Abstract
Mating and immunity are two major components of fitness and links between them have been demonstrated in a number of recent investigations. In Drosophila melanogaster, a seminal fluid protein, sex-peptide (SP), up-regulates a number of antimicrobial peptide (AMP) genes in females after mating but the resulting effect on pathogen resistance is unclear. In this study we tested 1) whether SP-induced changes in gene expression affect the ability of females to kill injected non-pathogenic bacteria and 2) how the injection process per se affects the expression of AMP genes relative to SP. The ability of virgin females and females mated to SP lacking or control males to clear bacteria was assayed using an established technique in which E. coli are injected directly into the fly body and the rate of clearance of the injected bacteria is determined. We found no repeatable differences in clearance rates between virgin females and females mated to SP producing or SP lacking males. However, we found that the piercing of the integument, as occurs during injection, up-regulates AMP gene expression much more strongly than SP. Thus, assays that involve piercing, which are commonly used in immunity studies, can mask more subtle and biologically relevant changes in immunity, such as those induced by mating.
Keywords: mating, immunity, sex-peptide, injection, antimicrobial
1. Introduction
Immunity and reproduction are important components of fitness and an increasing number of studies report interplay between the two processes (reviewed in Lawniczak et al., 2007). In some species mating and other reproductive processes appear to suppress aspects of immunity, thus broadly supporting a resource trade-off model (Sheldon & Verhulst, 1996). For example, in the flour beetle, Tenebrio molitor, mating suppresses an immune effector system (phenoloxidase) in both sexes (Rolff & Siva-Jothy, 2002), potentially reducing pathogen resistance. Reductions in measures of immunity resulting from mating or reproductive activity have also been detected in female damselflies, Matrona basilaris japonica (Siva-Jothy et al., 1998), female ground crickets, Allonemobious socius (Fedorka et al., 2004; Fedorka & Zuk, 2005) female pea aphids, Acyrthosiphon pisum (Gwynn et al., 2005), female ants, Atta colombica (Baer et al., 2006) and male Drosophila melanogaster (McKean & Nunney, 2001).
However, in some other species mating apparently increases aspects of immunity. For example, in females of the cricket Gryllus texensis, mating increases pathogen resistance (Shoemaker et al., 2006) and in Drosophila melanogaster a number of immune genes, particularly antimicrobial peptides (AMPs) are up-regulated for several hours after mating (Lawniczak & Begun, 2004; McGraw et al., 2004; Peng et al., 2005; Domanitskaya et al., 2007). This up-regulation of immune genes results from the actions of male accessory gland proteins (Acps) (McGraw et al., 2004) which are transferred to females in seminal fluid. One Acp in particular (Acp70A, the sex-peptide, SP), up-regulates several AMPs (Peng et al., 2005; Domanitskaya et al., 2007). However, increases in immune gene expression or other proxy measures of immunity do not necessarily result in an increase in pathogen resistance (Adamo, 2004a; Adamo, 2004b). McKean & Nunney (2005) found that D. melanogaster females showed no difference in their ability to clear injected non-pathogenic bacteria whether they were maintained with males or in single sex groups as virgins. Unexpectedly, Fedorka et al. (2007) found that, when AMPs were up-regulated in mated females (3 hrs post-mating) resistance to an injected pathogenic bacterium was lower than that of virgin females. Moreover, at 27 hrs post-mating, when several AMPs were down-regulated in mated females, pathogen resistance was similar to that of virgin females. Fedorka et al.’s (2007) study shows that there can be a disparity between proxy measures of immunity, such as gene expression, and the real ability of animals to fight infection.
There is currently no general pattern in the effects of mating and reproductive effort upon immunity in insects (Lawniczak et al., 2007). One potential reason for this is that a range of different techniques have been employed to measure aspects of immunity in insects: some are proxy measures and others are direct measures of pathogen resistance. Furthermore, several of these techniques involving piercing the integument to inject pathogens, non-pathogenic bacteria or foreign objects into the body (e.g. Siva-Jothy et al., 1998; McKean & Nunney, 2001; McKean & Nunney, 2005; Baer et al., 2006; Fedorka et al., 2007). However, in Drosophila it is not known how piercing the integument per se affects the expression of AMP genes or how any changes compare to those induced by mating. In this study we addressed this issue. Firstly we investigated whether SP-induced up-regulation of immune genes affects the ability of female D. melanogaster to kill injected bacteria. We used the immunity assay developed by McKean and Nunney, in which non-pathogenic bacteria are injected into females and the remaining live bacteria are retrieved after several days (McKean & Nunney, 2001; McKean & Nunney, 2005). We compared females that were virgin, mated to wild-type males or mated to SP knockdown males (which produce no detectable SP). Secondly, to examine whether the injection process per se affects female immunity and how any changes compare to those induced by SP we measured the expression of two AMP genes in females that were either virgin and pierced (with nothing injected), virgin and injected with Ringers solution, virgin and injected with synthetic SP solution, virgin and not pierced, mated to SP lacking males and not pierced, or mated to SP producing (control) males and not pierced.
2. Materials and Methods
2.1. Fly stocks and husbandry
All cultures were maintained at 25°C on a 12:12 h light: dark cycle. Flies for bacterial clearance assays were maintained on sugar-yeast food and flies for gene expression assays were maintained on cornmeal-yeast-agar food. Wild type stocks used were Dahomey, for bacterial clearance assays, and Oregon-R for gene expression assays. SP knockdown males were obtained by RNA interference as previously described (Chapman et al., 2003). These consist of two replicate, genetically matched, knockdown and control lines whereby SP1 knockdown is matched with control 1 and SP2 knockdown is matched with control 2 (Wigby & Chapman, 2005). SP0 and control (SP+) males were as described in (Liu & Kubli, 2003). SP0 males contain a mutant non-functional SP allele in place of the wild-type SP gene and produce no SP. SP+ control males contain both the mutant and wild-type genes and produce normal levels of SP (Liu & Kubli, 2003).
2.2. Injections and piercings
All injections and piercings were performed using pulled glass needles with the flies under ice or CO2 anaesthesia. Control flies (not pierced or injected) were anaesthetised in the same way to control for fly handling.
2.3. Bacterial clearance assay
The bacterial clearance assay was based on that used by McKean and Nunney (2001) with minor modifications. On the evening before the bacteria were injected, E. coli D21 (which is resistant to both ampicillin and streptomycin) were grown overnight in LB solution. The following morning the resulting population was centrifuged and re-suspended in Drosophila Ringers solution. The suspension was diluted and the cell concentration determined using a Helber counter. The suspension was diluted further to a concentration of ≈ 13 × 109 cells/ml. 74nL of the solution was injected into flies which equates to ≈ 106 cells per fly. Flies were injected in the thorax. Three days after injection the flies were assayed for the number of surviving E. coli D21. Individual flies were CO2 anaesthetized, placed in an Eppendorf and homogenised in 200 μL Ringers solution. The solution was diluted × 75 and 300μL of the resulting solution was spread on LB agar plates containing 50μg/ml streptomycin. The plates were stored overnight at 37°C and the number of colonies were counted manually.
2.4. The ability of virgin females and females mated to control or SP knockdown males to clear bacteria
To test the ability of females to clear bacteria after mating, three experiments were performed. For all bacterial clearance assays, wild-type Dahomey females were reared at standard density (Clancy & Kennington, 2001), collected as virgins within eight hours of eclosion using ice anaesthesia and housed for 4-5 days in groups of 10. Flies were maintained in vials with sugar-yeast food and added live yeast grains. In the first experiment the females were either kept as virgins or mated to wild-type Dahomey males which were derived from the same culture bottles as the females. For the mating treatment one female was aspirated, without anaesthesia, into a vial that already contained 2 males. Females were allowed to mate once and any pairs that mated for less than 10 minutes were discarded. Females that mated once for more than 10 minutes were aspirated into fresh vials in groups of 10. Mated and virgin females were randomly allocated to one of 2 treatments. One set of flies was injected with bacteria 4 hrs after the matings and the other set of flies was injected 24 hrs after the matings. At both time points a further 10 virgin females were injected with Ringers solution to act as negative controls. After the injections, females were housed, 10 per vial, in fresh vials. Each day after injections, females were transferred to fresh vials. Three days after injections, individual females were assayed for the number of living E. coli D21 remaining in them.
The second and third experiments were identical to the first except that females were mated to SP knockdown or control males and all injections were performed at 4 hrs post mating (there was no 24 hr treatment). In the second experiment all flies were assayed simultaneously whilst in the third experiment the two replicate knockdown lines were assayed at different times and hence there were two sets of virgin controls. SP knockdown in the males was confirmed by performing Western Blots.
2.5. Statistical analysis
To test for differences between treatments in the ability of females to clear bacteria, colony count data was compared between treatments using Kruskal-Wallis tests. Analyses were carried out using JMP 5.1.2 statistical software (SAS Institute Inc.).
2.6. Quantitative real-time PCR
Total RNA was prepared using Trizol, followed by DNase treatment to control for amplification of background genomic DNA in the RNA samples (Ambion, DNA-free). Total RNA was quantified with a spectrophotometer (NanoDrop® ND-1000 UV-Vis). 1 μg total RNA was used for cDNA synthesis using the Qiagen reverse transcription system (Qiagen, Cat. No. 205111). Reactions without reverse transcriptase were used to control for amplification of background genomic DNA in the RNA samples. Each QRT-PCR was performed using SYBR Green PCR Core Reagents (Applied Biosystems). Rpl32 (60S ribosomal protein L32), tubulin and actin were used as reference control genes. The QRT-PCR data were analyzed using the comparative CT method (Livak & Schmittgen, 2001). Briefly, the relative difference in cycle times, ΔCT, measured during the exponential phase of the reactions was standardised to the reference control genes (Rpl32, tubulin or actin). ΔΔCT was obtained by finding the difference between treatments. The fold change was calculated as FC=2-ΔΔCT. We took measurements from 3 replicate QRT-PCRs on each extraction to determine the variability in ΔΔCT arising from the methods we used. Confidence intervals were calculated and converted to the fold-change scale.
2.7. The effects of mating, sex-peptide and piercing the integument on antimicrobial peptide gene expression
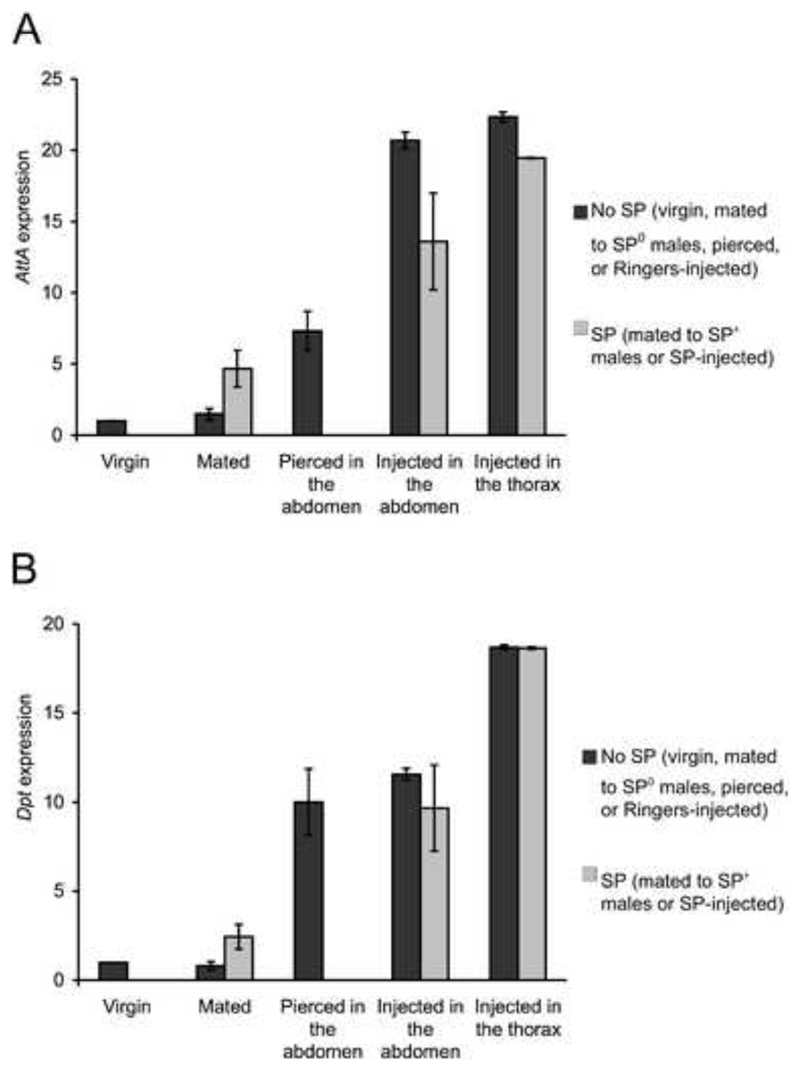
To test the relative effects of mating and piercing of the integument on immune gene expression in females QRT-PCRs were performed for Attacin-A and Diptericin. These AMPs show SP dependent expression in mated females (Fig 2; Peng et al., 2005; Domanitskaya et al., 2007). Wild-type Oregon-R females were collected as virgins within 5 hours of eclosion on ice anaesthesia. Five day-old females were assayed either as virgins or after mating to SP0 or control (SP+) males (Liu & Kubli, 2003). Virgin females were allocated to one of 6 treatments: 1) pierced in the abdomen (with nothing injected), 2) injected in the abdomen with 50nL Drosophila Ringers solution, 3) injected in the abdomen with 50nL synthetic SP (3pmol) dissolved in Drosophila Ringers solution, 4) injected in the thorax with 50nL Drosophila Ringers solution, 5) injected in the thorax with 50nL synthetic SP (3pmol) dissolved in Drosophila Ringers solution or 6) not pierced or injected. Synthetic SP was prepared as described in Schmidt et al. (1993). QRT-PCRs were performed on RNA extracted from the abdomens of females 4 hours after the injections, piercings or matings. RNA was pooled for 10-20 flies per treatment.
Fig. 2.
Expression (mean ± standard deviations of replicate QRT-PCRs) of A) AttA and B) Dpt in the abdomen of females. The values shown are the fold-change relative to virgin females (virgin value=1).
3. Results
3.1. The ability of virgin females and females mated to control or SP knockdown males to clear bacteria
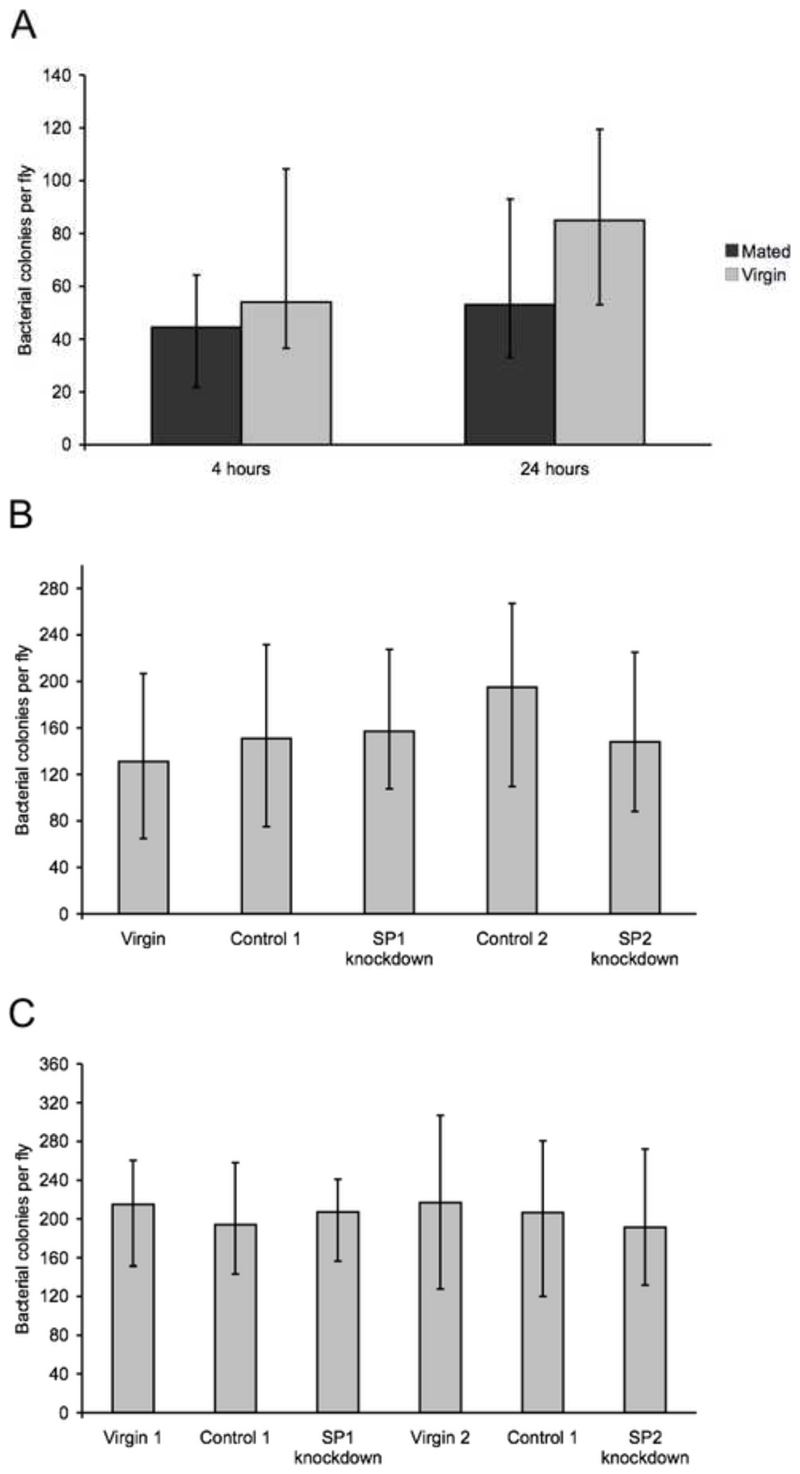
In all bacterial clearance assays control females injected with Ringers solution produced bacterial counts of 0, showing that there was no contamination from non-injected bacteria. In the first experiment, mated females injected at 4 hrs and at 24 hrs had significantly lower bacterial counts than virgin females (4 hrs, χ2 1 = 4.27, P = 0.039, 24 hrs, χ2 1 = 7.10 P = 0.008, Fig. 1a). However, in the second and third experiments, we found no significant differences in colony counts between virgin females, females mated to SP knockdown or females mated to control males in either knockdown line (experiment 2, Line 1, χ2 2 = 1.16, P = 0.56, Line 2, χ2 2 = 3.70, P = 0.158, Fig. 1b; experiment 3, Line 1, χ2 2 = 0.82, P = 0.665, Line 2, χ2 2 = 1.48, P = 0.478, Fig. 1c).
Fig. 1.
The level of E. coli infection in female flies 3 days after infection. Median (± inter-quartile range) bacterial colony counts per fly. Sample sizes were A) N = 38 and 41 for mated females and virgin controls injected at 4 hours post-mating, N = 47 and 48 for mated females and virgin females injected 24 hours post-mating; B) N = 44, 49, 43, 45 and 44 for virgin females, females mated to SP1 knockdown males, females mated to SP2 knockdown males, females mated to control 1 males and females mated to control 2 males; C) N = 53, 57, 57, 56, 60 and 57 for virgin 1 females, females mated to SP1 knockdown males, females mated to control 1 males, virgin 2 females, females mated to SP2 knockdown males and females mated to control 2 males.
3.2. The effects of mating, sex-peptide and piercing the integument on antimicrobial peptide gene expression
The expression data show, as expected, that mating with SP producing males up-regulated AMP gene expression in females (mean fold-change for AttA = 4.67 and for Dpt = 2.43, Fig 2) and that mating to SP0 males failed to produce this up-regulation (mean fold-change for AttA = 1.48 and for Dpt = 0.80, Fig 2). However, injection or piercing of the integument, either in the abdomen and in the thorax, up-regulated AttA and Dpt considerably more than mating and the presence of SP did not further increase this gene expression (mean fold change for females pierced in the abdomen, AttA = 7.31 and Dpt = 10.00, for females injected in the abdomen with Ringers solution, AttA = 20.69 and Dpt = 11.55, for females injected in the abdomen with SP AttA = 13.60 and Dpt = 9.66, for females injected in the thorax with Ringers solution, AttA = 22.34 and Dpt = 18.69 and for female injected in the thorax with SP, AttA = 19.46 and Dpt = 18.63, Fig 2). In females injected in the abdomen there was a trend for lower AMP expression when SP was injected compared to when Ringers alone was injected, in contrast to the effect seen when SP was delivered by mating.
4. Discussion
The results of the first part of this study show that a single mating, and specifically the receipt of SP from that mating, has no repeatable effect on the ability of females to clear injected E. coli. This is consistent with the findings of McKean and Nunney (2005) who found that females maintained with males (who were therefore likely to have mated at least once) do not clear bacteria at a different rate from virgin females. It is not clear why we found differences between virgin and mated females in the first experiment but not in subsequent experiments. One possibility is that we used males of different genotypes in experiment 1 (wild-type) vs experiments 2 and 3 (SP knockdown and controls). However, the control males used in experiments 2 and 3 are effective at inducing post mating responses (Chapman et al., 2003) so there is no reason to expect these males to be ineffective at inducing changes in immunity in females. It is clear that the effects seen in experiment 1 were not repeatable and are therefore unlikely to be of major biological importance.
The second part of our study highlights a potential caveat with immunity assays that involve piercing the integument. We found that the effect of mating, specifically of SP, on the expression of 2 AMP genes, was dwarfed by the effect of piercing with a needle. It was not possible to detect, using SP injection, the up-regulation of AttA and Dpt that occurs when SP is delivered via the natural method of mating (Peng et al., 2005 Domanitskaya et al., 2007; Fig. 2). Instead there was a trend for lower AMP gene expression in females injected in the abdomen with SP solution compared to females injected with Ringers only. Injection of SP has been shown to successfully stimulate 2 of the other major postmating responses: non-receptivity to mating and an increase in egg laying (Chen et al., 1988). Injected SP must therefore reach at least some of its natural targets. Instead, our results suggest that assays that involve piercing the integument of insects may be a poor method for examining subtle immune traits because of the potentially large effect of the piercing on immunity. Thus, we can not exclude the possibility that the lack of repeatable differences in the ability to clear bacteria between virgin and mated females in this study and in McKean and Nunney (2005) might be a result of any effects being masked by the effect of piercing on immunity.
We can also not exclude the possibility that the effects of piercing on AMP gene expression or the effects of mating on bacterial clearance might differ between fly stocks. We used Oregon-R females in the AMP gene expression assays but bacterial clearance experiments have been performed on females from the Dahomey stock (this study) and a stock from California (McKean & Nunney, 2005). It is therefore important that future studies examine the relationship between gene expression and phenotypic immunity, using the same flies and in the same experiment. It will also be important to connect gene expression and pathogen resistance to the levels of AMPs circulating in the haemolymph. In Drosophila, the upregulation of AMP genes are typically measured over the course of few hours to 1 day following mating or immune challenge but measures of pathogen resistance are taken days later. Levy et al. (Levy et al., 2004) found that the molecules induced by bacterial challenges show peak concentrations at 6 and 24 hrs post insult and most are at decreased concentrations by 2 days. A challenge for future research will be to determine the temporal relationship between changes in gene expression, AMP concentration and pathogen resistance.
Mating or reproduction induced changes in immunity have been detected using assays involving piercing the integument (e.g. Siva-Jothy et al., 1998; McKean & Nunney, 2001; McKean & Nunney, 2005; Baer et al., 2006; Fedorka et al., 2007) which clearly shows that such assays are not without value. McKean and Nunney (2001, 2005) detected changes in the bacterial clearance abilities of D. melanogaster males in response to sexual behaviour using the assay that we replicated in this study. Changes in male immunity in response to continued mating and reproductive behaviour may therefore be much larger than potential changes in female immunity after a single mating, and are thus not masked by piercing effects. Fedorka et al (2007) detected changes in female immunity after mating using an assay in which pathogenic bacteria were placed directly in the thorax by piercing the integument and measuring female survival times. It is not clear why the method used by Fedorka et al (2007) was able to detect mating induced immunity changes in females whereas the bacterial clearance assay used by McKean and Nunney (2005) and this study failed to. Fedorka et al’s (2007) assay is more immunologically challenging to flies (it results in death) than the injection of non-pathogenic bacteria used here and in McKean and Nunney (2001, 2005). It is possible that this difference might account for the contrast in results if stronger immune challenges are more effective at uncovering small differences in immunity. More generally, the use of non-pathogenic agents (e.g. E. coli, here and in McKean & Nunney, 2001; 2005) in immunity studies may result in important phenomena being overlooked. Recent studies have highlighted a strong degree of specificity in invertebrate immunity (reviewed in Little et al., 2005). The use of non-pathogenic microbes or general immunoelicitors in immunity studies might therefore yield little information about biologically relevant invertebrate immune responses.
In larger insects the effect of piercing in immunity may be ameliorated because the relative size the wound inflicted compared to the size of the insect decreases with increasing body size (given a fixed needle size). However, our finding that piercing produced much higher immune gene expression than mating in D. melanogaster suggests that investigators should explore ways of measuring immunity that do not require integument piercing. For example, insects can be exposed to entomopathogenic fungi (e.g. Metarhizium anisopliae, Barnes & Siva-Jothy, 2000; Moret & Siva-Jothy, 2003) to investigate immune function. With this type of system infection occurs naturally without the need for manual damage to the integument. Of particular value to investigations into mating and immunity would be to explore the fitness effects of sexually transmitted insect pathogens (reviewed in Knell & Webberley, 2004). For example, it would be interesting to examine the ability of virgin and mated individuals to fight pathogens that are commonly transmitted during mating to determine whether mating induced changes in immunity are adaptations to the risk of disease. This prospect is especially intriguing in light of the recent finding that copulatory wounding occurs in many species of Drosophila (Kamimura, 2007), a process that could potentially facilitate pathogen entry into the female haemolymph.
Acknowledgements
We would like to thank Immogen Dennis and Clare Heaviside for help with bacterial clearance assays. This research was supported by grants from the BBSRC (S.W. and T.C.), the Swiss National Foundation and the Kanton Zürich (E.K.).
References
- Adamo SA. Estimating disease resistance in insects: phenoloxidase and lysozyme-like activity and disease resistance in the cricket Gryllus texensis . Journal of Insect Physiology. 2004a;50:209–216. doi: 10.1016/j.jinsphys.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Adamo SA. How should behavioural ecologists interpret measurements of immunity? Animale Behaviour. 2004b;68:1443–1449. [Google Scholar]
- Baer B, Armitage SAO, Boomsma JJ. Sperm storage induces an immunity cost in ants. Nature. 2006;441:872–875. doi: 10.1038/nature04698. [DOI] [PubMed] [Google Scholar]
- Barnes AI, Siva-Jothy MT. Density-dependent prophylaxis in the mealworm beetle Tenebrio molitor L. (Coleoptera: Tenebrionidae): cuticular melanization is an indicator of investment in immunity. Proceedings of the Royal Society of London B. 2000;267:177–182. doi: 10.1098/rspb.2000.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, Smith HK, Partridge L. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PS, Stumm-Zollinger E, Aigaki T, Balmer J, Bienz M, Böhlen P. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster . Cell. 1988;54:291–298. doi: 10.1016/0092-8674(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Kennington WJ. A simple method to achieve consistent larval density in bottle cultures. Drosophila Information Service. 2001;84:168–169. [Google Scholar]
- Domanitskaya EV, Liu H, Chen S, Kubli E. The hydroxyproline motif of male sex peptide elicits the innate immune response in Drosophila females. FEBS Journal. 2007;274:5659–5668. doi: 10.1111/j.1742-4658.2007.06088.x. [DOI] [PubMed] [Google Scholar]
- Fedorka KM, Linder JE, Winterhalter W, Promislow D. Postmating disparity between potential and realized immune response in Drosophila melanogaster . Proceedings of the Royal Society of London B. 2007;274:1211–1217. doi: 10.1098/rspb.2006.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorka KM, Zuk M. Sexual conflict and female immune suppression in the cricket, Allonemobious socius . Journal of Evolutionary Biology. 2005;18:1515–1522. doi: 10.1111/j.1420-9101.2005.00942.x. [DOI] [PubMed] [Google Scholar]
- Fedorka KM, Zuk M, Mousseau TA. Immune suppression and the cost of reproduction in the ground cricket, Allonemobius socius . Evolution. 2004;58:2478–2485. doi: 10.1111/j.0014-3820.2004.tb00877.x. [DOI] [PubMed] [Google Scholar]
- Gwynn DM, Callaghan A, Gorham J, Walters KFA, Fellowes MDE. Resistance is costly: trade-offs between immunity, fecundity and survival in the pea aphid. Proceedings of the Royal Society of London B. 2005;272:1803–1808. doi: 10.1098/rspb.2005.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura Y. Twin intromittent organs of Drosophila for traumatic insemination. Biology Letters. 2007;3:401–404. doi: 10.1098/rsbl.2007.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knell RJ, Webberley KM. Sexually transmitted diseases of insects: distribution, evolution, ecology and host behaviour. Biological Reviews. 2004;79:557–581. doi: 10.1017/s1464793103006365. [DOI] [PubMed] [Google Scholar]
- Lawniczak MK, Begun DJ. A genome-wide analysis of courting and mating responses in Drosophila melanogaster females. Genome. 2004;47:900–910. doi: 10.1139/g04-050. [DOI] [PubMed] [Google Scholar]
- Lawniczak MKN, Barnes AI, Linklater JR, Boone JM, Wigby S, Chapman T. Mating and immunity in invertebrates. Trends in Ecology Evolution. 2007;22:48–55. doi: 10.1016/j.tree.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Levy F, Rabel D, Charlet M, Bulet P, Hoffmann JA, Ehret-Sabatier L. Peptidomic and proteomic analyses of the systemic immune response of Drosophila. Biochimie. 2004;86:607–616. doi: 10.1016/j.biochi.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Little TJ, Hultmark D, Read AF. Invertebrate immunity and the limits of mechanistic immunology. Nat Immunol. 2005;6:651–654. doi: 10.1038/ni1219. [DOI] [PubMed] [Google Scholar]
- Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster . Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McGraw LA, Gibson G, Clark AG, Wolfner MF. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster . Current Biology. 2004;14:1509–1514. doi: 10.1016/j.cub.2004.08.028. [DOI] [PubMed] [Google Scholar]
- McKean KA, Nunney L. Increased sexual activity reduces male immune function in Drosophila melanogaster . Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7904–7909. doi: 10.1073/pnas.131216398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean KA, Nunney L. Bateman’s principle and immunity: phenotypically plastic reproductive strategies predict changes in immunological sex differences. Evolution. 2005;59:1510–1517. [PubMed] [Google Scholar]
- Moret Y, Siva-Jothy MT. Adaptive innate immunity? Responsivemode prophylaxis in the mealworm beetle, Tenebrio molitor . Proceedings of the Royal Society of London B. 2003;270:2475–2480. doi: 10.1098/rspb.2003.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Zipperlen P, Kubli E. Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Current Biology. 2005;15:1690–1694. doi: 10.1016/j.cub.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Rolff J, Siva-Jothy MT. Copulation corrupts immunity: a mechanism for a cost of mating in insects. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9916–9918. doi: 10.1073/pnas.152271999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T, Choffat Y, Klauser S, Kubli E. The Drosophila melanogaster sex-peptide - a molecular analysis of structure-function-relationships. Journal of Insect Physiology. 1993;39:361–368. [Google Scholar]
- Sheldon BC, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends in Ecology Evolution. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Shoemaker KL, Parsons NM, Adamo SA. Mating enhances parasite resistance in the cricket Gryllus texensis . Animal Behaviour. 2006;71:371–380. [Google Scholar]
- Siva-Jothy MT, Tsubaki Y, Hooper RE. Decreased immune response as a proximate cost of copulation and oviposition in a damselfly. Physiological Entomology. 1998;23:274–277. [Google Scholar]
- Wigby S, Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster . Current Biology. 2005;15:316–321. doi: 10.1016/j.cub.2005.01.051. [DOI] [PubMed] [Google Scholar]