Abstract
Objective
Patients with systemic lupus erythematosus (SLE) have an increased risk of developing cardiovascular disease (CVD). Standard serum lipid measurements in clinical practice do not predict CVD risk in SLE patients. More detailed analysis of lipoprotein taxonomy could identify better predictors of CVD risk in SLE.
Approach and Results
Eighty women with SLE and no history of CVD underwent carotid and femoral ultrasound scans; 30 had atherosclerosis plaques (SLE-P) and 50 had no plaques (SLE-NP). Serum samples obtained at the time of the scan were analysed using a lipoprotein-focused metabolomics platform assessing 228 metabolites by nuclear magnetic resonance spectroscopy. Data were analysed using logistic regression (LR) and five binary classification models with 10-fold cross validation. SLE patients had global changes in complex lipoprotein profiles compared to healthy controls despite having clinical serum lipid levels within normal ranges. In the SLE cohort, univariate LR identified four metabolites associated with sub-clinical plaque; three subclasses of very low-density lipoprotein (VLDL) (free cholesterol in medium and large VLDL particles and phospholipids in chylomicrons and extremely large VLDL particles) and leucine. Together with age, these metabolites were also within the top features identified by the lasso LR (with and without interactions) and random forest machine learning models. LR with interactions differentiated between SLE-P and SLE-NP groups with the greatest accuracy (0.800). Notably, free cholesterol in large VLDL particles and age differentiated between SLE-P and SLE-NP patients in all models.
Conclusions
Serum metabolites are promising biomarkers to uncover and predict multi-metabolic phenotypes of sub-clinical atherosclerosis in SLE.
Keywords: Systemic lupus erythematosus, lipoprotein, atherosclerosis, metabolomics, machine learning
Introduction
Systemic Lupus Erythematosus (SLE) is a multi-system autoimmune condition that predominantly affects women (women to men ratio of 9:1) with a prevalence of approximately 1 in 1000 in the UK1. Patients with SLE have a 5-10 fold increased risk of developing cardiovascular disease (CVD) compared to healthy people of the same age and sex2. Strikingly, the presence of SLE in women between the ages of 35-44 increases the risk of coronary artery disease by 50 times2. Furthermore, in a large multinational study of 9547 patients with SLE, a quarter of deaths were attributed to CVD3. The precise mechanism of this increased CVD risk is yet to be fully elucidated. Whilst traditional risk factors such as high blood pressure, diabetes and high cholesterol contribute to the increased risk, they fail to account for it fully4. The risk is likely to be multifactorial, resulting from a complex interplay of SLE-driven immunological dysfunction and traditional CVD risk factors4.
Abnormalities in lipid profiles are a traditional risk factor for CVD and can also be affected by chronic inflammatory conditions such as SLE. Lipids are central to driving atherosclerosis, the main pathology underlying CVD. Various fractions of lipoproteins can be distinguished in blood on account of their size and density: High-Density Lipoprotein (HDL), Low-Density Lipoprotein (LDL) and Very Low Density Lipoprotein (VLDL). Dyslipidemias are present in over 70% of cases of premature coronary heart disease5 and elevated plasma concentrations of LDL and VLDL can induce the development of atherosclerosis in the absence of other risk factors6. In contrast, HDL has anti-atherogenic properties that include macrophage cholesterol efflux, anti-oxidation and protection against thrombosis7. Conversely, McMahon and colleagues have demonstrated the existence of a subpopulation of pro-inflammatory HDL in patients with SLE and rheumatoid arthritis that promotes atherosclerosis and could be a biomarker for increased risk of developing CVD8, 9.
Hypercholesterolaemia (defined as elevated plasma total cholesterol (TC) and/or LDL-cholesterol or non-HDL-cholesterol) is found in 34-51% of patients with SLE5 and is characterised by elevated levels of VLDL and triglycerides (TGs) and low HDL levels10. In addition, development of CVD in women with SLE has been found to be associated with smaller sub-fractions of LDL11. Studies in non-SLE patients with CVD suggest that the ratio between serum lipid-associated proteins, apolipoprotein-B:apolipoprotein-A1 (ApoB:ApoA1), is a more effective CVD predictor than routine cholesterol measurements. A higher ApoB:ApoA1 ratio is associated with increased cardiovascular risk12–16; however, the role of this ratio in the prediction of SLE CVD is still being assessed. Overall, dyslipidaemia detected in routine lipid screens available in clinical practice fails to fully account for the increased risk of CVD in patients with SLE4. Many SLE patients with normal serum lipid levels on standard assays also go on to have CVD. Therefore, more sensitive and specific lipid profiles need to be delineated in order to identify high CVD risk patients in SLE cohorts.
Here we used a Nuclear Magnetic Resonance (NMR) Spectroscopy metabolomics platform17 and machine learning (ML) analyses to assess the association of lipoprotein subclasses and lipid content and other low molecular weight serum metabolites with the presence of sub-clinical atherosclerotic plaque in patients with SLE.
Materials and Methods
All data have been made publicly available in Mendeley and can be accessed at http://dx.doi.org/10.17632/fmygdybj2h.1.
Patient cohort
Serum samples were collected from 80 non-fasting patients with SLE attending a rheumatology clinic at University College London Hospital (UCLH) and fulfilling the American College of Rheumatology classification criteria for lupus (1997)18. Patients had no previous history of CVD (defined as coronary artery disease, stroke, or myocardial infarction with confirmatory evidence from blood tests and/or imaging) and all underwent a vascular ultrasound scan between 2011-201319. Serum samples were also donated by 39 healthy female volunteers, which were used as controls. Demographic information was collected and summarized in Supplementary Table I. Carotid and femoral ultrasound scans were performed in order to document any evidence of early arterial wall changes including the presence and size of plaques. Demographic and clinical information for patients were recorded at the time of scan/blood sampling, including sex, age, ethnicity, blood pressure (mean arterial blood pressure: calculated as 2 x diastolic pressure + systolic pressure divided by 3), routine serology measures, treatment (including hydroxychloroquine, statins, ACE inhibitor, immunosuppressives, rituximab-number of treatment cycles and time since last rituximab cycle recorded, prednisolone (and dose), and aspirin), and disease activity assessed by the global British Isles Lupus Assessment Group (BILAG)-2004 index20 and SLE damage index Systemic Lupus International Collaborating Clinics damage index21(Supplementary Table II). Serum cytokine levels (IL6, IL10, IFN-γ and TNF-α) were measured in a subset of patients (SLE-NP n=19 and SLE-P n=17) from serum taken at the time of the scan: no significant differences were identified between two groups (Supplementary Table II). In total, four patients were not on any treatment at the time of the scan. All patients gave informed written consent and the study was approved by the combined University College London / University College London Hospital Research Ethics Committee (Reference 06/Q0505/79).
Plaque detection
A detailed description of the scanning protocol is in the supplementary methods. Briefly, the intima–media thickness (IMT) and the size and nature (stable or unstable) of plaques were measured objectively and noninvasively using vascular ultrasound scans of the common carotid artery, carotid bulb, carotid bifurcation, common femoral artery and femoral bifurcation, performed bilaterally using the Philips IU22 ultrasound computer and the L9-3 MHz probe and as described previously22, 23. Each carotid bifurcation was examined transversely, and then longitudinally to ensure optimal demonstration of the intima–media complex of both the near and far walls of the common carotid artery 1.5–2.0cm proximal to the carotid bulb. IMT measurements were performed using QLAB Advanced Quantification Software® version 7.1 (Philips Ultrasound, Bothell, USA). The presence of plaque was defined as “a focal thickening >1.2 mm that encroaches into the arterial lumen as measured from the media-adventitia interface to the lumen interface”24. Patients having at least one region fulfilling this description were included in the group with plaque (SLE-P).
Total plaque area (TPA) was defined as the sum of the cross-sectional areas of all plaques seen in longitudinal images (plaque area in mm2).
Grey Scale Median (GSM): Images were normalized using linear scaling with two reference points blood (grey scale=0) and adventitia (grey scale=190). After image normalization plaque echogenicity, a measure of plaque stability and lipid content, was expressed numerically by GSM value22. Lower GSM values signify more echolucent plaque associated with a large lipid core and high inflammatory cell content; whereas plaque with high GSM scores are associated with a small lipid core and high collagen content23, 25, 26.
Serum metabolomics
Measures of 228 serum biomarkers were acquired with an established NMR-spectroscopy platform (Nightingale Health)27, 28. These included both absolute concentrations (mmol/L), ratios, and percentages (%) of lipoprotein composition. Serum lipids measured included apolipoproteins and VLDL, LDL, intermediate density lipoprotein (IDL) and HDL particles of different sizes ranging from chylomicrons and extremely large (XXL), very large (XL), large (L), medium (M), small (S) and very small (XS). Lipids within each lipoprotein subclass included –total lipid, phospholipids (PL), total cholesterol (C), cholesterol esters (CE), free cholesterol (FC) and triglycerides (TG). Distribution of these lipids was expressed as a ratio or percentage (_%) of total lipid content for each lipoprotein subclass (for list of metabolites see Supplementary Table III).
Data analysis
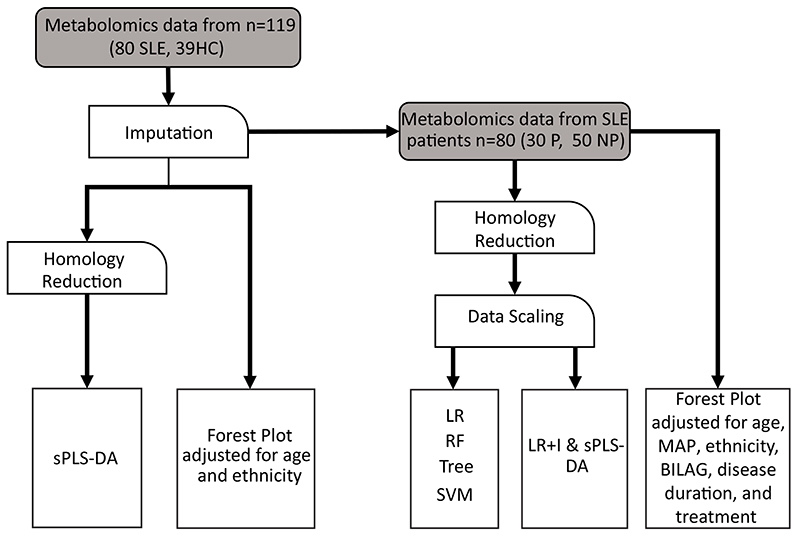
Data analysis plan is summarised in Figure 1. Data were analysed for association using logistic regression (LR) and for classification using five different supervised machine learning algorithms: support vector machine (SVM), LR with and without interactions (LR/LR+I), decision trees, and random forest (RF). Ten-fold cross-validation was used to evaluate model performance. Partial Least Squares Discriminant Analysis (sPLS-DA) was used to combine parameter selection and classification into one operation See Supplementary Methods for detailed description of the analysis and software/packages used. Demographic, clinical and treatment variables were adjusted for as appropriate and denoted in figure legends.
Figure 1. Data analysis workflow.
Flow chart depicting the data cleaning and processing steps taken prior data analysis using machine learning algorithms. Abbreviations: HC, healthy controls; SVM, support vector machine; LR, Logistic regression; RF, random forest; Tree, decision tree; LR+I, logistic regression with interactions; sPLS-DA, sparse partial least squares discriminant analysis; P, plaque; NP, no plaque; BILAG, British Isles Lupus Assessment Group-2004 disease activity score.
Statistical testing
Statistical tests were performed in Microsoft Excel and GraphPad Prism version 8.3.0 for Windows (GraphPad Software, San Diego, USA). Data was assessed for normality and analysed with parametric or non-parametric tests as appropriate. Details of statistical tests and parameters accounted for in the analyses are given in the figure legends. P-values < 0.05 were considered statistically significant.
Results
Serum metabolites can differentiate SLE patients from healthy controls
In order to establish the taxonomy of lipoproteins in patients with SLE compared to healthy volunteer controls (HC) detailed lipoprotein-based serum metabolomics was performed (Figure 1, Supplementary Tables I and III). While the routinely available clinical lipid measurements of SLE patients were within normal ranges (Supplementary Table IV), univariate logistic regressions of the serum metabolites adjusted for age and ethnicity demonstrated a significant difference in the metabolite profile between SLE and HC (Supplementary Data File I and II). Although age was significantly different between the two groups, it did not impact the results; the beta value (which expresses the importance of each variable) ranged between 0.02-0.12 (odds ratio 1.05) as opposed to the significant metabolites where the beta coefficient ranged from -5.92 (odds ratio 0.0027) and 7.88 (odds ratio 2645) (Supplementary Data Files I and II).
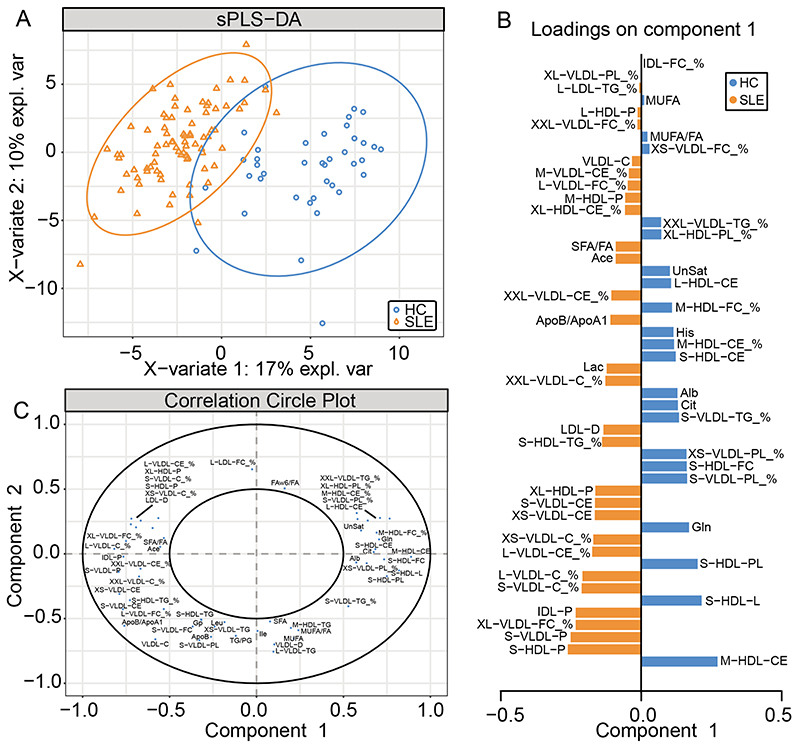
sPLS-DA, a supervised clustering ML model that combines parameter selection and classification into one operation, was then performed using 4 components and 50 metabolite measurements (following model optimisation, refer to Supplementary Methods) to rank and validate the metabolite features according to their distribution in SLE and HC. A significant separation between SLE patients and HCs was observed by plotting principal component (PC)-2 against PC-1 (Figure 2A). The 50 metabolite measurements were ranked by discriminating capability (Figure 2B, 2C). The top five ranked metabolites were M-HDL-CE, which was associated with a HC classification, and S-HDL-P, S-VLDL-P, XL-VLDL-FC_%, and IDL-P, which were associated with a SLE classification. Notably, age was not in the top 50 predictors for SLE in the sPLS-DA plot (Figure 2A-C) suggesting that metabolites rather than age differences were driving the separation of SLE patients from HCs. All 50 metabolites included in the sPLS-DA clustering (Figure 2B) were all also found to be significantly different between SLE and HC in the univariate logistic regressions (Supplementary Data File I and II) suggesting that patients with SLE have an underlying dyslipidaemia that is not detected by routine lipid assessments.
Figure 2. Partial least squares discriminate analysis able to differentiate SLE patients from HC.
Metabolomic data from 80 patients with SLE and 39 healthy controls (HC) were analysed using a NMR platform. (A) sPLS-DA performed on 50 metabolic markers following model optimisation separated SLE patients from healthy controls. (B) Features included in the sPLS-DA are plotted with their factor loading value. (C) Visualisation of the weighting and correlation of each metabolite in component 1 and 2 on the sPLS-DA model. Abbreviations: HDL, high density lipoprotein: LDL, low density lipoprotein: VLDL, very low density lipoprotein: IDL, intermediate density lipoprotein: XXL, chylomicrons and extremely large: XL, very large: L, large: M, medium: S, small: XS very small: PL, phospholipids: C, total cholesterol: CE, cholesterol esters: FC, free cholesterol: TG, triglycerides: P, particle: D, diameter: see Supplementary Table III for full list of metabolite abbreviations).
Serum metabolites can predict the presence of sub-clinical atherosclerotic plaque in SLE patients
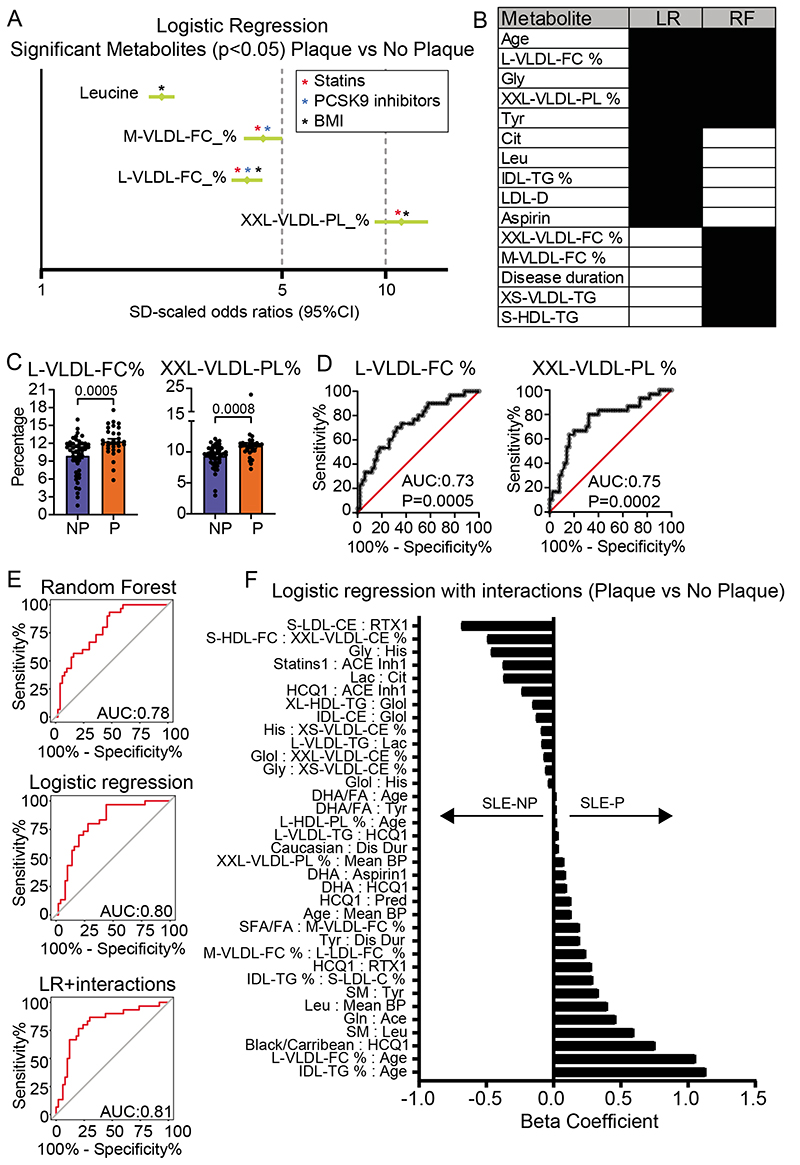
To assess whether the disrupted serum metabolite profile identified in patients with SLE could be associated with the presence of sub-clinical atherosclerosis, SLE patients were stratified based on the presence (SLE-P) or absence (SLE-NP) of plaque(s) detected by vascular ultrasound (Supplementary Tables II and III). Several analysis strategies were applied, and all models were adjusted for ethnicity, age, mean arterial blood pressure, disease duration, disease activity (global BILAG score), and treatment at the time of the scan (Figure 1). Firstly, univariate logistic regressions identified four metabolites which differentiated between SLE-P and SLE-NP patients; Leucine, M-VLDL-FC_%, L-VLDL-FC_% and XXL-VLDL-PL_% which were all increased in serum from SLE-P compared to SLE-NP patients (Figure 3A, Supplementary Data Files III and IV). Of note, these metabolites can be significantly affected by treatment with statins29, 30 and pro-protein convertase subtilisin/kexin type 9 (PCSK9) inhibitors31, or body mass index32, suggesting that abnormal serum lipid metabolite profiles in SLE-P patients, could be modified using available therapies or interventions (Figure 3A asterisks).
Figure 3. Identification of important metabolites separating patients with SLE-P from SLE-NP.
(A) Forest plot depicting statistically significant individual logistic regression results of metabolites in SLE-P (n=30) vs SLE-NP (n=50). Results given in Odds Ratio (95% confidence intervals). Logistic regressions were adjusted for age, ethnicity, mean arterial blood pressure, global BILAG-2004, disease duration, and treatments at the time of scan (Supplementary Table II). Coloured asterisks denote metabolite has previously been shown to be modified by statins, PCSK9 inhibitors or body mass index29, 30, 32, 31. (B) Best performing models were determined based on performance statistics (Table 1). The top ten features of these LR and RF models are listed to identify common features. (C-D) Metabolites which were significant in the individual logistic regression and featured in the top ten of the LR and RF models were further analysed using (C) bar charts showing mean, and p value (see Supplementary Figure IIA) and (D) ROC plots (see Supplementary Figure IIB). (E) ROC analysis of the metabolites and features contributing to the LR, RF and LR+I models, which utilise a combination of metabolomic and clinical features. (F) Beta coefficients from the logistic regressions with interactions are plotted for the SLE-P vs SLE-NP analysis. The sign indicates the direction of the effect of the predictor; a positive sign indicates an increased likelihood of a SLE-P prediction, while a negative sign indicates an increased likelihood of a SLE-NP prediction. See Supplementary Table III for metabolite abbreviations.
Next, five supervised ML models were developed and validated to predict the presence of plaque in SLE patients; LR, LR+I, SVM, RF and Decision tree. Since many of the metabolites measured were biologically interdependent, and therefore highly correlated, homology reduction was applied (see Supplementary Methods). The models were built using the homology reduced dataset (124 metabolites) and patient information (age, ethnicity, mean arterial blood pressure, disease duration, global BILAG index, and treatments at the time of first scan). Sex was not considered as all participants were female (Supplementary Data File V for full lists of the predictors contributing to each model).
The top three models, according to classification accuracy, specificity, and F1 scores, were LR, LR+I, and RF (Table 1). Performance metrics were based on predictions of the models, summarised in confusion matrices (Supplementary Figure I). LR and LR+I had a similar performance, correctly classifying 75% and 80% of SLE-P patients respectively. The RF model had the best specificity, identifying 45 out of 50 (90%) SLE-NP patients correctly.
Table 1. Comparison of predictive model performance.
Performance statistics for 5 predictive models based on serum metabolites at the time of the first scan. The models used were logistic regression (LR) with and without interactions (I), support vector machine (SVM), random forest (RF), and decision tree (Tree). The classification accuracy (CA) represents the proportion of correctly identified cases, in contrast to specificity, which is the true negative rate. F1 is the weighted average of the precision and recall (see Methods). Statistics are rounded to 3 decimal places.
| Model | F1 | Precision | Recall | Specificity | CA | AUC |
|---|---|---|---|---|---|---|
| LR | 0.630 | 0.708 | 0.567 | 0.860 | 0.750 | 0.802 |
| LR + I | 0.714 | 0.769 | 0.667 | 0.880 | 0.800 | 0.812 |
| SVM | 0.480 | 0.600 | 0.400 | 0.840 | 0.675 | 0.707 |
| RF | 0.542 | 0.722 | 0.433 | 0.900 | 0.725 | 0.779 |
| Tree | 0.464 | 0.500 | 0.433 | 0.740 | 0.625 | 0.630 |
The three models were further investigated to identify the top features in predicting plaque formation in SLE (Figure 3B-E). Four metabolites (XXL-VLDL-PL_%, L-VLDL-FC_%, glycine and tyrosine) and patient age were identified in both LR and RF models as important predictors for SLE-P (Figure 3B). Metabolites which were significant in individual logistic regressions and also featured in the top ten metabolites of the LR and RF model, were further investigated for differences between SLE-P and SLE-NP patients (Figure 3C and Supplementary Figure IIA). Receiver operating characteristic curve of the individual metabolites showed an area under the curve of 0.6810 (XXL-VLDL-PL %), 0.7523 (L-VLDL-FC %), 0.7337 (glycine), and 0.6300 (tyrosine) (Figure 3D and Supplementary Figure IIB). However, receiver operating characteristic curves based on the classification true positive rate (sensitivity) and false positive rate (1 – specificity) of the LR, RF and LR+I models had an improved area under the curve of 0.78, 0.80 and 0.81 respectively (Figure 3E). This suggested a stronger potential predictive ability when both metabolites and clinical and demographic features (including age and disease duration) were combined and considered together.
The top performing LR+I model (classification accuracy of 0.800) included interactions of each metabolite with all other metabolites and clinical features (assessing over 15,000 possible features) (Table 1). Using the lasso method of shrinkage and selection, only 35 interactions were identified as important and given a beta coefficient to describe the effect size and direction on the model (Figure 3F, Supplementary Table V). Features with larger beta coefficients had the greatest effect on the classification. Notably, L-VLDL-FC_% : age and IDL-triglyceride (TG)_%:age, Black/Caribbean ethnicity:hydroxychloroquine treatment and sphingomyelin:leucine were significantly associated with plaque; while S-LDL-CE:rituximab treatment, S-HDL-FC:XXL-VLDL-CE_%, glycine:histidine and statins:ACE inhibitor treatment were associated with the absence of plaques.
Importantly, although age and disease duration were significantly different between the two patient groups (Supplementary Table II), and contributed to separation between SLE-P and SLE-NP in the ML models (Supplementary Data File V), these factors did not individually influence metabolite concentrations (Supplementary Data File IV and Supplementary Data File VI), thus demonstrating that the metabolite features identified by the models were due to the presence of subclinical plaque.
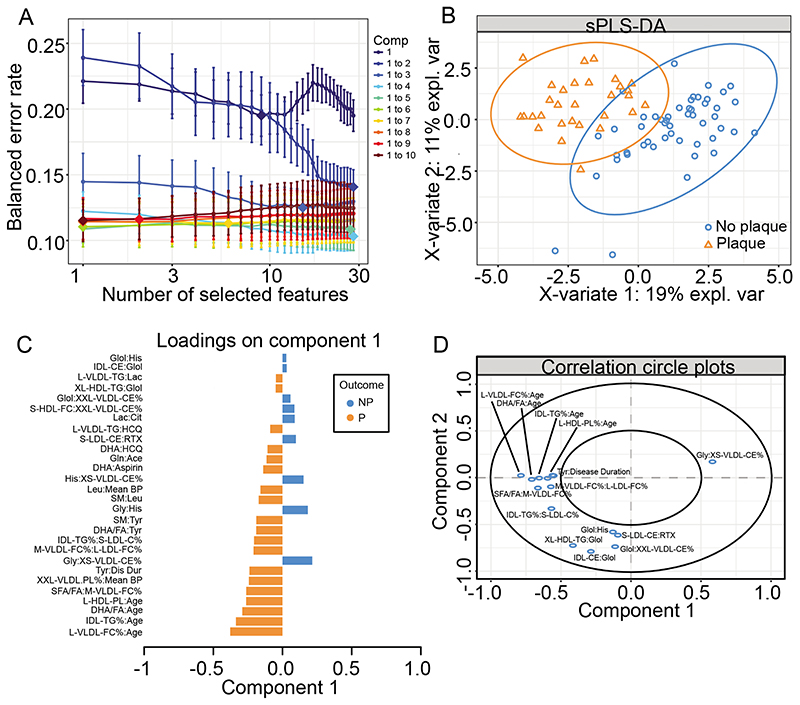
Metabolite interactions can differentiate between SLE-P and SLE-NP patients
Using the metabolite interaction features that were selected for the LR+I model (Figure 3F, Supplementary Table V), a sPLS-DA was performed to rank and validate the metabolite features by their distribution in patients with SLE-P and SLE-NP. By assessing the overall estimation error rate in ten-fold cross-validation, models with 4 components and a subset of 28 metabolite features were chosen for optimal model performance (Figure 4A). This analysis identified a significant separation between SLE-P and SLE-NP patients by plotting PC-2 against PC-1 (Figure 4B). The 28 selected metabolite interaction features were ranked by discriminating capability (Figure 4C, 4D). The two highest weighted features were L-VLDL-FC_%:Age and IDL-TG_%:Age, which were also the highest ranked features in the LR+I model (Figure 3F). L-VLDL-FC_% and age were both also included in the top ten features shared by LR and RF (Figure 3B) and were differentially expressed between SLE-P and SLE-NP patients (Figure 3A) suggesting their influential role in SLE-P patients.
Figure 4. Partial least squares discriminate analysis validated metabolites identified by logistic regression with interactions to predict SLE-P.
(A) Model optimisation – model with different components and features kept in the analysis were analysed, with each colour representing a different number of components (Comp), number of features kept in the analysis on the x-axis, and the overall error on the y-axis. (B) sPLS-DA plot to validate top hits from the logistic regression with interactions. sPLS-DA is a supervised clustering method which separates SLE-P from SLE-NP. (C) Features included in the sPLS-DA plotted with their factor loading value. (D) Visualisation of the weighting and correlation of each metabolite in component 1 and 2 on the sPLS-DA model. See Supplementary Table III for metabolite abbreviations.
Differential metabolites correlated with clinical features of SLE-P patients
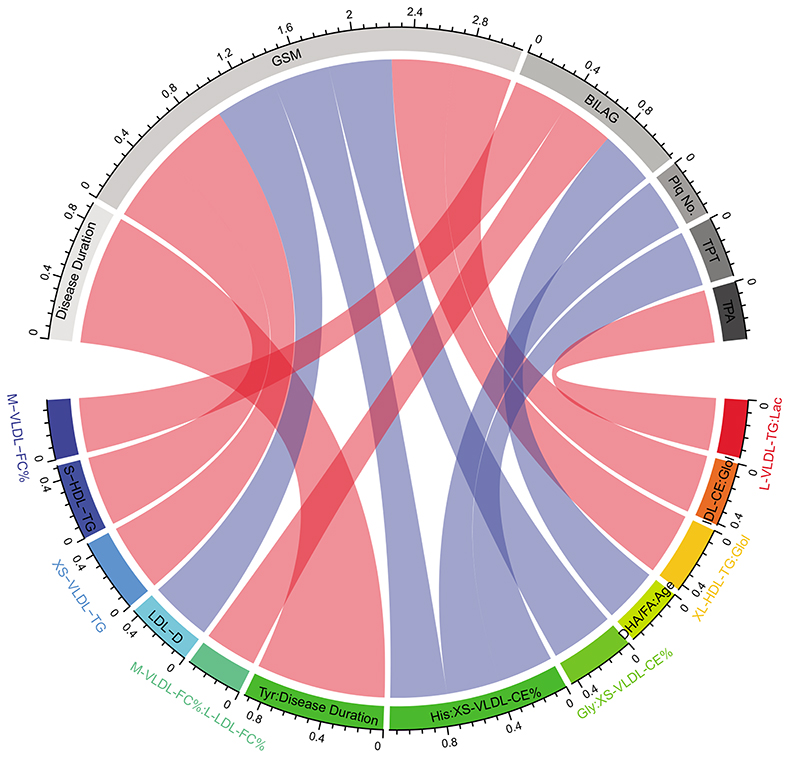
Finally, the top ten metabolites from LR, RF, and all metabolite interactions included in the LR+I models were correlated with clinical and plaque features (Figure 5, Supplementary Table VI). Significant correlations include GSM (grey scale median, measure of plaque stability and lipid content) correlated positively with S-HDL-TG, XS-VLDL-TG, XL-HDL-TG:glycerol and IDL-CE:glycerol and negatively with LDL-D, histidine:XS-VLDL-CE_% and Glycine:XS-VLDL-CE_%; Plaque number and plaque thickness negatively correlated with histidine:XS-VLDL-CE_%; TPA (total plaque area) positively correlated with L-VLDL-TG:lactate; and disease activity (BILAG-2004) positively correlated with M-VLDL-FC_% and M-VLDL-FC_%:L-LDL-FC_% and negatively with DHA-FA(22:6, docosahexaenoic acids to total fatty acids):age. The strongest correlation was between disease duration and tyrosine:disease duration.
Figure 5. Correlations of significant metabolites with SLE clinical markers.
Correlations between metabolites and patient clinical characteristics. Pearson’s product moment correlation coefficients are represented as connecting lines between the clinical characteristic section (grey) and metabolite section (rainbow)75. Only correlations with p value below 0.05 are shown. Red line=positive correlation and blue line=negative correlation. Width of lines represents the value of correlation coefficients (measured with scale). See Supplementary Table VI. GSM, grey scale median; BILAG, British Isles Lupus Assessment Group-2004 disease activity score; Plq No, plaque number; TPT, total plaque thickness; TPA, Total Plaque area (see Supplementary Table III for metabolite abbreviations).
Discussion
CVD risk in SLE patients is an important cause of mortality in a cohort of mostly female and relatively young patients compared to the general population. Despite the long-established link between SLE and increased CVD risk2, 4, 33 SLE specialists still lack accurate methods of predicting the risk in an individual patient. Traditional cardiovascular risk factors encapsulated in the Framingham equations underestimate the true risk in patients with SLE and fail to predict which patients will have cardiovascular events34, 35. A more recent comparison of the performance of eight different clinical risk scores to classify CVD risk in SLE concluded that most of the scores underestimated high CVD risk in SLE patients36. SLE specialists therefore have no means currently of accurately stratifying patients at high risk.
This study used serum metabolomics incorporating detailed lipoprotein subclass evaluation to differentiate between patients with SLE with and without confirmed sub-clinical atherosclerosis. Analysis using ML models identified an association between multiple VLDL subsets, amino acids leucine, glycine and tyrosine and clinical features including age with the presence of sub-clinical plaque, suggesting that more detailed lipoprotein and metabolomic measurements together with demographic information could help to better predict those patients at greatest CVD risk.
While age, disease duration and treatment are known contributors to cardiovascular disease risk in SLE, other metabolic factors also to contribute to the accelerated risk4, 37, 38. Our findings support this showing that the combination of both metabolites and age most strongly distinguished SLE-P from SLE-NP. VLDL subclasses featured predominantly in all the analysis models used suggesting its potential importance in predicting which patients with SLE go on to develop atherosclerosis. VLDL is known to be associated with increased CVD risk. It is the main carrier of TGs, which are an independent risk factor for CVD39, and VLDL particle concentrations have been positively associated with the risk of myocardial infarction40. In the JUPITER trial of CVD risk in nearly 12,000 patients, risk among placebo-allocated participants was associated with total VLDL particles, as well as ApoB, total cholesterol, and TGs41. Furthermore, pharmacological lowering of ApoB–containing lipoproteins, including VLDL, earlier in life is proposed to eliminate high risk of atherosclerotic-CVD in individuals with image-documented sub-clinical atherosclerosis, such as the SLE women in this study42.
Mechanistically, the interaction between lipoprotein profile, lupus and atherogenesis has remained elusive and likely involves a complex cross-regulation between lipoproteins, lupus specific factors (including female sex hormones and treatment) and an activated and dysregulated immune response4, 43. Lipoproteins play an important role in maintaining lipid homeostasis, both systemically and at a cellular level via lipid uptake (LDL/VLDL) and efflux (HDL). Lupus-related non-traditional risk factors likely contribute to dyslipidemia, for instance: chronic inflammation can induce reduced serum HDL and increased TGs due to increased hepatic VLDL production and reduced clearance of TG-rich lipoproteins44; lupus disease activity/damage and inflammatory mediators (including IL-6 and TNF-α), are independently associated with a pro-atherogenic lipid profile (elevated TGs and low HDL)45, 46; and lower LDL activity leading to the accumulation of TG-rich particles47, 48. Small dense LDL is also increased and more easily oxidized as the ability of HDL to prevent the oxidation of LDL is diminished49. Conversely, sub-clinical changes in circulating lipoprotein subclasses and their lipid content as described here between SLE patients with and without plaque, are likely to impact immune cell metabolism and function50. Cell mediated cholesterol efflux is impaired in SLE patients51 and LDL composition rather than total LDL levels promoted macrophage infiltration, aortic foam cell formation and vascular aging in experimental lupus models52. Furthermore, larger, typically TG-rich, ApoB-containing lipoproteins such as VLDL, may have difficulty leaving the intima because of their larger size or because they get entrapped by components in the sub-endothelial space53. Here, these lipoproteins undergo enzymatic modifications that accelerate accumulation and promote aggregation, which is influenced by lipoprotein quantity and composition54. Notably, the larger TG and cholesterol-rich lipoproteins appear to be more potent than LDL, the most common atherogenic lipoprotein, for provoking greater maladaptive immune activation55. This supports our previous work showing that VLDL from SLE-P patients could influence the phenotype of iNKT cells and monocytes, via altered lipid-antigen presentation19. Other mechanisms could include disrupted immune cell signaling due to changes in plasma membrane lipid rafts56. In addition, SLE auto-antibodies induce endothelial injury, inflammation and cell-adhesion, all mechanisms promoting atherosclerotic plaque formation.
Previous metabolomics studies in patients with SLE have not focused on cardiovascular risk but rather compared metabolomics profiles between healthy donors and SLE patients57. One study used mass spectroscopy rather than NMR to compare 20 patients with SLE and 9 healthy controls, identifying >100 differentially expressed metabolites, but did not assess lipoprotein particles and only one patient had CVD58. Another study using NMR, identified raised VLDL and LDL and reduced HDL in patients with SLE59. However, they did not report on lipoprotein subclasses and vascular ultrasound imaging was not performed. Guleria et al used metabolomics to identify metabolic signatures in different clinical subgroups within a cohort of lupus patients60, as we have done here for SLE-P versus SLE-NP patients. Another NMR metabolomics study compared patients with and without lupus nephritis, and healthy controls. Compared to healthy donors, this study reported lower VLDL and LDL in patients with SLE, though higher in the nephritis patients59. The study used patients from India, so ethnicity and lifestyle factors such as diet may have played a role in these results, which do not support other reports. In our more in-depth lipoprotein analysis, we show that patients with SLE have a significant alteration in the concentration and content of all lipoprotein subsets compared to healthy controls, with a specific increase of total lipid, cholesterol and cholesterol ester content in smaller VLDL subsets, but a reduction in HDL subsets. We found a significant increase in the ApoB:ApoA1 ratio in SLE patients compared to HCs; specifically, a decrease in ApoA1, but no difference in ApoB. This suggests that the increased ApoB:ApoA1 ratio in SLE could be due mainly to a reduction of HDL. Regarding these observations, other studies have reported increased ApoB concentrations43 and decreased ApoA161, 62, whilst some studies have associated these differences with autoantibodies against apolipoproteins in SLE63, 64.
We also found associations between metabolites and ultrasound scanning measurements. Interestingly, TGs in VLDL showed significant correlations with TPA and GSM. Measurements of TPA and echolucency have been shown to have good predictive value for coronary artery disease in women65, and thus pertinent in our all-female SLE cohort.
In addition to lipoproteins, leucine, glycine and tyrosine were also identified by the ML models to be important predictors of SLE-P. Leucine is a branched chain essential amino acid with a potential important role in regulating protein, glucose and lipid metabolism, in part via activation of the mTOR (mammalian target of rapamycin) protein kinase and promotion of leptin synthesis66. Studies in mice show that leucine supplementation improves diet-induced obesity, insulin resistance and atherosclerosis outcomes67, 68. Increased leucine could be associated with an early response to sub-clinical plaque development, as we have shown previously19. Glycine, shown here to be reduced in SLE-P, is also reported have a potential anti-inflammatory role via reducing nuclear factor-kappa B (NF-KB) activation in vascular endothelial cells69. Plasma glycine levels are inversely correlated with acute myocardial infarction in patients undergoing coronary angiography70. Tyrosine, in the form of 3-nitrotyrosine, is associated with oxidised HDL in the human artery wall and circulation in atherosclerosis, and may promote atherogenesis71. Further work is needed to confirm these findings and to understand fully the complex role of these metabolites in atherogenesis in SLE.
This study has some limitations. While we accounted for most important patient associated factors including treatment, age and disease duration, we did not have access to sex hormone levels which change with age and could also influence lipoprotein levels72. An important overall aim of this research was to define a clinical algorithm using serum metabolomics and clinical data to help identify SLE patients at elevated cardiovascular risk who would benefit most from therapeutic or lifestyle intervention. Efforts are ongoing to validate these metabolic signatures in additional larger, multi-centre cohorts which are beyond the scope of the current study. It will be important to establish this before clinical algorithms can be defined. Furthermore, while we have identified a number of metabolites associated with sub-clinical plaque, that have previously been associated with various features of atherosclerosis formation (see above), understanding the specific role these metabolites play in plaque development was beyond the scope of the current study.
In conclusion, the interrogation of lipid subclasses may hold the key to providing insights on how to better stratify CVD risk in SLE. Analysis of lipoproteins using NMR spectroscopy is of particular interest given this high throughput metabolomics analysis is rapid, can be carried out on serum, gives a larger amount of information from each sample and is potentially cost-effective73 depending on how many high-risk patients are identified and how the risk is managed. It is possible that a composite score of metabolomics with conventional risk factors may be the best way to assess CVD risk in patients with SLE74.
Supplementary Material
Highlights 3-5 bullet points that summarize the major findings of the study.
Lipoprotein-based metabolomics identified a significantly disrupted lipoprotein subclass taxonomy in women with SLE compared with health volunteers, characterised by increased total lipid, cholesterol and cholesterol ester content in various VLDL subsets, and reduced HDL subsets.
SLE patients with sub-clinical atherosclerosis plaques had a unique metabolomic profile associated with increased circulating VLDL subsets, leucine and tyrosine and reduced glycine.
Interactions between metabolites and patient demographic and treatment features were also important in discriminating between SLE patients with and without sub-clinical plaque.
This study suggests that a composite score of detailed metabolomics with conventional risk factors may be a better predictor of CVD risk in patients with SLE.
Acknowledgments
None
Sources of funding
This work was supported by NIHR University College London Hospital Biomedical Research Centre grant reference BRC531/III/IPT/101350 (IPT/ECJ). LC is supported by UCL & Birkbeck MRC Doctoral Training Programme. GAR was supported by a PhD studentship from Lupus UK and The Rosetrees Trust (M409). KEW was funded by a British Heart Foundation PhD Studentship (FS/13/59/30649). JP is supported by Versus Arthritis grant reference 21226.
Abbreviations
- Apo
apolipoprotein
- BILAG
British Isles Lupus Assessment Group
- CVD
cardiovascular disease
- C
total cholesterol
- CE
cholesterol esters
- FC
free cholesterol
- (GSM)
Grey Scale Median
- HC
healthy volunteer control
- HDL
High-Density Lipoprotein
- IDL
intermediate density lipoprotein
- IMT
intima–media thickness
- LDL
Low-Density Lipoprotein
- L
large
- LR
logistic regression
- LR+I
logistic regression with interactions
- M
medium
- NMR
Nuclear Magnetic Resonance
- PL
phospholipids
- RF
random forest
- S
small
- SLE
Systemic Lupus Erythematosus
- SLE-NP
SLE patients with no sub-clinical plaque
- SLE-P
SLE patients with sub-clinical plaque
- sPLS-DA
Sparse Partial Least Squares Discriminant Analysis
- SVM
support vector machine
- TG
triglycerides
- VLDL
Very Low Density Lipoprotein
- XL
very large
- XXL
chylomicrons and extremely large
- XS
very small.
Footnotes
Disclosure: The authors have declared that no conflict of interest exists.
References
- 1.Rees F, Doherty M, Grainge M, Davenport G, Lanyon P, Zhang W. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999-2012. Annals of the rheumatic diseases. 2016;75:136–41. doi: 10.1136/annrheumdis-2014-206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr, Jansen-McWilliams L, D’Agostino RB, Kuller LH. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–15. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 3.Bernatsky S, Boivin JF, Joseph L, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2550–7. doi: 10.1002/art.21955. [DOI] [PubMed] [Google Scholar]
- 4.Bruce IN. ‘Not only...but also’: factors that contribute to accelerated atherosclerosis and premature coronary heart disease in systemic lupus erythematosus. Rheumatology (Oxford, England) 2005;44:1492–502. doi: 10.1093/rheumatology/kei142. [DOI] [PubMed] [Google Scholar]
- 5.Wajed J, Ahmad Y, Durrington PN, Bruce IN. Prevention of cardiovascular disease in systemic lupus erythematosus--proposed guidelines for risk factor management. Rheumatology (Oxford, England) 2004;43:7–12. doi: 10.1093/rheumatology/keg436. [DOI] [PubMed] [Google Scholar]
- 6.Skalen K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, Innerarity TL, Boren J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–4. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- 7.Rosenson RS, Brewer HB, Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–19. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn BH, Grossman J, Ansell BJ, Skaggs BJ, McMahon M. Altered lipoprotein metabolism in chronic inflammatory states: proinflammatory high-density lipoprotein and accelerated atherosclerosis in systemic lupus erythematosus and rheumatoid arthritis. Arthritis research & therapy. 2008;10:213. doi: 10.1186/ar2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMahon M, Grossman J, Skaggs B, et al. Dysfunctional proinflammatory high-density lipoproteins confer increased risk of atherosclerosis in women with systemic lupus erythematosus. Arthritis and rheumatism. 2009;60:2428–37. doi: 10.1002/art.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borba EF, Bonfa E. Dyslipoproteinemias in systemic lupus erythematosus: influence of disease, activity, and anticardiolipin antibodies. Lupus. 1997;6:533–9. doi: 10.1177/096120339700600610. [DOI] [PubMed] [Google Scholar]
- 11.Nuttall SL, Heaton S, Piper MK, Martin U, Gordon C. Cardiovascular risk in systemic lupus erythematosus--evidence of increased oxidative stress and dyslipidaemia. Rheumatology (Oxford, England) 2003;42:758–62. doi: 10.1093/rheumatology/keg212. [DOI] [PubMed] [Google Scholar]
- 12.Sniderman AD, Williams K, Contois JH, Monroe HM, McQueen MJ, de Graaf J, Furberg CD. A Meta-Analysis of Low-Density Lipoprotein Cholesterol, Non-High-Density Lipoprotein Cholesterol, and Apolipoprotein B as Markers of Cardiovascular Risk. Circulation-Cardiovascular Quality and Outcomes. 2011;4:337–U144. doi: 10.1161/CIRCOUTCOMES.110.959247. [DOI] [PubMed] [Google Scholar]
- 13.Lawler PR, Akinkuolie AO, Ridker PM, Sniderman AD, Buring JE, Glynn RJ, Chasman DI, Mora S. Discordance between Circulating Atherogenic Cholesterol Mass and Lipoprotein Particle Concentration in Relation to Future Coronary Events in Women. Clinical Chemistry. 2017;63:870–879. doi: 10.1373/clinchem.2016.264515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation. 2005;112:3375–3383. doi: 10.1161/CIRCULATIONAHA.104.532499. [DOI] [PubMed] [Google Scholar]
- 15.McQueen MJ, Hawken S, Wang XY, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E, Kazmi K, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372:224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 16.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026–2033. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 17.Soininen P, Kangas AJ, Wurtz P, Suna T, Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet. 2015;8:192–206. doi: 10.1161/CIRCGENETICS.114.000216. [DOI] [PubMed] [Google Scholar]
- 18.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and Rheumatism. 1997;40:1725–1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 19.Smith E, Croca S, Waddington KE, Sofat R, Griffin M, Nicolaides A, Isenberg DA, Torra IP, Rahman A, Jury EC. Cross-talk between iNKT cells and monocytes triggers an atheroprotective immune response in SLE patients with asymptomatic plaque. Sci Immunol. 2016;1 doi: 10.1126/sciimmunol.aah4081. [DOI] [PubMed] [Google Scholar]
- 20.Yee CS, Cresswell L, Farewell V, et al. Numerical scoring for the BILAG-2004 index. Rheumatology (Oxford) 2010;49:1665–9. doi: 10.1093/rheumatology/keq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–9. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 22.Griffin M, Nicolaides A, Tyllis T, Georgiou N, Martin RM, Bond D, Panayiotou A, Tziakouri C, Doré CJ, Fessas C. Carotid and femoral arterial wall changes and the prevalence of clinical cardiovascular disease. Vasc Med. 2009;14:227–232. doi: 10.1177/1358863X08101542. [DOI] [PubMed] [Google Scholar]
- 23.Nicolaides AN, Kakkos SK, Kyriacou E, Griffin M, Sabetai M, Thomas DJ, Tegos T, Geroulakos G, Labropoulos N, Doré CJ, Morris TP, et al. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg. 2010;52:1486–1496.e1-5. doi: 10.1016/j.jvs.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Griffin M, Nicolaides AN, Belcaro G, Shah E. Cardiovascular risk assessment using ultrasound: the value of arterial wall changes including the presence, severity and character of plaques. Pathophysiology of haemostasis and thrombosis. 2002;32:367–370. doi: 10.1159/000073602. [DOI] [PubMed] [Google Scholar]
- 25.Spanos K, Tzorbatzoglou I, Lazari P, Maras D, Giannoukas AD. Carotid artery plaque echomorphology and its association with histopathologic characteristics. J Vasc Surg. 2018;68:1772–1780. doi: 10.1016/j.jvs.2018.01.068. [DOI] [PubMed] [Google Scholar]
- 26.Salem MK, Bown MJ, Sayers RD, West K, Moore D, Nicolaides A, Robinson TG, Naylor AR. Identification of patients with a histologically unstable carotid plaque using ultrasonic plaque image analysis. Eur J Vasc Endovasc Surg. 2014;48:118–25. doi: 10.1016/j.ejvs.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Soininen P, Kangas AJ, Würtz P, Tukiainen T, Tynkkynen T, Laatikainen R, Järvelin MR, Kähönen M, Lehtimäki T, Viikari J, Raitakari OT, et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst. 2009;134:1781–5. doi: 10.1039/b910205a. [DOI] [PubMed] [Google Scholar]
- 28.Würtz P, Kangas AJ, Soininen P, Lawlor DA, Davey Smith G, Ala-Korpela M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Large-Scale Epidemiology: A Primer on -Omic Technologies. American Journal of Epidemiology. 2017;186:1084–1096. doi: 10.1093/aje/kwx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wurtz P, Wang Q, Soininen P, et al. Metabolomic Profiling of Statin Use and Genetic Inhibition of HMG-CoA Reductase. Journal of the American College of Cardiology. 2016;67:1200–1210. doi: 10.1016/j.jacc.2015.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kofink D, Eppinga RN, van Gilst WH, Bakker SJL, Dullaart RPF, van der Harst P, Asselbergs FW. Statin Effects on Metabolic Profiles: Data From the PREVEND IT (Prevention of Renal and Vascular End-stage Disease Intervention Trial) Circulation-Cardiovascular Genetics. 2017;10:9. doi: 10.1161/CIRCGENETICS.117.001759. [DOI] [PubMed] [Google Scholar]
- 31.Sliz E, Kettunen J, Holmes MV, et al. Metabolomic Consequences of Genetic Inhibition of PCSK9 Compared With Statin Treatment. Circulation. 2018;138:2499–2512. doi: 10.1161/CIRCULATIONAHA.118.034942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bogl LH, Pietilainen KH, Rissanen A, Kangas AJ, Soininen P, Rose RJ, Ala-Korpela M, Kaprio J. Association between habitual dietary intake and lipoprotein subclass profile in healthy young adults. Nutrition Metabolism and Cardiovascular Diseases. 2013;23:1071–1078. doi: 10.1016/j.numecd.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of systemic lupus erythematosus. The American journal of medicine. 1976;60:221–5. doi: 10.1016/0002-9343(76)90431-9. [DOI] [PubMed] [Google Scholar]
- 34.Bessant R, Hingorani A, Patel L, MacGregor A, Isenberg DA, Rahman A. Risk of coronary heart disease and stroke in a large British cohort of patients with systemic lupus erythematosus. Rheumatology (Oxford, England) 2004;43:924–9. doi: 10.1093/rheumatology/keh213. [DOI] [PubMed] [Google Scholar]
- 35.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, Côte R, Grover SA, Fortin PR, Clarke AE, Senécal JL. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–7. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 36.Drosos GC, Konstantonis G, Sfikakis PP, Tektonidou MG. Underperformance of clinical risk scores in identifying vascular ultrasound-based high cardiovascular risk in systemic lupus erythematosus. European Journal of Preventive Cardiology. 0 doi: 10.1093/eurjpc/zwaa256. 2047487320906650. [DOI] [PubMed] [Google Scholar]
- 37.Erdozain J-G, Villar I, Nieto J, Ruiz-Arruza I, Ruiz-Irastorza G. Predictors of peripheral arterial disease in SLE change with patient’s age. Lupus Science & Medicine. 2017;4:e000190. doi: 10.1136/lupus-2016-000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMahon M, Skaggs BJ, Grossman JM, et al. A panel of biomarkers is associated with increased risk of the presence and progression of atherosclerosis in women with systemic lupus erythematosus. Arthritis Rheumatol. 2014;66:130–9. doi: 10.1002/art.38204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prenner SB, Mulvey CK, Ferguson JF, Rickels MR, Bhatt AB, Reilly MP. Very low density lipoprotein cholesterol associates with coronary artery calcification in type 2 diabetes beyond circulating levels of triglycerides. Atherosclerosis. 2014;236:244–50. doi: 10.1016/j.atherosclerosis.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren J, Grundy SM, Liu J, Wang W, Wang M, Sun J, Liu J, Li Y, Wu Z, Zhao D. Long-term coronary heart disease risk associated with very-low-density lipoprotein cholesterol in Chinese: the results of a 15-Year Chinese Multi-Provincial Cohort Study (CMCS) Atherosclerosis. 2010;211:327–32. doi: 10.1016/j.atherosclerosis.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 41.Lawler PR, Akinkuolie AO, Chu AY, Shah SH, Kraus WE, Craig D, Padmanabhan L, Glynn RJ, Ridker PM, Chasman DI, Mora S. Atherogenic Lipoprotein Determinants of Cardiovascular Disease and Residual Risk Among Individuals With Low Low-Density Lipoprotein Cholesterol. Journal of the American Heart Association. 2017;6 doi: 10.1161/JAHA.117.005549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson JG, Williams KJ, Gidding S, et al. Eradicating the Burden of Atherosclerotic Cardiovascular Disease by Lowering Apolipoprotein B Lipoproteins Earlier in Life. Journal of the American Heart Association. 2018;7:e009778. doi: 10.1161/JAHA.118.009778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szabó MZ, Szodoray P, Kiss E. Dyslipidemia in systemic lupus erythematosus. Immunol Res. 2017;65:543–550. doi: 10.1007/s12026-016-8892-9. [DOI] [PubMed] [Google Scholar]
- 44.Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–96. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Chung CP, Oeser A, Solus J, Avalos I, Gebretsadik T, Shintani A, Linton MF, Fazio S, Stein CM. Inflammatory mechanisms affecting the lipid profile in patients with systemic lupus erythematosus. J Rheumatol. 2007;34:1849–54. [PubMed] [Google Scholar]
- 46.Svenungsson E, Gunnarsson I, Fei G-Z, Lundberg IE, Klareskog L, Frostegård J. Elevated triglycerides and low levels of high-density lipoprotein as markers of disease activity in association with up-regulation of the tumor necrosis factor α/tumor necrosis factor receptor system in systemic lupus erythematosus. Arthritis & Rheumatism. 2003;48:2533–2540. doi: 10.1002/art.11264. [DOI] [PubMed] [Google Scholar]
- 47.Maury C, Teppo AM. Tumor necrosis factor in the serum of patients with systemic lupus erythematosus. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 1989;32:146–150. doi: 10.1002/anr.1780320206. [DOI] [PubMed] [Google Scholar]
- 48.Svenungsson E, Fei GZ, Jensen-Urstad K, de Faire U, Hamsten A, Frostegard J. TNF-alpha: a link between hypertriglyceridaemia and inflammation in SLE patients with cardiovascular disease. Lupus. 2003;12:454–61. doi: 10.1191/0961203303lu412oa. [DOI] [PubMed] [Google Scholar]
- 49.Feingold KR, Grunfeld C. Effect of inflammation on HDL structure and function. Curr Opin Lipidol. 2016;27:521–30. doi: 10.1097/MOL.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 50.Rhoads JP, Major AS, Rathmell JC. Fine tuning of immunometabolism for the treatment of rheumatic diseases. Nat Rev Rheumatol. 2017;13:313–320. doi: 10.1038/nrrheum.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ronda N, Favari E, Borghi MO, Ingegnoli F, Gerosa M, Chighizola C, Zimetti F, Adorni MP, Bernini F, Meroni PL. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 2014;73:609–15. doi: 10.1136/annrheumdis-2012-202914. [DOI] [PubMed] [Google Scholar]
- 52.Chan H-C, Chan H-C, Liang C-J, et al. Role of Low-Density Lipoprotein in Early Vascular Aging Associated With Systemic Lupus Erythematosus. Arthritis & Rheumatology. 2020;72:972–984. doi: 10.1002/art.41213. [DOI] [PubMed] [Google Scholar]
- 53.Nordestgaard BG. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ Res. 2016;118:547–63. doi: 10.1161/CIRCRESAHA.115.306249. [DOI] [PubMed] [Google Scholar]
- 54.Borén J, Williams KJ. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: a triumph of simplicity. Current Opinion in Lipidology. 2016;27:473–483. doi: 10.1097/MOL.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 55.Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Elevated Remnant Cholesterol Causes Both Low-Grade Inflammation and Ischemic Heart Disease, Whereas Elevated Low-Density Lipoprotein Cholesterol Causes Ischemic Heart Disease Without Inflammation. Circulation. 2013;128:1298–1309. doi: 10.1161/CIRCULATIONAHA.113.003008. [DOI] [PubMed] [Google Scholar]
- 56.Waddington KE, Papadaki A, Coelewij L, Adriani M, Nytrova P, Kubala Havrdova E, Fogdell-Hahn A, Farrell R, Dönnes P, Pineda-Torra I, Jury EC. Using Serum Metabolomics to Predict Development of Anti-drug Antibodies in Multiple Sclerosis Patients Treated With IFNβ. Front Immunol. 2020;11:1527. doi: 10.3389/fimmu.2020.01527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang T, Mohan C. Caution in studying and interpreting the lupus metabolome. Arthritis Research & Therapy. 2020;22:172. doi: 10.1186/s13075-020-02264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu T, Xie C, Han J, Ye Y, Weiel J, Li Q, Blanco I, Ahn C, Olsen N, Putterman C, Saxena R, et al. Metabolic disturbances associated with systemic lupus erythematosus. PLoS One. 2012;7:e37210. doi: 10.1371/journal.pone.0037210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ouyang X, Dai Y, Wen JL, Wang LX. (1)H NMR-based metabolomic study of metabolic profiling for systemic lupus erythematosus. Lupus. 2011;20:1411–20. doi: 10.1177/0961203311418707. [DOI] [PubMed] [Google Scholar]
- 60.Guleria A, Pratap A, Dubey D, Rawat A, Chaurasia S, Sukesh E, Phatak S, Ajmani S, Kumar U, Khetrapal CL, Bacon P, et al. NMR based serum metabolomics reveals a distinctive signature in patients with Lupus Nephritis. Scientific Reports. 2016;6:35309. doi: 10.1038/srep35309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarı RA, Polat MF, Taysi S, Bakan E, Çapoğlu İ. Serum Lipoprotein(a) Level and its Clinical Significance in Patients with Systemic Lupus Erythematosus. Clinical Rheumatology. 2002;21:520–524. doi: 10.1007/s100670200127. [DOI] [PubMed] [Google Scholar]
- 62.Machado D, Sarni RO, Abad TT, Silva SG, Khazaal EJ, Hix S, Correia MS, Suano-Souza FI, Len CA, Terreri MT. Lipid profile among girls with systemic lupus erythematosus. Rheumatol Int. 2017;37:43–48. doi: 10.1007/s00296-015-3393-z. [DOI] [PubMed] [Google Scholar]
- 63.Nigolian H, Ribi C, Courvoisier D, Pagano S, Álvarez M, Trendelenburg M, Huynh-Do U, Vuilleumier N, Dayer J, Chizzolini C, Roux-Lombard P. Anti-apolipoprotein A-1 autoantibodies correlate with disease activity in systemic lupus erythematosus. Rheumatology. 2019 doi: 10.1093/rheumatology/kez306. [DOI] [PubMed] [Google Scholar]
- 64.Svenungsson E, Engelbertsen D, Wigren M, Gustafsson JT, Gunnarsson I, Elvin K, Jensen-Urstad K, Fredrikson GN, Nilsson J. Decreased levels of autoantibodies against apolipoprotein B-100 antigens are associated with cardiovascular disease in systemic lupus erythematosus. Clin Exp Immunol. 2015;181:417–426. doi: 10.1111/cei.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnsen SH, Mathiesen EB, Joakimsen O, Stensland E, Wilsgaard T, Lochen ML, Njolstad I, Arnesen E. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow-up study of 6226 persons: the Tromso Study. Stroke; a journal of cerebral circulation. 2007;38:2873–80. doi: 10.1161/STROKEAHA.107.487264. [DOI] [PubMed] [Google Scholar]
- 66.Pedroso JAB, Zampieri TT, Donato J., Jr Reviewing the Effects of L-Leucine Supplementation in the Regulation of Food Intake, Energy Balance, and Glucose Homeostasis. Nutrients. 2015;7:3914–3937. doi: 10.3390/nu7053914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56:1647–54. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]
- 68.Zhao Y, Dai X-y, Zhou Z, Zhao G-x, Wang X, Xu M-j. Leucine supplementation via drinking water reduces atherosclerotic lesions in apoE null mice. Acta Pharmacologica Sinica. 2016;37:196–203. doi: 10.1038/aps.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hasegawa S, Ichiyama T, Sonaka I, Ohsaki A, Okada S, Wakiguchi H, Kudo K, Kittaka S, Hara M, Furukawa S. Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin Exp Immunol. 2012;167:269–274. doi: 10.1111/j.1365-2249.2011.04519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ding Y, Svingen GF, Pedersen ER, Gregory JF, Ueland PM, Tell GS, Nygård OK. Plasma Glycine and Risk of Acute Myocardial Infarction in Patients With Suspected Stable Angina Pectoris. J Am Heart Assoc. 2015;5 doi: 10.1161/JAHA.115.002621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pennathur S, Bergt C, Shao B, Byun J, Kassim SY, Singh P, Green PS, McDonald TO, Brunzell J, Chait A, Oram JF, et al. Human Atherosclerotic Intima and Blood of Patients with Established Coronary Artery Disease Contain High Density Lipoprotein Damaged by Reactive Nitrogen Species. Journal of Biological Chemistry. 2004;279:42977–42983. doi: 10.1074/jbc.M406762200. [DOI] [PubMed] [Google Scholar]
- 72.Stevenson JC, Tsiligiannis S, Panay N. Cardiovascular Risk in Perimenopausal Women. Curr Vasc Pharmacol. 2019;17:591–594. doi: 10.2174/1570161116666181002145340. [DOI] [PubMed] [Google Scholar]
- 73.Wurtz P, Soininen P, Kangas AJ, Makinen VP, Groop PH, Savolainen MJ, Juonala M, Viikari JS, Kahonen M, Lehtimaki T, Raitakari OT, et al. Characterization of systemic metabolic phenotypes associated with subclinical atherosclerosis. Mol Biosyst. 2011;7:385–93. doi: 10.1039/c0mb00066c. [DOI] [PubMed] [Google Scholar]
- 74.Wurtz P, Raiko JR, Magnussen CG, et al. High-throughput quantification of circulating metabolites improves prediction of subclinical atherosclerosis. European heart journal. 2012;33:2307–16. doi: 10.1093/eurheartj/ehs020. [DOI] [PubMed] [Google Scholar]
- 75.Gu Z, Gu L, Eils R, Schlesner M, Brors B. circlize Implements and enhances circular visualization in R. Bioinformatics. 2014;30:2811–2. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.