Summary
The response to antipsychotic treatment in schizophrenia appears to vary, and as such it has been proposed that different subtypes of schizophrenia exist, defined by treatment-response. This has not been formally examined using meta-analysis. Randomised controlled trials comparing placebo and antipsychotics in acute treatment of schizophrenia listed in PubMed, EMBASE and PsycINFO from inception until November 30, 2018 were examined. Relative variability of symptomatic improvement in antipsychotic-treated individuals compared to placebo-treated individuals was quantified using coefficient of variation ratio (CVR). Mean difference in symptom change was quantified using Hedges’ g. In addition, individual patient data from two clinical trials was examined in terms of both the distribution of total symptom change, and the variability of individual symptoms and symptom factors. 11,006 articles were identified. 66 met inclusion criteria, reporting on 17,202 patients. Compared with placebo, antipsychotic-treated patients demonstrated greater total symptom improvement (g=0.47, p<0.001) and reduced variability in symptomatic improvement for total (CVR=0.86, p<0.001), positive (CVR=0.89, p<0.001), and negative symptoms (CVR=0.86, p=0.001). Lower variability in antipsychotic-response was associated with studies published earlier (z=3.98, p<0.001), younger patients (z=3.07, p=0.002), higher dose treatments (z=-2.62, p=0.009), and greater mean-difference in symptom-change (z=-5.70, p<0.001). In the individual patient dataset (N=522 patients), antipsychotic treated patients did not show significantly increased variability for any individual symptom, and there was no evidence of a bimodal distribution of response. Compared to placebo, antipsychotic treatment shows greater improvement and lower variability of change in total, positive and negative symptoms. This is contrary to the hypothesis that there is a subtype of antipsychotic non-responsive schizophrenia. Instead our findings, provide evidence for a relatively homogeneous effect of antipsychotic treatment in improving symptoms of schizophrenia.
Introduction
Schizophrenia is a leading cause of global disease burden.1 The main treatments are antipsychotic drugs, which are all dopamine receptor blockers.2,3 Meta-analyses of double-blind randomised placebo controlled trials (RCT) have found a significantly greater mean improvement in symptoms in patients treated with antipsychotics compared to those treated with placebo, with medium to large standardised mean differences (SMD).2
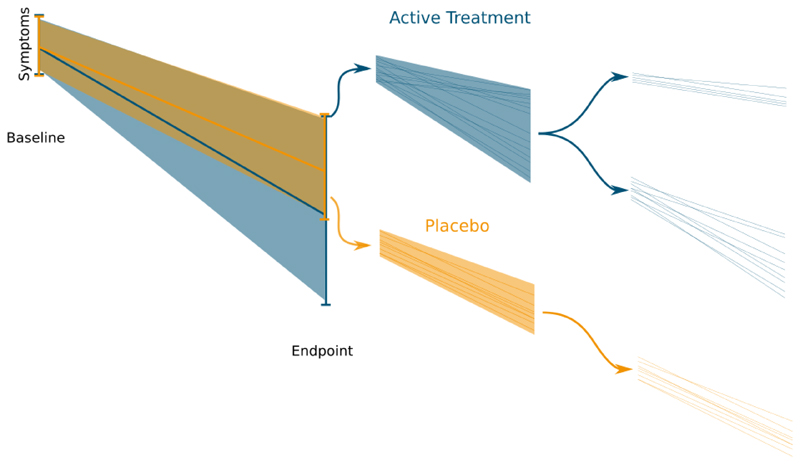
Response to antipsychotic treatment, is, however, heterogeneous, and whilst some patients show marked improvements, others appear to show little change with first-line (non-clozapine) antipsychotic treatment.4,5 Indeed, in around one third of patients, their illness shows a clinically insignificant response to first-line(non-clozapine) antipsychotic drugs,6,7 which has been termed treatment resistant schizophrenia.8 This may be explained by the hypothesis that there are at least two biological subtypes of schizophrenia, one characterised by striatal hyperdopaminergia and a good response to antipsychotic drugs, and a second subtype with a different underlying neurobiological basis that does not respond to antipsychotic treatment.9,10 A key prediction of this hypothesis is that first-line (non-clozapine) antipsychotics will have significant benefit only in some patients, who will show improvement beyond non-specific effects seen with a placebo, whereas, in the subtype of non-responsive schizophrenia, drug treatment is non-specific and any symptom change will be similar to that seen in the placebo group. This will lead to greater change in symptoms in the antipsychotic treatment group which includes both the specific effects of treatment and non-specific effects, whereas in the placebo group, which assesses non-specific effects alone, symptom change will be more uniform. This is shown in figure 1. Thus, in the context of a RCT, the subtype hypothesis predicts greater variability in response in the antipsychotic treatment group compared to the placebo group.
Figure 1.
Placebo and an active treatment are administered in a randomized controlled trial. Over the course of the trial the individuals receiving the active treatment show both a larger response, and more variability in their response. This implies that the active treatment is more effective in some individuals and less effective in others, whereas the placebo is associated with a more uniform response across individuals.
Determining if there is greater variability in symptom change with active treatment is important as it implies some patients are receiving ineffective treatment, and the need for personalised medicine. The variation in response seen in clinical practice is often taken as support for the subtype hypothesis, however it is important to note that much of this variation can result from within individual variability, in which case the appearance of subtypes may be illusory.11 This is an issue across medicine, but to our knowledge this has not previously been addressed in a systematic synthesis of RCT results.
Advances in meta-analytic techniques mean that, in addition to calculating summary SMDs, it is now possible to integrate information from multiple studies in order to quantify the magnitude of difference in variability between two treatments.12–14 We applied this approach to test the prediction that there is greater variability in treatment-response in patients receiving antipsychotics compared to those receiving placebo in all suitable RCTS comparing these interventions in schizophrenia. We also conducted sensitivity analyses and meta regressions to investigate potential moderating factors. We hypothesised that trials specifically excluding patients thought to be resistant would have lower variability in the antipsychotic arm, and trials specifically excluding placebo responders would have lower variability in the placebo arm. We also performed meta regressions examining the effect of publication year, size of mean difference, dose, length of study, baseline severity, age and gender.
In addition, we examined individual patient data from two randomised placebo controlled trials to determine if there is a bimodal distribution of response, as predicted by the sub-type hypothesis, and to determine if there are differences in variability between placebo and antipsychotic treated patients at the level of individual symptoms and symptom factors.
Methods
Search Strategy and Study Selection
We followed PRISMA guidelines (checklist available in supplementary materials), and registered the study on PROSPERO (CRD42018096693).15 We searched EMBASE, PsycInfo, and PubMed from inception until 1 May 2018, and checked references of included studies and previous reviews. Our search strategy was based on previously conducted systematic reviews,16 and full search terms are in supplementary information. In brief we searched for: (antipsychotic OR [generic/branded antipsychotic names]) AND placebo AND (schizo* OR psychot* or psychos*) AND (random* or clinic* trial). Screening and selection of studies was performed independently by four of the authors (A.M., H.P., T.P., & L.V.), with each study assessed by a minimum of two researchers. Disagreements were resolved via discussion with R.M.
Inclusion criteria consisted of: (1) randomised, double blind, placebo-controlled trials, (2) Monotherapy with antipsychotic medications licensed for the treatment of schizophrenia, (3) adults aged 18-65 with an acute exacerbation of schizophrenia or a related disorder (schizoaffective, schizophreniform and delusional disorders). Exclusion criteria included (1) grey literature (with the exception of clinical study reports), (2) relapse prevention studies with no acute treatment phase.
Data Extraction and Processing
We extracted both the mean and variance (standard deviation, standard error, or confidence intervals) of symptom change for total, positive, and negative symptoms ratings from each study for the active treatment and placebo groups, as measured using the Positive and Negative Syndrome Scale,17 Brief Psychiatric Rating Scale,18 Scale for the Assessment of Negative Symptoms,19 and Scale for the Assessment of Positive Symptoms.20 In studies where there were multiple active arms then the number of patients in the placebo group was divided by the number of arms.21 Where information was missing, the study authors were contacted to request the missing information. We also extracted details of year of publication, baseline symptom severity, study duration, antipsychotic, antipsychotic dose in olanzapine equivalents,22,23 participant age, and participant gender. We also determined whether studies used a placebo lead-in period to exclude placebo responders, and whether studies attempted to enrich for responders by excluding patients previously found to be non-responsive to antipsychotic treatment. The quality of included studies was rated using the Cochrane Risk of Bias Assessment Tool.21 Any discrepancies were resolved by consensus and discussion with the other authors.
Variability Outcome Measures
The relative variability between an antipsychotic treated group and a placebo treated group of patients can be quantified using the log variability ratio (VR):
Where and are the unbiased estimates of the population standard deviation for the change in symptoms score of the antipsychotic treated, and placebo treated groups respectively. Sa and Sp are the reported SDs, while na and np are the sample sizes.
In biological systems variance often scales with the mean.24 This can be adjusted for using the log coefficient of variation ratio (CVR) which adjusts the VR for mean differences between groups:
Where and are the mean symptom scores for antipsychotic treated and placebo treated groups respectively. The use of CVR to quantify group differences in variability is possible only where data have a true zero point.12 This is not the case for raw change scores which can be positive or negative, and we therefore converted values of mean change to a ratio scale (see supplementary information). f
A CVR above 1 indicates greater variability in the antipsychotic arm, while a value below 1 indicates greater variability in the placebo arm. We present CVR as our primary analysis, as otherwise any differences in variability may primarily reflect differences in mean, although the VR results are presented as a secondary analysis.
In addition to the variability measures calculated above we also calculated the standardised mean difference (Hedges’ g) between placebo and antipsychotic treated groups using a random effects model.
Individual Patient Data
Response in clinical trials is generally reported as change in total symptom severity or change in sub-domains such as positive symptoms. However, it is possible that antipsychotics have a variable effect on one or two specific symptoms which is obscured in the summary scores. To address this, we have conducted analyses at the symptom level using individual patient data. These complement the meta-analytic approach described above in a number of ways. First, it allows us to test the effect of treatment on the variability of response in individual symptoms, rather than at the level of sub-domains or total scores. Second, it allows for sensitivity analyses including only patients that have clearly received adequate antipsychotic treatment. Third, it allows assessment of the modal distribution of data, which is another method to detect the presence of subgroups.
We obtained individual patient data from two previously reported trials.25,26 We first examined whether individual symptoms might show variability differences that were obscured when solely looking at combined symptoms score. We used the individual symptom item ratings, and, in case variability was due to specific symptom factors, also used the Marder symptom domains, which is a widely used factor analysis of symptoms in schizophrenia.27 Symptom change was calculated using the last observation carried forward was calculated for each arm of each trial. The CVR for each arm was then calculated and entered into a random effects meta-analysis as described above. We also performed this analysis restricting to patients that had received at least four weeks of antipsychotic treatment.
We also examine whether there was any evidence of bimodality in the distribution of change scores that might suggest subgroups of responders and non-responder were present. After pooling antipsychotic arms within each clinical trial Hartigan’s Dip Test of Unimodality was used to test whether these distributions followed a unimodal distribution or whether there was evidence of bimodality.14,28
Statistical Analysis
All statistical analyses were carried out in the statistical programming language R (version 3.5.1), primarily using the “metafor” package (version 2.0.0).29 The correlation coefficient between studies’ mean and SD, and between moderating factors was weighted by study size with the “weights” package (version 1.0), while plots were generated using “ggplot2” (version 2.2.1). For the primary analysis of total symptoms a univariate random effects model was employed. However, a multivariate meta analytic model was used to compare CVR for positive and negative symptoms (see supplementary methods) because this accounts for the correlation between measures.
Sensitivity analyses were performed for studies that had use a placebo lead-in to exclude placebo responders, and studies that had attempted to exclude resistant patients. This was accomplished by performing the meta-analysis separately in e.g. the set of studies using a placebo lead-in and the set of studies that did not use a run in; the summary effect sizes calculated in these two subgroups were then compared in a Wald type test to assess significance. The effect sizes for different antipsychotics were also compared using the same approach.
Meta-regressions were performed to determine whether year of publication, magnitude of standardised mean difference, dose, length of study, baseline severity, age or gender moderated antipsychotic-placebo differences in CVR. Correlations between potential moderating factors was examined using correlation coefficients weighted by study size. Moderators that were found to be significant in the bivariate meta-regressions were then put into a single meta-regression model to see if they remained significant when taken into account simultaneously. The results of a meta-regression only allow one to see how the moderating factor is related to CVR – that is the ratio of antipsychotic and placebo variability, the results do not allow one to determine the effect within individual arms. Therefore, in order to determine whether the moderators primarily acted on antipsychotic or placebo arms, we examined the relationship between moderator and mean standardised variability in each placebo and active treatment arms separately (see supplementary information for further details regarding this variability measure):
Publication bias was assess by visual inspection of funnel plots and the use of Egger’s regression test.30 I 2 values were calculate to quantify between-study inconsistency.
Results
Study Selection
The search identified 11,006 articles, 66 of which met criteria for the meta-analysis (eFigure 1 and eTable1). The meta-analysis included data on 11,978 patients treated with antipsychotics and 5,224 treated with placebo. There were a total of 153 separate antipsychotic treatment arms and 66 placebo arms. Average age was 38.9 years, average duration of illness was 13.7 years, and males constituted 69% of trial participants.
Variability Differences between Antipsychotic and Placebo
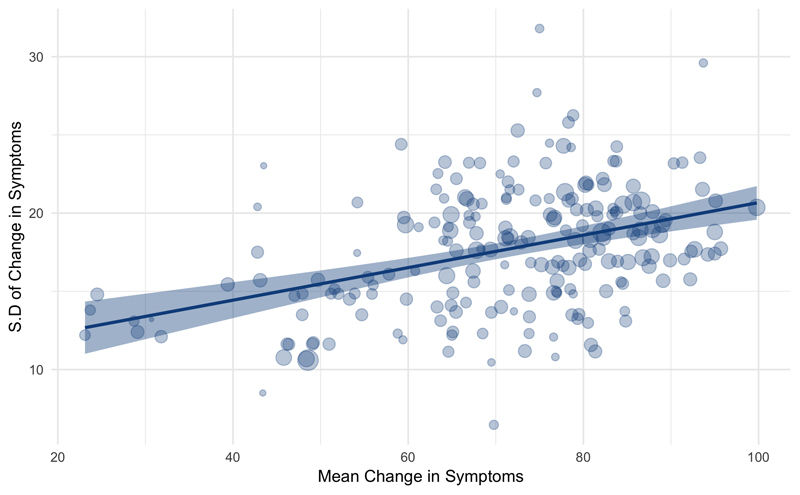
There was a positive relationship between mean change in total symptoms and standard deviation of change (weighted rp = 0.47, p<0.001, figure 2), indicating mean-scaling of variability. However, CVR, our primary outcome measure, adjusts for mean-scaling.12
Figure 2.
A significant correlation exists between mean change in symptoms and the standard deviation of change (weighted rp=0.45, p<0.001). The shaded area represents the 95% confidence interval, and the size of the dots is proportional to study size.
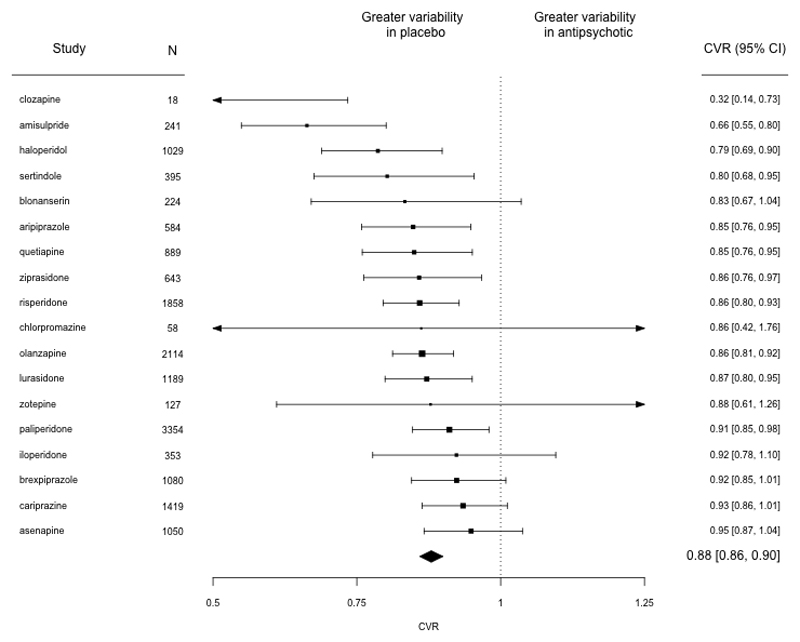
We found significantly reduced variability in the antipsychotic treated groups compared to placebo treated groups (CVR = 0.88, 95% CI 0.86-0.90, p<0.001, Figure 3). This result was present when different antipsychotics were examined individually, with all antipsychotics showing reduced variability numerically, and this reaching statistical significance for clozapine, amisulpride, haloperidol, sertindole, aripiprazole, quetiapine, ziprasidone, risperidone, olanzapine, lurasidone and paliperidone (Figure 3). When not accounting for mean, variability was also reduced in antipsychotic treated groups although this was not statistically significant (VR = 0.98, 95% CI 0.95-1.00, p=0.06).
Figure 3.
Forest plot showing the coefficient of variation ratio (CVR) for change in total PANSS score for placebo-controlled trials of antipsychotic treatment. CVR was significantly lower in antipsychotic treated individuals relative to placebo, indicating lower variability in symptom change in this group compared to the placebo group after accounting for differences in the mean change in symptoms. N=Number of participants, Trials = number of trials
Egger’s test did not suggest the possibility of publication bias (z=-1.67, p=0.10), although a trim and fill analysis suggested the presence of two missing studies (eFigure 2a). Repeating the analyses incorporating the putative missing estimates did not, however, make a significant change to the results (CVR = 0.88, 95% CI 0.86-0.90, p<0.001). I 2 was 0.01% indicating low levels of between-study inconsistency.
A multivariate analysis examining positive and negative symptoms showed that variability was significantly reduced in antipsychotic arms for both positive (CVR=0.89, 95%CI 0.87-0.91, p<0.001) and negative (CVR= 0.86, 95% CI 0.84-0.89, p<0.001) symptoms. A comparison of effect sizes demonstrated that this difference between CVR for positive and negative symptoms was not significant (z=1.78, p=0.08).
Individual Patient Data
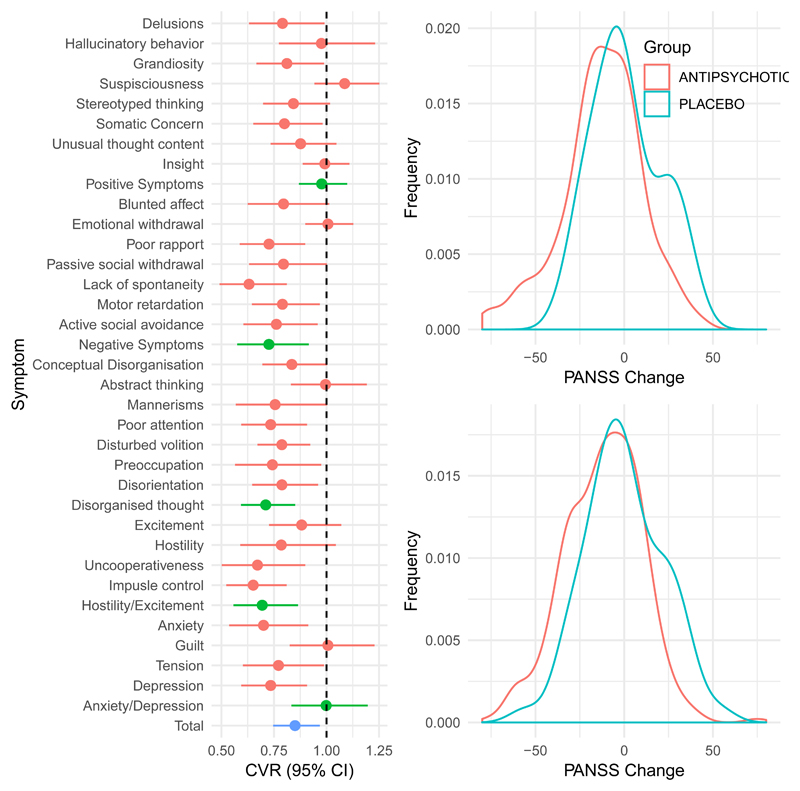
Individual patient data were available for 434 antipsychotic treated, and 88 placebo treated patients. One of these trials,26 showed overall relatively high variability in the antipsychotic arm (CVR=1.18), whereas the other,25 was close to the median CVR for all trials (CVR=0.89). For all symptoms and symptom dimensions, variability was either significantly greater in the placebo arm or no different from the antipsychotic arm (Figure 5A). We also performed the same analyses but considered only participants who had remained in the trial per protocol for at least four weeks, CVR remained lower in the antipsychotic treated arm in this analysis (see efigure 3).
Figure 5.
Individual patient data findings
(A) Showing the relative variability in response for each symptom rating item on the PANSS scale for two clinical trials combined. A coefficient of variability ratio CVR of less than 1 means that variability is reduced in the antipsychotic treated relative to the placebo arm. For both individual symptoms (shown in red), and summary symptom domains (shown in green) there is no evidence of increased variability in those treated with antipsychotics relative to placebo treatment. The CVR for total symptoms was significantly lower in the antipsychotic treated relative to the treated arm (p=0.01).
(B) Kernel density plots illustrating the distribution of symptom change in Chouniard et al (above) and Marder and Meibach (below) clinical trials. As can be seen in the figures, in both trials there was no evidence of significant bimodality in change in total symptoms following treatment (all p-values >0.2).
In addition, we examined whether there was any evidence of a bimodal distribution in terms of antipsychotic response (Figure 5b). In both trials, there was no evidence for bimodality when change in total symptoms following treatment was examined, in either the antipsychotic treated or placebo arms (Marder and Meibach trial25: antipsychotic arm p=0.78, placebo arm p=0.25; Chouinard et al26 trial: antipsychotic arm p=0.99, placebo arm p=0.67).
Efficacy Differences between Antipsychotic and Placebo
Antipsychotics showed greater improvements in symptoms than placebo (g=0.47, 95%CI 0.42-0.51, p<0.001, eFigure 4). Egger’s test suggested the possibility of publication bias (z=2.6647, p=0.008) and a trim and fill analysis suggested the presence of three missing studies (eFigure 2b). Including these putatively missing studies did not markedly change the results (g=0.46, 95% CI 0.41-0.50, p<0.001).
Sensitivity Analyses
When examining study quality, only eleven studies were classified as low risk in all domains of the Cochrane risk of bias tool. A sensitivity analysis examining only these studies showed significantly reduced variability in improvement in the antipsychotic relative to placebo group, consistent with our findings in the total dataset (CVR = 0.86, 95% CI 0.78-0.93, p<0.001).
We examined whether excluding placebo responders had an effect on CVR. Studies in which placebo responders were excluded, surprisingly showed numerically greater variability in the placebo arm (CVR = 0.85, 95%CI 0.81-0.90, p<0.001), than those that did not (CVR = 0.89, 95%CI 0.86-0.91, p<0.001). The difference between these effect sizes, however, was not statistically significant (z=-1.26, p=0.18).
We also investigated whether excluding individuals who were potentially treatment resistant affected CVR. Although the two sets of studies contained patients with symptoms of similar severity (t-test PANSS total p=0.95), this is not unexpected given that entry criteria specify that symptoms must be above a certain threshold, and as such resistance is defined on the basis of previous non-response. Studies that specified treatment resistance as an exclusion criteria had a lower variability in the antipsychotic arm (CVR=0.84, 95%CI 0.79-0.9, p<0.001) than those that did not (g=0.88, 95%CI 0.85-0.9, p<0.001). However, these differences were not significant (z=1.26, p=0.21).
We also compared CVR between different antipsychotics. Amisulpride showed significantly lower variability of symptom response than all other antipsychotic drugs apart from clozapine (see efigure 5). Clozapine and haloperidol also showed significantly lower variability of symptom response than a number of other antipsychotics (see eFigure 5). There were no other significant differences between drugs.
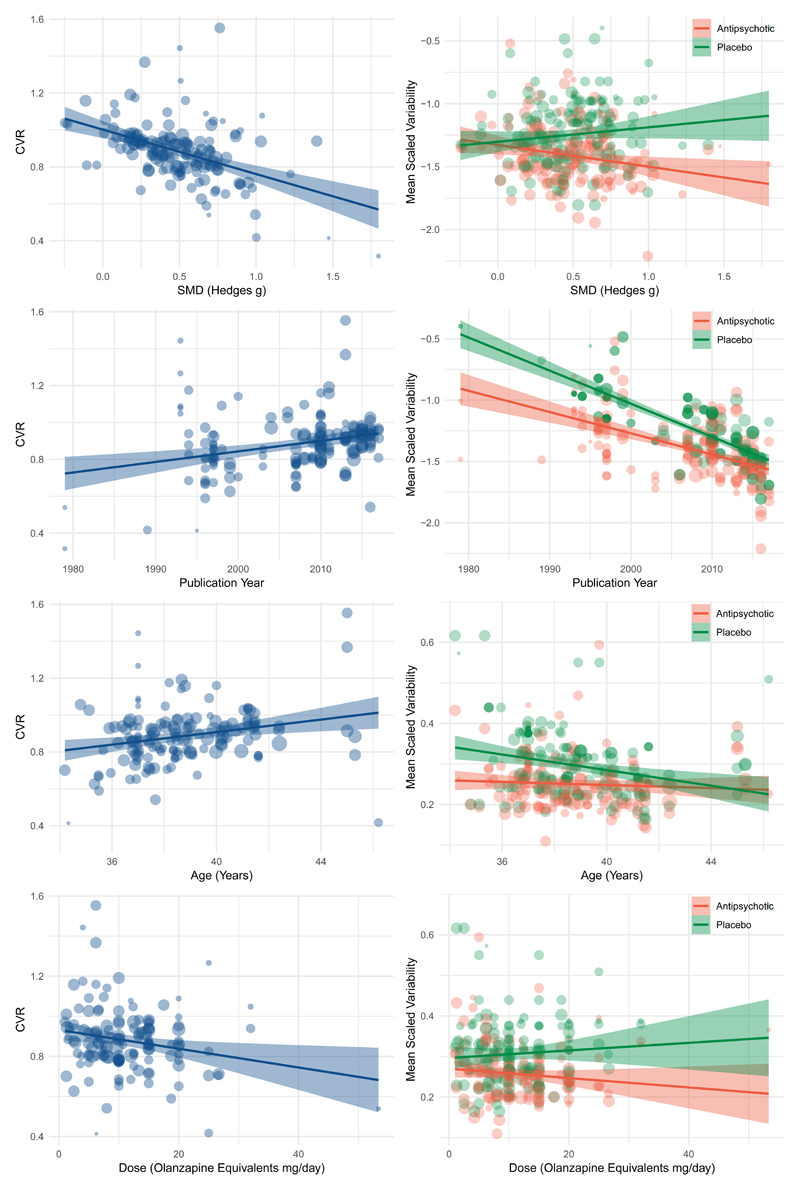
Meta-regression
We found that there was a negative relationship between the standardised mean difference calculated for a trial and its CVR (z=-5.70, p<0.001, Figure 4A). This indicates that trials demonstrating greater antipsychotic efficacy were associated with lower variability in the antipsychotic relative to placebo group. Further analysis of the individual trial arms showed placebo variability was not significantly associated with SMD (z=1.02, p=0.31), but there was a significant relationship between greater SMD and reduced antipsychotic variability (z=-2.70, p=0.01, Figure 4B).
Figure 4.
Figures to the left illustrate meta-regressions exploring the influence of trial characteristics on the coefficient of variation ration (CVR) for change in total symptoms. Figures to the right study the same trial characteristics but examine placebo and antipsychotic arms separately.
(A) Trials that show greater antipsychotic efficacy (greater mean symptom improvement with antipsychotic relative to placebo) show lower variability in antipsychotic treatment response relative to placebo (z=-5.7, p<0.001)
(B) Trials showing greater efficacy show reduced variability in antipsychotic treated arms (z=-2.7, p=0.01), but no significant relationship is apparent with regard to placebo variability (z=1.0, p=0.3).
(C) Older trials show lower variability in antipsychotic treatment response relative to placebo (z=4.0, p<0.001)
(D) Both placebo (z=-11.6, p<0.001) and antipsychotic (z=-7.7, p<0.001) arms show reduced variability in more recent trials.
(E) Trials with younger participants show lower variability in antipsychotic treatment response relative to placebo (z=3.1, p=0.002)
(F) Younger patients show greater variability in the placebo arm(z=2.79, p=0.005), while there is minimal effect on the antipsychotic arm(z=0.0, p=0.7).
(G) Trials using higher antipsychotic doses show lower variability in antipsychotic treatment response relative to placebo (z=-2.62, p=0.009)
(H) Neither antipsychotic (z=-0.4, p=0.68) nor placebo arms (z=0.79, p=0.43) independently show a significant relationship with dose.
A meta-regression of year of publication found that CVR was lower in older studies (z=3.98, p<0.001), indicating that it was these studies that showed the greatest difference in variability between antipsychotic and placebo arms (Figure 4B). Both placebo (z=-11.58, p<0.001) and antipsychotic (z=-7.70, p<0.001) variability has decreased with time, but this has occurred at a faster rate in placebo arms (Figure 4B).
The meta-regression for age was also significant (z=3.07, p=0.002), indicating that studies with younger patients showed lower variability in the antipsychotic relative to placebo arms than studies with older patients. Here placebo variability decreased with increasing age (z=-2.79, p<0.005), at a faster rate than antipsychotic variability (z=-0.00, p=0.68) (Figure 4C).
The meta-regression for duration of illness was significant (z=2.7, p=0.006), indicating that studies including patients with a shorter duration of illness displayed lower variability in the antipsychotic relative to placebo arms than studies with older patients. Here placebo variability remained relatively constant (z=0.07, p=0.95), while antipsychotic variability increased with duration of illness(z=2.6, p=0.009).
A meta-regression examining the effect of dose found that it was studies using higher doses that showed the lowest variability with antipsychotic relative to placebo treatment (z=-2.62, p=0.009). When the individual arms were examined, neither were independently significant (placebo z=0.79, p=0.43; antipsychotic z=-0.41, p=0.68) (Figure 4D).
Baseline symptom severity (PANSS z=-1.14, p=0.25; BPRS z=0.19, p=0.85), participant gender composition (z=-1.50, p=0.13), and study duration (z=0.12, p=0.91) did not did not display significant relationships with CVR.
A number of the variables found to be significant moderating factors in the meta regressions showed a degree of collinearity. Significant correlations were observed for year of publication and age (weighted rp=0.37, p<0.001), year of publication and dose (weighted rp=-0.20, p=0.01), age and dose (weighted rp=-0.18, p=0.03), dose and standardised mean difference (weighted rp=0.27, p=0.002), and age and duration of illness (weighted rp=0.67, p<0.001). In a meta-regression including all the statistically significant moderating factors, year of publication (z=2.49, p=0.01), SMD (z=-3.64, p<0.001) remained significant, but dose (z=-0.41, p=0.68), age (z=0.55, p=0.58), and duration of illness (z=1.93, p=0.053) were no longer significant.
Discussion
Our main finding is that antipsychotic treatment results in an overall greater and more homogenous improvement in symptoms compared to that seen with placebo. These findings extend previous meta-analyses of antipsychotic efficacy by showing, to our knowledge for the time, that antipsychotic response in schizophrenia is not only greater but also more uniform than placebo response. In addition, we found evidence that compared to placebo, antipsychotic-related improvement is more homogenous in studies where antipsychotics showed greater efficacy, in older studies, in younger patients, in those with a shorter duration of illness, and in studies employing higher doses. We were able to adjust for the fact that variability scales with mean by using CVR, a measure that accounts for this. Our meta-analytic findings were consistent with our results obtained using individual patient data, in which there was also no evidence of response subtypes. Our findings regarding efficacy were consistent with previous studies, and any discrepancies are due to the fact we could only include studies reporting measures of variance, leading to some studies that did not report this being excluded.
These findings are not consistent with our hypothesis that greater variability would exist with antipsychotic treatment due to subtypes of schizophrenia showing different response profiles. Furthermore, we show that this is the case for all antipsychotics, and all symptom domains examined; none of which showed greater variability in the antipsychotic arm. In fact, our findings suggest that heterogeneity of response is greater in placebo treated individuals. This lack of support for a subtype phenomenon is in keeping with recent suggestions that an emphasis on attempting to identify subtypes of treatment responsive individuals may be misguided.11 The fact that we did not find evidence for subtypes must be reconciled with the fact that it is clear clinically that a proportion of patients do not respond to treatment. However, a range in the magnitude of response is the norm for many medical treatments.31,32 This may be due to intraindividual variability and measurement noise, and as such it does not in itself imply that subtypes exist. To fully address the question of subtypes, future work will require markedly different trial designs such as repeated N-of-1 studies.33,34
A prediction of our non-dopaminergic sub-type hypothesis is that a selective D2 blocker such as amisulpride would show greater variability in treatment response than drugs with actions at a range of receptors such as olanzapine.35,36 However, in fact we found lower variability of symptom response with amisulpride treatment than all other first-line drugs. This is thus further evidence against the sub-type hypothesis. However, caution must be taken when drawing inferences from these between-drug comparisons because they were based on indirect comparisons. It would be useful to test this further in head-to-head comparisons between amisulpride and broad action drug such as olanzapine.
While no symptoms showed greater variability in the antipsychotic compared to placebo arm, there was a suggestion that the observed effect regarding reduced variance in those treated with antipsychotics may be slightly stronger for negative symptoms. This did not, however, reach statistical significance.
We found that variability of both placebo and antipsychotic arms has decreased with time, but that this has occurred at a faster rate in placebo arms(figure 4D). It has previously been demonstrated that the magnitude of placebo response in antipsychotic trials has increased with time.37,38 Taken together with our findings, this suggests that trials are increasingly including individuals who show a relatively homogenous high placebo response. This could be partially due to the fact that more recent trials tend to include older patients and use lower doses, both factors that we also demonstrated are associated with a more homogeneous placebo response compared to antipsychotics. Our findings are relevant when considering recruitment for clinical trials. Previous meta-analyses examining mean differences show that placebo lead-in periods do not improve the ability of trials to detect drug-placebo differences,37 and we extend these findings by showing that placebo lead-in does not reduce variability placebo-treatment differences either. Thus placebo lead-in phases do not seem to be effective.
The placebo effect is a well-recognised phenomenon across medicine, and significant advances have been made in determining mechanisms underlying the effect.39,40 Individual studies have previously demonstrated that there is significant heterogeneity in the magnitude of placebo response between individuals, and it has been suggested that subtypes of high and low placebo responders exist.41,42 Variability in the placebo group could also reflect heterogeneity in the natural course of the illness, which our findings suggest treatment ameliorates through reducing symptoms. Thus, there could be fluctuations in natural course as well as sub-types of high and low placebo responders contributing to our placebo findings. Placebo effects are often understood to refer to the response specifically engendered by aspects of treatment other than the direct pharmacological effects of the active comparator treatment, as opposed to phenomena such as regression to the mean. We are unable to directly distinguish between these aspects, although given that regression to the mean effects should be equal for both placebo and antipsychotic arms it is reasonable to conclude that the observed differences in variability refer to the more stringent definition of ‘placebo effect’.
Our study has some limitations. Despite contacting authors, we were unable to obtain variability data for a high proportion of older trials, and as such our findings are mostly based on RCTs of second-generation antipsychotics. However, given the lack of a pharmacological basis for the distinction between first and second generation drugs,3 and the fact that our finding was present when looking separately at either selective D2 blockers such as amisulpride, or those drugs with a wider spectrum of activity such as olanzapine, suggests that our findings are relevant for antipsychotics in general. Although one would expect publication bias to be less of an issue for our variability analyses given variability is not an outcome focused upon in RCTs, nevertheless the possibility still exists given the association we observed between CVR and SMD. Our analysis indicated the possibility of some publication bias with a small number of putatively missing studies. However, adjusting for this with a trim and fill analysis did not change the direction of results.
The trials generally used intention-to-treat analyses, which, as they include patients who may have discontinued drug treatment immediately after randomisation, could under-estimate the effect of treatment and possibly reduced variability. However, our additional analyses in the individual patient datasets restricted to patients that completed their allocated treatment indicated that antipsychotic treatment was still associated with reduced variability, indicating that this does not explain our variability findings. Another possibility is that the uniformity of response observed in the antipsychotic arm is an artefact of trial inclusion criteria which might be designed with the aim of recruiting individuals likely to respond to antipsychotics. However this is not supported by the sensitivity analyses excluding trials that specifically attempted to enrich for treatment responders. This showed that trials without this form of selection still showed significantly less variability in antipsychotic arms compared to placebo arms. Theoretically our meta-analytic results could show a multimodal distribution despite reduced variance. However, our analysis of the individual patient data did not show a multimodal distribution of response, indicating that this is unlikely. It is important to recognise, however, that while our findings are not consistent with our initial hypothesis that treatment subtypes exist, they cannot exclude the possibility that there is a subtype of schizophrenia that does not enter clinical trials. Relevant to this, it should be noted that, although shorter duration of illness was associated with reduced variability, none of the trials included were of first episode patients, and the mean duration of illness was 13.7 years. As such, it could be the case that first episode patients are qualitatively different and this warrants testing in future clinical trials. This is a potential limitation of clinical trial design relevant for efficacy analyses as well. It is should also be recognised that our findings are specific to treatment response, and so do not exclude the existence of sub-types of schizophrenia linked to factors other than treatment response.
Supplementary Material
In summary, we found antipsychotics were efficacious with moderate-large effect sizes and showed a more homogeneous response to treatment in those receiving antipsychotics compared to those receiving placebo. These findings indicate a relatively uniform effect of antipsychotic treatment that exceeds non-specific effects seen with placebo treatment. This suggests that antipsychotic effects are relevant to patients with schizophrenia in general rather than any subtype of patient.
Acknowledgements
RM’s work is supported by a clinical research training fellowship grant from the Wellcome trust (no. 200102/Z/15/Z). OH’s work is supported by Medical Research Council-UK (no. MC-A656-5QD30), Maudsley Charity (no. 666), Brain and Behavior Research Foundation, and Wellcome Trust (no. 094849/Z/10/Z) grants, and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Author Contributions
Study conception: RM, TP,YM, OH
Study design: RM, TP, YM, AM, HP, LV, OH
Data searching and extraction: RM, TP, YM, AM, HP, LV
Data analysis: RM
Data interpretation: RM, TP, YM, OH
Writing of manuscript: RM, TP, YM, AM, HP, LV, OH
Declaration of interest
RM, TP, AM,HP and LV declare no financial conflicts of interest. Dr. Mizuno has received manuscript fees or speaker’s honoraria from Sumitomo Dainippon Pharma and Yoshitomi Yakuhin, fellowship grants from Japan Society for the Promotion of Science, Astellas Foundation for Research on Metabolic Disorders, Japanese Society of Clinical Neuropsychopharmacology, and Mochida Memorial Foundation for Medical and Pharmaceutical Research, and consultant fees from Bracket within the past three years. ODH has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organised by Astra-Zeneca, Autifony, BMS, Eli Lilly, Heptares, Jansenn, Lundbeck, Lyden-Delta, Otsuka, Servier, Sunovion, Rand and Roche. Neither Dr Howes or his family have been employed by or have holdings/a financial stake in any biomedical company.
References
- 1.Howes O, Murray R. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;6736:1–11. doi: 10.1016/S0140-6736(13)62036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leucht S, Cipriani A, Spineli L, Mavridis D, Örey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;6736:1–12. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 3.Howes O, Egerton A, Allan V. Mechanisms underlying psychosis and antipsychotic treatment response in schizophrenia: insights from PET and SPECT imaging. Curr Pharm Des. 2009;15:2550–2559. doi: 10.2174/138161209788957528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agid O, Arenovich T, Sajeev G, Zipursky RB, Kapur S, Foussias G, et al. An algorithm-based approach to first-episode schizophrenia: Response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry. 2011;72:1439–1444. doi: 10.4088/JCP.09m05785yel. [DOI] [PubMed] [Google Scholar]
- 5.Marques TR, Arenovich T, Agid O, Sajeev G, Muthén B, Chen L, et al. The different trajectories of antipsychotic response: antipsychotics versus placebo. Psychol Med. 2011;41:1481–8. doi: 10.1017/S0033291710002035. [DOI] [PubMed] [Google Scholar]
- 6.Lally J, Ajnakina O, Di Forti M, Trotta A, Demjaha A, Kolliakou A, et al. Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol Med. 2016:1–10. doi: 10.1017/S0033291716002014. [DOI] [PubMed] [Google Scholar]
- 7.Demjaha A, Lappin JM, Stahl D, Patel MX, MacCabe JH, Howes OD, et al. Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol Med. 2017:1–9. doi: 10.1017/S0033291717000435. [DOI] [PubMed] [Google Scholar]
- 8.Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJ, Birnbaum ML, et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am J Psychiatry. 2017;174:216–229. doi: 10.1176/appi.ajp.2016.16050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howes OD, Kapur S. A neurobiological hypothesis for the classification of schizophrenia: Type a (hyperdopaminergic) and type b (normodopaminergic) Br J Psychiatry. 2014;205:1–3. doi: 10.1192/bjp.bp.113.138578. [DOI] [PubMed] [Google Scholar]
- 10.Crow TJ. The Two-syndrome Concept: Origins and Current Status. Schizophr Bull. 1985;11:471–488. doi: 10.1093/schbul/11.3.471. [DOI] [PubMed] [Google Scholar]
- 11.Senn S. Statistical pitfalls of personalized medicine. Nature. 2018;563:619–621. doi: 10.1038/d41586-018-07535-2. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa S, Poulin R, Mengersen K, Reinhold K, Engqvist L, Lagisz M, et al. Meta-analysis of variation: Ecological and evolutionary applications and beyond. Methods Ecol Evol. 2015;6:143–152. [Google Scholar]
- 13.Brugger SP, Howes OD. Heterogeneity and Homogeneity of Regional Brain Structure in Schizophrenia. JAMA Psychiatry. 2017;74:1104. doi: 10.1001/jamapsychiatry.2017.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillinger T, Osimo EF, Brugger S, Mondelli V, Mccutcheon RA, Howes OD. A Meta-analysis of Immune Parameters, Variability, and Assessment of Modal Distribution in Psychosis and Test of the Immune Subgroup Hypothesis. 2018:1–14. doi: 10.1093/schbul/sby160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Leucht S, Leucht C, Huhn M, Chaimani A, Mavridis D, Helfer B, et al. Sixty Years of Placebo-Controlled Antipsychotic Drug Trials in Acute Schizophrenia: Systematic Review, Bayesian Meta-Analysis, and Meta-Regression of Efficacy Predictors. Am J Psychiatry. 2017 doi: 10.1176/appi.ajp.2017.16121358. appi.ajp.2017.1. [DOI] [PubMed] [Google Scholar]
- 17.Kay SR, Flszbein A, Opfer LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 18.Overall JE, Gorham DoR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 19.Andreasen NC. Scale for the assessment of negative symptoms. University of Iowa; Iowa City: 1984. [Google Scholar]
- 20.Andreasen NC. Scale for the assessment of positive symptoms. University of Iowa; Iowa City: 1984. [Google Scholar]
- 21.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2008. [DOI] [Google Scholar]
- 22.Leucht S, Samara M, Heres S, Davis JM. Dose Equivalents for Antipsychotic Drugs: The DDD Method: Table 1. Schizophr Bull. 2016;42:S90–S94. doi: 10.1093/schbul/sbv167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci. 2015;69:440–447. doi: 10.1111/pcn.12275. [DOI] [PubMed] [Google Scholar]
- 24.Eisler Z, Bartos I, Kertész J. Fluctuation scaling in complex systems: Taylor’s law and beyond. Adv Phys. 2008;57:89–142. [Google Scholar]
- 25.Marder S, Meibach R. Risperidone in the treatment of schizophrenia. AmJPsychiatry. 1994;151:825–35. doi: 10.1176/ajp.151.6.825. [DOI] [PubMed] [Google Scholar]
- 26.Chouinard G, Jones B, Remington G, Bloom D, Addington D, Macewan GW, et al. A Canadian multicenter placebo-controlled study of fixed doses of risperidone and haloperidol in the treatment of chronic schizophrenic patients. J Clin Psychopharmacol. 1993;13:25–40. [PubMed] [Google Scholar]
- 27.Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58:538–46. doi: 10.4088/jcp.v58n1205. [DOI] [PubMed] [Google Scholar]
- 28.Hartigan J, Hartigan P. The Dip Test of Unimodality. Ann Stat. 1985;13:70–84. [Google Scholar]
- 29.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 30.Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 31.Vijverberg SJH, Farzan N, Slob EMA, Neerincx AH, Maitland-van der Zee AH. Treatment response heterogeneity in asthma: the role of genetic variation. Expert Rev Respir Med. 2018;12:55–65. doi: 10.1080/17476348.2018.1403318. [DOI] [PubMed] [Google Scholar]
- 32.Cantrell RA, Alatorre CI, Davis EJ, Zarotsky V, Le Nestour E, Carter GC, et al. A review of treatment response in type 2 diabetes: Assessing the role of patient heterogeneity. Diabetes Obes Metab. 2010;12:845–857. doi: 10.1111/j.1463-1326.2010.01248.x. [DOI] [PubMed] [Google Scholar]
- 33.Schork NJ. Personalized medicine: Time for one-person trials. Nature. 2015;520:609–611. doi: 10.1038/520609a. [DOI] [PubMed] [Google Scholar]
- 34.Marwick KFM, Stevenson AJ, Davies C, Lawrie SM. Application of n-of-1 treatment trials in schizophrenia: Systematic review. Br J Psychiatry. 2018;213:398–403. doi: 10.1192/bjp.2018.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Correll CU. From receptor pharmacology to improved outcomes: Individualising the selection, dosing, and switching of antipsychotics. Eur Psychiatry. 2010;25:S12–S21. doi: 10.1016/S0924-9338(10)71701-6. [DOI] [PubMed] [Google Scholar]
- 36.Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: An update for the 21 st century. J Psychopharmacol. 2015;29:97–115. doi: 10.1177/0269881114563634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutherford BR, Pott E, Tandler JM, Wall MM, Roose SP, Lieberman JA. Placebo Response in antipsychotic clinical trials ameta-analysis. JAMA Psychiatry. 2014;71:1409–1421. doi: 10.1001/jamapsychiatry.2014.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agid O, Siu CO, Potkin SG, Kapur S, Watsky E, Vanderburg D, et al. Meta-Regression Analysis of Placebo Response in Antipsychotic Trials, 1970–2010. Am J Psychiatry. 2013;170:1335–1344. doi: 10.1176/appi.ajp.2013.12030315. [DOI] [PubMed] [Google Scholar]
- 39.Wager TD, Atlas LY. The neuroscience of placebo effects: Connecting context, learning and health. Nat Rev Neurosci. 2015;16:403–418. doi: 10.1038/nrn3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaptchuk TJ, Miller FG. Placebo Effects in Medicine. N Engl J Med. 2015;373:8–9. doi: 10.1056/NEJMp1504023. [DOI] [PubMed] [Google Scholar]
- 41.Price DD, Finniss DG, Benedetti F. A Comprehensive Review of the Placebo Effect: Recent Advances and Current Thought. Annu Rev Psychol. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 42.Petrovic P. Placebo and Opioid Analgesia-- Imaging a Shared Neuronal Network. Science (80- ) 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.