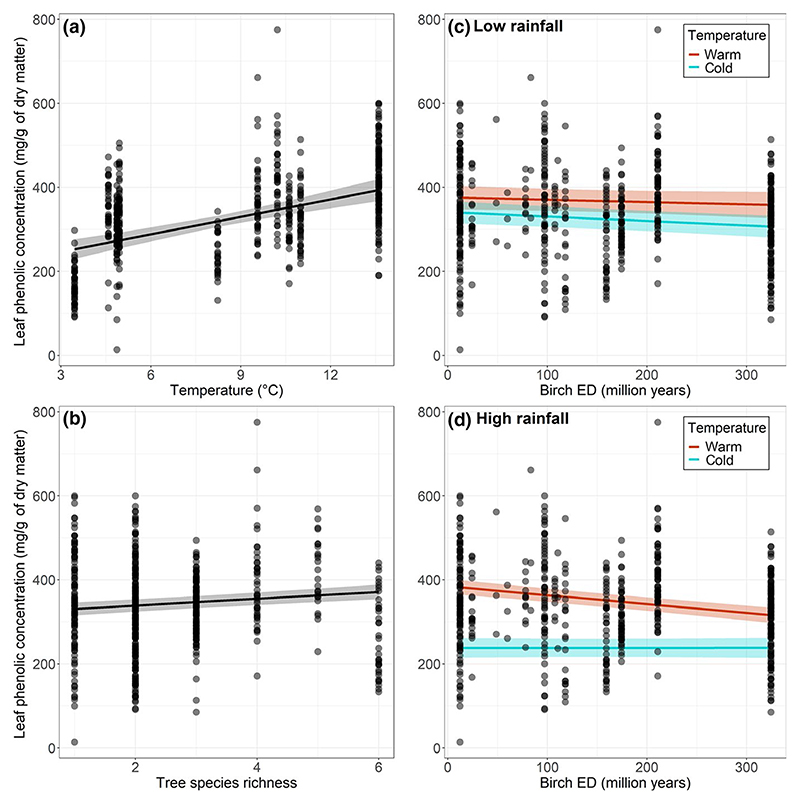
Leaf phenolic concentration significantly increased with increasing temperature and tended to decrease with increasing rainfall but not significantly (Table 2b; Figure 3a). In addition, leaf phenolic concentration significantly increased with species richness (Table 2b; Figure 3b) regardless of the climate (no significant interactions with rainfall or temperature). In contrast, the effect of birch ED on leaf phenolic concentration was contingent upon temperature and rainfall conditions (significant birch ED × temperature × rainfall interaction; Table 2b). Specifically, leaf phenolic concentration decreased with increasing birch phylogenetic distinctiveness independently of the temperature at low rainfall level (Figure 3c), but decreased more markedly with increasing phylogenetic distinctiveness of birch in warm conditions only at high rainfall level (Figure 3d). In the final simplified model, climate and tree species diversity collectively explained 46% of the variability in phenolic concentration of birch leaves (; ).
Figure 3.
Leaf phenolic concentration in birch leaves as a function of (a) temperature, (b) tree species richness and as a function of birch evolutionary distinctiveness (ED) for two contrasted levels of temperature under two contrasted levels of rainfall (c and d). The figure shows observed data (points) as well as model predictions (solid lines) and standard errors (shaded areas). ‘Warm’ and ‘cold’ temperature levels corresponded to 0.25 and 0.75 quartiles of the observed temperature range, respectively. ‘Low’ and ‘high’ rainfall levels corresponded to 0.25 and 0.75 quartiles of the observed rainfall range, respectively. The predictors that were not involved in the relationships shown were set at median values to compute predictions
3.4. Indirect trait-mediated effects of tree diversity and climate on herbivory
When we included leaf phenolic concentration as a covariate in the herbivory models—with either species richness or birch ED—we found that insect herbivory decreased with increasing leaf phenolic concentration in both cases (Table 3; Figure 4a). In the two models, the positive effect of temperature on insect herbivory remained significant (Table 3), which indicated that the effect of temperature on insect herbivory was not mediated by leaf phenolics.
Table 3.
Effects of leaf phenolic concentration on insect herbivory as a covariate of tree diversity, temperature and rainfall. Predictors that were excluded from the final model during simplification are not shown. Bold predictors have a significant effect
| Predictors | Standardized estimate ± SD | df | t value | p value | Random intercept effects (variance ± SD) | |||
|---|---|---|---|---|---|---|---|---|
| Site | Block:Site | Plot:Block:Site | ||||||
| Herbivory w/Sp. richness | 0.24 (0.58) | 1.77 ± 1.33 | 0.30 ± 0.55 | 0.42 ± 0.65 | ||||
| Intercept | 3.96 ± 0.43 | 9.30 | 9.20 | <0.001 | ||||
| Temperature | 1.41 ± 0.45 | 9.57 | 3.15 | 0.011 | ||||
| Phenolics | −0.29 ± 0.12 | 466.99 | −2.39 | 0.017 | ||||
| Herbivory w/Birch ED | 0.25 (0.59) | 1.84 ± 1.36 | 0.31 ± 0.55 | 0.35 ± 0.59 | ||||
| Intercept | 3.99 ± 0.44 | 9.29 | 9.11 | <0.001 | ||||
| Birch ED | 0.02 ± 0.10 | 111.88 | 0.22 | 0.826 | ||||
| Temperature | 1.41 ± 0.46 | 9.54 | 3.11 | 0.012 | ||||
| Phenolics | −0.25 ± 0.12 | 476.63 | −2.04 | 0.042 | ||||
| Birch ED × Phenolics | 0.23 ± 0.10 | 285.52 | 2.42 | 0.016 | ||||
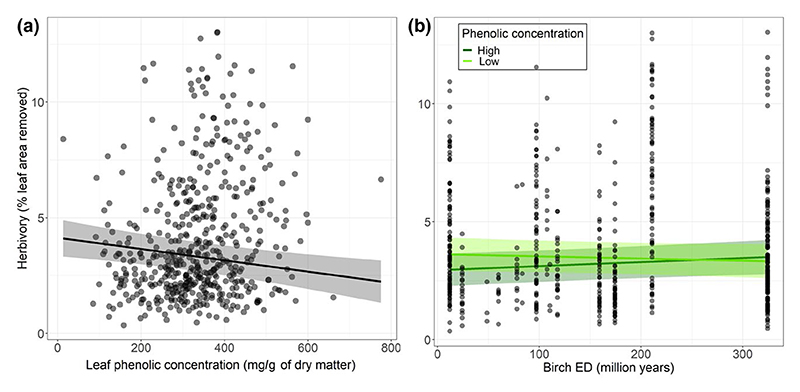
Figure 4.
Relationship (a) between insect herbivory and leaf phenolic concentration and (b) between insect herbivory and birch evolutionary distinctiveness (ED) for different levels of leaf phenolic concentration. The figure shows observed data (points) as well as model predictions (solid lines) and standard errors (shaded areas). ‘Low’ and ‘high’ phenolic concentration levels corresponded to 0.25 and 0.75 quartiles of the observed phenolic concentration range, respectively. The predictors that were not involved in the relationships shown were set at median values to compute predictions
In the herbivory model with species richness, we found that the effect of species richness—that was contingent upon temperature— became non-significant after including leaf phenolic concentration as a covariate (Table 3), indicating that the effect of tree species richness on insect herbivory was mediated by leaf phenolics. Temperature and leaf phenolic concentration collectively explained 24% of the variability in insect herbivory (; ).
In the herbivory model with birch ED, we found that birch ED effect on insect herbivory was contingent upon phenolic concentration in birch leaves (significant birch ED × phenolic concentration interaction, Table 3). In particular, when birch leaves had low phenolic concentration, insect herbivory decreased with increasing birch ED, while when birch leaves had high phenolic concentration insect herbivory increased with increasing birch ED (Figure 4b). Temperature, birch ED and leaf phenolic concentration collectively explained 25% of the variability in insect herbivory (; ).
4. Discussion
With this study, we showed that the effect of tree species diversity on insect herbivory on silver birch leaves, that is, associational effects, was climate-dependent and, in particular, varied with temperature. Our findings also showed that tree species diversity modified chemical defence levels in birch leaves and further suggested that such changes in leaf chemistry induced by heterospecific neighbours were partly climate-dependent. Finally, we found that associational effects were mediated by changes in defences under certain climatic conditions. Below, we discuss mechanisms underlying the observed patterns.
4.1. Effects of tree diversity on insect herbivory are climate dependent
We found no significant effects of either tree species richness or birch phylogenetic distinctiveness per se on background levels of insect herbivory on birch. In fact, we found evidence that tree diversity effects on herbivory were dependent on climate. This result could partly explain the variable effects of tree diversity on herbivory previously reported in the literature (Brezzi et al., 2017; Castagneyrol et al., 2014; Jactel et al., 2020; Kambach et al., 2016; Ratcliffe et al., 2017; Schuldt et al., 2010, 2014; Vehviläinen et al., 2007; Wein et al., 2016) including in studies focusing on birch trees (Castagneyrol et al., 2018; Haase et al., 2015; Muiruri et al., 2019; Setiawan et al., 2014). Specifically, we provided evidence for changes in associational effects along the mean annual temperature gradient: associational resistance of birch to insect herbivory occurred in cold conditions, whereas no associational effects could be detected in warm conditions. The mitigation of associational resistance with increasing temperature could be due to the higher proportion of generalist (versus specialist) herbivore species in warmer regions (Forister et al., 2015), that are less affected or even benefit from plant diversity (Castagneyrol et al., 2014). This finding might also be explained by the greater abundance and activity of herbivores in warmer climate that may, in turn, lower the resistance of mixed plant communities to herbivores. Supporting this view, associational resistance to the bean beetle Callosobruchus maculatus was found to decrease with the population density of this herbivore, likely because of conspecific avoidance behaviour (Merwin et al., 2017; but see Fernandez-Conradi et al., 2017). Higher herbivore density may also increase the probability for a host tree to be located and attacked, simply because proportionally more individuals will pass through the net of resistance mechanisms (e.g. resource concentration effect, host-finding disruption or predation by natural enemies), resulting in lower apparent resistance to herbivores. In support of this density dependence hypothesis, our results showed that background insect herbivory on birch leaves, although low, markedly increased with increasing mean annual temperature (Kozlov et al., 2015; Wang et al., 2016). Several mechanisms have been proposed to explain the positive effect of temperature on herbivory, including direct effects on herbivores’ developmental rate, winter survival and activity and indirect effects through reduced plant nutritional quality inducing compensatory feeding (Bale et al., 2002; Bauerfeind & Fischer, 2013; Garibaldi et al., 2011; Klapwijk et al., 2012).
We found no effect of rainfall on insect herbivory, neither directly nor through changes in the herbivore response to tree diversity. Yet, drought-induced water stress is known to increase tree susceptibility to defoliators (Carnicer et al., 2011; Jactel et al., 2012), and a previous study reported an increase in insect herbivory on birch under drought (Castagneyrol et al., 2018). We could not assess the effect of drought per se in the present study, and it is possible that annual rainfall does not reflect water availability to trees because of site-specific topology or edaphic conditions. Besides, ectophagous (skeletonizers and chewers) and endophagous (miners) herbivores may respond inconsistently to rainfall conditions because they live on the surface versus inside the leaves, which could also explain the absence of an overall response since we pooled the two groups. Therefore, the lack of effect of rainfall on herbivory in the present study should be interpreted with caution and this question should be further explored.
4.2. Levels of leaf defences are shaped by both tree diversity and climate
Our results showed that tree diversity modifies leaf chemistry of focal birches—and hence their quality for herbivores. Specifically, the concentration of leaf phenolics increased with increasing tree species richness, but decreased with increasing birch evolutionary distinctiveness, used as a proxy for birch functional distinctiveness in experimental plots (Srivastava et al., 2012). Positive effect of plant species diversity on plant chemical defences has been previously reported in birch (Castagneyrol et al., 2018; Muiruri et al., 2019) and other plant species (Bustos-Segura et al., 2017; Kostenko et al., 2017; Moreira et al., 2014). The underlying mechanisms however are poorly understood and the opposing effects of species richness and functional diversity suggest they are complex. On the one hand, defence induction in richer plant community could arise in response to greater herbivory (Karban & Baldwin, 1997) due to associational susceptibility. However, herbivore mediation of species richness effects on defences seems unlikely in our case since our results mainly report associational resistance (Figure 2b) and we found a negative association between herbivory and defence concentration (Figure 4a). On the other hand, it is plausible that the production of leaf phenolics reflected a trade-off between growth and defences. Indeed, assuming that ecologically important traits are phylogenetically conserved, increased allocation to growth in plots with functionally dissimilar species (e.g. through complementarity or facilitation) could lead to a concomitant reduction in defence investment (Bryant et al., 1983; Herms & Mattson, 1992). In this sense, studies have reported that experimental manipulation of resource availability (e.g. nutrients or water) can lead to concomitant and opposite modulations of growth and defence production (Gutbrodt et al., 2012; Lange et al., 2019). This process could be particularly strong in birch, a fast-growing, resource-acquisitive species. Consistently, a recent study found that tree species composition affected leaf chemistry in birch, with less defence compounds in phylogenetically more diverse mixtures (Castagneyrol et al., 2018).
A meta-analysis by Koricheva et al. (1998) supports the view that tree diversity primarily affects the local abiotic conditions (specifically nutrient, water or light availability) and that such effects subsequently shape plant secondary chemistry. In particular, studies have demonstrated that crown illumination can affect leaf chemical composition, with shading associated with lower carbohydrate and phenol concentrations in leaves of birch trees (Henriksson et al., 2003) and other species (Larsson et al., 1986; Mole et al., 1988). The opposing effects of tree species richness and birch phylogenetic distinctiveness on birch leaf phenolics could both relate to the relative heights or growth rates of the trees present in the plots and the light available to birch trees. Indeed, birch is a fast-growing, early successional species that is expected to be more shaded in monocultures or in plots where it is present at high density (self-shading), than in mixtures where it is present at lower density and mixed with slow-growing tree species. In our study, species richness increase was correlated with the probability to include broadleaved species growing slower than birch trees and with a reduction of birch proportion (Figures S2 and S3). Hence, the positive effect of species richness on leaf phenolic concentration in birch leaves might be explained by a reduction of shading in species-richer mixtures. On the opposite, the increase in birch phylogenetic distinctiveness was correlated with the proportion of fast-growing coniferous (vs. broadleaved) neighbours such as larches or pines (Figures S2 and S3) that were generally taller than birch trees. The decrease in leaf phenolic concentration with birch phylogenetic distinctiveness could therefore result from lower light availability in plots where birch is more phylogenetically isolated (mixed with a greater proportion of conifers). However, birches are able to adapt their crown architecture to better compete with their neighbours for light acquisition (Lintunen & Kaitaniemi, 2010), therefore potentially limiting the impact of neighbours on crown illumination and leaf chemistry and explaining the relatively low phenolic concentration changes observed along tree diversity gradients.
Because our study was not designed to determine the mechanisms underlying neighbour-induced changes in leaf chemical defences, nor did it include tree growth or abiotic factors measurements, our lines of arguments are mostly speculative. Few studies have explicitly addressed the implication of growth-defence trade-offs in associational effects and they were inconclusive (Moreira et al., 2014; Rosado-Sánchez et al., 2017). Future studies should specifically investigate the role that tree relative heights and architectures play in neighbour-induced changes of focal species chemistry.
We found that the concentration of chemical defences increased with temperature, which contrasts with the results of previous studies on oaks and birches in temperate and boreal biomes (Kuokkanen et al., 2001; Moreira, Castagneyrol, et al., 2018). Although there is ample literature on the variation of plant defences along climatic gradients, there is no consensus on the strength and direction of this relationship (Moles et al., 2011). Interestingly, we showed that climate also affected leaf phenolic concentration indirectly by modulating the tree diversity–defences relationships. Specifically, decrease in chemical defence levels of birch associated with greater tree phylogenetic diversity were stronger in warm and humid conditions. This indicates that climate and tree species composition jointly determined tree investment in chemical defences, likely through growth-defence trade-offs.
4.3. Do leaf chemical defences mediate effects of climate and diversity on insect herbivory?
We found a negative relationship between leaf phenolic concentration and insect herbivory, supporting the view that these secondary metabolites act as defences against herbivores (in addition to being involved in other physiological processes; Forkner et al., 2004; Harborne & Williams, 2000).
We found evidence that the effect of temperature on leaf herbivory was independent of the level of chemical defences. However, our results showed that the interactive effects of temperature and tree species richness on insect herbivory were mediated by changes in leaf chemical defence levels. This finding suggests that defence-mediated associational effects on insect herbivory are also climate-dependent. In our case, such effects were only observed in cold climates where chemical defences levels were low and where an increase in defences may have a stronger effect on background insect herbivory levels.
We found that the effect of birch phylogenetic distinctiveness on herbivory varied with the levels of chemical defences in birch leaves. Specifically, associational effects shifted from resistance to susceptibility with the increase in leaf phenolics concentration. This finding suggests that mechanisms involved in birch associational resistance against herbivores, other than chemical defence, might have been at play (e.g. host-finding disruption and resource dilution), and that an undetermined factor was simultaneously controlling the concentration of leaf chemical defences and interfering with these mechanisms. Forest structure, and more specifically relative heights of tree species, may for instance influence at the same time (a) leaf chemistry of a focal species by affecting crown illumination (Koricheva et al., 1998) and the synthesis of photo-protective flavonoids (Agati & Tattini, 2010) and (b) the apparency of this focal species to herbivores (Castagneyrol et al., 2019; Damien et al., 2016). In addition, nutrient availability may affect growth of trees and the concentration of carbon-based defences in leaves (Bryant et al., 1983; Koricheva et al., 1998). In turn, tree growth, as jointly determined by tree diversity (the relative competitive ability of the species) and nutrient availability, could affect apparency of the focal species to herbivores, as well as the abundance and diversity of canopy arthropods (Stone et al., 2010) with consequences for multitrophic interactions.
5. Conclusions
By taking advantage of an international network of tree diversity experiments and a standardized sampling protocol, we addressed the independent and interactive effects of tree species diversity and climate on tree–herbivore interactions in temperate and boreal forests. Altogether, our findings show that insect herbivory depends on a complex interplay between tree species diversity and climatic conditions, and that diversity effects on insect herbivory are partially mediated by neighbour-induced changes in leaf chemical defences. Our findings also confirm that tree species diversity can modify leaf chemistry of a focal species—and hence its quality for herbivores—but further suggest that such neighbour-induced changes are dependent on climate. Nevertheless, our approach remains correlative in essence and the ecological mechanisms underlying such patterns need to be further elucidated. We also acknowledge that a limitation of this study is that we could not well control for the influence of the position of the sites within the distribution range of birch (e.g. marginal or central), nor for their spatial correlation, which could however influence tree–insect interactions through local adaptation processes. Future studies should be specifically designed to investigate whether diversity and climate interactively shape leaf chemistry of a focal host plant because they jointly influence resource availability and their allocation to growth versus defences by trees. Our study also supports the view that the phylogenetic or functional diversity of tree species is complementary to species richness in predicting tree–herbivore relationships, likely because it accounts for additional information relative to niche differentiation and functional dissimilarities between tree species. Finally, our findings suggest that tree diversity effects on herbivory levels should be viewed as a balance between multiple processes arising from different attributes of tree diversity (interspecific variation of different traits). Future research should investigate which traits of tree species drive associational effects on herbivory and address simultaneously multiple underlying mechanisms. For instance, it would be particularly interesting to explore the role of forest structure and tree spatial arrangement in associational effects, as it may be implied in both neighbour-induced changes in chemical defences through effects on individual crown illumination, as well as in focal plant apparency. Importantly, the climatic context in which plant–herbivore interactions occur should be accounted for in future studies for a better understanding of the processes at play. By doing so, the study of tree diversity effects on tree resistance to insect herbivores interactions will move towards a more predictive framework.
Supplementary Material
Acknowledgements
We thank all the TreeDivNet partners for data collection. This study was funded by the ‘Diversity and Productivity of Trees in the context of Climate Change’ project (DiPTiCC, Grant ANR-16-CE32-0003-01). N.E., O.F. and F.G. acknowledge financial support by the German Centre for Integrative Biodiversity Research Halle–Jena–Leipzig, funded by the German Research Foundation (FZT 118), and N.E. and O.F. received support from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement no. 677232 to N.E.).
Funding information
German Research Foundation, Grant/Award Number: FZT 118; H2020, European Research Council, Grant/Award, Number: 677232; Agence Nationale de la Recherche, Grant/Award Number: ANR-16-CE32-0003-01
Footnotes
Authors’ Contributions
B.C. and H.J. designed the study; H.J., N.B., J.B., N.E., O.F., F.G., D.G., J.K., B.Ma., E.M., B.Mu., C.N., A.P., Q.P., M.S.-L., V.S., M.S., K.V. and B.C. collected the data; X.M. and M.F. performed the phenolics analysis; C.P. computed the extra data, extracted the climatic data and run the analysis; C.P., B.C., H.J., X.M. and J.K. wrote the first draft. All co-authors contributed substantially to subsequent revisions.
Data Availability Statement
Data are available from the Data INRAe repository https://doi.org/10.15454/SHCUXW.
References
- Agati G, Tattini M. Multiple functional roles of flavonoids in photoprotection: Letters. New Phytologist. 2010;186(4):786–793. doi: 10.1111/j.1469-8137.2010.03269.x. [DOI] [PubMed] [Google Scholar]
- Anstett DN, Chen W, Johnson MTJ. Latitudinal Gradients in Induced and Constitutive Resistance against Herbivores. Journal of Chemical Ecology. 2016;42(8):772–781. doi: 10.1007/s10886-016-0735-6. [DOI] [PubMed] [Google Scholar]
- Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, Butterfield J, Buse A, Coulson JC, Farrar J, Good JEG, et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Global Change Biology. 2002;8(1):1–16. doi: 10.1046/j.1365-2486.2002.00451.x. [DOI] [Google Scholar]
- Barbosa P, Hines J, Kaplan I, Martinson H, Szczepaniec A, Szendrei Z. Associational resistance and associational susceptibility: Having right or wrong neighbors. Annual Review of Ecology, Evolution, and Systematics. 2009;40(1):1–20. doi: 10.1146/annurev.ecolsys.110308.120242. [DOI] [Google Scholar]
- Barton K. MuMIn: Multi-model inference. 2018 R package version 1.42.1 (Version 1.42.1). Retrieved from https://CRAN.R-project.org/package=MuMIn. [Google Scholar]
- Bauerfeind SS, Fischer K. Increased temperature reduces herbivore host-plant quality. Global Change Biology. 2013;19(11):3272–3282. doi: 10.1111/gcb.12297. [DOI] [PubMed] [Google Scholar]
- Beck P, Caudullo G, de Rigo D, Tinner W. Betula pendula, Betula pubescens and other birches in Europe: Distribution, habitat, usage and threats. In: San-Miguel-Ayanz J, de Rigo D, Caudullo G, Houston Durrant T, Mauri A, editors. European Atlas of forest tree species. Publication Office of the European Union; 2016. pp. 70–73. [Google Scholar]
- Brezzi M, Schmid B, Niklaus PA, Schuldt A. Tree diversity increases levels of herbivore damage in a subtropical forest canopy: Evidence for dietary mixing by arthropods? Journal of Plant Ecology. 2017;10(1):13–27. doi: 10.1093/jpe/rtw038. [DOI] [Google Scholar]
- Bryant JP, Chapin FS, Klein DR. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos. 1983;40(3):357–368. doi: 10.2307/3544308. [DOI] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multi-modal inference: A practical information theoretic approach. 2nd. Vol. 2 Springer; 2002. [Google Scholar]
- Bustos-Segura C, Poelman EH, Reichelt M, Gershenzon J, Gols R. Intraspecific chemical diversity among neighbouring plants correlates positively with plant size and herbivore load but negatively with herbivore damage. Ecology Letters. 2017;20(1):87–97. doi: 10.1111/ele.12713. [DOI] [PubMed] [Google Scholar]
- Carnicer J, Coll M, Ninyerola M, Pons X, Sanchez G, Penuelas J. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(4):1474–1478. doi: 10.1073/pnas.1010070108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagneyrol B, Jactel H, Moreira X. Anti-herbivore defences and insect herbivory: Interactive effects of drought and tree neighbours. Journal of Ecology. 2018;106(5):2043–2057. doi: 10.1111/1365-2745.12956. [DOI] [Google Scholar]
- Castagneyrol B, Jactel H, Vacher C, Brockerhoff EG, Koricheva J. Effects of plant phylogenetic diversity on herbivory depend on herbivore specialization. Journal of Applied Ecology. 2014;51(1):134–141. doi: 10.1111/1365-2664.12175. [DOI] [Google Scholar]
- Castagneyrol B, Kozlov MV, Poeydebat C, Toïgo M, Jactel H. Associational resistance to a pest insect fades with time. Journal of Pest Science. 2019;93(1):427–437. doi: 10.1007/s10340-019-01148-y. [DOI] [Google Scholar]
- Chamberlain S. brranching: Fetch ‘phylogenies’ from many sources. 2018 R package version 0.3.0 (Version 0.3.0) [R]. Retrieved from https://CRAN.R-project.org/package=brranching. [Google Scholar]
- Damien M, Jactel H, Meredieu C, Regolini M, van Halder I, Castagneyrol B. Pest damage in mixed forests: Disentangling the effects of neighbor identity, host density and host apparency at different spatial scales. Forest Ecology and Management. 2016;378:103–110. doi: 10.1016/j.foreco.2016.07.025. [DOI] [Google Scholar]
- Dinnage R. Phylogenetic diversity of plants alters the effect of species richness on invertebrate herbivory. Peerj. 2013;1:e93. doi: 10.7717/peerj.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endara M-J, Coley PD. The resource availability hypothesis revisited: A meta-analysis. Functional Ecology. 2011;25(2):389–398. doi: 10.1111/j.1365-2435.2010.01803.x. [DOI] [Google Scholar]
- Fernandez-Conradi P, Jactel H, Hampe A, Leiva MJ, Castagneyrol B. The effect of tree genetic diversity on insect herbivory varies with insect abundance. Ecosphere. 2017;8(1):e01637. doi: 10.1002/ecs2.1637. [DOI] [Google Scholar]
- Forister ML, Novotny V, Panorska AK, Baje L, Basset Y, Butterill PT, Cizek L, Coley PD, Dem F, Diniz IR, Drozd P, et al. The global distribution of diet breadth in insect herbivores. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(2):442–447. doi: 10.1073/pnas.142304211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkner RE, Marquis RJ, Lill JT. Feeny revisited: Condensed tannins as anti-herbivore defences in leaf-chewing herbivore communities of Quercus. Ecological Entomology. 2004;29(2):174–187. doi: 10.1111/j.1365-2311.2004.0590.x. [DOI] [Google Scholar]
- Fox J, Weisberg S. An R companion to applied regression. 3rd. Sage Publications; 2018. [Google Scholar]
- Garibaldi LA, Kitzberger T, Ruggiero A. Latitudinal decrease in folivory within Nothofagus pumilio forests: Dual effect of climate on insect density and leaf traits? Global Ecology and Biogeography. 2011;20(4):609–619. doi: 10.1111/j.1466-8238.2010.00623.x. [DOI] [Google Scholar]
- Glassmire AE, Jeffrey CS, Forister ML, Parchman TL, Nice CC, Jahner JP, Wilson JS, Walla TR, Richards LA, Smilanich AM, Leonard MD, et al. Intraspecific phytochemical variation shapes community and population structure for specialist caterpillars. New Phytologist. 2016;212(1):208–219. doi: 10.1111/nph.14038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman JJ, Vanhellemont M, Barsoum N, Bauhus J, Bruelheide H, Castagneyrol B, Cavender-Bares J, Eisenhauer N, Ferlian O, Gravel D, Hector A, et al. Synthesis and future research directions linking tree diversity to growth, survival, and damage in a global network of tree diversity experiments. Environmental and Experimental Botany. 2018;152:68–89. doi: 10.1016/j.envexpbot.2017.12.015. [DOI] [Google Scholar]
- Gutbrodt B, Dorn S, Mody K. Drought stress affects constitutive but not induced herbivore resistance in apple plants. Arthropod-Plant Interactions. 2012;6(2):171–179. doi: 10.1007/s11829-011-9173-0. [DOI] [Google Scholar]
- Haase J, Castagneyrol B, Cornelissen JHC, Ghazoul J, Kattge J, Koricheva J, Scherer-Lorenzen M, Morath S, Jactel H. Contrasting effects of tree diversity on young tree growth and resistance to insect herbivores across three biodiversity experiments. Oikos. 2015;124(12):1674–1685. doi: 10.1111/oik.02090. [DOI] [Google Scholar]
- Hambäck PA, Inouye BD, Andersson P, Underwood N. Effects of plant neighborhoods on plant-herbivore interactions: Resource dilution and associational effects. Ecology. 2014;95(5):1370–1383. doi: 10.1890/13-0793.1. [DOI] [PubMed] [Google Scholar]
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55(6):481–504. doi: 10.1016/S0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- Henriksson J, Haukioja E, Ossipov V, Ossipova S, Sillanpaa S, Kapari L, Pihlaja K. Effects of host shading on consumption and growth of the geometrid Epirrita autumnata: Interactive roles of water, primary and secondary compounds. Oikos. 2003;103(1):3–16. doi: 10.1034/j.1600-0706.2003.12306.x. [DOI] [Google Scholar]
- Herms D, Mattson W. The dilemma of plants – To grow or defend. Quarterly Review of Biology. 1992;67(3):283–335. doi: 10.1086/417659. [DOI] [Google Scholar]
- Iverson AL, Marín LE, Ennis KK, Gonthier DJ, Connor-Barrie BT, Remfert JL, Cardinale BJ, Perfecto I. REVIEW: Do polycultures promote win-wins or trade-offs in agricultural ecosystem services? A meta-analysis. Journal of Applied Ecology. 2014;51(6):1593–1602. doi: 10.1111/1365-2664.12334. [DOI] [Google Scholar]
- Jactel H, Bauhus J, Boberg J, Bonal D, Castagneyrol B, Gardiner B, Gonzalez-Olabarria JR, Koricheva J, Meurisse N, Brockerhoff EG. Tree diversity drives forest stand resistance to natural disturbances. Current Forestry Reports. 2017;3(3):223–243. doi: 10.1007/s40725-017-0064-1. [DOI] [Google Scholar]
- Jactel H, Birgersson G, Andersson S, Schlyter F. Non-host volatiles mediate associational resistance to the pine processionary moth. Oecologia. 2011;166(3):703–711. doi: 10.1007/s00442-011-1918-z. [DOI] [PubMed] [Google Scholar]
- Jactel H, Gritti ES, Drössler L, Forrester DI, Mason WL, Morin X, Pretzsch H, Castagneyrol B. Positive biodiversityproductivity relationships in forests: Climate matters. Biology Letters. 2018;14(4) doi: 10.1098/rsbl.2017.0747. 20170747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jactel H, Moreira X, Castagneyrol B. Tree diversity and forest resistance to insect pests: Patterns, mechanisms and prospects. Annual Review of Entomology. 2020;66 doi: 10.1146/annurev-ento-041720-075234. [DOI] [PubMed] [Google Scholar]
- Jactel H, Petit J, Desprez-Loustau M-L, Delzon S, Piou D, Battisti A, Koricheva J. Drought effects on damage by forest insects and pathogens: A meta-analysis. Global Change Biology. 2012;18(1):267–276. doi: 10.1111/j.1365-2486.2011.02512.x. [DOI] [Google Scholar]
- Jactel H, Poeydebat C, van Halder I, Castagneyrol B. Interactive effects of tree mixing and drought on a primary forest pest. Frontiers in Forests and Global Change. 2019;2:1–12. doi: 10.3389/ffgc.2019.00077. [DOI] [Google Scholar]
- Johnson JB, Omland KS. Model selection in ecology and evolution. Trends in Ecology & Evolution. 2004;19(2):101–108. doi: 10.1016/j.tree.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Kambach S, Kühn I, Castagneyrol B, Bruelheide H. The impact of tree diversity on different aspects of insect herbivory along a global temperature gradient – A meta-analysis. PLoS ONE. 2016;11(11):e0165815. doi: 10.1371/journal.pone.0165815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karban R, Baldwin IT. Induced responses to herbivory. Chicago University Press; 1997. [Google Scholar]
- Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann NE, Linder HP, Kessler M. Data descriptor: Climatologies at high resolution for the earth’s land surface areas. Scientific Data. 2017;4 doi: 10.1038/sdata.2017.122. 170122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO. Picante: R tools for integrating phylogenies and ecology. Bioinformatics. 2010;26(11):1463–1464. doi: 10.1093/bioinformatics/btq166. [DOI] [PubMed] [Google Scholar]
- Klapwijk MJ, Ayres MP, Battisti A, Larsson S. Assessing the impact of climate change on outbreak potential. In: Barbosa P, Letourneau DK, Agrawal AA, editors. Insect outbreak revisited. Wiley-Blackwell; 2012. pp. 429–450. [Google Scholar]
- Koricheva J, Larsson S, Haukioja E, Keinänen M, Keinanen M. Regulation of woody plant secondary metabolism by resource availability: Hypothesis testing by means of meta-analysis. Oikos. 1998;83(2):212–226. doi: 10.2307/3546833. [DOI] [Google Scholar]
- Kostenko O, Mulder PPJ, Courbois M, Bezemer TM. Effects of plant diversity on the concentration of secondary plant metabolites and the density of arthropods on focal plants in the field. Journal of Ecology. 2017;105(3):647–660. doi: 10.1111/1365-2745.12700. [DOI] [Google Scholar]
- Kozlov MV, Lanta V, Zverev V, Zvereva EL. Global patterns in background losses of woody plant foliage to insects. Global Ecology and Biogeography. 2015;24(10):1126–1135. doi: 10.1111/geb.12347. [DOI] [Google Scholar]
- Kuokkanen K, Julkunen-Tiitto R, Keinänen M, Niemelä P, Tahvanainen J. The effect of elevated CO2 and temperature on the secondary chemistry of Betula pendula seedlings. Trees. 2001;15(6):378–384. doi: 10.1007/s004680100108. [DOI] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software. 2017;82(13):1–26. doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- Lange ES, Kyryczenko-Roth V, Johnson-Cicalese J, Davenport J, Vorsa N, Rodriguez-Saona C. Increased nutrient availability decreases insect resistance in cranberry. Agricultural and Forest Entomology. 2019;21(3):326–335. doi: 10.1111/afe.12335. [DOI] [Google Scholar]
- Larsson S, Wirén A, Lundgren L, Ericsson T, Wiren A. Effects of light and nutrient stress on leaf phenolic chemistry in Salix dasyclados and susceptibility to Galerucella lineola (Coleoptera) Oikos. 1986;47(2):205–210. doi: 10.2307/3566047. [DOI] [Google Scholar]
- Lintunen A, Kaitaniemi P. Responses of crown architecture in Betula pendula to competition are dependent on the species of neighbouring trees. Trees. 2010;24(3):411–424. doi: 10.1007/s00468-010-0409-x. [DOI] [Google Scholar]
- Magallón S, Gómez-Acevedo S, Sánchez-Reyes LL, Hernández-Hernández T. A metacalibrated time-tree documents the early rise of flowering plant phylogenetic diversity. New Phytologist. 2015;207(2):437–453. doi: 10.1111/nph.13264. [DOI] [PubMed] [Google Scholar]
- Merwin AC, Underwood N, Inouye BD. Increased consumer density reduces the strength of neighborhood effects in a model system. Ecology. 2017;98(11):2904–2913. doi: 10.1002/ecy.200. [DOI] [PubMed] [Google Scholar]
- Miller ET, Farine DR, Trisos CH. Phylogenetic community structure metrics and null models: A review with new methods and software. Ecography. 2017;40(4):461–477. doi: 10.1111/ecog.02070. [DOI] [Google Scholar]
- Mole S, Ross JAM, Waterman PG. Light-induced variation in phenolic levels in foliage of rain-forest plants. I. Chemical Changes. Journal of Chemical Ecology. 1988;14(1):1–21. doi: 10.1007/BF01022527. [DOI] [PubMed] [Google Scholar]
- Moles AT, Bonser SP, Poore AGB, Wallis IR, Foley WJ. Assessing the evidence for latitudinal gradients in plant defence and herbivory. Functional Ecology. 2011;25(2):380–388. doi: 10.1111/j.1365-2435.2010.01814.x. [DOI] [Google Scholar]
- Moreira X, Abdala-Roberts L, Parra-Tabla V, Mooney KA. Positive effects of plant genotypic and species diversity on anti-herbivore defenses in a tropical tree species. PLoS ONE. 2014;9(8):e105438. doi: 10.1371/journal.pone.0105438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira X, Abdala-Roberts L, Rasmann S, Castagneyrol B, Mooney KA. Plant diversity effects on insect herbivores and their natural enemies: Current thinking, recent findings, and future directions. Current Opinion in Insect Science. 2016;14:1–7. doi: 10.1016/j.cois.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Moreira X, Castagneyrol B, Abdala-Roberts L, Berny-Mier y Teran JC, Timmermans BGH, Bruun HH, Covelo F, Glauser G, Rasmann S, Tack AJM. Latitudinal variation in plant chemical defences drives latitudinal patterns of leaf herbivory. Ecography. 2018;41(7):1124–1134. doi: 10.1111/ecog.03326. [DOI] [Google Scholar]
- Moreira X, Galman A, Francisco M, Castagneyrol B, Abdala-Roberts L. Host plant frequency and secondary metabolites are concurrently associated with insect herbivory in a dominant riparian tree. Biology Letters. 2018;14(12) doi: 10.1098/rsbl.2018.0281. 20180281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muiruri EW, Barantal S, Iason GR, Salminen J, Perez-Fernandez E, Koricheva J. Forest diversity effects on insect herbivores: Do leaf traits matter? New Phytologist. 2019;221(4):2250–2260. doi: 10.1111/nph.15558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution. 2013;4(2):133–142. doi: 10.1111/j.2041-210x.2012.00261.x. [DOI] [Google Scholar]
- Paquette A, Hector A, Castagneyrol B, Vanhellemont M, Koricheva J, Scherer-Lorenzen M, Verheyen K. A million and more trees for science. Nature Ecology & Evolution. 2018;2(5):763–766. doi: 10.1038/s41559-018-0544-0. [DOI] [PubMed] [Google Scholar]
- Pineau X, David G, Peter Z, Sallé A, Baude M, Lieutier F, Jactel H. Effect of temperature on the reproductive success, developmental rate and brood characteristics of Ips sexdentatus (Boern.): Temperature effect on I. sexdentatus brood. Agricultural and Forest Entomology. 2017;19(1):23–33. doi: 10.1111/afe.12177. [DOI] [Google Scholar]
- R Core Development Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2013. Retrieved from http://www.R-project.org/ [Google Scholar]
- Ratcliffe S, Wirth C, Jucker T, van der Plas F, Scherer-Lorenzen M, Verheyen K, Allan E, Benavides R, Bruelheide H, Ohse B, Paquette A, et al. Biodiversity and ecosystem functioning relations in European forests depend on environmental context. Ecology Letters. 2017;20(11):1414–1426. doi: 10.1111/ele.12849. [DOI] [PubMed] [Google Scholar]
- Redding DW, Mooers AO. Incorporating evolutionary measure into conservation prioritization. Conservation Biology. 2006;20(6):1670–1678. doi: 10.1111/j.1523-1739.2006.00555.x. [DOI] [PubMed] [Google Scholar]
- Riipi M, Kause A, Haukioja E, Ossipov V, Ossipova S, Pihlaja K. Variable responses of folivorous sawflies to leaf quality of mountain birch. Canadian Journal of Forest Research. 2005;35(1):189–198. doi: 10.1139/x04-166. [DOI] [Google Scholar]
- Rodríguez-Castañeda G. The world and its shades of green: A meta-analysis on trophic cascades across temperature and precipitation gradients. Global Ecology and Biogeography. 2013;22(1):118–130. doi: 10.1111/j.1466-8238.2012.00795.x. [DOI] [Google Scholar]
- Rosado-Sánchez S, Parra-Tabla V, Betancur-Ancona D, Moreira X, Abdala-Roberts L. Tree species diversity alters plant defense investment in an experimental forest plantation in southern Mexico. Biotropica. 2017;50(2):246–253. doi: 10.1111/btp.12527. [DOI] [Google Scholar]
- Rosado-Sánchez S, Parra-Tabla V, Betancur-Ancona D, Moreira X, Abdala-Roberts L. Effects of tree species diversity on insect herbivory and leaf defences in Cordia dodecandra. Ecological Entomology. 2018;43(6):703–711. doi: 10.1111/een.12648. [DOI] [Google Scholar]
- Schielzeth H. Simple means to improve the interpretability of regression coefficients: Interpretation of regression coefficients. Methods in Ecology and Evolution. 2010;1(2):103–113. doi: 10.1111/j.2041-210X.2010.00012.x. [DOI] [Google Scholar]
- Schuldt A, Assmann T, Bruelheide H, Durka W, Eichenberg D, Härdtle W, Kröber W, Michalski SG, Purschke O. Functional and phylogenetic diversity of woody plants drive herbivory in a highly diverse forest. New Phytologist. 2014;202(3):864–873. doi: 10.1111/nph.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldt A, Baruffol M, Böhnke M, Bruelheide H, Härdtle W, Lang AC, Nadrowski K, Von Oheimb G, Voigt W, Zhou H, Assmann T. Tree diversity promotes insect herbivory in subtropical forests of south-east China. Journal of Ecology. 2010;98(4):917–926. doi: 10.1111/j.1365-2745.2010.01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan NN, Vanhellemont M, Baeten L, Dillen M, Verheyen K. The effects of local neighbourhood diversity on pest and disease damage of trees in a young experimental forest. Forest Ecology and Management. 2014;334:1–9. doi: 10.1016/j.foreco.2014.08.032. [DOI] [Google Scholar]
- Siemann E, Tilman D, Haarstad J, Ritchie M. Experimental tests of the dependence of arthropod diversity on plant diversity. The American Naturalist. 1998;152(5):738–750. doi: 10.2307/2463851. [DOI] [PubMed] [Google Scholar]
- Srivastava DS, Cadotte MW, MacDonald AAM, Marushia RG, Mirotchnick N. Phylogenetic diversity and the functioning of ecosystems. Ecology Letters. 2012;15(7):637–648. doi: 10.1111/j.1461-0248.2012.01795.x. [DOI] [PubMed] [Google Scholar]
- Stone AC, Gehring CA, Whitham TG. Drought negatively affects communities on a foundation tree: Growth rings predict diversity. Oecologia. 2010;164(3):751–761. doi: 10.1007/s00442-010-1684-3. [DOI] [PubMed] [Google Scholar]
- Vehviläinen H, Koricheva J, Ruohomaki K. Tree species diversity influences herbivore abundance and damage: Meta-analysis of long-term forest experiments. Oecologia. 2007;152(2):287–298. doi: 10.1007/s00442-007-0673-7. [DOI] [PubMed] [Google Scholar]
- Visakorpi K, Riutta T, Martinez-Bauer AE, Salminen J-P, Gripenberg S. Insect community structure covaries with host plant chemistry but is not affected by prior herbivory. Ecology. 2019;100:e02739. doi: 10.1002/bes2.1561. [DOI] [PubMed] [Google Scholar]
- Walter J, Hein R, Auge H, Beierkuhnlein C, Löffler S, Reifenrath K, Schädler M, Weber M, Jentsch A. How do extreme drought and plant community composition affect host plant metabolites and herbivore performance? Arthropod-Plant Interactions. 2012;6(1):15–25. doi: 10.1007/s11829-011-9157-0. [DOI] [Google Scholar]
- Wang X-F, Liu J-F, Gao W-Q, Deng Y-P, Ni Y-Y, Xiao Y-H, Kang F-F, Wang QI, Lei J-P, Jiang Z-P. Defense pattern of Chinese cork oak across latitudinal gradients: Influences of ontogeny, herbivory, climate and soil nutrients. Scientific Reports. 2016;6:27269. doi: 10.1038/srep27269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wein A, Bauhus J, Bilodeau-Gauthier S, Scherer-Lorenzen M, Nock C, Staab M. Tree species richness promotes invertebrate herbivory on congeneric native and exotic tree saplings in a young diversity experiment. PLoS ONE. 2016;11(12):e0168751. doi: 10.1371/journal.pone.0168751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yguel B, Bailey R, Tosh ND, Vialatte A, Vasseur C, Vitrac X, Jean F, Prinzing A. Phytophagy on phylogenetically isolated trees: Why hosts should escape their relatives. Ecology Letters. 2011;14(11):1117–1124. doi: 10.1111/j.1461-0248.2011.01680.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen HYH, Reich PB. Forest productivity increases with evenness, species richness and trait variation: A global meta-analysis. Journal of Ecology. 2012;100(3):742–749. doi: 10.1111/j.1365-2745.2011.01944.x. [DOI] [Google Scholar]
- Zúbrik M, Kunca A, Csóka G. Insects and diseases damaging trees and shrubs of Europe. N.A.P. Editions; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the Data INRAe repository https://doi.org/10.15454/SHCUXW.