Abstract
Purpose of Review
This current review summarizes the investigational and therapeutic applications of transcranial magnetic stimulation (TMS) in schizophrenia.
Recent Findings
Fairly consistent findings of an impaired cortical excitation-inhibition balance, cortical plasticity, and motor resonance have been reported in schizophrenia. Cortical connectivity impairments have also been demonstrated in motor and prefrontal brain regions. In terms of treatment, the best support is for 1-Hz TMS to the left temporoparietal cortex for the shortterm treatment of persistent auditory hallucinations. High-frequency TMS to the left prefrontal cortex improves negative and cognitive symptoms, but with inconsistent and small effects.
Summary
TMS combined with diverse brain mapping techniques and clinical evaluation can unravel critical brain-behavior relationships relevant to schizophrenia. These provide critical support to the conceptualization of schizophrenia as a connectopathy with anomalous cortical plasticity. Adaptive modulation of these aberrant brain networks in a neuroscience-informed manner drives short-term therapeutic gains in difficult-to-treat symptoms of schizophrenia.
Keyword: Brain stimulation, Neuromodulation, TMS, Connectivity, Treatment, Psychosis, Resistant schizophrenia
Introduction
Schizophrenia is a severe brain disorder with a lifetime prevalence of ~ 1% that typically begins in early adulthood, resulting in substantial disability, morbidity, and mortality, at considerable personal and societal costs [1, 2]. Antipsychotic medications discovered in the 1950s, which act via blocking dopamine receptors in the brain, are still the mainstay of treatment in schizophrenia [1]. They are effective in approximately 50% of patients [3] and help in correcting positive symptoms like hallucinations and delusions, with little impact on the more disabling negative and cognitive symptoms. It is therefore imperative to examine novel therapeutic avenues that not only target the resistant, positive symptoms but also improve negative and cognitive symptoms of schizophrenia. In order to develop new treatments, the current pathophysiological models need to be empirically examined using both in vivo and in vitro methods. It is here that the role of transcranial magnetic stimulation (TMS) plays a dual and vital role. First, TMS can be utilized as a neurophysiological probe to understand brain function, thus enabling better neurobiological characterization of disease processes and their evolution with treatment [4, 5••]. Next, when precisely delivered in trains of repeated pulses (rTMS), it can potentially have excitatory and inhibitory cortical network-level effects, depending on the pattern of stimulation. This property of TMS has been leveraged to drive short-term change in behavior based on prior understanding of the underlying cortical-level pathophysiological processes [6]. This current review will focus on both these applications of TMS—(a) an investigational probe for characterizing aberrant brain function in schizophrenia, and (b) a therapeutic tool for treating resistant symptoms of schizophrenia.
TMS as an Investigational Probe (Table 1)
Table 1. Summary of investigational TMS approaches in schizophrenia.
| TMS paradigm | Definition | Putative neurophysiology | Findings in SZ | |
|---|---|---|---|---|
| Consistent | Emerging | |||
| Cortical excitability | ||||
| Resting motor threshold (RMT) | The minimal stimulator intensity that produces a motor evoked potential of ≥ 50 μV in 5 of 10 trials in a relaxed muscle | Neuronal membrane activity regulated by voltage-gated Na+ channels & ionotropic non-NMDA glutamate receptors | ≈ * | – |
| Motor evoked potential (MEP) amplitude | The mean amplitude of hand muscle contraction evoked by a series of pulses applied at a consistent TMS intensity | Neuronal membrane activity governed by the excitation/inhibition balance through various neurotransmitter systems | ≈ * | – |
| Intracortical facilitation (ICF) | The % of MEP facilitation relative to suprathreshold test pulse MEP, elicited by pairing a subthreshold conditioning pulse (80% of RMT) 7-20 ms before the test pulse | NMDA-glutamate receptor-mediated excitability | ≈ * | – |
| Cortical inhibition | ||||
| Short-interval intracortical inhibition (SICI) | The % of MEP inhibition relative to suprathreshold test pulse MEP, elicited by pairing a subthreshold conditioning pulse (80% of RMT) 1-5 ms before the test pulse | GABAA receptor-mediated inhibitory neurotransmission | ↓ * | Non-specific to SZ, but related to cognitive dysfunction |
| Long-interval intracortical inhibition (LICI) | The % of MEP inhibition relative to suprathreshold test pulse MEP, elicited by pairing a suprathreshold conditioning pulse 50-200 ms before the test pulse | GABAB receptor-mediated inhibitory neurotransmission | ≈ * | ↑ LICI in mania—differential biomarker |
| Cortical silent period (CSP) | Time from MEP offset to the return of any voluntary EMG activity when a suprathreshold pulse is delivered to the motor cortex contralateral to a voluntarily contracted hand muscle | First 50 ms of the silent period is mediated by spinal mechanisms; the later part is mediated by GABAB receptor-driven motor cortical inhibition | ≈ * | Increases with clozapine treatment |
| Putative MNS activity Motor resonance |
The degree of motor cortical reactivity facilitation during action observation, relative to static image viewing | Driven by premotor MNS activity, possibly through premotor-motor connections | ↓ in untreated SZ, related to social cognition deficits | ↑ in untreated mania, modulated by context, but less so in SZ |
| Cortical connectivity | ||||
| Ipsilateral silent period (ISP) | Time from MEP offset to the return of any voluntary EMG activity when a suprathreshold pulse is delivered to the motor cortex ipsilateral to a voluntarily contracted hand muscle | Corpus callosum functional integrity | ↓ | – |
| Transcallosal inhibition (TCI) | The % of MEP inhibition relative to suprathreshold test pulse MEP from one hemisphere, when a suprathreshold conditioning pulse is delivered over the contralateral hemisphere 7-20 ms before the test pulse | Corpus callosum functional integrity | ↓ | – |
| Transcallosal facilitation (TCF) | The % of MEP facilitation relative to suprathreshold test pulse MEP from one hemisphere, when a low-intensity conditioning pulse is delivered over the contralateral hemisphere 4-5 ms before the test pulse | Corpus callosum functional integrity | ≈ | – |
| Parieto-motor connectivity | A subthreshold conditioning pulse applied to the posterior parietal cortex 4 and 15 ms prior facilitates the MEP obtained by administering a test pulse to the motor hand area on the same side | Superior longitudinal fasciculus functional integrity | – | ↓ |
| Premotor-motor connectivity | A subthreshold conditioning stimulus to the premotor cortex (8 ms prior) facilitates the MEP elicited by a suprathreshold test pulse in the contralateralmotor cortex | Transcallosal premotor-motor connectivity | – | ↓ |
| Cerebellar-cortical connectivity | A subthreshold conditioning stimulus to the cerebellum 5-15 ms prior to a suprathreshold test stimulus to the contralateral motor cortex inhibits the output of the test stimulus | Inhibitory influence of the cerebellum on the motor cortex through the cerebello-thalamo cortical connectivity | ↓ | |
| Cortical plasticity | ||||
| Motor cortical plasticity | The degree of facilitation or inhibition of cortical reactivity (most often measured as the MEP amplitude) with TMS/TES perturbation protocols | Long-term potentiation/depression (LTP/LTD)-like experience-dependent synaptic plasticity | ↓ LTP- and LTD-like plasticity* | – |
| Non-motor cortical reactivity and connectivity | ||||
| TMS-evoked potential (TEP) | ATMS pulse when administered to the cortex elicits an immediate response of positive and negative deflections visible on EEG | Cortical reactivity | – | ↓ prefrontal reactivity |
| Cortical connectivity | ATMS pulse when administered to the cortex elicits a delayed intra- and interhemispheric spread of activation that follows in spatial, temporal, and frequency domains | Effective connectivity | ↓ prefrontal connectivity | |
μV microvolts, ms milliseconds, NMDA N-methyl-D-aspartate, SZ schizophrenia, MNS mirror neuron system, TMS transcranial magnetic stimulation, TES transcranial electrical stimulation, EEG electroencephalography, EMG electromyography
Consistent findings are highlighted if data is available from meta-analyses (if available) or from at least two studies; emerging findings reflect more recent findings that are not replicated
Not different from healthy
Diminished as compared to healthy)
Enhanced as compared to healthy
The motivation behind the invention of TMS was primarily to use it as an investigational tool that could focally (and non-invasively) modulate brain activity [7]. Typically, a single stimulus (perturbation pulse) is applied over the scalp area corresponding to the motor cortex via a figure-of-eight coil that carries a large, brief pulse of current (~ 4000 A), generating a magnetic field, which passes effortlessly through a highresistance structure like the skull. Visible motor twitches in the contralateral limb arise from the resultant action potentials generated by ionic currents induced in the superficial brain by the rapid, time-varying magnetic field. Electromyography is typically used to measure the amplitude of this motor response.
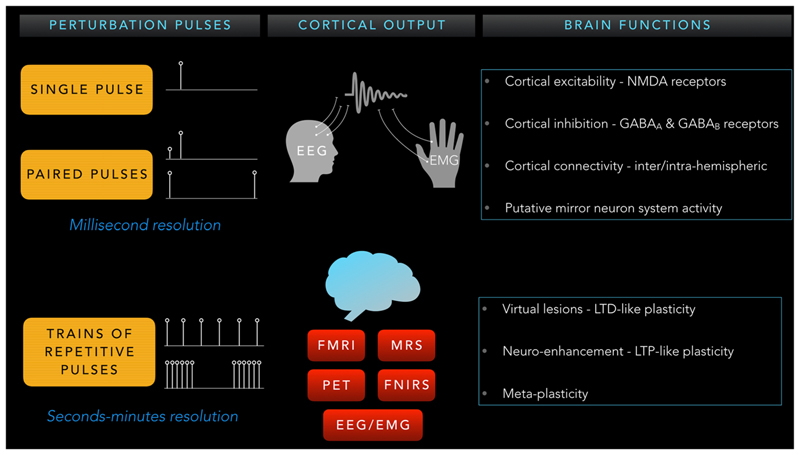
Depending on the number, frequency, and interval between pulses, diverse brain physiological systems can be probed to reveal both correlative and causal mechanisms of the schizophrenia pathophysiology (Fig. 1). Single and paired-pulse TMS paradigms are used to study functional brain dynamics through cortical reactivity (excitation and inhibition) and connectivity (inter- and intrahemispheric) at resting or active/task states with high temporal (order of milliseconds) resolution. In contrast, longer-lasting trains of rTMS pulses are used to manipulate neuronal activity to produce transient/virtual lesions or neuroenhancement, based on long-term depression (LTD)– or potentiation (LTP)-like plasticity effects. When applied in an offline manner, paired with an informed examination of cortical/ behavioral after-effects that last over several minutes, this technique permits the study of causal contributions of specific brain networks towards specific behaviors [5••].
Fig. 1. Schematic representation of different investigational TMS approaches.
Motor Cortical Reactivity
Balance of excitatory and inhibitory post-synaptic potentials generated through the stimulation of gamma-aminobutyric acid (GABA) and N-methyl-D-aspartate (NMDA) glutamate interneurons regulates pyramidal neuron firing. An optimal cortical excitation/inhibition balance is necessary for synchronized firing of neuronal networks that drive social behavior relevant to psychiatric disorders [8]. TMS can probe the functioning of these intracortical networks and their neurotransmitter systems using single/paired-pulse paradigms [4].
Cortical Excitability
Resting motor threshold (RMT), motor evoked potential (MEP) amplitude, and intracortical facilitation (ICF) capture cortical excitability. Voltage-gated Na+ channels and ionotropic non-NMDA glutamate receptors govern the transsynaptic effects of the TMS pulse on the corticospinal pyramidal neurons and spinal motoneurons, thus regulating RMT [9]. Trans-synaptic activation of pyramidal neurons via excitatory/inhibitory interneurons, and the moderating effects of dopamine, serotonin, norepinephrine, and acetylcholine, contribute to the MEP amplitude [9]. ICF reflects predominant facilitation triggered by stronger excitatory (glutamatergic; primarily NMDA) and weaker inhibitory (GABAergic; primarily GABAA) interneurons. A meta-analysis that examined pooled effect sizes in these excitability measures revealed no significant differences between schizophrenia patients and healthy comparison subjects [10•]. This indicates that the excitability of the motor cortex to an external perturbation TMS pulse is intact in schizophrenia.
Cortical Inhibition
Short-interval intracortical inhibition (SICI) and long-interval intracortical inhibition (LICI) are paired-pulse inhibitory TMS paradigms. Fast inhibitory post-synaptic potentials mediated by the rapid but less potent ionotropic GABAA receptors mediate SICI [11]. In contrast, slow inhibitory post-synaptic potentials mediated by the more potent metabotropic GABAB receptors mediate LICI [12]. GABAB receptor–driven motor cortical inhibition mediates the cortical silent period (CSP) [4]. While the observation of deficits in LICI and CSP (both mediated by GABAB receptors) was inconsistent, that of deficient SICI was consistent in schizophrenia (Hedge’s g = 0.47) [10•]. Diminished SICI is related to the strength of the prefrontal to motor cortex functional connectivity and the integrity of the connecting white matter tracts [13], suggesting a dysregulated top-down frontal inhibitory mechanism. Importantly, a deficit in SICI is not specific to schizophrenia; it is also observed in marginally greater magnitude (Hedge’s g ~ 0.6) in major depression and obsessive-compulsive disorder [10•]. SICI impairments in schizophrenia are related to deficits in both social [14] and non-social [15] cognition. A critical aspect of CSP in schizophrenia is that it is enhanced with clozapine treatment as compared to other antipsychotic medications [16, 17]; this not only provides insights into the mechanisms of clozapine but also provides a potential physiological marker of tracking symptom change. Recent studies in untreated patient populations [14, 18] have revealed that the balance between GABAa- and GABAB-mediated neurotransmission may differentiate schizophrenia (deficient GABAA and normal GABAB) patients from those with mania (deficient GABAA and elevated GABAB); however, these findings need replication.
Motor Resonance or Putative Mirror Neuron System Activity
The mirror neuron system (MNS) is a frontoparietal network of specialized nerve cells with dual properties—they discharge during action execution, as well as observation of the same action [19]. This dual property is thought to form an internal template to decode intentions underlying gestures, actions, and emotions in social interactions via an automatic reflexive mechanism referred to as embodied simulation [20]. Social behaviors like imitation and empathy are potential functional correlates of the MNS. TMS can be used to indirectly infer premotor MNS activity, via its posited connections with the motor cortex [21, 22]. Deficient MNS response was observed in un-medicated schizophrenia patients that had a significant association with the severity of their social cognition impairments [23, 24]. In contrast, an elevated MNS response (possible disinhibition) was demonstrated in unmedicated bipolar mania patients that correlated with hyperimitative behaviors (incidental echolalia) and manic symptom severity [25]. Evidence for such a deficit is not consistently reported in medicated schizophrenia patients [26, 27]. Recent investigations have attempted to examine MNS responses using more nuanced, social context–based action observation stimuli. Viewing actions within a context results in greater facilitation of motor cortical reactivity relative to neutral action observation in both patients and healthy subjects. However, this MNS response to context-based action observation was still blunted in schizophrenia patients treated with antipsychotic medications [28].
Cortical Connectivity
TMS-evoked cortical reactivity propagates to connected hubs of the network that is being stimulated. This propagation can inform about the integrity of the connecting white matter tracts, as well as examine functional properties (excitatory or inhibitory) of the stimulated networks.
Transcallosal Connectivity
The ipsilateral silent period (ISP) is a function of corpus callosum integrity [29] since it reflects transcallosal inhibition of the contralateral preactivated motor area by the motor area stimulated by TMS. This can also be evaluated by applying TMS pulses in close temporal approximation to both hemispheres. This transcallosal inhibition (TCI) is also cortical in origin and mediated by transcallosal motor fibers, thus providing a direct measure of optimal corpus callosum maturation/integrity [29]. TCI at shorter interstimulus intervals (7–10 ms) does not correlate with that at longer intervals (40 ms). However, the 40-ms TCI correlates with ISP duration, indicating both common and shared mechanisms [30]. Transcallosal facilitation (TCF) is the percentage of MEP facilitation relative to suprathreshold test pulse MEP from one hemisphere when a low-intensity conditioning pulse is delivered over the contralateral hemisphere 4–5 ms before the test pulse [31]. In schizophrenia, there is consistent evidence of diminished TCI and ISP [32–34], indicative of aberrant corpus callosum functioning. However, there is inconclusive evidence about interhemispheric facilitation in schizophrenia [33].
Motor Cortical Connectivity
Ipsilateral subthreshold parietal stimulation facilitates the MEP obtained from the motor cortex [35]. This twin-coil, paired-pulse TMS paradigm can provide a robust estimation of functional connectivity within the brain. This parieto-motor connectivity, structurally supported by the superior longitudinal fasciculus, is found to be deficient in patients with schizophrenia and has behavioral correlates with severity of the disabling negative symptoms [36]. On similar lines, premotor-motor connectivity is also found to be impaired in schizophrenia [37]—a subthreshold conditioning stimulus produces diminished facilitation of MEP elicited by a suprathreshold test pulse in the contralateral hemisphere in schizophrenia as compared to healthy subjects. Lastly, the inhibitory influence of the cerebellum on the motor cortex (via Purkinje cells) can also be examined with TMS, informing about the integrity of the cerebellar-thalamo-cortical connectivity. In schizophrenia, a diminished cerebellar inhibition of MEP was reported in a small study [38]. This is, however, in keeping with more recent findings of an association between negative symptom severity and aberrant cerebellar-prefrontal connectivity in schizophrenia [39•]. Delivering single TMS pulses to the motor cortex and examining the change in resting-state fMRI BOLD signals in the thalamus can reveal cortico-thalamic connectivity. The strength of this TMS-evoked fMRI response in the thalamus is significantly diminished in schizophrenia as compared to healthy subjects [40].
Motor Cortical Plasticity
Dysplasticity is a core pathophysiology of schizophrenia, which encompasses hypoplastic cognitive and volitional neural systems, as well as hyperplastic salience detection and emotion processing systems [41]. With the advent of diverse neuromodulatory techniques, we can now quantify and compare cortical plasticity in schizophrenia and healthy individuals [42–44]. TMS can be employed as both a neuromodulator—to induce plastic change, and, as a neurophysiological probe—to quantify the degree of plastic change, in combination with EMG, EEG, or fMRI. In schizophrenia, both TMS and transcranial electrical stimulation perturbation protocols have been used to study in vivo transitory cortical reactivity increases or decreases [45] that are thought to parallel the commonly observed in vitro longterm potentiation/depression (LTP/LTD)–like experience-dependent synaptic plasticity [46]. A recent meta-analysis of 16 datasets yielding data from 189 schizophrenia patients and 187 healthy controls [47•] to quantify the magnitude of such cortical plasticity impairments in schizophrenia revealed effect sizes ranging from 0.66 (LTP-like plasticity) to 0.68 (LTD-like plasticity). The consistency of these findings, despite the known clinical heterogeneity of schizophrenia, encourages the use of these biomarkers to characterize illness trajectories and treatment response. Future studies can also examine how the cortical excitation-inhibition balance influences cortical plasticity outcomes to the different neuromodulatory techniques [48]. Connectivity of the motor cortex to distinct premotor and parietal cortices can also be examined using such perturbation protocols. Delivering 1-Hz TMS to the premotor cortex resulted in suppression of motor cortical excitability in healthy individuals, but facilitation in schizophrenia patients [49]. In contrast, delivering 20-Hz TMS to the premotor/inferior frontal gyrus area enhanced MNS responsiveness in healthy individuals [50, 51]. This application of TMS has both heuristic (understanding the behavioral aftereffects of enhancing MNS activity) and translational (poten-tial treatment of social cognition deficits) applications when tested in patients with schizophrenia.
Cortical Reactivity and Connectivity in Non-motor Brain Regions
Earlier TMS investigations focused on the motor cortex. This limitation is overcome partly by the advent of TMS-EEG, which has ushered a new era of examining brain states and their dynamics in motor and non-motor regions [52••]. Cortical reactivity and effective connectivity can be recorded with EEG following a TMS pulse [53]. These responses are thought to reflect a summation of excitatory and inhibitory cortical pyramidal and intemeuron post-synaptic potentials [54]. Despite the tremendous challenges that come with the technique (especially handling artifacts and analyzing large data dimensions), there are important inferences from current TMS-EEG experiments that could be consolidated in the future. Schizophrenia patients demonstrated diminished TMS-evoked EEG gamma oscillations in the site of frontal stimulation; its propagation (connectivity) to other cortical regions was also restricted as compared to healthy subjects [55]. Slowing of the TMS-evoked EEG frequency in the prefrontal (and not in the parietal) cortex was associated with symptom severity and cognitive deficits in schizophrenia [56]. Similar aberrations in prefrontal reactivity were described in first-episode psychotic patients as well [57]. In a related investigation, schizophrenia patients demonstrated waves of recurrent excitation propagating throughout the cortex, as compared to a faster fading away in healthy subjects [58].
Further advances have been made in examining cortical inhibitory processes within the prefrontal cortex using TMS-EEG. SICE LICI, and ICF can now be determined in nonmotor areas, thus providing measurements of various excitatory and inhibitory intracortical circuit functioning. Inhibition or facilitation is measured as suppression or facilitation of specific positive (e.g., P30) and negative (e.g., N100) peaks of the TMS-evoked potentials (TEP) [59] or as a change in the average EEG amplitude [60]. Schizophrenia patients demonstrate diminished prefrontal LICI as compared to their unaffected relatives and healthy subjects [61]. They also revealed deficits in prefrontal SICI and ICF as compared to healthy subjects in some of the positive and negative peaks of the TEP [62]. Lastly, another inhibitory paradigm that has been examined using TMS-EEG is the short-latency afferent inhibition (SAI). Here, a somatosensory conditioning (electrical stimulation of a peripheral nerve) stimulus delivered at a short latency (20 ms) inhibits the MEP (motor cortex) or TEP (prefrontal cortex). This process is thought to be mediated by both cholinergic and GABAergic inputs [63]. The prefrontal (and not motor) SAI is seen to be deficient in schizophrenia and is related to their cognitive performance [64]. Nevertheless, these TMS-EEG results are very preliminary findings that require replication in future studies.
TMS in the Treatment of Schizophrenia
TMS has been used in the treatment of difficult-to-treat symptoms of schizophrenia, like persistent auditory hallucinations, negative symptoms, and cognitive deficits. There is a need for exploring alternative, neuroscience-informed treatments of these symptoms. Administering repetitive TMS treatment successively for several days can leverage the unique focal neuromodulatory property of TMS in bringing about behavioral change based on the site and pattern of stimulation, guided by our current understanding of the neurobiology of these symptom dimensions.
Conventional TMS can either increase or decrease cortical activity depending on the frequency used to administer magnetic pulses. High-frequency TMS (10 or 20 Hz) enhances cortical activity while low-frequency TMS (1 Hz) reduces it. Recently introduced theta burst stimulation (TBS) uses bursts of very high frequency (50 Hz) repeatedly in either intermitted or continuous pattern to bring about stimulation or inhibition of cortical neiuons, respectively [65]. TBS has advantages of being brief and has longer-lasting neuroplasticity changes and a theoretically lower risk of inducing seizures compared to conventional TMS.
Positive Symptoms
Apart from its effects on resistant auditory hallucinations, the utility of TMS in treating other positive symptoms like delusions, made phenomena, or formal thought disorder is limited, with the current stimulation site and parameters. Speech processing brain regions in the bilateral temporal lobes are hyperactive during ongoing auditory hallucinations [66]. Therefore, low-frequency (1-Hz) rTMS has been used to reduce this cortical hyper-activation to treat auditory hallucinations [67]. Recent meta-analyses on the therapeutic effects of rTMS for auditory hallucinations reveal significant benefits as compared to sham stimulation, with varying effect sizes: 0.29 [68•], 0.44 [69], 0.49 [70], and 0.51 [71••]. However, these come with several caveats. The effect sizes observed in more recent metaanalyses, which include larger samples, are lower than those reported in earlier analyses with smaller samples but stronger effects of 0.8 [72] to 1 [73]. There is a high degree of variability in the stimulation parameters, brain region targeted, the degree of treatment resistance, and duration of treatment. Concerns of publication bias, where negative trials may not have been published and unstable results on sensitivity analyses by removing individual trials [68•], also require consideration. The most common and perhaps most effective among all stimulation protocols is the low-frequency (1-Hz) stimulation over the left temporoparietal cortex [69]. This protocol, however, has limited benefits for other psychotic symptoms [69, 71••]. Interestingly, within-group (before and after treatment) effects of placebo (sham) treatment are also significant (effect size of 0.29) [74]; however, it must be noted that the effect sizes reported in the meta-analyses quoted above are for between-group differences (true versus sham). The durability of beneficial effects is short-lasting (4–6 weeks), and this underlines the importance of continued medical treatment with antipsychotics [69], as well as a systematic study of pragmatic maintenance treatment strategies [75].
Younger age, female gender [76], higher co-prescribed antipsychotic dose, short (< 3-week) trial duration [71 ••], shorter scalp-to-temporal cortex distance [77], and increased regional cerebral blood flow at the site of stimulation before treatment are associated with better response [78]. High emotional salience to die hallucinations predicts a better response to rightsided 1-Hz rTMS [79]. While some of these predictive findings are plausible from a neurophysiological perspective, others (e.g., shorter trial duration and greater improvement) are indeed counterintuitive, requiring more systematic investigation. Given the promising but variable results, investigators have attempted to examine means to enhance the efficacy and durability of rTMS effects. High-frequency (20-Hz) rTMS to the left temporoparietal cortex [80], high-frequency primed 1-Hz rTMS [81], structural [82] and functional [83] MRI guided or neuronavigational rTMS, theta burst stimulation [84], bilateral sequential rTMS [85], and deep TMS [86] have been used to enhance treatment outcomes, with mixed results. Indications other than treatment resistance are under-represented. For example, the potential benefits during early-course treatment of schizophrenia, its utility in children, pregnancy, and maintenance therapies have not been evaluated sufficiently.
Negative Symptoms
TMS is typically administered to enhance the “hypofrontality” of the dominant prefrontal cortex for improving negative symptoms. Results from two recent meta-analyses [71••, 87••] suggest a small-to-moderate benefit of true TMS over sham in improving negative symptoms (effect sizes 0.49 to 0.64). A third meta-analysis included studies examining only 10-Hz rTMS with more stringent selection criteria and did not show any significant benefit of true TMS [68•]. In a sensitivity analysis that excluded two studies with unusually high effects of true TMS, the overall benefits of true TMS over sham TMS persisted at a much lower (effect size 0.31) magnitude [87••]. Higher pulse frequency (10–20 Hz), stronger stimulus intensity (100–110% RMT), longer treatment duration (> 3 weeks), younger age (< 39 years), and shorter duration of illness (< 13 years) were associated with better outcomes [71••, 87••]. However, there was also a trend suggesting a worsening of positive symptoms with this treatment protocol for negative symptoms, thus necessitating stringent monitoring [71••]. Structural plasticity of the left hippocampus and precuneus also predicts negative symptom improvement following left prefrontal stimulation [88•].
Nevertheless, these are short-term effects, from small trials and influenced by heterogeneity in the recruited patients and the technique of TMS administration. Not all studies control for the moderating effects of improvement in depression since the stimulation protocol is similar [89]. Interestingly, sham stimulation also resulted in a significant within-group improvement with an effect size of 0.31 [87••]. Moreover, an adequately powered multi-center trial failed to demonstrate any beneficial effects of true left prefrontal TMS over sham in improving negative symptoms immediately post-treatment and at the 3-month follow-up [90•].
These observations have led to the investigation of stimulating newer sites (e.g., cerebellum) with different parameters. Prefrontal TBS [91], deep prefrontal TMS [92], and bilateral prefrontal TMS [93] are modifications of stimulation technique that have been examined for negative symptoms, with limited and mixed results. One promising alternative approach is to target prefrontal activity by stimulating its connections within the cerebellum. In a recent study, the functional connectivity of the cerebellum with the right prefrontal cortex was inversely associated with negative symptom severity; modulating the cerebellum using intermittent theta burst stimulation (iTBS) resulted in improvement of negative symptoms and reversal of this functional dysconnectivity in schizophrenia [39•]. These emerging findings provide further scientific rationale to explore cerebellar iTBS in the treatment of negative symptoms [94, 95]. A recent study from our facility randomized 60 patients to active and sham groups of intermittent TBS at 90% of RMT over the cerebellar vermis administered twice daily for 5 days. Both groups demonstrated a significant improvement in negative symptoms at the end of treatment and at the 6-week followup, but only the hue TMS group revealed a significant engagement of the cerebellar-prefrontal resting-state functional connectivity [96]. Future studies may examine more prolonged and potent activation of this neural circuit.
Cognitive Symptoms
Cognitive deficits across social and non-social domains are a significant hallmark of schizophrenia [97]. They persist during symptom remission [98] and are an important determinant of real-world functioning [99]. With the advent of rTMS as a viable therapeutic option for depression [100], there was an active call for monitoring its cognitive safety [101]. Over the years, it became apparent that TMS had limited detrimental effects on cognition, and could perhaps be leveraged to enhance cognition when delivered under strictly monitored conditions [102, 103]. Also, trials that examined the benefits of TMS for negative symptoms noticed improvements in cognition in the active TMS group [104]. Subsequently, trials have been designed with specific aims of evaluating the cognitive benefits of rTMS in schizophrenia. Given the critical role of the prefrontal cortex in cognitive dysfunction of schizophrenia [105], and the ease with which it can be targeted, most studies have attempted to deliver high-frequency rTMS to the left or bilateral prefrontal cortices.
A pilot randomized controlled trial of 20-Hz bilateral prefrontal rTMS over 4 weeks demonstrated significant improvement in working memory as compared to sham rTMS in schizophrenia [106]. Modulation of task-related frontal gamma oscillatory activity may drive this response [107]. Another trial examined 10-Hz bilateral prefrontal rTMS at subthreshold doses (90% RMT), but longer train duration (10 s); cognition improved only in one (verbal fluency) of the seven domains assessed [93]. Similar improvements in overall neurocognitive performance were observed in a more recent trial with a small sample receiving a similar bilateral 20-Hz stimulation, but for 2 weeks [108]. The improvement persisted even at the 2-week follow-up, and baseline left frontal cortical thickness correlated with treatment response. However, a large, multi-center study involving 156 schizophrenia patients found no benefits of 3-week left prefrontal 10-Hz stimulation as compared to sham stimulation [109]. Interestingly, 2-week left prefrontal 10-Hz stimulation improved a measure of social cognition (facial emotion recognition) in schizophrenia as compared to sham stimulation, thus opening a window of opportunity to explore newer neuromodulatory techniques to improve the disabling social cognition deficits in schizophrenia [110]. A recent meta-analysis of cognitive benefits with rTMS in schizophrenia reported a beneficial effect size of 0.34 in improving working memory as compared to sham stimulation [111•]; these benefits persisted when reassessments were performed approximately 1 month after stopping treatment.
Nevertheless, the modest degree of improvement in limited cognitive domains requires the study of novel stimulation patterns, targeting different brain networks. In this regard, hippocampal-functional connectivity–guided stimulation of the lateral parietal cortex has shown improvements in associative memory in healthy individuals [112•]; this technique holds promise for exploration in schizophrenia. Lastly, combining rTMS with other known therapies for cognitive enhancement may be able to yield more potent therapeutic gains [113]; future studies may explore this avenue. This field is still nascent, and important challenges regarding matching the best stimulation parameters for the specific cognitive deficits a given patient experiences still remain. In addition, safety, durability of benefits, their generalizability to real-world functioning, and dissemination to a larger community patient population require more refined study [114].
Conclusions and Future Directions
With its immense potential of (a) evoking brain responses with precision temporal resolution and (b) targeted modulation of cortical activity and therefore behavior with successive trains of stimulation, TMS is an invaluable tool for both neuroscientists and psychiatrists. Future studies must focus on consolidating the critical leads derived in the last three decades.
As for investigational applications, the field has gradually moved to incorporate multimodal strategies that combine TMS with functional neuroimaging [115] and high-density EEG [52••] studies. While these methods are technologically challenging, they have the potential to reveal brain dynamics in schizophrenia at rest and during tasks, with much better spatiotemporal accuracy. This may result in more accurate diagnostic and prognostic biomarkers. Important methodological challenges and variability in responses still remain, especially in terms of the stimulation parameters to elicit cortical reactivity/ connectivity. Systematic studies to ascertain the reliability and validity of these measurements will go a long way in bridging the translational application gap of these techniques [13, 116].
Therapeutic use of rTMS in schizophrenia has not achieved success as in depression. Larger, high-quality, multi-center studies are very few, and they do not support its unequivocal clinical utility. Nevertheless, meta-analyses suggest small to modest effect size benefits of rTMS over placebo treatment for all three symptom dimensions described above. The clinical applicability of small effect treatments appears discouraging. However, it must be noted that most of these trials have been conducted on patients with resistant or difficult-to-treat symptoms. This also emphasizes an urgent need to strategize and identify key thrust areas for research. Clinicians must work in tandem with basic neuroscientists and engineers to refine stimulation parameters (site, dose, duration, etc.) that would improve clinical benefits without compromising safety. The clinical effectiveness of newer treatments must be examined in large, multi-center trials that are sufficiently powered to yield reliable and consistent results. Clinical trials need to be supplemented by mechanistic studies that identify key processes driving clinical benefits. Lastly, future studies should explore demographic, clinical, genomic, and brain physiological reasons for variable treatment response. This could help identify the category of patients responding well to rTMS, and set the platform to explore next-generation personalized treatment approaches catered to a given person’s symptoms based on individualized biological markers.
Funding Information
UMM was supported by the Wellcome Trust/DBT India Alliance Early Career Fellowship, Grant/Award Number: IA/E/12/1/500755.
Compliance with Ethical Standards
Conflict of Interest Urvakhsh Meherwan Mehta serves as an Associate Editor at Schizophrenia Research and receives an honorarium from Elsevier for the same.
Shalini S Naik, Milind Vijay Thanki, and Jagadisha Thirthalli each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97. doi: 10.1016/S0140-6736(15)01121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr Res. 2008;102:1–18. doi: 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 3.de Araujo AN, de Sena EP, de Oliveira IR, Juruena MF. Antipsychotic agents: efficacy and safety in schizophrenia. Drug Healthc Patient Saf. 2012;4:173–80. doi: 10.2147/DHPS.S37429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClintock SM, Freitas C, Oberman L, Lisanby SH, Pascual-Leone A. Transcranial magnetic stimulation: a neuroscientific probe of cortical function in schizophrenia. Biol Psychiatry. 2011;70:19–27. doi: 10.1016/j.biopsych.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polania R, Nitsche MA, Ruff CC. Studying and modifying brain function with non-invasive brain stimulation. Nat Neurosci. 2018;21:174–87. doi: 10.1038/s41593-017-0054-4. [ •• This is a state-of-the-art review of using neuromodulation techniques like TMS and others for investigating brain-behavior relationships over the last three decades. ] [DOI] [PubMed] [Google Scholar]
- 6.Dougall N, Maayan N, Soares-Weiser K, McDermott LM, McIntosh A. Transcranial magnetic stimulation (TMS) for schizophrenia. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD006081.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;325:1106–7. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 8.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–8. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulus W, Classen J, Cohen LG, Large CH, Di Lazzaro V, Nitsche M, et al. State of the art: pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimulation. 2008;1:151–63. doi: 10.1016/j.brs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Radhu N, de Jesus DR, Ravindran LN, Zanjani A, Fitzgerald PB, Daskalakis ZJ. A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clin Neurophysiol. 2013;124:1309–20. doi: 10.1016/j.clinph.2013.01.014. [ • This is the only meta-analysis of investigational TMS studies in schizophrenia and other psychiatric disorders. ] [DOI] [PubMed] [Google Scholar]
- 11.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol Lond. 1993;471:501–19. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–64. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- 13.Du X, Choa F-S, Chiappelli J, Wisner KM, Wittenberg G, Adhikari B, et al. Aberrant middle prefrontal-motor cortex connectivity mediates motor inhibitory biomarker in schizophrenia. Biol Psychiatry. 2019;85:49–59. doi: 10.1016/j.biopsych.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta UM, Thirthalli J, Basavaraju R, Gangadhar BN. Association of intracortical inhibition with social cognition deficits in schizophrenia: findings from a transcranial magnetic stimulation study. Schizophr Res. 2014;158:146–50. doi: 10.1016/j.schres.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi S, Ukai S, Kose A, Hashimoto T, Iwatani J, Okumura M, et al. Reduction of cortical GABAergic inhibition correlates with working memory impairment in recent onset schizophrenia. Schizophr Res. 2013;146:238–43. doi: 10.1016/j.schres.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 16.Daskalakis ZJ, Christensen BK, Fitzgerald PB, Moller B, Fountain SI, Chen R. Increased cortical inhibition in persons with schizophrenia treated with clozapine. J Psychopharmacol. 2008;22:203–9. doi: 10.1177/0269881107084002. [DOI] [PubMed] [Google Scholar]
- 17.Liu SK, Fitzgerald PB, Daigle M, Chen R, Daskalakis ZJ. The relationship between cortical inhibition, antipsychotic treatment, and the symptoms of schizophrenia. Biol Psychiatry. 2009;65:503–9. doi: 10.1016/j.biopsych.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Basavaraju R, Sanjay TN, Mehta UM, Muralidharan K, Thirthalli J. Cortical inhibition in symptomatic and remitted mania compared to healthy subjects: a cross-sectional study. Bipolar Disord. 2017;19:698–703. doi: 10.1111/bdi.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. 1992;91:176–80. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- 20.Gallese V. Before and below “theory of mind”: embodied simulation and the neural correlates of social cognition. Philos Trans R Soc Lond Ser B Biol Sci. 2007;362:659–69. doi: 10.1098/rstb.2006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol. 1995;73:2608–11. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- 22.Mehta UM, Thirthalli J, Aneekaj D, Jadhav P, Gangadhar BN, Keshavan MS. Mirror neuron dysfunction in schizophrenia and its functional implications: a systematic review. Schizophr Res. 2014;160:9–19. doi: 10.1016/j.schres.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta UM, Thirthalli J, Basavaraju R, Gangadhar BN, Pascual-Leone A. Reduced mirror neuron activity in schizophrenia and its association with theory of mind deficits: evidence from a transcranial magnetic stimulation study. Schizophr Bull. 2014;40:1083–94. doi: 10.1093/schbul/sbt155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta UM, Ashok AH, Thirthalli J, Keshavan MS. Early motor resonance differentiates schizophrenia patients from healthy subjects and predicts social cognition performance. Prog Brain Res. 2019 doi: 10.1016/bs.pbr.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Basavaraju R, Mehta UM, Pascual-Leone A, Thirthalli J. Elevated mirror neuron system activity in bipolar mania: evidence from a transcranial magnetic stimulation study. Bipolar Disord. 2019;21:259–69. doi: 10.1111/bdi.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enticott PG, Hoy KE, Herring SE, Johnston PJ, Daskalakis ZJ, Fitzgerald PB. Reduced motor facilitation during action observation in schizophrenia: a mirror neuron deficit? Schizophr Res. 2008;102:116–21. doi: 10.1016/j.schres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Andrews SC, Enticott PG, Hoy KE, Thomson RH, Fitzgerald PB. No evidence for mirror system dysfunction in schizophrenia from a multimodal TMS/EEG study. Psychiatry Res. 2015;228:431–40. doi: 10.1016/j.psychres.2015.05.067. [DOI] [PubMed] [Google Scholar]
- 28.Bagewadi VI, Mehta UM, Naik SS, Govindaraj R, Varambally S, Arumugham SS, et al. Diminished modulation of motor cortical reactivity during context-based action observation in schizophrenia. Schizophr Res. 2019;204:222–9. doi: 10.1016/j.schres.2018.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–46. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen R, Yung D, Li J-Y. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–64. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- 31.Hanajima R, Ugawa Y, Machii K, Mochizuki H, Terao Y, Enomoto H, et al. Interhemispheric facilitation of the hand motor area in humans. J Physiol. 2001;531:849–59. doi: 10.1111/j.1469-7793.2001.0849h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry. 2002;59:347–54. doi: 10.1001/archpsyc.59.4.347. [DOI] [PubMed] [Google Scholar]
- 33.Hoy KE, Georgiou-Karistianis N, Laycock R, Fitzgerald PB. A transcranial magnetic stimulation study of transcallosal inhibition and facilitation in schizophrenia. J Clin Neurosci. 2008;15:863–7. doi: 10.1016/j.jocn.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Fitzgerald PB, Brown TL, Daskalakis ZJ, deCastella A, Kulkami J. A study of transcallosal inhibition in schizophrenia using transcranial magnetic stimulation. Schizophr Res. 2002;56:199–209. doi: 10.1016/s0920-9964(01)00222-5. [DOI] [PubMed] [Google Scholar]
- 35.Koch G, Fernandez Del Olrno M, Cheeran B, Ruge D, Schippling S, Caltagirone C, et al. Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J Neurosci. 2007;27:6815–22. doi: 10.1523/JNEUROSCI.0598-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koch G, Ribolsi M, Mori F, Sacchetti L, Codeca C, Rubino LA, et al. Connectivity between posterior parietal cortex and ipsilateral motor cortex is altered in schizophrenia. Biol Psychiatry. 2008;64:815–9. doi: 10.1016/j.biopsych.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 37.Ribolsi M, Mori F, Magni V, Codeca C, Kusayanagi H, Monteleone F, et al. Impaired inter-hemispheric facihtatory connectivity in schizophrenia. Clin Neurophysiol. 2011;122:512–7. doi: 10.1016/j.clinph.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Daskalakis ZJ, Christensen BK, Fitzgerald PB, Fountain SI, Chen R. Reduced cerebellar inhibition in schizophrenia: a preliminary study. Am J Psychiatry. 2005;162:1203–5. doi: 10.1176/appi.ajp.162.6.1203. [DOI] [PubMed] [Google Scholar]
- 39.Brady RO, Gonsalvez I, Lee I, Ongiir D, Seidman LJ, Schmahmann JD, et al. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. AJP Appi Ajp. 2019;2018 doi: 10.1176/appi.ajp.2018.18040429. 18040429. [• This article demonstrates the role of the cerebellar-prefrontal resting state network in negative symptoms of schizophrenia by using TMS to alter the connectivity and hence symptoms.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guller Y, Ferrarelli F, Shackman AJ, Sarasso S, Peterson MJ, Langheim FJ, et al. Probing thalamic integrity in schizophrenia using concurrent transcranial magnetic stimulation and functional magnetic resonance imaging. Arch Gen Psychiatry. 2012;69:662–71. doi: 10.1001/archgenpsychiatry.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keshavan MS, Mehta UM, Padmanabhan JL, Shah JL. Dysplasticity, metaplasticity, and schizophrenia: implications for risk, illness, and novel interventions. Dev Psychopathol. 2015;27:615–35. doi: 10.1017/S095457941500019X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhandari A, Voineskos D, Daskalakis ZJ, Rajji TK, Blumberger DM. A review of impaired neuroplasticity in schizophrenia investigated with non-invasive brain stimulation. Front Psychiatry. 2016;7 doi: 10.3389/fpsyt2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasan A, Falkai P, Wobrock T. Transcranial brain stimulation in schizophrenia: targeting cortical excitability, connectivity and plasticity. Curr Med Chem. 2013;20:405–13. [PubMed] [Google Scholar]
- 44.Voineskos D, Rogasch NC, Rajji TK, Fitzgerald PB, Daskalakis ZJ. A review of evidence linking disrupted neural plasticity to schizophrenia. Can J Psychiatr. 2013;58:86–92. doi: 10.1177/070674371305800205. [DOI] [PubMed] [Google Scholar]
- 45.Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129:1659–73. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- 46.Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–5. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- 47.Mehta UM, Thanki MV, Padmanabhan J, Pascual-Leone A, Keshavan MS. Motor cortical plasticity in schizophrenia: a meta-analysis of transcranial magnetic stimulation - electromyography studies. Schizophr Res. 2019;207:37–47. doi: 10.1016/j.schres.2018.10.027. [ • This article reports a meta-analytic quantification of cortical plasticity impairments in schizophrenia as assessed using TMS-EMG studies. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–88. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 49.Oxley T, Fitzgerald PB, Brown TL, de Castella A, Jeff Daskalakis Z, Kulkami J. Repetitive transcranial magnetic stimulation reveals abnormal plastic response to premotor cortex stimulation in schizophrenia. Biol Psychiatry. 2004;56:628–33. doi: 10.1016/j.biopsych.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 50.Meherwan Mehta U, Agarwal SM, Kalmady SV, Shivakumar V, Kumar CN, Venkatasubramanian G, et al. Enhancing putative mirror neuron activity with magnetic stimulation: a single-case functional neuroimaging study. Biol Psychiatry. 2013;74:e1–2. doi: 10.1016/j.biopsych.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Mehta UM, Waghmare AV, Thirthalli J, Venkatasubramanian G, Gangadhar BN. Is the human mirror neuron system plastic? Evidence from a transcranial magnetic stimulation study. Asian J Psychiatr. 2015;17:71–7. doi: 10.1016/j.ajp.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 52.Tremblay S, Rogasch NC, Premoli I, et al. Clinical utility and prospective of TMS-EEG. Clin Neurophysiol. 2019;130:802–44. doi: 10.1016/j.clinph.2019.01.001. [ •• This manuscript is an up-to-date review on basic principles, clinical utility and future applications of TMS-EEG studies in brain disorders. ] [DOI] [PubMed] [Google Scholar]
- 53.Dmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Naatanen R, et al. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8:3537–40. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- 54.Kirschstein T, Kohling R. What is the source of the EEG? Clin EEG Neurosci. 2009;40:146–9. doi: 10.1177/155005940904000305. [DOI] [PubMed] [Google Scholar]
- 55.Ferrarelli F, Massimini M, Peterson MJ, et al. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study. Am J Psychiatry. 2008;165:996–1005. doi: 10.1176/appi.ajp.2008.07111733. [DOI] [PubMed] [Google Scholar]
- 56.Ferrarelli F, Sarasso S, Guller Y, Riedner BA, Peterson MJ, Bellesi M, et al. Reduced natural oscillatory frequency of frontal thalamocortical circuits in schizophrenia. Arch Gen Psychiatry. 2012;69:766–74. doi: 10.1001/archgenpsychiatry.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferrarelli F, Kaskie RE, Graziano B, Reis CC, Casali AG. Abnormalities in the evoked frontal oscillatory activity of first-episode psychosis: a TMS/EEG study. Schizophr Res. 2019;206:436–9. doi: 10.1016/j.schres.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 58.Frantseva M, Cui J, Farzan F, Chinta LV, Perez Velazquez JL, Daskalakis ZJ. Disrupted cortical conductivity in schizophrenia: TMS-EEG study. Cereb Cortex. 2014;24:211–21. doi: 10.1093/cercor/bhs304. [DOI] [PubMed] [Google Scholar]
- 59.Rogasch NC, Daskalakis ZJ, Fitzgerald PB. Mechanisms underlying long-interval cortical inhibition in the human motor cortex: a TMS-EEG study. J Neurophysiol. 2013;109:89–98. doi: 10.1152/jn.00762.2012. [DOI] [PubMed] [Google Scholar]
- 60.Daskalakis ZJ, Farzan F, Barr MS, Mailer JJ, Chen R, Fitzgerald PB. Long-interval cortical inhibition from the dorsolateral prefrontal cortex: a TMS-EEG study. Neuropsychopharmacology. 2008;33:2860–9. doi: 10.1038/npp.2008.22. [DOI] [PubMed] [Google Scholar]
- 61.Radhu N, Dominguez LG, Greenwood TA, Farzan F, Semeralul MO, Richter MA, et al. Investigating cortical inhibition in first-degree relatives and probands in schizophrenia. Sci Rep. 2017;7:43629. doi: 10.1038/srep43629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noda Y, Barr MS, Zomorrodi R, Cash RFH, Farzan F, Rajji TK, et al. Evaluation of short interval cortical inhibition and intracortical facilitation from the dorsolateral prefrontal cortex in patients with schizophrenia. Sci Rep. 2017;7:17106. doi: 10.1038/s41598-017-17052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523:503–13. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noda Y, Barr MS, Zomorrodi R, Cash RFH, Rajji TK, Farzan F, et al. Reduced short-latency afferent inhibition in prefrontal but not motor cortex and its association with executive function in schizophrenia: a combined TMS-EEG study. Schizophr Bull. 2018;44:193–202. doi: 10.1093/schbul/sbx041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 66.Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57:1033–8. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- 67.Hoffman RE, Boutros NN, Berman RM, Roessler E, Belger A, Krystal JH, et al. Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated “voices.”. Biol Psychiatry. 1999;46:130–2. doi: 10.1016/s0006-3223(98)00358-8. [DOI] [PubMed] [Google Scholar]
- 68.He H, Lu J, Yang L, Zheng J, Gao F, Zhai Y, et al. Repetitive transcranial magnetic stimulation for treating the symptoms of schizophrenia: a PRISMA compliant meta-analysis. Clin Neurophysiol. 2017;128:716–24. doi: 10.1016/j.clinph.2017.02.007. [ • This is one of the recent meta-analysis of therapeutic value of TMS in schizophrenia. ] [DOI] [PubMed] [Google Scholar]
- 69.Slotema CW, Blom JTD, van Lutterveld R, Hoek HW, Sommer IEC. Review of the efficacy of transcranial magnetic stimulation for auditory verbal hallucinations. Biol Psychiatry. 2014;76:101–10. doi: 10.1016/j.biopsych.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 70.Otani VHO, Shiozawa P, Cordeiro Q, Uchida RR. A systematic review and meta-analysis of the use of repetitive transcranial magnetic stimulation for auditory hallucinations treatment in refiactory schizophrenic patients. Int J Psychiatry Clin Pract. 2015;19:228–32. doi: 10.3109/13651501.2014.980830. [DOI] [PubMed] [Google Scholar]
- 71.Kennedy NI, Lee WH, Frangou S. Efficacy of non-invasive brain stimulation on the symptom dimensions of schizophrenia: a metaanalysis of randomized controlled trials. Eur Psychiatry. 2018;49:69–77. doi: 10.1016/j.eurpsy.2017.12.025. [ •• This is the most recent meta-analysis of therapeutic value of TMS in schizophrenia. ] [DOI] [PubMed] [Google Scholar]
- 72.Aleman A, Sommer IE, Kahn RS. Efficacy of slow repetitive transcranial magnetic stimulation in the treatment of resistant auditory hallucinations in schizophrenia: a meta-analysis. J Clin Psychiatry. 2007;68:416–21. doi: 10.4088/jcp.v68n0310. [DOI] [PubMed] [Google Scholar]
- 73.Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108:11–24. doi: 10.1016/j.schres.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dollfus S, Lecardeur L, Morello R, Etard O. Placebo response in repetitive transcranial magnetic stimulation trials of treatment of auditory hallucinations in schizophrenia: a meta-analysis. Schizophr Bull. 2016;42:301–8. doi: 10.1093/schbul/sbv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thirthalli J, Bharadwaj B, Kulkami S, Gangadhar BN, Kharawala S, Andrade C. Successfid use of maintenance rTMS for 8 months in a patient with antipsychotic-refractory auditory hallucinations. Schizophr Res. 2008;100:351–2. doi: 10.1016/j.schres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 76.Koops S, Slotema CW, Kos C, Bais L, Aleman A, Blom JTD, et al. Predicting response to rTMS for auditory hallucinations: younger patients and females do better. Schizophr Res. 2018;195:583–4. doi: 10.1016/j.schres.2017.08.060. [DOI] [PubMed] [Google Scholar]
- 77.Nathou C, Simon G, Dollfus S, Etard O. Cortical anatomical variations and efficacy of rTMS in the treatment of auditory hallucinations. Brain Stimulation. 2015;8:1162–7. doi: 10.1016/j.brs.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 78.Homan P, Kindler J, Hauf M, Hubl D, Dierks T. Cerebral blood flow identifies responders to transcranial magnetic stimulation in auditory verbal hallucinations. Transl Psychiatry. 2012;2:e189–9. doi: 10.1038/tp.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hoffman RE, Wu K, Pittman B, Cahill JD, Hawkins KA, Fernandez T, et al. Transcranial magnetic stimulation of Wernicke’s and right homologous sites to curtail “voices”: a randomized trial. Biol Psychiatry. 2013;73:1008–14. doi: 10.1016/j.biopsych.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Weijer AD, Sommer IEC, Lotte Meijering A, Bloemendaal M, Neggers SFW, Daalman K, et al. High frequency rTMS; a more effective treatment for auditory verbal hallucinations? Psychiatry Res. 2014;224:204–10. doi: 10.1016/j.pscychresns.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 81.Blumberger DM, Christensen BK, Zipursky RB, Moller B, Chen R, Fitzgerald PB, et al. MRI-targeted repetitive transcranial magnetic stimulation of Heschl’s gyms for refractory auditory hallucinations. Brain Stimulation. 2012;5:577–85. doi: 10.1016/j.brs.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 82.Dollfus S, Jaafari N, Guillin O, Trojak B, Plaze M, Saba G, et al. High-frequency neuronavigated rTMS in auditory verbal hallucinations: a pilot double-blind controlled study in patients with schizophrenia. Schizophr Bull. 2018;44:505–14. doi: 10.1093/schbul/sbx127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Diederen KMJ, Charbonnier L, Neggers SFW, van Lutterveld R, Daalman K, Slotema CW, et al. Reproducibility of brain activation during auditory verbal hallucinations. Schizophr Res. 2013;146:320–5. doi: 10.1016/j.schres.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 84.Koops S, Dellen E, van Schutte MJL, Nieuwdorp W, Neggers SFW, Sommer IEC. Theta burst transcranial magnetic stimulation for auditory verbal hallucinations: negative findings from a double-blind-randomized trial. Schizophrenia Bulletin sbvlOO. 2015 doi: 10.1093/schbul/sbv100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Plewnia C, Zwissler B, Wasserka B, Fallgatter AJ, Klingberg S. Treatment of auditory hallucinations with bilateral theta burst stimulation: a randomized controlled pilot trial. Brain Stimulation. 2014;7:340–1. doi: 10.1016/j.brs.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 86.Rosenberg O, Gersner R, Klein LD, Kotler M, Zangen A, Dannon P. Deep transcranial magnetic stimulation add-on for the treatment of auditory hallucinations: a double-blind study. Ann General Psychiatry. 2012;11:13. doi: 10.1186/1744-859X-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aleman A, Enriquez-Geppert S, Knegtering H, Dlabac-de Lange JJ. Moderate effects of noninvasive brain stimulation of the frontal cortex for improving negative symptoms in schizophrenia: metaanalysis of controlled trials. Neurosci Biobehav Rev. 2018;89:1118. doi: 10.1016/j.neubiorev.2018.02.009. [ •• This study is a recent meta-analysis of TMS and TES treatments in negative symptoms of schizophrenia. ] [DOI] [PubMed] [Google Scholar]
- 88.Hasan A, Wobrock T, Guse B, et al. Structural brain changes are associated with response of negative symptoms to prefrontal repetitive transcranial magnetic stimulation in patients with schizophrenia. Mol Psychiatry. 2017;22:857–64. doi: 10.1038/mp.2016.161. [ • This study identifies structural brain markers (hippocampus and precuneus) that predict improvement in negative symptoms of schizophrenia. ] [DOI] [PubMed] [Google Scholar]
- 89.Lefaucheur J-P, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin Neurophysiol. 2014;125:2150–206. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 90.Wobrock T, Guse B, Cordes J, et al. Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry. 2015;77:979–88. doi: 10.1016/j.biopsych.2014.10.009. [ • This is a large multi-center trial of rTMS to the prefrontal cortex for treating negative symptoms in schizophrenia. ] [DOI] [PubMed] [Google Scholar]
- 91.Zhao S, Kong J, Li S, Tong Z, Yang C, Zhong H. Randomized controlled trial of four protocols of repetitive transcranial magnetic stimulation for treating the negative symptoms of schizophrenia. Shanghai Arch Psychiatry. 2014;26:15–21. doi: 10.3969/j.issn.1002-0829.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rabany L, Deutsch L, Levkovitz Y. Double-blind, randomized sham controlled study of deep-TMS add-on treatment for negative symptoms and cognitive deficits in schizophrenia. J Psychopharmacol. 2014;28:686–90. doi: 10.1177/0269881114533600. [DOI] [PubMed] [Google Scholar]
- 93.Dlabac-de Lange JJ, Bais L, van Es FD, Visser BGJ, Reinink E, Bakker B, et al. Efficacy of bilateral repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: results of a multicenter double-blind randomized controlled trial. Psychol Med. 2015;45:1263–75. doi: 10.1017/S0033291714002360. [DOI] [PubMed] [Google Scholar]
- 94.Demirtas-Tatlidede A, Freitas C, Cromer JR, Safar L, Ongur D, Stone WS, et al. Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophr Res. 2010;124:91–100. doi: 10.1016/j.schres.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garg S, Sinha VK, Tikka SK, Mishra P, Goyal N. The efficacy of cerebellar vermal deep high frequency (theta range) repetitive transcranial magnetic stimulation (rTMS) in schizophrenia: a randomized rater blind-sham controlled study. Psychiatry Res. 2016;243:413–20. doi: 10.1016/j.psychres.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 96.Basavaraju R, Ithal D, Thanki M, Hr A, Thirthalli J, Pascual-Leone A, et al. T79. Intermittent theta burst stimulation of cerebellar vermis in schizophrenia: impact on negative symptoms and brain connectivity. Schizophr Bull. 2019;45:S234–4. [Google Scholar]
- 97.Mehta UM, Thirthalli J, Subbakrishna DK, Gangadhar BN, Eack SM, Keshavan MS. Social and neuro-cognition as distinct cognitive factors in schizophrenia: a systematic review. Schizophr Res. 2013;148:3–11. doi: 10.1016/j.schres.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 98.Mehta UM, Thirthalli J, Naveen Kumar C, Keshav KJ, Gangadhar BN, Keshavan MS. Schizophrenia patients experience substantial social cognition deficits across multiple domains in remission. Asian J Psychiatr. 2013;6:324–9. doi: 10.1016/j.ajp.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 99.Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a metaanalysis. Neurosci Biobehav Rev. 2011;35:573–88. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 100.Pascual-Leone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–7. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- 101.Brown P. Shocking safety concerns. Lancet. 1996;348:959. doi: 10.1016/S0140-6736(05)65370-6. [DOI] [PubMed] [Google Scholar]
- 102.Hoy KE, Fitzgerald PB. Brain stimulation in psychiatry and its effects on cognition. Nat Rev Neurol. 2010;6:267–75. doi: 10.1038/nrneurol.2010.30. [DOI] [PubMed] [Google Scholar]
- 103.Guse B, Falkai P, Wobrock T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J Neural Transm. 2010;117:105–22. doi: 10.1007/s00702-009-0333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mogg A, Purvis R, Eranti S, Contell F, Taylor JP, Nicholson T, et al. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: a randomized controlled pilot study. Schizophr Res. 2007;93:221–8. doi: 10.1016/j.schres.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 105.Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, Noll DC, et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–8. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 106.Barr MS, Farzan F, Rajji TK, Voineskos AN, Blumberger DM, Arenovich T, et al. Can repetitive magnetic stimulation improve cognition in schizophrenia? Pilot data from a randomized controlled trial. Biol Psychiatry. 2013;73:510–7. doi: 10.1016/j.biopsych.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 107.Barr MS, Farzan F, Arenovich T, Chen R, Fitzgerald PB, Daskalakis ZJ. The effect of repetitive transcranial magnetic stimulation on gamma oscillatory activity in schizophrenia. PLoS One. 2011;6:e22627. doi: 10.1371/journal.pone.0022627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Francis MM, Hummer TA, Vohs JL, Yung MG, Visco AC, Mehdiyoun NF, et al. Cognitive effects of bilateral high frequency repetitive transcranial magnetic stimulation in early phase psychosis: a pilot study. Brain Imaging Behav. 2018;13:852–61. doi: 10.1007/s11682-018-9902-4. [DOI] [PubMed] [Google Scholar]
- 109.Hasan A, Guse B, Cordes J, Wolwer W, Winterer G, Gaebel W, et al. Cognitive effects of high-frequency rTMS in schizophrenia patients with predominant negative symptoms: results from a multicenter randomized sham-controlled trial. Schizophr Bull. 2016;42:608–18. doi: 10.1093/schbul/sbv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wolwer W, Lowe A, Brinkmeyer J, Streit M, Habakuck M, Agelink MW, et al. Repetitive transcranial magnetic stimulation (rTMS) improves facial affect recognition in schizophrenia. Brain stimul. 2014;7:559–63. doi: 10.1016/j.brs.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 111.Jiang Y, Guo Z, Xing G, He L, Peng H, Du F, et al. Effects of high-frequency transcranial magnetic stimulation for cognitive deficit in schizophrenia: a meta-analysis. Front Psychiatry. 2019;10:135. doi: 10.3389/fpsyt.2019.00135. [ • This is the only meta-analysis to examine the beneficial effects of rTMS in the treatment of cognitive deficits of schizophrenia. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang JX, Rogers LM, Gross EZ, Ryals AJ, Dokucu ME, Brandstatt KL, et al. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345:1054–7. doi: 10.1126/science.1252900. [• This elegant study describes how engaging parietal-hippocampal connectivity using rTMS can improve associative memory in humans.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sathappan AV, Luber BM, Lisanby SH. The dynamic duo: combining noninvasive brain stimulation with cognitive interventions. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;89:347–60. doi: 10.1016/j.pnpbp.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 114.Mehta UM, Keshavan MS. Cognitive rehabilitation and modulating neuroplasticity with brain stimulation: promises and challenges. Journal of Psychosocial Rehabilitation and Mental Health. 2015;2:5–7. [Google Scholar]
- 115.Navarro de Lara LI, Windischberger C, Kuehne A, Woletz M, Sieg J, Bestmann S, et al. A novel coil array for combined TMS/fMRI experiments at 3 T. Magn Reson Med. 2015;74:1492–501. doi: 10.1002/mrm.25535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Du X, Hong LE. Test-retest reliability of short-interval intracortical inhibition and intracortical facilitation in patients with schizophrenia. Psychiatry Res. 2018;267:575–81. doi: 10.1016/j.psychres.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]