Abstract
Background and purpose
Pre-stroke dementia prevalence is high and impacts outcome. Although the Informant Questionnaire for Cognitive Decline in the Elderly (IQCODE) is being used to assess pre-stroke cognition, data on its validity for pre-stroke dementia are lacking. We studied the accuracy of the short-form (16-item) IQCODE for pre-event dementia in a population-based study of all TIA/stroke.
Methods
All patients with TIA/stroke in a defined population of 92 728 (Oxford Vascular Study, 2002-2017) with IQCODE were included. IQCODE questionnaires were given to participants at baseline interview with instructions to pass to an informant for completion and return by post. Diagnosis of pre-event dementia was defined as prior diagnosis of dementia, or dementia by the DSM-IV criteria on study interview and hand-searching of the entire medical record blinded to IQCODE. Reliability of the IQCODE for dementia was determined by the area under the receiver operating curve (AUC), sensitivity and specificity, stratified by age, event severity and first ever stroke.
Results
Among 2059 interviewed survivors, IQCODE questionnaires were returned in 1068 (mean age/SD=72.9/12.3, 47% TIA, 52.3% male, 68 (6.4%) pre-event dementia). AUC for IQCODE for pre-event dementia was 0.94 (95% CI 0.90-0.97, p<0.001) with similar results by age: 0.92, 0.88-0.96, <65 years; 0.94, 0.83-1.00, 65-74 years; 0.95, 0.92-0.99, 75-84 years; 0.89, 0.82-0.96, ≥85 years. The optimal cut-off score overall was >3.48 (sensitivity=89.7%; specificity=84.2%) but was non-significantly higher for major stroke (NIHSS>3) than minor stroke/TIA (>3.85 versus >3.47). Performance was similar in patients with first ever stroke (AUC=0.92, 0.88-0.97; sensitivity=85.7%; specificity=84.8% for cut-off >3.48). All 16-IQCODE questions discriminated between dementia and no dementia (all p<0.001) with the greatest differences seen for finances, using gadgets, arithmetic and learning new things.
Conclusions
IQCODE has excellent accuracy for detecting pre-existing dementia in TIA and stroke with the pattern of deficits suggesting prominent executive dysfunction.
Keywords: IQCODE stroke TIA dementia pre-stroke OXVASC cognition
Subject Terms: Cerebrovascular Disease/Stroke, Cognitive impairment, Ischemic Stroke, Transient Ischemic Attack (TIA)
Introduction
Cognitive impairment is associated with increased risk of stroke and of more severe stroke.1,2 The prevalence of pre-stroke dementia ranges from 5% in TIA to 21% in severe major stroke (NIHSS>10).1 Pre-stroke cognitive decline also increases the risk of death from stroke and of post-stroke dementia.3–6
However, identifying pre-TIA/stroke cognitive status at the time of the index cerebrovascular event may be difficult. Whilst some patients will have a documented dementia diagnosis, rates of undiagnosed dementia are high: around one half of hospitalized people aged ≥75 years have moderate/severe cognitive impairment but only around 20% have a diagnosis of dementia.7,8 Pre-existing dementia can be excluded in patients with good cognitive function after TIA/stroke but for cognitively impaired patients, a retrospective assessment of cognition will be required. In such cases, indirect assessment via relatives or caregivers is necessary facilitated by structured tools including the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), Eight-item Informant Interview to Differentiate Aging and Dementia (AD-8) and the General Practitioner assessment of cognition (GP-Cog), the latter being used in primary care settings.9–14
The IQCODE measures global cognitive decline across a range of areas and is largely unaffected by occupation or education.9,10,12 It was developed to assess community dwelling older adults, in whom Alzheimer’s disease is the most common dementia subtype and amnestic deficits are prominent. Each IQCODE item is scored on an ordinal five-point scale (1-5) and the average score across all items is calculated. Higher scores indicate cognitive decline. There is no consensus on the optimal cut-off for dementia which may vary according to the population characteristics and as with all cognitive screening tests, there is a trade-off between sensitivity and specificity.10,12 The IQCODE is available as 16- and 26- item versions that have similar validity and high internal consistency and test-retest reliability in non-stroke settings.10,12 However, previous validation studies had possible selection bias and limited reporting of IQCODE application, missing data and incomplete questionnaires.10–12 There are also few data on differential item patterns across dementia subtypes.
The IQCODE has not been validated for pre-stroke dementia in cerebrovascular populations15 although it has been used to quantify or exclude pre-event dementia in stroke studies using variable cut-offs (3.30, 3.35, 4.00).4,5,16–20 We therefore assessed the accuracy of the IQCODE for pre-TIA/stroke dementia, and the effect of event severity and informant characteristics, in a population-based observational study of all TIA and stroke.
Methods
Study background
This study was conducted within the ongoing Oxford Vascular Study (OXVASC, 2002-) a longitudinal, prospective, population-based incidence study of all acute vascular events in a defined population of ~92,720 in nine primary care practices with around 100 general practitioners in Oxfordshire, United Kingdom.21,22 OXVASC is approved by the local Oxfordshire clinical research ethics committee (CO.043). Written consent is obtained at inclusion for study interview and follow-up, including review of all primary care and hospital records and death certificates. Where a patient is unable to give consent, assent from a relative is obtained.
Patients with TIA or minor stroke are referred by their primary care physician or the emergency department to the daily OXVASC emergency clinic staffed by a dedicated OXVASC clinical team. Patients with major stroke are admitted to the regional acute stroke unit at the John Radcliffe Hospital, Oxford, covering the study population area and are seen as soon as possible after admission by a member of the study team. Ascertainment of TIA and stroke approaches 100% of events reaching medical attention.21,22 Diagnosis and management are reviewed by the same senior neurologist (PMR) in all patients. Major stroke is defined as National Institutes of Health Stroke Score (NIHSS) of 3 or greater, as this corresponds broadly to the inpatient versus outpatient population.23
Participants
The current study included consecutive patients recruited during two periods when the IQCODE was included in the OXVASC study protocol (April 1, 2002 to February 31, 2004; March 31, 2010 to March 31, 2017). All patients with a stroke (ischemic and haemorrhagic) or definite/probable TIA, as adjudicated by PMR, were included.
Baseline interview was done using a structured proforma which included: medical history, risk factors, family history, medication, functional, and cognitive assessment. Reasons for lack of interview or cognitive test were documented. When a patient could not be interviewed (e.g. because of aphasia, delirium or dementia), a relative or caregiver was interviewed instead. Brain and vascular imaging were done at baseline.
The validated 16-item short IQCODE in English was given to the patient during the baseline interview.10 The patient was instructed to ask someone they had known for at least ten years to complete the form, and post it back. Patients were free to choose any informant. Informants were asked to score cognitive function over the last ten years up to just before the index event. Each item was scored on a 5-item scale: ‘1: Much improved’, ‘2: A bit improved’, ‘3: Not much change’, ‘4: A bit worse’, ‘5: Much worse’. Informants or patients were called by a research nurse if the IQCODE was not returned within two weeks, or if they had very low scores or missing items. From 2010 onwards, the IQCODE form included questions about the relationship of the informant to the patient.
Pre-event dementia diagnosis was made as described previously1 (and see also Supplemental Methods, please see https://www.ahajournals.org/journal/str) using the following information blinded to the IQCODE assessment: i) baseline clinical assessment by study clinician; ii) any dementia diagnosis, and related consultations and investigations, where available, in the primary care record, with hand-searching of the entire record including individual consultations, clinic letters, and hospitalisation documentation.1 The diagnosis of pre-event dementia was made by a senior study physician with expertise in dementia (STP) using the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria.1 Dementia subtype diagnoses were recorded where available (e.g. in the primary care or hospital records or clinic letters).
Statistical analysis
Demographic and clinical characteristics were compared for patients with vs without IQCODE, using Chi-square or ANOVA as appropriate. We included IQCODEs with missing items and calculated mean item scores by dividing by the number of completed items.
Performance of individual IQCODE items was evaluated by calculating the difference in mean item scores of patients with versus without dementia and statistical significance was tested with ANOVA. Items were subsequently ranked in order of the differences in mean item scores between subjects with versus without dementia.
Validation of the IQCODE for pre-event dementia was done according to STARDdem criteria.24 Reliability was determined using the area under the receiver operator curve (AUC). Optimal cut-offs were calculated using the Youden-index.25 Sensitivity, specificity, positive and negative predictive values (ppv, npv) were determined. AUC, accuracy and mean item scores were stratified by age, event severity, and dementia subtype (Alzheimer’s vs Vascular) and subgroups were compared using ANOVA. We evaluated the differences between AUCs with z-tests.26 We also compared mean item scores and AUC between patients who had the IQCODE but who were untestable with a direct-to-patient cognitive test (abbreviated mental test score (AMTS), Mini-Mental-State-Examination (MMSE) and Montreal Cognitive Assessment (MoCA)) and patients who had both IQCODE and a direct-to-patient test.
We performed sensitivity analyses omitting IQCODEs with missing items and where mean item scores were <3.00 (indicating cognitive improvement over 10-years). To exclude any possibility of incorporation bias, i.e. that IQCODE had been used in the pre-event dementia diagnosis, we also performed analyses including only those patients with a dementia subtype diagnosis since all such patients had received their dementia diagnosis from a specialist other than STP. Statistical significance was set at 0.05 and 95% CIs were derived as +/-1.96 times the standard error of the estimate. All analyses were done in SPSS for Windows 25.0.
Results
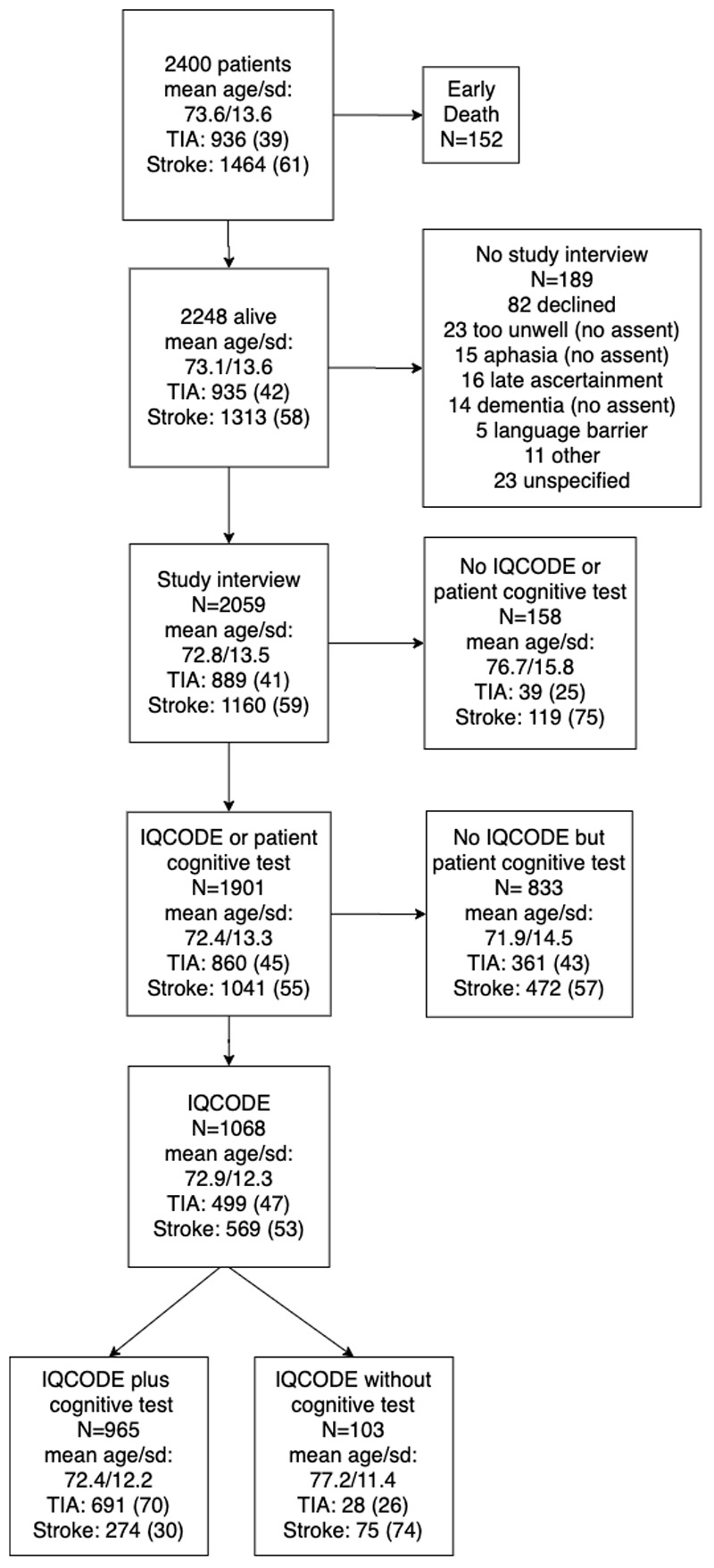
Of 2400 patients (mean age/SD=73.6/13.6 years, 1180 female) with a definite or probable TIA or stroke over the nine year study period, 2248 survived to ascertainment, of whom 2059 (92%) were interviewed and given an IQCODE questionnaire to pass on to a relative or carer to complete and return by post. Questionnaires were returned in 1068 (52%, Figure and Table 1). The mean/SD age of interviewed patients with IQCODE (72.9/12.3 years) was similar to that of the 991 interviewed patients without a returned IQCODE (72.6/14.8, p=0.70) but those without IQCODE had more stroke (591, 59.6% vs 569, 53.3%, p=0.004), major stroke (271, 27.0% vs 212, 19.9%, p<0.0001), previous stroke (141, 14.3% vs 70, 6.6%, p<0.0001), hospitalization for the event (508, 51.2% vs 367, 34.3%, p<0.0001) and low baseline cognitive score (313/831 vs 299/968, p<0.0001 of those who had a baseline cognitive test, supplementary table I, please see https://www.ahajournals.org/journal/str).
Table 1. Baseline characteristics of all patients with IQCODE stratified by whether they were untestable or testable using with a direct-to-patient cognitive test.
| All with IQCODE N=1068 |
Untestable with cognitive test* N=103 |
Testable with cognitive test* N=965 |
p | |
|---|---|---|---|---|
| Age | 72.9/12.2 | 77.2/11.4 | 72.4/12.2 | <0.0001 |
| Sex | 558 (52.2) | 52 (50.5) | 506 (52.4) | 0.71 |
| Education<12 yrs | 536 (50.2) | 22 (21.4) | 514 (53.3) | <0.0001 |
| TIA | 499 (46.7) | 30 (29.1) | 469 (48.6) | <0.0001 |
| Minor stroke | 364 (34.1) | 21 (20.4) | 343 (35.5) | |
| Major stroke | 205 (19.2) | 52 (50.5) | 153 (15.9) | |
| PICH | 28 (2.6) | 7 (6.8) | 21 (2.2) | <0.0001 |
| In-patient | 349 (32.7) | 75 (72.8) | 274 (28.4) | <0.0001 |
| Previous stroke | 74 (6.9) | 11 (10.7) | 63 (6.5) | 0.10 |
| Pre-event dementia | 68 (6.4) | 12 (11.7) | 56 (5.8) | 0.02 |
| IQCODE mean/SD | 3.23/0.55 | 3.27/0.77 | 3.23/0.52 | 0.48 |
| IQCODE >3.48 | 219 (20.5) | 29 (28.2) | 190 (19.7) | 0.04 |
| IQCODE >3.6 | 168 (15.7) | 22 (21.4) | 146 (15.1) | 0.10 |
Numbers are n (%) unless otherwise specified.
MMSE, MoCA or AMTS at baseline. IQCODE: Informant Questionnaire on Cognitive Decline in the Elderly. PICH: primary intracerebral haemorrhage. TIA: transient ischemic attack.
Of the 1068 completed IQCODEs, all 16 items were completed in 1025 (96%) and in the remaining 43, 30 (70%) had only one item missing. Most commonly left unanswered were items 14 -”handling financial matters” (n=12; 1.1%) and 1-”remembering things about family and friends” (n=11; 1.0%). The question about the nature of the informant was completed in 529 IQCODEs: 251 (65%) were spouses, 104 (27%) children, 20 friends (5%), 7 siblings (2%), 1 parent (0.2%) and 1 (0.2%) grandchild.
Mean/SD item score for all 1068 IQCODEs was 3.23/0.55 with median (IQR) 3.13 (3.00-3.38). The reference standard of a clinical pre-event dementia diagnosis was present in 68/1068 (6.4%) patients. Mean/SD item score in pre-event dementia was 4.34/0.59 versus 3.15/0.46 for no dementia (p<0.0001). AUC for IQCODE and pre-event dementia was 0.94 (0.90-0.97, p<0.001) with optimal cut-off >3.48 showing optimal trade-off between sensitivity and specificity (sensitivity=89.7%, 79.9-95.8%; specificity=84.2%, 81.8-86.4%, table 2). Results were similar by age (0.92, 0.88-0.96, <65 years; 0.94, 0.83-1.00, 65-74 years; 0.95, 0.92-0.99, 75-84 years; 0.89, 0.82-0.96, >84 years) and when analyses were restricted to patients with first ever stroke (AUC=0.92, 0.88-0.97, sensitivity=85.7%, 72.8-94.1%; specificity=84.8%, 82.4-87.0% for cut-off >3.48). Notably, the IQCODE had high negative predictive value with npv approaching 100 (table 2). In contrast, ppv was much lower reaching a high of 51.5 (44.0-58.8) for cut-offs of >4.
Table 2. IQCODE accuracy and 95% confidence intervals for pre-event dementia using different cut-offs for all patients, and stratified by TIA, minor and major (NIHSS>3) stroke.
| IQCODE accuracy for pre-event dementia | ||||||
|---|---|---|---|---|---|---|
| IQCODE Cut-off | Sensitivity | Specificity | PPV | NPV | False negative | False positive |
| All, N=1068 | ||||||
| >3.30 | 92.7 (83.7-97.6) | 71.8 (68.9-74.5) | 18.3 (16.6-20.1 | 99.3 (98.4-99.7) | 0.282 | 0.074 |
| >3.48 | 89.7 (79.9-95.8) | 84.2 (81.8-86.4) | 24.9 (24.7-31.3) | 99.2 (98.4-99.6) | 0.158 | 0.103 |
| >3.50 | 89.7 (79.9-95.8) | 84.2 (81.8-86.4) | 24.9 (24.7-31.3) | 99.2 (98.4-99.6) | 0.158 | 0.103 |
| >3.60 | 82.4 (71.2-90.5) | 88.8 (86.7-90.7) | 33.3 (28.9-38.1) | 98.7 (97.8-99.2) | 0.112 | 0.176 |
| >4.00 | 77.9 (66.2-87.1) | 95.0 (93.5-96.3) | 51.5 (44.0-58.8) | 98.5 (97.6-99.0) | 0.050 | 0.221 |
| TIA, N=499, optimal cut-off | ||||||
| >3.47 | 88.0 (68.8-97.5) | 86.1 (82.7-89.1) | 25.0 (20.3-30.3) | 99.3 (97.9-99.8) | 0.750 | 0.007 |
| Minor stroke, N=364, optimal cut-off | ||||||
| >3.47 | 94.1 (71.3-99.9) | 83.2 (78.8-87.0) | 21.6 (17.5-26.4) | 99.7 (97.7-100.0) | 0.784 | 0.003 |
| Major stroke, N=205, optimal cut-off | ||||||
| >3.85 | 84.6% (65.1-95.6) | 91.1% (85.9-94.8) | 57.9 (45.6-69.3) | 97.6 (94.3-99.0) | 0.421 | 0.024 |
TIA: transient ischemic attack. IQCODE: informant questionnaire on cognitive decline in the elderly. PPV: positive predictive value. NPV: negative predictive value.
Major stroke patients (n=205) had higher mean item scores than those with minor stroke (n=364) and TIA (n=499): mean/SD score=3.33/0.70 versus 3.19/0.51 and 3.22/0.50, p=0.01. Prevalence of pre-event dementia was 5.0% (25/499) in TIA, 4.7% (364/499) in minor stroke and 12.7% (26/205) in major stroke with AUCs of 0.92 (0.85-1.00, p<0.001), 0.95 (0.91-1.00, p<0.001) and 0.92 (0.87-0.97, p<0.001) respectively. Optimal cut-offs for identification of pre-event dementia were >3.47 for TIA and minor stroke and >3.85 for major stroke although differences were non-significant (table 2).
Among the 1068 patients with IQCODE, 103 were untestable (table 1, figure 1) with a direct-to-patient cognitive test (AMTS, MMSE or MoCA). Compared to the 965 testable patients, untestable patients were older (mean age/SD=77.2/11.4 vs 72.4/12.2 years, p<0.0001) with less TIA/minor stroke and more major stroke (p<0.0001), hospitalization (75, 72.8% vs 274, 28.4%, p<0.001), pre-event dementia (12, 11.7% vs 56, 5.8%, p=0.02), and IQCODEs scoring >3.48 (29, 28.2% vs 190,19.7%, p-0.04, table 1, figure 1). However, AUCs for pre-existing dementia were similar in testable and untestable patients: 0.93 (0.90-0.97) and 0.93 (0.87-1.00) respectively.
Figure 1. Flow chart showing the number and characteristics of all the TIA and stroke patients in the source population, and for all survivors, interviewed vs not interviewed, with vs without IQCODE, and with vs without direct-to-patient cognitive test (MMSE, MoCA, AMTS).
Mean item scores in incomplete IQCODEs were comparable to those of fully completed questionnaires (3.28 versus 3.23, p=0.52). Sensitivity analyses showed that test accuracy was unaffected by removal of IQCODEs with missing items: sensitivity= 88.9% and specificity=84.2% for cut-off of >3.48. The 117 patients with mean IQCODE score below 3.00 (indicating cognitive improvement over the last 10-years) were younger than those with mean scores ≥3.00: mean age/SD= 68.6/12.6 years versus 73.4/12.1 years (p<0.001). Excluding these 117 patients did not significantly change accuracy: sensitivity=91.0%, specificity=82.1% for cut-off of >3.48. Among 68 pre-event dementia cases, 40 had a prior diagnosed dementia subtype. To rule out possible effects of incorporation bias, we performed a further sensitivity analysis excluding dementia patients without a documented dementia subtype. Accuracy of the IQCODE did not significantly change: cut-off >3.48 had 92.5% sensitivity and 84.0% specificity.
All 16-IQCODE items differed significantly for patients with versus without pre-event dementia both overall and after stratification for event severity (all p<0.001, table 3, supplementary table II, please see https://www.ahajournals.org/journal/str). Items ‘14- finances’, ‘9-gadgets’, ‘15-arithmetic’ and ‘10-learning new things’ had the biggest mean differences between those with vs without pre-event dementia. The best discriminating item ‘14-handling financial matters’ alone had AUC of 0.89 (supplementary figure I, please see https://www.ahajournals.org/journal/str). When stratified by event severity, major stroke showed greatest differences between dementia vs no dementia in items that might be considered as requiring greater executive function (items 14, 9, 15, 10), whereas TIA and minor stroke showed a more mixed pattern (items 9, 15, 14, 10/2/3, supplementary table I, please see https://www.ahajournals.org/journal/str)
Table 3. Mean scores of each IQCODE item for pre-event dementia vs no dementia (p<0.0001 for all 16 items). Rank difference is rank based on mean difference from highest to lowest.
| Dementia Mean (95% CI) | No dementia Mean (95% CI) | Mean difference | Rank difference | |
|---|---|---|---|---|
| 1: Family | 4.24 (4.02-4.44) | 3.14 (3.10-3.18) | 1.10 | 13 |
| 2: Recent | 4.51 (4.37-4.66) | 3.28 (3.23-3.32) | 1.24 | 5 |
| 3: Conversation | 4.56 (4.41-4.71) | 3.33 (3.28-3.37) | 1.23 | 6 |
| 4: Address | 4.03 (3.82-4.24) | 3.00 (2.97-3.03) | 1.03 | 16 |
| 5: Day and month | 4.22 (4.03-4.41) | 3.07 (3.04-3.11) | 1.15 | 10 |
| 6: Things kept | 4.16 (3.97-4.36) | 3.14 (3.11-3.18) | 1.02 | 15 |
| 7: Different place | 4.56 (4.39-4.72) | 3.34 (3.30-3.38) | 1.22 | 7 |
| 8: Machines | 4.14 (3.94-4.33) | 3.04 (3.00-3.07) | 1.10 | 14 |
| 9: Gadget | 4.61 (4.45-4.76) | 3.25 (3.20-3.29) | 1.36 | 2 |
| 10: New things | 4.52 (4.33-4.70) | 3.26 (3.22-3.30) | 1.26 | 4 |
| 11: Story | 4.24 (4.04-4.43) | 3.11 (3.07-3.14) | 1.13 | 11 |
| 12: Decisions | 4.36 (4.17-4.55) | 3.17 (3.13-3.21) | 1.19 | 8 |
| 13: Money | 4.22 (4.02-4.43) | 3.03 (3.00-3.06) | 1.19 | 9 |
| 14: Finances | 4.48 (4.30-4.66) | 3.12 (3.08-3.16) | 1.36 | 1 |
| 15: Arithmetic | 4.44 (4.26-4.62) | 3.09 (3.05-3.12) | 1.35 | 3 |
| 16: Intelligence | 4.36 (4.17-4.55) | 3.22 (3.11-3.18) | 1.12 | 12 |
Among the 40 patients with known dementia subtypes, there were 17 (42.5%) with Alzheimer’s disease, 16 (40.0%) with vascular dementia, 3 (7.5%) with Lewy Body disease or Parkinson’s dementia, 2 (5.0%) with mixed dementia, and 2 (5.0%) with other. There were no significant differences in mean item scores across dementia subtypes (p=0.50). Although there were no significant differences in question performance between vascular and Alzheimer disease subgroups, Alzheimer patients were worse at remembering things about family and in doing arithmetic whereas vascular dementia patients were worse in handling money, dealing with finances, and making decisions (table 4).
Table 4. Mean item scores and 95% confidence intervals for pre-event dementia by diagnosed subtype (Alzheimer’s vs vascular dementia). Rank difference is rank based on mean difference from highest to lowest.
| Alzheimer’s dementia N=17 Mean (95% CI) |
Vascular dementia N=16 Mean (95% CI) |
p | Mean difference |
Rank difference |
|
|---|---|---|---|---|---|
| 1: Family | 4.44 (4.05-4.83) | 4.06 (3.61-4.52) | 0.19 | 0.38 | 1 |
| 2: Recent | 4.71 (4.40-5.01) | 4.50 (4.22-4.78) | 0.30 | 0.21 | 7 |
| 3: Conversation | 4.71 (4.40-5.01) | 4.69 (4.37-5.01) | 0.93 | 0.02 | 16 |
| 4: Address | 4.12 (3.68-4.56) | 4.00 (3.56-4.44) | 0.69 | 0.12 | 13 |
| 5: Day and month | 4.41 (4.05-4.78) | 4.25 (3.89-4.61) | 0.51 | 0.16 | 10 |
| 6: Things kept | 4.31 (3.85-4.78) | 4.13 (3.70-4.55) | 0.53 | 0.18 | 9 |
| 7: Different place | 4.76 (4.48-5.05) | 4.56 (4.23-4.90) | 0.34 | 0.20 | 8 |
| 8: Machines | 4.31 (3.89-4.74) | 4.19 (3.79-4.59) | 0.65 | 0.12 | 13 |
| 9: Gadget | 4.63 (4.24-5.01) | 4.69 (4.37-5.01) | 0.79 | 0.06 | 15 |
| 10: New things | 4.47 (4.06-4.88) | 4.69 (4.37-5.01) | 0.37 | 0.22 | 6 |
| 11: Story | 4.29 (3.86-4.73) | 4.44 (4.10-4.77) | 0.59 | 0.15 | 11 |
| 12: Decisions | 4.38 (3.95-4.80) | 4.63 (4.36-4.89) | 0.30 | 0.25 | 4 |
| 13: Money | 4.13 (3.65-4.60) | 4.50 (4.11-4.89) | 0.20 | 0.37 | 2 |
| 14: Finances | 4.44 (4.00-4.87) | 4.69 (4.37-5.01) | 0.33 | 0.25 | 4 |
| 15: Arithmetic | 4.71 (4.40-5.01) | 4.44 (4.16-4.71) | 0.25 | 0.27 | 3 |
| 16: Intelligence | 4.59 (4.22-4.95) | 4.44 (4.16-4.71) | 0.49 | 0.15 | 11 |
Discussion
The IQCODE had excellent accuracy for identifying pre-event dementia with an overall optimal cut-off of >3.48. Only 3% returned IQCODES were incomplete, and of these, two thirds had only one item missing. Missing items did not affect test accuracy. Patients with IQCODE who were untestable with a cognitive test were older and had more dementia suggesting that the IQCODE provided cognitive information in otherwise untestable patients and therefore mitigates selection bias. The pattern of deterioration across individual items suggested executive-type deficits especially in those with major stroke, in keeping with dementia with a vascular component.
To our knowledge this is the first study to validate the IQCODE against a reference standard of prior clinical diagnosis of pre-TIA/stroke dementia. The original IQCODE validation found an optimal cut-off >4.0 with sensitivity of 92.7% and specificity of 88.1% but compared moderate/severely affected Alzheimer patients with community volunteers and was at risk of bias.9 The cut-off of >4.0 has subsequently been used to exclude pre-existing dementia in stroke studies4,5,16–20 but our findings suggest that this will have resulted in some cases of dementia being included. The higher optimal cut-off we observed for major stroke than for TIA or minor stroke may be the result of more advanced/ more severe dementia in those presenting with major stroke.1 Just over a tenth of IQCODEs had a mean item score <3.00, indicating cognitive improvement. Studies in non-stroke populations rarely found low scores since aging is usually associated with stable or declining cognition.27 Mistakenly scoring early recovery after stroke as improvement might explain these low scores. Patients with low scores were younger and young patients are more likely to improve after stroke.28
IQCODE items about finances, money, arithmetic problems and learning new things discriminated best between TIA/stroke patients with and without pre-existing dementia but in previous non-vascular validation studies, memory items were also important.9,10 There have been few studies on IQCODE-item performance across different dementia subtypes. One study reported that executive items showed differences between fronto-temporal dementia and Alzheimer’s disease.29 However, none of the 16 items differentiated between Alzheimer’s disease and vascular dementia subtypes in our study, possibly because of small numbers although there were some qualitative differences: Alzheimer’s disease patients showed the biggest mean score difference in items associated with memory whereas vascular dementia patients scored worst on items requiring executive function.
Informants were most often spouses (60-65%) or children (27-30%) in keeping with the few studies that have reported informant details.30–32 The IQCODE was completed and returned in just over half of interviewed patients which is comparable to previous studies: lack of suitable informants may affect 12-40% in-patients.31–33 Since poor social interaction is associated with higher dementia incidence, this group might be at high risk resulting in a lower measured dementia rate in those with IQCODE vs the population as a whole.34
Overall, the IQCODE would appear a valid tool for use in stroke research to exclude patients with pre-stroke dementia in studies of the prevalence and predictors of post-stroke dementia. The IQCODE may also be useful in clinical practice to guide rehabilitation strategies: for example, patients with pre-existing dementia may not benefit from cognitive rehabilitation and could be identified using an IQCODE score favouring specificity over sensitivity to ensure that patients without dementia were not excluded inappropriately. The IQCODE may also provide clinically useful prognostic information: patients with pre-stroke dementia may be more likely to have a substantial Alzheimer’s disease component and therefore ongoing post-stroke decline than those with post-stroke dementia and normal IQCODE who may show a more fixed deficit pattern with slower progression. Similarly, patients with abnormal IQCODE not reaching the threshold for dementia are at greater risk of future dementia after stroke as shown in previous studies5,6,35 although it remains unclear whether this is independent of baseline cognitive test score. In terms of the implications for clinical practice, the IQCODE findings may help in counselling patients and carers regarding the cognitive prognosis, likelihood of recovery and response to rehabilitation. In addition, studies in Alzheimer’s disease suggest that cognitive decline may be slowed by treatment of vascular risk factors and the acute TIA/stroke event provides an opportunity to properly address these through robust secondary prevention measures to reduce both the risk of future cerebrovascular events and dementia.36
Strengths of our study include the large sample, population-based design, and limited exclusion criteria. We also performed a number of sensitivity analyses including for the effects of possible incorporation bias. Limitations include the fact that, despite the robust study design, the IQCODE was completed and returned less often in in-patients with more severe stroke and previous stroke in whom rates of pre-event dementia are higher, resulting in a lower rate of pre-event dementia (6.4%) in those with IQCODE than in the cohort overall (9.8%).1 We did not specifically examine feasibility, cost and acceptability in our study, but available data from elsewhere suggest that these are favorable.12 In addition, in keeping with previous studies, we analysed the IQCODE as a nominal mean item score which assumes that the difference between each item is equal, when in fact it is an ordinal 5-point scale.10–12 We did not screen informants for cognitive problems or depression, anxiety, stress or carer burden11,31 or stipulate any requirement regarding the amount of time informants should spend in the company of the patient.33 Finally, informant characteristics were only available for later subjects in our study.
In conclusion, the IQCODE has high accuracy for identifying pre-TIA/stroke dementia, with optimal cut-off >3.48. However, the cut-off used in practice will vary according to whether greater sensitivity or specificity is preferred, and whether major stroke versus minor stroke or TIA is being assessed. Further studies are required to examine the patterns of item performance in different dementia subtypes, the impact of informant characteristics, and the predictive value of the IQCODE for post-stroke cognitive decline.
Supplementary Material
Acknowledgments
STP is supported by the NIHR Oxford Biomedical Research Centre (BRC). PMR is supported by the NIHR Oxford BRC and the Wellcome Trust.
Sources of Funding
Wellcome Trust, Wolfson Foundation, British Heart Foundation, National Institute for Health Research (NIHR), and NIHR Oxford Biomedical Research Centre.
Footnotes
Disclosures
None.
Data availability
Any requests for data will be considered by PMR (peter.rothwell@ndcn.ox.ac.uk).
References
- 1.Pendlebury ST, Rothwell PM. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford Vascular Study. Lancet Neurol. 2019;18:248–258. doi: 10.1016/S1474-4422(18)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rostamian S, Mahinrad S, Stijnen T, Sabayan B, de Craen AJ. Cognitive impairment and risk of stroke: a systematic review and meta-analysis of prospective cohort studies. Stroke. 2014 May;45(5):1342–8. doi: 10.1161/STROKEAHA.114.004658. [DOI] [PubMed] [Google Scholar]
- 3.Hénon H, Durieu I, Lebert F, Pasquier F, Leys D. Influence of prestroke dementia on early and delayed mortality in stroke patients. J Neurol. 2003;250:10–6. doi: 10.1007/s00415-003-0917-3. [DOI] [PubMed] [Google Scholar]
- 4.Barba R, Morin M, del M, Cemillán C, Delgado C, Domingo J, Del Ser T. Previous and incident dementia as risk factors for mortality in stroke patients. Stroke. 2002;33:1993–1998. doi: 10.1161/01.str.0000017285.73172.91. [DOI] [PubMed] [Google Scholar]
- 5.Hénon H, Durieu I, Guerouaou D, Lebert F, Pasquier F, Leys D. Poststroke dementia: Incidence and relationship to prestroke cognitive decline. Neurology. 2001;57:1216–1222. doi: 10.1212/wnl.57.7.1216. [DOI] [PubMed] [Google Scholar]
- 6.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–18. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 7.Pendlebury ST, Klaus SP, Mather M, de Brito M, Wharton RM. Routine cognitive screening in older patients admitted to acute medicine: abbreviated mental test score (AMTS) and subjective memory complaint versus Montreal Cognitive Assessment and IQCODE. Age Ageing. 2015;44:1000–5. doi: 10.1093/ageing/afv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampson EL, Blanchard MR, Jones L, Tookman A, King M. Dementia in the acute hospital: prospective cohort study of prevalence and mortality. Br J Psychiatry. 2009;195:61–6. doi: 10.1192/bjp.bp.108.055335. [DOI] [PubMed] [Google Scholar]
- 9.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 10.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 11.Jorm AF. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): a review. Int Psychogeriatrics. 2004;16:275–293. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- 12.Harrison JK, Fearon P, Noel-Storr AH, McShane R, Stott DJ, Quinn TJ. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the diagnosis of dementia within a secondary care setting (Review) Cochrane Database Syst Rev Rev. 2014 doi: 10.1002/14651858.CD010772.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, Miller JP, Storandt M, Morris JC. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559–64. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 14.Brodaty H, Pond D, Kemp NM, Luscombe G, Harding L, Berman K, Huppert FA. The GPCOG: a new screening test for dementia designed for general practice. J Am Geriatr Soc. 2002;50:530–4. doi: 10.1046/j.1532-5415.2002.50122.x. [DOI] [PubMed] [Google Scholar]
- 15.McGovern A, Pendlebury ST, Mishra NK, Fan Y, Quinn TJ. Test Accuracy of Informant-Based Cognitive Screening Tests for Diagnosis of Dementia and Multidomain Cognitive Impairment in Stroke. Stroke. 2016;47:329–335. doi: 10.1161/STROKEAHA.115.011218. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee G, Wilson D, Ambler G, Appiah KOB, Shakeshaft C, Lunawat S, Cohen H, Yousry T, Habil M, Lip GYH, et al. Cognitive impairment before intracerebral hemorrhage is associated with cerebral amyloid angiopathy. Stroke. 2018;49:40–45. doi: 10.1161/STROKEAHA.117.019409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klimkowicz A, Dziedzic T, Polczyk R, Pera J, Słowik A, Szczudlik A. Factors associated with pre-stroke dementia: The Cracow stroke database. J Neurol. 2004;251:599–603. doi: 10.1007/s00415-004-0384-5. [DOI] [PubMed] [Google Scholar]
- 18.Laible M, Horstmann S, Möhlenbruch M, Schueler S, Rizos T, Veltkamp R. Preexisting cognitive impairment in intracerebral hemorrhage. Acta Neurol Scand. 2017;135:628–634. doi: 10.1111/ane.12646. [DOI] [PubMed] [Google Scholar]
- 19.Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz CL, Looi JCL, Berman K, Ross A, Wen W, Zagami AS. Clinical determinants of dementia and mild cognitive impairment following ischaemic stroke: The Sydney stroke study. Dement Geriatr Cogn Disord. 2006;21:275–283. doi: 10.1159/000091434. [DOI] [PubMed] [Google Scholar]
- 20.Lefebvre C, Deplanque D, Touzé E, Hénon H, Parnetti L, Pasquier F, Gallai V, Leys D. Prestroke dementia in patients with atrial fibrillation: Frequency and associated factors. J Neurol. 2005;252:1504–1509. doi: 10.1007/s00415-005-0900-2. [DOI] [PubMed] [Google Scholar]
- 21.Coull AJ, Silver LE, Bull LM, Giles MF, Rothwell PM. Direct assessment of completeness of ascertainment in a stroke incidence study. Stroke. 2004;35:2041–2045. doi: 10.1161/01.STR.0000137605.48864.2f. [DOI] [PubMed] [Google Scholar]
- 22.Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JNE, Bull LM, Welch SJV, Cuthbertson FC, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 23.Paul NLM, Koton S, Simoni M, Geraghty OC, Luengo-Fernandez R, Rothwell PM. Feasibility, safety and cost of outpatient management of acute minor ischaemic stroke: A population-based study. J Neurol Neurosurg Psychiatry. 2013;84:356–361. doi: 10.1136/jnnp-2012-303585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noel-Storr AH, McCleery JM, Richard E, Ritchie CW, Flicker L, Cullum SJ, Davis D, Quinn TJ, Hyde C, Rutjes AWS, et al. Reporting standards for studies of diagnostic test accuracy in dementia: The STARDdem Initiative. Neurology. 2014;83:364–373. doi: 10.1212/WNL.0000000000000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Xhou X-H, Obuchowski NA, McClish DK. Statistical Methods in Diagnostic Medicine. Second edition. Wiley; 2011. [Google Scholar]
- 27.Reichenheim M, Dos Santos Sanchez MA, Lourenço RA. Re-assessing the dimensional structure of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Empirical evidence for a shortened Brazilian version Neurology, stroke and cognition. BMC Geriatr. 2015;15:1–11. doi: 10.1186/s12877-015-0098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knoflach M, Matosevic B, Rücker M, Furtner M, Mair A, Wille G, Zangerle A, Werner P, Ferrari J, Schmidauer C, et al. Functional recovery after ischemic stroke - A matter of age: Data from the Austrian Stroke Unit Registry. Neurology. 2012;78:279–285. doi: 10.1212/WNL.0b013e31824367ab. [DOI] [PubMed] [Google Scholar]
- 29.Larner A. Can IQCODE differentiate Alzheimer’s disease and frontotemporal dementia? Age Ageing. 2010;39:389–392. doi: 10.1093/ageing/afq014. [DOI] [PubMed] [Google Scholar]
- 30.Tang WK, Chan SS, Chiu HF, Wong KS, Kwok TC, Mok V, Ungvari GS. Can IQCODE detect poststroke dementia? Int J Geriatr Psychiatry. 2003;18:706–10. doi: 10.1002/gps.908. [DOI] [PubMed] [Google Scholar]
- 31.Harald A, Nygaard Naik M, Geitung JT. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) is associated with informant stress. Int J Geriatr Psychiatry. 2009;24:1185–1191. doi: 10.1002/gps.2243. [DOI] [PubMed] [Google Scholar]
- 32.Bloomfield K, John N. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) completion on an acute care ward for the elderly: A brief study of informant characteristics. Int Psychogeriatrics. 2012;24:1700–1701. doi: 10.1017/S1041610212000415. [DOI] [PubMed] [Google Scholar]
- 33.Harwood DMJ, Hope T, Jacoby R. Cognitive impairment in medical inpatients. Age Ageing. 1997;26:37–39. doi: 10.1093/ageing/26.1.37. [DOI] [PubMed] [Google Scholar]
- 34.Kuiper JS, Zuidersma M, Oude Voshaar RC, Zuidema SU, van den Heuvel ER, Stolk RP, Smidt N. Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2015;22:39–57. doi: 10.1016/j.arr.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Leys D, Hénon H, Mackowiak-Cordoliani M-A, Pasquier F. Poststoke dementia. Lancet Neurol. 2005;4:752–759. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- 36.Deschaintre Y, Richard F, Leys D, Pasquier F. Treatment of vascular risk factors is associated with slower decline in Alzheimer disease. Neurology. 2009;73:674–80. doi: 10.1212/WNL.0b013e3181b59bf3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any requests for data will be considered by PMR (peter.rothwell@ndcn.ox.ac.uk).