Abstract
Cocaine is a naturally occurring and illicitly used psychostimulant drug. Cocaine acts at monoaminergic neurotransmitter transporters to block uptake of the monoamines, dopamine, serotonin and norepinephrine. The resulting increase of monoamines in the extracellular space underlies the positively reinforcing effects that cocaine users seek. In turn, this increase in monoamines underlies the development of addiction, and can also result in a number of severe side effects. Currently, cocaine is one of the most common illicit drugs available on the European market. However, cocaine is increasingly sold in impure forms. This trend is driven by cocaine dealers seeking to increase their profit margin by mixing (“cutting”) cocaine with numerous other compounds (“adulterants”). Importantly, these undeclared compounds put cocaine consumers at risk, because consumers are not aware of the additional potential threats to their health. This review describes adulterants that have been identified in cocaine sold on the street market. Their typical pharmacological profile and possible reasons why these compounds can be used as cutting agents will be discussed. Since a subset of these adulterants has been found to exert effects similar to cocaine itself, we will discuss levamisole, the most frequently used cocaine cutting agent today, and its metabolite aminorex.
Keywords: Levamisole, Aminorex, Neurotransmitter transporter, Cocaine, Adulterant
1. Introduction
Cocaine is the most commonly used psychostimulant drug in Europe, however since 2010 its abuse has been steadily rising (EMCDDA, 2016, UNODC, 2016).1 Cocaine is an alkaloid (Fig. 1) which is synthesized by and stored in the leaves of Erythroxylum coca to fend off insects and herbivores; the plant is native to South America, Mexico, the West Indies, and Indonesia (Goldstein et al., 2009). Cocaine use by humans has an interesting history. Ancient cultures used coca leaves not only for ceremonies and religious reasons, but also for the local anesthetic properties of cocaine (Jay, 2015). After the Spanish conquistadores invaded South America, they soon found out about cocaine and its “mind-altering” and aversive effects, and immediately banned its use (Goerig and van Zundert, 2012). However, cocaine’s beneficial effects were not discovered until much later, 1857–1859, when the Austrian frigate, Novara, sailed around the globe under the flag of the Austro-Hungarian empire, and the command of Commodore von Wüllerstorf-Urbair. Among a number of other scientifically important findings resulting from this expedition, a considerable amount of coca shrubs were brought, first to Triest, the sea harbor of the empire, and subsequently to Vienna. Some of the coca plants were then sent to Würzburg, Deutsches Reich, where a young scientist called Albert Niemann achieved the extraction of cocaine as part of his thesis work (Niemann, 1860). It took until the 1880s for the medical and psychoactive effects of cocaine to be widely recognized by Western medicine (Markel, 2011). The local anesthetic and psychoactive effects of cocaine were discovered by two Viennese scientists, the pharmacologist and physiologist Sigmund Freud, and the ophthalmologist, Karl Koller, respectively (Freud, 1885; Koller, 1884). Importantly, Koller, was the first to realize that cocaine would revolutionize cataract surgeries, which at that time were performed with the aide of a red-hot needle (Koller-Becker, 1963). The subsequent development of local anesthesia and improved local anesthetics revolutionized not only ophthalmology but medicine in general (Markel, 2011). However, cocaine’s use as a recreational drug, and increasingly abused drug, has become a major public health concern.
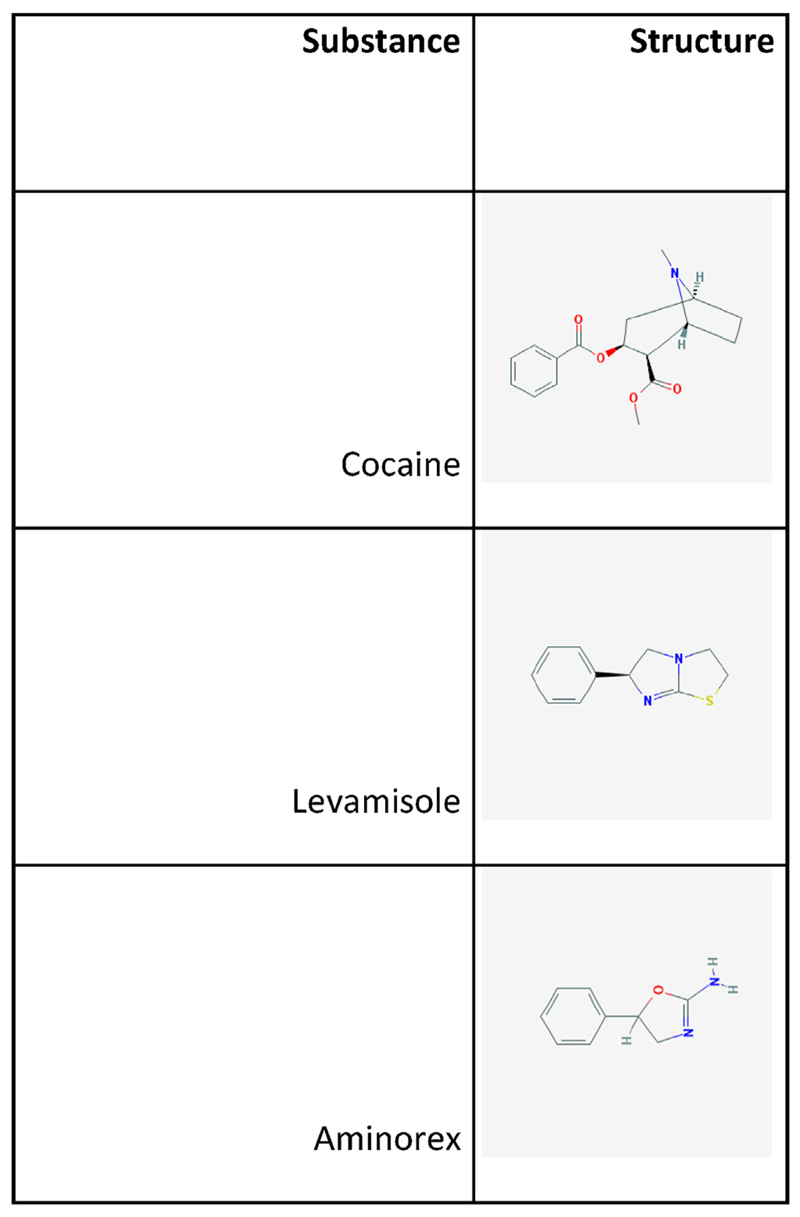
Fig. 1.
Structures of the three main compounds described in this article: cocaine, levamisole and aminorex. All structures have been retrieved from Pubchem (URL: http://pubchem.ncbi.nlm.nih.gov/).
This review focuses on cocaine as a recreational drug. Two distinct chemical forms of cocaine are typically abused: on the one hand, the water-soluble hydrochloride salt, and on the other hand, the water-insoluble cocaine base (“freebase”). The drug user typically snorts the hydrochloride salt, which is a powder, but it can also be readily dissolved and injected. Cocaine base is produced by “cooking” the extracted compound with ammonia or sodium bicarbonate (“baking soda”) and water to neutralize the hydrochloride and thereby produce the deprotonated species of cocaine, which can be smoked in vaporized form. Freebase cocaine is also sold under the street name “crack”, which stems from the crackling sound emerging during the burning of the mixture during the smoking procedure.
The main targets for cocaine are transporters for the neurotransmitters dopamine (DA), norepinephrine (NE) and serotonin (5HT). These belong to the Na+/Cl -neurotransmitter family of transporters (Kristensen et al., 2011). The substrate is eponymous for these transporters, naming them the serotonin transporter (SERT) for 5HT, the dopamine transporter (DAT) for DA and the norepinephrine transporter (NET) for NE. Each transporter is composed of 12 membrane spanning domains with their N- and C- terminals facing the intracellular side. Their function in the central nervous system is to clear the extracellular space of released neurotransmitters, thereby terminating signaling (Kristensen et al., 2011). Blockade of these transporters results in a prolonged monoamine signal at the corresponding pre- and post-synaptic receptors. Therefore, drugs affecting these transporters are used for clinical, but also for recreational purposes. Cocaine and similar compounds exert their pharmacological effects by simply blocking the transporters. Amphetamines on the other hand compete with the physiological substrate for uptake and induce non-exocytotic neurotransmitter release (Sitte and Freissmuth, 2015). While amphetamines and cocaine were synthesized or extracted, respectively, in the 19th century, many more psychostimulant drugs have appeared over the years. Nowadays, it is rather straightforward for talented chemists to synthesize new psychostimulant drugs (Baumann et al., 2013a). Recently, many of these so called “new psychoactive substances” (NPS) have flooded the illicit drug market. This creates a challenge for legal authorities because drug control legislation must keep up with the rapidly increasing number of new drugs. Chemists continue to synthesize new drugs to stay one step ahead of law enforcement. Sold under innocent sounding names like “bath salts” or “plant food”, this group consists of compounds similar to amphetamine and cathinones (Baumann et al., 2013b), but also hallucinogenic compounds (e.g. 2-CB (2,5-dimethoxy-4-bromophenethylamine), a partial agonist at 5HT-2A receptors) and cannabimimetic drugs (Baumann et al., 2013). However, cathinones and phenethylamines are the major types of drugs found on the European NPS market (Tyrkko et al., 2016). The expected effects and the tolerated side effects of the “old drugs”, like cocaine and amphetamines, are well known, but this does not hold true for new compounds. Deleterious dose-dependent side effects of substances like 4-methyl-N-methylca-thinone (mephedrone) or 3,4-methylenedioxypyrrovalerone (MDPV) (Dargan et al., 2011) are often unknown to users, and therefore pose unexpected risks of toxic effects (Meyer, 2016). Another risk for consumers results from the fact that consumers do not know the composition of the product and the concentration of the ingredients when buying drugs on the illicit street market (Rosenauer et al., 2013).
In Austria, the ‘checkIt!’ addiction prevention programme2 allows drug consumers to have their purchased substances analyzed anonymously and free of cost. This project has been implemented by the city of Vienna. A mobile analytical laboratory is brought to music events where customers can have their drugs analyzed. This analysis provides the mostly young, very often for the first time drug users, with information on the content of their drugs prior to use. This can be an eye-opening experience, which increases their awareness of unexpected risks encountered when buying drugs on the illicit market. To the big surprise of many customers, the drug they thought was pure contains not only a mixture a psychoactive substances in highly variable amounts, but also compounds that (at least at a first glance) are not expected to exert any psychostimulant effect. In the latter case, the purchaser is now made aware that they are not getting their “money’s worth”, 1. e. by paying for inclusion of compounds that will not give them the “high” they seek, rather than an equivalent amount of pure drug, in its “uncut” form. In recent publications we (Hofmaier et al., 2014) and others (Broseus et al., 2015; Lapachinske et al., 2015; Schneider and Meys, 2011) described some of these “adulterant” substances. These papers discuss why these substances, in particular, were used. We also addressed the additional health risks arising from recreational drug combinations. In addition, systematic surveys, which track the chemical profile of samples collected during drug seizures, provide useful information to analyze the structure and organization of illicit drug markets (Broseus et al., 2016). It is evident that the forensic intelligence gathered by this systematic approach reflects the supply side of the market. In contrast, the knowledge-seeking behavior of volunteers, who partake in the Viennese ‘checkIt!’ programme, offers a biased view into the effective demand of self-selected consumers (Hofmaier et al., 2014).
2. Adulterants in drug samples
In the years 2012 to 2015, 524 drug samples that were purchased as ‘cocaine’ were analyzed by the ‘checkIt!’ programme. Only about 10% of these samples (in numbers: 54) contained cocaine as the only active ingredient. On the other hand, 5% (25) did not contain cocaine at all. The remaining 445 samples consisted of cocaine plus 24 different substances used to ‘cut’ and therefore dilute the cocaine samples, as revealed by HPLC–MS analysis. These substances include paracetamol (=acetaminophen), phenacetine, caffeine, lidocaine and in almost 70% of the samples levamisole (363 out of 524 samples). In a pan-European study (Trans European Drug Information, TEDI), levamisole was clearly the most commonly found adulterant (Brunt et al., 2017). The amount and ratio of levamisole to cocaine was highly variable in the analyzed samples. While some of the samples contained only traces of levamisole (less than 1%), in others, there was even a higher amount of levamisole than cocaine (up to 1.25 times more). On average ‘cocaine’ samples contained 49.7% cocaine and 7.9% levamisole. It is known that users consume more and more of their drugs until the desired effect kicks in (Cole et al., 2011); therefore, it is likely that the more the cocaine is diluted, the more adulterant is – and must be – consumed.
While the Austrian sample was retrieved from drug users, who anonymously donated part of their material, other samples were partially based on seizures performed by police and other law enforcement agencies: for instance, a large study, performed in Switzerland, including 9 years of sample-collection was recently published by Broseus and colleagues (Broseus et al., 2015). These authors evaluated the evolution of ‘cutting agents’ found in heroin and cocaine samples. Interestingly, the median purity of cocaine showed a fluctuation over the years examined, but remained between 30% and 40% (Broseus et al., 2015). These values are somewhat lower than the values obtained from seizures in Luxembourg, which fluctuated between 43% and 54% (Schneider and Meys, 2011) and are comparable to the Austrian values. Importantly, levamisole was also found in increasing quantities in these surveys, and in a study examining samples from seizures in Brazil, which also reported the same adulterant compounds (Lapachinske et al., 2015). Of note, the Swiss study also highlights that less than approximately 10% of all samples contained only the ‘cutting agents’ and no cocaine at all; this is certainly more than was observed in Austria (~5%, see above). The ‘cutting agents’, which were used to adulterate cocaine in the Swiss study, were phenacetin, levamisole, lidocaine, caffeine, diltiazem, hydroxyzine, procaine, tetracaine, paracetamol, creatine and benzocaine.
3. Pharmacological mode of action of adulterants and the rationale for their use
When examining the properties of the various adulterants, it becomes clear that the rationale for their addition to the ‘main’ ingredient cocaine is multi-faceted. The simplest reason is to increase the bulk by diluting the expensive drug with inexpensive ones, thereby increasing profit margins of the drug dealing enterprise. However, this explanation does not address the question of why certain “adulterant” compounds are found at such high frequency. The choice is guided by the consideration that the adulterant should not be obvious to the consumer. In other words, as Broseus pointed out, a compound is useful if it is a “strategic” adulterant, i.e. it enhances or mimics the effects of illicit drugs, like cocaine. Accordingly, local anesthetics are a logical choice to adulterate of cocaine (Broseus et al., 2015). Even the customer with limited chemical knowledge can perform simple tests to check the “purity” of their purchase. For example, the well-known local anesthetic property of cocaine is easily detectable. In the so-called tongue test, small amounts of cocaine are added to the gingivolabial tissue, which results in a numb feeling due to blockade of nervous signal transmission. However, when cocaine is adulterated with local anesthetics, the impure nature of the consumer’s purchase escapes detection. In addition, adulterants are selected so as to mimic the well-known bitter taste of cocaine. Likewise, another simple test for the customer is to melt a small amount of the purchased drug. Pure cocaine hydrochloride (which is the most common form that can be found in the market) will melt at once In the case of adulterated mixes they will melt at a different time points, which is easily detected. Therefore, an adulterant should have a comparable melting point to make it indistinguishable from cocaine. Finally, certain compounds may also be more or less available, and even the illicit drug market follows the usual general principles of all markets: the higher the availability at a given time the more will be used/consumed (for example, lidocaine described by Cole and colleagues (Cole et al., 2011)).
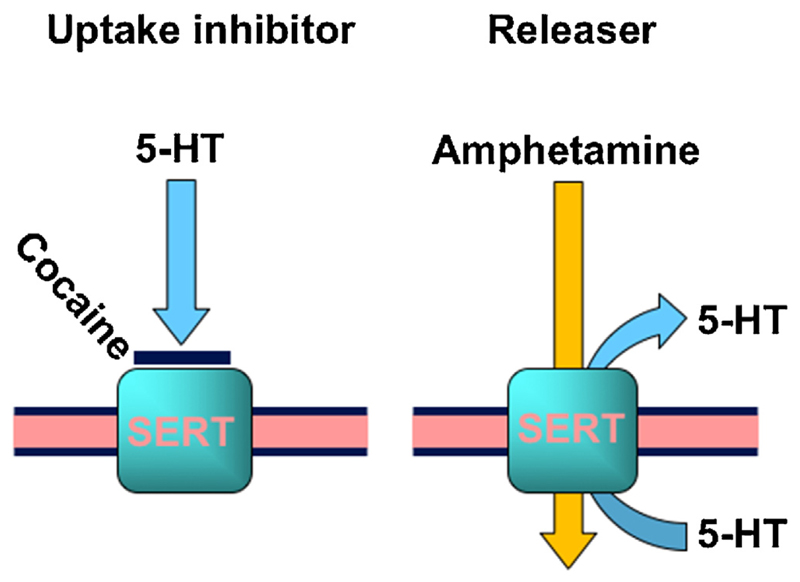
In addition to local anesthetics, other compounds such as amphetamines, synthetic cathinones, and analgesics, as well as substances like caffeine, hydroxizine and the anthelmintic drug levamisole have been found mixed with cocaine. Among these compounds, amphetamines and synthetic cathinones target the same monoamine transporters as cocaine itself. However, they have a different mode of action. They are substrates of monoamine transporters. Once inside the neuron, amphetamine and amphetamine-like compounds induce transporter-mediated release of monoamines, in contrast to cocaine, which non-competitively blocks the uptake of monoamines via these transporters (Fig. 2). Clearly, mixing an uptake blocker (e.g. cocaine) with a substrate (e.g. amphetamine) appears counterintuitive (Sitte and Freiss-muth, 2015). Levamisole is, as already mentioned, the most widely found adulterant in cocaine samples in Austria (Hofmaier et al., 2014), but also increasingly used in other countries (Broseus et al., 2015). In the following sections, we will briefly explain the rationale for typical individual cocaine adulterants, and then focus on levamisole, which is either the regionally most popular adulterant or on its way to number 1; this is a source of concern, because levamisole can cause dangerous side effects such as agranulocytosis (Indorato et al., 2016; Zhu et al., 2009).
Fig. 2. A simple illustration of uptake inhibition versus release.
On the left side, uptake inhibition by the prototypical, non-selective blocker cocaine is shown. The right side shows the releaser amphetamine which needs to be taken up to exert its releasing activity. In both cases, the illustration shows the serotonin transporter (SERT) and its cognate neurotransmitter serotonin, abbreviated as 5-HT.
3.1. Local anesthetics
Although local anesthetics have been shown to act at monoamine transporters in a way similar to cocaine (Sato et al., 2000), the potency of local anesthetics at monoamine transporters is too low to explain why these are used as ‘cutting agents’. It is more likely that the local anesthetic property of cocaine is the underlying rationale. Like other local anesthetics cocaine blocks voltage-gated sodium channels. Therefore, addition of local anesthetics, e.g. lidocaine, procaine or tetracaine, simulates higher concentrations of cocaine, and fools the customer, who relies on the tongue test to gauge the quality of his/her purchase.
3.2. Amphetamine and congeners
Cocaine increases the concentration of monoamines in the extracellular space by blocking their uptake via DAT, NET and SERT. Through competition at the same transporters, amphetamines can also inhibit the uptake of monoamines, however, amphetamines induce reverse transport of monoamines by DAT, NET and SERT, which results in an efflux of neurotransmitters (Sitte and Freissmuth, 2015). Therefore, amphetamines are not only used in medical therapy (e.g., for narcoleptic patients or patients with attention deficit hyperactivity disorder), but also have a long history of being used as illicit ‘recreational’ drugs (e.g., speed and methylene-dioxy-methamphetamine or ‘Ecstasy’). Nevertheless, with respect to cocaine, it is difficult to conceive why amphetamines should be used as adulterants because their action at monoamine transporters is inhibited by the concomitant presence of cocaine. However, (i) most amphetamines have a (substantially) longer half-life than cocaine, and (ii) analysis of behavioral data suggests that these drugs are more reinforcing when given together, compared to when given alone (Li et al., 2006).
3.3. Synthetic cathinones
At the beginning of this century, rogue chemists tried to avoid legislative restriction on psychoactive substances by synthesizing compounds that were not yet classified as illicit drugs (Rothman and Baumann, 2002). Synthetic cathinones were found among these, so-called “designer drugs” or, more accurately, “new psychoactive substances” (NPS). Cathinones are a class of compounds that stem from the naturally occurring cathinone (also called b-ketoamphetamine). They differ from the amphetamine-type psychostimulants by the functional keto-group. Cathinone itself is found in the African shrub ‘Catha edulis’ and cultivated in countries close to the Horn of Africa including Yemen; people in these countries chew the fresh cathinone-containing Cath-leaves as a recreational drug. The chemical modification or synthesis of cathinone-congeners is straightforward. Thus, nowadays, several different compounds are available. Their mechanism of action is very similar to amphetamines, leading to increased levels of monoamines in the synaptic cleft. Synthetic cathinones comprise the so-called first (e.g. mephedrone, methylone (Baumann et al., 2012), MPDV (Baumann et al., 2013b)) and second generation compounds (e.g. 4-MEC, see (Saha et al., 2015)). Their straightforward synthesis, and the fact that cathinones were legal for a long time, make them ideal adulterants. Considering the current model of psychostimulant action, co-administration of a releaser and a blocker is anticipated to reduce the efficacy of both by mutual competition, but again half-lives differ and this may be more important in the market place (see above for amphetamine and congeners).
3.4. Analgesics
While the substances mentioned above exert a psychostimulant effect by themselves, this is not the case for analgesic drugs, like acetyl-salicylic acid, paracetamol or phenacetine. Although there are reports mentioning a limited euphoric potency of paracetamol and its parent compound phenacetine, it seems unlikely that this is strong enough to qualify these drugs as ideal adulterants. For acetyl-salicylic acid, not even small euphoric effects have been reported. In all cases, it seems more plausible that these legal, over-the-counter medicines, are used to dilute cocaine because of their bitter taste or comparable chemical properties such as their similar melting points.
3.5. Caffeine
Caffeine is the most popular psychoactive compound worldwide. The known targets for caffeine are adenosine receptors (reviewed in (Fredholm et al.,1999)).The concentrations normally reached within the body are high enough to elicit physiologically relevant effects. In addition, caffeine is a low affinity non-selective inhibitor of cyclic nucleotide phosphodiesterases, and thus raises agonist-induced cAMP levels. This may be relevant in dopaminergic neurotransmission via D1-receptors. There are other stimulatory effects which have been proposed to be exerted via Ca2+-dependent release or inhibition of GABA-receptors; however, these effects would require concentrations in a toxic range (Fredholm et al.,1999). Most recently, several publications reported on the augmentation by caffeine of effects elicited by cocaine (Muniz et al., 2016; Prieto et al., 2016). This may explain, together with the bitter taste of caffeine, why caffeine is widely used as an adulterant.
3.6. Hydroxyzine
Hydroxyzine is found in slightly more than 1% of cocaine samples. Hydroxyzine was originally approved as an anti-allergic drug. As an H1-blocker of the first generation, hydroxyzine was also in use for the treatment of kinetosis. Hydroxyzine easily penetrates the blood-brain barrier, and blocks various dopamine and 5-HT receptors. Therefore sedative, hypnotic and anxiolytic effects are observed. Adverse effects of hydroxyzine in the central nervous system (CNS) include tinnitus, headache, and hypotension. For these reason, the hydroxyzine metabolite, cetirizine, is in clinical use as antihistaminic medicine nowadays. Even so, hydroxyzine is still approved for the treatment of allergic reactions, and as an anxiolytic medicine. Taken together, these effects do not explain why hydroxyzine should be used as a cocaine adulterant. Alternative explanations could be its mild, anti-muscarinic effects (Orzechowski et al., 2005) or more likely the fact that hydroxyzine was shown to block axonal conduction, therefore also displaying local anesthetic effects (Elliott and Quilliam, 1964).
4. Why levamisole differs from other adulterants
As outlined above, the anthelmintic drug levamisole is more and more frequently found as a cocaine adulterant. Analysis from a number of different studies, including the Austrian ‘checkIt!’-drug testing, showed that more than two thirds of all examined samples (69.3%) contained this drug (Buchanan et al., 2010; Chai et al., 2011).
We therefore analyzed its pharmacological properties to understand the reason for its preferred status as a cocaine adulterant. A survey of levamisole and its action at SERT, NET and DAT revealed that the compound exerts inhibitory activity at these transporters, but at much higher concentrations than cocaine (Hofmaier et al., 2014). Given these large differences, it was concluded that levamisole was highly unlikely to exert a significant pharmacological action on these transporters in vivo when administered in therapeutic and/or recreational doses. An orally administered amount of 50 mg of levamisole gives rise to peak blood plasma concentrations (cmax) of 368 mg/L on average (equivalent to about 1.5 μM) (Gwilt et al., 2000). Furthermore, there is a large intra-individual variation in its pharmacokinetic properties (Gwilt et al., 2000), and some uncertainty about nasal absorption, which is the most commonly used route of cocaine intake (and therefore also of the adulterant levamisole). Nevertheless, due to its lipophilic nature, levamisole can be expected to readily penetrate the blood-brain barrier leading to possibly higher concentrations of levamisole in the brain than cocaine. When consumed at excessive levels, as a consequence, this could potentially lead to enhanced and protracted inhibition of NET and DAT.
Levamisole and cocaine differ in their ability to inhibit DAT, NET and SERT (Hofmaier et al., 2014). Whereas cocaine inhibits these transporters to similar extents, levamisole is a more potent inhibitor of DAT and NET, with lesser affinity for SERT. Nevertheless, drugs like levamisole can act as cocaine-like inhibitors at transporters and trap them in their outward facing conformation, thereby precluding completion of the transport cycle. Alternatively, levamisole may act as an amphetamine-like releaser (Rothman et al., 2002), where an increase in extracellular monoamine concentration ensues the triggering of substrate efflux (Sitte and Freissmuth, 2015). However, the chemical structure of levamisole is distantly related to amphetamine (Fig. 1), and even concentrations of up to 100 mM do not induce any appreciable efflux. Based on these findings it is concluded that levamisole acts as a cocaine-like blocker without any releasing activity (Hofmaier et al., 2014).
However, it was found that levamisole is metabolised in the liver. Indeed, its metabolite, aminorex, was found in samples taken from race horses to check for doping (Barker, 2009). Aminorex, a well-known amphetamine-like compound, was not only found in race horses, but also in dogs and humans (Bertol et al., 2011; Hess et al., 2013) treated with the anthelmintic drug levamisole. Activity of aminorex was seen at DAT, NET and SERT, though it more strongly inhibited substrate uptake by NET and DAT, compared to SERT (Hofmaier et al., 2014). The pattern of inhibition (NET > DAT >> > SERT) was similar to that of the parent compound levamisole, however, the inhibitory potency of aminorex was in a range similar to cocaine. That notwithstanding, aminorex has also been shown to be an amphetamine-like, monoamine transporter substrate, and used as an appetite suppressant (akin to fenfluramine (Fen-Phen), also a substrate for monoamine transporters) (Roszkowski and Kelley, 1963; Zheng et al., 1997). In line with these reports, others have described that aminorex behaves like a substrate at SERT, and like an inhibitor at DAT, leading to increased extracellular levels of dopamine and 5-HT (Rothman et al., 1999). Yet others, using different experimental methods, report that aminorex functions as a substrate releaser via DAT, SERT and NET (Rothman et al., 2001). Upon testing the capability of aminorex to induce efflux, the compound was able to elicit release only in SERT expressing cells, and to a much lower extent in NET expressing cells. Cells that stably expressed DAT did not show any appreciable release (Hofmaier et al., 2014). In contrast, studies using rat synaptosomes as an assay system, revealed that DAT and NETcontaining synaptosomes displayed similar substrate efflux after aminorex exposure, while SERT containing synaptosomes only responded to higher concentrations of aminorex (Brandt et al., 2014; Rothman et al., 2001). The reason for this difference might be explained by differences in experimental approaches: synaptosomes versus stably expressing cell lines carrying different amounts of the human monoamine transporters. In addition, there are arguments both for and against species differences, which could be another explanation: Sealover and co-workers recently showed differences in the action of MDMA on human, drosophila and C.elegans SERT (Sealover et al., 2016), while no differences have been observed in a recent study employing rat and human DAT and SERT (Saha et al., 2015). Hence, there are multiple ways how the observed differences could be explained and thorough investigations are necessary to resolve these differences.
Regardless, these discrepancies in the literature, together with evidence that peripheral SERT activity is hampered by aminorex, and the risk for pulmonary hypertension, led to the withdrawal of aminorex from the market (Eddahibi and Adnot, 2002; Pollick, 1999). A subsequent study, using a pharmacoinformatic docking approach to understand the discrimination by aminorex against SERT, showed that the docking orientation was markedly different between SERT compared to DAT and NET (for all details, please refer to (Hofmaier et al., 2014)). This analysis provided an explanation for the cocaine-like effect of aminorex on human DAT and NET, and an amphetamine-like effect on human SERT. Therefore, it can be speculated that levamisole could potentially serve as an adulterant that extends the effects of cocaine. When the psychoactive effect of cocaine “fades out”, the aminorex-mediated effect “kicks in” due to of the longer systemic availability of aminorex.
Most all illicit drugs that are recreationally consumed are adulterated. These adulterants may not only be used to ‘cut’ (dilute) the drugs, but act as ‘cutting agents’ (enhancers), which are also pharmacologically active compounds. Sometimes the adulterants may also be used to extend the duration of the drug action, as discussed above with levamisole and its metabolite aminorex as an example. However, mostly, adulterants are used to increase the profitable margin of the drug dealer’s business. Cocaine must be available at a cheap price. The use of adulterants enables the market to profit by being undetectable to the consumer with respect to both their appearance and their chemical profile. However, the overall gain in return on investment for the drug market, may come at a considerable price to the consumer, i.e. an increased health risk and potentially life-threatening consequences. This scenario is exemplified by examples where adulterants are formerly marketed therapeutics, which have been withdrawn from the market because of discoveries revealing their serious toxicological effects. This underscores the importance of public outreach, to educate recreational drug users of the potential, serious, threats to their well-being by using drugs sold on the street market.
Acknowledgements
The work of HHS, TS and MF was supported by the Austrian Science Foundation/FWF (grant F35). The drug prevention project ‘checkiT!’ is financially supported by the Department of Addiction and Drug Coordination (SDW) of the City of Vienna, Austria. We are grateful to Dr. Lynette C. Daws for helpful comments and suggestions on the manuscript.
Footnotes
Conflict of interest
All authors declare no competing financial interests related to the publication of this paper.
References
- Barker SA. The formation of aminorex in racehorses following levamisole administration: a quantitative and chiral analysis following synthetic aminorex or levamisole administration vs. aminorex-positive samples from the field: a preliminary report. J Vet Pharmacol Ther. 2009;32:160–166. doi: 10.1111/j.1365-2885.2008.01015.x. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR. Psychoactive bath salts: not so soothing. Eur J Pharmacol. 2013a;698:1–5. doi: 10.1016/j.ejphar.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, et al. Powerful cocaine-like actions of 3,4-Methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘Bath salts’ products. Neuropsychopharmacology. 2013b;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertol E, Mari F, Milia MG, Politi L, Furlanetto S, Karch SB. Determination of aminorex in human urine samples by GC–MS after use of levamisole. J Pharm Biomed Anal. 2011;55:1186–1189. doi: 10.1016/j.jpba.2011.03.039. [DOI] [PubMed] [Google Scholar]
- Brandt SD, Baumann MH, Partilla JS, Kavanagh PV, Power JD, Talbot B, Twamley B, Mahony O, O’Brien J, Elliott SP, Archer RP, et al. Characterization of a novel and potentially lethal designer drug (+/-)-cis-para-methyl-4-methylaminorex (4,4’-DMAR, or ‘Serotoni’) Drug Test Anal. 2014;6:684–695. doi: 10.1002/dta.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broseus J, Gentile N, Bonadio Pont F, Garcia Gongora JM, Gaste L, Esseiva P. Qualitative, quantitative and temporal study of cutting agents for cocaine and heroin over 9 years. Forensic Sci Int. 2015;257:307–313. doi: 10.1016/j.forsciint.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Broseus J, Baechler S, Gentile N, Esseiva P. Chemical profiling: a tool to decipher the structure and organisation of illicit drug markets: an 8-year study in Western Switzerland. Forensic Sci Int. 2016;266:18–28. doi: 10.1016/j.forsciint.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Brunt TM, Nagy C, Bucheli A, Martins D, Ugarte M, Beduwe C, Ventura Vilamala M. Drug testing in Europe: monitoring results of the trans european drug information (TEDI) project. Drug Test Anal. 2017 Feb;9(2):188–198. doi: 10.1002/dta.1954. [DOI] [PubMed] [Google Scholar]
- Buchanan JA, Oyer RJ, Patel NR, Jacquet GA, Bornikova L, Thienelt C, Shriver DA, Shockley LW, Wilson ML, Hurlbut KM, Lavonas EJ. A confirmed case of agranulocytosis after use of cocaine contaminated with levamisole. J Med Toxicol. 2010;6:160–164. doi: 10.1007/s13181-010-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai PR, Bastan W, Machan J, Hack JB, Babu KM. Levamisole exposure and hematologic indices in cocaine users. Acad Emerg Med. 2011;18:1141–1147. doi: 10.1111/j.1553-2712.2011.01202.x. [DOI] [PubMed] [Google Scholar]
- Cole C, Jones L, McVeigh J, Kicman A, Syed Q, Bellis M. Adulterants in illicit drugs: a review of empirical evidence. Drug Test Anal. 2011;3:89–96. doi: 10.1002/dta.220. [DOI] [PubMed] [Google Scholar]
- Dargan PI, Sedefov R, Gallegos A, Wood DM. The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone) Drug Test Anal. 2011;3:454–463. doi: 10.1002/dta.312. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Adnot S. Anorexigen-induced pulmonary hypertension and the serotonin (5-HT) hypothesis: lessons for the future in pathogenesis. Respir Res. 2002;9(3) doi: 10.1186/rr181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott RC, Quilliam JP. Some actions of centrally active and other drugs on the transmission of single nerve impulses through the isolated superior cervical ganglion preparation of the rabbit. Br J Pharmacol Chemother. 1964;23:222–240. doi: 10.1111/j.1476-5381.1964.tb01582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Freud S. Über Coca. Centralblatt für die ges Therapie. 1885;2:289–314. [Google Scholar]
- Goerig MBD, van Zundert A. Carl koller, cocaine, and local anesthesia. Some less known and forgotten facts. Reg Anesth Pain Med. 2012;37:318–324. doi: 10.1097/AAP.0b013e31825051f3. [DOI] [PubMed] [Google Scholar]
- Goldstein RA, DesLauriers C, Burda AM. Cocaine: history, social implications, and toxicity–a review. Dis Mon. 2009;55:6–38. doi: 10.1016/j.disamonth.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Gwilt P, Tempero M, Kremer A, Connolly M, Ding C. Pharmacokinetics of levamisole in cancer patients treated with 5-fluorouracil. Cancer Chemother Pharmacol. 2000;45:247–251. doi: 10.1007/s002800050036. [DOI] [PubMed] [Google Scholar]
- Hess C, Ritke N, Broecker S, Madea B, Musshoff F. Metabolism of levamisole and kinetics of levamisole and aminorex in urine by means of LC-QTOF-HRMS and LC-QqQ-MS. Anal Bioanal Chem. 2013;405:4077–4088. doi: 10.1007/s00216-013-6829-x. [DOI] [PubMed] [Google Scholar]
- Hofmaier T, Luf A, Seddik A, Stockner T, Holy M, Freissmuth M, Ecker GF, Schmid R, Sitte HH, Kudlacek O. Aminorex, a metabolite of the cocaine adulterant levamisole, exerts amphetamine like actions at monoamine transporters. Neurochem Int. 2014;73:32–41. doi: 10.1016/j.neuint.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indorato F, Romano G, Barbera N. Levamisole-adulterated cocaine: two fatal case reports and evaluation of possible cocaine toxicity potentiation. Forensic Sci Int. 2016;265:103–106. doi: 10.1016/j.forsciint.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Jay M. Miracle or menace?: the arrival of cocaine 1860-1900. Int Rev Neurobiol. 2015;120:27–39. doi: 10.1016/bs.irn.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Koller C. Ueber die Verwendung des Cocain zur Anästhesierung am Auge. Wien Med Wochenschr. 1884;43:1276–1278. [Google Scholar]
- Koller-Becker H. Carl koller and cocaine. Psychoanal Q. 1963;32:309–373. [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- Lapachinske SF, Okai GG, dos Santos A, de Bairros AV, Yonamine M. Analysis of cocaine and its adulterants in drugs for international trafficking seized by the Brazilian Federal Police. Forensic Sci Int. 2015;247:48–53. doi: 10.1016/j.forsciint.2014.11.028. [DOI] [PubMed] [Google Scholar]
- Markel H. Uber coca: Sigmund Freud, Carl Koller, and cocaine. JAMA. 2011;305:1360–1361. doi: 10.1001/jama.2011.394. [DOI] [PubMed] [Google Scholar]
- Meyer MR. New psychoactive substances: an overview on recent publications on their toxicodynamics and toxicokinetics. Arch Toxicol. 2016;90:2421–2444. doi: 10.1007/s00204-016-1812-x. [DOI] [PubMed] [Google Scholar]
- Muniz JA, Gomez G, Gonzalez B, Rivero-Echeto MC, Cadet JL, Garcia-Rill E, Urbano FJ, Bisagno V. Combined effects of simultaneous exposure to caffeine and cocaine in the mouse striatum. Neurotox Res. 2016;29:525–538. doi: 10.1007/s12640-016-9601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann A. Ueber eine neue organische Base in den Cocablättern. Arch Pharm. 1860;153:129–155. [Google Scholar]
- Orzechowski RF, Currie DS, Valancius CA. Comparative anticholinergic activities of 10 histamine H1 receptor antagonists in two functional models. Eur J Pharmacol. 2005;506:257–264. doi: 10.1016/j.ejphar.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Pollick C. Aminorex to Fen/Phen: an epidemic foretold. Circulation. 1999;100:e147. doi: 10.1161/01.cir.100.25.e147. [DOI] [PubMed] [Google Scholar]
- Prieto JP, Scorza C, Serra GP, Perra V, Galvalisi M, Abin-Carriquiry JA, Piras G, Valentini V. Caffeine, a common active adulterant of cocaine, enhances the reinforcing effect of cocaine and its motivational value. Psychopharmacology (Berl) 2016;233:2879–2889. doi: 10.1007/s00213-016-4320-z. [DOI] [PubMed] [Google Scholar]
- Rosenauer R, Luf A, Holy M, Freissmuth M, Schmid R, Sitte HH. A combined approach using transporter-flux assays and mass spectrometry to examine psychostimulant street drugs of unknown content. ACS Chem Neurosci. 2013;4:182–190. doi: 10.1021/cn3001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszkowski AP, Kelley NM. A rapid method for assessing drug inhibition of feeding behavior. J Pharmacol Exp Ther. 1963;140:367–374. [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Serotonin releasing agents Neurochemical, therapeutic and adverse effects. Pharmacol Biochem Behav. 2002;71:825–836. doi: 10.1016/s0091-3057(01)00669-4. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Appetite suppressants as agonist substitution therapies for stimulant dependence. Ann N Y Acad Sci. 2002;965:109–126. doi: 10.1111/j.1749-6632.2002.tb04155.x. [DOI] [PubMed] [Google Scholar]
- Saha K, Partilla JS, Lehner KR, Seddik A, Stockner T, Holy M, Sandtner W, Ecker GF, Sitte HH, Baumann MH. Second-Generation’ mephedrone analogs, 4-MEC and 4-MePPP, differentially affect monoamine transporter function. Neuropsychopharmacology. 2015;40:1321–1331. doi: 10.1038/npp.2014.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Kitayama S, Morita K, Ikeda T, Dohi T. Changes in seizure susceptibility to local anesthetics by repeated administration of cocaine and nomifensine but not GBR12935: possible involvement of noradrenergic system. Jpn J Pharmacol. 2000;83:265–268. doi: 10.1254/jjp.83.265. [DOI] [PubMed] [Google Scholar]
- Schneider S, Meys F. Analysis of illicit cocaine and heroin samples seized in Luxembourg from 2005 to 2010. Forensic Sci Int. 2011;212:242–246. doi: 10.1016/j.forsciint.2011.06.027. [DOI] [PubMed] [Google Scholar]
- Sealover NR, Felts B, Kuntz CP, Jarrard RE, Hockerman GH, Barker EL, Henry LK. The external gate of the human and Drosophila serotonin transporters requires a basic/acidic amino acid pair for 3,4-methylenedioxymethamphetamine (MDMA) translocation and the induction of substrate efflux. Biochem Pharmacol. 2016;120:46–55. doi: 10.1016/j.bcp.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitte HH, Freissmuth M. Amphetamines, new psychoactive drugs and the monoamine transporter cycle. Trends Pharmacol Sci. 2015;36:41–50. doi: 10.1016/j.tips.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrkko E, Andersson M, Kronstrand R. The toxicology of new psychoactive substances: synthetic cathinones and phenylethylamines. Ther Drug Monit. 2016;38:190–216. doi: 10.1097/FTD.0000000000000263. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Russell B, Schmierer D, Laverty R. The effects of aminorex and related compounds on brain monoamines and metabolites in CBA mice. J Pharm Pharmacol. 1997;49:89–96. doi: 10.1111/j.2042-7158.1997.tb06758.x. [DOI] [PubMed] [Google Scholar]
- Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287–289. doi: 10.7326/0003-4819-150-4-200902170-00102. [DOI] [PubMed] [Google Scholar]