Abstract
Cyclin-dependent kinases 4 and 6 (CDK4/6) phosphorylate and inhibit retinoblastoma (RB) family proteins. Hyperphosphorylated RB releases E2F transcription factors, activating a transcriptional program that initiates S phase. Due to the critical role that this pathway has in regulating cell cycle progression, inhibiting CDK4/6 is an attractive therapeutic strategy. Indeed, CDK4/6 inhibitors in combination with anti-estrogens produce a significant benefit in patients with ER+/HER2- breast cancer. Clinical trials are currently investigating if the use of CDK4/6 inhibitors alone or in combination can be extended to other cancer types. Inhibition of CDK4/6 can result in different cell fates such as quiescence, senescence or apoptosis. Senescence is a stress response that can be induced by stimuli that include oncogenic activation, chemotherapy, irradiation and targeted therapies such as CDK4/6 inhibitors. Senescent cells undergo a stable cell cycle arrest and produce a bioactive secretome that remodels their microenvironment and engages the immune system. In this review, we analyze the therapeutic relevance of senescence induction by CDK4/6 inhibitors. We also discuss how different therapies, including checkpoint inhibitors and drugs targeting MEK or PI3K, can be used in combination with CDK4/6 inhibitors to reinforce or exploit senescence. Recently, a lot of effort has been put into identifying compounds that selectively kill senescent cells (termed senolytics). Thus, sequential treatment with senolytics might be an additional strategy to potentiate the antitumor effects of CDK4/6 inhibitors.
Keywords: Senescence, CDK4, CDK6, CDK4/6 inhibitors, Palbociclib, Abemaciclib, Ribociclib
CDK4/6 inhibitors: overview
Cyclin-dependent kinases 4 and 6 (CDK4/6) play a crucial role in mammalian cell proliferation. When enzymatically activated by D-type cyclins (D1, D2 and D3), CDK4/6 phosphorylate members of the retinoblastoma (RB) family proteins (Figure 1). These include the p110 retinoblastoma protein (encoded by RB1) and the related pocket proteins p107 (encoded by RBL1) and p130 (encoded by RB2). Sequential phosphorylation of RB1 by CDK4/6 and cyclin E-dependent CDK complexes leads to release of E2F transcription factors, initiation of S phase and commitment to the cell cycle. CDK4/6 can be inhibited by proteins belonging to the CIP/KIP(such as p21CIP, encoded by CDKN1A) and INK4 (such as p16INK4a, encoded by CDKN2A) families of cyclin dependent kinase inhibitors. Both,RB1 and p16INK4a, are tumor suppressors and their loss of function is frequent in cancer. Conversely, Cyclin D1acts as an oncogene and it is frequently amplified in tumors [1–3]. Overall, alterations of the “RB pathway” are a hallmark of cancer, whichhighlights the rationale for therapeutic intervention.
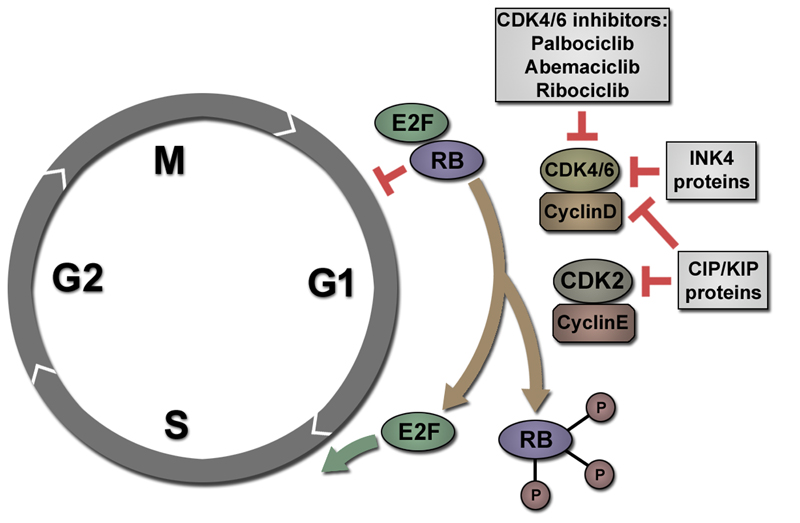
Figure 1. Inhibition of CDK4/6 induces cell cycle arrest in G1 phase.
After M phase, RB is hypophosphorylated and can bind and inhibit transcription factors of the E2F family. When activated by Cyclin D, CDK4/6 phosphorylate RB. RB can subsequently be phosphorylated by CDK2/Cyclin E. Hyperphosphorylated RB releases E2F transcription factors that activate a transcriptional programme promoting transition to S phase. Cyclin dependent kinase inhibitors of the CIP/KIP family (such as p21CIP) can inhibit both CDK2 and CDK4/6 activity. INK4 proteins, like p16INK4a, specifically inhibit CDK4/6 activity leading to cell cycle arrest in G1 phase. CDK4/6 inhibitors (such as Palbociclib, Abemaciclib or Ribociclib) exert similar effects, also inducing an arrest in G1.
First attempts to develop a CDK4 inhibitor started even before the RB pathway was completely understood in the early 1990s [4]. As there are 20 different human CDKs, identification of a specific CDK4/6 inhibitor turned out to be a critical challenge, and initial trials with pan-CDK inhibitors were problematic due to serious side effects [5, 6]. It took more than a decade to overcome these issues, but eventually a specific CDK4/6 inhibitor, Palbociclib (PD0332991), was shown to induce G1 cell cycle arrest in cell lines and mouse models with functional RB [7, 8]. In the years since, other oral CDK4/6 inhibitors such as Abemaciclib (LY2835219) and Ribociclib (LEE011) have been described [9, 10]. In 2015, the phase II PALOMA-1 trial led to provisional FDA-approval of Palbociclib and letrozole for patients with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negativemetastatic breast cancer [11]. As of today, Palbociclib, Abemaciclib and Ribociclibhave all been granted FDA-approval and other CDK4/6 inhibitors are being testedfor the treatment of ER+/HER2- breast cancer [5]. In addition, there are currently numerous clinical trials investigating the efficacy of CDK4/6 inhibitors alone or in combination to treat other cancers types.
Palbociclib, Abemaciclib and Ribociclib are all orally bioavailable and show great specificity for CDK4 and CDK6. Ribociclib is structurally very similar to Palbociclib, whereas the structure of Abemaciclib is different from the two other compounds [12]. Abemaciclib might also differ functionally, as multiomics profiling revealed a wider spectrum of secondary targets and a pan-CDK transcriptional signature [13]. Apart from that, there are differences in pharmacokinetics, dosing schedules and toxicity profiles between the three drugs [12]. Current preclinical and clinical research on CDK4/6 inhibitors focusses on a better understanding of treatment responses, identification of biomarkers to predict sensitivity or resistance, and designing combinations that could enhance their antiproliferative effects or engage the immune system for tumor clearance. The ongoing research might help to enhance patient stratification, allow for a wider but more targeted use of CDK4/6 inhibitors, and significantly improve patient outcome on the long term.
Senescence, quiescence, or resistance: biomarkers and cell fates
In cells with functional RB, CDK4/6 inhibitors arrest cells in the G1 phase of the cell cycle. Depending ondifferent factors, this arrest can lead to quiescence or senescence. Whereas quiescent cells retain the ability to exit the arrest in response to mitogenic signaling, senescent cells are characterized by a highly stable growth arrest accompanied by specific phenotypic alterations (e.g. a senescence-associated secretory phenotype/SASP andmetabolic, epigenetic and morphologic changes) [14] (Figure 2). In fact, CDK4/6 inhibitors have proven to induce quiescence or senescence in a variety of different cell types in vitro and in vivo: breast cancer [15–17], melanoma [18, 19], hepatocellular carcinoma [20], gastric and oesophageal cancer [21, 22], liposarcoma [23, 24], leukaemia [25] and neuroblastoma [26]. The extent to which senescent cancer cells can be found in patients after treatment with CDK4/6 inhibitors remains unknown, since broadly applicable diagnostic tests to detect senescent cells are still lacking.
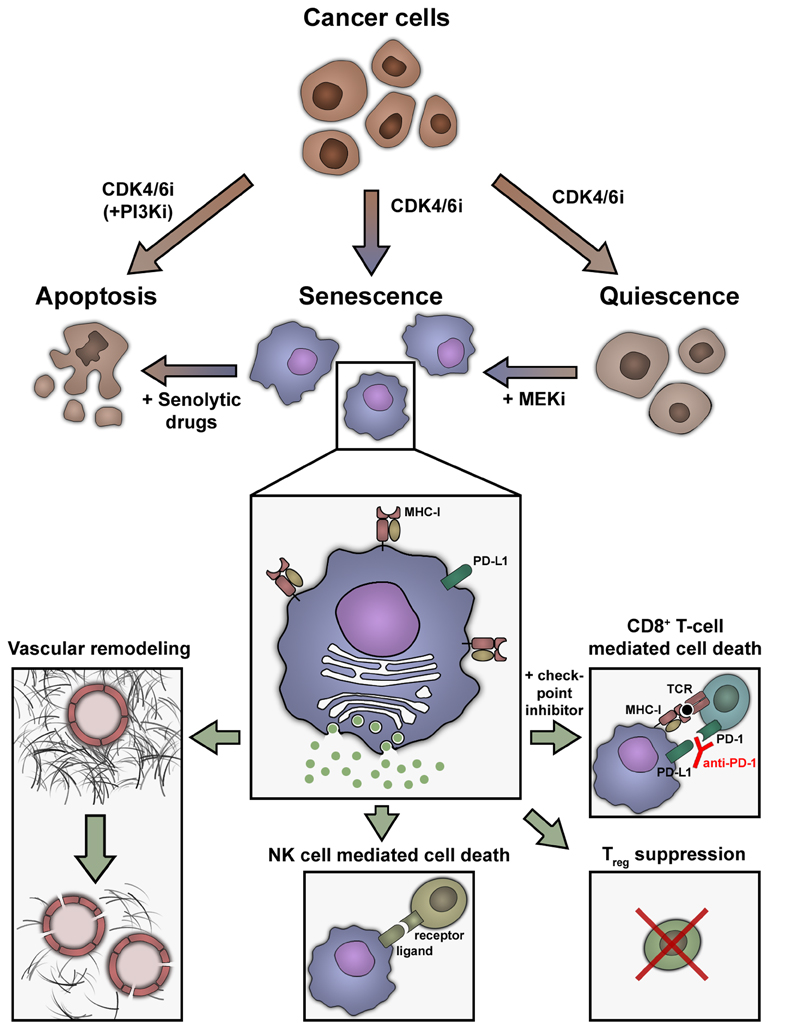
Figure 2. Different cell fates caused by CDK4/6 inhibitors influence treatment outcome.
In cancer cells with a functional RB pathway, treatment with CDK4/6 inhibitors usually induces quiescence or senescence. In some tumor types, senescence can be enforced by combined treatment with MEK inhibitors. The combination of a CDK4/6 inhibitor with a PI3K inhibitor can change the cell fate towards apoptosis, which is rarely seen in a monotherapy setting. Senescent cells have a characteristic phenotype that includes an enlarged and flattened morphology, increased senescence-associated β-Galactosidase activity (“blue cells”) and the senescence-associated secretory phenotype (SASP). The SASP promotes changes in the tumor microenvironment, like vascular remodeling (allowing for increased uptake of chemotherapy in pancreatic cancer) or recruitment of cytotoxic T cells. CDK4/6 inhibitors also induce enhanced expression of MHC-I complexes on cancer cells, facilitating activation of cytotoxic T cells. Combination treatment with checkpoint inhibitors (e.g. an anti-PD-1 antibody) can further improve the immune-mediated clearance of senescent cancer cells. In some tumors, treatment with CDK4/6 inhibitors can also reduce levels of immunosuppressive regulatory T cells or attract NK cells and promote NK-cell mediated cancer cell death.
Alongside quiescence and senescence, CDK4/6 inhibitors can also induce apoptosisin certain settings. In T-cell acute lymphoblastic leukemia (T-ALL), Palbociclib inhibits CDK6-mediated phosphorylation of glycolytic enzymes, ultimately leading to depletion of antioxidants, increased levels of reactive oxygen species (ROS), and apoptosis [27–29]. In tumors like T-ALL, where CDK6 is a major oncogenic driver, unique functions of CDK6 can influence treatment outcome of CDK4/6 inhibitors independent of RB. Apart from its enzymatic activity, CDK6 is also a transcriptional regulator and as such can promote expression of VEGF or p53 antagonists [30, 31]. It has been suggested that CDK4/6 inhibitor therapy might therefore favor the outgrowth of p53 mutant clones [30].
Whereas quiescence, senescence and apoptosis are favorable outcomes in cancer treatment, some cells/tumors are less sensitive or even resistant to CDK4/6 inhibitors. RB is the main biomarker to predict benefit of CDK4/6 inhibitor treatment and loss of RB function is a cause of primary and secondary resistance [7, 32, 33]. Other reported resistance mechanisms are activating alterations in AKT1, RAS, AURKA, CCNE2, ERBB2, and FGFR2, as well as high Cyclin E1 expression and CDK2 activity [15, 33–36]. Loss of p53 function might also be associated with reduced sensitivity to CDK4/6 inhibitors [34, 37]. Cells with specific genomic alterations, on the other hand, are particularly sensitive (CCND1 translocation, CCND1-3 3′UTR loss, and CCND2 or CCND3 amplification) [34]. Notably, high expression of Cyclin D1 alone, CCND1 amplification or loss of p16INK4a do not predict increased sensitivity [34]. A comprehensive review article about the mechanisms that underlie the sensitivity or resistance to treatment with CDK4/6 inhibitors has been published recently [5].
Senescence induced by CDK4/6 inhibitors
Cellular senescence was first described as a response limiting the proliferative capacityof human fibroblasts in culture [38]. Senescence is now considered as a stress response triggered by stimuli like telomere shortening (replicative senescence), oncogene activation, oxidative stress, irradiation or chemotherapy (therapy-induced senescence) [39]. Overall, senescence is a powerful mechanism of tumor suppression preventing the uncontrolled proliferation of preneoplasticcells [40].
In addition, induction of senescence in tumor cells is relevantduring cancer therapy. Irradiation, chemotherapeutic agents and targeted anti-cancer therapies, including CDK4/6 inhibitorscan induce senescence in different cancer types in vitro and in vivo [41, 42]. In a lymphoma mouse model, senescence was associated with a better prognosis after chemotherapy [43]. Senescent breast and lung cancer cells have also been found in biopsies of patients who had received neoadjuvant chemotherapy [44, 45]. Importantly, clinical studies have confirmed that senescence is a predictor of treatment outcome [46] and a low SASP correlates with resistance to cancer therapies [47].
Thesenescence-associated growth arrest is orchestratedby activation of the p53 and p16/RBtumor suppressor pathways[48]. Two CDK inhibitors, p21CIP1 and p16INK4a, are induced during senescence. p21CIP1 is upregulated by p53 as a result of the DNA damage associated with senescence induction [49]. The CDK4/6 inhibitors p16INK4a and p15INK4bare amongst the best characterized markers of senescence [50, 51]. p16INK4aand p15INK4b are expressed from the INK4/ARF locus together with ARF, which inhibits MDM2 and thereby activates p53. The INK4/ARF locus, epigenetically repressed by Polycomb proteins in normal cells, is activated during senescence [52].
Since the p16/RB pathway is a major component of the senescence machinery, it is not surprising that CDK4/6 inhibitionor overexpression of p16INK4aresult in senescence [18, 53–55]. Accordingly, CDK4 activity islinked to suppression of senescence and is necessary for Ras- or HER2-driven tumorigenesis [56, 57]. Senescence induction by CDK4/6 inhibition seems to depend on a functional RB. The transcription factor Forkhead Box M1 (FOXM1) is anothertarget of CDK4/6 [18]. Destabilization of FOXM1 upon CDK4/6 inhibition leads to elevated levels of ROS, priming cells for senescence induction.
Nevertheless, treatment with Palbociclib, Abemaciclib or Ribociclib does not uniformly lead to senescence in cancer cells. Complex genomic alterations and phenotypes in different tumorscould be responsible forthe particular treatment responses. CDK4/6 inhibitorsmight create a senescence permissive status, with additional intrinsic or external factors influencing the cell fate. The RAS and PI3K/AKT signaling pathways, for example, regulate the transcription of D cyclins, their assembly with CDK4/6 and their intracellular location [4]. Downregulation of MDM2 and repression of HRAS have been shown to be necessary for senescence induction in liposarcoma cells treated with CDK4/6 inhibitors [24]. In melanoma cells, mTOR inhibition has been demonstrated to be crucial for the implementation of senescence, and combining mTOR inhibition with Palbociclib intensified the cell cycle arrest [19, 58]. In another study, Palbociclib-induced senescence was associated with increased proteasome activity [59], further highlighting that multiple factors can influence cell fate. Uncovering the mechanisms that guide cells toundergo quiescence or senescence in response to CDK4/6 inhibition will help to further improve therapy planning and outcome.
Engaging the immune system
The stable growth arrest associated with senescence induction is appealing for cancer therapy. Although senescent cells have exited the cell cycle, they are highly bioactive cells. One of the most prominent characteristics of senescent cells, apart from the growth arrest, is the so-called senescence-associated secretory phenotype (SASP). The extent and composition of the cytokines and factors secreted by senescent cells can vary and is celltype- and stimulus-dependent [60]. The SASP of senescent stroma cells is associated with detrimental phenotypes such as the promotion of tumor growth, angiogenesis, metastasis and immunosuppression [61–63]. On the other hand, the SASP can induce senescence in autocrine and paracrine manners (thereby inhibiting proliferation) [64, 65] and stimulate the immune clearance of senescent cells [66]. Both the adaptive and the innate immune system are involved in the elimination of senescent (pre)malignant cells [66–69].
The positive outcomes achieved by checkpoint inhibitor treatment in clinical studieshave evoked great interest in therapies promoting immune clearance of cancer cells. So far, 7 antibodies have been FDA-approved (e.g. Ipilimumab, Nivolumab, Pembrolizumab) for the treatment of several different cancers. As for CDK4/6 inhibitors, a few studies have shown activation of the immune system and/or beneficial effects in combination with checkpoint inhibitors. Interestingly, these effects are at least partially related to their ability to induce senescence.
In a study investigating CDK4/6 inhibitor treatment in breast cancermodels, Abemaciclib and Palbociclib induced growth arrest and tumor regression in vivo, which was not attributable to apoptosis. Instead, the authors found that cytotoxic T cells were recruited into the tumors, which could be explained by increased antigen presentation on the tumor cells themselves and suppression of regulatory T cells (Figure 2). While the authors describe an increase in senescence-associated β-Galactosidase positive cells, expression of inflammatory SASP components like IL6, IL1A and IL1B was not detected [17]. Enhanced T-cell infiltration was also observed in other studies. In one of them, CDK4/6 inhibitors were identified as activators of PD-1 suppressed T cells in a small molecule screen [70]. Another study found synergistic and immunogenic effects when combining Ribociclib with the PI3Kα inhibitor Alpelisib [71]. Of note, CDK4/6 inhibition has been described to promote expression of PD-L1on cancer cells by inhibiting its proteasomal degradation [72]. Nonetheless, the combination of CDK4/6 inhibitors with checkpoint inhibitorsresulted in enhanced anti-tumor effects in all these studies. In addition, CDK4/6 inhibitors have been shown to repress an intracellular program associated with resistance to anti-PD-1 therapy [47]. Clinical trials are still ongoing (Table 1), but preclinical data give reason to hope that this treatment regimen could be transferred to the clinic.
Table 1. Clinical trials combining CDK4/6 inhibitors and checkpoint inhibitors.
| Drugs | Title | Condition(s) | Phase | NCT Number |
|---|---|---|---|---|
|
Palbociclib
Cetuximab (anti-EGFR) Avelumab (anti-PD-L1) |
Avelumab, Cetuximab, and Palbociclib in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma | Head and Neck Squamous Cell Carcinoma | Phase 1 | NCT03498378 |
|
Palbociclib
Tamoxifen Avelumab (anti-PD-L1) |
Neoadjuvant Tamoxifen, Palbociclib, Avelumab in Estrogen Receptor Positive Breast Cancer | Breast Cancer | Phase 2 | NCT03573648 |
|
Palbociclib
Letrozole Pembrolizumab (anti-PD-1) |
Pembrolizumab, Letrozole, and Palbociclib in Treating Postmenopausal Patients with Newly Diagnosed Metastatic Stage IV Estrogen Receptor Positive Breast Cancer | Breast Cancer | Phase 2 | NCT02778685 |
|
Palbociclib
Anastrozole Nivolumab (anti-PD-1) |
A Study of Neoadjuvant Nivolumab + Palbociclib + Anastrozole in Post-Menopausal Women and Men with Primary Breast Cancer | Breast Cancer | Phase 2 | NCT04075604 |
|
Abemaciclib
Nivolumab (anti-PD-1) |
Modulation of the Tumor Microenvironment by Abemaciclib in Operable HPV-Negative Head and Neck Cancer (HNC) | Head and Neck Squamous Cell Carcinoma | Phase 2 | NCT04169074 |
|
Abemaciclib
Nivolumab (anti-PD-1) |
Abemaciclib + Nivolumab in Patients with Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma That Progressed or Recurred Within Six Months After Platinum-based Chemotherapy | Head and Neck Squamous Cell Carcinoma | Phase 1/2 | NCT03655444 |
|
Abemaciclib
Atezolizumab (anti-PD-L1) |
Abemaciclib With or Without Atezolizumab in Metastatic Castration Resistant Prostate Cancer | Prostate Cancer | Phase 2 | NCT04272645 |
|
Abemaciclib
Nivolumab (anti-PD-1) |
Abemaciclib and Nivolumab for Subjects with Hepatocellular Carcinoma | Hepatocellular Carcinoma | Phase 2 | NCT03781960 |
|
Abemaciclib
Pembrolizumab (anti-PD-1) |
Abemaciclib + Pembrolizumab In Glioblastoma | Glioblastoma | Phase 2 | NCT04118036 |
|
Abemaciclib
Pembrolizumab (anti-PD-1) |
Pilot Study of Pembrolizumab Combined with Pemetrexed or Abemaciclib for High Grade Glioma | High Grade Glioma | Early Phase 1 | NCT04220892 |
|
Abemaciclib
Pembrolizumab (anti-PD-1) |
Abemaciclib and Pembrolizumab in Locally Advanced Unresectable or Metastatic Gastroesophageal Adenocarcinoma | Gastro-oesophageal Adenocarcinoma | Phase 2 | NCT03997448 |
|
Abemaciclib
Durvalumab (anti-PD-L1) |
Neoadjuvant Study of Abemaciclib, Durvalumab, and an Aromatase Inhibitor Early Stage Breast Cancer | Breast Cancer | Early Phase 1 | NCT04088032 |
|
Ribociclib
Spartalizumab (anti-PD-1) |
Ribociclib and Spartalizumab in R/M HNSCC | Head and Neck Squamous Cell Carcinoma | Phase 1 | NCT04213404 |
|
Ribociclib
Spartalizumab (anti-PD-1) |
Ribociclib + PDR001 (Spartalizumab) in Breast Cancer and Ovarian Cancer | Breast Cancer Ovarian Cancer |
Phase 1 | NCT03294694 |
While the combination with checkpoint inhibitors aims to boost the adaptive immune response, the innate immune system has been described to play a major role in lung cancer mouse models treated with Palbociclib and the MEK inhibitor Trametinib. Regardless of the p53 status of the cells, this combination led to tumor cell senescence and induction of a robust SASP [73]. The authors showed that SASP induction served to recruit and activate NK cells, enabling NK cell-mediated cytotoxicity and tumor regression (Figure 2). Another study of the same group showed different effects of the SASP on the tumor microenvironment in pancreatic cancer [74]. Like previously seen in lung cancer cells, treatment with Palbociclib and Trametinib induced senescence and a robust SASP in vitro and in vivo. However, this did not result in recruitment of NK cells, but led to increased vascularization, which improved delivery of conventional chemotherapeutics (gemcitabine) (Figure 2). In addition, the recruitment of cytotoxic T cells was enhanced, and combination with checkpoint inhibitors led to tumor regression and prolonged survival.
Taken together, there is clear evidence that CDK4/6 inhibitors alone or in combination with other drugs have immunogenic effects. Whereas induction of senescence might not be a general requirement therefore, it seems that senescent tumor cells can mediate profound changes in the tumor microenvironment through their SASP. This underlines that induction of senescence can yield expanded anti-tumor effects beyond cell cycle arrest, making it a desirable treatment outcome.
Other combination therapies
Despite the clear advantages of CDK4/6 inhibitors (interference with key regulators of proliferation, high specificity and bioavailability, manageable side effects), there is increasing understanding that combination therapies might be necessary to achieve significant anti-tumor effects. Potential benefits of combination therapieslie in improved inhibition of tumor growth, shiftingfrom quiescence toapoptosis or senescence, overcoming resistance, engaging the immune system or reducing side effects by lowering the single agent doses.
As the RAS/MEK/ERK and the PI3K/AKT signaling pathwaysare involved in Cyclin D regulation and CDK4/6 inhibitor resistance (see above), several studies have been focusing on combining CDK4/6 inhibitors with inhibition of these pathways. Activating mutations in RAS or RAF are frequent in cancer, especially in pancreatic cancer (KRAS, 91%), colorectal cancer (KRAS, 42%), NSCLC (KRAS, 33%) and malignant melanoma (BRAF, 66%; NRAS 27%) [75, 76]. The development of potent RAF inhibitors (e.g. Vemurafenib, Dabrafenib) and MEK inhibitors (e.g. Trametinib, Binimetinib) has opened new opportunities. Despite their beneficial effects in BRAF V600E mutant melanoma, resistance mediated by feedback loops is a major problem, urging the need to find novel combination therapies. In an NRAS-driven melanoma mouse model, genetic ablation of NRAS, but not treatment with a MEK inhibitor, led to tumor regression. Further analysis revealed that CDK4 was the main driver of continued cell proliferation after MEK inhibition. Combining Palbociclib and a MEK inhibitor was synergistic, resulting in apoptosis, cell cycle arrest and tumor regression [77]. Other studies also found that this combination caused apoptosis in melanoma but induced senescence in pancreatic and lung cancer models (Table 2) [73, 74, 78, 79]. First results of a phase Ib/II trial combining Ribociclib and Binimetinib in patients with NRAS mutant melanoma were promising, as it resulted in significant tumor regression, however, serious side effects were also reported [80]. More clinical trials are ongoing (Table 3).
Table 2. Preclinical studies combining CDK4/6 inhibitors and MEK or PI3K inhibitors.
| Cancer type | Drugs | Cell fate | Study design and overall outcome | Reference |
|---|---|---|---|---|
| Melanoma |
Palbociclib
MEK inhibitor (Selumetinib) |
quiescence andapoptosis | NRAS driven melanoma mouse model; combination leads to tumor regression | [77] |
| Melanoma |
CDK4i
(219476) MEK inhibitor (PD98059) |
apoptosis | combination leads to apoptosis in melanoma cell lines | [78] |
| Pancreatic cancer |
Palbociclib
MEK inhibitor (Trametinib) |
senescence | pancreatic cancer mouse models; combination leads to senescence and remodeling of the tumor microenvironment(see also Fig. 2 and “Engaging the immune system”) | [74] |
| Pancreatic cancer |
Palbociclib
MEK inhibitor (Selumetinib) |
senescence | combination leads to senescence in pancreatic cancer cell lines | [79] |
| Lung cancer |
Palbociclib
MEK inhibitor (Trametinib) |
senescence | lung cancer mouse model; combination leads to senescence, NK cell recruitment and tumor regression | [73] |
| Pancreatic cancer |
Palbociclib
PI3K/MTOR inhibitor (BEZ235) |
apoptosis | combination leads to apoptosis in vitro and suppression of tumor growth in patient-derived xenograft models of pancreatic cancer in vivo | [79] |
| Breast cancer |
Ribociclib
PI3K(α) inhibitor (Alpelisib and Pictilisib) |
primarily quiescence |
breast cancer cell lines and xenograft mouse models; combination is synergistic, tumor regression in PI3KCA mutant breast cancer | [83] |
| Breast cancer |
Ribociclib
PI3Kα inhibitor (Alpelisib) |
quiescence and apoptosis | combination is synergistic in vitro and in patient-derived xenograft models in vivo and stimulates the immune system (see also paragraph “Engaging the immune system”) | [71] |
| Breast cancer |
Palbociclib
PI3Kα inhibitor (Taselisib) |
apoptosis | breast cancer cell lines and xenograft mouse models; combination increases apoptosis in PI3KCA mutant cells in vitro, tumor regression in vivo | [84] |
| Breast Cancer (ER positive) |
Palbociclib
Fulvestrant PI3K inhibitor (Pictilisib) |
apoptosis | cell lines and patient-derived xenograft models; triple combination induces apoptosis and leads to tumor regression in vivo | [85] |
Table 3. Selection of ongoing clinical trials combining CDK4/6 inhibitors and MEK inhibitors or PI3K inhibitors.
| Drugs | Title | Condition(s) | Phase | NCT Number |
|---|---|---|---|---|
|
Palbociclib
Binimetinib (MEK inhibitor) |
Study of the CDK4/6 Inhibitor Palbociclib (PD-0332991) in Combination with the MEK Inhibitor Binimetinib (MEK162) for Patients with Advanced KRAS Mutant Non-Small Cell Lung Cancer | KRAS mutant NSCLC | Phase 1/2 | NCT03170206 |
|
Palbociclib
Trametinib (MEK inhibitor) |
A Study to Investigate the Safety, Pharmacokinetics, Pharmacodynamics, and Anti-Cancer Activity of Trametinib in Combination with Palbociclib in Subjects with Solid Tumors | Solid Tumours | Phase 1 | NCT02065063 |
|
Palbociclib
Binimetinib (MEK inhibitor) |
Binimetinib and Palbociclib or TAS-102 in Treating Patients with KRAS and NRAS Mutant Metastatic or Unresectable Colorectal Cancer | KRAS mutant or NRAS mutant Colorectal Cancer | Phase 2 | NCT03981614 |
|
Ribociclib
Trametinib (MEK inhibitor) |
Study of Safety and Efficacy of Ribociclib and Trametinib in Patients With Metastatic or Advanced Solid Tumors | Solid Tumours | Phase 1 | NCT02703571 |
|
Ribociclib
Binimetinib (MEK inhibitor) |
A Phase Ib/II Study of LEE011 in Combination with MEK162 in Patients with NRAS Mutant Melanoma | NRAS mutant melanoma | Phase 1/2 | NCT01781572 |
|
Ribociclib
Letrozole Alpelisib (PI3Kαinhibitor) |
Study of LEE011, BYL719 and Letrozole in Advanced ER+ Breast Cancer | Breast Cancer | Phase 1 | NCT01872260 |
|
Ribociclib
Fulvestrant Alpelisib (PI3Kα inhibitor) |
Study of LEE011 With Fulvestrant and BYL719 or BKM120 in Advanced Breast Cancer | Breast Cancer | Phase 1 | NCT02088684 |
|
Palbociclib
Fulvestrant GDC-0077 (PI3Kα inhibitor) |
A Study Evaluating the Efficacy and Safety of GDC-0077 + Palbociclib + Fulvestrant vs Placebo + Palbociclib + Fulvestrant in Patients with PIK3CA-Mutant, Hormone Receptor-Positive, Her2-Negative, Locally Advanced or Metastatic Breast Cancer | PIK3CA mutant breast cancer | Phase 2/3 | NCT04191499 |
|
Ribociclib
Letrozole Buparlisib (PI3K inhibitor) |
Dose Escalation Study of LEE011 in Combination with Buparlisib and Letrozole in HR+, HER2-negative Post-menopausal Women with Advanced Breast Cancer. | Breast Cancer | Phase 1 | NCT02154776 |
|
Abemaciclib
Fulvestrant Copanlisib (PI3Kα inhibitor) |
Testing the Addition of Copanlisib to Usual Treatment (Fulvestrant and Abemaciclib) in Metastatic Breast Cancer - Dose-Finding Study | Breast Cancer | Phase 1/2 | NCT04088032 |
|
Palbociclib
Gedatolisib (PI3K/mTOR inhibitor) |
Phase I Study of Combination of Gedatolisib With Palbociclib and Faslodex in Patients With ER+/HER2- Breast Cancer | Breast Cancer | Phase 1 | NCT02626507 |
|
Palbociclib
Gedatolisib (PI3K/mTOR inhibitor) |
A Study to Assess The Tolerability And Clinical Activity Of Gedatolisib In Combination With Palbociclib/Letrozole Or Palbociclib/Fulvestrant In Women With Metastatic Breast Cancer | Breast Cancer | Phase 1 | NCT02684032 |
|
Palbociclib
Gedatolisib (PI3K/mTOR inhibitor) |
Study of the CDK4/6 Inhibitor Palbociclib (PD-0332991) in Combination with the PI3K/mTOR Inhibitor Gedatolisib (PF-05212384) for Patients with Advanced Squamous Cell Lung, Pancreatic, Head & Neck and Other Solid Tumors | Solid Tumours | Phase 1 | NCT03065062 |
|
Palbociclib
Taselisib (PI3Kα inhibitor) OR Pictilisib (PI3K inhibitor) |
Combination of PI3 Kinase Inhibitors and Palbociclib | Solid Tumours | Phase 1 | NCT02389842 |
Like the RAS/MEK/ERK signaling pathway, the PI3K/AKT pathway is one of the signaling pathways most frequently activated in cancer [81]. 40% of patients with ER+/HER2- breast cancer have tumors with PI3KCA mutations. In 2019, the PI3Kα inhibitor Alpelisib was FDA-approved for the treatment of these tumors [82]. Testing the combination of CDK4/6 inhibitors and PI3K inhibitors therefore seems natural. Whereas many clinical trials are still ongoing (Table 3), preclinical studies have shown that CDK4/6 inhibitors sensitize PI3KCA mutant breast cancer cells to PI3K inhibitors and that the combination of both drugs is synergistic in vitro and in vivo [83, 84]. Another groupdemonstratedthat a PI3K inhibitor could preventthe resistance to CDK4/6 inhibitors caused by increased CDK2 activity [85]. Interestingly, the combination of PI3K inhibitors and CDK4/6 inhibitors has been reported to lead to cell cycle arrest and apoptosis rather than senescence (Table 2) [79, 85].
Whether CDK4/6 inhibitors can be combined with conventional chemotherapy or irradiation remains controversial, as many cytotoxic therapies rely on cycling cells and are effective in S or M phase. In fact, Palbociclib has been shown to reduce the effect of carboplatin in RB-competent breast cancer mouse models, but at the same time mitigated chemotherapy-induced myelotoxicity [86, 87]. On the other hand, there are reports that CDK4/6 could be combined with gemcitabine, taxanesor platinum-based compounds in pancreatic or ovarian cancer, respectively [74, 88, 89]. A recent study investigatedthe use of CDK4/6 inhibitors after cytotoxic chemotherapy in pancreatic cancer models [90]. Chemotherapy followed by treatment with CDK4/6 inhibitors led to prolonged inhibition of proliferation and improved therapeutic effects, as CDK4/6 inhibitors repressed the DNA-repair machinery, preventing cells from recovering from DNA damaging agents.
Two-Hit strategy: Inducing apoptosis in senescent cells
Despite all the benefits of CDK4/6-induced senescence described above, the aberrant persistence of senescent cells has detrimentaleffects that might affect outcome on the long term. Senescent cells within thyroid tumors, for example, have been reported to lead invasion [91]. Besides, cancer recurrence is a major concern, as therapy-induced senescence has been shown to provoke stem cell-like features leading to more aggressive tumorbehavior [92]. In addition, the induction of senescence in stromal cells and normal tissues contributes to the side effects of chemotherapy andpromotes chronic inflammation [93, 94].
Elimination of senescent cells -through stimulation of immune cell-mediated clearance orthrough senolytic approaches- can alleviate these detrimental effects. Targeted induction of apoptosis in senescent cells is an appealing treatment strategy. In 2011, it was first shown that the selective elimination of senescent cells can increase lifespan in a progeroid mouse model [95]. Since then, senolytic approaches have been demonstrated to be beneficial in a widerange of diseases [48]. Therapy-induced senescence can also prepare the ground for subsequent elimination of cancer cells. This was first demonstrated in a preclinical study investigating the phenotype of lymphoma cells treated with chemotherapy [96]. Senescent lymphomas were more sensitive to inhibition of glucose utilization or autophagy, and addition of an autophagy inhibitor after chemotherapy improved treatment outcome in mice. This “double-hit” strategy (Figure 3) has also proven effective in a recently published study in liver cancer [97]. Combination of a pro-senescence therapy with a senolytic drug resulted in marked reduction of tumor growth in vivo.
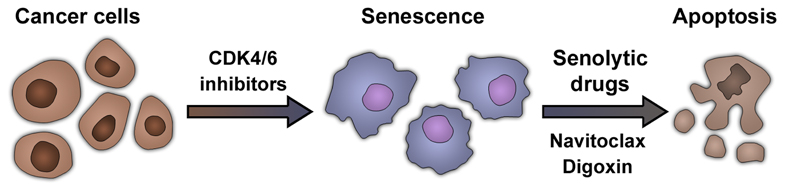
Figure 3. Sequential treatment with CDK4/6 inhibitors and senolytics.
Treatment with CDK4/6 inhibitors induces senescence in many cancer cells. Senescent cells share specific vulnerabilities that can be targeted by senolytic drugs. Examples of senolytic drugs include BCL2-family inhibitors such as navitoclax (ABT-263) or cardiac glycosides (e.g. digoxin). Senolytic drugs induce apoptosis in senescent cells. The stepwise combination of drugs able to induce senescence (pro-senescence therapy) such as CDK4/6 inhibitors and a senolytic drug constitutes an emerging therapeutic strategy.
ABT-263 (also known as navitoclax), a specific inhibitor of the anti-apoptotic proteins BCL-2 and BCL-xL, was the first described prototypical senolytic drug [98]. As its clinical use is limited by side effects, especially thrombocytopenia, there is a need to identify novel senolytic compounds. Cardiac glycosides (such as digoxin or digitoxin)are widely used in clinic and have been recently described as broad-spectrum senolytics [99]. Interestingly, they also induced apoptosis in different senescent cancer cells treated with Palbociclib and could therefore be used to improve the outcome of CDK4/6 inhibitor therapies.
Conclusion
In 2015, the FDA approved Palbociclib in combination with letrozole for the treatment ofER+/HER2-metastatic breast cancer. Since then, the possibility to expand the use of CDK4/6 inhibitors to other tumor types has contributed to build excitement in this family of drugs. While the potential is huge, the challenge resides in understanding what patients will respond to CDK4/6 inhibitors and what drug combinations might be better suited to achieve maximum benefit.
A frequent (but not universal) outcome of treatment with CDK4/6 inhibitors is the induction of senescence. Senescence is a powerful tumor suppressor mechanism and influences the outcome of chemo- and radiotherapy. Cells undergoing senescence not only implement a stable cell cycle arrest, but produce a secretome (the SASP) that directs their immune-mediated clearance. The SASP is a key mediator of the effects exerted by senescent cells. The composition of the SASP differs depending on cell type and how senescence is induced [60]. Characterizing the secretome of cancer cells treated with CDK4/6 inhibitors is therefore important.
Senescence has recently been seen as a favorable outcome during cancer treatment. The ability to target senescent cells with senolytic drugs makes the scenario even more attractive, as the detrimental effects associated with the aberrant accumulation of senescent cells can be eliminated. To be able to fully exploit this strategy, we will need techniquesto detect senescent cells in patients. While markers and guidelines to identify senescent cells in vitro and in vivo are clear [14], diagnostic tests or imaging methods for patients are lacking. Hopefully, the identification of novel markers and the development of non-invasive diagnostics to monitor senescent cells [100] will help to facilitate the translation of this approach to the clinic. Given the myriad of active clinical trials with CDK4/6 inhibitors, the next years will better define the utility of these drugs and clarify the relevance of senescence induction for their efficacy.
Acknowledgements
Grants from CRUK (C15075/A28647) and core support from MRC (MC_U120085810) funded this research in J. Gil’s laboratory. V. Wagner was funded by the German Cancer Aid Mildred-Scheel Postdoc Program.
Footnotes
Competing Interests
J. Gil has acted as a consultant for Unity Biotechnology and Geras Bio and Merck KGaA; owns equity in Unity Biotechnology and Geras Bio and is a named inventor in an MRC patent related to senolytic therapies.
References
- 1.Sherr CJ. Cancer cell cycles. Science. 274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 2.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 4.Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov. 2016;6:353–367. doi: 10.1158/2159-8290.CD-15-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez-Fernandez M, Malumbres M. Mechanisms of Sensitivity and Resistance to CDK4/6 Inhibition. Cancer Cell. 2020;37:514–529. doi: 10.1016/j.ccell.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nature Reviews Drug Discovery. 2015;14:130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–1438. [PubMed] [Google Scholar]
- 8.Toogood PL, Harvey PJ, Repine JT, Sheehan DJ, VanderWel SN, Zhou H, et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J Med Chem. 2005;48:2388–2406. doi: 10.1021/jm049354h. [DOI] [PubMed] [Google Scholar]
- 9.Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014;32:825–837. doi: 10.1007/s10637-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S, Loo A, Chopra R, Caponigro G, Huang A, Vora S, et al. Abstract PR02: LEE011: An orally bioavailable, selective small molecule inhibitor of CDK4/6– Reactivating Rb in cancer. Mol Cancer Ther. 2013;12:PR02–PR02. [Google Scholar]
- 11.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18)a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 12.Klein ME, Kovatcheva M, Davis LE, Tap WD, Koff A. CDK4/6 Inhibitors: The Mechanism of Action May Not Be as Simple as Once Thought. Cancer Cell. 2018;34:9–20. doi: 10.1016/j.ccell.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hafner M, Mills CE, Subramanian K, Chen C, Chung M, Boswell SA, et al. Multiomics Profiling Establishes the Polypharmacology of FDA-Approved CDK4/6 Inhibitors and the Potential for Differential Clinical Activity. Cell Chem Biol. 2019;26:1067–1080 e1068. doi: 10.1016/j.chembiol.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, et al. Cellular Senescence: Defining a Path Forward. Cell. 2019;179:813–827. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Vijayaraghavan S, Karakas C, Doostan I, Chen X, Bui T, Yi M, et al. CDK4/6 and autophagy inhibitors synergistically induce senescence in Rb positive cytoplasmic cyclin E negative cancers. Nature Communications. 2017;8:15916. doi: 10.1038/ncomms15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres-Guzman R, Calsina B, Hermoso A, Baquero C, Alvarez B, Amat J, et al. Preclinical characterization of abemaciclib in hormone receptor positive breast cancer. Oncotarget. 2017;8:69493–69507. doi: 10.18632/oncotarget.17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471–475. doi: 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM, et al. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell. 2011;20:620–634. doi: 10.1016/j.ccr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida A, Lee EK, Diehl JA. Induction of Therapeutic Senescence in Vemurafenib-Resistant Melanoma by Extended Inhibition of CDK4/6. Cancer Res. 2016;76:2990–3002. doi: 10.1158/0008-5472.CAN-15-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bollard J, Miguela V, Ruiz de Galarreta M, Venkatesh A, Bian CB, Roberto MP, et al. Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumour growth in preclinical models of hepatocellular carcinoma. Gut. 2017;66:1286–1296. doi: 10.1136/gutjnl-2016-312268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Pan J. Dual cyclin-dependent kinase 4/6 inhibition by PD-0332991 induces apoptosis and senescence in oesophageal squamous cell carcinoma cells. Br J Pharmacol. 2017;174:2427–2443. doi: 10.1111/bph.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valenzuela CA, Vargas L, Martinez V, Bravo S, Brown NE. Palbociclib-induced autophagy and senescence in gastric cancer cells. Exp Cell Res. 2017;360:390–396. doi: 10.1016/j.yexcr.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Kovatcheva M, Liao W, Klein ME, Robine N, Geiger H, Crago AM, et al. ATRX is a regulator of therapy induced senescence in human cells. Nat Commun. 2017;8:386. doi: 10.1038/s41467-017-00540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovatcheva M, Liu DD, Dickson MA, Klein ME, O'Connor R, Wilder FO, et al. MDM2 turnover and expression of ATRX determine the choice between quiescence and senescence in response to CDK4 inhibition. Oncotarget. 2015;6:8226–8243. doi: 10.18632/oncotarget.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tao YF, Wang NN, Xu LX, Li ZH, Li XL, Xu YY, et al. Molecular mechanism of G1 arrest and cellular senescence induced by LEE011, a novel CDK4/CDK6 inhibitor, in leukemia cells. Cancer Cell Int. 2017;17:35. doi: 10.1186/s12935-017-0405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rader J, Russell MR, Hart LS, Nakazawa MS, Belcastro LT, Martinez D, et al. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin Cancer Res. 2013;19:6173–6182. doi: 10.1158/1078-0432.CCR-13-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi YJ, Li X, Hydbring P, Sanda T, Stefano J, Christie AL, et al. The requirement for cyclin D function in tumor maintenance. Cancer. 2012;22:438–451. doi: 10.1016/j.ccr.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawai CM, Freund J, Oh P, Ndiaye-Lobry D, Bretz JC, Strikoudis A, et al. Therapeutic targeting of the cyclin D3:CDK4/6 complex in T cell leukemia. Cancer Cell. 2012;22:452–465. doi: 10.1016/j.ccr.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Nicolay BN, Chick JM, Gao X, Geng Y, Ren H, et al. The metabolic function of cyclin D3-CDK6 kinase in cancer cell survival. Nature. 2017;546:426–430. doi: 10.1038/nature22797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellutti F, Tigan AS, Nebenfuehr S, Dolezal M, Zojer M, Grausenburger R, et al. CDK6 Antagonizes p53-Induced Responses during Tumorigenesis. Cancer Discov. 2018;8:884–897. doi: 10.1158/2159-8290.CD-17-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kollmann K, Heller G, Schneckenleithner C, Warsch W, Scheicher R, Ott RG, et al. A Kinase-Independent Function of CDK6 Links the Cell Cycle to Tumor Angiogenesis. Cancer Cell. 2016;30:359–360. doi: 10.1016/j.ccell.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Condorelli R, Spring L, O'Shaughnessy J, Lacroix L, Bailleux C, Scott V, et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann Oncol. 2018;29:640–645. doi: 10.1093/annonc/mdx784. [DOI] [PubMed] [Google Scholar]
- 33.Wander SA, Cohen O, Gong X, Johnson GN, Buendia-Buendia JE, Lloyd MR, et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor positive metastatic breast cancer. Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-19-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong X, Litchfield LM, Webster Y, Chio LC, Wong SS, Stewart TR, et al. Genomic Aberrations that Activate D-type Cyclins Are Associated with Enhanced Sensitivity to the CDK4 and CDK6 Inhibitor Abemaciclib. Cancer Cell. 2017;32:761–776 e766. doi: 10.1016/j.ccell.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Turner NC, Liu Y, Zhu Z, Loi S, Colleoni M, Loibl S, et al. Cyclin E1 Expression and Palbociclib Efficacy in Previously Treated Hormone Receptor-Positive Metastatic Breast Cancer. J Clin Oncol. 2019;37:1169–1178. doi: 10.1200/JCO.18.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walter DM, Yates TJ, Ruiz-Torres M, Kim-Kiselak C, Gudiel AA, Deshpande C, et al. RB constrains lineage fidelity and multiple stages of tumour progression and metastasis. Nature. 2019;569:423–427. doi: 10.1038/s41586-019-1172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, et al. Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non-Small Cell Lung Cancer, and Other Solid Tumors. Cancer Discov. 2016;6:740–753. doi: 10.1158/2159-8290.CD-16-0095. [DOI] [PubMed] [Google Scholar]
- 38.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 39.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Chang BD, Broude EV, Dokmanovic M, Zhu H, Ruth A, Xuan Y, et al. A senescence-like phenotype distinguishes tumor cells that undergo terminal proliferation arrest after exposure to anticancer agents. Cancer Res. 1999;59:3761–3767. [PubMed] [Google Scholar]
- 42.Petrova NV, Velichko AK, Razin SV, Kantidze OL. Small molecule compounds that induce cellular senescence. Aging Cell. 2016;15:999–1017. doi: 10.1111/acel.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109:335–346. doi: 10.1016/s0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 44.Roberson RS, Kussick SJ, Vallieres E, Chen SY, Wu DY. Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers. Cancer Res. 2005;65:2795–2803. doi: 10.1158/0008-5472.CAN-04-1270. [DOI] [PubMed] [Google Scholar]
- 45.te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62:1876–1883. [PubMed] [Google Scholar]
- 46.Haugstetter AM, Loddenkemper C, Lenze D, Grone J, Standfuss C, Petersen I, et al. Cellular senescence predicts treatment outcome in metastasised colorectal cancer. Br J Cancer. 2010;103:505–509. doi: 10.1038/sj.bjc.6605784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jerby-Arnon L, Shah P, Cuoco MS, Rodman C, Su MJ, Melms JC, et al. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell. 2018;175:984–997 e924. doi: 10.1016/j.cell.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McHugh D, Gil J. Senescence and aging: Causes, consequences, and therapeutic avenues. J Cell Biol. 2018;217:65–77. doi: 10.1083/jcb.201708092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 50.Serrano M, Gomez-Lahoz E, DePinho RA, Beach D, Bar-Sagi D. Inhibition of ras-induced proliferation and cellular transformation by p16INK4. Science. 1995;267:249–252. doi: 10.1126/science.7809631. [DOI] [PubMed] [Google Scholar]
- 51.Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 52.Gil J, Peters G. Regulation of the INK4b–ARF–INK4a tumour suppressor locus: all for one or one for all. Nature Reviews Molecular Cell Biology. 2006;7:667–677. doi: 10.1038/nrm1987. [DOI] [PubMed] [Google Scholar]
- 53.Coppe JP, Rodier F, Patil CK, Freund A, Desprez PY, Campisi J. Tumor suppressor and aging biomarker p16INK4a induces cellular senescence without the associated inflammatory secretory phenotype. J Biol Chem. 2011;286:36396–36403. doi: 10.1074/jbc.M111.257071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zou X, Ray D, Aziyu A, Christov K, Boiko AD, Gudkov AV, et al. Cdk4 disruption renders primary mouse cells resistant to oncogenic transformation, leading to Arf/p53-independent senescence. Genes Dev. 2002;16:2923–2934. doi: 10.1101/gad.1033002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puyol M, Martin A, Dubus P, Mulero F, Pizcueta P, Khan G, et al. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell. 2010;18:63–73. doi: 10.1016/j.ccr.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 56.Reddy HK, Mettus RV, Rane SG, Grana X, Litvin J, Reddy EP. Cyclin-dependent kinase 4 expression is essential for neu-induced breast tumorigenesis. Cancer Res. 2005;65:10174–10178. doi: 10.1158/0008-5472.CAN-05-2639. [DOI] [PubMed] [Google Scholar]
- 57.Yu Q, Sicinska E, Geng Y, Ahnstrom M, Zagozdzon A, Kong Y, et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell. 2006;9:23–32. doi: 10.1016/j.ccr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 58.Damsky W, Micevic G, Meeth K, Muthusamy V, Curley DP, Santhanakrishnan M, et al. mTORC1 activation blocks BrafV600E-induced growth arrest but is insufficient for melanoma formation. Cancer Cell. 2015;27:41–56. doi: 10.1016/j.ccell.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miettinen TP, Peltier J, Hartlova A, Gierlinski M, Jansen VM, Trost M, et al. Thermal proteome profiling of breast cancer cells reveals proteasomal activation by CDK4/6 inhibitor palbociclib. EMBO J. 2018;37 doi: 10.15252/embj.201798359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basisty N, Kale A, Jeon OH, Kuehnemann C, Payne T, Rao C, et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18:e3000599. doi: 10.1371/journal.pbio.3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coppe JP, Kauser K, Campisi J, Beausejour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281:29568–29574. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- 62.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruhland MK, Loza AJ, Capietto AH, Luo X, Knolhoff BL, Flanagan KC, et al. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat Commun. 2016;7:11762. doi: 10.1038/ncomms11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 65.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 67.Eggert T, Wolter K, Ji J, Ma C, Yevsa T, Klotz S, et al. Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression. Cancer Cell. 2016;30:533–547. doi: 10.1016/j.ccell.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med. 2013;210:2057–2069. doi: 10.1084/jem.20130783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018;8:216–233. doi: 10.1158/2159-8290.CD-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teo ZL, Versaci S, Dushyanthen S, Caramia F, Savas P, Mintoff CP, et al. Combined CDK4/6 and PI3Kalpha Inhibition Is Synergistic and Immunogenic in Triple-Negative Breast Cancer. Cancer Res. 2017;77:6340–6352. doi: 10.1158/0008-5472.CAN-17-2210. [DOI] [PubMed] [Google Scholar]
- 72.Zhang J, Bu X, Wang H, Zhu Y, Geng Y, Nihira NT, et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature. 2018;553:91–95. doi: 10.1038/nature25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ruscetti M, Leibold J, Bott MJ, Fennell M, Kulick A, Salgado NR, et al. NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science. 2018;362:1416–1422. doi: 10.1126/science.aas9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ruscetti M, Morris JPt, Mezzadra R, Russell J, Leibold J, Romesser PB, et al. Senescence-Induced Vascular Remodeling Creates Therapeutic Vulnerabilities in Pancreas Cancer. Cell. 2020 doi: 10.1016/j.cell.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 76.Simanshu DK, Nissley DV, McCormick F. RAS Proteins and Their Regulators in Human Disease. Cell. 2017;170:17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwong LN, Costello JC, Liu H, Jiang S, Helms TL, Langsdorf AE, et al. Oncogenic NRAS signaling differentially regulates survival and proliferation in melanoma. Nature Medicine. 2012;18:1503–1510. doi: 10.1038/nm.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li J, Xu M, Yang Z, Li A, Dong J. Simultaneous inhibition of MEK and CDK4 leads to potent apoptosis in human melanoma cells. Cancer Invest. 2010;28:350–356. doi: 10.3109/07357900903286966. [DOI] [PubMed] [Google Scholar]
- 79.Franco J, Balaji U, Freinkman E, Witkiewicz AK, Knudsen ES. Metabolic Reprogramming of Pancreatic Cancer Mediated by CDK4/6 Inhibition Elicits Unique Vulnerabilities. Cell Rep. 2016;14:979–990. doi: 10.1016/j.celrep.2015.12.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schuler MH, Ascierto PA, Vos FYFLD, Postow MA, Herpen CML-V, Carlino MS, et al. Phase 1b/2 trial of ribociclib+binimetinib in metastatic NRAS-mutant melanoma: Safety, efficacy, and recommended phase 2 dose (RP2D) Journal of Clinical Oncology. 2017;35:9519–9519. [Google Scholar]
- 81.Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Annals of Medicine. 2014;46:372–383. doi: 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- 82.Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 83.Vora SR, Juric D, Kim N, Mino-Kenudson M, Huynh T, Costa C, et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell. 2014;26:136–149. doi: 10.1016/j.ccr.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Asghar US, Barr AR, Cutts R, Beaney M, Babina I, Sampath D, et al. Single-Cell Dynamics Determines Response to CDK4/6 Inhibition in Triple-Negative Breast Cancer. Clin Cancer Res. 2017;23:5561–5572. doi: 10.1158/1078-0432.CCR-17-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Early Adaptation and Acquired Resistance to CDK4/6 Inhibition in Estrogen Receptor-Positive Breast Cancer. Cancer Res. 2016;76:2301–2313. doi: 10.1158/0008-5472.CAN-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He S, Roberts PJ, Sorrentino JA, Bisi JE, Storrie-White H, Tiessen RG, et al. Transient CDK4/6 inhibition protects hematopoietic stem cells from chemotherapy-induced exhaustion. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roberts PJ, Bisi JE, Strum JC, Combest AJ, Darr DB, Usary JE, et al. Multiple Roles of Cyclin-Dependent Kinase 4/6 Inhibitors in Cancer Therapy. JNCI: Journal of the National Cancer Institute. 2012;104:476–487. doi: 10.1093/jnci/djs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dall'Acqua A, Sonego M, Pellizzari I, Pellarin I, Canzonieri V, D'Andrea S, et al. CDK6 protects epithelial ovarian cancer from platinum-induced death via FOXO3 regulation. EMBO Mol Med. 2017;9:1415–1433. doi: 10.15252/emmm.201607012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kumarasamy V, Ruiz A, Nambiar R, Witkiewicz AK, Knudsen ES. Chemotherapy impacts on the cellular response to CDK4/6 inhibition: distinct mechanisms of interaction and efficacy in models of pancreatic cancer. Oncogene. 2020;39:1831–1845. doi: 10.1038/s41388-019-1102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salvador-Barbero B, Alvarez-Fernandez M, Zapatero-Solana E, El Bakkali A, Menendez MDC, Lopez-Casas PP, et al. CDK4/6 Inhibitors Impair Recovery from Cytotoxic Chemotherapy in Pancreatic Adenocarcinoma. Cancer Cell. 2020;37:340–353 e346. doi: 10.1016/j.ccell.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 91.Kim YH, Choi YW, Lee J, Soh EY, Kim JH, Park TJ. Senescent tumor cells lead the collective invasion in thyroid cancer. Nat Commun. 2017;8:15208. doi: 10.1038/ncomms15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Milanovic M, Fan DNY, Belenki D, Dabritz JHM, Zhao Z, Yu Y, et al. Senescence-associated reprogramming promotes cancer stemness. Nature. 2018;553:96–100. doi: 10.1038/nature25167. [DOI] [PubMed] [Google Scholar]
- 93.Demaria M, O'Leary MN, Chang J, Shao L, Liu S, Alimirah F, et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017;7:165–176. doi: 10.1158/2159-8290.CD-16-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guan X, LaPak KM, Hennessey RC, Yu CY, Shakya R, Zhang J, et al. Stromal Senescence By Prolonged CDK4/6 Inhibition Potentiates Tumor Growth. Mol Cancer Res. 2017;15:237–249. doi: 10.1158/1541-7786.MCR-16-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dorr JR, Yu Y, Milanovic M, Beuster G, Zasada C, Dabritz JH, et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature. 2013;501:421–425. doi: 10.1038/nature12437. [DOI] [PubMed] [Google Scholar]
- 97.Wang C, Vegna S, Jin H, Benedict B, Lieftink C, Ramirez C, et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature. 2019;574:268–272. doi: 10.1038/s41586-019-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guerrero A, Herranz N, Sun B, Wagner V, Gallage S, Guiho R, et al. Cardiac glycosides are broad-spectrum senolytics. Nat Metab. 2019;1:1074–1088. doi: 10.1038/s42255-019-0122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.MunozEspin D, Rovira M, Galiana I, Gimenez C, Lozano-Torres B, Paez-Ribes M, et al. A versatile drug delivery system targeting senescent cells. EMBO Mol Med. 2018;10 doi: 10.15252/emmm.201809355. pii e9355. [DOI] [PMC free article] [PubMed] [Google Scholar]