Abstract
Background and Purpose
Cerebrovascular disease contributes to age-related cognitive decline, but the mechanisms underlying this phenomenon remain incompletely understood. We hypothesized that vascular risk factors would lead to cognitive impairment through the disruption of brain white matter network efficiency.
Methods
Participants were 19346 neurologically healthy individuals from UK Biobank that underwent diffusion MRI and cognitive testing (mean age = 62.6). Global efficiency, a measure of network integration, was calculated from white matter networks constructed using deterministic diffusion tractography. First, we determined whether demographics (age, sex, ethnicity, socioeconomic status, and education), vascular risk factors (hypertension, hypercholesterolemia, diabetes, smoking, body mass index), and white matter hyperintensities (WMH) were related to global efficiency using multivariate linear regression. Next, we used structural equation modeling (SEM) to model a multiple regression. The dependent variable was a latent cognition variable using all cognitive data, while independent variables were a latent factor including all vascular risk factors (vascular burden), demographic variables, WMH, and global efficiency. Finally, we used mediation analysis to determine whether global efficiency explained the relationship between vascular burden and cognition.
Results
Hypertension and diabetes were consistently associated with reduced global efficiency even after controlling for WMH. SEMs revealed that vascular burden was associated with cognition (P = 0.023), but not after adding global efficiency to the model (P = 0.09), suggesting a mediation effect. Mediation analysis revealed a significant indirect effect of global efficiency on cognition through vascular burden (P < 0.001), suggesting a partial mediation effect.
Conclusions
Vascular burden is associated with reduced global efficiency and cognitive impairment in the general population. Network efficiency partially mediates the relationship between vascular burden and cognition. This suggests that treating specific risk factors may prevent reductions in brain network efficiency and preserve cognitive functioning in the aging population.
Keywords: network analysis, vascular risk factors, cognitive impairment, cerebrovascular disease, stroke, diffusion tensor imaging, UK Biobank
Introduction
Cerebrovascular disease is a major cause of stroke and age-related cognitive decline.1,2 Cerebral small vessel disease (SVD) is an important factor linking vascular pathology to cognitive impairment.3 Vascular risk factors associated with SVD risk, such as hypertension, may contribute to SVD by remodeling the cerebral vasculature, which can include lowering vessel caliber, vessel rarefaction, and reduced cerebral blood flow.4 These may have ischemic consequences that can damage large-scale brain networks, which have been implicated as a factor underlying SVD-related cognitive decline.5
Structural brain networks can be quantitatively analyzed using diffusion tensor imaging (DTI) and network analysis.6 These networks can be constructed by estimating white matter connectivity between brain regions using DTI and tractography.7 Network measures such as global efficiency are sensitive to SVD-related pathology and are sensitive to cognitive decline.5 However, the impact of vascular risk factors on global efficiency remains relatively under-explored. Furthermore, no study has definitively shown a relationship between global efficiency and cognitive function in the general population.
We tested the relationships between vascular risk factors, global efficiency, and cognitive function in a large sample of healthy elderly. Based on previous research, we hypothesized that vascular risk factors would be related to global efficiency and cognition. We also hypothesized that global efficiency would play a key role in mediating the relationship between vascular risk factors and cognition.
Materials and Methods
Data availability statement
UK Biobank is an open access resource available to verified researchers upon application (ukbiobank.ac.uk).
Study participants
UK Biobank is a population-based cohort study comprising ~500,000 men and women recruited across Great Britain (England, Scotland, and Wales) between 2006 and 2010.8 Following an initial assessment, a subset of participants returned for brain magnetic resonance imaging (MRI) an average of 7.7 (SD = 1.4) years later. The data we analyze is from the visit for the MRI scan.
During the MRI visit, participants underwent a medical history review by a trained nurse. Participants were excluded if they reported a diagnosis of: Alzheimer's disease or any other dementia, Parkinson's disease, ischemic or hemorrhagic stroke, aneurysm, demyelinating disease including multiple sclerosis, chronic or degenerative neurological problems, neurological injury or trauma, head injury, infection of the nervous system, encephalitis, epilepsy, brain/intracranial abscess, cerebral palsy, or brain tumor.
UK Biobank received ethical approval from the Research Ethics Committee (reference 11/NW/0382), and all participants provided written informed consent. The present analyses were conducted under UK Biobank application 36509.
Demographic and vascular risk factor information
Sex and self-reported ethnic background were reported via touchscreen interview at the initial assessment. Ethnicity was recoded as a binary variable (White or not). The Townsend Deprivation Index (TDI) was used to measure socioeconomic status and was automatically assigned to participants based on their postal codes. All other information was reported during the MRI visit. Educational qualifications and smoking status were recorded via touchscreen. Qualifications were recoded as a binary variable (College/University degree or not), while smoking status was an ordinal variable with three levels (Never smoked/Ex-smoker/Current smoker). Body mass index (BMI) was calculated after removal of bulky items of clothing and shoes as participant weight (kg) / height2 (m). Diagnoses of diabetes, hypertension, and hypercholesterolemia were obtained from the medical history interview. Two seated automated measurements of blood pressure were taken using the Omron HEM-7015IT device. These two measurements were averaged to provide mean systolic blood pressure (SBP) and diastolic blood pressure.
Cognitive assessment
The cognitive tests used in our study were administered via touchscreen during the MRI visit. Fluid intelligence was assessed using thirteen questions about logic/reasoning, with scores reflecting the number of questions correct within a two-minutes time limit. Visual memory was assessed using a six card pairs-matching test and scored as the total number of incorrect matches made. Reaction time was assessed using a timed symbol matching test similar to the card game 'Snap' and scored as the mean response time in milliseconds across all trials containing matching pairs. Prospective memory was assessed by giving participants an instruction they had to remember later in the assessment, and scored as 1 if the participant remembered the instruction of their first try or 0 if not. Finally, a subset of participants completed a numeric memory test, which required participants to remember a two-digit number after a pause, with digits increasing by one until the participant made an error or reached twelve digits. The reliability and longitudinal stability of these tests has been previously reported.9
Brain image acquisition
All MRI data was acquired on a single Siemens Skyra 3T scanner using a standard Siemens 32-channel head coil. Full details on the acquisition parameters have been detailed elsewhere.10 In brief, the protocol included a T1-weighted anatomical image, a T2-weighted Fluid Attenuated Inversion Recovery (FLAIR) image to quantify white matter hyperintensities (WMH), and a diffusion MRI sequence to measure white matter microstructural properties. The diffusion MRI sequence included 105 volumes acquired at 2 mm3 resolution, with 5 at b = 0 s/mm2, 50 at b = 1000 s/mm2, and 50 at b = 2000 s/mm2.
Brain volumetry
Brain volumetry was calculated using tools from FSL as previously described.10 In brief, total brain volumes (TBV) were estimated from the T1-weighted images using SIENAX, while WMH volumes were automatically calculated from FLAIR images using BIANCA, TBV and WMH were then normalized for differences in head size by multiplying the raw volumes by a scaling parameter estimated in SIENAX.11
Diffusion MRI preprocessing and tractography
Raw diffusion data was corrected for eddy currents, head motion, outlier slices, and gradient distortion. Diffusion tensors were then fit using the b = 1000 s/mm2 shell, yielding a scalar fractional anisotropy (FA) image. This FA image was then non-linearly warped to standard space. Full details on the diffusion processing pipeline and the software used by the UK Biobank imaging team have been published elsewhere.10
The FA image in native diffusion space was used for deterministic diffusion tensor tractography using MRtrix3.12 Streamlines were seeded on a 0.5 mm grid for every voxel with FA > 0.15 and propagated in 0.5 mm steps using 4th-order Runge-Kutta integration.13 Termination criteria included: streamline length < 20 mm or > 250 mm, turning angle > 45°, or voxel FA < 0.15.
Network analysis
Following tractography, a connectivity matrix was generated for each participant using nodes derived from the Automated Anatomical Labeling (AAL) atlas.14 The AAL is widely used in network analysis studies and consists of 90 manually-labeled cortical and subcortical areas (45 per hemisphere) in standard space. These 90 regions exclude cerebellar regions in the AAL as tractography algorithms perform poorly in these areas.
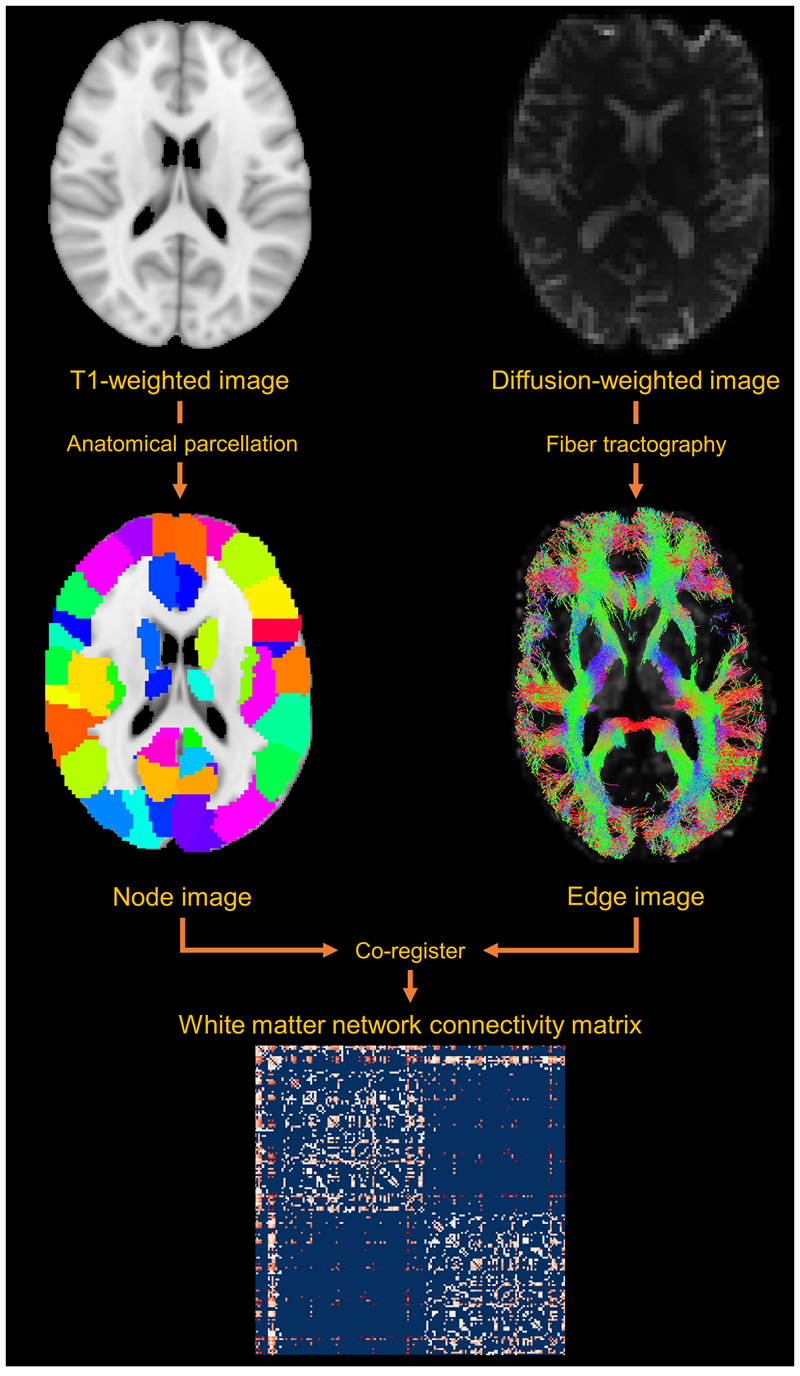
The warp field calculated from each participant's FA image to standard space was inverted and applied to the AAL using nearest neighbor interpolation, bringing the AAL into native diffusion space (where streamlines were computed). Two areas in the AAL were considered connected if joined by the endpoints of a reconstructed streamline, resulting in a non-zero edge in the connectivity matrix. Edges were weighted according to the number of streamlines connecting two regions multiplied by the inverse average streamline length, as longer streamlines are seeded multiple times.15 Streamlines that terminated in the same AAL region they were initiated in were excluded, and edge weights <1 were set to 0 to minimize noise-related false positives.16 This yielded a zero-diagonal symmetric 90x90 undirected connectivity matrix for each participant. An illustration of how connectivity matrices are derived is shown in Figure 1.
Figure 1.
White matter network construction. A network consists of nodes and connecting edges. Using MRI, nodes can be defined using an a priori neuroanatomical parcellation, while edges can be defined using tractography on diffusion tensor images. Co-registering the parcellation and tractography image yields information on how grey matter regions are connected by the white matter. This is the basis of the connectivity matrix, which represents white matter network connectivity.
Finally, network measures were calculated for each connectivity matrix using the Brain Connectivity Toolbox.17 We chose to focus on weighted global efficiency, Ew, which is the average inverse shortest path length, dw, between a pair of nodes, i and j, for the set of all nodes in a network, N, such that:
with n being the total number of nodes. A more formal mathematical description of this measure can be found elsewhere.17 The global efficiency of brain white matter networks has been shown to be a strong predictor of cognitive deficits, dementia, and mortality in patients with cerebrovascular disease.5,18
Statistical analysis
All analyses were conducted in R 3.6.2 with the lavaan package 0.6-5.19 Unless otherwise specified, all tests were two-tailed with α = 0.05. All reported P-values were adjusted for multiple comparisons on a model-wise basis using the false discovery rate (FDR).20 Variables with positive skew were transformed using log10 transformations, with the prior addition of a scalar constant if values were < 1. Variables transformed included TDI, BMI, reaction time, and visual memory.
Our analysis attempted to answer three inter-related questions: (1) are vascular risk factors related to global efficiency; (2) does global efficiency explain variance in general cognitive function after controlling for vascular risk factors; and (3) does global efficiency mediate the relationship between vascular risk factors and cognition?
To answer the first question, whether vascular risk factors were related to global efficiency, we modeled two multivariate linear regressions with global efficiency as the dependent variable. In the first model, age, sex, ethnicity, education, TDI, normalized TBV, and the vascular risk factors were regressed on global efficiency, allowing us to assess the individual contributions of each vascular risk factor on global efficiency. The second model included all the terms in the first while adding normalized WMH volume as an additional covariate to control for SVD severity. These models were then compared using a likelihood ratio test. A significant difference in these models would indicate that WMH explains additional variance in global efficiency beyond the vascular risk factors alone. Given the potential importance of blood pressure for affecting global efficiency, we repeated the analyses by replacing hypertension with SBP.
To answer the second question, whether global efficiency explained additional variance in general cognitive function after controlling for vascular risk factors, we used structural equation modeling (SEM). SEM allows for the modeling of directed (regression) and undirected (correlation) relationships between observed and unobserved (latent) variables. A latent variable is not directly measured in an experiment, but rather inferred through other measured variables. Latent variables can then be regressed on other observed or unobserved variables, making it a useful approach to dimensionality reduction.21
Two latent variables were defined: a vascular factor (henceforth referred to as vascular burden), which included smoking, BMI, hypertension, hypercholesterolemia, and diabetes; and a factor representing general cognitive function, which included numeric memory, prospective memory, fluid intelligence, reaction time, and visual memory. The psychometric characteristics and longitudinal stability of this single-factor cognitive construct have been previously validated in this cohort.9 These latent variables were then used in another series of multiple regressions, modeled within the SEM framework. In the first model, vascular burden, demographic variables, and WMH burden was regressed on the general cognitive factor. The second model included all the terms of the first but added global efficiency as a predictor of cognition.
To answer the third question, which was whether global efficiency mediated the relationship between vascular risk factors and cognition, we used mediation analysis, a technique used to determine if the association between variables can be partially explained by the effect of a third, or mediating, variable.22 In this mediation model, vascular burden was used as the independent variable, cognition the dependent variable, and global efficiency the mediating variable. In mediation analyses, the total effect (TE) is the original relationship between vascular burden and cognition, unadjusted for the mediator. The direct effect (DE) is the effect of vascular burden after adjusting for the mediator, while the indirect effect (IE), also called the mediation effect, is the effect of global efficiency on cognition through vascular burden. A significant IE is indicative of a mediation effect.
In order to account for sporadic missing data, all SEMs were fit using full information maximum likelihood (FIML). FIML uses all available information to estimate models, thereby leveraging information in partially complete cases without imputing missing values.23 All reported β's presented are standardized regression coefficients, and are thus directly comparable, while reported P-values have been adjusted for multiple comparisons on a model-wise basis using the FDR method.
Results
Following exclusions due to neurological diseases, MR scans of inadequate quality for analysis, and failures of the MRI analysis pipeline, a total of 19364 participants had usable diffusion MRI data. Descriptive statistics for this subset of participants are reported in Table 1.
Table 1. Descriptive statistics for included participants.
| Mean (SD) or Count (%) | N | |
|---|---|---|
| Demographic variables | ||
| Age | 62.6 (7.4) | 19346 |
| Sex, Female (%) | 9108 (47.1) | 19346 |
| Ethnicity, White (%) | 18786 (97.4) | 19285 |
| Education, College or University (%) | 9000 (46.5) | 19346 |
| Townsend Deprivation Index | -2.0 (2.6) | 19329 |
| Vascular risk factors | ||
| Smoking (Never/Ex/Current) | 11982 (62.6)/6444 (33.7)/724 (3.8) | 19150 |
| BMI, mm/kg2 | 26.6 (4.4) | 18871 |
| Hypertension (%) | 3924 (20.3) | 19346 |
| SBP, mm/Hg | 136.8 (17.8) | 16351 |
| Diabetes (%) | 891 (4.6) | 19346 |
| Hypercholesterolemia (%) | 2265 (11.7) | 19346 |
| Neuroimaging variables | ||
| TBV, mm3 | 1502645.4 (72588.0) | 19346 |
| WMH, mm3 | 4530.2 (5850.0) | 19303 |
| Global efficiency | 6.6 (1.3) | 19346 |
| Cognitive variables | ||
| Numeric memory | 6.8 (1.3) | 8509 |
| Fluid Intelligence | 6.8 (2.1) | 17437 |
| Reaction time, ms | 588.5 (106.1) | 17827 |
| Prospective memory, correct first try (%) | 15474 (86.5) | 17880 |
| Visual memory | 3.5 (2.8) | 17897 |
Note. SD = standard deviation; TDI = Townsend Deprivation Index; BMI = body mass index; SBP = systolic blood pressure; TBV = total brain volume; WMH = White matter hyperintensity volume. TBV and WMH have been adjusted for head size.
Vascular risk factors are associated with white matter network global efficiency
The results of the regression analyses on global efficiency are shown Table 2. The first regression, which included demographic variables, vascular risk factors, and TBV as covariates showed that all variables except hypercholesterolemia were associated with global efficiency. Age, TBV, and sex explained the most variability, with all other variables explaining relatively small amounts of variance.
Table 2. Multivariate linear regressions showing associations between risk factors and global efficiency, with and without WMH (n = 18,592).
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| β [95% CI] | P | β [95% CI] | P | |
| Age | -0.26 [-0.28, -0.25] | <0.001 | -0.14 [-0.16, -0.12] | <0.001 |
| Sex | 0.36 [0.33, 0.39] | <0.001 | 0.34 [0.32, 0.37] | <0.001 |
| Ethnicity | 0.28 [0.20, 0.36] | <0.001 | 0.26 [0.18, 0.33] | <0.001 |
| Education | 0.05 [0.03, 0.08] | <0.001 | 0.05 [0.02, 0.07] | <0.001 |
| log10(TDI) | -0.02 [-0.03, -0.01] | 0.002 | -0.02 [-0.03, -0.01] | 0.001 |
| Smoking | -0.02 [-0.03, -0.00] | 0.009 | -0.01 [-0.02, 0.01] | 0.29 |
| log10(BMI) | -0.03 [-0.05, -0.02] | <0.001 | -0.01 [-0.02, 0.00] | 0.12 |
| Hypertension | -0.17 [-0.20, -0.14] | <0.001 | -0.11 [-0.15, -0.08] | <0.001 |
| Diabetes | -0.14 [-0.20, -0.08] | <0.001 | -0.09 [-0.15, -0.03] | 0.003 |
| Hypercholesterolemia | -0.01 [-0.05, 0.03] | 0.71 | -0.01 [-0.05, 0.02] | 0.45 |
| TBV | 0.25 [0.23, 0.26] | <0.001 | 0.23 [0.22, 0.25] | <0.001 |
| WMH | -0.27 [-0.28, -0.25] | <0.001 | ||
| Adjusted R2 | 0.23 | 0.28 | ||
Note. β = standardized beta coefficient; CI = confidence interval; TDI = Townsend Deprivation Index; BMI = body mass index; TBV = total brain volume; WMH = white matter hyperintensities.
We then added WMH volume to this regression model to determine whether vascular risk factors were merely causing SVD, which in itself leads to network disruption. As predicted, WMH was associated with global efficiency after controlling for all other variables. The addition of WMH to the model attenuated many coefficients from the original, leading smoking and BMI to become non-significant. Hypertension and diabetes remained significant however, suggesting that some vascular risk factors predict global efficiency even after taking WMH into account. The inclusion of WMH increased the adjusted R2 of the model, and a likelihood ratio test confirmed that the full model was an improvement (F = 1224.3, P < 0.001). This pattern of results was identical after replacing hypertension with SBP (Table 3).
Table 3. Multivariate linear regressions with blood pressure instead of hypertension (n = 15,899).
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| β [95% CI] | P | β [95% CI] | P | |
| Age | -0.25 [-0.27, -0.24] | <0.001 | -0.13 [-0.15, -0.11] | <0.001 |
| Sex | 0.38 [0.35, 0.41] | <0.001 | 0.36 [0.33, 0.39] | <0.001 |
| Ethnicity | 0.29 [0.20, 0.37] | <0.001 | 0.25 [0.17, 0.34] | <0.001 |
| Education | 0.05 [0.03, 0.08] | <0.001 | 0.05 [0.02, 0.08] | <0.001 |
| log10(TDI) | -0.02 [-0.04, -0.01] | <0.001 | -0.03 [-0.04, -0.01] | <0.001 |
| Smoking | -0.01 [-0.03, -0.00] | 0.044 | -0.00 [-0.02, 0.01] | 0.53 |
| log10(BMI) | -0.03 [-0.05, -0.02] | <0.001 | -0.01 [-0.02, 0.01] | 0.17 |
| SBP | -0.06 [-0.08, -0.05] | <0.001 | -0.03 [-0.05, -0.02] | <0.001 |
| Diabetes | -0.18 [-0.25, -0.11] | <0.001 | -0.12 [-0.19, -0.06] | <0.001 |
| Hypercholesterolemia | -0.04 [-0.08, 0.00] | 0.08 | -0.04 [-0.08, 0.00] | 0.10 |
| TBV | 0.25 [0.23, 0.27] | <0.001 | 0.23 [0.22, 0.25] | <0.001 |
| WMH | -0.27 [-0.28, -0.25] | <0.001 | ||
| Adjusted R2 | 0.22 | 0.28 | ||
Note. β = standardized beta coefficient; CI = confidence interval; TDI = Townsend Deprivation Index; BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure; TBV = total brain volume; WMH = white matter hyperintensities.
Vascular burden is not associated with cognition after controlling for global efficiency
The results of the SEM-based regressions are in Table 4. Model 1 revealed that all variables, including vascular burden, demographic variables, and WMH were associated with the latent cognition factor. In Model 2, which added global efficiency to Model 1, all variables remained significant except vascular burden, suggesting that the relationship between vascular burden and cognition may be mediated in part by global efficiency.
Table 4. Structural equation models predicting cognition, with and without global efficiency.
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| β [95% CI] | P | β [95% CI] | P | |
| Vascular burden | -0.04 [-0.07, -0.01] | 0.023 | -0.03 [-0.06, 0.00] | 0.09 |
| Age | -0.21 [-0.24, -0.18] | <0.001 | -0.19 [-0.22, -0.16] | <0.001 |
| Sex | 0.15 [0.13, 0.18] | <0.001 | 0.14 [0.12, 0.16] | <0.001 |
| Ethnicity | 0.22 [0.20, 0.24] | <0.001 | 0.21 [0.19, 0.24] | <0.001 |
| log10(TDI) | -0.05 [-0.07, -0.03] | <0.001 | -0.05 [-0.07, -0.03] | <0.001 |
| Education | 0.30 [0.28, 0.32] | <0.001 | 0.30 [0.27, 0.32] | <0.001 |
| log10(WMH) | -0.10 [-0.13, -0.08] | <0.001 | -0.08 [-0.10, -0.05] | <0.001 |
| Global efficiency | 0.10 [0.07, 0.12] | <0.001 | ||
| AIC | 67384 | 67324 | ||
| BIC | 67671 | 67619 | ||
Note. β = standardized beta coefficient; CI = confidence interval; TDI = Townsend Deprivation Index; WMH = white matter hyperintensities; AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion. Lower AIC and BIC values indicate a better fitting model.
These models were then compared using the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC), two model selection indices where smaller values indicate better models.24 Model 2 minimized the AIC (Model 1: 67384, Model 2: 67324, ΔAIC = -60) and BIC (Model 1: 67671, Model 2: 67619, ΔBIC = -52), suggesting that it was the better overall model.
Global efficiency partially mediates the relationship between vascular risk factors and cognition
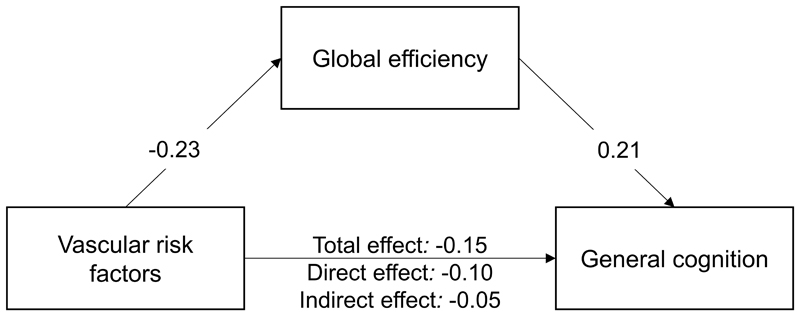
The results of the mediation analysis are shown in Figure 2. All regression paths were significant (P < 0.001). Mediation analysis showed that relationship between vascular burden and cognition was partially mediated by global efficiency, remaining significant even after controlling for global efficiency.
Figure 2.
Global efficiency partially mediates the relationship between vascular risk factors and cognition. All paths significant at P < 0.001. TE = total effect, DE = direct effect, IE = indirect effect.
Discussion
We investigated the relationships between vascular burden, the global efficiency of white matter networks, and cognitive function in a large population-based cohort of neurologically healthy individuals. Our study yields three major findings. First, we showed that specific vascular risk factors were associated with reduced global efficiency, even after controlling for demographic variables and WMH volumes. Second, we demonstrated that vascular burden was associated with general cognitive function after controlling for demographic variables and WMH, but not after adding global efficiency to the model. Third, we found that global efficiency partially mediated the relationship between vascular burden and cognition.
Analysis of individual vascular risk factors revealed that smoking, hypertension, diabetes, and BMI were associated with global efficiency, while hypercholesterolemia was not. This is consistent with research in smaller samples that show that hypertensive patients show reduced global efficiency, which may partially mediate the association between hypertension and executive function.25 Reduced global efficiency is also seen in diabetic patients.26 This suggests that treating specific vascular risk factors in the general population may prevent declines in global efficiency.
Furthermore, our study also showed that SVD-related pathology, as indicated by WMH volume, is associated with reduced global efficiency above and beyond the contributions of individual vascular risk factors. Indeed, the introduction of WMH to the model reduced many of the coefficients of individual vascular risk factors, leading to smoking status and BMI to become non-significant, suggesting that SVD-related processes play an important role in vascular alterations to white matter network topology. Previous studies have shown that WMH severity is associated with major symptoms of SVD, and that the relationship of WMH to these symptoms may be related to changes in the global efficiency of white matter networks.5,27,28 Importantly, however, WMH did not completely explain all the variance related to individual vascular risk factors, suggesting that vascular risk factors also result in network disruption via other mechanisms.
We also showed that vascular burden was related to general cognitive function, even after controlling for demographic variables and WMH volume. This relationship became non-significant after adding global efficiency to the model, suggesting a partial mediation effect which was supported by our subsequent mediation analysis. This suggests that the effects of vascular risk factors on cognitive function cannot be fully explained by differences in demographic variables such as age, education or socioeconomic status, nor by subsequent SVD-related pathology such as WMH. The global efficiency of white matter networks, in addition to the abovementioned variables, may be necessary for a more complete description of how vascular burden leads to cognitive impairment.
Taken together, our results suggest that an important area for future work lies in elucidating the mechanisms that lead from vascular risk factors to network function to cognitive impairment. It is important to stress that the answer to this question may lie beyond currently observed cerebrovascular pathology, given that these results were observed after excluding participants with ischemic or hemorrhagic stroke and after controlling for an aspect of SVD-related pathology. For instance, it is possible that hypertension and diabetes may lead to increased arterial tortuosity.29 Although mild vessel tortuosity is considered asymptomatic,29 it is possible that it may lead to subtle ischemic changes in the brain that are not radiologically visible, but still result in reduced global efficiency.
Increasing evidence suggests early management of vascular risk factors may delay or prevent white matter network damage, in turn delaying cognitive decline and possibly lowering dementia risk.30 Mounting evidence suggests that midlife vascular risk factor exposure accelerates rates of WMH volume progression, global brain and hippocampal atrophy, and late-life cognitive impairment.2,31 Our study suggest that white matter network integrity may be a central mechanism which underlies this association between vascular risk factors and cognition.
Future work may also expand on the potential interactions between vascular risk factors and demographic variables. For instance, we found that ethnicity was associated with both global efficiency and cognition after controlling for other variables. This effect may have been biased by our predominantly white sample, but may also be indicative, or related to, ethnic differences in vascular risk factors and subsequent cerebrovascular complications.32 In a similar vein, our participants were mostly older individuals. Given the established inter-relationships between age and changes in white matter organization, vascular risk, and cognition,33,34 it is possible that the pattern and magnitude of our findings differ across the lifespan. Replicating these findings in different populations with more balanced subgroups may therefore yield additional insights into these complex relationships.
There are some limitations in this study. First, cognitive tests in the UK Biobank were conducted by participants themselves using a touch screen interface, rather than being administered by a trained examiner. The tests themselves, while assessing a broad range of cognitive functions, are not typically administered in clinical research. Second, this study was cross-sectional in nature, and only focused on middle-aged to elderly participants. Longitudinal research may be able to better indicate whether vascular risk factors lead to a change in the global efficiency of white matter networks, and whether this has consequent effects on cognition. Furthermore, a longitudinal sample that included younger individuals would be able to better determine how these effects might change across the lifespan.
Summary
We have shown that community-dwelling individuals with higher vascular burden show increased white matter network disruption and cognitive impairment, and that white matter network disruption may mediate the association between vascular risk factors and cognitive impairment. This suggests that treating specific vascular risk factors may prevent reductions in white matter network global efficiency, potentially preserving cognitive function in aging individuals.
Acknowledgments
JS, HSM, and JT conceived of the study. DJT and JT analyzed the imaging data. JS and JT conducted the statistical analysis. JS and JT drafted the manuscript. JS, DJT, HSM, and JT revised the manuscript for intellectual content.
JS acknowledges the support of his supervisor Junjian Zhang in Zhongnan Hospital of Wuhan University.
Sources of Funding
UK Biobank was established by the Wellcome Trust Medical Charity, Medical Research Council, Department of Health, Scottish Government, and the Northwest Regional Development Agency. It has also had funding from the Welsh Assembly Government and the British Heart Foundation. The funders had no role in study design, data collection or management, analyses or interpretation of the data, or preparation, review or approval of the manuscript. JS was sponsored by China Scholarship Council. DJT is funded by a grant from the Medical Research Council (MR/N0268696/1). HSM receives infrastructural support from the Cambridge University Hospitals National Institute of Health Research Biomedical Research Centre and is supported by an NIHR Senior Investigator award. JT is supported by a scholarship from the Cambridge Trust.
Footnotes
Disclosures
HSM reports personal fees from BIBA outside the present study.
References
- 1.Pantoni L. Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 2.van Leijsen EM, Tay J, van Uden IW, Kooijmans EC, Bergkamp MI, van der Holst HM, et al. Memory decline in elderly with cerebral small vessel disease explained by temporal interactions between white matter hyperintensities and hippocampal atrophy. Hippocampus. 2019;29:500–510. doi: 10.1002/hipo.23039. [DOI] [PubMed] [Google Scholar]
- 3.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019;18:684–696. doi: 10.1016/S1474-4422(19)30079-1. [DOI] [PubMed] [Google Scholar]
- 4.Laurent S, Boutouyrie P. The structural factor of hypertension: Large and small artery alterations. Circ Res. 2015;116:1007–1021. doi: 10.1161/CIRCRESAHA.116.303596. [DOI] [PubMed] [Google Scholar]
- 5.Tuladhar AM, Tay J, van Leijsen E, Lawrence AJ, van Uden IWM, Bergkamp M, et al. Structural network changes in cerebral small vessel disease. J Neurol Neurosur Psychiatry. 2020;91:196–203. doi: 10.1136/jnnp-2019-321767. [DOI] [PubMed] [Google Scholar]
- 6.Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 7.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyall DM, Cullen B, Allerhand M, Smith DJ, Mackay D, Evans J, et al. Cognitive test scores in UK Biobank: Data reduction in 480,416 participants and longitudinal stability in 20,346 participants. PLoS One. 2016;11:e0154222. doi: 10.1371/journal.pone.0154222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alfaro-Almagro F, Jenkinson M, Bangerter NK, Andersson JL, Griffanti L, Douaud G, et al. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage. 2018;166:400–424. doi: 10.1016/j.neuroimage.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews P, Federico A, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 12.Tournier J-D, Calamante F, Connelly A. MRtrix: Diffusion tractography in crossing fiber regions. Int J Imaging Syst Technol. 2012;22:53–66. [Google Scholar]
- 13.Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 14.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 15.Hagmann P, Kurant M, Gigandet X, Thiran P, Wedeen VJ, Meuli R, et al. Mapping human whole-brain structural networks with diffusion MRI. PLoS One. 2007;2:e597. doi: 10.1371/journal.pone.0000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence AJ, Chung AW, Morris RG, Markus HS, Barrick TR. Structural network efficiency is associated with cognitive impairment in small-vessel disease. Neurology. 2014:10–1212. doi: 10.1212/WNL.0000000000000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence AJ, Zeestraten EA, Benjamin P, Lambert CP, Morris RG, Barrick TR, et al. Longitudinal decline in structural networks predicts dementia in cerebral small vessel disease. Neurology. 2018;90:e1898–e1910. doi: 10.1212/WNL.0000000000005551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosseel Y. lavaan: An R package for structural equation modeling and more. J Stat Softw. 2012;48:1–36. [Google Scholar]
- 20.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 21.Li R, Tsaih S-W, Shockley K, Stylianou IM, Wergedal J, Paigen B, et al. Structural model analysis of multiple quantitative traits. PLoS Genetics. 2006;2:e114. doi: 10.1371/journal.pgen.0020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equ Modeling. 2001;8:430–457. [Google Scholar]
- 24.Burnham KP, Anderson DR. Multimodel inference: Understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33:261–304. [Google Scholar]
- 25.Li X, Ma C, Sun X, Zhang J, Chen Y, Chen K, et al. Disrupted white matter structure underlies cognitive deficit in hypertensive patients. Eur Radiol. 2016;26:2899–2907. doi: 10.1007/s00330-015-4116-2. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Liu Z, Li Z, Wang Y, Chen Y, Li X, et al. Disrupted white matter network and cognitive decline in type 2 diabetes patients. J Alzheimers Dis. 2016;53:185–195. doi: 10.3233/JAD-160111. [DOI] [PubMed] [Google Scholar]
- 27.Tay J, Tuladhar AM, Hollocks MJ, Brookes RL, Tozer DJ, Barrick TR, et al. Apathy is associated with large-scale white matter network disruption in small vessel disease. Neurology. 2019;92:e1157–e1167. doi: 10.1212/WNL.0000000000007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tay J, Lisiecka-Ford DM, Hollocks MJ, Tuladhar AM, Barrick TR, Forster A, et al. Network neuroscience of apathy in cerebrovascular disease. Prog Neurobiol. 2020;188 doi: 10.1016/j.pneurobio.2020.101785. 101785. [DOI] [PubMed] [Google Scholar]
- 29.Han H-C. Twisted blood vessels: Symptoms, etiology and biomechanical mechanisms. J Vasc Res. 2012;49:185–197. doi: 10.1159/000335123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, et al. Effect of intensive vs standard blood pressure control on probable dementia: A randomized clinical trial. JAMA. 2019;321:553–561. doi: 10.1001/jama.2018.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debette S, Seshadri S, Beiser A, Au R, Himali J, Palumbo C, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sacco RL, Kargman D, Gu Q, Zamanillo M. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction: The Northern Manhattan Stroke Study. Stroke. 1995;26:14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy KM, Raz N. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res. 2009;1297:41–56. doi: 10.1016/j.brainres.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craik FI, Bialystok E. Cognition through the lifespan: Mechanisms of change. Trends Cogn Sci. 2006;10:131–138. doi: 10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
UK Biobank is an open access resource available to verified researchers upon application (ukbiobank.ac.uk).