Abstract
Objectives
The role of the “mirror neuron system” (MNS) in the pathophysiology of mood disorders is not well studied. Given its posited role in the often-impaired socio-emotional processes like intention detection, empathy, and imitation, we compared putative MNS-activity in patients with bipolar mania and healthy comparison sub-jects. We also examined the association between putative MNS-activity and hyper-imitative behaviors in patients.
Methods
We studied 39 medication-free individuals diagnosed with mania and 45 healthy comparison subjects. TMS-evoked motor cortical reactivity was measured via single- and paired-pulse stimuli (assessing SiCi—short and LiCi—long interval in-tracortical inhibition) while subjects viewed a static image and goal-directed actions.Manic symptom severity and imitative behaviors were quantified using the Young's Mania Rating Scale and a modification of the Echolalia Questionnaire.
Results
Two-way repeated measures analysis of variance demonstrated a significant group xtime interaction effect indicating greater facilitation of cortical reactivity during action-observation (putative MNS-activity) in the patient group as compared to the healthy group. While LiCi-mediated MNS-activity had a significant association with manic symptom severity (r = 0.35, P = 0.038), SiCi-mediated MNS-activity was significantly associated with incidental echolalia scores in a subgroup of 17 patients with incidental echolalia (r = 0.75, P < 0.001).
Conclusions
Our findings demonstrate that putative MNS-activity is heightened in mania, possibly because of disinhibition, and associated with behavioral conse-quences (incidental echolalia).
Keywords: bipolar disorder, corticospinal facilitation, echolalia, mirror neurons, motor resonance
1. Introduction
Bipolar disorder (BD) is a chronic psychiatric disorder with high rates of relapses and recurrences and is among the leading causes of disability worldwide.1 A sound understanding of its biological substrates will not only help device better treatments but also facilitate identifying a neuroscience-informed and psychopathology-dependent set of biomarkers. The current understanding of brain network disturbances in BD is centered on the theory of diminished prefrontal regulation of anterior limbic emotion processing structures.2,3 in bipolar mania, this is supported by the observations of a diminished functional connectivity between the prefrontal cortex/ anterior cingulate and the amygdala,3,4 along with an increased functional connectivity of the amygdala with the inferior frontal gyrus5 and the supplementary motor area.4 interestingly, both the inferior frontal gyrus6 and the supplementary motor area7 have “mirroring” properties—the functional attribute of discharging during both action execution and observation. This unique functional property may contribute to human imitation skills based on the direct matching hypothesis,8,9 which suggests that matching an observed action to its internal motor representation facilitates not just action understanding10,11 but also imitation. However, there is a top-down regulation of this mirror neuron system (MNS) exerted by the anterior frontomedian and temporoparietal cortices that are critical in controlling automatic mimicry.12 In fact, it has been proposed that a loss of this prefrontal inhibitory control over the MNS may result in abnormal hyper-imitative13 or hyper-empathetic14 behaviors. While there is limited empirical data15,16 to support the presence of these behaviors in mania, given the shared biological mechanisms (frontal disinhibition), it is possible that these behaviors are relevant to the expression of manic symptoms like increased goal-directed activities, social disinhibition, and overfamiliarity.
Interestingly, the MNS17,18 is thought to play an important functional role at a neuronal circuit level under the subconstruct of action perception of the “social processes” domain within the Research Domain Criteria (RDOC) framework.19,20 The behavioral manifestations of this subconstruct include imitation and empathy. Transcranial Magnetic Stimulation (TMS) is a neurophysiological probe that can be used to study motor cortical reactivity facilitation or motor resonance during action-observation, relative to rest states. The degree of this facilitation during action-observation, elicited from the motor cortex, is likely to be influenced by the premotor cortex mirror neuron responses.21,22 Therefore, TMS-derived motor cortical reactivity facilitation provides a putative, indirect index of premotor MNS-activity.21,23,24 While this method of quantifying MNS-activity using the degree of motor cortical reactivity facilitation is listed in the RDoC matrix,20 perhaps owing to its satisfactory millisecond-level temporal resolution,25 there are important limitations including the inter- and intraindividual variability with TMS-evoked motor potentials,26,27 the indirect estimate of MNS-activity, and the limited spatial resolution.
Other methods of estimating MNS-activity include electroencephalography (EEG) derived mu-wave suppression and functional magnetic resonance imaging (fMRI). One study employing EEG during an action-observation paradigm demonstrated reduced MNS-activity in euthymic BD patients compared to healthy subjects.28 Another study using fMRI demonstrated lower activations in the right anterior cingulate, insula, and inferior frontal gyrus—regions that partly overlap with the MNS, when processing emotional compared to neutral control conditions, in euthymic BD compared to healthy subjects.29 However, MNS-activity has not been studied in patients with BD during the manic state. This will inform specific state-related changes in putative MNS-activity secondary to the frontal disinhibition3 commonly observed in mania.
In this study, we primarily aimed to compare TMS-measured motor cortical reactivity facilitation during goal-directed action-observation, relative to rest states (a putative measure of MNS-activity), between medication-free/medication-naive patients with mania and healthy comparison subjects. As a secondary aim, we explored how putative MNS-activity related to manic symptom severity and hyper-imitative echo-phenomena in the patient group. We hypothesized that (a) putative MNS-activity will be high in the manic group compared to healthy subjects and (b) higher putative MNS-activity within the manic group will be positively associated with severity of manic symptoms and hyper-imitative echo-phenomena. We employed two single (of different intensities)- and two paired-pulse TMS paradigms assessing short (SICI) and long (LICI) interval intracortical inhibition, primarily to demonstrate consistency of motor cortical reactivity facilitation during action-observation across diverse physiological mechanisms of cortical excitability—these include cell membrane excitability30 (single-pulse) and GABA-inter-neuron effects on pyramidal neuronal output (SICI/LICI).31,32 Since our primary hypothesis was that the motor cortical reactivity facilitation during action-observation (relative to rest states) would be greater in patients with mania than in healthy subjects, we expected all the four stimulus paradigms to demonstrate this response. That is to say, we anticipated both neuronal membrane-driven and interneuron modulation-driven33,34 changes in cortical reactivity during action-observation to be higher in patients, indicating a state of disinhibition in mania that overrides different physiological systems of cortical excitability.
2. Materials and Methods
2.1. Study participants
Forty right-handed,35 medication-naive (n = 13; never treated with psychotropics) or medication-free (n = 27; not on any psychotropic medications for ≥2 months prior to assessments) patients diagnosed by a qualified psychiatrist as mania or hypomania according to the Diagnostic and Statistical Manual-IV36 and confirmed using the Mini-International Neuropsychiatric Interview37 were recruited for the study from the clinical services of a tertiary care neuropsychiatric hospital in South India. We did not have any symptom severity cut-offs for inclusion of patients. Patients were excluded if they had substance dependence in the last 6 months (other than nicotine), psychoactive substance use in the last 1 week, a clinically diagnosable or self-reported visual or auditory impairment, or need for acute intervention (eg, rescue medications) for severe problem behaviors. The presence of comorbid neurological disorder that predisposed participants for seizures or cognitive impairment, pregnancy, and subjects with metallic implants, cardiac pacemakers, or fulfilling any of the risk factors for TMS procedures as assessed using the TMS Adult Safety Screen38 were also excluded from the study. All the study participants provided a written informed consent for the study. Patient data were compared to the healthy comparison group recruited from the community for a previous study conducted at the same center with similar methodology.24 Efforts were made to recruit patients who would match the age, gender, and education profile of the healthy comparison group that was recruited for the earlier study. Of the patients who were screened, ~50% were ineligible as they were not able to consent and cooperate in the study. This experiment and the previous experiment (healthy subjects and schizophrenia)24 were approved by the institutional ethics committee.
2.2. Assessments
Clinical symptoms were assessed using the Young's Mania Rating Scale (YMRS),39 an 11-item clinician-rated instrument. Echo-phenomena (echolalia and echopraxia) were assessed using a modification of the Echolalia Questionnaire40 and the echopraxia item from the Bush Francis Catatonia Rating Scale.41 The echolalia questionnaire (Tables S1 & S2 in supporting information) involves observations for echolalia while the subject is asked a set of 15 questions (automatic or induced echolalia) or when his/her caregiver, sitting nearby, is asked similar 15 questions (ambient or incidental echolalia). Their responses to the 15 questions are rated on a scale from one to six, in an increasing order of severity of echolalia.40 All patients were rated for manic symptoms and echo-phenomena on the day of the TMS experiment and consenting process by the same clinician. These were assessed either before or after the TMS experiment, based on logistics. Healthy comparison subjects were not rated for manic symptoms and echo-phenomena.
2.3. TMS experiment to determine Putative MNS-activity
2.3.1. Setting
The MagPro R30 stimulator with MagOption (MagVenture, Farum, Denmark) was used for the current study as well as the earlier experiment involving the healthy group.24 Magnetic pulses were delivered with a noncooled figure-of-eight coil (MC-B70); data acquisition and analyses were done using Signal-4 Software (Cambridge Electronic Devices, Cambridge, UK). The subject was seated on a chair 50 cm away from the observation (13-inch laptop) monitor in a silent room. The area corresponding to the right first dorsal interosseous (FDI) was located in the left motor cortex and motor-evoked potentials (MEP) were recorded by disposable pre-gelled electrodes connected to a 1-channel electromyography amplifier mounted on the MagPro system (MEP-monitor). The minimum stimulation intensity (measured in percentage of maximum machine output) required to evoke a >50 μV (Resting Motor Threshold—RMT) and >1 mV (Stimulation intensity to elicit 1 mV MEP—SI1mV) MEP in the resting right FDI muscle in at least 6 of 10 consecutive trials42 was defined.
2.3.2. Cortical reactivity (excitation and inhibition) measurements
Four stimulus paradigms were used in the experimental sessions among which two were single-pulse and two were paired-pulse paradigms:
-
a
120%RMT: This is a single-pulse parameter. MEPs were recorded using a stimulus intensity of 120% RMT. This stimulus intensity has been most commonly implemented in studying putative MNS activity using TMS.43,44
-
b
SI1mV: This is a single-pulse parameter. MEPs were recorded using stimulus intensity to elicit MEPs of approximately 1 mV peak-to-peak amplitude (SI1mV).
-
c
Short Interval Intracortical Inhibition (SICI): This is a dual-pulse TMS paradigm. SICI tests the inhibitory effects of a subthreshold first stimulus at intensity of 80% RMT or the conditioning stimulus (CS) on the amplitude of the test MEP elicited by a suprathreshold second stimulus at SI1mV or the test stimulus (TS), delivered at short interstimulus intervals of 1-5 ms through the same stimulating coil, reflecting inhibitory capacity at the cortical level.33,45 SICI at Intervals of 2.5-4 ms reflects more purely Gamma Amino Butyric Acld-A (GABA-A) receptor-mediated synaptic Inhibition.46,47
-
d
Long Interval Intracortical Inhibition (LICI): This is a dual-pulse TMS paradigm. A suprathreshold conditioning stimulus (SIlmV) is given 100 ms before a suprathreshold test stimulus (SI1mV), thus inhibiting the MEP response to the test stimulus (conditioned MEP).48 It is mediated through the activation of GABA-B receptors.31
SICI and LICI were expressed as a percentage of the ratio between the conditioned MEPs and the nonconditioned MEPs with a stimulus intensity of SIlmV; that is, (conditioned MEP/nonconditioned MEP) x 100.49
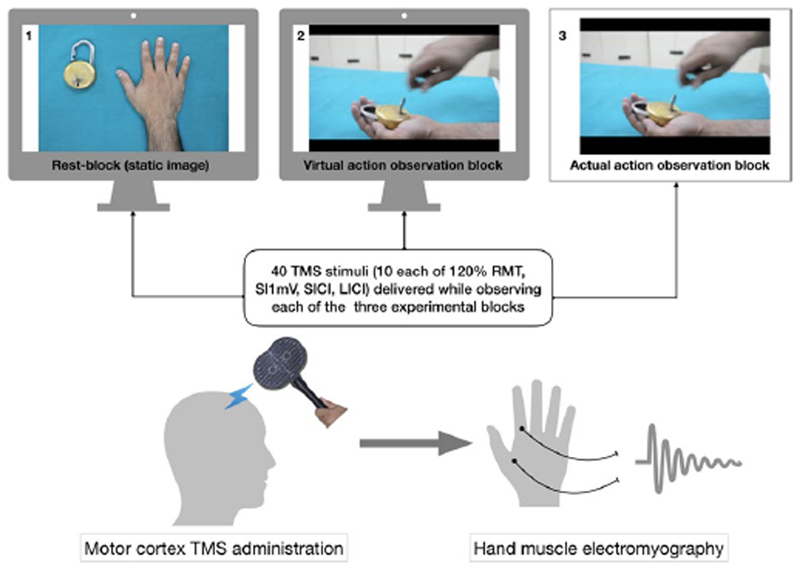
Ten MEP recordings, using SIlmV, 120%RMT’ and SICI and LICI stimulus paradigms (total of 40 recordings)’ were elicited in pseudorandom sequence with 5-second intervals while the subjects observed each of the following three experimental stimulus blocks (Figure l):
-
a
Actual block: Actual observation of an action being performed involving the FDI. This involved watching, locking, and unlocking of a lock performed by the experimenter with the right hand grasping the key in the lateral pinch grip (holding the key between the side of the index finger and the thumb). This action requires contraction of the FDI to abduct the index finger.50
-
b
Virtual block: The subjects observed a video showing the above action.
-
c
Rest block: The subjects observed a still image of a hand and a lock displayed on the monitor.
Figure 1. Illustration of the TMS experiment. Recording of TMS-evoked motor cortical reactivity while the subject observes three experimental blocks. SIlmV = Stimulus intensity to evoke l millivolt motor-evoked potential; RMT = Resting Motor Threshold; SICI and LICI are short and long interval intracortical inhibition.
The sequence of displaying these experimental states to each subject was randomized, with an approximately 5-minute interval between blocks to alter the display logistics. Each of the actual (experimenter performed) and virtual (video display on the laptop monitor) action blocks comprised of 5-second duration goal-directed actions involving the right FDI muscle (locking and unlocking of a lock with a key held in lateral pinch grip) that were looped or repeated 40 times for a total of 200 seconds. The first TMS-pulse was administered to coincide with the first maximum contraction of the right FDI muscle in the above-mentioned action. The preset timing of 5-second pulse-delivery set in the stimulator ensured time locking of the TMS-pulses with the subsequent maximal muscle contractions in the looped (40 times) display of goal-directed actions. The consistency of experimenter-performed actions was ensured by having an experienced experimenter who had performed these actions for our earlier experiment and was trained to maintain the same pace and intensity of the action.
2.3.3. Ensuring fidelity of the electromyography recordings
In order to guarantee optimal attention allocation during the TMS experiments, subjects were instructed to pay attention to all the stimuli throughout the experiment, also a second experimenter monitored the subjects' behavior. All participants were explicitly asked to remain as relaxed as possible during the TMS experiment. Movements during the recordings were monitored by visual observation; no auditory feedback was used. This was crucial to ensure fidelity of the electromyography recordings. Subjects who moved their hands or any other body parts did not continue the experiment and their data were excluded.
2.4. Main outcome
The principle used to measure putative MNS-activity is that relative to static image viewing, there is a change in motor cortical reactivity (increased cortical excitability or decreased cortical inhibition) while observing goal-directed actions of the particular muscle from which the electromyography is being recorded. This property of motor cortical reactivity (MCR) facilitation during action-observation (average across virtual and actual action-observation blocks) relative to rest block was the primary outcome measure compared between the two groups (see below under statistical analyses). A percentage of this facilitatory process was calculated for use in correlation analyses with symptom measures43 as follows:
2.5. Statistical analyses
To examine cortical reactivity facilitation (putative MNS-activity), we compared the MEP amplitudes (with the four stimulus paradigms described above) during rest and action-observation state using one-way repeated measures analysis of variance (RMANOVA)’ separately for the two groups. To compare putative MNS-activity between the two groups, we used two-way RMANOVA for each of the four stimulus paradigms. Secondary analyses were performed to examine differences in MNS-activity elicited using virtual- and actual-observation blocks separately. Finally, to examine the association of MNS-activity with YMRS symptom scores and echo-phenomena, we conducted a partial correlation analysis, covarying for baseline differences in cortical reactivity. All statistical tests were two-tailed and significance was set at an error probability of 0.05.
3. Results
3.1. Socio-demographic and clinical details
One patient was excluded from the analyses as there were artifacts in the electromyography recordings. The two groups showed no significant difference in age, gender, and marital status, but the healthy comparison group had higher years of education and lower rates of nicotine abuse (Table 1). Two patients of the 39 had hypomania; the rest had mania. Years of education did not have a significant association (Pearson's coefficients ranged from -0.06 to -0.1; all P values >0.3) with any of the measures of putative MNS-activity (percentage of motor cortical reactivity facilitation with action-observation). Therefore, “years of education” was not included as a covariate in further analyses to detect group differences in putative MNS-activity.51
Table 1. Socio-demographic and clinical characteristics.
| Variables | Patients (n = 39) | Healthy (n = 45) | t/x2 | P |
|---|---|---|---|---|
| Age in years | 32.82 (11.01) | 30.69 (9.58) | 0.949 | 0.345 |
| #Gender | ||||
| Male | 22 (56) | 23 (51) | 0.236 | 0.627 |
| Female | 17 (44) | 22 (49) | ||
| #Marital status | ||||
| Married | 25 (64.1) | 24 (53) | 4.067 | 0.254 |
| Unmarried | 12 (30.8) | 21 (47) | ||
| Divorced | 1(2.6) | 0 | ||
| Widowed | 1(2.6) | 0 | ||
| Education in years | 7.68 (4.82) | 13.13 (3.5) | −5.795 | <0.001 |
| #Nicotine abuse/ dependance | 10 (25.6) | 2 (4.4) | 7.66 | 0.006 |
| YMRS total score | 22.36 (7.1) | – | – | – |
| Duration of current episode (days) | 46.13 (48.68) | – | – | – |
| No. of past episodes | ||||
| Any | 2.76 (4.12) | – | – | – |
| Mania | 2.32 (3.64) | – | – | – |
| Depression | 0.45 (1.61) | – | – | – |
| #Drug-naïve patients | 13 (30) | – | – | – |
| #Drug-free patients | 27 (70) | – | – | – |
Note. All values in mean (SD), except #n(%),YMRS = Young's Mania Rating Scale, drug-naïve = first episode patients who were never treated with medications, drug-free = patients who had a relapse, and were off medications for 2 months or longer. Bold values indicate statistically significant results.
3.2. Comparison of baseline TMS parameters between groups
The resting motor threshold (% of maximum machine strength) was not significantly different between the two groups. However, while observing the rest block (static image), patients demonstrated significantly lower MEPs with both the single-pulse stimuli, lower SICI and higher LICI, than healthy subjects (Table 2). Cortical reactivity at baseline for each of the four TMS parameters of interest had a significant correlation (Pearson’s coefficients ranged from -0.25 to -0.65; all P values <0.05) with putative MNS-activity, and hence, these baseline parameters were used as covariates in the two-way RMANOVA to explore group differences.
Table 2. Baseline cortical reactivity of patients and healthy comparison subjects.
| Variables | Patients (n = 39) | Healthy (n = 45) | T | P |
|---|---|---|---|---|
| SI1mV | 53.59 (11.97) | 48.4 (11.43) | 2.03 | 0.046 |
| RMT | 39.36 (8.33) | 36.69 (7.23) | 1.573 | 0.12 |
| SICI | 84.32 (44.96) | 64.12 (27.40) | 2.429 | 0.018 |
| LICI | 24.99 (20.52) | 42.18 (45.38) | −2.285 | 0.026 |
| MEP (SI1mV) | 0.7 (0.29) | 0.89 (0.24) | −3.207 | 0.002 |
| MEP (120%RMT) | 0.52 (0.34) | 0.77 (0.32) | −3.475 | 0.001 |
Note. All values are expressed as mean (SD), SI1mV = stimulation Intensity In % machine output to elicit 1 millivolt motor-evoked potentials; RMT =Resting Motor Threshold in % machine output to elicit motor-evoked potentials of 50 microvolts, SICl and LICl are short and long interval intracortical inhibition and they were expressed as a percentage of the ratio between the conditioned MEPs and the nonconditioned MEPs with stimulus intensity of SI1mV; that is, (conditioned MEP/nonconditioned MEP) x 100; MEP with test pulse (SI1mV) and MEP with 120%RMT =motor-evoked potentials (millivolts) produced by the respective stimulation intensities while viewing the static-block. Bold values indicate statistically significant results.
3.3. Examining motor cortical reactivity facilitation during action-observation (putative MNS-activity)in the two groups
There was a significant facilitation of motor cortical reactivity during action-observation relative to rest states (putative MNS-activity) as determined by all the four stimulus paradigms in the patient group [one-way RMANOVA (df = 1.38): SI1mV (F = 25.1, P < 0.001); 120%RMT (F = 45.96, P < 0.001); SICI (F = 22.56, P < 0.001); and LICI (F = 10.27, P < 0.001)] even after applying a Bonferroni correction (P < 0.025). Similar facilitation was observed in the healthy comparison group (df = 1.44), [SI1mV (F = 6.54, P = 0.01); 120%RMT (F = 5.72, P = 0.02); SICI (F = 4.16, P = 0.04), and LICI (F = 0.02, P = 0.8)], albeit not significant for the SICI and LICI paradigms after applying a Bonferroni correction (P < 0.025).
3.4. Comparing motor cortical reactivity facilitation during action-observation (putative MNS-activity) between the two groups
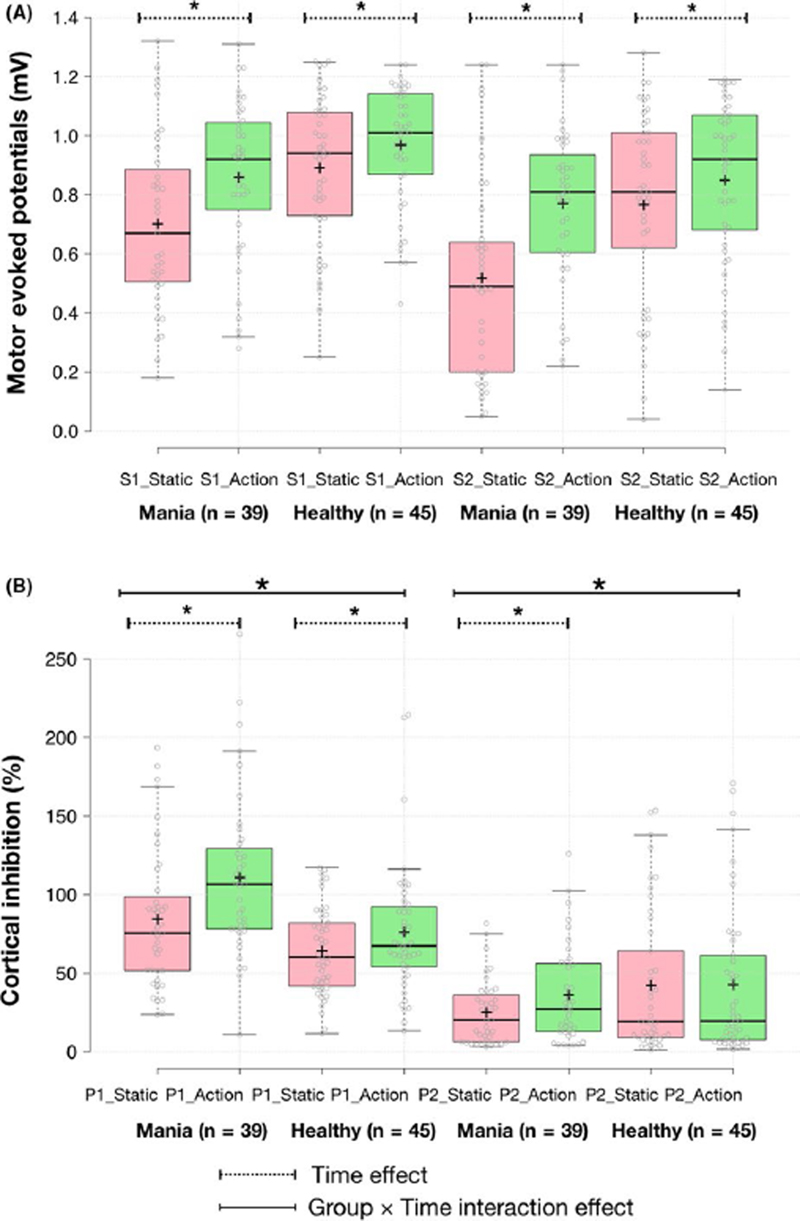
Putative MNS-activity was compared between the two groups using two-way RMANOVA with group status (patients or healthy subjects) as the between-subjects factor, cortical reactivity at rest and action-observation states as the within-subjects factor. This revealed a significant group xtime interaction effect for putative MNS-activity modulated by 120%RMT and LICI stimuli (Table 3) indicating greater putative MNS-activity in the patient group. Since there were baseline (rest block) cortical reactivity differences across all parameters (see Table 2), we controlled for these differences by including them as covariates in the two-way RMANOVA. A significant group xtime interaction effect was observed indicating higher putative MNS-activity mediation in patients than in healthy subjects by SlCl (P = 0.024, partial η2 = 0.061) and LlCl (P = 0.033, partial η2 = 0.055) paradigms after controlling for these baseline differences. Putative MNS-activity calculated using the Sl1mV and 120%RMT paradigms was not significantly different between the two groups after controlling for the baseline differences (Table 3 and Figure 2). Secondary analyses revealed greater putative MNS-activity for both virtual and actual action-observation experimental blocks in the patient group compared to healthy subjects, some of which reached trend-level statistical significance only (table S3 in supporting information).
Table 3. Motor cortical reactivity changes with action-observation in patients and healthy comparison subjects.
| Experimental paradigm | F statisticsa | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| TMS parameter | Group | Static | Action-observation | F 1 | P | F 2 | P | F 3 | P |
| MEP (SI1mV) | Patients | 0.70 (0.29) | 0.86 (0.27) | 56.11 | <0.001 | 3.14 | 0.08 | 0.002 | 0.96 |
| Healthy | 0.89 (0.25) | 0.97 (0.20) | |||||||
| MEP (120%RMT) | Patients | 0.52 (0.34) | 0.77 (0.26) | 87.65 | <0.001 | 10.9 | 0.001 | 2.132 | 0.14 |
| Healthy | 0.77 (0.32) | 0.85 (0.28) | |||||||
| SICI (%) | Patients | 84.32 (44.96) | 110.9 (51.31) | 15.25 | <0.001 | 3.25 | 0.075 | 5.292 | 0.02 |
| Healthy | 64.12 (27.90) | 76.01 (41.62) | |||||||
| LICI (%) | Patients | 24.99 (20.52) | 36.04 (30.95) | 4.237 | 0.043 | 5.38 | 0.023 | 4.683 | 0.03 |
| Healthy | 42.19 (45.39) | 42.64 (47.98) | |||||||
Note. All values in cells are expressed as mean (SD); MEP with test pulse (Sl1mV), and MEP with 120%RMT = motor-evoked potentials (in millivolts) produced by the respective stimulation intensities; SlCl and LlCl are short and long interval intracortical inhibition and they were expressed as a percentage of the ratio between the conditioned MEPs and the nonconditioned MEPs with stimulus intensity of Sl1mV; that is, (Conditioned MEP/ Nonconditioned MEP) x 100. Bold values indicate statistically significant results.
Two-way repeated measures ANOVA: F1 = Time effect, F2 = group xtime interaction effect without covarying for baseline differences with degrees of freedom = (1,82), F3 = group xtime interaction effect after covarying the effect of baseline cortical reactivity differences with degrees of freedom = (1,81)
Figure 2.
Cortical reactivity with action-observation in patients with mania (n = 39) and healthy subjects (n = 45). Boxplots of single (A)- and paired (B)-pulse cortical reactivity measures (SI = SIlmV or single pulse with stimulus intensity to evoke 1 millivolt motor-evoked potentials S2 = single pulse with 120% resting motor threshold, PI = paired pulse using short interval intracortical inhibition and P2 = long interval intracortical inhibition) during static image and goal-directed action-observation stimuli; *P < 0.05. Group xtime interaction effects after covarying for baseline differences were significant only for paired pulse TMS measures and not single pulse measures. In the boxplots, the central lines and crosses represent medians and means, respectively; box limits are at 25th and 75th percentiles; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles; outliers are represented by black open circles; data points are plotted as gray open circles; pink boxes represent static image observation; and green boxes represent action-observation (average of virtual and actual blocks)
3.5. Putative MNS-activity and clinical correlates
Partial correlations were performed to examine how putative MNS-activity related to symptoms, after covarying for the effects of baseline cortical reactivity measurements (rest block). Complete ratings of echo-phenomena were possible in 23 of the 39 patients. ln the rest, ratings were not possible because of various pragmatic difficulties including language barriers. While none of the patients demonstrated echopraxia, two patients demonstrated induced echolalia. lncidental echolalia was present in 17 of the 23 patients assessed (~74%). Scores ranged from 20 to 75, with a mean ± SD of 39.65 ± 17.07.
While LlCl-mediated putative MNS-activity correlated significantly with the total YMRS score, SlCl-mediated putative MNS-activity had a significant association with incidental echolalia scores; the latter association persisted even after applying Bonferroni correction (Table 4; scatter plots in Figure S1, supporting information).
Table 4. Relationship of putative MNS-activity with symptom severity of mania and incidental echolalia scores in the patient group.
| MNS-activity | YMRS score (n = 39) | Incidental echolalia score (n = 17) |
|---|---|---|
| MNS-activity_SI1mV | −0.15 (−0.444, 0.173) | 0.18 (−0.329, 0.608) |
| MNS-activity_120%RMT | −0.174 (−0.464, 0.149) | 0.04 (−0.449, 0.51) |
| MNS-activity_SICI | 0.165 (−0.158, 0.456) | 0.751** (0.423, 0.904) |
| MNS-activity_LICI | 0.35* (0.038, 0.599) | −0.013 (−0.491, 0.471) |
Note. YMRS, Youngs Mania Rating Scale; MNS, Mirror Neuron System, measured as a percentage of cortical reactivity facilitation from resting to action-observation state; SllmV = Stimulation intensity to elicit l mV motor-evoked potentials, 120%RMT =120% Resting Motor Threshold, SlCl, Short Interval Intracortical Inhibition and LlCl, Long Interval Intracortical Inhibition, respectively.
*P < 0.05.
**P < 0.001 (survives Bonferroni correction of P < 0.006); all values in cells are Pearson's correlation coefficients (95% confidence intervals), covaried for the respective baseline cortical reactivity measurements.
Since there was a significantly greater proportion of subjects with nicotine abuse or dependence in the patient group than in the healthy group, we examined if patients with or without nicotine abuse had different putative MNS-activity. None of the single/paired-pulse-mediated putative MNS-activity measures differed between the two patient groups (all T values <0.8; all P values >0.43).
4. Discussion
The primary finding from this experiment was that patients with mania had significantly greater putative MNS-activity when compared to healthy subjects when measured using paired-pulse TMS paradigms. In addition, in patients with mania, greater putative MNS-activity was related to more prominent incidental echolalic behavior.
The first inference was made based on the observation of consistent and significantly greater cortical reactivity facilitation during action-observation relative to static image viewing in the patient group. We considered the possibility that this conclusion could be an artifact of a possible overarching hyperexcitable state of mania. However, this is unlikely because generalized hyperexcitability would drive the cortical reactivity during static image viewing as well as during action-observation. Putative MNS-activity is primarily measured as the change in cortical reactivity between these two states. This effectively controls for and nullifies the effect of a possible overall hyperexcitable cortical state. Moreover, even after controlling for the baseline cortical reactivity group differences,52 patients demonstrated greater motor cortical facilitation during action-observation, during the SICI and LICI paradigms. That is to say, despite demonstrating the opposite patterns of baseline abnormalities (low SICI and high LICI), putative MNS-activity in the patient group was higher than that in the healthy subjects. This is a novel finding, which needs to be replicated in future studies. While we expected group differences in putative MNS-activity derived from all four TMS-measures, we found significantly elevated putative MNS-activity in patients when measured using the two paired-pulse paradigms only. Whether this differential between-group putative MNS-response, based on its method of elicitation (single vs paired pulse), is merely reflective of a ceiling effect with single-pulse paradigms or is an indication of a disease-specific frontal disinhibition resulting in GABA-interneuron dysregulation of MNS-activity (as inferred from paired-pulse results) can be examined in future studies.
Two other experiments have reported either reduced29 or normal28 activity within the MNS in patients with BD when given emotion attribution and action-observation tasks, respectively. Critically, these prior studies were done in patients with BD studied in euthymic states. Our finding of heightened mirroring response in mania, when put in perspective of these studies in euthymic states,28,29 suggests a possible state-dependent effect of an exaggerated mirroring response during manic phases of BD. Hence, it is imperative to examine how such neurophysiological measurements of putative MNS-activity relate to various clinical states of bipolar disorder. The relevance of our results is further increased by the fact that our patients were medication naive or off medications. Therefore, our findings cannot be accounted for by potential medication interactions.
In contrast, our earlier experiment using a similar experimental method in untreated schizophrenia patients demonstrated a deficient MNS response when compared to healthy subjects.24 If these findings are replicated, TMS-measured putative MNS-activity could potentially be used as a neuromarker to differentiate clinical states of mania from schizophrenia. Whether this excessive putative MNS-activity is related to the earlier described heightened affective empathy in bipolar disorder patients15,16 remains to be examined. More importantly, the findings of an exaggerated MNS response in mania add incrementally to the MNS dysfunction model of psychosis19 which suggests an inherent MNS deficit underlying the persistent negative and cognitive symptoms and a pathological excessive MNS-activity supporting the phasic affective and catatonic symptoms.
Consistent with this hypothesis, we demonstrate enhanced putative MNS-activity in mania compared to healthy subjects. Next, we also demonstrate a positive association between putative MNS-activity mediated by the LICI and SICI stimulus paradigms and global severity of mania and incidental echolalia, respectively; albeit only the latter relationship survived Bonferroni correction. It must be noted that examining clinical correlates of putative MNS-activity was a secondary aim of our study and these associations are to be deemed tentative requiring replication in larger studies. In our earlier analysis of a smaller (n = 20) subsample,53 we found significant associations between putative MNS-activity measured using all the four stimulus paradigms and symptom severity. We could demonstrate this trend (though not conclusively) only for the LICI paradigm in this larger sample (n = 39). There is a possibility of chance findings from our earlier analysis,53 owing to a small sample size, reduced power, and hence a potential for poor replicability.54 Nevertheless, given the heterogeneous nature of mania,55 it may be worthwhile to examine the relationship between LICl-mediated putative MNS-activity with diverse latent structures of manic symptoms in larger samples.56,57
We also observed that putative MNS-activity modulated by SICI, and not baseline SICI was related to incidental echolalia scores in a subset (n = 17) of the patient group. The high (~74%) prevalence of ambient or incidental echolalia, relative to automatic or induced echolalia (~0.08%), is also noteworthy. These two types of echolalia perhaps share distinct mechanisms—while a disconnection of the perisylvian language area from the temporoparietal cortex58 is associated with induced echolalia, incidental echolalia is associated with lesions in the medial frontal and anterior cingulate cortices leading to altered control of shared representations (self-other distinctions) and evaluation of outcomes.59 In addition, treatment of echolalia with lorazepam was associated with a reduction in putative MNS-activity as described in a single case report.60 Lorazepam primarily acts as a GABAA receptor facilitator. SICI is thought to be mediated by ionotropic GABAA receptor neurotransmission32,61 and was significantly reduced in the patient group. Hence, we surmise that a deficient inhibitory regulation of motor and premotor MNS subsequent to attenuated GABAA-mediated SICI could potentially result in an exaggerated MNS response and the hyper-imitative echo-phenomena thereof. In the context of the manic syndrome, incidental echolalia may be conceptualized as an environmental dependency phenomenon62 that manifests as a tendency to 'imitate' verbal stimuli in the absence of ongoing control/filtering processes, potentially leading to the more common communicative symptoms of pressured speech and overfamiliarity in an environmentally dependent manner.63 Such heightened imitative/utilization behaviors have also been described across a range of psychiatric disorders including melancholic depression,64 mania,65 schizophrenia,13 Tourette Syndrome,66 attention deficit disorder,67 and autism.40,68 Together, these findings suggest a need to examine such hyper-imitative behaviors and their relationship to deficient frontal reactive control processes that inhibit MNS-driven imitation after an action is observed69 within the RDoC initiative using a transdiagnostic approach.70,71
Important caveats need to be considered while interpreting our results. First, we employed an indirect measurement of motor cortical reactivity facilitation to infer MNS-activity. Despite providing excellent temporal resolution, TMS does neither provide a direct measurement of MNS-activity nor does it give information from extra-motor areas, many of which are intrinsic to the pathogenesis of mania. Also, since we used four different stimulus paradigms to elicit putative MNS-activity and found group differences in only two of the four paradigms, the influence of a potential type-I error cannot be ruled out. Second, the healthy group data have been taken from an experiment conducted 2 years prior, though from the same center using the same stimulator and by the same experimenters. However, the between-groups baseline cortical reactivity differences have been accounted for statistically, by including them as covariates for detecting MNS-activity group differences. Third, the mean YMRS score in the patient group was 22.36 ± 7.1 indicating mild to moderate symptomatology. This precludes generalizability of our findings to patients with severe mania. Fourth, there was no objective method to ensure attentiveness to the visual stimuli depicted on the monitor. Given the heightened MNS-response in the patient group, poor attentiveness is unlikely to have contributed to this observation. Fifth, an additional visual stimulus of nongoal-directed movement would have improved our inferences by controlling for cortical excitability in response to any movement. Sixth, nicotine use is known to alter cortical reactivity.72 Even though we have found no difference in putative MNS-activity between patients with and without nicotine abuse, we did not examine the impact of the recency and quantity of nicotine use. Lastly, we report only an association between high putative MNS-activity and incidental echolalia. This does not suggest a causal role for the MNS in hyper-imitative states, as this relationship could reflect an associative learning process73 that has been unmasked during mania.
A sufficiently large clinical sample, that is not under the influence of psychotropic medications, which can alter TMS cortical reactivity outputs,74 enhances the validity of our observations. Complementing TMS with functional neuroimaging and electroencephalography will further improve the validity of these findings and enable better temporospatial characterization of responsiveness of the MNS in disease states. The reliability of these findings of diametrically opposite putative MNS-activity across patients with mania and schizophrenia and their extended clinical correlates (eg, relationship to cognition and behavior) may be examined via replication studies. Furthermore, longitudinal studies across the different phases of bipolar disorder (euthymia and depression) and schizophrenia (symptomatic, remission, refractory) will inform how putative MNS-activity varies as a function of symptoms.
In summary, we provide evidence of greater facilitation of motor cortical output during action-observation relative to static image viewing, in drug-free/drug-naïve patients with mania using a TMS experiment. This indicates, albeit in an indirect manner, an aberrant exaggerated responsiveness of the premotor MNS in individuals with mania. These findings were pronounced, even after controlling for baseline cortical reactivity groups differences. Higher putative MNS-activity in the patient group was significantly associated with a greater tendency to verbally imitate parts of a social conversation between two individuals (incidental echolalia). The contribution of a disinhibited MNS to the pathophysiology of manic states is emphasized by demonstrating the first-time link between putative neurophysiological (heightened MNS-activity) and behavioral (incidental echolalia) markers of a disinhibited cortical regulatory state in mania.
Supplementary Material
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Acknowledgements
We acknowledge the contribution of Dr. C Naveen Kumar, Additional Professor of Psychiatry, NIMHANS, Bengaluru, who served as a coguide to RB during her MD thesis on this topic.
Funding information
We acknowledge funding from the Department of Biotechnology, Government of india Grant No. BT/PR14311/ Med/30/470/2010 to UMM for the project titled "investigating Mirror Neuron Basis of Social Cognition Deficits in Schizophrenia Patients Using TMS" from which the data of healthy subjects are taken.
Footnotes
Disclosures
UMM is supported by the Wellcome Trust/DBT India Alliance Early Career Fellowship, Grant/Award Number: IA/E/12/1/500755; RB is supported by the Wellcome Trust/DBT India Alliance Research Training Fellowship, Grant/Award Number: IA/RTF/15/1/1009. Dr APL was partly supported by the Sidney R. Baer Jr Foundation, the NIH (R01MH100186, R01HD069776, R01NS073601, R21 NS082870, R21 MH099196, R21 NS085491, R21 HD07616), the Football Players Health Study at Harvard University, and Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758). Drs. RB, UMM, and JT have no biomedical financial interests or potential conflicts of interest to declare. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIMHANS, Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Institutes of Health, or the Sidney R. Baer Jr Foundation. Dr APL is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging.
References
- 1.Salomon JA, Haagsma JA, Davis A, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3:e712–e723. doi: 10.1016/S2214-109X(15)00069-8. [DOI] [PubMed] [Google Scholar]
- 2.Wegbreit E, Cushman GK, Puzia ME, et al. Developmental metaanalyses of the functional neural correlates of bipolar disorder. JAMA Psychiatry. 2014;71:926. doi: 10.1001/jamapsychiatry.2014.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 4.Brady RO, Margolis A, Masters GA, Keshavan M, Öngür D. Bipolar mood state reflected in cortico-amygdala resting state connectivity: A cohort and longitudinal study. J Affect Disord. 2017;1:205–209. doi: 10.1016/j.jad.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerullo MA, Fleck DE, Eliassen JC, et al. A longitudinal functional connectivity analysis of the amygdala in bipolar I disorder across mood states. Bipolar Disord. 2012;14:175–184. doi: 10.1111/j.1399-5618.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev. 2012;36:341–349. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Mukamel R, Ekstrom AD, Kaplan J, lacoboni M, Fried I. Single-neuron responses in humans during execution and observation of actions. Curr Biol. 2010;27:750–756. doi: 10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iacoboni M. Cortical Mechanisms of Human Imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- 9.Heiser M, Iacoboni M, Maeda F, Marcus J, Mazziotta JC. The essential role of Broca's area in imitation. Eur J Neurosci. 2003;17:1123–1128. doi: 10.1046/j.1460-9568.2003.02530.x. [DOI] [PubMed] [Google Scholar]
- 10.Gallese V, Keysers C, Rizzolatti GA. unifying view of the basis of social cognition. Trends Cogn Sci. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Liepelt R, Cramon D, Brass M. What is matched in direct matching? Intention attribution modulates motor priming. J Exp Psychol Hum Percept Perfor. 2008;34:578–591. doi: 10.1037/0096-1523.34.3.578. [DOI] [PubMed] [Google Scholar]
- 12.Brass M, Ruby P, Spengler S. Inhibition of imitative behaviour and social cognition. Philos Trans R Soc B Biol Sci. 2009;364:2359–2367. doi: 10.1098/rstb.2009.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridmore S, Brune M, Ahmadi J, Dale J. Echopraxia in schizophrenia: possible mechanisms. Aust N Z J Psychiatry. 2008;42:565–571. doi: 10.1080/00048670802119747. [DOI] [PubMed] [Google Scholar]
- 14.Case LK, Abrams RA, Ramachandran VS. Immediate interpersonal and intermanual referral of sensations following anesthetic block of one arm. Arch Neurol. 2010;67:1521–1523. doi: 10.1001/archneurol.2010.290. [DOI] [PubMed] [Google Scholar]
- 15.Bodnar A, Rybakowski JK. Increased affective empathy in bipolar patients during a manic episode. Rev Bras Psiquiatr. 2017;39:342–345. doi: 10.1590/1516-4446-2016-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shamay-Tsoory S, Harari H, Szepsenwol O, Levkovitz Y. Neuropsychological evidence of impaired cognitive empathy in euthymic bipolar disorder. J Neuropsychiatry Clin Neurosci. 2009;21:59–67. doi: 10.1176/jnp.2009.21.1.59. [DOI] [PubMed] [Google Scholar]
- 17.Di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: A neurophysiological study. Exp Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- 18.Rizzolatti G, Fadiga L, Matelli M, et al. Localization of grasp representations in humans by PET: 1. Observation versus execution. Exp Brain Res. 1996 Sep;111:246–252. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- 19.Mehta UM, Thirthalli J, Aneelraj D, Jadhav P, Gangadhar BN, Keshavan MS. Mirror neuron dysfunction in schizophrenia and its functional implications: A systematic review. Schizophr Res. 2014;160:9–19. doi: 10.1016/j.schres.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NIMH RDoC Working Group. NIMH » Social Processes: Workshop Proceedings. [Accessed March 13, 2018];2012 [Internet]. https://www.nimh.nih.gov. https://www.nimh.nih.gov/research-priorities/rdoc/social-processes-workshop-proceedings.shtml.
- 21.Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action-observation: a magnetic stimulation study. J Neurophysiol. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- 22.Avenanti A, Bolognini N, Maravita A, Aglioti SM. Somatic and motor components of action simulation. Curr Biol. 2007;18:2129–2135. doi: 10.1016/j.cub.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 23.Maeda F, Chang VY, Mazziotta J, Iacoboni M. Experience-dependent modulation of motor corticospinal excitability during action-observation. Exp Brain Res. 2001;140:241–244. doi: 10.1007/s002210100827. [DOI] [PubMed] [Google Scholar]
- 24.Mehta UM, Thirthalli J, Basavaraju R, Gangadhar BN. Pascual-Leone A. Reduced mirror neuron activity in schizophrenia and its association with theory of mind deficits: evidence from a transcranial magnetic stimulation study. Schizophr Bull. 2014;40:1083–1094. doi: 10.1093/schbul/sbt155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gangitano M, Mottaghy FM. Pascual-Leone A. Modulation of premotor mirror neuron activity during observation of unpredictable grasping movements. Eur J Neurosci. 2004;20:2193–2202. doi: 10.1111/j.1460-9568.2004.03655.x. [DOI] [PubMed] [Google Scholar]
- 26.Burgess JD, Arnold SL, Fitzgibbon BM, Fitzgerald PB, Enticott PG. A transcranial magnetic stimulation study of the effect of visual orientation on the putative human mirror neuron system. Front Hum Neurosci. 2013;7:679. doi: 10.3389/fnhum.2013.00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson MT, St GL. Repetitive transcranial magnetic stimulation: a call for better data. Front Neural Circuits. 2016;10:57. doi: 10.3389/fncir.2016.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrews SC, Enticott PG, Hoy KE, Thomson RH, Fitzgerald PB. Reduced mu suppression and altered motor resonance in euthymic bipolar disorder: Evidence for a dysfunctional mirror system? Soc Neurosci. 2016;11:60–71. doi: 10.1080/17470919.2015.1029140. [DOI] [PubMed] [Google Scholar]
- 29.Kim E, Jung Y-C, Ku J, et al. Reduced activation in the mirror neuron system during a virtual social cognition task in euthymic bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1409–1416. doi: 10.1016/j.pnpbp.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry. 2002;59:347–354. doi: 10.1001/archpsyc.59.4.347. [DOI] [PubMed] [Google Scholar]
- 31.McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- 32.Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- 33.Kujirai T, Caramia MD, Rothwell JC, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strafella AP, Paus T. Modulation of cortical excitability during action-observation: a transcranial magnetic stimulation study. NeuroReport. 2000;14:2289–2292. doi: 10.1097/00001756-200007140-00044. [DOI] [PubMed] [Google Scholar]
- 35.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 36.Washington DC, editor. Diagnostic and statistical Manual of Mental Disorders. 4th edn. American Psychiatric Press: 1994. American Psychiatric Association. [Google Scholar]
- 37.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20) 22–33;quiz 34–57. [PubMed] [Google Scholar]
- 38.Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol. 2000;112:720. doi: 10.1016/s1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 39.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 40.Grossi D, Marcone R, Cinquegrana T, Gallucci M. On the differential nature of induced and incidental echolalia in autism. J Intellect Disabil ResJIDR. 2013;57:903–912. doi: 10.1111/j.1365-2788.2012.01579.x. [DOI] [PubMed] [Google Scholar]
- 41.Bush G, Fink M, Petrides G, Dowling F, Francis A, Catatonia I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93:129–136. doi: 10.1111/j.1600-0447.1996.tb09814.x. [DOI] [PubMed] [Google Scholar]
- 42.Wasserman E, Ziemann U, Epstein CM, editors. The Oxford handbook of transcranial stimulation. Oxford; NY: Oxford University Press; 2008. p. 747. Oxford handbooks series. [Google Scholar]
- 43.Enticott PG, Hoy KE, Herring SE, Johnston PJ, Daskalakis ZJ, Fitzgerald PB. Reduced motor facilitation during action-observation in schizophrenia: a mirror neuron deficit? Schizophr Res. 2008;102:116–121. doi: 10.1016/j.schres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Maeda F, Kleiner-Fisman G, Pascual-Leone A. Motor facilitation while observing hand actions: specificity of the effect and role of observer’s orientation. J Neurophysiol. 2002;87:1329–1335. doi: 10.1152/jn.00773.2000. [DOI] [PubMed] [Google Scholar]
- 45.Di Lazzaro V, Restuccia D, Oliviero A, et al. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- 46.Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143:240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- 47.Hanajima R, Furubayashi T, Iwata NK, et al. Further evidence to support different mechanisms underlying intracortical inhibition of the motor cortex. Exp Brain Res. 2003;151:427–434. doi: 10.1007/s00221-003-1455-z. [DOI] [PubMed] [Google Scholar]
- 48.Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- 49.Patuzzo S, Fiaschi A, Manganotti P. Modulation of motor cortex excitability in the left hemisphere during action-observation: a single-and paired-pulse transcranial magnetic stimulation study of self- and non-self-action-observation. Neuropsychologia. 2003;41:1272–1278. doi: 10.1016/s0028-3932(02)00293-2. [DOI] [PubMed] [Google Scholar]
- 50.Geere J, Chester R, Kale S, Jerosch-Herold C. Power grip, pinch grip, manual muscle testing or thenar atrophy - which should be assessed as a motor outcome after carpal tunnel decompression? A systematic review. BMC Musculoskelet Disord. 2007;8:114. doi: 10.1186/1471-2474-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kahan BC, Jairath V, Dore CJ, Morris TP. The risks and rewards of covariate adjustment in randomized trials: an assessment of 12 outcomes from 8 studies. Trials. 2014;15:139. doi: 10.1186/1745-6215-15-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basavaraju R, Sanjay TN, Mehta UM, Muralidharan K, Thirthalli J. Cortical inhibition in symptomatic and remitted mania compared to healthy subjects: A cross-sectional study. Bipolar Disord. 2017;19:698–703. doi: 10.1111/bdi.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehta UM, Basavaraju R, Thirthalli J. Mirror neuron activity and symptom severity in drug-naïve mania- A Transcranial Magnetic Stimulation Study. Brain Stimul. 2014;7:757–759. doi: 10.1016/j.brs.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Button KS, loannidis J, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Not Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 55.Post RM, Rubinow DR, Uhde TW, et al. Dysphoric mania. Clinical and biological correlates. Arch Gen Psychiatry. 1989;46:353–358. doi: 10.1001/archpsyc.1989.01810040059009. [DOI] [PubMed] [Google Scholar]
- 56.Ruggero CJ, Kotov R, Watson D, Kilmer JN, Perlman G, Liu K. Beyond a single index of mania symptoms: structure and validity of subdimensions. J Affect Disord. 2014;161:8–15. doi: 10.1016/j.jad.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 57.Cassidy F, Forest K, Murry E, Carroll BJ. A Factor Analysis of the Signs and Symptoms of Mania. Arch Gen Psychiatry. 1998:55–27. doi: 10.1001/archpsyc.55.1.27. [DOI] [PubMed] [Google Scholar]
- 58.Geschwind N, Quadfasel FA, Segarra J. Isolation of the speech area. Neuropsychologia. 1968;6:327–340. [Google Scholar]
- 59.Berthier ML, Torres-Prioris MJ, Lodpez-Barroso D. Thinking on treating echolalia in aphasia: recommendations and caveats for future research directions. Front Hum Neurosci. 2017;11:164. doi: 10.3389/fnhum.2017.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehta UM, Basavaraju R, Thirthalli J. Mirror neuron disinhibition may be linked with catatonic echo-phenomena: a single case TMS study. Brain Stimul. 2013;6:705–707. doi: 10.1016/j.brs.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Di Lazzaro V, Pilato F, Dileone M, et al. GABAA receptor subtype specific enhancement of inhibition inhuman motor cortex. J Physiol. 2006;575:721–726. doi: 10.1113/jphysiol.2006.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Human LF. autonomy and the frontal lobes. Part II: Patient behavior in complex and social situations: the “environmental dependency syndrome”. Ann Neurol. 1986;19:335–343. doi: 10.1002/ana.410190405. [DOI] [PubMed] [Google Scholar]
- 63.Raymond LC. Disorders of Thought Are Severe Mood Disorders: the Selective Attention Defect in Mania Challenges the Kraepelinian Dichotomy A Review. Schizophr Bull. 2007;34:109–117. doi: 10.1093/schbul/sbm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lhermitt F. Imitation and utilization behavior in major depressive states. Bull Acad Natl Med. 1993;177:883–90. discussion 890-892. [PubMed] [Google Scholar]
- 65.Abrams R, Catatonia T. A prospective clinical study. Arch Gen Psychiatry. 1976;33:579–581. doi: 10.1001/archpsyc.1976.01770050043006. [DOI] [PubMed] [Google Scholar]
- 66.Ganos C, Ogrzal T, Schnitzler A, Munchau A. The pathophysiology of echopraxia/echolalia: Relevance to Gilles De La Tourette syndrome. Mov Disord. 2012;27:1222–1229. doi: 10.1002/mds.25103. [DOI] [PubMed] [Google Scholar]
- 67.Archibald S. Physical Overactivity in Children with ADHD: Utilization Behavior. Victoria: BC, Canada; University of Victoria: 2001. [Doctoral Dissertation,] [Google Scholar]
- 68.Spengler S, Bird G, Brass M. Hyperimitation of Actions Is Related to Reduced Understanding of Others' Minds in Autism Spectrum Conditions. Biol Psychiatry. 2010;68:1148–1155. doi: 10.1016/j.biopsych.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 69.Cross KA, lacoboni M. Neural systems for preparatory control of imitation. Philos Trans R Soc B Biol Sci. 2014;369 doi: 10.1098/rstb.2013.0176. 20130176-20130176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 71.Foss-Feig JH, McPartland JC, Anticevic A, Wolf J. Re-conceptualizing ASD Within a Dimensional Framework: Positive, Negative, and Cognitive Feature Clusters. J Autism Dev Disord. 2016 Jan;46:342–351. doi: 10.1007/s10803-015-2539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strube W, Bunse T, Nitsche MA, et al. Smoking restores impaired LTD-like plasticity in schizophrenia: a transcranial direct current stimulation study. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2015;40:822–830. doi: 10.1038/npp.2014.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cook R, Bird G, Catmur C, Press C, Heyes C. Mirror neurons: From origin to function. Behav Brain Sci. 2014;37:177–192. doi: 10.1017/S0140525X13000903. [DOI] [PubMed] [Google Scholar]
- 74.Ziemann U, Reis J, Schwenkreis P, et al. TMS and drugs revisited 2014. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2015;126:1847–1868. doi: 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found online in the Supporting Information section at the end of the article.